Abstract
Background
To identify the potential biological target molecules and the corresponding interaction networks in primary open-angle glaucoma (POAG) development.
Methods
The microarray datasets of GSE138125 and GSE27276 concerning lncRNA and mRNA expression profiles in trabecular meshwork of POAG were downloaded from the Gene Expression Omnibus database. The R software was applied to identify differentially expressed (DE) lncRNAs and mRNAs in POAG, and to perform GO and KEGG functional enrichment analysis. Protein–protein interaction (PPI) network and module analysis, and lncRNA-miRNA-mRNA competing endogenous RNA (ceRNA) network were performed by Cytoscape software.
Results
A total of 567 DE-mRNAs were identified from GSE138125 and GSE27276, including 298 up-regulated and 269 down-regulated mRNAs, which were found enriching in biological processes of extracellular matrix organization and epidermis development, respectively. KEGG pathway enrichment analysis further revealed that module genes in PPI network were primarily involved in the AGE-PAGE, PI3K-Akt and TGF-β signaling pathways. Moreover, 897 up-regulated and 1036 down-regulated DE-lncRNAs were identified from GSE138125. Through literature review and databases searching, we obtained 712 lncRNA-miRNA and 337 miRNA-mRNA pairs based on the selected eight POAG-related miRNAs. After excluding 702 lncRNAs and 284 mRNAs that were not comprised in the DE-lncRNA and DE-mRNAs, a total of 53 lncRNA nodes, eight miRNA nodes, 10 mRNA nodes, and 78 edges were included in the final ceRNA network.
Conclusions
This study demonstrated the lncRNA and mRNA expression profiles of trabecular meshwork in POAG patients and the normal controls, and identified potentially ceRNAs and pathways which might improve the pathogenic understanding of this ocular disease.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12886-022-02368-0.
Keywords: Primary open-angle glaucoma, Differentially expressed gene, Competing endogenous RNA network, Bioinformatics analysis
Background
Glaucoma is currently the second leading cause of blindness in the world, affecting more than 60 million people [1]. According to whether the angle is closed or not, glaucoma can be divided into two categories: closed angle and open angle. Primary open-angle glaucoma (POAG), a common type of glaucoma, is characterized by progressive and irreversible degeneration of retinal ganglion cells and unique visual field loss [2]. It has been determined that a variety of risk factors may be related to the onset of POAG, such as age, elevated intraocular pressure (IOP), family history, etc. [3–5]. In addition, genetics has also been shown to play a key role in the pathogenesis of POAG [6, 7].
A non-coding RNA (ncRNA) is an RNA molecule that is not translated into protein. It has been found that ncRNAs are the main regulators involved in various biological pathological processes [8, 9]. Long non-coding RNA (lncRNA) is a typical ncRNA of more than 200 nucleotides, with fewer exons and tissue- or cell-specific characteristics [10]. LncRNA can regulate gene expression at different biological levels, such as gene translation and transcription [11, 12]. Recently, new evidence has shown that lncRNA is involved in the development of POAG [7, 13]; however, so far, the functions and related mechanisms of most lncRNAs have not been fully elucidated, and only a small portion being well-annotated. In addition, another ncRNA – microRNA (miRNA) has also been proved to play a vital role in the diagnosis and treatment of glaucoma [14–17]. MiRNA is a single-stranded nucleotide with a length of 18–23 bp, which could regulate the expression of target genes at the post-transcriptional level [18]. Salmena et al. put forward a hypothesis that lncRNAs were emerging as competing endogenous RNAs (ceRNAs), communicating with messenger RNA (mRNA) through competitive miRNA [19]. On the one hand, miRNAs can bind to their target mRNAs and inhibit their expression in the ceRNA network. On the other hand, lncRNA can share miRNA response elements (MREs) with mRNA, which can alleviate the inhibition of the miRNA-mediated gene-encoded protein level [20, 21].
Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) is a largest high-throughput database containing microarray- and sequence-based data from a variety of tissues. Mining and analyzing of the vast reliable gene expression data is helpful for revealing molecular changes of diseases [22]. Over the past decades, several studies have utilized the GEO data to identify the underlying molecular mechanisms of glaucoma pathogenesis [7, 23, 24]. Although certain protein-coding genes or miRNAs have been identified, all of them were found in aqueous humor or optic nerve head tissue but not in trabecular meshwork of POAG patients and the interaction network of these genes is seldom reported [25]. In this study, we first obtained differentially expressed RNAs (DE-RNAs), including DE-lncRNAs and DE-mRNAs, in trabecular meshwork of POAG patients by mining two GEO datasets. Subsequently, we performed functional enrichment and protein–protein interaction (PPI) analysis on the acquired DE-mRNA. Then, through the integration of relevant lncRNA, miRNA and mRNA, a regulatory ceRNA network related to POAG was successfully established. This new method of predicting disease-specific ceRNA networks can clarify the regulatory mechanism of lncRNA-mediated ceRNA in the development and prognosis of POAG, and identify new lncRNAs as potential diagnostic biomarkers or therapeutic targets.
Methods
All data of this study was conducted based on the public databases and performed in accordance with the ethical standards stated in the 2013 Declaration of Helsinki. The informed consent from each participant and the ethical approval was obtained in the original study.
Data collection and preprocessing
We searched the GEO database for studies comparing RNA expression of human TM between POAG and normal eyes. The RNA expression profile data of GSE27276 based on the GPL2507 platform (Sentrix Human-6 Expression BeadChip), and GSE138125 based on the GPL21827 platform (Agilent-079487 Arraystar Human LncRNA microarray V4), were selected for subsequent analysis. As the lncRNA microarray includes the expression results of lncRNA and mRNA, the lncRNA microarray of GSE138125 is divided into lncRNA array and mRNA array for further analysis. Finally, four pairs of POAG and normal samples in GSE138125 dataset, and 17 POAG samples and 19 normal samples in GSE27276 dataset, were enrolled in this study (Supplementary Tables 1–2). All TM tissues were obtained from POAG patients undertaking the conventional trabeculectomy and the donated normal eyes in both datasets [26].
Differential expression analysis of RNAs
The probes in gene expression matrix were annotated with corresponding gene symbols based on the platform annotation information. For a given gene mapping to several probes in one sample, the gene expression level was determined as the average of the detected probe values. We used the “limma” package in R to identify the DE-RNAs, and the p-value was adjusted to false discovery rate (FDR) by applying the Benjamini–Hochberg (BH) program [27]. FDR adjusted p-value < 0.05 and |log2Fold Change (FC)|> 0.58 were considered as the cutoff values for DE-RNAs screening. The “ggplot2” and “pheatmap” packages were applied for data visualization via volcano plots and two-way hierarchical clustering heat maps in R [28]. Moreover, the DE-mRNAs from both microarrays were subjected to Venn analysis using the “venn” package in R. Overlapping genes were considered as co-expression DE-mRNAs (Co-DE-mRNAs) in the following analyses.
