Abstract
Background
Several countries have authorised or begun using a booster vaccine dose against COVID-19. Policy makers urgently need evidence of the effectiveness of additional vaccine doses and its clinical spectrum for individuals with complete primary immunisation schedules, particularly in countries where the primary schedule used inactivated SARS-CoV-2 vaccines.
Methods
Using individual-level data, we evaluated a prospective, observational, national-level cohort of individuals (aged ≥16 years) affiliated with the Fondo Nacional de Salud insurance programme in Chile, to assess the effectiveness of CoronaVac (Sinovac Biotech), AZD1222 (Oxford-AstraZeneca), or BNT162b2 (Pfizer-BioNTech) vaccine boosters in individuals who had completed a primary immunisation schedule with CoronaVac, compared with unvaccinated individuals. Individuals administered vaccines from Feb 2, 2021, to the prespecified study end date of Nov 10, 2021, were evaluated; we excluded individuals with a probable or confirmed SARS-CoV-2 infection (RT-PCR or antigen test) on or before Feb 2, 2021, and individuals who had received at least one dose of any COVID-19 vaccine before Feb 2, 2021. We estimated the vaccine effectiveness of booster doses against laboratory-confirmed symptomatic COVID-19 (symptomatic COVID-19) cases and COVID-19 outcomes (hospitalisation, admission to the intensive care unit [ICU], and death We used inverse probability-weighted and stratified survival regression models to estimate hazard ratios, accounting for time-varying vaccination status and adjusting for relevant demographic, socioeconomic, and clinical confounders. We estimated the change in hazard from unvaccinated status to vaccinated status associated with the primary immunisation series and a booster vaccine.
Findings
11 174 257 individuals were eligible for this study, among whom 4 127 546 completed a primary immunisation schedule (two doses) with CoronaVac and received a booster dose during the study period. 1 921 340 (46·5%) participants received an AZD1222 booster, 2 019 260 (48·9%) received a BNT162b2 booster, and 186 946 (4·5%) received a homologous booster with CoronaVac. We calculated an adjusted vaccine effectiveness (weighted stratified Cox model) in preventing symptomatic COVID-19 of 78·8% (95% CI 76·8–80·6) for a three-dose schedule with CoronaVac, 96·5% (96·2–96·7) for a BNT162b2 booster, and 93·2% (92·9–93·6) for an AZD1222 booster. The adjusted vaccine effectiveness against COVID-19-related hospitalisation, ICU admission, and death was 86·3% (83·7–88·5), 92·2% (88·7–94·6), and 86·7% (80·5–91·0) for a homologous CoronaVac booster, 96·1% (95·3–96·9), 96·2% (94·6–97·3), and 96·8% (93·9–98·3) for a BNT162b2 booster, and 97·7% (97·3–98·0), 98·9% (98·5–99·2), and 98·1% (97·3–98·6) for an AZD1222 booster.
Interpretation
Our results suggest that a homologous or heterologous booster dose for individuals with a complete primary vaccination schedule with CoronaVac provides a high level of protection against COVID-19, including severe disease and death. Heterologous boosters showed higher vaccine effectiveness than a homologous booster for all outcomes, providing additional support for a mix-and-match approach.
Funding
Agencia Nacional de Investigación y Desarrollo through the Fondo Nacional de Desarrollo Científico y Tecnológico, Millennium Science Initiative Program, and Fondo de Financiamiento de Centros de Investigación en Áreas Prioritarias.
Introduction
The COVID-19 pandemic has had a major global impact, with more than 486 million cases and 6 million COVID-19-related deaths reported globally as of March 30, 2022.1 COVID-19 vaccines are now an essential component of the pandemic response to reduce disease burden and allow a safer reopening of society and economic recovery. 23 effective COVID-19 vaccines have been approved for use since the first vaccine tested in a large randomised clinical trial was approved in the UK on Dec 2, 2020,2 and several new vaccines are in the final testing stage. The efficacy, effectiveness, and safety profiles of numerous vaccine platforms are well supported by large-scale efficacy trials or observational studies. Many countries are currently running mass vaccination campaigns.3
Research in context.
Evidence before this study
We searched PubMed and medRxiv for research articles, with no language restrictions, using the search terms (“COVID-19” OR “SARS-CoV-2” OR “2019-nCoV” OR “coronavirus”) AND (“vaccine” OR “vaccination”) AND (“third dose” OR “booster”). We searched for studies published between Dec 1, 2020, and Dec 10, 2021. We identified five original clinical studies on the effectiveness of booster doses for SARS-CoV-2 vaccines. Four studies from Israel examined the effectiveness of a third dose of Pfizer-BioNTech's mRNA vaccine BNT162b2 compared with the primary vaccination series. One study estimated a 70–84% reduction in the probability of testing positive for SARS-CoV-2 infection among individuals who had received a third dose, but did not examine other outcomes. Another study found that the infection rate in the booster group was lower by a factor of 11·3 for confirmed infection and by a factor of 19·5 for severe illness, but did not adjust for comorbidities. The third study found a 90% lower COVID-19 related mortality rate among participants with a third dose. The fourth study estimated that the vaccine effectiveness of a third dose of BNT162b2 was 93% against hospital admission, 92% against severe disease, and 81% against death. Additionally, a preprint study found that, compared with demographically and clinically matched individuals with two doses, the effectiveness of a third dose in preventing SARS-CoV-2 infection and hospitalisation was 46% and 47%, respectively, for BNT162b2, and 47% and 50%, respectively, for Moderna's mRNA-1273 vaccine. All available evidence relates to mRNA vaccines. We found no studies examining the effectiveness of a homologous or heterologous booster dose for individuals with a complete primary vaccination schedule with an inactivated SARS-CoV-2 vaccine.
