Abstract
Introduction
Mixed connective tissue disease (MCTD) is a rare autoimmune condition characterized by Scleroderma, Polymyositis, and Systemic Lupus Erythematous (SLE). Though a possible relationship between COVID-19 and autoimmune diseases has been recently reported, its pathophysiological mechanism behind flares in Lupus Nephritis (LN), a complication of SLE, remains unknown.
Case presentation
A 22-year-old COVID-19 positive female presented with anemia, bilateral pitting edema, periorbital swelling, and posterior cervical lymphadenitis. Further inspection revealed lower abdominal striae, hepatosplenomegaly, and hyperpigmented skin nodules. Complete blood counts showed elevated inflammatory markers and excessively high protein creatinine ratio. Antinuclear antibody titers were elevated (anti-smith and U1 small nuclear ribonucleoprotein) and Rheumatoid Factor was positive. She was diagnosed with MCTD associated with a flare of LN. To control her lupus flare, a lower dose of steroids was initially administered, in addition to oral hydroxychloroquine and intravenous cyclophosphamide. Her condition steadily improved and was discharged on oral steroid maintenance medication.
Discussion
We present a rare phenomenon of newly diagnosed LN, a complication of SLE, with MCTD in a PCR-confirmed COVID-19 patient. The diagnostic conundrum and treatment hurdles should be carefully addressed when patients present with lupus and COVID-19 pneumonia, with further exploration of the immuno-pathophysiology of COVID-19 infection in multi-systemic organ dysfunction in autoimmune disorders.
Conclusion
In COVID-19 patients with LN and acute renal injury, it is critical to promptly and cautiously treat symptomatic flares associated with autoimmune disorders such as SLE and MCTD that may have gone unnoticed to prevent morbidity from an additional respiratory infection.
Highlights
-
•
SLE disease has been associated with COVID-19. However, there is a lack of data on LN in conjunction with MCTD in COVID-19 positive patients.
-
•
A possible relationship between Coronavirus disease 2019 (COVID-19) and autoimmune disease has been documented in many case reports.
-
•
Because of the overlapping clinical manifestations and laboratory findings between lupus and COVID-19 pneumonia, the diagnostic problems and treatment hurdles should be carefully addressed.
-
•
In COVID-19 patients with LN flare and acute renal injury, it is critical to resolve any reversible causes of the kidney injury and manage the COVID-19 before treating the LN.
1. Introduction
Mixed Connective Tissue Disease (MCTD) was initially identified in 1972 as a condition characterized by overlapping characteristics of systemic sclerosis, systemic lupus erythematosus (SLE), and polymyositis [1]. Because the signs and symptoms of these three diseases may not always emerge simultaneously, diagnosing MCTD can be difficult. SLE is a chronic inflammatory autoimmune disease that presents a wide range of clinical symptoms owing to its influence on several organ systems, with Lupus Nephritis (LN) being one of the disease manifestations. LN affects up to five out of ten people with SLE and can manifest clinically as weight gain, hypertension, and foamy urine [2]. Despite emerging developments in the treatment of Lupus Nephritis, guidelines for management are not definitive and only consist of symptomatic relief globally [3].
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which causes Coronavirus Disease-2019 (COVID-19), has been a global epidemic for the past two years. COVID-19 causes a broad spectrum of clinical symptoms that impact various body systems, often appearing with respiratory signs and symptoms, such as flu-like illness exacerbated by acute respiratory distress syndrome (ARDS) and lung failure [4,5]. Additional symptoms and risks include severe metabolic syndrome, acute renal injury, neurological diseases, cardiovascular and thromboembolic events such as encephalopathy, seizures, and stroke [[6], [7], [8], [9], [10]]. A possible relationship between COVID-19 and autoimmune diseases such as SLE has also been recently documented in many case reports within the literature [[11], [12], [13]]. However, there is a lack of data and knowledge on LN in conjunction with MCTD in COVID-19 positive patients. Given the clinical importance of COVID-19 during the ongoing pandemic, the present paper elucidates a rare case of newly diagnosed LN in combination with MCTD in a PCR-confirmed COVID-19 patient. A review of the literature was conducted to analyse all linked clinical case reports and case series to provide an in-depth understanding of the relationship between COVID-19 and renal manifestations of Lupus.
2. Case Presentation
A 22-year-old COVID-19 positive female presented to the emergency department via an ambulance with fever, weight loss (20 kg), shortness of breath, loose stools, and multiple skin lesions present for the previous eight months. The fever was mild, intermittent, and alleviated by antipyretics. This was accompanied by frequent bowel movements (4–5 times per day) and progressive shortness of breath at rest and during exertion. However, there were no reports of orthopnea or paroxysmal nocturnal dyspnea. She also complained of polyuria and hematuria for the last two days. Her past medical history was insignificant, and other aspects of her health, including menstrual health, were unremarkable. There is no history of chronic disease in her family.
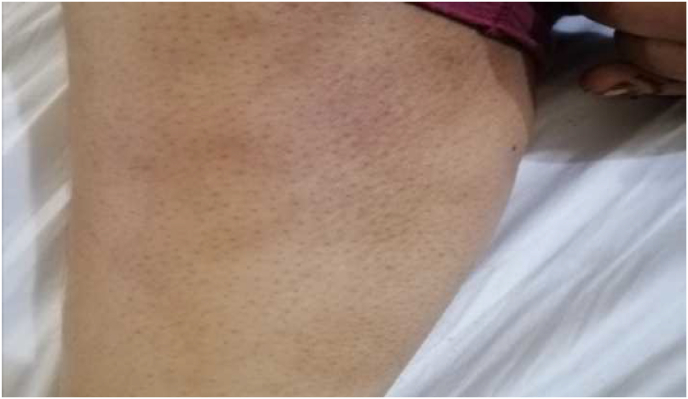
Upon presentation to the emergency department, she was a-febrile, tachypneic but hemodynamically stable, and well oriented to time and place. On inspection, there was a noticeable pallor, indicating a positive anemic state. Dehydration, bilateral pitting edema up to the shin, and periorbital swelling were also seen. Posterior cervical lymph nodes (less than 0.4cm) and a lymph node (1 cm) in the right axilla were palpable. Painful, itchy, indurated, and hyperpigmented lesions [Fig. 1] were observed in various places of her body, as well as a history of hair loss, mouth ulcers, and mouth dryness.