Functional enrichment analysis
To understand the potential biological functions of DE-mRNAs, the “clusterProfiler” package in R was utilized to identify the Gene Ontology (GO) biological functions and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways [29, 30]. The GO and KEGG analyses were performed for up-regulated and down-regulated DE-mRNAs, separately. Records with a p-value < 0.05 and enrichment > 2.0 were regarded as statistically significant in functional enrichment analysis. The “enrichplot” package in R was utilized to show the relationship between GO-terms and genes by chord plots.
Construction of PPI network
Search tool for the retrieval of interacting genes (STRING, http://string-db.org/), an online database that collects predicted and/or testified protein interactions, was used to construct the PPI interaction network of the identified DE-mRNAs [31]. After DE-mRNAs were entered into the database, important PPI interaction pairs were obtained from six sources: neighborhood, gene fusion, co-occurrence, co-expression, experiments, databases, and text mining. Cytoscape software was used to predict the association among these target genes with a combined confidence score ≥ 0.4 in the regulatory network analysis. In addition, the MCODE program in Cytoscape was used to identify important modules (degree cutoff ≥ 2, node score cutoff ≥ 0.2, K core ≥ 2 and maximum depth = 100). The clusters that contained ≥ 10 nodes and that possessed MCODE scores ≥ 10 were selected [32].
Identification and establishment of potential ceRNA interactions
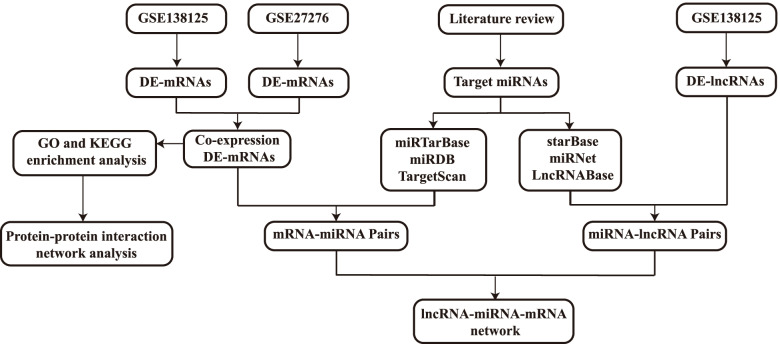
Based on the hypothesis that lncRNA can be anchored to MREs to prevent the binding of miRNA to its target mRNA, a ceRNA network was constructed [33]. First, we selected several miRNAs closely related to the pathogenesis of POAG through literature review. These miRNAs directly promote the development of POAG by participating in pathological processes such as TM extracellular matrix deposition or inflammation [17]. The miRNA-lncRNA interactions were predicted by the miRNet [34, 35] (https://www.mirnet.ca/), LncRNABase (https://starbase.sysu.edu.cn/starbase2/mirLncRNA.php) and starBase [36, 37] (https://starbase.sysu.edu.cn/). The miRDB (http://www.mirdb.org/), miRTarBase [38] (http://mirtarbase.mbc.nctu.edu.tw/) and TargetScan (http://www.targetscan.org/) were used for predicting target mRNAs of miRNA. The lncRNAs and mRNAs, existing in the DE-lncRNA and DE-mRNA groups, respectively, and also appearing in the above databases, were enrolled in the final ceRNA network (Fig. 1). Cytoscape software was used for the construction and visualization of the lncRNA-miRNA-mRNA ceRNA network [39].
Fig. 1.
The flowchart of the identification differentially RNAs and the subsequent construction of lncRNA-miRNA-mRNA network. DE-RNAs, differentially expressed mRNAs, DE-lncRNAs, differentially expressed lncRNAs
Results
Screening of significantly DE-RNAs in POAG
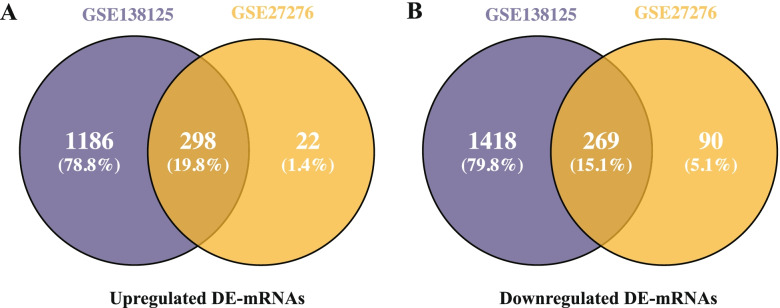
All microarray data is standardized after median normalization. The volcano plots and heat maps of both DE-mRNAs and DE-lncRNAs are shown in Fig. 2, showing that the DE-RNAs in both datasets could be easily distinguished from each of the samples, and suggesting an expressional heterogeneity among the samples. A total of 567 DE-mRNAs were identified after screening of GSE138125 and GSE27276, among which 298 were up-regulated and 269 were down-regulated (Fig. 3). Moreover, 897 up-regulated and 1036 down-regulated DE-lncRNAs were identified after screening of GSE138125. Table 1 shows the top 10 up- and down-regulated DE-mRNAs from GSE138125 and GSE27276, and top 10 up- and down-regulated DE-lncRNAs from GSE138125.
Fig. 2.
Overview of differentially expressed RNAs from microarray datasets. Volcano plots and heat maps of the differentially expressed mRNAs from GSE138125 A, B and GSE27276 C, D, and differentially expressed lncRNAs from GSE138125 E, F. Dash-dotted lines: vertical ones represent log transformed p value, and horizontal ones indicated the mean expression differences of genes between POAG and normal samples. Red dots are for up-regulated RNAs and blue dots for down-regulated ones. Color scale bar denotes the expression levels from high (dark red) to low (dark blue). |log2FC|> 0.58 and adj. p-value < 0.05 were set as the cut-off criteria
Fig. 3.
Venn diagrams of overlapped differentially expressed mRNAs (DE-mRNAs) in GSE138125 and GSE27276
Table 1.
