Abstract
Background
Thromboembolism is the third most common cardiovascular disorders and substantial worldwide health burden, with 1–2 instances per 1000 persons each year. This study aimed to describe the epidemiological characteristics, clinical relevance, risk factor and outcome of thromboembolic complications among COVID-19 infected patients.
Method
This is a retrospective, single-center, observational study using a hospital information system (HIS). The study included 46-patients with a confirmed diagnosis of pneumonia by SARS-CoV-2 admitted to a tertiary hospital.
Results
The incidence of cardiovascular thromboembolic events among COVID-19 infected patients was 41.3% (n = 19). Cerebrovascular accident was the most common thromboembolic events among COVID-19 infected patients about 15.2%, flowed by pulmonary embolism (13%), acute myocardial infract (8.7%), and deep venous thrombosis (4.4%). In generally, 63% (n = 29) were males, while 37% (n = 17) were females. The majority of those who suffered thromboembolic events were over 65 years old (p < 0.000**).
Patients with thromboembolic event were also more likely to have IHD (13.0% vs 0%, p = 0.003), diabetes (24% vs 13.0%, p = 0.025) and CL (10.9% vs 2.2%, p = 0.03) as precipitating factors when compared those without thromboembolic events.
According to the outcome, 19 examinees had thrombotic events: 11 (24%) patients had admitted to non ICU inpatient ward, 2 (43%) had admitted to ICU and remaining 6 (13%) patients had dead. There was significant statistical difference in the proportion of examinees with thrombotic and non-thrombotic events in relation to outcome (p = 0.000).
Conclusion
The incidence of thromboembolic complications among COVID19 infected patients were associated with elder (>65years), IHD, diabetes and CLD.
Keywords: Thromboembolic, Cardiovascular, Stroke, Myocardial infract, Pulmonary embolism, Deep venous thrombosis
Highlights
-
•
Thromboembolism is the third most common cardiovascular disorders and substantial worldwide health burden, with 1–2 instances per 1000 persons each year.
-
•
The incidence of cardiovascular thromboembolic among COVID19 infected patients is relatively high, and it is associated with elder (>65years), IHD, diabetes and chronic liver disease. There was significant statistical difference in the proportion of examinees with thrombotic and non-thrombotic events in relation to outcome (p = 0.000).
-
•
In our knowledge there is no study explored the role of incidence, risk factors and outcome of thromboembolic diseases among patients with SARSCoV-2 infection in Somalia. Similarly, limited data are currently available about the incidence of thromboembolic in Sub-Sahara African countries. This study aimed to describe the epidemiological characteristics, clinical relevance, risk factor and outcome of cardiovascular thromboembolic complications among COVID-19 infected patients.
1. Introduction
In December 2019, people in Wuhan, China, had reported patients presented with pneumonia like symptoms which was later on described as corona virus disease of 2019 (COVID-19) pneumonia caused by the severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) [1]. In March 2020, World Health Organization (WHO) was officially declared a pandemic [2].
Thromboembolism is the third most common cardiovascular disorders and substantial worldwide health burden, with 1–2 instances per 1000 persons each year [3].
Acute respiratory distress syndrome (ARDS) and end-organ failure are the most common causes of morbidity and mortality in COVID-19 patients, also cardiovascular complications such as myocardial infarction (MI), ischemic stroke, and pulmonary embolism (PE) can also cause disability and death in these patients [[4], [5], [6]].
In critically ill patients, the risk of thromboembolic is increased in the presence of COVID-19 infection. In addition to multiple mechanisms such as asystemic inflammatory condition, mechanical ventilation, central catheters, immobility, hemostatic abnormalities, and hepatic alterations enhance the risk of thromboembolic events [[7], [8], [9]]. A high prevalence of in situ micro-thrombosis suspected to be due to endothelial injury from direct viral infection has also been described [[10], [11], [12]].
Until now, in our knowledge there is no study explored the role of incidence, risk factors and outcome of thromboembolic diseases among patients with SARSCoV-2 infection in Somalia. Similarly, limited data are currently available about the incidence of thromboembolic in Sub-Sahara African countries. This study aimed to describe the epidemiological characteristics, clinical relevance, risk factor and outcome of cardiovascular thromboembolic complications among COVID-19 infected patients.