Fig. 1.
Hyperpigmented lesion on the leg.
Abdominal examinations revealed striae over the lower abdomen, a palpable spleen, and a liver with a 17-cm span. Furthermore, several cystic lesions were noticeable on breast examination, with the largest one measuring 1.4 × 0.9 cm in the right breast and 1.4 × 0.6 cm in the left breast. The lesions had a firmness and smooth edges.
Extensive investigations were carried out to rule out any potential diagnoses [Table 1]. A Complete Blood Count (CBC) profile revealed that the patient had low hemoglobin levels. An in-depth analysis of anemia resulted in the reporting of an increased reticulocyte count. Other cell lines were also deranged, with a high leukocyte count and thrombocytopenia. Based on the findings, further screening of inflammatory markers, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), revealed unusually increased serum levels for both.
Table 1.
Baseline Laboratory investigations.
| Test Name | Results | Normal Ranges |
|---|---|---|
| Complete blood count [CBC] | ||
| Hemoglobin | 6.3g/dl | 12–16 g/dl |
| HCT | 20.10 | 0.37–0.47 |
| MCV | 82 fl | 80-100 fl |
| MCH | 25.7 pg | 21–32 pg |
| MCHC | 31.3 Gm/dl | 33.4–35.5 Gm/dl |
| TLC | 17.5/uL | 3.6–110/uL |
| Neutrophils | 73% | 55–70% |
| Lymphocytes | 21% | 20–40% |
| Monocytes | 4% | 2–8% |
| Eosinophils | 2% | 1–4% |
| PLT | 660 × 10^6 mcL | 150–450 × 10^9 mcL |
| Reticulocyte Count | 1.3% | 0.2–2% |
| Inflammatory Markers | ||
| CRP | 153 mg/L | <5 mg/L |
| ESR | 102 mm/hr | 3–9 mm/hr |
| Fasting Lipid Profile | ||
| Cholesterol | 240 mg/dl | <200 mg/dl |
| Triglycerides | 612 mg/dl | 35–135 mg/dl |
| LDL | 140 mg/dl | <130 mg/dl |
| Total Lipid | 1202 mg/dl | <150 mg/dl |
| HDL | 26 mg/dl | <50 mg/dl |
| Protein creatinine ratio | 9.3g/day | <0.2/day |
| Urine Direct Report | ||
| Quantitiy | 40 ml | 800–2000 ml |
| Colour | Dark Yellow | Pale Yellow |
| Ph | 6.0 | 4.5–8 |
| Specific Gravity | 1.020 | 1.005–1.025 |
| Albumin | +++ | <30 mg/g |
| Sugars | Nil | 0–0.8 mmol/L |
| Blood (RBCs) | ++ | ≤3 |
| Red Cells (per hpf) | 12–13 | ≤2 |
| Pus cells | 2–4 | 0–4 |
| Nitrites | Nil | Nil |
| Granular Cast | ++ | Nil |
| Amorphous urate | ++ | – |
| Miscellaneous Tests | ||
| Total Protein | 7.6 g/dL | 6–8.3 g/dL |
| Serum Albumin | 1.3 g/dL | 3.4–5.4 g/dL |
| Serum Globulin | 6.3 g/dL | 2–3.5 g/dL |
| Albumin/Globulin ratio | 0.21 | 1.1–2.5 |
| D dimer | 0.2 | <0.5 |
| Lactose Dehydrogenase (LDH) | 514 U/L | 140–280 U/L |
Furthermore, the urine report was positive for protein, red blood cells, pus cells, granular casts, and urate but harmful for any infectious organism. The protein creatinine ratio was excessively high, indicating severe proteinuria. A comprehensive stool culture was also performed, which revealed the presence of mucus, red blood cells, pus cells, and yeast cells. Further investigation included viral markers, which were shown to be negative.
On suspicion of autoimmune disorders [Table 2], antinuclear antibodies (ANA) titers were carried which were found to be elevated. Along with this, Extractable Nuclear Antigen (ENA) profile revealed an increase in antibody titers to the anti-smith (Sm) and U1 small nuclear ribonucleoprotein (U1-RNP). Moreover, she was tested positive for Rheumatoid factor, while C3 and C4 complement levels were within range (see Table 3).
Table 2.
ANA-ENA Profile testing.
| Test Name | Results |
|---|---|
| ANA (Anti-nuclear antibodies) | Positive |
| ASMA | Negative |
| AMA | Negative |
| Serum Anti-dsDNA (IgG) | Negative |
| Rheumatoid Factor | Negative |
| Serum C3 | 1.21 |
| Serum C4 | 0.35 |
| Extractable Nuclear Antigen (ENA) PROFILE | |
| U1-RNP-Antibodies | 43.49 U/ml |
| SS-A/Ro- Antibodies | 0.54 U/ml |
| SS-B/La- Antibodies | 0.66 U/ml |
| Sm-Antibodies | >40 U/ml |
| Scl-70 Antibodies | 1.93 U/ml |
Table 3.
