Abstract
Objective/Background:
X-linked adrenoleukodystrophy (ALD) is a neurodegenerative disease that causes progressive gait and balance problems. Leg discomfort, sleep disturbances, and pain contribute to daily disability. We sought to investigate the prevalence and severity of Restless Legs Syndrome (RLS) in patients with ALD.
Patients/Methods:
We administered questionnaires and conducted diagnostic telephone interviews to assess RLS severity. We retrospectively extracted data from neurological examinations, functional gait measures, and laboratory assessments.
Results and Conclusions:
Thirty-two adults with ALD (21 female, 11 male) were recruited to participate. Thirteen patients (40.6%) had RLS (10/21 females and 3/11 males). The median age of RLS onset was 35 years [IQR = 22 – 54]. Patients with RLS had more signs and symptoms related to myelopathy, but not the brain demyelination seen in ALD. This pilot study suggests a high prevalence of RLS in adults with ALD, which may contribute to sleep problems and impair quality of life.
Keywords: adrenoleukodystrophy, adrenomyeloneuropathy, RLS, myelopathy
1. INTRODUCTION
X-linked adrenoleukodystrophy (ALD) is a progressive neurodegenerative disease caused by mutations in the ABCD1 gene, which result in dysfunctional ABCD1, a membrane protein that normally transports very long chain fatty acids (VLCFAs) into peroxisomes for degradation1. In men, mutations in ABCD1 lead to progressive spinal cord disease in all, primary adrenal insufficiency in 80%, and cerebral inflammatory disease in 60% across the lifespan2. As ALD is an X-linked disease, women were previously considered asymptomatic carriers. It is now known that although adrenal insufficiency and cerebral disease occur in less than 1% of females, more than 80% will eventually develop progressive spinal cord disease3,4.
Restless Legs Syndrome (RLS) is a sensory-motor neurological disorder characterized by an intense urge to move the legs, often with dysesthesias, which interferes with sleep and impairs quality of life5. It is more common in conditions with iron deficiency6, but has been associated with a variety of neurological disorders (Friedrich’s Ataxia7, peripheral neuropathy8, multiple sclerosis9, and Parkinson’s disease10). To our knowledge, no data has been published regarding RLS in patients with ALD. In our clinical experience, leg discomfort, sleep disturbance, and pain are prominent in both males and females with ALD and contribute to daily disability. While spasticity and neuropathy may contribute to leg discomfort, the degree of sleep disturbance at times appeared out of proportion to these symptoms alone. We therefore sought to investigate the prevalence, associations, and severity of RLS in this patient population.
2. MATERIALS AND METHODS
2.1. Design
The study was reviewed and approved by the Mass General Brigham Human Research Committee. All participants provided written informed consent prior to enrollment. Males and females over the age of 18 years with a genetically or biochemically confirmed diagnosis of ALD were recruited from the Leukodystrophy Clinic at Massachusetts General Hospital11. We included participants with genetically confirmed ALD, without radiographic evidence of inflammatory disease on brain MRI. We excluded individuals with other confounding neurological deficits that could affect participation in phone interviews or gait and balance assessments.
2.2. Diagnostic Interviews
Clinical telephone interviews were conducted using the Hopkins Telephone Diagnostic Interview (HTDI), a validated structured diagnostic RLS assessment12. Diagnostic criteria for RLS are based on a patient report of (1) urge to move the legs, usually accompanied or caused by uncomfortable leg sensations, which (2) begin or worsen during rest or inactivity and are (3) partially or totally relieved by movement. The symptoms above are (4) worse in the evening or night than during the day. The HTDI was independently administered by a board-certified sleep professional with expertise in RLS (J.W.) and a neurologist experienced in ALD (F.E.), who were blinded to each other’s assessments, and each decided whether the participant had “definite”, “probable”, “possible” or “not” RLS. Those with “definite” or “probable” RLS were considered RLS positive. Participants were identified as having “probable” rather than “definite” RLS if they satisfied all four characteristics of RLS but also met one of the following criteria: presence of leg discomfort with some features of neuropathy, the occurrence of symptoms only while sitting, or a morning onset from the beginning of symptom history. Differences in RLS diagnosis between the two clinicians were adjudicated in discussion and, if indicated, re-interview of the participant. An International Restless Legs Scale (IRLS) was administered to those with RLS, assessing severity of RLS symptoms in the past week13.