2. Method
This is a retrospective, single-center, observational, cohort study using a hospital information system (HIS). The study included 46 patients with a confirmed diagnosis of pneumonia by SARS-CoV-2 admitted to Mogadishu Somali Turkish Training and Research Hospital located in Mogadishu Somalia during the second wave of COVID-19 between January to February 2020.
One research resident collected data on demographics, comorbidities, clinical symptoms, laboratory and radiological analyses retrospectively. All patient.
Nineteen patients had fulfilled arterio-venous thromboembolic criteria of our study. The criteria included: a confirmed clinicoradiological diagnosis of SARS-CoV-2 pneumonia, according to the criteria proposed by the Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment or positive Real-time reverse transcriptase polymerase-chain-reaction (RT-PCR) assay test for SARS-CoV-2 in throat swab specimens. Pregnant women, patients who have cancer or taking anti-cancer drugs, patients who have known disease of thromboembolic with treatment including MI, PE, and DVT, and children (those younger than 18 years of age) were excluded from the study.
The arterio-venous thromboembolic complication of COVID-19 included; pulmonary embolism (PE) and deep vein thrombosis (DVT) those were diagnosed by imaging tests included whole-leg ultrasound and chest CT angiography with clinical features such as shortness of breath, chest pain, palpitation, and lower limb pain and swelling. Moreover, the cardiovascular thromboembolic complications included acute coronary syndrome/acute myocardial infarction and ischemic stroke was diagnosed by ECG and Brain CT scan, or MRI with clinical presentations included altered level of consciousness, focal neurologic deficit, shortness of breath, chest pain, and palpitation. The baseline characteristics of the cases included age and sex, and comorbidities included diabetes mellitus, hypertension, dyslipidemia, CAD, and organ failure. Pregnant women, patients having coagulopathies, patients taking anti-cancer drugs, and children (those younger than 18 years of age) were excluded from the study.
The study was approved by the medical ethical committee from the institutional Review Board of Mogadishu Somalia-Turkey Recep Tayyip Erdogan Training and Research Hospital, Mogadishu, Somalia (Ref. MSTH-4127), and patient's informed consent was waived by the Institutional Review Board and no harm could potentially be done to patients.
Continuous variables are represented by means and standard deviations, and categorical variables are represented by numbers and proportions. Fisher's exact test and independent t-test were used for categorical and continuous variables, respectively, in a two-group comparison (patients with and without thromboembolic complication). SPSS software version 23 was used to conduct the statistical analysis. P < 0.5 was considered statistically significant.
3. Results
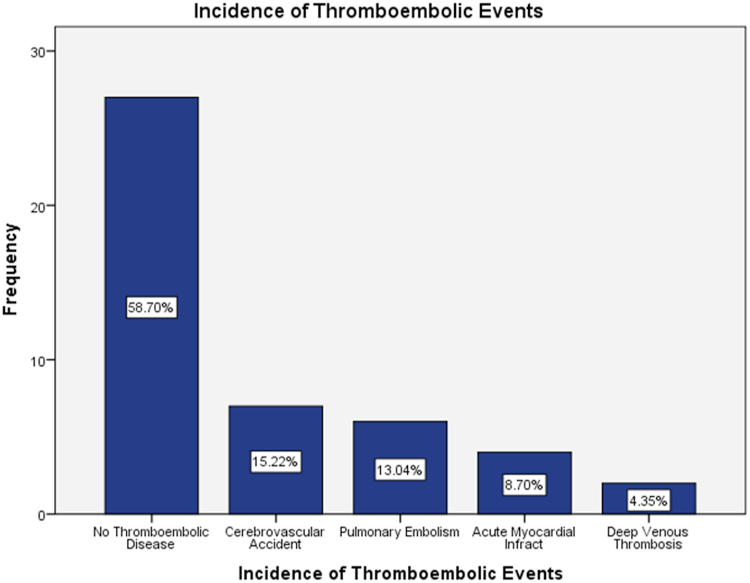
The prevalence of cardiovascular thromboembolic events among COVID-19 infected patients was 41.3% (n = 19). Characteristics and risk factors of both thromboembolic and non-thromboembolic patients hospitalized for COVID-19 pneumonia are shown in Table 1. Cerebrovascular accident was the most common thromboembolic events among COVID-19 infected patients about 15.2%, flowed by pulmonary embolism (13%), acute myocardial infract (8.7%), and deep venous thrombosis (4.4%) as show in Fig. 1.
