Abstract
Purpose of Review:
This review is intended to provide an up-to-date analysis of the structural and functional alterations of the kidneys that accompany healthy and unhealthy aging in humans. Macro- and micro- structural changes and glomerular filtration rate (whole kidney and single nephron) accompanying aging will be stressed.
Recent Findings:
Comparative findings concerning distribution of anatomic changes of the kidney healthy and unhealthy aging are reviewed. Challenges concerning definition of Chronic Kidney Disease (CKD) in otherwise healthy aging subjects is discussed. The complex interactions of CKD and aging is discussed. The role of podocyte dysbiosis in kidney aging is reviewed.
Summary:
Kidney aging is a complex phenomenon often difficult to distinguish from CKD. Nonetheless, phenotypes of healthy and unhealthy aging are evident. Much more information concerning the molecular characteristics of normal kidney aging and its relevance to chronic kidney disease is needed.
Keywords: Aging, Kidney Anatomy, Kidney Function, Glomerular Filtration Rate, Chronic Kidney Disease
INTRODUCTION and SCOPE:
The changes in the structure and function of the kidneys during the process of aging have been studied for many decades (1). The underlying biology of kidney aging remains elusive and likely involves genes, environment and chance (1, 2). We now have an appreciable understanding of the normal kidney aging (3). Animal experimentation in murine species has shed much light on the process of kidney aging (4), but important differences exist between human and murine aging at the descriptive level, so modeling of kidney aging from laboratory animals in forced captivity and a proscribed environment may not be translatable to humans. There are also differences between aging humans that are only indirectly related to the aging process per se. The kidneys can be affected by co-morbid features, such as obesity, diabetes and hypertension that commonly accompany aging (5). This complicates the analysis of how aging affects kidney structure and function; i.e, what differences exist in the kidneys with “healthy” aging (in the absence of overt co-morbidity) and unhealthy aging. Events in early intrauterine development, such as nephron under-endowment, may set in motion adaptive changes that affect structure and function of the kidneys later in life, even among apparently healthy subjects (6, 7). It is the intent of this review to analyze the processes in both healthy and unhealthy kidney aging in humans and to contrast these findings with those found in aging animals. Molecular studies will not be covered, as this topic has been covered in other recent reviews and original investigations (1, 8–10).
HEATHY AGING IN HUMANS
Structural changes- macroscopic and microscopic
The anatomy of kidney cortex and medulla does not change in parallel with healthy aging. Instead, up to age of 50 years, whereas medullary volume increases, cortex volume declines, providing a false impression that kidney does not change macroscopically with aging (11, 12). Only beyond age of 50 years, when the cortex further atrophies, can a decline in total kidney volume be fully appreciated. This age-related decline in cortex volume (but not medulla volume) has recently been demonstrated using novel deep-learning-based approach in a multicenter study of 1612 potential kidney donors (13).
