Abstract
Statins reduce cholesterol, prevent cardiovascular disease, and are among the most commonly prescribed medications in the world. Statin-associated musculoskeletal symptoms (SAMS) impact statin adherence and ultimately can impede the long-term effectiveness of statin therapy. There are several identified pharmacogenetic variants that impact statin disposition and adverse events during statin therapy. SLCO1B1 encodes a transporter (SLCO1B1; alternative names include OATP1B1 or OATP-C) that facilitates the hepatic uptake of all statins. ABCG2 encodes an efflux transporter (BCRP) that modulates the absorption and disposition of rosuvastatin. CYP2C9 encodes a Phase-I drug metabolizing enzyme responsible for the oxidation of some statins. Genetic variation in each of these genes alters systemic exposure to statins (i.e., simvastatin, rosuvastatin, pravastatin, pitavastatin, atorvastatin, fluvastatin, lovastatin), which can increase the risk for SAMS. We summarize the literature supporting these associations and provide therapeutic recommendations for statins based on SLCO1B1, ABCG2, and CYP2C9 genotype with the goal of improving the overall safety, adherence and effectiveness of statin therapy. This document replaces the 2012 and 2014 Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for SLCO1B1 and simvastatin-induced myopathy.
Keywords: pharmacogenetics, pharmacogenomics, myalgias, rhabdomyolysis, CPIC, SLCO1B1, ABCG2, CYP2C9, HMGCR, CYP3A, statins, SAMS, simvastatin, rosuvastatin, fluvastatin, rosuvastatin, atorvastatin, lovastatin, pitavastatin, pravastatin
INTRODUCTION
In 2012, the Clinical Pharmacogenetics Implementation Consortium (CPIC) published a gene-based prescribing guideline for simvastatin based on SLCO1B1 genotype (1), and this guideline was updated in 2014 (2). The current document replaces the original 2012 guideline and the 2014 update. New to this guideline are the addition of recommendations for CYP2C9 and ABCG2 and addition of recommendations for all statins.. We summarize literature supporting how SLCO1B1, ABCG2, and CYP2C9 genotype test results should be applied to optimize new or existing statin therapy to reduce the risk of statin-associated musculoskeletal symptoms (SAMS). This CPIC document serves as a guide for selecting the most appropriate statin and the optimal dose if pharmacogenetic test results are available (not whether to perform pharmacogenetic testing). Decisions concerning when, in whom and at what intensity statin therapy should be initiated are beyond the scope of this manuscript and are extensively reviewed elsewhere (3). Given the balance of SAMS risk versus known cardiovascular disease benefit, for patients who are candidates for new statin therapy, pharmacogenetic test results may provide additional useful information. For patients currently prescribed statin therapy, depending on how long the patient has been tolerating the statin, pharmacogenetic test results may be used as the basis for changing to another statin type or dose. Statin therapy should neither be discontinued nor avoided based on SLCO1B1, ABCG2, or CYP2C9 genotype results for patients with an indication for statin therapy, especially if the statin therapy is based on the shared decision making between patient and provider. Although evidence review included other outcomes such as the impact of genetic variation on lipid-lowering, the recommendations provided in this guideline are based on the effect of genetic variations on the risk of SAMS.
FOCUSED LITERATURE REVIEW AND UPDATE
A systematic literature review was conducted, focusing on associations of statin-related clinical endpoints (efficacy and toxicity) with gene variants of SLCO1B1, ABCG2, CYP2C9, CYP3A4, CYP3A5, and HMGCR (details in Tables S1-S5 and Supplement). Based on the evidence review and insufficient evidence to support clinical implementation, no recommendations are provided for HMGCR, CYP3A4 or CYP3A5 (see Tables S4 and S5 and the supplement text for details). Hence, this guideline will focus only on SLCO1B1, ABCG2, and CYP2C9 genetic variation as these have been shown to impact statin exposure and risk of SAMS. As the previous CPIC guideline focused only on SLCO1B1 and simvastatin, the SLCO1B1 recommendation provided in this guideline should be considered a replacement of the previous SLCO1B1 and simvastatin recommendations (2).
GENES: SLCO1B1, ABCG2, AND CYP2C9
Background
SLCO1B1.
SLCO1B1 (alternative protein names include OATP1B1, OATP-C) is used in this guideline to designate the protein product of the SLCO1B1 gene. SLCO1B1 facilitates the hepatic uptake of statins, as well as other exogenous and endogenous compounds (e.g., bilirubin and 17-beta-glucuronosyl estradiol) (4). Decreased function of this transporter (inherited through genetic variability or acquired through drug-mediated inhibition) can markedly increase the systemic exposure to statins, the putative causal factor underlying the link to SAMS (5). The SLCO1B1 gene locus occupies 109 kb on chromosome 12 (Chr 12p12.2) and, although many single nucleotide variants (SNVs) have been identified in this gene, only a few are known to have a clinically relevant functional impact (SLCO1B1 Allele Definition and Functionality Tables (6, 7)). The common c.521T>C variant, rs4149056, produces a p.V174A substitution and is contained within SLCO1B1*5 and *15 haplotypes. The SLCO1B1*17 haplotype also contains the c.521T>C variant; however, this allele designation no longer exists (the Pharmacogene Variation Consortium (PharmVar, (8)) recently merged this allele with SLCO1B1*15. The minor C allele at c.521T>C has been associated with decreased transport function in vitro and increased systemic exposure to several drugs in vivo (See Table S1). Differences in allele frequencies have been observed across multiple ancestries and geographically diverse groups (SLCO1B1 Allele Frequency Table (6, 7)).
ABCG2.
ABCG2, which encodes the transporter ATP-Binding Cassette G2 (also known as Breast Cancer Resistance Protein, BCRP) is expressed in many different tissues including liver, blood-brain barrier and intestine. ABCG2 facilitates the export of compounds into the extracellular space. The ABCG2 gene locus spans over 66 kb on chromosome 4 (Chr 4q22.1). The common variant p.Q141K (c.421C>A, rs2231142) has been studied extensively; the minor A allele is associated with 30 to 40% reduced protein expression compared with the reference allele and with increased plasma levels of rosuvastatin (Table S2) (ABCG2 Allele Definition and Functionality tables (6, 7)). Differences in allele frequencies have been observed across multiple geographically, racially and ethnically diverse groups (ABCG2 Allele Frequency Table (6, 7)).
CYP2C9.
The CYP2C9 enzyme contributes to the Phase-I metabolism of many drugs. CYP2C9 is one of the CYP2C genes clustered in a 500-kb region on 10q24 (Chr 10q23.33) The CYP2C9 gene is highly polymorphic, with at least 71 variant alleles (CYP2C9 Allele Definition Table (6, 7, 9)). Differences in allele frequencies have been observed across multiple geographically, racially- and ethnically-diverse groups (CYP2C9 Allele Frequency Table (6, 7)). The two most extensively studied variants are CYP2C9*2 (p.R144C; rs1799853) and CYP2C9*3 (p.I359L; rs1057910) (10), which reduce CYP2C9 function by approximately 30–40% and 80%, respectively, and lead to increased systemic exposure to fluvastatin (CYP2C9 Allele Functionality table (6, 7).
