Abstract
TMC1 is a causative gene for both autosomal dominant non-syndromic hearing loss (DFNA36) and autosomal recessive non-syndromic hearing loss (DFNB7/11). To date, 125 pathogenic variants in TMC1 have been reported. Most of the TMC1 variants are responsible for autosomal recessive hearing loss, with only 8 variants reported as causative for DFNA36. Here, we reported the prevalence of TMC1-associated hearing loss in a large non-syndromic hearing loss cohort of about 12,000 subjects. As a result, we identified 26 probands with TMC1-associated hearing loss, with the estimated prevalence of TMC1-associated hearing loss in the Japanese hearing loss cohort being 0.17% among all patients. Among the 26 probands with TMC1-associated hearing loss, 15 cases were identified from autosomal dominant hearing loss families. Based on the audiometric data from the probands, family members and previously reported cases, we evaluated hearing deterioration for DFNA36 patients. In addition, we performed haplotype analysis for 11 unrelated autosomal dominant hearing loss families carrying the same variant TMC1: NM_138691:c.1627G > A:p.Asp543Asn. The results clearly indicated that the same haplotype was present despite the families being unrelated, supporting the contention that this variant occurred by founder mutation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00439-021-02364-2.
Introduction
Hearing loss is one of the most common sensory disorders and, currently, approximately 120 genes have been reported as causative for non-syndromic hearing loss (The Hereditary Hearing Loss Homepage). TMC1 is a causative gene for both autosomal dominant non-syndromic hearing loss (ADNSHL) and autosomal recessive non-syndromic hearing loss (ARNSHL) as first reported by Kurima et al (2002). The encoding protein transmembrane channel-like protein 1 is highly expressed in the tips of stereocilia and plays a crucial role in mechano-electro-transduction (Liu et al. 2020).
TMC1 variants are a relatively common genetic cause of non-syndromic hearing loss, and accounts for 3.4% (19/557) of Pakistani ARNSHL patients (Kitajiri et al. 2007a, b), 2.4% (3/125) of Chinese ARNSHL patients (Yang et al. 2013), 0.69% (3/433) of Chinese hearing loss patients (Yuan et al. 2020), 3.1% (4/131) of Western European GJB2-negative ARNSHL patients (Sommen et al. 2016), 0.5% (1/200) of Dutch hearing loss patients (Seco et al. 2017), 0.8% (4/491) of Palestinian hearing loss patients (Abu Rayyan et al. 2020), 0.5% (1/197) of Czech hearing loss patients (Safka Brozkova et al. 2020), 4.3% (4/93) to 8.1% (7/86) of Turkish ARNSHL (Kalay et al. 2005; Sirmaci et al. 2009), 5.9% (5/85) of Tunisian ARNSHL (Tlili et al. 2008) and 0.9% (10/1119) of American (Sloan‐Heggen et al. 2016) hearing loss patients. Most cases of TMC1-associated hearing loss are identified as autosomal recessive inherited hearing loss, and only limited cases are identified as autosomal dominant. The clinical phenotypes of TMC1-associated hearing loss differ according to the inheritance mode. TMC1-associated ARNSHL cases show congenital severe-to-profound hearing loss, whereas ADNSHL cases show late-onset progressive hearing loss with predominant deterioration in the higher frequencies. To date, 125 pathogenic variants in TMC1 have been reported (HGMD Professional). Among the 125 pathogenic variants, only 8 variants were reported as causative for ADNSHL (DFNA36). The TMC1 gene variants associated with ADNSHL are p.Ile266Thr (Sloan-Heggen et al. 2016), p.Ser320Arg (Hassan et al. 2015), p.Tyr381Asn (Likar et al. 2018), p.Gly417Arg (Yang et al. 2010), p.Met418Lys (Zhao et al. 2014; Wang et al. 2018), p.Asp543Asn (Moteki et al. 2016), p.Asp572Asn (Kurima et al. 2002; Wang et al. 2018; Ramzan et al. 2020), and p.Asp572His (Kitajiri et al. 2007a, b). However, there is some conflict regarding the pathogenicity of the p.Asp572His variants (Azaiez et al. 2018). In addition, the p.Ile266Thr variant and p.Tyr381Asn variant were also reported as causative for TMC1-associated ARNSHL (Wang et al. 2018; Sommen et al. 2016). Therefore, only five variants identified from 8 families are reliably known to be the genetic cause of TMC1-associated ADNSHL. Based on this limited number of cases, the overall picture regarding the clinical phenotypes of TMC1-associated ADNSHL remains unclear.
Recently, autosomal dominant TMC1-associated hearing loss has received special attention as a candidate for gene therapy. A mouse model of TMC1-related hearing loss (Beethoven mice), generated by ENU mutagenesis, showed autosomal dominant inherited progressive hearing loss (Vreugde et al. 2002). This mouse model carries the Tmc1:c.1235T > A:p.Met412Lys variant, and subsequent to this report, ADNSHL patients with an orthologous TMC1 variant (TMC1 c.1253T > A:p.Met418Lys) were reported (Zhao et al. 2014). As the Beethoven mice showed a similar phenotype (progressive hearing loss with predominant deterioration in the higher frequencies) to human patients and carried the orthologous mutation identified in human ADNSHL patients, this mouse model is widely used for translational research for gene therapy (Askew et al. 2015; Shibata et al. 2016; Yoshimura et al. 2019; Gao et al. 2018; Nist-Lund et al. 2019; György et al. 2019; Wu et al. 2021). However, prior to the clinical application of gene therapies, the detailed phenotypes and prevalence information are essential.
