Abstract
Purpose
The aim of this study is to evaluate the efficacy, feasibility and acceptability of a co-designed lifestyle-focused text message intervention (EMPOWER-SMS) for breast cancer survivors’ self-efficacy, quality of life (QOL), mental (anxiety, depression, stress) and physical (endocrine therapy medication adherence, physical activity, BMI) health.
Methods
Single-blind randomised controlled trial (1:1) comparing EMPOWER-SMS to usual care at 6-months (intention-to-treat). Setting: public Breast Cancer Institute (Sydney, Australia). Eligibility criteria: adult (> 18 years) females, < 18-months post-active breast cancer treatment (stage I-III), owned a mobile phone, written informed consent. Primary outcome: Self-Efficacy for Managing Chronic Disease Scale at 6 months. Process data: message delivery analytics, cost, and post-intervention survey.
Results
Participants (N = 160; mean age ± SD 55.1 ± 11.1 years) were recruited 29th-March-2019 to 7th-May-2020 and randomised (n = 80 EMPOWER-SMS: n = 80 control). Baseline mean self-efficacy was high (I: 7.1 [95%CI 6.6, 7.5], C: 7.4 [7, 7.8]). Six-month follow-up: no significant differences between groups for self-efficacy (I: 7.6 [7.3, 7.9], C: 7.6 [7.3, 7.9], adjusted mean difference 0 (95%CI 0.4, 0.4), QOL, mental health, physical activity, or BMI. Significantly less EMPOWER-SMS participants missed ≥ 1 endocrine therapy medication doses compared to control (I: 3/42[7.1%], C: 8/47[17.0%], Adjusted RR 0.13 [95%CI 0.02, 0.91]). Text messages were delivered successfully (7925/8061, 98.3%), costing $13.62USD/participant. Participants strongly/agreed EMPOWER-SMS was easy-to-understand (64/64; 100%), useful (58/64; 90.6%), motivating for lifestyle change (43/64; 67.2%) and medication adherence (22/46; 47.8%).
Conclusion
EMPOWER-SMS was feasible, inexpensive, acceptable for delivering health information to breast cancer survivors between medical appointments, with minor improvements in medication adherence.
Implications for Cancer Survivors
Text messages offer a feasible strategy for continuity-of-care between medical appointments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11764-022-01209-9.
Keywords: Text messaging, Breast cancer, Cancer survivorship, Mobile health, Randomised controlled trial, Telemedicine
Introduction
Breast cancer is the most commonly diagnosed cancer among women worldwide and five-year survival rates are high (85–90% in high-income countries) [1]. However, the number of lost disability adjusted life years [DALYs] among breast cancer survivors is high globally (17,708,600 DALYs) [1]. The greatest contributors to DALYs are modifiable risk factors, including overweight and obesity, unhealthy diet, physical inactivity and smoking [1]. Managing modifiable risks can be challenging due to treatment and endocrine therapy medication side effects (e.g. hot flushes, fatigue) that negatively affect women’s mental and physical health [2]. Health education can improve self-efficacy (self-confidence) for managing health [3–6] and promote healthy lifestyles after active treatment (surgery, chemotherapy, radiation) [7]. However, survivorship education programs are scarce [8], resource-intensive, and rarely co-designed with end-users, which may reduce effectiveness [7]. Novel, scalable and co-designed post-treatment health programs are urgently needed.
Mobile health (mHealth) interventions (e.g. mobile applications, text messaging) are strategies for providing health information remotely [9]. The most accessible mHealth intervention is text messages, as over 5 billion people own mobile phones globally [10]. Randomised clinical trials (RCTs) show that health education via text messages is effective for improving various modifiable risks [11–14]. There is limited but growing evidence that text message interventions may improve modifiable risks for individuals with breast cancer, including adherence to endocrine therapy medication [12] and weight maintenance [13]. However, few interventions were co-designed with breast cancer survivors [15].
Our team co-designed the EMPOWER-SMS text message program with breast cancer survivors, health professionals and researchers, which aims to improve breast cancer survivors’ health self-efficacy, quality of life (QOL), mental (anxiety, depression, stress) and physical (BMI, physical activity, medication adherence) health [16]. We hypothesized that participants who received the six-month EMPOWER-SMS intervention would have improved self-efficacy, QOL and health outcomes compared to usual care (control group) at six-month follow-up.
Methods
Trial design
The study protocol is published [17]. Briefly, this study was a single-blind RCT (1:1) comparing EMPOWER-SMS to usual care for improving breast cancer survivors’ self-efficacy, QOL and mental and physical health at 6-month follow-up. The study followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines (Supplementary Material 1: CONSORT checklist). Ethics approval was received from Western Sydney Local Health District Human Ethics Research Committee (AU RED HREC/18/WMEAD/281) and all participants provided written informed consent. Clinical trial registration: ANZCTR12618002020268, http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12618002020268, 17-December-2018). University of Sydney was the study sponsor.
Study population
Participants were recruited from the Westmead Breast Cancer Institute in Western Sydney, Australia that serves a culturally and socioeconomically diverse population. Inclusion criteria: adult (> 18 years) females, diagnosed with early-stage (0–III) breast cancer, within 18 months of finishing active breast cancer treatment (could be taking endocrine therapy medication, e.g. Tamoxifen, Aromatase inhibitors) and owned a mobile phone. Exclusion criteria: diagnosed with metastatic breast cancer or insufficient English to provide consent.
Control group
The control group received usual medical care according to the breast cancer institute, which included access to breast care nursing and allied health support (psychologist, dietician, physiotherapist). Participants received one ‘welcome’ text message at baseline, containing their group allocation, and one ‘follow-up interview reminder’ text message at six-months.
Intervention group
EMPOWER-SMS aimed to provide supportive health education and was co-designed by breast cancer survivors, health professionals and researchers [16]. Participants received usual care, a ‘welcome’ text message, EMPOWER-SMS and a ‘follow-up interview reminder’ message. EMPOWER-SMS delivered four text messages per week for 6 months (104 messages total) regarding (i) physical activity and healthy diet, (ii) social and emotional wellbeing, (iii) medication adherence and side effects management and (iv) general breast cancer information. Participants taking endocrine therapy medications received 26 text messages from each topic. Other participants received messages regarding physical activity and healthy diet (39/104; 37.5%), social and emotional wellbeing (27/104; 26.0%) and general breast cancer information (38/104; 36.5%). Messages were positively toned, semi-personalised with the participant’s preferred name and designed to be appropriate for individuals with a Grade 7 (Flesch Kincaid) reading level [16]. One-third (33/104; 33.0%) of messages included weblinks to additional science-based information and free resources. Participants were advised not to reply (one-way delivery). However, for safety, an unblinded health counsellor monitored the automated text message delivery system for replies.
