Abstract
Durable and temporary mechanical circulatory support (MCS) use is growing for a range of cardiovascular indications. Kidney dysfunction is common in people evaluated for or receiving durable or temporary MCS and portends worse outcomes. This kidney dysfunction can be due to preexisting kidney chronic kidney disease (CKD), acute kidney injury (AKI) related to acute cardiovascular disease necessitating MCS, AKI due to cardiac procedures, and acute and chronic MCS effects and complications. Durable MCS, with implantable continuous flow pumps, is used for long-term support in advanced heart failure refractory to guideline-directed medical and device therapy, either permanently or as a bridge to heart transplantation. Temporary MCS—encompassing in this review intra-aortic balloon pumps (IABP), axial flow pumps, centrifugal flow pumps, and venoarterial ECMO—is used for diverse situations: high-risk percutaneous coronary interventions (PCI), acute decompensated heart failure, cardiogenic shock, and resuscitation after cardiac arrest. The wide adoption of MCS makes it imperative to improve understanding of the effects of MCS on kidney health/function and of kidney health/function on MCS outcomes. The complex structure and functions of the kidney, and the complex health states of individuals receiving MCS, makes investigations in this area challenging, and current knowledge is limited. Fortunately, the increasing nephrology toolbox of noninvasive kidney health/function assessments may enable development and testing of individualized management strategies and therapeutics in the future. We review technology, epidemiology, pathophysiology, clinical considerations, and future directions in MCS and nephrology.
Keywords: clinical nephrology, dialysis, durable, kidney, LVAD, mechanical circulatory support, nephrology, temporary
Introduction
Mechanical circulatory support (MCS) encompasses devices and strategies to provide or support circulation over time spans ranging from the duration of a cardiac catheterization to years. Durable MCS is used for long-term support for people with advanced heart failure (HF) refractory to guideline-directed medical and device therapy. Temporary MCS is used to provide hemodynamic support in several clinical situations: prophylactic hemodynamic support during high-risk percutaneous coronary interventions (PCI), acute decompensated HF, cardiogenic shock, and resuscitation after cardiac arrest.
Implantable left ventricular (LV) assist devices (LVADs) are the most commonly used durable MCS device type by far and will be the main focus of the durable MCS portion of this review (Table 1) (1). Currently used devices are small, long-lasting continuous flow devices, and in recent years centrifugal (third generation) pumps have begun to supplant axial flow (second-generation) pumps (1,2). Centrifugal flow pumps were designed to be smaller, more durable, and less thrombogenic and hemolytic than axial flow pumps (3). Although LV support is the most common durable MCS use in advanced HF, a smaller number of individuals—those with severe biventricular failure or predominant right ventricular (RV) disease with substantial LV failure—are treated with biventricular support (1). Durable biventricular support options include a total artificial heart and biventricular implantation of ventricular support devices (4). Finally, isolated durable RV support (RVAD) is used in a small number of individuals with isolated RV dysfunction but preserved LV function (5).
Table 1.
Mechanical circulatory support uses, device types, and considerations
| Durable Mechanical Circulatory Support | ||
|---|---|---|
| Use Categories | Uses | Considerations |
| Left ventricular support |
|
|
| Biventricular support |
|
|
| Biventricular replacement device |
|
|
| Right ventricular support |
|
|
| Device types | Considerations | |
| Axial flow devices |
|
|
| Centrifugal flow devices |
|
|
| Pneumatic displacement pump devices |
|
|
| Temporary Mechanical Circulatory Support | ||
| Device types | Uses | Considerations |
| Intra-aortic balloon pump |
|
|
| Axial flow pumps |
|
|
| Centrifugal flow pumps |
|
|
| Venoarterial ECMO |
|
|
MCS, mechanical circulatory support; LVAD, left ventricular assist device; BiVAD, biventricular assist device; TAH, total artificial heart; RVAD, right ventricular assist device; RV, right ventricular; BiV, biventricular; PCI, percutaneous coronary intervention; ECMO, extracorporeal membrane oxygenation.
Temporary MCS is used to improve systemic and myocardial perfusion, reduce cardiac filling pressures, and reduce cardiac workload in a less invasive manner than with durable devices (Table 1) (6). Intra-aortic balloon pumps (IABP) and venoarterial extracorporeal membrane oxygenation (ECMO) were the first temporary MCS devices, and IABP remains the most commonly used (7). Newer devices include impeller-driven axial flow pumps, a left atrial-to-femoral artery bypass pump, extracorporeal centrifugal pumps, and new ECMO devices.
The high burden of cardiovascular disease in the United States, along with technological innovations, have led to growing use of durable and temporary MCS (8,9). Kidney dysfunction is common in people receiving MCS due to systemic diseases and the reciprocal relationship between kidney and cardiovascular health, and it is strongly related to adverse outcomes (10,11).
Durable MCS
LVAD utilization has transformed from rare use, primarily for bridge to heart transplantation, to more common use, primarily for destination therapy, with 3198 LVAD implantations in 2019 per the Society of Thoracic Surgeons Intermacs database (9,12). Destination therapy was the goal in 73% of LVAD implantations in 2019 compared with bridge to transplantation in 9% (9). This was a substantial change from 2010, when the goal was destination therapy in 35% of implantations and bridge to transplantation in 29% (9). This evolution of LVAD use occurred with the widespread adoption of continuous flow pumps and Food and Drug Administration approval for destination therapy use (13). We separate nephrology considerations in durable MCS into the preimplantation, early postimplantation, and prolonged MCS periods.
Preimplantation Period
Epidemiology
Kidney dysfunction at the time of LVAD implantation is an important risk factor for mortality and has been incorporated into several prognostic scores. It is challenging in LVAD studies to assess the degree and chronicity of preexisting kidney disease accurately. In part, this is due to limitations in current kidney health assessments, limited largely to endogenous filtration markers and proteinuria. Filtration markers, particularly serum creatinine concentration and derived eGFR, are influenced by muscle mass and fluid balance, resulting in inaccurate estimates in advanced HF (14,15). Chronicity of kidney dysfunction is difficult to determine in large cohort studies and may be challenging to determine even in single-center studies where patient care may be transferred to LVAD implantation centers shortly before implantation. Despite these limitations, and highlighting the importance of kidney function to prognosis, preimplantation creatinine and eGFR-based categorizations correlate with adverse outcomes after LVAD implantation (16,17). One large cohort found that 36% of LVAD recipients had an eGFR <60 ml/min per 1.73 m2, and 6% had an eGFR <30 ml/min per 1.73 m2 (16). People requiring preimplantation dialysis, particularly those on dialysis classified as having kidney failure with replacement therapy (KFRT), have especially high mortality after LVAD implantation (18–21). The other widely assessed kidney biomarker, proteinuria, also relates strongly with adverse LVAD outcomes (22,23).
The relationship of preimplantation kidney dysfunction with changes in kidney function after LVAD implantation is variable. Among LVAD recipients, lower preimplantation eGFR is associated with a greater increase in eGFR after implantation (24,25). In an analysis of a national database of LVAD recipients, those with a preimplantation eGFR <60 ml/min per 1.73 m2 were more than twice as likely to experience a 50% eGFR rise at 1 month post implantation than those with an eGFR ≥60 ml/min per 1.73 m2 (52% versus 22%, P<0.001) (25). However, it is currently unclear to what extent this finding is driven by survival bias (with high short-term mortality in those with lower kidney function), regression to the mean (preimplantation eGFR estimates are often on the basis of single measures with susceptibility to large fluctuations), and by endogenous filtration markers’ confounding by fluid and body composition changes (25).
Severe CKD can be a contraindication to LVAD use, although guidance and clinical practices vary. A recent Centers for Medicare and Medicaid Services Medicare Coverage decision listed “irreversible renal disease” as an absolute contraindication (26). In contrast, the American College of Cardiology’s Heart Failure and Transplant Member Section and Leadership Council recently stated “dialysis-dependent renal failure is no longer an absolute contraindication to LVAD insertion” (27). Some centers use LVADs in advanced CKD for bridge to combined heart/kidney transplantation (28). Individuals with advanced CKD—including KFRT—have received devices (18,19). These individuals have lower survival than those without KFRT, although many achieved >1-year survival (18).