Added value of this study
Our study estimates the effectiveness of a homologous or heterologous booster dose for individuals with a complete primary vaccination schedule with an inactivated SARS-CoV-2 vaccine (CoronaVac), which is among the world's most widely used COVID-19 vaccines. Specifically, we used a prospective national cohort of 11·2 million people aged 16 years or older to assess the effectiveness of CoronaVac, AZD1222, or BNT162b2 vaccine boosters in individuals who had completed their primary immunisation schedule with CoronaVac. We assessed vaccine effectiveness against symptomatic COVID-19 and COVID-19 outcomes (hospitalisation, admission to the intensive care unit, and death) between treated individuals (three doses) and non-treated individuals (unvaccinated), adjusting for known demographic, socioeconomic, and clinical confounders by inverse probability of treatment weighting. We found that a homologous or heterologous booster dose significantly increased vaccine effectiveness against COVID-19 and COVID-19 outcomes.
Implications of all the available evidence
COVID-19 vaccines are an essential component of the pandemic response to reduce disease burden. However, increasing evidence suggests that vaccine protection against COVID-19 might be decreasing over time or have lower effectiveness against the delta variant (B.1.617.2). The decrease in vaccine protection is particularly worrying for inactivated vaccines, which offer lower protection than other vaccine technologies. Considering this emerging evidence, several countries have authorised or begun using a third dose. Our results suggest that a homologous or heterologous booster dose for individuals with a complete primary vaccination schedule with CoronaVac provides a high level of protection against COVID-19, including severe disease and death, with the heterologous boosters offering higher protection than the homologous booster.
Increasing evidence suggests that vaccine protection against COVID-19 might be waning over time, and newly emerging variants, such as delta and omicron, might evade vaccine-induced immune protection.4, 5 Although the correlates of protection from COVID-19 vaccines are not fully understood,6, 7 research has shown a time-dependent decrease in humoral immune responses after vaccination with any vaccine against SARS-CoV-2,8, 9 which might parallel decreasing protection against infection and disease. Research suggests that this decrease also occurs for the CoronaVac inactivated SARS-CoV-2 vaccine produced by Sinovac Biotech.10, 11 Additionally, increasing reports are describing breakthrough infections among vaccinated individuals.12, 13, 14 Recent research has shown that vaccine effectiveness might weaken over time, particularly for symptomatic illness.5, 15, 16 These studies examined data for Pfizer-BioNTech's mRNA vaccine (BNT162b2), and Oxford-AstraZeneca's ChAdOx1 nCov-19 vaccine (AZD1222). Whether protection against more severe disease has also decreased is unknown. A potential decrease in vaccine protection is particularly worrying for inactivated vaccines, which offer lower protection than other vaccine technologies, and account for about half the COVID-19 vaccine doses delivered globally thus far.17
In view of this emerging evidence, several high-income countries, including France, Germany, Israel, the UK, and the USA, have authorised or begun using a third vaccine dose.18 Most of these countries have restricted vaccine boosters to people at high risk of SARS-CoV-2 infection or related complications, including older adults, health-care workers, and individuals with underlying health conditions. Other countries that have relied on inactivated vaccines, including Cambodia, Chile, Uruguay, Thailand, and Turkey, are offering homologous and heterologous booster doses to individuals immunised with primary inactivated SARS-CoV-2 vaccines schedules.18 Policy makers urgently need evidence of the effectiveness of vaccine boosters against severe disease for individuals who have completed their primary immunisation schedules. Existing evidence for the effectiveness of boosters is limited to mRNA vaccines.19, 20, 21, 22, 23
On Feb 2, 2021, Chile began a mass vaccination campaign with four COVID-19 vaccines. The Chilean Ministry of Health organised vaccination rollout through a publicly available schedule at the national level, assigning specific dates to eligible groups.24 On Aug 11, 2021, the Ministry of Health began administering a booster dose for individuals fully vaccinated with CoronaVac. CoronaVac has been the campaign's backbone, with 20·5 million (59·0%) of all doses administered (n=34·8 million doses) being CoronaVac as of Nov 16, 2021. Pfizer-BioNTech's BNT162b2 vaccine represents 10·6 million (30·5%) doses, Oxford-AstraZeneca's AZD1222 vaccine represents 3·1 million (8·8%) doses, and CanSino Biologics’ Ad5-nCoV vaccine represents 0·57 million (1·7%) doses Nov 16, 2021.24
We used a comprehensive, individual-level administrative dataset representative of the Chilean population to assess the effectiveness of CoronaVac, AZD1222, and BNT162b2 vaccine boosters (third doses) in preventing COVID-19 cases, hospitalisations, intensive care unit (ICU) admissions, and deaths in individuals who completed their primary immunisation series with CoronaVac. We estimated vaccine effectiveness for homologous (three-dose schedule) and heterologous (mix-and-match) booster doses, adjusting for relevant demographic and clinical confounders of the association between vaccination and COVID-19 outcomes. Our results are relevant to policy makers considering a third dose for populations vaccinated with CoronaVac, one of the most widely used COVID-19 vaccines globally,17 and provide essential information on homologous and heterologous vaccine booster schedules.
Methods
Study design and participants
We used individual-level data from a prospective, observational, national-level cohort from the Fondo Nacional de Salud (FONASA). FONASA is the national health insurance programme in Chile that collects, manages, and distributes funds for the health care system. Eligibility criteria for inclusion in this study were being age 16 years or older, affiliation with FONASA with active insurance status (about 80% of the Chilean population), and vaccination with CoronaVac, BNT162b2, AZD1222, or Ad5-nCoV COVID-19 vaccines between Feb 2, 2021, and the prespecified study end date of Nov 10, 2021, or having not received any COVID-19 vaccination during that period. We excluded individuals with a probable or confirmed SARS-CoV-2 infection, detected by RT-PCR or antigen test, on or before Feb 2, 2021, and individuals who had received at least one dose of a COVID-19 vaccine before that date.