Outcomes
Study visits were in-person at baseline and 6 months [17]. Briefly, at baseline, participants provided self-reported demographics (age, sex, ethnicity, country of birth), medical history and chronic disease risk factors (confirmed in medical files). The primary outcome was self-efficacy measured by the Self-Efficacy for Managing Chronic Disease Scale [18] (Cronbach alpha range: 0.88–0.95) [19], which measures confidence for managing six domains (fatigue, physical discomfort, emotional distress, other health problems, achieving health management tasks and things other than medication) on a scale from 1 to 10 (not confident at all to totally confident). The mean score out of 10 across domains is calculated, with higher scores reflecting higher self-efficacy. Secondary outcomes included:
Clinical and lifestyle measures
BMI (healthy range: BMI ≤ 25kg/m2), body fat percentage and skeletal muscle mass measured using the Seca medical Body Composition Analyser (Seca GmbH & Co. KG, Hamburg, Germany) [20] and waist circumference (cm) measured using standard tape measurement [21]. Self-reported nutrition behaviours included number of fruits, vegetables, red meat and standard alcoholic drinks consumed in 7 days [22]. Self-reported physical activity was measured using the Global Physical Activity Questionnaire [GPAQ] [21] and validated using an ActiGraph™ GT3X+ (ActiGraph, Pensacola, FL) accelerometer and wear-time log-book with 32/160 (20%) participants [23]. Accelerometers were worn for 7 days after baseline and follow-up visits and then completed the GPAQ. Adherence to endocrine therapy medication was measured by self-reported missed doses within the last 7 days. Australian guideline cut points [24, 25] are presented in Table 2.
Table 2.
Primary and secondary outcomes at baseline and 6-month follow-up
| Baseline | Six-month follow-up | |||||
|---|---|---|---|---|---|---|
| EMPOWER-SMS (n = 78) | Control (n = 78) | EMPOWER-SMS (n = 78) | Control (n = 78) | |||
| Mean months (SD) | Mean months (SD) | Mean months (SD) | ||||
| Time from randomisation to follow-up | 7.1 (1.6) | 7.1 (1.5) | 7.1 (1.5) | |||
| Primary outcome | Mean (95%CI) | Mean (95%CI) | Mean difference (95%CI) | Adjusted mean (95%CI) | Adjusted mean (95%CI) | Adjusted mean difference (95%CI) |
| Self-efficacy | 7.1 (6.6, 7.5) | 7.4 (7, 7.8) | − 0.3 (− 1, 0.3) | 7.6 (7.3, 7.9) | 7.6 (7.3, 7.9) | 0 (− 0.4, 0.4) |
| Secondary outcomes | ||||||
| Quality of life | 69 (63.8, 74.2) | 70.4 (65.3, 75.6) | − 1.4 (− 8.8, 5.9) | 74.3 (70.9, 77.7) | 73 (69.8, 76.2) | 1.3 (− 3.3, 6) |
| Depressive symptoms | 18.8 (15.1, 22.4) | 17.6 (14.1, 21.2) | 1.1 (− 4, 6.2) | 17.8 (14.6, 21.1) | 18.5 (15.5, 21.5) | − 0.7 (− 5.1, 3.7) |
| Anxiety symptoms | 20.7 (16.4, 25) | 17.8 (13.6, 22) | 2.9 (− 3.1, 8.9) | 19.3 (15.8, 22.8) | 20.1 (16.8, 23.4) | − 0.8 (− 5.6, 4) |
| Stress symptoms | 17 (13.3, 20.7) | 14.5 (10.9, 18.2) | 2.5 (− 2.8, 7.7) | 14.5 (11.5, 17.6) | 15.6 (12.7, 18.4) | − 1 (− 5.2, 3.1) |
| Physical activity, METS$ | 1668.7 (1108.2, 2229.3) | 1805.4 (1244.9, 2366) | − 136.7 (− 929.5, 656) | 1940 (1344.7, 2535.4) | 1747.4 (1152, 2342.8) | 192.6 (− 649.5, 1034.8) |
| Clinical | ||||||
| BMI& kg/m2 | 33.7 (27.6, 39.9) | 27.6 (21.4, 33.7) (n = 77) | 6.2 (− 2.5, 14.9) (n = 155) | 29 (27.6, 30.4) (n = 60) | 27.5 (26.2, 28.9) (n = 73) | 1.4 (− 0.5, 3.4) |
| Waist circumference, cm | 95.3 (92.2, 98.3) | 91.4 (88.3, 94.4) (n = 76) | 3.9 (− 0.4, 8.2) (n = 154) | 93.1 (91.7, 94.5) (n = 56) | 92.1 (90.8, 93.3) (n = 68) | 1 (− 0.8, 2.9) |
| Fat Mass Percentage % | 42.7 (41.3, 44.1) (n = 74) | 41.4 (40, 42.9) (n = 71) | 1.3 (− 0.7, 3.3) (n = 145) | 41.9 (41, 42.9) (n = 25) | 42.9 (41.9, 43.9) (n = 27) | − 1 (− 2.4, 0.4) |
| Skeletal muscle mass Percentage % | 25.4 (24.8, 25.9) (n = 74) | 25 (24.3, 25.6) (n = 70) | 0.4 (− 0.4, 1.3) | 24.6 (24.1, 25.1) (n = 25) | 24.7 (24.2, 25.2) (n = 27) | − 0.1 (− 0.8, 0.6) |
| Lifestyle | ||||||
| Endocrine medication adherence (≥ 1 missed doses in the last 7 days) | 6/46 (13) | 6/51 (11.8) | 1.28 (− 11.87, 14.43)^ | 3/42 (7.1) | 8/47 (17) | 0.13 (0.02, 0.91)+* |
| Servings of fruit per day, mean (SD#) | 1.7 (1.5, 2) (n = 77) | 1.7 (1.5, 1.9) | 0.1 (− 0.3, 0.4) | 1.5 (1.3, 1.7) | 1.7 (1.5, 1.9) | − 0.2 (− 0.4, 0) |
| Servings of vegetables per day, mean (SD) | 4.5 (4.1, 4.9) (n = 77) | 3.7 (3.3, 4.1) | 0.8 (0.2, 1.4)** | 3.9 (3.5, 4.4) | 4.2 (3.8, 4.6) | − 0.3 (− 0.9, 0.3) |
| Servings of red meat per week, mean (SD) | 2.6 (2.1, 3) (n = 77) | 2 (1.6, 2.5) | 0.5 (− 0.1, 1.2) | 1.8 (1.4, 2.2) | 1.8 (1.5, 2.2) | 0 (− 0.6, 0.5) |
| ≥ 1 Standard Alcoholic drinks per week, n/N (%) | 32/77 (41.6) | 20/78 (25.6) | 15.92 (1.25, 30.58)^* | 22/65 (33.8) | 16/74 (21.6) | 0.88 (0.68, 1.15)+ |
| Mean number standard alcoholic drinks per week (95%CI) | 4.6 (3.2, 6.1) | 4.6 (2.8, 6.4) | 0.1 (− 2.2, 2.3) | 4.4 (2.8, 6) | 6.2 (4.5, 8) | − 1.8 (− 4.2, 0.5) |
| Takeaway meals/week | 1.9 (1.3, 2.5) (n = 77) | 1.3 (0.7, 1.9) | 0.6 (− 0.2, 1.4) | 1.1 (0.8, 1.5) (n = 65) | 1.2 (0.9, 1.5) (n = 74) | − 0.1 (− 0.5, 0.4) |
^Percent difference (95% confidence interval), +adjusted relative risk (95% confidence interval), #standard deviation, $metabolic equivalents, &body mass index
*p < 0.05; **p < 0.01
QOL and mental health
QOL (European Organization for Research and Treatment of Cancer QOL Questionnaire–Core [EORTC QLQ-C30]; and Breast Cancer subscale [EORTC QLQ-BR23]) [26]. Two questions about health and QOL during the past week (7-point Likert scale; 1: very poor to 7: excellent) form the global health status/QOL score that is transformed to a 0–100 scale; higher scores represent higher QOL. Depression Anxiety and Stress scale (DASS-21) measures occurrences of certain behaviours on a 4-point Likert scale (0 = does not apply to me, 3 = Applied to me most of the time) [27]. Depressive, anxiety and stress symptom subscales each have 7-items and scores are doubled (range 0–42); higher scores reflect higher depressive, anxiety and stress symptoms.