Mechanisms and Pathophysiology
The mechanistic underpinnings of the epidemiologic data are unclear, and discussion is dependent on extrapolation from studies of low cardiac output states, kidney ischemia, non-HF disease states, and conjecture. First, diverse disease processes may be present preimplantation. Chronic kidney parenchymal disease may be driven by type 2 diabetes and atherosclerotic vascular disease in many individuals, and proteinuria or albuminuria measurements provide some information about glomerular aspects of parenchymal kidney health, in addition to reflecting endothelial health (29). Chronic ischemic damage to the kidneys may be an important factor in some patients, with chronic tubular hypoxia caused by low cardiac output leading to inflammation and tubulointerstitial fibrosis (30,31). Elevated central venous pressure (CVP) may cause interstitial fibrosis in the kidneys by causing capillary pericytes to detach from vascular walls and transition to a profibrotic myofibroblast cell type (32). Neurohormonal responses to the failing heart likely play an important role in chronic damage, with activation of the renin-angiotensin-aldosterone system and sympathetic nervous system driving intrarenal inflammation and fibrogenesis (30,33).
AKI in the preimplantation period may be driven by low cardiac output and high renal venous pressures, particularly in patients in cardiogenic shock before LVAD implantation. In addition to causing reduced GFR, these flow disturbances can cause structural ischemia-reperfusion injury to the kidney (34,35). A large portion of LVAD recipients have cardiogenic shock severe enough to be managed with temporary MCS before durable LVAD implantation, presenting additional kidney risks discussed later. A particularly important question for nephrology is to what degree kidney dysfunction and damage drives adverse outcomes in LVAD recipients versus being a marker of severity of cardiac and systemic illness.
Clinical Considerations
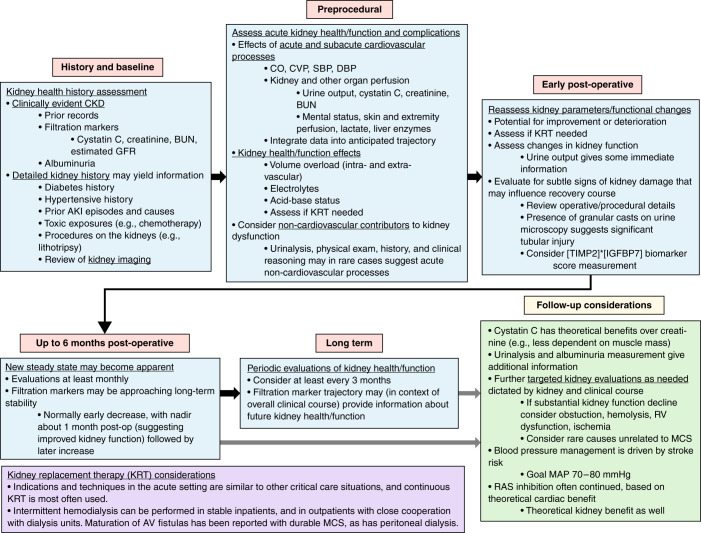
Effective methods to improve preimplantation kidney dysfunction currently lie in the years before a patient requires LVAD implantation, when therapies to improve kidney outcomes are available for many disease states. In the short-term preimplantation period, specific measures to improve kidney outcomes are not available beyond systemic hemodynamic optimization. Determination of potential reversibility of kidney dysfunction after LVAD implantation is often desired (26). However, prediction of kidney function improvement currently relies on clinical judgment. Comprehensive evaluation may provide information about the potential for improvement and susceptibility to potentially life-threatening kidney complications (Figure 1). Data about parenchymal kidney health and reserve can be obtained from a detailed history, including long-term eGFR and albuminuria trends, prior AKI episodes, review of any prior kidney procedures (e.g., lithotripsy, partial nephrectomy), urinalysis and sediment examination, and evaluation of the preimplantation period (trends in urine output and endogenous filtration markers, systemic perfusion). Direct information about kidney parenchymal injury in the preimplantation period could be provided by an approved kidney biomarker score calculated from urinary concentrations of the cell-cycle arrest markers insulin-like growth factor-binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinases-2 (TIMP2), although this biomarker score has not been validated in this setting (36).
Figure 1.
Kidney considerations throughout the timeline of mechanical circulatory support (MCS). The focus is on durable MCS, but many considerations will apply to temporary MCS as well. CO, cardiac output; CVP, central venous pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; BUN, blood urea nitrogen; KRT, kidney replacement therapy; RV, right ventricle; MAP, mean arterial pressure; MCS, mechanical circulatory support.
Future Investigations
Syndromic, filtration marker- and albuminuria-dependent AKI and CKD classifications are insufficient to understanding the complexity of kidney disease (37). This is particularly relevant to complex LVAD recipients. Investigations into cellular and molecular processes of kidney pathology are addressing this critical barrier to progress in many disease states, with analysis of kidney tissue and body fluids (37). Obtaining kidney tissue from LVAD recipients would be ideal for understanding kidney pathophysiology in this group, but kidney biopsy is seldom performed in these patients because of their tenuous states. Thus, improved noninvasive assessments of kidney health are crucial to this patient population. Growing availability of urine and blood biomarkers reflecting different aspects of kidney health/function, and growing use of omics-based systems investigations in many areas of medicine, may enable important noninvasive insights into kidney health in MCS (38,39). Pre-LVAD implantation parenchymal biomarkers may enable accurate assessment of kidney damage and differentiation of damage from flow-mediated functional reductions, yielding prognostic information on likelihood of kidney function recovery. Multi-omics studies of blood and urine samples in the preimplantation period have the potential to elucidate further the heterogeneity and mechanistic processes underlying kidney health states in these complex patients. Analysis of exosomes (membrane-derived, 50–200-nm-diameter vesicles) from the urine may provide a noninvasive window into kidney cellular processes (40,41). In addition to accurate diagnosis and prognosis, movement of kidney health understanding from broad syndromes to accurate molecular mechanistic understanding is a crucial step toward therapies (42).
Early Postimplantation Period
Epidemiology
LVAD recipients are at high risk of AKI in the early postoperative period. A recent meta-analysis estimated the incidence of AKI in more than 63,000 LVAD recipients at 37% when AKI was assessed using standardized criteria (21). The incidence of AKI treated with kidney replacement therapy was 13% (21). With AKI, odds of death more than tripled in the first 30 days and more than doubled over 1 year. With AKI treated with kidney replacement therapy, odds of death increased more than seven-fold in the first 30 days and more than five-fold in the first year (21).
Mechanisms and Pathophysiology
AKI in LVAD recipients has many potential contributing factors. LVAD implantation is a major cardiac surgery with prolonged cardiopulmonary bypass (43). Cardiac surgery is thought to damage the kidney through ischemia-reperfusion injury, inflammatory and oxidative injuries, microembolic injury, toxin-mediated injury, and neurohormonal activation (44). Much of this information has come from animal models, although noninvasive data from humans supporting many of these mechanisms is accruing (44–46). LVAD recipients, given their tenuous preimplantation status, may be particularly susceptible to these insults.
Another potential cause of AKI is RV dysfunction through elevated CVP. RV dysfunction is a common complication of LVAD implantation and can occur anytime, although early appearance is most common (43). LVAD support reduces LV volume, which can shift the interventricular septum, distorting RV geometry and reducing function (43). The severity can vary from mild (manageable with inotropes, inhaled nitric oxide, or intravenous vasodilators) to severe (requiring temporary or permanent RV MCS) (47).
