Abstract
Chondral defects are frequent and important causes of pain and disability. Cartilage has limited self-repair and regeneration capacity. The ideal approach for articular cartilage defects is the regeneration of hyaline cartilage with sustainable symptom-free constructs. Tissue engineering provides new strategies for the regeneration of functional cartilage tissue through optimized scaffolds with architectural, mechanical, and biochemical properties similar to the native cartilage tissue. In this review, the basic science of cartilage structure, interactions between proteins, stem cells, as well as biomaterials, scaffold characteristics and fabrication methods, as well as current and potential therapies in regenerative medicine will be discussed mostly from a biochemical point of view. Furthermore, the recent trends in scaffold-based therapies and supplementary factors in cartilage tissue engineering will be considered.
Key Words: Biochemical, Cartilage, Cartilage regeneration, Scaffold, Tissue engineering
Introduction
The human musculoskeletal system is comprised of connective tissues, such as elastic and fibrous tissues, as well as bones and cartilage. It is susceptible to injury through excessive loading forces or aging (1). Human articular cartilage keeps particular structural materials for specific physical functions that are necessary for joints (2). Diseases or traumatic injuries can affect both cartilage and subchondral bone. Bone and cartilage (osteochondral) defects are frequent and important causes of pain and disability. These lesions gradually progress to degenerative osteoarthritis (OA).
The clinical, social, and economic impacts of osteochondral lesions are impressive. Approximately, 10% of the U.S. adult population represents some kind of clinical OA. OA is the most important indication for joint replacement surgery. It was estimated that 905,000 knee and hip replacements were performed in 2009 at $42.3 billion (3).
Although the present routine therapies are partly effective in reducing pain, new and alternative methods for ultimate healing of cartilage are under discussion and demand (4, 5). Current treatment strategies, such as microfracture or chondroplasty can decrease pain but form a kind of low-lasting fibrocartilage and eventually result in joint degeneration. There is a correlation between age-related changes in articular cartilage, such as the function of chondrocytes and the development and incidence of OA (6, 7). Furthermore, patient factors, such as age and activity level, should also be considered (8). In comparison with native articular hyaline cartilage, fibrocartilage has less toughness and elasticity, as well as poor wear characteristics (9, 10). The ideal approach for articular cartilage defects is the regeneration of hyaline cartilage which is more elastic, painless, and long-lasting (11). Regenerative medicine combines advances in tissue engineering and molecular biology towards replacing or regeneration of human cells, tissues, or organs with the goal of reestablishing normal function subsequent to loss due to injury, disease, or aging (12). Understanding the normal cartilage tissue formation and development is indispensable for successful clinical cartilage regeneration (13).
Cartilage Structure and Function
Cartilage tissue is avascular and aneural, and therefore, has a limited repair capacity. In adults, water composes approximately 70%-80% of the cartilage weight. The principal functional constituents of the dry matrix are collagen (50%-75% w) for tensile strength and proteoglycan (15%-30% w) for compressive stiffness, load distribution, and resilience (14). Articular cartilage consists of hyaline cartilage with about 2 to 4 mm thickness and four various zones, including the superficial, middle, deep, and calcified zones. The main components include collagen fibers, proteoglycans (PGs), glycosaminoglycans (GAG), and chondrocytes. The integrity of the thin superficial zone is vital for protecting the deeper layers from shear, tensile, and compressive stresses, as well as the maintenance of a high number of compacted chondrocytes. Collagen fibers in this zone are mostly types II and IX. The middle zone contains PGs and thicker collagen fibrils and is the first line of resistance to compressive forces. The deep zone contains the highest proteoglycan content and the lowest water concentration for resistance to compressive stress. Chondrocytes in the calcified zone are scarce and hypertrophic.
Cartilage extracellular matrix (ECM) is composed of biochemical components including the network type II collagen (Col II) and an interlocking mesh of fibrous proteins and PGs, hyaluronic acid (HA), and chondroitin sulfate (CS). HA and CS influence the proliferation and differentiation of chondrocytes. Scaffolds composed of Col II, CS, and HA may create an environment that can preserve the normal phenotype of cells to promote the regeneration of cartilage-like constructs. Integrins have two main functions, including (1) cell-ECM attachment, and (2) signal transduction (8).
The mechanical loading modifies the proteoglycan content in the articular cartilage, while overloading causes biochemical damage to the collagen network and proteoglycan loss. Age may act as a prompting factor for OA, while mechanical overloading is a trigger of this condition (15, 16). ECM plays an essential role in the maintenance and renewal of the native tissues (17). In addition to providing a cell niche and structural support, the ECM acts as a special source of biochemical signals, a reservoir for growth factors, induction of mechanical signals, and many other complex features that are related to cellular behaviors (18). ECM homeostasis is a dynamic process. Changes in the matrix affect the biochemical and biophysical properties and alter normal tissue function (19). Bioactive scaffolds for regenerative medicine are meant to mimic these conditions.
Biochemical Content of ECM cartilage
Chondrocytes
Chondrocytes are high special residents in articular cartilage. They are differentiated from mesenchymal stem cells and diverge in form, number, and size, depending on the zone. Chondrocytes are metabolically active cells that play an exclusive role in the development, maintenance, and repair of the microenvironment and turnover of the surrounding ECM. Communication between chondrocytes and direct cell-to-cell signal transduction is rare. However, a variety of stimulants, such as growth factors, mechanical loads, and hydrostatic pressures are responded. Articular cartilage experiences intricate loading conditions, including compression, shear, friction, and tension. Chondrocytes have limited intrinsic reproduction capacity, and their survival depends on an optimal biochemical and mechanical environment (20, 21).
Chondrocytes and their surrounding ECM have a close affiliation with a distinct duty that is necessary for the homeostasis of articular cartilage. Chondrocytes makeup, repair, and sustain their ECM, whereas the ECM maintains their optimal properties and prevents the cells from the pending physical and biochemical stress. This relationship suggests that transferring the chondroinducible cells into a precisely engineered 3D scaffold may differentiate them into chondrocytes and result in the secretion of cartilage ECM. This approach is a potentially promising method of articular cartilage therapy (22).
Collagen
Collagen makes about 60% of the dry weight of cartilage and is the most abundant structural macromolecule in the ECM. Collagen type II is the most dominant form in the articular cartilage ECM that forms fibrils and fibers tangled with PGs. The minor collagen types I, IV, V, VI, and IX help to arrange and steady the type II collagen fibril network. Collagen polypeptide chains are mainly comprised of glycine, proline, and hydroxyproline amino acids that provide a triple helix structure and maintain stability with hydrogen bonds along the length of the molecule to resist against shear and tensile forces (23, 24). Aggrecan is the major proteoglycan in the articular cartilage ECM. Accordingly, the expression of collagen type II and aggrecan are usually the recognized signs of chondrogenesis. Collagen type I is the most abundant form in fibrocartilage, skin, tendon, ligament, and bone tissues; moreover, it is well known as a negative marker that is mostly down-regulated during chondrogenesis (25). Furthermore, collagen type X is an indicator of hypertrophic cartilage formation. Therefore, collagen types I and X are conventional negative markers in chondrogenesis (26).
Proteoglycans and Glycosaminoglycans
PGs are glycosylated proteins consisting of a core protein with one or more covalently attached GAG chains that are synthesized and secreted into the ECM by chondrocytes. PGs are the second most abundant group of macromolecules in articular cartilage ECM and contain aggrecan, decorin, biglycan, and fibromodulin. Aggrecan has rod shape fibers with charge repulsion that fill the interfibrillar spaces in the cartilage ECM and is responsible for osmotic properties. It can absorb water which is critical to resist the compressive loads in cartilage tissue. PG metabolism is associated with regulatory peptides and growth factors (27, 28). GAG varieties depend on the location of the ECM, age, and gender. These linear polysaccharides promote water retention and contribute to the gel-like properties of the matrix. GAGs specifically interact with other biological molecules, such as chemokines, cytokines, and growth factors, thereby preserving them within the ECM (29, 30). The biological functions of PGs are critically assessed in development. Small leucine-rich PGs bind with various collagens, tyrosine kinase receptors, and innate immune receptors; in addition, they participate a role in several biological functions, such as migration, proliferation, innate immunity, apoptosis, and autophagy (31). Articular chondrocytes simultaneously receive signals from the surrounding microenvironment and synthesize the ECM biochemical components, such as types II, IX, and XI collagens, PGs, growth factors, and enzymes (32). Cartilage regeneration is a dynamic process parallel with the ECM turnover and always depends on alterations in the immediate microenvironment and signals received.
Cell-PCM-ECM
Chondrocytes are distinguished from the ECM by their surrounding pericellular matrix (PCM). The chondrocyte along with its PCM forms the chondron structure which is polarized and oriented depending on the structure depth in cartilage. This matrix can be used as a template for cell growth, modulation, and matrix degradation. The thin structure of the PCM is distinct from the biochemical and biomechanical characteristics of the ECM (e.g., the presence of collagen type VI). Due to the lack of cartilaginous veins, the chondrocyte environment (PCM) will play a very important role in regulating cell activity so that the matrix is considered a receptor for the transduction of biochemical and biomechanical signals to chondrocytes. In pathogenic conditions, such as OA, PCM alterations result in chondrocyte changes in response to cellular interactions and matrix protein which subsequently affects the biochemical and biomechanical properties of ECM, and therefore, the articular cartilage. In general, consideration of interactions (cell-PCM-ECM) is essential for a better understanding of tissue behavior (33).
Nourishment of articular cartilage cells is prepared by synovial fluid and is dependent on anaerobic metabolism due to the lack of blood vessels and lymphatics. Progress of OA disease is associated with alterations in cartilage metabolism. Physiological imbalance follows extreme synthesis of ECM components or extreme degradation by proteinase enzymes involved in cartilage turnover, including metalloproteinases (collagenase, gelatinase, stromelysin) and cathepsins (34). Changes in chondrocytes activity are regulated by age-related mechanisms. As a result of aging, the aggrecan synthesis rate decreases through a decreased addition of sulphate into GAG chains and decreased link protein (hyaluronan and proteoglycan) by transcriptional or post-transcriptional mechanisms (35). Chondrocytes in older people remain viable but changes occur in phenotype, β-galactosidase, telomere length, and expression of enzymes involved in cartilage destruction, such as matrix metalloproteinases (MMP). The expression of collagenases MMP-1,-8 and -13, as well as tissue inhibitor of metalloproteinases (TIMP) are altered in OA articular cartilage (36, 37). The engineered cartilage must fulfill the most exact biochemical and mechanical requirements.
