Abstract
Early detection of endometrial cancer, especially its precancers, remains a critical and evolving issue in patient management and the quest to decrease mortality due to endometrial cancer. Due to many factors such as specimen fragmentation, the confounding influence of endogenous or exogenous hormones, and variable or overlapping histologic features, identification of bona fide endometrial precancers and their reliable discrimination from benign mimics remains one of the most challenging areas in diagnostic pathology. At the same time, the diagnosis of endometrial precancer, or the presence of suspicious but subdiagnostic features in an endometrial biopsy, can lead to long clinical follow-up with multiple patient visits and serial endometrial sampling, emphasizing the need for accurate diagnosis. Our understanding of endometrial precancers and their diagnosis has improved due to systematic investigations into morphologic criteria, the molecular genetics of endometrial cancer and their precursors, the validation of novel biomarkers and their use in panels, and more recent methods such digital image analysis. Although precancers for both endometrioid and non-endometrioid carcinomas will be reviewed, emphasis will be placed on the former. We review these advances and their relevance to the histopathologic diagnosis of endometrial precancers, and the recently updated 2020 World Health Organization (WHO) Classification of Female Genital Tumors.
Keywords: endometrial cancer, endometrial precancer, endometrial atypical hyperplasia (AH), endometrioid intraepithelial neoplasia (EIN), Pax2, Pten, β-catenin
Introduction
Endometrial cancer (EC) is the most common gynecological cancer and the third most common cancer in women1, 2. The incidence of EC has been on the rise in the past decade and poses a major threat to public health3, 4. Earlier and more accurate diagnosis of EC, and particular its histologic precursors, represents an outstanding opportunity for prevention and improved patient management of this commonly-encountered malignancy. The diagnosis of endometrial atypical hyperplasia/endometrioid intraepithelial neoplasia (AH/EIN), the accepted precancerous lesion for endometrioid adenocarcinomas, remains challenging and subjective, with variable histologic criteria and differences of opinion among gynecologic pathologists, potentially leading to under- or overtreatment, thereby delaying diagnosis or adding to healthcare costs5–8. In this review, and in accordance to prior literature, we draw a distinction between “precancer” and “precursor”. A precancer is a precursor with a high risk for progression to a malignancy (typically necessitating clinical intervention), whereas a precursor is an entity believed to precede the eventual malignancy but not necessarily with a high risk to do so. Thus, “precursor” encompasses a wider range of lesions than “precancer”. We consider Pten or Pax2-deficient clones in otherwise normal endometria (discussed below) to be “precursors” whereas AH/EIN are “precancers”. These definitions are somewhat fluid and subject to refinements as more is learned. Serous endometrial intraepithelial carcinoma (SEIC) and its relationship to endometrial serous carcinoma will also be briefly reviewed.
The practical challenges in reliable diagnosis are well-known to pathologists and include 1) specimen fragmentation/limited tissue; 2) variations in glandular architecture, density, and cytology during normal cycling; 3) treatment with hormonal agents that obscure architectural and cytologic features; 4) presence of fragments of endometrial polyps, which can normally exhibit considerable gland crowding—but conversely often harbor precancers, and 5) gradual variations in gland architecture can make it difficult to identify or clearly demarcate definitive regions of neoplasia. Naturally, there has been sustained interest in the refinement of histologic criteria for the diagnosis of endometrial precancer, and more recently, in the use of other approaches such as specific immunohistochemical markers as adjuncts in AH/EIN diagnosis.
The molecular pathways and genetic driver lesions underlying endometrioid adenocarcinoma are now well established. Building upon past studies, the EC genome atlas project (TCGA, National Cancer Institute)9 revealed the pathways most frequently misregulated via mutations in genes such as PTEN, CTNNB1 (β-catenin), or ARID1A. These refined views of molecular genetics of EC combined with numerous studies of early endometrial neoplasia provide a rational basis for the systematic exploration and validation of biomarkers that could be useful in the diagnosis of AH/EIN. In this review, we summarize the gradual evolution of WHO criteria. We then present several individual biomarkers of potential diagnostic use, and discuss efforts to define combinations of these biomarkers that work well together in limited immunohistochemistry panels. Finally, we close with a brief discussion of other methods that merit further investigation as promising future approaches to the diagnosis of endometrial precancers.
Changes in the diagnostic system for the precancer of endometrioid adenocarcinoma from WHO 1994 to 2020 classifications
WHO 1994 4-tier classification system.
In WHO 1994, hyperplasia was classified into 4 diagnostic categories based on 1) the degree of architectural complexity (simple vs. complex) and 2) nuclear atypia of the lesional glands (presence vs. absence). In this system, all presumptive lesions were divided into 2 binary categories leading to 4 entities: hyperplasia without atypia (simple or complex) and hyperplasia with atypia a.k.a. atypical hyperplasia (simple or complex) based on a seminal 1985 study showing that the 4 categories were predictive of risk of progression to invasive adenocarcinoma10. This diagnostic scheme was a major advance, as it was a more systematic and unified nomenclature and classification system than the pre-existing and non-uniform terminology.
Development of 2-tier endometrioid intraepithelial neoplasia (EIN) scheme.
On the other hand, over time, some limitations of the WHO 1994 became apparent. One challenge was the lack of specific, reliable, and reproducible cutoffs for architectural complexity and nuclear atypia, or at least, difficulties in their application in day-to-day cases. Another limitation was the creation of 4 diagnostic categories that did not clearly align with clinical management options (e.g. treatment vs. continued follow-up) including one category that remains controversial or rarely seen (simple atypical hyperplasia). These perceived limitations led to the advent of a distinct 2-tier scheme with different histologic criteria and terminology: endometrioid intraepithelial neoplasia/EIN11, 12. While early literature used the term “endometrial intraepithelial neoplasia”13, this was later modified to endometrioid intraepithelial neoplasia to emphasize the distinct endometrioid pathway of carcinogenesis (e.g. vs. serous). This 2-tier EIN scheme dropped atypia and subgrading of glandular complexity as formal criteria. Another point stressed by the proponents of EIN was that the term “neoplasia” is preferable to “hyperplasia” when referring to a bona fide cancer precancer, because hyperplasias have generally been defined as physiologic i.e. non-clonal, non-neoplastic processes. In the EIN classification system, lesions were subclassified into 2 categories: “hyperplasia without atypia” (i.e. non-neoplastic) and “EIN”. The diagnostic criteria for EIN are 1) glandular crowding (gland/stroma ratio>1); 2) cytology differs between architecturally crowded focus and background; 3) size of lesion >1 mm; and 4) exclusion of carcinoma or mimics such as endometrial polyp or artifactual crowding. The EIN system emphasizes “cytologic distinctiveness” (nuclear and/or cytoplasmic features) relative to entrapped or adjacent normal glands that serve as internal morphologic controls for assessing putatively neoplastic foci. Inherent to the EIN scheme was the argument that nuclear atypia is subjective, and hence not highly reproducible, whereas comparison of normal vs. putative neoplastic glands provides a more reliable and readily applied diagnostic criterion. The adoption of this 2-tier system has led to better reproducibility among pathologists, according to some studies14, 15.
WHO 2014 and 2020: Incorporation of EIN system, continued refinement of the diagnostic criteria for atypical hyperplasia/EIN, and use of biomarkers.
In the 2000s and 2010s, the differences and advantages of the atypical hyperplasia (AH) vs. EIN schema were debated, and some pathology departments began using the EIN nomenclature. The existence of 2 competing schema with different criteria may have been somewhat disconcerting, a less than ideal state of affairs requiring some resolution. To a large extent, WHO 2014 was conciliatory and largely (if not explicitly) merged the AH and EIN systems into one 2-tiered system16 where AH and EIN were considered equal: “AH/EIN”. Per WHO 2014, “cytologic atypia superimposed on endometrial hyperplasia defines AH/EIN”. The influence of the EIN conceptual framework is clearly evident in the collapse to 2 categories, while at the same time the concept of atypia intrinsic to the AH system was maintained. While this could seem counterintuitive, it may make sense in light of the fact that pathologists using the AH system were likely aware of normal glands and their cytologic distinctiveness, even if this had not been emphasized as a specific diagnostic criterion10. The WHO 2020 classification continued with the combined AH/EIN terminology, specifying as essential diagnostic criteria crowded glandular architecture with altered epithelial cytology distinct from non-neoplastic glands. The initial 1 mm size cutoff in the WHO 2014 system, based on morphometric studies (10) to exclude artifact crowding, was modified to “sufficient size that artifact can be excluded”. Therefore, some small lesions that were subdiagnostic in WHO 2014 could be classified as AH/EIN in the WHO 2020 system. Notably, WHO 2020 also specified loss of immunoreactivity for Pten, Pax2, or mismatch repair proteins as “desirable” diagnostic criteria. This was a significant development, as it was the first time that incorporation of biomarkers was recommended in the diagnostic workflows for endometrial precancers. The use of biomarkers in the diagnosis of AH/EIN is discussed at length below. The evolving criteria in the WHO systems are summarized in Table 1.
Table 1.
The evolution of WHO criteria for the diagnosis of endometrial precancers.
| Diagnostic criteria | WHO 1994 | |
|---|---|---|
| Hyperplasia |
|
|
| Nuclear atypia* |
|
|
| Architecture |
|
|
|
|
|
*Features of nuclear atypia:
| ||
| Diagnostic criteria | WHO 2014 | WHO 2020 |
|---|---|---|
| Hyperplasia without atypia | ||
Same in WHO 2014 and 2020.
| ||
| AH/EIN | ||
| Hyperplasia | Gland to stroma ratio >1 | |
| Cytological atypia |
|
|
| Size |
|
|
| Exclusions |
|
|
| Biomarkers* | N/A |
|
| *Biomarkers listed in WHO 2020 included Pten, Pax2 and mismatch repair proteins (MMR), with the latter not further specified | ||
Disordered proliferative endometrium (DPE) and hyperplasia without atypia
The WHO diagnostic criteria for “non-atypical” hyperplasia has not explicitly changed over the years. In the current WHO 2-tiered system, hyperplasia without atypia is considered a “benign” hyperplasia resulting from a physiological polyclonal proliferation typically caused by prolonged unopposed estrogen, which is common in the perimenopause. The range of histopathological features [so-called benign hyperplasia sequence17] is likely dependent on the quality and duration of unopposed estrogen in the perimenopause or other anovulatory conditions such as polycystic ovarian syndrome.
Changes at the lower end of the histological spectrum are referred to as “disordered proliferative endometrium” (DPE), which describes a proliferative endometrium (PE) lacking the usual regularity of gland size and spacing. Instead, DPE is characterized by irregularly shaped, cystically dilated glands producing a disordered arrangement. True gland cribriforming is not seen and the epithelium remains as a single layer without stratification. Metaplastic changes are common, including tubal or eosinophilic syncytial metaplasia18. Other changes associated with endometrial stromal breakdown are common. Most importantly, the endometrium retains a relatively normal gland to stroma ratio. When the gland:stroma area exceeds 50% (1:1), the term “hyperplasia without atypia” may be used, although this threshold is somewhat arbitrary and does not necessarily signify a clear-cut biological or clinical distinction. Of note, the changes associated with hyperplasia without atypia involve the entire endometrium (global changes), best appreciated at low magnification. In contrast, local admixtures of irregularly shaped glands have variable appearances among different fields at medium magnification. This combination of low magnification uniformity and medium magnification variability has been described as “regularly irregular”17. A feature of hyperplasia without atypia is that cytological features of crowded glands are unchanged from field to field. This global change serves as a helpful criterion for the distinction between hyperplasia without atypia (presumed non-clonal) and AH/EIN (clonal).