Clinical Considerations
Specific interventions to reduce the risk of AKI and improve kidney outcomes in the early postoperative period are not available. Critical care nephrology practices are essential, with review of operative details, close monitoring of urine output and volume status, and frequent measurement of electrolytes and acid-base status to detect impending kidney failure or severe derangements (Figure 1). Diuretic use or ultrafiltration may be required for volume management. Urine microscopy may provide information because the presence of numerous granular casts may suggest severe acute tubular necrosis. Urine cell-cycle arrest biomarker score measurement ([TIMP2]×[IGFBP7]) may provide additional information about parenchymal kidney injury.
Future Investigations
Detailed noninvasive assessments of parenchymal health using biomarker panels may enable accurate diagnosis of kidney injury sustained during surgery. In general, cardiac surgery settings the [TIMP2]×[IGFBP7] score has enabled early identification and targeted management of AKI (48–50). Other biomarkers (basic fibroblast growth factor, kidney injury molecule-1, N-terminal pro-B-type natriuretic peptide, and tumor necrosis factor receptor) measured early in the postoperative period are associated with development of CKD after general cardiac surgery (51). Multi-omics approaches hold the promise of more granular insights into kidney health (38). Improved understanding of the molecular mechanisms of kidney dysfunction in the early postoperative period has the potential to inform therapeutic development and to identify individuals who may benefit from specific treatments.
Prolonged MCS Period
Epidemiology
LVADs tend to improve macrocirculatory parameters, increasing cardiac output and decreasing CVP, and disordered macrocirculation may be the primary driver of preimplantation kidney dysfunction in some patients (52,53). Despite these macrocirculatory improvements, LVADs often do not cause substantial sustained kidney function improvement (54–56). Group-average eGFR changes in LVAD recipients shows an early increase in eGFR, a peak eGFR around 1 month after implantation, followed by a decline (25,54). Relationships between changes in kidney function and outcomes are nonlinear (25).
Mechanisms and Pathophysiology
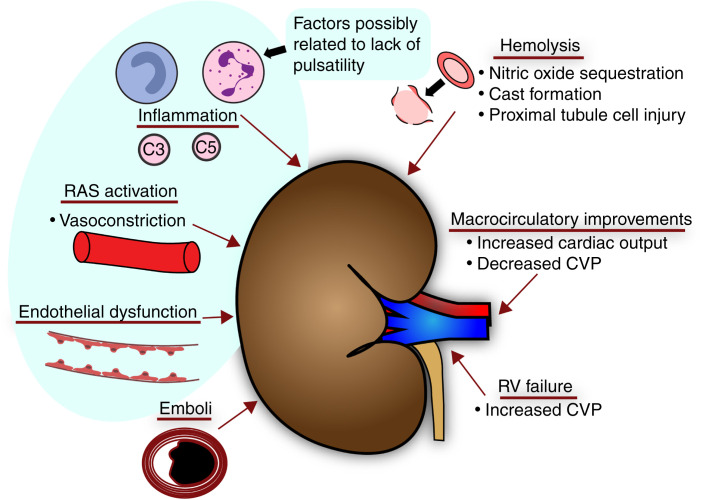
Numerous potential mechanisms have been proposed (Figure 2) for adverse kidney outcomes with prolonged MCS. A long-standing concern is over lack of pulsatility. For a time, continuous flow was thought to induce periarteritis in the kidneys on the basis of an animal study (57); however, this periarteritis has not been found in humans (58). Evidence is stronger for continuous flow causing endothelial dysfunction from reduced endothelial release of nitric oxide, increased secretion of endothelin-1, and other factors (59–62). Continuous flow may activate the sympathetic nervous system and renin-angiotensin system systemically and in the kidneys (63–66). Intravascular hemolysis resulting in free hemoglobin in the plasma can harm the kidneys through vasoconstriction from nitric oxide sequestration, tubular casts, and proximal tubular heme toxicity (67,68). Although hemolysis appears very limited with newer devices, this is a possible mechanism for chronic and acute kidney damage in some cases (69). Chronic RV impairment after LVAD implantation is another potential factor driving long-term kidney dysfunction (70).
Figure 2.
Potential mechanisms for left ventricular assist device effects (beneficial and harmful) on the kidneys. RAS, renin-angiotensin system.
Clinical Considerations
Interventions to improve kidney health with long-term LVAD support are unknown, and limitations of endogenous filtration marker-based kidney function estimates make it difficult to assess kidney health accurately in this setting (71). Nevertheless, large changes in serum creatinine or cystatin C levels or eGFR, or clear trajectories, may allow inferences about health and prognosis. Given the apparent renin-angiotensin system activation with continuous flow, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use is attractive for kidney preservation; however, the risks and benefits in this setting are uncertain. These medications are often continued after LVAD implantation (72).
Special management is necessary for maintenance dialysis in people with LVADs. Earlier devices were quite sensitive to reductions in preload, complicating ultrafiltration (73). Modern devices, however, have limited preload sensitivity, facilitating volume removal (74). Several case series of outpatient hemodialysis and case reports of outpatient peritoneal dialysis have been published (75–77). A theme of these reports is close cooperation between the dialysis providers and MCS teams, including initial education about interpreting LVAD device information (pump speed, flow rate, and pulsatility index) and availability of immediate communication. Blood pressure measurement is important and depends on whether the aortic valve continues to open during the cardiac cycle, causing a detectable pulse pressure. Aortic valve opening is influenced by native heart contractility and pump flow. If the aortic valve opens, systolic and diastolic blood pressure may be measurable with the usual techniques (78). If the aortic valve does not open, blood pressure can be measured using a Doppler ultrasound device and manual sphygmomanometer, and the mean arterial pressure (MAP) is obtained from the pressure at which flow is detected. The goal MAP is generally 70–80 mm Hg; MAP >90 mm Hg is avoided, given the increased risk of stroke (79–81). Vascular access for maintenance hemodialysis is an important consideration. Arteriovenous access placement, and avoidance of long-term tunneled catheter use, is desirable given the elevated risk of bloodstream infections with dialysis catheters (82). Several cases of successful arteriovenous fistula placement, maturation, and use have been reported (83,84). Concerns remain, however, about arteriovenous fistula maturation in the setting of low pulsatile blood flow, and others have advocated for arteriovenous grafts as the preferred access (85). Peritoneal dialysis is an option because modern LVADs do not compromise the peritoneal space (43,77). Both KFRT treated with dialysis and LVAD use alone have high complication rates related to underlying disease factors and treatment factors. Patients presenting with both are therefore at very high risk (86).
Future Investigations
The growing availability of techniques discussed in prior sections may enable important insights into the effects of prolonged MCS on kidney health. Longitudinal assessments of biomarker panels and multi-omics data and accurate, comprehensive assessments of clinical characteristics in large cohorts would be needed to assess the multiple dimensions of kidney health in LVAD recipients accurately and to draw inferences about mechanistic effects of durable LVAD support on the kidneys. It seems clear now that the idea of a single, predominant salutary effect on kidney function with macrocirculatory parameter improvement with LVAD support does not capture most patients’ experiences, and that molecular mechanisms rather than macrocirculatory mechanisms underlie much of cardiac-LVAD-kidney interactions.
Temporary MCS
Several temporary MCS devices are available for use in high-risk PCI, acute decompensated HF, cardiogenic shock, and cardiac arrest resuscitation (Table 1). The evidence basis to guide usage decisions is limited, and decisions rely on expert opinion and experience, pathophysiological considerations, and observational studies. A recent analysis of administrative data found an increase in the use of temporary MCS for postcardiac surgery cardiogenic shock (87). Temporary MCS use before durable MCS has also increased (88). ST-elevation myocardial infarction was complicated by cardiogenic shock in 9% of cases in an analysis of data from 2003 to 2012, and temporary MCS was used in more than half of these cases (89).