At the time of our study, all people aged 16 years or older were eligible to receive a COVID-19 vaccine in Chile. Estimates of the effectiveness of the primary immunisation schedule with CoronaVac from Feb 2 to May 1 (inclusive), 2021, including a description of vaccination rollout, the Chilean health-care system, and vaccine security profile, are available elsewhere.24 In our analysis, we focused on the effectiveness of homologous and heterologous booster doses for individuals who completed their primary series with CoronaVac. On Aug 11, 2021, the Chilean Ministry of Health began administering a booster dose according to a publicly available national vaccination schedule (appendix pp 3–6). By programme indication, for the booster vaccination, individuals aged 55 years or older could receive one standard dose of AZD1222, and those younger than 55 years could receive one standard dose of BNT162b2. An alternative booster of one standard dose of CoronaVac was available for all age groups (based on individual preference or medical prescription). Recipients of the Ad5-NCov vaccine were younger because most people older than 30 years had already started another vaccine schedule and became eligible for heterologous booster doses later in the year (Oct 12, 2021). We therefore do not have the data to provide these vaccine effectiveness estimates. Individuals who received Ad5-NCov, and those who did not complete their primary Coronavac schedule, were not studied further. In our analysis, we classified the cohort participants into the following groups: unvaccinated individuals, individuals vaccinated with two CoronaVac doses (≥14 days after receipt of the second vaccine dose and before the third dose), and individuals vaccinated with three doses (≥14 days after receipt of the third vaccine dose using a homologous regimen with CoronaVac, or a heterologous booster with either AZD1222 or BNT162b2). Our main focus was on the comparative effectiveness of booster doses in individuals vaccinated with CoronaVac versus no vaccination. However, we also evaluated the effectiveness of the homologous and heterologous boosters compared with the primary two-dose vaccination schedule with CoronaVac.
The research protocol was approved by the ethics committee of Clínica Alemana Universidad del Desarrollo (Santiago, Chile). No human health risks as a result of our study were identified because we analysed the administrative dataset and the study was considered exempt from informed consent by the same ethics committee.
Outcomes and covariates
We estimated the vaccine effectiveness of booster doses using four primary outcomes of interest: laboratory-confirmed COVID-19 cases, hospitalisation, admission to the ICU, and death. We considered the time from the beginning of follow-up, on Feb 2, 2021, to the onset of symptoms as the endpoint for the four outcomes. The Chilean Ministry of Health requires that all suspected COVID-19 cases are notified to health authorities via an online platform and undergo confirmatory laboratory testing. We defined COVID-19 cases as laboratory-confirmed infections (92% RT-PCR and 8% antigen test), and related deaths by corresponding code U07.1 (COVID-19, virus identified) in the ICD-10-CM. Our analysis included several individual characteristics associated with the probability of getting a vaccination, including booster doses and infection or severity of COVID-19 outcomes. These variables were age, sex, region of residence, individual-level income, nationality (Chilean or non-Chilean), and whether the patient had underlying conditions that have been associated with severe COVID-19 illness.24 These conditions were chronic kidney disease, diabetes, cardiovascular disease, stroke, chronic obstructive pulmonary disease, haematological disease, autoimmune disease, HIV, and Alzheimer's and other dementias.24
Statistical analysis
We determined vaccine effectiveness by estimating the hazard ratio (HR) between treated individuals (three doses) and non-treated individuals (unvaccinated), on the basis of observed time to onset of symptoms, during the period from Feb 2 to Nov 10, 2021. To estimate HRs, we used an extension of the Cox hazards model that allowed accounting for the time-varying vaccination status of participants (appendix pp 14–16).24, 25 We adjusted for differences in observed individual characteristics by inverse probability of treatment weighting as in marginal structural models,26 estimating the weights non-parametrically on the basis of observed characteristics.27 To account for the time-varying vaccination status and show that our results do not depend on model specification, we report estimates of the HRs adjusted for age, sex, region of residence, nationality, individual-level income, and underlying conditions under both standard and stratified versions of the Cox hazards model, stratifying by the variables of interest (appendix pp 8–9).24, 25
We estimated vaccine effectiveness as one minus the corresponding HR (expressed as a percentage). We show the results for the standard and stratified versions of the Cox hazards model using inverse probability of treatment weighting and without weighting as a robustness check. Inference was based on a partial likelihood approach. The comparison of the risk of an event for individuals who had received a booster dose and for those who were unvaccinated was made at the same calendar time. Each term in the partial likelihood of the effectiveness regression coefficient corresponds to the conditional probability of an individual expressing the outcome of interest from the risk set at a given calendar time. Bonferroni adjusted Pearson's χ2 test was used to compare descriptive data. Standard 95% Wald confidence intervals (95% CI) were computed for the estimates. Finally, to show the added benefit of the booster doses, we compared the vaccine effectiveness of the homologous and heterologous boosters in preventing COVID-19 outcomes compared with the primary two-dose CoronaVac vaccination schedule. We used the survival package for R (version 4.0.5) for the analyses (appendix pp 14–16).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report, or in the decision to submit the paper for publication.
