INTRODUCTION
The term “mild” in mild traumatic brain injury (mTBI) greatly understates the significance of injury when considering its major implications to patients and society. Up to 30% of patients who have suffered mTBI do not recover in the first 3 months postinjury and may suffer long-term or permanent deficits.1,2 It is estimated that 70% to 90% of all hospital-treated head injuries are mild, with an incidence of approximately 300 in 100,0003; however, the true incidence is likely higher considering that many patients with mild injuries are either not treated at hospitals or not treated at all.2,3
Early recognition of mTBI is important because prompt medical treatment and rehabilitation are believed to improve outcome.1 Unfortunately, standard central nervous system imaging, such as computed tomography (CT) and conventional MR imaging, has limited sensitivity for mTBI, often failing to detect brain damage despite the presence of known injury.1,2,4 This has motivated the search for imaging biomarkers of injury in this group of patients, particularly markers that reflect patient symptoms and predict patient outcome. One of the major known forms of parenchymal injury in mTBI is axonal injury,1 which makes advanced diffusion-weighted (DWI) MR imaging of particular interest because changes in diffusion reflect underlying changes in microstructure. Over the past decade, much has been learned regarding diffusion MR imaging, mTBI, and the use of diffusion MR imaging to detect and evaluate injury in these patients.1,2,5
DIFFUSION-WEIGHTED IMAGING
Restricted diffusion (ie, reduced diffusivity) on DWI can be seen in several processes, most notably cytotoxic edema, which occurs when ischemia is associated with cellular edema, typically in acute infarction.6 There are, however, other causes of cytotoxic edema, particularly excitotoxic edema. Excitotoxic edema is triggered by the release of a high concentration of glutamate, which then results in intramyelinic edema, a reversible cause of cytotoxic edema. Restricted diffusion may occur acutely after white matter injury7 (Fig. 1), although the exact mechanism is still uncertain. Some of the proposed theories for the presence of transient restricted diffusion in traumatic axonal injury include the release of glutamate from injury, resulting in excitotoxic edema6; the presence of associated hypoxia and hypotension, leading to trauma-induced ischemia8; or cytoskeletal collapse of the injured axons that would further hinder the motion of free water molecules.9 In contrast, increased diffusivity is seen in the setting of increased extracellular water such as in with vasogenic edema.1 Lesions from traumatic axonal injury, therefore, may show variable signal on DWI, depending on timing after injury and, because of the aforementioned mechanisms, may exhibit either increased or decreased diffusivity.7
Fig. 1.
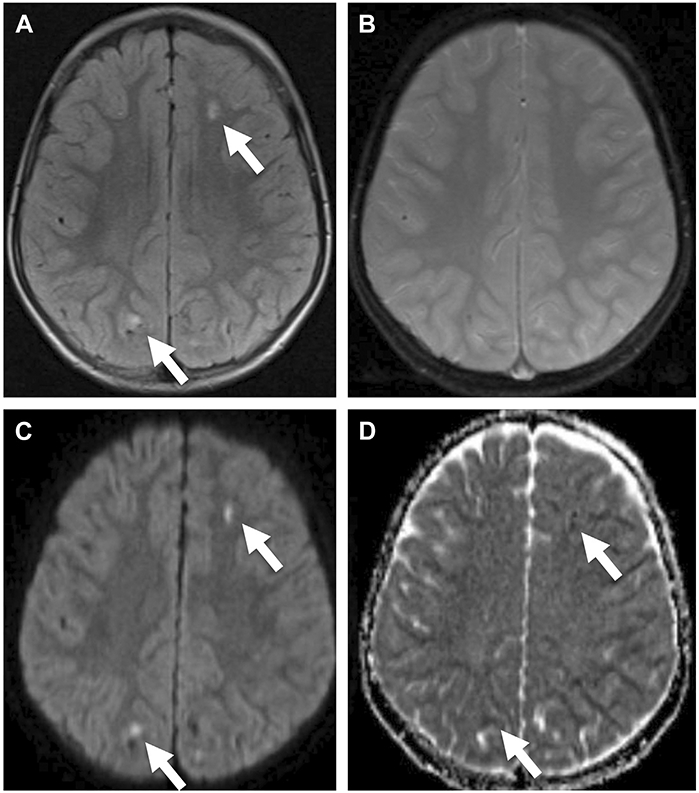
A 5-year-old boy with traumatic axonal injury at the level of the centrum semiovale. The lesions (arrows) are hyperintense on fluid-attenuated inversion recovery (FLAIR) (A), do not demonstrate appreciate susceptibility on T2*-weighted images (B), and show restricted diffusion on DWI (C) and apparent diffusion coefficient maps (D), reflecting acute injury.