In this study, we sought to (1) elucidate the prevalence of hearing loss (HL) caused by TMC1 variants in a large cohort of non-syndromic hearing loss patients, (2) analyze the rate of HL deterioration in TMC1-associated ADNSHL patients, and (3) carry out haplotype analysis of the TMC1: NM_138691:c.1627G > A:p.Asp543Asn variant identified from 11 unrelated ADNSHL families to confirm whether the mutation occurred by founder mutation or in a mutational hotspot.
Methods
Subjects
We performed target re-sequencing analysis for 12,139 Japanese non-syndromic sensorineural hearing loss patients and controls (2462 autosomal dominant or mitochondrial inheritance cases, 6912 autosomal recessive inheritance or sporadic cases, 2220 unknown family history cases, 212 cases with unilateral hearing loss, and 333 normal hearing control subjects) from 90 otorhinolaryngology departments spread across Japan enrolled in this study. In addition, we also analyzed 187 cochlear implant patients or electric acoustic stimulation patients enrolled from 10 cochlear implantation centers listed below: Antwerp University Hospital, Belgium (Prof. Paul Van de Heyning); Hospital Universitario La Paz, Spain (Prof. Javier Gavilán); Klinikum der Universität München, German (Prof. Joachim Müller); Karolinska University Hospital, Sweden (Prof. Eva Karltorp); Institute of Physiology and Pathology of Hearing, Poland (Dr. Henryk Skarzynski and Dr. Piotr Skarzynski); King Abdulaziz University Hospital, Saudi Arabia (Prof. Abdulrahman Hagr), ENT Super Speciality Institute and Research Center, India (Dr. Manikoth Manoj); University of Western Australia, Australia (Prof. Gunesh Rajan); Kansas University, USA (Prof. Hinrich Staecker); and Allende Sanatorio, Argentina (Dr. Mario Zernotti).
Informed written consent was obtained from all subjects (or guardians in the case of minors) prior to participation. This study was approved by the Shinshu University Ethics Committee (Approval number: 576) and the respective ethics committees of all other participating institutions.
Next-generation sequencing and bioinformatic analysis
Next-generation sequencing was performed for the 63 genes reported to cause non-syndromic hearing loss as described in a previous report (Nishio et al. 2015). In brief, amplicon libraries were prepared using the Ion AmpliSeq Custom Panel, with the Ion AmpliSeq Library Kit 2.0 and the Ion Xpres Barcode Adapter 1-96 Kit (Life Technologies) according to the manufacturer’s instructions. After amplicon library preparation, equal amounts of libraries for 45 patients were pooled for 1 sequence reaction and next-generation sequencing was performed by Ion Proton system with an Ion P1 chip or Ion S5 system with an Ion 540 chip according to the manufacturer’s instructions. The sequence data were aligned to the human reference genome sequence (build GRCh37/hg19) by the Torrent Mapping Alignment Program (TMAP) and, subsequently, DNA variants were piled up with the Torrent Variant Caller plug-in software including in the Torrent Suit (Life Technologies).
The effects of the variants were analyzed using ANNOVAR software (Wang et al. 2010). The missense, nonsense, insertion/deletion, and splicing variants were selected among the identified variants. Variants were further selected as < 1% of several control database including the 1000 genome database (http://www.1000genomes.org/), the 6500 exome variants (http://evs.gs.washington.edu/EVS/), The Genome Aggregation Database (https://gnomad.broadinstitute.org), the human genetic variation database (dataset for 1208 Japanese exome variants) (http://www.genome.med.kyoti-u.ac.jp/SnpDB/index.html), the 8300 Japanese genome variation database (https://jmorp.megabank.tohoku.ac.jp/202102/) and the 333 in-house Japanese normal hearing controls. All filtering procedures were performed using original database software described previously (Nishio and Usami 2017). The pathogenicity of the identified variants was evaluated in accordance with the American College of Medical Genetics (ACMG) standards and guidelines (Richards et al. 2015) with the ClinGen hearing loss clinical domain working group expert specification (Oza et al. 2018). We performed Sanger sequencing analysis to validate the identified variants using PCR and exon-specific custom primers according to the manufacturer’s instructions. All primers were designed using the web version Primer 3 plus software (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi).
Haplotype analysis
The haplotype pattern within the 3 Mbp region surrounding the frequent Japanese variation TMC1: NM_138691:c.1627G > A identified in this study was analyzed using a set of 47 single-nucleotide polymorphisms (SNPs) (21 sites for upstream and 26 sites for downstream). For this analysis, we selected 15 individuals (including 11 affected and 4 un-affected family members) from 5 families. Haplotype analysis was performed by Sanger sequencing. The mutation-linked haplotype was determined by family member segregation analysis with multiple family member samples, and compared among unrelated families with the same mutations.
