Abstract
Backgrounds & Aims:
Polycystic liver disease (PLD) is characterized by defective cholangiocyte cilia that regulate progressive growth of hepatic cysts. Because formation of primary cilia is influenced by autophagy through degradation of proteins involved in ciliogenesis, we hypothesized that ciliary defects in PLD cholangiocytes (PLDC) originate from autophagy-mediated depletion of ciliogenic proteins, ARL3 and ARL13B, and ARL-dependent mislocation of a ciliary-localized bile acid receptor, TGR5, activation of which enhances hepatic cystogenesis. The aims here were to determine: i) if ciliogenesis is impaired in PLDC, is associated with increased autophagy, and involves autophagy-mediated depletion of ARL3 and ARL13B; ii) if depletion of ARL3 and ARL13B in PLDC cilia impacts ciliary localization of TGR5; and iii) if pharmacological inhibition of autophagy reestablishes cholangiocyte cilia, ciliary localization of ARL3, ARL3B and TGR5, and reduces hepatic cystogenesis.
Approach & Results:
By using liver tissue from healthy individuals and patients with PLD, in vitro and in vivo models of PLD, and in vitro models of ciliogenesis, we demonstrated that in PLDC: ciliogenesis is impaired; autophagy is enhanced; ARL3 and ARL13B are ubiquitinated by HDAC6, depleted in cilia and present in autophagosomes; depletion of ARL3 and ARL13B impacts ciliary localization of TGR5; and pharmacologic inhibition of autophagy with mefloquine and verteporfin reestablishes cholangiocyte cilia, ciliary localization of ARL3, ARL13B and TGR5, and reduces hepatic cystogenesis.
Conclusion:
The intersection between autophagy, defective cholangiocyte cilia, and enhanced hepatic cystogenesis contributes to PLD progression and can be considered a novel target for therapeutic interventions.
Keywords: Polycystic liver disease, cholangiocytes, primary cilia, ciliogenesis
INTRODUCTION
Polycystic liver disease (PLD) is a group of genetic disorders characterized by development and progressive growth of cholangiocyte-derived, fluid-filled hepatic cysts. PLD is the most common hepatic manifestation of Autosomal Dominant (ADPKD) and Autosomal Recessive (ARPKD) Polycystic Kidney Diseases, and rarely occurs as Autosomal Dominant Polycystic Liver Disease (ADPLD).(1–5) ADPKD affects between 1/400 to 1/1000 people in the general population, and about 94% of patients with ADPKD develop PLD.(4) There are no regulatory approved drugs for PLD largely because the molecular and cellular mechanisms of hepatic cystogenesis are complex and obscure. Thus, studies on the mechanisms of PLD with a potential therapeutic application are crucial.
PLD is a cholangiociliopathy - a pathologic phenotype characterized by defects in the structure and sensory/signaling functions of cholangiocyte primary cilia.(1, 6, 7) In healthy cholangiocytes, cilia extend from the apical plasma membrane into the intrahepatic bile duct lumen to detect, amplify, integrate, and transmit diverse extracellular stimuli into intracellular signals and functional responses.(8–10) In contrast, PLD cholangiocytes (PLDC) have morphologically abnormal cilia with altered sensory and signaling functions.(8, 11, 12)
What accounts for the formation of defective primary cilia is poorly understood. Recent studies suggest that structural and functional properties of primary cilia are positively or negatively regulated by autophagy through degradation of proteins required for ciliogenesis and ciliary functions,(13–17) which we refer to as “ciliogenic” proteins. Importantly, autophagy-facilitated alterations in ciliogenesis contribute to ciliopathies such as focal cortical dyslamination,(18) Huntington’s disease,(19) thyroid hurthle cell tumors,(20) oral-facial digital syndrome,(14), and others. Considering that PLDC have both enhanced autophagy (21) and defective primary cilia,(8, 11) we hypothesized that: i) structural and functional alterations in PLDC cilia are autophagy-facilitated via depletion of selective ciliogenic and signaling proteins; and ii) inhibition of autophagy restores PLDC cilia, replaces key structural and signaling proteins, and reduces hepatic cyst progression.
Although many proteins are involved in ciliogenesis and the functions of primary cilia, we focus on two small G-proteins of the ARL family, ARL13B and ARL3, and a G protein-coupled bile acid receptor, TGR5, which are required for the structural and functional integrity of cholangiocyte cilia and PLD progression.
