Abstract
The present study examined temporal patterns of symptom change during treatment for comorbid posttraumatic stress disorders (PTSD) and substance use disorders (SUDs). We hypothesized that PTSD symptom severity would predict subsequent-session substance use and that this association would be particularly strong among patients who received an integrated treatment versus SUD-only treatment. Participants were 81 United States military veterans with current PTSD and an SUD who were enrolled in a 12-week, randomized controlled trial examining the efficacy of an integrated treatment called Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure (COPE) compared with cognitive behavioral relapse prevention therapy (RP). Lagged multilevel models indicated that PTSD symptom improvement did not significantly predict the likelihood of next-session substance use (likelihood of use: B = 0.03, SE = 0.02, p = .141; percentage of days using B = −0.02, SE = 0.01, p = .172. Neither substance use, B = 1.53, SE = 1.79, p = .391, nor frequency of use, B = 0.26, SE = 0.50, p = .612, predicted next-session PTSD symptom severity in either treatment condition. Stronger associations between PTSD symptoms and next-session substance use were expected given the self-medication hypothesis. Additional research is needed to better understand the temporal dynamics of symptom change as well as the specific mediators and mechanisms underlying symptom change.
Posttraumatic stress disorder (PTSD) and substance use disorders (SUDs) are highly prevalent, debilitating disorders that commonly co-occur (Grant et al., 2015; Pietrzak et al., 2011); compared with the general population, military veterans incur a heightened risk for co-occurring PTSD and SUD. For example, PTSD and SUD co-occur in approximately 40% of veterans who served in the Vietnam era or later (Petrakis et al., 2011). This complex comorbidity is particularly concerning due to its tremendous economic burden on the health care system as well as the increased risk of associated problems, including suicidality, poor physical health, increased mortality, unemployment, violence, more chronic substance use, and more episodes of treatment (Norman et al., 2018; Pietrzak et al., 2009).
The self-medication hypothesis (Khantzian, 1997) suggests that individuals are commonly motivated to use substances to ameliorate PTSD symptoms (e.g., to sleep and not remember nightmares, to “numb out” and forget a traumatic event; Haller & Chassin, 2014; Kehle et al., 2011; Simpson et al., 2012; Swendsen et al., 2010). Indeed, empirical findings indicate that PTSD onset typically precedes that of SUDs (Back et al., 2005; Breslau et al., 2003). Findings from daily diary studies have demonstrated that individuals with PTSD are more likely to drink on the days they experience more severe PTSD symptoms (Possemato et al., 2015; Simpson et al., 2014), and there is preliminary evidence that coping-related drinking may partially account for the association between PTSD and problem drinking (O’Hare & Sherrer, 2011). Although other hypotheses exist to account for the co-occurrence of PTSD and SUD, the self-medication hypothesis has received the most conceptual and empirical support (McCauley et al., 2012). Recent literature has applied this theory to examine predictors of change in treatment for PTSD and SUDs (Badour et al., 2017; Brady et al., 2001; Soder et al., 2019; Tripp et al., 2020).
Despite the abundant literature demonstrating that individuals with co-occurring PTSD and SUDs sustain a more complicated clinical course and less successful treatment outcomes compared to those with either diagnosis alone (Cohen & Hien, 2014; Hien et al., 2010), evidence-based treatments that concurrently target PTSD and SUDs have only recently begun to receive thorough attention. Emerging reviews and clinical practice guidelines converge to suggest that the most effective approach for treating co-occurring PTSD and SUD involves a combination of trauma-focused exposure therapy for PTSD and cognitive behavioral therapy for SUDs (Ostacher & Cifu, 2019; RAND Corporation, 2020; Roberts et al., 2016; Simpson et al., 2017; Torchalla et al., 2012). Research is now needed to identify temporal patterns of symptom change during integrated treatments (i.e., do PTSD symptoms improve prior to reductions in substance use?), a first step toward identifying mediators (i.e., one or more factors that statistically account for the association between an intervention and a given treatment outcome) and mechanisms (i.e., specific processes through which an intervention leads to a given outcome) through which such integrated treatments result in symptom change (Kazdin, 2007).
Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure (COPE; Back et al., 2015) is an integrated treatment with empirical support for reducing PTSD symptoms and substance use in multiple populations (Brady et al., 2001; Mills et al., 2012; Persson et al., 2017; Ruglass et al., 2017), including in military veterans with co-occurring PTSD and SUDs (Back et al., 2019; Norman et al., 2019). COPE is a manualized treatment that synthesizes an efficacious cognitive behavioral therapy for SUDs (Carroll et al., 2000; Kadden et al., 1992) with prolonged exposure (PE) therapy for PTSD (Foa et al., 2007). Although the scientific literature supporting COPE’s efficacy continues to grow, important questions remain about the specific therapeutic changes achieved in integrated treatments. Central to the current investigation is the question of whether (a) improvement in PTSD symptoms temporally precedes subsequent improvement in substance use, in line with the self-medication hypothesis; (b) changes in substance use temporally precede subsequent improvement in PTSD symptoms; or (c) this association is bidirectional. Existing evidence to support these questions is mixed.
Some findings from treatment studies support the supposition that PTSD symptoms should predict, and perhaps drive, the subsequent change in substance use behavior during treatment. In an early test of this hypothesis, Hien and colleagues (2010) found that changes in PTSD symptoms predicted subsequent change in substance use in a multisite randomized controlled trial (RCT) of women with comorbid PTSD and SUD who participated in Seeking Safety—a skills-focused intervention that does not involve trauma-focused exposure or processing (Najavits et al., 2010)—versus a health education control treatment; the findings did not support the converse association (i.e., substance use predicting subsequent PTSD symptom severity). Similarly, in a sample of individuals with PTSD and alcohol use disorders who received supportive counseling and either PE, naltrexone, or a combination of PE and naltrexone, Kaczkurkin and colleagues (2016) found that PTSD symptoms prospectively predicted alcohol craving, but the percentage of days drinking during treatment did not predict subsequent PTSD symptom severity. Peirce and colleagues (2020) found that prior-week substance use was moderately associated with more severe PTSD symptoms during treatment, yet weekly PTSD symptom severity did not predict subsequent-week self-reported substance use in a sample of patients receiving PE for PTSD while also receiving methadone maintenance.
Two studies have considered this question in relation to patients receiving COPE (Hien et al., 2018; Tripp et al., 2020). Hien and colleagues (2018) found that continued substance use during treatment subsequently predicted higher PTSD symptom severity at the end of treatment, regardless of condition (i.e., COPE vs. Relapse Prevention [RP] for SUD). The authors did not examine the reverse association (i.e., PTSD symptom severity predicting subsequent substance use) and did not consider week-to-week changes in PTSD symptoms and substance use. In a sample of veterans with PTSD and alcohol use disorder who received either COPE or Seeking Safety, Tripp and colleagues (2020) examined session-by-session data and found a bidirectional association between PTSD symptoms and drinking during treatment such that more severe PTSD symptoms predicted subsequent-session alcohol use (i.e., number of drinking days * number of drinks per day) and vice versa, although the size of the effect was stronger for PTSD symptoms predicting subsequent session alcohol use. These effects did not differ as a function of treatment condition.