Results
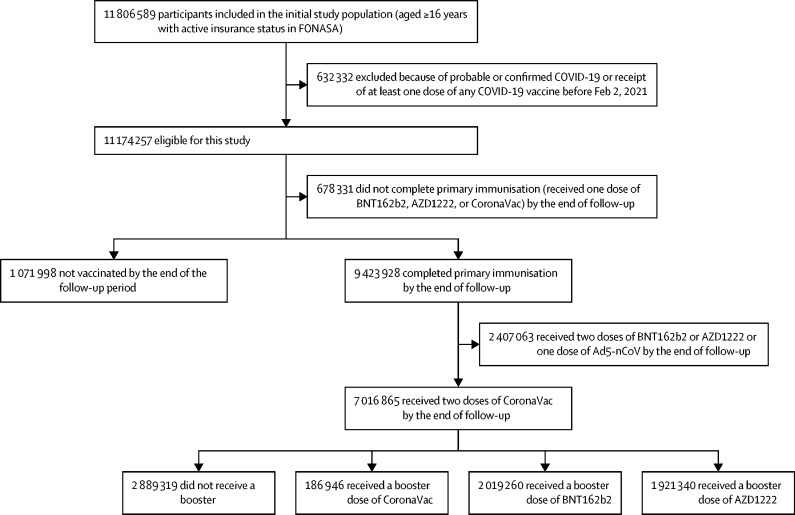
Our cohort initially included 11 806 589 individuals aged 16 years or older and affiliated with FONASA, of whom 11 174 257 were eligible for this study. As of Nov 10, 2021, 1 071 998 participants were unvaccinated, 678 331 had received one COVID-19 vaccine dose (BNT162b2, AZD1222, or CoronaVac), and 9 423 928 had completed their primary immunisation against COVID-19 (two doses of BNT162b2, AZD1222, or CoronaVac or one dose of Ad5-nCoV). Among individuals who had completed primary immunisation, 7 016 865 (74·5%) received two doses of CoronaVac separated by at least 28 days. Of these, 186 946 received a third dose of CoronaVac, 2 019 260 received a BNT162b2 booster dose, and 1 921 340 received an AZD1222 booster dose, as of Nov 10, 2021 (figure ). We summarised descriptive statistics for the study cohort (table 1 ). We found statistically significant differences (p<0·0001) both in the incidence of COVID-19 and according to vaccination status (unvaccinated, vaccinated with one dose or two doses, or vaccinated with a booster) by sex, age group, comorbidities, nationality, region of residence, and income. These differences justified inclusion of the variables in the model.
Figure.
Study participants and cohort eligibility, Feb 2 to Nov 10, 2021
Participants were aged 16 years or older, affiliated with FONASA, the public national health-care insurance system, and vaccinated with CoronaVac, BNT162b2, AZD1222, or Ad5-nCoV COVID-19 vaccines, or had not received any COVID-19 vaccination, between Feb 2, and Nov 10, 2021. We excluded individuals who had probable or confirmed COVID-19 according to RT-PCR assay for SARS-Cov-2 or antigen test on or before Feb 2, 2021. FONASA=Fondo Nacional de Salud.
Table 1.
Characteristics of the study cohort of FONASA affiliates, Feb 2 to Nov 10, 2021
| All participants | Laboratory-confirmed COVID-19 | Unvaccinated | Vaccination status: one dose | Vaccination status: two doses | Vaccination status: three doses | ||
|---|---|---|---|---|---|---|---|
| Overall population | 11 174 257 (100·0%) | 534 314 (4·8%) | 1 071 988 (9·6%) | 678 341 (6·1%) | 4 754 538 (42·6%) | 4 669 390 (41·8%) | |
| Sex | |||||||
| Female | 5 993 736 (54·0%) | 292 040 (4·9%) | 505 607 (8·4%) | 298 962 (5·0%) | 2 432 234 (40·6%) | 2 756 933 (46·0%) | |
| Male | 5 180 521 (46·0%) | 242 274 (4·7%) | 566 381 (10·9%) | 379 379 (7·3%) | 2 322 304 (44·8%) | 1 912 457 (36·9%) | |
| Age group, years | |||||||
| 16–19 | 736 905 (6·6%) | 32 034 (4·3%) | 62 457 (8·5%) | 127 731 (17·3%) | 514 370 (69·8%) | 32 347 (4·4%) | |
| 20–29 | 2 121 616 (19·0%) | 127 418 (6·0%) | 241 502 (11·4%) | 238 566 (11·2%) | 1 318 987 (62·2%) | 322 561 (15·2%) | |
| 30–39 | 2 001 611 (18·0%) | 116 321 (5·8%) | 243 211 (12·2%) | 144 776 (7·2%) | 1 059 332 (52·9%) | 554 292 (27·7%) | |
| 40–49 | 1 735 067 (16·0%) | 88 973 (5·1%) | 165 463 (9·5%) | 84 411 (4·9%) | 769 601 (44·4%) | 715 592 (41·2%) | |
| 50–59 | 1 795 580 (16·0%) | 79 308 (4·4%) | 136 770 (7·6%) | 51 346 (2·9%) | 548 956 (30·6%) | 1 058 508 (59·0%) | |
| 60–69 | 1 421 931 (13·0%) | 49 711 (3·5%) | 97 548 (6·9%) | 15 666 (1·1%) | 289 219 (20·3%) | 1 019 498 (71·7%) | |
| 70–79 | 881 220 (7·9%) | 26 116 (3·0%) | 65 071 (7·4%) | 8657 (1·0%) | 148 063 (16·8%) | 659 429 (74·8%) | |
| ≥80 | 480 327 (4·3%) | 14 433 (3·0%) | 59 966 (7·2%) | 7188 (1·5%) | 106 010 (22·1%) | 307 163 (64·0%) | |
| Comorbidities* | |||||||
| None | 7 586 853 (68·0%) | 361 575 (4·8%) | 832 456 (11·0%) | 558 203 (7·4%) | 3 611 727 (47·6%) | 2 584 467 (34·1%) | |
| ≥1 | 3 587 404 (32·0%) | 172 739 (4·8%) | 239 532 (6·7%) | 120 138 (3·4%) | 1 142 811 (31·9%) | 2 084 923 (58·1%) | |
| Nationality | |||||||
| Chilean | 10 427 613 (93·3%) | 501 394 (4·8%) | 895 370 (8·6%) | 616 986 (5·9%) | 4 417 917 (42·4%) | 4 497 340 (43·1%) | |
| Non-Chilean | 746 644 (6·7%) | 32 920 (4·4%) | 176 618 (23·7%) | 61 355 (8·2%) | 336 621 (45·1%) | 172 050 (23·0%) | |
As well as the listed characteristics, the main analysis model (table 2) also included individual-level income (seven groups) and location (16 regions; appendix pp 8–9). Using the χ2 test, we found statistically significant differences (p<0·001) both in the incidence of COVID-19 and according to vaccination status by sex, age group, comorbidities, nationality, region of residence, and income. More detailed cohort data are provided in the appendix (pp 8–10). The COVID-19 vaccines include AZD1222, Ad5-nCov, BNT162b2, and CoronaVac.