ARL13B and ARL3 are enriched in primary cilia and function in a coordinated fashion to determine the morphology of cilia and ciliary localization of sensory/signaling proteins, including G protein-coupled receptors (GPCRs).(22–25) It has been shown, for example, that Arl13b mutant mouse embryos have short primary cilia with morphological defects and abnormal Sonic hedgehog (Shh) signaling.(26) Furthermore, studies on mouse embryonic fibroblasts demonstrated that Arl13b regulates ciliary length and Shh signaling.(23, 27) In Arl3−/− mice, loss of Arl3 resulted in abnormal development of renal, hepatic, and pancreatic epithelial tubular structures similar to characteristics of autosomal recessive polycystic kidney disease.(28) Based on the observed mislocalization of rhodopsin in Arl3−/− rod cell bodies, the authors hypothesized that Arl3 plays an important role in ciliary abnormalities in the kidney, liver and pancreas. (28) However, no direct evidence to support this hypothesis was presented.
TGR5, a G-protein coupled bile acid receptor linked to the intracellular cAMP signaling pathway, is one of the key sensory/signaling proteins in cholangiocyte cilia. In healthy cholangiocytes, TGR5 is localized to cilia and its activation suppresses intracellular cAMP signaling.(29, 30) In contrast, in PLDC, TGR5 resides at the apical plasma membrane, and its activation increases the levels of cAMP and promotes cAMP-dependent hepatic cystogenesis. (31) However, it remains unclear if TGR5-dependent hepatic cystogenesis is linked to alterations in the ciliary localization of TGR5.
Thus, the aims of this work were to determine: i) if ciliogenesis is impaired in PLDC, is associated with increased autophagy, and involves autophagy-mediated depletion of ARL3 and ARL13B; ii) if depletion of ARL3 and ARL13B in PLDC cilia impacts ciliary localization of TGR5; and iii) if pharmacological inhibition of autophagy reestablishes cholangiocyte cilia, ciliary localization of ARL3, ARL3B and TGR5, and reduces hepatic cystogenesis.
MATERIALS AND METHODS
Human liver tissues, cell cultures, animals, and drugs.
Liver tissues from healthy humans and patients with PLD were provided by the Mayo Clinic Center for Cell Signaling in Gastroenterology Clinical Core. Normal (NRC) and PCK rat cholangiocytes (PCKC), normal human (NHC) and PLD cholangiocytes (PLDC) were maintained as we previously described.(32) NRC and PCKC were stably transfected with LC3-GFP construct (Invitrogen). Age (3–4 weeks old) - and sex-matched wild type (WT, Harlan Sprague Dawley) and PCK rats (Harlan Sprague Dawley background) were housed on a 12 hours light/dark cycle, maintained on a standard diet and water ad lib. After anesthesia (pentobarbital, 50 mg/kg) livers were removed, fixed and paraffin embedded. The protocol was approved by the Mayo Clinic Institutional Animal Care and Use Committee. Mefloquine (MQ) and Verteporfin (VP) were purchased from Sigma Aldrich.
Conventional and inducible models of ciliogenesis.
Ciliogenesis in normal and PLDC was evaluated by using two models - a non-inducible model of ciliogenesis in which cholangiocytes cultured under standard conditions develop mature cilia in 7–10 days after they reach 100% confluence,(12) and an inducible model of rapid ciliogenesis in which cilia assembly is induced by a reduction of fetal bovine serum (FBS) in culture medium from 10% to 0.5% for 24 h, followed by an increase of FBS from 0.5% to standard 10% for the next 24 h to induce cilia disassembly.(33) NHC and PLDC were fixed and the length of cilia was assessed by immunofluorescence confocal microscopy.
Treatment of cultured cholangiocytes with Mefloquine (MQ) and Verteporfin (VP).
NHC and PLDC were incubated with 10 μM MQ or 20 μM VP for 24 hours. NHC and PLDC were fixed and the length of cilia was assessed by immunofluorescence confocal microscopy.
Treatment of PCK rats with MQ and VP.
PCK rats (4–6 weeks old, n=12 females, n=12 males) were divided into three groups: i) MQ-treated (n=4 females, n=4 males), ii) VP-treated (n=4 females, n=4 males), and iii) treated with DMSO, control (n=4 females, n=4 males). MQ (10 mg/kg body weight) and VP (5mg/kg body weight) were dissolved in DMSO and injected intraperitoneally (IP) twice a week; doses of both drugs were adjusted to the rat weight every other week. Rats were sacrificed after 6 weeks of treatment. After anesthesia (pentobarbital, 50 mg/kg) livers were removed, fixed and paraffin-embedded for future analysis. Cystic areas of liver in untreated and drug-treated PCK rats were analyzed as previously described.(32)
Statistical analysis.
The data are expressed as the Mean±SD. Statistical analysis was performed by Student’s t-test and results were considered statistically significant at P<0.05.
