Abstract
Purpose:
To determine if persistent hyper-transmission defects (hyperTDs), previously shown to have a greatest linear dimension (GLD) ≥ 250μm on en face swept source OCT (SS-OCT) images, serve as a stand-alone early biomarker for the future formation geographic atrophy (GA).
Design:
Post-hoc cohort study using a subgroup of a prospective study.
Participants:
Patients with intermediate age-related macular degeneration (iAMD).
Methods:
All subjects underwent 6X6mm SS-OCT raster scans at baseline and during their follow-up period. En face images were generated using a slab with segmentation boundaries positioned 64μm to 400μm beneath Bruch’s membrane. Two graders independently evaluated all en face structural images for the presence of hyperTDs with a GLD ≥ 250μm and GA.
Results:
A total of 190 eyes were included with a mean follow up of 31(SD: 13.2) months. At baseline, 31 eyes (16%) had at least one hyperTD ≥ 250μm and 13 eyes (42%) progressed to GA. In those eyes without a hyperTD ≥ 250μm at baseline, 42 (26%) developed hyperTDs ≥ 250μm during their follow-up and 11 eyes (7%) progressed to GA. At the last available follow-up visit, 25 eyes (13%) progressed to GA and of these 25 eyes, 24 eyes had a prior hyperTD ≥ 250μm detected before GA formed. A time-dependent Cox-survival regression analysis estimated an 80-fold (95% CI: 10.7 – 614, p<0.001) increased risk of developing GA once a hyperTD ≥ 250μm appeared.
Conclusions:
Persistent hyperTDs detected on en face OCT images were shown to serve as an early stand-alone OCT biomarker for the future formation of GA.
Graphical Abstract
Persistent hyper-transmission defects detected on en face swept source OCT images can serve as stand-alone early biomarkers for the future formation of geographic atrophy in eyes with intermediate age-related macular degeneration.
INTRODUCTION
Geographic atrophy (GA) is the late stage of non-exudative age-related macular degeneration (AMD) characterized by the progressive loss of photoreceptors, retinal pigmented epithelium (RPE), and choriocapillaris. GA is also known as complete retinal pigment epithelium and outer retinal atrophy (cRORA), and this consensus term arose from the Classification of Atrophy Meetings (CAM) in which GA was used specifically when referring to late cRORA in AMD. 1 Due to the irreversible progressive loss of visual function wherever GA appears, early detection of precursor lesions that predict where GA may arise and enlarge is important not only for managing patient expectations but also for designing clinical trials to test therapies that could prevent the onset and progression of GA.2 Currently, two precursor lesions that precede the onset of GA have been proposed: nascent GA (nGA) and incomplete RPE and outer retinal atrophy (iRORA).3,4
nGA was first described by Wu and colleagues (Wu et al.) after following 181 eyes with intermediate AMD (iAMD).5 From their study, two features observed on averaged spectral domain (SD) optical coherence tomography (OCT) B-scan images were found to serve as potential GA biomarkers. These features were the subsidence of the outer plexiform layer (OPL) and inner nuclear layer (INL) and the formation of a hypo-reflective wedge-shaped band within the boundaries of the OPL. More recently, an international group of experts in the diagnosis, management, and imaging of AMD held a consensus meeting and defined iRORA as a precursor of GA.3 iRORA was also defined by averaged OCT B-scan images and required the presence of choroidal hypertransmission (hyperT), which arises when the RPE is attenuated or disrupted resulting in increased light transmission into the choroid, along with the presence of overlying photoreceptor degeneration. Although both nGA and iRORA are important biomarkers for disease progression, they are limited by the requirements that highly averaged B-scans need to be performed and experienced graders are needed to interpret these scans.6 In addition, the review of all macular B-scans from an OCT raster scan is a tedious process and requires meticulous review of each averaged B-scan to assess whether a region on a B-scan, usually the size of a druse or larger, exceeds a given threshold of change to fulfill the criteria for the classification of nGA or iRORA. Moreover, the detection of nGA and iRORA also requires that the OCT scan be performed by using tightly spaced B-scans. If a large druse has a minimum size of 125μm, then the spacing between B-scans should not exceed this distance in a macular raster scan, and ideally, the distance should be at least half the size of a large druse to be sure the lesion is captured. For a standard macular raster scan with a 6X6mm field of view, this requirement would be achieved by using the current default setting of 97 averaged B-scans (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany), which results in a B-scan spacing of about 62μm. Not only is this grading process laborious, but as the Classification of Atrophy Meeting (CAM) consensus grading exercises have shown, it may be difficult to reach a definitive classification as the lesions evolve, so determining when these lesions cross a grading threshold requires a high-level of expertise and training.1,3,6
Previously, we proposed an alternative to reviewing averaged B-scans for the grading of nGA or iRORA, and this alternative takes advantage of the choroidal hypertransmission defects (hyperTDs) that are a feature of both iRORA and GA.3,7-13 Rather than reviewing each B-scan separately, we suggested the use of an en face OCT imaging strategy using raster scans with B-scan spacing intervals that are sufficiently close together. In practice, scan patterns with spacing between 12μm and 46μm have been employed. This en face approach allows for the review of the entire volumetric scan at a single glance. The hyperTDs appear as bright regions on an en face OCT image derived from a sub-RPE slab with segmentation boundaries positioned 64 and 400μm below Bruch’s membrane. When using this slab, the region of the hyperTD appears bright compared with the surrounding intact RPE because of the increased light transmission into the choroid where the RPE is attenuated or absent.14 When we first proposed the use of the hyperTDs as an early precursor to GA, we did not require a minimum size for these hyperTDs.11 However, we subsequently reported that a minimum size requirement is necessary because small pinpoint foci with hyperTDs were found to be transient.13
To establish a minimum size for persistent hyperTDs, Shi et al. studied the persistence of these hyperTDs in a prospective, longitudinal study that included the use of both averaged B-scans and en face volumetric scans.13 In this study, the presence of nGA was determined from the averaged B-scans and the size of the hyperTDs was determined from en face images. They found that the greatest linear dimension (GLD) on an en face image for hyperTDs that persisted over 36 months was between 250μm and 300 μm. Moreover, Shi et al. found a strong correlation between the presence of these persistent choroidal hyperTDs and the diagnosis of nGA based on averaged B-scans, thus validating the detection of hyperTDs as potential precursor for the future formation of GA.13
To validate the use of hyperTDs with a minimum size of 250μm as a useful stand-alone precursor for the future formation of GA in eyes with iAMD and drusen, we performed a separate natural history study using swept source OCT (SS-OCT) raster scans of AMD eyes with drusen but with no evidence of GA. In the current report, we establish that hyperTDs with a minimum size of 250μm, when detected on en face images of densely spaced SS-OCT B-scans, are highly predictive of future formation of GA.
