Key Points
The relative efficacy of each specific sodium-glucose co-transporter 2 inhibitor compared with the other in affecting electrolytes has rarely assessed in head-to-head trials.
The study aimed to maximize statistical power to summarize direct and indirect evidence using both pairwise and network meta-analyses.
Sodium-glucose co-transporter 2 inhibitors significantly increased serum magnesium and phosphate levels, supporting a class effect of sodium-glucose co-transporter 2 inhibition.
Keywords: diabetes and the kidney, calcium, diabetes mellitus, electrolytes, magnesium, meta-analysis, phosphate, potassium, SGLT2 inhibitor, sodium, type 2 diabetes
Visual Abstract
Abstract
Background
Previous studies have reported that sodium-glucose co-transporter 2 (SGLT2) inhibitors (SGLT2is) affect levels of serum electrolytes, especially magnesium. This study aimed to integrate direct and indirect trial evidence to maximize statistical power to clarify their overall and comparative effects in patients with type 2 diabetes (T2D).
Methods
We systematically searched PubMed, EMBASE, CENTRAL, and ClinicalTrials.gov up to January 2021 to identify eligible randomized controlled trials (RCTs) of SGLT2is that reported mean changes in serum electrolytes, including magnesium, sodium, potassium, phosphate, and calcium. We performed both random-effects pairwise and network meta-analyses to calculate the weighted mean difference (WMD) and 95% confidence intervals (CI).
Results
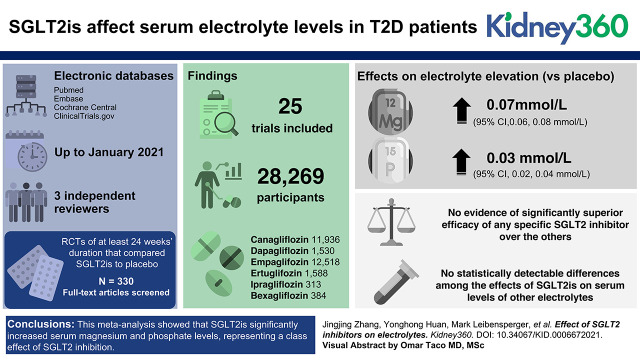
In total, we included 25 RCTs involving 28,269 patients with T2D and 6 SGLT2is. Compared with placebo, SGLT2is were significantly associated with elevations in serum magnesium by 0.07 mmol/L (95% CI, 0.06 to 0.08 mmol/L) and serum phosphate by 0.03 mmol/L (95% CI, 0.02 to 0.04 mmol/L). Our network meta-analysis showed no evidence of significantly superior efficacy of any specific SGLT2 inhibitor over the others, although dapagliflozin was associated with a larger increment in serum magnesium (WMD=0.16 mmol/L) compared with other SGLT2is. Similarly, no statistically detectable differences among the effects of SGLT2is on serum levels of other electrolytes were detected.
Conclusions
SGLT2is significantly increased serum magnesium and phosphate levels, consistent with a class effect of SGLT2 inhibition. However, further investigations of long-term efficacy and safety in patients with T2D with different clinical phenotypes are needed.
Introduction
Sodium-glucose cotransporter (SGLT) 2 inhibitors (SGLT2is) are a novel class of glucose-lowering agents that are indicated for the treatment of type 2 diabetes (T2D). SGLT2is selectively inhibit renal glucose reabsorption and increase urinary glucose excretion (1). Besides hypoglycemic effects in patients with T2D, SGLT2is have been considered an effective treatment option for renal and cardiovascular protection in diabetic patients with CKD (2–4). More recently, dapagliflozin was shown to extend its renal-protective effect to patients without diabetes (5).
SGLT2 is mainly expressed in renal tissue (6) but not in the human heart (7), where only SGLT1 is expressed at low levels. Therefore, the potential benefit of SGLT2is is likely through the kidney. SGLT2is blocks reabsorption of sodium and other electrolytes coupled with sodium, and although the effects of SGLT2is on electrolyte balance may play a critical role in improving cardiovascular and kidney outcomes, those contentions will need to be confirmed by more evidence.
The mechanisms underlying renal and cardiovascular protection by SGLT2is remain unresolved. The data on renal electrolyte handling in individuals with T2D using SGLT2is are limited. Several previous studies have examined the head-to-head effects of different SGLT2is in conventional pairwise meta-analyses. Yet, many of these direct comparison results lacked statistical power to test a SGLT2is class effect or a specific drug effect on electrolytes (8). Given the fact that few of comparisons between any two of SGLT2i have been studied in head-to-head trials, the relative efficacy of a given SGLT2 inhibitor compared with others in influencing electrolytes has never been systematically or quantitatively assessed. The current study was designed to maximize statistical power to examine whether and to what extent SGLT2is affect serum electrolyte levels in patients with T2D. With accumulating trial data, we conducted both pairwise and network meta-analyses to summarize both direct and indirect evidence on the basis of available data from randomized controlled trials (RCTs).
Materials and Methods
Search Strategy and Selection of Articles
We searched PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Clinical Trials.gov up to January 2021 to identify eligible RCTs using relevant search terms without restriction of language or year of publication. We included parallel RCTs of at least 24 weeks’ duration that compared SGLT2is with placebo in adult patients with T2D and reported mean postintervention changes in electrolyte levels from baseline in each group or the data that allowed us to estimate the mean changes and their variances. Our outcomes included serum levels of magnesium, phosphate, calcium, sodium, and potassium. Six commonly prescribed SGLT2is were chosen for study: canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, ipragliflozin, and bexagliflozin.
Data Extraction and Quality Assessment
We collected the following information from each eligible RCT: first author, publication year, study characteristics (country of origin, design, and funding), patients’ characteristics (inclusion criteria, background treatments, mean age, race, baseline glycated hemoglobin [HbA1c], mean eGFR, and body mean index), interventions (type and dose of SGLT2is), and the mean values (electrolyte level), variance measure, and the number of participants in the treatment and control arms for all reported periods.
The Cochrane risk-of-bias tool was used to assess the quality of RCTs on the basis of five domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding (performance bias and detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). Three reviewers independently extracted the data; they were all blinded to the authors and institutions of the studies undergoing review. Any disagreements were resolved by consensus or referral to a third reviewer.
Statistical Analyses
For pairwise meta-analyses, we applied the classic DerSimonian and Laird’s method using inverse variance weights to combine the weighted mean difference (WMD) estimates as reported or derived from the original reports. Heterogeneity between studies was assessed by the Cochrane Q (P value) and I2 statistics. The Q statistic is a chi-squared test for heterogeneity, and the I2 is the percentage of observed variance in effect sizes across studies. A value of 0% indicates no observed heterogeneity. Heterogeneity can be quantified as low, moderate, high, or considerably high, with ranges of 0%–25%, 25%–50%, 50%–75%, and 75%–100% for I2, respectively.
