Abstract
The therapeutic efficacy of human neutrophil peptide 1 (HNP-1) against experimental tuberculosis in mice on the basis of numbers of CFU has been examined. Mice infected with 1.5 × 104 CFU of Mycobacterium tuberculosis H37Rv and treated with different doses of HNP-1 injected subcutaneously exhibited significant clearance of bacilli from lungs, livers, and spleens. There were time- and dose-dependent decreases in the bacillary load in lungs, livers, and spleens of the HNP-1-treated animals compared to that in controls (untreated animals). These observations strongly suggest the therapeutic activity of HNP-1 against tuberculosis.
The recent resurgence in the incidence of tuberculosis and its association with human immunodeficiency virus infection and AIDS warrants the development of new therapeutic strategies for the effective control of tuberculosis. Despite the outbreaks of multidrug-resistant strains of Mycobacterium tuberculosis and calls for new drug development, truly novel compounds, which would significantly improve treatment, continue to elude us. Antibiotic peptides from higher eukaryotes have gained considerable attention as an alternative to conventional antibiotics owing to their potent antimicrobial activities in vitro (for a recent review see reference 2). The defensins are a family of small antimicrobial peptides having six highly conserved cysteine residues, resulting in three disulfide linkages. Human neutrophil peptide 1 (HNP-1) is one of the four types of defensins present in the azurophilic granules of polymorphonuclear neutrophils. HNP-1 has been demonstrated to exhibit potent in vitro bactericidal activity against a wide variety of microbes, including mycobacterial species (5). Recently, we have reported the ability of HNP-1 to kill M. tuberculosis in vitro as well as ex vivo (6, 7). To date, no study has been carried out to investigate the therapeutic efficacy of HNP-1 against M. tuberculosis infections. In the present study, the in vivo therapeutic potential of HNP-1 against experimental tuberculosis in a mouse model was investigated.
Six- to eight-week-old pathogen-free Laca (inbred) strain mice were infected intravenously with 1.5 × 104 CFU of M. tuberculosis H37Rv per mouse. After 15 days of challenge, establishment of infection was confirmed by Ziehl-Neelsen staining of whole-tissue homogenates of lungs, livers, and spleens from four animals. Chemically synthesized HNP-1 with the same primary structure and the same disulfide linkages (i.e., between Cys-2 and Cys-30, Cys-4 and Cys-19, and Cys-9 and Cys-29) as native HNP-1 (obtained from Peptide Institute Inc., Osaka, Japan) was used in this study. It was dissolved in 0.01% acetic acid and stored as a stock solution of 100 μg/ml at −20°C (dissolved peptide was used within 3 weeks, as the aqueous solution was stable for a few weeks only). To investigate the therapeutic potential of HNP-1, infected animals were divided into three groups (at least four animals in each group) and were injected subcutaneously with two different doses of HNP-1, i.e., 1 and 5 μg per mouse once weekly. Control animals were injected with 0.01% acetic acid subcutaneously. The therapy was given for 1, 2, and 4 weeks. Animals were sacrificed 7 days after the completion of therapy. The organs (lungs, livers, and spleens) were removed aseptically and homogenized in hand homogenizers containing 3 ml of sterile phosphate-buffered saline. Serial 10-fold dilutions of individual whole-organ homogenates were plated on modified Youman's solid medium containing 1% bovine serum albumin. Plates were incubated at 37°C for 4 to 6 weeks, and the bacterial load in each organ was determined by CFU enumeration. Statistical analysis was performed by two-way analysis of variance using the MINITAB computer program. (Minitab Inc., State College, Pa.)
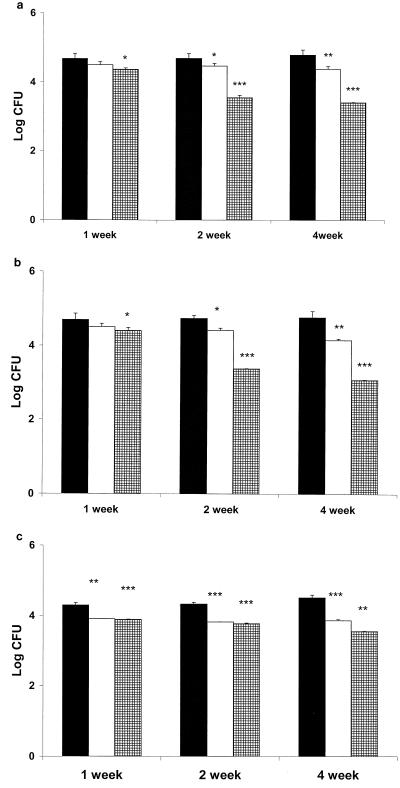
The results of this study are presented in Fig. 1. There were concentration- and time-dependent decreases in bacillary load in all the organs studied, i.e., lungs, livers, and spleens. A therapeutic dose of HNP-1 of 5 μg given subcutaneously resulted in a significant decrease in CFU (P < 0.05) from lungs after 1 week of therapy compared to controls. Further, increase in the therapy for 2 and 4 weeks resulted in more than a 1-log-unit decrease (P < 0.001) in CFU compared to controls. Therapy with 1 μg of HNP-1 also resulted in a significant decrease in CFU from lungs after 2 and 4 weeks (P < 0.01 for both time points) compared to controls. Clearance of bacillary load similar to that observed in lungs was seen in livers after each time point at both the therapeutic doses of HNP-1. In spleens, greater clearance of bacilli was observed after 1 week of therapy even with 1 μg of HNP-1 (P < 0.01) than in controls, whereas at the same time point, there was a nonsignificant decrease in log CFU in lungs and livers compared to controls.