Genetic Test Interpretation
SLCO1B1.
The assignment of the predicted SLCO1B1 phenotype, based on star (*) allele diplotypes, has been summarized in Table 1. SLCO1B1 haplotypes are often named using star allele nomenclature, representing various SNVs alone or in combination (PharmVar (8) and SLCO1B1 Allele Definition Table (6, 7, 11)) that are associated with altered SLCO1B1 protein expression or function (Allele Functionality Table (6, 7)). The combination of alleles is used to determine a patient’s diplotype (often also referred to as genotype), which can then be used to infer an individual’s predicted phenotype (Table 1; SLCO1B1 Diplotype to Phenotype table (6, 7)). Individuals with two increased function alleles (SLCO1B1*14/*14) have a SLCO1B1 increased function phenotype. Individuals with only normal function alleles (SLCO1B1*1/*1) or a normal function allele plus an increased function allele (SLCO1B1*1/*14) have a SLCO1B1 normal function phenotype, while individuals with one no function allele (e.g., SLCO1B1*5) and one normal or increased function allele have a SLCO1B1 decreased function phenotype and individuals with two no function alleles (e.g., SLCO1B1*5/*5) have an SLCO1B1 poor function phenotype.
TABLE 1.
ASSIGNMENT OF PREDICTED SLCO1B1, ABCG2, AND CYP2C9 LIKELY PHENOTYPE BASED ON GENOTYPE
| Gene | Phenotypea,b | Activity score (if applicable) | Genotype | Examples of diplotypes |
|---|---|---|---|---|
| SLCO1B1 | Increased function | n/a | An individual carrying two increased function alleles | *14/*14 |
| Normal function | n/a | An individual carrying two normal function alleles or one normal plus one increased function allele | *1/*1, *1/*14 | |
| Decreased function | n/a | An individual carrying one normal or increased function allele plus one no function allele | *1/*5, *1/*15, | |
| Possible Decreased Function |
An individual carrying one no function allele plus one uncertain/unknown function allele | *5/*6, *15/*10, *5/*43 | ||
| Poor function | n/a | An individual carrying two no function alleles | *5/*5, *5/*15, *15/*15 | |
| Indeterminate | n/a | An individual carrying one normal function allele plus one uncertain or unknown function allele OR allele combinations with uncertain and/or unknown function alleles | *1/*7, *1/*10, *7/*10 | |
| ABCG2 | Normal function | n/a | An individual carrying two normal function alleles | c.421 C/C (rs2231142) |
| Decreased function | n/a | An individual carrying one normal function allele plus one decreased function allele | c.421 C/A (rs2231142) | |
| Poor function | n/a | An individual carrying two decreased function alleles | c.421 A/A (rs2231142) | |
| CYP2C9 | Normal Metabolizer | 2 | An individual carrying two normal function alleles | *1/*1 |
| Intermediate Metabolizer |
1.5 1 |
An individual carrying one normal function allele plus one decreased function allele OR one normal function allele plus one no function allele OR two decreased function alleles |
*1/*2
*1/*3, *2/*2 |
|
| Poor Metabolizer | 0.5 0 |
An individual carrying one no function allele plus one decreased function allele OR two no function alleles |
*2/*3
*3/*3 |
|
| Indeterminate | n/a | An individual carrying allele combinations with uncertain and/or unknown function alleles | *1/*7, *1/*10, *7/*10 |
The most common and well-studied variant in SLCO1B1 is c.521T>C (rs4149056), and can be genotyped alone (e.g., PCR-based single SNV assay) or multiplexed on a variety of array-based platforms. All SLCO1B1 genetic tests should interrogate c.521T>C; however, while other less common variants in this gene may have limited evidence to guide action, they may also be important (SLCO1B1 Allele Definition and Functionality Tables (6, 7)).
ABCG2.
Unlike SLCO1B1 and CYP2C9, there is no star allele nomenclature to represent ABCG2 variants at this time. Assignment of the predicted ABCG2 phenotype is summarized in Table 1. An individual carrying one normal function allele plus one decreased function allele (rs2231142; c.421C>A) has ABCG2 decreased function and an individual with two decreased function alleles has ABCG2 poor function. rs2231142 can be genotyped alone (e.g., PCR-based single SNP assay) or multiplexed on a variety of array-based platforms. Various commercial genotyping platforms include rs2231142 in panels of pharmacogenetic variants (12).
CYP2C9.
Most clinical laboratories reporting CYP2C9 genotype use the star (*) allele nomenclature which can be found at PharmVar (8)and in the CYP2C9 Allele Definition Table (6, 7). The combination of alleles is used to determine a patient’s diplotype, which can then be used to infer an individual’s predicted metabolizer phenotype (Table 1; CYP2C9 Diplotype to Phenotype Table (6, 7)). Each allele’s functional status is assigned an activity value ranging from 0 to 1 (e.g., 0 for no function, 0.5 for decreased function, and 1.0 for normal function), which are summed to calculate the activity score (AS) for each diplotype (CYP2C9 Allele Functionality Table (6, 7)). The CYP2C9 AS is then translated into phenotype: individuals with an AS of 0 or 0.5 are poor metabolizers (PMs), those with an AS of 1 or 1.5 are intermediate metabolizers (IMs), and those with an AS of 2 are normal metabolizers (NMs) (Table 1; CYP2C9 Diplotype to Phenotype Table (6, 7)). Because reference laboratories providing clinical CYP2C9 genotyping may use varying methods to assign phenotypes, it is advisable to note a patient’s CYP2C9 diplotype and to refer to the CYP2C9 Diplotype to Phenotype Table online for a complete list of possible diplotypes and phenotype assignments before making therapeutic decisions.
Available Genetic Test Options
See the Genetic Testing Registry (www.ncbi.nlm.nih.gov/gtr/) for more information on commercially available clinical testing options.
Incidental findings
Genetic variability in SLCO1B1 influences the hepatic uptake of other drugs (e.g., methotrexate) (13, 14) as well as important endogenous compounds (e.g., bilirubin) (15). Complete SLCO1B1 and SLCO1B3 deficiency is associated with Rotor Syndrome (15). Genetic polymorphisms in ABCG2 influence absorption and disposition of many drugs including anti-cancer drugs and anti-viral drugs (16). In addition, genomewide association studies reveal that ABCG2 variants influence serum uric acid levels, risk for gout and response to the anti-gout medication, allopurinol (17, 18). In addition, null ABCG2 expression is associated with the Junior blood group, which determines presence of the Jr(a) antigen (19). No diseases or conditions have been consistently or strongly linked to variation in CYP2C9 independent of drug metabolism and response. CYP2C9 IMs and PMs may be predisposed to serious bleeding during warfarin therapy and increased risk of phenytoin- and non-steroidal anti-inflammatory drug (NSAID)-related toxicities (20–23).
Other considerations
All studies in this literature review investigated each gene individually for SAMS. As high throughput genotyping and more sequence-based analyses become more widely available, it is important to consider higher order interactions of these (and other) genes, in addition to epigenetic, drug-drug-gene and gene-environment interactions in statin therapies.