In the absence of direct comparisons between two treatments, a network meta-analysis can be used to integrate a network of available evidence to allow for both direct and indirect comparisons between treatments for a specific outcome (9,10). For comparative effects of different SGLT2is on each electrolyte, a network meta-analysis in a frequentist framework using multivariate meta-analysis and meta-regression was constructed so that the effects of different SGLT2is on the same electrolyte were compared indirectly. The network meta-analysis was performed with STATA v16.1 (StataCorp, College Station, TX) using the “mvmeta” command and programmed STATA routines (9,10). To rank the SGLT2is for a specified outcome, we estimated the relative ranking probabilities of each treatment using surface under the cumulative ranking curve (SUCRA) probabilities and mean ranks. Higher SUCRA probability and lower mean rank indicate a larger intervention (11). The heterogeneity variance (τ) estimated by a restricted maximum likelihood method was used to quantify between-study heterogeneity for each outcome (12). In addition, a comparison-adjusted funnel plot was used to assess small-study effects within a network of interventions, with symmetry around the summary effect line indicating the absence of small-study effects (13). The result revealed no small-study effects, which indicated the absence of any over- or underestimate of the effect of SGLT2is.
All analyses were conducted using STATA/SE v16.1 for Windows (StataCorp). P values are based on two-sided hypothesis tests. A P value of <0.05 was considered statistically significant.
Results
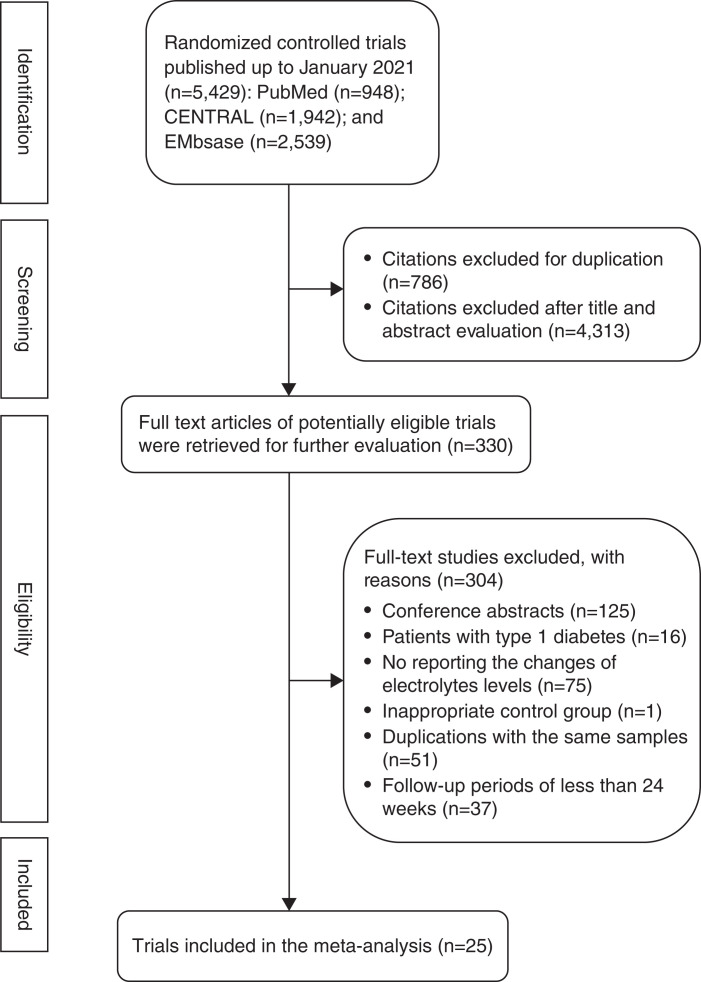
Of the 5429 articles identified from electronic databases up to January 2021, after titles and abstracts were screened and duplicate studies were removed, 330 full-text articles were reviewed for further assessment. Twenty-five trials comparing SGLT2is with placebo met inclusion criteria and were included in the meta-analysis (Figure 1), encompassing a total of 28,269 participants with T2D (Table 1).
Figure 1.
Flow chart of the identification of eligible trials.
Table 1.
Characteristics of included studies
| First Author (Year) | NCT | SGLT2i | Control | Sample Size (n) | Background Therapy | Mean Age (Years) | Race (Primary) | Mean HbA1c (%) | Mean BMI (kg/m2) | Mean eGFR (ml/min per 1.73 m2) | Follow-up (Weeks) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yale (2014) (45) | NCT01064414 | CANA | PLA | 269 | SU or INS | 68.5 | White | 8 | 33 | 39.4 | 52 |
| Wilding (2013) (46) | NCT01106625 | CANA | PLA | 469 | MET+SU | 56.8 | White | 8.1 | 33.1 | NR | 52 |
| Bode (2015) (47) | NCT01106651 | CANA | PLA | 714 | OAD | 63.6 | White | 7.7 | 31.6 | 77.5 | 104 |
| Forst (2014) (48) | NCT01106690 | CANA | PLA | 342 | MET+PIOG | 57.4 | White | 7.9 | 32.5 | 86.4 | 26 |
| Zhou (2019) (49) | NCT01032629, NCT01989754 | CANA | PLA | 10142 | Standard care | 63.3 | White | 8.3 | 32 | 76.5 | 130 |
| Ferrannini (2010) (50) | NCT00528372 | DAPA | PLA | 274 | Naïve treatment | 52.2 | White | 7.9 | 32.6 | NR | 24 |
| Wilding (2012) (51) | NCT00673231 | DAPA | PLA | 800 | INS±OAD | 59.3 | White | 8.5 | 33.1 | NR | 48 |
| Bolinder (2014) (52) | NCT00855166 | DAPA | PLA | 182 | MET | 60.7 | White | 7.2 | 31.9 | 84.3 | 102 |
| Bailey (2015) (53) | NCT00528372 | DAPA | PLA | 274 | MET | 56.5 | White | 8 | NR | 85.9 | 102 |
| Roden (2013) (54) | NCT01177813 | EMPA | PLA AND SIT | 899 | Naïve treatment | 55 | Asian | 7.9 | 28.4 | 87.4 | 76 |
| Barnett (2014) (55) | NCT01164501 | EMPA | PLA | 741 | OAD | 63.9 | White | 8 | 30.7 | 53.2 | 52 |
| Häring (2014) (56) | NCT01159600 | EMPA | PLA | 638 | MET | 55.7 | White | 7.9 | 29.2 | 89 | 24 |
| Haering (2015) (57) | NCT01159600 | EMPA | PLA | 666 | MET+SU | 57.1 | Asian | 8.1 | 28.2 | 87.2 | 76 |