MATERIAL AND METHODS
AMD patients were enrolled in a prospective, observational, SS-OCT imaging study at the Bascom Palmer Eye Institute. The institutional review board (IRB) of the University of Miami Miller School of Medicine approved the study (Protocol 201220997) and all patients signed an informed consent for this prospective SS-OCT study. The study was performed in accordance with the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act of 1996.
A retrospective review of this prospective observational SS-OCT imaging study included eyes from patients followed from April 2016 to February 2021. All patients included in this study had at least one eye diagnosed with iAMD defined by the presence of at least one large druse with a minimal diameter of 125μm (equivalent to an approximate minimal volume of 0.001 mm3), in a 5 mm circle centered on the fovea. Patients also had to have at least one-year follow-up between their baseline visit, when they qualified for the study, and their last available visit. The frequency of evaluation was determined by the clinical stage of the disease, the status of the fellow eye, and patients’ availability. For the purpose of this study, all available images from all visits were considered. Exclusion criteria included a history of exudative macular neovascularization (MNV) or GA, diabetic retinopathy, any concomitant retinal diseases associated with drusen-like deposits such as Stargardt disease, cuticular drusen, vitelliform dystrophy, any vitreoretinal interface disease causing marked distortion of the macular anatomy, and a history of previous vitreoretinal surgery.
All eyes underwent SS-OCT imaging (PLEX® Elite 9000, Carl Zeiss Meditec, Dublin, CA) at baseline and during follow-up and these scans were acquired by one of two trained imaging technicians. All patients were imaged using the SS-OCT angiography (SS-OCTA) 6x6 mm scan pattern centered on the fovea. This scan pattern consists of 500 A-scans per B-scan, with each B-scan repeated twice at each position to generate the angiographic signal, and 500 B-scan positions along the slow axis, resulting in a uniform spacing of 12 μm between A-scans. Each A-scan had a depth of 3 mm consisting of 1536 pixels per A-scan. The complex optical microangiographic algorithm known as OMAGC was used to generate flow information from the variations in both the OCT signal magnitude and phase information between sequential B-scans acquired at the same position.15, 16 Each volumetric scan was reviewed for quality and signal strength, and scans with a signal strength less than 7 based on the instrument’s output, as well as scans with significant motion artifacts, were excluded. If more than one scan of a given type was available at a visit, the scan with the best quality was chosen. The drusen volume (mm3) and the RPE elevation maps were generated and validated using the Advanced RPE Analysis Algorithm version 0.10 (Carl Zeiss Meditec, USA) as described by Jiang et al.17
All eyes were screened for the presence of non-exudative MNV. MNV was identified using two SS-OCTA slabs. One slab extended from the outer retina to the choriocapillaris, known as the ORCC slab, and was used primarily to identify type 2 and type 3 MNV. The second slab extended from the RPE to the RPE fit, and this slab was used primarily to detect type 1 MNV. In addition, corresponding structural B-scans and flow encoded B-scans were used to further identify evidence of low-lying irregular RPE elevations consistent with the presence of non-exudative type 1 MNV. Evidence of nonexudative type 3 MNV was obtained by reviewing the ORCC slab and flow encoded B-scans in suspicious areas with increased retinal thickening. All scans were reviewed to assess for evidence of exudation. Exudation was defined as the appearance of any subretinal or intraretinal fluid on structural OCT B-scans and on the retinal thickness maps.
The presence of a hyperTD was determined by reviewing the structural en face images that were prepared by using a sub-RPE slab positioned 64 to 400μm beneath Bruch’s membrane as previously reported.2,13 Two separated graders, RL and YS, independently evaluated all en face images for the presence of these lesions. HyperTDs were defined as areas of increased focal brightness corresponding to the hyper-transmission of light into the choroid. Multiple hyperTDs could be identified in any eye. Each grader then measured the GLD for each hyperTD using the caliper from the instrument. In a previous study, a persistent hyperTD was defined as having a GLD ≥ 250μm.13 Once hyperTDs ≥ 250μm were identified on the sub-RPE OCT slabs, B-scans through the lesions were reviewed to confirm the presence of a choroidal hyperTD. At each hyperTD measuring at least 250μm, the presence of GA was assessed. GA was defined on the OCT B-scans as the complete loss of the RPE along with the complete loss of the photoreceptors in the outer nuclear layer, which required the complete loss of the photoreceptor ellipsoid zone, also known as the inner segment/outer segment region.
Consensus gradings of the hyperTDs and GA were reached between the two graders for each lesion in each eye at each visit. Any remaining disagreement was adjudicated by a senior grader (PJR). For each eye, all the available visits were reviewed and annotated for the development of the first hyperTD with a GLD ≥ 250μm. For the subsequent visits, we tracked the persistence of the hyperTD ≥ 250μm, the progression of the hyperTD ≥ 250μm to GA, the appearance of additional hyperTDs ≥ 250μm and their progression to GA, and the development of nonexudative and exudative MNV.
Statistical Analysis
Cumulative incidence of hyperTDs ≥ 250μm, exudative MNV, and GA were calculated with Kaplan-Meier survival methods. Data were censored at the last available visit date or when exudation occurred. The influence of hyperTDs ≥ 250μm on incident GA, incident exudation, and incident late AMD, defined by either GA or exudative MNV, were assessed with the Cox proportional hazards regression model in which the observation of hyperTDs ≥ 250μm was included as a time-dependent covariate. Since data from two eyes of some patients were included in the analysis, standard errors were estimated with the robust sandwich method.18 Statistical analyses were performed with IBM SPSS Statistics for Windows, Version 25.0 (IBM Corporation, Armonk, NY) and SAS Version 9.4 (SAS Institute, Cary NC) with a p value of < 0.05 considered statistically significant.