DPE and hyperplasia without atypia represent a spectrum of continuous histological change caused by excess estrogen exposure and are not considered precancers. Because there are no well-established criteria for distinguishing the 2 entities (the use of 1:1 ratio as a threshold notwithstanding), the separation of these 2 diagnoses is essentially subjective19, highly variable among individual pathologists, and thus of questionable value. The diagnosis of “hyperplasia without atypia” might imply an entity more worrisome than “DPE”, or one more likely to represent a bona fide endometrial precancer requiring management. This is particularly the case because the term hyperplasia persists in the AH/EIN nomenclature, potentially causing confusion among clinicians and possibly leading to overly aggressive clinical management in some cases. On the other hand, some lesions diagnosed as simple hyperplasias, perhaps at the higher end of the spectrum, might be more significant precursor lesions20, a possibility that warrants investigation and is further discussed below. Similarly, there is no generally accepted criterion to separate PE from DPE. In our practice, the presence of ≥10% dilated or architecturally irregular glands is sufficient for the diagnosis of DPE.
Molecular genetics of AH/EIN
The integrated genomic analysis of EC by The Cancer Genome Atlas (TCGA) has provided a more complete understanding view of the genetic EC landscape21. Per this analysis, ECs can be divided into 4 categories based on patterns of genomic instability: 1) POLE/ultramutated 2) microsatellite unstable and mismatch repair deficient/hypermutated 3) copy-number low, and 4) copy-number high, largely corresponding to TP53-mutant serous and high-grade endometrioid carcinomas. In addition to the original publication in 2013, there have been several outstanding reviews22–24 about these discoveries and their implications for patient management. Frequent tumor driver genes include PTEN, CTNNB1, PIK3CA, ARID1A and KRAS. Genetic changes lead to frequent disruption of diverse pathways including the PI3K pathway (PI3K–PTEN–AKT–mTOR, RAS–MEK–ERK), WNT–β-catenin) and the SWI/SNF chromatin remodeling complex, which includes ARID1A. Immunohistochemical loss of PAX2 is also frequently observed in EC and AH/EIN, suggesting that PAX2 loss is a very early event, although interestingly, this does not appear to be due to mutational inactivation21, 25–35.
Recent genome-wide mutation profiling34, 36–38 in paired AH/EIN and EC cast new light on the clonal evolution of endometrioid carcinoma. While stepwise acquisition of cancer driver mutations and progressive accumulation of tumor mutation burden have been observed in some cases, private mutations (i.e., present in only one sample) are not uncommon. Thus, beside an idealized linear pathway which involves stepwise accumulation of molecular events, more complex pathways in which AH/EIN and/or carcinoma diverge early or develop independently—leading to considerable genetic heterogeneity—may also exist (see example case described below).
Potential immunohistochemical biomarkers in the diagnosis of AH/EIN
Several factors that participate in early endometrial neoplasia are aberrantly expressed in some AH/EIN, stimulating interest in their use as practical biomarkers in the diagnosis of AH/EIN. Below, we briefly present each of these protein markers, followed by a discussion of their potential use together as a group (i.e. in panels). Photomicrographs showing normal and aberrant patterns of expression are presented in Figs. 1 and 2.
Figure 1. Normal patterns of expression of biomarkers capable of identifying AH/EIN.
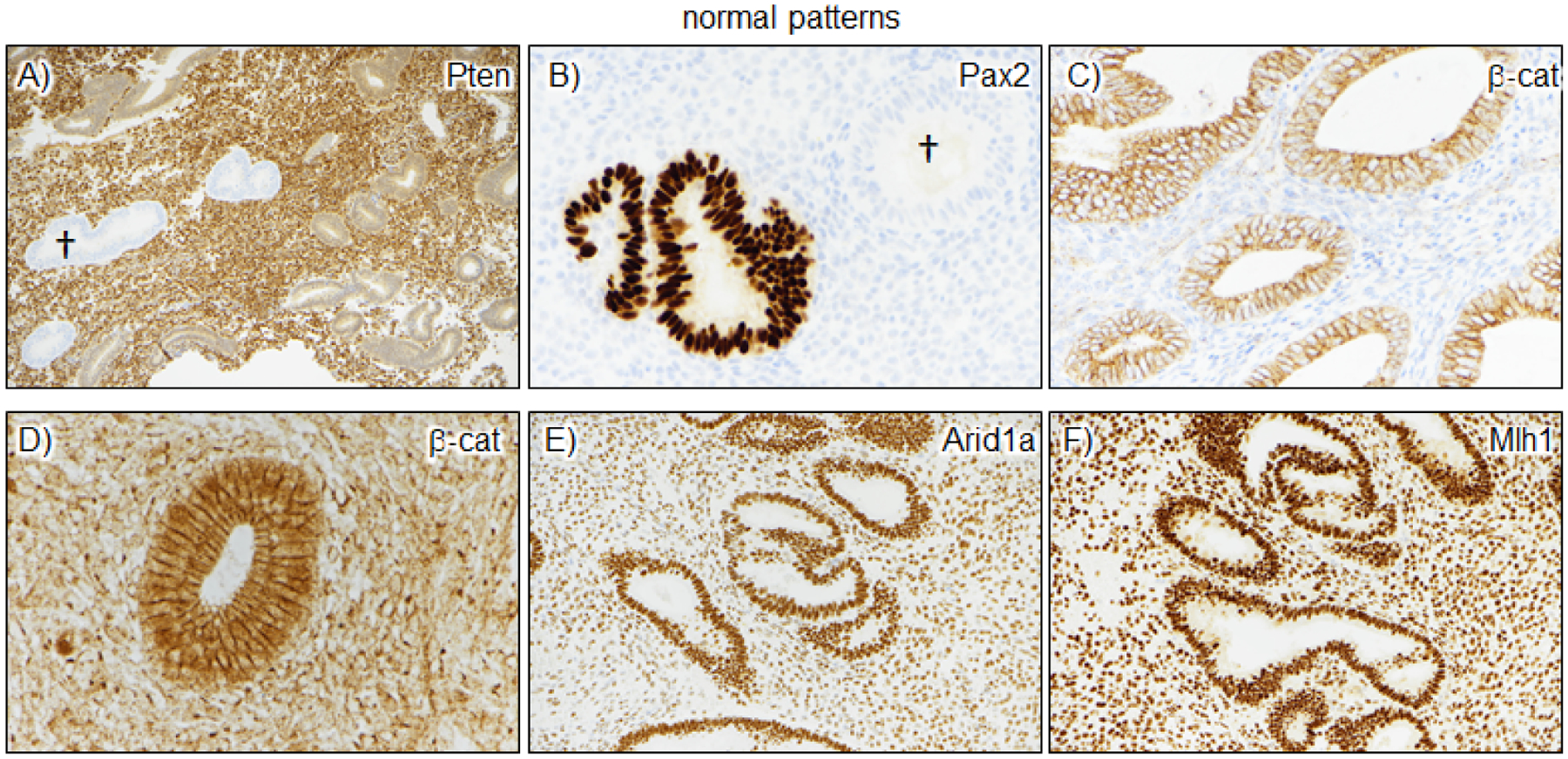
A-B) Pten and Pax2 panels show examples of marker loss in scattered glands. The loss is striking and easily identified. Such loss is common in normal endometrial, but usually in <1% of the entire sample, and is thus usually readily distinguishable from the much more extensive loss seen in specimens with AH/EIN; †=glands with marker loss. C-D) β-catenin panels show the range of normal β-catenin expression, which can range from faint and entirely membranous to somewhat stronger expression with some nuclear localization. However, in normal endometria, the nuclear β-catenin expression is not stronger than the intervening cell membranes, a useful feature distinguishing it from mutant/aberrant patterns of β-catenin expression. E-F) Arid1a and Mlh1 are rarely lost in normal endometria; the two panels show the typical strong expression in glands and stroma, with the latter serving as a useful internal control.
Figure 2. Aberrant patterns of expression in AH/EIN.
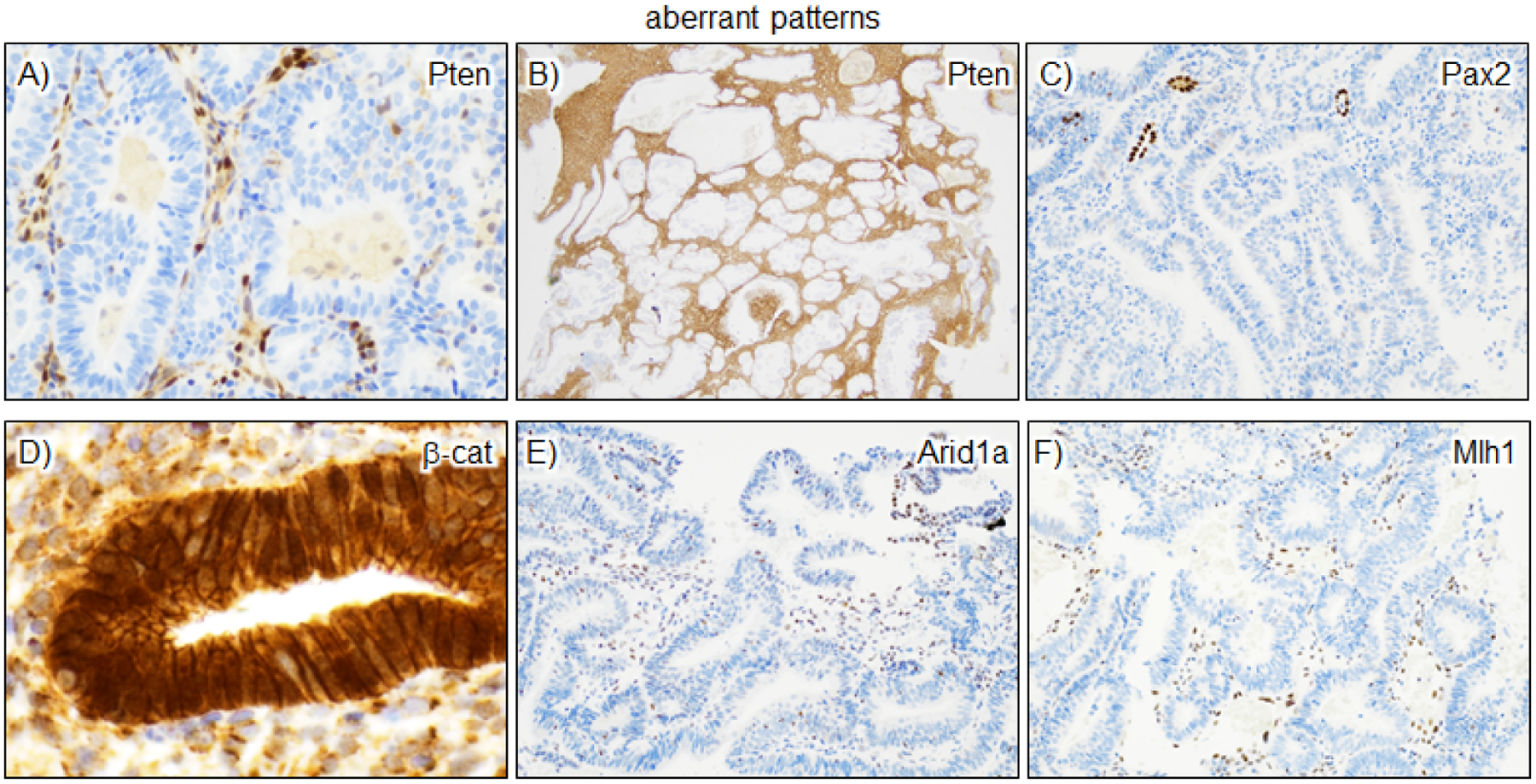
Pax2, Pten, and β-catenin are the most useful markers and comprise the most efficient panel for the detection of AH/EIN. A-B) For Pten, distinct loss produces a “punched-out” appearance of glands. Stroma and intraluminal macrophages/other leukocytes retain Pten. C) Pax2 loss is also usually easily scored, although the lack of expression in stroma or leukocytes means that other internal or external IHC controls are needed. Often scattered normal glands are present, providing an internal control, as in this example. D) β-catenin expression is readily scored by the presence of strong nuclear expression greater than that observed in the intervening cell membranes. E-F) Arid1a and Mlh1 loss in epithelium can be easily scored but are less useful in practice due to a lower incidence of biomarker aberrancy. In our study of n=111 AH/EIN, all cases were diagnosed with the 3-marker panel and inclusion of Arid1a and Mlh1 did not lead to the identification of any additional cases.
