Abstract
Introduction
Hyperuricemia and diabetes mellitus (DM) are associated with increased mortality risk in patients with chronic kidney disease (CKD). Here we aimed to evaluate the independent and joint risks of these two conditions on mortality and end stage kidney disease (ESKD) in CKD-patients.
Methods
This retrospective cohort study enrolled 4380 outpatients (with CKD stage 3–5) with mortality and ESKD linkage during a 7-year period (from 2007 to 2013). All-causes mortality and ESKD risks were analyzed by multivariable-adjusted Cox proportional hazards models (adjusted for age, sex, smoke, previous coronary arterial disease, blood pressure, and medications for hyperlipidemia, hyperuricemia and renin–angiotensin system inhibitors).
Results
Overall, 40.5% of participants had DM and 66.4% had hyperuricemia. In total, 356 deaths and 932 ESKD events occurred during the 7 years follow-up. With the multivariate analysis, increased risks for all-cause mortality were: hyperuricemia alone, HR = 1.48 (1–2.19); DM alone, and HR = 1.52 (1.02–2.46); DM and hyperuricemia together, HR = 2.12 (1.41–3.19). Similar risks for ESKD were: hyperuricemia alone, HR = 1.34 (1.03–1.73); DM alone, HR = 1.59 (1.15–2.2); DM and hyperuricemia together, HR = 2.46 (1.87–3.22).
Conclusions
DM and hyperuricemia are strongly associated with higher all-cause mortality and ESKD risk in patients with CKD stage 3–5. Hyperuricemia is similar to DM in terms of risk for all-cause mortality and ESKD. DM and hyperuricemia when occurred together further increase both risks of all-cause mortality and ESKD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-022-02755-1.
Keywords: Hyperuricemia, Diabetes mellitus, dialysis, Mortality, Chronic kidney disease
Introduction
Chronic kidney disease (CKD) is a public health burden worldwide due to its rapidly expanding patient populations, high risk of progression into end-stage kidney disease (ESKD), and poor prognosis of morbidity and mortality [1, 2]. The leading mortality of these patients is cardiovascular (CV) related deaths. With CKD progression, the CV outcomes become worse, including CV death, re-infarction, congestive heart failure, stroke, and resuscitation [3]. The most common cause of CKD is diabetes mellitus (DM) [4]. The 2002 National Cholesterol Education Program report designated DM a coronary heart disease risk equivalent, and DM is placed in the highest risk category [5]. Furthermore, DM and CKD are both potent independent risk factors for CV events and progression to ESKD [6, 7]. DM has huge burden of atherosclerosis related intimal thickening and CKD also causes medial calcification [8]. Therefore, patients with both conditions at the same time are therefore at exceedingly high risk of adverse events and would end with poor patient outcome.
The serum level of uric acid (UA) is also a risk factor for kidney disease [9], cardiovascular disease (CVD) [10–12], and atherosclerosis [13]. Serum UA is an independent risk factor for CKD, even in those without diabetes [14, 15]. Two large epidemiologic studies showed that UA is a major predictor for the incidence of renal disease [15, 16]. Moreover, hyperuricemia is often prevalent in CKD patients, and that is associated with a higher incidence of ESKD [16]. A number of studies showed that UA independently predicts the development of type 2 DM [17–19] and the progression of CKD [20]. For about 20 years, UA is known to be a potential risk factor for CKD and CVD with pathological implications [21, 22, 23]. Given the complex interplay among hyperuricemia, DM and the progression of CKD, we are interested to explore the complicated interactions regarding renal and patients outcomes. Here, we aimed to investigate the effects of DM and hyperuricemia on patient mortality and the development of ESKD in a large cohort of CKD patients.
Methods
Study cohort and definition
In this retrospective cohort study, we enrolled 4380 patients with CKD from the outpatients clinic of the nephrology department, Taichung Veterans General hospital (TVGH), Taiwan. Our hospital, a medical center with 1500 beds, is the referral hospital for the critically ill and difficult cases in central Taiwan. During the past 30 years, the CKD care program treated > 10,000 outpatients with CKD. CKD was defined as estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73m2 for > 3 months irrespective of the cause. The eGFR equation was from Modification of Diet in Renal Disease (ml/min/1.732m2) [24]. DM was confirmed according the diagnosis of medical records. Hyperuricemia was defined as UA levels > 7.0 mg/dl for men, or > 6.0 mg/dl for women [21, 25]. These laboratory data were measured in our institute (TVGH).
Data collections
We enrolled patients (> 20 years old) with CKD 3–5 from 2007 to 2013 in this study. After follow-up (2.5 years of mean duration) (end data of this study: 31-December-2015), the outcomes were analyzed by mortality and participants received regular dialysis at least 3 months or renal transplantation (ESKD). Their baseline variables were collected from medical records, including age, gender, stages of CKD, systolic blood pressure (SBP) (baseline and 1 year mean value), and diastolic blood pressure (DBP) (baseline and 1 year mean value), history of coronary artery disease, history of ever smoker, UA (baseline and 1 year mean value), baseline total cholesterol, usage of statin and usage of angiotensin-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB). The stage of CKD was based on the baseline renal function (the first laboratory data during the recruitment period of time). We chose the Modification of Diet in Renal Disease (MDRD) formula, instead of the Cockcroft and Gault formula, due to its superior accuracy in diabetic patients with impaired renal functions [26]. Although CKD-EPI (Epidemiology Collaboration) is more accurate than the MDRD equation for subjects with eGFR > 60 ml/min/1.73 m2, the MDRD formula is the one applied in the Taiwan National Database to evaluate dialysis initiation and CKD prevalence [27–29].
Ethical approval and consent to participate
The study was approved by the institutional review board of the Taichung Veterans General Hospital approved the study (IRB TCVGH No: CE16235A-3) and all methods were carried out in accordance with relevant guidelines and regulations. The Informed consent was waived by the above ethics committee due to retrospective nature of the study.
Statistical analysis
Data were presented as the mean ± standard deviation for continuous variables and proportions for categorical variables. An independent two-tailed t test was used for the comparison of continuous variables, and the differences between nominal variables were compared with the Chi-square test. Cox proportional hazards model was used to compare the hazard ratios (HRs) of all-cause mortality and dialysis event (adjusted for age, sex, ever smoke, CKD stage, 1 year mean SBP, use of statin, hyperuricemia drug usage, and ACEi/ARB usage.). Joint effects of hyperuricemia and DM on all-cause mortality and dialysis were also evaluated by Cox proportional hazards model, with adjusted for important covariates known to be associated with the predictors and outcomes of interests (adjusted for age, sex, ever smoke, CKD stage, 1 year mean SBP, use of statin, hyperuricemia drug usage, and ACEi/ARB usage.). In addition, since mortality was a competing event with dialysis, an extended Cox proportional hazards model was used to calculate the subdistribution hazard ratio (SHR) of dialysis as a sensitivity test [30]. We also analzyed the effect of DM and hyperuricemia on all-cause mortality and ESKD according to different stages of CKD. Statistical significance was set at p < 0.05. Statistical analyses were all carried out by using SPSS 22.0 (SPCC, Chicago, Illinois). Extended Cox proportional hazards model was analyzed by SAS software (version 9.4; SAS Institute, Inc., Cary, NC, USA).