RESULTS
A total of 190 eyes from 134 patients with iAMD in at least one eye were enrolled. The mean age was 77.5±7.2 years and 64% were women. Eyes were followed for a mean of 31 (Standard Deviation (SD):13.2) months (median: 29; range 6-57). Ten percent of eyes were followed for at least 12 months, 26% for at least 24 months, 32% for at least 36 months and the remaining 32% were followed over 37 months. Regarding the frequency of follow-up, on average, 18% of eyes had an annual visit, 46% of eyes had between 2 and 3 annual visits, and 36% of eyes had more than four annual visits. At baseline, the average drusen volume within the 3mm and 5mm circles centered on the fovea were 0.138±0.150mm3 and 0.173±0.191mm3, respectively.
Overall, at the last available follow-up visit, 25 eyes (13%) progressed to GA and in 24 (96%) of these 25 eyes, a hyperTD ≥ 250μm was detected prior to the formation of GA. Figure 1 show the Kaplan-Meier cumulative proportion of iAMD eyes that developed GA over time, as well as the cumulative proportion for iAMD eyes with hyperTDs ≥ 250μm. Table 1 reports the cumulative proportions at 36 months. By 36 months, 19.0% of all eyes had developed GA from baseline, while 61.9% of eyes with a prior hyperTDs ≥ 250μm developed GA within 36 months after the first hyperTD was seen. In eyes without hyperTDs ≥ 250μm at baseline or follow-up, the cumulative proportion of all iAMD eyes developing GA was1.3%.
Figure 1.

Kaplan-Meier analysis showing the cumulative proportion of eyes developing geographic atrophy (GA). (A) All subjects (n=190 eyes). (B). Subjects with at least one hyper-transmission defect (hyperTD) measuring at least 250μm in greatest linear dimension. Abbreviations: GA: geographic atrophy; hyperTD: hyper-transmission defect.
Table1.
Kaplan-Meier Cumulative Proportion of Eyes Progressing to HyperTDs and GA by 36 Months.
| Cumulative Proportion of Eyes Developing hyperTDs |
Cumulative Proportion of Eyes Developing GA |
|
|---|---|---|
| All iAMD Eyes at Baseline (N=190) | 38.7% | 19.0% |
| iAMD Eyes without HyperTDs at Baseline or Follow-up (N=117) | N/A | 1.28% |
| iAMD Eyes with HyperTDs at Baseline or Follow-up N=73) | N/A | 61.90% |
Abbreviations: HyperTD: hyper-transmission defect; GA: geographic atrophy; iAMD: intermediate age-related macular degeneration; N/A: not applicable
Figure 2A shows the Kaplan-Meier cumulative proportions over time to the development of at least one hyperTD ≥ 250μm. By 36 months, 38.7% of eyes had developed at least one hyperTD ≥ 250μm. Figure 2B shows the cumulative proportion of eyes with at least one hyperTD ≥ 250μm accumulating additional hyperTDs ≥ 250μm. By 36 months after the detection of the first hyperTD ≥ 250μm, 38.8% of eyes had developed additional lesions.
Figure 2.

Kaplan-Meier analysis showing the cumulative proportion of eyes developing hyperTDs ≥ 250μm. (A) Eyes developing at least one hyperTD ≥ 250μm. (B) Cumulative proportion of eyes developing additional hyperTDs ≥ 250μm after the first one was detected. Abbreviations: hyperTD: hyper-transmission defect.
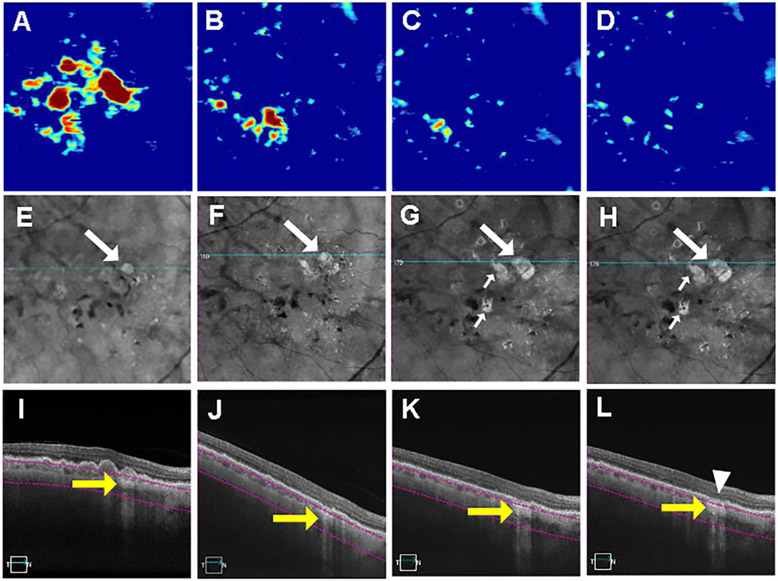
At baseline, 31 (16%) eyes had at least one hyperTD ≥ 250μm, with a cumulative proportion of 56.3% of these eyes progressing to GA by 36 months. Twelve of these eyes developed GA at the same location where the first hyperTD ≥ 250μm was detected, while one eye developed GA at the location where a subsequent hyperTD ≥ 250μm developed. Figure 3 shows an example of an eye with a hyperTD ≥ 250μm at baseline that progressed to GA. In this figure, the hyperTD is evident on the en face structural sub-RPE slab image, and as the lesion enlarged, GA was confirmed on the corresponding B-scans by the complete loss of the RPE and loss of the outer retina photoreceptor layer.
Figure 3.
