Abstract
The cachexia syndrome in cancer is characterized by weight loss due to the combination of anorexia and atrophy of adipose and skeletal muscle. For decades, inflammatory circulatory factors have been identified to regulate wasting, yet inhibitors of these factors have not yielded the same clinical benefit as in animal models. Therefore, additional mediators of cachexia likely regulate this syndrome, and such factors might be more suitable for targeted intervention. Here, we highlight several anorexia-cachexia signaling mediators, including activin A, myostatin, GDF15, and lipocalin-2. Current evidence is discussed that these factors associate with cachexia in cancer patients and translational efforts including essential early phase clinical trials are summarized. We conclude with thoughts on targeted and personalized approaches for future anti-cachexia treatments.
Keywords: cachexia, anorexia, cytokines, inflammation, pancreatic cancer
The Cancer Cachexia Syndrome
Cachexia is a common clinical syndrome of cancer patients that is characterized by involuntary weight loss due to depletion of skeletal muscle and adipose tissue [1, 2]. Because of the frequency with which cachexia occurs in conjunction with anorexia, or decreased appetite, it is often referred to as the anorexia- cachexia syndrome. Although patients with weight loss greater than 5% are generally considered cachectic, it is not uncommon for patients to lose 20–25% of their pre-illness weight [3, 4]. Continual weight loss associates with a poor prognosis, and patients with cachexia are often weak and prone to fatigue, which lowers their quality of life and complicates their care. Nutritional intervention promotes some weight gain in patients, but this weight is mostly transient in the form of adipose tissue, while the loss of lean muscle is thought not to be reversable [5]. Therefore, there remains an urgent need to develop effective cachexia therapies that can translate to better quality of life, improved outcomes, and when given in combination with anti-tumor therapies, increased survival.
The cachexia syndrome does not present equally among tumor types. Pancreatic ductal adenocarcinoma (PDAC) patients have amongst the highest incidence of cachexia, estimated at 70% [1, 6, 7]. In addition, while an increased incidence of cachexia is associated with advanced disease, a significant proportion of PDAC patients already meet cachexia criteria at the time of cancer diagnosis. Other risk groups include esophageal, lung, liver, colon, gastric, and head and neck cancers, while breast, melanoma, prostate, and thyroid cancers present with the lowest incidence [1]. The current basis for these differences remains to be determined.
Over the decades that the cachexia syndrome has been studied in cancer, much attention has been given to the role of pro-inflammatory cytokines, such as tumor necrosis factor (TNF) and interleukin-6 (IL-6). Both in vitro studies and animal models of cancer cachexia support that these factors can directly promote the catabolism of adipose and skeletal muscle tissues [8]. However, pharmacological inhibitors of these cytokines have yet to prove effective in restoring or maintaining body weight or improving muscle function in cancer patients with cachexia [9–13]. Such outcomes in human studies have led to an appreciation that additional mediators besides TNF and IL-6 are likely to be involved in the regulation of the cancer cachexia syndrome. In this review, we discuss recent studies that have placed new attention on signaling mediators such as activin A and myostatin, and their association with cancer cachexia [14], as well GDF15 and lipocalin-2 which have provided fresh insight on the underlying mechanism of anorexia in response to cancer and the side effects of platinum-based chemotherapeutic drugs [15–17].
Associating cancer cachexia with activin and myostatin in rodents and patients
Activin A and myostatin are members of the tumor growth factor-beta (TGF-β) family of ligands. The signaling of these ligands is controlled through the binding to the type II receptor in skeletal muscle (activin: ACVR2B or ACVR2A; myostatin: ACVR2B predominantly) leading to the phosphorylation and dimerization of SMAD2 and SMAD3 transcription factors that are translocated from the cytoplasm to the nuclei to regulate gene expression [18–20]. Increases in circulating levels of myostatin and activin A are sufficient to induce muscle wasting [21, 22] [Figure 1]. Tumor studies in mice show that elevated levels of activin signaling is associated with increased metastases and shorter survival, and with respect to cancer cachexia, increased weight loss [23, 24]. Consistent with these findings, cancer patients with increases in circulating levels of activin A also present with weight loss [14, 25–27]. Specifically for ovarian and pancreatic cancers, the source of circulating activin A appears to reside within the tumors themselves, perhaps explaining the high rates of cachexia in these cancers [24, 28–30]. With regards to myostatin, the association between its circulating levels and cachexia is less clear. In fact, Loumaye et. al. reported that circulating levels of myostatin are reduced, not elevated, in colorectal and lung cancer patients with cachexia [14].