| Kovacs (2014) (58) | NCT01210001 | EMPA | PLA | 498 | PIOG±MET | 54.5 | Asian | 8.1 | 29.2 | 85.7 | 24 |
| Rosenstock (2014) (59) | NCT01306214 | EMPA | PLA | 563 | INS | 56.7 | White | 8.3 | 34.8 | 84 | 52 |
| Lewin (2015) (60) | NCT01422876 | EMPA±LINA | LINA | 667 | MET | 54.6 | White | 8 | 31.6 | 88.9 | 52 |
| Rosenstock (2015) (61) | NCT01011868 | EMPA | PLA | 494 | INS±OAD | 58.8 | White | 8.2 | 32.2 | 84 | 78 |
| Søfteland (2017) (62) | NCT01734785 | EMPA | PLA | 332 | MET+LINA | 55.2 | White | 7.97 | 30.2 | 92.3 | 24 |
| Aronson (2018) (63) | NCT01958671 | ERTU | PLA | 461 | Naïve treatment | 56.4 | NR | 8.2 | 33 | 87.7 | 52 |
| Rosenstock (2018) (64) | NCT02033889 | ERTU | PLA | 621 | MET | 56.6 | White | 8.1 | 30.9 | 90.5 | 26 |
| Ji (2019) (65) | NCT02630706 | ERTU | PLA | 506 | MET | 56.5 | Asian | 8.1 | 26 | 99.3 | 26 |
| Lu (2016) (66) | NCT01505426 | IPRA | PLA | 170 | MET | 53 | Asian | 7.7 | 26.8 | 149.3 | 24 |
| Han (2018) (67) | NCT02452632 | IPRA | PLA | 143 | MET_SITA | 57.5 | Asian | 7.9 | 25.8 | 90 | 24 |
| Halvorsen (2019) (68) | NCT03115112 | BEXA | sitagliptin | 384 | MET | 59.4 | White | 8 | 31.7 | NR | 24 |
| Zinman (2015) (69) | NCT01131676 | EMPA | PLA | 7020 | Standard care | 63.2 | White | 8.1 | 30.6 | 74 | 160 |
NR, not reported; BMI, body mass index; EMPA, empagliflozin; ERTU, ertugliflozin; DAPA, dapagliflozin; CANA, canagliflozin; BEXA, bexagliflozin; PLA, placebo; MET, metformin; SIT, sitagliptin; SAXA, saxagliptin; LINA, linagliptin; SU, sulfonylureas; OAD, oral antidiabetic drugs; INS, insulin. PIOG, pioglitazone; IPRA, ipragliflozin.
Baseline characteristics of the included trials are shown in Table 1. All trials enrolled participants with T2DM and compared SGLT2is with their respective placebo groups. Overall, six different SGLT2is were studied; there were five trials of 11,936 (42%) participants for canagliflozin, four trials of 1530 (5%) participants for dapagliflozin, 10 trials of 12,518 (44%) participants for empagliflozin, three trials of 1588 (6%) participants for ertugliflozin, two trials of 313 (1%) participants for ipragliflozin, and one trial of 384 participants for bexagliflozin. The mean age of participants was 58 years, and the median trial duration was 58.7 weeks.
Risk-of-Bias Assessment
The overall risk of bias is presented in Supplemental Figure 1. The generation of random numbers was well performed in most trials. One open-label trial was assessed as having a high risk of performance bias for blinding of participants and personnel. Because electrolyte levels were measured in the lab (which is seldom influenced by outcome evaluators), the domain of blinding of outcome assessment was assessed as low risk. The risk of selective reporting (reporting bias) for all trials was considered low due to electrolyte outcomes reported in these trials.
Direct Head-to-Head Evidence from Pairwise Meta-Analysis
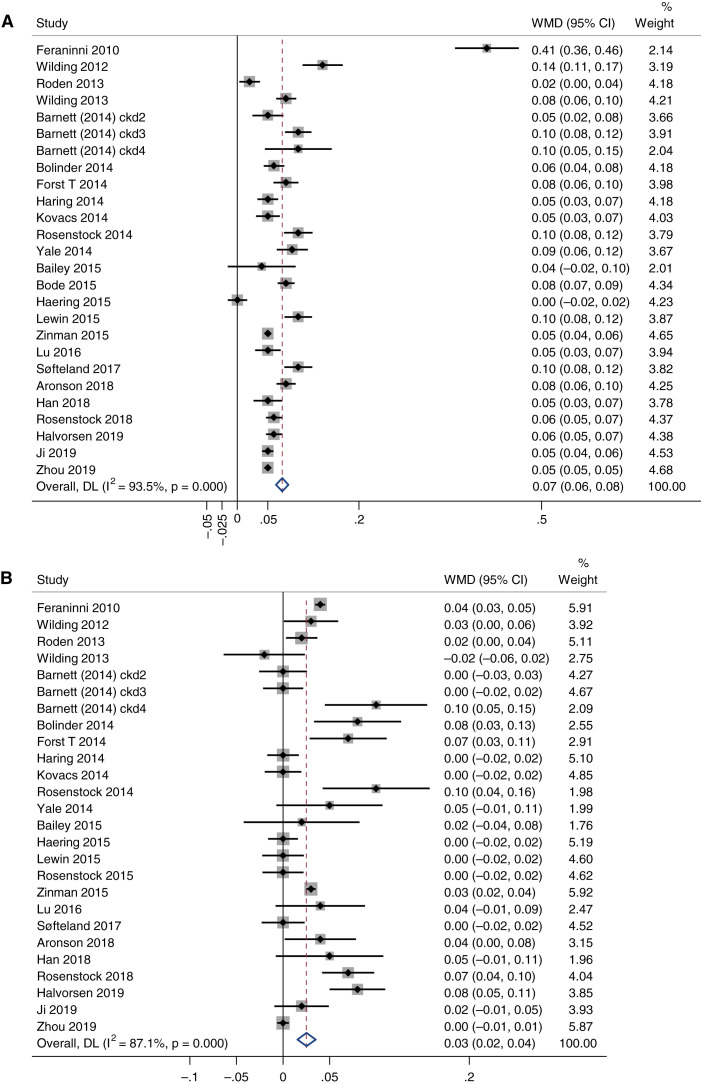
Despite significant between-trial heterogeneity (I2=94%), most trials (except two) showed significantly elevated magnesium levels among patients using SGLT2is (Figure 2A). Overall, SGLT2is were significantly associated with increases in magnesium of 0.07 mmol/L (95% confidence interval [CI], 0.06 to 0.08 mmol/L).
Figure 2.
Pairwise meta-analyses of the effects of sodium-glucose cotransporter (SGLT) 2 inhibitors (SGLT2is) on magnesium and phosphate. (A) Blood magnesium levels (mmol/L) and (B) blood phosphate levels (mmol/L). Each square indicates the WMD in each trial. The horizontal line represents the 95% CI. The pooled WMD and 95% CI is indicated by the dashed line and diamond. The black vertical line represents the null hypothesis. Heterogeneity between studies was assessed by the I2 statistics and Cochrane Q (P value). WMD, weighted mean difference; CI, confidence interval.