Results
Baseline characteristics
Of the 4380 CKD patients, their median follow-up duration was 7 years. Among them, 40.5% had DM and 66.4% had hyperuricemia. Their mean age was 71 years and 63% were men. Baseline clinical and characteristics of patients are shown in Table 1 for the population, and in subpopulations according to DM or hyperuricemia. Of them, the mean age was 71 ± 14.8 years old. Patients with CKD were mostly in the stage 3 (47.4%). Mean SBP and DPP were 134 ± 18.7 and 75 ± 11.1 mmHg. Baseline and one-year later UA were both around 8 mg/dl (8.1 ± 2.5 and 8.0 ± 2.1 mg/dl, respectively). In DM related CKD patients with hyperuricemia were younger (71 ± 12.2 vs. 73 ± 18.8, p = 0.001), of later stages of CKD (p < 0.001), more frequent smokers (43.4% vs. 35.4%, p = 0.001), higher baseline SBP (138 ± 19.5 vs. 135 ± 18.1 mmHg, p = 0.013) and DBP (75 ± 10.8 vs. 74 ± 10.3 mmHg, p = 0.043), and higher one-year-later mean UA (9.1 ± 2.1 vs. 5.9 ± 0.9 mg/dl, p < 0.001). For CKD patients without DM, those with hyperuricemia were younger (70 ± 16.8 vs. 72 ± 15.3, p < 0.001), more male gender (65.7 vs. 59.1%, p < 0.001), of later stages of CKD (p < 0.001), more frequent smokers (38.6 vs. 34.1%, p = 0.027), higher one-year-later mean UA (8.9 ± 1.5 vs. 5.9 ± 0.9 mg/dl, p < 0.001), and more with ACEi/ARB usage (52.7% vs. 48.6%, p = 0.045).
Table 1.
Basic characteristics stratified by DM and uric acid level
| DM | No DM | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Overall (N = 4380) | UA > 7 (n = 1174) | UA < 7 (n = 602) | p-value | UA > 7 (n = 1737) | UA < 7 (n = 867) | p-value |
| Age (y) | 71 ± 14.8 | 71 ± 12.2 | 73 ± 11.8 | 0.001a | 70 ± 16.8 | 72 ± 15.3 | < 0.001a |
| Male sex | 2747(62.7) | 741(63.1) | 352(58.5) | 0.057b | 1142(65.7) | 512(59.1) | < 0.001b |
| CKD stage | < 0.001b | < 0.001b | |||||
| 3 | 2076(47.4) | 461(39.3) | 323(53.7) | 803(46.2) | 489(56.4) | ||
| 4 | 1364(31.1) | 414(35.3) | 200(33.2) | 530(30.5) | 220(25.4) | ||
| 5 | 940(21.5) | 299(25.5) | 79(13.1) | 404(23.3) | 158(18.2) | ||
| Ever smoke | 1688(38.5) | 509(43.4) | 213(35.4) | 0.001b | 670(38.6) | 296(34.1) | 0.027b |
| Previous CAD | 225(5.1) | 86(7.3) | 31(5.1) | 0.08b | 69(4.0) | 39(4.5) | 0.526b |
| Baseline SBP (mmHg) | 134 ± 18.7 | 138 ± 19.5 | 135 ± 18.1 | 0.013a | 133 ± 18.5 | 132 ± 17.9 | 0.40a |
| 1 year mean SBP (mmHg) | 134 ± 16.1 | 137 ± 16.4 | 135 ± 15.6 | 0.014a | 133 ± 16.1 | 131 ± 15.3 | 0.118a |
| Baseline DBP (mmHg) | 75 ± 11.1 | 75 ± 10.8 | 74 ± 10.3 | 0.043a | 75 ± 11.4 | 75 ± 11.2 | 0.34a |
| 1 year mean DBP (mmHg) | 74 ± 9.5 | 75 ± 8.9 | 74 ± 8.9 | 0.116a | 75 ± 10.0 | 74 ± 9.4 | 0.092a |
| Baseline UA (mg/dl) | 8.1 ± 2.5 | 9.2 ± 3.1 | 6.0 ± 1.1 | < 0.001a | 9.1 ± 1.8 | 6.0 ± 1.1 | < 0.001a |
| 1 year mean UA (mg/dl) | 8.0 ± 2.1 | 9.1 ± 2.1 | 5.9 ± 0.9 | < 0.001a | 8.9 ± 1.5 | 5.9 ± 0.9 | < 0.001a |
| Baseline cholesterol (mmol/l) | 185.2 ± 51.4 | 183.5 ± 53.0 | 180.1 ± 50.8 | 0.214a | 187.9 ± 50.1 | 185.7 ± 51.9 | 0.325a |
| Statin usage | 1522(34.7) | 544(46.3) | 279(46.3) | 0.997b | 473(27.2) | 226(26.1) | 0.528b |
| ACEi/ARB usage | 2540(58.0) | 802(68.3) | 401(66.6) | 0.468b | 916(52.7) | 421(48.6) | 0.045b |
| Uric acid-lowering drugs | 1228 (28.0) | 306 (26.1) | 107 (17.8) | < 0.0001 | 599 (34.5) | 216 (24.9) | < 0.0001 |
Continuous variables are expressed as mean ± SD
Categorical data are presented as numbers (percentages). aIndependent T-Test. bChi-Square Test
Association between hyperuricemia and all-cause mortality and ESKD
During the follow-up periods, 356 (8.1%) deaths occurred and 932 (21.3%) participants received regular dialysis at least 3 months (ESKD). Hazard ratios (HRs) with 95% confidence intervals (CIs) for each combination of predictors are summarized in Table 2. For all-cause mortality, the univariate analysis showed the following associated factors: DM (HR = 1.49, 95%CI = 1.21–1.83)(p = 0.0002), hyperuricemia (HR = 1.39, 95%CI = 1.1–1.75)(p = 0.0058), older age (HR = 1.06, 95%CI = 1.05–1.07)(p < 0.0001), male gender (HR = 1.46, 95%CI = 1.16–1.84)(p = 0.0012), ever smoker (HR = 1.74, 95%CI = 1.41–2.14) (p < 0.0001), CKD stage 4 compared to stage 3 (HR = 1.61, 95%CI = 1.27–2.05)(p < 0.0001), stage 5 compared to stage 3 (HR = 1.56, 95%CI = 1.19–2.05)(p = 0.0015), medication for hyperuricemia (HR = 1.33, 95%CI = 1.07–1.65) (p = 0.0105), and statin (HR = 0.71, 95%CI = 0.56–0.9)(p = 0.004). Further multivariate analysis for all-cause mortality still showed the following associated factors: DM (HR = 1.46, 95%CI = 1.13–1.88) (p = 0.004), hyperuricemia (HR = 1.44, 95%CI = 1.09–1.91)(p = 0.0114), older age (HR = 1.05, 95%CI = 1.04–1.06) (p < 0.0001), ever smoker (HR = 1.58, 95%CI = 1.16–2.16) (p = 0.0042), CKD stage 4 compared to stage 3 (HR = 1.88, 95%CI = 1.42–2.49) (p < 0.0001), CKD stage 5 compared to stage 3(HR = 1.85, 95%CI = 1.3–2.63) (p = 0.0007), and statin (HR = 0.67, 95%CI = 0.5–0.9) (p = 0.0085).