Key Figure. Schematic of emerging signaling mediators in the cancer anorexia-cachexia syndrome.
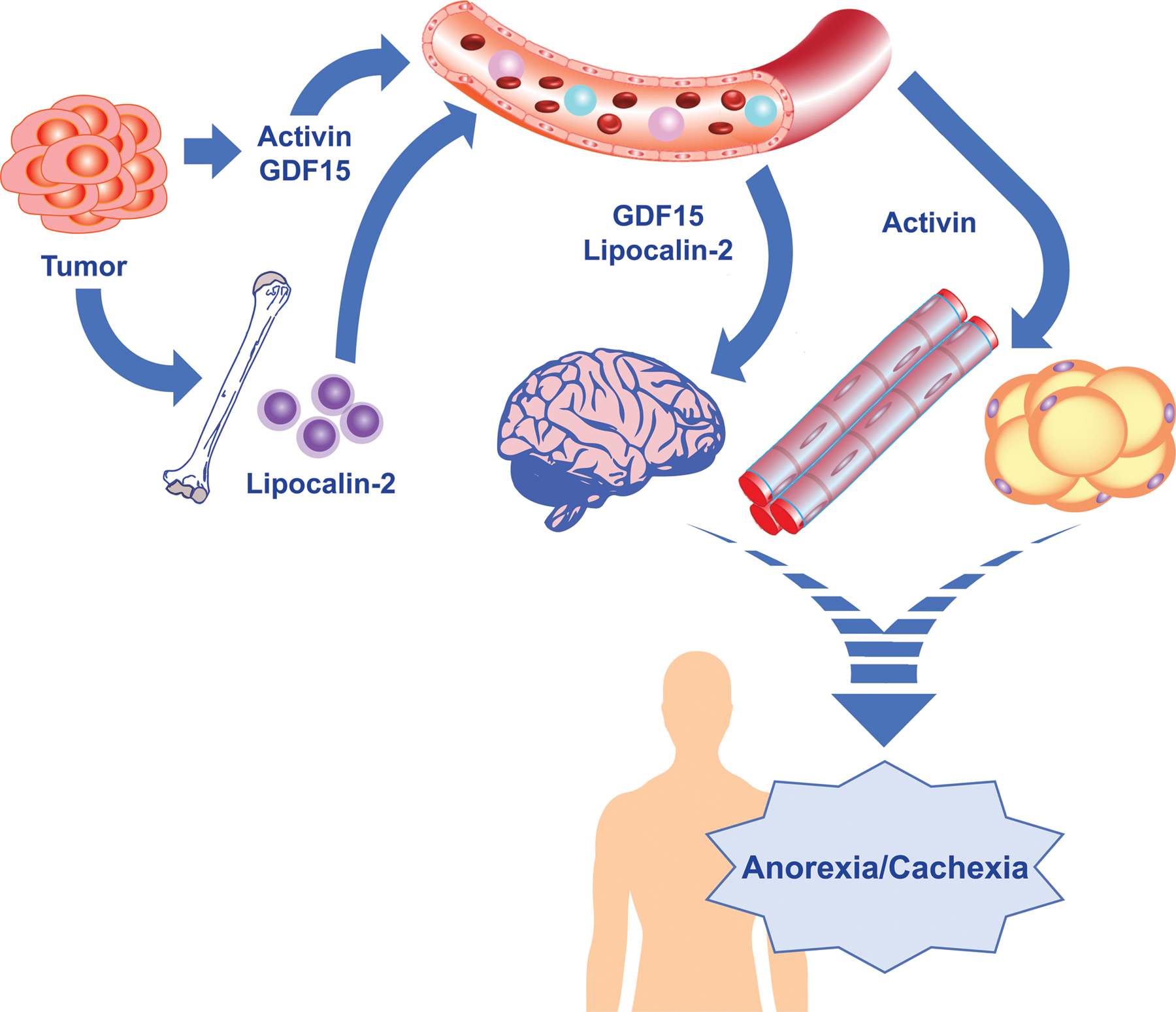
The signaling mediators activin A, GDF15, and lipocalin-2 and their roles in the cancer anorexia-cachexia syndrome are displayed. Activin and GDF15 are believed to be synthesized and secreted largely from tumors and circulate to peripheral organs to regulate feeding (GDF15), and adipose and skeletal muscle atrophy (activin A). Lipocalin-2 is generated from bone marrow-derived neutrophils and circulates to the brain to regulate anorexia. The human outline is meant to portray the translational potential of targeting these signaling mediators as a therapy for cancer cachexia.