For serum phosphate levels, nearly half the trials (n=11) showed a significant increase in phosphate among participants using SGLT2is, while the others (n=15) showed no effect or nonsignificant increments (Figure 2B). Overall, there is a statistical trend toward phosphate levels being elevated by SGLT2is (WMD=0.03 mmol/L; 95% CI, 0.02 to 0.04 mmol/L), although heterogeneity was significant (I2=87%).
Of 19 trials with calcium data, only three showed a significant association with increased calcium, all of which used empagliflozin (Supplemental Figure 2A). Obviously, the final meta-analysis result was heavily weighted by the two trials that contributed to 67% of the weight. Overall, the SGLT2is were significantly associated with elevated levels of calcium by 0.01 mmol/L (95% CI, 0 to 0.01 mmol/L), but the result was not significant after removing these two trials.
In contrast, the pairwise meta-analysis results did not show significant effects of SGLT2is on serum levels of potassium (WMD=0; 95% CI, –0.02 to 0.02) or sodium (WMD=0.16; 95% CI, –0.02 to 0.35; Supplemental Figure 2, B and C). Compared with serum magnesium, phosphate, and calcium, the trials of serum sodium and potassium showed more heterogenous results in terms of the association direction and magnitude and statistical significance.
Comparative Evidence from Network Meta-Analysis
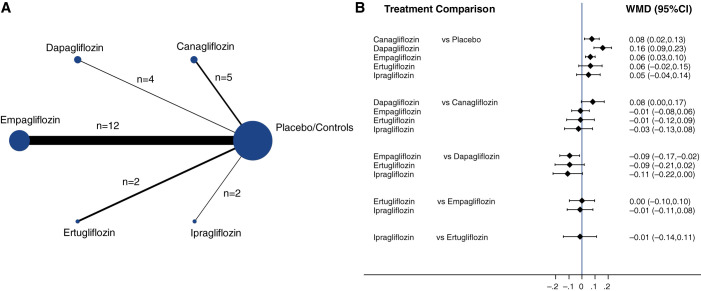
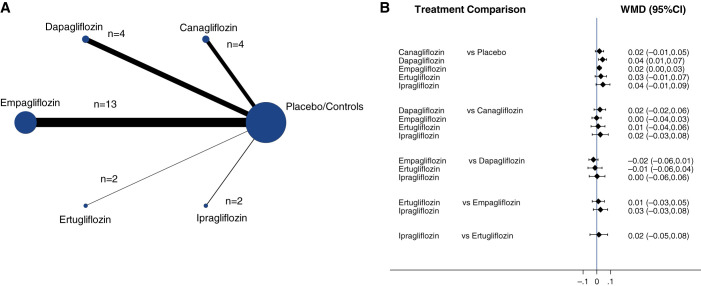
Figure 3A shows the network diagrams of direct comparison of specific classes of SGLT2is for magnesium levels reflected by the solid lines, the number of studies by the size of the nodes, and the number of patients by the thickness of the lines. In total, there were 25 direct comparisons between five different SGLT2is and their respective placebo/control groups. Empagliflozin compared with placebo/control had the highest number of trials (n=12), with the largest contribution in the estimation to the entire network for response in magnesium levels.
Figure 3.
Network meta-analysis results of the effect of SGLT2is on blood magnesium levels. (A) Network of eligible comparisons for the multiple-SGLT2is meta-analysis for effects on blood magnesium levels. Each node represents one treatment. The directly compared treatments are linked with a solid line; the width of the lines is proportional to the number of randomized participants (sample size), and the size of every node is proportional to the number of trials comparing every pair of treatments. (B) Network meta-analysis combining direct and indirect evidence within a network of eligible trials for the effects of SGLT2is on blood magnesium levels (mmol/L). The black solid lines represent the confidence intervals for WMD of blood magnesium levels for each comparison, and the blue line is the line of no effect (WMD=0).
We found a significant increase in serum magnesium among those taking canagliflozin (WMD=0.08; 95% CI, 0.02 to 0.13), dapagliflozin (WMD=0.16; 95% CI, 0.09 to 0.23), and empagliflozin (WMD=0.06; 95% CI, 0.03 to 0.1) compared with those taking placebo (Figure 3B). There were similar increasing trends among those taking either ertugliflozin or ipragliflozin, although statistical significance was not reached due to low power. Overall, there was no significant difference between these SGLT2is, although the use of dapagliflozin appeared to have a larger effect than canagliflozin.
Similarly, there is available information on 25 pairwise comparisons of five SGLT2is for serum phosphate levels (Figure 4A). The comparison with the highest number of included studies was empagliflozin (n=13). Some SGLT2is were significantly associated with elevations in serum phosphate (Figure 4B). Compared with placebo, dapagliflozin (WMD=0.04; 95% CI, 0.01 to 0.07) and empagliflozin (WMD=0.02; 95% CI, 0 to 0.03) were significantly associated with a trend toward increases in serum phosphate levels. There were similar small increases in phosphate among those taking canagliflozin (WMD=0.02; 95% CI, –0.01 to 0.05), ertugliflozin (WMD=0.03; 95% CI, –0.01 to 0.07), and ipragliflozin (WMD=0.04; 95% CI, –0.01 to 0.09) but without statistical significance. There was no significant difference between any SGLT2is.
Figure 4.
Network meta-analysis results of the effect of SGLT2is on blood phosphate levels. (A) Network of eligible comparisons for the multiple-SGLT2is meta-analysis for effects on blood phosphate levels. Each node represents one treatment. The directly compared treatments are linked with a solid line; the width of the lines is proportional to the number of randomized participants (sample size), and the size of every node is proportional to the number of trials comparing every pair of treatments. (B) Network meta-analysis combining direct and indirect evidence within a network of eligible trials for the effects of SGLT2is on blood phosphate levels (mmol/L). The black solid lines represent the CIs for WMD of blood magnesium levels for each comparison, and the blue line is the line of no effect (WMD=0).
Levels of serum sodium were significantly higher among patients taking empagliflozin compared with those taking placebo (WMD=0.28; 95% CI, 0.06 to 0.49; Supplemental Figure 3). There was a modest increase in sodium among patients with ertugliflozin, and decreases with both canagliflozin and dapaliflozin, but without statistical significance. The overall effect of SGLT2is on serum sodium levels was not statistically significant. Again, there was no significant difference between any SGLT2is.
There were no significant differences in serum potassium levels; small increases in serum calcium levels were not clinically meaningful (Supplemental Figures 4 and 5).
Discussion
In this large meta-analysis of 25 RCTs involving 28,269 patients with T2D and six different SGLT2is, we found that SGLT2is significantly increased serum magnesium and phosphate levels, consistent with a class effect of SGLT2 inhibition. In contrast, there was no statistical evidence of differences in serum levels of other electrolytes produced by SGLT2is or specific SGLT2 inhibitor drugs.
