Abstract
Background:
Immunotherapy, specifically immune checkpoint inhibitors (ICIs), including anti-programmed cell death 1 (anti-PD1), have recently received clinical approval for the treatment of adult hepatocellular carcinoma (HCC). However, the safety and efficacy of ICIs prior to solid organ transplant are unknown, especially in pediatrics. Safety reports are variable in adults, with some series describing subsequent allograft rejection and loss while others report successful transplants without allograft rejection. As ICIs stimulate the immune system by blocking the interaction between PD1 and the ligand-receptor pair programmed cell death-ligand 1 (PDL1), the downstream effects of T cell activation increase the risk of graft rejection.
Methods:
Here, we present a case of an adolescent with moderately differentiated non-fibrolamellar HCC treated with pembrolizumab, an anti-PD1 therapy, who subsequently underwent successful orthotopic liver transplantation (OLT).
Results:
Our patient received an OLT 138 days from the last pembrolizumab dose with graft preservation. The patient has no evidence of recurrent disease or any episode of allograft rejection 48 months post OLT. Staining of tumor and normal tissues from longitudinal specimens finds PDL1 positive Kupffer cells present in normal liver and peritumoral areas with no changes post anti-PD1 therapy. In contrast, tumor cells were negative for PDL1.
Conclusion:
This case represents a basis for optimism in potential use of anti-PD1 therapy in liver transplant candidates and supports further investigation of immune checkpoint inhibitors use in this unique patient population.
Keywords: adolescent, liver transplantation, immune checkpoint inhibitor, pediatric, pembrolizumab, anti-pd1
Introduction
Hepatocellular carcinoma (HCC) is a rare pediatric tumor. Therapeutic interventions in children include chemotherapy and surgical resection, but the overall 5-year survival rate is only 24% for the pediatric population.(1) Immunotherapy, specifically immune checkpoint inhibitors (ICIs), including anti-programmed cell death 1 (anti-PD1), has recently received clinical approval for the treatment of adult HCC with durable responses in at least 25% of patients having advanced disease.(2) A Phase 1 study evaluating the safety and efficacy of anti-PD1 therapy in children with programmed cell death-ligand 1 (PDL1) positive refractory solid tumors, including HCC, is ongoing.(3) In the interim analysis of the trial, pembrolizumab, an anti-PD1 agent, was well tolerated in children but showed rare anti-tumor efficacy as a single agent in most refractory solid tumors including HCC, suggesting monotherapy with pembrolizumab may not be sufficient.(3)
In organ transplant recipients or candidates for future transplantation, the safety and efficacy of ICIs are highly debatable. ICIs stimulate the immune system by blocking the interaction between PD1 and PDL1. The downstream effects of T cell activation increase the risk of graft rejection.(4) Here, we present a case of an adolescent with recurrent HCC after primary surgical resection and chemotherapy and treatment with pembrolizumab, who underwent successful orthotopic liver transplantation (OLT) 138 days from last dose of anti-PD1.
Case Report
A fourteen-year-old male presented with abdominal asymmetry and pain. Initial workup included abdominal magnetic resonance imaging (MRI) demonstrating a large mass confined to the liver, an alpha-fetoprotein (AFP) level of 36,876 ng/mL, and a liver biopsy revealing moderately differentiated non-fibrolamellar HCC. No extrahepatic disease was demonstrated on further imaging. He underwent three cycles of cisplatin/doxorubicin/dexrazoxane (PLADO) chemotherapy and drug-eluting bead transarterial chemoembolization (DEB-TACE) with doxorubicin. He subsequently had an extended left tri-segmentectomy after which AFP levels normalized (Figure 1). At 14 months, a follow-up MRI revealed recurrence with three new small hepatic nodules. AFP was 55. Biopsy demonstrated a moderately differentiated non-fibrolamellar HCC with tumor staining positive for PDL1. Patient then received three cycles of 2 mg/kg every 3 weeks of pembrolizumab (anti-PD1). He tolerated the therapy well with no side effects. Repeat MRI, however, revealed disease progression; therefore, pembrolizumab was discontinued. Subsequently, he was given another round of DEB-TACE. Despite decreased AFP levels, repeat imaging showed a small remaining hepatic lesion with no major vascular invasion and no extrahepatic involvement.