Total number and volume of lesions on DWI (both with increased and decreased diffusivity) in the acute phase after head injury is shown to strongly correlate with memory deficits in mild trauma,10 as well as with the modified Rankin Scale in moderate to severe trauma.7 Importantly, however, although DWI has proven to detect some additional lesions not evident on T2* or fluid-attenuated inversion recovery (FLAIR), its sensitivity for lesion detection remains limited because it may not depict all of the injuries seen on other sequences.11
DIFFUSION TENSOR IMAGING
Diffusion tensor imaging (DTI) is a diffusion MR imaging method that measures directional diffusion of water molecules in vivo and is, therefore, of particular interest in disorders of white matter. Notably, individual axons are not resolved using DTI. Instead, the generalized diffusion properties of a white matter fiber bundle can be depicted and, with that, information about the direction and course of WM bundles can be inferred.2 Based on this principle, DTI can indirectly reveal changes to axonal microstructure after mTBI.12
FRACTIONAL ANISOTROPY
The main quantitative metric derived from DTI is fractional anisotropy (FA), a measure of anisotropic diffusion.2 Higher FA is associated with homogeneity in fiber orientation, increased fiber density or axonal diameter, and increased ratio of intracellular or extracellular space.4 Decreased white matter FA may occur due to a variety of histopathologic changes, including damage to myelin, damage to axon membranes, reduced number of axons, decreased axonal coherence, and increased edema.
Alterations in FA have been found in patients with mTBI in both acute (time from injury <2 weeks) and chronic phases. In the chronic phase after mTBI, most reports are consistent and reveal various areas of reduced FA.1,5,13-15 Inglese and colleagues16 reported reduced FA in multiple areas of the brain, including corpus callosum, internal capsule, and centrum semiovale, through grouped region of interest (ROI) analysis in 26 subjects with chronic mTBI. Studies by Salmond and colleagues,17 Kraus and colleagues,18 and Little and colleagues19 evaluated 16, 20, and 12 subjects with chronic mTBI, respectively. They also found decreased FA in multiple regions of the brain using grouped voxel-based analysis, ROI, and histogram analysis. Both grouped and individual voxel-based analysis performed by Lipton and colleagues20 on 17 subjects with chronic mTBI demonstrated similar reductions in FA.
The reported FA values in the acute phase after mTBI are variable. FA values may be increased and/or decreased.5,13,14,16,20-27 Fig. 2 gives representative examples of the differences in FA changes reported in the literature (Fig. 3). Several metaanalyses and reviews of the literature agree that both elevated and reduced FA can be seen in subjects in the acute phase of injury after mTBI.1,4,5,13,15 In a 2014 metaanalysis of mTBI subjects, Eierud and colleagues13 reviewed 122 publications reporting DTI results after mTBI over the past 21 years and concluded that increased FA in the acute phase was reported more frequently than decreased FA. This could be due to a variety of reasons, including axonal injury in areas of crossing fibers and significantly reduced extracellular diffusivity in the setting of cytotoxic edema.1 Wilde and colleagues4 demonstrated a more complex, heterogeneous pattern of anisotropy in which FA fluctuated in the acute stage. These investigators performed serial DTI in 8 subjects with mTBI at 4 different time points within the first week after injury, noting acute transient increases of FA in the left cingulum bundle at variable time points.
Fig. 2.
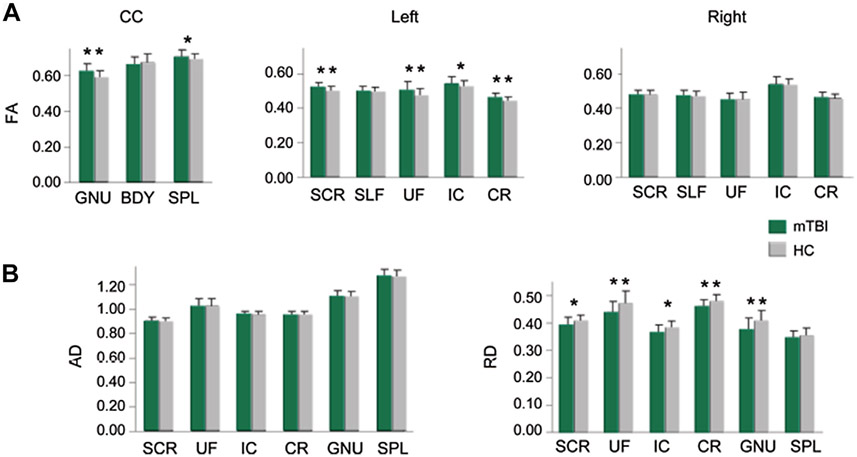
Mean FA values (A) from ROI in the genu (GNU), body (BDY), and splenium (SPL) of the corpus callosum (CC), superior corona radiata (SCR), superior longitudinal fasciculus (SLF), uncinate fasciculus (UF), corona radiata (CR), and internal capsule (IC). Patients with mTBI (green bars) showed mostly higher FA values than healthy controls (gray bars). Significance is indicated with double asterisks, statistical trends with single asterisk. Axial diffusivity and radial diffusivity (B) measurements from mTBI patients and healthy controls for regions with statistical differences in FA. (From Mayer AR, Ling J, Mannell MV, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 2010;74(8):643–50; with permission.)
Fig. 3.