Another common macroscopic feature of an aging kidney is a higher prevalence, number, and size of benign simple cysts (14). Computerized tomography (CT) studies in potential kidney donors have revealed that focal cortical scars, fibromuscular dysplasia, parenchymal calcifications, atherosclerosis of kidney arteries (15), and kidney surface roughness (16) also increase with older age.
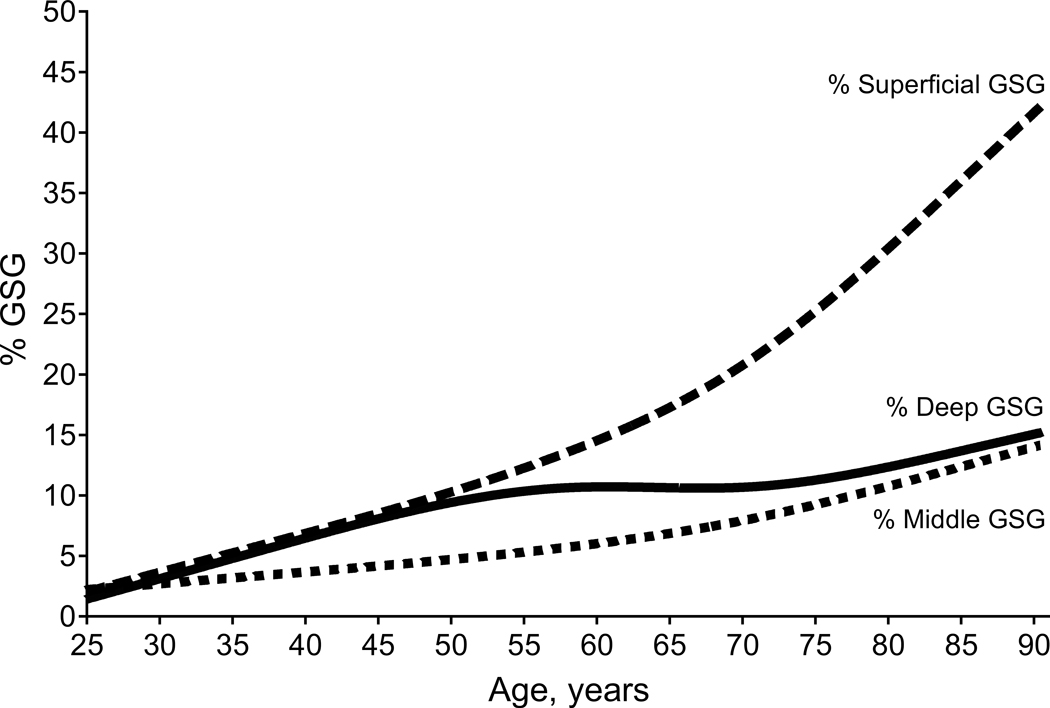
One of the most frequently described micro-anatomical changes in healthy aging is an age-dependent increase in the frequency of focal and global glomerulosclerosis (FGGS). Virtually all studies, including autopsy and studies in living kidney donors, agree that even in the absence of an overt kidney disease, the prevalence of focal and global (but not focal and segmental) glomerulosclerosis increases with older age (17–20). While FGGS increases, the number of healthy/viable glomeruli decreases. A study in living kidney donors ranging from 18 to 75 years of age, demonstrated that nephron loss in the oldest individual can be significantly underappreciated by the commonly reported metric of the percent of globally sclerosed glomeruli in kidney biopsies. This may be due to progressive atrophy and eventual reabsorption of globally sclerosed glomeruli (20). Over a 50+ year span of aging about 50% of the original nephron endowment is lost by physiological aging (20, 21). While enumerating nephrons by combinations of volumetric CT scans for cortical volume and kidney biopsy stereology for glomerular density is not precise in individual patients (22), reasonably accurate population-level patterns can be described in large cohorts (20, 23–25). Another interesting aspect of age-related global glomerulosclerosis is that it increases with age primarily in the superficial cortex (Figure 1 and 2), whereas global glomerulosclerosis associated with underlying disease occurs more diffusely throughout the cortex (26).
Figure 1.
Age-related global glomerulosclerosis primarily occurs in superficial cortical regions.
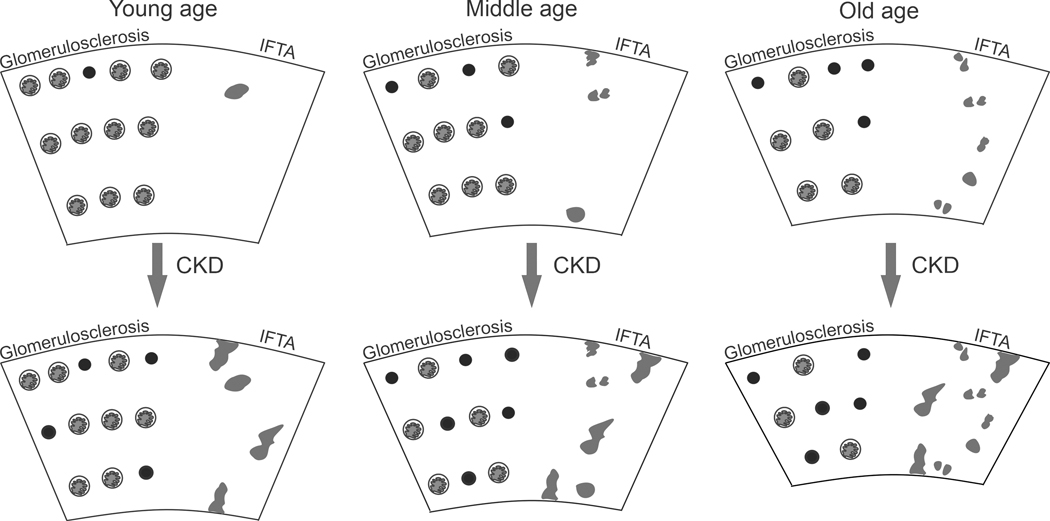
Figure 2.
With healthy aging, there is an increase in global glomerulosclerosis (predominately in the superficial cortex) and loss of glomeruli from young age to old age. There is minimal IFTA (gray areas) that atrophies over time with contraction of the cortex and an increase in IFTA density. Superimposed chronic changes from CKD can occur at any age resulting in more global glomerulosclerosis and more IFTA. Black circles denote globally sclerosed glomeruli.
In addition to glomerulosclerosis, studies have shown that biopsies from older individuals have more ischemic-appearing glomeruli (27), more foci of interstitial fibrosis and tubule atrophy (IFTA) (16, 28), as well as more arteriosclerosis and arteriolar hyalinosis (16). These anatomic changes are likely inter-related as stenosis of small to medium arteries can cause ischemic changes both in glomeruli and tubules. As individuals age, IFTA foci develop from the attached tubules of sclerosing glomeruli and over time these foci progressively result in contraction of the cortex volume such that the IFTA density increases with age (Figure 2). Indeed, IFTA density increases with age independent of the overall fraction of cortex that contains IFTA (28). Onset of CKD at any age is superimposed on this process with increased glomerulosclerosis and IFTA beyond age-expected levels (Figure 2).