Coexisting conditions included chronic kidney disease, diabetes, cardiovascular disease (ie, hypertension, myocardial infarction), stroke, chronic obstructive pulmonary disease, haematological disease (ie, lymphoma, leukaemia, myeloma), autoimmune disease (ie, rheumatoid arthritis, juvenile idiopathic arthritis, systemic lupus erythematosus), HIV, and Alzheimer's disease and other dementias.
The Chilean Ministry of Health administered 4 127 546 booster doses during the study period to individuals with a complete primary immunisation schedule (two doses) with CoronaVac. Most booster doses (3 940 600 [95·4%]) were heterologous, with 1 921 340 (46·5%) participants receiving an AZD1222 booster and 2 019 260 (48·9%) participants receiving a BNT162b2 booster. Only 186 946 (4·5%) participants received a homologous booster with CoronaVac (figure).
We calculated vaccine effectiveness for booster doses in preventing symptomatic SARS-CoV-2 infection among participants with a complete CoronaVac primary immunisation schedule (table 2 ). Vaccine effectiveness was compared between treated individuals (three doses) and non-treated individuals (unvaccinated). According to the weighted stratified version of the Cox model, the adjusted vaccine effectiveness against symptomatic COVID-19 was 78·8% (95% CI 76·8–80·6) for a homologous booster with CoronaVac, 96·5% (96·2–96·7) for a BNT162b2 booster, and 93·2% (92·9–93·6) for an AZD1222 booster. We also assessed the vaccine effectiveness of booster doses in preventing severe COVID-19 outcomes (hospitalisation, ICU admission, and death) among participants with primary immunisation with CoronaVac (table 2). The adjusted vaccine effectiveness (weighted stratified model) against hospitalisation was 86·3% (83·7–88·5) for a homologous booster with CoronaVac, 96·1% (95·3–96·9) for a BNT162b2 booster, and 97·7% (97·3–98·0) for an AZD1222 booster. The adjusted vaccine effectiveness against ICU admission was 92·2% (88·7–94·6) for a homologous booster with CoronaVac, 96·2% (94·6–97·3) for a BNT162b2 booster, and 98·9 (98·5–99·2) for an AZD1222 booster. The adjusted vaccine effectiveness against COVID-19-related death was 86·7% (80·5–91·0) for a homologous booster with CoronaVac, 96·8% (93·9–98·3) for a BNT162b2 booster, and 98·1% (97·3–98·6) for an AZD1222 booster.
Table 2.
Effectiveness of COVID-19 vaccine CoronaVac, BNT162b2, and AZD1222 boosters in preventing COVID-19 outcomes among cohort participants according to immunisation status, Feb 2 to Nov 10, 2021*
|
Study population* |
Vaccine effectiveness, % (95% CI) |
||||||
|---|---|---|---|---|---|---|---|
| Overall person-days | COVID-19 case number | COVID-19 incidence rate, 1000 person-days | Unweighted, adjusted for all covariates† | Weighted, adjusted for all covariates† | Unweighted, stratified analysis†‡ | Weighted, stratified analysis†‡ | |
| Laboratory-confirmed COVID-19 | |||||||
| Unvaccinated | 107 933 645 | 6021 | 0·0559 | .. | .. | .. | .. |
| CoronaVac booster (≥14 days after third dose) | 8 795 237 | 323 | 0·0367 | 75·6% (72·7–78·1) | 78·1% (76·1–79·9) | 77·3% (74·6–79·8) | 78·8% (76·8–80·6) |
| BNT162b2 booster (≥14 days after third dose) | 34 755 396 | 334 | 0·0096 | 95·6% (95·1–96·1) | 96·3% (96·1–96·5) | 95·8% (95·3–96·2) | 96·5% (96·2–96·7) |
| AZD1222 booster (≥14 days after third dose) | 96 601 030 | 969 | 0·0100 | 92·8% (92·4–93·3) | 93·2% (92·8–93·5) | 93·1% (92·6–93·5) | 93·2% (92·9–93·6) |
| COVID-related hospitalisation | |||||||
| Unvaccinated | 111 515 766 | 1134 | 0·0102 | .. | .. | .. | .. |
| CoronaVac booster (≥14 days after third dose) | 8 999 341 | 89 | 0·0099 | 83·4% (79·5–86·6) | 84·7% (81·8–87·1) | 87·2% (84·1–89·7) | 86·3% (83·7–88·5) |
| BNT162b2 booster (≥14 days after third dose) | 35 941 136 | 55 | 0·0015 | 95·3% (93·8–96·4) | 96·4% (95·6–97·0) | 95·7% (94·4–96·7) | 96·1% (95·3–96·9) |
| AZD1222 booster (≥14 days after third dose) | 98 599 509 | 139 | 0·0014 | 97·3% (96·7–97·7) | 97·5% (97·1–97·8) | 97·8% (97·4–98·2) | 97·7% (97·3–98·0) |
| COVID-related ICU admission | |||||||
| Unvaccinated | 112 043 195 | 428 | 0·0038 | .. | .. | .. | .. |
| CoronaVac booster (≥14 days after third dose) | 9 046 214 | 21 | 0·0023 | 89·4% (83·6–93·1) | 91·1% (87·1–93·9) | 92·5% (88·3–95·2) | 92·2% (88·7–94·6) |
| BNT162b2 booster (≥14 days after third dose) | 36 034 118 | 16 | 0·0004 | 96·1% (93·7–97·7) | 96·3% (94·8–97·4) | 96·6% (94·4–98·0) | 96·2% (94·6–97·3) |
| AZD1222 booster (≥14 days after third dose) | 98 801 374 | 26 | 0·0003 | 98·6% (98·0–99·1) | 98·8% (98·3–99·1) | 99·0% (98·5–99·3) | 98·9% (98·5–99·2) |
| Confirmed COVID-19-related death | |||||||
| Unvaccinated | 111 912 342 | 192 | 0·0017 | .. | .. | .. | .. |
| CoronaVac booster (≥14 days after third dose) | 9 059 669 | 18 | 0·0020 | 85·8% (77·2–91·2) | 83·7% (76·6–88·7) | 88·9% (82·1–93·2) | 86·7% (80·5–91·0) |