Pax2
Pax2 is a DNA-binding transcription factor localized exclusively within nuclei. Loss of nuclear expression of PAX2 is observed in endocervical adenocarcinoma39, EC, and AH/EIN35. Frequent immunohistochemical loss of Pax2 in AH/EIN was first reported by George Mutter in 201035. Pax2 is usually strongly expressed in all endometrial epithelial nuclei. Loss of expression, when it occurs, appears complete (i.e. “all or none” within individual cells) in AH/EIN. Such complete Pax2 loss relative to the strong and uniform expression in control glands makes Pax2 an easily-scored and attractive AH/EIN marker, with several investigations confirming its utility and the robustness of Pax2 IHC11, 35, 40–43. Widespread loss of nuclear expression of Pax2 in AH/EIN is specific and demonstrates high sensitivity (~80% of AH/EIN exhibit significant Pax2 loss, Figs. 3, 4) as also confirmed in a recently published meta- analysis44. Interestingly, as noted above, the mechanism(s) for Pax2 loss are not fully understood, although some studies have implicated epigenetic misregulation, perhaps due to hypermethylation of the PAX2 promoter45.
Figure 3. Parts-of-whole representations for most useful AH/EIN biomarkers.
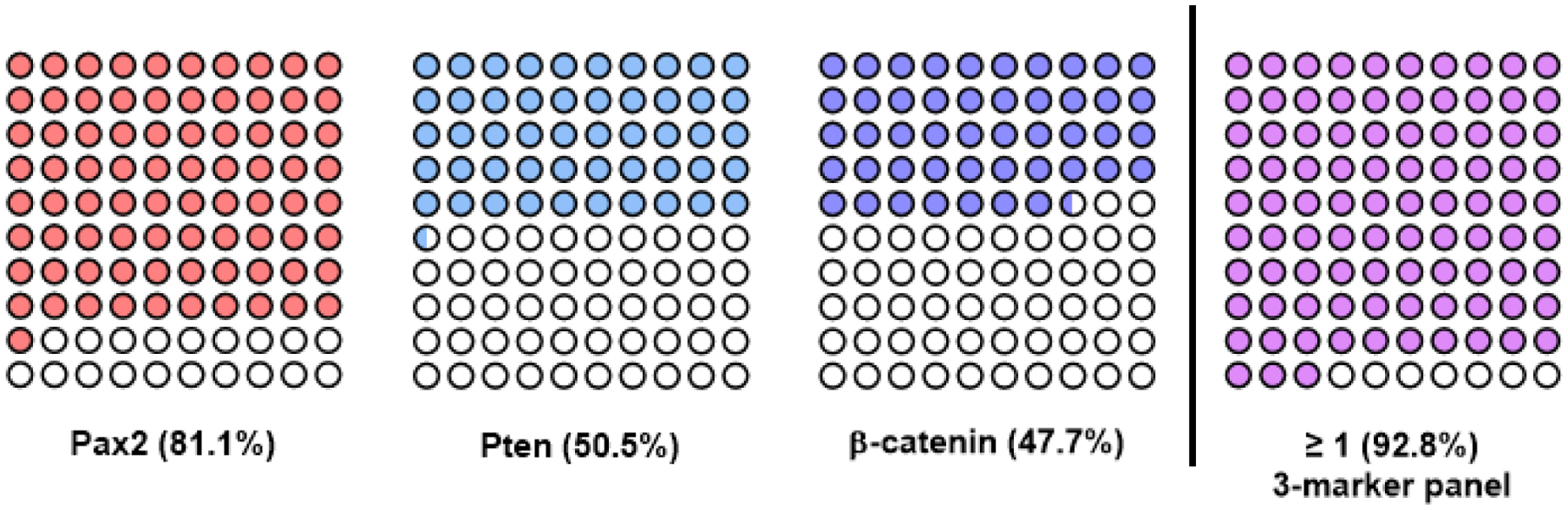
Graphs show individual cases (among idealized set of 100 patients) detected (filled circles) by each of the 3 markers individually and by ≥1 marker when all 3 markers are used. Data is based on a previous analysis of n=111 patients77.
Figure 4. Quantitative analyses of biomarker aberrance in AH/EIN including group performance.
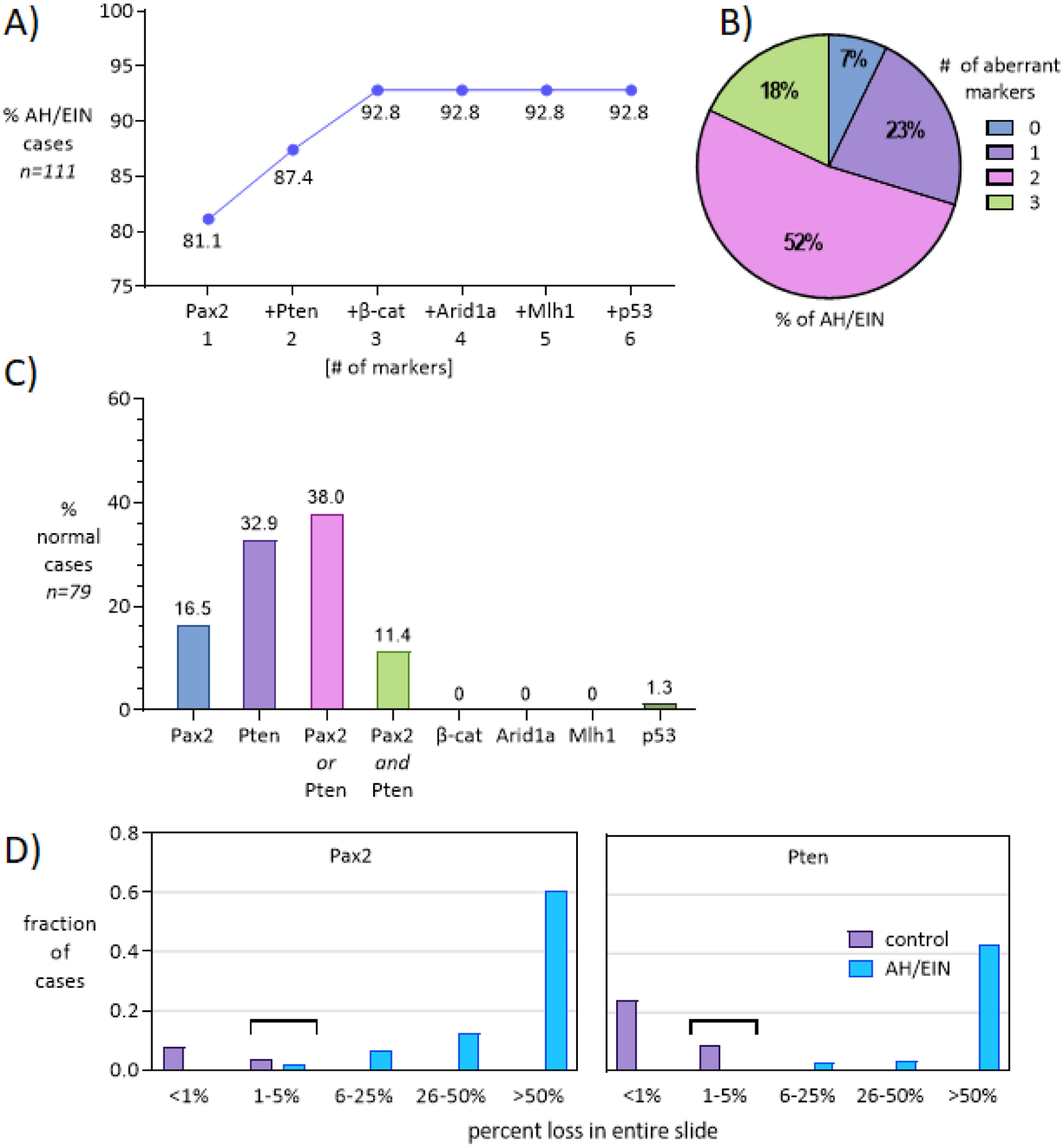
A) Diagnostic yields following addition of individual markers, in order of greatest to least likelihood of aberrancy. Arid1a, Mlh1, or p53 did not lead to the identification of any additional AH/EIN cases, pointing to Pax2/Pten/β-catenin as the most effective and compact panel. B) Pie chart showing the number of aberrant markers (0–3) among AH/EIN. Aberrance for 2 or more markers (which occurs in >50% of cases) can enhance diagnostic confidence. C) Percent of normal endometrial controls showing aberrancy for each marker. The numbers are not comparable to (A), because aberrancy within a single gland of normal endometrium is shown, whereas aberrancy within AH/EIN was characterized large areas involving many glands. Nonetheless, the findings indicate that evaluation of markers must always occur in the context of histologic features. D) Fraction (%) of control (purple) or AH/EIN (blue) cases exhibiting loss of Pax2 or Pten across different categories based on estimated overall loss on slide (<1%, 1–5%, 6–25%, 26–50%, or >50%).
Pten
Pten is a ubiquitously-expressed lipid phosphatase present throughout the cell in the nucleus, cytoplasm, and cell membrane46. PTEN is among the most frequently mutated genes in EC, usually as an early driver event9, 22. The first studies documenting Pten loss in AH/EIN were, as for Pax2, performed by Mutter’s group35, 47, 48. Pten was not immediately adopted as an AH/EIN biomarker, with some relatively recent practice consensus publications arguing against its routine use49. Resistance to its acceptance may stem from the existence of diverse commercially monoclonal antibodies, some of which may be suboptimal for formalin-fixed paraffin-embedded tissue. However, optimal commercial monoclonal antibodies are reliable in the clinical pathology laboratory (Figs. 1, 2). Although the frequent loss (~50%) of Pten protein in AH/EIN (Figs. 3, 4) is consistent with the high frequency of PTEN point mutations in EC, the frequent immunohistochemical loss of Pten protein is not yet entirely explained at the molecular level. For example, to fully rationalize complete loss of the Pten protein, biallelic mutations would have to be identified (or a single mutation with documented loss-of-heterozygosity), and each mutation would have to be predicted or demonstrated to lead to protein loss (i.e. premature stop, frameshift, etc.). Molecular studies to date have not been systematically carried out at this level of resolution. Thus, it remains possible that diverse mechanisms contribute to Pten loss, such as nonsense-mediated decay, destabilization of the protein by point mutations, intragenic or larger deletions not readily detectable by standard (short-read) next-generation sequencing methods, or epigenetic/autoregulatory mechanisms operating at the transcriptional level.