Translating activin A and myostatin therapies from mice to cachexia patients
Evidence from a number of studies performed in mouse models of cancer cachexia has demonstrated that reducing activin A and myostatin signaling either through ligand trap or receptor blockade approaches is sufficient to rescue muscle wasting [24, 31–35]. These respective treatments resulted in the downregulation of SMAD2/3 phosphorylation, validating the mechanism of action and suggesting that downstream inhibition of SMAD2/3 can prevent muscle wasting in cancer cachexia [31, 36]. Such studies point to the activin/myostatin signaling pathways as viable therapeutic targets for the treatment of cancer cachexia. Indeed, clinical trials have been conducted with a series of new compounds specifically aimed at reducing activin A and myostatin activities through either acting as ligand traps for myostatin and activin A or blocking agents for the ACVR2B receptor. Despite the apparent anti-cachectic potential for these compounds, the vast majority of clinical trials have been conducted to treat muscle wasting associated with neuromuscular conditions or sarcopenia [37].
Specific to cancer cachexia, the anti-myostatin antibody Landogrozumab (LY2495655) progressed to a Phase II trial in pancreatic cancer patients (NCT01505530)i (Table 1), although it was not deemed to be superior to placebo in improving outcome measures related to muscle wasting [38]. Concerningly, patients treated with the study’s higher dose of LY2495655 demonstrated shorter overall survival compared to the placebo-treated group. STM 434, a ligand trap designed for activin A, was recently tested in a Phase I trial (NCT02262455)ii (Table 1). In ovarian and other cancer patients, researchers identified some indications of improved lean body mass at higher doses, which were associated with improved 6-minute walk test times in a number of patients [39]. However, very few patients in this study were found to have reductions in tumor burden following treatment, contributing to concerns that activin A inhibition might promote tumor growth. Furthermore, bleeding was a significant complication for a number of patients. Bleeding, particularly of the nose and gums, has been the most consistent and problematic complication of attempts to reduce activin A and myostatin signaling in humans. Bleeding complications have been documented in healthy adult individuals, boys with Duchenne muscular dystrophy, and cancer patients [19, 39]. Because of these complications, in addition to testing different molecules for the current ligand trap and receptor blockade approaches, alternative strategies are currently being pursued to reduce activin A signaling in skeletal muscle without exhibiting similar associated toxicities. One such strategy includes flooding the receptor with inactive propeptide to prevent active activin A from reaching its receptor [21].
Table 1.
Summary of clinical trials for signaling mediators of cancer cachexia
| Clinical Trial | Agent | Target | Population | Phase | Status |
|---|---|---|---|---|---|
| NCT01505530 i | Landogrozumab (LY2495655) | myostatin | pancreatic cancer patients | II | Completed |
| NCT02262455 ii | STM 434 | Activin A | ovarian cancer patients | I | Completed |
| NCT04068896 iii | NGM120 | GDF15 | advanced solid tumor and pancreatic cancer patients | I/II | Recruiting |
| NCT04803305 iv | PF-06946860 | GDF15 | certain advanced solid tumor patients with anorexia | I | Recruiting |
| NCT04815551 v | AV-380 | GDF15 | healthy subjects | I | Active, not recruiting |
| NCT04725474 vi | CTL-002 | GDF15 | advanced solid tumor patients | I | Recruiting |
Roman numerals following the Clinical Trial Identifier refer to additional information under Resources.