The top 10 up- and down-regulated DE-mRNAs and DE-lncRNAs
| mRNA | Log2FC | p Value | lncRNA | Log2FC | p Value | Change |
|---|---|---|---|---|---|---|
| HBA2 | 4.65 | 3.42E-11 | AC068282.1 | 9.51 | 1.92E-05 | UP |
| HBB | 4.51 | 9.67E-14 | AC105916.1 | 7.95 | 2.02E-03 | UP |
| HBD | 2.89 | 3.90E-08 | AC022517.1 | 7.43 | 9.72E-03 | UP |
| MGP | 2.08 | 5.19E-07 | LINC02428 | 7.20 | 3.22E-03 | UP |
| HLA-DPA1 | 1.74 | 1.87E-08 | NTN5 | 7.04 | 6.55E-09 | UP |
| ECRG4 | 1.60 | 2.28E-07 | DPP9-AS1 | 6.61 | 3.58E-04 | UP |
| PTGDS | 1.54 | 3.18E-08 | LINC02682 | 6.16 | 2.32E-03 | UP |
| CYTL1 | 1.51 | 4.24E-06 | AC079790.2 | 6.00 | 2.32E-02 | UP |
| GRP | 1.48 | 1.16E-05 | AC096577.1 | 5.53 | 5.49E-04 | UP |
| HBG1 | 1.43 | 4.89E-06 | LINC02489 | 5.39 | 3.17E-03 | UP |
| KRT13 | -2.69 | 4.95E-07 | AL138787.2 | -11.67 | 7.66E-09 | DOWN |
| KRT19 | -2.68 | 4.26E-04 | AC006305.1 | -9.68 | 7.69E-10 | DOWN |
| LCN2 | -2.30 | 3.00E-06 | AC136443.4 | -9.49 | 5.53E-08 | DOWN |
| S100A9 | -2.27 | 6.62E-06 | POM121L4P | -9.46 | 2.03E-08 | DOWN |
| SLPI | -2.27 | 1.37E-06 | AC002454.1 | -9.40 | 1.65E-07 | DOWN |
| PAX6 | -2.24 | 4.12E-06 | AL358876.2 | -9.22 | 1.90E-07 | DOWN |
| S100A8 | -2.10 | 3.75E-04 | AC008758.6 | -8.85 | 2.22E-04 | DOWN |
| TNNT3 | -2.08 | 3.67E-08 | PDE6B | -8.82 | 2.94E-06 | DOWN |
| TGM1 | -1.97 | 5.53E-06 | PARD6G-AS1 | -8.78 | 7.15E-07 | DOWN |
| KRT5 | -1.86 | 4.17E-05 | LINC02008 | -8.25 | 6.24E-08 | DOWN |
Functional enrichment analysis
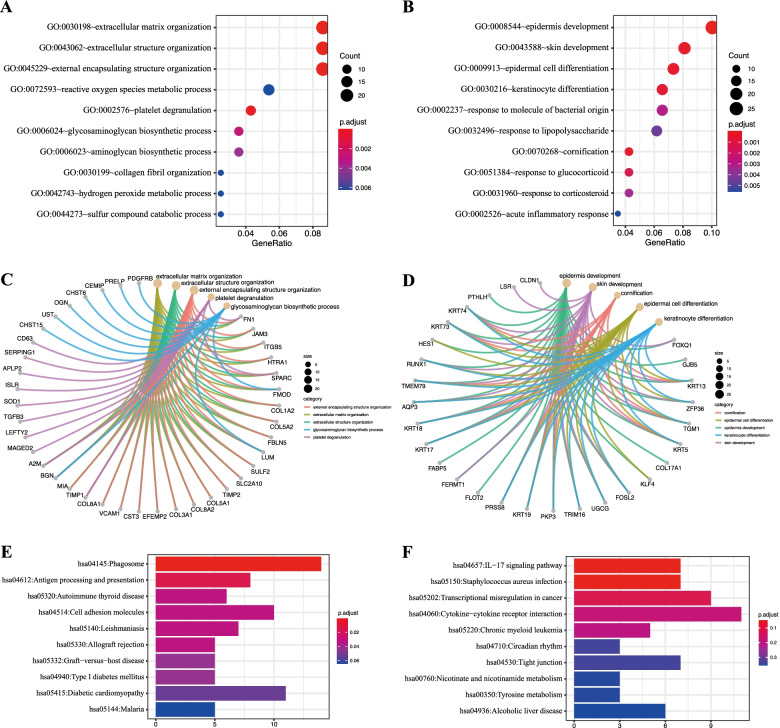
The complete results of GO analysis, including biological process (BP), cellular component (CC) and molecular function (MF), and KEGG analysis are presented in Supplementary Table 3. The top 10 GO-BP terms of DE-mRNAs were visualized in the bubble diagram (Fig. 4A-B). The top three enriched GO term of up-regulated DE-mRNAs were the extracellular matrix organization (GO:0,030,198), extracellular structure organization (GO:0,043,062) and external encapsulating structure organization (GO:0,045,229). The epidermis development (GO:0,008,544), skin development (GO:0,043,588) and epidermal cell differentiation (GO:0,009,913) were the top three enriched pathways of down-regulated DE-mRNAs. GO chord plot shows the relationship between GO term and genes (Fig. 4C-D). In addition, the top 10 KEGG pathways enriched are visualized in the bar plots (Fig. 4E-F), which shows that immunity and infection probably played an important role in POAG.
Fig. 4.
Functional enrichment analysis of the 567 overlapped differentially expressed mRNAs (DE-mRNAs). A and B, The top 10 enriched biological processes for the up-regulated and down-regulated DE-mRNAs via GO analysis; C and D, Chord plots revealed the detailed relationships between GO-terms and up-regulated and down-regulated DE-mRNAs; E and F, The top 10 enriched KEGG pathways for the up-regulated and down-regulated DE-mRNAs. (KEGG pathway database is available at www.kegg.jp/feedback/copyright.html)
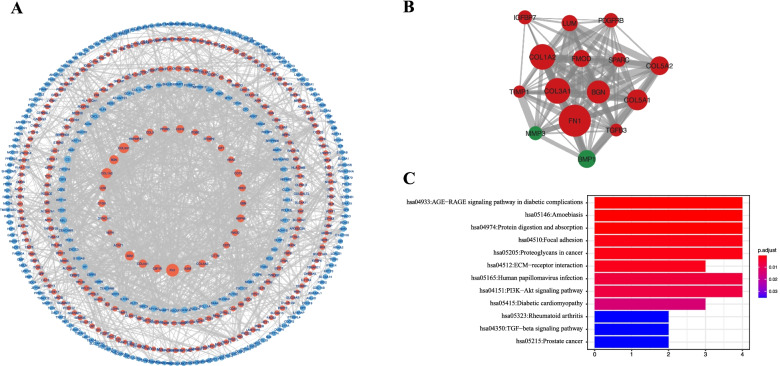
PPI network analysis
PPI network of the significantly DE-mRNAs was constructed with 133 nodes and 259 edges being mapped from STRING database (Fig. 5A). Only one cluster was selected from the PPI network by MCODE analysis based on the criterial of nodes ≥ 10 and scores ≥ 10 (Fig. 5B). The cluster consisted of eight up-regulated genes (FN1, COL1A2, COL3A1, BGN, COL5A1, COL5A2, FMOD, LUM, PDGFRB, SPARC, IGFBP7, TGFB3, TIMP1) and two down-regulated genes (BMP1, MMP3). KEGG pathway enrichment analysis revealed that these module genes were primarily involved in the AGE-PAGE, PI3K-Akt and TGF- β signaling pathway (Fig. 5C).