β-catenin.
In endometrial neoplasia, mutations in the CTNNB1 gene encoding β-catenin are frequent early events27, 50 occurring in ~50% of ECs, most commonly in copy-number low endometrioid adenocarcinomas9, 51. Most CTNNB1 mutations alter specific residues within exon 3 that form a β-catenin protein degradation motif. These mutations inhibit proteasomal degradation and lead to stabilization of β-catenin, resulting in increased protein levels and abnormal relocalization from the membrane/cytoplasm to the nucleus51–53. Strong nuclear β-catenin localization, usually associated with overall overexpression, is a reliable indicator of β-catenin activation in AH/EIN or endometrial adenocarcinoma and such aberrancy occurs in ~48% of cases (Figs. 3, 4)43, 53, 54. Studies of ECs showed that nuclear β-catenin staining has ≥90% specificity and sensitivity for CTNNB1 mutations52, 53, 55.
Although abnormal nuclear localization of β-catenin in AH/EIN associated with CTNNB1 mutations has been documented since 199927, 56, 57 only recently have studies begun to formally explore the utility of β-catenin as a practicable diagnostic AH/EIN marker58, 59. Unlike Pten or Pax2, where loss of expression is the feature indicating aberrance, relocalization of β-catenin to the nucleus is the principal immunohistochemical finding indicating an underlying molecular defect. The presence of strong, distinctively nuclear expression in glands observed in many AH/EIN cases, even when focally present, makes scoring such cases straightforward. Morular squamous metaplasia, which has been associated with underlying CTNNB1 mutations, always exhibits nuclear β-catenin60, and β-catenin should be assessed in endometrial epithelium without obvious morules. When morules are present, adjacent epithelium usually exhibits distinctive nuclear localization in some non-morular epithelial cells. Characteristically, nuclear localization occurs in scattered cells within AH/EIN glands, and is not uniform among all cells in a gland.
Mlh1 and other mismatch repair (MMR) factors.
The use of Mlh1 as an AH/EIN marker is analogous to its use in the standard 4-marker panel for MMR deficiency and Lynch Syndrome screening in newly-diagnosed EC61–63. Mlh1 could be an attractive AH/EIN marker, at least in principle, in that 1) pathologists are experienced in its use 2) it is the most commonly aberrant MMR marker in AH/EIN and 3) MMR defects are early if not initiating defects in endometrioid carcinogenesis. AH/EINs with Mlh1 protein deficiency are easily scored, with the strong stromal nuclear staining normally present in endometrial stroma serving as an internal control (Figs. 1, 2)64.
However, studies investigating MMR expression in biopsies with AH/EIN have reported that only 5–10% of AH/EIN demonstrated loss of Mlh1 expression by immunohistochemistry (IHC)64, 65 vs. the 29% incidence of Mlh1 loss in EC66. Thus, the prevalence of MMR deficiency in AH/EIN appears to be much lower than in EC, perhaps because MMR deficient AH/EIN have a shorter transition time to carcinoma relative to MMR proficient AH/EIN67. More rapid progression may be a general feature of hypermutant (MMR deficient) and ultramutant (POLE) ECs20, 50. In our previous study, most AH/EIN cases with loss expression of MMR proteins other than Mlh1 were patients with Lynch syndrome. However, the low prevalence of MMR deficiency in unselected AH/EIN is comparable to the reported 2–4% incidence of LS in unselected EC cases66, 68, 69. Therefore, MMR testing in AH/EIN appears to be highly useful in the early detection of LS, even if Mlh1 or other MMR factors were not found to be highly effectual markers in the diagnosis of AH/EIN.
Arid1a.
ARID1A (a.k.a. BAF250A), which encodes a component of the SWI/SNF nucleosome-remodeling complex, is one of the most frequently mutated genes in endometrioid adenocarcinomas, leading to complete immunohistochemical loss of the protein in some mutant cases70. Arid1a protein is, like Mlh1, nuclear and ubiquitously expressed. Although Arid1a IHC is not available in most pathology laboratories, robust IHC protocols with monoclonal antibodies are available71. Scoring of Arid1a loss in AH/EIN and identification of Arid1a-deficient cases is analogous to Mlh171, 72, making it also a potentially useful marker (Figs. 1, 2). A recently published meta-analysis of ARID1A in AH/EIN also showed that ARID1A loss is highly specific as a diagnostic marker for AH/EIN73, with loss occurring in 5–10% of cases. Arid1a protein loss is clearly associated with ARID1A mutations, although as with PTEN, it is not always obvious why some mutations lead to protein loss while others do not, or if mutations must be biallelic for protein loss to occur.
Foxo1 and phospho-Akt.
Pten loss leads to hyperphosphorylation of the kinase Akt, which can be immunohistochemically detected by phospho-specific AKT antibodies. Phosphorylated Akt in turn phosphorylates the forkhead transcription factor Foxo1, leading to its export from the nucleus to the cytoplasm and hence its functional inactivation. Foxo1 and phospho-Akt are aberrant in some AH/EIN, but both markers appear less suitable as practicable biomarkers due to challenges in detecting phospho-proteins in paraffin-embedded tissues, or the influence of steroid hormone variation during normal cycling that impact levels of Foxo1 and or phospho-Akt. Nonetheless, these markers may merit further investigation59.
p53.
The use of p53 as a marker of endometrial serous cancers is well-known, and pathologists are familiar with identification of p53 mutant patterns74. Although p53 is typically considered as a marker of serous EC precancers, it is also mutated in some endometrioid adenocarcinomas, particularly those that are high grade. It is mentioned here for completeness, but the fact that p53 mutations occur very late in disease progression (i.e. in the progression from well- to poorly-differentiated invasive adenocarcinoma) makes it unlikely to be a useful AH/EIN marker. However, some studies have identified aberrant p53 expression in at least rare AH/EIN cases75.
Systematic evaluation of potential biomarkers for AH/EIN and selection of an optimal panel
In recognition of the challenges in the reliable diagnosis of AH/EIN, and the promise of at least some of the above biomarkers, the 2020 WHO Classification of Female Genital Tumors stated that “loss of immunoreactivity for Pten, Pax2, or MMR proteins is desirable” in the diagnosis of AH/EIN76. This statement implies that a panel of immunostains is desirable in the diagnosis of AH/EIN. However, this raised critical questions. How many markers should be employed? And which ones? To address these unresolved issues, we recently performed a systematic analysis of the performance characteristics of 6 immunohistochemical markers (Pax2, Pten, β-catenin, Arid1a, Mlh1, and p53) both individually and in combinations in AH/EIN and normal controls, and the findings are reviewed below.
As single markers, Pax2, Pten, or β-catenin were aberrant in a high percentage of AH/EIN cases (Pax2, 81.1%; Pten, 50.5%; β-catenin, 47.7%) (Fig. 3). Arid1a, Mlh1, or p53 were aberrant in a significant, but much smaller percentage of cases (7.2%, 4.5%, and 2.7% respectively). With a hypothetical panel consisting of 6 markers, at least 1 of the markers was aberrant in 92.8% of AH/EIN (Fig. 4A). The 5 non-Pax2 markers identified 83.0% of cases, while Pten and β-catenin combined identified 78.4% of cases. The additive effects of each marker in order of aberrancy in AH/EIN provides a useful way to assess the value of including additional markers. A panel consisting of only Pax2, Pten, and β-catenin identified 92.8% of cases. Inclusion of Arid1a or Mlh1 did not increase the diagnostic yield further because all of the cases detected by Arid1a/Mlh1 were already scored as aberrant by Pax2, Pten, or β-catenin. p53 did not prove useful, because of the rarity of p53 mutant clones in AH/EIN, and the occurrence of rare p53-overexpressing clones in normal control endometria. Also of note, most AH/EIN cases were aberrant for 2 or 3 markers, further increasing diagnostic confidence (Fig. 4B).
One challenge in the use of Pten or Pax2 as practical AH/EIN biomarkers is the surprisingly common occurrence of sporadic Pax2 or Pten negative glands in normal endometria, also first described by Mutter. Pten-null glands were considered to represent extremely early “latent precancers” (i.e. precursors) for ECs, although their high incidence in biopsies (>40% of histologically normal PE) clearly indicates that most do not progress to EC, and do not represent a critical rate-limiting step in endometrial carcinogenesis35. That such Pten-null glands are indeed very early precursors to AH/EIN and cancer is an appealing idea, and a recent serial genomic analysis of early EC progression provided additional support for this notion. In some patients the same point mutations were identified in the pre-precancerous lesions as in the eventual endometrioid adenocarcinomas, many years apart20. The possibility that Pax2-null glands in normal endometria represent precursors also seems likely, although interestingly, Pax2 and Pten loss do not generally occur in overlapping patterns in normal endometria35. While the presence of rare Pax2 or Pten null glands is an important fact to be aware of, it does not greatly limit the utility of either marker in the diagnosis in AH/EIN. This is because the sporadic loss in normal endometria occurs in very rare and isolated glands, usually just 1 or a few in an entire section (<1% of the sample), whereas AH/EIN are typified by loss over large areas comprising >>5% of the sample. Thus the patterns of Pax2 and Pten loss in normal endometria and AH/EIN are quite different and usually resolvable. However, loss in the 1–5% range should be interpreted with caution and in the context of other histologic features and sampling (Fig. 4C, D).
In summary, in addition to refining specific criteria for scoring these markers, our findings demonstrated that a panel of only 3 markers (Pax2, Pten, or β-catenin) had optimal performance characteristics and is practical, feasible, efficient, and of considerable utility in the diagnosis of AH/EIN77. Also, the approach serves as a useful template for assessing any additional markers that may be discovered or further considered in the future78.
Other benefits to the general use of the Pax2/Pten/β-catenin panel
Most cases of AH/EIN can be confidently diagnosed without the use of immunostains. Nonetheless, there should be considerable benefit to the routine use of the 3-marker AH/EIN biomarker panel. First, routine use of the panel will help pathologists refine their diagnostic accuracy and skills48. Second, and more importantly, many women with AH/EIN undergo conservative management with long-term progestin administration. This necessitates routine surveillance with repeat endometrial samplings, and yet, progestin profoundly masks the histologic features of AH/EIN, making surveillance difficult in practice79, 80. A large recent longitudinal investigation of Pax2 and Pten expression patterns in serial biopsies from women treated with progestin found that expression patterns in pretreatment AH/EIN were consistently recapitulated by AH/EIN present following treatment78. β-catenin patterns are also likely to be recapitulated following treatment20. Thus, in addition to facilitating the initial diagnosis of AH/EIN, establishment of baseline expression patterns should be useful diagnostically in follow-up biopsies in the setting of progestin treatment. Additional investigations will be needed to determine the incidence and patterns of marker aberrance in mimics of AH/EIN, including endometrial polyps, DPE, or non-atypical hyperplasia. The utility of the IHC panel in the diagnosis of AH/EIN in progestin-treated endometrial specimens is discussed in another article in this issue (see Chang et al).
In addition, the diagnosis of AH/EIN is challenging in other situations, such as minute lesions of questionable significance, secretory AH/EIN, AH/EIN within endometrial polyps or in the background of polyps (including atypical polypoid adenomyoma). Pathologists often find it difficult to diagnose AH/EIN when secretory change is present because 1) glandular crowding criteria are difficult to apply since glandular crowding is a feature of secretory endometrium; and 2) under the influence of high circulating levels of progesterone/progestins, nuclear atypia is less prominent or absent81, especially when the background endometrium is also secretory type. The diagnosis of AH/EIN within a polyp or polyp background remains difficult in that both glandular crowding and metaplasia are common in benign endometrial polyps. Thus, in practice it is often difficult to exclude endometrial polyp, or reliably identify AH/EIN in a polyp. In these diverse and challenging situations, the 3-marker panel is particularly useful (Figs. 5, 6).