RESULTS
CILIOGENESIS IS IMPAIRED IN PLDC
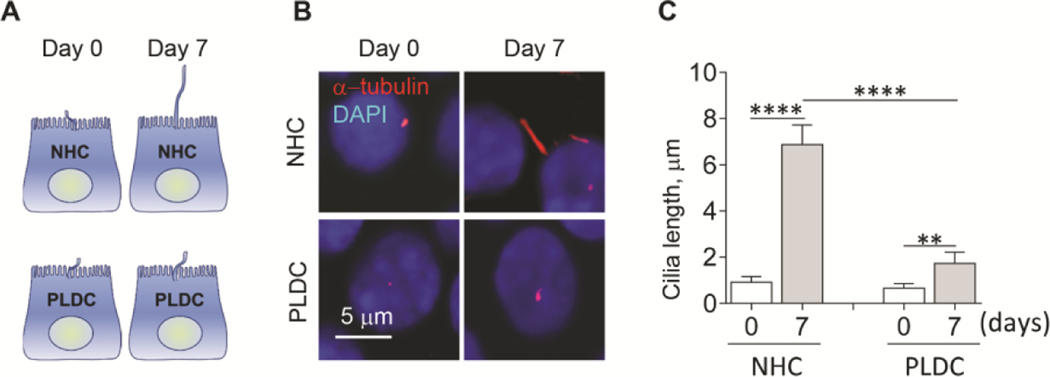
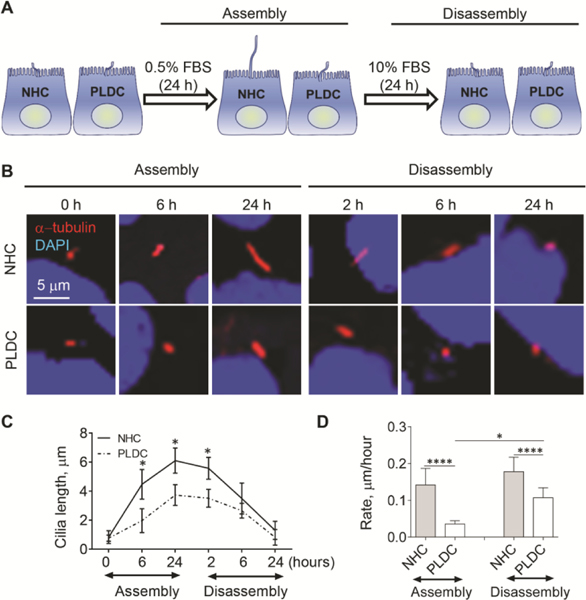
To evaluate ciliogenesis in human PLDC, we employed two in vitro models - a conventional model of ciliogenesis in which cholangiocytes develop mature cilia after 7 days post-confluence (12) (Fig. 1A), and an inducible model of rapid ciliogenesis in which cilia assembly is induced by a reduction of fetal bovine serum (FBS) in culture medium from 10% to 0.5% for 24 h, followed by an increase of FBS from 0.5% to 10% for the next 24 h to induce cilia disassembly (33) (Fig. 2A). In both models, human PLDC cilia were 55% shorter (day 7 post-confluence in the conventional model [Fig. 1B, C] and after 6–24 hours of inducible ciliogenesis [Fig. 2B, C]) as compared to normal human cholangiocyte (NHC) cilia. The rate of cilia assembly was reduced 3-fold (i.e., from 0.15±0.05 to 0.05±0.02 μm/hour) in PLDC compared to NHC, and the rate of cilia disassembly was reduced by 1.6-fold (i.e., from 0.18±0.04 to 0.11±0.03 μm/hour) (Fig. 2D).
FIG. 1.
Ciliogenesis is impaired in PLDC grown under conventional conditions. (A) A schematic outline of a conventional model of ciliogenesis in vitro. (B) Representative immunofluorescence confocal microscopy (IF) images and (C) a quantitative analysis show that PLDC cilia (detected by a ciliary marker, acetylated α-tubulin, stained in red) at day 7 post-confluence are shorter compared to NHC cilia. Nuclei (blue, DAPI). Data are presented as Mean±SD. n=10 cilia for each experimental condition. **p<0.001; ****p<0.0001.
FIG. 2.
Ciliogenesis is impaired in PLDC grown under conditions of induced ciliogenesis. (A) A schematic outline of an inducible model of ciliogenesis in vitro. (B) Representative conditions of IF images of cholangiocyte cilia assembly and disassembly (detected by a ciliary marker, acetylated α-tubulin, stained in red) and a quantitative analysis (C) show that ciliogenesis in PLDC results in shorter cilia compared to NHC cilia. (D) The rates of cilia assembly and disassembly are reduced in PLDC compared to NHC. Nuclei (blue, DAPI). Data are presented as Mean±SD. n=10 cilia for each experimental condition. *p<0.05; ****p<0.0001.