2.3. Retrospective Chart Review
To assess the extent of myelopathy-associated signs and symptoms, we retrospectively reviewed medical charts of patients who participated in the HTDI phone interviews. We extracted data on 12 major neurological signs and symptoms including the presence of muscle weakness, spasticity, impaired coordination, hyperreflexia, lower extremity sensory loss, positive Romberg test, gait/balance difficulty, paresthesia, neuropathic pain, bladder dysfunction, bowel dysfunction, and erectile dysfunction (in male patients). Neurological examinations were conducted by two neurologists with expertise in neuromuscular disorders (F.E. and R.S.). We also collected data from laboratory testing for ferritin levels. Neither limb actigraphy or polysomnography, either home or in-laboratory, was performed. Therefore, objective data on sleep or periodic limb movements in sleep was not available.
2.4. Functional Gait Assessment
Participants performed functional gait assessments including the 25-Foot Walk test (25-FW), the Timed Up and Go test (TUG), and Six Minute Walk test (6MW). The average of two trials was recorded for both the 25FW and TUG.
2.5. Statistical Analysis
Contingency tables were created and Fisher’s exact test statistics were used to assess the relationship between RLS and presence of neurological signs and symptoms.
2.6. Data Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
3. RESULTS
We evaluated 32 patients (21 female, 11 male) with ALD (Table 1). The median age of our cohort was 45.9 years [IQR=36.4–57.5] (females, 49.5 [IQR=43.7–58.6]; males, 36.0 [IQR=21.7–40.0]). Of the 27 patients with symptoms of myelopathy, the median age of onset was 34 years [IQR=30–38]. Eleven patients used walking aid for ambulation. Median scores for EDSS, 6MW, TUG, and 25-FW were 2.5 [IQR=1.5–3.5], 460 meters [IQR=359–549], 8.1 seconds [IQR=7.0–9.1], and 5.6 seconds [IQR=5.0–6.5], respectively. Patients with RLS had more neurological signs and symptoms, many related to their myelopathy (Figure 1). These myelopathy symptoms encompassed not only motor and sensory symptoms, but also bladder and bowel dysfunction as well as erectile dysfunction. Presence of RLS was significantly associated with patient reports of gait/balance difficulty (p=0.024) as well as pain (p=0.024). Seven patients had non-inflammatory brain lesions affecting the white matter. Nine patients (all male) had adrenal insufficiency.
Table 1.
Individual characteristics of patients in our cohort
| Pt | Sex | Age (yrs) | IRLS | RLS meds1 | AD meds2 | EDSS | Walking aid | Brain Lesion3 | AI | ID | AMN | AMN onset (yrs) | RLS onset (yrs) | 6MW (m) | TUG (s) | 25-FW (s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| RLS + | ||||||||||||||||
|
| ||||||||||||||||
| 3 | F | 41.6 | 26 (6 w/ med) | + | + | 2 | + | − | − | − | + | 30 | 35 | 333.8 | 9.3 | 7.6 |
| 4 | F | 58.7 | 11 | + | − | 3.5 | + | − | − | − | + | 32 | 56 | 395.3 | 8.7 | 6.3 |