Although it seems intuitive that with glomerular loss, the size of remaining functional glomeruli will increase, this is not the case with truly healthy aging in humans (16, 20). The physiological reasons why remaining glomeruli do not undergo hypertrophy in healthy aging in humans, while such hypertrophy does seem to occur in aging (often inbred) murine species (often accompanied by lesions of focal and segmental glomerulosclerosis) (4) is not well understood, but may be due to fundamental differences in the biology of aging between humans and murine species.
Functional Changes:
An analysis of measured GFR (mGFR) studies in healthy adults suggests a decline in mGFR begins after the age of 40 years (29); However, the lack of a universal method for determining mGFR, and biological differences in mGFR in healthy persons exist across different mGFR methods (30). Given methodological differences and lack of standardization, method-specific, age-based reference limits are often needed for mGFR (31–33). In practice, there is often a reliance on estimated GFR (eGFR), but age-specific reference ranges are still needed and are typically lower than those based on mGFR (34).
Cross-sectional studies of mGFR with aging are often criticized as they cannot distinguish within-person from between-person variation in mGFR with age. However, they also can have the advantage of careful ascertainment of kidney health and absence of CKD risk factors, data often lacking in longitudinal studies. Indeed, longitudinal studies with health only required at baseline show a more rapid decline in GFR of 1.26 ml/min/1.73 m2 per year(35) compared to cross-sectional studies where the decline is typically <1.0 ml/min/1.73m2 per year (36, 37). Notably, cross-sectional studies of healthy kidney donors have consistently shown a decline in, not only the average GFR by age, but the upper (e.g. 97.5th) and lower (e.g., 2.5th) percentiles with age (38). If the decline in mGFR with aging is not universal but due to a subset developing clinically covert kidney disease, then the population-level variability in GFR would become wider with older age from those with covert disease having a decline in their GFR. However, this finding is not evident. Rather, the population-level variability in GFR does not increase with age but rather remains relatively constant (37). Further, the 2.5th percentile in a healthy 50-year-old woman and the 97.5th percentile in a healthy 90-year-old woman are both about 78 ml/min/1.73 m2 (37), making it highly implausible for there to be a stable GFR with healthy aging.
As previously mentioned, an age-related increase in FGGS is evident, so an assumption is that glomerulosclerosis is linked with a decline in whole kidney GFR. However, a study of healthy kidney donors did not find evidence that age-related decline in whole kidney GFR associated with the age-related increase in nephrosclerosis or glomerulosclerosis on kidney biopsy (16, 19). More recently, this was confirmed in a cohort of patients who underwent radical nephrectomy, where authors found a decline in whole kidney GFR with age even among those with minimal amounts of nephrosclerosis (39). Since the functional nephron number declines with age and the whole kidney GFR declines in parallel, then the single nephron GFR remains relatively constant at least until about age 70 years with healthy human aging (21).
It is worth noting that kidney function has been largely defined by the glomerular filtration rate (GFR). However, other dimensions of kidney function include preventing protein loss during filtration and reabsorbing filtered proteins; reabsorbing water, electrolytes, and metabolites; preventing crystal precipitation in supersaturated urine, and endocrine/hormonal functions such as erythropoietin production. With healthy aging, there is no evidence for age-dependent protein leak into the urine (19) or increased urine crystallization with stone formation. There is evidence of a reduced range of urine diluting and urine concentrating capacity with aging (40).
UNHEALTHY AGING IN HUMANS
Structure and Function:
Unhealthy functional changes with aging can be grouped into two categories. Abnormal GFR for age and other evidence of kidney dysfunction from comorbidities and diseases that are common with aging. In particular, other evidence of kidney dysfunction includes abnormal proteinuria (or albuminuria) as this does not occur with healthy aging and should always prompt an evaluation for CKD. Unhealthy changes in GFR with aging can either be high GFR (e.g., GFR >97.5th percentile for age) or CKD (e.g., GFR <2.5th percentile for age). High GFR is an imperfect surrogate for glomerular hyperfiltration and often simply reflects a higher nephron number (38). But high GFR, especially when based on body surface area uncorrected values of mGFR (in ml/min), can be evidence of glomerular hyperfiltration as it associated with glomerular enlargement, obesity, and albuminuria (38).