Swept source optical coherence tomography (SS-OCT) imaging of a persistent hyper-transmission defect (hyperTD) detected on sub-RPE en face imaging at baseline that evolved into geographic atrophy (GA). (A-D) Drusen maps. (E-H) En face structural images derived from a slab under the retinal pigment epithelium (sub-RPE slab) showing bight areas (white arrow) consistent with hyperTDs. (I-L) B-scans corresponding to the blue line in panels E-H crossing the hyperTD shown by the yellow arrows. The purple segmentation lines on the B-scans depict the segmentation boundaries for the sub-RPE slab which is located 64 to 400μm beneath Bruch’s membrane. At baseline (A, E, I), a hyperTD can be seen in panels E and I that corresponds to a druse seen in panels A and I. After 7 months (B, F, J), there is a loss of drusen volume as seen in panels B and J with persistence of the hyperTD. Seven months later (14 months from baseline), we see a further decrease in drusen volume (C) with enlargement of the original hyperTD (G) along with the appearance of additional hyperTDs (small white arrows) and further atrophy of the outer retina as seen on the B-scan (K). Five months later, 19 months from baseline, drusen volume continued to decrease (D) while the hyperTDs increased in size of (H) and atrophy of the RPE and outer retina consistent with the formation of GA can be seen on a B-scan through the site of the initial hyperTD can (L, white arrowhead). Drusen volume in a 5mm fovea-centered circle: A: 0.43mm3; B: 0.095 mm3; C: 0.036 mm3; D: 0.023 mm3.
Among the eyes without a hyperTD ≥ 250μm at baseline (159 eyes, 84%), the cumulative proportion of eyes developing a hyperTD by 36 months was 26.8%, and the proportion of eyes developing GA by 36 months was 12.3%. Of the eyes that developed hyperTDs, 42 (58%) developed at least one additional hyperTD ≥ 250μm during the observation period (Figure 2B), and 29 (69%) of these eyes had further follow up with 11 (7%) progressing to GA. In our study population, the longest interval between the observation of hyperTDs and progression to GA was 31.5 months in our population. Ten of these eyes developed GA at the same location where the first hyperTD ≥ 250μm was detected, while one eye developed GA at the location where a subsequent hyperTD ≥ 250μm was detected. Figures 4, 5 and 6 show eyes developing hyperTDs ≥ 250μm that later progressed to GA. As in Figure 3, the GA developed at the site of the enlarging hyperTD. Of note, the drusen volume maps and the corresponding B-scans show that the RPE elevations corresponding to the drusen decreased as the hyperTDs enlarged and developed into GA. In the corresponding B-scans, the progressive loss of the RPE and outer retinal layers can be appreciated as GA developed. However, as shown in Figure 7, the collapse of drusen associated with persistent hyperTD ≥ 250μm may result in a persistent hyperTD with outer retinal changes, but these lesions may not always progress to GA, as defined by the graders, during the follow-up visits that were available.
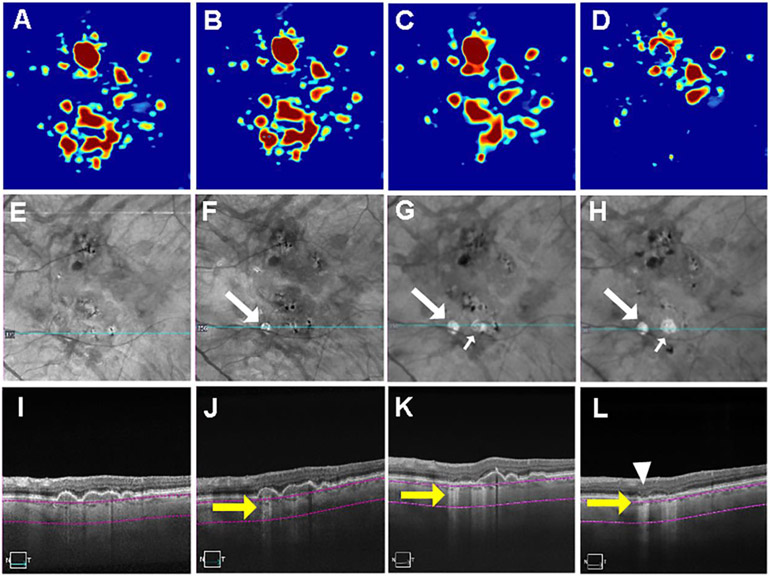
Figure 4.
Swept source optical coherence tomography (SS-OCT) imaging of a persistent hyper-transmission defect (hyperTD) detected on sub-RPE en face imaging after six months of follow-up and evolving into geographic atrophy (GA). (A-D) Drusen maps. (E-H) En face structural images derived from a slab under the retinal pigment epithelium (sub-RPE slab) showing bight areas (F-H, white arrow) consistent with hyperTDs. (I-L) B-scans corresponding to the blue line in panels E-H crossing the hyperTD shown by the yellow arrows (J-L). The purple segmentation lines on the B-scans depict the segmentation boundaries for the sub-RPE slab which is located 64 to 400μm beneath Bruch’s membrane. A hyperTD was first detected at 6 months of follow-up (B, F, J). The hyperTD can be seen in panels F and J in correspondence to a druse seen in panels B and J. Twelve months later (18 months from baseline), the persistent original hyperTD enlarged (G) and disruption of the outer retinal layers can be seen in the corresponding B-scan (K). Twelve months after (D, H, L), at 30 months from baseline, there is a loss of drusen volume as seen in panels D and L with persistence and further enlargement of the original hyperTD (H) along with atrophy of the RPE and outer retina consistent with the formation of GA (L, white arrowhead). Drusen volume in a 5mm fovea-centered circle: A: 0.63mm3; B: 0.75 mm3; C: 0.89 mm3; D: 0.13 mm3.