Illness perceptions
Brief Illness Perception Questionnaire [BIPQ] [28] is scored on a 10-point Likert scale across eight domains: disease consequences, timeline, personal control, treatment effectiveness, symptoms, concern, illness understanding, affected emotionally. The final question asks what participants believe were the three most important causes of their breast cancer (free-text response), which were analysed thematically.
Sample size
A mean difference of 1 (SD 2.05) on the Self-Efficacy for Managing Chronic Disease Scale (6 items) between EMPOWER-SMS and control at 6 months was considered clinically meaningful [18]. A total of 160 participants (80 EMPOWER-SMS: 80 control) were needed to achieve 80% power, with a 5% type I error rate and 20% dropout rate.
Randomisation and masking
Participants were randomised in a 1:1 (EMPOWER-SMS: control) allocation ratio, using a secured central computer-based randomisation service (R statistical software version 3.6.1; ©The R Foundation). Group allocation was automatically concealed using computer software (Research Electronic Data Capture [REDCap]), which revealed codes ‘Group A’ or ‘Group B’ to the researcher, maintaining researcher blinding. A subsample of 32/160 (20%) participants were randomised to wear an accelerometer, stratified by group (16 EMPOWER-SMS; 16 control), which notified researchers using a computer-generated notification. On the Monday after enrolment, the blinded researcher submitted the ‘Group A’ or ‘Group B’ allocation into the text message software, which automatically sent the ‘welcome’ text message containing participants’ group allocation (EMPOWER-SMS or control). Participants were instructed not to share their group allocation with the research team. A blinded researcher conducted the follow-up interview.
Statistical methods
Analyses were pre-specified and performed according to the intention-to-treat principle by a blinded statistician [17]. Primary and secondary outcomes were summarised as means and corresponding 95% confidence intervals (CIs) or standard deviations (SD), or if the distribution was skewed, as medians and interquartile intervals (IQI) for continuous variables and as frequencies and percentages for categorical variables. The outcomes were compared between EMPOWER-SMS and control groups at six-months, adjusting for the baseline measure of the outcome, with a significance level of 0.05. Dichotomous outcomes were analysed using log-binomial regression and for continuous outcomes, the analysis of covariance (ANCOVA).
To validate the GPAQ, metabolic equivalents (MET) minutes/day for moderate-to-vigorous physical activity (MVPA) were compared between the self-reported and accelerometer-assessed physical activity. Freedson and colleagues (1998) [23] cut-points were used to define accelerometer MVPA. Total MVPA minutes were divided by the number of days participants wore the accelerometer and MET minutes/day was estimated: minutes of moderate activity × 4 METs plus minutes of vigorous activity × 8 METs. The median MET minutes/day and IQI for accelerometer-assessed and self-reported physical activity were reported, and Spearman correlation coefficients were used to assess the correlation between the measurements. MET minutes/day at follow up was compared between EMPOWER-SMS and control groups using exact Wilcoxon rank sum test.
Process evaluation
Program delivered as planned
Automated text message delivery software (April 2019–November 2020) collected the number of text messages that were sent, delivered successfully or unsuccessfully (‘bounced’), resulted in an ‘opt-out’ or a reply (number, content, and type [text, photo, ‘reaction’, ‘emoji’]). ‘Reactions’ are when a participant clicks on a text message, then clicks that they ‘liked’, ‘loved’ or ‘laughed at’ the message.
Program delivery costs
Text message delivery data were used to estimate the cost per person of intervention delivery. Total staff time dedicated to intervention monitoring was estimated.
Program acceptability and utility
End-of-study feedback survey data were collected, including 13 questions: ten 5-point Likert-scale items (1 = strongly disagree to 5 = strongly agree) and three yes/no. Question topics included participants’ perceived acceptability and usefulness of repeated text messages, delivery timing, and content suitability for breast cancer survivors.
Results
Characteristics of the participants
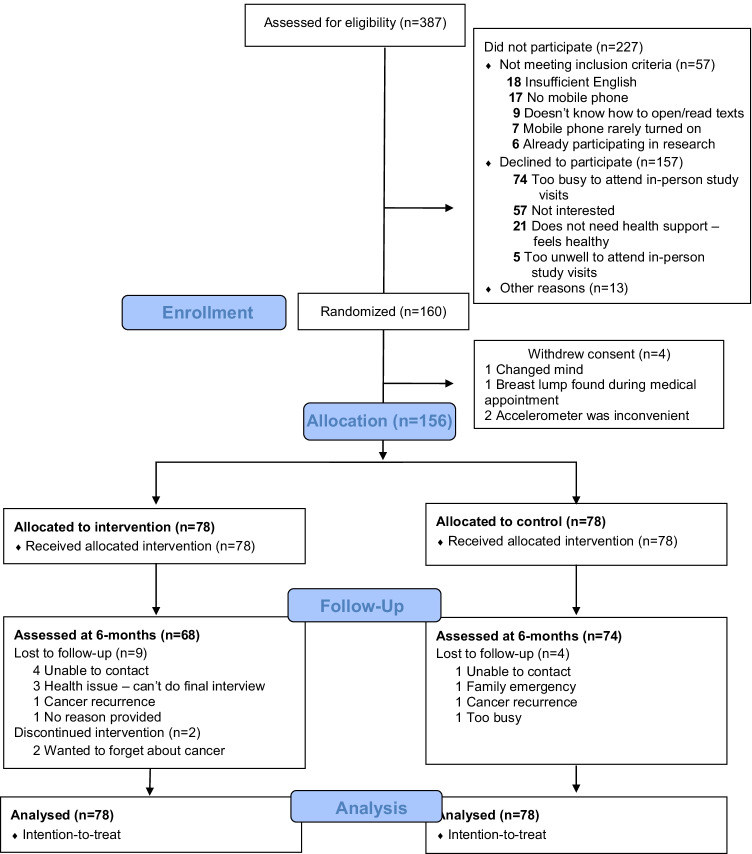
From March 2019 to May 2020, 387 patients were assessed for eligibility and approached; 227 did not participate (157 declined; 57 did not meet inclusion criteria) and 160 enrolled and were randomised (see Fig. 1). Due to COVID-19 restrictions, 4/160 (2.5%) participants were recruited and 79/160 (49%) completed follow-up visits over the phone and provided self-reported weight, waist circumference and height measurements based on detailed instructions from the research team. Before completing the baseline visit or receiving the text message with the group allocation, four participants withdrew consent (reasons provided in Fig. 1). Therefore, 156 were allocated to EMPOWER-SMS (n = 78) or control (n = 78) groups and were included in the analysis. At 6 months, 14/156 (9.0%) participants did not complete the follow-up visit and 2/78 (2.5%) discontinued the intervention (Fig. 1).