DRUGS: STATINS (HMG-CoA Reductase Inhibitors)
Background
One in four Americans aged 40 and older use a statin (24). In 2018, atorvastatin and simvastatin were the #1 and #10 most commonly prescribed drugs in the US, respectively. Statins have a wide therapeutic index. The most common statin-related adverse drug reaction (ADR) is skeletal muscle toxicity which manifest as SAMS (25). SAMS include a range of clinical entities from the most common (about 1 in 10), myalgia (pain without evidence of muscle degradation., i.e. creatine kinase levels < 3x normal); less common (about 1 in 2000), myopathy (evidence of muscle degradation with or without myalgia, i.e. creatine kinase levels ≥3x normal); and rare (less than 1 in 10,000), rhabdomyolysis (severe muscle damage with risk for acute kidney injury)(26). Based on extrapolation from dose-response and drug-drug interaction data, most SAMS cases are likely statin concentration-dependent (27) due to direct statin myotoxicity. An alternative form of SAMS stems from an autoimmune-mediated necrotizing myopathy characterized by autoantibodies against HMGCR and is not considered further in this guideline’s reference to SAMS.
The frequency of SAMS in clinical practice is higher than observed in blinded, placebo-controlled trials for reasons that can be attributed to differences in the types of patients enrolled in clinical trials versus practice, the use of “run-in” periods in clinical trials, as well as a potential “nocebo” effect of statins. Nevertheless, patients and providers frequently report SAMS in clinical practice and data from the National Health and Nutrition Examination Survey (NHANES) suggesting that the ‘number needed to harm’ maybe as high as 17 (28). Although described as “mild”, SAMS frequently leads to statin discontinuation, thus leading to higher cholesterol levels and a higher risk for cardiovascular disease if statins are not re-initiated (29, 30).
Linking genetic variability to variability in drug-related phenotypes
We applied a systematic approach to reviewing the evidence underlying the clinical validity of genetic associations with statin-related phenotypes including statin pharmacokinetics (in vivo and in vitro), SAMS, hepatotoxicity, lab-based efficacy (cholesterol lowering) and clinical efficacy (vascular event reduction). Statins evaluated included simvastatin, rosuvastatin, pravastatin, pitavastatin, atorvastatin, fluvastatin, and lovastatin. We reviewed the evidence for SLCO1B1, ABCG2, CYP2C9, CYP3A4/5, and HMGCR, and applied a grading system for each piece of evidence that evaluated an association between genotype and phenotype (Tables S1-S5). We found the highest levels of evidence for SLCO1B1 (all statins), ABCG2 (rosuvastatin), and CYP2C9 (fluvastatin), and this evidence forms the basis for therapeutic recommendations in the current guideline. Evidence tables for CYP3A4/5 and HMGCR are provided in the supplement (Tables S4 and S5). Based on weak evidence and the lack of conclusive clinical action based on genotype, no recommendations are provided for statins and CYP3A4/5 and HMGCR. See section “Linking genetic variability to variability in drug-related phenotypes” in the supplement for discussion of evidence.
Therapeutic Recommendations
SLCO1Bl.
The American College of Cardiology and the American Heart Association issued an updated clinical practice guideline for the management of blood cholesterol in 2018. In those guidelines, statins at various daily doses are classified as high-, medium- or low-intensity statins based on expected ranges of LDL-cholesterol lowering. For example, they recommend initiation of high-intensity statins in patients with evidence of clinical atherosclerotic cardiovascular disease (ASCVD) which may include atorvastatin at 40 or 80 mg once daily or rosuvastatin at 20 or 40 mg once daily (3). Figure 1 is designed to be used in conjunction with the aforementioned guideline, as it provides statin recommendations, including preferred statin intensity and statin dose, stratified by SLCO1B1 phenotype (i.e., decreased or poor function). Statin and statin doses indicated in the light grey boxes can be prescribed with the lowest risk for SAMS. Statin and statin doses indicated in dark grey boxes should be used with caution (possible increased risk for SAMS) and statin and statin doses indicated in black boxes should be avoided as the available evidence suggests that they are associated with increased risk of harm. The recommendations are based on the combination of available pharmacokinetic and SAMS-risk data, in most cases, and are informed by the number of available statin options within each intensity. Some statins and doses in Figure 1 were derived based on pharmacokinetic data only (see Figure 1 legend). Full recommendations can be found in Tables 2.
FIGURE 1.
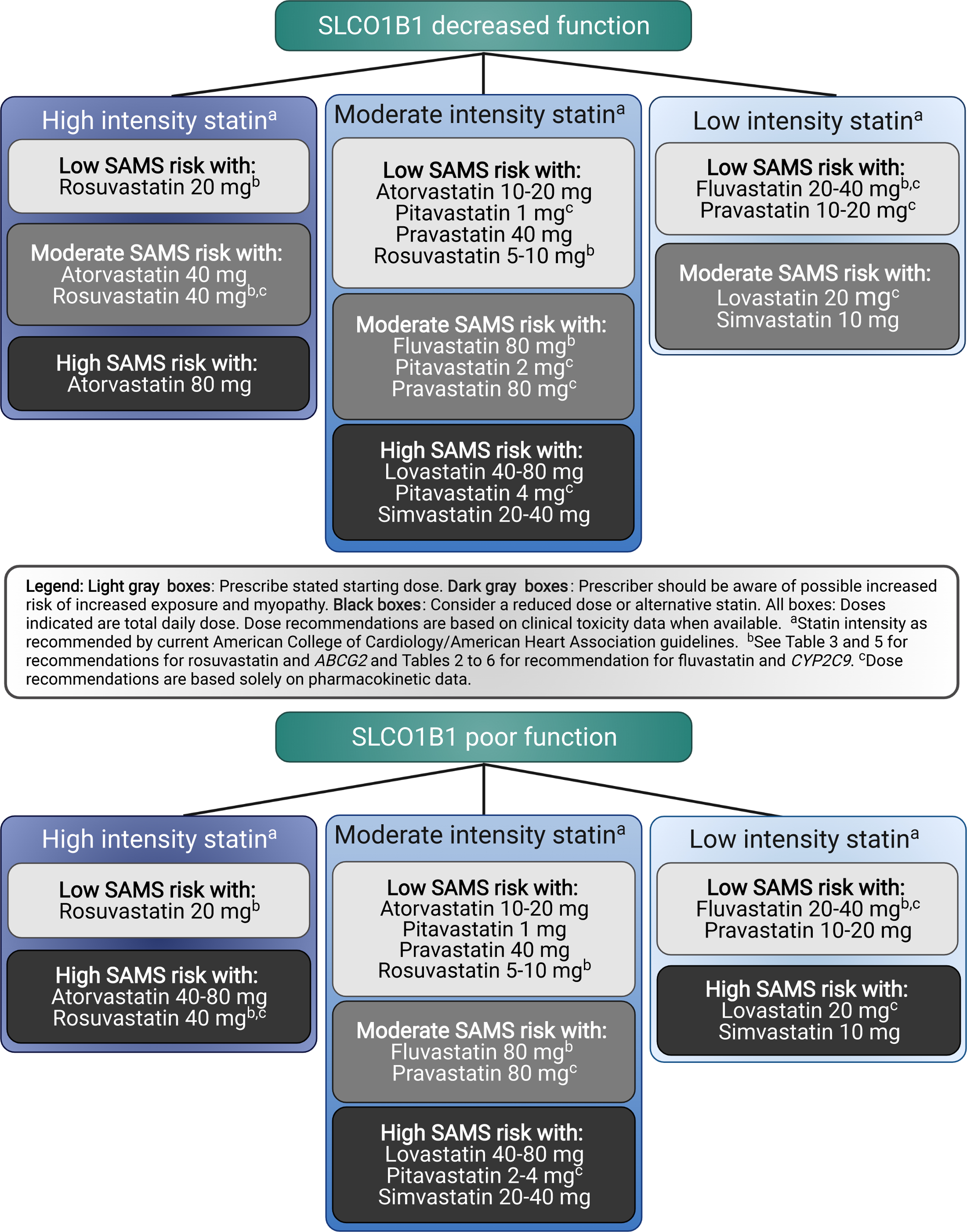
SLCO1B1 recommendations with intensity and statin dose stratified by SLCO1B1 phenotype; all doses assume adult dosing.