Cell-Based Therapies
Current treatment methods with potential advantages include autologous chondrocyte implantation, osteocho-ndral autograft transfer, and osteochondral allograft transplantation (8). Although cell-based therapies for cartilage regeneration have been introduced since 1994, the clinical applications remain restricted due to several disadvantages, such as potential contamination, latent immune rejection, and suspected carcinogenesis, as well as concerns about cell storage and transportation (38, 39). Tissue engineering approaches through the combination of cells with a 3D scaffold can potentially overcome the aforementioned limitations (40).
Stem Cells
Stem cells have the potential for multiple differentiation and self-renewal upon demand, making them an ideal choice for use in cartilage tissue engineering. Embryonic stem cells (ESCs), induced pluripotent stem cells, and adult stem cells are promising sources for cartilage regeneration (41, 42). Cell source is an important concern for successful outcomes in stem cell therapies (43). Due to the ethical concerns over ESCs, adult stem cells residing in native microenvironments (niches) can potentially provide balanced outcomes in avoiding tissue overgrowth, cancer, and aging (44).
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are one of the first migrating cells to the damaged site after injury. They can potentially differentiate into adipocytes, chondrocytes, and osteoblasts (45). Bone marrow, adipose tissue, umbilical cord blood, peripheral blood, amniotic fluid, dental pulp, synovium, and muscle are the potential MSCs sources for cartilage tissue engineering applications (46-53). Dominant characteristics including growth, proliferation, integration, availability, and chondrogenic efficiency have been reported in many studies for bone marrow mesenchymal stem cells (BM-MSCs). Higher collagen expression and GAG deposition represent BM-MSCs as a superior choice for cartilage tissue engineering (54-57). BM-MSCs are also known as environmentally-responsive cells capable of influencing their microenvironment by secreting different growth factors, anti-inflammatory mediators, and anti-catabolic or immunomodulatory factors. However, the bone marrow has limitations in the volume and number of stem cells harvested (58, 59).
BM-MSCs are characterized by their ability to adhere to plastic cell culture plates and expression of CD105, CD73, CD90 (95% or more of the cells of a colony must express), CD29, and CD44, while they do not express CD45, CD14, CD11b, CD34, and CD79a or CD19 markers (60-62).
Stem cells are highly sensitive to physicochemical changes in their microenvironment. Biochemical and biomechanical modification of the microenvironment can alter the fate of stem cells. This is a very important point for stem cell research and applications (63). The cells continually sense and respond to the biomechanical changes, such as shear and stretching stresses through mechanotransduction processes (64). The sensitivity characteristic of stem cells can be a potential advantage when combined with optimized scaffold architecture in the fabrication of a functional repair cartilage tissue (65).
Cartilage Tissue Engineering
Due to the avascular and aneural nature, as well as containing only one type of cell (chondrocytes), the cartilage tissue has been one of the first candidates for tissue engineering and a suitable target for the earliest efforts for producing living and functional tissue constructs in vitro (66). The impacts of the neighboring tissues should be considered in orthopedic tissue engineering. Although bone and cartilage are two different tissues, their development is interrelated. The transcription factor Sox9 is expressed in chondrocytes and regulates chondrogenesis. It also suppresses the later stages of osteochondral bone formation by downregulation of vasculogenesis (67).
Chondrocyte dedifferentiation is a major concern in cartilage tissue engineering. Human articular chondrocytes regularly dedifferentiate when grown in monolayer cultures. They show decreased expression of PGs and type II collagen simultaneously with increased expression of collagen I and vimentin (68). Some studies have recommended co-culture systems (chondrocytes+stem cells) to address many issues encountered by monocultures in cartilage tissue engineering. Supplementary investigations have suggested the combination of co-culture with three-dimensional (3D) biomaterial scaffolds (69). The advanced strategy of the 3D culture of chondrocytes on hydrogel scaffolds has been shown to prevent chondrocyte dedifferentiation and preserve chondrogenic phenotype (70-72).
3D scaffolds have shown a great influence on MSCs to undergo chondrogenesis as the stem cells in 3D culture are induced and characterized by the mechanical environment, hydrostatic pressure, tensile strain, cell-cell interactions, growth factor gradients, and other factors that are formed by cells as in the embryonic development (73, 74). The design and fabrication of scaffolds is an intricate process with a crucial role in tissue engineering approaches. In fact, scaffolds provide the appropriate 3D microenvironment for cells to attach, grow, proliferate, differentiate, and secrete their own specific ECM for appropriate tissue composition (75).
Scaffolds for tissue regeneration should contain particular criteria, including (1) appropriate architecture indicating the porosity, pore size, and interconnectivity to support cell penetration, migration, proliferation, and differentiation, as well as allowing the diffusion of oxygen and nutrients and waste disposal; (2) mechanical properties similar to the original cartilage tissue; (3) biocompatibility in terms of lack of toxicity, prevention of cellular stress and immune response, and scarring; and (4) biodegradability in terms of the proportional decomposition rate of the scaffold coincidently with new tissue formation (75, 76).
Hydrogels are hydrated 3D networks formed by cross-linked hydrophilic polymers. They can absorb water or biological fluids up to thousands of times their dry weight and can be potentially used as the prime candidates for cell encapsulation, biosensors, drug delivery, injectable structures, and carriers or matrices for cells in tissue engineering (77, 78). Applied external stimuli and mechanical signals are transmitted to the tissue, cellular, and molecular levels. Consequently, the cells activate biochemical pathways that define the functional properties of the resulting engineered tissue (79). Biochemical interactions and regulatory responses of cells have key effects on cell attachment, orientation, shape, movement, distribution, proliferation, and differentiation (80). Scaffolds synthesized by binding peptide sequences (arginine, glycine, aspartic acid) secreted from fibronectin in the ECM can be used for attraction and adhesion of MSCs to the lesion site and repair the tissue (81). Collagen/HA-based hydrogel constructs demonstrate an almost ideal 3D support for in vitro chondrogenesis of MSCs, decreased hypertrophy, and increased ECM production (82).
Each of the scaffold fabrication techniques has advantages and drawbacks, depending on the type of biomaterial applied, desired texture, and application. Freeze-drying (lyophilization), electrospinning, solvent casting and particulate leaching, gas foaming, sol-gel, self-assembling, and additive manufacturing are some of these techniques (83).
Electrospinning is a versatile and cost-efficient method to fabricate nonwoven mats or conduits with high interconnected porosity and controlled fiber diameter in the range of a few nanometers to microns. However, the distance between fibers (pore size) cannot be exactly controlled (84-86). Although the impact of process parameters has been widely studied, it is difficult to predict and obtain fibers characteristics, such as porosity, stiffness, and uniform diameter without globules for some materials (beads) (87, 88).
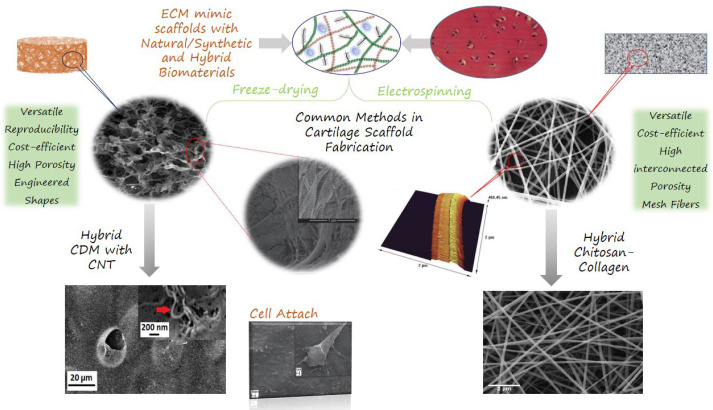
Freeze-dry processing or phase separation, is a simple dehydration method for fabricating porous scaffolds. The possibility to obtain several engineered shapes is an advantage of the freeze-drying method. Similar to electrospinning and self-assembling, freeze-dried scaffolds expose low mechanical properties. These constructs can be cross-linked to increase mechanical properties and enzymatic resistance (89). Freeze-dried fibrous scaffolds have high porosity and interconnected uneven pores, which can potentially enhance cell and tissue infiltration (90). Freeze-dried materials exhibit higher protein adsorption, compared to bulk/solid materials, which is due to their high surface to mass ratio. Due to their advantages, freeze-dried scaffolds have been widely used in tissue engineering, including wound healing, spinal cord, nerve, cartilage, and tendon repair (25, 91-97). Common methods of scaffold fabrication for cartilage tissue engineering applications are shown in [Figure 1].
Figure 1.
Common methods of scaffold fabrication in cartilage tissue engineering with FESEM images of morphology and fiber distribution. CNT, carbon nanotubes; CDM, cartilage derived matrix. Original illustration designed and provided by the authors
Biomaterials in Cartilage Tissue Engineering
Natural/synthetic scaffolds
Material, structure, and fabrication methods are three factors that determine scaffold characteristics. The wide range of materials used in tissue engineering applications consists of natural and/or synthetic polymers. Hydrogels from natural polymers containing one or more molecules of the ECM (e.g., collagen, GAG, HA, agarose, alginate, fibrin, gelatin, chitosan, and silk fibroin) are often called “ECM mimics” and have been extensively characterized. The most commonly used synthetic polymers include polyglycolic acid, polylactic acid, polylactide-co-glycolide, polycaprolactone, and polyethylene glycol (83, 98-100).
Natural hydrogels containing collagen, gelatin, and HA are known to be degradable by cell-secreted enzymes, such as collagenase, gelatinase, and hyaluronidase, respectively (101). Scaffolds with natural materials for this purpose are largely used. Collagen scaffolds present an ideal platform for cell therapy approaches including the delivery of stem cells (102). In a comparison among collagen, agarose, alginate, and matrigel scaffolds, collagen hydrogel scaffolds have been reported to be better for chondrocyte and phenotypic cells. This is probably due to their similarity to the natural chondrocyte ECM (103).
In contrast to biocompatibility and large modification, low mechanical strength and unmanageable degradation rates are the characteristics of natural materials (104). Synthetic polymers have superior control of physicochemical properties, excellent mechanical manner, and process facility. Conversely, synthetic polymers may elicit unwanted inflammation and tissue formation (105). Designing scaffolds with proper biochemical and biomechanical properties for cartilage tissue engineering is a supreme challenge. Combinations of natural and synthetic polymers with different concentrations have been reported. A summary of natural/synthetic and hybrid biomaterial scaffolds for cartilage tissue engineering is presented in [Table 1].
Table 1.