Fig. 1.
CONSORT flow diagram
At baseline, participants were mean ± SD of 8.0 ± 5.0 months post-active treatment, mean age ± SD 55.0 ± 11.0 years and characteristics were not significantly different between groups, except the EMPOWER-SMS group had more smokers and ex-smokers compared to control (Table 1). No significant differences in primary or secondary outcomes between groups were found at baseline, except EMPOWER-SMS group had higher mean vegetable intake, and higher proportion of people who consumed > 1 standard alcoholic drink per week compared to the control group (Table 2). Overall, few participants met post-treatment secondary prevention guidelines at baseline or were within healthy ranges for depression, anxiety or stress, and significantly fewer EMPOWER-SMS participants met guidelines for waist circumference than controls (Supplementary Material 2). Baseline QOL subscales revealed EMPOWER-SMS participants had significantly lower scores for body image and higher sexual enjoyment than controls (Supplementary Material 3).
Table 1.
Baseline characteristics
| Characteristics | No./Total (%) | ||
|---|---|---|---|
| EMPOWER-SMS (n = 78) | Control (n = 78) | Total (N = 156) | |
| Time between finishing active treatment to enrolment, mean months (SD+) | 8 (5) | 8.1 (5) | 8 (5) |
| Demographics | |||
| Age (years), mean (SD) | 53.8 (9.6) | 55.7 (12.1) | 54.8 (10.9) |
| Ethnicity | |||
| Caucasian | 37/78 (47.4) | 32/78 (41.0) | 69/156 (44.2) |
| South Asian (Bangladesh, India, Nepal, Pakistan, Sri Lanka) | 8/78 (9.0) | 13/78 (16.7) | 21/156 (13.4) |
| Other Asian | 10/78 (12.8) | 20/78 (25.6) | 56/156 (35.9) |
| Other | 18/78 (23.1) | 13/78 (16.7) | 31/156 (19.9) |
| Country or region of birth | |||
| Australia | 37/78 (47.4) | 27/78 (34.6) | 64/156 (41) |
| Europe/United Kingdom | 6/78 (7.8) | 8/78 (10.2) | 14/78 (17.9) |
| India | 5/78 (6.4) | 8/78 (10.2) | 13/78 (16.7) |
| Middle East (Including Pakistan, Afghanistan, Egypt) | 3/78 (3.8) | 7/78 (9.0) | 10/78 (12.8) |
| Southeast Asia (Philippines, Thailand, Laos) | 11/78 (14.1) | 10/78 (12.8) | 21/78 (26.9) |
| Pacific Islands (Including New Zealand, Tonga, Fiji) | 9/78 (11.5) | 6/78 (7.7) | 15/78 (19.2) |
| Other | 7/78 (9.0) | 12/78 (15.4) | 19/156 (24.0) |
| Education | |||
| Year (Grade) 12 or below | 26/76 (34.3) | 18/78 (23.1) | 44/154 (28.6) |
| Diploma/technical degree | 22/76 (28.9) | 21/78 (26.9) | 43/154 (27.9) |
| Undergraduate/postgraduate degree | 28/76 (36.8) | 39/78 (50.0) | 67/154 (43.5) |
| Marital status | |||
| Single/widowed | 16/77 (20.8) | 12/78 (15.4) | 28/155 (18.1) |
| DeFacto/married | 50/77 (64.9) | 55/78 (70.5) | 105/155 (67.7) |
| Separated/divorced | 11/77 (14.3) | 11/78 (14.1) | 22/155 (14.2) |
| Employment status | |||
| Working full/part time | 49/75 (65.3) | 44/78 (56.4) | 93/153 (60.8) |
| Unemployed | 9/75 (12.0) | 13/78 (16.7) | 22/153 (14.4) |
| Retired | 14/75 (18.7) | 18/78 (23.1) | 32/153 (20.9) |
| Other | 3/75 (4) | 3/78 (3.8) | 6/153 (3.9) |
| Children, # yes | 61/78 (78.2) | 68/78 (87.2) | 129/156 (82.7) |
| Medical history | |||
| Tumour removal surgery | 78/78 (100) | 77/78 (98.7) | 155/156 (99.4) |
| Radiotherapy | 69/78 (88.5) | 69/78 (88.5) | 138/156 (88.5) |
| Chemotherapy | 48/77 (62.3) | 50/78 (64.1) | 98/155 (63.2) |
| Endocrine therapy | 52/78 (66.7) | 55/78 (70.5) | 107/156 (68.6) |
| Targeted therapy | 12/78 (15.4) | 14/77 (18.2) | 26/155 (16.8) |
| High cholesterol diagnosis | 11/78 (14.1) | 18/78 (23.1) | 29/156 (18.6) |
| High blood pressure diagnosis | 17/78 (21.8) | 27/78 (34.6) | 44/156 (28.2) |
| CVD diagnosis | 3/78 (3.8) | 4/78 (5.1) | 7/156 (4.5) |
| Smoking status | |||
| Current smoker | 8/78 (10.3) | 4/78 (5.1)* | 12/156 (7.7) |
| Ex-smoker | 31/78 (39.7) | 15/78 (19.2)* | 46/156 (29.5) |
| Never smoked | 39/78 (50) | 59/78 (75.6)* | 98/156 (62.8) |
+Standard deviation; *p < 0.05
Effectiveness of the intervention
Self-efficacy data were available for 138/158 (87.3%) randomised participants (66/78 [84.6%] EMPOWER-SMS; 72/78 [92.3%] control). There were no significant differences in self-efficacy at 6 months between EMPOWER-SMS and control groups (7.6 [95% CI 7.3, 7.9] and 7.6 [7.3, 7.9], respectively, Adjusted mean difference 0 [95% CI − 0.4, 0.4], p = 0.924). A sensitivity analysis using complete case analysis was conducted to confirm the results, as there was more than 5% missing (completely-at-random) data and revealed no significant differences between EMPOWER-SMS and control groups (7.6 [95% CI 7.3, 7.9] and 7.6 [95% CI 7.3, 7.9], respectively, p = 0.925). Overall, few participants missed ≥ 1 doses of endocrine therapy medication. However, there was a significant difference between groups, with EMPOWER-SMS participants missing less doses than control (I: 3/42 [7.1%], C: 8/47 [17.0%], Adjusted RR 0.13 [95% CI 0.02, 0.91], p = 0.040). There were no other significant differences between groups (Table 2; Supplementary Material 3). The proportion of participants meeting guideline recommendations was low and there were no significant differences between groups (Supplementary Material 2). Only 5/78 (6.4%) participants in the EMPOWER-SMS group and 3/78 (3.8%) in the control group were ‘current smokers’ at follow-up.