Notably, the physiological healthy age-related decline in GFR is not associated with mortality. In population-based studies, a chronic eGFR of 45–59 ml/min/1.73 m2 without significant albuminuria meets KDIGO criteria for CKD (41) but this is not associated with an increased risk of mortality for age >65 years (42, 43). A chronic eGFR of 60–74 ml/min/1.73 m2 does not meet KDIGO criteria for CKD but is associated with an increased risk of mortality for ages 18–45 years (42). These studies, in our opinion, highlight the failure of a single eGFR threshold to identify unhealthy age-related GFR decline. Rather, age-based thresholds that can be continuous (e.g. <2.5th percentile for age in healthy adults) or simplified into categories (<45 ml/min/1.73 m2 age >65 years, <60 ml/min/1.73 m2 for age 40 to 65 years, and <75 ml/min/1.73 m2 for age <40 years) have been proposed (44). However, eGFR thresholds may need to be adjusted for newer equations (45, 46).
With unhealthy aging, comorbidities and overt CKD affect the microstructural changes that impact GFR. In particular, obesity and diabetes, that are more common with older age relate to micro-structural changes, as evident by hypertrophy of glomeruli and corresponding tubules. In contrast to the normal age-related “obsolescent” glomerulosclerosis, the solidification type of glomerulosclerosis is always pathologic (47–49). When FGGS of any type is more severe than expected for age among patients with nephrotic syndrome then progressive CKD is much more likely to be observed (50). Any finding of a FSGS lesion should be regarded as a sign of unhealthy aging in humans. In addition, abnormal albuminuria (excretion rate >30mg/day), as noted above, is not a feature of normal healthy aging, even when whole kidney GFR has declined to less than 60ml/min/1.73 m2 (but above 45ml/min/1.73 m2) in a subject over 65 years of age (51). High GFR for age may be the consequence of concomitant obesity (52, 53) and diabetes-related hyperfiltration (54) and represent examples of unhealthy aging.
THE INTER-RELATIONSHIPS of KIDNEY AGING AND CHRONIC KIDNEY DISEASE
With the current definition of CKD, any adult person having a GFR (eGFR or mGFR) of <60ml/min/1.73 m2 for 3 months or longer can be regarded as having CKD (41). Many apparently healthy subjects over age 65 years will meet the definition of Category 3A CKD (GFR between 45 and 59ml/min/1.73 m2 and a uACR of <30mg/g) (44, 55). Any relationship of such a Category of CKD to excess risk of cardiovascular disease and all- cause mortality is inconsistent and very modest at best (56, 57) and likely explained by comorbidity and not GFR itself. This discrepancy has led many investigators, mainly from Europe, to suggest that these criteria for defining CKD on GFR criteria (either eGFR or mGFR) be age-adapted (43, 44). Such an age-adapted schema would lead to a substantial reduction in the estimated global burden of CKD (55). Recent studies give much support to the notion that the absence of age-adapted thresholds of GFR for defining CKD leads to overdiagnosis of this condition in older adults (42, 43, 58, 59).
To be sure, the processes involved in the generation of CKD and kidney aging share many elements in common (60). CKD may accelerate the processes involved in healthy aging and vice-versa, however, disentangling the cause-and-effect aspects can be very difficult (61).
The assessment of GFR in aging humans can be challenging. Creatinine formation and excretion decreases with age, likely a consequence of sarcopenia (62). These changes are, in part adjusted for by the age exponent in the eGFR-creatinine equations. But a simple exponential adjustment may be insufficient to account for extremes in muscle mass loss observed in frail, chronically ill elderly subjects. This can lead to an over-estimation of true GFR by eGFR-creatinine equations. The use of a Cystatin C based eGFR or a creatinine + Cystatin C based equation may improve the prediction accuracy (as assessed by the P30 metric), as Cystatin C generation/degradation seems to be much less affected by age than creatinine generation (63) at any given level of mGFR. Nevertheless, Cystatin C serum levels can also be affected by non-GFR related determinants, such as diabetes, obesity and chronic inflammation that can commonly be found in elderly subjects (64). Endothelial dysfunction, found in unhealthy aging, may also lead to a discrepancy between the values of eGFR-creatinine and eGFR-Cystatin C due to reduced permeability of Cystatin C (Molecular weight= 13,000 Daltons) compared to Creatinine (Molecular weight=113 Daltons) at the glomerular capillary level. This hypothesis is also known as the “shrunken pore syndrome” (65), first described by Anders Grubb (66).
As mentioned above, a low nephron endowment at birth (signaled by a low birth weight of <2.5kg) or exposure to potential nephrotoxins in the peri-natal period, can lead to the occurrence of a GFR <60ml/min/1.73 m2 earlier in life (7). The post-natal adaptation to such nephron under-endowment partially obscures the effect when whole kidney GFR measurements or estimates are used to assess kidney function. These adaptive processes lead to an increased single-nephron GFR (hyperfiltration) and glomerular hypertrophy, which can collectively cause maladaptive glomerular (podocyte) injury (often manifested by higher systemic arterial blood pressure and/or increasing albuminuria) to the remaining nephrons and thereby facilitate an accelerated loss of nephron number compared to individual with a normal nephron endowment at birth (7, 67).
