Abstract
Purpose
Myopes have a reduced ability to elicit transient axial eye shortening after imposed positive defocus, which may be due to changes in the biochemical signaling cascade controlling choroidal thickness. We have investigated whether reading with inverted text contrast can still elicit transient axial eye shortening in myopes, like it has been shown in emmetropes.
Methods
Changes in axial length before and after reading were measured with the Lenstar LS-900. Text with inverted contrast was read from a large screen at 2 m distance (angular subtense 35.9°, screen luminance matched in all conditions to 86 ± 7 cd/m²) for 30 minutes. Moreover, we investigated the effects of letter sizes. Two text sizes were tested: “small” text (letter height 13.75 arcmin) and “large” text (letter height 34.39 arcmin).
Results
Reading text with inverted contrast induced eye shortening (–10.2 ± 9.5 µm) in myopic eyes (n = 11; refraction –3.5 ± 1.9 diopters [D]), showing that an inhibitory signal was still generated by the retina as in emmetropes. In 15 subjects (refraction +1.7 to –4.2 D) we found that small text does not elicit significant differences in axial length (P = 0.09). However, with large text, changes in axial length were clearly different for the both contrast polarities (standard contrast, +1.7 ± 9.0 µm; inverted contrast, –9.7 ± 8.9 µm; P = 0.0017).
Conclusions
Although positive defocus may not be an effective intervention to inhibit further eye growth in myopes, other visual stimuli can still trigger choroidal thickening and possibly generate signals to decrease myopia progression.
Translational Relevance
Our results have shown the optimized text features, which may have a positive impact on myopia control.
Keywords: myopia, ON/OFF pathways, text contrast polarity, defocus, emmetropization
Introduction
There is extensive experimental evidence that the feedback mechanism of emmetropization (the developmental matching of focal length and eye length) uses retinal image defocus as an error signal when optimizing refractive state (review1). However, central questions remain unresolved: if there is a closed loop feedback mechanism in operation, why does it not block the development of myopia? And why do children become myopic even though there is no consistent defocus on their retina? It is clear also that other factors, in addition to retinal image defocus, feed into the emmetropization mechanism. Examples include limited exposure to outdoor lighting as well as extensive near work indoors; both are well-known risk factors of myopia development (recent review2). However, it remains unexplained why the retina stops triggering emmetropization under these conditions. There are two main options: (1) the visual information necessary for the retina to emmetropize the eye is altered or insufficient, or (2) the myopic retina undergoes functional changes early in myopia development which compromises correct retinal growth control, despite appropriate visual input.
-
(1)
In favor of the first assumption is that emmetropization is affected by restrictions of the spectral composition of light (i.e., tree shrews3; rhesus monkeys4; chickens5), to lower average illuminances (i.e., human6; rhesus monkeys7,8; mice9). It was also proposed that spatial features of the visual environment, like the “greenness of the residential area”10 or changes in the spatial frequency spectra can affect emmetropization.11 It was also proposed that printed text represents an imbalance in ON/OFF pathway activation which was found to change choroidal thickness and may therefore affect emmetropization.12
-
(2)
In favor of the second assumption is the observation that none of the known interventions to control myopia could fully stop its progression and that imposed positive defocus no longer elicits axial eye shortening in myopic subjects.13 There may be functional changes in the myopic retina that cannot be recovered by optimizing visual input or by pharmacological means. It is clear, however, that optically corrected myopes have otherwise normal visual function, at least when their myopia is moderate.14 The nature and mechanism of the presumed changes in the retina remain therefore obscure.
A major progress in myopia research was the finding that retinal responses to different visual stimuli can be studied in short-term experiments by measuring miniature changes in choroidal thickness (by optical coherence tomography) or axial length (by low coherence interferometry).15–19 A basic assumption, made by several authors, is that these short-term changes have predictive power for future myopia development.15,18,20 It remains unknown how long the effects of visual stimulation persist and whether they remain stable over time. The hypothesis can probably be best supported by long-term studies in children,21,22 although it may be difficult to finally determine what is cause or consequence.13,23–25
To learn more about what might have changed in the myopic retina, we asked young adult subjects to read text with standard contrast (dark text on bright background) or with inverted contrast (bright text on dark background) with matched luminance. The procedure was previously shown to trigger bidirectional changes in choroidal thickness,12 which show up as small changes in measured axial length. Two questions were studied: (1) Knowing that the choroid in our myopic cohort was not responsive to imposed positive defocus in our previous study,13 can reading with inverted text contrast elicit axial eye shortening in myopic subjects? (2) Are the effects dependent on letter size?