AUTOPHAGY IS ENHANCED IN PLDC WITH IMPAIRED CILIOGENESIS
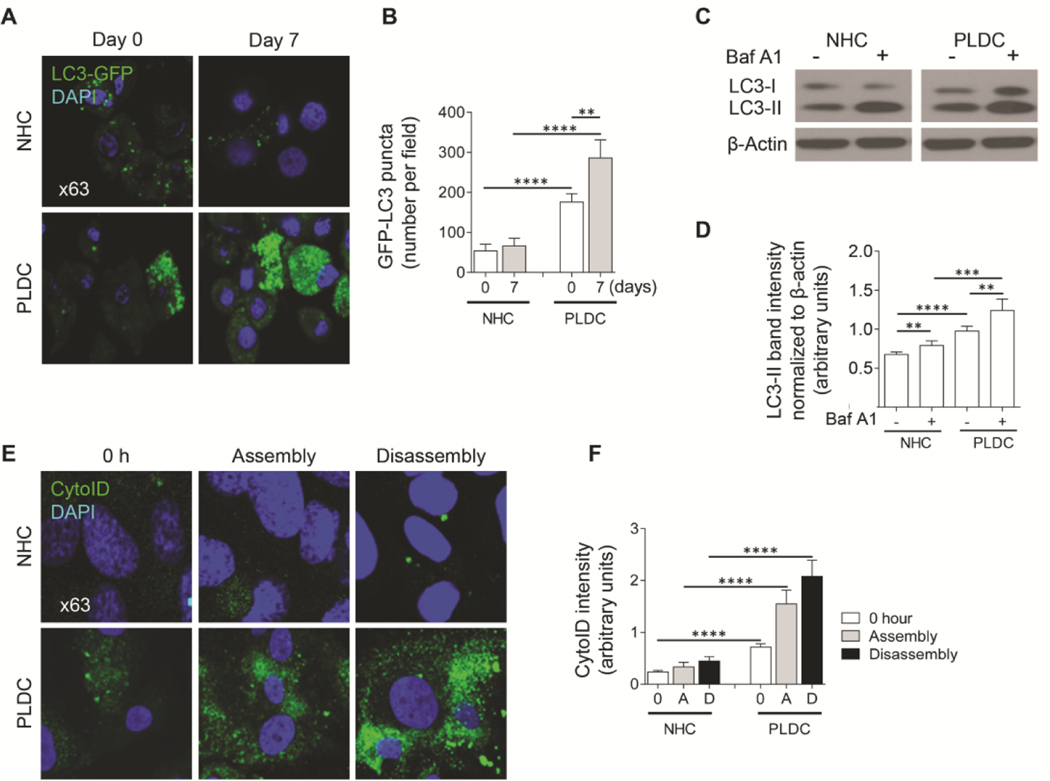
To monitor autophagy, we transfected NHC and PLDC with a plasmid containing autophagy marker LC3 tagged with GFP (GFP-LC3) and assessed autophagy through direct fluorescence microscopy by measuring punctate GFP-LC3 that represents the quantity of newly formed autophagosomes (Fig. 3A). GFP-LC3-transfected cells were cultured under standard conditions, and punctate GFP-LC3 was compared at day 0, when cilia start to grow, to day 7 post-confluence, when cholangiocytes possess mature cilia. At day 7 post-confluence, NHC revealed a similar number of punctate GFP-LC3 (i.e., 66±19) as at day 0 (i.e., 54±10) (Fig. 3B) consistent with a basal level of autophagy in NHC during ciliogenesis. However, the number of punctate GFP-LC3 in PLDC was 3.3 times greater at day 0 (i.e., 176±21) and 4.3 times higher at day 7 (i.e., 286±45) compared to NHC (Fig. 3B) demonstrating enhanced autophagy.
FIG. 3.
Autophagy is enhanced in PLDC with impaired ciliogenesis. (A) Representative IF images and (B) a quantitative analysis show a greater GFP-LC3 puncta in PLDC compared to NHC at day 0, and an increase at day 7. (C) Western blot of LC3 and (D) a quantitative analysis show increased autophagic flux in PLDC vs NHC [i.e., the levels of LC3-II normalized to β-actin is greater and increased in the presence of autophagy inhibitor, Bafilomycin A1 (Baf A1)]. (E) IF images and (F) a quantitative analysis show increased fluorescence of CYTO-ID (green) that selectively labels autophagosomes. In PLDC, CYTO-ID fluorescence increased during both cilia assembly and disassembly. Nuclei (blue, DAPI). Data are presented as Mean±SD. n=3 for each condition; **p<0.05; ***p<0.001; ****p<0.0001.