Table 1.
Differences in demographic characteristics, clinical Manifestations, and risk factors between Thromboembolic and Non-Thromboembolic patients with COVID-19 pneumonia.
| Variables | Thromboembolic event |
Non Thromboembolic event |
P-Value | |||
|---|---|---|---|---|---|---|
| Frequency | Percentage (%) | Frequency | Percentage (%) | |||
| Sex | Male | 10 | 21.7 | 19 | 41.3 | 0.180 |
| Female | 9 | 19.6 | 8 | 17.4 | ||
| Age | 15-24? | 2 | 4.3 | 0 | 0 | 0.000** |
| 25–39 | 1 | 2,2 | 15 | 32.6 | ||
| 40–65 | 6 | 13 | 9 | 19.6 | ||
| >65 | 10 | 21.7 | 3 | 6.5 | ||
| Risk Factors | Smoker | 8 | 17.4 | 7 | 15.2 | 0.495 |
| IHD | 6 | 13 | 0 | 0 | 0.003** | |
| Hypertention | 3 | 6.5 | 3 | 6.5 | 0.484 | |
| Diabetes | 11 | 24 | 6 | 13 | 0.015* | |
| Hyperlipidemia | 0 | 0 | 2 | 4.3 | 0.339 | |
| COPD | 3 | 6.5 | 3 | 6.5 | 0.484 | |
| CLD | 5 | 11 | 1 | 2.2 | 0.03* | |
| CRD | 1 | 2.2 | 4 | 8.7 | 0.302 | |
| Malignancy | 2 | 4.3 | 1 | 2.2 | 0.368 | |
| Heart Failure | 3 | 6.5 | 1 | 2.2 | 0.184 | |
| Clinical Features | Cough | 12 | 26.1 | 24 | 52.2 | 0.043* |
| SOB | 15 | 32.6% | 20 | 43.5 | 0.492 | |
| Fever | 12 | 26.1 | 19 | 41.3 | 0.421 | |
| Headache | 10 | 21.7 | 6 | 13 | 0.035* | |
| Chest pain | 13 | 28.3 | 2 | 4.3 | 0.000* | |
| Hemiplegia | 7 | 15.2 | 1 | 2.2 | 0.001** | |
| ALOC | 8 | 17.4 | 2 | 4.3 | 0.021* | |
| Fatigue | 3 | 6.5 | 6 | 13 | 0.441 | |
| Other symptoms | 2 | 4.3 | 2 | 4.3 | 0.423 | |
IHD; Ischemic heart disease, COPD; Chronic abstractive pulmonary disease, CLD; Chronic liver disease, CRD; Chronic renal disease, SOB; Shortness of breath, ALOC; Altered level of conscious.
Fig. 1.
The incidence of cardiovascular thromboembolic complications among patients with COVID-19 pneumonia.
In generally, 63% (n = 29) were males, while 37% (n = 17) were females. According to the thromboembolic and thromboembolic events no difference between the two genders (male = 52.6%; n = 10, vs female = 47.4%; n = 9). There was no significant statistical difference in the proportion of examinees with thromboembolic events and non-thromboembolic events in relation to gender (p = 0.180).
The majority of the overall participants (34.8%) were in the age group of 25–39 years old. There are more non-thromboembolic COVID-19 pneumonia patients in this age group than thromboembolic COVID-19 pneumonia patients. Among COVID-19 infected individuals, the majority of those who suffered thromboembolic events were over 65 years old (p < 0.000**).
Thromboembolic event among COVID-19 infected patients were also more likely to have ischemic heart disease (IHD) (13.0% vs 0%, p = 0.003), diabetes (24% vs 13.0%, p = 0.025) and chronic liver disease (CLD) (10.9% vs 2.2%, p = 0.03) as precipitating factors of thromboembolic events among COVID-19 infected patients. This cohort had also more frequently comorbidities, such as hypertension, smoker, malignancy, as well as, heart failure and chronic renal disease.
As compared to those with thromboembolic events, participants with non-thromboembolic events mostly had presented headache (21.7% versus 13%, p < 0.035*), chest pain (28.3% versus 4.3%, p < 0.000*), hemiplegia (15.2% versus 2.2% versus 21.6%%, p = 0.001), and altered level of conscious (17.4% versus 2.2% versus 4.3%%, p = 0.021), but a lower proportion of participants with cough (26.1% versus 52.2%, p < 0.043*), shortness of breath (32.6% versus 63% versus 43.5%, p < 0.492), fever (26.1% versus 63% versus 41.3%, p < 0.421), and fatigue (6.5% versus 63% versus 13%, p < 0.423).
