Abstract
Fetal neurodevelopment in utero is profoundly shaped by both systemic maternal immunity and local processes at the maternal–fetal interface. Immune pathways are a critical participant in the normal physiology of pregnancy and perturbations of maternal immunity due to infections during this period have been increasingly linked to a diverse array of poor neurological outcomes, including diseases that manifest much later in postnatal life. While experimental models of maternal immune activation (MIA) have provided groundbreaking characterizations of the maternal pathways underlying pathogenesis, less commonly examined are the immune factors that serve pathogen-independent developmental functions in the embryo and fetus. In this review, we explore what is known about the in vivo role of immune factors in fetal neurodevelopment during normal pregnancy and provide an overview of how MIA perturbs the proper orchestration of this sequence of events. Finally, we discuss how the dysregulation of immune factors may contribute to the manifestation of a variety of neurological disorders.
Keywords: pregnancy, immune system, cytokines, complement, neurodevelopment, maternal immune activation
1. INTRODUCTION
The maternal immune system has been increasingly recognized as a critical participant in the processes that propel embryonic and fetal development (PrabhuDas et al. 2015). Long mischaracterized as a host-versus-graft response, the rich immunological landscape of the maternal–fetal interface facilitates the establishment and support of the conceptus throughout all stages of pregnancy, from the priming of the uterus for blastocyst implantation to the promotion of immune tolerance to the initiation of parturition (Mor et al. 2017).
In parallel to immune pathways in the mother, the fetus itself is now recognized to express a diverse array of immunological factors from the earliest stages of development. The expression of Toll-like receptors (TLRs), cytokines, and other molecules with important functions in immunity can be detected in both embryonic and extraembryonic tissues with distinct spatial and temporal patterns of expression (Abrahams & Mor 2005, Coulthard et al. 2018b). These observations suggest a role for various immune pathways in normal development; however, a functional understanding of these contributions and their relative importance is only recently emerging.
The complexity of studying these maternal and fetal microenvironments is compounded not only by their adjacency and interactions but also by the rapid developmental changes taking place on each side. Nevertheless, epidemiological and experimental studies have together demonstrated that infections and inflammation during pregnancy can lead to poor maternal and fetal outcomes (Knuesel et al. 2014, Yockey & Iwasaki 2018), with many pathologies being traced to cross talk at or across the interface.
Early interest in the relationship between maternal immunity and fetal neurodevelopment can be traced to the ever-growing body of epidemiological studies linking infections during pregnancy to neurological and psychiatric diseases in offspring. The 1964 rubella epidemic in the United States produced multiple studies that found an extremely high rate of autism in children with known in utero exposure (Chess 1971). Interestingly, these pioneering studies document a range of coinciding neurological abnormalities in rubella-affected neonates including seizures, cerebral palsy (CP), and microcephaly (Desmond et al. 1967).
Fittingly, the first categorical descriptions of TORCH [Toxoplasma gondii, others (including Treponema pallidum, Listeria monocytogenes, varicella-zoster virus, and parvovirus B19), rubella virus, cytomegalovirus, and herpes simplex virus] pathogens and their shared clinical sequelae were also released during this time (Nahmias et al. 1971), with central nervous system (CNS) abnormalities notably documented as the most frequent presentation of in utero TORCH infections. The introduction of the term TORCH into the lexicon formally canonized the common observation that a diverse collection of pathogens can elicit similar fetal pathologies.
Numerous additional studies have since linked public health outbreaks of other infectious diseases (e.g., influenza and measles) to subsequent increases in the incidence of neuropsychiatric disorders. Concurrently, the number of pathogens within the TORCH classification known to cause congenital neurological anomalies continues to rise. The most obvious example from the recent decade is the Zika virus (ZIKV) epidemic that swept the Americas from 2015 to 2016 and captured a high degree of public attention due to its dramatic association with microcephaly. While some features of infection are specific to the virus, ZIKV shares remarkable similarities with other TORCH pathogens in terms of its biology and the spectrum of congenital defects displayed following in utero exposure (Coyne & Lazear 2016).
Indeed, the dizzying collection of diverse pathogens capable of producing neurodevelopmental disease, whether autism spectrum disorder (ASD) or microcephaly, is puzzling when each pathogen is considered individually. Advances in our understanding of both sides of the maternal–fetal interface have coalesced into new breakthroughs that instead shift focus onto a conserved component of these wide-ranging insults: the maternal immune system.
Maternal immune activation (MIA; see the sidebar titled Maternal Immune Activation) has now been implicated in a broad range of distinct disorders in offspring, and an outsized proportion of these are, strikingly, brain pathologies long suspected to have developmental origins (Estes & McAllister 2016, Knuesel et al. 2014). The capacity of pathogens to induce MIA during pregnancy suggests a pathway to developmental disease distinct from that of direct infection and damage. These emerging principles can not only be applied to TORCH infections such as ZIKV but also can more widely inform an assessment of the risks posed by the current novel coronavirus (COVID-19) pandemic and future pathogens. While the potential for vertical transmission remains an important question for any novel pathogen, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), studies of MIA suggest that systemic perturbations of maternal immunity can be detrimental to the fetus even without transfer of the infection across the maternal–fetal interface.
Given that the foundations of brain development are established in utero and that the complexity of these processes render them uniquely sensitive to disruption, it is not surprising that the pathological impacts of MIA are overrepresented by brain disorders. Nonetheless, the sheer diversity of neurological manifestations in response to this spectrum of insults is intriguing. In this review, we discuss the prenatal immunology shaping neurodevelopment with a specific focus on fetal immune pathways during normal brain development in utero and the role of maternal immunity in driving risk for various neurological diseases. We highlight a multidimensional range of work suggesting that mechanisms of maternal and fetal immunity play a more active role in promoting neurodevelopment throughout all stages of pregnancy than previously appreciated, and that disruptions in the orchestration of these processes can prime the brain for abnormalities not just perinatally but also potentially well into adulthood. This emerging understanding allows us to create a more inclusive and broadly applicable framework of the immunological contributions to early life that can be used to approach the assessment, prevention, and treatment of complex neurodevelopmental disorders that arise in utero.
2. OVERVIEW OF IMMUNE MEDIATORS AT THE MATERNAL–FETAL INTERFACE
The maternal–fetal interface is centrally composed of the decidua of the maternal uterus and the extraembryonic tissues of the developing fetus (Ander et al. 2019, Erlebacher 2013). Formation of the interface is initiated with blastocyst invasion of the uterine mucosa, during which the endometrial lining of the maternal uterus undergoes a dramatic remodeling process called decidualization (see the sidebar titled Decidualization) that is marked by a significant influx of immune cells into the environment (Ander et al. 2019). Other maternal cell types such as decidual stromal and endothelial cells are also being established and expanded during this time (Erlebacher 2013).
The immunological renovation establishing the maternal–fetal interface is not confined to the maternal tissues, however; fetal trophoblasts invading the maternal tissue are also potent immune signalers. Later in pregnancy, these trophoblasts form the placenta, where early fetus-derived immune populations from the yolk sac also reside. Together, the interactions of these maternal and extraembryonic tissues dictate immunological communication across the interface.
Extensive overviews of the maternal–fetal interface, including conserved and divergent features between mouse and humans, have been discussed elsewhere (Erlebacher 2013, Georgiades et al. 2002, Mor et al. 2017). Here, we briefly discuss some of the major immune players in the maternal decidua and in the extraembryonic fetal compartments most relevant to this review.
2.1. Maternal Decidualization and Immune Cell Subsets
From the first stages of pregnancy, the maternal decidua is characterized by a remarkable proportion of leukocytes. Following decidualization, leukocytes ultimately comprise 30–40% of all cells in the decidua in the first trimester (Bulmer et al. 1991, 2010). Natural killer (NK) cells predominate in early pregnancy, comprising approximately 70% of leukocytes in the decidua basalis (Erlebacher 2013). Macrophages make up roughly 20% of the population, with T cell numbers varying from around 3% to 10%; as in other tissues, dendritic cells are relatively infrequent, comprising 1–2% in the maternal decidua during pregnancy (Liu et al. 2017, Mor et al. 2017). After the first trimester, these proportions shift dramatically, with NK cell and macrophage numbers decreasing and T cells becoming the predominant immune cell subset by the end of the third trimester (Bulmer et al. 1991, Williams et al. 2009).
Importantly, cytokines play a critical role in the infiltration and development of these populations at the maternal–fetal interface. Nonleukocytes such as stromal and endothelial cells in the decidua also directly contribute to this regulation through the release of cytokines and chemokines (for more on this subject, see Erlebacher 2013).
2.2. Fetal Trophoblasts and Macrophages
The fetus is typically regarded as highly vulnerable to infections in utero due to the absence or immaturity of the prenatal immune system. However, a deeper examination of the fetal immune system during pregnancy paints a more complex picture than mere deficiency. Although commonly overlooked, leukocytes derived from both fetal and extraembryonic structures are present and display a surprising degree of maturity and functionality prior to birth (Bulmer et al. 2010, Davies et al. 1992, Holt & Jones 2000, Rechavi et al. 2015).
Indeed, a significant body of recent work has emphasized the critical importance of fetal microglia (see the sidebar titled Microglia) in shaping neurodevelopment both during and after pregnancy. In addition, placental macrophages (also known as Hofbauer cells in humans; see the sidebar titled Hofbauer Cells: Placental Macrophages) play a significant role in immune regulation and disease pathogenesis, particularly during infections and inflammatory processes.
A central source of immune signaling also stems from nonhematopoietic cell types. Trophoblasts serve as both immune sensors and signal propagators long before the formation of the definitive placenta, which is itself now considered an immune organ. A comprehensive list can be found elsewhere (Abrahams & Mor 2005, Bowen et al. 2002), but virtually all known TLRs and cytokines are expressed in placental tissues throughout gestation. Trophoblasts secrete numerous factors, including cytokines, complement factors, and exosomes, that establish distinct spatial and temporal patterns of immunity at the maternal–fetal interface (Bulla et al. 2009, Delorme-Axford et al. 2013, Hsi et al. 1991, Saito 2000). They confer protection against pathogens even to nontrophoblast cells in the local environment (Bayer et al. 2015, 2016; Delorme-Axford et al. 2013; Stefanski et al. 2019) and modulate the migration and function of maternal leukocytes (Erlebacher 2013). In addition to these baseline functions in pregnancy, trophoblasts also exhibit differential responses to immune stimulation in a pathogen-associated molecular pattern (PAMP)-specific manner (Abrahams et al. 2004, 2005, 2006).
While the hormonal and physiological functions of the placenta are well known to impact fetal development, fewer studies have explored the importance of converging immune signals at the placenta. By facilitating maternal–fetal cross talk, the placenta is a key crossroads capable of directly impacting fetal neurodevelopmental outcomes, as further described in Section 4.