List of selected natural/synthetic and hybrid biomaterials in cartilage tissue engineering including their advantages and research phase
| Scaffold Type | Biomaterials | Methods | Cell type | Effect |
Study
Type |
Ref. |
|---|---|---|---|---|---|---|
| Natural hydrogels |
collagen | Gelatinization, Freeze-drying |
chondrocyte | Manipulation with slurry concentrations Chondrogenic capacity of human OA chondrocytes More compliant response Trigger of cellular development and matrix deposition |
In vitro/ In vivo | (184, 185) |
| Bio inspired hydrogel |
poly (γ-glutamic acid)/ (γ-PGA-SH-MA) | Chemical reaction | BMSCs | Good mechanical properties and superior biocompatible Gelation time, mechanical property, porous structure, swelling, and degradation process can be modulated easily |
In vitro/ In vivo | (186) |
| Natural hydrogels |
agarose | explant model chondrocyte-laden hydrogels |
chondrocyte | Development integration with native cartilage Optimal Young’s modulus Implanted constructs |
In vitro | (164) |
| Micro/nano composite hydrogels |
maleilated chitosan- methacrylated silk fibroin | Photopolymerization Chemical reaction |
mouse articular chondrocytes | Biocompatible Support cells attachment well Compressive modulus in the range of articular cartilage |
in vitro | (103) |
| Hybrid nanocomposite scaffolds |
PCL-gelatin MWNTs |
electrospinning | Rabbit Chondrocyte | Hybrid nanocomposite More bioactivity and slower degradation rate |
In vitro | (187) |
| Coaxial nanofibers | PGS/PCL | Coaxial electrospinning | BMSCs | Kartogenin-loaded coaxial nanofibers Enhanced cell proliferation and chondrogenic differentiation More controlled and sustained small molecule release |
In vitro | (58) |
| Hybrid nano-micro scaffolds |
P3HB-chitosan-MWNTs/silk | Electrospinning | chondrocytes | Increase in tensile strength Appropriate for a long-term |
In vitro | (188) |
| CDM | Decellularization Bovine, porcine cartilage matrix |
Decellularization, freeze-drying |
ASCs, Fibroblast, Human chondrocyte |
Maintenance of mechanical properties Increase in bioactive component Nonimmunogenic biomechanically Compatible decellularized tissue |
In vitro | (98, 99, 118, 122) |
| Hybrid CDM |
CDM- carbon nanotubes | Decellularization, freeze-drying |
ASCs | Reinforced CDM Enhance the mechanical properties Retaining biocompatibility |
In vitro | (97) |
| Nanofibrillated Hydrogel | cellulose/alginate | 3D-bioprinting Cell-laden |
BMSCs/ nasal chondrocytes/ co-culture |
Bioprinted cell-laden scaffold In vivo chondrogenesis Good mechanical properties Long term structural integrity |
In vitro/ In vivo | (189, 190) |
| Bioink Hybrid scaffold |
alginate/gelatin/fibrinogen | 3D-bioprinting Cell-laden |
BMSCs | Combining innovative bioink with low cell density Cell encapsulation Safety of bioextrusion Hypoxic conditions |
In vitro | (178) |
| GelMA/PCL | 3D-bioprinting Cell-laden |
chondrocyte | Mechanical properties to nasal cartilage Method for fabricating implants for nose reconstruction |
In vitro | (180) | |
| Hyaluronic acid/ PLA | 3D-bioprinting | chondrocyte | Suitable biological and mechanical properties Co-printing bioink and thermoplastic polymer Support load forces |
In vitro | (181) |
MWNTs (multi-walled carbon nanotubes), P3HB(3-hydroxybutyrate), GelMA (gelatin methacrylate), CDM(Cartilage Derived Matrix), PCL(poly caprolactone), PGS(glycerol sebacate), MA(methacrylate), SH(cysteamine group), BMSCs (Bone marrow stem cells), ASCs (Adipose tissue stem cells).
Autografts have been believed to be the golden standard for orthopedic tissue regeneration; however, a promising technique for scaffold fabrication is the use of decellularized bone, cartilage, ligament, and tendon allografts or xenografts. Growth, proliferation, and differentiation of new cells onto natural ECM-derived scaffolds can be considered an appropriate alternative for autograft transplantation (106).
Decellularized ECM as a Scaffold
Increasing efforts have been made to develop ECM-derived scaffolds by decellularizing the native tissues that represent biological superiority in terms of preferred cellular activities and minimized immune responses (107). The decellularization process is the removal of native cells and antigens from tissue, leaving behind a 3D ultrastructure of ECM while preserving the biochemical and biomechanical cues of the tissue (108, 109). ECM-derived scaffolds provide specific niches for cells and a diverse class of biomaterials. Decellularized ECM (dECM) has been established as a biomaterial that preserves the native tissue environment and promotes cell growth, viability, proliferation, and differentiation. Unlike transplanted tissues, dECM has a minor risk for an immune response since nearly all the cellular DNA is removed (110). There are various decellularization procedures involving a combination of physical, enzymatic, and chemical processes according to the tissue type. This flexibility allows dECM particles to be incorporated into various types of constructs, including hydrogels, electrospun, and 3D-printed scaffolds. Elimination of DNA from dECM while preserving the mechanical properties is the gold standard for successful decellularization (110). Decellularized ECM from a variety of tissues, including heart, heart valves, blood vessels, and cartilage has been studied (96, 111-118).
In general, similarity to native cartilage matrix can ensure a great chance of achieving suitable engineered cartilage. Cartilage-derived matrix (CDM) scaffolds from various animal and human sources have recently drawn attention as providing structural and biochemical signals, as well as matrix components at the same time seems perfectly rational (116). Porous CDM scaffolds have already been examined for articular chondrocyte growth, matrix accumulation, and mechanical properties and have resulted in neocartilage formation in the absence of exogenous growth factors (119). The chondroinductive potential of bovine porous CDM scaffolds on human dermal fibroblasts has also been investigated in different research. The upregulation of chondrogenic genes with sulfated GAG production confirmed the chondrogenic potential of the construct (25).
CDM scaffolds are fabricated from human, porcine, ovine, and bovine sources (96, 116, 119, 120). Each of the aforementioned sources has some advantages and drawbacks. Apart from the ethical concerns, human articular cartilage is significantly thicker than other specimens and stiffer than bovine cartilage. However, studies have proved no relationship between animal weight and cartilage stiffness. Human cartilage has less permeability than porcine and bovine cartilage. Porcine cartilage has the highest GAG and the lowest level of collagen. Bovine cartilage contains more GAG, compared to human cartilage, which is considered a positive factor during the decellularization process (121, 122).
Crosslinking treatments influence the mechanical properties and chondrogenic differentiation. These effects are mediated by the modifications in cell-matrix interactions. The common physical crosslinking methods include ultraviolet light (UV) irradiation and dehydrothermal treatment, while chemical cross-linkers are carbodiimide (CAR), glutaraldehyde, and genipin. Physical crosslinking treatments have been shown to preserve epitopes that participate in cell-matrix interactions and support greater chondrogenic induction than chemically crosslinked scaffolds (96, 116). Moreover, the residual chemicals may diminish biocompatibility.
A combination of hydrogels derived from decellularized tissues with natural or synthetic materials (hybrid hydrogels) provides mechanical reinforcement and enhanced biological activity. This approach is promising for the development of biologically relevant hybrid hydrogels that incorporate nano/microstructures with potential applications in regenerative medicine (95, 123, 124). Significantly different methods of dECM tissue processing and standard clinical treatments are the main issues with dECM.
Evaluation techniques
Several techniques are used for the evaluation of scaffolds for cartilage tissue engineering, including biochemical assays, immunohistochemistry, mechanical tests, micro-CT, MRI, SEM, EDX, TEM, confocal microscopy, AFM, DSC, TGA, FTIR, gene expression, and molecular genetics (95, 125). SEM micrographs can show the presence of cells in the construct, organization, porosity, pore size, and interwoven networks of fibers (126). Furthermore, mass spectrometry (MS), high-performance liquid chromatography interfaced with tandem mass spectroscopy (LC-MS/MS), and proteomics provide efficient methods to monitor the complete profile of ECM molecules from a sample of engineered cartilage tissue, compared to native cartilage (127, 128).
Discussion
Alteration of the structural and biochemical matrix composition affects cell growth, morphogenesis, differentiation, migration, communication, and survival (129). Attention to biochemical and biomechanical properties of scaffold corresponding ECM is essential for the cartilage repair process. In addition to scaffold planning, several factors that need to be considered in cartilage tissue engineering contain genes, impact proteins, growth factors, signaling pathways, culture conditions, and biochemical factors.
Impact factors involved in chondrogenesis
Gene Effects
Thrombospondin-5 also known as cartilage oligomeric matrix protein (COMP) is a multidomain 524 kD glycoprotein that functions at cell surfaces and ECM. COMP is located in the interterritorial matrix of adult articular cartilage, where it interacts with the collagen networks. It plays a significant role in matrix assembly which is important in maintaining the structural integrity of cartilage. In vitro chondrogenesis of MSCs is affected by COMP; moreover, it has been introduced as a biomarker to monitor the cartilage degradation progress (130). Sex-determining region Y box-9 (SOX9) is a master transcription factor in the regulation of chondrocyte development and differentiation of MSCs into chondrocytes. Mutations in SOX9 cause severe disease of cartilage characterized by the hypoplasia of endochondral bone. SOX9 regulates multiple genes in chondrocytes, including genes encoding ECM proteins (e.g., COL2A1, COL9A1, ACAN, and CD-rap), ECM modification enzymes, receptors, and transporters. Fibroblast growth factor (FGF) upregulates SOX9, and parathyroid hormone increases SOX9 activity, whereas Wnt/β-catenin inhibits SOX9 activity (131). Patients with OA have shown decreased SOX9 mRNA (132). Conditional knockout of SOX9 in adult mice causes an OA-like phenotype leading to the loss of COL2A and ACAN expression which increases the hypertrophic differentiation (133). Overexpression of SOX9 has been shown to result in cartilage repair in mice models and ex-vivo human OA tissue (134).
Oxygen effects
A hypoxia-induced transcriptional profile plays an important role in the chondrogenic differentiation of hBM-MSC and is mediated by the hypoxia-inducible factor (HIF) complex through the upregulation of HIF target genes (135). In multiple studies, chondrocytic gene expressions of SOX9, collagen II, and aggrecan were elevated in hypoxic (2%-5% oxygen), compared to standard (20% oxygen) culture conditions, whereas the expression of collagens I and X (degradation and hypertrophy markers) was suppressed. Hypoxia also caused significantly greater GAG retention and HA synthesis in ECM (136-138).