Methods
Subjects
In total, 21 young subjects participated in the experiment. The first part of the study (question 1) included 11 myopic subjects (2 males; aged 24.4 ± 2.6 years; average refraction, –3.4 ± 1.4 diopters [D]; range, –1.25 to –6.00 D). The second part (questions 2) included 15 subjects (2 males; aged 25.7 ± 3.1 years): 8 emmetropic, not needing any distance correction (average refraction, 0.0 ± 0.3 D; range, +0.25 to –0.50), 5 myopic (average refraction, –3.4 ± 0.8 D; range, –2.50 to –4.25 D), and 2 hyperopic (average refraction, +1.4 ± 0.5 D; range, +1.0 to +1.7 D). Myopic and hyperopic subjects wore their habitual corrections during the experiments. One emmetropic female subject could not take a part in the second appointment (reading large text) owing to restrictions related to the coronavirus disease-2019 pandemic. All subjects were in good general health and had no history of previous ocular pathologies, other than moderate refractive errors. They had normal far and near visual acuity and no accommodative anomalies. None of the subjects had astigmatism or anisometropia of more than 1 D, amblyopia, or squint. Subjects signed a consent form before participating in the experiments. The study was conducted in accordance with the tenets of the Declaration of Helsinki and approved by the Swiss Research Ethics Committee (EKNZ, reference 2020-01576).
Experimental Protocol
Question 1: Can Reading With Inverted Text Contrast Still Elicit Axial Eye Shortening in Myopic Subjects?
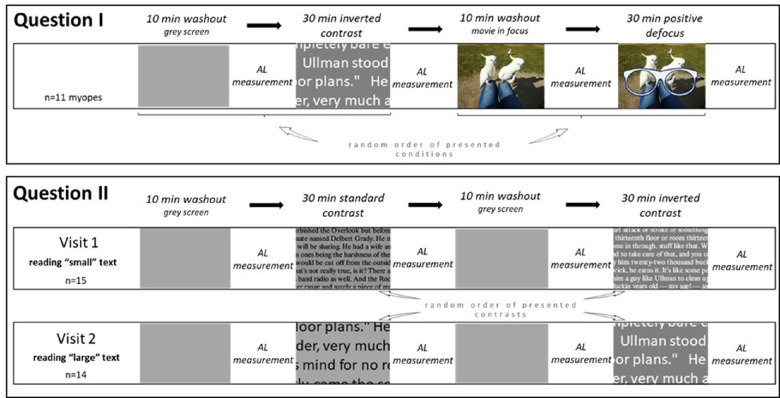
We have previously found that myopes, different from emmetropes, develop longer eyes when they watch a movie with imposed positive defocus.13 Eleven myopic subjects were asked to watch a movie binocularly with +2.5 D of optical defocus, as previously described,13 and subsequently to read “large” text on the screen with inverted contrast (bright letters on the darker background) wearing their habitual corrections (Calibri Body font, sentence case, 34.39 arcmin). Experimental stimuli were presented on a large TV screen at 2 m distance (LG OLED65C9, 65 inch, 4K resolution 3840 × 2160 pixels, subtending 35.9° of the visual field), with an average screen luminance of 86 ± 7 cd/m². The distance was selected to stimulate a large retinal area in the visual field (35.9°). The visual angle was calculated as an arctangent of ratio of letter height to screen distance in millimeters. The room was illuminated only by the screen with no other light sources in the room. Before each task, subjects underwent 10 minutes of a washout period where they had to look at an empty gray screen before the reading task, with the screen luminance matching the average luminance of text displays, or they had to watch a movie in focus before imposing optical defocus. Axial length was measured with the Lenstar LS-900 before and after 30 minutes of movie watching or text reading. The sequence of movie watching, and text reading was randomized and is illustrated in Figure 1. After the reading period, subjects were asked about a content of the text that they had read to confirm that attention was paid to the screen content and visual stimulation had lasted for the entire period.
Figure 1.
Schematic illustration of the study protocol for two parts of the experiment. Question 1 included one visit, where myopic subjects read inverted contrast “large” text and watched a movie with imposed positive defocus. Question 2 included two visits for separate testing the effects of “small” and “large” text, presented at standard and inverted contrast polarity. Before each task, a washout period of 10 minutes was imposed to eliminate possible influences of previous activities. Before and after each viewing task, axial length was measured. The order of the visits and viewing tasks was randomized. The spectacle symbol denotes conditions where positive defocus was imposed.
Question 2: Are the Effects of Text Contrast Polarity on Axial Length Dependent on Text Size?