3. FETAL IMMUNE PATHWAYS IN NORMAL NEURODEVELOPMENT
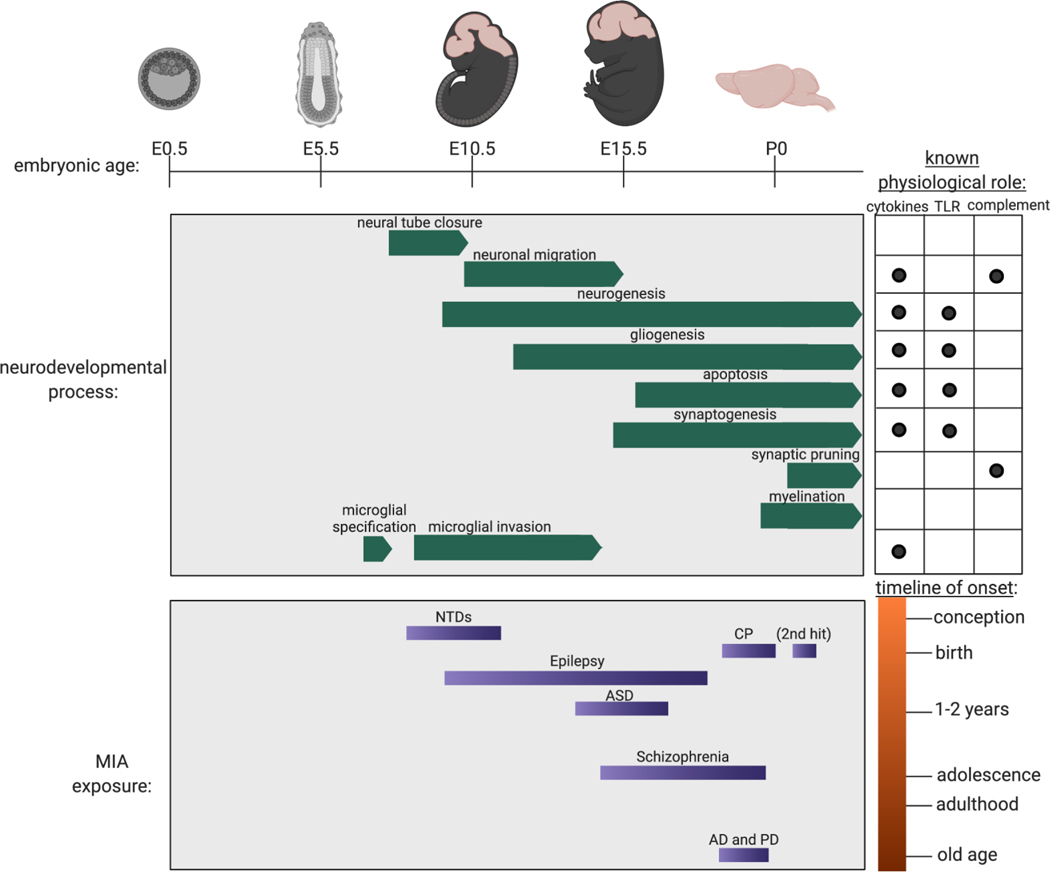
A striking but underappreciated aspect of prenatal brain development is the regulated expression of numerous genes associated with immune signaling (Figure 1). The fragmented representation of immunity-associated pathways can be detected from the blastocyst stage onward in distinct spatiotemporal patterns and is highly conserved even in oviparous model organisms such as Xenopus and zebrafish. Why are these classic immunological factors expressed so early in development without clear correlates to their adult functions? Although the expression of some factors may confer intrinsic immune protection, others are clearly not involved in pathogen defense; their pleiotropic roles in fetal development are apparent particularly at the CNS (Supplemental Table 1). Here we review the fetal expression of immune pathways in relation to neurodevelopment in utero and explore potential physiological functions.
Figure 1.
Fetal neurodevelopment and MIA throughout the timeline of murine pregnancy. Gestational age is denoted by embryonic day (E) starting at E0.5, designated as the morning that the seminal plug is observed. Postnatal day (P) 0 is designated as the day of birth. Immune pathways are known to participate in normal physiological processes of brain formation and development. The timing, character, and intensity of MIA relative to the timing of development dictate fetal vulnerability to a spectrum of neurological diseases. Abbreviations: AD, Alzheimer’s disease; ASD, autism spectrum disorder; CP, cerebral palsy; MIA, maternal immune activation; NTD, neural tube defect; PD, Parkinson’s disease; TLR, Toll-like receptor. Figure adapted from images created with Biorender.com.
3.1. Physiological Role of Toll-Like Receptors in Normal Brain Development
Originally discovered in Drosophila, the Toll gene was long known to dictate dorsal–ventral patterning during development before it was later demonstrated to play a role in antimicrobial defense (Hoffmann 2003). Akin to this latter functionality, the mammalian homologs of Toll, the TLRs, play a critical role in host recognition of microbial signatures or PAMPs, and their importance in innate immunity has been well characterized.
Far less explored, however, is the involvement and function of TLRs in early development, despite the knowledge that TLRs and their adaptor proteins MyD88 and TRIF are highly expressed within the nascent embryonic brain in distinct patterns through time (Alvarado & Lathia 2016, Kaul et al. 2012). Furthermore, seemingly separate from their central role in mediating the host response to infectious insult, TLRs have been implicated in a spectrum of noninfectious CNS diseases impacting neurogenesis and cognitive development (Hanke & Kielian 2011). Although TLR deficiencies are known to impact adult neurogenesis and postnatal behaviors (Okun et al. 2011, Rolls et al. 2007), in many studies it is impossible to discern the relative contributions of prenatal versus postnatal TLR deficiency or the impact of compensatory mechanisms engaged in the absence of TLRs to respond to microbial infections. However, shared features in the behavioral abnormalities of TLR-knockout mice and models of MIA during pregnancy suggest that prenatal TLR signaling plays a major role in shaping adult phenotypes. In this section, we focus on studies examining prenatal TLR deficiency with an emphasis on in vivo evidence.
One study (Park et al. 2015) has found that Tlr2−/− mice exhibit an increase in both schizophrenia-associated behaviors and apoptotic cell death in multiple regions of the brain as well as ventricle enlargement. Although TLR2 activation leads to distinct effects in vitro and in vivo, TLR2 deficiency does not affect the proliferation or differentiation of embryonic-stage neural progenitor cells (NPCs) (Okun et al. 2010b). Notably, the role of TLR2 during development is different from that in the adult because NPCs derived from adult Tlr2−/− mice exhibit altered differentiation (Rolls et al. 2007).
TLR3 signaling in the embryonic period provides proliferative control, as TLR3-deficient NPCs show increased proliferation (Lathia et al. 2008) and Tlr3−/− mice exhibit increased hippocampal neurogenesis including increased dentate gyrus volumes (Okun et al. 2010a). Tlr3−/− mice also display reductions in cued fear conditioning and anxiety-associated behaviors (Okun et al. 2010a). Similar to findings in Tlr3−/− mice, one study found enhanced acquisition of spatial memory and impairments in fear learning in Tlr4−/− mice, which were different from the effects of TLR4 antagonism in adults (Okun et al. 2012). However, other studies of Tlr4−/− mice have reported contrasting findings, such as increased anxiety-like behaviors, reduced social interactions, and attenuated drug-reward learning (Femenia et al. 2018, Kashima & Grueter 2017).
Studies using a combination of in vitro culture and in utero electroporation in mouse models have shown that TLR7 and TLR8 deficiencies result in abnormalities in the differentiation and maturation of embryonic-stage neurons, even in the absence of infection or immune stimulation. Tlr7−/− neurons displayed increased dendritic and axonal outgrowth in vitro, which was attenuated by exogenous TLR7 expression (Liu et al. 2013). These observations were dependent on interleukin (IL)-6 and MyD88 signaling and were associated with decreased exploratory behaviors in Tlr7−/− mice (Liu et al. 2013). TLR7 and TLR8 knockdown using microRNA (miRNA) delivered by in utero electroporation were also shown to impact dendritic arborization in postnatally analyzed neurons, albeit at distinct timescales for each gene (Hung et al. 2018b).
In addition, various behavioral alterations have been reported in Tlr7−/− mice, including reduced aggression and impairments in contextual memory (Hung et al. 2018a). A potential caveat in interpreting the link between TLRs and neurodevelopment is that TLR-deficient mice often exhibit outgrowth or dysbiosis of the virome and microbiome (Ubeda et al. 2012), as evidenced by the emergence of endogenous retroviruses in Tlr7−/− mice (Young et al. 2012, Yu et al. 2012). Whether changes in the virome and microbiome are responsible for the various neurologic phenotypes observed in TLR-deficient mice needs to be explored in the future.
3.2. Physiological Role of Cytokines in Normal Brain Development
Cytokines are expressed during normal neurodevelopment in prenatal life and play multifaceted roles in the process of brain formation, among them (a) the modulation of proliferation, apoptosis, and survival programs; (b) the induction, differentiation, and maturation of neural precursors; and (c) the direction of neuronal migration paths (Deverman & Patterson 2009). The final outcome of these pleiotropic functions is informed by the developmental stage at the time of exposure and other environmental cues. Comprehensive reviews detailing the function of cytokines across all stages of neurodevelopment, including postnatal life, and a significant body of in vitro studies can be found elsewhere (Bilbo & Schwarz 2012, Borsini et al. 2015, Deverman & Patterson 2009, Jiang et al. 2018, Pronovost & Hsiao 2019, Stolp 2013). We instead highlight a selection of in vivo work implicating cytokines and chemokines in the functions of normal brain development during the in utero period.
Cytokines exhibit specificity in temporal and spatial expression within the developing brain during pregnancy (Burns et al. 1993, Deverman & Patterson 2009, Pousset 1994, Zhao & Schwartz 1998). While numerous in vivo data demonstrate fundamental roles for specific cytokines in shaping CNS development, many key mouse knockouts in cytokines such as interferon (IFN)-γ and IL-1β do not exhibit overt neurological deficits. However, this may be due at least in part to significant redundancy and synergism in these cytokine networks (Zhao & Schwartz 1998). For example, transforming growth factor (TGF)-β signaling is required for NPC maturation into dopaminergic neurons in the murine E14.5 midbrain; this reduction was significantly higher in Tgf-β2−/− Tgf-β3−/− double-knockout mice compared to that in mice carrying a single competent allele in either gene (Roussa et al. 2006). TGF-β2 appears to be more broadly important for CNS development, however, as Tgf-β2−/− mice are perinatally unviable due to synaptic brain stem dysfunction (Heupel et al. 2008). Downstream of TGF-β, both bone morphogenetic proteins (BMPs) and the Smad family of transcription factors have been extensively studied for their wide-ranging functions in embryonic CNS development and severe knockout phenotypes, including many that are lethal in utero (Goumans & Mummery 2000, Hegarty et al. 2013). Among their many functions, various Smad proteins as well as interacting partners such as Sip1 have been implicated in neural crest specification, primary neurogenesis, neural tube closure, oligodendrocyte differentiation, astrogliogenesis, interneuron specification, and axon growth (Hegarty et al. 2013). For example, Smad2- or Smad4-deficient mice each exhibit abnormalities in anterior development, including craniofacial and forebrain defects (Goumans & Mummery 2000), while the knockout of BMP family members in mice results in a range of aberrant cerebellar phenotypes (Hegarty et al. 2013). Cytokines in other signaling pathways show distinctive effects; TNFα−/− mice, for example, exhibit reduced motor and sensory neuron death (Barker et al. 2001, Sedel et al. 2004).
The gp130 family of cytokines, also known as the IL-6 family, is a group of structurally and functionally related proteins that includes IL-6, IL-11, leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), and cardiotrophin (CT)-1. These cytokines are known to have pleiotropic and redundant functions, and the receptor complexes for all family members include gp130 as a common signal transducer, typically activating signal transducer and activator of transcription 3 (STAT3). Signaling pathways mediated by gp130 are important for the maintenance of radial glial cell (RGC)–NPC populations during embryonic brain development. Knockdown of STAT3 at E13.5–14.5 leads to increased neuronal differentiation with increased expression of β-tubulin III and decreased expression of RGC markers (Hong & Song 2015, Yoshimatsu 2006). In addition, mice deficient in either gp130 or LIF receptor beta (LIFRβ) expression exhibit forebrain hypoplasia and a reduction in the number of mitotic RGCs at E15 and E12.5, respectively (Gregg & Weiss 2005, Hatta et al. 2002). Human studies have supported these findings established in mice; a recent single-cell sequencing study (Pollen et al. 2015) of the human neocortex found that outer RGCs selectively express the LIFR components, and signaling through this pathway is necessary for cell cycle progression and support of the neural stem cell niche in the outer subventricular zone.
Astrogliogenesis is also impacted by the loss of endogenous gp130 signaling pathways. Mice deficient in either gp130 or LIFRβ exhibit decreased astrogliogenesis in late gestation (Barnabé-Heider et al. 2005, Koblar et al. 1998, Nakashima et al. 1999). Similarly, neonatal mice lacking CT-1 exhibit reduced astrocyte numbers, suggesting this cytokine may contribute to the activation of downstream gp130 pathways in utero (Barnabé-Heider et al. 2005).