It is proposed that the effects of O2 on cartilage are mediated partly through reactive oxygen species (ROS). Alterations in O2 levels and fall in ROS in hypoxia reduce the ability of articular chondrocytes to regulate pH and inhibit NHE activity (including Na+/H+ exchanger, NHE, and Na+/K+ pump) via changes in protein phosphorylation (139). In contrast, elevated levels of oxidative stress products consequently decrease antioxidant capacity and defenses and may be involved in the progress of pathology and disease (140). Both pH and oxygen tension influence chondrocyte metabolism and marker expression in mRNA and protein levels. Expression of aggrecan, type II collagen, and HIF1A are pH-independent. Sophisticated pH and oxygen control allow revising new venues in cartilage tissue engineering (141).
Growth factors
Growth factors are the major regulators of cell behavior. They promote cell proliferation, migration, and differentiation by specific receptor bindings that stimulate cellular signal transduction pathways. Growth factors (soluble signals) are involved in several physiological and pathological processes, such as tissue repair and hemostasis. Growth factors can be released from ECM by the degradation of ECM proteins, GAGs, or PGs (142, 143). Several growth factors and cytokines have been suggested to be involved in chondrogenesis. Transforming growth factor β (TGF-βs) family that include TGF-β-1, -2, -3, and bone morphogenic proteins (BMPs), have a prominent role in chondrocyte ECM metabolism and activity as a major inducer of collagen synthesis and tissue homeostasis. Signaling by this protein family uniquely activates SMAD-dependent signaling and transcription and also activates SMAD-independent signaling via MAPKs, such as ERK and TAK1 (144, 145). TGF-β is able to induce SOX9 synthesis, as well as promote aggrecan, collagen type II, and ECM synthesis through activation of the SMAD2/3 phosphorylation pathway leading to articular cartilage repair (146). BMPs stimulate cartilage synthesis and decrease the activity of catabolic cytokines, such as IL-1, IL-6, IL-8, MMP-1, and MMP-13. Moreover, BMP-7 may reduce the degradation of articular cartilage in OA (147). Some studies have investigated new approaches with combinations of the IGF-I/FGF-2/ TGF-β/ BMPs/SOX for cell-based articular cartilage repair (148, 149).
Signaling pathways
Chondrocyte differentiation is regulated by multiple signal transduction pathways which form a complex transcriptional network. Understanding these signaling pathways will help us comprehend the process of chondrogenesis and cartilage repair in the future (150). Recent studies have identified several critical signaling pathways as key regulators and activators of cellular and molecular processes to be abnormally activated or suppressed during chondrogenesis and OA development (151). The key signaling pathways which regulate chondrogenesis have been known as wnt, nitric oxide (NO), and retinoic acid (RA) signal, as well as protein kinase C (PKC). PKC is a crucial regulator of chondrogenesis and exerts promoting effects via the ERK-MAPK pathway. PKCs regulate the chondrocyte phenotype through the actin cytoskeleton. Moreover, they mediate the effects of IGF-1 and EGF during chondrogenesis (152).
Many proteins are involved in cell-matrix mechanotransduction, among which, the integrin subtypes have prominent biological roles in biomaterials engineering. Through integrin, the signaling cells are able to adhere to their ECM and respond to biochemical signals, adjust cytoskeletal organization, cell shape, mechanotransduction, and cell proliferation. It also senses the biomechanical properties of the ECM and mediates force transmission in focal adhesion complexes applied for tissue engineering (153, 154).
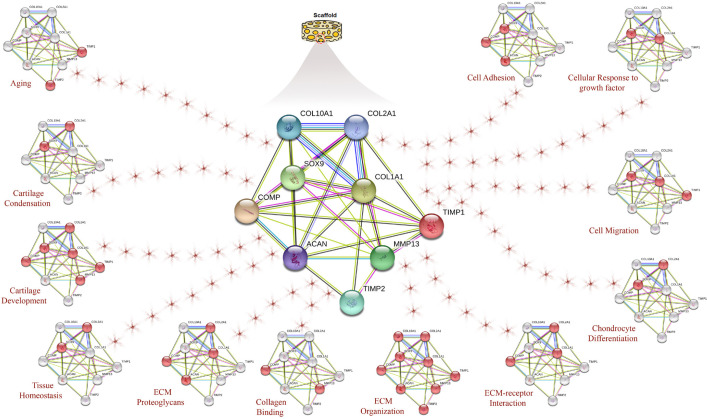
Cell-ECM forces interact through proteins that can activate or inhibit enzymes, increase or decrease protein-protein interactions, activate or inhibit protein stratum, induce catch bonds, and regulate interactions with membranes or nucleic acids. Under normal conditions, an increased stiffness is detected and activates complex intracellular signaling cascades which affect ECM, while decreasing the trigger pathways in mechanobiology that result in ECM hardening. Forces are supposed to initiate biochemical signals by unfolding certain protein domains and changing binding affinities which alter protein interactions and motivate signaling pathways important in mechanosensing (19, 155). Figure 2 describes the interactions between the genes and proteins in the scaffold under the impact of biological processes and molecular functions serving the STRING database. Each globule indicates an active protein at the stated biological process.
Figure 2.
STRING database analysis involves genes and proteins in hyaline cartilage which describes the interactions between genes and proteins in scaffold under the impact of biological processes and molecular functions. Red globules indicate active proteins at the mentioned biological process. Original illustration designed and provided by the authors
Conclusion and Perspective
Although over the last two decades cartilage tissue engineering has developed, there is still a scarceness of valid clinical treatment (156).
Unsolved problems and challenges in cartilage repair
Tissue integration
Most researchers in cartilage tissue engineering have failed to consider the integration of the engineered tissue into the recipient’s joint after implantation. Strong and stable connections between the graft and native tissues are essential for the functional integrity of the implant. Tissue degradation, mechanical weakness, and undurability of the joint might cause poor integration. The formation of hematomas and migration of MSCs provides an inferior and transient fibrocartilagenous replacement for hyaline cartilage (157, 158). Several other factors are known to contribute toward poor cartilage integration, including low chondrocyte viability at the graft and host interfaces. Moreover, adult cartilage is avascular and potentially inhibitory compounds in native cartilage and synovial fluid may be involved (159). The key factor influencing tissue integration is cell migration leading to cell and matrix accumulation at the interfacial zone. Chondrocytes migrating to the graft interface may originate from either the engineered or host tissue. Furthermore, integration with native cartilage is modulated by substrate elasticity which is important in the long-term survival of the in vivo regenerated tissue (160, 161). Enhancement of chondrocyte migration using microRNA, gene delivery, gene- or protein-level induction by signaling molecules, and transcription factors have been promoted as a strategy to improve chondrogenesis and integration outcomes (162, 163).
Maintenance of cell phenotype
Long-term chondrocyte morphology maintenance is the basic criterion for cartilage tissue engineering that has not been exactly elucidated so far. Recently micropatterned 3D culture platforms, such as 3D scaffolds, organoids, microspheres, spheroids, and cellwell strategy, have been shown to promote the maintenance of chondrocyte phenotype (164-168).
Scaffold-based therapy
Scaffold-based gene therapy through transfecting the cells to enhance the constant expression of the proteins in chondrogenesis or silencing target genes associated with bone and joint disease provides a promising strategy for cartilage tissue engineering. Genes have been transfected into MSCs, ADSCs, or chondrocytes to improve their phenotypic characteristics (169). Scaffolds combined with microRNAs (miRNAs) have been developed for osteochondral repair in the early stages. MiRNAs are ~22 nucleotide single-stranded RNAs that regulate post-transcriptional gene expression. They can regulate chondrocyte signaling and epigenetic functions (170). Yan et al. have prepared a summary of the gene therapy and miRNAs studies in osteochondral tissue repair (169). Recent studies using single-cell RNA sequencing (scRNA-seq), gene expression, and transcription factor activity can define deconstruct gene regulatory networks in hMSC differentiation during chondrogenesis and revealed the trajectories of maturation (171, 172).
Bioprinted Scaffolds for Cartilage Tissue Engineering
3D bioprinting is a fast-growing technology that has been broadly used in tissue engineering, disease studies, and drug screening. This technology is expected to address organ-shortage concerns in the future (173). Bioprinting creates three-dimensional, free-form, and computer-designed scaffolds using biomaterials, biomolecules, and/or cells (174).
Novel advances in 3D bioprinting offer interesting opportunities for 3D cartilage tissue printing by providing living cells with applicable scaffolds. Biological advances in bioinks are currently promising for scaffold printing and cell encapsulation, particularly on MSCs through chondrogenic differentiation (175, 176). Multi-material 3D bioprinted porous scaffolds with the combination of mechanical and biological properties in a single construct could be applied for cartilage tissue engineering. However, in the cell-laden bioprinting method, consideration is necessary for 3D manufacturing parameters, including temperature, needle gauge, UV exposure time, and cell carrier formulation on the viability and functionality of the cells in bioprinted constructs (177, 178). Ongoing studies in this field are concentrating on issues, such as the types of bio- nano/macromolecular cartilage scaffolds, standardization of cell culture protocols, cell density, bioink formulation, and 3D bioprinting technology (179, 180).
A key goal of functional cartilage tissue engineering is to optimize constructs with biochemical and mechanical properties approaching those of the native cartilage tissue. Silver bullet as an ideal scaffold for cartilage tissue engineering should be fulfilled with such necessities as biocompatible, biodegradable, highly porous, suitable for cell attachment, proliferative and differentiative, chondroinductive, noncytotoxic, nonantigenic, flexible, and elastic. In the near future, engineered cartilage may present routine treatment for cartilage injury.
Conflicts of interest:
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Acknowledgements
The authors would like to appreciate the help and support from the Clinical Research Development Unit, Ghaem Hospital, Mashhad University of Medical Sciences, Mashhad, Iran.