FIG. 1.
CFU enumeration in the lungs, livers, and spleens of infected mice treated with HNP-1. Mice were infected with 1.5 × 104 M. tuberculosis H37Rv organisms via the tail vein. HNP-1 at 1 (□) and 5 μg per mouse was given subcutaneously for 1, 2, and 4 weeks (▪, control). Seven days after the completion of therapy, CFU were enumerated in lungs (a), livers (b), and spleens (c). Error bars represent standard deviations for four animals in each group at each time point. The statistical analysis was performed by two-way analysis of variance. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
In the present study, significantly low concentrations of HNP-1 (1 and 5 μg per mouse) were found to clear mycobacteria in vivo, relative to those required in in vitro (50 μg/ml) (4) and ex vivo (40 μg/ml) (7) studies dealing with antimycobacterial activities. These findings suggest that some events other than the direct killing of bacilli are involved in the reduction of viable mycobacteria in infected organs in vivo. Recently, Welling et al. (10) showed that the antibacterial activity of HNP-1 in experimental infections in mice is accompanied by increased leukocyte accumulation. As little as 4 ng of HNP-1 was found to significantly decrease the bacterial counts in experimental peritoneal Klebsiella pneumoniae infections. HNP-1 has been shown to be chemotactic to T cells (1) and monocytes (8), and it also induces the production of interleukin 8 in airway epithelium (9). Thus, it can be hypothesized that HNP-1 may act as an immunostimulatory molecule in vivo in tuberculosis-infected mice, where it is recognized as an effector molecule of innate immunity (3).
Although in vivo studies regarding the role of HNP-1 as an antimicrobial agent against other bacterial infections are limited, this is the first report indicating the therapeutic potential of HNP-1 against experimental tuberculosis. However, further studies are required to investigate the in vivo immunostimulatory role of HNP-1 and its mechanism of action.
REFERENCES
- 1.Chertov O, Michiel D F, Xu L, Wang J M, Tani K, Murphy W J, Longo D L, Taub D D, Oppenhein J J. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271:2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 2.Ganz T, Lehrer R I. Antibiotic peptides from higher eukaryotes: biology and applications. Mol Med Today. 1999;5:292–297. doi: 10.1016/s1357-4310(99)01490-2. [DOI] [PubMed] [Google Scholar]
- 3.Levy O. Antibiotic proteins of polymorphonuclear leukocytes. Eur J Haematol. 1996;56:1407–1410. doi: 10.1111/j.1600-0609.1996.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 4.Miayakawa Y, Ratnakar P, Rao A G, Costello M E, Costello O M, Lehrer R I, Catanzaro A. In vitro activity of the antimicrobial peptides human and rabbit defensins and porcine leukocyte protein against Mycobacterium tuberculosis. Infect Immun. 1996;64:926–932. doi: 10.1128/iai.64.3.926-932.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogata K, Lizer B A, Zuberi R I, Ganz T, Leherer R I, Catanzaro A. Activity of defensins from human neutrophilic granulocyte against Mycobacterium avium-Mycobacterium intracellulare. Infect Immun. 1992;60:4720–4725. doi: 10.1128/iai.60.11.4720-4725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma S, Verma I, Khuller G K. Biochemical interaction of HNP-1 with Mycobacterium tuberculosis H37Ra. Arch Microbiol. 1999;171:338–342. doi: 10.1007/s002030050719. [DOI] [PubMed] [Google Scholar]
- 7.Sharma S, Verma I, Khuller G K. Antibacterial activity of human neutrophil peptide-1 against Mycobacterium tuberculosis H37Rv: in vitro and ex vivostudy. Eur Respir J. 2000;16:112–117. doi: 10.1034/j.1399-3003.2000.16a20.x. [DOI] [PubMed] [Google Scholar]
- 8.Territo M C, Ganz T, Selsted M E, Lehrer R L. Monocyte chemotactic activity of defensins from human neutrophils. J Clin Investig. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Wetering S, Mannesse-Lazeroms S P G, van Sterkenburg M A J A, Daha M R, Dijkman J H, Hiemstra P S. Effects of defensins on IL-8 synthesis in airway epithelial cells. Am J Physiol. 1997;272:888–896. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- 10.Welling M M, Hiemstra P S, van den Barsellar M T, Paulusma-Annema A, Nibbering P H, Pauwels E K J, Calame W. Antibacterial activity of human neutrophil defensins in experimental infections in mice is accompanied by increased leukocyte accumulation. J Clin Investig. 1998;102:1583–1590. doi: 10.1172/JCI3664. [DOI] [PMC free article] [PubMed] [Google Scholar]