SPECULATIONS ON PATHOPHYSIOLOGY: Ischemia vs Podocyte dysbiosis
It is generally accepted that visceral glomerular epithelial cells (podocytes) are post-mitotic, terminally differentiated cells, with a minimal regeneration potential. A study has shown how podocyte nuclear density was around 3-fold smaller in older kidneys compared to young kidney, although it is unclear what is the real effect of older age because not all studied individuals were healthy (68). The authors did not find evidence that ischemic glomerulopathy correlated with arteriosclerosis, but instead it correlated with higher rate of podocyte detachment. This was especially evident in glomeruli with signs of wrinkled glomerular basement membrane and glomerular collapse.
The same group showed a similar age-related decline in podocyte density in rats with hypertrophic stress(69), however, this was associated with FSGSs, a feature not found in human healthy aging. More recently, it was proposed that FSGS lesions(70), and glomerulosclerosis(71) are a consequence of a mismatch between glomerulomegaly and the capacity of podocytes to compensate and adequately expand filtration surface. The authors found that calorie restriction ameliorated glomerular growth, which in turn significantly reduced progression of FSGS lesions or slowed progression of ESKD. This finding is consistent with increased prevalence of FSGS lesions in humans with increasing obesity prevalence in the last several decades in many countries (70, 72). It seems likely that studying age-related changes in podocyte biology in both humans and murine species is hampered by concurrent obesity and comorbidities. Inbred animal raised in captivity and nurtured by a diet different from conditions in the wild might experience a form of unhealthy aging, akin to that seen in some humans.
CONCLUSIONS AND RESEARCH INITIATIVES
This review has highlighted the structural and functional changes (stressing GFR in the latter dimension) that occur in normal physiological (healthy) and co-morbidity associated aging in humans. The detailed study of function and anatomy among healthy living donors has greatly amplified our knowledge of the kidney alterations accompanying physiological human aging, but we cannot yet pinpoint the precise mechanisms involved in these processes. Co-morbidity, such as diabetes, obesity and hypertension accompanying aging, seems to substantially alter the phenotype of the aging kidney but much deeper phenotyping and “multi-omics” will be required to better understand the mechanisms underlying these changes in the aging kidney. Chronic kidney disease and kidney aging intersect at multiple levels- including diagnosis, pathophysiology and prognosis. More detailed analysis of the tissue distribution of anatomical and pathological changes as well as molecular phenotyping may give better insight into the two-way interaction of CKD with aging. The use of a single, absolute threshold of GFR (mGFR or eGFR) to define CKD in all adults, regardless of age, needs to be re-examined.
KEY POINTS:
The kidney cortex and medulla volumes do not change in parallel with healthy aging, as up to 50 years of age cortex volume declines whereas medulla volume increases.
Microstructural features of healthy aging include nephron loss, increase in globally sclerosed and ischemic-appearing glomeruli (predominantly in superficial cortex), and a higher density of small interstitial fibrosis and tubular atrophy foci.
The single nephron GFR remains relatively constant, at least until about age 70 years.
With unhealthy aging, comorbidities such as obesity and diabetes and overt chronic kidney disease accelerate microstructural pathology seen in aging, as well as contribute disease-specific pathology such as the solidification form of glomerulosclerosis.
The use of a single, absolute threshold of GFR to define chronic kidney disease in all adults, regardless of age, does not account for the microstructural differences evident between healthy aging and chronic kidney disease.
Acknowledgements
Financial support
This study was supported with funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK090358).
Footnotes
Conflict of Interest
There are no conflicts of interest.
REFERENCES
- 1.Schmeer C, Kretz A, Wengerodt D, Stojiljkovic M, Witte OW. Dissecting Aging and Senescence-Current Concepts and Open Lessons. Cells. 2019;8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finch CE, Kirkwood TBL. Chance, Development and Aging. 1 ed. New York: Oxford University Press; 2000. 278 p. [Google Scholar]
- 3.Glassock RJ, Denic A, Rule AD. The Physiology and Pathophysiology of the Kidneys in Aging. Brenner and Rector’s The Kidney: Elsevier; 2019. p. 710–30. [Google Scholar]
- 4.Shankland SJ, Wang Y, Shaw AS, Vaughan JC, Pippin JW, Wessely O. Podocyte Aging: Why and How Getting Old Matters. J Am Soc Nephrol. 2021;32(11):2697–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevalier RL. Evolution, kidney development, and chronic kidney disease. Semin Cell Dev Biol. 2019;91:119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luyckx VA, Brenner BM. Clinical consequences of developmental programming of low nephron number. Anat Rec (Hoboken). 2020;303(10):2613–31. [DOI] [PubMed] [Google Scholar]
- 8.Fang Y, Gong AY, Haller ST, Dworkin LD, Liu Z, Gong R. The ageing kidney: Molecular mechanisms and clinical implications. Ageing Res Rev. 2020;63:101151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franzin R, Stasi A, Ranieri E, Netti GS, Cantaluppi V, Gesualdo L, et al. Targeting Premature Renal Aging: from Molecular Mechanisms of Cellular Senescence to Senolytic Trials. Front Pharmacol. 2021;12:630419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randles M, Lausecker F, Kong Q, Suleiman H, Reid G, Kolatsi-Joannou M, et al. Identification of an Altered Matrix Signature in Kidney Aging and Disease. J Am Soc Nephrol. 2021. 28;32(7):1713–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bax L, van der Graaf Y, Rabelink AJ, Algra A, Beutler JJ, Mali WP. Influence of atherosclerosis on age-related changes in renal size and function. Eur J Clin Invest. 2003;33(1):34–40. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Vrtiska TJ, Avula RT, Walters LR, Chakkera HA, Kremers WK, et al. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int. 2014;85(3):677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Korfiatis P, Denic A, Edwards ME, Gregory AV, Wright DE, Mullan AF, et al. Automated Segmentation of Kidney Cortex and Medulla in CT Images: A Multisite Evaluation Study. J Am Soc Nephrol. 2021(in press). *Study used deep-learning to automatically quantify kidney cortex and medulla volumes separately.