Figure 5. Aberrant patterns of expression in AH/EIN within an endometrial polyp.
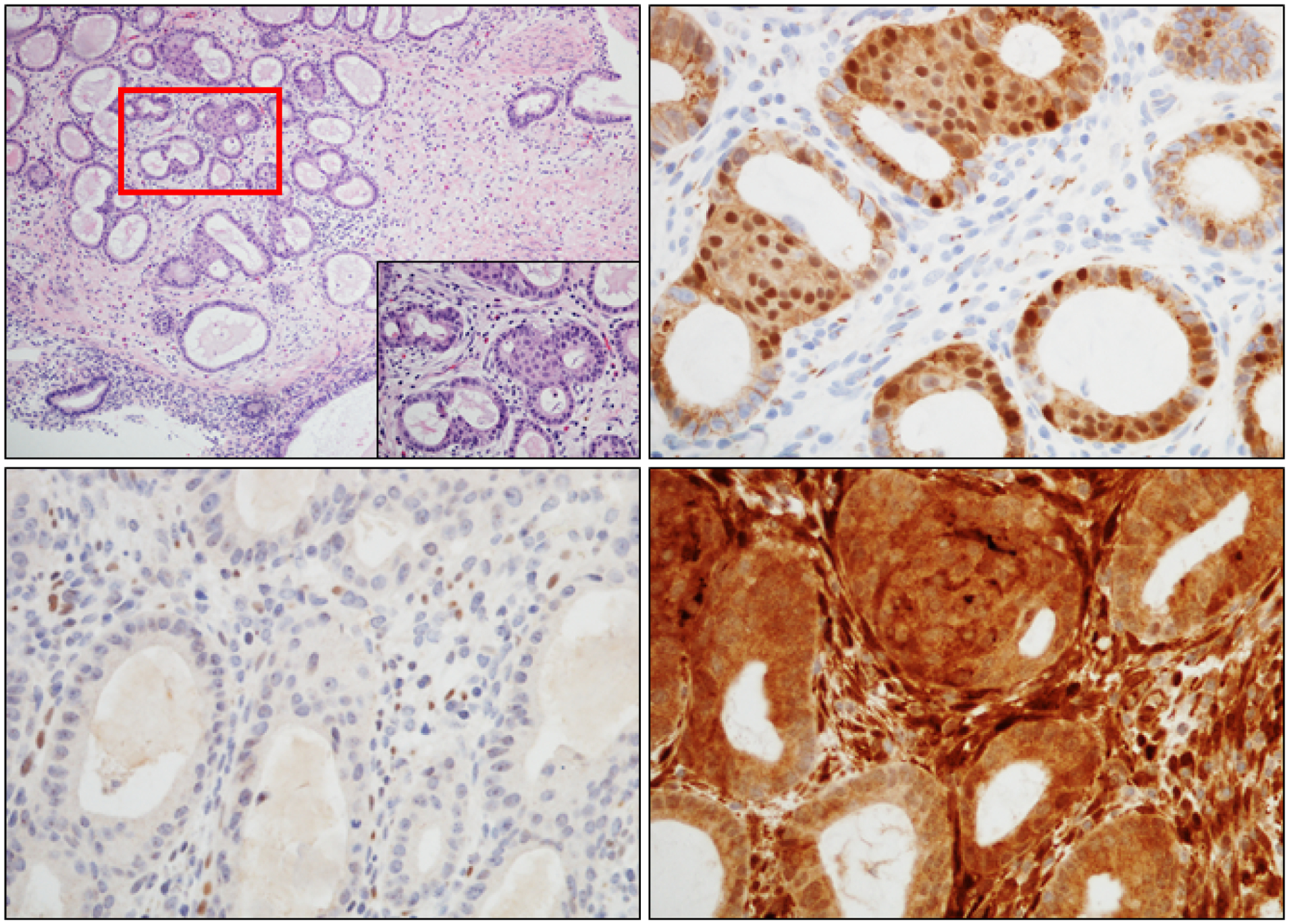
A) H&E, inset=higher magnification of boxed area; background glands are located at the periphery. B) β-catenin nuclear staining in both glandular and squamous morule components of AH/EIN. C) Loss of Pax2 expression. Intact expression in stromal cells serves as good internal control; D) Intact expression of Pten. Thus, the 3-marker panel supports the diagnosis of AH/EIN despite modest gland crowding (Pax2 and β-catenin aberrant) and the aberrancy of 2 markers enhances diagnostic confidence.
Figure 6. Aberrant patterns of expression in secretory AH/EIN.
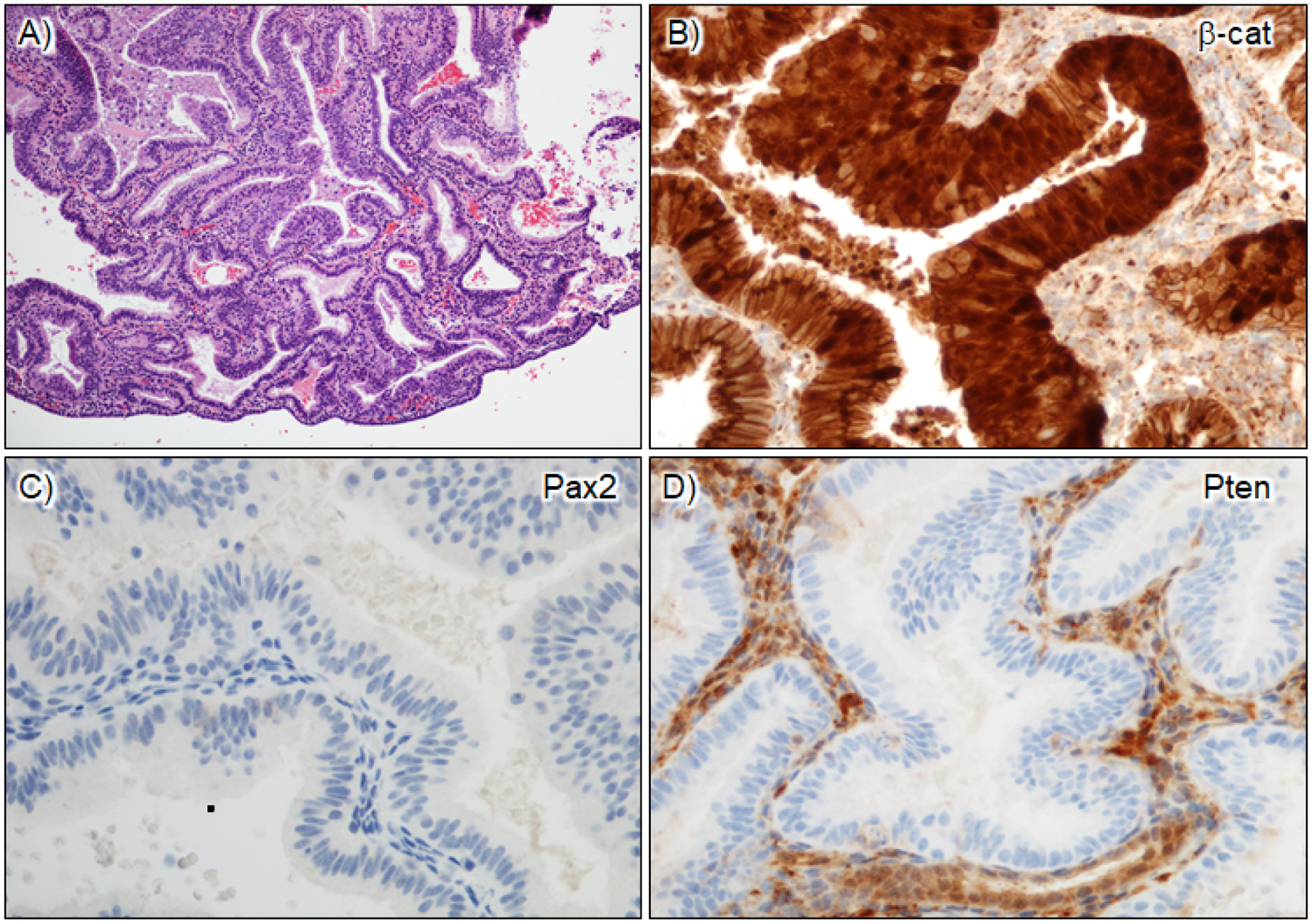
A) H&E. B) β-catenin nuclear staining in AH/EIN; strong overexpression is also observed. C) Loss of Pax2 expression; D) Loss of Pten expression. Intact expression in stromal cells serves as an internal control. This case is thus Pax2/Pten aberrant.
In closing, we present a case (Fig. 7) that—though unusual—illustrates the power of the 3-marker panel in providing more refined views of AH/EIN. In this initial biopsy, the 3 marker panel yielded unexpected results, revealing 2 distinct EINs. In the first, Pax2 and Pten were lost, but β-catenin was wild-type (membrane-associated). In the second, Pax2 and Pten were retained but β-catenin was diffusely mutant with strong overexpression/nuclear localization. Intriguingly, the 2 EINs were histologically distinct, with the second exhibiting less impressive architectural features. These distinct EINs cannot be readily explained as sharing a common precursor, suggesting that the 2 EINs are likely to be clonally independent.
Figure 7. An unusual endometrial biopsy harboring 2 separate AH/EIN lesions with entirely distinct biomarker profiles and different histologic features.
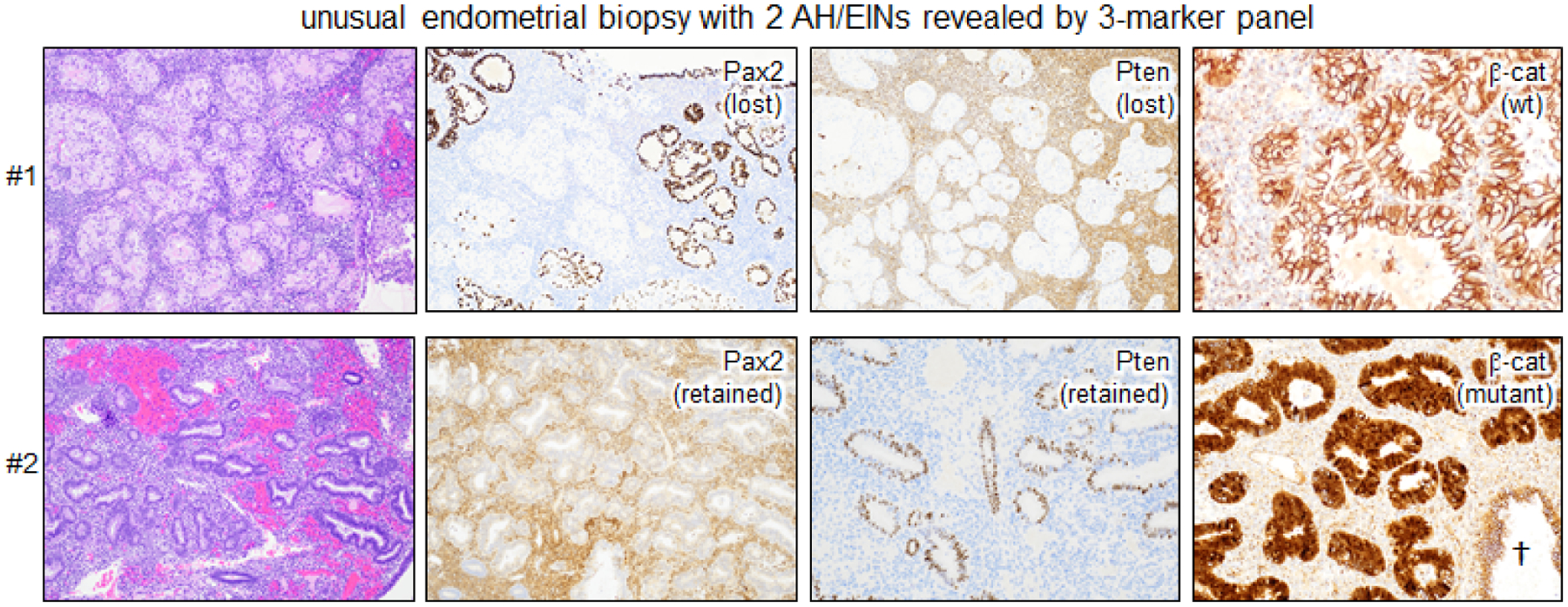
†=normal entrapped gland with wild-type membranous pattern of β-catenin. EIN #1 is Pax2 and Pten aberrant, whereas EIN #2 is β-catenin aberrant.