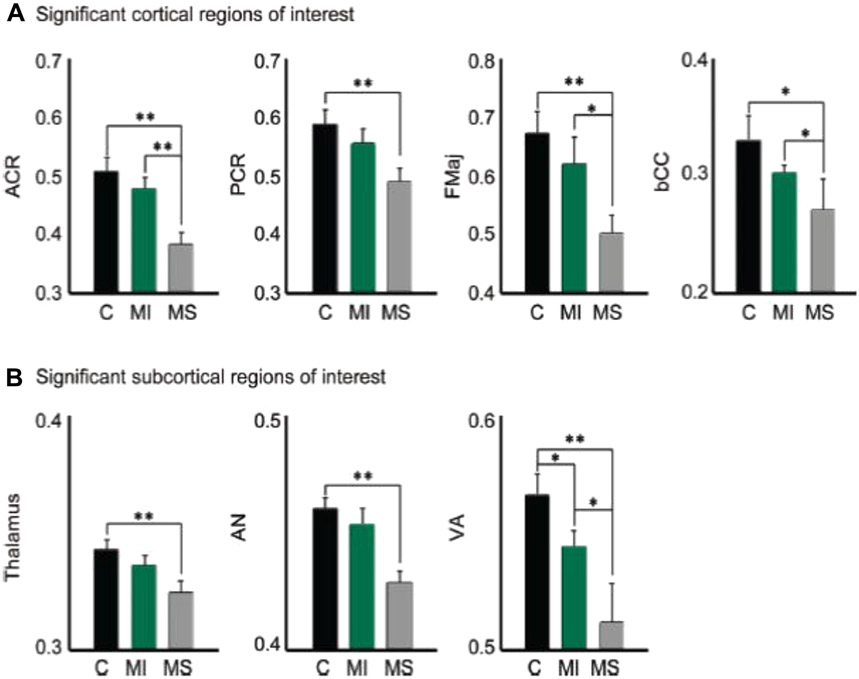
Mean FA extracted from the (A) anterior corona radiata (ACR), posterior corona radiata (PCR), forceps major (fMaj), and body of the corpus callosum (bCC), as well as from the (B) thalamus, anterior thalamic nucleus (AN) and ventral anterior thalamic nucleus (VA). Patients with mTBI (MI) showed lower FA than controls (C), but higher than moderate/severe TBI (MS). Significance is indicated (*P = .05; **P = .01). (From Little DM, Kraus MF, Joseph J, et al. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology 2010;74(7):558–64; with permission.)
Only a few longitudinal studies have been performed in subjects with mTBI.14,27-31 These longitudinal data, though limited, corroborate the presence of variable changes in FA in brain parenchyma. Normalization, partial normalization, and worsening abnormal FA values compared with healthy controls have all been reported in longitudinal studies and may reflect tissue repair, partial tissue recovery, and continued tissue damage.28 Mayer and colleagues14 examined 15 healthy controls and 10 subjects in the semiacute stage after mTBI, 12 days after injury, and 3 to 5 months later. They found partial normalization of previously abnormal FA values in the splenium and corona radiata of subjects on follow-up. Arfanakis and colleagues27 evaluated 2 subjects 24 hours after injury and 1 month after injury and found partial and complete normalization of previously decreased FA values in several white matter regions on follow-up. In a longitudinal study on mTBI, Grossman and colleagues28 studied 16 controls and 20 subjects with mTBI 1 month and 9 months after injury and reported variable changes in different brain regions for DTI, diffusion kurtosis imaging (DKI), and arterial spin labeling (ASL) measures with time. MacDonald and colleagues29 studied 63 subjects from military personnel with mTBI within 90 days after injury and after 6 to 12 months for follow-up. Using ROIs in grouped and individual analysis compared with controls, regions of low FA were reported in the acute phase with partial normalization on the follow-up scans. Eighteen concussed athletes within 6 days and at 6 months after injury were evaluated by Henry and colleagues31 using grouped voxel-based analysis. They observed higher FA, higher axial diffusivity (AD), and lower mean diffusivity (MD) values in the corpus callosum and corticospinal tracts of concussed subjects in the acute and chronic setting, without significant change between acute versus chronic stages.
AXIAL AND RADIAL DIFFUSIVITY
Other metrics that can be quantified using DTI data include AD and radial diffusivity (RD). AD refers to the magnitude of diffusion along the long axis of the fiber tract, thought to be affected by pathologic processes involving axons. RD refers to the diffusivity perpendicular to the fiber tract, thought to be affected by pathologic processes involving myelin.1,2,15 These metrics have also been explored in an effort to improve the sensitivity of DTI to mTBI. FA, MD, AD, and RD are mutually related13; however, AD and RD show different patterns when assessing patients with traumatic brain injury (TBI) in terms of time since injury and severity of injury.15 According to the current literature,14,25 in the acute and subacute phase of mTBI, AD seems to remain comparable to that of controls, whereas RD has been reported to be both elevated25 and reduced.14 In the study by Kumar and colleagues,25 elevated RD values were observed in the genu and splenium of 26 subjects with mTBI compared with 33 healthy controls, using ROI analysis in the acute period (within 5–14 days) after injury. In contrast, Mayer and colleagues14 observed low RD in the genu and several left hemispheric white matter tracts in 22 subjects with mTBI, using ROI analysis during the acute to subacute stages of trauma (within 21 days, mean of 12).
In the chronic phase after injury, Kraus and colleagues18 found elevated AD values throughout all severities of trauma, whereas RD changes seemed to depend on injury severity. The investigators found no significant difference in RD in 20 subjects with mild chronic injury when compared with controls but higher RD in chronic moderate or severe TBI. These differences in RD are likely due to the intrinsic diversity of neuroinflammatory mechanisms in TBI15 and possibly irreparable damage to myelin with greater degrees of injury.