Fig. 5.
Protein–protein interaction analysis. A, Protein–protein interaction network of the differentially expressed mRNAs from GSE138125 and GSE27276 datasets; B, The sub-network module genes analyzed by MCODE in Cytoscape software; C, KEEG pathways analysis of the genes in the sub-network module genes
Construction of a ceRNA network
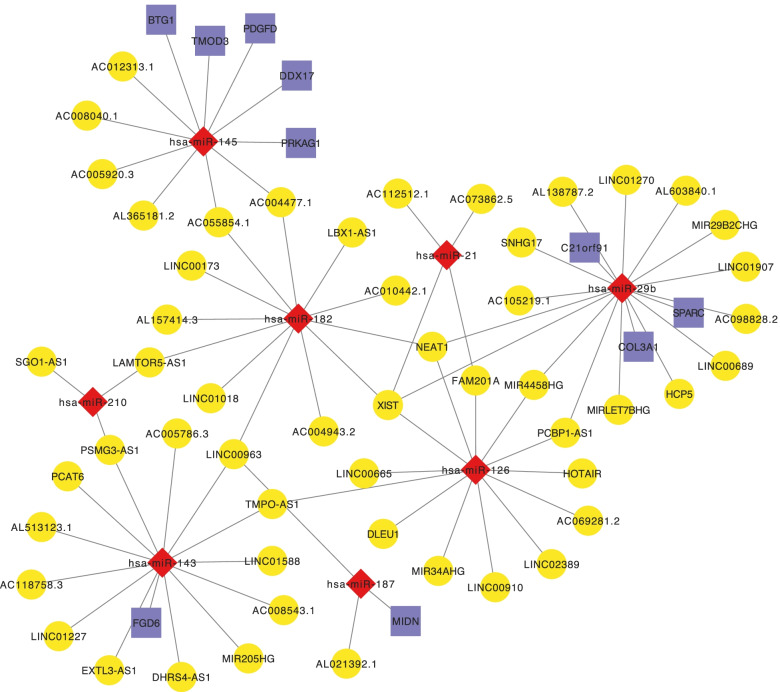
To further understand how lncRNA regulates mRNA through binding with miRNA in human POAG, a lncRNA-miRNA-mRNA (ceRNA) network was constructed. By literature review, we first identified eight miRNAs that may be related to the pathogenesis of POAG, including hsa-miR-29b, hsa-miR-143/145, hsa-miR-21, hsa-miR-210, hsa-miR-126, hsa-miR-182 and hsa-miR-187. Then we found 712 lncRNAs interacting with the eight miRNAs via searching the miRNet, LncRNABase and starBase databases. However, only 53 of these lncRNAs were overlapped with the DE-lncRNAs of GSE138125. Similarly, through searching miRDB, miRTarBase and TargetScan databases, we found 10 mRNAs interacting with the target miRNAs as well as contained in the Co-DE-mRNAs between GSE138125 and GSE27276. As shown in Fig. 6, the preliminarily lncRNA-miRNA-mRNA network was built based on the miRNA-mRNA and miRNA-lncRNA pairs, which was composed of 53 lncRNA nodes, eight miRNA nodes, 10 mRNA nodes, and 78 edges.
Fig. 6.
Competing endogenous RNA network in human POAG. Red nodes denote miRNAs, blue for mRNAs and yellow for lncRNAs
Discussion
POAG is a progressive optic neuropathy, and it is estimated that by 2040, there will be 111.8 million glaucoma patients worldwide [40]. The major risk factor of POAG is the increased IOP, which could compress the structures in and around the optic nerve head to disturb the axoplasmic transport of nerve fibers [41]. This can lead to the death of retinal ganglion cells and their axons, resulting in thinning of the edge of the neural retina and depression of the optic nerve head [42]. Injury related to elevated IOP is mainly manifested as the occurrence of TM degeneration [43]. TM is a key component of the aqueous humor outflow pathway and constitutes most of the outflow resistance [44]. In POAG, a series of pathological changes occur in the TM, leading to increased outflow resistance and elevated IOP [45]. In the past few years, considerable efforts have been made to explore the molecular mechanisms of POAG [46, 47]. However, most studies have focused on protein-coding genes or miRNAs [15, 48]. Neither GO or KEGG analysis of key genes nor the lncRNA expression profile of the TM in POAG was established. Therefore, we analyzed two published microarray data from GEO databases, including the lncRNA microarray from GSE138125 and mRNA microarray from GSE138125 and GSE27276, and constructed a possible ceRNA network based on the DE-mRNAs and DE-lncRNAs. To our knowledge, this study is the largest comparison of lncRNA and mRNA expressions in the TM of POAG group and normal group.