Colony-stimulating factor 1 receptor (CSF1R) expression is required for the development of all macrophage lineages, but unlike bone marrow–derived macrophages, microglia are not dependent on CSF-1 and instead require IL-34 for their establishment (Wang et al. 2012). Nonetheless, CSF-1-null mice exhibit cortical processing deficits, and neonates show blunted responses to external visual or auditory stimuli (Michaelson et al. 1996, Pollard 1997). Csf1r−/− mice, however, exhibit a loss of microglia and major perturbations in brain architecture as well as olfaction (Erblich et al. 2011).
Other cytokines have also been implicated in promoting microglial functions during neurodevelopment. IL-33-deficient mice exhibit behavioral abnormalities, including decreased anxiety-associated behaviors and impaired social novelty recognition (Dohi et al. 2017). Although there are conflicting reports regarding whether microglia express IL-33 (Fairlie-Clarke et al. 2018, Yasuoka et al. 2011), the production of IL-33 by developing astrocytes directly impacts microglial functions such as synapse engulfment (Vainchtein et al. 2018). Learning and memory impairments have also been documented in mice deficient in IL-2 or IL-6 (Bialuk et al. 2018, Petitto et al. 1999), but it remains undetermined whether these abnormalities are the long-term consequence of perturbations in early development or are due to adult-specific cytokine functions in mediating learning, memory, and social behaviors as has been attributed to other cytokines such as IL-4 and IFN-γ, in which exogenous addition of the missing cytokine in Il4−/− or Ifng−/− mice can rescue observed defects (Derecki et al. 2010, Filiano et al. 2016).
Chemokines can influence the development of brain progenitors by modulating migration, survival, and proliferation activities. C-X-C motif chemokine 12 (CXCL12), also known as stromal cell–derived factor 1 (SDF-1), binds to the CXCR4 receptor; this is the only known interaction for each (Lazarini et al. 2003). CXCR4 is expressed by neurons, astrocytes, and microglia of the CNS, in which levels peak during embryonic brain development; SDF-1 serves as a chemoattractant for these CXCR4-expressing progenitors (Lazarini et al. 2003). CXCR4-deficient mice exhibit abnormalities in dentate gyrus and cerebellar development due to reduced proliferation and inhibition of the normal migratory pathways of granule cells (Bagri et al. 2002, Lu et al. 2002, Ma et al. 1998, Zhu et al. 2009, Zou et al. 1998). Sdf1−/− mice display similar phenotypes to Cxcr4−/− mice, including defects in the migration of cerebellar neurons (Ma et al. 1998, Zhu et al. 2002).
CXCR4 deficiency in mice also leads to reductions in the number and migratory capacity of gonadotropin-releasing hormone neurons in the vomeronasal organ (Schwarting et al. 2006) and oligodendrocyte progenitors in the spinal cord (Dziembowska et al. 2005) during embryonic life. In the peripheral nervous system, CXCR4-deficient mice show a profound loss of spinal cord motor neurons and dorsal root ganglion neurons leading to reduced innervation of the developing fetal limbs (Ödemis et al. 2005). Finally, CXCR4 plays role in determining axon trajectories of both sensory and motor neurons in the spinal cord (Chalasani et al. 2003, Lieberam et al. 2005).
3.3. Physiological Role of Complement in Normal Brain Development
Separate from its well-known roles in host defense, the complement system (see the sidebar titled Complement System) has recently been recognized to have distinct expression patterns and functions in development (Coulthard et al. 2018b, Lee et al. 2019, Stephan et al. 2012). While much of the work describing complement in neurodevelopment has focused on its role in peri- or postnatal synaptic pruning, complement factors serve distinct functions in the prenatal period as well. Complement factors are expressed at high concentrations in NPCs in addition to their demonstrated presence on all mature neuronal and glial cells of the brain. The importance of complement pathways across brain development has been well reviewed elsewhere (Coulthard et al. 2018b, Lee et al. 2019, Stephan et al. 2012), but here we summarize some of the most salient work implicating complement in embryonic neurodevelopmental processes during pregnancy.
Developmental expression of complement pathway components appears to be highly regulated from the earliest stages of brain formation and maturation. During murine neurulation (E7.5–E10.5), a wide range of complement factors are expressed in the neural tube with distinct temporal and spatial distributions. Notably, each of the complement pathways are represented in a piecemeal manner, and the absence of key initiating factors precludes complement activation (Jeanes et al. 2015). Supporting the existence of novel, immune-independent roles in promoting normal development at this stage, the deficiency or pharmacological antagonism of the complement C5a–C5aR axis has been demonstrated to increase the risk of neurulation defects in mouse models. C5ar1−/− mice do not exhibit impaired fertility or congenital abnormalities; however, in combination with dietary folate deficiency, loss of C5a-mediated signaling using either genetic C5ar1-knockout mice or treatment with a C5aR antagonist at E4.5 leads to a high rate of neural tube defects (NTDs) in the resulting offspring (Denny et al. 2013).
Concordant with these findings, pharmacological C5aR1 blockade results in reduced NPC proliferation, while endogenous or exogenous C5a exposure leads to increased proliferation of NPCs both in vitro and in vivo (Coulthard et al. 2017). These models also reveal impacts on NPC polarity: The C5a–C5aR pathway promotes the organization and maintenance of NPCs into neural rosettes in culture, while injection of a C5aR antagonist into the ventricles of embryos in utero alters NPC division planes (Coulthard et al. 2017). C5aR1 is localized to the apical surface of the ventricular zone of the developing brain at E14.5, and the C5a concentration in embryonic cerebrospinal fluid is significantly higher than that in normal adult cerebrospinal fluid at this time, further suggesting a developmental function (Coulthard et al. 2017). Perturbations of C5a–C5aR signaling during embryonic life are furthermore associated with postnatal motor and behavioral abnormalities. C5ar1−/− mice display impairments in short-term memory (Gong et al. 2013), while acute antagonism of C5aR1 from E12.5–E14.5 leads to impaired motor coordination and increases in anxious and depressive behaviors (Coulthard et al. 2017).
Interestingly, different complement signaling pathways appear to have differential effects on NPCs. At E14.5, C3aR exhibits a pattern of distribution similar to that of C5aR at the apical pole of NPCs in the ventricular zone but exerts opposite effects on NPC proliferation (Coulthard et al. 2018a). Injection of a C3aR antagonist into the embryonic ventricle leads to increased proliferation, while C3aR agonist injection leads to reduced proliferation (Coulthard et al. 2018a), potentially by driving NPC differentiation (Lee et al. 2019, Shinjyo et al. 2009). C3ar−/− mice also do not display overt abnormalities in viability or development but exhibit cognitive deficits in spatial memory in adulthood (Coulthard et al. 2018a).
The C3-mediated pathway has also been demonstrated to dictate the migration patterns of neurons and neural crest cells along with other complement factors. In mice, knockdown of C3, mannose-associated serine protease 1 (Masp1), and Masp2 using short hairpin RNAs (shRNAs) delivered by in utero electroporation at E14.5 resulted in impaired neuronal migration in the neocortex, a defect that was rescued by mimics of C3 cleavage products or pharmacological activation of downstream C5aR or C3aR (Gorelik et al. 2017). Studies in Xenopus and zebrafish models, which also exhibit high levels of complement expression strikingly restricted to neural structures, have allowed a more detailed elucidation of the mechanism by which complement-mediated interactions coordinate complex migratory paths. In both Xenopus and zebrafish, the C3–C3aR1 axis was shown to drive the coattractive forces necessary for collective cell migration of neural crest cells. Loss of these factors leads to significant disruptions in the self-organizing properties and coordinated movement of these cells (Carmona-Fontaine et al. 2011).
4. MATERNAL IMMUNE ACTIVATION: MODELS AND DISEASE ASSOCIATIONS
The physiological involvement of various innate immune effectors in brain development makes the nervous system susceptible to disorders when the levels and timing of such effectors are altered by inflammation. Human epidemiological studies have uncovered a multitude of associations between maternal inflammatory signatures and poor fetal outcomes, but these links are challenging to explore in depth because the manifestations of MIA relevant to neurodevelopmental risk can be mild or subclinical in pregnant women, and the outcomes in offspring are highly variable with regard to both onset and phenotype. Studies of maternal infections are further complicated by the need to consider the contributions of two confounding potential sources of damage: the pathogen and the maternal immune response.
Rodent models of MIA have thus provided a breakthrough in our ability to isolate the effects of maternal immunity and attain a more mechanistic understanding of the processes underlying neurodevelopmental pathologies stemming from pregnancy (Figure 1). The most common models of MIA invoke the use of PAMPs, including polyinosinic:polycytidylic acid [poly(I:C)], a TLR3 agonist that stimulates antiviral responses, and lipopolysaccharide (LPS), which induces antibacterial responses through TLR4. Although some models of MIA use direct infection of pregnant dams with live pathogens such as influenza virus and Escherichia coli, the relative contributions of immune activation versus pathogen-inflicted damage cannot easily be ascertained. Thus, in this review, we focus almost exclusively on models of MIA induced by exposure to the TLR ligands poly(I:C) and LPS, and refer to other reviews for all-encompassing discussions of models of pathogenic MIA (Careaga et al. 2017, Knuesel et al. 2014, Solek et al. 2017).
4.1. Autism Spectrum Disorder
The most established body of work on MIA has focused on the induction of behavioral abnormalities linked to ASD and schizophrenia. Epidemiological and clinical data have long suggested that maternal infection and inflammatory conditions increase the risk for offspring with ASD and schizophrenia, as reviewed in Abdallah et al. (2012), Atladóttir et al. (2010), Brown et al. (2014), Keil et al. (2010), Krakowiak et al. (2012), and Xiang et al. (2015). These disorders share striking commonalities in their genetic and environmental origins: A recent meta-analysis of genome-wide single-nucleotide polymorphism (SNP) data across eight neuropsychiatric conditions including ASD and schizophrenia revealed significant overlaps in genetic risk, with approximately three-quarters of significant SNPs associated with more than one disorder. These pleiotropic genes were also enriched in associations with neurodevelopmental function (Cross-Disord. Group Psychiatr. Genom. Consort. 2019).
In support of these observations, recent work using MIA models has critically allowed the identification of specific cytokines driving these aberrant phenotypes during pregnancy and in utero fetal development. There are numerous models of MIA-induced ASD, and the heterogeneity of these approaches may contribute to variability in reproducibility and conclusions (Kentner et al. 2019). Extensive reviews summarizing the time line and dosages used in various MIA models of ASD and schizophrenia have been published by others (Careaga et al. 2017, Solek et al. 2017), some of which we address here.
Intraperitoneal injection of poly(I:C) to pregnant dams mid-gestation (e.g., E12.5) leads to transient increases in maternal IL-6 levels and offspring defects in prepulse inhibition, latent inhibition, and social and exploratory behaviors commonly associated with both ASD and schizophrenia. Il6−/− mice and wild-type mice treated with neutralizing antibodies to IL-6, however, are protected from this phenotype (Smith et al. 2007). These findings align with reports in humans that correlate increased maternal IL-6 levels during pregnancy with changes in amygdala volume and connectivity and lower impulse control in offspring at 2 years of age (Graham et al. 2018). Further changes in neural network structure have been described in mouse models of MIA-induced ASD-like behavioral abnormalities, in particular changes to the dysgranular zone of the primary somatosensory cortex (S1DZ) (Shin Yim et al. 2017).
Although human and rodent studies suggest that IL-6 can cross the placenta (Dahlgren et al. 2006, Zaretsky et al. 2004), fetal trophoblasts are central to the development of MIA-driven behavioral abnormalities. Maternal IL-6 induces distinct immune and endocrine changes in the placenta (Hsiao & Patterson 2011), and IL-6 responsiveness in placental trophoblasts is required for the development of MIA-associated behaviors (Wu et al. 2017).