TABLE 2.
DOSING RECOMMENDATIONS FOR STATINS BASED ON SLCO1B1 PHENOTYPE IN ADULTS
| Phenotype | Implications | Dosing Recommendations | Classification of Recommendationsa | Considerations |
|---|---|---|---|---|
| All Statins | ||||
| SLCO1B1 Increased Function | Typical myopathy risk and statin exposure | Prescribe desired starting dose and adjust doses based on disease-specific guidelines. | Strong | The potential for drug-drug interactions and dose limits based on renal and hepatic function and ancestry should be evaluated prior to initiating a statin. |
| SLCO1B1 Normal Function | Typical myopathy risk and statin exposure | Prescribe desired starting dose and adjust doses based on disease-specific guidelines. | Strong | The potential for drug-drug interactions and dose limits based on renal and hepatic function and ancestry should be evaluated prior to initiating a statin. |
| Atorvastatin | ||||
| SLCO1B1 Decreased Function Or SLCO1B1 Possible Decreased Function | Increased atorvastatin exposure as compared to normal function which may translate to increased myopathy risk | Prescribe ≤40mg as a starting dose and adjust doses of atorvastatin based on disease-specific guidelines. Prescriber should be aware of possible increased risk for myopathy especially for 40mg dose. If dose >40mg needed for desired efficacy, consider combination therapy (i.e., atorvastatin plus non-statin guideline directed medical therapy) (3). | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| SLCO1B1 Poor Function | Increased atorvastatin exposure as compared to normal and decreased function which may translate to increased myopathy risk. | Prescribe ≤20mg as a starting dose and adjust doses of atorvastatin based on disease-specific guidelines. If dose >20mg is needed for desired efficacy, consider rosuvastatin or combination therapy (i.e., atorvastatin plus non-statin guideline directed medical therapy) (3). | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| Fluvastatin | ||||
| SLCO1B1 Decreased Function Or SLCO1B1 Possible Decreased Function | Increased fluvastatin exposure as compared to normal function; Typical myopathy risk with ≤40 mg. | Prescribe desired starting dose and adjust doses of fluvastatin based on disease-specific guidelines. Prescriber should be aware of possible increased risk for myopathy especially for doses >40mg per day. | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| SLCO1B1 Poor Function | Increased fluvastatin exposure as compared to normal and decreased function; Typical myopathy risk with doses less ≤40 mg. | Prescribe ≤40mg per day as a starting dose and adjust doses of fluvastatin based on disease-specific guidelines. If patient is tolerating 40mg per day but higher potency is needed, a higher dose (>40mg) or an alternative statin (see Figure 1 for recommendations for alternative statins) or combination therapy (i.e. fluvastatin plus non-statin guideline directed medical therapy)(3) could be considered. Prescriber should be aware of possible increased risk for myopathy with fluvastatin especially with doses >40mg per day. | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| Lovastatin | ||||
| SLCO1B1 Decreased Function Or SLCO1B1 Possible Decreased Function | Increased lovastatin acid exposure as compared to normal function which may translate to increased myopathy risk | Prescribe an alternative statin depending on the desired potency (see Figure 1 for recommendations for alternative statins). If lovastatin therapy is warranted, limit dose to ≤20mg/day. | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| SLCO1B1 Poor Function | Increased lovastatin acid exposure as compared to normal and decreased function which may translate to increased myopathy risk | Prescribe an alternative statin depending on the desired potency (see Figure 1 for recommendations for alternative statins). | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| Pitavastatin | ||||
| SLCO1B1 Decreased Function Or SLCO1B1 Possible Decreased Function | Increased pitavastatin exposure as compared to normal function which may translate to increased myopathy risk | Prescribe ≤ 2mg as a starting dose and adjust doses of pitavastatin based on disease-specific guidelines. Prescriber should be aware of possible increased risk for myopathy especially for doses >1mg. If dose >2mg needed for desired efficacy, consider an alternative statin (see Figure 1 for recommendations for alternative statins) or combination therapy (i.e. pitavastatin plus non-statin guideline directed medical therapy) (3). | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| SLCO1B1 Poor Function | Increased pitavastatin exposure as compared to normal and decreased function which may translate to increased myopathy risk. | Prescribe ≤1mg as a starting dose and adjust doses of pitavastatin based on disease-specific guidelines. If dose >1mg needed for desired efficacy, consider an alternative statin (see Figure 1 for recommendations for alternative statins) or combination therapy (i.e. pitavastatin plus non-statin guideline directed medical therapy)(3). | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| Pravastatin | ||||
| SLCO1B1 Decreased Function Or SLCO1B1 Possible Decreased Function | Increased pravastatin exposure as compared to normal function; Typical myopathy risk with doses ≤40 mg. | Prescribe desired starting dose and adjust doses of pravastatin based on disease-specific guidelines. Prescriber should be aware of possible increased risk for myopathy with pravastatin especially with doses >40mg per day. | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| SLCO1B1 Poor Function | Increased pravastatin statin exposure as compared to normal and decreased function; Typical myopathy risk with doses ≤40 mg. | Prescribe ≤40mg as a starting dose and adjust doses of pravastatin based on disease-specific guidelines. If patient is tolerating 40mg dose but higher potency is needed, a higher dose (>40mg) or an alternative statin (see Figure 1 for recommendations for alternative statins) or combination therapy (i.e. pravastatin plus non-statin guideline directed medical therapy)(3) could be considered. Prescriber should be aware of possible increased risk for myopathy especially with pravastatin doses >40mg. | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| Rosuvastatin | ||||
| SLCO1B1 Decreased Function Or SLCO1B1 Possible Decreased Function | Increased rosuvastatin exposure as compared to normal function; Typical myopathy risk with doses ≤20 mg. | Prescribe desired starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. Prescriber should be aware of possible increased risk for myopathy especially for doses >20mg. | Strong | The potential for drug-drug interactions and dose limits based on renal and hepatic function and Asian ancestry should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| SLCO1B1 Poor Function | Increased rosuvastatin exposure as compared to normal function and decreased function; Typical myopathy risk with doses ≤20 mg. | Prescribe ≤20mg as a starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines If dose >20mg needed for desired efficacy, consider combination therapy (i.e. rosuvastatin plus non-statin guideline directed medical therapy) (3). | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function and Asian ancestry should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| Simvastatin | ||||
| SLCO1B1 Decreased Function Or SLCO1B1 Possible Decreased Function | Increased simvastatin acid exposure as compared to normal function; increased risk of myopathy | Prescribe an alternative statin depending on the desired potency (see Figure 1 for recommendations for alternative statins). If simvastatin therapy is warranted, limit dose to <20mg/day. | Strong | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| SLCO1B1 Poor Function | Increased simvastatin acid exposure compared to normal and decreased function; highly increased myopathy risk | Prescribe an alternative statin depending on the desired potency (see Figure 1 for recommendations for alternative statins). | Strong | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
Rating scheme described in the Supplemental Material.