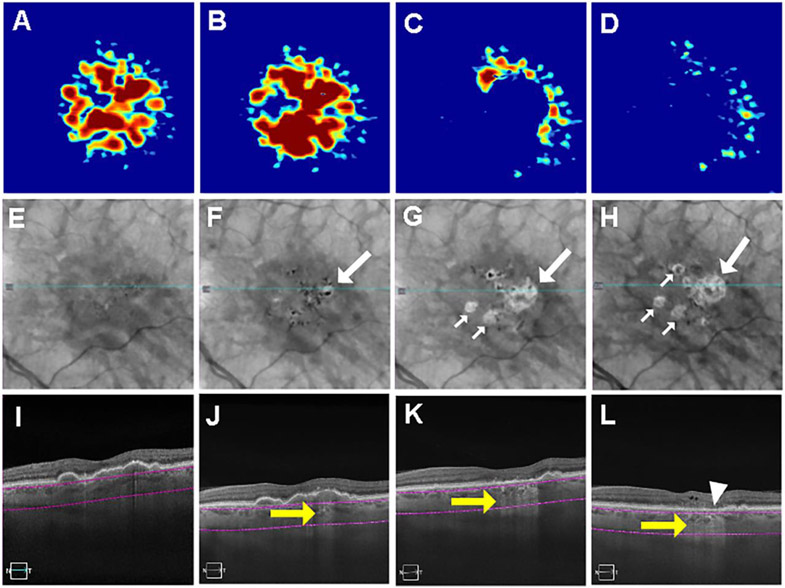
Figure 5.
Swept source optical coherence tomography (SS-OCT) imaging of a persistent hyper-transmission defect (hyperTD) detected on sub-RPE en face imaging after six months of follow-up and evolving into geographic atrophy (GA). (A-D) Drusen maps. (E-H) En face structural images derived from a slab under the retinal pigment epithelium (sub-RPE slab) showing bight areas (F-H, white arrow) consistent with hyperTDs. (I-L) B-scans corresponding to the blue line in panels E-H crossing the hyperTDs shown by the yellow arrows (J-L). The purple segmentation lines on the B-scans depict the segmentation boundaries for the sub-RPE slab which is located 64 to 400μm beneath Bruch’s membrane. A hyperTD was first detected at 6 months of follow-up (B, F, J). The hyperTDs can be seen in panels F and J corresponding to a druse seen in panels B and J. After 12 months (C, G, K), 18 months from baseline, there is a loss of drusen volume as seen in panels C and K with persistence and enlargement of the original hyperTD and appearance of a second hyperTD (G and H, small white arrow). Ten months later (28 months from baseline), there’s a further decrease in drusen volume (D) with enlargement of the original hyperTD (H) and atrophy of RPE and the outer retina consistent with the formation of GA at the site of the initial hyperTD (L, white arrowhead). Drusen volume in a 5mm fovea-centered circle: A: 0.39 mm3; B: 0.43 mm3; C: 0.41 mm3; D: 0.18 mm3.
Figure 6.
Swept source optical coherence tomography (SS-OCT) imaging of a persistent hyper-transmission defect (hyperTD) detected on sub-RPE en face imaging after twelve months of follow-up and evolving into geographic atrophy (GA). (A-D) Drusen maps. (E-H) En face structural images derived from a slab under the retinal pigment epithelium (sub-RPE slab) showing bight areas (F-H, white arrow) consistent with hyperTDs. (I-L) B-scans corresponding to the blue line in panels E-H crossing the hyperTDs shown by the yellow arrows (J-L). The purple segmentation lines on the B-scans depict the segmentation boundaries for the sub-RPE slab which is located 64 to 400μm beneath Bruch’s membrane. A hyperTD was first detected at 12 months of follow-up (B, F, J). The hyperTDs can be seen in panels F and J corresponding to a druse seen in panels B and J. After 18 months (C, G, K), or 30 months from baseline, there is a loss of drusen volume as seen in panels C and K with persistence and enlargement of the original hyperTD and appearance of other hyperTDs (G and H, small white arrows). Five months later, 35 months from baseline, there’s a further decrease in drusen volume (D) with enlargement of the original hyperTD (H) and atrophy of the RPE and outer retina consistent with GA at the site of the initial hyperTD (L, white arrowhead). Drusen volume in a 5mm fovea-centered circle: A: 0.46 mm3; B: 0.59 mm3; C: 0.14 mm3; D: 0.05 mm3.
Figure 7.
Swept source optical coherence tomography (SS-OCT) imaging of a persistent hyper-transmission defect (hyperTD) detected on sub-RPE en face imaging after thirty-seven months of follow-up and not evolving into geographic atrophy (GA). (A-D) Drusen maps. (E-H) En face structural images derived from a slab under the retinal pigment epithelium (sub-RPE slab) showing bight areas (G-H, white arrow) consistent with hyperTDs. (K-L) B-scans corresponding to the blue line in panels E-H crossing the hyperTDs shown by the yellow arrows (K-L). The purple segmentation lines on the B-scans depict the segmentation boundaries for the sub-RPE slab which is located 64 to 400μm beneath Bruch’s membrane. A hyperTD was first detected at 37 months follow-up (C, G, K). The hyperTDs can be seen in panels G and K corresponding to a druse seen in panels C and K. Four months later, 41 months from baseline at the last available visit (D, H, L), there is a loss of drusen volume as seen in panels D and L with persistence and enlargement of the original hyperTD (H) and some atrophy of the outer retinal layers but GA was not yet diagnosed. Drusen volume in a 5mm fovea-centered circle: A: 0.047 mm3; B: 0.052 mm3; C: 0.065 mm3; D: 0.036 mm3.
We observed only one eye that developed GA without the prior detection of a hyperTD ≥ 250μm. In this case, GA developed following the collapse of a drusenoid retinal pigment epithelium detachment (PED) and a hyperTD ≥ 250μm was not detected at the visit prior to this collapse, but this prior visit was 9 months before the GA developed. In addition, three hyperTDs ≥ 250μm were found to be non-persistent. In the case shown in Figure 8, a hyperTD ≥ 250μm was detected at month-23 of follow up. This lesion was replaced by an area associated with a choroidal hypo-transmission defect (hypoTD), and this hypoTD developed by an apparent thickening of RPE layer that appeared to develop into a plaque located at the top of the druse. This hypoTD was associated with the loss of the outer nuclear layer. In the second case, a hyperTD ≥ 250μm was noted at baseline and a similar process occurred with the hyperTD lesion evolving into a choroidal hypoTD corresponding to a thickened RPE layer or plaque associated with the druse. The third case corresponded to a transitory hyperTD noted at month-18 of follow-up that resolved when the druse collapsed.
Figure 8.