| 5 | F | 57.6 | 25 (20 w/ med) | + | + | 2 | − | − | − | + | + | 37 | 43 | 534.3 | 6.6 | 5.6 |
| 17 | F | 57.7 | 13 | − | + | 1 | − | − | − | − | + | NR | 57 | 603.5 | 6.8 | 4.8 |
| 10 | M | 26.0 | 0 | − | − | 2 | + | + | + | − | + | 23 | 22 | 452.0 | 7.4 | 5.6 |
| 22 | F | 39.7 | 13 | − | + | 1.5 | − | − | − | − | + | 31 | 20 | NR | NR | NR |
| 24 | M | 37.6 | 23 | − | − | 3.5 | + | − | − | + | + | 25 | 30 | 368.8 | 13.7 | 8.6 |
| 25 | M | 39.1 | 0 | − | + | 3 | + | + | + | − | + | 30 | 35 | 358.7 | 8.8 | 7.2 |
| 26 | F | 73.3 | 26 | + | + | 4 | + | − | − | − | + | 68 | 68 | 568.1 | 7.0 | 5.0 |
| 27 | F | 46.3 | 10 | − | − | 1.5 | − | − | − | − | + | 38 | 45 | NR | NR | NR |
| 28 | F | 58.5 | 0 | − | + | 3 | − | − | − | − | + | 50 | 54 | NR | NR | NR |
| 30 | F | 35.4 | 21 | − | − | 1.5 | − | − | − | + | + | 21 | 20 | NR | NR | NR |
| 32 | F | 45.9 | 34 (19 w/ med) | + | + | 2.5 | − | − | − | − | + | 38 | 17 | NR | NR | NR |
|
| ||||||||||||||||
| RLS − | ||||||||||||||||
|
| ||||||||||||||||
| 1 | F | 79.2 | − | − | − | 4 | + | − | − | − | + | 58 | − | 345.9 | 9.5 | 6.5 |
| 2 | F | 45.8 | − | − | − | 0 | − | − | − | + | − | n/a | − | 502.6 | 8.1 | 5.6 |
| 6 | F | 34.1 | − | + | − | 3 | − | − | − | − | + | 26 | − | 272.5 | 13.3 | 8.2 |
| 7 | M | 26.2 | − | − | − | 0 | − | − | − | − | − | n/a | − | 269.7 | 7.5 | 4.8 |
| 8 | M | 36.0 | − | + | − | 3.5 | + | + | + | − | + | 31 | − | 440.1 | 9.0 | 5.7 |
| 9 | F | 60.9 | − | − | − | 1.5 | − | − | − | + | + | NR | − | 487.7 | 6.8 | 5.2 |
| 11 | M | 20.7 | − | − | − | 1 | − | − | + | − | − | n/a | − | 518.2 | 5.0 | 4.4 |
| 12 | F | 49.4 | − | + | − | 1.5 | − | − | − | − | + | NR | − | 548.6 | 5.6 | 4.4 |
| 13 | M | 43.7 | − | − | − | 2.5 | − | + | + | − | + | 37 | − | 548.6 | 7.6 | 6.3 |
| 14 | M | 50.8 | − | − | − | 3.5 | − | + | + | − | + | 39 | − | 548.6 | 8.2 | 5.5 |
| 15 | F | 49.8 | − | + | − | 3 | − | − | − | + | + | 37 | − | 377.6 | 8.3 | 6.5 |
| 16 | M | 21.7 | − | − | + | 1.5 | − | − | + | − | − | n/a | − | 479.1 | 7.8 | 5.2 |
| 18 | M | 21.6 | − | − | − | 4 | − | + | + | − | + | 21 | − | 576.1 | 7.6 | 4.7 |
| 19 | F | 40.9 | − | − | − | 0 | − | − | − | − | − | n/a | − | NR | NR | NR |
| 20 | F | 49.5 | − | + | − | 3 | + | − | − | − | + | 30 | − | 330.4 | 14.9 | 10.5 |
| 21 | M | 40.0 | − | − | − | 3.5 | − | + | + | − | + | 31 | − | 460.6 | 9.1 | 5.6 |
| 23 | F | 64.8 | − | + | − | 6.5 | + | − | − | − | + | 35 | − | NR | NR | NR |
| 29 | F | 47.9 | − | + | − | 2 | − | − | − | − | + | 35 | − | NR | NR | NR |
| 31 | F | 57.4 | − | + | − | 6 | + | − | − | − | + | 42 | − | NR | NR | NR |
includes baclofen, gabapentin, and pramipexole
includes venlafaxine, escitalopram oxalate, bupropion, trazodone, citalopram, desvenlafaxine succinate
includes self-halted and stable brain lesions
AD = anti-depressant; AI = adrenal insufficiency, AMN = adrenomyeloneuropathy; ID = iron deficiency or anemia; NR = not recorded; TUG = timed up and go test; 25-FW = 25-foot walk test; 6MW = 6-minute walk test
Figure 1.