A second experiment was done to find out whether letter sizes were important to elicit the bidirectional changes in axial length during reading of text with different contrast polarities. Moreover, it was of interest whether changes in myopes were different from those in emmetropes. In all cases, reading with one contrast polarity served as a control for reading with the other contrast polarity, separated by washout periods of looking at a gray screen with matched luminance.
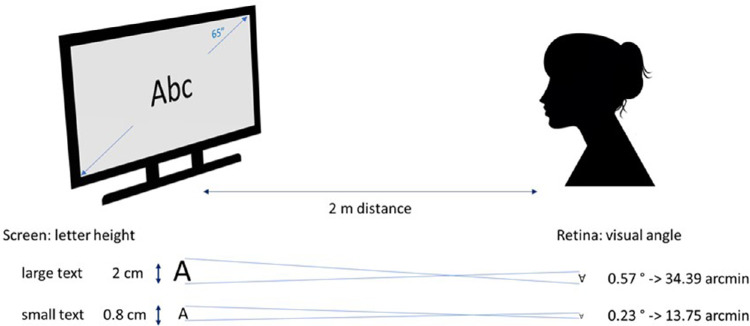
The effects of reading small or large text were studied on 2 separate days. Using the same screen and setup as in the experiments above, 15 subjects were asked to read text binocularly for 30 minutes (Calibri Body font, sentence case) with standard contrast (dark letters on the brighter background) or with inverted contrast (bright letters on the darker background). As in the experiments above, to determine the baseline axial length, subjects were asked to look at an empty gray screen with matched luminance for 10 minutes before each reading task. Presentation sequences of different text sizes and contrast polarities were randomized (Fig. 1). Two text sizes were presented (1) small text with a capital letter height of 8 mm on the screen, subtending 0.23° (13.75 arcmin) in the visual field and (2) large text with capital letter height of 20 mm high, subtending 0.57° (34.39 arcmin) (Fig. 2).26
Figure 2.
Experimental set-up to study the effect of reading of text with different contrast polarities on axial length. The large 65” screen was placed at 2 m distance so that its angular subtense was 35.9° in the visual field.
Screen luminance was carefully matched for the four types of texts (86 ± 7 cd/m²) by digitally adjusting the pixel brightness of the text displays (140.5 ± 4.8 px) and by matching the number of dark and bright pixels for the two text sizes with the same type of contrast (standard contrast, ratio dark to bright pixels 1:5–1:6; inverted contrast, ratio dark to bright pixels 5:1–6:1). The relative strength of the ON or OFF stimulation for the different text types was analyzed with the software that was published by Aleman et al.12 and confirmed that both small and large standard contrast texts overstimulated OFF-center receptive fields, whereas both small and large inverted contrast texts overstimulated ON-center receptive fields.12
Measurements of Axial Length and Refractive Errors
Ocular biometry was measured with a commercial low coherence interferometer, Lenstar (Lenstar LS 900 with autopositioning system; Haag-Streit, Koeniz, Switzerland).27 Axial length measurements of the right eyes were taken from each subject before and immediately (<1 minute) after each experimental session. The Lenstar LS 900 provides only 2 digits behind the decimal and is measuring in millimeters. This means that the effect sizes are outside the scale and can be resolved only by repeating measurements and taking averages. Averages of five repeated measurements were accepted for further analysis when the standard deviation was less than 10 µm. In our study, average standard deviation was 7 µm. All experiments were performed in the morning between 9:00 AM and 12:00 PM, at the same time for each individual subject to decrease the risk of effects of diurnal factors.
Refractive errors (denoted as spherical equivalents) were measured without cycloplegia using a commercially available infrared photorefractor (plusoptiX A12R binocular autorefractor, PlusOptix, Nuremberg, Germany) before the experiment.28
Data Analysis
Data analysis was performed by using a freely available software for statistical computing, “R” (version R 4.0.1; R Core Team, R Foundation for Statistical Computing, Vienna, Austria). Pairwise comparisons were calculated to evaluate effect of reading standard and inverted contrast text on change in axial length. The relationship between axial length changes after reading texts with different contrast polarity and refractive errors was estimated by Pearson's correlation coefficient. Axial length data from individual subjects are presented as the averages of five repeated measurements ± their standard deviation. Averaged data from all subjects are presented with standard errors. P values of less than 0.05 were considered statistically significant.
Results
Question 1: Can Reading With Inverted Text Contrast Still Elicit Axial Eye Shortening in Myopic Subjects?