Table 2.
Hazard ratios (HRs) (95% CI) on all-cause mortality and ESKD (univariate and multivariate analysis)
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Mortality | p value | ESKD | p value | Mortality | p value | ESKD | p value | |
| DM at least | 1.49(1.21–1.83) | 0.0002 | 1.49(1.31–1.7) | < 0.0001 | 1.46(1.13–1.88) | 0.004 | 1.77(1.49–2.10) | < 0.0001 |
| Hyperuricemia at least | 1.39(1.1–1.75) | 0.0058 | 1.71(1.48–1.99) | < 0.0001 | 1.44(1.09–1.91) | 0.0114 | 1.43(1.19–1.73) | 0.0002 |
| Age | 1.06(1.05–1.07) | < 0.0001 | 0.97(0.97–0.98) | < 0.0001 | 1.05(1.04–1.06) | < 0.0001 | 0.97(0.97–0.98) | < 0.0001 |
|
Gender (male v.s. female) |
1.46(1.16–1.84) | 0.0012 | 0.65(0.58–0.74) | < 0.0001 | 0.96(0.67–1.37) | 0.8161 | 1.01(0.82–1.24) | 0.9394 |
|
Ever smoker (yes v.s. no) |
1.74(1.41–2.14) | < 0.0001 | 0.9(0.78–1.03) | 0.1124 | 1.58(1.16–2.16) | 0.0042 | 1.16(0.95–1.43) | 0.1537 |
|
CKD stage (stage4 v.s. stage3) |
1.61(1.27–2.05) | < 0.0001 | 4.13(3.33–5.14) | < 0.0001 | 1.88(1.42–2.49) | < 0.0001 | 4.33(3.29–5.70) | < 0.0001 |
|
CKD stage (stage5 v.s. stage3) |
1.56(1.19–2.05) | 0.0015 | 22.43(18.29–27.52) | < 0.0001 | 1.85(1.30–2.63) | 0.0007 | 24.16(18.59–31.38) | < 0.0001 |
| Mean SBP (mmHg) | 1(0.99–1.00) | 0.3511 | 1.02(1.02–1.03) | < 0.0001 | 1(0.99–1.00) | 0.2826 | 1.02(1.01–1.02) | < 0.0001 |
|
Medications for hyperuricemia (yes v.s. no) |
1.33(1.07–1.65) | 0.0105 | 0.93(0.80–1.07) | 0.2845 | 1.2(0.92–1.56) | 0.1828 | 0.84(0.70–1.02) | 0.0746 |
|
Statin (yes v.s. no) |
0.71(0.56–0.90) | 0.004 | 1.08(0.94–1.24) | 0.2621 | 0.67(0.50–0.90) | 0.0085 | 1.18(0.99–1.40) | 0.0646 |
|
ACEi or ARB (yes v.s. no) |
1.02(0.82–1.26) | 0.8836 | 1.03(0.91–1.18) | 0.6346 | 1.13(0.87–1.47) | 0.3488 | 0.94(0.80–1.12) | 0.4984 |
Note: adjusted for age, sex, ever smoke, CKD stage, 1 year mean SBP, use of statin, hyperuricemia drug usage, ACEi/ARB usage
For ESKD, the univariate analyses showed the following associated factors: DM (HR = 1.49, 95%CI = 1.31–1.7)(p < 0.0001), hyperuricemia (HR = 1.71, 95%CI = 1.48–1.99) (p < 0.0001), younger age (HR = 0.97, 95%CI = 0.97–0.98) (p < 0.0001), male gender (HR = 0.65, 95%CI = 0.58–0.74) (p < 0.0001), CKD stage 4 compared to stage 3 (HR = 4.13, 95%CI = 3.33–5.14) (p < 0.0001), and stage 5 compared to stage 3 (HR = 22.43, 95%CI = 18.29–27.52) (p < 0.0001). Further multivariate analysis for ESKD still showed the following associated factors: DM (HR = 1.77, 95%CI = 1.49–2.1) (p < 0.0001), hyperuricemia (HR = 1.43, 95%CI = 1.19–1.73) (p = 0.0002), older age (HR = 0.97, 95%CI = 0.97–0.98) (p < 0.0001), CKD stage 4 compared to stage 3 (HR = 4.33, 95%CI = 3.29–5.7) (p < 0.0001), CKD stage 5 compared to stage 3(HR = 24.16, 95%CI = 18.59–31.38) (p < 0.0001), and mean SBP (HR = 1.02, 95%CI = 1.01–1.02) (p < 0.0001).