ABCG2.
Recommendations for ABCG2 are specific to rosuvastatin (Table 3). For individuals who have ABCG2 poor function, a rosuvastatin starting dose of ≤20mg is recommended; however, if a dose greater than 20mg is needed for desired efficacy, an alternative statin or combination therapy (e.g., statin + ezetimibe) is recommended. Although the risk of myopathy is unknown, rosuvastatin exposure (AUC) was 144% greater in those with the c.421AA genotype than the c.421CC genotype (wild-type) (31); thus, the recommendation is based primarily on pharmacokinetic data. Likely because of the higher hepatic exposure, the ABCG2 c.421A variant has also been associated with improved cholesterol lowering response to rosuvastatin in large genomewide association studies (32). Selection and dosing of rosuvastatin should also consider Asian ancestry (Table 3, see the Supplemental Material for more discussion). Atorvastatin pharmacokinetics are also affected by ABCG2 genetic variation; however, at this time, there is insufficient evidence to provide a recommendation (no recommendation, CPIC level C). As noted previously, there is also limited evidence for providing recommendations for other statins based on genetic variation in ABCG2.
TABLE 3.
DOSING RECOMMENDATIONS FOR ROSUVASTATIN BASED ON ABCG2 PHENOTYPE IN ADULTS
| Phenotype | Implications | Dosing Recommendations | Classification of Recommendationsa | Considerations |
|---|---|---|---|---|
| Normal Function | Typical myopathy risk and rosuvastatin exposure | Prescribe desired starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. | Strong | The potential for drug-drug interactions and dose limits based on renal and hepatic function and Asian ancestry should be evaluated prior to initiating rosuvastatin. |
| Decreased Function | Increased rosuvastatin exposure as compared to normal function; unknown risk for myopathy; increased lipid lowering effects | Prescribe desired starting dose and adjust doses of rosuvastatin based on disease-specific guidelines and specific population guidelines. | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function and Asian ancestry should be evaluated prior to initiating rosuvastatin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| Poor function | Increased rosuvastatin exposure compared to normal and decreased function; unknown myopathy risk; increased lipid-lowering effects | Prescribe ≤20mg as a starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. If dose >20mg needed for desired efficacy, consider an alternative statin or combination therapy (i.e., rosuvastatin plus non-statin guideline directed medical therapy)(3). | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function and Asian ancestry should be evaluated prior to initiating rosuvastatin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
Rating scheme described in the Supplemental Material.
CYP2C9.
Recommendations for fluvastatin based on CYP2C9 phenotype are available in Table 4. Genetic variations in CYP2C9 are associated with increased exposure to fluvastatin (Table S3), but the pharmacokinetics or pharmacodynamics of other statins are not affected.
TABLE 4.
DOSING RECOMMENDATIONS FOR FLUVASTATIN BASED ON CYP2C9 PHENOTYPE IN ADULTS
| Phenotype | Implication | Dosing recommendations | Classification of Recommendationsa | Considerations |
|---|---|---|---|---|
| CYP2C9 Normal Metabolizer | Normal exposure | Prescribe desired starting dose and adjust doses of fluvastatin based on disease-specific guidelines. | Strong | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. |
| CYP2C9 Intermediate Metabolizer AS of 1 and 1.5 | Increased fluvastatin exposure as compared to normal metabolizer which may translate to increased myopathy risk | Prescribe ≤40mg per day as a starting dose and adjust doses of fluvastatin based on disease-specific guidelines. If dose >40mg needed for desired efficacy, consider an alternative statin or combination therapy (i.e., fluvastatin plus non-statin guideline directed medical therapy) (3). | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
| CYP2C9 Poor Metabolizer AS 0.5 and 0 | Increased fluvastatin exposure as compared to normal and intermediate metabolizer which may translate to increased myopathy risk. | Prescribe ≤20mg per day as a starting dose and adjust doses of fluvastatin based on disease-specific guidelines. If dose >20mg needed for desired efficacy, consider an alternative statin or combination therapy (i.e., fluvastatin plus non-statin guideline directed medical therapy) (3). | Moderate | The potential for drug-drug interactions and dose limits based on renal and hepatic function should be evaluated prior to initiating a statin. The effects of drug-drug interactions may be more pronounced resulting in a higher risk of myopathy. |
Rating scheme described in the Supplemental Material.
CYP2C9 IMs should avoid fluvastatin doses greater than 40mg while CYP2C9 PMs should avoid doses greater than 20mg. If higher doses are required for desired efficacy, an alternative statin should be considered. If fluvastatin therapy is warranted, consider combination therapy of fluvastatin (40mg in IMs and 20mg in PMs) plus a non-statin lipid-lowering agent.
Combinatorial gene-based recommendations.
Although specific combinations of SLCO1B1 with ABCG2 or CYP2C9 genotypes are likely to result in additive effects on the pharmacokinetic properties of rosuvastatin or fluvastatin, respectively, little information is available on how to adjust initial doses based on combined genotype information (33). Combinatorial gene-based recommendations generated by extrapolating evidence supporting the single gene associations and assuming that they are additive, are provided for rosuvastatin in Table 5 and fluvastatin in Table 6. Because there are limited clinical or pharmacokinetic data regarding these combinatorial phenotypes, pharmacotherapy recommendations are classified as optional for the high-risk phenotype groups (e.g., SLCO1B1 no function plus ABCG2 no function). In the case of fluvastatin recommendations for CYP2C9 poor metabolizers who also have SLCO1B1 decreased or poor function, we recommend prescribing an alternative agent rather than prescribing a lower dose based on the available dosage forms (no dosage form less than 20 mg is available for fluvastatin).
TABLE 5.