Subsequent studies have identified the importance of IL-17a as a downstream mediator of ASD-associated phenotypes. IL-17a deficiency or the administration of blocking antibodies protects the offspring of poly(I:C)-treated dams from the development of behavioral deficits. Direct intraventricular injection of IL-17a into fetal brains at E14.5 is sufficient to induce ASD-like behaviors in offspring (Choi et al. 2016). The source of IL-17a in susceptible pregnancies was traced to the activity of maternal T helper 17 (Th17) cells, a RORγt-expressing subset of CD4+ T cells (Choi et al. 2016). Interestingly, the microbiome composition was key in modulating susceptibility to MIA because IL-17a is secreted by a memory Th17 population induced in the mother’s gut only in the presence of segmented filamentous bacteria (Kim et al. 2017, Lammert et al. 2018).
Finally, while maternal complement factor levels have classically been tied to schizophrenia risk (as described in the next section), recent evidence also links elevated C-reactive protein (CRP) levels during pregnancy to increased ASD risk in offspring (Brown et al. 2014).
4.2. Schizophrenia
Despite the considerable etiological overlap with ASD, schizophrenia also exhibits distinct differences in risk factors and disease pathogenesis. Genetic risk factors for schizophrenia reveal links to both neurodevelopmental and immune genes. The most striking genetic association of schizophrenia is with the major histocompatibility complex (MHC) locus, which spans several megabases on chromosome 6 (Schizophr. Work. Group Psychiatr. Genom. Consort. 2014). This association is explained at least in part by copy number variation and isotype structure at the complement factor C4 genes, C4A and C4B. Increased C4A copy number and expression is linked to increased schizophrenia risk. Interestingly, this association was especially pronounced for structural variants containing a human endogenous retrovirus (HERV) embedded in an intron of the C4 isotypes (C4-HERV); an increased copy number of C4-HERV was associated with a highly significant increase in risk (Sekar et al. 2016). Further studies are warranted to explore why dysfunction at the C4 locus is associated with schizophrenia risk; most of the prevailing hypotheses have centered on the potential effects on postnatal synaptic pruning, but the existing links between C4 and increased risk for infection and autoimmunity may also provide potential clues from an immunological framework of development (Nimgaonkar et al. 2017).
Epidemiological studies further support the link between levels of various maternal immune factors and subsequent schizophrenia risk in offspring (Benros et al. 2012, Brown 2006). For example, elevated maternal levels of the complement factors (e.g., CRP, C1q) (Canetta et al. 2014, Nimgaonkar et al. 2017, Severance et al. 2014) and cytokines (e.g., IL-8/CXCL8, TNF-α) (Brown et al. 2004, Buka et al. 2001) during pregnancy have each been associated with significantly increased risk for psychosis in offspring. Analysis by MRI of a cohort of human patients exposed during their fetal life in the second or third trimester to maternal IL-8 also showed a dose-dependent association with structural brain changes commonly associated with schizophrenia (Ellman et al. 2010).
In MIA models, other cytokines, including IL-1β and IL-10, have additionally been linked to vulnerability of offspring to schizophrenia (Fineberg & Ellman 2013, Miller et al. 2013). Constitutive overexpression of IL-10 by macrophages prevents the development of long-term behavioral abnormalities in a poly(I:C)-induced mouse model of schizophrenia (Meyer et al. 2008). However, intriguingly, normal pregnancies are negatively impacted by IL-10 overexpression; in the absence of MIA, macrophage-driven IL-10 overproduction alone results in aberrant behaviors, suggesting the critical importance of cytokine balance in neurodevelopment (Meyer et al. 2008). As in ASD, there is evidence for persistent structural changes in the brains of offspring following transient exposure to MIA during early in utero development.
4.3. Neural Tube Defects
While most models of MIA have been focused on behavioral abnormalities associated with neuropsychiatric disorders such as ASD and schizophrenia, the recent ZIKV outbreak has generated increased interest in other neurodevelopmental outcomes of infection and perturbed maternal immunity (Coyne & Lazear 2016, Meyer 2019). Indeed, murine models of LPS- and CpG-treated pregnancy have demonstrated the teratogenic potential of MIA in producing NTDs, the cause of which remains mysterious (Copp et al. 2013, Greene & Copp 2014).
Administration of CpG oligodeoxynucleotide at E6.5 in mice leads to the development of NTDs in addition to craniofacial and distal limb defects (Prater et al. 2006, Thaxton et al. 2009). CNS malformations in response to LPS exposure have also been characterized in gestational day 8.5 treatment of the golden hamster (Collins et al. 1994, Lanning et al. 1983) and multiple treatments across gestational days 7 to 16 in the rat (Ornoy & Altshuler 1976). In mouse models, either a single subcutaneous injection of LPS on E7.5 or a series of intraperitoneal LPS injections from E8.5 to E12.5 leads to an increased incidence of severe NTDs such as exencephaly (Chua et al. 2006, Zhao et al. 2008). LPS administration results in an increase in cytokines such as TNF-α, and direct administration of TNF-α during early pregnancy has been shown to recapitulate the types of NTDs seen after LPS administration in mice (Taubeneck et al. 1995).
Although complement factors have been implicated in the processes of embryonic neurulation, few studies have explored the role of complement activation or depletion during MIA. One study (McDonald et al. 2015) of maternal malarial infection during pregnancy showed that such infection led to neurodevelopmental abnormalities in uninfected offspring, with cognitive defects in memory and affective depression-like behaviors observed. Genetic deficiency or functional blockade of the C5a–C5aR axis, using C5ar1−/− mice or maternal treatment with C5a antisera, respectively, protected malaria-exposed pregnancies from the development of these neurocognitive deficits (McDonald et al. 2015).
Interestingly, while MIA can increase the risk of birth defects in the context of normal pregnancy, there is also evidence suggesting it can serve a potentially protective role in certain high-risk environments produced by exposure to known teratogens (Holladay et al. 2002). The medication valproic acid is known to cause a high incidence of NTDs in offspring exposed in utero, but the appearance of exencephaly was effectively blocked in mice by pretreatment with immunogenic agents such as pyran copolymer, bacillus Calmette-Guérin (BCG), or Freund’s complete adjuvant (FCA) (Holladay et al. 2000). These studies underscore the complexity inherent in the investigation of MIA influences on fetal development and emphasize the importance of considering the specific balance of signals integrated through various immune pathways rather than a simplistic binary of activation or quiescence.
4.4. Cerebral Palsy
Although historically attributed to perinatal hypoxia, the etiology of CP is thought to be multifactorial. Risk factors include birth-related events (e.g., premature birth, low birth weight, or multiple births) and maternal infections by pathogens including TORCH agents. Amniotic fluid levels of cytokines such as IL-1β, IL-6, and TNF-α have been linked to white matter lesions commonly seen in CP (Jacobsson & Hagberg 2004; Yoon et al. 1997, 2000); however, there is limited knowledge regarding the source or maternal contributions to CP pathogenesis.
While most animal models of CP employ hypoxia–ischemia to induce brain injury and do not directly target maternal immunity, more recent MIA-based models demonstrate that maternal immune responses can act as a potential driver of CP-associated outcomes (Bashiri et al. 2005, Knuesel et al. 2014). These CP models couple maternal PAMP exposure in late gestation with either postnatal induction of hypoxia (e.g., carotid arterial ligation) or direct intracerebral injection of ibotenate, a neurotoxin and brain-lesioning agent (Larouche et al. 2005, Rousset et al. 2008, Stridh et al. 2013).
Intraperitoneal or intrauterine exposure to LPS or poly(I:C) late in pregnancy (ranging from E17.5 to E20.5) leads to neuronal injury, reduced white matter myelination, and glial changes (Bell & Hallenbeck 2002; Larouche et al. 2005; Paintlia et al. 2004; Rousset et al. 2006, 2008). In addition, LPS administration has been associated with apoptosis and increased TNF-α expression in white matter regions of the cortex (Bell & Hallenbeck 2002). Another study (Girard et al. 2010) of LPS exposure in pregnant rats from gestational days 18.5 to 20.5 showed that white matter damage and microgliosis was attenuated by coadministration of an IL-1 receptor antagonist. Pre- and postnatal immune mediators of CP risk appear to be conserved, as a study of postnatal LPS treatment similarly demonstrated the induction of neuronal self-injury through the production of IL-1β, which could be prevented by IL-1R antagonism (Savard et al. 2015).
LPS exposure alone, however, is insufficient to induce long-term motor impairment, emphasizing the importance of a secondary insult following MIA priming in the precipitation of CP phenotypes. One study (Rousset et al. 2013) found that maternal exposure to LPS led to transient motor dysfunction in neonates but that this deficiency disappeared by 5 weeks postexposure. Another study (Poggi et al. 2005) showed in an LPS-only model that while histological white matter lesions could be detected following MIA, these lesions were not correlated with clinical behavioral signs of neurological lesion or disease.
4.5. Epilepsy
Maternal infections are epidemiologically linked to an increased risk of childhood epilepsy in offspring (Sun et al. 2008, 2010; Whitehead et al. 2006), and mouse models also suggest that seizure susceptibility is increased in mice prenatally exposed to MIA. In one model, pregnant dams were subjected to restraint stress for seven consecutive days starting on E14.5. On postnatal day 14, pups were subjected to injection of LPS and kainic acid (KA), an excitotoxic analog of glutamate used to induce acute seizures. Offspring that had been exposed to maternal stress in utero exhibited exaggerated seizure responses with increased plasma IL-1β levels (Qulu et al. 2012). Supporting a potential role for the IL-1β pathway in affecting the seizure threshold, a single dose of poly(I:C) at E9.5 exacerbated seizure responses to KA and induced transient increases in IL-1β and IL-6 serum levels that are absent in IL-1 receptor type I knockout (Il1r1−/−) mice (Corradini et al. 2018). Another group (Pineda et al. 2013), however, reported that IL-6 but not IL-1β is involved in mediating increased hippocampal excitability and seizure susceptibility in a poly(I:C)-driven model of seizure and behavioral deficits. In summation, IL-1β and IL-6 have each been proposed to mediate an MIA-induced vulnerability to seizures, but major differences in the MIA model employed, experimental design, and method of seizure induction likely contribute to conflicting conclusions.
4.6. Adult-Onset Neurological Disease and Aging: Alzheimer’s Disease and Parkinson’s Disease
Given the large latency period implicit between the time of insult and disease onset, the role of MIA in aging-associated neurological diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) has remained largely unexplored. However, a long-standing recognition of the role of inflammatory mediators in these diseases has led some groups to explore the contribution of perinatal immune priming to vulnerability in aging-associated progressive neurodegenerative diseases such as AD and PD.
Given that both genetic and epigenetic risk factors for AD and PD have been traced to immune genes (Dzamko et al. 2015, Gjoneska et al. 2015, Hoeijmakers et al. 2016, Holmans et al. 2013), it is conceivable that developmental MIA priming could shape susceptibility to neurodegenerative diseases far later in life. In a mouse model, maternal poly(I:C) exposure during late gestation at E17.5 led to distinct cytokine changes and the accumulation of APP (amyloid precursor protein) components in the hippocampus later in adulthood at 15 months of age, reminiscent of AD neuropathology (Krstic et al. 2012). Increased rates of mislocalization and hyperphosphorylation of another hallmark AD protein, tau protein, was also observed (Krstic et al. 2012). Secondary immune challenge with poly(I:C) in adulthood exacerbated this phenotype, which was accompanied by persistent changes in microglial activation and cognitive deficits (Krstic et al. 2012). Degenerative structural abnormalities in the hippocampus were also detected following maternal poly(I:C) treatment; while these phenotypes were not unique to MIA-exposed offspring, they were observed significantly earlier compared to controls, suggesting accelerated aging-related damage (Doehner et al. 2012).
In rat models of PD pathogenesis, LPS treatment of pregnant females at approximately gestational day 10.5 led to the loss of dopamine (DA) neurons during secondary challenge in adulthood accompanied by an exacerbated loss of DA neurons (Ling et al. 2001, 2006). LPS treatment has also been associated with an increase in TNF-α (Ling et al. 2001) and with cognitive deficits in adulthood that increased in significance with age (Hao et al. 2010). In further support of a multiple-hit model for PD, a model of poly(I:C)-induced MIA at E17.5 demonstrated an impairment to dopaminergic neurons of the midbrain that was compounded by genetic deficiency in NR4A2, a gene that encodes the NURR1 receptor and is important for dopaminergic neuron development as well as the neuroprotective function of glia responding to inflammatory insult (Glass et al. 2010, Saijo et al. 2009, Vuillermot et al. 2012).