References
- 1.Casanellas I, Garcia-Lizarribar A, Lagunas A, Samitier J. Producing 3D Biomimetic Nanomaterials for Musculoskeletal System Regeneration. Front Bioeng Biotechnol. 2018;6:128. doi: 10.3389/fbioe.2018.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunziker EB, Quinn TM, Hauselmann HJ. Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage. 2002;10(7):564–72. doi: 10.1053/joca.2002.0814. [DOI] [PubMed] [Google Scholar]
- 3.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective: A population-based review of the fourth most common cause of hospitalization in U. S. adults. Orthop Nurs. 2012;31(2):85–91. doi: 10.1097/NOR.0b013e31824fcd42. [DOI] [PubMed] [Google Scholar]
- 4.Vilela CA, da Silva Morais A, Pina S, Oliveira JM, Correlo VM, Reis RL, et al. Clinical Trials and Management of Osteochondral Lesions. Adv Exp Med Biol. 2018;1058:391–413. doi: 10.1007/978-3-319-76711-6_18. [DOI] [PubMed] [Google Scholar]
- 5.Bicho D, Pina S, Reis RL, Oliveira JM. Commercial Products for Osteochondral Tissue Repair and Regeneration. Adv Exp Med Biol. 2018;1058:415–28. doi: 10.1007/978-3-319-76711-6_19. [DOI] [PubMed] [Google Scholar]
- 6.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 7.Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477–86. [PubMed] [Google Scholar]
- 8.Camp CL, Stuart MJ, Krych AJ. Current concepts of articular cartilage restoration techniques in the knee. Sports Health. 2014;6(3):265–73. doi: 10.1177/1941738113508917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gudas R, Gudaite A, Mickevicius T, Masiulis N, Simonaityte R, Cekanauskas E, et al. Comparison of osteochondral autologous transplantation, microfracture, or debridement techniques in articular cartilage lesions associated with anterior cruciate ligament injury: a prospective study with a 3-year follow-up. Arthroscopy. 2013;29(1):89–97. doi: 10.1016/j.arthro.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Richter DL, Schenck RC Jr, Wascher DC, Treme G. Knee Articular Cartilage Repair and Restoration Techniques: A Review of the Literature. Sports Health. 2016;8(2):153–60. doi: 10.1177/1941738115611350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver-Welsh L, Griffin JW, Meyer MA, Gitelis ME, Cole BJ. Deciding How Best to Treat Cartilage Defects. Orthopedics. 2016;39(6):343–50. doi: 10.3928/01477447-20161020-03. [DOI] [PubMed] [Google Scholar]
- 12.Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med. 2008;3(1):1–5. doi: 10.2217/17460751.3.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Decker RS. Articular cartilage and joint development from embryogenesis to adulthood. Seminars in cell & developmental biology . 2017;62:50–56. doi: 10.1016/j.semcdb.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Hu J, Athanasiou KA. The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng. 2009;37(1-2):1–57. doi: 10.1615/critrevbiomedeng.v37.i1-2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorgensen AEM, Kjaer M, Heinemeier KM. The Effect of Aging and Mechanical Loading on the Metabolism of Articular Cartilage. J Rheumatol. 2017;44(4):410–7. doi: 10.3899/jrheum.160226. [DOI] [PubMed] [Google Scholar]
- 16.Elder BD, Athanasiou KA. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng Part B Rev. 2009;15(1):43–53. doi: 10.1089/ten.teb.2008.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu M, Li W, Dong X, Yuan X, Midgley AC, Chang H, et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nature communications. 2019;10(1):1–4. doi: 10.1038/s41467-019-12545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundelacruz S, Kaplan DL. Stem cell-and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Seminars in cell & developmental biology . 2009;20(6):646–655. doi: 10.1016/j.semcdb.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–12. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 21.Patel JM, Wise BC, Bonnevie ED, Mauck RL. A Systematic Review and Guide to Mechanical Testing for Articular Cartilage Tissue Engineering. Tissue Eng Part C Methods. 2019;25(10):593–608. doi: 10.1089/ten.tec.2019.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashita A, Tamamura Y, Morioka M, Karagiannis P, Shima N, Tsumaki N. Considerations in hiPSC-derived cartilage for articular cartilage repair. Inflammation and Regeneration. 2018;38(1):1–7. doi: 10.1186/s41232-018-0075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatari H. The structure, physiology, and biomechanics of articular cartilage: injury and repair. Acta orthopaedica et traumatologica turcica. 2007;41:1–5. [PubMed] [Google Scholar]
- 24.Yari D, Ehsanbakhsh Z, Validad MH, Langroudi FH. Association of TIMP-1 and COL4A4 Gene Polymorphisms with Keratoconus in an Iranian Population. J Ophthalmic Vis Res. 2020;15(3):299–307. doi: 10.18502/jovr.v15i3.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moradi A, Ataollahi F, Sayar K, Pramanik S, Chong PP, Khalil AA, et al. Chondrogenic potential of physically treated bovine cartilage matrix derived porous scaffolds on human dermal fibroblast cells. J Biomed Mater Res A. 2016;104(1):245–56. doi: 10.1002/jbm.a.35561. [DOI] [PubMed] [Google Scholar]
- 26.Mehlhorn AT, Niemeyer P, Kaiser S, Finkenzeller G, Stark GB, Sudkamp NP, et al. Differential expression pattern of extracellular matrix molecules during chondrogenesis of mesenchymal stem cells from bone marrow and adipose tissue. Tissue Eng. 2006;12(10):2853–62. doi: 10.1089/ten.2006.12.2853. [DOI] [PubMed] [Google Scholar]
- 27.Vincent TL, Wann AK. Mechanoadaptation: articular cartilage through thick and thin. The Journal of physiology. 2019;597(5):1271–81. doi: 10.1113/JP275451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roughley PJ, Lee ER. Cartilage proteoglycans: structure and potential functions. Microsc Res Tech. 1994;28(5):385–97. doi: 10.1002/jemt.1070280505. [DOI] [PubMed] [Google Scholar]
- 29.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harbor perspectives in biology. 2011;3(7):a004952. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neves MI, Araújo M, Moroni L, da Silva RM, Barrias CC. Glycosaminoglycan-inspired biomaterials for the development of bioactive hydrogel networks. Molecules. 2020;25(4):978. doi: 10.3390/molecules25040978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Driessen BJH, Logie C, Vonk LA. Cellular reprogramming for clinical cartilage repair. Cell Biol Toxicol. 2017;33(4):329–49. doi: 10.1007/s10565-017-9382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014;39:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carballo CB, Nakagawa Y, Sekiya I, Rodeo SA. Basic Science of Articular Cartilage. Clin Sports Med. 2017;36(3):413–25. doi: 10.1016/j.csm.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Bolton MC, Dudhia J, Bayliss MT. Age-related changes in the synthesis of link protein and aggrecan in human articular cartilage: implications for aggregate stability. Biochem J. 1999;337(1):77–82. [PMC free article] [PubMed] [Google Scholar]
- 36.Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1(1):57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 37.Saravani R, Yari D, Saravani S, Hasanian-Langroudi F. Correlation between the COL4A3, MMP-9, and TIMP-1 polymorphisms and risk of keratoconus. Jpn J Ophthalmol. 2017;61(3):218–22. doi: 10.1007/s10384-017-0503-3. [DOI] [PubMed] [Google Scholar]
- 38.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 39.Lepperdinger G, Brunauer R, Jamnig A, Laschober G, Kassem M. Controversial issue: is it safe to employ mesenchymal stem cells in cell-based therapies? Exp Gerontol. 2008;43(11):1018–23. doi: 10.1016/j.exger.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Mendelson A, Frank E, Allred C, Jones E, Chen M, Zhao W, et al. Chondrogenesis by chemotactic homing of synovium, bone marrow, and adipose stem cells in vitro. FASEB J. 2011;25(10):3496–504. doi: 10.1096/fj.10-176305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo Monaco M, Merckx G, Ratajczak J, Gervois P, Hilkens P, Clegg P, et al. Stem Cells for Cartilage Repair: Preclinical Studies and Insights in Translational Animal Models and Outcome Measures. Stem Cells Int. 2018;2018:9079538. doi: 10.1155/2018/9079538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang M, Yuan Z, Ma N, Hao C, Guo W, Zou G, et al. Advances and Prospects in Stem Cells for Cartilage Regeneration. Stem Cells Int. 2017;2017:4130607. doi: 10.1155/2017/4130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacob G, Shimomura K, Krych AJ, Nakamura N. The Meniscus Tear: A Review of Stem Cell Therapies. Cells. 2019;9:1. doi: 10.3390/cells9010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gur-Cohen S, Yang H, Baksh SC, Miao Y, Levorse J, Kataru RP, et al. Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science. 2019;366(6470):1218–25. doi: 10.1126/science.aay4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang WG, Lou SQ, Ju XD, Xia K, Xia JH. In vitro chondrogenesis of human bone marrow-derived mesenchymal progenitor cells in monolayer culture: activation by transfection with TGF-beta2. Tissue Cell. 2003;35(1):69–77. doi: 10.1016/s0040-8166(02)00106-4. [DOI] [PubMed] [Google Scholar]
- 46.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–7. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 47.Mahmoudifar N, Doran PM. Chondrogenic differentiation of human adipose-derived stem cells in polyglycolic acid mesh scaffolds under dynamic culture conditions. Biomaterials. 2010;31(14):3858–67. doi: 10.1016/j.biomaterials.2010.01.090. [DOI] [PubMed] [Google Scholar]
- 48.Pievani A, Scagliotti V, Russo FM, Azario I, Rambaldi B, Sacchetti B, et al. Comparative analysis of multilineage properties of mesenchymal stromal cells derived from fetal sources shows an advantage of mesenchymal stromal cells isolated from cord blood in chondrogenic differentiation potential. Cytotherapy. 2014;16(7):893–905. doi: 10.1016/j.jcyt.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang SJ, Jiang D, Zhang ZZ, Huang AB, Qi YS, Wang HJ, et al. Chondrogenic Potential of Peripheral Blood Derived Mesenchymal Stem Cells Seeded on Demineralized Cancellous Bone Scaffolds. Sci Rep. 2016;6:36400. doi: 10.1038/srep36400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuliani CC, Bombini MF, Andrade KC, Mamoni R, Pereira AH, Coimbra IB. Micromass cultures are effective for differentiation of human amniotic fluid stem cells into chondrocytes. Clinics (Sao Paulo). 2018;73:e268. doi: 10.6061/clinics/2018/e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Longoni A, Utomo L, van Hooijdonk IE, Bittermann GK, Vetter VC, Kruijt Spanjer EC, et al. The chondrogenic differentiation potential of dental pulp stem cells. Eur Cell Mater. 2020;39:121–35. doi: 10.22203/eCM.v039a08. [DOI] [PubMed] [Google Scholar]