Summary of all the case reports and case series related to lupus in association with COVID-19.
| Authors | Country | Age, Gender | Disease duration | Lupus system involvement | Lupus medications | Severity of COVID-19 | |
|---|---|---|---|---|---|---|---|
| 14 | Watchmake J.M. et al. | United States | 60 years, F | 33 days | Respiratory, neurological | Steroids, rituximab, methotrexate, remdesivir, apixaban | Mild |
| 15 | Kreuter, A. et al. | Germany | 79 years, M | NA | Cutaneous, Musculoskeletal | hydroxychloroquine 200 mg twice daily and tapered intravenous glucocorticosteroid therapy | Not infected but vaccinated |
| 16 | Brockman, T. et al. | United States | 71 years, F | 90 days | Renal, respiratory, cardiac | Initially, Clopidogrel and heparin (discountinued later) followed by aspirin and colchicine | Severe |
| 17 | Muyldermans, A. et al. | Belgium | 56 years, M | 127 days | Respiratory, gastrointestinal | hydroxychloroquine 200 mg twice a day | Moderate |
| 18 | Roncati, L. et al. | Italy | 44 years, M | 8 days | Respiratory, neurologic | N.A | Moderate |
| 19 | Patil, S. et al. | India | 22 years, F | N.A | Musculoskeletal, cutaneous | prednisolone (50 mg daily) (tapered later) hydroxychloroquine (400 mg daily), mycophenolate mofetil (2 g daily), furosemide (20 mg daily), telmisartan (20 mg daily), folic acid, calcium, and vitamin D3 | (SLE) following COVID-19 vaccination with Covishield |
| 20 | Nespola, M. et al. | Italy | 47 years, F | 25 days | Vascular | low-dose oral corticosteroids |
Severe |
| 21 | Karsulovic, C. et al. | Chile | 28 years, M | 3 weeks | Respiratory, cutaneous | Hydroxychloroquine, Mycophenolate Mofetil 2 g a day Prednisone 20 mg a day with descending tapering | Mild |
| Karsulovic, C. et al. | Chile | 25 years, F | 4 weeks | Articular, hematologic and cutaneous | Hydroxychloroquine, Mycophenolate Mofetil 1 g a day (reinitiated) Prednisone 40 mg a day with descending tapering | Mild | |
| Karsulovic, C. et al. | Chile | 68 years, F | 4 weeks | Articular and cutaneous | Hydroxychloroquine, Prednisone 20 mg a day with descending tapering | Mild | |
| 22 | Yusuf, A.S. et al. | Malaysia | 30 years, F | 2 weeks | Renal, respiratory, cutaneous | Methylprednisolone 50mg daily) and oral hydroxychloroquine 200mg once daily | Mild |
| 23 | Hali, F. et al. | Morocco | 25 years, F | 19 days | Cutaneous, musculoskeletal, ophthalmic, cardiovascular and hematological | Methylprednisolone | Mild |
| 24 | El Aoud, S. et al. | France | 62 years, M | 39 days | Respiratory, renal, musculoskeletal, neurologic | methylprednisolone 120 mg IV for 2 repeated doses, tocilizumab (TCZ) at 600 mg, and Tazocilline. Two days later, corticoids were decreased to 80 mg for 2 days then 40 mg for 2 more days | Severe |
| 25 | Bahramnezhad, F. et al. | Iran | 56 years, M | N.A | Vascular | dexamethasone 8 mg three times daily (intravascular), hydroxychloroquine tablets 200 mg twice daily, remdesivir injection 200 mg on day 1 and 100 mg from day 2 to day 5, and interferon-beta 250 mg every 48 hours (subcutaneous) | Mild |
| 26 | Kincaid, K.J. et al. | United States | 43 F |
N.A | Hematological, Neurological |
mycophenolate and hydroxychloroquine | Mild |
| 27 | Smeele, H.T et al. | Netherlands | 31 years, F Gravida 1, para 0, gestational age of 38 weeks F |
N.A | Musculoskeletal | azathioprine (25 mg/day), hydroxychloroquine (200 mg/day), prednisone (5 mg/day). Prophylactic acetyl sialic acid was initiated after pregnancy was confirmed | Mild |
| Smeele, H.T et al. | Netherlands | 39 years, F | N.A | Musculoskeletal, renal | Hydroxychloroquine, azathioprine and etanercept. Prophylactic acetyl sialic acid was initiated after pregnancy was confirmed. | Mild | |
| 28 | Gracia-Ramos, A.E. et al. | Mexico | 45 years, M | N.A | Hematological, Musculoskeletal, Respiratory |
Pulse methylprednisolone therapy (1 g IV for 5 days) and chloroquine 150 mg per day | Moderate |
| 29 | Plotz, B. et al. | United States | 27 years, F | N.A | Cutaneous, gastrointestinal, Vascular |
Enoxaparin, Apixaban | Mild |
| 30 | Zamani, B. et al. | Iran | 39 years, M | 6 weeks | Cutaneous, renal and neurological | Pulse methylprednisolone (1000 mg for three consecutive days) continued with hydroxychloroquine and prednisolone | Mild |
| 31 | Domínguez-Rojas, J. et al. | Peru | 11 years, M | N.A | Musculoskeletal, gastrointestinal, cutaneous | IV immunoglobulin, acetylsalicylic acid and methylprednisolone acetate. Post biopsy: chemotherapy including etoposide, cyclosporine, dexamethasone, and methotrexate | Moderate |
| 32 | Cohen, M.K. et al. | Israel | 62 years, F | 2 months | Gastrointestinal, renal | low-dose prednisone, hydroxychloroquine, eltroxin, pregabalin, rosuvastatin, carbamazepine, ramipril, and clopidogrel | Mild |
| 33 | Pang, J.H.Q. et al. | Singapore | 30 years, M | 7 days | Gastrointestinal, Vascular |
low-molecular-weight heparin at 1 mg/kg, enoxaparin sodium injections | Mild |
| 34 | Ghafouri, S. et al. | United States | 89 years, M | N.A | Musculoskeletal | Patient non-compliant with medications | Critical |
| 35 | Shoskes, A. et al. | United States | 69 years, M | N.A | Cutaneous, renal and neurological | N.A | Mild |
| 36 | Guven, F. et al. | Turkey | 43 years, F | N.A | Neurological, hematological | N.A | Mild |
| 37 | Araten, D.J. et al. | United States | 39 years, F | 9 days | Vascular | eculizumab since the age of 28 | Mild |
| Araten, D.J. et al. | United States | 54 years, F | 3 months | Gastrointestinal, Vascular, Hematological |