Swept source optical coherence tomography (SS-OCT) imaging of a persistent hyper-transmission defect (hyperTD) detected on sub-RPE en face imaging and evolving into a thickened hyper-reflective layer consistent with a pigment cap overlying a druse after twenty-three months of follow-up in the subsequent visits. (A-D) Drusen maps. (E-H) En face structural images derived from a slab under the retinal pigment epithelium (sub-RPE slab) showing a bight area (F, white arrow) consistent with hyperTDs. (K-L) B-scans corresponding to the blue line in panels E-H crossing the hyperTD shown by the yellow arrows (J, K). The purple segmentation lines on the B-scans depict the segmentation boundaries for the sub-RPE slab which is located 64 to 400μm beneath Bruch’s membrane. A hyperTD was first detected at 23 months of follow-up (B, F, J). The hyperTD can be seen in panels F and J corresponding to a druse seen in panels B and J. Three months later, 26 months from baseline (C, G, K), this hyperTD appears smaller (G, K), and is no longer detectable at the last available visit (H, L) It is replaced by a hypo-transmission defect caused by a thickened RPE cap on the druse seen in panel L (green arrow), and also seen as seen by the dark area in panel H (green arrow). Drusen volume in a 5mm fovea-centered circle: A: 0.34 mm3; B: 0.56 mm3; C: 0.57 mm3; D: 0.78 mm3.
At 36 months, the cumulative incidence of GA was 19.0% in all eyes versus 68.9% in eyes that developed a prior hyperTD ≥ 250μm (Figure 1). A time-dependent Cox-survival regression analysis showed that the appearance of a hyperTD ≥ 250μm increased the risk of GA formation 80.4 times (95% confidence interval (CI): 10.7 - 614, p<0.001) compared with eyes without hyperTDs ≥ 250μm. Although the estimated hazard ratio is not precise as only one eye had GA without a prior observed hyperTD ≥ 250μm, the lower estimate based on the 95% confidence interval indicates the increased risk of developing GA is at least 10-fold when a hyperTD ≥ 250μm is present.
In addition, 17 eyes (9%) progressed to exudative MNV, with eight having type 1 MNV and nine having type 3 MNV. Thirteen of the eyes that progressed to exudative MNV had evidence of non-exudative MNV at the visit before exudation and 5 had a previous hyperTDs ≥ 250μm. Two of the four eyes that did not present with a nonexudative neovascular lesions before exudation had prior visits with large intervals of more than 20 months. The third case was a type 3 neovascular lesion and there was no sign of an intraretinal neovascular process when the subject was seen six months before exudation developed. The fourth eye had a type 1 lesion and no detectable nonexudative MNV was found 3 months before exudation developed.
A time-dependent Cox-survival regression showed that the appearance of a hyperTD ≥ 250μm increased the risk of late AMD, which included exudative MNV or GA, 7.64 times (95% CI: 4.0, 14.1; p<001). However, in a Cox-survival regression analysis when considering each endpoint of exudative MNV and censoring follow up if GA was detected, the appearance of a hyperTD ≥ 250μm was not a risk factor for exudative MNV (hazard ratio=1.46, 95%CI 0.51-4.24; p=0.48), in contrast to what was observed for GA (hazard ratio=80.4, 95%CI 10.7 - 614, p<0.001).
DISCUSSION
In this longitudinal study of eyes with iAMD, we demonstrated that the presence of persistent hyperTDs, defined as having a GLD ≥250μm on en face SS-OCT images from sub-RPE slabs, could serve as a stand-alone high-risk precursor sign for the future formation of GA. These hyperTDs were defined as bright areas seen on en face SS-OCT images because of increased choroidal light transmission through regions where the highly scattering melanin granules contained within the RPE are lost as the RPE undergoes atrophy. This current study builds on our previous successful efforts to detect and measure GA using en face images derived from a sub-RPE slab with segmentation boundaries from 64 to 400μm below the Bruch’s membrane.7-10 This strategy for the detection and measurement of GA has been favorably compared with measurements of GA obtained from autofluorescence imaging.8
While en face imaging of large choroidal hyperTDs has been routinely used to detect and measure areas of GA,7-10 en face imaging can also be used in eyes with iAMD without GA to detect small hyperTDs before the onset of GA, and these hyperTDs represent an early sign of impending RPE loss that could serve as a precursor biomarker for disease progression when following drusen as they progress to drusen-related atrophy.11,13 The major limitation of this strategy was that very small hyperTDs were not always persistent, and these transient lesions probably correspond to foci of RPE dysfunction or disruption that could be repaired. However, as the size of these lesions increased, the likelihood of their reversal or disappearance decreased when being followed with en face imaging during follow-up visits. We used this observation to define the concept of persistent hyperTDs, which represent hyperTDs large enough to correspond to a point-of-no return for RPE disruption. In our previous 36-month follow-up study, we found that persistence was achieved when the hyperTD reached a minimum GLD of between 250μm and 300μm, and the presence of these persistent hyperTDs were highly correlated with the onset of nGA.13 In contrast to the laborious process of grading every averaged B-scan for the appearance of nGA in a densely spaced SD-OCT raster scan, we found the use of en face imaging provided a faster and a more convenient alternative in which the cursor on the review station could be easily positioned on the hyperTD and the B-scan rapidly reviewed to confirm that the en face hyperTD corresponded to a B-scan location with the expected outer retinal and RPE defect. Another advantage of using en face imaging instead of B-scans is that we are not limited to evaluating the disease in only the horizontal dimension as with B-scans. En face imaging allows for two-dimensional monitoring of disease progression with the ability to monitor the progression of hyperTDs in the horizontal, vertical, and diagonal dimensions. While B-scans provide additional cross-sectional depth information and should be used selectively in conjunction with en face images, they only allow evaluation of disease progression in a horizontal dimension.