GDF15 and GFRAL signaling regulates body weight in cancer cachexia
A recently described cytokine associated with cancer cachexia is Growth Differentiation Factor-15 (GDF15), otherwise referred to macrophage inhibitory cytokine 1 (MIC-1) or nonsteroidal anti-inflammatory drug activated gene-1 (NAG-1) [17]. GDF15 is a distant member of the TGF-β superfamily of growth factors. Circulating levels of GDF15 are substantially elevated in chronic illnesses such as cardiovascular disease, diabetes, and cancer [17]. Such regulation has led to the classification of GDF15 as a risk factor for cardiac failure and as a potential prognostic marker for certain cancers. Plasma levels of GDF15 are among the highest in PDAC, which is also the population that predominantly suffers from weight loss and cachexia [1, 40].
In animal studies, the administration of recombinant GDF15 promoted weight loss due to anorexia, and implantation of GDF15-producing tumor cells in mice accentuated adipose and skeletal muscle catabolism, together highlighting the multifaceted functions of GDF15 in cancer cachexia [41, 42] [Figure 1]. The mechanism by which GDF15 signals to regulate the anorexia-cachexia syndrome was elucidated in 2017, when several laboratories simultaneously identified the GDF15 receptor, named glial cell-derived neurotrophic factor receptor alpha-like (GFRAL) [43–46].
The GFRAL receptor is expressed in the central nervous system (CNS), specifically in the hindbrain region that controls appetite, and complete signaling activity requires a complex containing the GDF15 ligand, the GFRAL receptor, and the more promiscuously expressed co-receptor, c-Ret. When GFRAL is genetically ablated in mice, animals exposed to a high fat diet gain an excessive amount of weight. Such unambiguous results led to targeting the GDF15/GFRAL signaling axis as a therapeutic strategy for cancer cachexia. Indeed, the generation of separate neutralizing antibodies against GDF15 and GFRAL was efficacious in reversing weight loss in tumor-bearing mice [41, 42], and significantly the GFRAL antibody, NGM120, is currently in a Phase Ia/Ib clinical trial (NCT04068896)iii (Table 1) for the treatment of cancer and the cancer anorexia-cachexia syndrome. In mice, the GDF15 antibody, PF-06946860, was recently shown to be effective in attenuating the side effects of platinum-based chemotherapy drugs, including anorexia and nausea [15]. GDF15 antibody therapy is also currently being tested in a Phase I clinical trial (NCT04803305)iv (Table 1) to improve cachexia-anorexia symptoms in patients with advanced cancer. Two additional GDF15 antibodies are in earlier phase clinical trials (NCT04815551 and NCT04725474)v, vi (Table 1).
Lipocalin 2 and the type 4 melanocortin receptor mediate anorexia in cancer cachexia
Another secreted factor recently described in the cancer cachexia literature that also appears to function as a potent regulator of the feeding response is lipocalin-2 (LCN2). This protein has previously been linked to the innate immune system for its anti-bacterial properties, but recently was shown to function in the CNS and exhibit a neurotoxic activity [47]. In the context of cancer, authors showed that pancreas tumors induced LCN2 production from bone marrow-derived neutrophils [16].These innate immune cells circulated to the CNS and LCN2 bound to the type 4 melanocortin receptor (MC4R), which had previously been shown to be an essential regulator of appetite [48] [Figure 1]. Thus, similar to pharmacological inhibition of the melanocortin receptor that mitigates the anorexic response in tumor-bearing mice [49, 50], deletion of LCN2 restores appetite in PDAC-induced cachexia. Significantly, LCN2 function in the CNS also regulates the catabolism of adipose and skeletal muscle [16].