Clarity regarding the temporal sequencing of change in PTSD and substance use during COPE is needed. Moreover, it is important to understand whether the association between PTSD change and substance use differs as a function of treatment condition (i.e., COPE vs. SUD-only treatment). A better understanding of the timing of therapeutic changes in integrated treatments will inform much-needed future research aimed at identifying specific mediators and mechanisms of change in both PTSD and substance use, which can help optimize treatments for co-occurring PTSD and SUD.
Given the nascent stage of the scientific literature examining treatments for co-occurring PTSD and SUDs and the importance of developing and refining such treatments to maximize patient recovery efforts, the goal of the present study was to conduct a preliminary test to examine the extent to which (a) changes in PTSD symptom severity temporally preceded changes in substance use and (b) changes in substance use temporally preceded changes in PTSD symptom severity among a sample of military veterans who received COPE versus RP, an evidence-based, cognitive behavioral therapy that targets substance use only (Carroll et al., 2000; Kadden et al., 1992), as part of a recently completed randomized controlled trial (Back et al., 2019). Consistent with the self-medication hypothesis, we hypothesized that PTSD symptom severity would predict subsequent-session substance use in both treatment conditions. We hypothesized that PTSD symptoms would more strongly predict substance use for participants who received COPE given that COPE specifically targets reductions in PTSD. Theoretical and empirical support for the possibility that substance use would predict subsequent-session PTSD symptoms is less strong despite the common therapeutic model that requires individuals seeking treatment for PTSD to first obtain SUD-only treatment; as such, we examined this potential association as an exploratory hypothesis. This study was the first of which we are aware to investigate how (a) week-to-week PTSD symptoms predict subsequent-week substance use, and vice versa, during COPE compared to RP treatment.
METHOD
Participants
Participants were 81 United States military veterans (90.1% male) with current diagnoses of PTSD and SUD (Mage = 40.4 years, SD = 10.7) who participated in a larger RCT (Back et al., 2019). Participants reported mean educational attainment of 13.92 years (SD = 2.0). In total, 60.5% of participants identified as White, 37.0% identified as Black, and 2.5% identified as multiracial or listed their race as “other.” Hispanic ethnicity was endorsed by 3.7% of the sample. Most participants served in support of recent operations in Iraq and Afghanistan (64.6%), and the most distressing traumatic event occurred during military service (e.g., combat exposure, accident, military sexual trauma) for 81.0% of participants. With regard to SUD diagnoses, the majority of participants (63.0%) met the criteria for an alcohol use disorder only, 27.2% met the criteria for an alcohol use disorder plus at least one drug use disorder, and 9.9% met the criteria for a drug use disorder only. Additional information about the sample and can be obtained elsewhere (Back et al., 2019).
Procedure
Inclusion and exclusion criteria
All procedures were approved by the Institutional Review Board at the Medical University of South Carolina, and participants provided written informed consent prior to participation. To be included in the RCT, individuals needed to (a) be a United States military veteran aged 18–65 years; (b) meet the diagnostic criteria for current PTSD, per the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), and report a score of 50 or higher on the Clinician-Administered PTSD Scale for DSM-IV (CAPS; Blake et al., 1995); and (c) meet the DSM-IV diagnostic criteria for a current SUD as evaluated using the Mini-International Neuropsychiatric Interview (M.I.N.I.; Sheehan et al., 1998), with use in the past 90 days. Participants were excluded based upon (a) enrollment in another treatment for PTSD or SUD, (b) psychiatric conditions that would likely require a higher level of care (e.g., significant suicidal ideation with intent, psychotic disorder), and (c) severe cognitive impairment as evidenced by a score of 21 or lower on the Mini-Mental Status Examination (MMSE; Folstein et al., 1975). If a participant was taking psychotropic medications, they were required to maintain a stable dose for at least 4 weeks before therapy initiation.
After the informed consent procedure, participants completed a battery of interview and self-report assessments. Participants were stratified by baseline PTSD symptoms severity and substance use (Wei & Lachin, 1988) and were randomized 2:1 to COPE (n = 54) or RP (n = 27). Participants were compensated for participation in each assessment visit.
Treatment
COPE is a manualized, integrated, trauma-focused cognitive behavioral therapy for comorbid PTSD and SUD that consists of 12 weekly, individual, 90-min sessions (Back et al., 2015). Sessions 1 and 2 focus on psychoeducation related to PTSD and SUD, methods for coping with cravings, awareness of triggers for use, and the rationale for PE. In vivo (Sessions 3–12) and imaginal exposures (Sessions 4–11) are key components of the therapy and are integrated with SUD coping skills throughout treatment.
RP is a manualized, cognitive behavioral therapy for the treatment of SUD, which consists of 12 weekly, individual, 90-min sessions (Carroll et al., 2000; Kadden et al., 1992). Each session focuses on a topic related to common issues experienced during recovery (e.g., managing cravings and thoughts about using, drink and drug refusal skills, planning for emergencies, anger management, seemingly irrelevant decisions). RP targets the substance use problem and does not include any PTSD-specific or trauma-focused components.
Treatments were delivered by six master’s- or doctoral-level clinicians who participated in a 2–3-day training that included a didactic review of intervention-specific theory, techniques, and role-playing exercises. In both treatment modalities, abstinence was encouraged but not required. Clinicians received 1-hr weekly supervision during the study. All therapy sessions were recorded, and approximately 25% were randomly selected and evaluated to ensure adequate adherence to the manuals and competency (Brady et al., 2001).
Measures
PTSD symptoms
The PTSD Checklist–Military Version (PCL-M; Weathers et al., 1991), is a 17-item measure that was used to assess past-week DSM-IV PTSD symptoms at each treatment session. Respondents rate each item on a Likert-type scale ranging from 1(not at all) to 5 (extremely). Total symptom severity is calculated by summing the scores on individual items, with higher scores representing more severe symptoms. The PCL-M has demonstrated good internal consistency adequate test–retest reliability, and strong convergent validity with both clinician and self-report measures of PTSD (Wilkins et al., 2011). In the current sample, Cronbach’s alpha for baseline PCL-M score was .86.
Substance use
The Timeline Follow-back (TLFB; Sobell & Sobell, 1992), which uses a calendar-assisted, structured interview method for collecting self-report assessments of substance use, was used to assess drug and alcohol use 60-days prior to baseline as well as weekly during treatment. Scores on the TLFB have demonstrated high test–retest correlations, and the measure has been shown to be strongly associated with self-reported and other reports of drug and alcohol use (Carey, 1997). A calculation of any use (“yes” or “no”) and the percentage of days using among individuals who reported any use (i.e., frequency) served as our measure of substance use between therapy sessions. The percentage of days using was calculated by dividing the number of days the participant reported using any substances by the total number of days in the reference period.