Strengths and limitations of AH vs. EIN schema: a reassessment.
Although there has been some conciliation of the AH and EIN systems, at least in the recent WHO classifications, they do have different conceptual underpinnings and debate will continue as to their relative merits. On one hand, the EIN approach is pragmatic in that it seeks to provide a defined cutoff to distinguish benign from precancerous/neoplastic in a manner aligned to clinical decision making (i.e. no follow-up needed vs. extensive follow-up and/or treatment). Indeed, the EIN system is geared towards providing an optimal definition of this cutoff based on a precise percentage, currently set at >1:1 gland:stroma ratio. Even if the 50% (1:1) cutoff is the most practical from a diagnostic and clinical perspective, what comprises an EC precancer must be more nuanced. For most cancer types, high-grade in situ precancers (i.e. ductal carcinoma of the breast, prostatic intraepithelial neoplasia, pancreatic intraepithelial neoplasia, etc.) already harbor most of the mutations evident in their frankly invasive malignant counterparts82–84. This is also the case in AH/EIN, which harbor a similar spectrum of mutations as EC36, 37, 85, 86. Thus, AH/EINs must progress from “pre-precancer” precursors, which could include Pten or Pax2 null but morphologically normal glands, or other intermediates, pointing to practical limits of purely histologic evaluation. Also, we often encounter cases that are subdiagnostic for EIN, but raise considerable suspicion for a significant lesion. In some cases, there are diffuse architectural changes that may not fit well into the conception of a focal or clonal EIN, resulting in considerable diagnostic ambiguity.
Such pre-precancerous lesions might be found among DPEs and hyperplasias without atypia, and there is already considerable evidence for this. First, the work of Mutter identified “latent precancers” that are entirely normal histologically yet harbor mutations in PTEN, leading to its apparently complete genetic inactivation. We recently performed a retrospective analysis of women who developed EC but for whom preceding biopsies were available, including at least one prior to the histologic diagnosis of AH/EIN. In most cases, ≥1 mutation present in the invasive cancer could be identified in biopsies preceding AH/EIN, and in most such cases, at least ≥1 mutation could be confirmed by IHC to Pten, Arid1a, β-catenin or Mlh134. Also, “focal gland crowding” that is diagnostically ambiguous and/or falls short of diagnostic criteria for AH/EIN has been identified as an endometrial biopsy finding associated with a significant risk of AH/EIN or carcinoma in subsequent biopsies (23% and 4% respectively with 1.5 year average follow-up)7. This finding further supports the idea that there is a currently ill-defined subset of endometrial biopsies that fall short of AH/EIN but nonetheless carry a significant risk of subsequent neoplasia.
Thus, the AH system remains appealing in that it embraces the notion that there is in fact a continuum of cancer progression risk based on histologic and/or molecular features. The diagnosis of EIN on a biopsy signifies a 45-fold increase in the risk of EC >1 year after the diagnosis of EIN (excluding cases likely to have concurrent EC and EIN)11. However, 45x may be too stringent a criterion that risks not identifying patients with lower, but still substantial risk of EC. It would be desirable to be able to perform better risk stratification in the evaluation of endometrial biopsies and identify women with a lower but still significant risk of EC (e.g. 10–30x).
Early forms of endometrial serous and clear cell carcinoma.
Serous endometrial intraepithelial carcinoma (SEIC) is a recognized entity related to endometrial serous carcinoma (ESC). SEIC epithelium shows cytological features typical of serous carcinoma (marked nucleomegaly and pleomorphism) and p53 mutant patterns by IHC characteristic of ESC. SEIC may represent an early variant of ESC, as SEIC lesions are typically relatively small with less extensive involvement of the endometrium than ESC. However, SEIC is strongly associated with extra-uterine spread and should be considered as a malignancy even when invasion is not identified87–90. Thus, in the WHO 2020 classification, SEIC is considered as early stage serous carcinoma. Zheng et al proposed that ESC is preceded by a sequence similar to that of tubo-ovarian high-grade serous carcinoma [endometrial p53 signature lesion→endometrial glandular dysplasia (EmGD)→SEIC→ESC]89, 91, 92. In this sequence. EmGD is most likely an earlier precancer of SEIC/ESC93–98. EmGD is usually found adjacent to ESC and SEIC, and only rarely associated with endometrioid adenocarcinoma99. It is defined by endometrial glands with nuclear atypia falling short of SEIC, but distinct compared to background endometrium. In addition, it has an IHC profile bridging benign resting endometrium and SEIC (i.e. p53 and p16 diffusely strongly positive, increased IMP3 expression, and reduced ER/PR expression).
Similarly, a spectrum of atypical endometrial glandular and surface changes is often found adjacent to clear cell carcinoma. The atypical glands and surface epithelia are cytologically distinct from the background benign endometrium and the adjacent endometrial clear cell carcinoma. Lining epithelia show clear cytoplasm, moderate nuclear atypia (less than clear cell carcinoma) and occasional hobnailing. Fadare et al have proposed the term “clear cell EmGD” as the precancer of endometrial clear cell carcinoma100. More research is needed to better define the histologic and molecular features of early precancers for non-endometrioid ECs including clear cell, serous, and carcinosarcomas.
Future direction 1: Use of next-generation sequencing (NGS) for refining the diagnosis of endometrial precancer.
NGS assays have become standard-of-care, including circulating tumor DNA assays that highlight the extraordinary sensitivity of such assays and their ability to detect rare mutations. NGS on selected endometrial biopsies might someday be useful in clinical practice, e.g. to 1) establish a lesion as a bona fide precancer signifying an increased cancer risk, 2) identify class-defining mutations such as POLE or in MMR factors earlier in clinical progression, which could further guide management, and 3) diagnose hereditary cancer syndromes earlier, which would trigger earlier surveillance and further enhance clinical management. One major challenge would be the need to establish thresholds capable of distinguishing age-related mutations i.e. in PTEN, which clearly occur in normal aging endometria101. Other and arguably even more ambitious approaches have included NGS analysis of lavage specimens during hysteroscopy, or pap smears102, 103. Much more research is needed to establish the utility and practicability of such approaches with respect to patient management and assess their cost-effectiveness8, 104.
Future direction 2: Image analysis-aided assessment of endometrial biopsies.
There is a long history of investigations of quantitative or computerized morphometric image analysis in the assessment of normal, hyperplastic, and malignant glands in the endometrium105, 106. Indeed, quantitative image analysis of diverse potential classifiers led to the identification of volume percentage stroma as the best predictor of progression to adenocarcinoma107. Efforts to refine such image analysis methods continue108 and are likely to accelerate with the advent of deep learning/artificial intelligence methodologies for pathology image analysis109. The key questions are if 1) such algorithms can be efficiently used by pathologists or 2) if there are limits of even refined computational analysis, such that other methods like biomarker panels or NGS that add layers of non-morphologic information will ultimately prove more valuable.
Future direction 3: Refinement of 3-marker panel.
While the 3-marker panel (Pax2, Pten, β-catenin) is of demonstrated utility in the diagnosis of AH/EIN77, future refinements can be envisioned. The 3-marker panel detects >90% of AH/EIN but raising the sensitivity even further is desirable, so long as the panel remains “compact” and specificity is not sacrificed. There may be markers yet to be discovered that operate on new principles, or detect aberrancy of other signaling pathways involved in EC genesis. Alternatively, future markers may be identified that more reliably detect aberrations in their respective pathways than e.g. Pten or β-catenin.
Conclusion
The accurate diagnosis of AH/EIN remains a common challenge frequently encountered by pathologists in their daily clinical practice. Reflecting these challenges, diagnostic schema based on histologic features have evolved considerably in the last 25 years. We believe that the recent adoption of select biomarkers, as introduced in WHO 2020 and as reinforced by studies showing the utility of the 3-marker panel (Pax2, Pten, β-catenin), also represent a significant advance. Our understanding of the molecular pathways and aberrations characterizing early endometrial cancers/precancers/precursors is rapidly moving forward, and these future insights should be continually applied to refine diagnostic approaches and improve risk stratification for this common premalignancy of women.
Highlights.