Nephrology Considerations and Kidney Pathophysiology
Temporary MCS devices improve macrocirculatory parameters that affect kidney function, and clinical experience of marked kidney function improvement in some individuals is clear. However, there are limited published data on kidney outcomes. A case series of five patients who underwent placement of a left atrial-to-femoral artery bypass pump found no change in serum creatinine, although urine output more than doubled after device placement (90). Observational studies suggest that impeller-driven axial flow pumps are associated with a lower risk of AKI during high-risk PCI (11). A small study of 15 patients with cardiogenic shock found that increasing axial flow pump rate decreased renal resistive index without changing blood pressure, suggesting kidney perfusion may have increased (91). The effects of venoarterial ECMO on kidney function are challenging to assess, given it is reserved for situations involving respiratory failure or cardiac arrest where numerous processes affecting kidney health/function are at play (92,93). Studies of long-term kidney outcomes are not available.
There are several explanations for why kidney function improvement is often not observed with temporary MCS. The limits of routine kidney assessments mean that preexisting subclinical kidney damage with loss of reserve capacity and sensitivity to insults may be underestimated. This, combined with the perturbations of the acute process necessitating temporary MCS, puts these patients at high risk for damage regardless of macrocirculatory improvement, and assessment of kidney microcirculation and parenchymal markers may improve understanding.
The inflammatory environment in temporary MCS may be damaging to the kidneys. Systemic inflammation is well documented in settings that can lead to temporary MCS use—such as acute myocardial infarction or acute HF decompensation—in clinical and experimental settings (94). Although systemic and cardiac inflammatory environments have been most studied, it is likely that the kidneys are affected by systemic and intrarenal inflammation as well (30,94). Temporary MCS may exacerbate inflammation through procedural factors and exposure of the blood to artificial material. This has been most noticeable with ECMO, where a systemic inflammatory response syndrome often develops, attributed to activation of the innate immune system with extensive blood exposure to artificial material, in addition to a pro-inflammatory effect of nonpulsatile blood flow (95,96). Experimental studies have demonstrated that ECMO in healthy animals causes systemic inflammatory response syndrome with mobilization of cellular stores of inflammatory cytokines (97). In contrast to ECMO, IABP use has been reported to reduce inflammation in some settings, perhaps due to pulsatility (98,99).
Nephrology Clinical Management Considerations
Nephrology evaluation of acutely ill people on temporary MCS includes assessing their pre-episode kidney health/function, their recent exposures to kidney insults, and their current status, with particular attention to urine output, electrolytes, and likely trajectory (Figure 1). Routine nephrology critical care assessments, including consideration of a wide differential diagnosis for causes and contributors to kidney injury, in addition to clinical judgment on how the acute cardiac process is affecting the presentation, are appropriate. Frequent monitoring of urine output, electrolytes, and acid-base status is crucial as well to enable optimal supportive care and assess the need for dialysis, with close communication with the cardiovascular and intensivist teams.
Patients requiring temporary MCS who develop severe AKI may require dialysis. Given the hemodynamic instability and risk for severe acid-base, electrolyte, and volume disturbances, continuous renal replacement therapy is usually the modality of choice. Management considerations are similar to other situations, with volume management paramount. Catheter placement location may be influenced by the temporary MCS method if venous access is used for MCS, such as with venoarterial ECMO or percutaneous RV support devices. With venoarterial ECMO, attachment of the continuous renal replacement therapy machine to the ECMO circuit is often possible, making an additional catheter unnecessary. In all forms of temporary MCS, systemic anticoagulation is used, and thus regional or systemic anticoagulation specifically for dialysis is not necessary.
Future Investigations
To understand the effects of temporary MCS on the kidneys, the improved noninvasive assessments of kidney function discussed in the context of durable MCS will be crucial. Temporary MCS devices differ greatly in function, uses, and potential risks, leading to great heterogeneity in potential kidney effects—heterogeneity that will require detailed noninvasive molecular techniques to understand and characterize. This will enable individualized diagnosis and prognosis and development of targeted therapeutic and management strategies to improve kidney and overall outcomes.
Conclusions
The high burden of cardiovascular disease in the United States, improving MCS technologies, and changing use patterns, combined with the common coexistence of kidney and cardiovascular disease, mean that the need for nephrology care for patients receiving MCS will continue to increase. Nephrologists play an important role in ensuring patient safety and improving outcomes in this setting. Although current kidney-related management strategies in MCS are mostly opinion and experience based, the rapid development of tools and techniques for granular, individualized kidney assessment hold the promise of improved diagnostic tools and development of targeted management strategies and therapies in the future.
Disclosures
A.B. Civitello reports consultancy agreements with Medtronic; honoraria from Abbott and Medtronic; and being a scientific advisor for Medtronic Titan Medical Advisory Board. S.D. Navaneethan reports consultancy agreements with Bayer, Boehringer Ingelheim, Tricida, and Vifor; research funding from Keryx; honoraria from Bayer, Boehringer Ingelheim, Tricida, and Vifor; and being a scientific advisor for AJKD, CJASN, CardioRenal Medicine, Current Opinion in Nephrology and Hypertension, American Journal of Nephrology, and KDIGO (guideline writing committee member). The remaining authors have nothing to disclose.
Funding
C.P. Walther is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant/award number: K23DK122131).
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or Veterans Administration.
Author Contributions
S.D. Navaneethan and C.P. Walther conceptualized the study; S.D. Navaneethan was responsible for supervision; C.P. Walther was responsible for visualization and wrote the original draft of the manuscript; and all authors reviewed and edited the manuscript.
References
- 1.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB: Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 34: 1495–1504, 2015. 10.1016/j.healun.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, Ransom J, Ewald GA, Itoh A, Raval NY, Silvestry SC, Cogswell R, John R, Bhimaraj A, Bruckner BA, Lowes BD, Um JY, Jeevanandam V, Sayer G, Mangi AA, Molina EJ, Sheikh F, Aaronson K, Pagani FD, Cotts WG, Tatooles AJ, Babu A, Chomsky D, Katz JN, Tessmann PB, Dean D, Krishnamoorthy A, Chuang J, Topuria I, Sood P, Goldstein DJ; MOMENTUM 3 Investigators : A fully magnetically levitated left ventricular assist device—Final report. N Engl J Med 380: 1618–1627, 2019. 10.1056/NEJMoa1900486 [DOI] [PubMed] [Google Scholar]