| BNT162b2 booster (≥14 days after third dose) | 36 060 324 | 6 | 0·0002 | 96·8% (92·7–98·5) | 96·4% (93·2–98·0) | 97·3% (93·9–98·8) | 96·8% (93·9–98·3) |
| AZD1222 booster (≥14 days after third dose) | 98 845 182 | 22 | 0·0002 | 98·0% (96·4–98·7) | 97·9% (97·0–98·5) | 98·4% (97·5–98·9) | 98·1% (97·3–98·6) |
The 13 days between vaccine administration and partial or full immunisation were excluded from the at-risk person-time. We show the results for the standard and stratified versions of the extended Cox hazards model with inverse probability of treatment weighting, and without weighting as a robustness check.
The number of person-days, number of COVID-19 cases, and COVID-19 incidence rate for the unvaccinated group were computed for participants at risk between Aug 11, 2021 (when the Chilean Ministry of Health began administering booster doses) and Nov 10, 2021.
Adjusted for age group, sex, region of residence, income group, nationality, and whether the patient had underlying conditions that have been associated with severe COVID-19, as described in table 1 and the appendix (pp 8–9).
A stratified version of the extended Cox proportional hazards model was fit to test the robustness of the estimates to model assumptions, stratifying by age group, sex, region of residence, income group, nationality, and whether the patient had underlying conditions that have been associated with severe COVID-19, as described in table 1 and the appendix (pp 8–9).
In our evaluation of the effectiveness of the homologous and heterologous boosters in preventing COVID-19 outcomes compared with the primary two-dose vaccination schedule with CoronaVac, the adjusted vaccine effectiveness (weighted stratified model) for a homologous booster with CoronaVac was 63·8% (60·4–67·0) against laboratory-confirmed COVID-19, 59·3% (51·5–65·9) against hospitalisation, 71·2% (58·1–80·2) against ICU admission, and 62·7% (44·9–74·7) against death (appendix pp 17–18). Compared with the primary CoronaVac vaccination schedule, the adjusted vaccine effectiveness for a heterologous booster with BNT162b2 was 93·5% (93·1–93·9) against COVID-19, 86·6% (83·6–89·1) against hospitalisation, 84·1% (77·4–88·8) against ICU admission, and 90·7% (82·2–95·1) against death. Adjusted vaccine effectiveness for a heterologous booster with AZD1222 was 88·4% (87·8–89·0) against COVID-19, 93·0% (91·9–93·9) against hospitalisation, 95·9% (94·3–97·0) against ICU admission, and 94·7% (92·5–96·3) against death.
Discussion
Our findings show high effectiveness with homologous (CoronaVac) and heterologous (BNT162b2 or AZD1222) booster schedules in preventing COVID-19 and related outcomes. Compared with no vaccination, the adjusted vaccine effectiveness with a homologous booster was 78·8% against laboratory-confirmed COVID-19, 86·3% against hospitalisation, 92·2% against ICU admission, and 86·7% against death. The adjusted vaccine effectiveness with a heterologous BNT162b2 booster dose was 96·5% against COVID-19, 96·1% against hospitalisation, 96·2% against ICU admission, and 96·8% against death. Effectiveness with an AZD1222 booster dose was 93·2% against COVID-19, 97·7% against hospitalisation, 98·9% against ICU admission, and 98·1% against death. These results exceed the early effectiveness findings for the CoronaVac primary series previously reported by our group,24 suggesting that a three-dose schedule should be considered for primary immunisation with inactivated vaccines.
Four studies in Israel have examined the effectiveness of a homologous BNT162b2 booster compared with the primary vaccination series. One study estimated a 70–84% reduction in the probability of testing positive for SARS-CoV-2 infection among individuals who had received a BNT162b2 booster, although other clinical outcomes were not examined.19 Another study found that the rate of infection in the booster group was lower by a factor of 11·3 for confirmed infection and by a factor of 19·5 for severe illness, but did not adjust for clinical confounders.20 A third study found a 90% lower COVID-19-related mortality rate among participants with a third vaccine dose.21 The fourth study found that the effectiveness of the third dose of BNT162b2 was 93% (95% CI 88–97) against hospitalisation, 92% (82–97) against severe COVID-19, and 81% (59–97) against COVID-19-related death.22 A preprint study in the USA found lower risk of infection and hospitalisation with a third dose of BNT162b2 (46% and 47%, respectively) or Moderna's mRNA-1273 (47% and 50%).23 Although not directly comparable, our vaccine effectiveness estimates against COVID-19-related hospitalisation, ICU admission, and deaths for the third dose of BNT162b2 are higher than estimates in the USA,23 but similar to those in Israel considering estimated CIs.