Criteria for the diagnosis of endometrial precancers has evolved with each iteration of WHO guidelines, incorporating concepts and terminology from 4- and 2-tier systems
Despite refined histologic criteria, diagnosis of atypical hyperplasia/endometrioid intraepithelial neoplasia (AH/EIN) remains a common diagnostic dilemma faced by pathologists
In WHO 2020, for the first time, use of biomarkers was specified as desirable
A panel consisting of three biomarkers—Pax2, Pten, and β-catenin—has demonstrated utility in the diagnosis of AH/EIN
Additional methods such as image or molecular analysis of endometrial biopsies represent future research directions in the refined diagnosis of AH/EIN with improved risk stratification
Abbreviations
- AH
atypical hyperplasia
- DPE
disordered proliferative endometrium
- EC
endometrial cancer
- EmGD
endometrial glandular dysplasia
- ESC
endometrial serous cancer
- EIN
endometrioid intraepithelial neoplasia
- IHC
immunohistochemistry
- MMR
mismatch repair
- NGS
next generation sequencing
- PE
proliferative endometrium
- SEIC
serous endometrial intraepithelial carcinoma
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 3.Constantine GD, Kessler G, Graham S, Goldstein SR. Increased Incidence of Endometrial Cancer Following the Women’s Health Initiative: An Assessment of Risk Factors. J Womens Health (Larchmt). 2019;28:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Gong TT, Liu FH, et al. Global, Regional, and National Burden of Endometrial Cancer, 1990–2017: Results From the Global Burden of Disease Study, 2017. Front Oncol. 2019;9:1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allison KH, Reed SD, Voigt LF, Jordan CD, Newton KM, Garcia RL. Diagnosing endometrial hyperplasia: why is it so difficult to agree? Am J Surg Pathol. 2008;32:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanderson PA, Critchley HO, Williams AR, Arends MJ, Saunders PT. New concepts for an old problem: the diagnosis of endometrial hyperplasia. Hum Reprod Update. 2017;23:232–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang EC, Mutter GL, Crum CP, Nucci MR. Clinical outcome in diagnostically ambiguous foci of ‘gland crowding’ in the endometrium. Mod Pathol. 2010;23:1486–1491. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health. 2007;10:183–194. [DOI] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research N, Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403–412. [DOI] [PubMed] [Google Scholar]
- 11.Jarboe EA, Mutter GL. Endometrial intraepithelial neoplasia. Semin Diagn Pathol. 2010;27:215–225. [DOI] [PubMed] [Google Scholar]
- 12.Baak JP, Mutter GL. EIN and WHO94. J Clin Pathol. 2005;58:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutter GL. Endometrial intraepithelial neoplasia (EIN): will it bring order to chaos? The Endometrial Collaborative Group. Gynecol Oncol. 2000;76:287–290. [DOI] [PubMed] [Google Scholar]
- 14.Hecht JL, Ince TA, Baak JPA, Baker HE, Ogden MW, Mutter GL. Prediction of endometrial carcinoma by subjective endometrial intraepithelial neoplasia diagnosis. Modern Pathology. 2005;18:324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baak JP, Mutter GL, Robboy S, et al. The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer. 2005;103:2304–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurman RJ, Carcangiu ML, Young RH, Herrington CS. WHO Classification of Tumours of Female Reproductive Organs. 4th ed. Lyon, France: International Agency for Research on Cancer; 2014. [Google Scholar]
- 17.Mutter GL, Zaino RJ, Baak JP, Bentley RC, Robboy SJ. Benign endometrial hyperplasia sequence and endometrial intraepithelial neoplasia. Int J Gynecol Pathol. 2007;26:103–114. [DOI] [PubMed] [Google Scholar]
- 18.Carlson JW, Mutter GL. Endometrial intraepithelial neoplasia is associated with polyps and frequently has metaplastic change. Histopathology. 2008;53:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCluggage WG. My approach to the interpretation of endometrial biopsies and curettings. J Clin Pathol. 2006;59:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguilar M, Zhang H, Zhang M, et al. Serial genomic analysis of endometrium supports the existence of histologically indistinct endometrial cancer precursors. J Pathol. 2021;254:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine DA, Getz G, Gabriel SB, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell DW, Ellenson LH. Molecular Genetics of Endometrial Carcinoma. Annu Rev Pathol. 2019;14:339–367. [DOI] [PubMed] [Google Scholar]
- 23.McAlpine J, Leon-Castillo A, Bosse T. The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J Pathol. 2018;244:538–549. [DOI] [PubMed] [Google Scholar]
- 24.Jamieson A, Bosse T, McAlpine JN. The emerging role of molecular pathology in directing the systemic treatment of endometrial cancer. Ther Adv Med Oncol. 2021;13:17588359211035959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jovanovic AS, Boynton KA, Mutter GL. Uteri of women with endometrial carcinoma contain a histopathological spectrum of monoclonal putative precancers, some with microsatellite instability. Cancer Res. 1996;56:1917–1921. [PubMed] [Google Scholar]
- 26.Mutter GL, Ince TA, Baak JP, Kust GA, Zhou XP, Eng C. Molecular identification of latent precancers in histologically normal endometrium. Cancer Res. 2001;61:4311–4314. [PubMed] [Google Scholar]
- 27.Moreno-Bueno G, Hardisson D, Sarrio D, et al. Abnormalities of E- and P-cadherin and catenin (beta-, gamma-catenin, and p120ctn) expression in endometrial cancer and endometrial atypical hyperplasia. J Pathol. 2003;199:471–478. [DOI] [PubMed] [Google Scholar]
- 28.Levine RL, Cargile CB, Blazes MS, van Rees B, Kurman RJ, Ellenson LH. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Res. 1998;58:3254–3258. [PubMed] [Google Scholar]
- 29.Maxwell GL, Risinger JI, Gumbs C, et al. Mutation of the PTEN tumor suppressor gene in endometrial hyperplasias. Cancer Res. 1998;58:2500–2503. [PubMed] [Google Scholar]
- 30.Duggan BD, Felix JC, Muderspach LI, Tsao JL, Shibata DK. Early mutational activation of the c-Ki-ras oncogene in endometrial carcinoma. Cancer Res. 1994;54:1604–1607. [PubMed] [Google Scholar]
- 31.Mutter GL, Wada H, Faquin WC, Enomoto T. K-ras mutations appear in the premalignant phase of both microsatellite stable and unstable endometrial carcinogenesis. Mol Pathol. 1999;52:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki H, Nishii H, Takahashi H, et al. Mutation of the Ki-ras protooncogene in human endometrial hyperplasia and carcinoma. Cancer Res. 1993;53:1906–1910. [PubMed] [Google Scholar]
- 33.Werner HM, Berg A, Wik E, et al. ARID1A loss is prevalent in endometrial hyperplasia with atypia and low-grade endometrioid carcinomas. Mod Pathol. 2013;26:428–434. [DOI] [PubMed] [Google Scholar]
- 34.Aguilar M, Zhang H, Zhang M, et al. Serial genomic analysis of endometrium supports the existence of histologically indistinct endometrial cancer precursors. J Pathol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monte NM, Webster KA, Neuberg D, Dressler GR, Mutter GL. Joint loss of PAX2 and PTEN expression in endometrial precancers and cancer. Cancer Res. 2010;70:6225–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Yue P, Song Q, et al. Genome-wide mutation analysis in precancerous lesions of endometrial carcinoma. J Pathol. 2021;253:119–128. [DOI] [PubMed] [Google Scholar]
- 37.Russo M, Broach J, Sheldon K, et al. Clonal evolution in paired endometrial intraepithelial neoplasia/atypical hyperplasia and endometrioid adenocarcinoma. Hum Pathol. 2017;67:69–77. [DOI] [PubMed] [Google Scholar]
- 38.Chapel DB, Patil SA, Plagov A, et al. Quantitative next-generation sequencing-based analysis indicates progressive accumulation of microsatellite instability between atypical hyperplasia/endometrial intraepithelial neoplasia and paired endometrioid endometrial carcinoma. Mod Pathol. 2019;32:1508–1520. [DOI] [PubMed] [Google Scholar]
- 39.Rabban JT, McAlhany S, Lerwill MF, Grenert JP, Zaloudek CJ. PAX2 distinguishes benign mesonephric and mullerian glandular lesions of the cervix from endocervical adenocarcinoma, including minimal deviation adenocarcinoma. Am J Surg Pathol. 2010;34:137–146. [DOI] [PubMed] [Google Scholar]
- 40.Joiner AK, Quick CM, Jeffus SK. Pax2 expression in simultaneously diagnosed WHO and EIN classification systems. Int J Gynecol Pathol. 2015;34:40–46. [DOI] [PubMed] [Google Scholar]
- 41.Owings RA, Quick CM. Endometrial intraepithelial neoplasia. Arch Pathol Lab Med. 2014;138:484–491. [DOI] [PubMed] [Google Scholar]
- 42.Quick CM, Laury AR, Monte NM, Mutter GL. Utility of PAX2 as a marker for diagnosis of endometrial intraepithelial neoplasia. Am J Clin Pathol. 2012;138:678–684. [DOI] [PubMed] [Google Scholar]
- 43.Strickland AL, Rivera G, Lucas E, John G, Cuevas I, Castrillon DH. PI3K Pathway Effectors pAKT and FOXO1 as Novel Markers of Endometrioid Intraepithelial Neoplasia. Int J Gynecol Pathol. 2018. [DOI] [PubMed] [Google Scholar]
- 44.Raffone A, Travaglino A, Saccone G, et al. PAX2 in endometrial carcinogenesis and in differential diagnosis of endometrial hyperplasia: A systematic review and meta-analysis of diagnostic accuracy. Acta Obstet Gynecol Scand. 2019;98:287–299. [DOI] [PubMed] [Google Scholar]
- 45.Wu H, Chen Y, Liang J, et al. Hypomethylation-linked activation of PAX2 mediates tamoxifen-stimulated endometrial carcinogenesis. Nature. 2005;438:981–987. [DOI] [PubMed] [Google Scholar]
- 46.Liu T, Wang Y, Wang Y, Chan AM. Multifaceted Regulation of PTEN Subcellular Distributions and Biological Functions. Cancers (Basel). 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mutter GL, Lin MC, Fitzgerald JT, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–930. [DOI] [PubMed] [Google Scholar]
- 48.Nucci MR, Castrillon DH, Bai H, et al. Biomarkers in diagnostic obstetric and gynecologic pathology: a review. Adv Anat Pathol. 2003;10:55–68. [DOI] [PubMed] [Google Scholar]
- 49.Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16–41. [DOI] [PubMed] [Google Scholar]
- 50.Li HD, Lu C, Zhang H, et al. A PoleP286R mouse model of endometrial cancer recapitulates high mutational burden and immunotherapy response. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao C, Wang Y, Broaddus R, Sun L, Xue F, Zhang W. Exon 3 mutations of CTNNB1 drive tumorigenesis: a review. Oncotarget. 2018;9:5492–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costigan DC, Dong F, Nucci MR, Howitt BE. Clinicopathologic and Immunohistochemical Correlates of CTNNB1 Mutated Endometrial Endometrioid Carcinoma. Int J Gynecol Pathol. 2020;39:119–127. [DOI] [PubMed] [Google Scholar]
- 53.Kim G, Kurnit KC, Djordjevic B, et al. Nuclear beta-catenin localization and mutation of the CTNNB1 gene: a context-dependent association. Mod Pathol. 2018;31:1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright MF, Fitzlaff S, Wyeth A, et al. Nuclear Beta-Catenin Expression in Endometrioid Intraepithelial Neoplasia (Atypical Hyperplasia) Does Not Predict Carcinoma on Subsequent Hysterectomy. Int J Gynecol Pathol. 2021;40:240–247. [DOI] [PubMed] [Google Scholar]
- 55.Kurnit KC, Kim GN, Fellman BM, et al. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod Pathol. 2017;30:1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nei H, Saito T, Yamasaki H, Mizumoto H, Ito E, Kudo R. Nuclear localization of beta-catenin in normal and carcinogenic endometrium. Mol Carcinog. 1999;25:207–218. [PubMed] [Google Scholar]
- 57.Saegusa M, Okayasu I. Frequent nuclear beta-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J Pathol. 2001;194:59–67. [DOI] [PubMed] [Google Scholar]
- 58.Travaglino A, Raffone A, Saccone G, et al. Nuclear expression of beta-catenin in endometrial hyperplasia as marker of premalignancy. APMIS. 2019;127:699–709. [DOI] [PubMed] [Google Scholar]