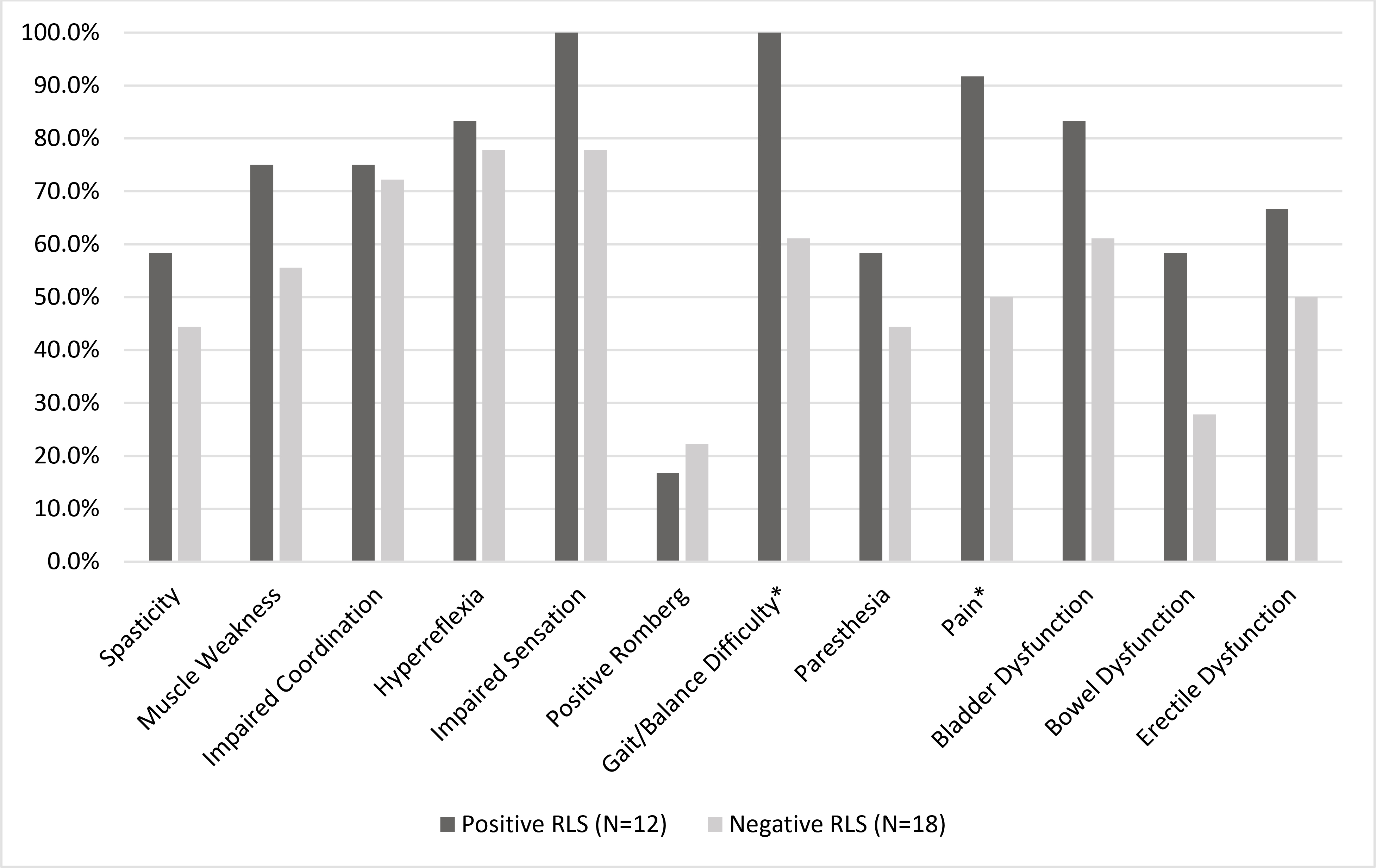
Percentage of patients in our cohort presenting with each neurological sign and symptom
*p<0.05
Thirteen patients (40.6%) were diagnosed with RLS by the HDTI interviews (10/21 [47.6%] females; 3/11 [27.3%] males) (Table 1). The median age of RLS onset was 35 years [IQR=22–54] (females, 44 [IQR=24–56]; males, 30 [IQR=26–33]). In the 7 patients with brain lesions, only two had a diagnosis of RLS; interestingly, these patients displayed very mild RLS (Table 1). Of note, none of the females in our study developed brain disease. Of the 13 patients with RLS, 6 (46.2%) had tried a medication to relieve their symptoms. Nine patients (8 with RLS, 1 without RLS) had a history of antidepressant use.