Accelerometer data were available for 26/32 (81.2%) participants at baseline (14/16; 87.5% EMPOWER-SMS, 12/16; 75.0% control) and 20/32 (62.5%) participants at follow-up (10/16; 62.5% EMPOWER-SMS, 10/16; 62.5% control). Overall, self-reported physical activity was over-estimated compared to accelerometer data at follow-up with a very weak negative correlation (median GPAQ MET minutes/day [IQR] 300.00 [220.0, 1080.0], median accelerometer MET minutes/day [IQR] 168.39 [124.0, 227.1], r = − 0.09, p = 0.691) and was over-estimated in the EMPOWER-SMS group (strong negative, but not significant, correlation 240.00 [220.0, 1080.0] vs. 187.02 [146.5, 227.2]. r = − 0.61, p = 0.062) and control group (weak positive correlation 480.00 [120.0, 1650.0] vs. 164.8 [101.5, 201.4], r = 0.36, p = 0.307). Wilcox rank sum test found that accelerometer METS did not differ between groups (median [IQI] 187 [146.5, 227.2] and 164.8 [101.5, 201.4], respectively, p = 0.796).
At follow-up, there were no significant differences between groups for participants’ illness perceptions across the 8 domains (BIPQ; Supplementary Material 4). Overall, participants reported that breast cancer minimally affected their life (mean ± SD 4.3 ± 3.0) and did not experience breast cancer symptoms much (3.8 ± 2.8). Participants felt that their treatment could help their breast cancer (7.8 ± 2.3) and they felt that they understand their breast cancer well (8.1 ± 1.7). However, they felt they had moderate control over their breast cancer (5.0 ± 3.0), concerns about their breast cancer (5.7 ± 2.9) and reported that their breast cancer affected them emotionally (5.0 ± 3.0). When participants were asked what they thought caused their breast cancer (free-text), several common themes emerged: ‘stress’, ‘genetics/family history’, ‘not sure’, ‘bad luck/chance’, ‘age’, ‘oestrogen/hormones’ and ‘unhealthy lifestyle’ (included subthemes unhealthy diet, physical inactivity, obesity, smoking and alcohol). Participants ranked the three most important causes of their breast cancer as (1) unhealthy lifestyle, (2) stress and (3) not sure.
Process evaluation
Evaluation of program delivery
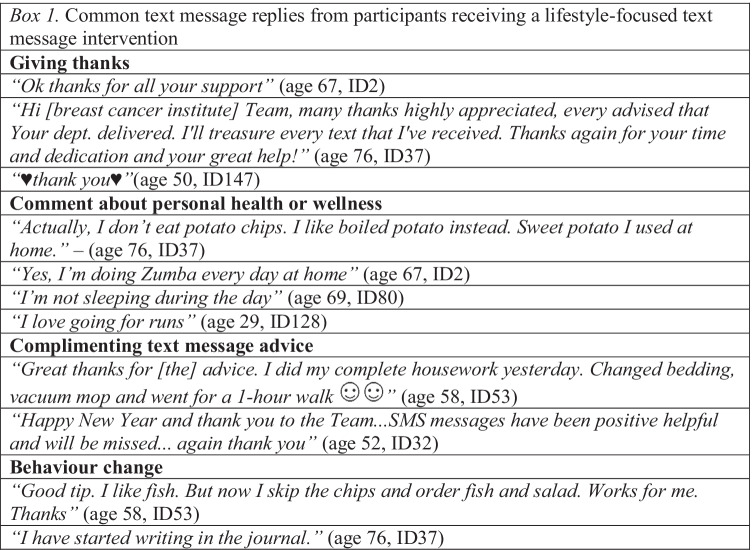
A total of 8061 text messages were sent; 7925/8061 (98.3%) delivered successfully and 136/8061 (1.7%) bounced. Despite being instructed not to reply, participants replied 130 times (median per participant = 1; range = 1–80; outlier participant ID53 n = 80; next highest n = 17). She reported that ‘it’s nice to reply, so you get feedback’ (age 58). The most common replies were giving thanks, comments about personal health or complimenting EMPOWER-SMS (Box 1). One photo and 12 ‘reactions’ (2 ‘like’, 10 ‘loved’; ID160, age 40) were received. Thirteen messages included an emoji; usually a smiley face or heart. The most common themes that triggered a reply were self-care or managing side effects (30/53;57%), practical health tips (27/53; 50%), exercise (9/53; 17%) or diet (9/53; 17%).
Program delivery costs
The cost to send one text message was $0.07USD and access automated delivery software for 20 months was $1523USD. Therefore, delivering 106 text messages to 76 EMPOWER-SMS participants and 2 text messages to 78 control participants cost $2097USD or $13.62USD per participant. Staff time to monitor incoming messages was estimated at 30 min/week.
Program acceptability and utility
Most (64/78; 82%) EMPOWER-SMS participants completed the intervention feedback survey (Table 3). The majority (57/64; 89%) read 75–100% of the messages and agreed or strongly agreed that the messages were easy to understand (64/64; 100%), useful (58/64; 90%) and motivated them to change their lifestyle (43/64; 67%). Half reported messages helped remind them to take their endocrine therapy medication (22/46; 48%). Most (51/64; 80%) participants thought that the six-month program length was just right and 10/64 (14%) it was too short or much too short. Most participants (46/64, 72%) saved the messages. However, only 28/64 (44%) shared them with family/friends and 7/64 (11%) forwarded the messages to others (Table 3), because it felt like ‘a personal experience’ [29].
Table 3.