To monitor autophagic flux in NHC and PLDC at day 7 post-confluence, we assesed the ratio of the lipidated form of LC3 (LC3-II) to β-actin in the prersence/absence of autophagy inhibitor, Bafilomycin A1 (Baf A1) by western blot. An increase in the ratio of LC3-II/β-actin in the presence of Baf A1 in PLDC showed enhanced autophagic flux in PLDC compared to NHC (Fig. 3C, D).
In addition, we stained NHC and PLDC with the CYTO-ID Dye (Enzo Life Sciences) that selectively labels autophagosomes, and assessed CYTO-ID fluorescence during induced cilia assembly and disassembly. The results show that CYTO-ID fluorescence was 3 times greater in PLDC compared to NHC at time 0 (i.e., 0.72±0–7 vs. 0.24±0.03 arbitrary units); and increased by 2.2 times (i.e., to 1.55±0.26 arbitrary units) during cilia assembly and by 2.9 times (i.e., to 2.08±0.31 arbitrary units) during cilia disassembly (Fig. 3E, F). CYTO-ID fluorescence in PLDC vs. NHC were on average 4.6-fold greater during both cilia assembly (i.e., 1.55±0.26 vs. 0.34±0.09 arbitrary units) and cilia disassembly (i.e., 2.08±0.31 vs. 0.45±0.09 arbitrary units).
ARL13B AND ARL3 ARE DEPLETED IN PLDC CILIA AND ARL13B IS PRESENT IN AUTOPHAGOSOMES
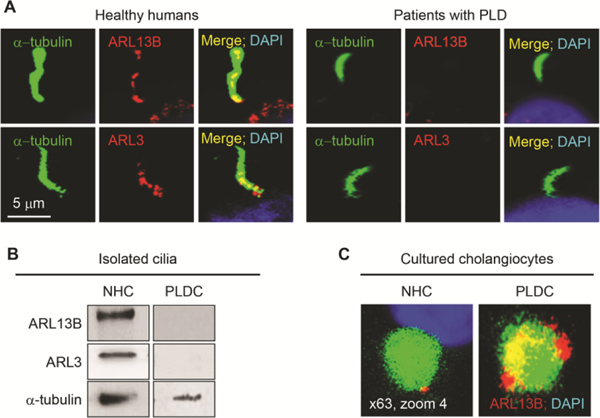
In liver tissue of healthy humans, ARL13B and ARL3 are expressed in cholangiocyte cilia; however, the expression of these two proteins is not detected in cholangiocyte cilia from patients with ADPKD-associated PLD (Fig. 4A). Similarly, by western blot, ARL13B and ARL3 are present in cilia isolated from NHC but not detected in cilia isolated from PLDC (Fig. 4B). Moreover, ARL13B, while depleted in PLDC cilia, is localized to autophagosomes in PLDC but not in NHC (Fig. 4C), suggesting that depletion of ARL3 and ARL13B from cilia in PLDC is due to their targeting to autophagosomes.
FIG. 4.
ARL13B and ARL3 are depleted in PLDC cilia and ARL13B is present in autophagosomes. (A) ARL13B and ARL3 (both, red) are localized to cholangiocyte cilia (green) in healthy humans but not in patients with ADPKD-associated PLD. (B) ARL13B and ARL3 are present in isolated NHC cilia but not detected in cilia isolated from PLDC. (C) Enlarged images of autophagosomes labeled with CytoID (green) in NHC and PLDC. ARL13B (red) co-localizes with Cyto ID. Yellow indicates the presence of ARL13B in PLDC autophagosomes. Nuclei (blue, DAPI).