Six patients (5 females, 1 male) with RLS reported a history of anemia or iron deficiency. Ferritin levels were available for 14 patients (8 females with RLS, mean ferritin level of 74.0±71.6 ug/L; 4 females and 2 males without RLS, mean ferritin level of 99.5±79.2 ug/L).
4. DISCUSSION
In this pilot study, we found RLS in 40% of adults with ALD, with females more commonly affected than males. This is substantially higher than that in the general adult population, in which the prevalence is 5–10%14. Those with RLS also had more signs and symptoms of myelopathy, suggesting that ALD’s pathologic effect on spinal pathways could contribute to RLS.
The myelopathy of ALD is associated with neuronal loss and shrinkage in the dorsal root ganglia and the Clarke column of the spinal cord15. Such spinal sensory dysregulation may also be implicated in the genesis of RLS (see reviews16). In multiple sclerosis, in which roughly 25% of patients have RLS, spinal pathology is thought to play a role in RLS pathophysiology17. Further, transcutaneous spinal cord direct stimulation has shown promise as a treatment for RLS18.
Consistent with patterns observed in the general population5, risk factors for RLS in this cohort of adults with ALD included female gender, increased age, lower iron indices, and use of serotonergic antidepressants. Presence of myelopathy signs/symptoms was more frequent in those with RLS than in those without RLS. By contrast, brain disease associated with ALD did not predispose to RLS, as even the 2 patients with cerebral disease diagnosed with RLS had the mildest severity seen (IRLS=0). Therefore, it may be that ALD patients affected by the phenotype of adrenomyeloneuropathy are more predisposed to RLS symptoms.
Both idiopathic and secondary RLS are independently associated with substantial long-term detrimental effects on health, cognition, quality of life, psychiatric morbidity, and all-cause mortality19. For instance, in multiple sclerosis, RLS is associated with reductions in functional capacity as well as physical and psychological health-related quality of life, sleep quality, fatigue, depression, and anxiety20,21. Thus, sleep disturbance associated with RLS may, in our cohort, also have functional consequences and worsen the manifestations of myelopathy in ALD.
In RLS, CNS iron deficiency has consistently been identified, using CSF, transcranial Doppler, MRI and pathological investigations 22. Low serum ferritin is inversely correlated with RLS severity. Oral and IV iron treatment both improve symptoms in patients with RLS, the former in those with serum ferritin levels below 75 ug/L6. Little is known about iron metabolism in X-ALD, although mitochondrial dysfunction, oxidative stress, and bioenergetic failure play major roles in the pathogenesis23.
Our data supports a high prevalence of RLS in patients with ALD. Although the high rate of RLS in our patients could be an artifact of their serotonergic antidepressant use, it may be just as likely that RLS predated their mood disturbance, as depression is known to have a bidirectional relationship to RLS, potentially related to sleep disturbance or to the chronic discomfort24. Awareness of RLS in patients with ALD would allow for its effective treatment25 which may improve the functional impairments as well as quality of life, mood, and anxiety issues in those with ALD.
Supplementary Material
Highlights:
Restless Legs Syndrome (RLS) is prevalent in adults with adrenoleukodystrophy (ALD)
ALD patients with RLS have more neurological signs and symptoms
RLS is more common in females with ALD than in males
Awareness of RLS in patients with ALD would allow for its effective treatment
ACKNOWLEDGMENT
We acknowledge Nurse Practitioner Catherine Becker for her support in recruitment and care of patients with ALD.