At baseline and before each therapy session, breathalyzer tests were used to measure blood alcohol concentration (BAC), with a value higher than 0.01 g/dl considered positive, and urine drug screen (UDS) tests (CLIAwaived Inc.; Carlsbad, CA) were used to assess for the presence of cocaine, marijuana, benzodiazepines, opioids, and amphetamines. Given the low percentages of positive BAC and UDS screens at baseline (i.e., BAC = 1.2%, UDS = 25.9%) and across all treatment sessions (i.e., BAC = 1.5%, UDS = 16.2% across all sessions of COPE; BAC = 2.5%, UDS = 19.1% across all sessions of RP), self-reported substance use was the focus of the present investigation.
Data analysis
A series of independent-samples t tests and chi-squared tests were used to compare baseline characteristics and treatment adherence variables across treatment condition. Intraclass correlation coefficients (ICCs) were calculated to provide the percentage of variance attributable to between-person differences. The primary hypotheses were examined using a series of multilevel linear models with robust maximum likelihood estimation. This approach utilizes all available responses for every participant, thus enabling a full intention-to-treat analysis (Chakraborty & Gu, 2009). All Level 1 data (i.e., PCL-M, TLFB) included 13 time points (i.e., baseline, Sessions 1–12) nested within participants. A linear time variable was created by setting the baseline session to equal 0. Minimal missingness (i.e., less than 1%) on individual items within the PCL-M was handled by using a “last observation carried forward” approach. In the first model, lagged PTSD symptoms were included as a predictor of substance use; in the second model, lagged substance use was included as a predictor of PTSD symptoms. Between-person variance in continuous lagged variables was separated using the cluster means feature in Mplus. Treatment condition (COPE = 0, RP = 1) was included as a Level 2 covariate in the moderated multilevel models (See Figure 1 and Figure 2). In prior analyses of these data, including in the main outcomes paper (Back et al., 2019), substance use variables were not linearly distributed; similar to these studies, we used two-part modeling to examine substance use in the present study. Two-part modeling separates nonnormally distributed outcomes into two parts: the likelihood of endorsing a particular behavior (i.e., substance use) and the conditional percentage of days using substances. The Bayesian information criterion (BIC) was provided for all models, with lower values indicating better model fit. The total sample size of 81, with 12 observations for each participant, is consistent with sample size recommendations for multilevel models showing that correct and unbiased estimates at Level 2 are generally estimated with 50 participants or more, and estimates for Level 1 are generally found to be more accurate than Level 2 estimates (Maas & Hox, 2005).
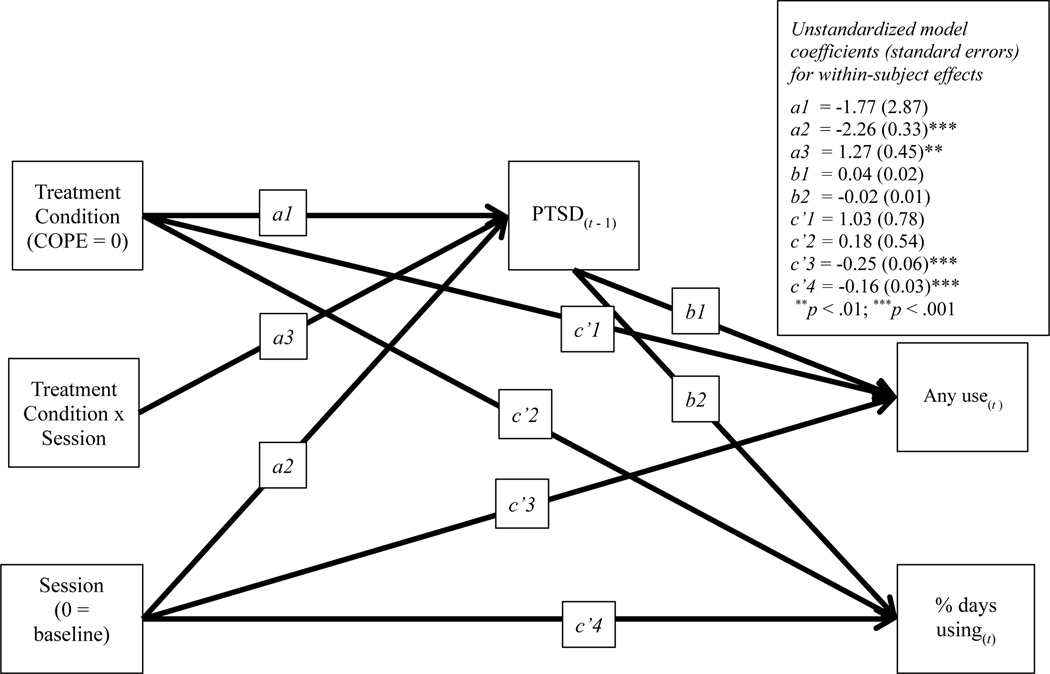
Figure 1.
Model Testing Session, Condition, and Session x Condition Interaction Predicting Change in Posttraumatic Stress Disorder (PTSD) Symptoms and Change in Likelihood of Substance Use and Percent Days Using as well as PTSD Symptoms Predicting Substance Use
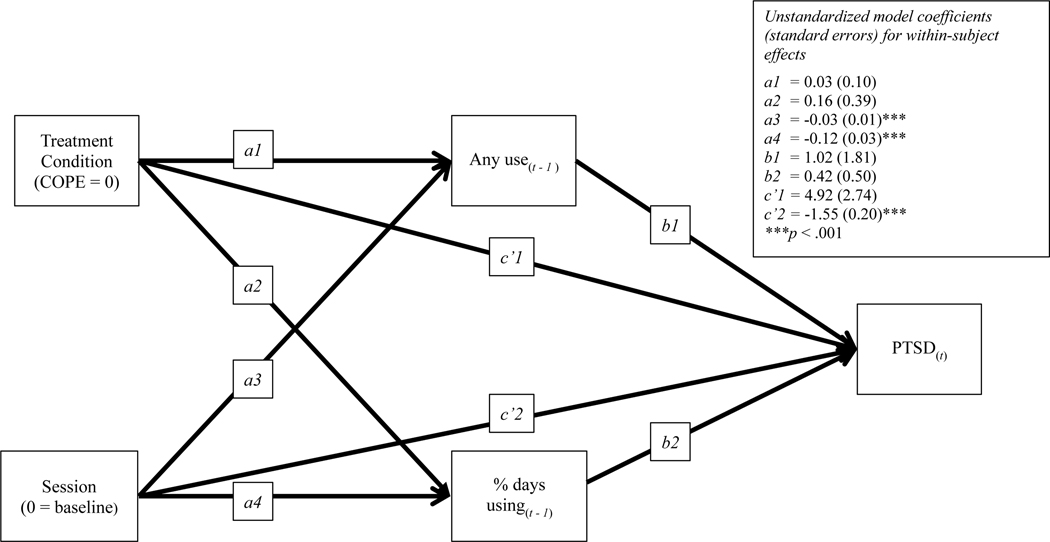
Figure 2.