Regarding laboratory findings on admission, COVID-19 infected patients with thromboembolic events were presented more often with WBC, platelets, CRP, AST, D-dimer, and Troponin levels and less frequently with urea, creatinine, ALT, D-dimer, and troponin than their non-thromboembolic counterpart (Table 2).
Table 2.
Differences in laboratory findings of COVID-19 pneumonia between with thromboembolic events and those without this condition.
| Variables | Thromboembolic event |
Non Thromboembolic event |
P-Value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Laboratory findings | WBC | 11.4 ± 6.7 | 8.7 ± 3.6 | 0.112 |
| Neutropen | 70.1 ± 17.8 | 49.6 ± 18.7 | 0.412 | |
| Lymphocytes | 13.4 ± 5.6 | 13.3 ± 17.3 | 0.361 | |
| Hemoglobin | 12 ± 2.6 | 12.3 ± 2.7 | 0.459 | |
| Platelets | 343.7 ± 218.5 | 365.9 ± 343.5 | 0.018* | |
| CRP | 81.2 ± 76.6 | 46.1 ± 36.3 | 0.51 | |
| Urea | 38. ±24.8 | 49.7 ± 48.2 | 0.323 | |
| Creatinine | 0.98 ± 0.31 | 2.08 ± 1.9 | 0.851 | |
| AST | 56.6 ± 48.2 | 33.6 ± 28.7 | 0.211 | |
| ALT | 35.1 ± 29 | 39.9 ± 20.2 | 0.417 | |
| D-dimer | 4.26 ± 4 | 0.8 ± 0.62 | 0.192 | |
| Troponin | 2.2 ± 6.2 | 0.5 ± 0.3 | 0.221 | |
WBC; White blood cells, CRP; C-reactive protein, AST; Aspartate transaminase, ALT; Alanine aminotransferase.
Table 3 summarizes the chest CT findings of the study population, participants with thromboembolic events had a lower prevalence of GCO (40% versus 43.4%, p = 0.180), consolidation (13% vs 14.2% P = 0.462), air bronchogram (11% vs 15.2% P = 0.618), and crazy paving (8.7% versus 11%, p < 0.559) but a higher extent of linear opacities (13% versus 2.2% p = 0.015), pleural effusion (11% versus 4.3% p = 0.912), lymphadenopathy (8.7% versus 6.5% p = 0.303), and cavitation (8.7% versus 2.2% p = 0.085) compared to those with non-thromboembolic events.
Table 3.
Differences in chest CT scan of COVID-19 pneumonia between with thromboembolic events and those without this condition.
| Variables | Thromboembolic event |
Non Thromboembolic event |
P-Value | |||
|---|---|---|---|---|---|---|
| Frequency | Percentage (%) | Frequency | Percentage (%) | |||
| Chest CT scan Findings | GCO | 17 | 40 | 20 | 43.4 | 0.180 |
| Consolidation | 6 | 13 | 7 | 15.2 | 0.462 | |
| Air bronchogram | 5 | 11 | 7 | 15.2 | 0.618 | |
| Crazy paving | 4 | 8.7 | 5 | 11 | 0.559 | |
| Linear Opacities | 6 | 13 | 1 | 2.2 | 0.015* | |
| Pleural effusion | 5 | 11 | 2 | 4.3 | 0.912 | |
| Lymphadenopathy | 4 | 8.7 | 3 | 6.5 | 0.303 | |
| Cavitation | 4 | 8.7 | 1 | 2.2 | 0.085 | |
GCO; Ground class opacities.
In accordance with therapeutic modalities, patients with thromboembolic event had higher prevalence for administration of anticoagulant (41.3%, n = 19, p < 0.000), oxygen 19.6%, n = 9, p = 0.118 and invasive mechanical ventilation (19.6%, n = 9, p < 0,008) when compared to the patients with non-thromboembolic events (Table 4).
Table 4.