6. IMPLICATIONS AND FUTURE WORK
MIA models have provided an important paradigm for the consideration of immunity during pregnancy (Figure 2). While major inroads have been made in characterizing the immune landscape of the maternal decidua, a comprehensive atlas of leukocyte composition and the expression of immune molecules at the maternal–fetal interface across time is absent. A better understanding of the pathways regulating leukocyte access and immune activation in the pregnant uterus would help pinpoint the relative contributions of systemic versus local immunity to specific fetal outcomes. Similarly, the ability of maternal cytokines and other circulating immune factors to cross the maternal–fetal interface is poorly characterized but has important implications for fetal pathogenesis. In many MIA models, it is still unknown whether PAMPs or systemic maternal cytokines cross the maternal–fetal interface to directly impact fetal pathways of immunity, or if these factors trigger secondary pathways of signal transduction mediated by fetal tissues such as the placenta. Heterozygous crosses can be used in future studies to determine the relative contributions of maternal and fetal immune pathways to neurodevelopmental pathogenesis.
Figure 2.
MIA alters immune programs at the maternal–fetal interface to directly impact fetal neurodevelopment. Various maternal conditions (e.g., inflammation, infection, autoimmune disease) can lead to perturbations in immune factor expression at the maternal–fetal interface, leading to disruptions in neurodevelopment. Abbreviations: CNS, central nervous system; MIA, maternal immune activation; TLR, Toll-like receptor. Figure adapted from images created with Biorender.com.
The expression of TLRs in the developing fetus, particularly in the tissues of the brain, clearly suggests that neural precursors may be responsive to PAMPs. A major unanswered question remains regarding the identity and source of the endogenous TLR ligands that guide neural development as well as what factors control access to these ligands. A comprehensive molecular characterization of which precursors are expressing or coexpressing TLRs at each stage of development is also missing. There is evidence suggesting these TLR pathways intersect and interact with one another during neurogenesis, further complicating an in-depth study of their functions in development (Hung et al. 2018b, Rolls et al. 2007). Similarly, the function of complement factors expressed in the developing brain and their potential role in precursor migration or other programs remains to be elucidated. However, new technologies and even existing studies in the field of developmental neurobiology offer an opportunity to uncover new details regarding immune expression in early brain formation, for example in established single-cell sequencing data sets (Li et al. 2018, Polioudakis et al. 2019, Zhong et al. 2018).
A deeper consideration of developmental timing is also critical to unraveling the mysteries behind the diverse manifestations of neurodevelopmental abnormalities. The rapid pace of changes on both sides of the maternal–fetal interface generates enormous variability and represents a major barrier to the execution, interpretation, and sharing of studies; details of embryonic dating and timing remain poorly standardized and reported in studies of prenatal development and MIA, even in the most widely utilized experimental mouse models. The character and intensity of exposures also contribute to differential outcomes in neurodevelopmental risk of disease and heterogeneity in MIA studies (Kentner et al. 2019, Meyer 2019). Finally, the complexity of modulating maternal factors such as the microbiome can profoundly impact pathogenesis; the relative impact of other contributors to maternal immune status must be better understood and standardized in future studies (Kentner et al. 2019). Ideally this would also include a characterization and comparison of MIA stemming from diverse triggers, from invading pathogens to environmental exposures to autoimmune disease.
Regardless of these obstacles, the lifelong consequences of perturbed immunity in prenatal life provide a compelling drive for the continued expansion of this highly intersectional field. Indeed, investigations of MIA have the potential to synergize with other fields to explain enduring mysteries, such as the sex biases observed in various neuropsychiatric diseases (Haida et al. 2019). Emerging evidence suggests that these skews may be driven by sex-based differences in prenatal responses to MIA rather than by purely hormonal or endocrine pathways as widely presumed (Klein & Flanagan 2016).
A better understanding of the contributions of MIA to neuropathogenesis is directly relevant to the development of strategies for prevention, intervention, and treatment. The incorporation of an immune perspective to our understanding of risk for neurological disease would certainly have the power to inform a myriad of clinical scenarios—not only practices of prenatal screening and management but also postnatal practices when considering the care of high-risk, MIA-exposed neonates.
Supplementary Material
SUMMARY POINTS
The maternal–fetal interface is a unique immunological context that both supports and actively shapes fetal development in utero.
The composition of the maternal–fetal interface changes dynamically throughout the stages of pregnancy; the interface consists of a diverse collection of immunologically active subsets unique to pregnancy, including important cell types such as trophoblasts that are not classically considered immune cells.
Immune pathway signaling through Toll-like receptors (TLRs), cytokines, and complement pathways plays physiological roles in promoting normal fetal neurodevelopment (see Supplemental Table 1), but our understanding of these processes remains incomplete.
Maternal immune activation (MIA) leads to a diverse array of neurological diseases that exhibit distinct characteristics in response to variations in timing, intensity, and category of stimulus.
Animal models of MIA have provided an experimental approach to determining the relative contributions of systemic and local maternal immunity to fetal development.
The connection between the immune signaling present in normal fetal neurodevelopment and the outcomes of MIA is still emerging, but this area provides new opportunities to address a wide range of disorders traced to perturbations during prenatal neurodevelopment.
FUTURE ISSUES
A comprehensive catalog and atlas of immune players and molecular expression at the maternal–fetal interface throughout the time line of pregnancy would provide critical context to studies of poor outcomes following MIA.
Many studies fail to differentiate prenatal from postnatal processes of neurodevelopment, which limits our understanding of whether the effects of specific maternal perturbations are executed exclusively in the prenatal period or whether these influences prime vulnerability far later into life (e.g., by altering microglial states).
The endogenous ligands for TLRs during development have yet to be identified.
The identities of cells at the maternal–fetal interface and in the developing fetal brain expressing various immune receptors and molecules have yet to be determined in molecular detail, but represent essential information needed to understand the circuits of immune signaling relevant to both normal and abnormal prenatal development.
The impact of other alterations in maternal immune status (e.g., microbiome status, environmental exposure, metabolic disease, or autoimmunity) is poorly characterized but could provide insights into the mechanisms linking immune conditions to fetal neurodevelopmental outcomes.
In terms of maternal versus fetal mechanisms of immune propagation across the maternal–fetal interface, are maternal components necessary for fetal outcomes or is the fetus directly sensing immune perturbations such as TLR ligands?
What areas are open to be impacted and targeted for potential prevention, intervention, and treatment?
SIDEBARS.
MATERNAL IMMUNE ACTIVATION
Maternal immune activation (MIA) is a broad term that refers to a systemic increase in inflammatory mediators during pregnancy, classically triggered by exposure to pathogens such as viruses. MIA is associated with poor outcomes in pregnancy and for the developing fetus.
While early studies of MIA relied on large-scale epidemiological data following epidemics or experimental models of immune responses driven by specific pathogens such as influenza or Escherichia coli, the field has evolved to focus on the study of MIA in isolation, independent of infection. Experimental MIA, sometimes referred to as sterile inflammation, induces immune responses by exposing the pregnant host to immunogens such as pathogen-associated molecular patterns (PAMPs).
DECIDUALIZATION
Upon blastocyst implantation into the maternal endometrium, the uterine mucosa undergoes a dramatic transformation into the decidua. Decidualization includes the processes of vascular and uterine gland transformation, the infiltration of immune cells, and the transformation of stromal cells in the endometrium into decidual cells with highly specialized and distinctive functions. This very specialized maternal decidual layer is maintained throughout pregnancy until it is shed following parturition.
MICROGLIA
Microglia are the resident macrophages of the central nervous system and arise from primitive macrophages in the yolk sac early in development. Microglia are known to play a central role in the pruning of synapses in the brain postnatally, but these macrophages have also been implicated in a number of prenatal neurodevelopmental processes.
The earliest noted activities of microglia in the brain occur at the site of the developing neural tube, where microglia have been shown to accumulate in the surrounding mesenchyme and phagocytose dying cells. Later in cortical neurogenesis, microglial phagocytosis is directed at the elimination of neural precursor cells. Microglia also direct dopaminergic axonal outgrowth and interneuron positioning within the murine forebrain. Importantly, the early actions of these neuroimmune cells in the developing brain are directly influenced by maternal signals, both in normal development and following maternal immune activation.
Microglial functions have been covered elsewhere in a number of excellent and comprehensive reviews (Bilimoria & Stevens 2015, Lenz & Nelson 2018, Paolicelli & Ferretti 2017).
HOFBAUER CELLS: PLACENTAL MACROPHAGES
Hofbauer cells are a heterogeneous population of fetal macrophages. After their initial appearance at roughly 4 weeks postconception in humans, they reside in the placenta throughout pregnancy. While they are known to be fetally derived, their precise developmental origins remain unknown, and it is possible that early waves of macrophages from the yolk sac are later replaced by macrophages derived from the fetal hematopoietic compartment. Hofbauer cells typically display an M2 or M2-like phenotype, and their major function is presumed to be in placental morphogenesis and homeostasis. Despite their macrophage identity, Hofbauer cells have not been shown to exhibit antimicrobial properties and conversely are highly vulnerable to infection by invading pathogens, serving as a potential reservoir for vertical transmission.
COMPLEMENT SYSTEM
The complement system is an evolutionarily ancient component of innate immunity best known for its canonical effector roles in the elimination of pathogens and compromised cells. It encompasses a vast array of soluble and membrane-bound factors arranged in sequential cleavage cascades that can be initiated by one of three pathways to activation: classical, alternative, and lectin. All three routes converge on the cleavage of complement component C3, the activated fragments of which subsequently drive effector functions leading to the destruction and clearance of targets via opsonization and/or formation of the lytic membrane attack complex. In addition, activated complement fragments can serve as signaling molecules that recruit other arms of the immune system to the local site. Regulation of complement is introduced at multiple levels; complement components circulate as inactive zymogens that require proteolytic processing for effector functions, and a large group of regulatory factors serves to protect the host from inappropriate activation.
ACKNOWLEDGMENTS
A.L.-C. is supported by the National Institutes of Health under Medical Scientist Training Program grant T32GM007205 and National Institute of Child Health and Development grant F30HD093350. A.I. is an investigator of the Howard Hughes Medical Institute.