- 14.Rule AD, Sasiwimonphan K, Lieske JC, Keddis MT, Torres VE, Vrtiska TJ. Characteristics of renal cystic and solid lesions based on contrast-enhanced computed tomography of potential kidney donors. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;59(5):611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenz EC, Vrtiska TJ, Lieske JC, Dillon JJ, Stegall MD, Li X, et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clinical journal of the American Society of Nephrology : CJASN. 2010;5(3):431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denic A, Alexander MP, Kaushik V, Lerman LO, Lieske JC, Stegall MD, et al. Detection and Clinical Patterns of Nephron Hypertrophy and Nephrosclerosis Among Apparently Healthy Adults. Am J Kidney Dis. 2016;68(1):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl. 2003(83):S31–7. [DOI] [PubMed]
- 18.Vazquez Martul E, Veiga Barreiro A. Importance of kidney biopsy in graft selection. Transplant Proc. 2003;35(5):1658–60. [DOI] [PubMed] [Google Scholar]
- 19.Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152(9):561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P, et al. The Substantial Loss of Nephrons in Healthy Human Kidneys with Aging. J Am Soc Nephrol. 2017;28(1):313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP, et al. Single-Nephron Glomerular Filtration Rate in Healthy Adults. N Engl J Med. 2017;376(24):2349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morozov D, Parvin N, Conaway M, Oxley G, Baldelomar E, Cwiek A, et al. Estimating Nephron Number from Biopsies: Impact on Clinical Studies. J Am Soc Nephrol. 2021. *Study uses virtual (by means of magnetic resonance imaging) and needle kidney biopsies from nephrectomy specimens to assess variability in estimated nephron number.
- 23.Sasaki T, Tsuboi N, Okabayashi Y, Haruhara K, Kanzaki G, Koike K, et al. Estimation of nephron number in living humans by combining unenhanced computed tomography with biopsy-based stereology. Sci Rep. 2019;9(1):14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuboi N, Sasaki T, Okabayashi Y, Haruhara K, Kanzaki G, Yokoo T. Assessment of nephron number and single-nephron glomerular filtration rate in a clinical setting. Hypertens Res. 2021;44(6):605–17. [DOI] [PubMed] [Google Scholar]
- 25.Denic A, Elsherbiny H, Rule AD. In-vivo techniques for determining nephron number. Curr Opin Nephrol Hypertens. 2019;28(6):545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Denic A, Ricaurte L, Lopez CL, Narasimhan R, Lerman LO, Lieske JC, et al. Glomerular Volume and Glomerulosclerosis at Different Depths within the Human Kidney. J Am Soc Nephrol. 2019;30(8):1471–80. * Human study that demonstrates different glomerular size, and global glomerulosclerosis at different depths of a kidney cortex.
- 27.Denic A, Mathew J, Nagineni VV, Thompson RH, Leibovich BC, Lerman LO, et al. Clinical and Pathology Findings Associate Consistently with Larger Glomerular Volume. J Am Soc Nephrol. 2018;29(7):1960–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ricaurte L, Denic A, Mullan A, Narasimhan R, Bogojevic M, Thompson RH, et al. A Higher Foci Density of Interstitial Fibrosis and Tubular Atrophy Predicts Progressive Chronic Kidney Disease after a Radical Nephrectomy for Tumor. Journal of the American Society of Nephrology. 2021:32(10):2623–2633. * Study develops concept of IFTA density and demontrates its association with age and importance in predicting CKD progression independent of %IFTA.