Model Testing Session and Condition Predicting Change in Substance Use and Posttraumatic Stress Disorder (PTSD) Symptoms and Substance Use Change Predicting PTSD Symptoms Symptoms and Change in Likelihood of Substance Use and Percent Days Using as well as PTSD Symptoms Predicting Substance Use
RESULTS
At baseline, participants assigned to COPE versus RP did not differ regarding any demographic characteristics, the severity of PTSD symptoms, or the percentage of days using substances at baseline. However, more participants in the COPE condition met the criteria for a current major depressive episode at baseline (COPE: 38.9%; RP: 11.1%). Average CAPS scores at baseline were in the severe to extreme range (M = 79.8, SD = 2.02). The overwhelming majority of participants reported at least 1 day of any substance use on the TLFB during the baseline assessment period (90.1%). Individuals who endorsed substance use during the baseline period reported using one or more substances more than half of the days assessed (percentage of days using: M = 52.8, SD = 33.7 for COPE; M = 54.1, SD = 32.2 for RP), with the use of alcohol being most common (percentage of days using alcohol: M = 53.6, SD = 30.4 for COPE; M = 46.0, SD = 31.5 for RP). The average number of drinks per drinking day at baseline exceeded the cutoff for heavy alcohol use (COPE: M = 8.5, SD = 7.0; RP: M = 9.7, SD = 6.7; U.S. Department of Health and Human Services, n.d.). At the baseline assessment, only one participant had a positive breathalyzer test, and 25.9% had a positive UDS test, with marijuana the most commonly detected drug on positive UDS tests at baseline (55.6%; see Back et al., 2019, for the distribution of PTSD and SUD scores at baseline, midtreatment, and posttreatment. On average, participants attended eight out of 12 treatment sessions, and this number did not differ by treatment group. Participants were classified a priori as treatment completers if they completed at least eight of the 12 sessions (Brady et al., 2001). Completion rates did not differ as a function of treatment condition (66.7% for COPE vs. 55.6% for RP). Over half (53.7%) of the participants completed all 12 sessions of COPE, and 48.1% completed all 12 sessions of RP. The ICC for PTSD symptoms was .60, and the ICC for substance use, modeled continuously, was .57.
Do PTSD symptoms predict next-session substance use?
As expected, two-part modeling best captured the distribution of substance use as an outcome. The first model tested excluded treatment condition to assess the effects of lagged PTSD symptoms on substance use, BIC = 8,310. Fixed-effects lagged multilevel modeling was conducted to examine a model in which session predicted (a) lagged PTSD symptoms and (b) the likelihood of substance use (i.e., any vs. none) and the percentage of days using, conditional on use. Finally, lagged PTSD symptoms predicted substance use outcomes, after controlling for session. The significant effects of session on lagged PTSD symptoms, B = −1.88, SE = 0.26, p < .001, demonstrated that PTSD symptoms decreased significantly across treatment sessions. Further, the significant effect of session on substance use likelihood, B = −0.25, SE = 0.06, p < .001, odds ratio (OR) = 0.78, and conditional percentage of days using, B = −0.16, SE = 0.03, p < .001, demonstrated decreased substance use, both in the likelihood of using and the percentage of days using. However, the effect of lagged PTSD symptoms (t-1) on the likelihood of using, B = 0.03, SE = 0.02, p = .141, and percentage of days using, B = −0.02, SE = 0.01, p = .172, before the subsequent session (t) was not significant.
Next, we included treatment condition and Treatment Condition x Session effects in the model, BIC = 8,300. The interaction term of treatment condition by lagged PTSD symptoms was not a significant predictor of the likelihood of substance use or the percentage of days using. This parameter was, therefore, dropped from the model for parsimony (see Figure 1). The effect of session predicting lagged PTSD symptoms remained significant, Path a2: B = −2.26, SE = 0.33, p < .001. Further, this effect was moderated by treatment condition, Path a3: B = 1.27, SE = 0.45, p = .005. Path a1 reflects the average reduction in PTSD symptoms per session for participants in the COPE condition. The effect of lagged PTSD symptoms predicting the likelihood of using by the next session did not reach the threshold for statistical significance, Path b1: B = 0.04, SE = 0.02, p = .084, although the odds ratio for this effect was 1.04, 95% CI [1.00, 1.08], indicating the odds of using was 4% greater than the odds of not using for every 1-unit increase in PTSD symptoms at the prior session. To provide additional context, this path was examined for COPE and RP individually. The effect of session on the likelihood of substance use at one session via prior session reductions in PTSD symptoms did not reach the threshold for significance among participants in the COPE group, B = −0.08, SE = 0.05, p = .092, OR = 0.92, 95% CI [0.84, 1.01], or the RP group, B = −0.04, SE = 0.03, p = .197, OR = 0.97, 95% CI [0.91, 1.02]. The only significant predictor of the percentage of days using was session, B = −0.16, SE = 0.03, p < .001.
Does substance use predict next-session PTSD symptom change?
A second multilevel model was examined, this time including session and lagged (a) likelihood of using any substance (t-1) and (b) percentage of days using substances, conditional on use (t-1) predicting PTSD symptoms (t), BIC = 10,226. Session significantly predicted lagged substance use likelihood, B = −0.13, SE = 0.03, p < .001, and lagged percentage of days using substances, B = −0.18, SE = 0.03, p < .001, as well as PTSD symptoms, Path c’: B = −1.55, SE = 0.21, p < .001. Neither lagged substance use likelihood, B = 1.53, SE = 1.79, p = .391, nor lagged percentage of days using substances, Path b2: B = 0.26, SE = 0.50, p = .612, predicted PTSD symptoms. There were no significant effects of condition as a moderator. Therefore, the final model included group as an additional covariate (see Figure 2), BIC = 10,866. The results were consistent with the model that did not control for group, although the lower BIC in the model that did not control for group indicates that including group status did not improve the model fit.
DISCUSSION
Despite mounting literature supporting the efficacy of integrated treatments for co-occurring PTSD and SUD (Back et al., 2019; Badour et al., 2017; Brady et al., 2001; McCauley et al., 2012; Mills et al., 2012; Roberts et al., 2016), important questions remain about how symptoms change during treatment and the potential mediators and mechanisms that may drive this change. The present study represents a first step toward this line of inquiry through the investigation of the temporal relations between changes in PTSD symptoms and substance use among veterans participating in an RCT comparing the efficacy of COPE, a trauma-focused, integrated treatment for PTSD and SUD, versus RP, a treatment that targets SUDs only. Consistent with the self-medication hypothesis, we expected that PTSD symptoms would predict subsequent-session substance use during treatment and that this association would be stronger among participants who received COPE. However, although the pattern of data was generally consistent with PTSD symptoms predicting next-session likelihood of use, the strength of this association was weak and nonsignificant, and there was no effect of PTSD symptoms on subsequent-session frequency of use. Moreover, although COPE resulted in significantly more PTSD symptom improvement compared with RP, such improvement did not correspond to larger symptom reductions, only comparable reduction, in substance use among individuals receiving COPE. If the primary mechanism of change in substance use behavior during COPE was the reduction of PTSD symptoms, we would have expected to observe a stronger association between PTSD symptom severity and subsequent-session substance use. There was also no evidence that (a) whether someone used substances or (b) the frequency of their use in a given week predicted PTSD symptoms at the next session.