- 52.To K, Zhang B, Romain K, Mak C, Khan W. Synovium-Derived Mesenchymal Stem Cell Transplantation in Cartilage Regeneration: A PRISMA Review of in vivo Studies. Front Bioeng Biotechnol. 2019;7:314. doi: 10.3389/fbioe.2019.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andriamanalijaona R, Duval E, Raoudi M, Lecourt S, Vilquin JT, Marolleau JP, et al. Differentiation potential of human muscle-derived cells towards chondrogenic phenotype in alginate beads culture. Osteoarthritis Cartilage. 2008;16(12):1509–18. doi: 10.1016/j.joca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 54.Huang JI, Kazmi N, Durbhakula MM, Hering TM, Yoo JU, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. J Orthop Res. 2005;23(6):1383–9. doi: 10.1016/j.orthres.2005.03.008.1100230621. [DOI] [PubMed] [Google Scholar]
- 55.Contentin R, Demoor M, Concari M, Desance M, Audigie F, Branly T, et al. Comparison of the Chondrogenic Potential of Mesenchymal Stem Cells Derived from Bone Marrow and Umbilical Cord Blood Intended for Cartilage Tissue Engineering. Stem Cell Rev Rep. 2020;16(1):126–43. doi: 10.1007/s12015-019-09914-2. [DOI] [PubMed] [Google Scholar]
- 56.Silva JC, Udangawa RN, Chen J, Mancinelli CD, Garrudo FFF, Mikael PE, et al. Kartogenin-loaded coaxial PGS/PCL aligned nanofibers for cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2020;107:110291. doi: 10.1016/j.msec.2019.110291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moura CS, Silva JC, Faria S, Fernandes PR, da Silva CL, Cabral JMS, et al. Chondrogenic differentiation of mesenchymal stem/stromal cells on 3D porous poly (epsilon-caprolactone) scaffolds: Effects of material alkaline treatment and chondroitin sulfate supplementation. J Biosci Bioeng. 2020;129(6):756–64. doi: 10.1016/j.jbiosc.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Kohli N, Wright KT, Sammons RL, Jeys L, Snow M, Johnson WE. An In Vitro Comparison of the Incorporation, Growth, and Chondrogenic Potential of Human Bone Marrow versus Adipose Tissue Mesenchymal Stem Cells in Clinically Relevant Cell Scaffolds Used for Cartilage Repair. Cartilage. 2015;6(4):252–63. doi: 10.1177/1947603515589650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satue M, Schuler C, Ginner N, Erben RG. Intra-articularly injected mesenchymal stem cells promote cartilage regeneration, but do not permanently engraft in distant organs. Sci Rep. 2019;9(1):10153. doi: 10.1038/s41598-019-46554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Secunda R, Vennila R, Mohanashankar AM, Rajasundari M, Jeswanth S, Surendran R. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology. 2015;67(5):793–807. doi: 10.1007/s10616-014-9718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baghaei K, Hashemi SM, Tokhanbigli S, Asadi Rad A, Assadzadeh-Aghdaei H, Sharifian A, et al. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol Hepatol Bed Bench. 2017;10(3):208–13. [PMC free article] [PubMed] [Google Scholar]
- 62.Grskovic B, Ruzicka K, Karimi A, Qujeq D, Muller MM. Cell cycle analysis of the CD133+ and CD133- cells isolated from umbilical cord blood. Clin Chim Acta. 2004;343(1-2):173–8. doi: 10.1016/j.cccn.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 63.Park D, Lim J, Park JY, Lee SH. Concise Review: Stem Cell Microenvironment on a Chip: Current Technologies for Tissue Engineering and Stem Cell Biology. Stem Cells Transl Med. 2015;4(11):1352–68. doi: 10.5966/sctm.2015-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jansen KA, Donato DM, Balcioglu HE, Schmidt T, Danen EH, Koenderink GH. A guide to mechanobiology: Where biology and physics meet. Biochim Biophys Acta. 2015;1853(11 Pt B):3043–52. doi: 10.1016/j.bbamcr.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Kim IG, Gil CH, Seo J, Park SJ, Subbiah R, Jung TH, et al. Mechanotransduction of human pluripotent stem cells cultivated on tunable cell-derived extracellular matrix. Biomaterials. 2018;150:100–11. doi: 10.1016/j.biomaterials.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 66.Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28(1):5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Hattori T, Muller C, Gebhard S, Bauer E, Pausch F, Schlund B, et al. SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development. 2010;137(6):901–11. doi: 10.1242/dev.045203. [DOI] [PubMed] [Google Scholar]
- 68.Schnabel M, Marlovits S, Eckhoff G, Fichtel I, Gotzen L, Vecsei V, et al. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage. 2002;10(1):62–70. doi: 10.1053/joca.2001.0482. [DOI] [PubMed] [Google Scholar]
- 69.Zou J, Bai B, Yao Y. Progress of Co-culture Systems in Cartilage Regeneration. Expert Opin Biol Ther. 2018;18(11):1151–8. doi: 10.1080/14712598.2018.1533116. [DOI] [PubMed] [Google Scholar]
- 70.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215–24. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 71.Okubo R, Asawa Y, Watanabe M, Nagata S, Nio M, Takato T, et al. Proliferation medium in three-dimensional culture of auricular chondrocytes promotes effective cartilage regeneration in vivo. Regen Ther. 2019;11:306–15. doi: 10.1016/j.reth.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin GZ, Kim HW. Efficacy of collagen and alginate hydrogels for the prevention of rat chondrocyte dedifferentiation. J Tissue Eng. 2018;9:2041731418802438. doi: 10.1177/2041731418802438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puetzer JL, Petitte JN, Loboa EG. Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng Part B Rev. 2010;16(4):435–44. doi: 10.1089/ten.TEB.2009.0705. [DOI] [PubMed] [Google Scholar]
- 74.Schumann D, Kujat R, Nerlich M, Angele P. Mechanobiological conditioning of stem cells for cartilage tissue engineering. Biomed Mater Eng. 2006;16(4 Suppl):S37–52. [PubMed] [Google Scholar]
- 75.Zhang S, Vijayavenkataraman S, Lu WF, Fuh JYH. A review on the use of computational methods to characterize, design, and optimize tissue engineering scaffolds, with a potential in 3D printing fabrication. J Biomed Mater Res B Appl Biomater. 2019;107(5):1329–51. doi: 10.1002/jbm.b.34226. [DOI] [PubMed] [Google Scholar]
- 76.Jafari M, Paknejad Z, Rad MR, Motamedian SR, Eghbal MJ, Nadjmi N, et al. Polymeric scaffolds in tissue engineering: a literature review. J Biomed Mater Res B Appl Biomater. 2017;105(2):431–59. doi: 10.1002/jbm.b.33547. [DOI] [PubMed] [Google Scholar]
- 77.Chai Q, Jiao Y, Yu X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels. 2017;3:1. doi: 10.3390/gels3010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bordbar S, Lotfi Bakhshaiesh N, Khanmohammadi M, Sayahpour FA, Alini M, Baghaban Eslaminejad M. Production and evaluation of decellularized extracellular matrix hydrogel for cartilage regeneration derived from knee cartilage. J Biomed Mater Res A. 2020;108(4):938–46. doi: 10.1002/jbm.a.36871. [DOI] [PubMed] [Google Scholar]
- 79.Thompson WR, Scott A, Loghmani MT, Ward SR, Warden SJ. Understanding Mechanobiology: Physical Therapists as a Force in Mechanotherapy and Musculoskeletal Regenerative Rehabilitation. Phys Ther. 2016;96(4):560–9. doi: 10.2522/ptj.20150224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marrella A, Lee TY, Lee DH, Karuthedom S, Syla D, Chawla A, et al. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater Today (Kidlington). 2018;21(4):362–76. doi: 10.1016/j.mattod.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24(24):4385–415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 82.Amann E, Wolff P, Breel E, van Griensven M, Balmayor ER. Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co-cultures. Acta Biomater. 2017;52:130–44. doi: 10.1016/j.actbio.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 83.Cheng A, Schwartz Z, Kahn A, Li X, Shao Z, Sun M, et al. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Eng Part B Rev. 2019;25(1):14–29. doi: 10.1089/ten.teb.2018.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu W, Thomopoulos S, Xia Y. Electrospun nanofibers for regenerative medicine. Adv Healthc Mater. 2012;1(1):10–25. doi: 10.1002/adhm.201100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amiri N, Rozbeh Z, Afrough T, Sajadi Tabassi SA, Moradi A, Movaffagh J. Optimization of Chitosan-Gelatin Nanofibers Production: Investigating the Effect of Solution Properties and Working Parameters on Fibers Diameter. BioNanoScience. 2018;8(3):778–89. [Google Scholar]
- 86.Amiri N, Moradi A, Tabasi SAS, Movaffagh J. Modeling and process optimization of electrospinning of chitosan-collagen nanofiber by response surface methodology. Materials Research Express. 2018;5(4):045404. [Google Scholar]
- 87.Braghirolli DI, Steffens D, Pranke P. Electrospinning for regenerative medicine: a review of the main topics. Drug Discov Today. 2014;19(6):743–53. doi: 10.1016/j.drudis.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 88.Kundu B, Rajkhowa R, Kundu SC, Wang X. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65(4):457–70. doi: 10.1016/j.addr.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 89.Friess W. Collagen--biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45(2):113–36. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 90.Ma PX, Zhang R. Synthetic nano-scale fibrous extracellular matrix. J Biomed Mater Res. 1999;46(1):60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 91.Garcia Y, Wilkins B, Collighan RJ, Griffin M, Pandit A. Towards development of a dermal rudiment for enhanced wound healing response. Biomaterials. 2008;29(7):857–68. doi: 10.1016/j.biomaterials.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 92.Movaffagh J, Fazly Bazzaz BS, Yazdi AT, Sajadi-Tabassi A, Azizzadeh M, Najafi E, et al. Wound Healing and Antimicrobial Effects of Chitosan-hydrogel/Honey Compounds in a Rat Full-thickness Wound Model. Wounds. 2019;31(9):228–35. [PubMed] [Google Scholar]
- 93.Stokols S, Tuszynski MH. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials. 2006;27(3):443–51. doi: 10.1016/j.biomaterials.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 94.Bozkurt A, Lassner F, O’Dey D, Deumens R, Bocker A, Schwendt T, et al. The role of microstructured and interconnected pore channels in a collagen-based nerve guide on axonal regeneration in peripheral nerves. Biomaterials. 2012;33(5):1363–75. doi: 10.1016/j.biomaterials.2011.10.069. [DOI] [PubMed] [Google Scholar]