Funding Sources:
Dr. Eichler receives research support from NINDS (U54 Global Leukodystrophy Initiative Clinical Trials Network (GLIA-CTN)), the European Leukodystrophy Association, the Arrivederci Foundation, the Leblang Foundation and the Hammer Family Fund for ALD Research and Therapies for Women. These funding sources had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Abbreviations:
- ALD
adrenoleukodystrophy
- HTDI
Hopkins Telephone Diagnostic Interview
- IRLS
International Restless Legs Scale
- RLS
restless legs syndrome
- TUG
Timed Up and Go test
- VLCFAs
very long chain fatty acids
- 25-FW
25-Foot Walk test
- 6MW
6-Minute Walk test
Footnotes
Declaration of interest: John W. Winkelman reports consultancies or honoraria from Advance Medical, Avadel, Disc Medicine, Eisai, Emalex, Idorsia, Noctrix, and UpToDate, research contracts with Merck Pharmaceuticals; and research support from NIDA (1R21DA052861-01) and the Baszucki Brain Research Foundation. Natalie R. Grant, Francine Molay, and Reza Sadjadi have nothing to disclose. Christopher D. Stephen reports a sponsored research contract with SwanBio Therapeutics. Florian S. Eichler reports sponsored research contracts with Minoryx Therapeutics and consultancies with Minoryx Therapeutics and SwanBio Therapeutics.
Credit author statement
John W. Winkelman: Conceptualization; Methodology; Investigation; Writing – Original Draft; Supervision
Natalie R. Grant: Investigation; Data Curation; Writing – Original Draft; Visualization
Francine Molay: Investigation; Data Curation; Writing – Review & Editing; Project administration
Christopher D. Stephen: Formal Analysis; Writing – Review & Editing
Reza Sadjadi: Formal Analysis; Data Curation; Writing – Review & Editing
Florian S. Eichler: Conceptualization; Methodology; Investigation; Writing – Original Draft; Supervision; Funding acquisition
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mosser J, Douart A-M, Sarde C-O, et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 1993; 361: 25. [DOI] [PubMed] [Google Scholar]
- 2.Engelen M, Kemp S, de Visser M, et al. X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis 2012; 7: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelen M, Barbier M, Dijkstra IM, et al. X-linked adrenoleukodystrophy in women: a cross-sectional cohort study. Brain 2014; 137(3): 693–706. [DOI] [PubMed] [Google Scholar]
- 4.Habekost CT, Pereira FS, Vargas CR, et al. Progression rate of myelopathy in X-linked adrenoleukodystrophy heterozygotes. Metab Brain Dis 2015; 30(5): 1279–1284. [DOI] [PubMed] [Google Scholar]
- 5.Allen RP, Picchietti DL, Garcia-Borreguero D, et al. ; International Restless Legs Syndrome Study Group. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med 2014; 15(8): 860–873. [DOI] [PubMed] [Google Scholar]
- 6.Allen RP, Picchietti DL, Auerbach M, et al. ; International Restless Legs Syndrome Study Group (IRLSSG). Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-Ekbom disease in adults and children: an IRLSSG task force report. Sleep Med 2018; 41: 27–44. [DOI] [PubMed] [Google Scholar]
- 7.Frauscher B, Hering S, Högl B, Gschliesser V, Ulmer H, Poewe W, Boesch SM. Restless legs syndrome in Friedreich ataxia: a polysomnographic study. Mov Disord 2011;26(2):302–6. [DOI] [PubMed] [Google Scholar]
- 8.Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, Agúndez JAG. Association between restless legs syndrome and peripheral neuropathy: A systematic review and meta-analysis. Eur J Neurol 2021; 28(7): 2423–2442. [DOI] [PubMed] [Google Scholar]
- 9.Manconi M, Fabbrini M, Bonanni E, Filippi M, Rocca M, Murri L, Ferini-Strambi L. High prevalence of restless legs syndrome in multiple sclerosis. Eur J Neurol 2007; 14(5): 534–539. [DOI] [PubMed] [Google Scholar]