The self-medication hypothesis is one of the most widely accepted theories to explain the high comorbidity of PTSD and SUDs; however, important nuances undergirding this theory should be considered in the context of the present results. First, the self-medication hypothesis offers an explanation for the development of PTSD–SUD comorbidity, wherein trauma exposure and PTSD symptoms temporally precede and contribute to the onset or exacerbation of coping-related substance use to mitigate the distressing symptoms of PTSD. Repeated and increased substance use to obtain or maintain one’s desired reduction in PTSD symptom–related distress can lead to long-term harmful substance use patterns, including the emergence of SUDs (Haller & Chassin, 2014; Hawn et al., 2020). However, once PTSD–SUD comorbidity has been established, it is less clear if this self-medication process remains the best explanation for why the co-occurrence of these symptoms persists. The mutual maintenance hypothesis (Stewart et al., 1998) is another theory that acknowledges there are likely multiple interacting risk factors that contribute to the development of comorbid PTSD and SUD. Once established, this theory suggests that PTSD and SUD maintain one another over time such that PTSD symptoms lead to the continued use of substances, and substance use leads to the maintenance or worsening of PTSD symptoms over time by interfering with trauma processing (Kaysen et al., 2011; McFarlane et al., 2009) and sensitizing reward and stress response systems in the brain (María-Ríos & Morrow, 2020).
The present findings appear counter to those expected by the self-medication hypothesis if, indeed, this hypothesis extends to understanding maintenance and treatment of PTSD/SUD comorbidity. Specifically, if a primary mechanism maintaining ongoing substance use is negative reinforcement obtained through a temporary reduction in distress associated with PTSD symptoms, PTSD symptom improvement should result in a reduced need for substance use, and a subsequent reduction of use should be observed. Further, we would expect the larger reduction in PTSD symptoms seen among participants who received COPE relative to RP to also lead to larger improvements in substance use. Similarly, if PTSD symptoms and substance use behavior are maintaining or exacerbating one another over time, in line with the mutual maintenance model, PTSD symptoms should be expected to predict subsequent substance use behavior, and vice versa, across the course of treatment. However, we failed to observe these expected associations when PTSD symptoms and substance use were assessed week-to-week. It is possible that session-to-session intervals are too brief to observe the expected prospective association between PTSD symptom improvement and subsequent substance use; however, in the parent trial for this study (Back et al., 2019), differences in substance use frequency also failed to emerge as a function of treatment condition at posttreatment and 3- and 6-month follow-ups despite significantly larger improvements in PTSD symptoms during the treatment window among participants who received COPE versus those who received RP.
It is notable that the present results diverge from those of a recently published trial of COPE versus Seeking Safety in veterans with PTSD and co-occurring alcohol use disorder, in which PTSD symptom severity was found to predict subsequent-session alcohol use, and vice versa, in both treatment conditions (Tripp et al., 2020). Importantly, participants were included in the study by Tripp and colleagues based on the presence of PTSD or subclinical PTSD and alcohol use disorder comorbidity, whereas nearly 40% of participants in the present trial presented with at least one substance use disorder other than or in addition to alcohol use disorder. Moreover, in the present study, week-to-week associations between PTSD symptoms and substance use considered the use of all substances (i.e., alcohol and drugs), as polysubstance use was common.
Despite the broad acceptance of the self-medication hypothesis as a leading explanation for PTSD/SUD comorbidity, most empirical studies that have tested this hypothesis have focused specifically on the link between PTSD and alcohol use, been limited by the use of cross-sectional designs, and relied on self-report measures of the tendency to engage in coping-related drinking rather than directly measuring proximal associations between PTSD symptoms and substance use behavior (Hawn et al., 2020). This theory has also been critiqued based on evidence that the successful treatment of psychiatric symptoms among dual-diagnosis patients tends not to result in large, sustained improvements in substance use outcomes in the absence of addiction-focused treatment (Lembke, 2012). This is not to say that additional research on the potential role of the self-medication hypothesis is not warranted in comorbid PTSD and SUD. Indeed, the inconsistent findings emerging from the few studies that have considered the temporal association between PTSD symptoms and substance use during treatment (e.g., Hien et al., 2010, 2018; Kackurkin et al., 2016; Peirce et al., 2010; Tripp et al., 2020) underscore the need to better understand both the unique and shared mediators and mechanisms of change in these two symptom dimensions in response to single-disorder and integrated treatment protocols.
Given the heterogeneity in presentation among patients with comorbid PTSD and SUDs, it is also likely that there are important moderators of change processes that need to be considered. For example, among individuals who report heavy or chronic use of substances that lead to physiological dependence, it may be that short-term fluctuations in PTSD symptom severity have less of an influence on substance use patterns compared with individuals who are not physiologically dependent on one or more substances. Moreover, the use of some illicit substances is likely restricted by the availability of access at a given time; thus, the use of some drugs might not be as directly tied to fluctuations in symptoms, as might be expected for alcohol. Finally, other significant stressors that frequently impact this population, such as housing and employment instability, interpersonal relationship conflict, and financial resource strain, may have a more robust influence on proximal substance use behavior during the course of treatment, compared to week-to-week fluctuations in PTSD symptoms. Ongoing stressors such as these are rarely considered in the context of examining the temporal relations between PTSD symptoms and substance use.
The present findings represent an important next step aimed at understanding how integrated treatments may function differently than single-disorder protocols to improve the symptoms of both PTSD and SUDs. The use of an integrated treatment may simultaneously reduce the symptoms of both PTSD and SUDs, resulting in improvements in each disorder in the same amount of time when compared with treatment that focuses on one disorder only. Moreover, the present findings converge with a growing literature suggesting superior improvements in PTSD symptoms occur via trauma-focused integrated treatments compared to disorder-specific treatments for SUD, such as RP. However, considerable gaps remain in our understanding of (a) the temporal dynamics of PTSD and SUD symptom change, (b) the purported mediators that may account for observed symptom change, and (c) the specific mechanisms that drive both PTSD and substance use symptom change in each of these treatments. Such research is necessary to optimize treatment delivery to obtain the best results for individual patients. Although more research is needed on integrated treatments, the initial findings regarding safety, acceptability, and efficacy are promising, and integrated treatment is recommended in the U.S. Department of Veterans Affairs/Department of Defense Clinical Practice Guidelines (Ostacher & Cifu, 2019).
Several study limitations should be noted. First, this study presents secondary analyses from a parent RCT that was adequately powered to detect group differences in primary outcomes of PTSD and substance change across 12 weeks of treatment. As such, this dataset was underpowered to conduct formal tests of mediation, and, thus, we were only able to address questions regarding the temporal ordering of symptom change. Future studies should consider recruiting adequate sample sizes or pooling samples across similar trials to conduct further investigations regarding mediators and mechanisms of change. In addition, the present sample consisted of veterans, most of whom were male, reported military-related trauma exposure, and had an alcohol use disorder alone or an alcohol use disorder plus at least one other drug use disorder. The current findings may differ among civilians or individuals who have primarily drug use disorders. Further, although there was some racial diversity in the current sample, there was limited ethnic diversity and not enough power to examine differences based on racial or ethnic group. Self-report measures were used, which are subject to various biases, including recall and social desirability. Although similar to those reported for other integrated treatments (Roberts et al., 2016), the attrition rate in this study was relatively high. This patient population is characterized by significant challenges to treatment adherence, highlighting the need for additional research on effective implementation strategies to ensure that patients are able to access adequate doses of integrated therapies. Although participants were equally likely to complete treatment in either condition, the association between PTSD and SUD symptom change may differ among individuals who drop out of treatment prior to completion. The addition of a standalone PTSD arm or alternative integrated treatment to further investigate and improve the generalization of findings for integrated treatments should be considered to move this line of research forward.