Intervention participants’ perceived acceptability and usefulness of the EMPOWER-SMS intervention
| Characteristic | No./Total (%)a |
|---|---|
| Usefulness and understandingb | |
| Found messages useful | 58/64 (90) |
| Majority of messages were easy to understand | 64/64 (100) |
| Influence on motivation and behaviour changeb | |
| Messages motivated lifestyle change | 43/64 (67) |
| I increased by physical activity levels because of the messages | 33/64 (52) |
| Messages helped remind me to take my medicinesc | 22/46 (48) |
| Message saving and sharingd | |
| Saved messages | 46/64 (72) |
| Showed messages to family or friends | 28/64 (44) |
| Forwarded messages to family or friends | 7/64 (11) |
| Acceptability of program and message content | |
| Read 75-100% of messages | 57/64 (89) |
| Number of messages per week was appropriate or ‘just right’e | 57/63 (90) |
| Language of the messages was appropriate or ‘just right’f | 56/62 (90) |
| 6-month program was appropriate or ‘just right’e | 51/64 (80) |
| 6-month program was ‘too short’ or ‘much too short’e | 10/64 (14) |
| Time of day receiving messages (9 am, 12 pm, 3 pm or 6 pm) was appropriateb | 51/64 (80) |
aResponse rate was 64/78 (82%) of the intervention participants
bResponse options were ‘strongly disagree, disagree, neutral, agree, strongly agree’. Reported the proportion that agree and strongly agree.
cResponses from participants taking endocrine therapy tablets.
dResponses were Yes or No. Reported proportion of participants who responded ‘Yes’
eResponses were Much too few/short, too few/short, just right, too many/long, much too many/long
fResponses were Too casual, casual, just right, formal, too formal
Discussion
Accessible health education and support after breast cancer treatment remains a challenge in survivorship care [8, 30]. Despite participants’ perceptions that unhealthy lifestyle caused their breast cancer, this study highlighted that few participants were meeting secondary prevention guidelines; most participants had overweight or obesity, high body fat percentage, poor nutrition behaviours and poor mental health (anxiety, depression, stress), supporting a strong need for wide-reaching health support strategies. The EMPOWER-SMS RCT implemented a low-cost strategy to support self-efficacy, QOL and health outcomes via semi-personalised text messages for 6 months. Although EMPOWER-SMS was delivered as planned, it did not improve the primary outcome (self-efficacy). Adherence to endocrine therapy medication was high, but there was a significant difference between groups, favouring EMPOWER-SMS. Moreover, most participants rated EMPOWER-SMS easy-to-understand (100%), useful (91%), motivating (67%). Importantly, many participants felt the program duration was appropriate or wanted it to continue.
In terms of the primary outcome (self-efficacy), it is possible that the chosen scale (self-efficacy for managing chronic disease) was too broad to identify a change in this population. This scale combines self-rated self-efficacy across multiple domains including managing fatigue, pain, emotional distress and ‘things other than medications’. A previous study with cancer survivors found that self-efficacy can vary greatly across domains [18], indicating that evaluating them together may dilute their individual interpretation. Domain-specific baseline scores ranged from mean ± SD of 5.83 ± 2.56 for fatigue self-efficacy to 6.84 ± 2.23 for ‘things other than medications’ [18]. Comparatively, the current study’s baseline self-efficacy scores were high. A recent systematic review with meta-analyses of non-clinical populations found that digital health interventions had a small but positive impact on domain-specific self-efficacy for smoking but not healthy eating or physical activity [31] and only two were text message interventions (both not significant). Therefore, a domain-specific self-efficacy scale that aligned with EMPOWER-SMS content (physical activity, nutrition, fatigue, medication adherence) may have been more appropriate.
Despite the positive data relating to usefulness and motivation, it is also possible that text messages alone are insufficient to produce a change in self-efficacy for female breast cancer survivors. A systematic review with meta-analysis of healthy adults found that the effect of digital health interventions on self-efficacy decreased as the number of women included in the study increased [32]. Moreover, cancer survivors’ self-efficacy can be impacted by high levels of pain, depression and negative perceptions of cancer [18]. As there are no known similar text message studies targeting breast cancer survivors’ self-efficacy, direct comparison with previous research is not possible. However, a recent study with women of reproductive age found that text messages improved knowledge of breast cancer and breast self-examination but not self-efficacy for breast self-examination [33]. A recent systematic review with meta-analyses found that some eHealth interventions, namely interactive websites, improved breast cancer survivors’ self-efficacy [15], whereas another did not [34]. Further large-scale trials are needed to elucidate the relationship between text message interventions and domain-specific self-efficacy for breast cancer survivors.
The current study provided preliminary evidence that a lifestyle-focused text message intervention can improve endocrine therapy medication adherence compared to usual care. This result mirrors findings of increased medication adherence compared to control for patients with coronary heart disease [35], HIV positive youth taking anti-retroviral medication among [36], patients taking anti-diabetes oral tablets and beta-blockers [37] and asthma treatment [38]. One systematic review of 2742 patients with chronic diseases found that text message reminders doubled medication adherence compared to usual care [39]. Contrary to the EMPOWER-SMS intervention, which sent one, one-way (no replies) medication-related message per week, most previous interventions delivered daily text message reminders, and some required a response (two-way communication) [35–39]. A systematic review found 33–50% of women are non-adherent to their endocrine therapy tablets largely due to side effects (e.g. hot flushes, joint pain), but receiving social support and good clinician-patient communication can increase adherence [40]. Moreover, a pre-post study of 100 breast cancer survivors found evidence that daily medication reminder text messages with option to report side effects to a health professional was helpful for identifying adherence barriers and convenient for patients [41]. Qualitative results (focus groups, text message replies, free-text feedback survey responses) of the EMPOWER-SMS RCT found that the messages helped women feel supported and connected to their medical team between clinic visits [29]. To our knowledge, EMPOWER-SMS is the first lifestyle-focused text message intervention with only one medication-related message per week and found evidence of improved adherence to endocrine therapy medication. Since overestimation of self-reported adherence to endocrine therapy tablets compared to objective measures is common [42] and may limit study findings, further robust research is needed with a larger sample size of patients taking endocrine therapy medication to understand the full clinical potential of this resource-light intervention.
This study’s results are confounded by the global COVID-19 pandemic. In March 2020, COVID-19 restrictions in Sydney Australia included a stay-at-home order, which mandated working from home and closure of non-essential services [43]. For breast cancer survivors, telehealth replaced in-person clinic visits to lower chances of disease transmission [44]. This period was extremely stressful [45]. Pre-pandemic research estimated the incidence of anxiety and depression among breast cancer patients to be 18–33% [46] and 9–66% [46, 47], respectively and symptoms consistently improve across the first 2 years post-surgery [48]. In contrast, this study found that anxiety and depressive symptoms were above healthy ranges for 90/133 (68%) and 78/133 (58.6%) participants who were 1–2 years post-surgery. A systematic review of RCTs, including 764 adults diagnosed with depression, found that interventions that delivered 1–5 daily text messages were effective for improving depressive symptoms [49]. The current study, on the other hand, did not specifically recruit patients with depression or anxiety and message content targeted general lifestyle support, rather than daily support for depressive or anxiety symptoms. Despite this, this study found a text message intervention was feasible, low-cost, and acceptable for delivering health information and support to breast cancer survivors, even during COVID19 lockdowns.