- 10.Peralta CM, Frauscher B, Seppi K, Wolf E, Wenning GK, Högl B, Poewe W. Restless legs syndrome in Parkinson’s disease. Mov Disord. 2009; 24(14): 2076–2080. [DOI] [PubMed] [Google Scholar]
- 11.Godbole NP, Sadjadi R, DeBono MA, et al. Gait difficulties and postural instability in adrenoleukodystrophy. Front Neurol 2021; 12: 684102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hening WA, Allen RP, Washburn M, Lesage S, Earley CJ. Validation of the Hopkins telephone diagnostic interview for restless legs syndrome. Sleep Med 2008; 9(3): 283–289. [DOI] [PubMed] [Google Scholar]
- 13.Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, Trenkwalder C; International Restless Legs Syndrome Study Group. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003; 4(2): 121–132. [DOI] [PubMed] [Google Scholar]
- 14.Allen RP, Walters AS, Montplaisir J, Hening W, Myers A, Bell TJ, Ferini-Strambi L. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med 2005;165(11):1286–92 [DOI] [PubMed] [Google Scholar]
- 15.Powers JM, DeCiero DP, Cox C, Richfield EK, Ito M, Moser AB, Moser HW. The dorsal root ganglia in adrenomyeloneuropathy: neuronal atrophy and abnormal mitochondria. J Neuropathol Exp Neurol 2001; 60(5): 493–501. [DOI] [PubMed] [Google Scholar]
- 16.Dafkin C, McKinon W, Kerr S. Restless legs syndrome: Clinical changes in nervous system excitability at the spinal cord level. Sleep Med Rev 2019; 47: 9–17. [DOI] [PubMed] [Google Scholar]
- 17.Monschein T, Schestak C, Schillerwein-Kral C, Leutmezer F, Berger T, Bsteh G, Seidel S. Restless Legs Syndrome in Multiple Sclerosis: Risk factors and effect on sleep quality - a case-control study. Mult Scler Relat Disord 2021; 51: 102916. [DOI] [PubMed] [Google Scholar]
- 18.Heide AC, Winkler T, Helms HJ, Nitsche MA, Trenkwalder C, Paulus W, Bachmann CG. Effects of transcutaneous spinal direct current stimulation in idiopathic restless legs patients. Brain Stimul 2014; 7(5): 636–642. [DOI] [PubMed] [Google Scholar]
- 19.Gao X, Ba DM, Bagai K, Liu G, Ma C, Walters AS. Treating restless legs syndrome was associated with low risk of cardiovascular disease: a cohort study with 3.4 years of follow-up. J Am Heart Assoc 2021;10(4):e018674. doi: 10.1161/JAHA.120.018674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cederberg KLJ, Jeng B, Sasaki JE, Braley TJ, Walters AS, Motl RW. Restless legs syndrome and health-related quality of life in adults with multiple sclerosis. J Sleep Res 2020; 29(3). doi: 10.1111/jsr.12880. [DOI] [PubMed] [Google Scholar]
- 21.Giannaki CD, Aristotelous P, Stefanakis M, et al. Restless legs syndrome in multiple sclerosis patients: a contributing factor for fatigue, impaired functional capacity, and diminished health-related quality of life. Neurol Res 2018; 40(7): 586–592. [DOI] [PubMed] [Google Scholar]
- 22.Frauscher B, Gschliesser V, Brandauer E, et al. The severity range of restless legs syndrome (RLS) and augmentation in a prospective patient population: association with ferritin levels. Sleep Med 2009; 10: 611–615. [DOI] [PubMed] [Google Scholar]
- 23.van de Beek MC, Ofman R, Dijkstra I, Wijburg F, Engelen M, Wanders R, Kemp S. Lipid-induced endoplasmic reticulum stress in X-linked adrenoleukodystrophy. Biochim Biophys Acta Mol Basis Dis 2017; 1863(9): 2255–2265. [DOI] [PubMed] [Google Scholar]
- 24.Mackie S, Winkelman JW. Restless legs syndrome and psychiatric disorders. Sleep Med Clin 2015; 10(3): 351–357. [DOI] [PubMed] [Google Scholar]
- 25.Winkelman JW, Armstrong MJ, Allen RP, et al. Practice guideline summary: Treatment of restless legs syndrome in adults: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2016; 87(24): 2585–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