The present study was the first of which we are aware to investigate the week-to-week temporal dynamics of PTSD and SUD symptom change during an integrated treatment for PTSD and SUD versus treatment for SUD alone in a sample of military veterans with co-occurring PTSD and SUD. Although some PTSD symptom improvement emerged, predicting the next-session likelihood of substance use, but not the frequency of use, during the course of integrated treatment (COPE), this finding was weak and nonsignificant. Substance use also did not predict subsequent-session PTSD symptoms. The self-medication hypothesis would suggest stronger temporal associations between PTSD symptoms and subsequent-session substance use. Significant gaps remain regarding the field’s understanding of how symptom change occurs during integrated versus single-disorder treatments.
Open Practices Statement
The study reported in this article was not formally preregistered. Neither the data nor the materials have been made available on a permanent third-party archive; requests for the data or materials should be sent via email to the lead author at backs@musc.edu.
Table 1.
Means, Standard Deviations, Frequencies, and Percentages of Outcome and Mediator Variables
| PCL-M | TLFB (any use) | TLFB (% days using, conditional on use) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| COPE | RP | COPE | RP | COPE | RP | |||||||
|
|
|
|
|
|
|
|||||||
| Session | M | SD | M | SD | n | % | n | % | M | SD | M | SD |
|
| ||||||||||||
| Baseline | 62.17 | 11.03 | 64.26 | 8.93 | 48 | 88.9 | 25 | 92.6 | 52.85 | 33.72 | 54.06 | 32.23 |
| Session 1 | 58.59 | 11.43 | 58.21 | 11.49 | 33 | 61.1 | 17 | 63.0 | 55.78 | 34.04 | 61.85 | 30.21 |
| Session 2 | 57.86 | 13.38 | 57.64 | 11.16 | 31 | 67.4 | 16 | 59.3 | 54.23 | 33.50 | 53.42 | 27.32 |
| Session 3 | 53.89 | 12.66 | 55.19 | 14.86 | 27 | 50.0 | 12 | 44.4 | 52.72 | 31.90 | 55.19 | 28.60 |
| Session 4 | 53.07 | 14.58 | 55.16 | 14.78 | 28 | 51.9 | 12 | 44.4 | 52.03 | 27.06 | 50.74 | 35.94 |
| Session 5 | 50.41 | 15.64 | 56.31 | 14.62 | 23 | 42.6 | 10 | 37.0 | 50.99 | 32.12 | 52.10 | 31.74 |
| Session 6 | 45.54 | 15.59 | 58.00 | 18.47 | 25 | 46.3 | 10 | 37.0 | 33.64 | 26.15 | 43.32 | 26.07 |
| Session 7 | 44.21 | 18.00 | 56.93 | 13.96 | 22 | 40.7 | 9 | 33.3 | 38.03 | 24.34 | 29.42 | 11.93 |
| Session 8 | 42.25 | 16.84 | 52.53 | 13.91 | 19 | 35.2 | 5 | 18.5 | 42.07 | 31.69 | 35.93 | 14.51 |
| Session 9 | 41.37 | 17.40 | 49.43 | 12.27 | 17 | 31.5 | 5 | 18.5 | 45.40 | 23.92 | 47.54 | 13.00 |
| Session 10 | 39.90 | 18.23 | 49.14 | 15.09 | 15 | 27.8 | 9 | 33.3 | 39.91 | 20.80 | 47.12 | 26.40 |
| Session 11 | 39.93 | 17.72 | 54.00 | 13.24 | 13 | 24.1 | 6 | 22.2 | 31.68 | 21.22 | 58.89 | 25.60 |
| Session 12 | 36.57 | 17.20 | 53.07 | 14.49 | 14 | 25.9 | 9 | 33.3 | 43.33 | 26.66 | 42.27 | 36.23 |
Note. The percentage of each group reporting any substance use at each session is based on the number of participants in the intent-to-treat sample (n =54 in COPE; n = 27 in RP).PTSD = posttraumatic stress disorder; COPE = Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure; PCL-M = PTSD Checklist–Military Version; RP = relapse prevention; TLFB = timeline follow-back.
Acknowledgments
The authors would like to acknowledge support from the National Institute on Drug Abuse (NIDA; R01DA030143: Sudie E. Back; R25DA020537: Sudie E. Back, Kathleen T. Brady; K12DA035150: Christal L. Badour; K23DA042935: Amanda K. Gilmore), National Institute on Alcohol Abuse and Alcoholism (K23AA023845: Julianne C. Flanagan; R01 AA025086-03S1: Sudie E.Back), the Department of Veteran Affairs (VA) (CX000845: Daniel F. Gros), NIDA and Office of Research on Women’s Health in partnership with the Medical University of South Carolina (U54 DA016511: Delisa G. Brown), and resources at the Ralph H. Johnson VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of NIDA, NIAAA, National Institute of Mental Health, VA, or the U.S. Government. Sudie E. Back and Therese Killeen are co-authors of the COPE therapy manuals. This study is registered at ClinicalTrials.gov (NCT01338506).