DIFFUSION KURTOSIS IMAGING
The model used for DTI at typical maximal b-values of 1000 s/mm2 is based on assuming a Gaussian distribution of diffusion. At higher b-values, however, diffusion displacement in white matter is known to be non-Gaussian. DKI uses multishell imaging with multiple b-values beyond 1000 to quantify this non-Gaussian behavior (Fig. 4).32,33 Kurtosis is a measure of the deviation from a Gaussian distribution and is a measure of tissue microstructural complexity.2,34 Thus, DKI has theoretic advantages in detecting subtle injury in tissue and may be of specific utility in areas normally shown to have relatively low FA in which the potential of DTI metrics to detect injury may be lower.
Fig. 4.
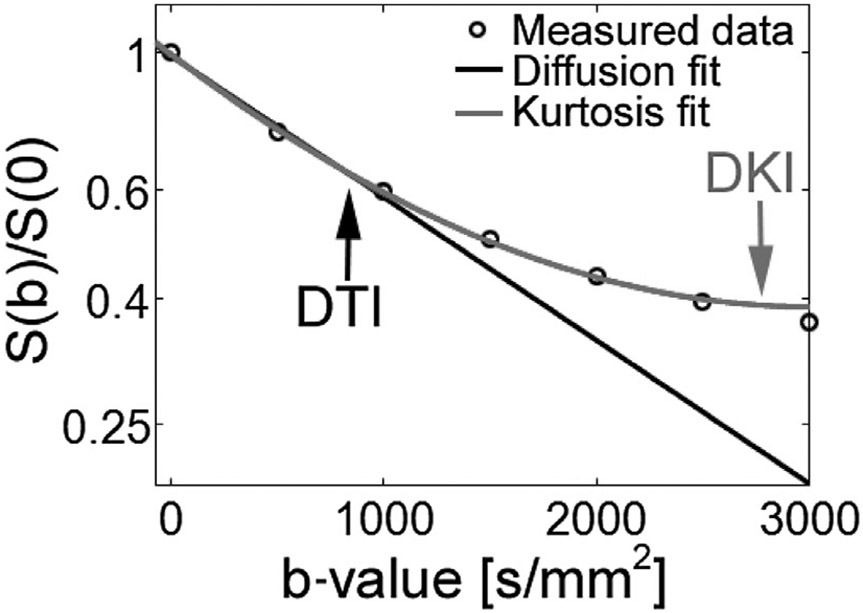
Signal intensity versus diffusion b-value. Graph of signal intensity against diffusion b-value demonstrating improved modeling of DKI compared with DTI at higher b-values. S(b)/S(0), normalized signal.
DKI results in the literature are consistent with DTI findings in longitudinal studies and may provide additional information about DTI.28,35 In a longitudinal study by Grossman and colleagues,28 20 subjects with mTBI demonstrated significant differences from 16 controls in DTI, DKI, and ASL measures in the thalamus and white matter within 1 month and 9 months after injury, correlating with cognitive performance. Fig. 5, reprinted from this longitudinal study, illustrates differences in mean kurtosis (MK) in the thalamus of impaired and unimpaired subjects with mTBI compared with controls. Results show that impaired subjects with TBI had lower MK values than unimpaired subjects and controls (see Fig. 5). Reduced MK and radial kurtosis but no significant changes in DTI were reported in the anterior internal capsule of 24 subjects with mTBI when examined in a longitudinal study by Stokum and colleagues35 at 10 days, 1 month, and 6 months postinjury. Improvements in cognition 1 to 6 months after injury correlated with changes in MK and radial kurtosis in the thalamus, internal capsule, and corpus callosum. Lancaster and colleagues36 performed DTI and DKI in 26 high school and college athletes with sports-related concussion within 24 hours from injury and at 8 days. They found higher axial kurtosis in the corpus callosum and several white matter tracts in subjects compared with controls, which also positively correlated with symptoms.
Fig. 5.
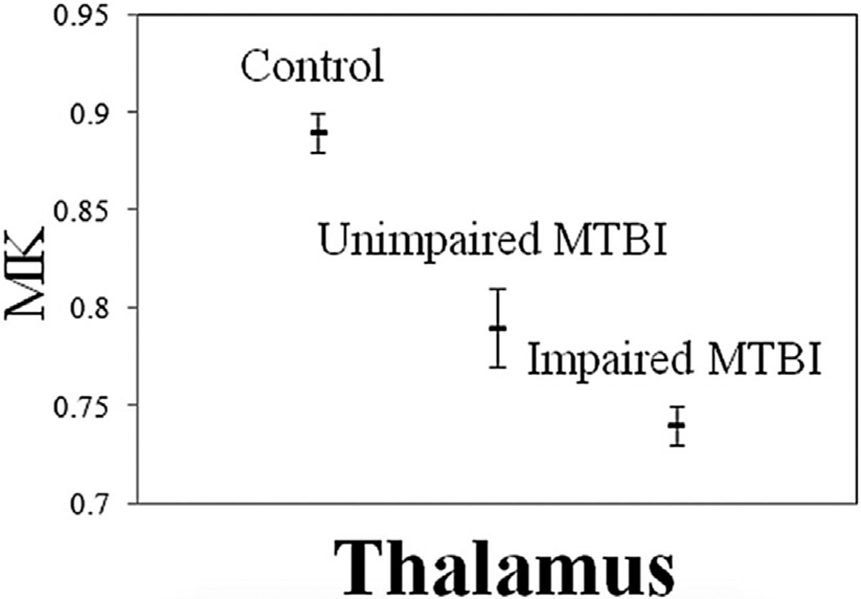
Mean (hash marks) and standard error (lines) for thalamic mean kurtosis (MK) in controls, and for cognitively normal and cognitively impaired subjects. Cognitively impaired subjects show significantly lower MK in the thalamus than unimpaired subjects and controls. (From Grossman EJ, Jensen JH, Babb JS, et al. Cognitive impairment in mild traumatic brain injury: a longitudinal diffusional kurtosis and perfusion imaging study. AJNR Am J Neuroradiol 2013;34(5):951–7, 955; with permission.)