Analyses of effects of DM and hyperuricemia on all-cause mortality, and ESKD
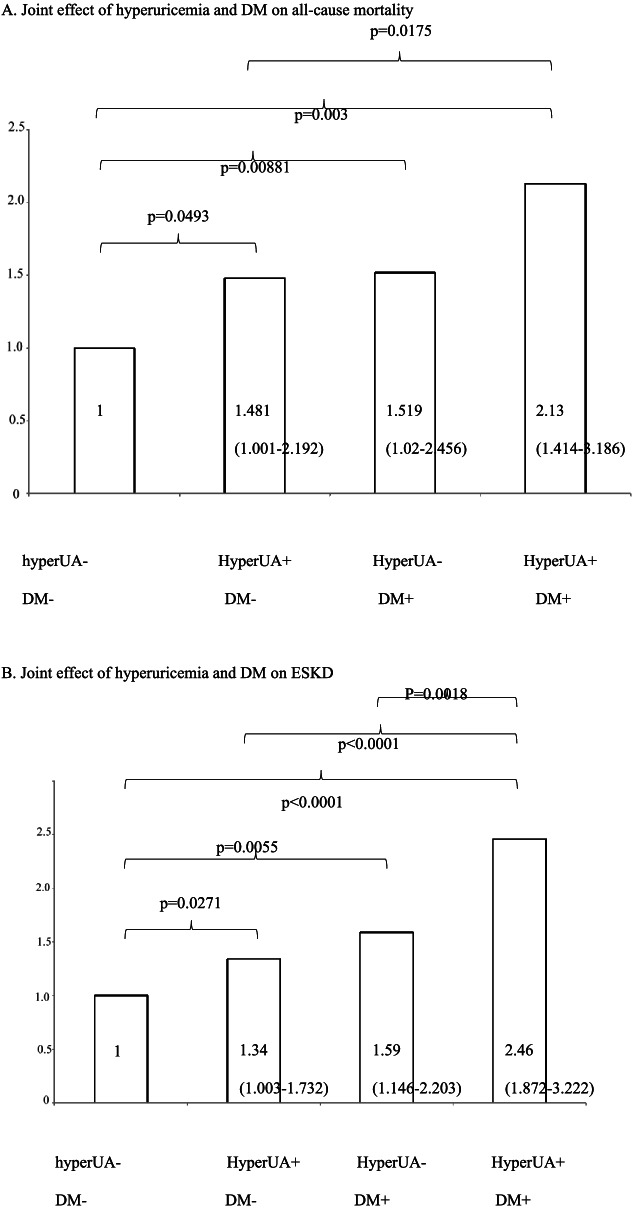
Further analysis of DM, hyperuricemia and both DM and hyperuricemia on all-cause mortality, ESKD and CV death are shown in supplementary Table 1. Results of Cox proportional hazard models are shown in Table 3 (univariate and multivariate analyses). All were compared with the reference (neither DM nor hyperuricemia). In univariate analyses, hyperuricemia alone, DM alone, and DM and hyperuricemia together showed higher risk for all-cause mortality (HR = 1.46, 95%CI = 1.05–2.02 (p = 0.0228); HR = 1.59, 95%CI = 1.07–2.36 (p = 0.023); HR = 2.13, 95%CI = 1.53–2.98 (p < 0.0001)). The higher risks for all-cause mortality were still found in the multivariate analysis: hyperuricemia alone, HR = 1.48, 95%CI = 1.2–2.19 (p = 0.0493); DM alone, HR = 1.52, 95%CI = 1.02–2.46 (p = 0.0088); DM and hyperuricemia together, HR = 2.12, 95%CI = 1.41–3.19 (p = 0.0003). We found similar results with ESKD. The univariate analysis still showed the following associated factors: hyperuricemia alone, HR = 1.72, 95%CI = 1.40–2.10 (p < 0.0001); DM alone, HR = 1.48, 95%CI = 1.14–1.92 (p = 0.003); hyperuricemia and DM together, HR = 2.61, 95%CI = 2.11–3.22 (p = < 0.0001). The multivariate analysis revealed the following assocaited factors: hyperuricemia alone, HR = 1.34, 95%CI = 1.03–1.73 (p = 0.0271); DM alone, HR = 1.59, 95%CI = 1.15–2.2 (p = 0.0055); hyperuricemia and DM together, HR = 2.46, 95%CI = 1.87–3.22 (p < 0.0001). This joint effects of DM and hyperuricemia on ESKD and all-cause mortality are also shown in Fig. 1, including all-cause mortality (Fig. 1A) and ESKD (Fig. 1B). The Kaplan-Meier plot for all-cause mortality or ESKD were also showed similar results (Supplementary Fig. 1A for ESKD and Fig. 1B for mortality).
Table 3.
Cox Proportional Hazards Models for patient all-cause mortality and ESKD divided by DM and hyperuricemia
| No. of Events | Univariate 95% CI |
p-value | Multivariate 95% CIa | p-value | |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| Neither DM nor Hyperuricemia | 49 | REF. | REF. | – | |
| Hyperuricemia only | 142 | 1.46(1.05–2.02) | 0.0228 | 1.48(1–2.19) | 0.0493 |
| DM only | 48 | 1.59(1.07–2.36) | 0.023 | 1.52(1.02–2.46) | 0.0088 |
| Both DM and Hyperuricemia | 117 | 2.13(1.53–2.98) | <.0001 | 2.12(1.41–3.19) | 0.0003 |
| ESKD | |||||
| Neither DM nor Hyperuricemia | 121 | REF. | REF. | – | |
| Hyperuricemia only | 388 | 1.72 (1.40–2.10) | <.0001 | 1.34(1.03–1.73) | 0.0271 |
| DM only | 107 | 1.48 (1.14–1.92) | 0.003 | 1.59(1.15–2.2) | 0.0055 |
| Both DM and Hyperuricemia | 316 | 2.61 (2.11–3.22) | <.0001 | 2.46(1.87–3.22) | <.0001 |
| Dialysis with considering competing risk of mortality | |||||
| Neither DM nor Hyperuricemia | 121 | REF. | REF. | ||
| Hyperuricemia only | 388 | 1.671(1.366–2.044) | <.0001 | 1.266(0.97–1.651) | 0.0825 |
| DM only | 107 | 1.428(1.106–1.844) | 0.0064 | 1.529(1.064–2.197) | 0.0218 |
| Both DM and Hyperuricemia | 316 | 2.467(2.004–3.036) | <.0001 | 2.251(1.702–2.978) | <.0001 |
Note: adjusted for age, sex, ever smoke, previous CAD, 1 year mean SBP, use of statin, hyperuricemia drug usage, ACEi/ARB usage
Fig. 1.