- 3.Gregoric ID, Arabia FA: Current types of devices for durable mechanical circulatory support. In: Mechanical Circulatory Support: A Companion to Braunwald’s Heart Disease, edited by Kirklin JK, Rogers JG, Philadelphia, Elsevier, 2020, pp 109–119 10.1016/B978-0-323-56699-5.00010-3 [DOI] [Google Scholar]
- 4.Arabía FA, Cantor RS, Koehl DA, Kasirajan V, Gregoric I, Moriguchi JD, Esmailian F, Ramzy D, Chung JS, Czer LS, Kobashigawa JA, Smith RG, Kirklin JK: Interagency registry for mechanically assisted circulatory support report on the total artificial heart. J Heart Lung Transplant 37: 1304–1312, 2018. 10.1016/j.healun.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 5.Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, Higgins RS, Stevenson LW, Stehlik J, Atluri P, Grady KL, Kirklin JK: The Society of Thoracic Surgeons Intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant 38: 114–126, 2019. 10.1016/j.healun.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 6.Aaronson KD, Pagani FD: Temporary mechanical circulatory support. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine, 11th Ed., edited by Zipes DP, Philadelphia, Elsevier, 2019, pp 573–575 [Google Scholar]
- 7.Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, Tu T; Society for Cardiovascular Angiography and Interventions (SCAI)Heart Failure Society of America (HFSA)Society of Thoracic Surgeons (STS)American Heart Association (AHA), and American College of Cardiology (ACC) : 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol 65: e7–e26, 2015. 10.1016/j.jacc.2015.03.036 [DOI] [PubMed] [Google Scholar]
- 8.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee : Heart disease and stroke statistics—2021 update: A report from the American Heart Association. Circulation 143: e254–e743, 2021. 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 9.Molina EJ, Shah P, Kiernan MS, Cornwell WK 3rd, Copeland H, Takeda K, Fernandez FG, Badhwar V, Habib RH, Jacobs JP, Koehl D, Kirklin JK, Pagani FD, Cowger JA: The Society of Thoracic Surgeons Intermacs 2020 annual report. Ann Thorac Surg 111: 778–792, 2021. 10.1016/j.athoracsur.2020.12.038 [DOI] [PubMed] [Google Scholar]
- 10.Franz D, Stedman M, Myers S, Naftel DC, Silver SA, Banerjee D, Chang TI: Long-term changes in kidney function after left ventricular assist device implant: An analysis of the STS Intermacs database. J Heart Lung Transplant 38: S89–S90, 2019. 10.1016/j.healun.2019.01.206 [DOI] [Google Scholar]
- 11.Flaherty MP, Moses JW, Westenfeld R, Palacios I, O’Neill WW, Schreiber TL, Lim MJ, Kaki A, Ghiu I, Mehran R: Impella support and acute kidney injury during high-risk percutaneous coronary intervention: The Global cVAD Renal Protection Study. Catheter Cardiovasc Interv 95: 1111–1121, 2020. 10.1002/ccd.28400 [DOI] [PubMed] [Google Scholar]
- 12.Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC: Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 36: 1080–1086, 2017. 10.1016/j.healun.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 13.Ward ST, Liang Q, Pagani FD, Zhang M, Kormos RL, Aaronson KD, Althouse AD, Nallamothu BK, Likosky DS: A roadmap for evaluating the use and value of durable ventricular assist device therapy. J Heart Lung Transplant 37: 146–150, 2018. 10.1016/j.healun.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolsrud O, Ricksten SE, Holmberg E, Felldin M, Karason K, Hammarsten O, Samuelsson O, Dellgren G: Measured and not estimated glomerular filtration rate should be used to assess renal function in heart transplant recipients. Nephrol Dial Transplant 31: 1182–1189, 2016. 10.1093/ndt/gfv338 [DOI] [PubMed] [Google Scholar]
- 15.Kervella D, Lemoine S, Sens F, Dubourg L, Sebbag L, Guebre-Egziabher F, Bonnefoy E, Juillard L: Cystatin C versus creatinine for GFR estimation in CKD due to heart failure. Am J Kidney Dis 69: 321–323, 2017. 10.1053/j.ajkd.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 16.Kirklin JK, Naftel DC, Kormos RL, Pagani FD, Myers SL, Stevenson LW, Givertz MM, Young JB: Quantifying the effect of cardiorenal syndrome on mortality after left ventricular assist device implant. J Heart Lung Transplant 32: 1205–1213, 2013. 10.1016/j.healun.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 17.Cowger J, Sundareswaran K, Rogers JG, Park SJ, Pagani FD, Bhat G, Jaski B, Farrar DJ, Slaughter MS: Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol 61: 313–321, 2013. 10.1016/j.jacc.2012.09.055 [DOI] [PubMed] [Google Scholar]
- 18.Bansal N, Hailpern SM, Katz R, Hall YN, Kurella Tamura M, Kreuter W, O’Hare AM: Outcomes associated with left ventricular assist devices among recipients with and without end-stage renal disease. JAMA Intern Med 178: 204–209, 2018. 10.1001/jamainternmed.2017.4831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walther CP, Niu J, Winkelmayer WC, Cheema FH, Nair AP, Morgan JA, Fedson SE, Deswal A, Navaneethan SD: Implantable ventricular assist device use and outcomes in people with end-stage renal disease. J Am Heart Assoc 7: 1–10, 2018. 10.1161/JAHA.118.008664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Eckardt KU, Dorman NM, Christiansen SL, Hoorn EJ, Ingelfinger JR, Inker LA, Levin A, Mehrotra R, Palevsky PM, Perazella MA, Tong A, Allison SJ, Bockenhauer D, Briggs JP, Bromberg JS, Davenport A, Feldman HI, Fouque D, Gansevoort RT, Gill JS, Greene EL, Hemmelgarn BR, Kretzler M, Lambie M, Lane PH, Laycock J, Leventhal SE, Mittelman M, Morrissey P, Ostermann M, Rees L, Ronco P, Schaefer F, St Clair Russell J, Vinck C, Walsh SB, Weiner DE, Cheung M, Jadoul M, Winkelmayer WC: Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int 97: 1117–1129, 2020. 10.1016/j.kint.2020.02.010 [DOI] [PubMed] [Google Scholar]
- 21.Thongprayoon C, Lertjitbanjong P, Cheungpasitporn W, Hansrivijit P, Fülöp T, Kovvuru K, Kanduri SR, Davis PW, Vallabhajosyula S, Bathini T, Watthanasuntorn K, Prasitlumkum N, Chokesuwattanaskul R, Ratanapo S, Mao MA, Kashani K: Incidence and impact of acute kidney injury on patients with implantable left ventricular assist devices: A meta-analysis. Ren Fail 42: 495–512, 2020. 10.1080/0886022X.2020.1768116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muslem R, Caliskan K, Akin S, Sharma K, Gilotra NA, Brugts JJ, Houston B, Whitman G, Tedford RJ, Hesselink DA, Bogers AJJC, Manintveld OC, Russell SD: Pre-operative proteinuria in left ventricular assist devices and clinical outcome [erratum appears in J Heart Lung Transplant 37: 535, 2018 10.1016/j.healun.2018.02.001]. J Heart Lung Transplant 37: 124–130, 2018. 10.1016/j.healun.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 23.Topkara VK, Coromilas EJ, Garan AR, Li RC, Castagna F, Jennings DL, Yuzefpolskaya M, Takeda K, Takayama H, Sladen RN, Mancini DM, Naka Y, Radhakrishnan J, Colombo PC: Preoperative proteinuria and reduced glomerular filtration rate predicts renal replacement therapy in patients supported with continuous-flow left ventricular assist devices. Circ Heart Fail 9: e002897, 2016. 10.1161/CIRCHEARTFAILURE.115.002897 [DOI] [PubMed] [Google Scholar]
- 24.Hasin T, Topilsky Y, Schirger JA, Li Z, Zhao Y, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, Edwards BS, Pereira NL, Joyce L, Daly R, Park SJ, Kushwaha SS: Changes in renal function after implantation of continuous-flow left ventricular assist devices. J Am Coll Cardiol 59: 26–36, 2012. 10.1016/j.jacc.2011.09.038 [DOI] [PubMed] [Google Scholar]