INJURY LOCALIZATION
Despite variations in methodology, group analysis with DTI points to several specific anatomic regions of frequent injury in mTBI,13 suggesting vulnerability of these areas.2 Frequently reported areas of white matter injury include frontal lobe, corpus callosum, posterior limb of internal capsule, cingulum, and fronto-occipital fasciculus.5,37 In addition to white matter injury, thalamic injury has been described in mTBI.19,37 Although this information is applicable for group analyses, reliably detecting specific areas of injury in individual subjects remains a work in progress.38
DIFFUSION TRACTOGRAPHY
Diffusion tractography relates diffusion profiles across voxels and reconstructs structural diffusion pathways in the brain corresponding to major visualized white matter fiber bundles.
Limitations include lack of standardization of tractography algorithms and potential low sensitivity for detecting small lesions.15,39 Deterministic tractography, currently among the most popular methods of tractography available on vendor platforms, does not account for crossing fibers and tracts that have broad, multidimensional direction.2 Research efforts to solve these problems include high angular resolution diffusion imaging (HARDI) and diffusion spectrum imaging (DSI).1,15
DIFFUSION AND COGNITION
Conventional imaging findings in mTBI do not correlate with neurocognitive performance. In contradistinction, the global burden of disease in mTBI measured by DTI (ie, number of DTI lesions detected or average FA over multiple ROIs) is reported to correlate with executive function and cognitive processing speed.15 Miles and colleagues26 studied 17 subjects with mTBI and found a positive correlation between low FA and poor executive function, as measured in the Prioritization Form B test. Results from a study of Niogi and colleagues40 of 34 subjects with mTBI suggest negative correlation between overall number of DTI lesions (defined as FA <2.5 SD from the average in a set of predetermined ROIs) and the mean reaction time in the cognitive tasks performed (attention network task).
In addition to global burden of injury, current literature suggests that injuries in specific areas of the brain, such as frontal white matter, correlate with poor attention, memory, learning, and executive function.13,28,41 Lipton and colleagues41 reported a positive correlation between low FA in the frontal white matter and poor performance on tests of executive function (continuous performance task and the executive maze task) in 34 subjects with mTBI studied 2 to 14 days after the trauma. There is an increasing body of literature associating alterations of thalamic diffusion with altered neuropsychological studies in mTBI.37 Little and colleagues19 found a correlation between low thalamic FA and lower scores on neurocognitive tests of attention, memory, and executive function performed in 12 subjects with mTBI within 12 months from the injury. A DKI study performed by Grossman and colleagues37 evaluating 20 subjects with mTBI, also demonstrated lower MK and FA in the thalamus and internal capsule of subjects with cognitive impairment compared with subjects without cognitive impairment, within and after 1 year from mild trauma.
Altered performance in neuropsychological tests may variably correlate with FA values. In a metaanalysis of mTBI, Eierud and colleagues13 found poor neuropsychological performance correlated with high FA in the acute phase and low FA in the chronic phase. Others; for example, Wilde and colleagues,4 studied 8 subjects with mTBI within 8 days postinjury and found inconsistent correlation between FA values and performance on memory tasks. The pattern of FA was variable (sometimes transiently elevated), independent of the task performance.
SPECIAL POPULATIONS: PEDIATRIC PATIENTS
Children, age 0 to 4 years, and adolescents, age 15 to 19, are at high risk for TBI,28,42 though they are less likely to report postconcussive symptoms and have better outcomes than older patients, possibly due to greater neuroplasticity.43,44 Despite being a high-risk group for head injury, few studies specifically address the pediatric population.21,24,45 The available studies mirror some of the results derived from adult data with elevated FA, decreased MD, and decreased RD in the acute phase involving corpus callosum and cingulum bundle.21,24,45
Changes in FA and RD in adolescents with mTBI have also been reported to correlate with both performance on neuropsychological tests and postconcussive symptoms.21,45 A DTI study by Wu and colleagues45 on 12 adolescents (range 14–17 years) 1 to 6 days post-mTBI showed a correlation between high FA in the left cingulum bundle with poor episodic verbal learning and memory task. Wilde and colleagues21 performed DTI in the corpus callosum of 10 adolescents with mTBI 1 to 6 days after trauma and found that increased FA and decreased RD in the corpus callosum correlates with the severity of postconcussion symptoms. High FA in the frontal and supracallosal white matter was also found in the chronic stage of mTBI 6 to 12 months postinjury in 24 subjects of ages 10 to 18 years, compared with 24 controls in a study performed by Wozniak and colleagues.46
To the authors’ knowledge, there are no current published studies using DKI in pediatric patients with mTBI.