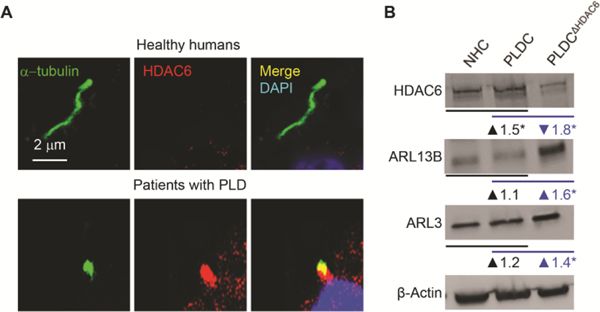
IN PLDC, HDAC6 IS OVEREXPRESSED, LOCALIZED TO CILIA, AND UBIQUTINATES ARL13B AND ARL3
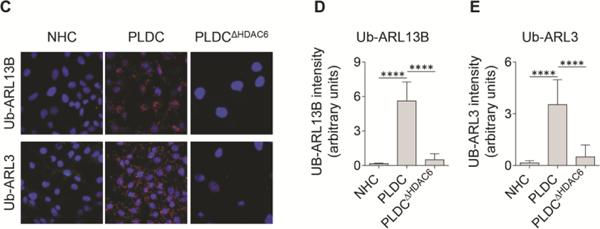
To understand the mechanisms of ARL13B and ARL3 depletion in PLDC cilia, we considered previous discoveries that: i) ciliary levels of ARL13B and ARL3 are regulated by histone deacetylate 6 (HDAC6); (34) ii) HDAC6 has a DAC1 (deacetylase 1) domain that functions as ubiquitin E3 ligase, (35), and iii) ubiquitination is often a first step in protein targeting to autophagosomes.(36) Thus, we tested if in PLDC, ARL13B and ARL3 are ubiquitinated by HDAC6. Our data show that in cholangiocytes of PLD patients but not in healthy humans, HDAC6 is overexpressed and localized to primary cilia (Fig 5A-B). We searched the protein post-translational modification comprehensive database (https://www.phosphosite.org/homeAction.action) and found that ARL13B has two and ARL3 has four ubiquitination sites, thus could be ubiquitinated. By the proximity ligation assay (PLA), ubiquitination of ARL13B was increased in PLDC vs NHC by 50-fold, and ubiquitination of ARL3 was increased by 35-fold (Fig. 5C-E). To demonstrate that an increase in ARL13B and ARL3 ubiquitination in PLDC is HDAC6-mediated, we reduced HDAC6 expression with a Dox-inducible HDAC6 shRNA (Fig. 5B) and found that ubiquitination of ARL13B and ARL3 in HDAC6-deficient PLDC was diminished (Fig. 5C-E), whereas the expression levels of ARL13B and ARL3 were increased (Fig. 5B). These data suggest that ubiquitination of ARL13B and ARL3 by HDAC6 might be the initial step in the mechanisms of defective ciliogenesis in PLDC.
FIG. 5.
In PLDC, HDAC6 is overexpressed, localized to cilia and ubiquitinates ARL13B and ARL3. (A) In cholangiocytes of healthy humans, HDAC6 (red) is absent in cilia (green) and is expressed in cytoplasm at a low level. In cholangiocytes of PLD patients, HDAC6 is overexpressed and localized to cilia. (B) By Western blot, in PLDC vs. NHC, HDAC6 is overexpressed, whereas the levels of ARL13B and ARL3 are not changed. Reduction of HDAC6 expression in PLDC by Dox-inducible HDAC6-shRNA (PLDCΔHDAC6) results in increased expression of ARL13B and ARL3 demonstrating that the levels of ciliogenic proteins in PLDC are mediated by HDAC6. Changes in protein expression (fold increase/decrease) in PLDC vs NHC are shown in black, and in PLDCΔHDAC6 vs PLDC in blue. (C) Proximity Ligation Assay (PLA) between ubiquitin and ARL13B (Ub-ARL13B, red) and between ubiquitin and ARL3 (Ub-ARL3, red) shows increased Ub-ARL13B (D) and UbARL3 in PLDC (E). In PLDCΔHDAC6 cholangiocytes, ubiquitination of ARL3 and ARL13B was abolished. Nuclei (blue, DAPI). Mean±SD, n=3 for each condition.* p<0.05; **** p<0.0001.