Figure 1: Timeline of Patient diagnosis, follow-up, and therapeutic interventions.
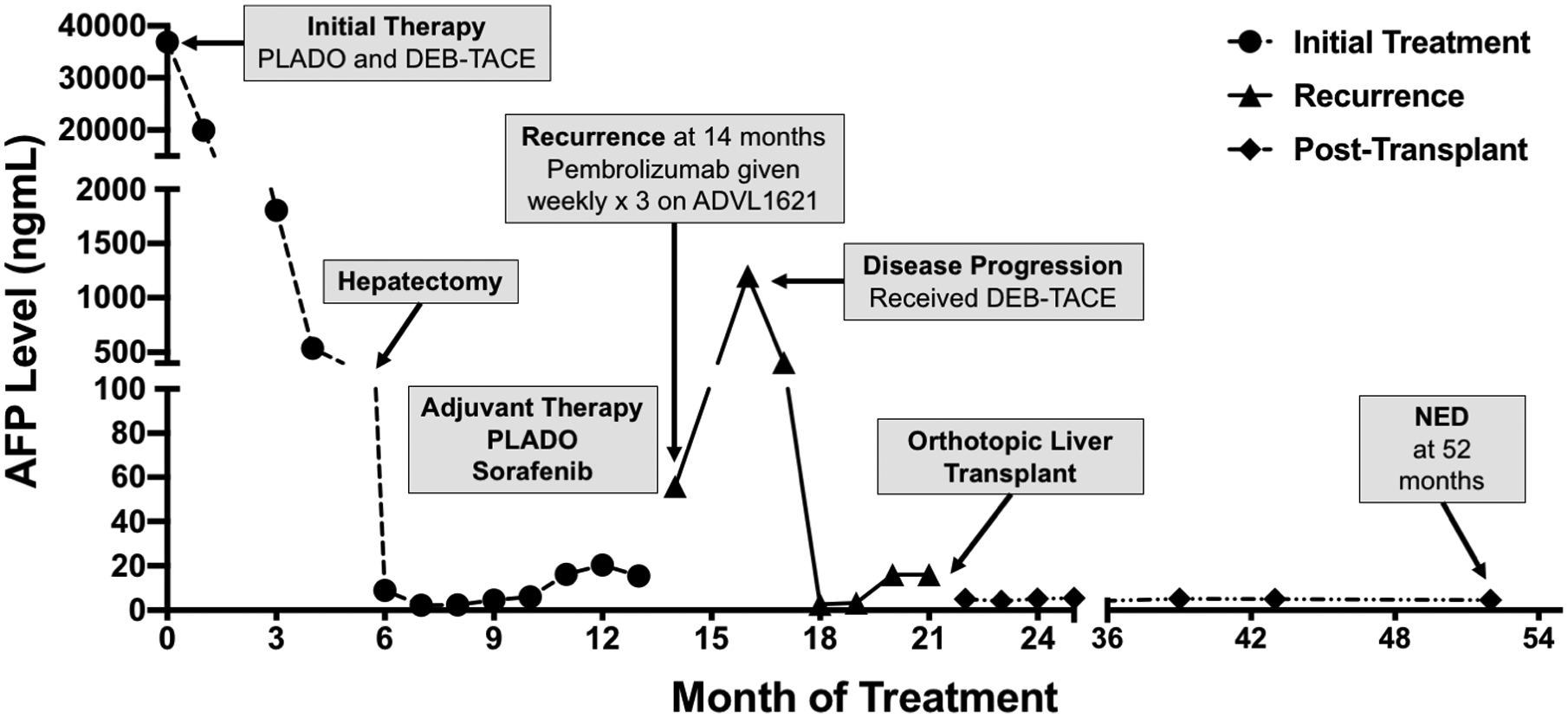
Despite neoadjuvant and adjuvant chemotherapy with PLADO, DEB-TACE, and hepatic resection, the disease recurred. Following pembrolizumab therapy (2mg/kg every 3 weeks × 3) radiological studies demonstrated disease progression without major vascular invasion and no extrahepatic involvement. DEB-TACE therapy was administered as a bridge to transplantation. The patient underwent liver transplantation 21 months after initial presentation. Implantation was complicated by hepatic artery dissection, requiring retransplantation. PLADO = Cisplatin/doxorubicin/dexrazoxane chemotherapy, DEB-TACE = Drug-eluting bead transarterial chemoembolization with doxorubicin, anti-PD1 = anti-programmed cell death protein 1, NED = No evidence of disease
Since total hepatectomy was the only curative option, 138 days from the last pembrolizumab dose, the patient underwent an OLT (Figure 1). Unfortunately, due to dissection of the hepatic artery, he required a second OLT 6 days later. Now 4 years from transplantation, the patient is doing well on sirolimus and tacrolimus with no graft rejection episodes or disease recurrence.