In this study, a total of 567 significantly DE-mRNAs were found in POAG patients compared to normal beings, including 298 up-regulated and 269 down-regulated DE-mRNAs. Functional analysis further showed that the main enrichment pathways of the up-regulated DE-mRNAs resided in cell organization, such as extracellular matrix organization involving genes of fibromodulin (FMOD, log2FC = 1.40), biglycan (BGN, log2FC = 1.25) and HtrA serine peptidase 1 (HTRA1, log2FC = 1.16), etc. Specifically, HTRA1 and FMOD are responsible for the degradation and reconstruction of the extracellular matrix [49], while BGN is positively correlated with collagen fibril assembly in multiple tissues [50, 51]. Previous study indicated that both FMOD and BGN play a role in the pathogenesis of POAG, in which BGN participate in the extracellular matrix remodeling and axonal damage in the lamina cribrosa of the optic nerve head in glaucomatous optic neuropathy, and FMOD might be associated with susceptibility to glaucoma damage [52–54]. In addition, it was reported that HTRA1 participate in the extracellular deposits of proteins and lipids on the basal side of retinal pigment epithelium, which contribute to the pathogenesis of age-related macular degeneration [55]. For the down-regulated DE-mRNAs, development related pathways were mainly enriched. The pathway of epidermis development, for example, involved genes of keratin 13 (KRT13, log2FC = -2.69), keratin 19 (KRT19, log2FC = -2.68) and transglutaminase 1 (TGM1, log2FC = -1.97), etc. Both KRT13 and KRT19 are members of the keratin family which is a group of intermediate filament proteins responsible for the structural integrity of epithelial cells. And TGM1 is also involved in the process of keratinization. The above three mRNAs mainly exist in epithelial cells, which may be involved in the process of corneal epithelial conjunctivalisation, and few studies have investigated their relationship with POAG [56]. These down-regulated DE-mRNAs may be considered as potential targets for drug therapy of POAG. Moreover, the PPI network of DE-mRNAs outlined their functional connections and revealed a total of 15 hub genes: FN1, COL1A2, COL3A1, BGN, COL5A1, COL5A2, FMOD, LUM, PDGFRB, SPARC, IGFBP7, TGFB3 and TIMP1 amongst the up-regulated genes, and BMP1, MMP3 amongst the down-regulated genes. Among them, the top DE-mRNA was fibronectin (FN1), a glycoprotein within the extracellular matrix. It has been proved that FN1 was upregulated in tears, tenon's capsule and aqueous humor samples in pseudoexfoliation glaucoma, and that inhibiting FN1 would promote the proliferation and invasion of TM cells [57, 58]. Two other highly expressed mRNAs, COL1A2 and COL3A1, have also been shown to be genetic and biochemical biomarkers of POAG [59, 60]. All above significantly dysregulated genes involved in extracellular matrix organization and epidermis development may play a vital role in the pathogenesis of POAG and thus deserve more exploration.
Moreover, 897 up-regulated and 1036 down-regulated DE-lncRNAs were identified by screening the GSE138125 database. Compared with protein-coding gene and miRNA, lncRNA has significant advantages as a prognostic biomarker or therapeutic target [61, 62]. LncRNA regulates the level of gene-encoded proteins by competitively binding to MREs to regulate cell activities [63]. For example, the lncRNA TGFβ2-AS1 could promote the production of extracellular matrix production through targeting TGF-β2 in human TM cells, suggesting that lncRNA TGFβ2-AS1 may be a potential treatment target for POAG [64]. Moreover, lncRNAs are also important components of the ceRNA network, which plays an important role in the post-transcriptional regulation of genes. It has reported that the ceRNA was associated with the molecular mechanisms of eye disease [65]. In these circumstances, a ceRNA network based on the differentially expressed genes in TM tissue can be helpful to understand the underlying molecular mechanisms of POAG development. In order to construct the lncRNA-miRNA-mRNA network, we selected a total of eight miRNAs that have been functionally proved in cells and animal models to be associated with the pathogenesis of POAG, including hsa-miR-29b, hsa-miR-143/145, hsa-miR-21, hsa-miR-210, hsa-miR-126, hsa-miR-182 and hsa-miR-187. All these miRNAs are involved in IOP elevation or TM damage. For example, the hsa-miR-29b could inhibit the expression of collagen I/III/IV through PI3K/Akt/Sp1 signaling pathway, leading to the deposition of extracellular matrix in the TM [66]. And the hsa-miR-143/145 primarily promotes the phosphorylation of myosin in TM cells, which in turn promotes the contraction of TM cells and thus results in an elevated IOP [67, 68].
In this study, all above eight miRNAs were applied to predict the possible miRNA-lncRNA pairs and miRNA-mRNA pairs within the public databases. These identified lncRNAs or mRNAs presented both in the pairs and in the DE-lncRNAs or DE-mRNAs groups were enrolled in the final ceRNA network. Consequently, 53 lncRNAs, eight miRNAs, and 10 mRNAs were selected for the ceRNA network analysis. Among them, the most up-regulated lncRNA in the ceRNA network is HOTAIR, which is an antisense lncRNA that has been reported to play a critical role in multiple complex diseases, such as the progression of Parkinson’s disease, psoriasis and cancers, by targeting hsa-miR-126 and its downstream pathways [69–71]. In POAG patients, hsa-miR-126 may be down-regulated under chronic hypoxia conditions, resulting in retinal ganglion cells injury through targeting VEGF-Notch signaling pathway [72]. We speculated that this can be related to the competitive binding of up-regulated HOTAIR to the hsa-miR-126 response element. Besides, the ceRNA network also shown that LINC00173 and LBX1-AS1 could sponge to the hsa-miR-182. Recent studies suggested that hsa-miR-182 was up-regulated in aging TM cells and aqueous humor of POAG patients to regulate IOP and protect retinal ganglion cells from oxidative stress [73, 74]. Thus, we thought the elevated IOP and optic nerve damage of POAG would be associated with the up-regulation of LINC00173 and LBX1-AS1. XIST is another notable lncRNA in the ceRNA network, which is interacted with four miRNAs including hsa-miR-29b, hsa-miR-21, hsa-miR-126 and hsa-miR-182. It has been reported that XIST was involved in the epithelial-mesenchymal transitions of retinoblastoma and also participated in the apoptosis and migration of retinal pigment epithelial cells subjected to hyperglycemia [75, 76]. However, few studies have reported the function of XIST as miRNA sponges in the development of POAG.
Conclusions
In summary, current research demonstrated the lncRNA and mRNA differential expression profiles of TM between POAG patients and the normal controls by bioinformatics methods. We identified several potential lncRNAs and mRNAs that may be involved in the pathogenesis of POAG. And the lncRNA-miRNA-mRNA ceRNA network is successfully constructed, showing complex interactions among the lncRNAs, miRNAs and mRNAs during the development of POAG. This may help reveal the unknown pathogenesis and potential therapeutic targets of POAG. Future research effort should focus more on lncRNA exploration to clarify the molecular mechanisms concerning the pathogenesis of POAG.
Supplementary Information
Additional file1: Supplementary Table 1. Clinical data of the enrolled patients in GSE27276 dataset. Supplementary Table 2. Clinical data of the enrolled patients in GSE138125 dataset. Supplementary Table 3. Enriched functional annnotation terms associated with significantly up-regulated genes in primary open-angle glaucoma.
Acknowledgements
Not applicable.