This suggests that LCN2 activity extends beyond the regulation of anorexia, which results from clinical trials had previous shown is a process in cachexia that is uncoupled from weight loss induced by chronic peripheral tissue wasting. Thus, LCN2 in addition to MC4R, adds to a list of exciting new therapeutic candidates for the treatment of the anorexia cachexia syndrome in cancer. Although a number of clinical trials have been conducted with agonists of MC4R [51], similar efforts to reduce circulating LCN2 or antagonize MC4R activity remain to be tested in humans.
Implications for future therapeutic approaches
The recently published European Society of Medical Oncology (ESMO) guidelines state: “Given the complex and multifaceted contributors to cachexia, anti-cachexia treatment must be based on a comprehensive assessment of the patient’s situation and an evaluation of reasonable, available treatment options, resulting in a personalised, multitargeted and multimodal approach” [52]. Emerging evidence suggests that similar to a more personalized treatment approach being pursued in precision oncology, anti-cachexia therapy may also benefit from an individualized treatment strategy. Factors such as age, sex, obesity, performance status, disease stage, or tissue of tumor origin may hold important information that can influence potential strategies to treat cancer-induced cachexia and anorexia. Particularly attractive targets for an individualized treatment are circulating factors that accumulate during cancer, positively associate with weight loss in patients, and whose neutralization in animal studies restores appetite, lean mass, and body weight (see Outstanding Questions Box).
Outstanding Questions.
Will advanced pre-clinical strategies to modulate circulating levels of activin A, myostatin, GDF15, or LCN2 ultimately translate to more effective treatments to address cancer-associated cachexia or anorexia?
Would cancer cachexia be best addressed by individualized approaches analogous to current practices in precision oncology?
What circulating tumor and host factors beyond activin A, myostatin, GDF15, and LCN2 be identified that regulate appetite and tissue catabolism, and serve as therapeutic targets?
One caveat to targeting circulating factors initially identified in animal models of cancer cachexia is that in many instances, careful attention has not been paid to if the concentrations of circulating factors in rodent models mirror concentrations found in cachectic humans. For example, circulating levels of IL-6 in the Colon-26 tumor model often range from 250 to more than 1000 pg/mL, which 10-fold higher than concentrations measured in cachectic cancer patients [25, 53–56]. Levels of TNF in the Lewis Lung carcinoma model appear to be more in line with humans at 10–40 pg/mL [25, 53, 57], although others have reported much higher concentrations [58]. For activin A, circulating levels in cancer patients that associate with cachexia have been measured between 400 to 650 pg/ml [14, 25]. While also within a comparable range, levels of circulating activin A in mouse cachexia models seem to be two to five times higher, with levels measured in the KPC genetic mouse model of pancreatic cancer at ~2,500 pg/mL and in the Colon-26 mouse model of cancer cachexia at ~1,000 pg/mL [24, 59]. Given that the concentrations of activin A in the circulation in these rodent models of cancer cachexia do not extensively exceed those found in humans, any successful future pre-clinical therapy targeting circulating activin A would have the potential to be translated to patients. Similar comparable serum concentrations were reported with GDF15 between PDAC patients and animal models of cancer cachexia [41, 42], suggesting that neutralization of this circulating signaling mediator might also be a candidate for individualized therapy. As additional human data are obtained for LCN2, the anticipation is that similar translational strategies can be adapted for this circulating signaling mediator as well.
Concluding Remarks and Future Perspectives
It is evident from recent literature that our understanding of the underlying mechanisms driving weight loss in cancer patients continues to grow. It is also becoming more the norm rather than the exception that studies validate their animal model findings in patient samples. This last point is key, and it is one of the important features we have attempted to highlight in this review. Is it our viewpoint that successful translational studies that will ultimately benefit cancer patients with cachexia will not only depend on elucidating the mechanism of action of a particular signaling mediator, but in addition that animal models used to translate new therapies are able to recapitulate the human phenotype as closely as possible. Inhibition of activin A, GDF15, and LCN2 in various tumor models of cachexia have been efficacious in restoring or maintaining body weight and lean muscle mass, and importantly, the circulating levels of these factors did not dramatically differ between animals and patients, indicating the potential to achieve a therapeutic dose that might exhibit equal efficacy in the clinic. With these thoughts in mind, the serum concentration of a specific signaling mediator of cachexia could be considered in future clinical trials as an inclusion criterion for enrollment.