Results
Identified variants, prevalence, and the clinical features of TMC1-associated hearing loss
As a result of the large cohort next-generation sequencing analysis, we identified 26 probands with TMC1-associated hearing loss (Table 1 and Supplemental Fig. 1). The pedigrees and audiometry results are shown in Supplemental Fig. 1. Among the 26 probands, 15 were identified from ADNSHL or maternally inherited cases, whereas 11 were identified from ARNSHL or sporadic cases. No other candidate pathogenic variants in the other 62 deafness genes were identified from these 26 probands. When we restricted analysis to Japanese bilateral non-syndromic hearing loss patients, the prevalence of TMC1-associated hearing loss was 0.17% (20/11,594) for all patients, 0.61% (15/2462) for ADNSHL and 0.07% (5/6912) for ARNSHL or sporadic hearing loss cases.
Table 1.
TMC1-associated hearing loss cases identified in this study
| ID | Inheritance | Variant 1 | Variant 2 | Ethnicity | Type of HL | Severity of HL | Progression | Tinnitus | Vertigo | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Base change | AA change | Base change | AA change | ||||||||
| O4886 | AD | c.1627G > A | p.Asp543Asn | Japanese | Flat | Profound | Yes | Yes | Yes | ||
| O4091 | AD | c.1627G > A | p.Asp543Asn | Japanese | Flat | Profound | Yes | Yes | No | ||
| O5030 | AD | c.1627G > A | p.Asp543Asn | Japanese | Flat | Moderate | Yes | Yes | No | ||
| HL2672 | AD | c.1627G > A | p.Asp543Asn | Japanese | Flat | Profound | Yes | Yes | No | ||
| O0487 | AD | c.1627G > A | p.Asp543Asn | Japanese | NA | Profound | Yes | NA | NA | ||
| HL6536 | AD | c.1627G > A | p.Asp543Asn | Japanese | High freq | Severe | Yes | NA | NA | ||
| HL9117 | AD | c.1627G > A | p.Asp543Asn | Japanese | High freq | Moderate | Yes | No | No | ||
| HL9205 | AD | c.1627G > A | p.Asp543Asn | Japanese | NA | Profound | Yes | NA | NA | ||
| HL9597 | AD | c.1627G > A | p.Asp543Asn | Japanese | High freq | Severe | Yes | Yes | No | ||
| HL4994 | AD | c.1627G > A | p.Asp543Asn | Japanese | NA | NA | NA | NA | NA | ||
| HL6717 | AD | c.1627G > A | p.Asp543Asn | Japanese | NA | NA | NA | NA | NA | ||
| HL3819 | AD | c.1714G > A | p.Asp572Asn | Japanese | High freq | Moderate | NA | NA | NA | ||
| HL4498 | AD | c.1714G > A | p.Asp572Asn | Japanese | NA | NA | NA | NA | NA | ||
| HL8588 | AD | c.1714G > A | p.Asp572Asn | Japanese | NA | NA | NA | NA | NA | ||
| HL7492 | AD | c.1714G > A | p.Asp572Asn | Japanese | NA | NA | NA | NA | NA | ||
| HL3123 | Sporadic | c.100C > T | p.Arg34Ter | c.884 + 1G > A | splicing | Japanese | Flat | Profound | No | No | No |
| HL3604 | Sporadic | c.210delG | p.Arg71GlyfsTer5 | c.1592A > T | p.Asp531Val | Japanese | Flat | Profound | No | NA | No |
| HL7927 | Sporadic | c.741 + 1_ + 4del | splicing | c.1333C > T | p.Arg445Cys | Japanese | Flat | Severe | NA | NA | No |
| HL4017 | Sporadic | c.1165C > T | p.Arg389Ter | c.1165C > T | p.Arg389Ter | Japanese | Flat | Profound | No | No | Yes |
| HL8573 | AR | c.2047_2048del | p.His683ArgfsTer169 | c.2047_2048del | p.His683ArgfsTer169 | Japanese | Flat | Profound | NA | NA | No |
| MED473 | Sporadic | c.247_249del | p.Glu83del | c.247_249del | p.Glu83del | Germany | NA | NA | No | No | No |
| MED214 | Sporadic | c.338T > C | p.Met113Thr | c.1534C > T | p.Arg512Ter | Swedish | High freq | Severe | NA | NA | NA |
| MED131 | Sporadic | c.674C > T | p.Pro225Leu | c.1333C > T | p.Arg445Cys | Polish | Flat | Profound | No | No | No |
| MED097 | Sporadic | c.1235delT | p.Met413CysfsTer 4 | c.1764G > A | p.Trp588Ter | Polish | High freq | Profound | No | No | No |
| MED138 | AR | c.1764G > A | p.Trp588Ter | c.1764G > A | p.Trp588Ter | Polish | High freq | Profound | No | No | No |
| MED430 | Sporadic | c.2176_2177del | p.Ala726GlufsTer 126 | c.2176_2177del | p.Ala726GlufsTer 126 | Indian | Flat | Profound | No | No | No |
Severity of HL: pure-tone average calculated from the audiometric thresholds at four frequencies (0.5, 1, 2, and 4 kHz) was categorized into mild (PTA: 21–40 dB HL), moderate (41–70 dB HL), severe (71–95 dB HL), or profound (> 95 dB HL)
AA amino acid, AD autosomal dominant, AR autosomal recessive, NA not available
*All variants are indicated on NM_138691
The variants identified in this study are summarized in Table 2. In this study, we identified 17 candidate TMC1 variants, 7 of which were novel variants and 10 were previously reported. Based on ACMG guidelines and ClinGen HLCDWG expert specifications, 5 were classified as “pathogenic” variants and 2 were classified as of “uncertain significance”. Interestingly, TMC1:c.1627G > A:p.Asp543Asn variants and TMC1:c.1714G > A:p.Asp572Asn variants were identified from 11 and 4 unrelated families with ADNSHL, respectively. Both variants were only identified from ADNSHL patients and were not identified from 6912 autosomal recessive inheritance or sporadic cases, or 2220 unknown family history cases. In addition, these variants were not identified in the gnomAD database or 8.3KJPN (Japanese 8380 genomic variant database). Taken together, the above results strongly supported the pathogenicity of these variants as causative for TMC1-associated ADNSHL.