In the current longitudinal study, we aimed to test the proposal that hyperTDs ≥ 250μm detected on en face imaging predicted the formation of GA in order to firmly establish the importance of hyperTDs as an early biomarker for disease progression from iAMD to GA. We found these persistent hyperTDs to be a risk factor for GA development and, in a time-dependent Cox-survival regression analysis, the appearance of a persistent hyperTD increased the risk of GA formation 80 times (95%CI 10.7 - 614, p<0.001) compared with eyes without hyperTDs. Since the precision of our risk estimation was affected by the fact that only one case developed GA in the absence of a previous hyperTD, we estimated that even under the worst scenario, the risk of developing GA after a hyperTD was detected in our study increased 10-fold compared with eyes that didn’t have these hyperTDs.
We were able to appreciate the evolution from drusen, to the formation of hyperTDs, and finally to the formation of GA. As seen in Figures 4-6, the hyperTDs were frequently associated with a decrease in drusen volume. As the hyperTDs enlarged, the RPE and outer retinal layers were lost, culminating in the development of GA. However, as shown in Figure 7, the collapse of a druse in association with a persistent hyperTD ≥ 250μm does not always result in the formation of GA during the available follow-up visits. As we showed previously, it is possible for drusen volume to decrease significantly without the commensurate formation of GA.13,19 Additional follow-up is needed to determine if these lesions will ever develop into GA.
We did observe that GA appeared in one eye in which a hyperTD ≥ 250μm was not detected, however, in this eye a drusenoid PED was present prior to the appearance of GA and there was a long interval (9 months) between visits. Most likely, had we seen this patient more frequently, the PED collapsing into GA would have been associated with the formation of a hyperTD ≥ 250μm. Another observation in this longitudinal study is the evolution of hyperTDs into hypoTDs that are associated with lesions that become hyper-reflective, and this hyper-reflectivity appear to result from a thickened RPE layer associated with the drusen. These areas appear as thickened RPE caps and are associated with the loss of the outer retina. Moreover, these areas of increased RPE reflectivity corresponded to the presence of hypoTDs in the choroid that appear dark on the en face sub-RPE slab. These hypoTDs may also be useful as indicators of future disease progression and are under investigation. Although there are a few exceptions to the presence of a hyperTD≥ 250μm leading to GA, most of these lesions do indicate near-term disease progression with a minimum 10-fold increased risk of progression compared with eyes that don’t harbor these lesions, and this increased risk of progression is most likely even greater.
Even though both iRORA and nGA are important biomarkers for disease progression from drusen to GA, the detection of both requires the use of closely spaced, highly averaged B-scans and each B-scan needs to be graded for the anatomic changes associated with both biomarkers, which requires experienced graders.6 In the latest publication from the CAM group, inter-reader agreement for iRORA was calculated using twelve trained readers from six reading centers. Even after intense training, the results showed only a moderate agreement between readers. The conclusions of this CAM group study suggest that there’s a need for a more robust biomarker that is easier to grade when studying disease progression from iAMD to late AMD, especially if this biomarker is to be used in future clinical trials. In contrast to iRORA and nGA, the use of en face OCT imaging is less labor intensive and graders can be easily trained. All that’s needed is the ability to detect a bright spot and measure its GLD. If a bright area is identified and measured on en face images, then it is straightforward to select the corresponding B-scan to confirm the grading. Future studies will need to explore the inter-reader agreement for detecting hyperTDs ≥ 250μm as done for iRORA, but based on our experience, en face images will have better inter-reader agreement compared with iRORA and will not only useful for rapid screening of suspicious hyperTD lesions, but also for the detection of multiple lesions and the ability to track these lesions reliably in two dimensions at subsequent visits using the retinal vessels as landmarks. This en face screening strategy would be particularly useful when attempting to enroll patients with iAMD into a clinical trial where the goal of the therapy would to prevent the formation of GA.2 One strategy might be to exclude eyes with a hyperTDs ≥ 250μm since it might not be possible to prevent GA from forming once these lesions are present. Another possibility would be to stratify for these lesions at baseline to see if these persistent hyperTDs could be prevented from developing GA once treated with the therapy in question. While a potential overall endpoint might be to prevent the formation of GA in these eyes, another possible endpoint might be to prevent the onset of hyperTDs in eyes without hyperTDs at baseline or the ability to test if a treatment could reverse hyperTDs once present. One of the most important advantages of using the appearance of hyperTDs as an endpoint in iAMD trials would be the ability to run shorter trials with fewer patients compared with those using GA as the endpoint. Another advantage of using the detection of hyperTDs as an endpoint is the possibility of developing an automated algorithms to detect and measure them, something that is currently under investigation. In a recent paper, Wu et al20 assessed several risk factors for determining the progression of iAMD to GA. While they found a similar predictive power between an OCT and a color fundus based predictive-models (area under the curve – AUC of 0.85 and 0.83, respectively), the presence of hyperTDs was not among the risk factors included. We hypothesize that adding the presence of a hyperTDs ≥ 250μm to such a model would increase the predictive power of GA formation, thus improving the ability to detect eyes at risk for disease progression at an earlier stage.
The current study is limited by its retrospective design and the variable follow-up intervals. While the number of participants in this study were adequate to make meaningful conclusions, a larger number of patients, with a uniform follow-up schedule, would have generated more robust data. An ongoing prospective SS-OCT natural history study with defined follow-up intervals of every 3 months should provide the necessary dataset to help validate the findings from this current study. Another limitation of this study is the absence of color fundus imaging and autofluorescence imaging. Routinely, we don’t perform these additional imaging tests to assess GA because we previously showed that en face OCT imaging was highly correlated with both color and autofluorescence imaging,10 so these additional tests are redundant and uncomfortable for the patient. In addition, the CAM consensus group agreed that OCT should be used as the primary imaging modality to assess GA since it provides three-dimensional volumetric images that all the macular layer scan be imaged.1,3,12
In summary, we demonstrated that persistent choroidal hyperTDs, defined as having a GLD ≥250μm on sub-RPE en face OCT images, were a strong predictor of GA formation. Our method constitutes a simplified, faster strategy for the detection of an early OCT biomarker in iAMD that predicts the future appearance of GA, and graders can be easily trained to use it. This en face imaging method will be particularly useful as a screening strategy to enroll subjects into clinical trials and as a clinical trial endpoint in studies designed to test new therapies to prevent disease progression in eyes with iAMD.