Highlights.
Recent findings have revealed new information on the likely roles of activin A and myostatin and identified GDF15 and lipocalin-2 as new signaling mediators of the cancer cachexia syndrome.
These developments have led to a number of translational efforts including early-phase clinical trials.
Additional data from cancer patients and late-stage clinical trials are needed to determine if activin A, myostatin, GDF15, or lipocalin-2 will ultimately translate to actionable targets to treat cancer cachexia.
The future of anti-cachexia treatment may lie in strategies involving more targeted and personalized approaches.
Acknowledgments
EET is supported by an NIH grant R00 AR071508 and DCG is supported by an NIH grant R01 AR072714. We are grateful for the assistance provided by Dr. David Wang in preparing our figure for this review.
Disclosures/ Ethics Statement:
D.C.G. receives funding support from Pfizer Inc.; E.E.T. declares no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baracos VE, et al. (2018) Cancer-associated cachexia. Nat Rev Dis Primers 4, 17105. [DOI] [PubMed] [Google Scholar]
- 2.Fearon KC, et al. (2012) Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 16, 153–166 [DOI] [PubMed] [Google Scholar]
- 3.Argiles JM, et al. (2014) Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 14, 754–762 [DOI] [PubMed] [Google Scholar]
- 4.Argiles JM, et al. (2010) Consensus on cachexia definitions. J Am Med Dir Assoc 11, 229–230 [DOI] [PubMed] [Google Scholar]
- 5.Evans WK et al. (1985) Limited impact of total parenteral nutrition on nutritional status during treatment for small cell lung cancer. Cancer Res. 45, 3347–3353 [PubMed] [Google Scholar]
- 6.Ozola Zalite I, et al. (2015) Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: a systematic review. Pancreatology : official journal of the International Association of Pancreatology (IAP) … [et al.] 15, 19–24 [DOI] [PubMed] [Google Scholar]
- 7.Nemer L, et al. (2017) Predictors of Pancreatic Cancer-Associated Weight Loss and Nutritional Interventions. Pancreas 46, 1152–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argiles JM, et al. (2009) The role of cytokines in cancer cachexia. Current opinion in supportive and palliative care 3, 263–268 [DOI] [PubMed] [Google Scholar]
- 9.Monk JP, et al. (2006) Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 24, 1852–1859 [DOI] [PubMed] [Google Scholar]
- 10.Wu C, et al. (2013) Disrupting cytokine signaling in pancreatic cancer: a phase I/II study of etanercept in combination with gemcitabine in patients with advanced disease. Pancreas 42, 813–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prado BL and Qian Y (2019) Anti-cytokines in the treatment of cancer cachexia. Ann Palliat Med 8, 67–79 [DOI] [PubMed] [Google Scholar]
- 12.Narsale AA and Carson JA (2014) Role of interleukin-6 in cachexia: therapeutic implications. Current opinion in supportive and palliative care 8, 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayliss TJ, et al. (2011) A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin Biol Ther 11, 1663–1668 [DOI] [PubMed] [Google Scholar]
- 14.Loumaye A, et al. (2015) Role of Activin A and myostatin in human cancer cachexia. The Journal of clinical endocrinology and metabolism 100, 2030–2038 [DOI] [PubMed] [Google Scholar]
- 15.Breen DM, et al. (2020) GDF-15 Neutralization Alleviates Platinum-Based Chemotherapy-Induced Emesis, Anorexia, and Weight Loss in Mice and Nonhuman Primates. Cell metabolism 32, 938–950 e936 [DOI] [PubMed] [Google Scholar]
- 16.Olson B, et al. (2021) Lipocalin 2 mediates appetite suppression during pancreatic cancer cachexia. Nat Commun 12, 2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai VWW, et al. (2018) The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell metabolism 28, 353–368 [DOI] [PubMed] [Google Scholar]
- 18.Hulmi JJ, et al. (2021) Targeting the Activin Receptor Signaling to Counteract the Multi-Systemic Complications of Cancer and Its Treatments. Cells 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto Y, et al. (2016) Molecular Pathways: Cachexia Signaling-A Targeted Approach to Cancer Treatment. Clinical cancer research : an official journal of the American Association for Cancer Research 22, 3999–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ries A, et al. (2020) Activin A: an emerging target for improving cancer treatment? Expert Opin Ther Targets 24, 985–996 [DOI] [PubMed] [Google Scholar]