Table 2.
TMC1 variants identified in this study
| Base change | AA change | Inheritance | SIFT | PP2 | MutTaster | REVEL | CADD | 8.3KJPN | gnomAD | AD_MAF | AR_MAF | ClinGenHL2018 | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c.100C > T | p.Arg34Ter | AR | – | – | A | – | 36 | 0 | 0.000056 | 0 | 0.00018 | Kurima et al. (2002) | |
| c.210delG | p. Arg71GlyfsTer5 | AR | – | – | – | – | – | 0.0001 | 0 | 0 | 0.00018 | Pathogenic | This study |
| c.247_249del | p.Glu83del | AR | – | – | – | – | – | 0 | 0 | 0 | 0.00036 | Sloan-Heggen et al. (2016) | |
| c.338 T > C | p.Met113Thr | AR | D | P | D | 0.263 | 24.8 | 0 | 0.000004 | 0 | 0.00018 | VUS | This study |
| c.674C > T | p.Pro225Leu | AR | T | D | D | 0.4 | 27.2 | 0 | 0.000044 | 0 | 0.00018 | Brownstein et al. (2020) | |
| c.741 + 1_ + 4del | spl | AR | – | – | – | – | – | 0 | 0 | 0 | 0.00036 | Pathogenic | This study |
| c.884 + 1G > A | spl | AR | – | – | D | – | 27.2 | 0 | 0.000012 | 0 | 0.00012 | Kurima et al. (2002) | |
| c.1165C > T | p.Arg389Ter | AR | – | – | A | – | 38 | 0 | 0.000068 | 0 | 0.00054 | Meyer et al. (2005) | |
| c.1235delT | p.Met413CysfsTer4 | AR | – | – | - | – | - | 0 | 0 | 0 | 0.00018 | Pathogenic | This study |
| c.1333C > T | p.Arg445Cys | AR | D | D | D | 0.662 | 35 | 0 | 0.000072 | 0 | 0.00036 | Sirmaci et al. (2009) | |
| c.1534C > T | p.Arg512Ter | AR | – | – | A | – | 42 | 0 | 0.0003 | 0 | 0.00018 | Kurima et al. (2002) | |
| c.1592A > T | p.Asp531Val | AR | D | D | D | 0.861 | 25.7 | 0 | 0 | 0 | 0.00018 | VUS | This study |
| c.1627G > A | p.Asp543Asn | AD | D | D | D | 0.472 | 32 | 0 | 0 | 0.0082 | 0 | Moteki et al. (2016) | |
| c.1714G > A | p.Asp572Asn | AD | T | D | D | 0.465 | 29.7 | 0 | 0 | 0.0045 | 0 | Kurima et al. (2002) | |
| c.1764G > A | p.Trp588Ter | AR | – | – | A | – | 42 | 0 | 0.000012 | 0 | 0.00054 | Tlili et al. (2008) | |
| c.2047_2048del | p.His683ArgfsTer169 | AR | – | – | – | – | – | 0 | 0 | 0 | 0.00036 | Pathogenic | This study |
| c.2176_2177del | p.Ala726GlufsTer126 | AR | – | – | – | – | – | 0 | 0 | 0 | 0.00036 | Pathogenic | This study |
AA amino acid, AD autosomal dominant, AR autosomal recessive, PP2 PolyPhen2, MutTaster Mutation Taster, AD_MAF minor allele frequency in ADNSHL cases, AR_MAF minor allele frequency in ARNSHL cases
In terms of clinical features, TMC1-associated ARNSHL patients showed congenital onset severe-to-profound hearing loss, whereas the TMC1-associated ADNSHL patients showed late-onset progressive hearing loss (Table 1). The severity of hearing loss in ADNSHL patients varied from moderate to severe hearing loss depending on patient age. In addition, 3 family members of family #O4886 who carried TMC1:c.1627G > A:p.Asp543Asn variants showed normal hearing (Supplemental Fig. 1). Most of the ADNSHL cases complained of the progression of hearing loss and tinnitus; however, only two patients suffered episodes of vertigo.