Discussion
We present the use of anti-PD1 pre-transplant with successful graft preservation in a pediatric patient with recurrent non-fibrolamellar HCC. Limited data exist on the use of ICIs in organ transplant patients, given the risk of organ rejection and frequent exclusion of organ transplant recipients from clinical trials. In a review of 91 adult organ transplant recipients who received ICI therapy, an overall allograft rejection rate of 60% was reported following ICIs treatment.(4) Amongst liver transplant patients, there was a 36% allograft rejection rate. Notably, immunosuppressive drug regimens were highly variable between patients. In a case series of nine adult patients with HCC, patients received nivolumab, another anti-PD1 agent, as part of their pretransplant tumor treatment between 1–252 days before transplant and successfully underwent transplantation without severe rejection or graft loss. (5) Timing of anti-PD1 administration appears to be critical, as a report of an adult who received nivolumab had an OLT only eight days from the last dose of anti-PD1 and unfortunately had a fatal acute hepatic necrosis immediately post-surgery.(6) Our case is the first to report safe use of ICI prior to liver transplant in a pediatric patient with HCC.
Given the risk of allograft rejection due to immune activation by ICIs, the timing of ICIs administration is crucial. While pembrolizumab has a half-life of 27 days, the length of the immunologic impact of the drug is unclear.(7) Alterations in PD-1 expression on T cells have been reported beyond the expected time frame for drug clearance. No biomarkers have been studied to predict the risk of rejection or the ideal window to proceed with liver transplant.(8) A small case series in adult liver transplant patients has reported administration of anti-PD1 therapy four weeks prior to undergoing transplant with no subsequent rejection.(5) However, given such limited data in pediatrics, future studies will need to be performed to assess for safe immunological and oncological windows to administer anti-PD1 therapy to transplant candidates. While our patient did not successfully respond to anti-PD1 therapy, he tolerated pembrolizumab well with his last dose administered four months prior to undergoing liver transplantation. At that point, minimal drug levels would be expected with lower risk of residual circulating antibodies affecting graft survival.
Even though the role of PDL1 immunohistochemistry (IHC) is currently not established in HCC, positive PDL1 staining in other malignancies has been shown to correlate with poor prognosis. It may also be associated with higher response rates to anti-PD1.(3) To evaluate PDL1 positivity in our patient, we performed PDL1 IHC staining on specimens from three separate time points using PDL1 (clone 73–10, Ventana Benchmark Ultra Platform) (Figure 2). While the clinical significance of our patient’s tissue staining is unclear, we found PDL1 positive Kupffer cells present in normal liver and unchanged post anti-PD1 therapy (Figure 2A–F) as per pathologist’s review (HR). Staining in tumor specimens was restricted to the peritumoral periphery (Figure 2B–C) with no intratumoral expression of PDL1 in immune or tumor cells.