Eculizumab, tacrolimus, mycophenolate, low doses of prednisone, and hydroxychloroquine | Mild | |
| Araten, D.J. et al. | United States | 60 years, F | N.A | Vascular | Eculizumab | Mild | |
| 38 | Bonometti, R. et al. | Italy | 85 years, F | N.A | Hematological, Renal, Neurological |
hydroxychloroquine | Moderate |
| 39 | He, F. et al. | China | 39 years, F | 32 days | Hematological, Renal, Musculoskeletal |
Prednisone, hydroxychloroquine, mycophenolate mofetil | Severe |
| 40 | Cardoso, E.M. et al. | United States | 18 years, F | 17 days | Renal, Hematological |
ceftazidime, vancomycin, azithromycin, and hydroxychloroquine | Severe |
| 41 | Gemcioglu, E. et al. | Turkey | 34 years, F | N.A | Neurological | acetyl salicylic acid, enoxaparin, favipiravir, hydroxychloroquine and azithromycin | Moderate |
| 42 | Yarlagadda, K. et al. | United States | 31 years, M | N.A | Respiratory, Hematological |
N.A | Moderate |
| 43 | Cho, J. et al. | Japan | 58 years, F | N.A | Hematological | prednisolone | Asymptomatic |
| Cho, J. et al. | Philippines | 32 years, F | N.A | Renal | hydroxychloroquine, mycophenolate mofetil and prednisolone | Moderate | |
| Cho, J. et al. | Philippines | 29 years, F | N.A | Renal | hydroxychloroquine, azathioprine and low-dose prednisolone | Moderate | |
| 44 | Arpali, E. et al. | Turkey | 28 years, F | N.A | Renal | Cyclophosphamide 500 mg/m2/mo for 7 months, mycophenolate mofetil, oral corticosteroids | Mild |
| 45 | Grimminck, K. et al. | Netherlands | 31- years, F G1P0, 38 + 1 weeks pregnant |
N.A | N.A | Methyldopa, prednisolone and azathioprine | Mild |
| 46 | Kichloo, A. et al. | United States | 22 years, F | 5 days | Respiratory, Renal and Cardiac |
Hydroxychloroquine, mycophenolic acid | Moderate |
Legends: N.A: Not Available, M: Male, F: Female, mg: milligram.
Ultrasonography was performed to thoroughly assess breast tissue, which revealed several cystic regions in the right breast, primarily in the upper quadrant. One measured 16.2 × 9.4 mm and extended into the retro-areolar area, displaying diffuse internal echoes. Multiple large lymph nodes measuring 16.0 × 9.4 mm were seen in the right axilla, along with hilar thinning. Multiple cystic regions were found dispersed throughout the parenchyma of the left breast, one of them being next to the areolar edge and measuring 15.8 × 6.8 mm. The discovered cysts were most likely complicated cysts. The left axilla showed a few swollen lymph nodes measuring 22.0 × 10.0 mm, as well as thinning of the hilum.
Echocardiography was performed to rule out cardiac involvement, which was expected. Along with an endoscopy, a color Doppler of the lower limbs was performed. Endoscopy revealed minor pangastritis, and a biopsy was performed (results are awaited). A Doppler examination of the lower limbs revealed no indications of stenosis, occlusion, or thrombosis. However, it did indicate bilateral soft tissue edema and a benign-looking inguinal lymph node on the right side.
The on-call nephrologist ordered a renal biopsy for further confirmation, and the results are still pending. Based on the clinical findings and laboratory investigations, the patient was diagnosed with MCTD associated with a flare of LN.
Despite the initial concerns regarding the commencement of steroids in an active COVID-19 infection, the management team decided to control her lupus flare with a lower steroid dose (intravenous methylprednisolone 50mg once daily) throughout hospitalization, in addition to oral hydroxychloroquine 200mg once daily. The patient was also given 1g intravenous cyclophosphamide once a month. Her condition steadily improved, and she was stable on the 7th day of her hospitalization. She was discharged on oral steroid maintenance medication with a follow-up appointment. At the follow-up appointment, the patient continues to do well with no evidence of recent flare-up and a complete resolution of her acute symptoms.
The present paper has been reported in accordance with the SCARE guidelines [14].
3. Methods
We conducted a thorough review of the literature and collated all clinical cases of LN and/or MCTD linked with COVID-19 infection, taking into account their place of origin, age, sex, body systems involving the disease, its associated medical regimen, and the severity of COVID-19 condition. We conducted a literature search on Pubmed using the terms ‘lupus nephritis', ‘systemic lupus erythematosus, ‘SLE,’ ‘Mixed Connective Tissue Disease,’ ‘MCTD,’ ‘COVID-19′, and ‘SARS-CoV-2'. The study included all case reports and case series. Articles that lacked extractable clinical data and a description of individual data were eliminated. The titles and abstracts of the retrieved publications were used to determine their eligibility. The eligibility criteria were met by a total of 33 papers involving 37 patients (Table 2).
4. Results
Out of the total papers, eleven articles were from Asia [19,22,25,30,32,33,36,39,41,43,44], eight from Europe [15,17,18,20,24,27,38,45], eleven from North America [14,16,26,28,29,34,35,37,40,42,46], two from South America [21,31] and one from Africa [23].
These Lupus patients were predominantly female (female/male ratio: 27:10). Fourteen of the cases had underlying LN. At the same time, there was only one patient who had underlying MCTD [21]. Moreover, most of the cases had musculoskeletal involvement [15,19,23,24,27,28,31,34,39].
For lupus management, more than half (56.7%) of the patients were on hydroxychloroquine therapy. Moreover, about half of the patients were given corticosteroids, while only nine were on mycophenolate mofetil.