Our results from both network and pairwise meta-analysis showed consistent evidence that SGLT2is significantly increase serum magnesium, further supporting our previous meta-analysis of available results from earlier trials (8). Collectively, previous trials documented the effect of SGLT2is on increasing serum magnesium. One post hoc analysis of 10 clinical trials showed that dapagliflozin corrected low magnesium in patients with T2D (14). Another report showed that three different SGLT2is relieved refractory hypomagnesemia in three patients, likely by blocking urinary magnesium wasting (15). SLGT2is also appeared to correct hypomagnesemia in patients with kidney transplant on tacrolimus (16). This treatment might also provide a benefit to kidney transplant recipients who experience chronic hypomagnesemia and improve renal- and cardiovascular-related outcomes. In addition, correcting hypomagnesemia may help glycemic control in diabetes but not vice versa (17).
The mechanism underlying the renal benefit of SGLT2is is likely to be independent of glucose levels and may possibly stem from a reduction in intraglomerular pressure (18) and other possible mechanisms presently being studied (19). SGLT2is can normalize proximal reabsorption via tubular glomerular feedback, which should have particular effects on glomerular hemodynamics, eliminate diabetic hyperfiltration, and improve hard renal end points (20,21).
Regulation of magnesium transport in the kidney occurs primarily in the thick ascending limb and distal convoluted tubules (22). In the thick ascending limb, both magnesium and calcium can activate the calcium-sensing receptor on the basolateral membrane and modulate paracellular magnesium transport (23). Other factors control magnesium transport through changes in the voltage and/or permeability of the paracellular pathway. SGLT inhibitors increased delivery of Na to the loop of Henle, which may increase magnesium absorption (24).
Serum magnesium levels are usually relatively stable, within a narrow range of 0.7–1 mmol/L in healthy adults. It is possible that even very small changes in serum magnesium levels are associated with an increased risk of renal and cardiovascular outcomes. In a meta-analysis of 48 randomized trials using oral magnesium supplements, an elevation in circulating magnesium of 0.05 mmol/L was observed in response to a wide range of doses of oral magnesium supplementation (25). Our study showed that SGLT2is produced 0.07 mmol/L increases in magnesium levels, which seems a minimal increment but is actually clinically meaningful. In 2016, an analysis of the prospective, population-based Rotterdam Study showed an inverse association between serum magnesium levels and mortality; a 0.1 mmol/L (0.24 mg/dl) increment of this ion was associated with 18% reduction in risk of coronary heart disease after 8.7 years follow-up (hazard ratio=0.82; 95% CI, 0.7 to 0.96) (26). The clinical significance of minimal change was also evident in a prospective study of 3525 British participants, which showed an inverse association between serum magnesium level and risk of incident heart failure. Lower magnesium levels were associated with impaired glycemic control, hypertension, and vascular calcification (27). Vice versa, higher magnesium levels can be beneficial for cardiovascular health, such as improvement of cellular respiration and cardiac output, and reduction in myocardial fibrosis (28).
We showed in a previous meta-analysis that blood phosphate was elevated in patients taking dapafliglozin (8). The current report, which includes more trial data and incorporates both direct and indirect evidence, shows similar results of SGLT2is on phosphate. The mechanism of phosphate elevation by SGLT2is is likely via the stimulation of renal proximal tubular reabsorption of phosphate through type 2 sodium-phosphate cotransporters (29,30). A small pharmacodynamic study showed that canagliflozin induced a prompt increase in serum phosphate, which triggered downstream changes in FGF23, 1,25-dihydroxyvitamin D, and parathyroid hormone in healthy volunteers (30). Decreases in 1,25-dihydroxyvitamin D levels with or without elevated parathyroid hormone were used to explain the possible risk of bone fracture associated with SGLT2is, but longer-term observation and additional data showed no evidence of increased risk of fracture (31,32). For individuals at high risk of fracture, phosphorus, calcium, and 25-dihydroxvitamin D levels should be monitored regularly, and a DEXA bone-density scan should also be performed regularly. Increased FGF23 levels may be an independent risk factor for cardiovascular outcomes (33), but most SGLT2is trials with potentially elevated FGF23 have shown improvements in cardiovascular outcomes. The discrepancy is likely due to the brevity of the pharmacodynamic study; FGF23 levels did not seem elevated after 24 weeks, even though phosphate was slightly higher. There is evidence of elevated cardiovascular mortality risk in patients with hyperphosphatemia (34–36). However, the balance between magnesium and phosphate may be more important for cardiovascular health and deserves further investigation (37).
SGLT2is rarely cause hypercalcemia. Our finding of increased calcium levels is mainly driven by two studies. A case report of a patient having hypercalcemia and diagnosed with primary hyperparathyroidism after dapagliflozin treatment is alarming (38). However, since primary hyperparathyroidism is a common condition, it remains unclear whether it could be caused by SGLT2is.
Our study showed no significant effect of SGLT2is on blood sodium or potassium levels, and the effects were similar across SGLT2is. These findings are consistent with previous reports on SGLT2is on blood sodium and potassium (39–42). Normally, kidneys reabsorb >99% of filtered sodium, with 4%–5% of filtered sodium acting as cotransporter of glucose through SGLT2. SGLT2 expression is increased in diabetes, contributing to a higher rate of glucose and sodium reabsorption. Inhibition of SGLT2 decreases reabsorption of sodium and glucose and leads to natriuresis and glycosuria. A randomized trial showed that empagliflozin led to a larger increase in blood sodium compared with placebo in treatment of hyponatremia due to the syndrome of inappropriate antidiuresis (43,44). However, the effect of empagliflozin on blood sodium levels was largely dependent on the severity of baseline hyponatremia, and the main difference between empagliflozin and placebo was severe hyponatremia, with blood sodium level <125 mmol/L. There were no clinically meaningful effects of SGLT2is on blood potassium level observed in prior trials of canagliflozin (42), dapagliflozin (40), or empagliflozin (44). Patients with diabetes were more likely to have hyperkalemia, especially when they also had reduced kidney function. Dapagliflozin was approved for patients with CKD without diabetes to slow down the progression of kidney disease on the basis of the lack of evidence of significant hyperkalemia in the SGLT2is arm of that trial (5).
Although our meta-analysis included a large number of available SGLT2is trials with electrolyte data, some limitations deserve consideration. First, many patients with T2D may also have hypertension. It is possible that they were more likely to be taking ACEI/ARB and diuretics, which could affect serum electrolytes. Due to randomized trial design, the percentage of such patients would likely be similar between treatment and control groups. Thus, inclusion of hypertensive patients would not invalidate the comparison but might have attenuated the precision of genuine effect estimates. Our analysis does not stratify the data on those medications. Second, although the effect of SGLT2is on magnesium may vary depending on different baseline magnesium status, most of the clinical trials included in the study do not have clear information on magnesium insufficiency/deficiency. Finally, because most of the trials did not recruit patients with impaired kidney function, the results are not to be generalized to patients with CKD.