- 25.Brisco M, Kimmel S, Coca S, Putt ME, Jessup M, Tang WHW, Parikh CR, Testani JM: Prevalence and prognostic importance of changes in renal function following mechanical circulatory support. J Heart Lung Transplant 32: S72, 2013. 10.1016/j.healun.2013.01.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Medicare and Medicaid Services : Decision Memo for Artificial Hearts and Related Devices, Including Ventricular Assist Devices for Bridge-to-transplant and Destination Therapy (CAG-00453N). Woodlawn, Department of Health and Human Services, 2020, pp 1–47 [Google Scholar]
- 27.Guglin M, Zucker MJ, Borlaug BA, Breen E, Cleveland J, Johnson MR, Panjrath GS, Patel JK, Starling RC, Bozkurt B; ACC Heart Failure and Transplant Member Section and Leadership Council : Evaluation for heart transplantation and LVAD implantation: JACC council perspectives. J Am Coll Cardiol 75: 1471–1487, 2020. 10.1016/j.jacc.2020.01.034 [DOI] [PubMed] [Google Scholar]
- 28.Gaffey AC, Chen CW, Soopan RV, Trubelja A, Acker MA, Atluri P: Bridge with a left ventricular assist device to a simultaneous heart and kidney transplant: Review of the United Network for Organ Sharing Database. J Am Coll Surg 221: S23, 2015. 10.1016/j.jamcollsurg.2015.07.041 [DOI] [PubMed] [Google Scholar]
- 29.Schlöndorff D, Wyatt CM, Campbell KN: Revisiting the determinants of the glomerular filtration barrier: What goes round must come round. Kidney Int 92: 533–536, 2017. 10.1016/j.kint.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 30.Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S: Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat Rev Nephrol 12: 610–623, 2016. 10.1038/nrneph.2016.113 [DOI] [PubMed] [Google Scholar]
- 31.Eirin A, Textor SC, Lerman LO: Emerging paradigms in chronic kidney ischemia. Hypertension 72: 1023–1030, 2018. 10.1161/HYPERTENSIONAHA.118.11082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimada S, Hirose T, Takahashi C, Sato E, Kinugasa S, Ohsaki Y, Kisu K, Sato H, Ito S, Mori T: Pathophysiological and molecular mechanisms involved in renal congestion in a novel rat model. Sci Rep 8: 16808, 2018. 10.1038/s41598-018-35162-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartupee J, Mann DL: Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol 14: 30–38, 2017. 10.1038/nrcardio.2016.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verbrugge FH, Guazzi M, Testani JM, Borlaug BA: Altered hemodynamics and end-organ damage in heart failure: Impact on the lung and kidney. Circulation 142: 998–1012, 2020. 10.1161/CIRCULATIONAHA.119.045409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh S, Kanwar A, Sundaragiri PR, Cheungpasitporn W, Truesdell AG, Rab ST, Singh M, Vallabhajosyula S: Acute kidney injury in cardiogenic shock: An updated narrative review. J Cardiovasc Dev Dis 8: 88, 2021. 10.3390/jcdd8080088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erstad BL: Usefulness of the biomarker TIMP-2•IGFBP7 for acute kidney injury assessment in critically ill patients: A narrative review. Ann Pharmacother 56: 83–92, 2022. 10.1177/10600280211005425 [DOI] [PubMed] [Google Scholar]
- 37.de Boer IH, Alpers CE, Azeloglu EU, Balis UGJ, Barasch JM, Barisoni L, Blank KN, Bomback AS, Brown K, Dagher PC, Dighe AL, Eadon MT, El-Achkar TM, Gaut JP, Hacohen N, He Y, Hodgin JB, Jain S, Kellum JA, Kiryluk K, Knight R, Laszik ZG, Lienczewski C, Mariani LH, McClelland RL, Menez S, Moledina DG, Mooney SD, O’Toole JF, Palevsky PM, Parikh CR, Poggio ED, Rosas SE, Rosengart MR, Sarwal MM, Schaub JA, Sedor JR, Sharma K, Steck B, Toto RD, Troyanskaya OG, Tuttle KR, Vazquez MA, Waikar SS, Williams K, Wilson FP, Zhang K, Iyengar R, Kretzler M, Himmelfarb J; Kidney Precision Medicine Project : Rationale and design of the Kidney Precision Medicine Project. Kidney Int 99: 498–510, 2021. 10.1016/j.kint.2020.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boerschel C, Geelhoed B, Conradi L, Girdauskas E, Mueller C, Reichenspurner H, Blankenberg S, Zeller T, Schnabel RB: Multi-omics approach to post-CABG renal impairment. Eur Heart J 41: 2671, 2020. 10.1093/ehjci/ehaa946.2671 [DOI] [Google Scholar]
- 39.Eddy S, Mariani LH, Kretzler M: Integrated multi-omics approaches to improve classification of chronic kidney disease. Nat Rev Nephrol 16: 657–668, 2020. 10.1038/s41581-020-0286-5 [DOI] [PubMed] [Google Scholar]
- 40.van Balkom BW, Pisitkun T, Verhaar MC, Knepper MA: Exosomes and the kidney: Prospects for diagnosis and therapy of renal diseases. Kidney Int 80: 1138–1145, 2011. 10.1038/ki.2011.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Fekih R, Hurley J, Tadigotla V, Alghamdi A, Srivastava A, Coticchia C, Choi J, Allos H, Yatim K, Alhaddad J, Eskandari S, Chu P, Mihali AB, Lape IT, Lima Filho MP, Aoyama BT, Chandraker A, Safa K, Markmann JF, Riella LV, Formica RN, Skog J, Azzi JR: Discovery and validation of a urinary exosome mRNA signature for the diagnosis of human kidney transplant rejection. J Am Soc Nephrol 32: 994–1004, 2021. 10.1681/ASN.2020060850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poyan Mehr A, Tran MT, Ralto KM, Leaf DE, Washco V, Messmer J, Lerner A, Kher A, Kim SH, Khoury CC, Herzig SJ, Trovato ME, Simon-Tillaux N, Lynch MR, Thadhani RI, Clish CB, Khabbaz KR, Rhee EP, Waikar SS, Berg AH, Parikh SM: De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat Med 24: 1351–1359, 2018. 10.1038/s41591-018-0138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schumer EM, Slaughter MS, Dembitsky WP: Operative techniques and intraoperative management. In: Mechanical Circulatory Support: A Companion to Braunwald’s Heart Disease, edited by Kirklin JK, Rogers JG, 2nd Ed., Philadelphia, Elsevier, 2020, pp 121–129 10.1016/B978-0-323-56699-5.00011-5 [DOI] [Google Scholar]
- 44.Wang Y, Bellomo R: Cardiac surgery-associated acute kidney injury: Risk factors, pathophysiology and treatment. Nat Rev Nephrol 13: 697–711, 2017. 10.1038/nrneph.2017.119 [DOI] [PubMed] [Google Scholar]
- 45.Moledina DG, Mansour SG, Jia Y, Obeid W, Thiessen-Philbrook H, Koyner JL, McArthur E, Garg AX, Wilson FP, Shlipak MG, Coca SG, Parikh CR: Association of T cell–derived inflammatory cytokines with acute kidney injury and mortality after cardiac surgery. Kidney Int Rep 4: 1689–1697, 2019. 10.1016/j.ekir.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenberg JH, Parsons M, Zappitelli M, Jia Y, Thiessen- Philbrook HR, Devarajan P, Everett AD, Parikh CR: Cardiac biomarkers for risk stratification of acute kidney injury after pediatric cardiac surgery. Ann Thorac Surg 111: 191–198, 2021. 10.1016/j.athoracsur.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lampert BC, Teuteberg JJ: Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant 34: 1123–1130, 2015. 10.1016/j.healun.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 48.Oezkur M, Magyar A, Thomas P, Stork T, Schneider R, Bening C, Störk S, Heuschmann PU, Leyh RG, Wagner M: TIMP-2*IGFBP7 (Nephrocheck®) measurements at intensive care unit admission after cardiac surgery are predictive for acute kidney injury within 48 hours. Kidney Blood Press Res 42: 456–467, 2017. 10.1159/000479298 [DOI] [PubMed] [Google Scholar]