The CoronaVac vaccine and Sinopharm's BBIBP-CorV vaccine account for about half of the COVID-19 vaccine doses delivered globally and have been administered in 110 primarily low-income and middle-income countries as of Oct 14, 2021.17 Our results suggest that a three-dose vaccination schedule for CoronaVac, one of the most commonly used COVID-19 vaccines globally,17 substantially increases protection against severe illness. However, protection is significantly higher for individuals who receive a heterologous vaccine booster compared with a homologous booster with CoronaVac. Our data are consistent with a recent study in Brazil showing that homologous and heterologous (BNT162b2 and AZD1222) boosters following a two-dose CoronaVac vaccination schedule were safe and substantially enhanced humoral immune responses.28 However, anti-spike IgG responses at day 28 were superior in all heterologous regimens compared with homologous regimens, suggesting enhanced protection. Our results for heterologous vaccine booster schedules with BNT162b2 and AZD1222 booster doses also show encouraging results for individuals with a complete primary immunisation schedule with CoronaVac, providing additional support for using a mix-and-match approach. These findings might be crucial for policymakers, particularly in low-resource settings.
Global debate is ongoing about the use of booster doses. Preliminary evidence suggests that the effectiveness of COVID-19 vaccines decreases over time,5, 15, 16 although there is no consensus on the speed of this decrease, and whether protection against severe COVID-19 also decreases. Although the priority should be to ensure that vulnerable individuals across the globe are vaccinated, particularly considering that some COVID-19 vaccines probably provide enough protection against severe disease for the most prevalent SARS-CoV-2 lineages,29 our results suggest that booster doses substantially increase vaccine effectiveness for a CoronaVac primary series. These results are consistent with evidence for BNT162b2 in Israel.19, 20, 21, 22 Rolling out booster vaccine doses parallel to primary immunisation campaigns might become a powerful strategy to reduce SARS-CoV-2 infections and mitigate its consequences. Our results are aligned with the recommendation of WHO's Strategic Advisory Group of Experts, to provide a third dose to people aged 60 years or older,30 without neglecting primary immunisation coverage also recommended by WHO.
The main strengths of our study include the use of a diverse cohort of 11·2 million individuals, with combined vaccination and administrative health-care data and representing about 80% of the Chilean population. We collected data on demographic variables, residence, income, nationality, and comorbidities, in addition to data on testing, health-care use, vital statistics, and vaccination. The large sample size allowed us to non-parametrically estimate inverse probability of treatment weights and fit a stratified extended Cox proportional hazards model for the different outcomes of interest (given that each combination of predictors has a specific hazard function), adding robustness to our statistical approach. In addition, we assessed the performance of homologous and heterologous booster doses, which provides valuable evidence to policy makers globally, particularly for countries that have used CoronaVac and are considering booster doses. The availability of a diverse matrix of highly effective booster alternatives should avoid potential vaccine supply shortages, helping countries implement and sustain COVID-19 vaccination efforts over time.
The study also has limitations. First, as an observational study, our results might have been subject to selection and misclassification biases. Selection bias might have occurred if, for example, vaccinated and unvaccinated individuals had systematic differences. We adjusted our estimates for known confounders that could affect vaccine effectiveness estimates, such as age, sex, region of residence, individual-level income, nationality, and whether the patient had underlying conditions associated with severe COVID-19. Although our model adjusted for known confounders that could affect the probability of vaccination, infection, or developing severe COVID-19, we cannot account for potentially systematic, unobservable behavioural or health differences between the study groups. The factors that could explain vaccine hesitancy are complex and context-specific. They might include concern about vaccine side-effects, distrust in government or pharmaceutical companies, religious or other personal beliefs, misinformation, health-care inequalities, and ineffective public health messaging.31 We cannot be sure in which direction, if any, these systematic differences affect vaccine effectiveness estimates. For example, publicly reported effectiveness results for the CoronaVac vaccine in Chile during May, 2021, might have resulted in fully vaccinated people taking more risks for acquiring SARS-CoV-2 infection than unvaccinated individuals.24 As we have described (appendix pp 3–6), the Chilean Ministry of Health organised a vaccination rollout through a publicly available vaccination schedule. In the current strategy, individuals need to attend the nearest vaccination site with a form of personal identification; no appointments are required. The risk for misclassification bias with the exposure or the outcomes is low. Chile has a single, electronic, and centralised immunisation registry, and SARS-CoV-2 testing is free and widely available.
A second limitation concerns SARS-CoV-2 variants. The Chilean Ministry of Health has incorporated SARS-CoV-2 genomic surveillance into an existing network of respiratory virus monitoring centres since Dec 22, 2020, and, as of Nov 15, 2021, reported the circulation of four variants of concern (alpha, beta, gamma, and delta; appendix pp 12–13), which might influence vaccine effectiveness. Research suggests that COVID-19 vaccines have lower effectiveness against delta,4, 5 the predominant circulating variant in Chile at the time of our analysis. We do not have representative data to estimate the true prevalence of these variants and their effect on vaccine effectiveness, which could be highly relevant to control the pandemic. A third limitation is that individuals younger than 55 years were not eligible for AZD1222 booster doses, so results cannot be directly compared among the different vaccines. Giving the AZD1222 boosters to older individuals at increased risk of COVID-19 and related outcomes might have resulted in lower estimated vaccine effectiveness compared with a situation in which all ages were eligible to receive AZD1222 boosters. A final limitation is that we estimated vaccine effectiveness within 3 months after the booster doses became available, but increasing evidence suggests that vaccine protection might decrease over time.