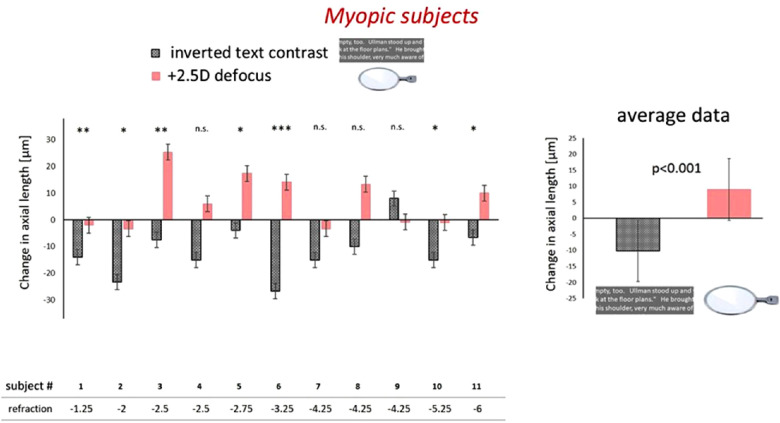
To find out whether the retina in myopic subjects was generally unable to trigger eye shortening, or whether this deficiency was limited to imposed positive defocus, myopic subjects were recruited to either watch movies with imposed positive defocus or read large text with inverted contrast. Interestingly, the eyes of the myopic subjects responded differently under both conditions. They became longer with the positive lenses, but shorter with text with inverted contrast (Fig. 3). Ten of 11 myopic subjects responded with eye shortening to text reading with inverted contrast, whereas positive defocus had opposite or no effect on axial lengths in those subjects. Averaged over all subjects, there was a highly significant difference in axial length change after reading text with inverted contrast or watching movies with positive defocus (–10.2 ± 9.5 µm vs. 8.9 ± 9.6 µm, respectively; P < 0.001) (Fig. 3). The result suggests that functional differences in the retina of emmetropes and myopes were limited to the responses to positive defocus.
Figure 3.
Myopic subjects responded to imposed positive defocus with elongation of the eye or displayed no changes (pink bars). However, their eyes became still shorter when they read text with inverted text contrast (dark gray bars), like in emmetropes. Left: comparisons between reading with inverted contrast and wearing positive lenses in individual myopic subjects. Right: average data, showing significantly opposite responses to both stimuli, inverted text contrast and positive defocus (p < 0.001).
Question 2: Are the Effects of Text Contrast Polarity on Axial Length Dependent on Text Size?
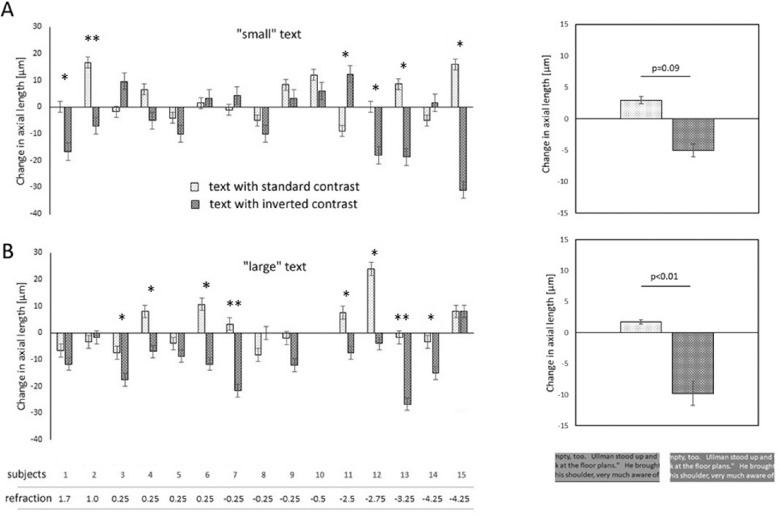
Although some subjects also showed a transient increase in axial length when reading the small text with standard contrast (6 of 15), and a decrease in axial length when reading the small text with inverted contrast (8 of 15), the average effect over 15 subjects showed similar trend as reported by Aleman et al.,12 but did not reach significance (+2.9 ± 7.9 µm vs. –5.0 ± 12.3 µm, respectively; P = 0.09) (Fig. 4A). However, large text with inverted contrast generated consistent effects across the subjects and causes shorter axial lengths (12 of 14 subjects). Only six subjects developed longer eyes when they read large text with standard contrast (Fig. 4B). In average, the differences in axial lengths were significant between the two contrast polarities (standard contrast: +1.7 ± 9.0 µm vs. inverted contrast: –9.7 ± 8.9 µm; n = 14; P = 0.0017), in line with previous findings by Aleman et al.12 Analyzing significances in individual subjects, 12 of the 14 showed decreased axial lengths after reading inverted contrast large text. Moreover, in 8 of the 14 subjects, the difference in axial length change between both text contrast polarities was found to be significant (Fig. 4B). Subjects who did respond to the contrast polarity of the text did not differ in age, refractive error, or gender from nonresponding subjects. It remains unexplained why some subjects responded more to the small text and others to the large text.