A The significance of HRs for mortality: DM and hyperuricemia together (HR = 2.13) > DM alone (HR = 1.52) = hyperuricemia alone (HR = 1.48) > neither DM nor hyperuricemia (HR = 1). B For ESKD, the significance of HRs: DM and hyperuricemia together (HR = 2.46) > DM alone (HR = 1.59) = hyperuricemia alone (HR = 1.34) > neither DM nor hyperuricemia (HR = 1)
Cox proportional multivariate hazards ratio for patient all-cause mortality and ESKD divided by DM and hyperuricemia ind different stages of CKD (strage 3, 4, and 5) were shown in Supplementary Table 2 (all-cause mortality) and Table 3 (ESKD). As for all-cause mortality (Supplementary Table 2), we found the following assciated facotrs: hyperuricemia alone in CKD stage 4, HR = 2.32, 95%CI = 1.03–5.21 (p = 0.0418); DM alone in CKD stage 4, HR = 3.282, 95%CI = 1.35–7.97 (p = 0.0087); DM and hyperuricemia together in CKD stage 4, HR = 3.99, 95%CI = 1.77–8.99 (p = 0.0009); DM and hyperuricemia in CKD stage 5, HR = 3.105, 95%CI = 1.04–9.30 (p = 0.0429). As for ESKD (Supplementary Table 3), we found the following assciated facotrs: DM alone in CKD stage 3, HR = 3.061, 95%CI = 1.235–7.586 (p = 0.0157); DM and hyperuricmie together in CKD stage 3, HR = 3.556, 95%CI = 1.616–7.824 (p = 0.0016); DM alone in CKD stage 4, HR = 2.396, 95%CI = 1.310–4.383 (p = 0.0046); DM and hyperuricemia together in CKD stage 4, HR = 2.862, 95%CI = 1.668–4.910 (p = 0.0001); DM and hyperuricemia together in CKD stage 5, HR = 1.815, 95%CI = 1.296–2.541 (p = 0.0005).
Discussion
The principal finding of this study is that the conditions hyperuricemia and DM when occurred together further increased risk of ESKD and all-cause mortality compared with the conditions existed alone. Results were independent of traditional risk factors such as age, gender, BP, and smoking in patients with CKD. Results are consistent with previous studies [15, 31, 32] showing that hyperuricemia is an independent risk factor for ESKD in the general population and in patients with CKD [33–36]. The higher risk on all-cause mortality and ESKD remained significant after adjustment for multiple confounding factors. However, other epidemiologic studies revealed uncertain conclusion because of differences in methodologies and impact on serum UA concentrations by even subtle changes in kidney function in the general population [37]. The causal role of serum UA in kidney disease, hypertension, or DM remains debatable regarding the general population [37]. For patients of CKD stage 3 to 5, we reported earlier in a retrospective study that hyperuricemia is associated with higher risk of incident renal replacement therapy and all-cause mortality [33]. The potential mechanisms that hyperuricemia contributes to CKD progression include a poorer renal perfusion via stimulation of afferent arteriolar vascular smooth muscle cell proliferation [16, 38–40]. Hyperuricemia may lead to acute UA nephropathy [41], chronic urate nephropathy [42], gout related renal injury and anesthesia related nephropathy. Many conditions associated with hyperuricemia in CKD patients could also contribute the progression of CKD.
To our knowledge, this is the first study to show that hyperuricemia is a risk equivalent to DM for all-cause mortality (HR = 1.48 vs. HR = 1.52) and ESKD (HR = 1.34 vs. HR = 1.59) in patients with CKD stage 3 to 5. Gout is a risk factor for CVD [43], CV mortality [44] and all-cause mortality [44, 45]. The possible mechanism is related to hyperuricemia [46]. Hyperuricemia in the absence of gout has a risk of stroke 1.47 times higher [47] and a risk of coronary heart disease 1.34 times higher [48]. From a retrospective study on claims database study, gout has a risk equivalent to DM for the incidence of stroke [46]. Hyperuricemia is linked to impaired production of nitric oxide [49, 50] the activation of renin-angiotensin system [51]. Both of the above factors cause endothelial dysfunction [52, 53], and further contribute to hypertension [54, 55] and hyperuricemia [56, 57]. Studies showed that UA stimulates the proliferation of vascular smooth muscle cells [58–60]. Hyperuricemia-related monosodium urate crystals [61, 62] may cause atherosclerosis with more coagulation [63]. Both hyperuricemia and DM are linked to CVD and all-cause mortality. In addition, hyperuricemia-related gout usually requires treatment with nonsteroidal anti-inflammatory drug (NSAID). NSAID is also associated with higher CV mortality and all-cause mortality [64–66]. Finally, the hyperuricemia may be related to the use of diuretics, a medication which is typically used in patients with heart failure with pulmonary edema and unstable heart function. Both low cardiac output and diuretic therapy reduce UA excretion. Hence hyperuricemia is likely a good maker for poor heart function and higher risk for mortality [67]. In summary, regarding all-cause mortality, hyperuricemia has a risk equivalent to DM.
Hyperuricemia was also a risk equivalent to DM for ESKD in this CKD cohort in Taiwan, which has the highest incidence of ESKD worldwide [68]. In addition to potential mechanisms that hyperuricemia contributes to CKD progression by reducing renal perfusion via stimulated proliferation of afferent arteriolar vascular smooth muscle cells [16, 38–40], and the over-use of NSAID for gout attack also threatened CKD progression. Once gout attack, patients got used to taking NSAIDs for pain relief even if definite evidence of renal toxicity of NSAIDs. From a Nationwide study in Taiwan (109,400 incident chronic ESKD patients from 1998 to 2009) [69], adjusted odds ratio (OR) was 2.73 (95% CI: 2.62–2.84) for nonselective NSAIDs and 2.17 (95% CI: 1.83–2.57) for celecoxib. Compared with the non-users, users of oral NSAID were 3.74 times more likely to develop dialysis-required ESKD. This severe renal risk could be even greater for people who had recently used the parenteral form of NSAIDs (adjusted OR: 8.66) [69]. About 30% dialytic patients still took NSAID 1 year before the initiation of dialysis (2018 Annual Report on Kidney Disease, Taiwan) [70]. Moreover, the number of patients taking NSAID was likely under-estimated because its over-the-counter availability in Taiwan.