References
- Back S, Foa E, Killeen T, Mills K, Teeson M, Cotton B, Carroll K, & Brady K. (2015). Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure (COPE). Therapist Manual. Oxford University Press. 10.1093/med:psych/9780199334513.001.0001 [DOI] [Google Scholar]
- Back S, Jackson J, Sonne S, & Brady K. (2005). Alcohol dependence and posttraumatic stress disorder: Differences in clinical presentation and response to cognitive-behavioral therapy by order of onset. Journal of Substance Abuse Treatment, 29(1), 29–37. 10.1016/j.jsat.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Back S, Killeen T, Badour C, Flanagan J, Allan N, Santa Ana E, Lozano B, Korte K, Foa E, & Brady K. (2019). Concurrent treatment of substance use disorders and PTSD using prolonged exposure: A randomized clinical trial in military veterans. Addictive Behaviors, 90, 369–377. 10.1016/j.addbeh.2018.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badour C, Flanagan J, Gros D, Killeen T, Pericot-Valverde I, Korte K, Allan N, & Back S. (2017). Habituation of distress and craving during treatment as predictors of change in PTSD symptoms and substance use severity. Journal of Consulting and Clinical Psychology, 85(3), 274–281. 10.1037/ccp0000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S, Killeen T, Badour C, Flanagan J, Allan N, Santa Ana E, Lozano B, Korte K, Foa E, & Brady K. (2019). Concurrent treatment of substance use disorders and PTSD using prolonged exposure: A randomized clinical trial in military veterans. Addictive Behaviors, 90, 369–377. 10.1016/j.addbeh.2018.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D, Weathers F, Nagy L, Kaloupek D, Gusman F, Charney D, & Keane T. (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90. 10.1007/BF02105408 [DOI] [PubMed] [Google Scholar]
- Brady K, Dansky B, Back S, Foa E, & Carroll K. (2001). Exposure therapy in the treatment of PTSD among cocaine-dependent individuals: Preliminary findings. Journal of Substance Abuse Treatment, 21(1), 47–54. 10.1016/s0740-5472(01)00182-9 [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis G, & Schultz L. (2003). Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Archives of General Psychiatry, 60(3), 289–294. 10.1001/archpsyc.60.3.289 [DOI] [PubMed] [Google Scholar]
- Carey K. (1997). Reliability and validity of the time-line follow-back interview among psychiatric outpatients: A preliminary report. Psychology of Addictive Behaviors, 11(1), 26–33. 10.1037/0893-164X.11.1.26 [DOI] [Google Scholar]
- Carroll K, Nich C, Sifry R, Nuro K, Frankforter T, Ball S, Fenton B, & Rounsaville B. (2000). A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence, 57(3), 225–238. 10.1016/s03768716(99)00049-6 [DOI] [PubMed] [Google Scholar]
- Chakraborty H, & Gu H. (2009). A mixed-model approach for intent-to-treat analysis in longitudinal clinical trials with missing values. RTI Press. 10.3768/rtipress.2009.mr.0009.0903 [DOI] [PubMed] [Google Scholar]
- Cohen L, & Hien D. (2014). Treatment outcomes for women with substance abuse and PTSD who have experienced complex trauma. Psychiatric Services, 57(1), 100–106. 10.1176/appi.ps.57.1.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E, Hembree E, & Rothbaum B. (2007). Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences therapist guide. Oxford University Press. [Google Scholar]
- Folstein M, Folstein S, & McHugh P. (1975). “Mini-mental state:” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Grant B, Goldstein R, Saha T, Chou S, Jung J, Zhang H, Pickering R, Ruan J, Smith S, Huang B, & Hasin D. (2015). Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry, 72(8), 757–766. 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller M, & Chassin L. (2014). Risk pathways among traumatic stress, posttraumatic stress disorder symptoms, and alcohol and drug problems: A test of four hypotheses. Psychology of Addictive Behaviors, 28(3), 841–851. 10.1037/a0035878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawn S, Cusack S, & Amstadter A. (2020). A systematic review of the self-medication hypothesis in the context of posttraumatic stress disorder and comorbid problematic alcohol use. Journal of Traumatic Stress, 33(5), 699–708. 10.1002/jts.22521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien D, Jiang H, Campbell A, Hu M, Miele G, Cohen L, Brigham G, Capstick C, Kulaga A, Robinson J, Suarez-Morales L, & Nunes E. (2010). Do treatment improvements in PTSD severity affect substance use outcomes? A secondary analysis from a randomized clinical trial in NIDA’s Clinical Trials Network. American Journal of Psychiatry, 167(1), 95–101. 10.1176/appi.ajp.2009.09091261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien D, Smith K, Owens M, López-Castro T, Ruglass L, & Papini S. (2018). Lagged effects of substance use on PTSD severity in a randomized controlled trial with modified prolonged exposure and relapse prevention. Journal of Consulting and Clinical Psychology, 86(10), 810–819. 10.1037/ccp0000345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin A, Asnaani A, Alpert E, & Foa E. (2016). The impact of treatment condition and the lagged effects of PTSD symptom severity and alcohol use on changes in alcohol craving. Behaviour Research and Therapy, 79, 7–14. 10.1016/j.brat.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadden R, Carroll K, Donovan D, Cooney N, Monti P, Abrams D, Litt M, & Hester R. (1992). Cognitive–behavioral coping skills therapy manual. National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- Kaysen D, Atkins D, Moore S, Lindgren K, Dillworth T, & Simpson T. (2011). Alcohol use, problems, and the course of posttraumatic stress disorder: A prospective study of female crime victims. Journal of Dual Diagnosis, 7(4), 262–279. 10.1080/15504263.2011.620449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE (2007). Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology, 3, 1–17. 10.1146/annurev.clinpsy.3.022806.091432 [DOI] [PubMed] [Google Scholar]
- Kehle S, Reddy M, Ferrier-Auerbach A, Erbes C, Arbisi P, & Polusny M. (2011). Psychiatric diagnoses, comorbidity, and functioning in National Guard troops deployed to Iraq. Journal of Psychiatric Research, 45(1), 126–132. 10.1016/j.jpsychires.2010.05.013 [DOI] [PubMed] [Google Scholar]
- Khantzian E. (1997). The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry, 4(5), 231–244. 10.3109/10673229709030550 [DOI] [PubMed] [Google Scholar]
- Lembke A. (2012). Time to abandon the self-medication hypothesis in patients with psychiatric disorders. The American Journal of Drug and Alcohol Abuse, 38(6), 524–529. 10.3109/00952990.2012.694532 [DOI] [PubMed] [Google Scholar]
- Maas C, & Hox J. (2005). Sufficient sample sizes for multilevel modeling. Methodology: European Journal of Research Methods for the Behavioral and Social Sciences, 1(3), 86–92. 10.1027/1614-2241.1.3.86 [DOI] [Google Scholar]
- María-Ríos C, & Morrow J. (2020). Mechanisms of shared vulnerability to post-traumatic stress disorder and substance use disorders. Frontiers in Behavioral Neuroscience, 14. 10.3389/fnbeh.2020.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley J, Killeen T, Gros D, Brady K, & Back S. (2012). Posttraumatic stress disorder and co-occurring substance use disorders: Advances in assessment and treatment. Journal of Clinical Psychology, 19(3), 283–304. 10.1111/cpsp.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane A, Browne D, Bryant R, O’Donnelle M, Silove D, Creamer M, & Horsley K. (2009). A longitudinal analysis of alcohol consumption and the risk of posttraumatic symptoms. Journal of Affective Disorders, 118, 166–172. 10.1016/j.jad.2009.01.017 [DOI] [PubMed] [Google Scholar]