Figure 4.
(A) Transient axial length changes in 15 subjects reading “small” text for 30 minutes. Light bars denote standard text contrast, and dark bars are inverted text contrast. Although some subjects displayed bidirectional changes in axial lengths depending on contrast polarities (2, 13, and 15), the average effect was not significant (see plot of the right). (B) With “large” text, most subjects developed shorter eyes (no data for subject 10), resulting in significant differences in axial length changes (plot on the right). Refractions refer to the spherical equivalents. * P < 0.05, ** P < 0.01. D, diopters.
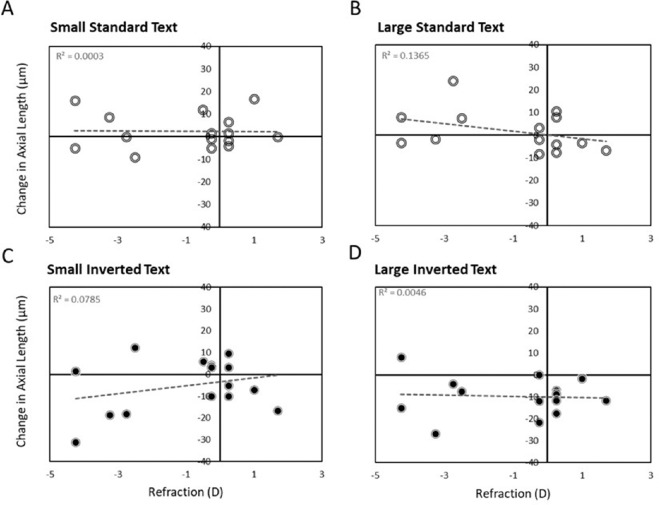
Figure 5 shows that the effects of reading with inverted text contrast on axial length did not depend on refractive error. All subjects responded similarly, no matter whether they were hyperopic, emmetropic or myopic. Figure 5 also shows that eye shortening could only be elicited by large text with inverted contrast (Fig. 5D).
Figure 5.
Effects of text contrast polarity were not dependent on refractive errors. Only inverted text contrast (C and D) caused, on average, a decrease in axial length, which was significant only for the large text fonts (“large, inverted text”, D). There were no correlations with refractive state.
Discussion
The most striking finding of this study was that the myopic retina responded like the emmetropic retina when text was read with inverted contrast, triggering the development of shorter eyes. In contrast, when positive lenses were applied, the myopic retina triggered axial eye elongation and the emmetropic retina induced axial eye shortening, which confirmed results from the previous study.13 There seems to be a difference in retinal responses in myopes when positive defocus is imposed. It is, however, difficult to determine what it might be, without further experiments. The problem is that the retinal tricks to detect the sign of defocus have still to be uncovered. At present we only know that a similar trick may also be used to guide accommodation. Del Aguila-Carrasco et al.29 have carefully studied visual cues controlling accommodation and concluded that “accommodation responds to optical vergence and not defocus blur alone.”
Speculations About the Nature of “the” Inhibitory Signal for Axial Eye Growth
A striking observation in the chicken model was that the retina can distinguish positive from negative defocus, even if the retinal images are continuously and heavily out of focus, containing no high spatial frequencies. Two laboratories placed chickens individually in the center of a large drum, covered inside with pictures, so that only one viewing distance was possible for the chickens.1,30 Lenses were placed in front of the eyes so that similar amounts of defocus were presented to the retina, although with a different sign. In the experiment by Diether and Schaeffel,30 chickens were cyclopleged in addition so that they could not refocus their retinal images. Even under these conditions, eyes started to compensate appropriately for the imposed positive and negative defocus by changing their axial lengths in different directions. Apparently, growth-inhibiting signals were generated, even in severely low-pass filtered retinal images that would normally induce deprivation myopia, except for the case when low-pass filtering resulted from positive defocus. Similarly, the human retina can generate a growth-inhibiting signal with continuously low-pass filtered retinal images, combined with a positive defocus.13 Emmetropic subjects developed shorter eyes when they watched movies with continuous positive defocus but myopes did not. We speculate that lack of the (yet undefined) inhibitory signal in the myopic retina is responsible for myopia development in general and not the low-pass filtering itself. Interestingly, hyperopia induced by positive lenses in animal models is based on a different biochemical signaling cascade, because it cannot be blocked by atropine (31 and our own unpublished observations), has different time kinetics,32 and activates a different set of genes in marmosets.33 Furthermore, the genetic networks responsible for a visual acuity do not match the ones controlling emmetropization in mice,34 in line with our conclusion that high spatial frequencies and high visual acuity are not required for emmetropization. To interfere with myopia development, we believe that this inhibitory signal requires more studies since it may be the key element responsible for myopia development. This cannot be done by studying the signaling cascade for deprivation myopia or myopia-induced by negative lenses.