We have analyzed and classified COVID-19 based on its severity, including asymptomatic, mild, moderate, severe, or critical. The majority of the patients (83.7%) were infected with mild to moderate COVID-19. In contrast, seven (18.9%) of the patients had severe to serious COVID-19. Except for 14 individuals, everyone was given systemic steroid therapy. Eculizumab was administered to three of the patients [37]. Tocilizumab IV was administered to a single patient [24]. Furthermore, for acute renal injury, only one patient required hemodialysis [40]. COVID-19 was linked to seven cases of thromboembolic events [20,25,29,33,37,41].
The clinical symptoms of active SLE and COVID-19 infection are often overlapping. Fever, rash, arthralgia, malaise, acute renal damage, and cytopenias are also symptoms of both disorders. Only four instances were documented to have a flare of lupus during the COVID-19 infection, according to our research [21,22,27,46].
5. Discussion
The relation of acute exacerbations of rheumatic and connective tissue diseases with viral infections like HIV, poliomyelitis, and influenza [47,48]. Because of the current COVID-19 pandemic, attention has been drawn to the possible flare-ups seen in patients with SLE and MCTD associated with mild COVID-19 infection, including diffuse lymphadenopathy [21] and full-blown SLE vasculitis [38]. A study by Jose L Pablos et al. statistically demonstrated how severe COVID-19 infection was a risk factor in diagnosing connective tissue disease, omitting inflammatory arthritis [49]. Moreover, Cheng Chen et al. reported in their study that during the COVID-19 pandemic, patients diagnosed with SLE abruptly ceased taking immunosuppressive therapy, which led to rapid flare-ups in their autoimmune conditions [50].
In this case, the patient had SLE and MCTD symptoms that were not recognized until she experienced a suspected flare-up of LN. During her active course of COVID-19 infection, she developed new-onset hematuria, proteinuria, bilateral pitting pedal edema, and periorbital edema, all of which were suggestive of Lupus Nephritis flare-up. Our patient was tested for autoimmune serology and found to have elevated levels of Anti-SM Antibodies, as well as ANA and Anti-U1 RNP Antibodies. Certain clinical features that confirm the diagnosis of SLE with MCTD include posterior cervical lymphadenitis, rheumatoid skin nodules, elevated inflammatory markers (ESR, CRP), and a deranged cell lineage. Though our patient was not commenced on immunosuppressive therapy during her illness, it did not affect her normal daily activities. This created the notion that COVID-19 infection may be associated with flare-ups in autoimmune disorders such as SLE and MCTD, which has not previously been documented in the literature.
The literature search primarily yielded case reports and case series involving the aforesaid patient population. The cohort size in the included studies was mainly limited to individual cases given the dearth of data and evolving COVID-19 literature. Furthermore, the follow-up duration for all of the studies was noted to be homogenous. The ongoing debate regarding the plight of SLE diagnosed individuals for an increased risk of acquiring COVID-19 infection due to immune dysregulation has already been assessed in a study of more than 900 patients (91% females) with the negative outcome of this hypothesis in which SLE diagnosed patients taking immunosuppressant like hydroxychloroquine and mycophenolate mofetil were not found to have an increase in COVID-19 infectivity rate [51]. Similarly, another study showed the same results, stating that patients with Lupus and the general population share the same COVID-19 hospitalization risk factors [52]. However, Giuseppe A. Ramirez et al. [48] concluded that COVID-19 could have a moderately increased morbidity in patients suffering from SLE, even though the study had certain limitations and selection bias, rendering the possibility controversial. In addition, another complication arising from overlap in symptoms of rheumatic flare and COVID-19 was observed in a retrospective study conducted in Tongji hospital, which stated that the overlapped symptoms were a cause of increased morbidity due to delayed diagnosis in patients presenting with respiratory infection due to COVID-19 [53]. Our patient, who presented with COVID-19 results but was later identified with chronic MCTD associated with LN, was a case that was somewhat but not entirely similar.
The onset of post-COVID-19 vaccine-associated SLE has also been reported in a case study by Miranda et al. [54], supporting the fact that COVID-19, as a multisystemic infection, has possible immune dysregulation mechanisms and antigen-autoantibody interactions, supporting the evidence of new-onset kidney disease in genetically susceptible individuals such as our patient. Our case is the first in our region to describe a newly diagnosed nephritic illness coupled with SLE and MCTD in a PCR-confirmed COVID-19 infected woman. The rarity of this occurrence suggests that it should be included in the literature.
6. Conclusion
We presented a case report of a PCR-confirmed COVID-19 positive patient with LN in association with SLE and MCTD. Because of the overlapping clinical manifestations and laboratory findings between lupus and COVID-19 pneumonia, the diagnostic problems and treatment hurdles should be carefully addressed. In COVID-19 patients with LN and acute renal injury, it is critical to promptly treat symptomatic flares associated with autoimmune disorders such as SLE and MCTD that may have gone unnoticed to prevent morbidity from the addition of a respiratory infection. However, the commencement of steroids at lower doses to treat lupus flare should be considered with caution in an active COVID-19 infection. To validate or reject the current findings, more extensive prospective studies are needed.
Ethical approval
NA.
Sources of funding
N/A.
Author contribution
SA, TA, UZ, FA, SS, FS: conceived the idea, designed the study, and drafted the manuscript, RT, MA, IA, MA, BS, ATA: Curated the literature review table and revised the first draft of the paper critically, MA, VRN, AS, MMA, MA: conducted literature search and screened the studies to fit the inclusion and exclusion criteria for the paper, JR, JR, HH: revised the manuscript critically and refined the literature review table based on reviewer comments, QSN, KAK, SK, SA, TA: revised the final version of the manuscript critically and gave the final approval.<a name = "Line_manuscript_48">
Registration of research studies
Name of the registry: NA.
Unique Identifying number or registration ID: NA.