- 95.Ghassemi T, Saghatolslami N, Matin MM, Gheshlaghi R, Moradi A. CNT-decellularized cartilage hybrids for tissue engineering applications. Biomed Mater. 2017;12(6):065008. doi: 10.1088/1748-605X/aa8435. [DOI] [PubMed] [Google Scholar]
- 96.Rowland CR, Lennon DP, Caplan AI, Guilak F. The effects of crosslinking of scaffolds engineered from cartilage ECM on the chondrogenic differentiation of MSCs. Biomaterials. 2013;34(23):5802–12. doi: 10.1016/j.biomaterials.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shen W, Chen X, Hu Y, Yin Z, Zhu T, Hu J, et al. Long-term effects of knitted silk-collagen sponge scaffold on anterior cruciate ligament reconstruction and osteoarthritis prevention. Biomaterials. 2014;35(28):8154–63. doi: 10.1016/j.biomaterials.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 98.Qujeq D, Abassi R, Faeizi F, Parsian H, Faraji AS, Taheri H, et al. Effect of granulocyte colony-stimulating factor administration on tissue regeneration due to carbon tetrachloride-induced liver damage in experimental model. Toxicol Ind Health. 2013;29(6):498–503. doi: 10.1177/0748233712440136. [DOI] [PubMed] [Google Scholar]
- 99.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 100.Zhou Y, Liang K, Zhao S, Zhang C, Li J, Yang H, et al. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int J Biol Macromol. 2018;108:383–90. doi: 10.1016/j.ijbiomac.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 101.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6(1):386–91. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Joanne P, Kitsara M, Boitard SE, Naemetalla H, Vanneaux V, Pernot M, et al. Nanofibrous clinical-grade collagen scaffolds seeded with human cardiomyocytes induces cardiac remodeling in dilated cardiomyopathy. Biomaterials. 2016;80:157–68. doi: 10.1016/j.biomaterials.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 103.Miao Z, Lu Z, Wu H, Liu H, Li M, Lei D, et al. Collagen, agarose, alginate, and Matrigel hydrogels as cell substrates for culture of chondrocytes in vitro: A comparative study. J Cell Biochem. 2017 doi: 10.1002/jcb.26411. [DOI] [PubMed] [Google Scholar]
- 104.Stoppel WL, Ghezzi CE, McNamara SL, Black LD, 3rd , Kaplan DL. Clinical applications of naturally derived biopolymer-based scaffolds for regenerative medicine. Ann Biomed Eng. 2015;43(3):657–80. doi: 10.1007/s10439-014-1206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chan G, Mooney DJ. New materials for tissue engineering: towards greater control over the biological response. Trends Biotechnol. 2008;26(7):382–92. doi: 10.1016/j.tibtech.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 106.Cheng CW, Solorio LD, Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv. 2014;32(2):462–84. doi: 10.1016/j.biotechadv.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morris AH, Stamer DK, Kyriakides TR. The host response to naturally-derived extracellular matrix biomaterials. Semin Immunol. 2017;29:72–91. doi: 10.1016/j.smim.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 108.Hoshiba T, Lu H, Kawazoe N, Chen G. Decellularized matrices for tissue engineering. Expert Opin Biol Ther. 2010;10(12):1717–28. doi: 10.1517/14712598.2010.534079. [DOI] [PubMed] [Google Scholar]
- 109.Gupta SK, Mishra NC, Dhasmana A. Decellularization Methods for Scaffold Fabrication. Methods Mol Biol. 2018;1577:1–10. doi: 10.1007/7651_2017_34. [DOI] [PubMed] [Google Scholar]
- 110.Kim YS, Majid M, Melchiorri AJ, Mikos AG. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng Transl Med. 2019;4(1):83–95. doi: 10.1002/btm2.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johnson TD, Hill RC, Dzieciatkowska M, Nigam V, Behfar A, Christman KL, et al. Quantification of decellularized human myocardial matrix: A comparison of six patients. Proteomics Clin Appl. 2016;10(1):75–83. doi: 10.1002/prca.201500048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanchez PL, Fernandez-Santos ME, Costanza S, Climent AM, Moscoso I, Gonzalez-Nicolas MA, et al. Acellular human heart matrix: A critical step toward whole heart grafts. Biomaterials. 2015;61:279–89. doi: 10.1016/j.biomaterials.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 113.VeDepo MC, Buse EE, Quinn RW, Williams TD, Detamore MS, Hopkins RA, et al. Species-specific effects of aortic valve decellularization. Acta Biomater. 2017;50:249–58. doi: 10.1016/j.actbio.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 114.Pellegata AF, Asnaghi MA, Stefani I, Maestroni A, Maestroni S, Dominioni T, et al. Detergent-enzymatic decellularization of swine blood vessels: insight on mechanical properties for vascular tissue engineering. Biomed Res Int. 2013;2013:918753. doi: 10.1155/2013/918753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ghassemi T, Saghatoleslami N, Mahdavi-Shahri N, Matin MM, Gheshlaghi R, Moradi A. A comparison study of different decellularization treatments on bovine articular cartilage. J Tissue Eng Regen Med. 2019;13(10):1861–71. doi: 10.1002/term.2936. [DOI] [PubMed] [Google Scholar]
- 116.Moradi A, Pramanik S, Ataollahi F, Abdul Khalil A, Kamarul T, Pingguan-Murphy B. A comparison study of different physical treatments on cartilage matrix derived porous scaffolds for tissue engineering applications. Sci Technol Adv Mater. 2014;15(6):065001. doi: 10.1088/1468-6996/15/6/065001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Garrigues NW, Little D, Sanchez-Adams J, Ruch DS, Guilak F. Electrospun cartilage-derived matrix scaffolds for cartilage tissue engineering. J Biomed Mater Res A. 2014;102(11):3998–4008. doi: 10.1002/jbm.a.35068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cheng NC, Estes BT, Young TH, Guilak F. Genipin-crosslinked cartilage-derived matrix as a scaffold for human adipose-derived stem cell chondrogenesis. Tissue Eng Part A. 2013;19(3-4):484–96. doi: 10.1089/ten.tea.2012.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheng NC, Estes BT, Young TH, Guilak F. Engineered cartilage using primary chondrocytes cultured in a porous cartilage-derived matrix. Regen Med. 2011;6(1):81–93. doi: 10.2217/rme.10.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mao Y, Block T, Singh-Varma A, Sheldrake A, Leeth R, Griffey S, et al. Extracellular matrix derived from chondrocytes promotes rapid expansion of human primary chondrocytes in vitro with reduced dedifferentiation. Acta Biomater. 2019;85:75–83. doi: 10.1016/j.actbio.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 121.Fermor HL, McLure SW, Taylor SD, Russell SL, Williams S, Fisher J, et al. Biological, biochemical and biomechanical characterisation of articular cartilage from the porcine, bovine and ovine hip and knee. Biomed Mater Eng. 2015;25(4):381–95. doi: 10.3233/BME-151533. [DOI] [PubMed] [Google Scholar]
- 122.Taylor SD, Tsiridis E, Ingham E, Jin Z, Fisher J, Williams S. Comparison of human and animal femoral head chondral properties and geometries. Proc Inst Mech Eng H. 2012;226(1):55–62. doi: 10.1177/0954411911428717. [DOI] [PubMed] [Google Scholar]
- 123.Palmese LL, Thapa RK, Sullivan MO, Kiick KL. Hybrid hydrogels for biomedical applications. Curr Opin Chem Eng. 2019;24:143–57. doi: 10.1016/j.coche.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grover GN, Rao N, Christman KL. Myocardial matrix-polyethylene glycol hybrid hydrogels for tissue engineering. Nanotechnology. 2014;25(1):014011. doi: 10.1088/0957-4484/25/1/014011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mansour JM, Welter JF. Multimodal evaluation of tissue-engineered cartilage. J Med Biol Eng. 2013;33(1):1–16. doi: 10.5405/jmbe.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou S, Wang Y, Zhang K, Cao N, Yang R, Huang J, et al. The Fabrication and Evaluation of a Potential Biomaterial Produced with Stem Cell Sheet Technology for Future Regenerative Medicine. Stem Cells Int. 2020;2020:9567362. doi: 10.1155/2020/9567362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vincourt JB, Lionneton F, Kratassiouk G, Guillemin F, Netter P, Mainard D, et al. Establishment of a reliable method for direct proteome characterization of human articular cartilage. Mol Cell Proteomics. 2006;5(10):1984–95. doi: 10.1074/mcp.T600007-MCP200. [DOI] [PubMed] [Google Scholar]
- 128.De Ceuninck F, Marcheteau E, Berger S, Caliez A, Dumont V, Raes M, et al. Assessment of some tools for the characterization of the human osteoarthritic cartilage proteome. J Biomol Tech. 2005;16(3):256–65. [PMC free article] [PubMed] [Google Scholar]
- 129.Gaudet AD, Popovich PG. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp Neurol. 2014;258:24–34. doi: 10.1016/j.expneurol.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Acharya C, Yik JH, Kishore A, Van Dinh V, Di Cesare PE, Haudenschild DR. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: interaction, regulation and role in chondrogenesis. Matrix Biol. 2014;37:102–11. doi: 10.1016/j.matbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 131.Oh CD, Lu Y, Liang S, Mori-Akiyama Y, Chen D, de Crombrugghe B, et al. SOX9 regulates multiple genes in chondrocytes, including genes encoding ECM proteins, ECM modification enzymes, receptors, and transporters. PLoS One. 2014;9(9):e107577. doi: 10.1371/journal.pone.0107577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tew SR, Clegg PD, Brew CJ, Redmond CM, Hardingham TE. SOX9 transduction of a human chondrocytic cell line identifies novel genes regulated in primary human chondrocytes and in osteoarthritis. Arthritis Res Ther. 2007;9(5):R107. doi: 10.1186/ar2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Henry SP, Liang S, Akdemir KC, de Crombrugghe B. The postnatal role of Sox9 in cartilage. J Bone Miner Res. 2012;27(12):2511–25. doi: 10.1002/jbmr.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim Y, Murao H, Yamamoto K, Deng JM, Behringer RR, Nakamura T, et al. Generation of transgenic mice for conditional overexpression of Sox9. J Bone Miner Metab. 2011;29(1):123–9. doi: 10.1007/s00774-010-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taheem DK, Foyt DA, Loaiza S, Ferreira SA, Ilic D, Auner HW, et al. Differential Regulation of Human Bone Marrow Mesenchymal Stromal Cell Chondrogenesis by Hypoxia Inducible Factor-1alpha Hydroxylase Inhibitors. Stem Cells. 2018;36(9):1380–92. doi: 10.1002/stem.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jahr H, Gunes S, Kuhn AR, Nebelung S, Pufe T. Bioreactor-Controlled Physoxia Regulates TGF-beta Signaling to Alter Extracellular Matrix Synthesis by Human Chondrocytes. Int J Mol Sci. 2019;20:7. doi: 10.3390/ijms20071715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Markway BD, Cho H, Johnstone B. Hypoxia promotes redifferentiation and suppresses markers of hypertrophy and degeneration in both healthy and osteoarthritic chondrocytes. Arthritis Res Ther. 2013;15(4):R92. doi: 10.1186/ar4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schrobback K, Malda J, Crawford RW, Upton Z, Leavesley DI, Klein TJ. Effects of oxygen on zonal marker expression in human articular chondrocytes. Tissue Eng Part A. 2012;18(9-10):920–33. doi: 10.1089/ten.TEA.2011.0088. [DOI] [PubMed] [Google Scholar]