Effects of Letter Size
We found that contrast polarity of small text (letter size 0.23° of visual angle – comparable with the text on a smartphone) had, averaged over 15 subjects, less effect on axial length than contrast polarity of large text (letter size 0.57° – comparable with the text in a textbook). Interestingly, the large text was in the range of best readability as described by Legge and Bigelow,35 and the small text was in a range where reading speed starts to decline. That large text is more effective is in line with the view that not foveal, but parafoveal, receptive ON /OFF fields are involved. It has recently been shown by Panorgias et al.36 that the retinal area between 6° and 12° eccentricity were most responsive to blur in electroretinogram recordings with the phase-reversing dead leave paradigm. Assuming that line thickness is one-fifth of the letter height (as in a Snellen chart), receptive field sizes of the assumed underlying ON and OFF cells should be in the range of 0.1° to 0.2°, resulting in spatial frequencies around 5 cyc/deg. Also, Charman37 concluded that text sizes in regular textbooks require a resolution of 5 cyc/deg to be read. Furthermore, it has been shown by Sanz Diez et al.38 that accommodation is driven by information in the low spatial frequency range approximately 5 cyc/deg. Swiatczak and Schaeffel13 had found that emmetropization uses visual cues in the low spatial frequency range between 1 and 10 cyc/deg. The question arises as to why emmetropization should use low spatial frequencies for a highly precise task like controlling the growth of the eye. A possible reason is that the depth of focus decreases with increasing spatial frequencies. If only high spatial frequencies were to be analyzed, contrast at these spatial frequencies would decrease sharply already with small amounts of defocus and the dioptric range of possible regulation would become narrow. In contrast, with low spatial frequencies, contrast declines continuously over a wide range of defocus, providing a continuous signal for emmetropization.39 Perhaps for this reason also accommodation is controlled by low spatial frequency information at less than 10 cyc/deg.
Evidence for axial eye shortening in response to a reading text with inverted contrast polarity was previously found by Aleman et al.,12 although, in their study choroidal thickness was measured rather than axial length. However, because thicker choroids cause a shorter axial length, both measures should at least partially reflect the same intraocular changes. A difference to the work by Aleman et al.12 was that bidirectional changes in choroidal thickness were found after 60 minutes of reading, whereas in the current study only contrast-inverted text elicited significant changes in axial length after 30 minutes. Possible explanations include (1) choroidal thinning was too small to be reliably detected by low coherence interferometry, (2) reading tasks lasted too short time or, that, (3) in the current study, screen luminance was higher (82 cd/m²) than in the previous experiments (35 cd/m²). Different luminance may partially explain the different outcomes, although it was shown that looking at an empty screen at three different screen luminance (35, 48, and 62 cd/m²) did not influence on axial length change.12 In line with the previous study is that “choroidal thickening with ON stimulation was not correlated with refractive errors,” we also found that the effects of reading text with inverted contrast were not dependent of refractive error (Fig. 4). Recently, Hoseini-Yazdi et al. found that reading text with standard contrast, stimulating predominantly the OFF pathways, caused choroidal thinning that was additive to the choroidal thinning induced by accommodation (Hoseini-Yazdi H RS, et al. ARVO Abstract. 2021;2021).40 Again, there was no correlation noted with refractive error, in line with our findings and findings by Aleman et al.12 Also, recently, Hogue and Taylor found that the individual sensitivity to ON or OFF stimuli is variable and, interestingly, it was related to axial length as well (Hogue WTC, et al. IOVS. 2021;2021:ARVO Abstract).41
As in other studies, it should be kept in mind that effect sizes in our study of around 10 µm are not clinically relevant for vision (note that this corresponds to changes in refractive state of approximately 0.027 D42,43), but that the study was designed to learn about the output of the retina, taking choroidal thickness changes as a measure.
These results suggest that the functional changes in the myopic retina are limited to the detection of positive defocus, whereas other visual stimuli can still shorten axial length and probably inhibit myopia development. Extrapolating our results to myopia correction paradigms, it becomes clearer why undercorrection may have inconsistent effects on myopia progression. However, other visual stimuli may still be effective to inhibit myopia.