Hyperlink to your specific registration (must be publicly accessible and will be checked): NA.
Guarantor
Talal Almas, RCSI University of Medicine and Health Sciences, 123 St. Stephen's Green, Dublin 2, Ireland, Talalalmas.almas@gmail.com.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Provenance and peer-review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
N/A.
References
- 1.Venables P.J. Mixed connective tissue disease. Lupus. 2006;15(3):132–137. doi: 10.1191/0961203306lu2283rr. [DOI] [PubMed] [Google Scholar]
- 2.Maidhof W., Hilas O. Lupus: an overview of the disease and management options. P T. 2012;37(4):240–249. [PMC free article] [PubMed] [Google Scholar]
- 3.Diagnosing and treating lupus | CDC. Cdc.gov. 2022. https://www.cdc.gov/lupus/basics/diagnosing.htm Retrieved 4 February 2022, from.
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Zavascki A.P., Falci D.R. Clinical characteristics of covid-19 in China. N. Engl. J. Med. 2020;382:1859. doi: 10.1056/NEJMc2005203. [DOI] [PubMed] [Google Scholar]
- 6.Khan I., Sarwar A., Ahmed Z. Atypical case of COVID-19 associated Kawasaki disease in an eight-year-old Pakistani boy. Cureus. 2020;12 doi: 10.7759/cureus.10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh R., Kashyap R., Hutton A., Sharma M., Surani S. A review of cardiac complications in coronavirus disease 2019. Cureus. 2020;12 doi: 10.7759/cureus.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon T., Gandhi S.A.Q., Tariq W., et al. Impact of chronic kidney disease on severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Cureus. 2021;13 doi: 10.7759/cureus.14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon T., Sharma R., Kataria S., et al. The association of acute kidney injury with disease severity and mortality in COVID- 19: a systematic review and meta-analysis. Cureus. 2021;13 doi: 10.7759/cureus.13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh R., Shiza S.T., Saadat R., Dawe M., Rehman U. Association of Guillain-Barre syndrome with COVID- 19: a case report and literature review. Cureus. 2021;13 doi: 10.7759/cureus.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joo Y.B., Lim Y.H., Kim K.J., Park K.S., Park Y.J. Respiratory viral infections and the risk of rheumatoid arthritis. Arthritis Res. Ther. 2019;21:199. doi: 10.1186/s13075-019-1977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gartshteyn Y., Askanase A.D., Schmidt N.M., et al. COVID-19 and systemic lupus erythematosus: a case series. Lancet Rheumatol. 2020;2:e452–e454. doi: 10.1016/S2665-9913(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalid M., Rogers S., Fatima A., et al. July 02 A flare of systemic lupus erythematosus disease after COVID-19 infection: a case of lupus cerebritis. Cureus. 2021;13(7) doi: 10.7759/cureus.16104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Kreuter A., Licciardi-Fernandez M.J., Burmann S.N., Burkert B., Oellig F., Michalowitz A.L. Induction and exacerbation of subacute cutaneous lupus erythematosus following mRNA-based or adenoviral vector-based SARS-CoV-2 vaccination. Clin. Exp. Dermatol. 2022;47(1):161–163. doi: 10.1111/ced.14858. Jan. Epub 2021 Sep 13. PMID: 34291477; PMCID: PMC8444843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brockman T., Hernandez L., Mehta T., Thapa B. Pericarditis as a secondary complication of COVID-19 in a renal transplant patient. Wis. Med. J. 2021;120(4):313–315. Dec. PMID: 35025181. [PubMed] [Google Scholar]
- 17.Muyldermans A., Maes P., Wawina-Bokalanga T., Anthierens T., Goldberg O., Bartiaux M., Soetens O., Wybo I., Van den Wijngaert S., Piérard D. Symptomatic severe acute respiratory syndrome coronavirus 2 reinfection in a lupus patient treated with hydroxychloroquine: a case report. J. Med. Case Rep. 2021;15(1):572. doi: 10.1186/s13256-021-03159-9. Nov 26. PMID: 34836543; PMCID: PMC8620303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roncati L., Corsi L., Barbolini G. Abnormal immunothrombosis and lupus anticoagulant in a catastrophic COVID-19 recalling Asherson's syndrome. J. Thromb. Thrombolysis. 2021;52(4):1043–1046. doi: 10.1007/s11239-021-02444-0. Nov. Epub 2021 Apr 12. PMID: 33844151; PMCID: PMC8040358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patil S., Patil A. Systemic lupus erythematosus after COVID-19 vaccination: acase report. J. Cosmet. Dermatol. 2021;20(10):3103–3104. doi: 10.1111/jocd.14386. Oct. Epub 2021 Aug 21. PMID: 34418261; PMCID: PMC8661983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nespola M., Sirignano P., Fermani N., Battocchio C., Tosti F., Pranteda C., Taurino M. Treatment-resistant acute upper limb Ischemia in a patient with systemic lupus erythematous and concomitant SARS-CoV-2 infection: a case report. Ann. Vasc. Surg. 2021;76:289–292. doi: 10.1016/j.avsg.2021.05.012. Oct. Epub 2021 Jun 25. PMID: 34182111; PMCID: PMC8233045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karsulovic C., Hojman L.P., Seelmann D.L., Wurmann P.A. Diffuse lymphadenopathy syndrome as a flare-up manifestation in lupus and mixed connective tissue disease following mild COVID-19. Am J Case Rep. 2021;22 doi: 10.12659/AJCR.932751. Sep. 10. PMID: 34504052; PMCID: PMC8445385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusuf A.S., Cheong X.K., Rozita M., Periyasamy P., Ruslinda M. A case of lupus nephritis flare-up in severe COVID-19 infection. Med. J. Malaysia. 2021;76(5):757–761. Sep. PMID: 34508391. [PubMed] [Google Scholar]