- Mills K, Teesson M, Back S, & Brady K. (2012). Integrated exposure-based therapy for co-occurring posttraumatic stress disorder and substance dependence: A randomized clinical trial. JAMA, 308(7), 690–699. 10.1001/jama.2012.9071 [DOI] [PubMed] [Google Scholar]
- Najavits L, Norman S, Kivlahan D, & Kosten T. (2010). Improving PTSD/substance abuse treatment in the VA: A survey of providers. The American journal on addictions. The American Journal on Addictions, 19(3), 257–263. 10.1111/j.1521-0391.2010.00039.x [DOI] [PubMed] [Google Scholar]
- Norman S, Haller M, Hamblen J, Southwick S, & Pietrzak R. (2018). The burden of co-occurring alcohol use disorder and PTSD in U.S. Military veterans: Comorbidities, functioning, and suicidality. Psychology of Addictive Behaviors, 32(2), 224–229. 10.1037/adb0000348 [DOI] [PubMed] [Google Scholar]
- Norman S, Trim R, Haller M, Davis B, Myers U, Colvonen P, Blanes E, Lyons R, Siegel E, Angkaw A, Norman G, & Mayes T. (2019). Efficacy of integrated exposure therapy vs integrated coping skills therapy for comorbid posttraumatic stress disorder and alcohol use disorder: A randomized clinical trial. JAMA Psychiatry, 76(8), 791–799. 10.1001/jamapsychiatry.2019.0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare T, & Sherrer M. (2011). Drinking motives as mediators between PTSD symptom severity and alcohol consumption in persons with severe mental illnesses. Addictive Behaviors, 36(5), 465–469. 10.1016/j.addbeh.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Ostacher M, & Cifu A. (2019). Management of posttraumatic stress disorder. JAMA, 321(2), 200–201. 10.1001/jama.2018.19290 [DOI] [PubMed] [Google Scholar]
- Persson A, Back S, Killeen T, Brady K, Schwandt M, Heilig M, & Magnusson A. (2017). Concurrent treatment of PTSD and substance use disorders using prolonged exposure (COPE): A pilot study in alcohol-dependent women. Journal of Addiction Medicine, 11(2), 119–125. 10.1097/ADM.0000000000000286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce J, Schacht R, & Brooner R. (2020). The effects of prolonged exposure on substance use in patients with posttraumatic stress disorder and substance use disorders. Journal of Traumatic Stress, 33(4), 465–476. 10.1002/jts.22546 [DOI] [PubMed] [Google Scholar]
- Petrakis I, Rosenheck R, & Desai R. (2011). Substance use comorbidity among veterans with posttraumatic stress disorder and other psychiatric illness. American Journal of Addictions, 20(3), 185–189. 10.1111/j.1521-0391.2011.00126.x [DOI] [PubMed] [Google Scholar]
- Pietrzak R, Goldstein R, Malley J, Johnson D, & Southwick S. (2009). Subsyndromal posttraumatic stress disorder is associated with health and psychosocial difficulties in veterans of Operations Enduring Freedom and Iraqi Freedom. Depression and Anxiety, 26(8), 739–744. 10.1002/da.20574 [DOI] [PubMed] [Google Scholar]
- Possemato K, Maistro S, Wade M, Barrie K, McKenzie S, Lantinga L, & Ouimette P. (2015). Ecological momentary assessment of PTSD symptoms and alcohol use in combat veterans. Psychology of Addictive Behaviors, 29(4), 894–905. 10.1037/adb0000129 [DOI] [PubMed] [Google Scholar]
- RAND Corporation. (2020). Improving substance use care: Addressing barriers to expanding integrated treatment options for post-9/11 veterans. Author. https://www.rand.org/pubs/research_reports/RR4354.html [Google Scholar]
- Roberts N, Roberts P, Jones N, & Bisson J. (2016). Psychological therapies for post traumatic stress disorder and comorbid substance use disorder. Cochrane Database of Systematic Reviews, 4, CD010204. 10.1002/14651858.CD010204.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruglass L, Lopez-Castro T, Papini S, Killeen T, Back S, & Hien D. (2017). Concurrent treatment with prolonged exposure for co-occurring full or subthreshold posttraumatic stress disorder and substance use disorders: A randomized clinical trial. Psychotherapy and Psychosomatics, 86, 150–161. 10.1159/000462977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Harnett-Sheehan K, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, & Dunbar G. (1998). The M.I.N.I. International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview. Journal of Clinical Psychiatry, 59(Suppl 20), 22–23. [PubMed] [Google Scholar]
- Simpson T, Lehavot K, & Petrakis I. (2017). No wrong doors: Findings from a critical review of behavioral randomized clinical trials for individuals with co-occurring alcohol/drug problems and posttraumatic stress disorder. Alcoholism: Clinical and Experimental Research, 41(4), 681–702. 10.1111/acer.13325 [DOI] [PubMed] [Google Scholar]
- Simpson T, Stappenbeck C, Luterek J, Lehavot K, & Kaysen D. (2014). Drinking motives moderate daily relationships between PTSD symptoms and alcohol use. Journal of Abnormal Psychology, 123(1), 237–247. 10.1037/a0035193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson T, Stappenbeck C, Varra A, Moore S, & Kaysen D. (2012). Symptoms of posttraumatic stress predict craving among alcohol treatment seekers: Results of a daily monitoring study. Psychology of Addictive Behaviors 26(4), 724–733. 10.1037/a0027169 [DOI] [PubMed] [Google Scholar]
- Sobell L, & Sobell M. (1992). Timeline follow-back: A technique for assessing self-reported ethanol consumption. In Allen J. & Litten R. (Eds.), Measuring alcohol consumption: Psychosocial and biological methods (pp. 41–72). Humana Press. 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- Soder H, Wardle M, Schmitz J, Lane S, Green C, & Vujanovic A. (2019). Baseline resting heart rate variability predicts post-traumatic stress disorder treatment outcomes in adults with co-occurring substance use disorders and post-traumatic stress. Psychophysiology, 56(8), e13377. 10.1111/psyp.13377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S, Pihl R, Conrod P, & Dongier M. (1998). Functional associations among trauma, PTSD, and substance-related disorders. Addictive Behaviors, 23(6), 797–812. 10.1016/s0306-4603(98)00070-7 [DOI] [PubMed] [Google Scholar]
- Swendsen J, Conway K, Degenhardt L, Glantz M, Jin R, Merikangas K, Simpson N, & Kessler R. (2010). Mental disorders as risk factors for substance use, abuse, and dependence: Results from the 10-year follow-up of the National Comorbidity Survey. Addiction, 105(6), 1117–1128. 10.1111/j.1360-0443.2010.02902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchalla I, Nosen L, Rostam H, & Allen P. (2012). Integrated treatment programs for individuals with concurrent substance use disorders and trauma experiences: A systematic review and meta-analysis. Journal of Substance Abuse Treatment, 42(1), 65–77. 10.1016/j.jsat.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Tripp J, Worley M, Straus E, Angkaw A, Trim R, & Norman S. (2020). Bidirectional relationship of posttraumatic stress disorder (PTSD) symptom severity and alcohol use over the course of integrated treatment. Psychology of Addictive Behaviors, 34(4), 506–511. 10.1037/adb0000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (n.d.). Drinking levels defined. National Institute on Alcohol Abuse and Alcoholism. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. [Google Scholar]
- Weathers F, Huska J, & Keane T. (1991). PTSD Checklist–Military Version for DSM-IV. National Center for PTSD–Behavioral Science Division. [Google Scholar]
- Wei LJ, & Lachin JM (1988). Properties of the urn randomization in clinical trials. Controlled Clinical Trials, 9(4), 345–364. 10.1016/0197-2456(88)90048-7 [DOI] [PubMed] [Google Scholar]
- Wilkins K, Lang A, & Norman S. (2011). Synthesis of the psychometric properties of the PTSD Checklist (PCL) military, civilian, and specific versions. Depression and Anxiety, 28(7), 596–606. 10.1002/da.20837 [DOI] [PMC free article] [PubMed] [Google Scholar]