Overall, our results suggest that a homologous or heterologous booster vaccine dose for individuals with a complete primary vaccination schedule with CoronaVac results in a high level of protection against COVID-19, including severe disease and death. Heterologous boosters showed higher vaccine effectiveness than a homologous booster for all clinical outcomes considered, providing additional support for use of a mix-and-match approach.
Data sharing
Our use of data follows Chilean Law 19.628 on personal data protection. Owing to this data privacy law, the individual-level data used in this study cannot be shared. Aggregate data on vaccination and COVID-19 incidence are publicly available online.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This research was supported by the Agencia Nacional de Investigación y Desarrollo (ANID) through the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) grant number 1180640 to AJ; Millennium Science Initiative Program - Millennium Nucleus Center for the Discovery of Structures in Complex Data (MiDaS) grant number NCN17_059 to AJ; Advanced Center for Chronic Diseases (ACCDiS) ANID FONDAP grant number 15130011 to RA; and Research Center for Integrated Disaster Risk Management (CIGIDEN) ANID FONDAP grant number 15110017 to EU. AJ, CG, AP, JA, KL, FP, TB, VV, MM, FL, IP, PL, PS, JC, HG-E, RA are employees of the Chilean Ministry of Health.
Contributors
AJ and RA conceived and designed the study. JRZ provided support for the study design and analytic approach. AJ, FP, TB, and RA managed and analysed the data. AJ, RA, and EU wrote the first draft of the manuscript. AJ, EU, JRZ, CG, AP, JA, KL, FP, TB, VV, MM, FL, IP, PL, PS, JCR, HGE, and RA, critically reviewed and edited the manuscript. AJ, RA, CG, AP, JA, KL, FP, TB, VV, MM, FL, IP, PL, PS, JCR, and HGE had full access to study data. VV, HGE, and RA had access to vaccine safety data. RA and HGE had access to genomic surveillance data. The author group was entirely responsible for study design, data collection, and data analysis. All authors vouch for the accuracy and completeness of the data and accept responsibility for publication.
Supplementary Material
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ledford H, Cyranoski D, Van Noorden R. The UK has approved a COVID vaccine—here's what scientists now want to know. Nature. 2020;588:205–206. doi: 10.1038/d41586-020-03441-8. [DOI] [PubMed] [Google Scholar]
- 3.Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- 4.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pouwels KB, Pritchard E, Matthews PC, et al. Effect of delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27:2127–2135. doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 8.Sauré D, O'Ryan M, Torres JP, Zuniga M, Santelices E, Basso LJ. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study. Lancet Infect Dis. 2022;22:56–63. doi: 10.1016/S1473-3099(21)00479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mok CKP, Cohen CA, Cheng SMS, et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong. Respirology. 2021 doi: 10.1111/resp.14191. published online Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng G, Wu Q, Pan H, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2021;22:483–495. doi: 10.1016/S1473-3099(21)00681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas SJ, Moreira ED, Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergwerk M, Gonen T, Lustig Y, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowlkes A, Gaglani M, Groover K, et al. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617. 2 (delta) variant predominance—eight US locations, December 2020–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1167–1169. doi: 10.15585/mmwr.mm7034e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feikin D, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallapaty S. China's COVID vaccines have been crucial—now immunity is waning. Nature. 2021;598:398–399. doi: 10.1038/d41586-021-02796-w. [DOI] [PubMed] [Google Scholar]
- 18.Mahase E. COVID-19 booster vaccines: what we know and who's doing what. BMJ. 2021;374 doi: 10.1136/bmj.n2082. [DOI] [PubMed] [Google Scholar]
- 19.Patalon T, Gazit S, Pitzer VE, Prunas O, Warren JL, Weinberger DM. Odds of testing positive for SARS-CoV-2 following receipt of 3 vs 2 doses of the BNT162b2 mRNA vaccine. JAMA Intern Med. 2022;182:179–184. doi: 10.1001/jamainternmed.2021.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to COVID-19. N Engl J Med. 2021;385:2413–2420. doi: 10.1056/NEJMoa2115624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma A, Oda G, Holodniy M. Effectiveness of a third dose of 23 or mRNA-1273 vaccine for preventing post-vaccination COVID-19 infection: an observational study. medRxiv. 2021 doi: 10.1101/2021.11.29.21266777. published online Nov 30. (preprint). [DOI] [Google Scholar]
- 24.Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 26.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa Clemens SA, Weckx L, Clemens R, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399:521–529. doi: 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altmann DM, Boyton RJ, Beale R. Immunity to SARS-CoV-2 variants of concern. Science. 2021;371:1103–1104. doi: 10.1126/science.abg7404. [DOI] [PubMed] [Google Scholar]
- 30.Strategic Advisory Group of Experts . World Health Organization; Oct 10, 2021. Highlights from the meeting of the Strategic Advisory Group of Experts (SAGE) on immunization, 4–7 October 2021.https://cdn.who.int/media/docs/default-source/immunization/sage/2021/october/sage_oct2021_meetinghighlights.pdf?sfvrsn=3dcae610_15 [Google Scholar]
- 31.Solís Arce JS, Warren SS, Meriggi NF, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. 2021;27:1385–1394. doi: 10.1038/s41591-021-01454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our use of data follows Chilean Law 19.628 on personal data protection. Owing to this data privacy law, the individual-level data used in this study cannot be shared. Aggregate data on vaccination and COVID-19 incidence are publicly available online.