Figure 2: PDL1 immunohistochemistry (IHC) staining in liver:
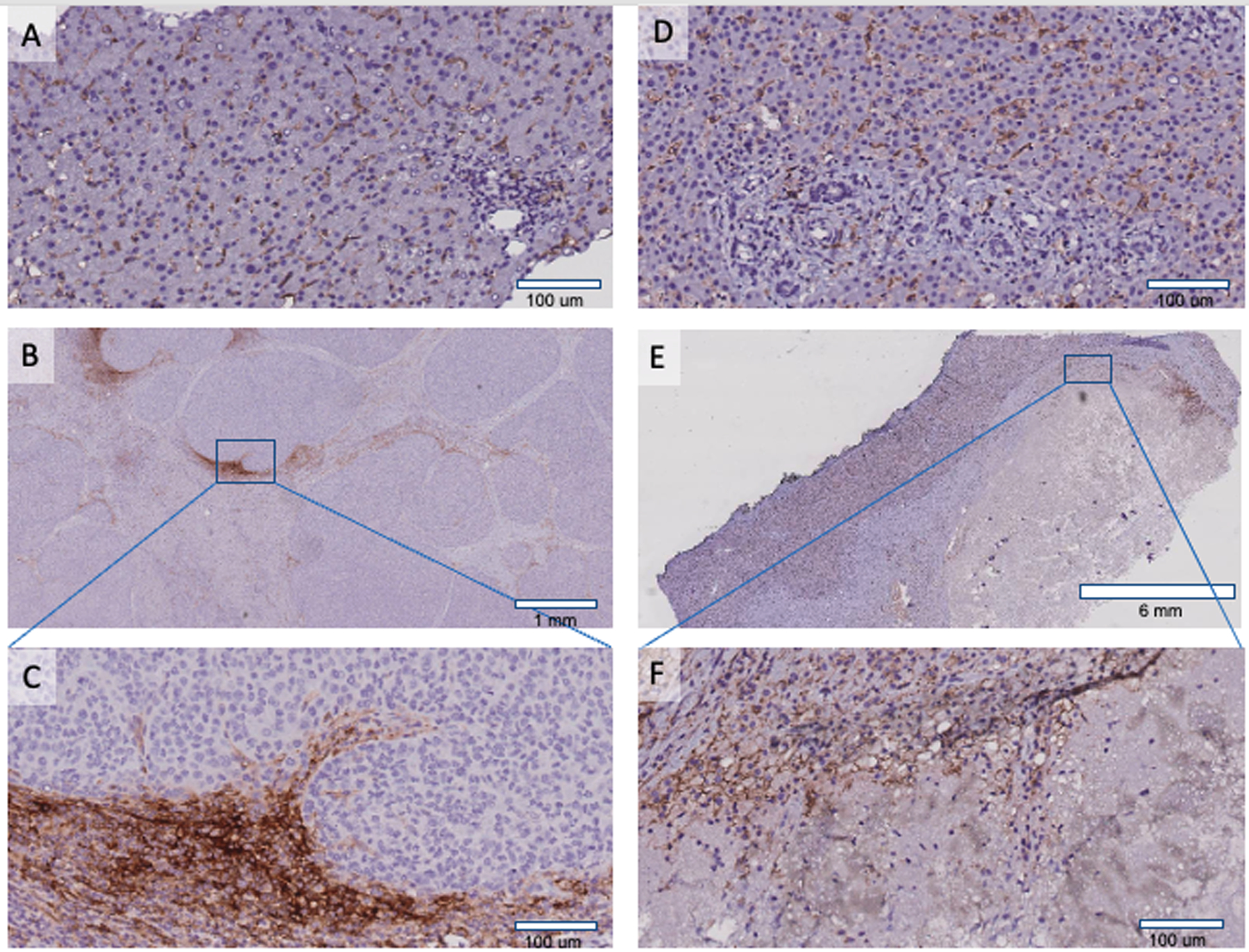
A. PDL1 positive Kupffer cells in normal liver. B. Primary tumor hepatectomy (20X) showing PDL1 staining in the peritumoral periphery. Square represents zoomed (200X) peritumoral area C showing PDL1 staining in Kupffer cells and macrophages. D-F are post anti-PD1 treatment in patient’s liver explant. D. Normal liver with PDL1 positive Kupffer cells. E. Whole section of tumor showing extensive tumor necrosis. Square represents zoomed (200X) area F revealing necrotic tumor and PDL1 positive macrophages.