Acknowledgments
The authors thank all subjects for their time and effort, and the IOB for funding the study.
Author contributions: BS – study design, measurements, data analysis, statistics, figures, writing the manuscript; FS – study design, software development, writing the manuscript.
Disclosure: B. Swiatczak, None; F. Schaeffel, None
References
- 1. Wallman J, Winawer J.. Homeostasis of eye growth and the question of myopia. Neuron. 2004; 43: 447–468. [DOI] [PubMed] [Google Scholar]
- 2. Baird PN, Saw SM, Lanca C, et al.. Myopia. Nat Rev Dis Primers. 2020; 6: 99. [DOI] [PubMed] [Google Scholar]
- 3. Norton TT, Khanal S, Gawne TJ.. Tree shrews do not maintain emmetropia in initially-focused narrow-band cyan light. Exp Eye Res. 2021; 206: 108525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hung LF, Arumugam B, She Z, Ostrin L, Smith EL 3rd. Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Exp Eye Res. 2018; 176: 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin G, Taylor C, Rucker F.. Effect of duration, and temporal modulation, of monochromatic light on emmetropization in chicks. Vision Res. 2020; 166: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lingham G, Yazar S, Lucas RM, et al.. Time spent outdoors in childhood is associated with reduced risk of myopia as an adult. Sci Rep. 2021; 11: 6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. She Z, Hung LF, Arumugam B, Beach KM, Smith EL 3rd. Effects of low intensity ambient lighting on refractive development in infant rhesus monkeys (Macaca mulatta). Vision Res. 2020; 176: 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. She Z, Hung LF, Arumugam B, Beach KM, Smith EL 3rd. The development of and recovery from form-deprivation myopia in infant rhesus monkeys reared under reduced ambient lighting. Vision Res. 2021; 183: 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Landis EG, Park HN, Chrenek M, et al.. Ambient light regulates retinal dopamine signaling and myopia susceptibility. Invest Ophthalmol Vis Sci. 2021; 62: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang L, Schmid KL, Zhang J, et al.. Association between greater residential greenness and decreased risk of preschool myopia and astigmatism. Environ Res. 2021; 196: 110976. [DOI] [PubMed] [Google Scholar]
- 11. Flitcroft DI, Harb EN, Wildsoet CF.. The spatial frequency content of urban and indoor environments as a potential risk factor for myopia development. Invest Ophthalmol Vis Sci. 2020; 61: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aleman AC, Wang M, Schaeffel F.. Reading and myopia: contrast polarity matters. Sci Rep. 2018; 8: 10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swiatczak B, Schaeffel F.. Emmetropic, but not myopic human eyes distinguish positive defocus from calculated blur. Invest Ophthalmol Vis Sci. 2021; 62: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Z, Hu Y, Yu H, Li J, Yang X.. Effect of age and refractive error on quick contrast sensitivity function in Chinese adults: a pilot study. Eye (Lond). 2021; 35: 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Read SA, Collins MJ, Sander BP.. Human optical axial length and defocus. Invest Ophthalmol Vis Sci. 2010; 51: 6262–6269. [DOI] [PubMed] [Google Scholar]
- 16. Delshad S, Collins MJ, Read SA, Vincent SJ.. The time course of the onset and recovery of axial length changes in response to imposed defocus. Sci Rep. 2020; 10: 8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiang ST, Chen TL, Phillips JR.. Effect of optical defocus on choroidal thickness in healthy adults with presbyopia. Invest Ophthalmol Vis Sci. 2018; 59: 5188–5193. [DOI] [PubMed] [Google Scholar]
- 18. Chiang ST, Phillips JR, Backhouse S.. Effect of retinal image defocus on the thickness of the human choroid. Ophthalmic Physiol Opt. 2015; 35: 405–413. [DOI] [PubMed] [Google Scholar]
- 19. Hoseini-Yazdi H, Vincent SJ, Read SA, Collins MJ.. Astigmatic defocus leads to short-term changes in human choroidal thickness. Invest Ophthalmol Vis Sci. 2020; 61: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chakraborty R, Read SA, Collins MJ.. Hyperopic defocus and diurnal changes in human choroid and axial length. Optom Vis Sci. 2013; 90: 1187–1198. [DOI] [PubMed] [Google Scholar]
- 21. Read SA, Alonso-Caneiro D, Vincent SJ, Collins MJ.. Longitudinal changes in choroidal thickness and eye growth in childhood. Invest Ophthalmol Vis Sci. 2015; 56: 3103–3112. [DOI] [PubMed] [Google Scholar]