- 29.Pottel H, Hoste L, Yayo E, Delanaye P. Glomerular Filtration Rate in Healthy Living Potential Kidney Donors: A Meta-Analysis Supporting the Construction of the Full Age Spectrum Equation. Nephron. 2017;135(2):105–19. [DOI] [PubMed] [Google Scholar]
- 30.Sterner G, Frennby B, Mansson S, Nyman U, Van Westen D, Almen T. Determining ‘true’ glomerular filtration rate in healthy adults using infusion of inulin and comparing it with values obtained using other clearance techniques or prediction equations. Scand J Urol Nephrol. 2008;42(3):278–85. [DOI] [PubMed] [Google Scholar]
- 31.Pottel H, Delanaye P, Weekers L, Selistre L, Goffin K, Gheysens O, et al. Age-dependent reference intervals for estimated and measured glomerular filtration rate. Clin Kidney J. 2017;10(4):545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fenton A, Montgomery E, Nightingale P, Peters AM, Sheerin N, Wroe AC, et al. Glomerular filtration rate: new age- and gender- specific reference ranges and thresholds for living kidney donation. BMC Nephrol. 2018;19(1):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautam S. Age-specific normal reference ranges for (99m)Tc-DTPA glomerular filtration rate to use with two-sample slope-intercept method and Jodal Brochner-Mortensen correction. Phys Eng Sci Med. 2021. 44(4):1331–1340. [DOI] [PubMed] [Google Scholar]
- 34.Poggio ED, Rule AD, Tanchanco R, Arrigain S, Butler RS, Srinivas T, et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney international. 2009;75(10):1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kasiske BL, Anderson-Haag TL, Duprez DA, Kalil RS, Kimmel PL, Pesavento TE, et al. A prospective controlled study of metabolic and physiologic effects of kidney donation suggests that donors retain stable kidney function over the first nine years. Kidney Int. 2020;98(1):168–75. *A longitudinal prospective study of GFR measurement in controls matched to donors.
- 36.Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43(1):112–9. [DOI] [PubMed] [Google Scholar]
- 37. Eriksen BO, Palsson R, Ebert N, Melsom T, van der Giet M, Gudnason V, et al. GFR in Healthy Aging: an Individual Participant Data Meta-Analysis of Iohexol Clearance in European Population-Based Cohorts. J Am Soc Nephrol. 2020;31(7):1602–15. **Demonstrates that both the mean and range of GFR in healthy persons declines with aging.
- 38. Chakkera HA, Denic A, Kremers WK, Stegall MD, Larson JJ, Ravipati H, et al. Comparison of high glomerular filtration rate thresholds for identifying hyperfiltration. Nephrol Dial Transplant. 2020;35(6):1017–26. *Compares differnet thresholds for high GFR to optimally detect glomerular hyperfiltration.
- 39. Li P, Gupta S, Mothi SS, Rennke HG, Leaf DE, Waikar SS, et al. Histopathologic Correlates of Kidney Function: Insights From Nephrectomy Specimens. Am J Kidney Dis. 2021;77(3):336–45. ** Shows that prevalence of nephrosclerosis increases with age and that GFR still declines strongly with age even when minimal nephrosclerosis detected.
- 40.Kittanamongkolchai W, Vaughan LE, Enders FT, Dhondup T, Mehta RA, Krambeck AE, et al. The Changing Incidence and Presentation of Urinary Stones Over 3 Decades. Mayo Clin Proc. 2018;93(3):291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Summary of Recommendation Statements. Kidney International Supplements. 2013;3(1):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jonsson AJ, Lund SH, Eriksen BO, Palsson R, Indridason OS. The prevalence of chronic kidney disease in Iceland according to KDIGO criteria and age-adapted estimated glomerular filtration rate thresholds. Kidney Int. 2020;98(5):1286–95. **Lower prevalence of CKD and improved prognostic implications when age-adapted eGFR used to defined CKD.
- 43. Liu P, Quinn RR, Lam NN, Elliott MJ, Xu Y, James MT, et al. Accounting for Age in the Definition of Chronic Kidney Disease. JAMA Intern Med. 2021;181(10):1359–66. ** Lower prevalence of CKD and improved prognostic implications when age-adapted eGFR used to defined CKD.
- 44.Delanaye P, Jager KJ, Bokenkamp A, Christensson A, Dubourg L, Eriksen BO, et al. CKD: A Call for an Age-Adapted Definition. J Am Soc Nephrol. 2019;30(10):1785–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med. 2021;385(19):1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pottel H, Bjork J, Courbebaisse M, Couzi L, Ebert N, Eriksen BO, et al. Development and Validation of a Modified Full Age Spectrum Creatinine-Based Equation to Estimate Glomerular Filtration Rate : A Cross-sectional Analysis of Pooled Data. Ann Intern Med. 2021;174(2):183–91. * A full age spectrum creatinine-based equation to estimate GFR.