- 21.Walton KL, et al. (2019) Activin A-Induced Cachectic Wasting Is Attenuated by Systemic Delivery of Its Cognate Propeptide in Male Mice. Endocrinology 160, 2417–2426 [DOI] [PubMed] [Google Scholar]
- 22.Zimmers TA, et al. (2002) Induction of cachexia in mice by systemically administered myostatin. Science 296, 1486–1488 [DOI] [PubMed] [Google Scholar]
- 23.Togashi Y, et al. (2015) Activin signal promotes cancer progression and is involved in cachexia in a subset of pancreatic cancer. Cancer letters 356, 819–827 [DOI] [PubMed] [Google Scholar]
- 24.Zhong X, et al. (2019) The systemic activin response to pancreatic cancer: implications for effective cancer cachexia therapy. J Cachexia Sarcopenia Muscle 10, 1083–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lerner L, et al. (2015) Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. Journal of cachexia, sarcopenia and muscle 6, 317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paajanen J, et al. (2020) Elevated Circulating Activin A Levels in Patients With Malignant Pleural Mesothelioma Are Related to Cancer Cachexia and Reduced Response to Platinum-based Chemotherapy. Clin Lung Cancer 21, e142–e150 [DOI] [PubMed] [Google Scholar]
- 27.Lerner L, et al. (2016) Growth differentiating factor-15 (GDF-15): A potential biomarker and therapeutic target for cancer-associated weight loss. Oncology letters 12, 4219–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pressoir M, et al. (2010) Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. British journal of cancer 102, 966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez-Lara K, et al. (2013) Gastrointestinal symptoms and weight loss in cancer patients receiving chemotherapy. Br J Nutr 109, 894–897 [DOI] [PubMed] [Google Scholar]
- 30.Do TV, et al. (2008) The role of activin A and Akt/GSK signaling in ovarian tumor biology. Endocrinology 149, 3809–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, et al. (2010) Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142, 531–543 [DOI] [PubMed] [Google Scholar]
- 32.Gallot YS, et al. (2014) Myostatin gene inactivation prevents skeletal muscle wasting in cancer. Cancer research 74, 7344–7356 [DOI] [PubMed] [Google Scholar]
- 33.Busquets S, et al. (2012) Myostatin blockage using actRIIB antagonism in mice bearing the Lewis lung carcinoma results in the improvement of muscle wasting and physical performance. Journal of cachexia, sarcopenia and muscle 3, 37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy KT, et al. (2011) Antibody-directed myostatin inhibition enhances muscle mass and function in tumor-bearing mice. Am J Physiol Regul Integr Comp Physiol 301, R716–726 [DOI] [PubMed] [Google Scholar]
- 35.Huot JR, et al. (2020) ACVR2B antagonism as a countermeasure to multi-organ perturbations in metastatic colorectal cancer cachexia. Journal of cachexia, sarcopenia and muscle 11, 1779–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonetto A, et al. (2011) STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PloS one 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodgers BD and Ward CW (2021) Myostatin/Activin Receptor Ligands In Muscle And The Development Status Of Attenuating Drugs. Endocr Rev [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golan T, et al. (2018) LY2495655, an antimyostatin antibody, in pancreatic cancer: a randomized, phase 2 trial. J Cachexia Sarcopenia Muscle 9, 871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tao JJ, et al. (2019) First-in-Human Phase I Study of the Activin A Inhibitor, STM 434, in Patients with Granulosa Cell Ovarian Cancer and Other Advanced Solid Tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 25, 5458–5465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X et al. (2014) Macrophage inhibitory cytokine 1 (MIC-1/GDF15) as a novel diagnostic serum biomarker in pancreatic ductal adenocarcinoma. BMC Cancer 14, 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lerner L, et al. (2016) MAP3K11/GDF15 axis is a critical driver of cancer cachexia. Journal of cachexia, sarcopenia and muscle 7, 467–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suriben R, et al. (2020) Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat Med 26, 1264–1270 [DOI] [PubMed] [Google Scholar]