Differences in therapeutic modalities and outcomes of COVID-19 pneumonia during hospitalization between with thromboembolic events and those without this condition.
| Variables | Thromboembolic event |
Non Thromboembolic event |
P-Value | |||
|---|---|---|---|---|---|---|
| Frequency | Percentage (%) | Frequency | Percentage (%) | |||
| Therapeutic Modalities | Anticoagulant | 19 | 41.3 | 7 | 15.2 | 0.000** |
| Aspirin | 9 | 19.6 | 14 | 30.4 | 0.500 | |
| Antibiotics | 11 | 24 | 12 | 26.1 | 0.275 | |
| Oxygen | 9 | 19.6 | 7 | 15.2 | 0.118 | |
| Steroid | 7 | 15.2 | 7 | 15.2 | 0.319 | |
| IMV | 9 | 19.6 | 3 | 6.5 | 0.008** | |
| Outcome | Discharge to Home | 0 | 0 | 21 | 46.7 | 0.000** |
| Admitted to Non ICU IP | 11 | 24 | 3 | 6.5 | ||
| Admitted to ICU | 2 | 4.3 | 2 | 4.3 | ||
| Dead | 6 | 13 | 1 | 2.2 | ||
ICU; Intensive care unit, IP; In patient.
According to the outcome, most of the patients with non-thromboembolic events had been discharged to home (46.7%, n = 21), flowed by 3 (6.5%) patients admitted to non ICU inpatient ward, 2 (4.3%) patients admitted to ICU, and 1 (2.2%) patients had dead, while 19 examinees had thrombotic events: 11 (24%) patients had admitted to non ICU inpatient ward, 2 (4.3%) had admitted to ICU and remaining 6 (13%) patients had dead. There was significant statistical difference in the proportion of examinees with thrombotic and non-thrombotic events in relation to outcome (p = 0.000).
4. Discussion
Limited data are currently available about the incidence of thromboembolic in Sub-Sahara African countries and in our knowledge there is no study explored the role of incidence, risk factors and outcome of thromboembolic diseases among patients with SARSCoV-2 infection in Somalia. This study aimed to describe the epidemiological characteristics, clinical relevance, risk factor and outcome of thromboembolic complications among COVID-19 infected patients.
Recent studies have found a significant frequency of thrombotic events in viral infections including corona-virus [[13], [14], [15]]. A meta-analysis included 8271 cases have reported thromboembolic rates in the range of 20–30% while others have reported rates as high as 40–70% [16].
In an evaluation of 184 severely ill COVID-19 patients, 31% of the patients suffered thrombotic complications, the most common of which was pulmonary embolism [17]. Similarly, another retrospective cohort study of 388 cases found that COVID-19 patients had a significant rate of venous thromboembolism (21%) [18].
The present study showed that the incidence of thrombotic events among hospitalized patients with COVID-19 was 41.3% (n = 19). This significant variability is likely attributable to differences in the clinical and socio-demographic features of the study participants, as well as sample size and study design. The inflammatory process, cytokine storm, lung injury, and endothelial injury that enhance the risk of hypercoagulable condition in hospitalized COVID-19 patients are likely to be the cause of this high prevalence of thrombotic events [19].
In the current study, cerebrovascular accident was the most common thromboembolic events among COVID-19 infected patients about 15.2%, flowed by pulmonary embolism (PE) (13%), acute myocardial infract (AMI) (8.7%), and deep venous thrombosis (4.4%).
A study done by Lodigiani et al. conducted that the incidence of PE, AMI and acute stroke were 33%, 2.5% and 1.1%, respectively [17]. Of 221 patients with COVID-19 at a hospital in Wuhan, 11 (5%) developed acute ischemic stroke [20].
PE was found to have an incidence rate of 8.3% in a major French retrospective multicentre observational investigation, which is lower than the current research [21]. These results are contrast to those of Li et al. [22]. A cross-sectional survey of 143 hospitalized patients with COVID-19 in Wuhan, China, found that 46% developed lower extremity deep venous thrombosis [23].
Since the outbreak of the corona-virus, only one case of thromboembolic complication has been reported in Somalia [24].
In this study, males were higher frequently affected, which is contrast to the previous studies [25,26]. A prospective multicenter observational study of 812 patients reported that thromboembolic events were high prevalence female sex [27].
According to the age, the majority of COVID-19 infected individuals those who suffered thromboembolic events were over 65 years old (p < 0.000**). A recent analysis from an academic hospital in Italy showed that the median age of thromboembolic complications in 388COVID- 19 patients were 66 years [18].