Progression of hearing loss in subjects with TMC1-associated ADNSHL
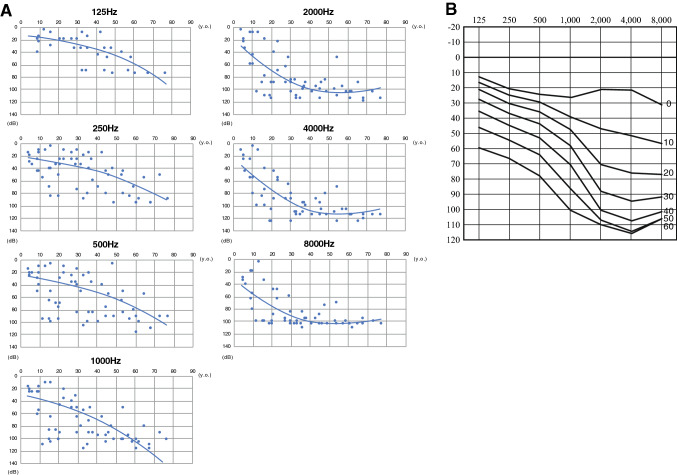
Most of the TMC1-associated ARNSHL patients showed congenital severe-to-profound hearing loss. On the other hand, TMC1-associated ADNSHL patients showed late-onset progressive hearing loss (Table 1). To elucidate the progression of hearing deterioration for TMC1-associated ADNSHL, we performed regression analysis of age and hearing thresholds of 125, 250, 500, 1000, 2000, 4000 and 8000 Hz (Fig. 1). For this analysis, we used the hearing thresholds for all TMC1-associated ADNSHL patients and their affected family members (10 probands and 13 family members) identified in this study and shown in Supplemental Fig. 1. In addition, we also included all available hearing threshold data (34 hearing threshold data) for 24 affected individuals with TMC1-associated ADNSHL from previous reports (Kurima et al. 2002; Yang et al. 2010; Zhao et al. 2014; Wang et al. 2018). As shown in Fig. 1, the hearing levels in the higher frequencies deteriorate more rapidly than those in the lower frequencies. The estimated hearing deterioration in terms of pure-tone average (average of 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz) was 1.0 dB per year. The estimated age-related typical audiogram (ARTA) was calculated based on the previously reported method (Huygen et al. 2003) with some modification to allow the use of exponential approximation or logarithmic approximation.
Fig. 1.
Detailed progression analysis of DFNA36 patients. A Hearing thresholds from audiograms (the better ear) of the patients identified in this study and those previously reported were plotted for each frequency. B Estimated age-related typical audiogram (ARTA) demonstrating the progression of hearing loss for DFNA36
Haplotype analysis
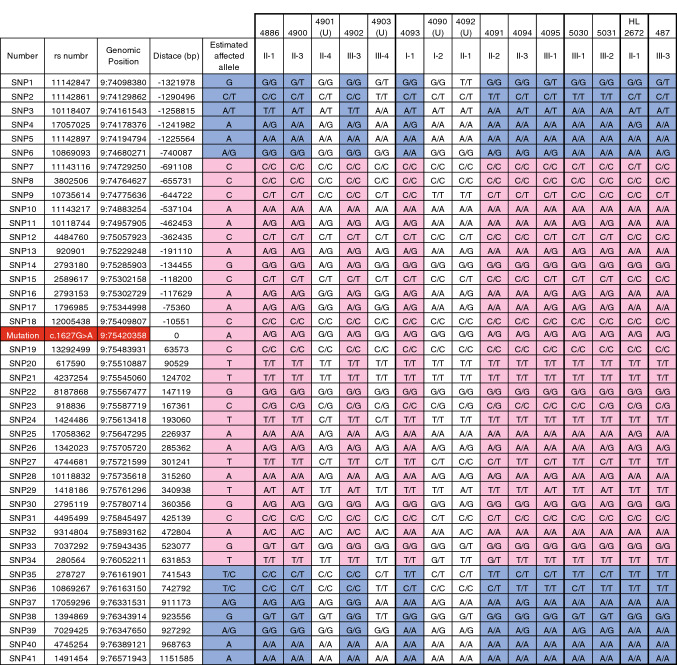
Interestingly, 11 unrelated Japanese ADNSHL families carried the same variant (TMC1: NM_138691:c.1627G > A:p.Asp543Asn). We, therefore, carried out haplotype analysis to confirm whether this mutation occurred by founder mutation or in a mutational hotspot. Figure 2 shows the haplotype patterns for four unrelated families who carried the same TMC1: NM_138691:c.1627G > A variant. As a result, the four unrelated families were found to carry the same haplotype in the 1.3 Mbp region surrounding this mutation (the preserved region ranged from 0.7 Mbp upstream to 0.6 Mbp downstream), suggesting that this mutation occurred and spread as a founder mutation in Japanese populations.
Fig. 2.