TERMS AND DEFINITIONS
- Maternal-fetal interface
in utero site of interaction and exchange between the decidua of the maternal uterus and trophoblasts of fetal extraembryonic tissues
- Lipopolysaccharide (LPS)
component of the outer cell wall of Gram-negative bacteria and TLR4 agonist that stimulates antibacterial immune responses
- Polyinosinic:polycytidylic acid (poly(I:C))
a synthetic long double-stranded RNA and TLR3 agonist that stimulates antiviral responses
- Radial glial cells (RGCs)
a heterogenous classification of progenitor cells derived from the neuroepithelium that appear transiently during neurogenesis with diverse functions in development
- Autism spectrum disorder (ASD)
a complex group of neurodevelopmental disorders characterized by hallmark deficits in social interaction and repetitive patterns of behavior
- Schizophrenia
a severe neuropsychiatric disorder characterized by chronic or recurrent periods of psychosis
- Pre-pulse inhibition (PPI)
measurement of the ability of a weak pre-stimulus to reduce startle response to a loud acoustic stimulus; used to assess sensorimotor gating at early attentional stages
- Latent inhibition (LI)
measurement of the ability to ignore irrelevant stimuli; used to assess gating at later processing stages of associative learning
- Epilepsy
a neurological disease marked by recurrent, non-provoked seizures
- Cerebral palsy (CP)
a heterogenous group of early-onset, non-progressive conditions that affect the developing fetal or infant brain and result in lifelong motor disability
- Neural tube defects (NTDs)
a collection of congenital CNS defects arising from a failure in formation of the neural tube in early embryogenesis
- Alzheimer’s disease (AD)
a progressive neurodegenerative disease of uncertain etiology characterized by selective memory and cognitive impairments; the most common cause of dementia
- Parkinson’s disease (PD)
a progressive neurodegenerative disease that affects the motor system, most classically characterized by tremor, bradykinesia, rigidity, and postural instability
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abdallah MW, Larsen N, Grove J, Nørgaard-Pedersen B, Thorsen P, et al. 2012. Amniotic fluid chemokines and autism spectrum disorders: an exploratory study utilizing a Danish Historic Birth Cohort. Brain Behav. Immun 26(1):170–76 [DOI] [PubMed] [Google Scholar]
- Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, et al. 2004. Divergent trophoblast responses to bacterial products mediated by TLRs. J. Immunol 173(7):4286–96 [DOI] [PubMed] [Google Scholar]
- Abrahams VM, Mor G. 2005. Toll-like receptors and their role in the trophoblast. Placenta 26(7):540–47 [DOI] [PubMed] [Google Scholar]
- Abrahams VM, Schaefer TM, Fahey JV, Visintin I, Wright JA, et al. 2006. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I: C). Hum. Reprod 21(9):2432–39 [DOI] [PubMed] [Google Scholar]
- Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G. 2005. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J. Immunol 175(12):8096–104 [DOI] [PubMed] [Google Scholar]
- Alvarado AG, Lathia JD. 2016. Taking a Toll on self-renewal: TLR-mediated innate immune signaling in stem cells. Trends Neurosci. 39(7):463–71 [DOI] [PubMed] [Google Scholar]
- Ander SE, Diamond MS, Coyne CB. 2019. Immune responses at the maternal-fetal interface. Sci. Immunol 4(31):eaat6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladóttir HÓ, Thorsen P, Østergaard L, Schendel DE, Lemcke S, et al. 2010. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord 40(12):1423–30 [DOI] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou Y-R, Littman DR, et al. 2002. The chemokine SDF1 regulates migration of dentate granule cells. Development 129(18):4249–60 [DOI] [PubMed] [Google Scholar]
- Barker V, Middleton G, Davey F, Davies AM. 2001. TNFα contributes to the death of NGF-dependent neurons during development. Nat. Neurosci 4(12):1194–98 [DOI] [PubMed] [Google Scholar]
- Barnabé-Heider F, Wasylnka JA, Fernandes KJL, Porsche C, Sendtner M, et al. 2005. Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron 48(2):253–65 [DOI] [PubMed] [Google Scholar]
- Bashiri A, Burstein E, Mazor M. 2005. Cerebral palsy and fetal inflammatory response syndrome: a review. J. Perinat. Med 34(1):277–78 [DOI] [PubMed] [Google Scholar]
- Bayer A, Delorme-Axford E, Sleigher C, Frey TK, Trobaugh DW, et al. 2015. Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am. J. Obstet. Gynecol 212(1):71.e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, et al. 2016. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 19(5):705–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MJ, Hallenbeck JM. 2002. Effects of intrauterine inflammation on developing rat brain. J. Neurosci. Res 70(4):570–79 [DOI] [PubMed] [Google Scholar]
- Benros ME, Mortensen PB, Eaton WW. 2012. Autoimmune diseases and infections as risk factors for schizophrenia. Ann. N. Y. Acad. Sci 1262(1):56–66 [DOI] [PubMed] [Google Scholar]
- Bialuk I, Taranta A, Winnicka MM. 2018. IL-6 deficiency alters spatial memory in 4- and 24-month-old mice. Neurobiol. Learn. Mem 155:21–29 [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. 2012. The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol 33(3):267–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilimoria PM, Stevens B. 2015. Microglia function during brain development: new insights from animal models. Brain Res. 1617:7–17 [DOI] [PubMed] [Google Scholar]
- Borsini A, Zunszain PA, Thuret S, Pariante CM. 2015. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 38(3):145–57 [DOI] [PubMed] [Google Scholar]
- Bowen JM, Chamley L, Mitchell MD, Keelan JA. 2002. Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta 23(4):239–56 [DOI] [PubMed] [Google Scholar]
- Brown AS. 2006. Prenatal infection as a risk factor for schizophrenia. Schizophr. Bull 32(2):200–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, et al. 2004. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am. J. Psychiatry 161(5):889–95 [DOI] [PubMed] [Google Scholar]
- Brown AS, Sourander A, Hinkka-Yli-Salomäki S, McKeague IW, Sundvall J, Surcel H-M. 2014. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol. Psychiatry 19(2):259–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. 2001. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav. Immun 15(4):411–20 [DOI] [PubMed] [Google Scholar]
- Bulla R, Bossi F, Agostinis C, Radillo O, Colombo F, et al. 2009. Complement production by trophoblast cells at the feto-maternal interface. J. Reprod. Immunol 82(2):119–25 [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Morrison L, Longfellow M, Ritson A, Pace D. 1991. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum. Reprod 6(6):791–98 [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Williams PJ, Lash GE. 2010. Immune cells in the placental bed. Int. J. Dev. Biol 54(2–3):281–94 [DOI] [PubMed] [Google Scholar]
- Burns TM, Clough JA, Klein RM, Wood GW, Berman NE. 1993. Developmental regulation of cytokine expression in the mouse brain. Growth Factors 9(4):253–58 [DOI] [PubMed] [Google Scholar]
- Canetta S, Sourander A, Surcel H-M, Hinkka-Yli-Salomäki S, Leiviskä J, et al. 2014. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am. J. Psychiatry 171(9):960–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Murai T, Bauman MD. 2017. Maternal immune activation and autism spectrum disorder: from rodents to nonhuman and human primates. Biol. Psychiatry 81(5):391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Theveneau E, Tzekou A, Tada M, Woods M, et al. 2011. Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev. Cell 21(6):1026–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani SH, Sabelko KA, Sunshine MJ, Littman DR, Raper JA. 2003. A chemokine, SDF-1, reduces the effectiveness of multiple axonal repellents and is required for normal axon pathfinding. J. Neurosci 23(4):1360–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess S. 1971. Autism in children with congenital rubella. J. Autism Child Schizophr 1:33–47 [DOI] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, et al. 2016. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351(6276):933–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua JSC, Rofe AM, Coyle P. 2006. Dietary zinc supplementation ameliorates LPS-induced teratogenicity in mice. Pediatr. Res 59(3):355–58 [DOI] [PubMed] [Google Scholar]
- Collins JG, Smith MA, Arnold RR, Offenbacher S. 1994. Effects of Escherichia coli and Porphyromonas gingivalis lipopolysaccharide on pregnancy outcome in the golden hamster. Infect. Immun 62(10):4652–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disord. Group Psychiatr. Genom. Consort. 2019. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179(7):1469–82.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Stanier P, Greene NDE. 2013. Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol. 12(8):799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini I, Focchi E, Rasile M, Morini R, Desiato G, et al. 2018. Maternal immune activation delays excitatory-to-inhibitory gamma-aminobutyric acid switch in offspring. Biol. Psychiatry 83(8):680–91 [DOI] [PubMed] [Google Scholar]
- Coulthard LG, Hawksworth OA, Conroy J, Lee JD, Woodruff TM. 2018a. Complement C3a receptor modulates embryonic neural progenitor cell proliferation and cognitive performance. Mol. Immunol 101:176–81 [DOI] [PubMed] [Google Scholar]
- Coulthard LG, Hawksworth OA, Li R, Balachandran A, Lee JD, et al. 2017. Complement C5aR1 signaling promotes polarization and proliferation of embryonic neural progenitor cells through PKCζ. J. Neurosci 37(22):5395–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulthard LG, Hawksworth OA, Woodruff TM. 2018b. Complement: the emerging architect of the developing brain. Trends Neurosci. 41(6):373–84 [DOI] [PubMed] [Google Scholar]
- Coyne CB, Lazear HM. 2016. Zika virus—reigniting the TORCH. Nat. Rev. Microbiol 14(11):707–15 [DOI] [PubMed] [Google Scholar]
- Dahlgren J, Samuelsson A-M, Jansson T, Holmäng A. 2006. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr. Res 60(2):147–51 [DOI] [PubMed] [Google Scholar]
- Davies NP, Buggins AG, Snijders RJ, Jenkins E, Layton DM, Nicolaides KH. 1992. Blood leucocyte count in the human fetus. Arch. Dis. Child 67(4):399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme-Axford E, Donker RB, Mouillet J-F, Chu T, Bayer A, et al. 2013. Human placental trophoblasts confer viral resistance to recipient cells. PNAS 110(29):12048–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny KJ, Coulthard LG, Jeanes A, Lisgo S, Simmons DG, et al. 2013. C5a receptor signaling prevents folate deficiency–induced neural tube defects in mice. J. Immunol 190(7):3493–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, et al. 2010. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med 207(5):1067–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond MM, Wilson GS, Melnick JL, Singer DB, Zion TE, et al. 1967. Congenital rubella encephalitis: course and early sequelae. J. Pediatr 71(3):311–31 [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. 2009. Cytokines and CNS development. Neuron 64(1):61–78 [DOI] [PubMed] [Google Scholar]
- Doehner J, Genoud C, Imhof C, Krstic D, Knuesel I. 2012. Extrusion of misfolded and aggregated proteins—a protective strategy of aging neurons? Eur. J. Neurosci 35(12):1938–50 [DOI] [PubMed] [Google Scholar]
- Dohi E, Choi EY, Rose IVL, Murata AS, Chow S, et al. 2017. Behavioral changes in mice lacking interleukin-33. eNeuro 4(6):ENEURO.0147–17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamko N, Geczy CL, Halliday GM. 2015. Inflammation is genetically implicated in Parkinson’s disease. Neuroscience 302:89–102 [DOI] [PubMed] [Google Scholar]
- Dziembowska M, Tham TN, Lau P, Vitry S, Lazarini F, Dubois-Dalcq M. 2005. A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors. Glia 50(3):258–69 [DOI] [PubMed] [Google Scholar]
- Ellman LM, Deicken RF, Vinogradov S, Kremen WS, Poole JH, et al. 2010. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr. Res 121(1–3):46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. 2011. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLOS ONE 6(10):e26317. Erratum. 2012. PLOS ONE 7(1). 10.1371/annotation/5dc1b3a6-ac3c-4c63-951d-cf355ffe7b48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A. 2013. Immunology of the maternal-fetal interface. Annu. Rev. Immunol 31:387–411 [DOI] [PubMed] [Google Scholar]
- Estes ML, McAllister AK. 2016. Maternal immune activation: implications for neuropsychiatric disorders. Science 353(6301):772–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairlie-Clarke K, Barbour M, Wilson C, Hridi SU, Allan D, Jiang H-R. 2018. Expression and function of IL-33/ST2 axis in the central nervous system under normal and diseased conditions. Front. Immunol 9:2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femenia T, Qian Y, Arentsen T, Forssberg H, Diaz Heijtz R. 2018. Toll-like receptor-4 regulates anxiety-like behavior and DARPP-32 phosphorylation. Brain Behav. Immun 69:273–82 [DOI] [PubMed] [Google Scholar]
- Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, et al. 2016. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 535(7612):425–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM. 2013. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol. Psychiatry 73(10):951–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades P, Ferguson-Smith AC, Burton GJ. 2002. Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23(1):3–19 [DOI] [PubMed] [Google Scholar]
- Girard S, Tremblay L, Lepage M, Sébire G. 2010. IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J. Immunol 184(7):3997–4005 [DOI] [PubMed] [Google Scholar]
- Gjoneska E, Pfenning AR, Mathys H, Quon G, Kundaje A, et al. 2015. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature 518(7539):365–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. 2010. Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Pan Y, Zhao W, Knable L, Vempati P, et al. 2013. IVIG immunotherapy protects against synaptic dysfunction in Alzheimer’s disease through complement anaphylatoxin C5a-mediated AMPA-CREB-C/EBP signaling pathway. Mol. Immunol 56(4):619–29 [DOI] [PubMed] [Google Scholar]
- Gorelik A, Sapir T, Haffner-Krausz R, Olender T, Woodruff TM, Reiner O. 2017. Developmental activities of the complement pathway in migrating neurons. Nat. Commun 8:15096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans MJ, Mummery C. 2000. Functional analysis of the TGFβ receptor/Smad pathway through gene ablation in mice. Int. J. Dev. Biol 44(3):253–65 [PubMed] [Google Scholar]
- Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, et al. 2018. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol. Psychiatry 83(2):109–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene NDE, Copp AJ. 2014. Neural tube defects. Annu. Rev. Neurosci 37:221–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Weiss S. 2005. CNTF/LIF/gp130 receptor complex signaling maintains a VZ precursor differentiation gradient in the developing ventral forebrain. Development 132(3):565–78 [DOI] [PubMed] [Google Scholar]
- Haida O, Al Sagheer T, Balbous A, Francheteau M, Matas E, et al. 2019. Sex-dependent behavioral deficits and neuropathology in a maternal immune activation model of autism. Transl. Psychiatry 9:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke ML, Kielian T. 2011. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin. Sci 121(9):367–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao LY, Hao XQ, Li SH, Li XH. 2010. Prenatal exposure to lipopolysaccharide results in cognitive deficits in age-increasing offspring rats. Neuroscience 166(3):763–70. Corrigendum. 2010. Neuroscience 169(1):555–57 [DOI] [PubMed] [Google Scholar]
- Hatta T, Moriyama K, Nakashima K, Taga T, Otani H. 2002. The role of gp130 in cerebral cortical development: in vivo functional analysis in a mouse ex utero system. J. Neurosci 22(13):5516–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty SV, O’Keeffe GW, Sullivan AM. 2013. BMP-Smad 1/5/8 signalling in the development of the nervous system. Prog. Neurobiol 109:28–41 [DOI] [PubMed] [Google Scholar]
- Heupel K, Sargsyan V, Plomp JJ, Rickmann M, Varoqueaux F, et al. 2008. Loss of transforming growth factor-beta 2 leads to impairment of central synapse function. Neural Dev. 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers L, Heinen Y, van Dam A-M, Lucassen PJ, Korosi A. 2016. Microglial priming and Alzheimer’s disease: a possible role for (early) immune challenges and epigenetics? Front. Hum. Neurosci 10:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann JA. 2003. The immune response of Drosophila. Nature 426(6962):33–38 [DOI] [PubMed] [Google Scholar]
- Holladay SD, Sharova LV, Punareewattana K, Hrubec TC, Gogal RM, et al. 2002. Maternal immune stimulation in mice decreases fetal malformations caused by teratogens. Int. Immunopharmacol 2(2–3):325–32 [DOI] [PubMed] [Google Scholar]
- Holladay SD, Sharova L, Smith BJ, Gogal RM, Ward DL, Blaylock BL. 2000. Nonspecific stimulation of the maternal immune system. I. Effects on teratogen-induced fetal malformations. Teratology 62(6):413–19 [DOI] [PubMed] [Google Scholar]
- Holmans P, Moskvina V, Jones L, Sharma M, Vedernikov A, et al. 2013. A pathway-based analysis provides additional support for an immune-related genetic susceptibility to Parkinson’s disease. Hum. Mol. Genet 22(5):1039–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PG, Jones CA. 2000. The development of the immune system during pregnancy and early life. Allergy 55(8):688–97 [DOI] [PubMed] [Google Scholar]
- Hong S, Song M-R. 2015. Signal transducer and activator of transcription-3 maintains the stemness of radial glia at mid-neurogenesis. J. Neurosci 35(3):1011–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi BL, Hunt JS, Atkinson JP. 1991. Differential expression of complement regulatory proteins on subpopulations of human trophoblast cells. J. Reprod. Immunol 19(3):209–23 [DOI] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. 2011. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav. Immun 25(4):604–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Y-F, Chen C-Y, Li W-C, Wang T-F, Hsueh Y-P. 2018a. Tlr7 deletion alters expression profiles of genes related to neural function and regulates mouse behaviors and contextual memory. Brain Behav. Immun 72:101–13 [DOI] [PubMed] [Google Scholar]
- Hung Y-F, Chen C-Y, Shih Y-C, Liu HY, Huang C-M, Hsueh Y-P. 2018b. Endosomal TLR3, TLR7, and TLR8 control neuronal morphology through different transcriptional programs. J. Cell Biol 217(8):2727–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson B, Hagberg G. 2004. Antenatal risk factors for cerebral palsy. Best Pract. Res. Clin. Obstet. Gynaecol 18(3):425–36 [DOI] [PubMed] [Google Scholar]
- Jeanes A, Coulthard LG, Mantovani S, Markham K, Woodruff TM. 2015. Co-ordinated expression of innate immune molecules during mouse neurulation. Mol. Immunol 68(2, Part A):253–60 [DOI] [PubMed] [Google Scholar]
- Jiang NM, Cowan M, Moonah SN, Petri WA. 2018. The impact of systemic inflammation on neurodevelopment. Trends Mol. Med 24(9):794–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima DT, Grueter BA. 2017. Toll-like receptor 4 deficiency alters nucleus accumbens synaptic physiology and drug reward behavior. PNAS 114(33):8865–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul D, Habbel P, Derkow K, Krüger C, Franzoni E, et al. 2012. Expression of Toll-like receptors in the developing brain. PLOS ONE 7(5):e37767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Daniels JL, Forssen U, Hultman C, Cnattingius S, et al. 2010. Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology 21(6):805–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner AC, Bilbo SD, Brown AS, Hsiao EY, McAllister AK, et al. 2019. Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology 44(2):245–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim H, Shin Yim Y, Ha S, Atarashi K, et al. 2017. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549(7673):528–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nat. Rev. Immunol 16(10):626–38 [DOI] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, et al. 2014. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol 10(11):643–60 [DOI] [PubMed] [Google Scholar]
- Koblar SA, Turnley AM, Classon BJ, Reid KL, Ware CB, et al. 1998. Neural precursor differentiation into astrocytes requires signaling through the leukemia inhibitory factor receptor. PNAS 95(6):3178–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, et al. 2012. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129(5):e1121–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, et al. 2012. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J. Neuroinflammation 9:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert CR, Frost EL, Bolte AC, Paysour MJ, Shaw ME, et al. 2018. Cutting edge: critical roles for microbiota-mediated regulation of the immune system in a prenatal immune activation model of autism. J. Immunol 201(3):845–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanning JC, Hilbelink DR, Chen LT. 1983. Teratogenic effects of endotoxin on the golden hamster. Teratog. Carcinog. Mutagen 3(2):145–49 [DOI] [PubMed] [Google Scholar]
- Larouche A, Roy M, Kadhim H, Tsanaclis AM, Fortin D, Sébire G. 2005. Neuronal injuries induced by perinatal hypoxic-ischemic insults are potentiated by prenatal exposure to lipopolysaccharide: animal model for perinatally acquired encephalopathy. Dev. Neurosci 27(2–4):134–42 [DOI] [PubMed] [Google Scholar]
- Lathia JD, Okun E, Tang S-C, Griffioen K, Cheng A, et al. 2008. Toll-like receptor 3 is a negative regulator of embryonic neural progenitor cell proliferation. J. Neurosci 28(51):13978–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. 2003. Role of the α-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia 42(2):139–48 [DOI] [PubMed] [Google Scholar]
- Lee JD, Coulthard LG, Woodruff TM. 2019. Complement dysregulation in the central nervous system during development and disease. Semin. Immunol 45:101340 [DOI] [PubMed] [Google Scholar]
- Lenz KM, Nelson LH. 2018. Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Front. Immunol 9:0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Santpere G, Imamura Kawasawa Y, Evgrafov OV, Gulden FO, et al. 2018. Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science 362(6420):eaat7615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberam I, Agalliu D, Nagasawa T, Ericson J, Jessell TM. 2005. A Cxcl12-CXCR4 chemokine signaling pathway defines the initial trajectory of mammalian motor axons. Neuron 47(5):667–79 [DOI] [PubMed] [Google Scholar]
- Ling Z, Gayle DA, Ma SY, Lipton JW, Tong CW, et al. 2001. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov. Disord 17(1):116–24 [DOI] [PubMed] [Google Scholar]
- Ling Z, Zhu Y, Tong CW, Snyder JA, Lipton JW, Carvey PM. 2006. Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp. Neurol 199(2):499–512 [DOI] [PubMed] [Google Scholar]
- Liu H-Y, Hong Y-F, Huang C-M, Chen C-Y, Huang T-N, Hsueh Y-P. 2013. TLR7 negatively regulates dendrite outgrowth through the Myd88–c-Fos–IL-6 pathway. J. Neurosci 33(28):11479–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Diao L, Huang C, Li Y, Zeng Y, Kwak-Kim JYH. 2017. The role of decidual immune cells on human pregnancy. J. Reprod. Immunol 124:44–53 [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. 2002. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. PNAS 99(10):7090–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, et al. 1998. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. PNAS 95(16):9448–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Cahill LS, Ho KT, Yang J, Kim H, et al. 2015. Experimental malaria in pregnancy induces neurocognitive injury in uninfected offspring via a C5a-C5a receptor dependent pathway. PLOS Pathog. 11(9):e1005140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U. 2019. Neurodevelopmental resilience and susceptibility to maternal immune activation. Trends Neurosci. 42(11):793–806 [DOI] [PubMed] [Google Scholar]
- Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. 2008. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol. Psychiatry 13(2):208–21 [DOI] [PubMed] [Google Scholar]
- Michaelson MD, Bieri PL, Mehler MF, Xu H, Arezzo JC, et al. 1996. CSF-1 deficiency in mice results in abnormal brain development. Development 122(9):2661–72 [DOI] [PubMed] [Google Scholar]
- Miller BJ, Culpepper N, Rapaport MH, Buckley P. 2013. Prenatal inflammation and neurodevelopment in schizophrenia: a review of human studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 42:92–100 [DOI] [PubMed] [Google Scholar]
- Mor G, Aldo P, Alvero AB. 2017. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol 17(8):469–82 [DOI] [PubMed] [Google Scholar]
- Nahmias AJ, Walls KW, Stewart JA, Herrmann KL, Flynt WJ. 1971. The ToRCH complex-perinatal infections associated with toxoplasma and rubella, cytomegol- and herpes simplex viruses. Pediatr. Res 5(8):405–6 [Google Scholar]
- Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, et al. 1999. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J. Neurosci 19(13):5429–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimgaonkar VL, Prasad KM, Chowdari KV, Severance EG, Yolken RH. 2017. The complement system: a gateway to gene–environment interactions in schizophrenia pathogenesis. Mol. Psychiatry 22(11):1554–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ödemis V, Lamp E, Pezeshki G, Moepps B, Schilling K, et al. 2005. Mice deficient in the chemokine receptor CXCR4 exhibit impaired limb innervation and myogenesis. Mol. Cell. Neurosci 30(4):494–505 [DOI] [PubMed] [Google Scholar]
- Okun E, Barak B, Saada-Madar R, Rothman SM, Griffioen KJ, et al. 2012. Evidence for a developmental role for TLR4 in learning and memory. PLOS ONE 7(10):e47522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Griffioen K, Barak B, Roberts NJ, Castro K, et al. 2010a. Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. PNAS 107(35):15625–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Mattson MP. 2011. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 34(5):269–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Son TG, Lee J-H, Roberts NJ, et al. 2010b. TLR2 activation inhibits embryonic neural progenitor cell proliferation. J. Neurochem 114(2):462–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy A, Altshuler G. 1976. Maternal endotoxemia, fetal anomalies, and central nervous system damage: a rat model of a human problem. Am. J. Obstet. Gynecol 124(2):196–204 [DOI] [PubMed] [Google Scholar]