This case report does not demonstrate efficacy of pembrolizumab in treatment of hepatocellular carcinoma, evidenced by rising AFP and absence of tumor shrinkage. However, it emphasizes the safety of anti-PD1 therapy in the pre-transplant setting for potential liver transplant recipients. More studies are needed to better understand the duration of the immunologic impact of anti-PD1 on patients prior to transplant. In addition, the benefits of anti-tumor immune response using ICIs pre-transplant should be carefully weighed against the risk of graft rejection in patients for whom transplantation may be the only sustainable option.
Supplementary Material
Acknowledgments
We would like to thank our patient and his family and the pediatric oncology clinical trial team at New York Presbyterian/Columbia University Irving Medical Center especially, Dr. Alice Lee and Rebecca Zylber. We would also like to acknowledge the Children’s Oncology Group and MERCK for their guidance and support in advancing the clinical care of our patients.
Financial Support
The National Institutes of Health supported the authors of this publication through Grant Numbers R01FD006108 (YS) and KL2TR001874 (RG). This research was funded in part through the NIH/NCI Cancer Center Support Grant P30CA013696. The content is solely the authors’ responsibility and does not necessarily represent the official views of the NIH. An Irving Assistant Professorship also supports YS at Columbia University’s NIH/NCATS CTSA Program hub: UL1TR001873. RG is also supported by Swim Across America and Hyundai Hope on Wheels Hope Scholar Award. DY is supported by the Alex’s Lemonade Stand Foundation Phase I/II Infrastructure Grant. The funding sources had no role in preparing the manuscript or the decision to submit it for publication.
Abbreviations
- ICIs
immune checkpoint inhibitors
- Anti-PD1
anti-programmed cell death 1
- HCC
hepatocellular carcinoma
- ICI
immune checkpoint inhibition
- PDL1
programmed cell death-ligand 1
- OLT
orthotopic liver transplantation
- MRI
magnetic resonance imaging
- AFP
alpha-fetoprotein
- PLADO
cisplatin/doxorubicin/dexrazoxane
- DEB-TACE
drug-eluting bead transarterial chemoembolization
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Bibliography
- 1.Allan BJ, Wang B, Davis JS, Parikh PP, Perez EA, Neville HL, Sola JE. A review of 218 pediatric cases of hepatocellular carcinoma. J Pediatr Surg 2014;49:166–171; discussion 171. [DOI] [PubMed] [Google Scholar]
- 2.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–952. [DOI] [PubMed] [Google Scholar]
- 3.Geoerger B, Kang HJ, Yalon-Oren M, Marshall LV, Vezina C, Pappo A, Laetsch TW, et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1–2 trial. Lancet Oncol 2020;21:121–133. [DOI] [PubMed] [Google Scholar]
- 4.Delyon J, Zuber J, Dorent R, Poujol-Robert A, Peraldi MN, Anglicheau D, Lebbe C. Immune Checkpoint Inhibitors in Transplantation-A Case Series and Comprehensive Review of Current Knowledge. Transplantation 2021;105:67–78. [DOI] [PubMed] [Google Scholar]
- 5.Tabrizian P, Florman SS, Schwartz ME. PD-1 inhibitor as bridge therapy to liver transplantation? Am J Transplant 2021;21:1979–1980. [DOI] [PubMed] [Google Scholar]
- 6.Nordness MF, Hamel S, Godfrey CM, Shi C, Johnson DB, Goff LW, O’Dell H, et al. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: Are checkpoint inhibitors safe for the pretransplant patient? Am J Transplant 2020;20:879–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longoria TC, Tewari KS. Evaluation of the pharmacokinetics and metabolism of pembrolizumab in the treatment of melanoma. Expert Opin Drug Metab Toxicol 2016;12:1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merryman RW, Kim HT, Zinzani PL, Carlo-Stella C, Ansell SM, Perales M-A, Avigdor A, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood 2017;129:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