Haplotype analysis of the TMC1 recurrent variant c.1627G > A:p.Asp543Asn. The estimated haplotypes surrounding the 3 Mbp region of this variant are indicated. The pink area was conserved between unrelated families. The pale blue area was not conserved
Discussion
In this study, we identified 26 probands with TMC1-associated hearing loss and the prevalence of TMC1-associated hearing loss in Japanese hearing loss patients was 0.17% for all patients. The prevalence of TMC1-associated hearing loss in other countries is 0.5–8.1% and varies among ethnic populations as described above in the introduction. These differences may be caused by the carrier frequencies of commonly observed mutations. In most previous studies, TMC1-associated hearing loss was observed more commonly in ARNSHL patients than in ADNSHL patients, and common mutations which may be caused by founder mutation were involved in these cases. On the other hand, in our Japanese hearing loss cohort, ADNSHL cases were more commonly observed than ARNSHL cases. In addition, all identified variants from Japanese TMC1-associated ARNSHL cases differed among patients and no common mutations were identified.
Similar to previous studies, TMC1-associated ARNSHL patients showed congenital onset severe-to-profound hearing loss, whereas the TMC1-associated ADNSHL patients showed late-onset progressive hearing loss. Indeed, 3 younger agers in family # O4886 showed normal hearing although they carried the same mutation as the other affected family members (Supplemental Fig. 1), supporting the late-onset nature of their hearing loss. In addition, we also clarified the progression of hearing loss for DFNA36 using the hearing threshold data obtained in this study and previous reports, and revealed the hearing deterioration in terms of pure-tone average was 1.0 dB per year. Most of the TMC1-associated HL patients identified in this study did not have vestibular symptoms and only two patients had episodes of vertigo. Thus, vestibular symptoms may not be associated with TMC1-associated HL cases.
Toward the clinical application of gene therapy for hereditary hearing loss, TMC1-associated ADNSHL is believed to be a good candidate, as the late-onset and progressive hearing loss phenotype can be stopped or slowed down by gene therapy prior to hearing deterioration. In addition, ENU-induced model mice with the orthologous mutation identified in human ADNSHL patients are widely used for translational research for gene therapy (Askew et al. 2015; Shibata et al. 2016; Yoshimura et al. 2019; Gao et al. 2018; Nist-Lund et al. 2019; György et al. 2019; Wu et al. 2021). In most of these gene therapy studies, the gene delivering vector, adeno associated virus (AAV), was administrated into the inner ear of neonate mice, allowing prevention of hearing deterioration. However, this timing is equivalent to the developmental stage of the inner ear of the human fetus and makes clinical application difficult. Recently, Yoshimura et al. (2019) reported gene therapy for 2- to 8-week-old mice and prevented hearing deterioration in these model mice, suggesting the appropriate time-window for gene therapy will be wider than previously thought. In this study, we indicated that the hearing deterioration in DFNA36 patients started from their 1st or 2nd decade (teenagers) and this result also supports the notion that the therapeutic time-window for gene therapy to prevent hearing deterioration in human patients might be wider than previously thought.
In this study, we identified 11 unrelated Japanese ADNSHL families that carried same the variant (TMC1: NM_138691:c.1627G > A:p.Asp543Asn). Haplotype analysis of TMC1: NM_138691:c.1627G > A:p.Asp543Asn showed the same haplotype among the families with the same mutation. This result suggested that this mutation occurred in one common ancestor and was subsequently spread by founder mutation rather than in a mutational hot spot (a mutation which frequently occurs in a specific DNA position). This hypothesis was supported by the fact that this mutation was only identified from Japanese hearing loss patients. This is the first report of a founder mutation identified in DFNA36. Based on the higher prevalence (11 patients carried this mutation in our 11,594 hearing loss subjects), this mutation will be a good candidate for the clinical study of gene therapy for DFNA36. On the other hand, the c.1714G > A:p.Asp572Asn variant observed in this study may be caused by a mutational hotspot. The p.Asp572Asn variant was identified from four Japanese ADNSHL patients in this study, but this variant was also identified from North American, Chinese and Saudi patients (Kurima et al. 2002; Wang et al. 2018; Ramzan et al. 2020; Yuan et al. 2020). The observations of patients from different ethnic backgrounds also support the fact that this variant was caused by a mutational hotspot.
In summary, next-generation sequencing analysis successfully identified 10 previously reported mutations and 7 novel variants for TMC1-associated hearing loss. The estimated prevalence of TMC1-associated hearing loss in the Japanese hearing loss cohort was 0.17% for all patients, 0.61% for ADNSHL and 0.07% for ARNSHL or sporadic hearing loss cases. This large cohort study of hearing loss patients provided valuable new insights, particularly with regard to hearing deterioration in DFNA36 patients. This information will be useful baseline data for future therapeutics including gene therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all participants in the present study and our collaborators for providing samples and clinical information as listed below; Dr. Tsukasa Ito and Dr. Toshinori Kubota (Yamagata University), Dr. Mayuri Okami (Tokai University), Dr. Mariko Kakudo (Hyougo Medical University), Dr. Kenji Ohyama and Dr. Kiyoshi Oda (Touhoku Rousai Hospital), Dr. Tomoko Esaki (Aichi Children Medical Center), Dr. Jun Nakayama (Shiga University), Dr. Yasushi Naito and Dr. Hiroshi Yamazaki (Kobe City Medical Center General Hospital), Dr. Yumi Ohta (Osaka University), Dr. Tetsuya Tono (Miyazaki University), Dr. Ikuyo Miyanohara (Kagoshima University), Dr. Manoj Manikoth (ENT Super Speciality Institute and Research Center, India), Dr. Joachim Müller (Klinikum der Universität München, German), Dr. Eva Karltorp (Karolinska University Hospital, Sweden), and Dr. Henryk Skarzynski, and Dr. Piotr Skarzynski (Institute of Physiology and Pathology of Hearing, Poland).