Similarly to our study, diabetes (9.7%), cardiovascular disease (16.4%), and hypertension (17.1%) were among the prevalent comorbid illnesses described in a meta-analysis of six published Chinese studies involving 1527 COVID-19 patients [20].
Unfractionated heparin and low molecular weight heparin (LMWH) have both been used prophylactically and therapeutically in these patients [28]. Patients with COVID-19 who have a severe thromboembolic event, such as PE, without any additional risk factors should be regarded to have had a “provoked thromboembolic event” and may require anticoagulation for 3–6 months [[29], [30]]. In the present study, COVID-19 infected patients with thromboembolic events had been administered anticoagulants were 100%, while those without thromboembolic complication had administered 30%.
However, we found that presence of major cardiovascular thromboembolism had the strongest association with adverse outcomes, including symptomatic VTE, worsening chest CT findings, required invasive mechanical ventilation, and death.
There are several limitations in our report. First, this is a retrospective, single-center, and small sample study. Second, in the absence of data on thromboembolic testing at the national level because there may be cases of hidden thromboembolic that could not be detected in this study. No satisfied or specific number on the incidence of thromboembolic events in patients with COVID-19 pneumonia in Somalia and this is due to lack of previous study publication regarding this subject.
Further studies are needed to uncover systemic thrombosis risk factors, such as genetic variables and coexisting medical disorders. To assess the incidence of thromboembolic consequences in mild, moderate, and severe COVID19 cases, larger sample sizes are required.
5. Conclusion
The incidence of cardiovascular thromboembolic among COVID19 infected patients is relatively high, and it is associated with elder (>65years), IHD, diabetes and chronic liver disease. There was significant statistical difference in the proportion of examinees with thrombotic and non-thrombotic events in relation to outcome (p = 0.000).
Authors’ contributions
M.F·Y.M: brought the idea of the study, performed data collection, prepared the manuscript, and final revision.
M.S.M: participated in the manuscript preparation, data collection, and final revision. All authors read and approved the manuscript before submitting.
Funding
We declare that we have no funding source.
Availability of data and materials
The data is available from the corresponding author and can be accessed if requested.
Ethics approval and consent to participate
This study was reviewed and approved by Mogadishu Somali Turkish Training and Research Hospital ethic committee (Ref. MSTH/6386), patient's informed consent was waived by the Institutional Review Board, and the patient's data confidentiality was respected.
Consent for publication
All authors gave their consent for this case study to be published.
Competing interests
We declare that we have any no competing of interest.
Provenance and peer review
Not commissioned, externally peer reviewed.
Acknowledgements
We thank the patient who gave her consent to be published in this case report.
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . 2020. https://www.who.int/dg/speeches/detail/who-directorgeneral-s-opening-remarks-at-the-mediabriefing-on-covid-19-11-march-2020 (WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020). (accessed March 11, 2020) [Google Scholar]
- 3.Di Nisio M., van Es N., Büller H.R. Deep vein thrombosis and pulmonary embolism. Lancet. 2016 Dec 17;388(10063):3060–3073. doi: 10.1016/S0140-6736(16)30514-1. [DOI] [PubMed] [Google Scholar]
- 4.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C.D., Ageno W., Madjid M., Guo Y., Tang L.V. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020 Jun 16;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonow R.O., Fonarow G.C., O'Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA cardiology. 2020 Jul 1;5(7):751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 6.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G., Brown T.S., Der Nigoghossian C., Zidar D.A., Haythe J., Brodie D. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020 May 12;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivas David, et al. Recomendaciones sobre el tratamiento antitrombótico durante la pandemia COVID-19. Posicionamiento del Grupo de Trabajo de Trombosis Cardiovascular de la Sociedad Española de Cardiología. Rev. Española Cardiol. 2020;73(9):749–757. doi: 10.1016/j.recesp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Ning, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemostasis. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattaneo Marco, et al. 2020. Pulmonary Embolism or Pulmonary Thrombosis in COVID-19? Is the Recommendation to Use High-Dose Heparin for Thromboprophylaxis Justified? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020 Jul 9;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May 2;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., Burdelski C. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020 Aug 18;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Wahab N., Talathi S., Lopez-Olivo M.A., Suarez-Almazor M.E. Risk of developing antiphospholipid antibodies following viral infection: a systematic review and meta-analysis. Lupus. 2018 Apr;27(4):572–583. doi: 10.1177/0961203317731532. [DOI] [PubMed] [Google Scholar]