COMBINED RECOMMENDATION FOR ROSUVASTATIN BASED ON SLCO1B1 AND ABCG2 PHENOTYPE IN ADULTS
| ABCG2 Normal Function | ABCG2 Decreased Function | ABCG2 Poor Function | |
|---|---|---|---|
| SLCO1B1 Increased Function | Prescribe desired starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. STRONG | Prescribe desired starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. MODERATE | Prescribe ≤20mg as a starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. If dose >20mg needed for desired efficacy, consider an alternative statin or combination therapy (i.e., rosuvastatin plus non-statin guideline directed medical therapy)(3). OPTIONAL |
| SLCO1B1 Normal Function | Prescribe desired starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. STRONG | Prescribe desired starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. MODERATE | Prescribe ≤20mg as a starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. If dose >20mg needed for desired efficacy, consider an alternative statin or combination therapy (i.e., rosuvastatin plus non-statin guideline directed medical therapy)(3). OPTIONAL |
| SLCO1B1 Decreased Function OR Possible SLCO1B1 Decreased Function | Prescribe desired starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. Prescriber should be aware of possible increased risk for myopathy especially for doses >20mg. STRONG | Prescribe desired starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. Prescriber should be aware of possible increased risk for myopathy especially for doses >20mg. MODERATE | Prescribe ≤10mg as a starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. If dose >10mg needed for desired efficacy, consider an alternative statin or combination therapy (i.e., rosuvastatin plus non-statin guideline directed medical therapy)(3).OPTIONAL |
| SLCO1B1 Poor Function | Prescribe ≥20mg as a starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. If dose > 20mg needed for desired efficacy, consider combination therapy (i.e., rosuvastatin plus non-statin guideline directed medical therapy) (3). MODERATE | Prescribe ≥20mg as a starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. If dose >20mg needed for desired efficacy, consider combination therapy (i.e., rosuvastatin plus non-statin guideline directed medical therapy) (3). MODERATE | Prescribe ≤10mg as a starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines. If dose >10mg needed for desired efficacy, consider combination therapy (i.e., rosuvastatin plus non-statin guideline directed medical therapy)(3). OPTIONAL |
Rating scheme described in the Supplemental Material
TABLE 6.
COMBINED RECOMMENDATION FOR FLUVASTATIN BASED ON SLCO1B1 AND CYP2C9 PHENOTYPE IN ADULTS
| CYP2C9 Normal Metabolizer | CYP2C9 Intermediate Metabolizer | CYP2C9 Poor Metabolizer | |
|---|---|---|---|
| SLCO1B1 Increased Function | Prescribe desired starting dose and adjust doses of fluvastatin based on disease-specific guidelines. STRONG | Prescribe ≤40mg per day as a starting dose and adjust doses of fluvastatin based on disease-specific guidelines. If dose >40mg needed for desired efficacy, consider an alternative statin or combination therapy (i.e., fluvastatin plus non-statin guideline directed medical therapy) (3). MODERATE | Prescribe ≤20mg per day as a starting dose and adjust doses of fluvastatin based on disease-specific guidelines. If dose >20mg needed for desired efficacy, consider an alternative statin or combination therapy (i.e., fluvastatin plus non-statin guideline directed medical therapy) (3). MODERATE |
| SLCO1B1 Normal Function | Prescribe desired starting dose and adjust doses of fluvastatin based on disease-specific guidelines. STRONG | Prescribe ≤40mg per day as a starting dose and adjust doses of fluvastatin based on disease-specific guidelines. If dose >40mg needed for desired efficacy, consider an alternative statin or combination therapy (i.e., | Prescribe ≤20mg per day as a starting dose and adjust doses of fluvastatin based on disease-specific guidelines. If dose >20mg needed for desired efficacy, consider an alternative statin or combination therapy (i.e., fluvastatin plus non- |
| fluvastatin plus non-statin guideline directed medical therapy) (3). MODERATE | statin guideline directed medical therapy) (3). MODERATE | ||
| SLCO1B1 Decreased Function OR Possible Decreased Function | Prescribe desired starting dose and adjust doses of fluvastatin based on disease-specific guidelines. Prescriber should be aware of possible increased risk for myopathy especially for doses >40mg per day. MODERATE | Prescribe ≤20mg per day as a starting dose and adjust doses of fluvastatin based on disease-specific guidelines. If dose >20mg needed for desired efficacy, consider an alternative statin or combination therapy (i.e., fluvastatin plus non-statin guideline directed medical therapy) (3). OPTIONAL | Prescribe an alternative statin depending on the desired potency (see Figure 1 for recommendations for alternative statins). OPTIONAL |
| SLCO1B1 Poor Function | Prescribe ≤40mg per day as a starting dose and adjust doses of fluvastatin based on disease-specific guidelines. If patient is tolerating 40mg per day but higher potency is needed, a higher dose (>40mg) or an alternative | Prescribe an alternative statin depending on the desired potency (see Table 2 and Figure 1 for recommendations for alternative statins). OPTIONAL | Prescribe an alternative statin depending on the desired potency (see Table 2 and Figure 1 for recommendations for alternative statins). OPTIONAL |
Rating scheme described in the Supplemental Material
General guidance for patients already receiving statin therapy.
The therapeutic recommendations described herein predominately apply to a new or a revision (dose or type) to statin prescription. However, given the increasing shift towards panel-based testing for multiple pharmacogenes, and the vast number of individuals already receiving statin therapy, an important issue to consider is how to manage statin therapy for patients that may already be receiving statin therapy, and then receive a genotype result, particularly for those whose genotype indicates that they are in a higher risk category based on the currently prescribed statin (i.e., moderate or high SAMS risk in Figure 1). For patients with SLCO1B1 genotype-statin dose combinations that fall within the moderate SAMS risk categories in Figure 1 who have already been on a stable statin and dose for at least 4 weeks without any symptoms suggestive of SAMS, then it is reasonable to continue that statin and dose long term (34). If those patients have been receiving that statin therapy for less than 4 weeks, then clinicians may consider changing to a lower SAMS risk statin/dose in order to prevent the development of SAMS. For patients that fall into the high SAMS risk categories, and they have been taking that statin therapy for at least 1 year without any negative effects, then it is deemed safe to continue that statin therapy long term. If those patients have been taking statin therapy for less than 1 year, then clinicians may consider changing to a lower SAMS risk statin/dose in order to reduce the risk for development of SAMS. These recommendations for the minimum duration of statin therapy for continued safe use long term are primarily based on expert opinion and the onset of SAMS observed for simvastatin in different SLCO1B1 genotypes in a single prospective clinical trial(34).
Pediatrics.
At the time of this writing, there are no data available regarding SLCO1B1 genotype effects on statin response or myopathy in pediatric patients. However, pharmacokinetic data show that the rs4149056 SNV in SLCO1B1 may affect the disposition of simvastatin more in children compared to adults, and the variant has equivalent impact on pravastatin and rosuvastatin pharmacokinetics between children and adults (35–37).
Recommendations for Incidental Findings
CPIC has published guidelines for utilizing CYP2C9 genotype for prescribing phenytoin, NSAIDs and warfarin (20–23).
Other Considerations
Other factors influencing SAMS.
Other factors known to influence a patient’s risk for developing SAMS include increased statin dose, drug interactions, advanced age, small body mass index, female gender, metabolic co-morbidities (e.g., hypothyroidism), intense physical exercise, and Asian or African ancestry (25, 38–41) (see Supplement). Because polypharmacy is common in the elderly, the association with age is often partly attributed to drug-drug interactions (see below) as well as increases in the frequency of chronic renal or hepatic disease (42).