In conclusion, our meta-analysis of randomized trial data showed that SGLT2is significantly increased serum magnesium and phosphate levels, representing a class effect of SGLT2 inhibition. These results call for long-term examination of efficacy and safety in patients with T2D with different clinical phenotypes in RCTs and real-world settings.
Disclosures
All authors have nothing to disclose.
Funding
None
Author Contributions
Y. Huan, M. Leibensperger, B. Seo, and J. Zhang curated the data; Y. Huan, M. Leibensperger, B. Seo, Y. Song, and J. Zhang were responsible for validation; Y. Huan, M. Leibensperger, B. Seo, and J. Zhang wrote the original draft of the manuscript; B. Seo was responsible for funding acquisition; B. Seo and Y. Song were responsible for methodology; Y. Song and J. Zhang conceptualized the study; and Y. Song was responsible for the investigation, software, and supervision, and reviewed and edited the manuscript.
Supplemental Material
This article contains supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0006672021/-/DCSupplemental.
Risk-of-bias assessments of individual trials. Download Supplemental Figure 1, PDF file, 226 KB (226KB, pdf)
(A) Pairwise meta-analysis results of SGLT2 inhibitors on blood calcium levels. (B) Pairwise meta-analysis results of SGLT2 inhibitors on blood potassium levels. (C) Pairwise meta-analysis results of SGLT2 inhibitors on blood sodium levels. Download Supplemental Figure 2, PDF file, 226 KB (226KB, pdf)
(A) Network of eligible comparisons for the multiple-SGLT2 inhibitors meta-analysis for effects on blood sodium levels. (B) Network meta-analysis of eligible trials for the effects of SGLT2 inhibitors on blood sodium levels (mmol/L). Download Supplemental Figure 3, PDF file, 226 KB (226KB, pdf)
(A) Network of eligible comparisons for the multiple-SGLT2 inhibitors meta-analysis for effects on blood potassium levels. (B) Network meta-analysis of eligible trials for the effects of SGLT2 inhibitors on blood potassium levels (mmol/L). Download Supplemental Figure 4, PDF file, 226 KB (226KB, pdf)
(A) Network of eligible comparisons for the multiple-SGLT2 inhibitors meta-analysis for effects on blood calcium levels. (B) Network meta-analysis of eligible trials for the effects of SGLT2 inhibitors on blood calcium levels (mmol/L). Download Supplemental Figure 5, PDF file, 226 KB (226KB, pdf)
References
- 1.Ferrannini E, Solini A: SGLT2 inhibition in diabetes mellitus: Rationale and clinical prospects. Nat Rev Endocrinol 8: 495–502, 2012. 10.1038/nrendo.2011.243 [DOI] [PubMed] [Google Scholar]
- 2.Zinman B, Lachin JM, Inzucchi SE: Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 374: 1092–1094, 2016. 10.1056/NEJMc1600827 [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators : Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347–357, 2019. 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 4.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators : Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 5.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators : Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Williams S, Ho S, Loraine H, Hagan D, Whaley JM, Feder JN: Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther 1: 57–92, 2010. 10.1007/s13300-010-0006-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Franco A, Cantini G, Tani A, Coppini R, Zecchi-Orlandini S, Raimondi L, Luconi M, Mannucci E: Sodium-dependent glucose transporters (SGLT) in human ischemic heart: A new potential pharmacological target. Int J Cardiol 243: 86–90, 2017. 10.1016/j.ijcard.2017.05.032 [DOI] [PubMed] [Google Scholar]
- 8.Tang H, Zhang X, Zhang J, Li Y, Del Gobbo LC, Zhai S, Song Y: Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: A meta-analysis of randomised controlled trials. Diabetologia 59: 2546–2551, 2016. 10.1007/s00125-016-4101-6 [DOI] [PubMed] [Google Scholar]
- 9.White IR, Barrett JK, Jackson D, Higgins JP: Consistency and inconsistency in network meta-analysis: Model estimation using multivariate meta-regression. Res Synth Methods 3: 111–125, 2012. 10.1002/jrsm.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G: Graphical tools for network meta-analysis in STATA. PLoS One 8: e76654, 2013. 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salanti G: Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: Many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods 3: 80–97, 2012. 10.1002/jrsm.1037 [DOI] [PubMed] [Google Scholar]
- 12.Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP: Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 41: 818–827, 2012. 10.1093/ije/dys041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaimani A, Salanti G: Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods 3: 161–176, 2012. 10.1002/jrsm.57 [DOI] [PubMed] [Google Scholar]
- 14.Toto RD, Goldenberg R, Chertow GM, Cain V, Stefánsson BV, Sjöström CD, Sartipy P: Correction of hypomagnesemia by dapagliflozin in patients with type 2 diabetes: A post hoc analysis of 10 randomized, placebo-controlled trials. J Diabetes Complications 33: 107402, 2019. 10.1016/j.jdiacomp.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 15.Ray EC, Boyd-Shiwarski CR, Liu P, Novacic D, Cassiman D: SGLT2 inhibitors for treatment of refractory hypomagnesemia: A case report of 3 patients. Kidney Med 2: 359–364, 2020. 10.1016/j.xkme.2020.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song CC, Brown A, Winstead R, Yakubu I, Demehin M, Kumar D, Gupta G: Early initiation of sodium-glucose linked transporter inhibitors (SGLT-2i) and associated metabolic and electrolyte outcomes in diabetic kidney transplant recipients. Endocrinol Diab Metab 4: e00185, 2020, 10.1002/edm2.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Bommel EJM, Geurts F, Muskiet MHA, Post A, Bakker SJL, Danser AHJ, Touw DJ, van Berkel M, Kramer MHH, Nieuwdorp M, Ferrannini E, Joles JA, Hoorn EJ, van Raalte DH: SGLT2 inhibition versus sulfonylurea treatment effects on electrolyte and acid-base balance: Secondary analysis of a clinical trial reaching glycemic equipoise: Tubular effects of SGLT2 inhibition in Type 2 diabetes. Clin Sci (Lond) 134: 3107–3118, 2020. 10.1042/CS20201274 [DOI] [PubMed] [Google Scholar]
- 18.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M: Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014. 10.1161/CIRCULATIONAHA.113.005081 [DOI] [PubMed] [Google Scholar]
- 19.Vallon V, Thomson SC: The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol 16: 317–336, 2020. 10.1038/s41581-020-0256-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson SC, Vallon V: Renal effects of sodium-glucose co-transporter inhibitors. Am J Cardiol 124: S28–S35, 2019. 10.1016/j.amjcard.2019.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H: Glomerular hyperfiltration in experimental diabetes mellitus: Potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576, 1999. 10.1681/ASN.V10122569 [DOI] [PubMed] [Google Scholar]