- 23.Hali F., Jabri H., Chiheb S., Hafiani Y., Nsiri A. A concomitant diagnosis of COVID-19 infection and systemic lupus erythematosus complicated by a macrophage activation syndrome: a new case report. Int. J. Dermatol. 2021;60(8):1030–1031. doi: 10.1111/ijd.15592. Aug. Epub 2021 Apr 17. PMID: 33864388; PMCID: PMC8251349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Aoud S., Morin C., Lorriaux P., Obert J., Sorial D., Chaabouni T., Thomas L. COVID-19 presenting as lupus erythematosus-like syndrome. Disaster Med. Public Health Prep. 2021;15(4):e12–e15. doi: 10.1017/dmp.2020.358. Aug. Epub 2020 Sep 10. PMID: 32907688; PMCID: PMC7642503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bahramnezhad F., Ghorbani B., Ghaedrahamt M., Jamaati H. Coronavirus- disease-2019-induced antiphospholipid-like syndrome: a case report. J. Med. Case Rep. 2021;15(1):408. doi: 10.1186/s13256-021-02966-4. Jul 29. PMID: 34321077; PMCID: PMC8318629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kincaid K.J., Simpkins A.N. Failure of anticoagulation to prevent stroke in context of lupus-associated anti-phospholipid syndrome and mild COVID-19. J. Stroke Cerebrovasc. Dis. 2021;30(7) doi: 10.1016/j.jstrokecerebrovasdis.2021.105817. Jul. Epub 2021 Apr 12. PMID: 33933349; PMCID: PMC8041145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smeele H.T., Perez-Garcia L.F., Grimminck K., Schoenmakers S., Mulders A.G., Dolhain R.J. Systemic lupus erythematosus and COVID-19 during pregnancy. Lupus. 2021;30(7):1188–1191. doi: 10.1177/09612033211002270. Jun. Epub 2021 Mar 14. PMID: 33715506; PMCID: PMC8120627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gracia-Ramos A.E., Saavedra-Salinas M.Á. Can the SARS-CoV-2 infection trigger systemic lupus erythematosus? A case-based review. Rheumatol. Int. 2021;41(4):799–809. doi: 10.1007/s00296-021-04794-7. Apr. Epub 2021 Feb 4. PMID: 33543338; PMCID: PMC7861004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plotz B., Castillo R., Melamed J., Magro C., Rosenthal P., Belmont H.M. Focal small bowel thrombotic microvascular injury in COVID-19 mediated by the lectin complement pathway masquerading as lupus enteritis. Rheumatology. 2021;60(2):e61–e63. doi: 10.1093/rheumatology/keaa627. Feb 1. Erratum in: Rheumatology (Oxford). 2021 Jul 1;60(7):3485. PMID: 33147605; PMCID: PMC7665776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zamani B., Moeini Taba S.M., Shayestehpour M. Systemic lupus erythematosus manifestation following COVID-19: a case report. J. Med. Case Rep. 2021;15(1):29. doi: 10.1186/s13256-020-02582-8. Ja 25. PMID: 33494816; PMCID: PMC7832415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domínguez-Rojas J., Atamari-Anahui N., Chonlon-Murillo K., Tello M., Coronado-Muñoz Á. Systemic lupus erythematosus complicated with macrophage activation syndrome mimicking COVID-19 multisystemic inflammatory syndrome in children. Bol. Med. Hosp. Infant. Mex. 2021;78(6):642–646. doi: 10.24875/BMHIM.21000064. English. PMID: 34934208. [DOI] [PubMed] [Google Scholar]
- 32.Kornowski Cohen M., Sheena L., Shafir Y., Yahalom V., Gafter-Gvili A., Spectre G. An early unexpected immune thrombotic thrombocytopenic purpura relapse associated with SARS-CoV-2 infection: a case report and literature review. Acta Haematol. 2021;144(6):678–682. doi: 10.1159/000514283. Epub 2021 Apr 23. PMID: 33895748; PMCID: PMC8247821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pang J.H.Q., Tang J.H., Eugene-Fan B., Lee C.L., Low J.K. A peculiar case of small bowel stricture in a coronavirus disease 2019 patient with congenital adhesion band and superior mesenteric vein thrombosis. Ann. Vasc. Surg. 2021;70:286–289. doi: 10.1016/j.avsg.2020.08.084. Jan. Epub 2020 Aug 28. PMID: 32861849; PMCID: PMC7453213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghafouri S., Rettig M., Kahlon K.S. An 89-year-old man with COVID-19-associated coagulopathy presenting with a prolonged partial thromboplastin time, lupus anticoagulant, and a high titer of factor VIII Inhibitor. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.926728. Oct 30. PMID: 33122620; PMCID: PMC7610155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kornowski Cohen M., Sheena L., Shafir Y., Yahalom V., Gafter-Gvili A., Spectre G. An early unexpected immune thrombotic thrombocytopenic purpura relapse associated with SARS-CoV-2 infection: a case report and literature review. Acta Haematol. 2021;144(6):678–682. doi: 10.1159/000514283. Epub 2021 Apr 23. PMID:33895748; PMCID: PMC8247821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guven F., Ogul H., Turgut A., Tezcan A., Kantarci M. Leptomeningeal involvement in a patient with systemic lupus erythematosus infected by COVID-19. Joint Bone Spine. 2020;87(5):495. doi: 10.1016/j.jbspin.2020.06.002. Oct. Epub 2020 Jun 10. PMID: 32534206; PMCID: PMC7286267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araten D.J., Belmont H.M., Schaefer-Cutillo J., Iyengar A., Mattoo A., Reddy R. Mild clinical course of COVID-19 in 3 patients receiving therapeutic monoclonal antibodies targeting C5 complement for hematologic disorders. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.927418. Sep. 12. PMID: 32917848; PMCID: PMC7508305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonometti R., Sacchi M.C., Stobbione P., Lauritano E.C., Tamiazzo S., Marchegiani A., Novara E., Molinaro E., Benedetti I., Massone L., Bellora A., Boverio R. The first case of systemic lupus erythematosus (SLE) triggered by COVID-19 infection. Eur. Rev. Med. Pharmacol. Sci. 2020;24(18):9695–9697. doi: 10.26355/eurrev_202009_23060.PMID:33015814. Sep. [DOI] [PubMed] [Google Scholar]