Our patients with both DM and hyperuricemia had more increased risk of ESKD (HR = 2.46) than either DM or hyperuricemia alone. The joint effect was greater than the additive HR (1.59*1.34 = 2.13) of DM and hyperuricemia. The discrepancy may be due to synergistic or potentiating effects. First, DM and hyperuricemia share some similar mechanism for renal injury in patients with CKD, but other UA associated mechanisms for renal injury may be independent from DM (like acute UA nephropathy [41], chronic urate nephropathy [42], gout related renal injury and NSAID related nephropathy [69]). Such additional mechanisms of hyperuricemia and gout-related renal injury could lead to higher risk of ESKD in DM-related CKD. Therefore, even hyperuricemia and DM shared similar mechanisms for CKD progression, the combined risks of DM and hyperuricemia for ESKD appeared higher than DM or hyperuricemia alone. Second, there are several kinds of synergistic effects between DM and hyperuricemia on CKD progression. Initial hyperuricemia is an independent risk factor for the progression of diabetic kidney disease (DKD) [71]. High serum UA levels potentiate CKD progression in patients with type 2 DM [72]. Initially, activation of the renin-angiotensin system causes glomerular hyperfiltration [51, 73], a finding characterizes diabetic kidney disease (DKD) and CKD. Thus, hyperfiltration is potentiated under hyperuricemia in patients with both CKD and type 2 DM. In addition, UA stimulates proliferation of vascular smooth muscle cells and their oxidative stress [59], leading to progression of CKD. The oxidative stress and inflammation are typical findings of DKD [74, 75]. Furthermore, UA-related alleles of SLC2A9 rs11722228, SLC2A9 rs3775948, ABCG2 rs2231142 affect DKD susceptibility in the Chinese patients with type 2 DM [76]. A clinical study on 15-year follow-up supported the contribution of hyperuricemia on CKD progression [71]. In another clinical study, febuxostat preserves eGFR in patients of DKD, at levels beyond glycemic control [77]. Low-doses allopurinol reduce the severity of proteinuria in type 2 DM, probably through decreased serum UA [78]. Therefore, UA can be considered as a mediator of DKD [79] and the joint effect of hyperuricemia and DM on ESKD could be synergistic.
There are some limitations of our present study. First, causal effect of hyperuricemia on ESKD and all-cause mortality cannot be established. Second, we did not record the gout condition and the usage of NSAID. Third, our results cannot be generalized to patients covering all stages of CKD. Finally, for patients with CKD under critical status, the result cannot be generalized to this population, neither. Despite these limitations, DM and hyperuricemia having joint effect on ESKD and all-cause mortality remains a robust finding.
Conclusions
Both DM and hyperuricemia are strongly associated with more all-cause mortality and ESKD risk in patients with CKD stage 3–5. In these patients, hyperuricemia has same effect as DM on risk of all-cause mortality and ESKD. Joint effects of DM and hyperuricemia further increase risk of all-cause mortality and ESKD.
Supplementary Information
Acknowledgements
nil.
Abbreviations
- ACEi
Angiotensin- converting enzyme inhibitors
- ARB
Angiotensin II receptor blockers
- CI
Confidence interval
- CKD
Chronic kidney disease
- CKD-EPI
Chronic kidney disease-Epidemiology Collaboration
- CV
Cardiovascular
- DKD
Diabetic kidney disease
- DM
Diabetes Mellitus
- eGFR
Estimated glomerular filtration rate
- ESKD
End-stage kidney disease
- HR
Hazard ratio
- MDRD
Modification diet of renal disease
- NSAID
Nonsteroidal anti-inflammatory drug
- UA
Uric acid
Authors’ contributions
CHL, CLL, YCH, CHC, and MJW wrote the draft and SFT revised this manuscript. CHL, CLL, YCH, CHC, MJW, and SFT all did literatures review. CHL, CLL, YCH, CHC, MJW, and SFT conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Funding
This study was supported by grant from Taichung Veterans General Hospital: TCVGH-1093602B, and TCVGH-1093605D.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
The institutional review board of the Taichung Veterans General Hospital approved the study (IRB TCVGH No: CE16235A-3). The Informed consent was waived by the above ethics committee due to retrospective nature of the study.
Consent for publication
No applicable.
Competing interests
The authors declared no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meguid El Nahas A, Bello AK: Chronic kidney disease: the global challenge. Lancet (London, England) 2005:365(9456):331–340. [DOI] [PubMed]
- 2.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from kidney disease improving global outcomes. Kidney Int. 2007;72(3):247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 3.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351(13):1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 4.Stengel B, Billon S, Van Dijk PC, Jager KJ, Dekker FW, Simpson K, Briggs JD. Trends in the incidence of renal replacement therapy for end-stage renal disease in Europe, 1990-1999. Nephrol Dial Transpl. 2003;18(9):1824–1833. doi: 10.1093/ndt/gfg233. [DOI] [PubMed] [Google Scholar]
- 5.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106(25):3143–3421. [PubMed]
- 6.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet (London, England) 2010, 375(9731):2073–2081. [DOI] [PMC free article] [PubMed]
- 7.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 8.Vervloet M, Cozzolino M. Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int. 2017;91(4):808–817. doi: 10.1016/j.kint.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Merjanian R, Budoff M, Adler S, Berman N, Mehrotra R. Coronary artery, aortic wall, and valvular calcification in nondialyzed individuals with type 2 diabetes and renal disease. Kidney Int. 2003;64(1):263–271. doi: 10.1046/j.1523-1755.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 10.Zoppini G, Targher G, Negri C, Stoico V, Perrone F, Muggeo M, Bonora E. Elevated serum uric acid concentrations independently predict cardiovascular mortality in type 2 diabetic patients. Diabetes Care. 2009;32(9):1716–1720. doi: 10.2337/dc09-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louwe MC, Lammers B, Frias MA, Foks AC, de Leeuw LR, Hildebrand RB, Kuiper J, Smit JWA, Van Berkel TJC, James RW, et al. Abca1 deficiency protects the heart against myocardial infarction-induced injury. Atherosclerosis. 2016;251:159–163. doi: 10.1016/j.atherosclerosis.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K, Hara S, Kushiyama A, Ubara Y, Yoshida Y, Mizuiri S, Aikawa A, Kawatzu S. Risk of macrovascular disease stratified by stage of chronic kidney disease in type 2 diabetic patients: critical level of the estimated glomerular filtration rate and the significance of hyperuricemia. Clin Exp Nephrol. 2011;15(3):391–397. doi: 10.1007/s10157-011-0420-6. [DOI] [PubMed] [Google Scholar]