- 47.Jennette JC, Charles L, Grubb W. Glomerulomegaly and focal segmental glomerulosclerosis associated with obesity and sleep-apnea syndrome. Am J Kidney Dis. 1987;10(6):470–2. [DOI] [PubMed] [Google Scholar]
- 48.Bailey RR, Lynn KL, Burry AF, Drennan C. Proteinuria, glomerulomegaly and focal glomerulosclerosis in a grossly obese man with obstructive sleep apnea syndrome. Aust N Z J Med. 1989;19(5):473–4. [DOI] [PubMed] [Google Scholar]
- 49.Fogo AB. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol. 2015;11(2):76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hommos MS, Zeng C, Liu Z, Troost JP, Rosenberg AZ, Palmer M, et al. Global glomerulosclerosis with nephrotic syndrome; the clinical importance of age adjustment. Kidney Int. 2018;93(5):1175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inker LA, Okparavero A, Tighiouart H, Aspelund T, Andresdottir MB, Eiriksdottir G, et al. Midlife Blood Pressure and Late-Life GFR and Albuminuria: An Elderly General Population Cohort. Am J Kidney Dis. 2015;66(2):240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denic A, Glassock RJ. Obesity-Related Glomerulopathy and Single-Nephron GFR. Kidney Int Rep. 2020;5(8):1126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Okabayashi Y, Tsuboi N, Sasaki T, Haruhara K, Kanzaki G, Koike K, et al. Single-Nephron GFR in Patients With Obesity-Related Glomerulopathy. Kidney Int Rep. 2020;5(8):1218–27. * A study from Japan shows increase single nephron hyperfiltration in patients with obesity-related glomerulopathy.
- 54.Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH, et al. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J Am Soc Nephrol. 2017;28(4):1023–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13(2):104–14. [DOI] [PubMed] [Google Scholar]
- 56.Delanaye P, Glassock RJ. Glomerular Filtration Rate and Aging: Another Longitudinal Study--A Long Time Coming! Nephron. 2015;131(1):1–4. [DOI] [PubMed] [Google Scholar]
- 57.Warnock DG, Delanaye P, Glassock RJ. Risks for All-Cause Mortality: Stratified by Age, Estimated Glomerular Filtration Rate and Albuminuria. Nephron. 2017;136(4):292–7. [DOI] [PubMed] [Google Scholar]
- 58.Ren Q, Zhou Y, Chen G, Li X, Ye W. Age-adapted definition of chronic kidney disease based on Chronic Kidney Disease Epidemiology Collaboration and full age spectrum equation. Kidney Int. 2020;98(5):1350–2. [DOI] [PubMed] [Google Scholar]
- 59.O’Hare AM, Rodriguez RA, Rule AD. Overdiagnosis of Chronic Kidney Disease in Older Adults-An Inconvenient Truth. JAMA Intern Med. 2021;181(10):1366–8. [DOI] [PubMed] [Google Scholar]
- 60.Denic A, Glassock R, Rule A. The Kidney in Normal Aging. Clin J Am Soc Nephrol. 2022;17(1):137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ebert T, Pawelzik SC, Witasp A, Arefin S, Hobson S, Kublickiene K, et al. Inflammation and Premature Ageing in Chronic Kidney Disease. Toxins (Basel). 2020;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Epstein M Aging and the kidney. J Am Soc Nephrol. 1996;7(8):1106–22. [DOI] [PubMed] [Google Scholar]
- 63.Potok OA, Ix JH, Shlipak MG, Katz R, Hawfield AT, Rocco MV, et al. The Difference Between Cystatin C- and Creatinine-Based Estimated GFR and Associations With Frailty and Adverse Outcomes: A Cohort Analysis of the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis. 2020;76(6):765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu X, Foster MC, Tighiouart H, Anderson AH, Beck GJ, Contreras G, et al. Non-GFR Determinants of Low-Molecular-Weight Serum Protein Filtration Markers in CKD. Am J Kidney Dis. 2016;68(6):892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grubb A. Glomerular filtration and shrunken pore syndrome in children and adults. Acta Paediatr. 2021;110(9):2503–8. [DOI] [PubMed] [Google Scholar]
- 66.Grubb A, Lindstrom V, Jonsson M, Back SE, Ahlund T, Rippe B, et al. Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: ‘Shrunken pore syndrome’. Scand J Clin Lab Invest. 2015;75(4):333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mackenzie HS, Brenner BM. Fewer nephrons at birth: a missing link in the etiology of essential hypertension? Am J Kidney Dis. 1995;26(1):91–8. [DOI] [PubMed] [Google Scholar]
- 68.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O’Connor C, et al. Glomerular Aging and Focal Global Glomerulosclerosis: A Podometric Perspective. J Am Soc Nephrol. 2015;26(12):3162–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, et al. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16(10):2953–66. [DOI] [PubMed] [Google Scholar]
- 70.Nishizono R, Kikuchi M, Wang SQ, Chowdhury M, Nair V, Hartman J, et al. FSGS as an Adaptive Response to Growth-Induced Podocyte Stress. J Am Soc Nephrol. 2017;28(10):2931–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, et al. Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol. 2012;23(8):1351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D’Agati VD, Chagnac A, de Vries AP, Levi M, Porrini E, Herman-Edelstein M, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12(8):453–71. [DOI] [PubMed] [Google Scholar]