- 59.Strickland AL, Rivera G, Lucas E, John G, Cuevas I, Castrillon DH. PI3K Pathway Effectors pAKT and FOXO1 as Novel Markers of Endometrioid Intraepithelial Neoplasia. Int J Gynecol Pathol. 2019;38:503–513. [DOI] [PubMed] [Google Scholar]
- 60.Brachtel EF, Sanchez-Estevez C, Moreno-Bueno G, Prat J, Palacios J, Oliva E. Distinct molecular alterations in complex endometrial hyperplasia (CEH) with and without immature squamous metaplasia (squamous morules). Am J Surg Pathol. 2005;29:1322–1329. [DOI] [PubMed] [Google Scholar]
- 61.Okoye EI, Bruegl AS, Fellman B, Luthra R, Broaddus RR. Defective DNA Mismatch Repair Influences Expression of Endometrial Carcinoma Biomarkers. Int J Gynecol Pathol. 2016;35:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu KH, Broaddus RR. Endometrial Cancer. N Engl J Med. 2020;383:2053–2064. [DOI] [PubMed] [Google Scholar]
- 63.Bartley AN, Luthra R, Saraiya DS, Urbauer DL, Broaddus RR. Identification of cancer patients with Lynch syndrome: clinically significant discordances and problems in tissue-based mismatch repair testing. Cancer Prev Res (Phila). 2012;5:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lucas E, Chen H, Molberg K, et al. Mismatch Repair Protein Expression in Endometrioid Intraepithelial Neoplasia/Atypical Hyperplasia: Should We Screen for Lynch Syndrome in Precancerous Lesions? Int J Gynecol Pathol. 2019;38:533–542. [DOI] [PubMed] [Google Scholar]
- 65.Vierkoetter KR, Kagami LA, Ahn HJ, Shimizu DM, Terada KY. Loss of Mismatch Repair Protein Expression in Unselected Endometrial Adenocarcinoma Precursor Lesions. Int J Gynecol Cancer. 2016;26:228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goodfellow PJ, Billingsley CC, Lankes HA, et al. Combined Microsatellite Instability, MLH1 Methylation Analysis, and Immunohistochemistry for Lynch Syndrome Screening in Endometrial Cancers From GOG210: An NRG Oncology and Gynecologic Oncology Group Study. J Clin Oncol. 2015;33:4301–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huvila J, Pors J, Thompson EF, Gilks CB. Endometrial carcinoma: molecular subtypes, precursors and the role of pathology in early diagnosis. J Pathol. 2021;253:355–365. [DOI] [PubMed] [Google Scholar]
- 68.Hampel H, Frankel W, Panescu J, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810–7817. [DOI] [PubMed] [Google Scholar]
- 69.Ollikainen M, Abdel-Rahman WM, Moisio AL, et al. Molecular analysis of familial endometrial carcinoma: a manifestation of hereditary nonpolyposis colorectal cancer or a separate syndrome? J Clin Oncol. 2005;23:4609–4616. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Hoang L, Ji JX, Huntsman DG. SWI/SNF Complex Mutations in Gynecologic Cancers: Molecular Mechanisms and Models. Annu Rev Pathol. 2020;15:467–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mao TL, Ardighieri L, Ayhan A, et al. Loss of ARID1A expression correlates with stages of tumor progression in uterine endometrioid carcinoma. Am J Surg Pathol. 2013;37:1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ayhan A, Mao TL, Suryo Rahmanto Y, et al. Increased proliferation in atypical hyperplasia/endometrioid intraepithelial neoplasia of the endometrium with concurrent inactivation of ARID1A and PTEN tumour suppressors. J Pathol Clin Res. 2015;1:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raffone A, Travaglino A, Saccone G, et al. Diagnostic and prognostic value of ARID1A in endometrial hyperplasia: a novel marker of occult cancer. APMIS. 2019;127:597–606. [DOI] [PubMed] [Google Scholar]
- 74.Kobel M, Ronnett BM, Singh N, Soslow RA, Gilks CB, McCluggage WG. Interpretation of P53 Immunohistochemistry in Endometrial Carcinomas: Toward Increased Reproducibility. Int J Gynecol Pathol. 2019;38 Suppl 1:S123–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Georgescu TA, Cirstoiu M, Costache M, Lazaroiu A, Dumitru A, Sajin M. Histopathological, Immunohistochemical and Therapeutical Assessment of Premalignant Endometrial Lesions in a Hospital Based Series of Cases. Maedica (Bucur). 2016;11:115–121. [PMC free article] [PubMed] [Google Scholar]
- 76.Mutter GL, Lax SF. Endometrial atypical hyperplasia / endometrioid intraepithelial neoplasia. In: WHO Classification of Tumours Editorial Board FGT, ed. WHO Classification of Tumours Series, 5th edition. Vol 4. 5th ed. Lyon, France: International Agency for Research on Cancer; 2020:250–251. [Google Scholar]
- 77.Aguilar M, Chen H, Rivera-Colon G, et al. Reliable Identification of Endometrial Precancers Through Combined Pax2, beta-Catenin, and Pten Immunohistochemistry. Am J Surg Pathol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen H, Lucas E, Strickland AL, et al. Specific Biomarker Expression Patterns in the Diagnosis of Residual and Recurrent Endometrial Precancers After Progestin Treatment: A Longitudinal Study. Am J Surg Pathol. 2020;44:1429–1439. [DOI] [PubMed] [Google Scholar]
- 79.Wheeler DT, Bristow RE, Kurman RJ. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol. 2007;31:988–998. [DOI] [PubMed] [Google Scholar]
- 80.Zaino RJ, Brady WE, Todd W, et al. Histologic effects of medroxyprogesterone acetate on endometrioid endometrial adenocarcinoma: a Gynecologic Oncology Group study. Int J Gynecol Pathol. 2014;33:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deligdisch L Hormonal pathology of the endometrium. Mod Pathol. 2000;13:285–294. [DOI] [PubMed] [Google Scholar]
- 82.Khani F, Wobker SE, Hicks JL, et al. Intraductal carcinoma of the prostate in the absence of high-grade invasive carcinoma represents a molecularly distinct type of in situ carcinoma enriched with oncogenic driver mutations. J Pathol. 2019;249:79–89. [DOI] [PubMed] [Google Scholar]
- 83.Hata T, Suenaga M, Marchionni L, et al. Genome-Wide Somatic Copy Number Alterations and Mutations in High-Grade Pancreatic Intraepithelial Neoplasia. Am J Pathol. 2018;188:1723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Casasent AK, Edgerton M, Navin NE. Genome evolution in ductal carcinoma in situ: invasion of the clones. J Pathol. 2017;241:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gibson WJ, Hoivik EA, Halle MK, et al. The genomic landscape and evolution of endometrial carcinoma progression and abdominopelvic metastasis. Nat Genet. 2016;48:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Yu M, Yang JX, et al. Genomic Comparison of Endometrioid Endometrial Carcinoma and Its Precancerous Lesions in Chinese Patients by High-Depth Next Generation Sequencing. Front Oncol. 2019;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hou JY, McAndrew TC, Goldberg GL, Whitney K, Shahabi S. A clinical and pathologic comparison between stage-matched endometrial intraepithelial carcinoma and uterine serous carcinoma: is there a difference? Reproductive sciences. 2014;21:532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soslow RA, Pirog E, Isacson C. Endometrial intraepithelial carcinoma with associated peritoneal carcinomatosis. Am J Surg Pathol. 2000;24:726–732. [DOI] [PubMed] [Google Scholar]
- 89.Zheng W, Xiang L, Fadare O, Kong B. A proposed model for endometrial serous carcinogenesis. Am J Surg Pathol. 2011;35:e1–e14. [DOI] [PubMed] [Google Scholar]
- 90.Baergen RN, Warren CD, Isacson C, Ellenson LH. Early uterine serous carcinoma: clonal origin of extrauterine disease. Int J Gynecol Pathol. 2001;20:214–219. [DOI] [PubMed] [Google Scholar]
- 91.Fadare O, Zheng W. Insights into endometrial serous carcinogenesis and progression. Int J Clin Exp Pathol. 2009;2:411–432. [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X, Liang SX, Jia L, et al. Molecular identification of “latent precancers” for endometrial serous carcinoma in benign-appearing endometrium. Am J Pathol. 2009;174:2000–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liang SX, Chambers SK, Cheng L, Zhang S, Zhou Y, Zheng W. Endometrial glandular dysplasia: a putative precursor lesion of uterine papillary serous carcinoma. Part II: molecular features. International journal of surgical pathology. 2004;12:319–331. [DOI] [PubMed] [Google Scholar]
- 94.Zheng W, Liang SX, Yi X, Ulukus EC, Davis JR, Chambers SK. Occurrence of endometrial glandular dysplasia precedes uterine papillary serous carcinoma. Int J Gynecol Pathol. 2007;26:38–52. [DOI] [PubMed] [Google Scholar]
- 95.Jia L, Liu Y, Yi X, et al. Endometrial glandular dysplasia with frequent p53 gene mutation: a genetic evidence supporting its precancer nature for endometrial serous carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:2263–2269. [DOI] [PubMed] [Google Scholar]
- 96.Fadare O, Zheng W. Endometrial Glandular Dysplasia (EmGD): morphologically and biologically distinctive putative precursor lesions of Type II endometrial cancers. Diagnostic pathology. 2008;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yi X, Zheng W. Endometrial glandular dysplasia and endometrial intraepithelial neoplasia. Curr Opin Obstet Gynecol. 2008;20:20–25. [DOI] [PubMed] [Google Scholar]
- 98.Fadare O, Zheng W. Endometrial serous carcinoma (uterine papillary serous carcinoma): precancerous lesions and the theoretical promise of a preventive approach. American journal of cancer research. 2012;2:335–339. [PMC free article] [PubMed] [Google Scholar]
- 99.Zheng W, Liang SX, Yu H, Rutherford T, Chambers SK, Schwartz PE. Endometrial glandular dysplasia: a newly defined precursor lesion of uterine papillary serous carcinoma. Part I: morphologic features. International journal of surgical pathology. 2004;12:207–223. [DOI] [PubMed] [Google Scholar]
- 100.Fadare O, Liang SX, Ulukus EC, Chambers SK, Zheng W. Precursors of endometrial clear cell carcinoma. Am J Surg Pathol. 2006;30:1519–1530. [DOI] [PubMed] [Google Scholar]
- 101.Moore L, Leongamornlert D, Coorens THH, et al. The mutational landscape of normal human endometrial epithelium. Nature. 2020;580:640–646. [DOI] [PubMed] [Google Scholar]
- 102.Wang Y, Li L, Douville C, et al. Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci Transl Med. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nair N, Camacho-Vanegas O, Rykunov D, et al. Genomic Analysis of Uterine Lavage Fluid Detects Early Endometrial Cancers and Reveals a Prevalent Landscape of Driver Mutations in Women without Histopathologic Evidence of Cancer: A Prospective Cross-Sectional Study. PLoS Med. 2016;13:e1002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Medverd JR, Dubinsky TJ. Cost analysis model: US versus endometrial biopsy in evaluation of peri- and postmenopausal abnormal vaginal bleeding. Radiology. 2002;222:619–627. [DOI] [PubMed] [Google Scholar]
- 105.Diegenbach PC, Baak JP. Quantitative nuclear image analysis: differentiation between normal, hyperplastic, and malignant appearing uterine glands in a paraffin section. II. Computer assisted recognition by discriminant analysis. Eur J Obstet Gynecol Reprod Biol. 1977;7:389–394. [DOI] [PubMed] [Google Scholar]
- 106.Baak JP. The role of computerized morphometric and cytometric feature analysis in endometrial hyperplasia and cancer prognosis. J Cell Biochem Suppl. 1995;23:137–146. [DOI] [PubMed] [Google Scholar]
- 107.Baak JP, Nauta JJ, Wisse-Brekelmans EC, Bezemer PD. Architectural and nuclear morphometrical features together are more important prognosticators in endometrial hyperplasias than nuclear morphometrical features alone. J Pathol. 1988;154:335–341. [DOI] [PubMed] [Google Scholar]
- 108.Papke DJ, Jr., Lohmann S, Downing M, Hufnagl P, Mutter GL. Computational augmentation of neoplastic endometrial glands in digital pathology displays. J Pathol. 2021;253:258–267. [DOI] [PubMed] [Google Scholar]
- 109.Salvi M, Acharya UR, Molinari F, Meiburger KM. The impact of pre- and post-image processing techniques on deep learning frameworks: A comprehensive review for digital pathology image analysis. Comput Biol Med. 2021;128:104129. [DOI] [PubMed] [Google Scholar]