Abbreviations
- BH
Benjamini–Hochberg
- BP
Biological process
- CC
Cellular component
- CeRNA
Competing endogenous RNA
- Co-DE
Co-expression differentially expressed
- DE
Differentially expressed
- FC
Fold change
- FDR
False discovery rate
- GEO
Gene Expression Omnibus
- GO
Gene Ontology
- IOP
Intraocular lens pressure
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LncRNA
Long non-coding RNA
- MF
Molecular function
- MiRNA
MicroRNA
- MRE
MicroRNA response element
- MRNA
Messenger RNA
- POAG
Primary open-angle glaucoma
- PPI
Protein-protein interaction
- TM
Trabecular meshwork
Authors’ contributions
HYL carried out the project, performed data analysis, complied the figures and the tables, and drafted the manuscript. HYL, ZY and ZHL conceived the study and its design as well as revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82070937; 81870640) and the National Science Foundation for Young Scientists of China (82101097).
Availability of data and materials
The datasets supporting the conclusions of this study are available in the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/). (GSE138125: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE138125; GSE27276: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE27276).
Declarations
Ethics approval and consent to participate
All data of this study was conducted based on the public databases and performed in accordance with the ethical standards stated in the 2013 Declaration of Helsinki. The written informed consent from each participant was obtained in the original study. The research of GSE138125 was approved by the Ethics Committee of The Second Xiangya Hospital of Central South University, and the research of GSE27276 was approved by the Institutional Review Board at Duke University Medical Center.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zi Ye, Email: yeziclover@163.com.
Zhaohui Li, Email: zhaohuili202104@163.com.
References
- 1.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma Lancet. 2017;390(10108):2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 2.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 3.Kreft D, Doblhammer G, Guthoff RF, Frech S. Prevalence, incidence, and risk factors of primary open-angle glaucoma - a cohort study based on longitudinal data from a German public health insurance. BMC Public Health. 2019;19(1):851. doi: 10.1186/s12889-019-6935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Baskaran M, Foo RC, Cheng CY, Narayanaswamy AK, Zheng YF, Wu R, et al. The Prevalence and Types of Glaucoma in an Urban Chinese Population: The Singapore Chinese Eye Study. JAMA Ophthalmol. 2015;133(8):874–880. doi: 10.1001/jamaophthalmol.2015.1110. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Allingham RR. Molecular genetics in glaucoma. Exp Eye Res. 2011;93(4):331–339. doi: 10.1016/j.exer.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie L, Mao M, Wang C, Zhang L, Pan Z, Shi J, et al. Potential Biomarkers for Primary Open-Angle Glaucoma Identified by Long Noncoding RNA Profiling in the Aqueous Humor. Am J Pathol. 2019;189(4):739–752. doi: 10.1016/j.ajpath.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Palazzo AF, Lee ES. Non-coding RNA: what is functional and what is junk? Front Genet. 2015;6:2. doi: 10.3389/fgene.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hombach S, Kretz M. Non-coding RNAs: Classification, Biology and Functioning. Adv Exp Med Biol. 2016;937:3–17. doi: 10.1007/978-3-319-42059-2_1. [DOI] [PubMed] [Google Scholar]
- 10.Silva A, Bullock M, Calin G. The Clinical Relevance of Long Non-Coding RNAs in Cancer. Cancers. 2015;7(4):2169–2182. doi: 10.3390/cancers7040884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Panni S, Lovering RC, Porras P, Orchard S. Non-coding RNA regulatory networks. Biochim Biophys Acta Gene Regul Mech. 2020;1863(6):194417. doi: 10.1016/j.bbagrm.2019.194417. [DOI] [PubMed] [Google Scholar]
- 13.Pasquale LR, Loomis SJ, Kang JH, Yaspan BL, Abdrabou W, Budenz DL, et al. CDKN2B-AS1 genotype-glaucoma feature correlations in primary open-angle glaucoma patients from the United States. Am J Ophthalmol. 2013;155(2):342–53.e5. doi: 10.1016/j.ajo.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatzikyriakidou A, Founti P, Melidou A, Minti F, Bouras E, Anastasopoulos E, et al. MicroRNA-related polymorphisms in pseudoexfoliation syndrome, pseudoexfoliative glaucoma, and primary open-angle glaucoma. Ophthalmic Genet. 2018;39(5):603–609. doi: 10.1080/13816810.2018.1509352. [DOI] [PubMed] [Google Scholar]
- 15.Ghanbari M, Iglesias AI, Springelkamp H, van Duijn CM, Ikram MA, Dehghan A, et al. A Genome-Wide Scan for MicroRNA-Related Genetic Variants Associated With Primary Open-Angle Glaucoma. Invest Ophthalmol Vis Sci. 2017;58(12):5368–5377. doi: 10.1167/iovs.17-22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayaram H, Phillips JI, Lozano DC, Choe TE, Cepurna WO, Johnson EC, et al. Comparison of MicroRNA Expression in Aqueous Humor of Normal and Primary Open-Angle Glaucoma Patients Using PCR Arrays: A Pilot Study. Invest Ophthalmol Vis Sci. 2017;58(7):2884–2890. doi: 10.1167/iovs.17-21844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Niu L, Zhao J, Wang M, Li K, Zheng Y. An update: mechanisms of microRNA in primary open-angle glaucoma. Brief Funct Genomics. 2021;20(1):19–27. doi: 10.1093/bfgp/elaa020. [DOI] [PubMed] [Google Scholar]
- 18.Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. 2019;20(1):5–20. doi: 10.1038/s41580-018-0059-1. [DOI] [PubMed] [Google Scholar]
- 19.Militello G, Weirick T, John D, Döring C, Dimmeler S, Uchida S. Screening and validation of lncRNAs and circRNAs as miRNA sponges. Brief Funct Genomics. 2017;18(5):780–788. doi: 10.1093/bib/bbw053. [DOI] [PubMed] [Google Scholar]