- 22. Jin P, Zou H, Xu X, et al.. Longitudinal changes in choroidal and retinal thicknesses in children with myopic shift. Retina. 2019; 39: 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fontaine M, Gaucher D, Sauer A, Speeg-Schatz C.. Choroidal thickness and ametropia in children: a longitudinal study. Eur J Ophthalmol. 2017; 27: 730–734. [DOI] [PubMed] [Google Scholar]
- 24. Wang D, Chun RK, Liu M, et al.. Optical defocus rapidly changes choroidal thickness in schoolchildren. PLoS One. 2016; 11: e0161535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mathis U, Feldkaemper MP, Schaeffel F.. Effects of single and repeated intravitreal applications of atropine on choroidal thickness in alert chickens. Ophthalmic Res. 2021; 64: 664–674. [DOI] [PubMed] [Google Scholar]
- 26. Joynson RB. The problem of size and distance. Q J Exp Psychol. 1949; 1: 119–135. [Google Scholar]
- 27. Drexler W, Findl O, Schmetterer L, Hitzenberger CK, Fercher AF.. Eye elongation during accommodation in humans: differences between emmetropes and myopes. Invest Ophthalmol Vis Sci. 1998; 39: 2140–2147. [PubMed] [Google Scholar]
- 28. Ghahghaei S, Reed O, Candy TR, Chandna A.. Calibration of the PlusOptix PowerRef 3 with change in viewing distance, adult age and refractive error. Ophthalmic Physiol Opt. 2019; 39: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Del Aguila-Carrasco AJ, Marin-Franch I, Bernal-Molina P, et al.. Accommodation responds to optical vergence and not defocus blur alone. Invest Ophthalmol Vis Sci. 2017; 58: 1758–1763. [DOI] [PubMed] [Google Scholar]
- 30. Diether S, Schaeffel F.. Long-term changes in retinal contrast sensitivity in chicks from frosted occluders and drugs: relations to myopia? Vision Res. 1999; 39: 2499–2510. [DOI] [PubMed] [Google Scholar]
- 31. Swiatczak B, Feldkaemper M, Schraermeyer U, Schaeffel F.. Demyelination and shrinkage of axons in the retinal nerve fiber layer in chickens developing deprivation myopia. Exp Eye Res. 2019; 188: 107783. [DOI] [PubMed] [Google Scholar]
- 32. Zhu X, Wallman J.. Temporal properties of compensation for positive and negative spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009; 50: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tkatchenko TV, Troilo D, Benavente-Perez A, Tkatchenko AV.. Gene expression in response to optical defocus of opposite signs reveals bidirectional mechanism of visually guided eye growth. PLoS Biol. 2018; 16: e2006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tkatchenko TV, Tkatchenko AV.. Genetic network regulating visual acuity makes limited contribution to visually guided eye emmetropization. Genomics. 2021; 113: 2780–2792. [DOI] [PubMed] [Google Scholar]
- 35. Legge GE, Bigelow CA.. Does print size matter for reading? A review of findings from vision science and typography. J Vis. 2011; 11:10.1167/11.5.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Panorgias A, Aigbe S, Jeong E, Otero C, Bex PJ, Vera-Diaz FA.. Retinal responses to simulated optical blur using a novel dead leaves ERG stimulus. Invest Ophthalmol Vis Sci. 2021; 62: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Charman WN. Near vision, lags of accommodation and myopia. Ophthalmic Physiol Opt. 1999; 19: 126–133. [DOI] [PubMed] [Google Scholar]
- 38. Sanz Diez P, Ohlendorf A, Schaeffel F, Wahl S.. Effect of spatial filtering on accommodation. Vision Res. 2019; 164: 62–68. [DOI] [PubMed] [Google Scholar]
- 39. Schaeffel F, Glasser A, Howland HC.. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988; 28: 639–657. [DOI] [PubMed] [Google Scholar]
- 40. Hoseini-Yazdi H RS, Alonso-Caneiro D, Collins MJ.. Retinal OFF pathway activation leads to greater accommodation-induced choroidal thinning. ARVO Abstract. 2021; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hogue WTC. Axial length is associated with individual differences in ON- and OFF- pattern detection. ARVO Abstract. 2021; 2021. [Google Scholar]
- 42. Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G.. A 3-year randomized clinical trial of MiSight lenses for myopia control. Optom Vis Sci. 2019; 96: 556–567. [DOI] [PubMed] [Google Scholar]
- 43. Jones LA, Mitchell GL, Mutti DO, Hayes JR, Moeschberger ML, Zadnik K.. Comparison of ocular component growth curves among refractive error groups in children. Invest Ophthalmol Vis Sci. 2005; 46: 2317–2327. [DOI] [PubMed] [Google Scholar]