DEPLETION OF ARL13B AND ARL3 IMPACTS CILIARY LOCALIZATION OF A G-PROTEIN COUPLED BILE ACID RECEPTOR, TGR5
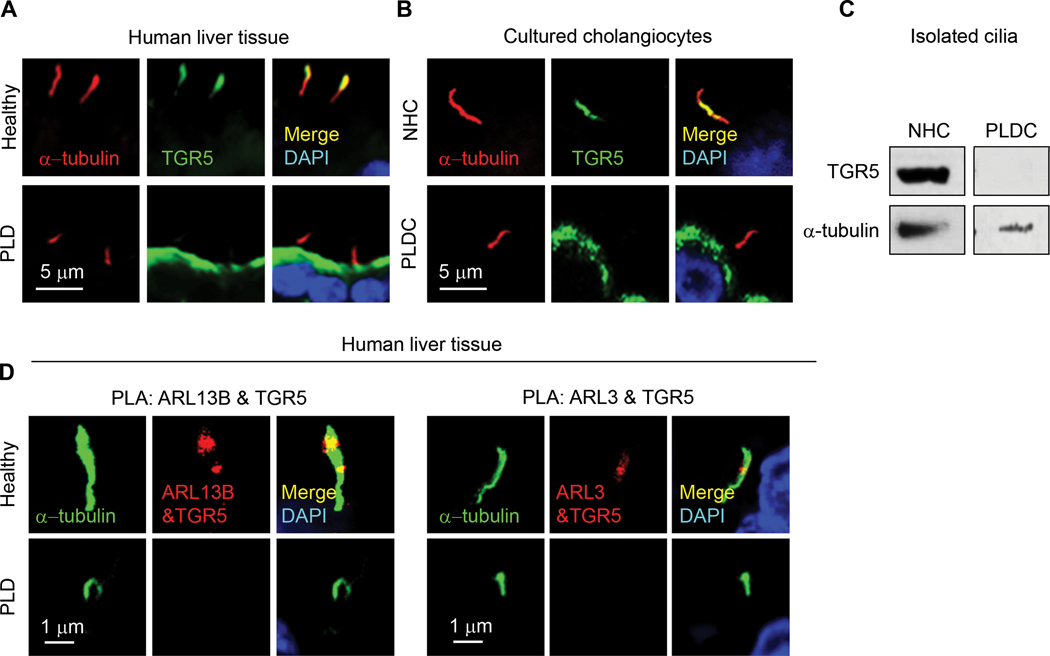
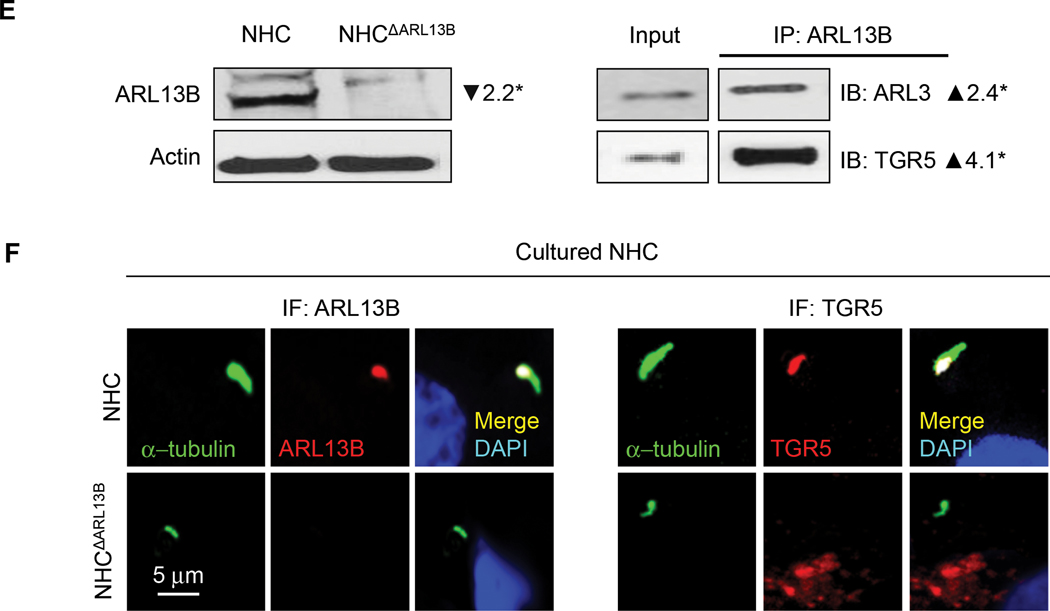
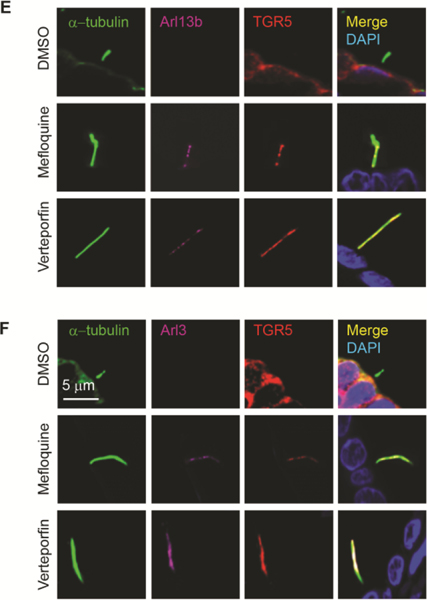
Next, we studied if depletion of ARL13B and ARL3 impacts ciliary localization of TGR5, a GPCR that is essential for cAMP-mediated hepatic cystogenesis.(31) We found that TGR5 is predominantly localized to primary cilia in cholangiocytes of healthy human liver tissue and cultured NHC (Fig. 6A, B). In contrast, in patients with ADPKD-associated PLD and cultured PLDC, TGR5 is depleted in cholangiocyte cilia but is overexpressed on the apical plasma membrane (Fig. 6A, B). Western blot analysis confirmed the expression of TGR5 in cilia isolated from NHC, but TGR5 expression was not in cilia isolated from PLDC (Fig. 6C). By PLA, TGR5 directly interacts with ARL13B and ARL3 in cholangiocyte cilia of healthy human liver tissue but not in PLD patients (Fig. 6D). Interaction in NHC between ARL3, ARL13B and TGR5 was further confirmed by IP (Fig. 6E). Transfection of NHC with Dox-inducible ARL13B shRNA (NHCΔARL13B) reduced ARL13B expression by 85% (Fig. 6E) resulting in shortened by 80% cilia deficient in TGR5 (Fig. 6F). These data show that ARL13B and ARL3 are invoved in targeting of TGR5 to NHC cilia, whereas depletion of ARL13B and ARL3 causes PLDC cilia deficient in TGR5.