- 20.Li F, Huang C, Li Q, Wu X. Construction and Comprehensive Analysis for Dysregulated Long Non-Coding RNA (lncRNA)-Associated Competing Endogenous RNA (ceRNA) Network in Gastric Cancer. Med Sci Monit. 2018;24:37–49. doi: 10.12659/MSM.905410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y, Xiang B, Liu Y, Wang Y, Kan H. LncRNA CDKN2B-AS1 promotes tumor growth and metastasis of human hepatocellular carcinoma by targeting let-7c-5p/NAP1L1 axis. Cancer Lett. 2018;437:56–66. doi: 10.1016/j.canlet.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41:D991–5. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou M, Lu B, Tan W, Fu M. Identification of lncRNA-miRNA-mRNA regulatory network associated with primary open angle glaucoma. BMC Ophthalmol. 2020;20(1):104. doi: 10.1186/s12886-020-01365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Gong K, Li H, Wang C, Qu C, Li H. Gene Expression Profiling of the Optic Nerve Head of Patients with Primary Open-Angle Glaucoma. J Ophthalmol. 2017;2017:6896390. doi: 10.1155/2017/6896390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Yu T, Zhang X, Cai X, Sun H. Network Integration Analysis and Immune Infiltration Analysis Reveal Potential Biomarkers for Primary Open-Angle Glaucoma. Front Cell Dev Biol. 2021;9:793638. doi: 10.3389/fcell.2021.793638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Allingham RR, Qin X, Layfield D, Dellinger AE, Gibson J, et al. Gene expression profile in human trabecular meshwork from patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2013;54(9):6382–6389. doi: 10.1167/iovs.13-12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maag JLV. gganatogram: An R package for modular visualisation of anatograms and tissues based on ggplot2. F1000Res. 2018;7:1576. doi: 10.12688/f1000research.16409.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walter W, Sánchez-Cabo F, Ricote M. GOplot: an R package for visually combining expression data with functional analysis. Bioinformatics. 2015;31(17):2912–2914. doi: 10.1093/bioinformatics/btv300. [DOI] [PubMed] [Google Scholar]
- 30.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–52. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandettini WP, Kellman P, Mancini C, Booker OJ, Vasu S, Leung SW, et al. MultiContrast Delayed Enhancement (MCODE) improves detection of subendocardial myocardial infarction by late gadolinium enhancement cardiovascular magnetic resonance: a clinical validation study. J Cardiovasc Magn Reson. 2012;14(1):83. doi: 10.1186/1532-429X-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo LL, Song CH, Wang P, Dai LP, Zhang JY, Wang KJ. Competing endogenous RNA networks and gastric cancer. World J Gastroenterol. 2015;21(41):11680–11687. doi: 10.3748/wjg.v21.i41.11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan Y, Siklenka K, Arora SK, Ribeiro P, Kimmins S, Xia J. miRNet - dissecting miRNA-target interactions and functional associations through network-based visual analysis. Nucleic Acids Res. 2016;44(W1):W135–W141. doi: 10.1093/nar/gkw288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan Y, Xia J. miRNet-Functional Analysis and Visual Exploration of miRNA-Target Interactions in a Network Context. Methods Mol Biol. 2018;1819:215–233. doi: 10.1007/978-1-4939-8618-7_10. [DOI] [PubMed] [Google Scholar]
- 36.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database issue):D92–7. [DOI] [PMC free article] [PubMed]
- 37.Yang JH, Li JH, Shao P, Zhou H, Chen YQ, Qu LH. starBase: a database for exploring microRNA-mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res. 2011;39:D202–9. doi: 10.1093/nar/gkq1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou CH, Shrestha S, Yang CD, Chang NW, Lin YL, Liao KW, et al. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46(D1):D296–d302. doi: 10.1093/nar/gkx1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharts-Hopko NC, Glynn-Milley C. Primary open-angle glaucoma. Am J Nurs. 2009;109(2):40–47. doi: 10.1097/01.NAJ.0000345434.37734.ee. [DOI] [PubMed] [Google Scholar]
- 41.Maddineni P, Kasetti RB, Patel PD, Millar JC, Kiehlbauch C, Clark AF, et al. CNS axonal degeneration and transport deficits at the optic nerve head precede structural and functional loss of retinal ganglion cells in a mouse model of glaucoma. Mol Neurodegener. 2020;15(1):48. doi: 10.1186/s13024-020-00400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanagi M, Kawasaki R, Wang JJ, Wong TY, Crowston J, Kiuchi Y. Vascular risk factors in glaucoma: a review. Clin Exp Ophthalmol. 2011;39(3):252–258. doi: 10.1111/j.1442-9071.2010.02455.x. [DOI] [PubMed] [Google Scholar]
- 43.Tektas OY, Lütjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88(4):769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 44.Tabak S, Schreiber-Avissar S, Beit-Yannai E. Crosstalk between MicroRNA and Oxidative Stress in Primary Open-Angle Glaucoma. Int J Mol Sci. 2021;22(5):2421. doi: 10.3390/ijms22052421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao W, Millar JC, Wang WH, Silverman SM, Liu Y, Wordinger RJ, et al. Existence of the canonical Wnt signaling pathway in the human trabecular meshwork. Invest Ophthalmol Vis Sci. 2012;53(11):7043–7051. doi: 10.1167/iovs.12-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong B, Zhang H, Huang L, Chen Y, Shi Y, Tam PO, et al. Mutant RAMP2 causes primary open-angle glaucoma via the CRLR-cAMP axis. Genet Med. 2019;21(10):2345–2354. doi: 10.1038/s41436-019-0507-0. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Allingham RR. Major review: Molecular genetics of primary open-angle glaucoma. Exp Eye Res. 2017;160:62–84. doi: 10.1016/j.exer.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raga-Cervera J, Bolarin JM, Millan JM, Garcia-Medina JJ, Pedrola L, Abellán-Abenza J, et al. miRNAs and Genes Involved in the Interplay between Ocular Hypertension and Primary Open-Angle Glaucoma. Oxidative Stress, Inflammation, and Apoptosis Networks. J Clin Med. 2021;10(11):2227. doi: 10.3390/jcm10112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clausen T, Kaiser M, Huber R, Ehrmann M. HTRA proteases: regulated proteolysis in protein quality control. Nat Rev Mol Cell Biol. 2011;12(3):152–162. doi: 10.1038/nrm3065. [DOI] [PubMed] [Google Scholar]
- 50.Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, et al. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284(13):8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson KA, Sun M, Barnum CE, Weiss SN, Huegel J, Shetye SS, et al. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biol. 2017;64:81–93. doi: 10.1016/j.matbio.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirwan RP, Leonard MO, Murphy M, Clark AF, O'Brien CJ. Transforming growth factor-beta-regulated gene transcription and protein expression in human GFAP-negative lamina cribrosa cells. Glia. 2005;52(4):309–324. doi: 10.1002/glia.20247. [DOI] [PubMed] [Google Scholar]
- 53.Kirwan RP, Fenerty CH, Crean J, Wordinger RJ, Clark AF, O'Brien CJ. Influence of cyclical mechanical strain on extracellular matrix gene expression in human lamina cribrosa cells in vitro. Mol Vis. 2005;11:798–810. [PubMed] [Google Scholar]