- 49.Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, Görlich D, Kellum JA, Zarbock A: Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One 9: e93460, 2014. 10.1371/journal.pone.0093460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, Zarbock A: Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial [erratum published in 43: 1749, 2017 10.1007/s00134-017-4735-y]. Intensive Care Med 43: 1551–1561, 2017. 10.1007/s00134-016-4670-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menez S, Moledina DG, Garg AX, Thiessen-Philbrook H, McArthur E, Jia Y, Liu C, Obeid W, Mansour SG, Koyner JL, Shlipak MG, Wilson FP, Coca SG, Parikh CR: Results from the TRIBE-AKI Study found associations between post-operative blood biomarkers and risk of chronic kidney disease after cardiac surgery. Kidney Int 99: 716–724, 2021. 10.1016/j.kint.2020.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uriel N, Sayer G, Addetia K, Fedson S, Kim GH, Rodgers D, Kruse E, Collins K, Adatya S, Sarswat N, Jorde UP, Juricek C, Ota T, Jeevanandam V, Burkhoff D, Lang RM: Hemodynamic ramp tests in patients with left ventricular assist devices. JACC Heart Fail 4: 208–217, 2016. 10.1016/j.jchf.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 53.Morgan JA, Paone G, Nemeh HW, Murthy R, Williams CT, Lanfear DE, Tita C, Brewer RJ: Impact of continuous-flow left ventricular assist device support on right ventricular function. J Heart Lung Transplant 32: 398–403, 2013. 10.1016/j.healun.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 54.Yalcin YC, Muslem R, Veen KM, Soliman OI, Hesselink DA, Constantinescu AA, Brugts JJ, Manintveld OC, Fudim M, Russell SD, Tomashitis B, Houston BA, Hsu S, Tedford RJ, Bogers AJJC, Caliskan K: Impact of continuous flow left ventricular assist device therapy on chronic kidney disease: A longitudinal multicenter study. J Card Fail 26: 333–341, 2020. 10.1016/j.cardfail.2020.01.010 [DOI] [PubMed] [Google Scholar]
- 55.Wettersten N, Estrella M, Brambatti M, Horiuchi Y, Adler E, Pretorius V, Murray PT, Shlipak M, Ix JH: Kidney function following left ventricular assist device implantation: An observational cohort study. Kidney Med 3: 378–385.e1, 2021. 10.1016/j.xkme.2021.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WW, Parikh CR, Testani JM: Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail 7: 68–75, 2014. 10.1161/CIRCHEARTFAILURE.113.000507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ootaki C, Yamashita M, Ootaki Y, Kamohara K, Weber S, Klatte RS, Smith WA, Massiello AL, Emancipator SN, Golding LA, Fukamachi K: Reduced pulsatility induces periarteritis in kidney: Role of the local renin-angiotensin system. J Thorac Cardiovasc Surg 136: 150–158, 2008. 10.1016/j.jtcvs.2007.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tromp TR, Nguyen TQ, de Jonge N, Joles JA: Absence of structural lesions in human renal arcuate arteries after LVAD implantation: Response to a letter regarding “Left ventricular assist devices: A kidney’s perspective.” Heart Fail Rev 20: 753–754, 2015. 10.1007/s10741-015-9507-6 [DOI] [PubMed] [Google Scholar]
- 59.von Horn C, Minor T: Isolated kidney perfusion: The influence of pulsatile flow. Scand J Clin Lab Invest 78: 131–135, 2018. 10.1080/00365513.2017.1422539 [DOI] [PubMed] [Google Scholar]
- 60.Nguyen KT, Donoghue L, Giridharan GA, Naber JP, Vincent D, Fukamachi K, Kotru A, Sethu P: Acute response of human aortic endothelial cells to loss of pulsatility as seen during cardiopulmonary bypass [published online ahead of print February 25, 2021]. Cells Tissues Organs 10.1159/000512558 [DOI] [PubMed] [Google Scholar]
- 61.Nakano T, Tominaga R, Nagano I, Okabe H, Yasui H: Pulsatile flow enhances endothelium-derived nitric oxide release in the peripheral vasculature. Am J Physiol Heart Circ Physiol 278: H1098–H1104, 2000. 10.1152/ajpheart.2000.278.4.H1098 [DOI] [PubMed] [Google Scholar]
- 62.Onorati F, Rubino AS, Nucera S, Foti D, Sica V, Santini F, Gulletta E, Renzulli A: Off-pump coronary artery bypass surgery versus standard linear or pulsatile cardiopulmonary bypass: Endothelial activation and inflammatory response. Eur J Cardiothorac Surg 37: 897–904, 2010. 10.1016/j.ejcts.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 63.Saito S, Westaby S, Piggot D, Dudnikov S, Robson D, Catarino PA, Clelland C, Nojiri C: End-organ function during chronic nonpulsatile circulation. Ann Thorac Surg 74: 1080–1085, 2002. 10.1016/S0003-4975(02)03846-8 [DOI] [PubMed] [Google Scholar]
- 64.Cornwell WK 3rd, Tarumi T, Stickford A, Lawley J, Roberts M, Parker R, Fitzsimmons C, Kibe J, Ayers C, Markham D, Drazner MH, Fu Q, Levine BD: Restoration of pulsatile flow reduces sympathetic nerve activity among individuals with continuous-flow left ventricular assist devices. Circulation 132: 2316–2322, 2015. 10.1161/CIRCULATIONAHA.115.017647 [DOI] [PubMed] [Google Scholar]
- 65.Markham DW, Fu Q, Palmer MD, Drazner MH, Meyer DM, Bethea BT, Hastings JL, Fujimoto N, Shibata S, Levine BD: Sympathetic neural and hemodynamic responses to upright tilt in patients with pulsatile and nonpulsatile left ventricular assist devices. Circ Heart Fail 6: 293–299, 2013. 10.1161/CIRCHEARTFAILURE.112.969873 [DOI] [PubMed] [Google Scholar]
- 66.Welp H, Rukosujew A, Tjan T, Hoffmeier A, Kösek V, Scheld HH, Drees G: Effect of pulsatile and non-pulsatile left ventricular assist devices on the renin-angiotensin system in patients with end-stage heart failure. Thorac Cardiovas Surg 58: S185–S188, 2010. 10.1055/s-0029-1240709 [DOI] [PubMed] [Google Scholar]
- 67.Qian Q, Nath KA, Wu Y, Daoud TM, Sethi S: Hemolysis and acute kidney failure. Am J Kidney Dis 56: 780–784, 2010. 10.1053/j.ajkd.2010.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rother RP, Bell L, Hillmen P, Gladwin MT: The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA 293: 1653–1662, 2005. 10.1001/jama.293.13.1653 [DOI] [PubMed] [Google Scholar]
- 69.Rodrigues J, Alam A, Bernard C, Giannetti N, Podymow T: Secondary hemosiderosis on kidney biopsy in a patient with a left ventricular assist device. Am J Med Sci 347: 172–173, 2014. 10.1097/MAJ.0000000000000221 [DOI] [PubMed] [Google Scholar]
- 70.Takeda K, Takayama H, Colombo PC, Yuzefpolskaya M, Fukuhara S, Han J, Kurlansky P, Mancini DM, Naka Y: Incidence and clinical significance of late right heart failure during continuous-flow left ventricular assist device support. J Heart Lung Transplant 34: 1024–1032, 2015. 10.1016/j.healun.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 71.Peura JL, Colvin-Adams M, Francis GS, Grady KL, Hoffman TM, Jessup M, John R, Kiernan MS, Mitchell JE, O’Connell JB, Pagani FD, Petty M, Ravichandran P, Rogers JG, Semigran MJ, Toole JM; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia : Recommendations for the use of mechanical circulatory support: device strategies and patient selection: A scientific statement from the American Heart Association. Circulation 126: 2648–2667, 2012. 10.1161/CIR.0b013e3182769a54 [DOI] [PubMed] [Google Scholar]
- 72.Khazanie P, Hammill BG, Patel CB, Kiernan MS, Cooper LB, Arnold SV, Fendler TJ, Spertus JA, Curtis LH, Hernandez AF: Use of heart failure medical therapies among patients with left ventricular assist devices: Insights from INTERMACS. J Card Fail 22: 672–679, 2016. 10.1016/j.cardfail.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moreno L, Leblanc M, Gurley M, McCarthy P, Paganini EP: Dialytic support in patients with acute renal failure with implantable left ventricular assist devices. J Intensive Care Med 12: 33–39, 1997. 10.1177/088506669701200104 [DOI] [Google Scholar]