- 13.Tavil Y, Kaya MG, Oktar SO, Sen N, Okyay K, Yazici HU, Cengel A. Uric acid level and its association with carotid intima-media thickness in patients with hypertension. Atherosclerosis. 2008;197(1):159–163. doi: 10.1016/j.atherosclerosis.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Yang C, Zhao Y, Zeng X, Liu F, Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: a systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014;15:122. doi: 10.1186/1471-2369-15-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19(12):2407–2413. doi: 10.1681/ASN.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44(4):642–650. doi: 10.1016/S0272-6386(04)00934-5. [DOI] [PubMed] [Google Scholar]
- 17.Lv Q, Meng XF, He FF, Chen S, Su H, Xiong J, Gao P, Tian XJ, Liu JS, Zhu ZH, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One. 2013;8(2):e56864. doi: 10.1371/journal.pone.0056864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viazzi F, Leoncini G, Vercelli M, Deferrari G, Pontremoli R. Serum uric acid levels predict new-onset type 2 diabetes in hospitalized patients with primary hypertension: the MAGIC study. Diabetes Care. 2011;34(1):126–128. doi: 10.2337/dc10-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, Ishimoto T, Sautin YY, Lanaspa MA. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307–3315. doi: 10.2337/db12-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang DH, Nakagawa T. Uric acid and chronic renal disease: possible implication of hyperuricemia on progression of renal disease. Semin Nephrol. 2005;25(1):43–49. doi: 10.1016/j.semnephrol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim IY, Lee DW, Lee SB, Kwak IS. The role of uric acid in kidney fibrosis: experimental evidences for the causal relationship. Biomed Res Int. 2014;2014:638732. doi: 10.1155/2014/638732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang CS, Shin DM, Jo EK. The role of NLR-related protein 3 Inflammasome in host defense and inflammatory diseases. Int Neurourol J. 2012;16(1):2–12. doi: 10.5213/inj.2012.16.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Chuang SY, Chen JH, Yeh WT, Wu CC, Pan WH. Hyperuricemia and increased risk of ischemic heart disease in a large Chinese cohort. Int J Cardiol. 2012;154(3):316–321. doi: 10.1016/j.ijcard.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 26.Rigalleau V, Lasseur C, Raffaitin C, Perlemoine C, Barthe N, Chauveau P, Combe C, Gin H. Glucose control influences glomerular filtration rate and its prediction in diabetic subjects. Diabetes Care. 2006;29(7):1491–1495. doi: 10.2337/dc06-0407. [DOI] [PubMed] [Google Scholar]
- 27.Teo BW, Xu H, Wang D, Li J, Sinha AK, Shuter B, Sethi S, Lee EJ. GFR estimating equations in a multiethnic Asian population. Am J Kidney Dis. 2011;58(1):56–63. doi: 10.1053/j.ajkd.2011.02.393. [DOI] [PubMed] [Google Scholar]
- 28.Hsu CC, Hwang SJ, Wen CP, Chang HY, Chen T, Shiu RS, Horng SS, Chang YK, Yang WC. High prevalence and low awareness of CKD in Taiwan: a study on the relationship between serum creatinine and awareness from a nationally representative survey. Am J Kidney Dis. 2006;48(5):727–738. doi: 10.1053/j.ajkd.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC. Taiwan Society of N: impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol Dial Transpl. 2010;25(8):2616–2624. doi: 10.1093/ndt/gfq308. [DOI] [PubMed] [Google Scholar]
- 30.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301–2308. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19(6):1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu P, Liu Y, Han L, Xu G, Ran JM. Serum uric acid is associated with incident chronic kidney disease in middle-aged populations: a meta-analysis of 15 cohort studies. PLoS One. 2014;9(6):e100801. doi: 10.1371/journal.pone.0100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CL, Wang JS: Effects of hyperuricemia on incident renal replacement therapy and all-cause mortality among patients with chronic kidney disease stages 3-5: a retrospective cohort study. Sao Paulo Med J = Rev Paul Med 2019:137(6):523–529. [DOI] [PMC free article] [PubMed]
- 34.Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Menon V. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53(5):796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ. The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis. 2008;51(2):212–223. doi: 10.1053/j.ajkd.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh TR, Choi HS, Kim CS, Bae EH, Ma SK, Sung SA, Kim YS, Oh KH, Ahn C, Kim SW. Hyperuricemia has increased the risk of progression of chronic kidney disease: propensity score matching analysis from the KNOW-CKD study. Sci Rep. 2019;9(1):6681. doi: 10.1038/s41598-019-43241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Sanchez Lozada LG, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis. 2018;71(6):851–865. doi: 10.1053/j.ajkd.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohno I, Hosoya T, Gomi H, Ichida K, Okabe H, Hikita M. Serum uric acid and renal prognosis in patients with IgA nephropathy. Nephron. 2001;87(4):333–339. doi: 10.1159/000045939. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Lozada LG, Tapia E, Santamaria J, Avila-Casado C, Soto V, Nepomuceno T, Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67(1):237–247. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 40.Bellomo G, Venanzi S, Verdura C, Saronio P, Esposito A, Timio M. Association of uric acid with change in kidney function in healthy normotensive individuals. Am J Kidney Dis. 2010;56(2):264–272. doi: 10.1053/j.ajkd.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 41.Kjellstrand CM, Cambell DC, 2nd, von Hartitzsch B, Buselmeier TJ. Hyperuricemic acute renal failure. Arch Intern Med. 1974;133(3):349–359. doi: 10.1001/archinte.1974.00320150023002. [DOI] [PubMed] [Google Scholar]
- 42.Johnson RJ, Kivlighn SD, Kim YG, Suga S, Fogo AB. Reappraisal of the pathogenesis and consequences of hyperuricemia in hypertension, cardiovascular disease, and renal disease. Am J Kidney Dis. 1999;33(2):225–234. doi: 10.1016/S0272-6386(99)70295-7. [DOI] [PubMed] [Google Scholar]
- 43.Abbott RD, Brand FN, Kannel WB, Castelli WP. Gout and coronary heart disease: the Framingham study. J Clin Epidemiol. 1988;41(3):237–242. doi: 10.1016/0895-4356(88)90127-8. [DOI] [PubMed] [Google Scholar]
- 44.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116(8):894–900. doi: 10.1161/CIRCULATIONAHA.107.703389. [DOI] [PubMed] [Google Scholar]
- 45.Cohen SD, Kimmel PL, Neff R, Agodoa L, Abbott KC. Association of incident gout and mortality in dialysis patients. J Am Soc Nephrol. 2008;19(11):2204–2210. doi: 10.1681/ASN.2007111256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh JA, Ramachandaran R, Yu S, Yang S, Xie F, Yun H, Zhang J, Curtis JR. Is gout a risk equivalent to diabetes for stroke and myocardial infarction? A retrospective claims database study. Arthritis Res Ther. 2017;19(1):228. doi: 10.1186/s13075-017-1427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(7):885–892. doi: 10.1002/art.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2010;62(2):170–180. doi: 10.1002/acr.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW, Kim KC, Lee J, Lee MY, Lee SM, Kang DH, et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 2014;28(7):3197–3204. doi: 10.1096/fj.13-247148. [DOI] [PubMed] [Google Scholar]