- 22.Curry JN, Yu ASL: Magnesium handling in the kidney. Adv Chronic Kidney Dis 25: 236–243, 2018. 10.1053/j.ackd.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J: Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 31: 1999–2012, 2012. 10.1038/emboj.2012.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP: Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285: 103–106, 1999. 10.1126/science.285.5424.103 [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Del Gobbo LC, Hruby A, Rosanoff A, He K, Dai Q, Costello RB, Zhang W, Song Y: The circulating concentration and 24-h urine excretion of magnesium dose- and time-dependently respond to oral magnesium supplementation in a meta-analysis of randomized controlled trials. J Nutr 146: 595–602, 2016. 10.3945/jn.115.223453 [DOI] [PubMed] [Google Scholar]
- 26.Kieboom BC, Niemeijer MN, Leening MJ, van den Berg ME, Franco OH, Deckers JW, Hofman A, Zietse R, Stricker BH, Hoorn EJ: Serum magnesium and the risk of death from coronary heart disease and sudden cardiac death. J Am Heart Assoc 5: e002707, 2016. 10.1161/JAHA.115.002707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yee J: Magnesium: An important orphan. Adv Chronic Kidney Dis 25: 217–221, 2018. 10.1053/j.ackd.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 28.Ray EC: Evolving understanding of cardiovascular protection by SGLT2 inhibitors: Focus on renal protection, myocardial effects, uric acid, and magnesium balance. Curr Opin Pharmacol 54: 11–17, 2020. 10.1016/j.coph.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor SI, Blau JE, Rother KI: Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol 3: 8–10, 2015. 10.1016/S2213-8587(14)70227-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blau JE, Bauman V, Conway EM, Piaggi P, Walter MF, Wright EC, Bernstein S, Courville AB, Collins MT, Rother KI, Taylor SI: Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight 3: e99123, 2018. 10.1172/jci.insight.99123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang HL, Li DD, Zhang JJ, Hsu YH, Wang TS, Zhai SD, Song YQ: Lack of evidence for a harmful effect of sodium-glucose co-transporter 2 (SGLT2) inhibitors on fracture risk among type 2 diabetes patients: A network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab 18: 1199–1206, 2016. 10.1111/dom.12742 [DOI] [PubMed] [Google Scholar]
- 32.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group : Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 33.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011. 10.1001/jama.2011.826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Louvet L, Büchel J, Steppan S, Passlick-Deetjen J, Massy ZA: Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant 28: 869–878, 2013. 10.1093/ndt/gfs520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz-Tocados JM, Peralta-Ramirez A, Rodríguez-Ortiz ME, Raya AI, Lopez I, Pineda C, Herencia C, Montes de Oca A, Vergara N, Steppan S, Pendon-Ruiz de Mier MV, Buendía P, Carmona A, Carracedo J, Alcalá-Díaz JF, Frazao J, Martínez-Moreno JM, Canalejo A, Felsenfeld A, Rodriguez M, Aguilera-Tejero E, Almadén Y, Muñoz-Castañeda JR: Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int 92: 1084–1099, 2017. 10.1016/j.kint.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Iseki K, Tsubakihara Y, Isaka Y; Committee of Renal Data Registry of the Japanese Society for Dialysis Therapy : Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: A cohort study. PLoS One 9: e116273, 2014. 10.1371/journal.pone.0116273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakaguchi Y, Hamano T, Isaka Y: Magnesium and progression of chronic kidney disease: Benefits beyond cardiovascular protection? Adv Chronic Kidney Dis 25: 274–280, 2018. 10.1053/j.ackd.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 38.Akhanlı P, Hepsen S, Ucan B, Saylam G, Cakal E: Hypercalcemic patient diagnosed with primary hyperparathyroidism after dapagliflozin treatment. AACE Clin Case Rep 6: e319–e321, 2020. 10.4158/ACCR-2020-0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cianciolo G, De Pascalis A, Capelli I, Gasperoni L, Di Lullo L, Bellasi A, La Manna G: Mineral and electrolyte disorders with SGLT2i therapy. JBMR Plus 3: e10242, 2019. 10.1002/jbm4.10242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yavin Y, Mansfield TA, Ptaszynska A, Johnsson K, Parikh S, Johnsson E: Effect of the SGLT2 inhibitor dapagliflozin on potassium levels in patients with type 2 diabetes mellitus: A pooled analysis. Diabetes Ther 7: 125–137, 2016. 10.1007/s13300-015-0150-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC: Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: The RECEDE-CHF trial. Circulation 142: 1713–1724, 2020. 10.1161/CIRCULATIONAHA.120.048739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weir MR, Slee A, Sun T, Balis D, Oh R, de Zeeuw D, Perkovic V: Effects of canagliflozin on serum potassium in the CANagliflozin cardioVascular Assessment Study (CANVAS) program. Clin Kidney J 14: 1396–1402, 2020. 10.1093/ckj/sfaa133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Refardt J, Imber C, Sailer CO, Jeanloz N, Potasso L, Kutz A, Widmer A, Urwyler SA, Ebrahimi F, Vogt DR, Winzeler B, Christ-Crain M: A randomized trial of empagliflozin to increase plasma sodium levels in patients with the syndrome of inappropriate antidiuresis. J Am Soc Nephrol 31: 615–624, 2020. 10.1681/ASN.2019090944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators : Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 45.Yale JF, Bakris G, Cariou B, Nieto J, David-Neto E, Yue D, Wajs E, Figueroa K, Jiang J, Law G, Usiskin K, Meininger G; DIA3004 Study Group : Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 16: 1016–1027, 2014. 10.1111/dom.12348 [DOI] [PubMed] [Google Scholar]
- 46.Wilding JP, Charpentier G, Hollander P, González-Gálvez G, Mathieu C, Vercruysse F, Usiskin K, Law G, Black S, Canovatchel W, Meininger G: Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: A randomised trial. Int J Clin Pract 67: 1267–1282, 2013. 10.1111/ijcp.12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bode B, Stenlöf K, Harris S, Sullivan D, Fung A, Usiskin K, Meininger G: Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55–80 years with type 2 diabetes. Diabetes Obes Metab 17: 294–303, 2015. 10.1111/dom.12428 [DOI] [PubMed] [Google Scholar]
- 48.Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, Stein P: Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab 16: 467–477, 2014. 10.1111/dom.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Z, Jardine M, Perkovic V, Matthews DR, Mahaffey KW, de Zeeuw D, Fulcher G, Desai M, Oh R, Simpson R, Watts NB, Neal B: Canagliflozin and fracture risk in individuals with type 2 diabetes: Results from the CANVAS Program. Diabetologia 62: 1854–1867, 2019. 10.1007/s00125-019-4955-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF: Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: A randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 33: 2217–2224, 2010. 10.2337/dc10-0612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S; Dapagliflozin 006 Study Group : Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: A randomized trial. Ann Intern Med 156: 405–415, 2012. 10.7326/0003-4819-156-6-201203200-00003 [DOI] [PubMed] [Google Scholar]