Statin dose is the strongest independent predictor of myopathy risk. The risk of SAMS is approximately 6-fold higher in patients on high-dose than lower-dose statin therapy (43). Among all statins, a growing body of evidence suggests that the influence of dose may be greatest for simvastatin (44). The exact molecular mechanism of SAMS is unclear, and evidence supports both direct and indirect myotoxic effects of statins on skeletal muscle, possibly mediated through changes in the balance of isoprenoids accompanying the inhibition of skeletal muscle HMG CoA reductase (45–47).
Drug-Drug Interactions.
In the context of statin monotherapy, myopathy rates are low (48). The frequency of this ADR increases with co-administration of medications altering the pharmacokinetics of statins (e.g., co-administration with cyclosporine [SLCO1B1 and ABCG2 interaction], gemfibrozil [SLCO1B1 and CYP2C8 (fluvastatin only) interaction] or calcium channel blockers [CYP3A4/5 interaction]). See the Supplemental Material for more information. A list of inhibitors for CYP3A, CYP2C9, SLCO1B1,ABCG2, CYP3A4 and CYP2C8 is available on the US FDA site (49).
POTENTIAL BENEFITS AND RISKS FOR THE PATIENT
Based on the highly prevalent use of statins, one potential benefit of preemptive SLCO1B1, ABCG2, and CYP2C9 testing may be a reduction in the incidence of SAMS, by identifying those at significant risk and recommending a lower statin dose or an alternative statin with lower SAMS risk. While prospective data showing that prescribing based on genetic testing results alter SAMS incidence are lacking, there are emerging data demonstrating an improvement in patient’s perceptions of statins, appropriate statin prescribing, neutral data on patient-reported adherence, and mixed data on reducing LDL-cholesterol levels (50, 51) as other potential benefits of applying SLCO1B1 testing to clinical practice.
A possible risk could be an error in genotyping. Because genotypes are lifelong test results, any such error could stay in the medical record for the life of the patient. An error in genotyping could result in a decrease in statin dose that was not otherwise necessary and could result in inadequate lipid lowering therapy. However, this risk can be minimized by 1) monitoring to ensure that the appropriate LDL-cholesterol reduction is achieved for the intended statin intensity and 2) using an alternative statin with a similar statin intensity based on the recommendation in Figure 1. Another potential risk is that a patient or provider may inappropriately stop or avoid statin therapy, and this could cause higher LDL-cholesterol and increased cardiovascular risk.
CAVEATS: APPROPRIATE USE AND/OR POTENTIAL MISUSE OF GENETIC TESTS
As with any diagnostic test, genetic variation is just one factor that clinicians should consider when prescribing statins. Furthermore, rare variants may not be included in the genotype test used, and patients with rare variants that reduce SLCO1B1 function may be incorrectly assigned a normal phenotype based on a default to wild-type (*1) test result.
In summary, statins are a powerful class of medications for lowering LDL cholesterol and cardiovascular risk with an established track record of safety and efficacy. However, statin- related musculoskeletal symptoms are the most frequently cited reason for discontinuing statin therapy. Although clinicians are well-tuned to trial stopping and later reinitiating statin therapy in those who develop SAMS, in many patients statin therapy is never restarted. As a result, LDL cholesterol values are higher as is their risk for cardiovascular disease. We applied a rigorous approach evaluating the collective evidence around SLCO1B1, ABCG2, and CYP2C9 on systemic drug exposure and risk of SAMS. Our evidenced-based recommendations for genotype-guided statin therapy are focused on reducing the risk of SAMS. Based on this foundation, future research can evaluate the extent to which implementation of these guidelines impacts prescribing, SAMS risk, statin adherence, LDL cholesterol levels, and risk for cardiovascular events in patients prescribed statin therapy.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the critical input of Dr. Mary V. Relling (St Jude Children’s Research Hospital) and the members of the Clinical Pharmacogenetics Implementation Consortium (CPIC). This work was funded by the National Institutes of Health (NIH) for CPIC (R24GM115264 and U24HG010135) and PharmGKB (R24GM61374). Additional author support includes P50GM115318 (RMK), HL143161 (S.T.), R01GM117163 (KMG, SWY), and K08HL146990 (J.A.L). M.N. is funded by a European Research Council ERC Consolidator Grant (Grant agreement 725249).
FUNDING
This work was funded by the National Institutes of Health (NIH) for CPIC (R24GM115264 and U24HG010135) and PharmGKB (U24 HG010615). Additional author support includes P50GM115318 (RMK), HL143161 (S.T.), U01HG007269 (RMC-D), R01GM117163 (KMG, SWY), and K08HL146990 (J.A.L). M.N. is funded by a European Research Council ERC Consolidator Grant (Grant agreement 725249).
Footnotes
DISCLAIMER
Publisher's Disclaimer: Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines reflect expert consensus based on clinical evidence and peer-reviewed literature available at the time they are written and are intended only to assist clinicians in decision-making, as well as to identify questions for further research. New evidence may have emerged since the time a guideline was submitted for publication. Guidelines are limited in scope and are not applicable to interventions or diseases not specifically identified. Guidelines do not account for all individual variation among patients and cannot be considered inclusive of all proper methods of care or exclusive of other treatments. It remains the responsibility of the health care provider to determine the best course of treatment for the patient. Adherence to any guideline is voluntary, with the ultimate determination regarding its application to be solely made by the clinician and the patient. CPIC assumes no responsibility for any injury to persons or damage to property related to any use of CPIC’s guidelines, or for any errors or omissions.
CONFLICTS OF INTEREST
THE AUTHORS DECLARED NO COMPETING INTERESTS FOR THIS WORK.
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/CPT.2557
REFERENCES
- (1).Wilke RA et al. The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin Pharmacol Ther 92, 112–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Ramsey LB et al. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin Pharmacol Ther 96, 423–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Grundy SM et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 73, 3168–209 (2019). [DOI] [PubMed] [Google Scholar]
- (4).Niemi M, Pasanen MK & Neuvonen PJ Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev 63, 157–81 (2011). [DOI] [PubMed] [Google Scholar]
- (5).Turner RM & Pirmohamed M Statin-Related Myotoxicity: A Comprehensive Review of Pharmacokinetic, Pharmacogenomic and Muscle Components. J Clin Med 9, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).CPIC. CPIC guideline for statins and SLCO1B1, ABCG2 and CYP2C9. <https://cpicpgx.org/guidelines/cpic-guideline-for-statins/>. Accessed August 1, 2021 2021.
- (7).PharmGKB. PGx Gene-specific Information Tables.
- (8).PharmVar. Pharmacogene Variation Consortium. <https://www.pharmvar.org/>. Accessed October 18 2021.