Although the study had many strengths, including successfully testing a new co-designed digital health intervention in a high quality RCT with a population of culturally and ethnically diverse breast cancer survivors, several limitations should also be considered. Due to COVID-19 restrictions, half of the participants were followed-up by phone. Body composition data (body fat percentage, muscle mass percentage) were therefore unavailable and weight and waist-circumference measurements were self-reported, which limited power and accuracy [50]. Also, participants’ were a mean of 8 months post-treatment. Research suggests that continuity of patient care during and after treatment is important [30]. It is possible that earlier implementation would have been more beneficial to assist in the acute phase of transitioning from active treatment to independent health self-management. Despite this, participants found EMPOWER-SMS acceptable, useful and motivating for behaviour change and some wanted it to continue beyond 6 months. Future studies should consider beginning digital health survivorship support closer to completion of active treatment and include longer-term follow-ups.
Conclusion
A semi-personalised lifestyle-focused text message intervention for women post-active breast cancer treatment was not associated with improvements in the primary (self-efficacy) or secondary outcomes but did have a small significant improvement on endocrine therapy medication adherence. Moreover, the program was deemed useful, acceptable, and motivating for behaviour change and medication adherence from a socioeconomically diverse population. The program provides a feasible, inexpensive, and easily scalable strategy for providing post-treatment health information and support remotely, including during COVID-19 lockdowns. The program could benefit from implementation closer to the end of active treatment and longer-term follow-up.
Supplementary Information
(DOC 91 kb)
(DOCX 20 kb)
(DOCX 20 kb)
(RTF 101 kb)
Acknowledgements
A warm thank you to the breast cancer consumers representatives from Breast Cancer Network Australia and Westmead Breast Cancer Institute (WBCI) and all staff and patients at the WBCI for their involvement in the study, especially administration staff for their support (Mrs Kerry Prior, Mrs Seena Jayaraj, Mrs Lalitha Chemudapati, Mrs Zenaida Bables, Mrs Swapna Paravalappil). A special thank you to consumer representatives Christine Mitchell and Fiona Neill and breast care nurses Jenny Cooper and Sarah Laffin. Thank you to staff members of the Engagement and Co-design Research Hub and Westmead Applied Research Centre for their research support, especially Caroline Wu. Thank you to the Westmead Hospital Dietetics and Nutrition department for use of their SECA body composition machine, especially Peter Talbot and Ashwini Chand.
Author contributions
AS, JR, SRP, and EE contributed to the study conception and design. AS, RR, MH, CCK, AT, KM, KAS, EE, SRP, and JR contributed to the intervention development. AS and RR supported participant recruitment and data collection. AS, RR, JTK, and KH contributed to the analyses of the results. AS, JR, SRP, and RR drafted the manuscript. All authors commented on previous versions of the manuscript and have read and approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This work was supported by a New South Wales Agency for Clinical Innovation Research Grant Scheme to fund a research assistant to conduct blinded follow-up interviews. Several team members are supported by scholarships and fellowships. AS’s stipend is provided by the University of Sydney’s Australian Government Research Training Program Scholarship and a Supplementary Postgraduate Research Scholarship in Breast Cancer. SRP is supported by a National Health and Medical Research Council Early Career Fellowship [APP1157438] and National Heart Foundation Postdoctoral Fellowship [HF102164]. KH is suported by a NHMRC Investigator Grant (Emerging leadership 1) [APP1196724]. JR is supported by a National Health and Medical Research Council Career Development Fellowship [APP1143538]. CKC is supported by a NHMRC Investigator Grant [APP1195326].
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Western Sydney Local Health District Human Research Ethics Committee (HREC; AU RED HREC/18/WMEAD/281).
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ji P, et al. The burden and trends of breast cancer from 1990 to 2017 at the global, regional, and national levels: results From the Global Burden of Disease Study 2017. Front Oncol. 2020, 10(650). [DOI] [PMC free article] [PubMed]
- 2.Mols F, et al. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer. 2005;41(17):2613–2619. doi: 10.1016/j.ejca.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Strecher VJ, et al. The role of self-efficacy in achieving health behavior change. Health Educ Q. 1986;13(1):73–92. doi: 10.1177/109019818601300108. [DOI] [PubMed] [Google Scholar]
- 4.de Jongh T, et al. Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev. 2012;12:Cd007459. doi: 10.1002/14651858.CD007459.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grembowski D, et al. Self-efficacy and health behavior among older adults. J Health Soc Behav. 1993;34(2):89–104. doi: 10.2307/2137237. [DOI] [PubMed] [Google Scholar]
- 6.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037/0033-295X.84.2.191. [DOI] [PubMed] [Google Scholar]
- 7.Downe-Wamboldt BL, et al. The effects and expense of augmenting usual cancer clinic care with telephone problem-solving counseling. Cancer Nurs. 2007;30(6):441–453. doi: 10.1097/01.NCC.0000300164.90768.ec. [DOI] [PubMed] [Google Scholar]
- 8.Keesing S, McNamara B, Rosenwax L. Cancer survivors’ experiences of using survivorship care plans: a systematic review of qualitative studies. J Cancer Surviv. 2015;9(2):260–268. doi: 10.1007/s11764-014-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rathbone AL, Prescott J. The use of mobile apps and SMS messaging as physical and mental health interventions: systematic review. J Med Internet Res. 2017;19(8):e295. doi: 10.2196/jmir.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Communications, G.G.S.f.M., Number of mobile subscribers worldwide hits 5 Billion. https://www.gsma.com/newsroom/press-release/number-mobile-subscribers-worldwide-hits-5-billion/, 2017. Accessed 25 June 2021.
- 11.Agyapong VIO, et al. Changes in stress, anxiety, and depression levels of subscribers to a daily supportive text message program (Text4Hope) during the COVID-19 pandemic: cross-sectional survey study. JMIR Ment Health. 2020;7(12):e22423. doi: 10.2196/22423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hershman DL, et al. Randomized trial of text messaging to reduce early discontinuation of adjuvant aromatase inhibitor therapy in women with early-stage breast cancer: SWOG S1105. J Clin Oncol. 2020;38(19):2122–2129. doi: 10.1200/JCO.19.02699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spark LC, et al. Efficacy of a text message-delivered extended contact intervention on maintenance of weight loss, physical activity, and dietary behavior change. JMIR mHealth uHealth. 2015;3(3):e88. doi: 10.2196/mhealth.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow CK, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA. 2015;314(12):1255–1263. doi: 10.1001/jama.2015.10945. [DOI] [PubMed] [Google Scholar]
- 15.Singleton AC, Tat-Ko J, Sum SCM, Hafiz N, Partridge SR, Hyun KK, Sherman K, Elder E, Redfern J. eHealth strategies to improve health outcomes among patients with breast cancer: a systematic review and meta-analysis. International Psychosocial Oncology Society's 22nd World Congress of Psycho-Oncology and Psychosocial Academy, 2021.