- 54.Steinhart MR, Cone-Kimball E, Nguyen C, Nguyen TD, Pease ME, Chakravarti S, et al. Susceptibility to glaucoma damage related to age and connective tissue mutations in mice. Exp Eye Res. 2014;119:54–60. doi: 10.1016/j.exer.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin MK, Yang J, Hsu CW, Gore A, Bassuk AG, Brown LM, et al. HTRA1, an age-related macular degeneration protease, processes extracellular matrix proteins EFEMP1 and TSP1. Aging cell. 2018;17(4):e12710. doi: 10.1111/acel.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubelowski AK, Latta L, Katiyar P, Stachon T, Käsmann-Kellner B, Seitz B, et al. HCE-T cell line lacks cornea-specific differentiation markers compared to primary limbal epithelial cells and differentiated corneal epithelium. Graefes Arch Clin Exp Ophthalmol. 2020;258(3):565–575. doi: 10.1007/s00417-019-04563-0. [DOI] [PubMed] [Google Scholar]
- 57.Sahay P, Reddy S, Prusty BK, Modak R, Rao A. TGFβ1, MMPs and cytokines profiles in ocular surface: Possible tear biomarkers for pseudoexfoliation. PloS One. 2021;16(4):e0249759. doi: 10.1371/journal.pone.0249759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin R, Chen X. Regulatory effect of miR-144-3p on the function of human trabecular meshwork cells and fibronectin-1. Exp Ther Med. 2019;18(1):647–653. doi: 10.3892/etm.2019.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu H, Zhu B, Ni S. Identification of genes associated with primary open-angle glaucoma by bioinformatics approach. Int Ophthalmol. 2018;38(1):19–28. doi: 10.1007/s10792-017-0704-2. [DOI] [PubMed] [Google Scholar]
- 60.Williams SE, Carmichael TR, Allingham RR, Hauser M, Ramsay M. The genetics of POAG in black South Africans: a candidate gene association study. Sci Rep. 2015;5:8378. doi: 10.1038/srep08378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chandra Gupta S, Nandan TY. Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int J Cancer. 2017;140(9):1955–1967. doi: 10.1002/ijc.30546. [DOI] [PubMed] [Google Scholar]
- 62.Matsui M, Corey DR. Non-coding RNAs as drug targets. Nat Rev Drug Discov. 2017;16(3):167–179. doi: 10.1038/nrd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing MiRNA-LncRNA Interactions. Methods Mol Biol. 2016;1402:271–286. doi: 10.1007/978-1-4939-3378-5_21. [DOI] [PubMed] [Google Scholar]
- 64.Lv Y, Zhang Z, Xing X, Liu A. lncRNA TGFβ2-AS1 promotes ECM production via TGF-β2 in human trabecular meshwork cells. Biochem Biophys Res Commun. 2020;527(4):881–888. doi: 10.1016/j.bbrc.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Su Y, Yi Y, Li L, Chen C. circRNA-miRNA-mRNA network in age-related macular degeneration: From construction to identification. Exp Eye Res. 2021;203:108427. doi: 10.1016/j.exer.2020.108427. [DOI] [PubMed] [Google Scholar]
- 66.Yu J, Luo H, Li N, Duan X. Suppression of Type I Collagen Expression by miR-29b Via PI3K, Akt, and Sp1 Pathway, Part II: An In Vivo Investigation. Invest Ophthalmol Vis Sci. 2015;56(10):6019–6028. doi: 10.1167/iovs.15-16558. [DOI] [PubMed] [Google Scholar]
- 67.Li X, Zhao F, Xin M, Li G, Luna C, Li G, et al. Regulation of intraocular pressure by microRNA cluster miR-143/145. Sci Rep. 2017;7(1):915. doi: 10.1038/s41598-017-01003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamagishi-Kimura R, Honjo M, Komizo T, Ono T, Yagi A, Lee J, et al. Interaction Between Pilocarpine and Ripasudil on Intraocular Pressure, Pupil Diameter, and the Aqueous-Outflow Pathway. Invest Ophthalmol Vis Sci. 2018;59(5):1844–1854. doi: 10.1167/iovs.18-23900. [DOI] [PubMed] [Google Scholar]
- 69.Guo YP, Wang ZF, Li N, Lei QQ, Cheng Q, Shi LG, et al. Suppression of lncRNA HOTAIR alleviates RCC angiogenesis through regulating miR-126/EGFL7 axis. Am J Physiol Cell Physiol. 2021;320(5):C880–C891. doi: 10.1152/ajpcell.00459.2019. [DOI] [PubMed] [Google Scholar]
- 70.Lin Q, Hou S, Dai Y, Jiang N, Lin Y. LncRNA HOTAIR targets miR-126-5p to promote the progression of Parkinson's disease through RAB3IP. Biol Chem. 2019;400(9):1217–1228. doi: 10.1515/hsz-2018-0431. [DOI] [PubMed] [Google Scholar]
- 71.Zha W, Guo B, Chen S, Lu J, Shan Y. Role of the long non-coding RNA HOTAIR/miR-126 axis in an in vitro psoriasis model. Exp Ther Med. 2021;21(5):450. doi: 10.3892/etm.2021.9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang LJ, Wang XZ, Li ZM, Kou D, Zhang D, Xu ZZ. MiR-126 facilitates apoptosis of retinal ganglion cells in glaucoma rats via VEGF-Notch signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(17):8635–8641. doi: 10.26355/eurrev_202009_22800. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y, Bailey JC, Helwa I, Dismuke WM, Cai J, Drewry M, et al. A Common Variant in MIR182 Is Associated With Primary Open-Angle Glaucoma in the NEIGHBORHOOD Consortium. Invest Ophthalmol Vis Sci. 2016;57(10):4528–4535. doi: 10.1167/iovs.16-19688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X, Wang Q, Ren Y, Wang X, Cheng H, Yang H, et al. Tetramethylpyrazine protects retinal ganglion cells against H2O2-induced damage via the microRNA-182/mitochondrial pathway. Int J Mol Med. 2019;44(2):503–512. doi: 10.3892/ijmm.2019.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng Y, Chang Q, Zheng B, Xu J, Li H, Wang R. LncRNA XIST promotes the epithelial to mesenchymal transition of retinoblastoma via sponging miR-101. Eur J Pharmacol. 2019;843:210–216. doi: 10.1016/j.ejphar.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 76.Dong Y, Wan G, Peng G, Yan P, Qian C, Li F. Long non-coding RNA XIST regulates hyperglycemia-associated apoptosis and migration in human retinal pigment epithelial cells. Biomed Pharmacother. 2020;125:109959. doi: 10.1016/j.biopha.2020.109959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file1: Supplementary Table 1. Clinical data of the enrolled patients in GSE27276 dataset. Supplementary Table 2. Clinical data of the enrolled patients in GSE138125 dataset. Supplementary Table 3. Enriched functional annnotation terms associated with significantly up-regulated genes in primary open-angle glaucoma.
Data Availability Statement
The datasets supporting the conclusions of this study are available in the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/). (GSE138125: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE138125; GSE27276: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE27276).