SEX DIFFERENCES
The impact of sex and progesterone on TBI risk, severity, and outcome remains a controversy, in view of the limited literature and conflicting results. Several studies using neuropsychological testing and symptom scale questionnaires44,47-49 suggest women may have worse outcome compared with men. Contrary to some of the existing literature, a DTI study of mTBI subjects by Fakhran and colleagues50 concluded that male sex was an independent risk factor for persistent postconcussive symptoms at 3 months after injury. In addition, a Cochrane review of the literature, including 8 studies that investigated the effects of progesterone in TBI, did not find sufficient evidence to prove that progesterone could reduce mortality or disability in subjects with TBI.51
ONGOING RESEARCH
Creation of a normative atlas is considered a fundamental step in the future of mTBI imaging research, which would allow the establishment of normative values and an accurate comparison between subjects and healthy subjects.12
Efforts are underway to pursue larger, multicenter trials with standardized methodology. The National Institute of Neurologic Disorders and Stroke (NINDS)-funded, multicenter Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study aims to collect and analyze detailed clinical data on 3000 subjects at 11 US sites, across the injury spectrum, along with CT or MR imaging, blood biospecimens, and detailed clinical outcomes (https://tracktbi.ucsf.edu). The TRACK-TBI Pilot dataset is the first to populate the Federal Interagency Traumatic Brain Injury Research (FITBIR) repository and, with the current TRACK-TBI data, is compatible with the International Initiative for Traumatic Brain Injury Research (InTBIR), a collaborative effort of the European Commission (EC), the Canadian Institutes of Health Research (CIHR) and the National Institutes of Health (NIH). The Federal Interagency TBI Research (FITBIR) Informatics System (https://fitbir.nih.gov/) is the result of a collaboration that began in 2011 between the NIH and the US Department of Defense. Its purpose is to create a national resource for archiving and sharing clinical data from research studies on TBI, along with appropriate control data.38
Efforts to improve sensitivity and specificity of diffusion MR imaging for detecting injury in mTBI12 are ongoing. Promising technical developments are being made on acquisition and postprocessing, as well as modeling. Multidimensional, multishell acquisitions, such as HARDI and DSI, allow for resolution of crossing fibers by measuring intravoxel diffusion in multiple directions.52 Multitensor models allow tracing of small, peripheral fiber bundles.53 Track-density imaging is a postprocessing technique using super resolution to obtain intravoxel information derived by whole-brain probabilistic streamline tractography.54 This allows for visualization of smaller pathways that are typically not identified using conventional DTI and can work toward solving the problem of crossing fibers (Fig. 6).55
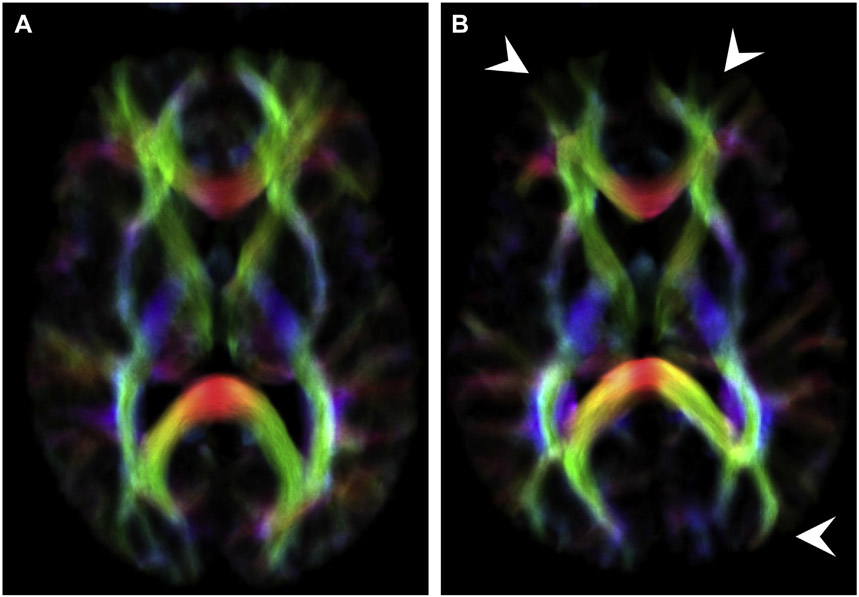
Fig. 6.
Directionally encoded color track-density imaging maps in a 37-year-old man with mTBI (B) and a healthy control matched for age and sex (A) show global relative paucity of peripheral tracts extending to the subcortical region (arrowheads) compared with the healthy control.
Newer, quantitative analyses use compartment-specific modeling of diffusion in both intracellular and extracellular spaces. This allows the derivation of modeled metrics believed to have greater biophysical meaning than traditional empirical DTI and DKI metrics. Such modeled metrics include axonal water fraction (a marker of axon density),56 intraaxonal diffusivity (a marker of intra-axonal injury),57 extraaxonal axial and radial diffusivities (eg, markers of changes in extraaxonal space associated with gliosis, astrocytosis, extracellular inflammation), and extraaxonal tortuosity (a marker of myelination or alignment of fibers).58 Extraaxonal RD would also be sensitive to demyelination.56 These newer metrics provide more information beyond the traditional empiric diffusion metrics such as FA.
SUMMARY
Remarkable advances have been made in the last decade in the use of diffusion MR imaging to study mTBI.1,2,5,13 Diffusion shows differences between mTBI subjects and healthy control groups in multiple different metrics using a variety of techniques, supporting the notion that there are microstructural injuries in mTBI patients that radiologists have previously been insensitive to.