ACKNOWLEDGMENTS/DISCLOSURES:
a. Funding/Support:
Research supported by grants from Carl Zeiss Meditec, Inc. (Dublin, CA), the Salah Foundation, the National Eye Institute Center Core Grant (P30EY014801) and Research to Prevent Blindness (unrestricted Grant) to the Department of Ophthalmology, University of Miami Miller School of Medicine. The funding organization had no role in the design or conduct of this research.
b. Financial Disclosures:
Giovanni Gregori and Philip J. Rosenfeld received research support from Carl Zeiss Meditec, Inc. Giovanni Gregori and the University of Miami co-own a patent that is licensed to Carl Zeiss Meditec, Inc. Philip J. Rosenfeld also received research funding from Stealth BioTherapeutics and Gyroscope Therapeutics. He is also a consultant for Apellis, Bayer, Boehringer-Ingelheim, Carl Zeiss Meditec, Chengdu Kanghong Biotech, InflammX Therapeutics, Ocudyne, Regeneron Pharmaceuticals, and Unity Biotechnology. He also has equity interest in Apellis, Valitor Verana Health, and Ocudyne. The remaining authors have no disclosures.
Abbreviations and Acronyms:
- AMD
age-related macular degeneration
- CAM
Classification of Atrophy Meetings
- CI
confidence interval
- cRORA
complete retinal pigment epithelium and outer retinal atrophy
- GA
geographic atrophy
- GLD
greatest linear dimension
- hyperT
hyper-transmission
- hyperTD
hyper-transmission defect
- hypoTD
hypo-transmission defect
- iAMD
intermediate age-related macular degeneration
- INL
inner nuclear layer
- iRORA
incomplete retinal pigment epithelium and outer retinal atrophy
- MNV
macular neovascularization
- nGA
nascent geographic atrophy
- OPL
outer plexiform layer
- PED
pigmented epithelium detachment
- RPE
retinal pigment epithelium
- SD-OCT
spectral domain optical coherence tomography
- SS-OCT
swept-source optical coherence tomography
- SS-OCTA
swept-source OCT angiography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: ARVO Annual Meeting 2021
REFERENCES
- 1.Sadda SR, Guymer R, Holz FG, et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology. 2018;125(4):537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaal KB, Rosenfeld PJ, Gregori G, Yehoshua Z, Feuer WJ. Anatomic Clinical Trial Endpoints for Nonexudative Age-Related Macular Degeneration. Ophthalmology. 2016;123(5):1060–1079. [DOI] [PubMed] [Google Scholar]
- 3.Guymer RH, Rosenfeld PJ, Curcio CA, et al. Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy in Age-Related Macular Degeneration: Classification of Atrophy Meeting Report 4. Ophthalmology. 2020;127(3):394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, Luu CD, Hodgson LAB, et al. Prospective Longitudinal Evaluation of Nascent Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmol Retina. 2020;4(6):568–575. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z, Luu CD, Ayton LN, et al. Optical coherence tomography-defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology. 2014;121(12):2415–2422. [DOI] [PubMed] [Google Scholar]
- 6.Wu Z, Pfau M, Blodi BA, et al. Optical Coherence Tomography Signs of Early Atrophy in Age-related Macular Degeneration: Inter-Reader Agreement. CAM Report 6. Ophthalmol Retina. 2021. [DOI] [PubMed] [Google Scholar]
- 7.Yehoshua Z, Rosenfeld PJ, Gregori G, et al. Progression of geographic atrophy in age-related macular degeneration imaged with spectral domain optical coherence tomography. Ophthalmology. 2011;118(4):679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yehoshua Z, Garcia Filho CA, Penha FM, et al. Comparison of geographic atrophy measurements from the OCT fundus image and the sub-RPE slab image. Ophthalmic Surg Lasers Imaging Retina. 2013;44(2):127–132. [DOI] [PubMed] [Google Scholar]
- 9.Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology. 2014;121(3):693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al. Comparison of Geographic Atrophy Growth Rates Using Different Imaging Modalities in the COMPLETE Study. Ophthalmic Surg Lasers Imaging Retina. 2015;46(4):413–422. [DOI] [PubMed] [Google Scholar]
- 11.Schaal KB, Gregori G, Rosenfeld PJ. En Face Optical Coherence Tomography Imaging for the Detection of Nascent Geographic Atrophy. Am J Ophthalmol. 2017;174:145–154. [DOI] [PubMed] [Google Scholar]
- 12.Jaffe GJ, Chakravarthy U, Freund KB, et al. Imaging Features Associated with Progression to Geographic Atrophy in Age-Related Macular Degeneration: CAM Report 5. Ophthalmol Retina. 2020. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Yang J, Feuer W, Gregori G, Rosenfeld PJ. Persistent Hyper-Transmission Defects on En Face OCT Imaging as a Stand-Alone Precursor for the Future Formation of Geographic Atrophy. Ophthalmol Retina. 2021. [DOI] [PubMed] [Google Scholar]
- 14.Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121(5):1079–1091. [DOI] [PubMed] [Google Scholar]
- 15.Wang RK, An L, Francis P, Wilson DJ. Depth-resolved imaging of capillary networks in retina and choroid using ultrahigh sensitive optical microangiography. Opt Lett. 2010;35(9):1467–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang A, Zhang Q, Chen CL, Wang RK. Methods and algorithms for optical coherence tomography-based angiography: a review and comparison. J Biomed Opt. 2015;20(10):100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang X, Shen M, Wang L, et al. Validation of a Novel Automated Algorithm to Measure Drusen Volume and Area using Swept Source Optical Coherence Tomography Angiography. Trans. Vis. Sci. Tech 2021;10(4):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gharibvand L, Liu L. Analysis of Survival Data with Clustered Events. 2009. [Google Scholar]
- 19.Yehoshua Z, Wang F, Rosenfeld PJ, Penha FM, Feuer WJ, Gregori G. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology. 2011;118(12):2434–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, Bogunovic H, Asgari R, Schmidt-Erfurth U, Guymer RH. Predicting Progression of Age-Related Macular Degeneration Using OCT and Fundus Photography. Ophthalmol Retina. 2021;5(2):118–125. [DOI] [PubMed] [Google Scholar]