- 39.He F., Luo Q., Lei M., Fan L., Shao X., Hu K., Qin S., Yu N., Cao J., Yang L. Successful recovery of recurrence of positive SARS-CoV-2 RNA in COVID-19 patient with systemic lupus erythematosus: a case report and review. Clin. Rheumatol. 2020;39(9):2803–2810. doi: 10.1007/s10067-020-05230-0. Sep. Epub 2020 Jul 28. PMID: 32725351; PMCID: PMC7385201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani Cardoso E., Hundal J., Feterman D., Magaldi J. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin. Rheumatol. 2020;39(9):2811–2815. doi: 10.1007/s10067-020-05310-1. Sep. Epub 2020 Jul 28. PMID: 32720260; PMCID: PMC7384868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gemcioglu E., Erden A., Davutoglu M., Karabuga B., Kucuksahin O. Acute Ischemic stroke in a lupus anticoagulant-positive Woman with COVID-19. J. Clin. Rheumatol. 2020;26(6):236–237. doi: 10.1097/RHU.0000000000001565. Sep. PMID: 32694351; PMCID: PMC7437427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarlagadda K., Mi K., Sendil S., Koons C.L., Komanduri S., Cinicola J.T. A 31-year- old man with COVID-19-associated empyema and lupus anticoagulant. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.926623. Aug 18. PMID: 32807764; PMCID: PMC7458696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho J., Kandane-Rathnayake R., Louthrenoo W., Hoi A., Golder V., Chen Y.H., Luo S.F., Wu Y.J., Hamijoyo L., Lau C.S., Navarra S., Zamora L., Tee M., Flora A., Jr., Li Z.G., An Y., Sockalingam S., Katsumata Y., Harigai M., Hao Y., Zhang Z., Kikuchi J., Takeuchi T., Basnayake D., Goldblatt F., Chan M., Ng K.P.L., Bae S.C., Oon S., O'Neill S., Gibson K., Kumar S., Law A.H.N., Tugnet N., Tanaka Y., Nikpour M., Morand E., Lateef A. COVID-19 infection in patients with systemic lupus erythematosus: data from the Asia Pacific Lupus Collaboration. Int J Rheum Dis. 2020;23(9):1255–1257. doi: 10.1111/1756-185X.13937. Aug. Epub 2020 Aug 25. PMID: 32841510; PMCID: PMC7461525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arpali E., Akyollu B., Yelken B., Tekin S., Turkmen A., Kocak B. Case report: a kidney transplant patient with mild COVID-19. Transpl. Infect. Dis. 2020;22(4) doi: 10.1111/tid.13296. Aug. Epub 2020 May 4. PMID: 32301198; PMCID: PMC7235513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grimminck K., Santegoets L.A.M., Siemens F.C., Fraaij P.L.A., Reiss I.K.M., Schoenmakers S. No evidence of vertical transmission of SARS-CoV-2 after induction of labour in an immune-suppressed SARS-CoV-2-positive patient. BMJ Case Rep. 2020;13(6) doi: 10.1136/bcr-2020-235581. Jun 30. PMID: 32606133; PMCID: PMC7358094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kichloo A., Aljadah M., Albosta M., Wani F., Singh J., Solanki S. COVID-19 and acute lupus pneumonitis: diagnostic and treatment dilemma. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620933438. Jan-Dec. PMID: 32500773; PMCID: PMC7277418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arora G., Kassir M., Jafferany M., et al. The COVID-19 outbreak and rheumatologic skin diseases. Dermatol. Ther. 2020;33(4) doi: 10.1111/dth.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramirez G., Gerosa M., Beretta L., Bellocchi C., Argolini L., Moroni L., Della Torre E., Artusi C., Nicolosi S., Caporali R., Bozzolo E., Dagna L. COVID-19 in systemic lupus erythematosus: data from a survey on 417 patients. Semin. Arthritis Rheum. 2020;50(5):1150–1157. doi: 10.1016/j.semarthrit.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pablos J.L., Galindo M., Carmona L. RIER Investigators Group, et alClinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort studyAnnals of the. Rheumatic Diseases. 2020;79:1544–1549. doi: 10.1136/annrheumdis-2020-218296. [DOI] [PubMed] [Google Scholar]
- 50.Chen C., Yao B., Yan M., Su K., Wang H., Xu C. The plight of patients with lupus nephritis during the outbreak of COVID-19 in Wuhan, China. J. Rheumatol. 2020;47(9) doi: 10.3899/jrheum.200452. 1452.2-1452. [DOI] [PubMed] [Google Scholar]
- 51.Favalli E.G., Gerosa M., Murgo A., et al. alAre patients with systemic lupus erythematosus at increased risk for COVID-19? Ann. Rheum. Dis. 2021;80:e25. doi: 10.1136/annrheumdis-2020-217787. [DOI] [PubMed] [Google Scholar]
- 52.NYU Langone News Studies show similar COVID-19 risk factors for patients with lupus or inflammatory arthritis as general population. 2022. https://nyulangone.org/news/studies-show-similar-covid-19-risk-factors-patients-lupus-or-inflammatory-arthritis-general-population [online] Available at:
- 53.Ye C., et al. Clinical features of rheumatic patients infected with COVID-19 in Wuhan, China. Ann. Rheum. Dis. 2020;79:1007–1013. doi: 10.1136/annrheumdis-2020-217627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zavala-Miranda M., González-Ibarra S., Pérez-Arias A., Uribe-Uribe N., Mejia-Vilet J. New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int. 2021;100(6):1340–1341. doi: 10.1016/j.kint.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]