Important future areas of discovery in diffusion MR imaging and mTBI include larger longitudinal studies to improve the understanding of the evolution of injury after mTBI and unravel the biophysical meaning of what detected changes in diffusion MR imaging may represent.
KEY POINTS.
Advanced diffusion-weighted MR imaging is of particular interest in the study of mild traumatic brain injury because changes in diffusion reflect underlying changes in microstructure.
Diffusion-weighted imaging, diffusion tensor imaging, and diffusion kurtosis imaging can reveal alterations in the brain parenchyma in acute and chronic mTBI that are not identified with standard central nervous system imaging.
Specific areas of the brain have shown vulnerability to damage after mild injury and may correlate with altered cognitive performance.
Promising technical developments are being made and efforts are underway to pursue larger, multicenter trials with standardized methodology to better understand what detected changes in diffusion MR imaging represent.
REFERENCES
- 1.Grossman EJ, Inglese M, Bammer R. Mild traumatic brain injury: is diffusion imaging ready for primetime in forensic medicine? Top Magn Reson Imaging 2010;21(6):379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 2012;6(2):137–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004;(43 Suppl):28–60. [DOI] [PubMed] [Google Scholar]
- 4.Wilde EA, McCauley SR, Barnes A, et al. Serial measurement of memory and diffusion tensor imaging changes within the first week following uncomplicated mild traumatic brain injury. Brain Imaging Behav 2012;6(2):319–28. [DOI] [PubMed] [Google Scholar]
- 5.Hulkower MB, Poliak DB, Rosenbaum SB, et al. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol 2013;34(11):2064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Brashdi YH, Albayram MS. Reversible restricted-diffusion lesion representing transient intramyelinic cytotoxic edema in a patient with traumatic brain injury. Neuroradiol J 2015;28(4):409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer PW, Huisman TA, Sorensen AG, et al. Diffusion-weighted MR imaging in closed head injury: high correlation with initial Glasgow coma scale score and score on modified Rankin scale at discharge. Radiology 2004;233(1):58–66. [DOI] [PubMed] [Google Scholar]
- 8.Ito J, Marmarou A, Barzo P, et al. Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J Neurosurg 1996; 84(1):97–103. [DOI] [PubMed] [Google Scholar]
- 9.Povlishock JT, Christman CW. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma 1995;12(4):555–64. [DOI] [PubMed] [Google Scholar]
- 10.Kurca E, Sivak S, Kucera P Impaired cognitive functions in mild traumatic brain injury patients with normal and pathologic magnetic resonance imaging. Neuroradiology 2006;48(9):661–9. [DOI] [PubMed] [Google Scholar]
- 11.Ezaki Y, Tsutsumi K, Morikawa M, et al. Role of diffusion-weighted magnetic resonance imaging in diffuse axonal injury. Acta Radiol 2006;47(7):733–40. [DOI] [PubMed] [Google Scholar]
- 12.Koerte IK, Hufschmidt J, Muehlmann M, et al. Advanced neuroimaging of mild traumatic brain injury. In: Laskowitz D, Grant G, editors. Translational research in traumatic brain injury. Boca Raton (FL): CRC Press; 2016. p. 277–98. [PubMed] [Google Scholar]
- 13.Eierud C, Craddock RC, Fletcher S, et al. Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin 2014;4:283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer AR, Ling J, Mannell MV, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 2010;74(8):643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil 2010;25(4):241–55. [DOI] [PubMed] [Google Scholar]
- 16.Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg 2005;103(2):298–303. [DOI] [PubMed] [Google Scholar]
- 17.Salmond CH, Menon DK, Chatfield DA, et al. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage 2006;29(1):117–24. [DOI] [PubMed] [Google Scholar]
- 18.Kraus MF, Susmaras T, Caughlin BP, et al. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 2007;130(Pt 10):2508–19. [DOI] [PubMed] [Google Scholar]
- 19.Little DM, Kraus MF, Joseph J, et al. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology 2010;74(7):558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipton ML, Gellella E, Lo C, et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J Neurotrauma 2008;25(11):1335–42. [DOI] [PubMed] [Google Scholar]
- 21.Wilde EA, McCauley SR, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 2008;70(12):948–55. [DOI] [PubMed] [Google Scholar]
- 22.Bazarian JJ, Zhong J, Blyth B, et al. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma 2007;24(9):1447–59. [DOI] [PubMed] [Google Scholar]
- 23.Yallampalli R, Wilde EA, Bigler ED, et al. Acute white matter differences in the fornix following mild traumatic brain injury using diffusion tensor imaging. J Neuroimaging 2013;23(2):224–7. [DOI] [PubMed] [Google Scholar]
- 24.Chu Z, Wilde EA, Hunter JV, et al. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am J Neuroradiol 2010;31(2):340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar R, Gupta RK, Husain M, et al. Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Inj 2009;23(7):675–85. [DOI] [PubMed] [Google Scholar]
- 26.Miles L, Grossman RI, Johnson G, et al. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj 2008;22(2):115–22. [DOI] [PubMed] [Google Scholar]
- 27.Arfanakis K, Haughton VM, Carew JD, et al. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol 2002;23(5):794–802. [PMC free article] [PubMed] [Google Scholar]