- 43.Emmerson PJ, et al. (2017) The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med 23, 1215–1219 [DOI] [PubMed] [Google Scholar]
- 44.Hsu JY, et al. (2017) Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 550, 255–259 [DOI] [PubMed] [Google Scholar]
- 45.Mullican SE, et al. (2017) GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med 23, 1150–1157 [DOI] [PubMed] [Google Scholar]
- 46.Yang L, et al. (2017) GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med 23, 1158–1166 [DOI] [PubMed] [Google Scholar]
- 47.Olson B, et al. (2021) Chronic cerebral lipocalin 2 exposure elicits hippocampal neuronal dysfunction and cognitive impairment. Brain Behav Immun 97, 102–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mosialou I, et al. (2017) MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature 543, 385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dallmann R, et al. (2011) The orally active melanocortin-4 receptor antagonist BL-6020/979: a promising candidate for the treatment of cancer cachexia. Journal of cachexia, sarcopenia and muscle 2, 163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu X, et al. (2020) Melanocortin-4 receptor antagonist TCMCB07 ameliorates cancer- and chronic kidney disease-associated cachexia. The Journal of clinical investigation 130, 4921–4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeo GSH, et al. (2021) The melanocortin pathway and energy homeostasis: From discovery to obesity therapy. Mol Metab 48, 101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arends J, et al. (2021) Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines(). ESMO Open 6, 100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talbert EE, et al. (2018) Circulating monocyte chemoattractant protein-1 (MCP-1) is associated with cachexia in treatment-naive pancreatic cancer patients. Journal of cachexia, sarcopenia and muscle [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yasumoto K, et al. (1995) Molecular analysis of the cytokine network involved in cachexia in colon 26 adenocarcinoma-bearing mice. Cancer research 55, 921–927 [PubMed] [Google Scholar]
- 55.Talbert EE, et al. (2017) Dual Inhibition of MEK and PI3K/Akt Rescues Cancer Cachexia through both Tumor-Extrinsic and -Intrinsic Activities. Molecular cancer therapeutics 16, 344–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujimoto-Ouchi K, et al. (1995) Establishment and characterization of cachexia-inducing and -non-inducing clones of murine colon 26 carcinoma. Int J Cancer 61, 522–528 [DOI] [PubMed] [Google Scholar]
- 57.Llovera M, et al. (1998) Role of TNF receptor 1 in protein turnover during cancer cachexia using gene knockout mice. Molecular and cellular endocrinology 142, 183–189 [DOI] [PubMed] [Google Scholar]
- 58.Zhang G, et al. (2017) Toll-like receptor 4 mediates Lewis lung carcinoma-induced muscle wasting via coordinate activation of protein degradation pathways. Scientific reports 7, 2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen JL, et al. (2016) Differential Effects of IL6 and Activin A in the Development of Cancer-Associated Cachexia. Cancer research 76, 5372–5382 [DOI] [PubMed] [Google Scholar]
Resources
- i.This study is registered with ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT01505530)
- ii.This study is registered with ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT02262455)
- iii.This study is registered with ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT04068896)
- iv.This study is registered with ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT04803305)
- v.This study is registered with ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT04815551)
- vi.This study is registered with ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT04725474)