Funding
This research was funded by a Health and Labor Sciences Research Grant for Research on Rare and Intractable Diseases and Comprehensive Research on Disability Health and Welfare from the Ministry of Health, Labor and Welfare of Japan (S.U. 20FC1048), and a Grants-in-Aid from Japan Agency for Medical Research and Development (AMED) (S.U.: 17kk0205010h0002, 18ek0109363h0001).
Declarations
Conflict of interest
All authors declare no conflicts of interest in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abu Rayyan A, Kamal L, Casadei S, et al. Genomic analysis of inherited hearing loss in the Palestinian population. Proc Natl Acad Sci USA. 2020;117:20070–20076. doi: 10.1073/pnas.2009628117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew C, Rochat C, Pan B, et al. Tmc gene therapy restores auditory function in deaf mice. Sci Transl Med. 2015;7:295ra108. doi: 10.1126/scitranslmed.aab1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azaiez H, Booth KT, Ephraim SS, et al. Genomic landscape and mutational signatures of deafness-associated genes. Am J Hum Genet. 2018;103:484–497. doi: 10.1016/j.ajhg.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein Z, Gulsuner S, Walsh T et al (2020) Spectrum of genes for inherited hearing loss in the Israeli Jewish population, including the novel human deafness gene ATOH1. Clin Genet 98:353–364 [DOI] [PMC free article] [PubMed]
- Gao X, Tao Y, Lamas V, et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature. 2018;553:217–221. doi: 10.1038/nature25164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- György B, Nist-Lund C, Pan B, et al. Allele-specific gene editing prevents deafness in a model of dominant progressive hearing loss. Nat Med. 2019;25:1123–1130. doi: 10.1038/s41591-019-0500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MA, Shah AA, Szmida E, et al. A TMC1 (transmembrane channel-like 1) mutation (p. S320R) in a Polish family with hearing impairment. J Appl Genet. 2015;56:311–316. doi: 10.1007/s13353-014-0263-4. [DOI] [PubMed] [Google Scholar]
- Huygen PLM, Pennings RJE, Cremers CWRJ. Characterizing and distinguishing progressive phenotypes in nonsyndromic autosomal dominant hearing impairment. Audiol Med. 2003;1:37–46. doi: 10.1080/16513860310003049. [DOI] [Google Scholar]
- Kalay E, Karaguzel A, Caylan R, et al. Four novel TMC1 (DFNB7/DFNB11) mutations in Turkish patients with congenital autosomal recessive nonsyndromic hearing loss. Hum Mutat. 2005;26:591. doi: 10.1002/humu.9384. [DOI] [PubMed] [Google Scholar]
- Kitajiri S, Makishima T, Friedman TB, Griffith AJ. A novel mutation at the DFNA36 hearing loss locus reveals a critical function and potential genotype-phenotype correlation for amino acid-572 of TMC1. Clin Genet. 2007;71:148–152. doi: 10.1111/j.1399-0004.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- Kitajiri SI, McNamara R, Makishima T, et al. Identities, frequencies and origins of TMC1 mutations causing DFNB7/B11 deafness in Pakistan. Clin Genet. 2007;72:546–550. doi: 10.1111/j.1399-0004.2007.00895.x. [DOI] [PubMed] [Google Scholar]
- Kurima K, Peters LM, Yang Y, et al. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet. 2002;30:277–284. doi: 10.1038/ng842. [DOI] [PubMed] [Google Scholar]
- Likar T, Hasanhodžić M, Teran N, et al. Diagnostic outcomes of exome sequencing in patients with syndromic or non-syndromic hearing loss. PLoS ONE. 2018;13:e0188578. doi: 10.1371/journal.pone.0188578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wang S, Zou L, Xiong W (2020) Mechanisms in cochlear hair cell mechano-electrical transduction for acquisition of sound frequency and intensity. Cell Mol Life Sci. Online ahead of print [DOI] [PMC free article] [PubMed]
- Meyer Christian G., Gasmelseed Nagla M., Mergani Adil, Magzoub Mubarak M.A., Muntau Birgit, Thye Thorsten, Horstmann Rolf D. NovelTMC1 structural and splice variants associated with congenital nonsyndromic deafness in a Sudanese pedigree. Human Mutation. 2005;25(1):100–100. doi: 10.1002/humu.9302. [DOI] [PubMed] [Google Scholar]