- 14.Wijarnpreecha K., Thongprayoon C., Panjawatanan P., Ungprasert P. Hepatitis C virus infection and risk of venous thromboembolism: a systematic review and meta-analysis. Ann. Hepatol. 2017 Nov 6;16(4):514–520. doi: 10.5604/01.3001.0010.0279. [DOI] [PubMed] [Google Scholar]
- 15.Kefale B., Tegegne G.T., Degu A., Tadege M., Tesfa D. Prevalence and risk factors of thromboembolism among patients with coronavirus disease-19: a systematic review and meta-analysis. Clin. Appl. Thromb. Hemost. 2020 Oct 15;26 doi: 10.1177/1076029620967083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020 Dec 1;29 doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klok F.A., Kruip M.J., Van der Meer N.J., Arbous M.S., Gommers D.A., Kant K.M., Kaptein F.H., van Paassen J., Stals M.A., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020 Jul 1;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.D., Sacco C., Bertuzzi A., Sandri M.T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020 Jul 1;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan I.H., Savarimuthu S., Leung M.S., Harky A. The need to manage the risk of thromboembolism in COVID-19 patients. J. Vasc. Surg. 2020 Sep 1;72(3):799–804. doi: 10.1016/j.jvs.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., Bi Z., Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020 May;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fauvel C., Weizman O., Trimaille A., Mika D., Pommier T., Pace N., Douair A., Barbin E., Fraix A., Bouchot O., Benmansour O. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur. Heart J. 2020 Aug 21;41(32):3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y., Li M., Wang M., Zhou Y., Chang J., Xian Y., Wang D., Mao L., Jin H., Hu B. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke and vascular neurology. 2020 Sep 1;5(3) doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L., Feng X., Zhang D., Jiang C., Mei H., Wang J. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome [published correction appears in Circulation. 2020; Jul 14; 142 (2): e33] Circulation. 2020;142(2):114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 24.Yusuf Mohamud M.F., Mukhtar M.S. SAGE Open Medical Case Reports; 2022. ‘Incidental Finding of Mild COVID-19 Pneumonia with Multiple Thromboembolic Disease: A Case Report. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hameed S., Wasay M., Soomro B.A., Mansour O., Abd-Allah F., Tu T., Farhat R., Shahbaz N., Hashim H., Alamgir W., Iqbal A. Cerebral venous thrombosis associated with COVID-19 infection: an observational, multicenter study. Cerebrovascular Diseases Extra. 2021;11(2):55–60. doi: 10.1159/000516641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wasay M., Kaul S., Menon B., Dai A.I., Saadatnia M., Malik A., Khalifa A., Borhani-Haghighi A., Mehndiratta M., Khan M., Bhowmik N.B. Asian study of cerebral venous thrombosis. J. Stroke Cerebrovasc. Dis. 2019 Oct 1;28(10) doi: 10.1016/j.jstrokecerebrovasdis.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Duman T., Uluduz D., Midi I., Bektas H., Kablan Y., Goksel B.K., Milanlioglu A., Orken D.N., Aluclu U., Colakoglu S., Tüfekci A. A multicenter study of 1144 patients with cerebral venous thrombosis: the VENOST study. J. Stroke Cerebrovasc. Dis. 2017 Aug 1;26(8):1848–1857. doi: 10.1016/j.jstrokecerebrovasdis.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Thachil J. The versatile heparin in COVID‐19. J. Thrombos. Haemost. 2020 May;18(5):1020–1022. doi: 10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belen-Apak F.B., Sarialioglu F. The old but new: can unfractioned heparin and low molecular weight heparins inhibit proteolytic activation and cellular internalization of SARS-CoV2 by inhibition of host cell proteases? Med. Hypotheses. 2020 Sep 1;142 doi: 10.1016/j.mehy.2020.109743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Streiff M.B., Agnelli G., Connors J.M., Crowther M., Eichinger S., Lopes R., McBane R.D., Moll S., Ansell J. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J. Thromb. Thrombolysis. 2016 Jan;41(1):32–67. doi: 10.1007/s11239-015-1317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available from the corresponding author and can be accessed if requested.