- (9).Sangkuhl K et al. PharmVar GeneFocus: CYP2C9. Clin Pharmacol Ther 110, 662–76 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Lee CR, Goldstein JA & Pieper JA Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics 12, 251–63 (2002). [DOI] [PubMed] [Google Scholar]
- (11).Gaedigk A et al. The Evolution of PharmVar. Clin Pharmacol Ther 105, 29–32 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Scott SA et al. Development and Analytical Validation of a 29 Gene Clinical Pharmacogenetic Genotyping Panel: Multi-Ethnic Allele and Copy Number Variant Detection. Clin Transl Sci 14, 204–13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ramsey LB et al. Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Res 22, 1–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ramsey LB et al. Genome-wide study of methotrexate clearance replicates SLCO1B1. Blood 121, 898–904 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).van de Steeg E et al. Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J Clin Invest 122, 51928 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Heyes N, Kapoor P & Kerr ID Polymorphisms of the Multidrug Pump ABCG2: A Systematic Review of Their Effect on Protein Expression, Function, and Drug Pharmacokinetics. Drug Metab Dispos 46, 1886–99 (2018). [DOI] [PubMed] [Google Scholar]
- (17).Eckenstaler R & Benndorf RA The Role of ABCG2 in the Pathogenesis of Primary Hyperuricemia and Gout-An Update. Int J Mol Sci 22, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Brackman DJ et al. Genome-Wide Association and Functional Studies Reveal Novel Pharmacological Mechanisms for Allopurinol. Clin Pharmacol Ther 106, 623–31 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Saison C et al. Null alleles of ABCG2 encoding the breast cancer resistance protein define the new blood group system Junior. Nat Genet 44, 174–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Caudle KE et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clin Pharmacol Ther 96, 542–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Johnson JA et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Pharmacogenetics-Guided Warfarin Dosing: 2017 Update. Clin Pharmacol Ther 102, 397–404 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Karnes JH et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C9 and HLA-B Genotypes and Phenytoin Dosing: 2020 Update. Clin Pharmacol Ther 109, 302–9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Theken KN et al. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clin Pharmacol Ther 108, 191–200 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Gu Q, Paulose-Ram R, Burt V & Kit B Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012. In: NCHS data brief, no 177. (Hyattsville, MD: National Center for Health Statistics, 2014). [PubMed] [Google Scholar]
- (25).Wilke RA et al. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nat Rev Drug Discov 6, 904–16 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Alfirevic A et al. Phenotype standardization for statin-induced myotoxicity. Clin Pharmacol Ther 96, 470–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Abd TT & Jacobson TA Statin-induced myopathy: a review and update. Expert Opin Drug Saf 10, 373–87 (2011). [DOI] [PubMed] [Google Scholar]
- (28).Buettner C, Rippberger MJ, Smith JK, Leveille SG, Davis RB & Mittleman MA Statin use and musculoskeletal pain among adults with and without arthritis. Am J Med 125, 176–82 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Serban MC et al. Statin Intolerance and Risk of Coronary Heart Events and All-Cause Mortality Following Myocardial Infarction. J Am Coll Cardiol 69, 1386–95 (2017). [DOI] [PubMed] [Google Scholar]
- (30).Cohen JD, Brinton EA, Ito MK & Jacobson TA Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol 6, 208–15 (2012). [DOI] [PubMed] [Google Scholar]
- (31).Keskitalo JE, Zolk O, Fromm MF, Kurkinen KJ, Neuvonen PJ & Niemi M ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther 86, 197–203 (2009). [DOI] [PubMed] [Google Scholar]
- (32).Chasman DI, Giulianini F, MacFadyen J, Barratt BJ, Nyberg F & Ridker PM Genetic determinants of statin-induced low-density lipoprotein cholesterol reduction: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Circ Cardiovasc Genet 5, 257–64 (2012). [DOI] [PubMed] [Google Scholar]
- (33).Wilke RA, Reif DM & Moore JH Combinatorial pharmacogenetics. Nat Rev Drug Discov 4, 911–8 (2005). [DOI] [PubMed] [Google Scholar]
- (34).Group SC et al. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med 359, 789–99 (2008). [DOI] [PubMed] [Google Scholar]
- (35).Wagner JB et al. Impact of SLCO1B1 Genetic Variation on Rosuvastatin Systemic Exposure in Pediatric Hypercholesterolemia. Clin Transl Sci 13, 628–37 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Wagner JB et al. Impact of Genetic Variation on Pravastatin Systemic Exposure in Pediatric Hypercholesterolemia. Clin Pharmacol Ther 105, 1501–12 (2019). [DOI] [PubMed] [Google Scholar]
- (37).Wagner JB et al. Impact of SLCO1B1 Genotype on Pediatric Simvastatin Acid Pharmacokinetics. J Clin Pharmacol 58, 823–33 (2018). [DOI] [PubMed] [Google Scholar]
- (38).de Lemos JA et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 292, 1307–16 (2004). [DOI] [PubMed] [Google Scholar]
- (39).Chung JY et al. Effect of OATP1B1 (SLCO1B1) variant alleles on the pharmacokinetics of pitavastatin in healthy volunteers. Clin Pharmacol Ther 78, 342–50 (2005). [DOI] [PubMed] [Google Scholar]
- (40).Lee E et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther 78, 330–41 (2005). [DOI] [PubMed] [Google Scholar]
- (41).Hippisley-Cox J & Coupland C Individualising the risks of statins in men and women in England and Wales: population-based cohort study. Heart 96, 939–47 (2010). [DOI] [PubMed] [Google Scholar]
- (42).Thompson PD, Clarkson P & Karas RH Statin-associated myopathy. JAMA 289, 168190 (2003). [DOI] [PubMed] [Google Scholar]
- (43).McClure DL, Valuck RJ, Glanz M, Murphy JR & Hokanson JE Statin and statinfibrate use was significantly associated with increased myositis risk in a managed care population. J Clin Epidemiol 60, 812–8 (2007). [DOI] [PubMed] [Google Scholar]
- (44).Link E et al. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N.Engl.J.Med. 359, 789–99 (2008). [DOI] [PubMed] [Google Scholar]
- (45).Ananthakumar A, Liu Y, Fernandez CE, Truskey GA & Voora D Modeling statin myopathy in a human skeletal muscle microphysiological system. PLoS One 15, e0242422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Osaki Y et al. Skeletal muscle-specific HMG-CoA reductase knockout mice exhibit rhabdomyolysis: A model for statin-induced myopathy. Biochem Biophys Res Commun 466, 536–40 (2015). [DOI] [PubMed] [Google Scholar]
- (47).Schirris TJ et al. Statin-Induced Myopathy Is Associated with Mitochondrial Complex III Inhibition. Cell Metab 22, 399–407 (2015). [DOI] [PubMed] [Google Scholar]
- (48).Graham DJ et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipidlowering drugs. JAMA 292, 2585–90 (2004). [DOI] [PubMed] [Google Scholar]
- (49).FDA. Drug Development and Drug Interactions | Table of Substrates, Inhibitors and Inducers. <https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-druginteractions-table-substrates-inhibitors-and-inducers>. Accessed Aug. 30 2021.
- (50).Vassy JL et al. Effect of Pharmacogenetic Testing for Statin Myopathy Risk vs Usual Care on Blood Cholesterol: A Randomized Clinical Trial. JAMA Netw Open 3, e2027092 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Peyser B et al. Effects of Delivering SLCO1B1 Pharmacogenetic Information in Randomized Trial and Observational Settings. Circ Genom Precis Med 11, e002228 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