- Paintlia MK, Paintlia AS, Barbosa E, Singh I, Singh AK. 2004. N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. J. Neurosci. Res 78(3):347–61 [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Ferretti MT. 2017. Function and dysfunction of microglia during brain development: consequences for synapses and neural circuits. Front. Synaptic Neurosci 9:0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Lee JY, Kim SJ, Choi S-Y, Yune TY, Ryu JH. 2015. Toll-like receptor-2 deficiency induces schizophrenia-like behaviors in mice. Sci. Rep 5:8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitto JM, McNamara RK, Gendreau PL, Huang Z, Jackson AJ. 1999. Impaired learning and memory and altered hippocampal neurodevelopment resulting from interleukin-2 gene deletion. J. Neurosci. Res 56(4):441–46 [DOI] [PubMed] [Google Scholar]
- Pineda E, Shin D, You SJ, Auvin S, Sankar R, Mazarati A. 2013. Maternal immune activation promotes hippocampal kindling epileptogenesis in mice. Ann. Neurol 74(1):11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi SH, Park J, Toso L, Abebe D, Roberson R, et al. 2005. No phenotype associated with established lipopolysaccharide model for cerebral palsy. Am. J. Obstet. Gynecol 192(3):727–33 [DOI] [PubMed] [Google Scholar]
- Polioudakis D, de la Torre-Ubieta L, Langerman J, Elkins AG, Shi X, et al. 2019. A single-cell transcriptomic atlas of human neocortical development during mid-gestation. Neuron 103(5):785–801.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. 1997. Role of colony-stimulating factor-1 in reproduction and development. Mol. Reprod. Dev 46(1):54–61 [DOI] [PubMed] [Google Scholar]
- Pollen AA, Nowakowski TJ, Chen J, Retallack H, Sandoval-Espinosa C, et al. 2015. Molecular identity of human outer radial glia during cortical development. Cell 163(1):55–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pousset F. 1994. Developmental expression of cytokine genes in the cortex and hippocampus of the rat central nervous system. Dev. Brain Res 81(1):143–46 [DOI] [PubMed] [Google Scholar]
- PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, et al. 2015. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat. Immunol 16(4):328–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prater MR, Johnson VJ, Germolec DR, Luster MI, Holladay SD. 2006. Maternal treatment with a high dose of CpG ODN during gestation alters fetal craniofacial and distal limb development in C57BL/6 mice. Vaccine 24(3):263–71 [DOI] [PubMed] [Google Scholar]
- Pronovost GN, Hsiao EY. 2019. Perinatal interactions between the microbiome, immunity, and neurodevelopment. Immunity 50(1):18–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qulu L, Daniels WMU, Mabandla MV. 2012. Exposure to prenatal stress enhances the development of seizures in young rats. Metab. Brain Dis 27(3):399–404 [DOI] [PubMed] [Google Scholar]
- Rechavi E, Lev A, Lee YN, Simon AJ, Yinon Y, et al. 2015. Timely and spatially regulated maturation of B and T cell repertoire during human fetal development. Sci. Transl. Med 7(276):276ra25 [DOI] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Ziv Y, Ronen A, et al. 2007. Toll-like receptors modulate adult hippocampal neurogenesis. Nat. Cell Biol 9(9):1081–88 [DOI] [PubMed] [Google Scholar]
- Roussa E, Wiehle M, Dünker N, Becker-Katins S, Oehlke O, Krieglstein K. 2006. Transforming growth factor β is required for differentiation of mouse mesencephalic progenitors into dopaminergic neurons in vitro and in vivo: ectopic induction in dorsal mesencephalon. Stem Cells 24(9):2120–29 [DOI] [PubMed] [Google Scholar]
- Rousset CI, Chalon S, Cantagrel S, Bodard S, Andres C, et al. 2006. Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatr. Res 59(3):428–33 [DOI] [PubMed] [Google Scholar]
- Rousset CI, Kassem J, Aubert A, Planchenault D, Gressens P, et al. 2013. Maternal exposure to lipopolysaccharide leads to transient motor dysfunction in neonatal rats. Dev. Neurosci 35(2–3):172–81 [DOI] [PubMed] [Google Scholar]
- Rousset CI, Kassem J, Olivier P, Chalon S, Gressens P, Saliba E. 2008. Antenatal bacterial endotoxin sensitizes the immature rat brain to postnatal excitotoxic injury. J. Neuropathol. Exp. Neurol 67(10):994–1000 [DOI] [PubMed] [Google Scholar]
- Saijo K, Winner B, Carson CT, Collier JG, Boyer L, et al. 2009. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137(1):47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S. 2000. Cytokine network at the feto-maternal interface. J. Reprod. Immunol 47(2):87–103 [DOI] [PubMed] [Google Scholar]
- Savard A, Brochu M-E, Chevin M, Guiraut C, Grbic D, Sébire G. 2015. Neuronal self-injury mediated by IL-1β and MMP-9 in a cerebral palsy model of severe neonatal encephalopathy induced by immune activation plus hypoxia-ischemia. J. Neuroinflammation 12:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophr. Work. Group Psychiatr. Genom. Consort. 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511(7510):421–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting GA, Henion TR, Nugent JD, Caplan B, Tobet S. 2006. Stromal cell-derived factor-1 (chemokine C-X-C motif ligand 12) and chemokine C-X-C motif receptor 4 are required for migration of gonadotropin-releasing hormone neurons to the forebrain. J. Neurosci 26(25):6834–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedel F, Béchade C, Vyas S, Triller A. 2004. Macrophage-derived tumor necrosis factor α, an early developmental signal for motoneuron death. J. Neurosci 24(9):2236–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, et al. 2016. Schizophrenia risk from complex variation of complement component 4. Nature 530(7589):177–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Buka SL, Cannon TD, Yolken RH. 2014. Maternal complement C1q and increased odds for psychosis in adult offspring. Schizophr. Res 159(1):14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Yim Y, Park A, Berrios J, Lafourcade M, Pascual LM, et al. 2017. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 549(7673):482–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinjyo N, Ståhlberg A, Dragunow M, Pekny M, Pekna M. 2009. Complement-derived anaphylatoxin C3a regulates in vitro differentiation and migration of neural progenitor cells. Stem Cells 27(11):2824–32 [DOI] [PubMed] [Google Scholar]
- Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. 2007. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci 27(40):10695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solek CM, Farooqi N, Verly M, Lim TK, Ruthazer ES. 2017. Maternal immune activation in neurodevelopmental disorders. Dev. Dyn 247(4):588–619 [DOI] [PubMed] [Google Scholar]
- Stefanski AL, Martinez N, Peterson LK, Callahan TJ, Treacy E, et al. 2019. Murine trophoblast-derived and pregnancy-associated exosome-enriched extracellular vesicle microRNAs: implications for placenta driven effects on maternal physiology. PLoS ONE 14(2):e0210675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B. 2012. The complement system: an unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci 35:369–89 [DOI] [PubMed] [Google Scholar]
- Stolp HB. 2013. Neuropoietic cytokines in normal brain development and neurodevelopmental disorders. Mol. Cell. Neurosci 53:63–68 [DOI] [PubMed] [Google Scholar]
- Stridh L, Mottahedin A, Johansson ME, Valdez RC, Northington F, et al. 2013. Toll-like receptor-3 activation increases the vulnerability of the neonatal brain to hypoxia-ischemia. J. Neurosci 33(29):12041–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Vestergaard M, Christensen J, Nahmias AJ, Olsen J. 2008. Prenatal exposure to maternal infections and epilepsy in childhood: a population-based cohort study. Pediatrics 121(5):e1100–7 [DOI] [PubMed] [Google Scholar]
- Sun Y, Vestergaard M, Christensen J, Olsen J. 2010. Prenatal exposure to elevated maternal body temperature and risk of epilepsy in childhood: a population-based pregnancy cohort study. Paediatr. Perinat. Epidemiol 25(1):53–59 [DOI] [PubMed] [Google Scholar]
- Taubeneck MW, Daston GP, Rogers JM, Gershwin ME, Ansari A, Keen CL. 1995. Tumor necrosis factor-α alters maternal and embryonic zinc metabolism and is developmentally toxic in mice. J. Nutr 125(4):908–19 [DOI] [PubMed] [Google Scholar]
- Thaxton JE, Romero R, Sharma S. 2009. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J. Immunol 183(2):1144–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C, Lipuma L, Gobourne A, Viale A, Leiner I, et al. 2012. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J. Exp. Med 209(8):1445–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, et al. 2018. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 359(6381):1269–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillermot S, Joodmardi E, Perlmann T, Ögren SO, Feldon J, Meyer U. 2012. Prenatal immune activation interacts with genetic Nurr1 deficiency in the development of attentional impairments. J. Neurosci 32(2):436–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, et al. 2012. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol 13(8):753–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead E, Dodds L, Joseph KS, Gordon KE, Wood E, et al. 2006. Relation of pregnancy and neonatal factors to subsequent development of childhood epilepsy: a population-based cohort study. Pediatrics 117(4):1298–306 [DOI] [PubMed] [Google Scholar]
- Williams PJ, Searle RF, Robson SC, Innes BA, Bulmer JN. 2009. Decidual leucocyte populations in early to late gestation normal human pregnancy. J. Reprod. Immunol 82(1):24–31 [DOI] [PubMed] [Google Scholar]
- Wu W-L, Hsiao EY, Yan Z, Mazmanian SK, Patterson PH. 2017. The placental interleukin-6 signaling controls fetal brain development and behavior. Brain Behav. Immun 62:11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, et al. 2015. Association of maternal diabetes with autism in offspring. JAMA 313(14):1425–34. Correction. 2017. JAMA 317(5):538 [DOI] [PubMed] [Google Scholar]
- Yasuoka S, Kawanokuchi J, Parajuli B, Jin S, Doi Y, et al. 2011. Production and functions of IL-33 in the central nervous system. Brain Res. 1385:8–17 [DOI] [PubMed] [Google Scholar]
- Yockey LJ, Iwasaki A. 2018. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 49(3):397–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BH, Jun JK, Romero R, Park KH, Gomez R, et al. 1997. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1β, and tumor necrosis factor-α), neonatal brain white matter lesions, and cerebral palsy. Am. J. Obstet. Gynecol 177(1):19–26 [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, et al. 2000. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am. J. Obstet. Gynecol 182(3):675–81 [DOI] [PubMed] [Google Scholar]
- Yoshimatsu T. 2006. Non-cell-autonomous action of STAT3 in maintenance of neural precursor cells in the mouse neocortex. Development 133(13):2553–63 [DOI] [PubMed] [Google Scholar]
- Young GR, Eksmond U, Salcedo R, Alexopoulou L, Stoye JP, Kassiotis G. 2012. Resurrection of endogenous retroviruses in antibody-deficient mice. Nature 491(7426):774–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Lübben W, Slomka H, Gebler J, Konert M, et al. 2012. Nucleic acid-sensing Toll-like receptors are essential for the control of endogenous retrovirus viremia and ERV-induced tumors. Immunity 37(5):867–79 [DOI] [PubMed] [Google Scholar]
- Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. 2004. Transfer of inflammatory cytokines across the placenta. Obstet. Gynecol 103(3):546–50 [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen Y-H, Wang H, Ji Y-L, Ning H, et al. 2008. Reactive oxygen species contribute to lipopolysaccharide-induced teratogenesis in mice. Toxicol. Sci 103(1):149–57 [DOI] [PubMed] [Google Scholar]
- Zhao B, Schwartz JP. 1998. Involvement of cytokines in normal CNS development and neurological diseases: recent progress and perspectives. J. Neurosci. Res 52(1):7–16 [DOI] [PubMed] [Google Scholar]
- Zhong S, Zhang S, Fan X, Wu Q, Yan L, et al. 2018. A single-cell RNA-seq survey of the developmental landscape of the human prefrontal cortex. Nature 555(7697):524–28 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Matsumoto T, Mikami S, Nagasawa T, Murakami F. 2009. SDF1/CXCR4 signalling regulates two distinct processes of precerebellar neuronal migration and its depletion leads to abnormal pontine nuclei formation. Development 136(11):1919–28 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yu T, Zhang X-C, Nagasawa T, Wu JY, Rao Y. 2002. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat. Neurosci 5(8):719–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y-R, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. 1998. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393(6685):595– [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.