- 28.Grossman EJ, Jensen JH, Babb JS, et al. Cognitive impairment in mild traumatic brain injury: a longitudenal diffusional kurtosis and perfusion imaging study. AJNR Am J Neuroradiol 2013;34(5):951–7. S951–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mac Donald CL, Johnson AM, Cooper D, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med 2011;364(22):2091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messe A, Caplain S, Paradot G, et al. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum Brain Mapp 2011;32(6):999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry LC, Tremblay J, Tremblay S, et al. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma 2011;28(10):2049–59. [DOI] [PubMed] [Google Scholar]
- 32.Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 2010;23(7):698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wintermark M, Sanelli PC, Anzai Y, et al. , American College of Radiology Head Injury Institute. Imaging evidence and recommendations for traumatic brain injury: advanced neuro- and neurovascular imaging techniques. AJNR Am J Neuroradiol 2015;36(2):E1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fieremans E, Jensen JH, Helpern JA. White matter characterization with diffusional kurtosis imaging. Neuroimage 2011;58(1):177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stokum JA, Sours C, Zhuo J, et al. A longitudinal evaluation of diffusion kurtosis imaging in patients with mild traumatic brain injury. Brain Inj 2015;29(1):47–57. [DOI] [PubMed] [Google Scholar]
- 36.Lancaster MA, Olson DV, McCrea MA, et al. Acute white matter changes following sport-related concussion: a serial diffusion tensor and diffusion kurtosis tensor imaging study. Hum Brain Mapp 2016;37(11):3821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grossman EJ, Ge Y, Jensen JH, et al. Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. J Neurotrauma 2012;29(13):2318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wintermark M, Coombs L, Druzgal TJ, et al. Traumatic brain injury imaging research roadmap. AJNR Am J Neuroradiol 2015;36(3):E12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardner A, Kay-Lambkin F, Stanwell P, et al. A systematic review of diffusion tensor imaging findings in sports-related concussion. J Neurotrauma 2012;29(16):2521–38. [DOI] [PubMed] [Google Scholar]
- 40.Niogi SN, Mukherjee P, Ghajar J, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol 2008;29(5):967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipton ML, Gulko E, Zimmerman ME, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology 2009;252(3):816–24. [DOI] [PubMed] [Google Scholar]
- 42.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006;21(5):375–8. [DOI] [PubMed] [Google Scholar]
- 43.Necajauskaite O, Endziniene M, Jureniene K. The prevalence, course and clinical features of postconcussion syndrome in children. Medicina (Kaunas) 2005;41(6):457–64. [PubMed] [Google Scholar]
- 44.Preiss-Farzanegan SJ, Chapman B, Wong TM, et al. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R 2009;1(3):245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu TC, Wilde EA, Bigler ED, et al. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. J Neurotrauma 2010;27(2):303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wozniak JR, Krach L, Ward E, et al. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin Neuropsychol 2007;22(5):555–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponsford J, Willmott C, Rothwell A, et al. Factors influencing outcome following mild traumatic brain injury in adults. J Int Neuropsychol Soc 2000;6(5):568–79. [DOI] [PubMed] [Google Scholar]
- 48.Farace E, Alves WM. Do women fare worse: a meta-analysis of gender differences in traumatic brain injury outcome. J Neurosurg 2000;93(4):539–45. [DOI] [PubMed] [Google Scholar]
- 49.Bazarian JJ, Blyth B, Mookerjee S, et al. Sex differences in outcome after mild traumatic brain injury. J Neurotrauma 2010;27(3):527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fakhran S, Yaeger K, Collins M, et al. Sex differences in white matter abnormalities after mild traumatic brain injury: localization and correlation with outcome. Radiology 2014;272(3):815–23. [DOI] [PubMed] [Google Scholar]
- 51.Ma J, Huang S, Qin S, et al. Progesterone for acute traumatic brain injury. Cochrane Database Syst Rev 2012;(10):CD008409. [DOI] [PubMed] [Google Scholar]
- 52.O’Donnell LJ, Westin CF. An introduction to diffusion tensor image analysis. Neurosurg Clin N Am 2011;22(2):185–96, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malcolm JG, Shenton ME, Rathi Y. Filtered multitensor tractography. IEEE Trans Med Imaging 2010; 29(9):1664–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calamante F, Tournier JD, Jackson GD, et al. Track-density imaging (TDI): super-resolution white matter imaging using whole-brain track-density mapping. Neuroimage 2010;53(4):1233–43. [DOI] [PubMed] [Google Scholar]
- 55.Hoch MC, Chung S, Fatterpekar GM, et al. Track density imaging of hypertrophic olivary degeneration from multiple sclerosis plaque. BJR Case Rep 2016;2(4):20150299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jelescu IO, Zurek M, Winters KV, et al. In vivo quantification of demyelination and recovery using compartment-specific diffusion MRI metrics validated by electron microscopy. Neuroimage 2016;132:104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grossman EJ, Kirov II, Gonen O, et al. N-acetylaspartate levels correlate with intra-axonal compartment parameters from diffusion MRI. Neuroimage 2015;118:334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jelescu IO, Veraart J, Adisetiyo V, et al. One diffusion acquisition and different white matter models: how does microstructure change in human early development based on WMTI and NODDI? Neuroimage 2015;107:242–56. [DOI] [PMC free article] [PubMed] [Google Scholar]