- 74.Fukamachi K, Shiose A, Massiello A, Horvath DJ, Golding LA, Lee S, Starling RC: Preload sensitivity in cardiac assist devices. Ann Thorac Surg 95: 373–380, 2013. 10.1016/j.athoracsur.2012.07.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franz DD, Hussein WF, Abra G, Diskin CD, Duggal V, Teuteberg JJ, Chang TI, Schiller B: Outcomes among patients with left ventricular assist devices receiving maintenance outpatient hemodialysis: A case series. Am J Kidney Dis 77: 226–234, 2021. 10.1053/j.ajkd.2020.04.018 [DOI] [PubMed] [Google Scholar]
- 76.Quader MA, Kumar D, Shah KB, Fatani YI, Katlaps G, Kasirajan V: Safety analysis of intermittent hemodialysis in patients with continuous flow left ventricular assist devices. Hemodial Int 18: 205–209, 2014. 10.1111/hdi.12073 [DOI] [PubMed] [Google Scholar]
- 77.Guglielmi AA, Guglielmi KE, Bhat G, Siemeck R, Tatooles AJ: Peritoneal dialysis after left ventricular assist device placement. ASAIO J 60: 127–128, 2014. 10.1097/MAT.0000000000000020 [DOI] [PubMed] [Google Scholar]
- 78.Holman WL, Kociol RD, Pinney S: Postoperative VAD management: Operating room to discharge and beyond: Surgical and medical considerations. In: Mechanical Circulatory Support: A Companion to Braunwald’s Heart Disease, 2nd Ed., edited by Kirklin JK, Rogers JG, Philadelphia, Elsevier, 2020, pp 131–143 10.1016/B978-0-323-56699-5.00012-7 [DOI] [Google Scholar]
- 79.Castagna F, Stöhr EJ, Pinsino A, Cockcroft JR, Willey J, Reshad Garan A, Topkara VK, Colombo PC, Yuzefpolskaya M, McDonnell BJ: The unique blood pressures and pulsatility of LVAD patients: Current challenges and future opportunities. Curr Hypertens Rep 19: 85, 2017. 10.1007/s11906-017-0782-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD, Starling RC, Chen L, Boyle AJ, Chillcott S, Adamson RM, Blood MS, Camacho MT, Idrissi KA, Petty M, Sobieski M, Wright S, Myers TJ, Farrar DJ; HeartMate II Clinical Investigators : Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant 29: S1–S39, 2010. 10.1016/j.healun.2010.01.011 [DOI] [PubMed] [Google Scholar]
- 81.Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, Gregoric ID, Mallidi H, Leadley K, Aaronson KD, Frazier OH, Milano CA: Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 376: 451–460, 2017. 10.1056/NEJMoa1602954 [DOI] [PubMed] [Google Scholar]
- 82.Poinen K, Quinn RR, Clarke A, Ravani P, Hiremath S, Miller LM, Blake PG, Oliver MJ: Complications from tunneled hemodialysis catheters: A Canadian observational cohort study. Am J Kidney Dis 73: 467–475, 2019. 10.1053/j.ajkd.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 83.Khawaja A, Lim HS, Howell NJ, Inston N: Arteriovenous fistula creation in a patient without a pulse: Vascular access in patients with left ventricular assist devices. J Vasc Access 20: 760–762, 2019. 10.1177/1129729819826029 [DOI] [PubMed] [Google Scholar]
- 84.Chin AI, Tong K, McVicar JP: Successful hemodialysis arteriovenous fistula creation in a patient with continuous-flow left ventricular assist device support. Am J Kidney Dis 69: 314–316, 2017. 10.1053/j.ajkd.2016.07.032 [DOI] [PubMed] [Google Scholar]
- 85.Safaya A, Bhuta K, Rajdeo H: Considerations for long-term dialysis access in patients with left ventricular assist devices. Ann Vasc Surg 70: 568.e13–568.e17, 2021. 10.1016/j.avsg.2020.08.120 [DOI] [PubMed] [Google Scholar]
- 86.Walther CP, Winkelmayer WC, Deswal A, Niu J, Navaneethan SD: Readmissions after acute kidney injury during left ventricular assist device implantation hospitalization. Am J Nephrol 51: 172–181, 2020. 10.1159/000505772 [DOI] [PubMed] [Google Scholar]
- 87.Vallabhajosyula S, Arora S, Sakhuja A, Lahewala S, Kumar V, Shantha GPS, Egbe AC, Stulak JM, Gersh BJ, Gulati R, Rihal CS, Prasad A, Deshmukh AJ: Trends, predictors, and outcomes of temporary mechanical circulatory support for postcardiac surgery cardiogenic shock. Am J Cardiol 123: 489–497, 2019. 10.1016/j.amjcard.2018.10.029 [DOI] [PubMed] [Google Scholar]
- 88.Vallabhajosyula S, Arora S, Lahewala S, Kumar V, Shantha GPS, Jentzer JC, Stulak JM, Gersh BJ, Gulati R, Rihal CS, Prasad A, Deshmukh AJ: Temporary mechanical circulatory support for refractory cardiogenic shock before left ventricular assist device surgery. J Am Heart Assoc 7: e010193, 2018. 10.1161/JAHA.118.010193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agarwal S, Sud K, Martin JM, Menon V: Trends in the use of mechanical circulatory support devices in patients presenting with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 8: 1772–1774, 2015. 10.1016/j.jcin.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 90.Bruckner BA, Jacob LP, Gregoric ID, Loyalka P, Kar B, Cohn WE, La Francesca S, Radovancevic B, Frazier OH: Clinical experience with the TandemHeart percutaneous ventricular assist device as a bridge to cardiac transplantation. Tex Heart Inst J 35: 447–450, 2008 [PMC free article] [PubMed] [Google Scholar]
- 91.Markus B, Patsalis N, Chatzis G, Luesebrink U, Ahrens H, Schieffer B, Karatolios K: Impact of microaxillar mechanical left ventricular support on renal resistive index in patients with cardiogenic shock after myocardial infarction: A pilot trial to predict renal organ dysfunction in cardiogenic shock. Eur Heart J Acute Cardiovasc Care 9: 158–163, 2020. 10.1177/2048872619860218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Villa G, Katz N, Ronco C: Extracorporeal membrane oxygenation and the kidney. Cardiorenal Med 6: 50–60, 2015. 10.1159/000439444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kilburn DJ, Shekar K, Fraser JF. The complex relationship of extracorporeal membrane oxygenation and acute kidney injury: Causation or association? Biomed Res Int 2016, 1094296, 2016. 10.1155/2016/1094296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adamo L, Rocha-Resende C, Prabhu SD, Mann DL: Reappraising the role of inflammation in heart failure [erratum published in Nat Rev Cardiol 18: 735, 2021 10.1038/s41569-021-00534-3]. Nat Rev Cardiol 17: 269–285, 2020. 10.1038/s41569-019-0315-x [DOI] [PubMed] [Google Scholar]
- 95.Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF: The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit Care 20: 387, 2016. 10.1186/s13054-016-1570-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Keebler ME, Haddad EV, Choi CW, McGrane S, Zalawadiya S, Schlendorf KH, Brinkley DM, Danter MR, Wigger M, Menachem JN, Shah A, Lindenfeld J: Venoarterial extracorporeal membrane oxygenation in cardiogenic shock. JACC Heart Fail 6: 503–516, 2018. 10.1016/j.jchf.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 97.McILwain RB, Timpa JG, Kurundkar AR, Holt DW, Kelly DR, Hartman YE, Neel ML, Karnatak RK, Schelonka RL, Anantharamaiah GM, Killingsworth CR, Maheshwari A: Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab Invest 90: 128–139, 2010. 10.1038/labinvest.2009.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prondzinsky R, Lemm H, Swyter M, Wegener N, Unverzagt S, Carter JM, Russ M, Schlitt A, Buerke U, Christoph A, Schmidt H, Winkler M, Thiery J, Werdan K, Buerke M: Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: The prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med 38: 152–160, 2010. 10.1097/CCM.0b013e3181b78671 [DOI] [PubMed] [Google Scholar]
- 99.Gu J, Hu W, Xiao H, Feng X, Song Z, Chen Y, Zhang D: Prophylactic intra-aortic balloon pump reduces C-reactive protein levels and early mortality in high-risk patients undergoing percutaneous coronary intervention. Acta Cardiol 66: 499–504, 2011. 10.1080/AC.66.4.2126599 [DOI] [PubMed] [Google Scholar]