- 139.Milner PI, Wilkins RJ, Gibson JS. The role of mitochondrial reactive oxygen species in pH regulation in articular chondrocytes. Osteoarthritis Cartilage. 2007;15(7):735–42. doi: 10.1016/j.joca.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 140.Yari D, Saravani R, Saravani S, Ebrahimian K, Galavi HR. Genetic Polymorphisms of Catalase and Glutathione Peroxidase-1 in Keratoconus. Iran J Public Health. 2018;47(10):1567–74. [PMC free article] [PubMed] [Google Scholar]
- 141.Das RH, van Osch GJ, Kreukniet M, Oostra J, Weinans H, Jahr H. Effects of individual control of pH and hypoxia in chondrocyte culture. J Orthop Res. 2010;28(4):537–45. doi: 10.1002/jor.20994. [DOI] [PubMed] [Google Scholar]
- 142.Mitchell AC, Briquez PS, Hubbell JA, Cochran JR. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016;30:1–12. doi: 10.1016/j.actbio.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Belair DG, Le NN, Murphy WL. Design of growth factor sequestering biomaterials. Chem Commun (Camb). 2014;50(99):15651–68. doi: 10.1039/c4cc04317k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thielen NGM, van der Kraan PM, van Caam APM. TGFbeta/BMP Signaling Pathway in Cartilage Homeostasis. Cells. 2019;8:9. doi: 10.3390/cells8090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu X, Zheng L, Yuan Q, Zhen G, Crane JL, Zhou X, et al. Transforming growth factor-beta in stem cells and tissue homeostasis. Bone Res. 2018;6:2. doi: 10.1038/s41413-017-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Coricor G, Serra R. TGF-beta regulates phosphorylation and stabilization of Sox9 protein in chondrocytes through p38 and Smad dependent mechanisms. Sci Rep. 2016;6:38616. doi: 10.1038/srep38616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Badlani N, Oshima Y, Healey R, Coutts R, Amiel D. Use of bone morphogenic protein-7 as a treatment for osteoarthritis. Clin Orthop Relat Res. 2009;467(12):3221–9. doi: 10.1007/s11999-008-0569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shi S, Mercer S, Eckert GJ, Trippel SB. Regulation of articular chondrocyte catabolic genes by growth factor interaction. J Cell Biochem. 2019;120(7):11127–39. doi: 10.1002/jcb.28389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shi S, Mercer S, Eckert GJ, Trippel SB. Growth factor transgenes interactively regulate articular chondrocytes. J Cell Biochem. 2013;114(4):908–19. doi: 10.1002/jcb.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li J, Dong S. The Signaling Pathways Involved in Chondrocyte Differentiation and Hypertrophic Differentiation. Stem Cells Int. 2016;2016:2470351. doi: 10.1155/2016/2470351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Y, Fan X, Xing L, Tian F. Wnt signaling: a promising target for osteoarthritis therapy. Cell Commun Signal. 2019;17(1) doi: 10.1186/s12964-019-0411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gao Y, Liu S, Huang J, Guo W, Chen J, Zhang L, et al. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed Res Int. 2014;2014:648459. doi: 10.1155/2014/648459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mas-Moruno C, Fraioli R, Rechenmacher F, Neubauer S, Kapp TG, Kessler H. alphavbeta3- or alpha5beta1-Integrin-Selective Peptidomimetics for Surface Coating. Angew Chem Int Ed Engl. 2016;55(25):7048–67. doi: 10.1002/anie.201509782. [DOI] [PubMed] [Google Scholar]
- 154.Jansen KA, Atherton P, Ballestrem C. Mechanotransduction at the cell-matrix interface. Semin Cell Dev Biol. 2017;71:75–83. doi: 10.1016/j.semcdb.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 155.Hu X, Margadant FM, Yao M, Sheetz MP. Molecular stretching modulates mechanosensing pathways. Protein Sci. 2017;26(7):1337–51. doi: 10.1002/pro.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, et al. Tissue engineering for articular cartilage repair--the state of the art. Eur Cell Mater. 2013;25:248–67. doi: 10.22203/ecm.v025a18. [DOI] [PubMed] [Google Scholar]
- 157.Di Bella C, Duchi S, O’Connell CD, Blanchard R, Augustine C, Yue Z, et al. In situ handheld three-dimensional bioprinting for cartilage regeneration. J Tissue Eng Regen Med. 2018;12(3):611–21. doi: 10.1002/term.2476. [DOI] [PubMed] [Google Scholar]
- 158.Khan IM, Gilbert SJ, Singhrao SK, Duance VC, Archer CW. Cartilage integration: evaluation of the reasons for failure of integration during cartilage repair. A review. Eur Cell Mater. 2008;16:26–39. doi: 10.22203/ecm.v016a04. [DOI] [PubMed] [Google Scholar]
- 159.Yang YH, Ard MB, Halper JT, Barabino GA. Type I collagen-based fibrous capsule enhances integration of tissue-engineered cartilage with native articular cartilage. Ann Biomed Eng. 2014;42(4):716–26. doi: 10.1007/s10439-013-0958-4. [DOI] [PubMed] [Google Scholar]
- 160.Pabbruwe MB, Esfandiari E, Kafienah W, Tarlton JF, Hollander AP. Induction of cartilage integration by a chondrocyte/collagen-scaffold implant. Biomaterials. 2009;30(26):4277–86. doi: 10.1016/j.biomaterials.2009.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang YK, Ogando CR, Barabino GA. In Vitro Evaluation of the Influence of Substrate Mechanics on Matrix-Assisted Human Chondrocyte Transplantation. J Funct Biomater. 2020;11:1. doi: 10.3390/jfb11010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gurusinghe S, Strappe P. Gene modification of mesenchymal stem cells and articular chondrocytes to enhance chondrogenesis. Biomed Res Int. 2014;2014:369528. doi: 10.1155/2014/369528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Celik E, Bayram C, Denkbas EB. Chondrogenesis of human mesenchymal stem cells by microRNA loaded triple polysaccharide nanoparticle system. Mater Sci Eng C Mater Biol Appl. 2019;102:756–63. doi: 10.1016/j.msec.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 164.Jimenez G, Venkateswaran S, Lopez-Ruiz E, Peran M, Pernagallo S, Diaz-Monchon JJ, et al. A soft 3D polyacrylate hydrogel recapitulates the cartilage niche and allows growth-factor free tissue engineering of human articular cartilage. Acta Biomater. 2019;90:146–56. doi: 10.1016/j.actbio.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 165.Takada E, Mizuno S. Reproduction of Characteristics of Extracellular Matrices in Specific Longitudinal Depth Zone Cartilage within Spherical Organoids in Response to Changes in Osmotic Pressure. Int J Mol Sci. 2018;19(5) doi: 10.3390/ijms19051507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yeung P, Cheng KH, Yan CH, Chan BP. Collagen microsphere based 3D culture system for human osteoarthritis chondrocytes (hOACs) Sci Rep. 2019;9(1):12453. doi: 10.1038/s41598-019-47946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Udomluck N, Kim SH, Cho H, Park JY, Park H. Three-dimensional cartilage tissue regeneration system harnessing goblet-shaped microwells containing biocompatible hydrogel. Biofabrication. 2019;12(1):015019. doi: 10.1088/1758-5090/ab5d3e. [DOI] [PubMed] [Google Scholar]
- 168.Saraswat R, Ratnayake I, Perez EC, Schutz WM, Zhu Z, Ahrenkiel SP, et al. Micropatterned Biphasic Nanocomposite Platform for Maintaining Chondrocyte Morphology. ACS Appl Mater Interfaces. 2020;12(13):14814–24. doi: 10.1021/acsami.9b22596. [DOI] [PubMed] [Google Scholar]
- 169.Yan X, Chen YR, Song YF, Yang M, Ye J, Zhou G, et al. Scaffold-Based Gene Therapeutics for Osteochondral Tissue Engineering. Front Pharmacol. 2019;10:1534. doi: 10.3389/fphar.2019.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cong L, Zhu Y, Tu G. A bioinformatic analysis of microRNAs role in osteoarthritis. Osteoarthritis Cartilage. 2017;25(8):1362–71. doi: 10.1016/j.joca.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 171.Wolock SL, Krishnan I, Tenen DE, Matkins V, Camacho V, Patel S, et al. Mapping Distinct Bone Marrow Niche Populations and Their Differentiation Paths. Cell Rep. 2019;28(2):302–11 e5. doi: 10.1016/j.celrep.2019.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huynh N, Kelly N, Katz D, Pham M, Guilak F. Single Cell RNA Sequencing Reveals Heterogeneity of Human MSC Chondrogenesis: Lasso Regularized Logistic Regression to Identify Gene and Regulatory Signatures. bioRxiv. . 2019:854406. [Google Scholar]
- 173.Zhang B, Gao L, Ma L, Luo Y, Yang H, Cui Z. 3D Bioprinting: A Novel Avenue for Manufacturing Tissues and Organs. Engineering. 2019;5(4):777–94. [Google Scholar]
- 174.Derby B. Printing and prototyping of tissues and scaffolds. Science. 2012;338(6109):921–6. doi: 10.1126/science.1226340. [DOI] [PubMed] [Google Scholar]
- 175.Henrionnet C, Pourchet L, Neybecker P, Messaoudi O, Gillet P, Loeuille D, et al. Combining Innovative Bioink and Low Cell Density for the Production of 3D-Bioprinted Cartilage Substitutes: A Pilot Study. Stem Cells International. 2020;2020:1–16. doi: 10.1155/2020/2487072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Francis SL, Di Bella C, Wallace GG, Choong PFM. Cartilage Tissue Engineering Using Stem Cells and Bioprinting Technology-Barriers to Clinical Translation. Front Surg. 2018;5:70. doi: 10.3389/fsurg.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ruiz-Cantu L, Gleadall A, Faris C, Segal J, Shakesheff K, Yang J. Multi-material 3D bioprinting of porous constructs for cartilage regeneration. Mater Sci Eng C Mater Biol Appl. 2020;109:110578. doi: 10.1016/j.msec.2019.110578. [DOI] [PubMed] [Google Scholar]
- 178.Antich C, de Vicente J, Jimenez G, Chocarro C, Carrillo E, Montanez E, et al. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020;106:114–23. doi: 10.1016/j.actbio.2020.01.046. [DOI] [PubMed] [Google Scholar]
- 179.Zhao Z, Fan C, Chen F, Sun Y, Xia Y, Ji A, et al. Progress in Articular Cartilage Tissue Engineering: A Review on Therapeutic Cells and Macromolecular Scaffolds. Macromol Biosci. 2020;20(2):e1900278. doi: 10.1002/mabi.201900278. [DOI] [PubMed] [Google Scholar]
- 180.Semba J, Mieloch A, Rybka J. Introduction to the state-of-the-art 3D bioprinting methods, design, and applications in orthopedics. Bioprinting. 2019;18:e00070. [Google Scholar]