- 50.Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids. 2008;27(8):967–978. doi: 10.1080/15257770802257952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28(6):1234–1242. doi: 10.1097/HJH.0b013e328337da1d. [DOI] [PubMed] [Google Scholar]
- 52.Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 2005;25(1):39–42. doi: 10.1016/j.semnephrol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez-Lozada LG, Lanaspa MA, Cristobal-Garcia M, Garcia-Arroyo F, Soto V, Cruz-Robles D, Nakagawa T, Yu MA, Kang DH, Johnson RJ. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. 2012;121(3–4):e71–e78. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feig DI, Nakagawa T, Karumanchi SA, Oliver WJ, Kang DH, Finch J, Johnson RJ. Hypothesis: uric acid, nephron number, and the pathogenesis of essential hypertension. Kidney Int. 2004;66(1):281–287. doi: 10.1111/j.1523-1755.2004.00729.x. [DOI] [PubMed] [Google Scholar]
- 55.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 56.Valle M, Martos R, Canete MD, Valle R, van Donkelaar EL, Bermudo F, Canete R. Association of serum uric acid levels to inflammation biomarkers and endothelial dysfunction in obese prepubertal children. Pediatr Diabetes. 2015;16(6):441–447. doi: 10.1111/pedi.12199. [DOI] [PubMed] [Google Scholar]
- 57.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41(6):1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 58.Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem. 1991;266(13):8604–8608. doi: 10.1016/S0021-9258(18)93017-6. [DOI] [PubMed] [Google Scholar]
- 59.Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26(2):269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 60.Chao HH, Liu JC, Lin JW, Chen CH, Wu CH, Cheng TH. Uric acid stimulates endothelin-1 gene expression associated with NADPH oxidase in human aortic smooth muscle cells. Acta Pharmacol Sin. 2008;29(11):1301–1312. doi: 10.1111/j.1745-7254.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 61.Buckland J. Crystal arthropathies: the NET is closing in on inflammation in gout. Nat Rev Rheumatol. 2014;10(6):319. doi: 10.1038/nrrheum.2014.74. [DOI] [PubMed] [Google Scholar]
- 62.Dalbeth N, Haskard DO. Mechanisms of inflammation in gout. Rheumatology (Oxford) 2005;44(9):1090–1096. doi: 10.1093/rheumatology/keh640. [DOI] [PubMed] [Google Scholar]
- 63.Tiong AY, Brieger D. Inflammation and coronary artery disease. Am Heart J. 2005;150(1):11–18. doi: 10.1016/j.ahj.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 64.Gislason GH, Rasmussen JN, Abildstrom SZ, Schramm TK, Hansen ML, Fosbol EL, Sorensen R, Folke F, Buch P, Gadsboll N, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169(2):141–149. doi: 10.1001/archinternmed.2008.525. [DOI] [PubMed] [Google Scholar]
- 65.Lai KM, Chen TL, Chang CC, Chen HH, Lee YW. Association between NSAID use and mortality risk in patients with end-stage renal disease: a population-based cohort study. Clin Epidemiol. 2019;11:429–441. doi: 10.2147/CLEP.S204322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jo HA, Kim DK, Park S, Kim Y, Han SS, Yang BR, Choi SH, Kim MS, Lee J, Lee H et al: Cardiovascular risk of nonsteroidal anti-inflammatory drugs in dialysis patients: a nationwide population-based study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2020. [DOI] [PubMed]
- 67.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105(22):2619–2624. doi: 10.1161/01.CIR.0000017502.58595.ED. [DOI] [PubMed] [Google Scholar]
- 68.Tsai MH, Hsu CY, Lin MY, Yen MF, Chen HH, Chiu YH, Hwang SJ. Incidence, prevalence, and duration of chronic kidney disease in Taiwan: results from a community-based screening program of 106,094 individuals. Nephron. 2018;140(3):175–184. doi: 10.1159/000491708. [DOI] [PubMed] [Google Scholar]
- 69.Chang YK, Liu JS, Hsu YH, Tarng DC, Hsu CC. Increased risk of end-stage renal disease (ESRD) requiring chronic Dialysis is associated with use of nonsteroidal anti-inflammatory drugs (NSAIDs): Nationwide case-crossover study. Medicine (Baltimore) 2015;94(38):e1362. doi: 10.1097/MD.0000000000001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.2018 Annual Report on Kidney Disease in Taiwan [https://www.tsn.org.tw/UI/L/L002.aspx].
- 71.Bartakova V, Kuricova K, Pacal L, Nova Z, Dvorakova V, Svrckova M, Maluskova D, Svobodova I, Rehorova J, Svojanovsky J, et al. Hyperuricemia contributes to the faster progression of diabetic kidney disease in type 2 diabetes mellitus. J Diabetes Complicat. 2016;30(7):1300–1307. doi: 10.1016/j.jdiacomp.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 72.Chang YH, Lei CC, Lin KC, Chang DM, Hsieh CH, Lee YJ. Serum uric acid level as an indicator for CKD regression and progression in patients with type 2 diabetes mellitus-a 4.6-year cohort study. Diabetes Metab Res Rev. 2016;32(6):557–564. doi: 10.1002/dmrr.2768. [DOI] [PubMed] [Google Scholar]
- 73.Hisatome I, Kuwabara M. Hyperuricemia plays pivotal role in progression of kidney disease. Circ J. 2016;80(8):1710–1711. doi: 10.1253/circj.CJ-16-0605. [DOI] [PubMed] [Google Scholar]
- 74.Pichler R, Afkarian M, Dieter BP, Tuttle KR. Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am J Physiol Renal Physiol. 2017;312(4):F716–f731. doi: 10.1152/ajprenal.00314.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hojs R, Ekart R, Bevc S, Hojs N. Markers of inflammation and oxidative stress in the development and progression of renal disease in diabetic patients. Nephron. 2016;133(3):159–162. doi: 10.1159/000447434. [DOI] [PubMed] [Google Scholar]
- 76.Yan D, Wang J, Jiang F, Zhang R, Sun X, Wang T, Wang S, Peng D, He Z, Bao Y, et al. Association between serum uric acid related genetic loci and diabetic kidney disease in the Chinese type 2 diabetes patients. J Diabetes Complicat. 2016;30(5):798–802. doi: 10.1016/j.jdiacomp.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 77.Mukri MNA, Kong WY, Mustafar R, Shaharir SS, Shah SA, Abdul Gafor AH, Mohd R, Abdul Cader R, Kamaruzaman L. Role of febuxostat in retarding progression of diabetic kidney disease with asymptomatic hyperuricemia: a 6-months open-label, randomized controlled trial. EXCLI J. 2018;17:563–575. doi: 10.17179/excli2018-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Momeni A, Shahidi S, Seirafian S, Taheri S, Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4(2):128–132. [PubMed] [Google Scholar]
- 79.Mauer M, Doria A. Uric acid and diabetic nephropathy risk. Contrib Nephrol. 2018;192:103–109. doi: 10.1159/000484284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.