- 52.Bolinder J, Ljunggren Ö, Johansson L, Wilding J, Langkilde AM, Sjöström CD, Sugg J, Parikh S: Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 16: 159–169, 2014. 10.1111/dom.12189 [DOI] [PubMed] [Google Scholar]
- 53.Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF: Efficacy and safety of dapagliflozin monotherapy in people with type 2 diabetes: A randomized double-blind placebo-controlled 102-week trial. Diabet Med 32: 531–541, 2015. 10.1111/dme.12624 [DOI] [PubMed] [Google Scholar]
- 54.Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, Broedl UC; EMPA-REG MONO trial investigators : Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 1: 208–219, 2013. 10.1016/S2213-8587(13)70084-6 [DOI] [PubMed] [Google Scholar]
- 55.Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC; EMPA-REG RENAL trial investigators : Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2: 369–384, 2014. 10.1016/S2213-8587(13)70208-0 [DOI] [PubMed] [Google Scholar]
- 56.Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Broedl UC, Woerle HJ; EMPA-REG MET Trial Investigators : Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 37: 1650–1659, 2014. 10.2337/dc13-2105 [DOI] [PubMed] [Google Scholar]
- 57.Haering HU, Merker L, Christiansen AV, Roux F, Salsali A, Kim G, Meinicke T, Woerle HJ, Broedl UC; EMPA-REG EXTEND™ METSU Investigators : Empagliflozin as add-on to metformin plus sulphonylurea in patients with type 2 diabetes. Diabetes Res Clin Pract 110: 82–90, 2015. 10.1016/j.diabres.2015.05.044 [DOI] [PubMed] [Google Scholar]
- 58.Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, Broedl UC; EMPA-REG PIO™ Trial Investigators : Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: A 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab 16: 147–158, 2014. 10.1111/dom.12188 [DOI] [PubMed] [Google Scholar]
- 59.Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, Broedl UC; EMPA-REG MDI Trial Investigators : Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care 37: 1815–1823, 2014. 10.2337/dc13-3055 [DOI] [PubMed] [Google Scholar]
- 60.Lewin A, DeFronzo RA, Patel S, Liu D, Kaste R, Woerle HJ, Broedl UC: Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes [published correction appears in Diabetes Care 38: 394–402, 2015 10.2337/dc15-er06a]. Diabetes Care 38: 394–402, 2015. 10.2337/dc14-2365 [DOI] [PubMed] [Google Scholar]
- 61.Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ; EMPA-REG BASALTM Trial Investigators : Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: A 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 17: 936–948, 2015. 10.1111/dom.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Søfteland E, Meier JJ, Vangen B, Toorawa R, Maldonado-Lutomirsky M, Broedl UC: Empagliflozin as add-on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: A 24-week randomized, double-blind, parallel-group trial. Diabetes Care 40: 201–209, 2017. 10.2337/dc16-1347 [DOI] [PubMed] [Google Scholar]
- 63.Aronson R, Frias J, Goldman A, Darekar A, Lauring B, Terra SG: Long-term efficacy and safety of ertugliflozin monotherapy in patients with inadequately controlled T2DM despite diet and exercise: VERTIS MONO extension study. Diabetes Obes Metab 20: 1453–1460, 2018. 10.1111/dom.13251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenstock J, Frias J, Páll D, Charbonnel B, Pascu R, Saur D, Darekar A, Huyck S, Shi H, Lauring B, Terra SG: Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET) [published correction appears in Diabetes Obes Metab 20: 2708, 2018 10.1111/dom.13533]. Diabetes Obes Metab 20: 520–529, 2018. 10.1111/dom.13103 [DOI] [PubMed] [Google Scholar]
- 65.Ji L, Liu Y, Miao H, Xie Y, Yang M, Wang W, Mu Y, Yan P, Pan S, Lauring B, Liu S, Huyck S, Qiu Y, Terra SG: Safety and efficacy of ertugliflozin in Asian patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: VERTIS Asia. Diabetes Obes Metab 21: 1474–1482, 2019. 10.1111/dom.13681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu CH, Min KW, Chuang LM, Kokubo S, Yoshida S, Cha BS: Efficacy, safety, and tolerability of ipragliflozin in Asian patients with type 2 diabetes mellitus and inadequate glycemic control with metformin: Results of a phase 3 randomized, placebo-controlled, double-blind, multicenter trial. J Diabetes Investig 7: 366–373, 2016. 10.1111/jdi.12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han KA, Chon S, Chung CH, Lim S, Lee KW, Baik S, Jung CH, Kim DS, Park KS, Yoon KH, Lee IK, Cha BS, Sakatani T, Park S, Lee MK: Efficacy and safety of ipragliflozin as an add-on therapy to sitagliptin and metformin in Korean patients with inadequately controlled type 2 diabetes mellitus: A randomized controlled trial. Diabetes Obes Metab 20: 2408–2415, 2018. 10.1111/dom.13394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halvorsen YD, Lock JP, Zhou W, Zhu F, Freeman MW: A 24-week, randomized, double-blind, active-controlled clinical trial comparing bexagliflozin with sitagliptin as an adjunct to metformin for the treatment of type 2 diabetes in adults. Diabetes Obes Metab 21: 2248–2256, 2019. 10.1111/dom.13801 [DOI] [PubMed] [Google Scholar]
- 69.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators : Empagliflozin, cardiovascular outcomes, and mortality in type 2 Diabetes. N Engl J Med 373: 2117-2128, 2015. 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk-of-bias assessments of individual trials. Download Supplemental Figure 1, PDF file, 226 KB (226KB, pdf)
(A) Pairwise meta-analysis results of SGLT2 inhibitors on blood calcium levels. (B) Pairwise meta-analysis results of SGLT2 inhibitors on blood potassium levels. (C) Pairwise meta-analysis results of SGLT2 inhibitors on blood sodium levels. Download Supplemental Figure 2, PDF file, 226 KB (226KB, pdf)
(A) Network of eligible comparisons for the multiple-SGLT2 inhibitors meta-analysis for effects on blood sodium levels. (B) Network meta-analysis of eligible trials for the effects of SGLT2 inhibitors on blood sodium levels (mmol/L). Download Supplemental Figure 3, PDF file, 226 KB (226KB, pdf)
(A) Network of eligible comparisons for the multiple-SGLT2 inhibitors meta-analysis for effects on blood potassium levels. (B) Network meta-analysis of eligible trials for the effects of SGLT2 inhibitors on blood potassium levels (mmol/L). Download Supplemental Figure 4, PDF file, 226 KB (226KB, pdf)
(A) Network of eligible comparisons for the multiple-SGLT2 inhibitors meta-analysis for effects on blood calcium levels. (B) Network meta-analysis of eligible trials for the effects of SGLT2 inhibitors on blood calcium levels (mmol/L). Download Supplemental Figure 5, PDF file, 226 KB (226KB, pdf)