- 16.Singleton A, et al. Co-designing a lifestyle-focused text message intervention for women after breast cancer treatment: mixed methods study. J Med Internet Res. 2021;23(6):e27076. doi: 10.2196/27076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singleton A, et al. A text message intervention to support women’s physical and mental health after breast cancer treatments (EMPOWER-SMS): a randomised controlled trial protocol. BMC Cancer. 2019;19(1):660. doi: 10.1186/s12885-019-5886-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster C, et al. Cancer survivors’ self-efficacy to self-manage in the year following primary treatment. J Cancer Surviv. 2015;9(1):11–19. doi: 10.1007/s11764-014-0384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritter PL, Lorig K. The English and Spanish self-efficacy to manage chronic disease scale measures were validated using multiple studies. J Clin Epidemiol. 2014;67(11):1265–1273. doi: 10.1016/j.jclinepi.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Peine S, et al. Generation of normal ranges for measures of body composition in adults based on bioelectrical impedance analysis using the seca mBCA. Int J Body Comp Res. 2013;11:67–76. [Google Scholar]
- 21.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health. 2009;6(6):790–804. doi: 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
- 22.Council, N.H.a.M.R., Australian dietary guidelines. Canberra: National Health and Medical Research Council., 2013.
- 23.Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, Inc. accelerometer. Med Sci Sports Exerc, 1998. 30(5). [DOI] [PubMed]
- 24.Centre, C.A.a.N.B.a.O.C., Recommendations for follow-up of women with early breast cancer. 2010. https://www.canceraustralia.gov.au/sites/default/files/publications/fueg-follow-up-of-women-with-early-breast-cancer_504af0340ef02.pdf: p. 1-23.
- 25.Australia, C.o., Management of early breast cancer. National Breast Cancer Centre Clincal practice guideliens for management of early breast cancer: Second edition, 2001. https://www.canceraustralia.gov.au/sites/default/files/publications/cpg-clinical-practice-guidelines-management-early-breast-cancer_504af03111a52.pdf.
- 26.Sprangers M, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 27.Lovibond SH, Lovibond PF. Manual for the depression anxiety stress scales. Psychology Foundation of Australia; 1996. [Google Scholar]
- 28.Basu S, Poole J. The brief illness perception questionnaire. Occup Med (Lond) 2016;66(5):419–420. doi: 10.1093/occmed/kqv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singleton AC, Raeside R, Partridge SR et al. Supporting breast cancer survivors via text messages: reach, acceptability, and utility of EMPOWER-SMS. J Cancer Surviv. 2021;1–11. 10.1007/s11764-021-01106-7 [DOI] [PMC free article] [PubMed]
- 30.Stricker CT, O'Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;18. [DOI] [PubMed]
- 31.Gell NM, Wadsworth DD. The use of text messaging to promote physical activity in working women: a randomized controlled trial. J Phys Act Health. 2015;12(6):756. doi: 10.1123/jpah.2013-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newby K, et al. Do automated digital health behaviour change interventions have a positive effect on self-efficacy? A systematic review and meta-analysis. Health Psychol Rev. 2021;15(1):140–158. doi: 10.1080/17437199.2019.1705873. [DOI] [PubMed] [Google Scholar]
- 33.Labrague LJ, et al. Effects of mobile text messaging on breast cancer and breast self-examination (BSE) knowledge, BSE self-efficacy, and BSE frequency: a randomised controlled trial. Scand J Caring Sci. 2021;35(1):287–296. doi: 10.1111/scs.12849. [DOI] [PubMed] [Google Scholar]
- 34.Ventura F, et al. Challenges of evaluating a computer-based educational programme for women diagnosed with early-stage breast cancer: a randomised controlled trial. Eur J Cancer Care. 2017;26(5):e12534. doi: 10.1111/ecc.12534. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y-Y, et al. The effect of text message reminders on medication adherence among patients with coronary heart disease: a systematic review and meta-analysis. Medicine. 2019;98(52):e18353–e18353. doi: 10.1097/MD.0000000000018353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garofalo R, et al. A randomized controlled trial of personalized text message reminders to promote medication adherence among HIV-positive adolescents and young adults. AIDS Behav. 2016;20(5):1049–1059. doi: 10.1007/s10461-015-1192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foreman KF, et al. Impact of a text messaging pilot program on patient medication adherence. Clin Ther. 2012;34(5):1084–1091. doi: 10.1016/j.clinthera.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Strandbygaard U, Thomsen SF, Backer V. A daily SMS reminder increases adherence to asthma treatment: a three-month follow-up study. Respir Med. 2010;104(2):166–171. doi: 10.1016/j.rmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Thakkar J, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med. 2016;176(3):340–349. doi: 10.1001/jamainternmed.2015.7667. [DOI] [PubMed] [Google Scholar]
- 40.Moon Z, et al. Barriers and facilitators of adjuvant hormone therapy adherence and persistence in women with breast cancer: a systematic review. Patient preference and adherence. 2017;11:305–322. doi: 10.2147/PPA.S126651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mougalian SS, et al. Bidirectional text messaging to monitor endocrine therapy adherence and patient-reported outcomes in breast cancer. JCO Clin Cancer Inform. 2017;1:1–10. doi: 10.1200/CCI.17.00015. [DOI] [PubMed] [Google Scholar]
- 42.Bright EE, Stanton AL. Correspondence between objective and self-reported endocrine therapy adherence among women with breast cancer. Ann Behav Med. 2018;53(9):849–857. doi: 10.1093/abm/kay094. [DOI] [PubMed] [Google Scholar]
- 43.Fisher JR, et al. Mental health of people in Australia in the first month of COVID-19 restrictions: a national survey. Med J Aust. 2020;213(10):458–464. doi: 10.5694/mja2.50831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinkove R, et al. Managing haematology and oncology patients during the COVID-19 pandemic: interim consensus guidance. Med J Aust. 2020;212(10):481–489. doi: 10.5694/mja2.50607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edge R, et al. Psychosocial impact of COVID-19 on cancer patients, survivors, and carers in Australia: a real-time assessment of cancer support services. Support Care Cancer. 2021;29(9):5463–5473. doi: 10.1007/s00520-021-06101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maass SWMC, et al. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: a systematic review. Maturitas. 2015;82(1):100–108. doi: 10.1016/j.maturitas.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Pilevarzadeh M, et al. Global prevalence of depression among breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;176(3):519–533. doi: 10.1007/s10549-019-05271-3. [DOI] [PubMed] [Google Scholar]
- 48.Taira N, et al. Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast Cancer Res Treat. 2011;128(3):735–747. doi: 10.1007/s10549-011-1631-y. [DOI] [PubMed] [Google Scholar]
- 49.Senanayake B, et al. Effectiveness of text messaging interventions for the management of depression: a systematic review and meta-analysis. J Telemed Telecare. 2019;25(9):513–523. doi: 10.1177/1357633X19875852. [DOI] [PubMed] [Google Scholar]
- 50.Nawaz H, et al. Self-reported weight and height: implications for obesity research. Am J Prev Med. 2001;20(4):294–298. doi: 10.1016/S0749-3797(01)00293-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 91 kb)
(DOCX 20 kb)
(DOCX 20 kb)
(RTF 101 kb)
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.