- Moteki H, Azaiez H, Booth KT, et al. Comprehensive genetic testing with ethnic-specific filtering by allele frequency in a Japanese hearing-loss population. Clin Genet. 2016;89:466–472. doi: 10.1111/cge.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio SY, Usami SI. The clinical next-generation sequencing database: a tool for the unified management of clinical information and genetic variants to accelerate variant pathogenicity classification. Hum Mutat. 2017;38:252–259. doi: 10.1002/humu.23160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio SY, Hayashi Y, Watanabe M, Usami S. Clinical application of a custom AmpliSeq library and ion torrent PGM sequencing to comprehensive mutation screening for deafness genes. Genet Test Mol Biomarkers. 2015;19:209–217. doi: 10.1089/gtmb.2014.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nist-Lund CA, Pan B, Patterson A, et al. Improved TMC1 gene therapy restores hearing and balance in mice with genetic inner ear disorders. Nat Commun. 2019;10:236. doi: 10.1038/s41467-018-08264-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza AM, DiStefano MT, Hemphill SE, et al. Expert specification of the ACMG/AMP variant interpretation guidelines for genetic hearing loss. Hum Mutat. 2018;39:1593–1613. doi: 10.1002/humu.23630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramzan K, Al-Owain M, Al-Numair NS, et al. Identification of TMC1 as a relatively common cause for nonsyndromic hearing loss in the Saudi population. Am J Med Genet B Neuropsychiatr Genet. 2020;183:172–180. doi: 10.1002/ajmg.b.32774. [DOI] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safka Brozkova D, Poisson Marková S, Mészárosová AU, et al. Spectrum and frequencies of non GJB2 gene mutations in Czech patients with early non-syndromic hearing loss detected by gene panel NGS and whole-exome sequencing. Clin Genet. 2020;98:548–554. doi: 10.1111/cge.13839. [DOI] [PubMed] [Google Scholar]
- Seco CZ, Wesdorp M, Feenstra I, et al. The diagnostic yield of whole-exome sequencing targeting a gene panel for hearing impairment in The Netherlands. Eur J Hum Genet. 2017;25:308–314. doi: 10.1038/ejhg.2016.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata SB, Ranum PT, Moteki H, et al. RNA interference prevents autosomal-dominant hearing loss. Am J Hum Genet. 2016;98:1101–1113. doi: 10.1016/j.ajhg.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirmaci A, Duman D, Oztürkmen-Akay H, et al. Mutations in TMC1 contribute significantly to nonsyndromic autosomal recessive sensorineural hearing loss: a report of five novel mutations. Int J Pediatr Otorhinolaryngol. 2009;73:699–705. doi: 10.1016/j.ijporl.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Sloan-Heggen CM, Bierer AO, Shearer AE, et al. Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Hum Genet. 2016;135:441–450. doi: 10.1007/s00439-016-1648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommen M, Schrauwen I, Vandeweyer G, et al. DNA diagnostics of hereditary hearing loss: a targeted resequencing approach combined with a mutation classification system. Hum Mutat. 2016;37:812–819. doi: 10.1002/humu.22999. [DOI] [PubMed] [Google Scholar]
- Tlili A, Rebeh IB, Aifa-Hmani M, et al. TMC1 but not TMC2 is responsible for autosomal recessive nonsyndromic hearing impairment in Tunisian families. Audiol Neurootol. 2008;13:213–218. doi: 10.1159/000115430. [DOI] [PubMed] [Google Scholar]
- Vreugde S, Erven A, Kros CJ, et al. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat Genet. 2002;30:257–258. doi: 10.1038/ng848. [DOI] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wu K, Guan J, et al. Identification of four TMC1 variations in different Chinese families with hereditary hearing loss. Mol Genet Genomic Med. 2018;6:504–513. doi: 10.1002/mgg3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Solanes P, Nist-Lund C, et al. Single and dual vector gene therapy with AAV9-PHP.B rescues hearing in Tmc1 mutant mice. Mol Ther. 2021;29:973–988. doi: 10.1016/j.ymthe.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Kahrizi K, Bazazzadeghan N, et al. A novel mutation adjacent to the Bth mouse mutation in the TMC1 gene makes this mouse an excellent model of human deafness at the DFNA36 locus. Clin Genet. 2010;77:395–398. doi: 10.1111/j.1399-0004.2009.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Wei X, Chai Y, et al. Genetic etiology study of the non-syndromic deafness in Chinese Hans by targeted next-generation sequencing. Orphanet J Rare Dis. 2013;8:85. doi: 10.1186/1750-1172-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H, Shibata SB, Ranum PT, et al. Targeted allele suppression prevents progressive hearing loss in the mature Murine model of Human TMC1 deafness. Mol Ther. 2019;27:681–690. doi: 10.1016/j.ymthe.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Li Q, Su Y, et al. Comprehensive genetic testing of Chinese SNHL patients and variants interpretation using ACMG guidelines and ethnically matched normal controls. Eur J Hum Genet. 2020;28:231–243. doi: 10.1038/s41431-019-0510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wang D, Zong L, et al. A novel DFNA36 mutation in TMC1 orthologous to the Beethoven (Bth) mouse associated with autosomal dominant hearing loss in a Chinese family. PLoS ONE. 2014;9:e97064. doi: 10.1371/journal.pone.0097064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.