FIG. 6.
Depletion of ARL13B and ARL3 impacts ciliary localization of a G-protein coupled bile acid receptor TGR5. (A) TGR5 (green) is localized to cholangiocyte cilia (red) in healthy humans but not in patients with ADPKD-associated PLD and (B) in cultured NHC but not in PLDC where it is overexpressed on the apical membrane. Nuclei (blue, DAPI). (C) TGR5 was not detected in isolated PLDC cilia. (D) By PLA, ARL13B and TGR5 (red) and ARL3 and TGR5 (red) interact in cholangiocyte cilia (green) of healthy humans but not in cilia of patients with PLD. (E) Transfection of NHC with doxycycline-inducible ARL13B (NHCΔARL13B) reduced ARL13B expression by 2.2-fold. NHC transfected with ARL13B-GFP were immunoprecipitated (IP) for ARL13B using GFP-Trap agarose beads and then immunoblotted (IB) for ARL3 and TGR5 demonstrating their interaction (2.4-fold and 4.1-fold increase, respectively). (F) NHCΔARL13B have shortened cilia (green) and TGR5 (red) is absent in cilia. n=3 for each experiment.
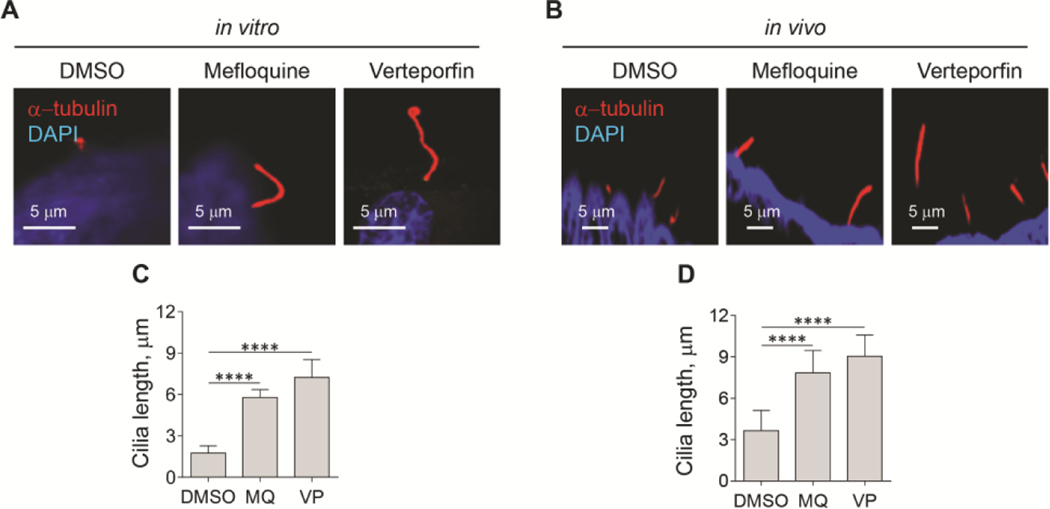
INHIBITION OF AUTOPHAGY BY MEFLOQUINE AND VERTEPORFIN REESTABLISHES PLDC CILIA, CILIARY LOCALIZATION OF Arl3, Arl13b AND TGR5, AND REDUCES HEPATIC CYSTOGENESIS
To address if inhibition of autophagy can restore PLDC cilia and ciliary localization of ARL13B, ARL3, and TGR5, and is beneficial for PLD progression, we performed in vitro studies on isolated human PLDC, and in vivo studies on an animal model of PLD, PCK rats. We inhibited autophagy in cultured human PLDC at the level of autophagosome-lysosome fusion with mefloquine (MQ), and at the level of autophagosome formation by verteporfin (VP). Our data show that both drugs increased the length of cilia in cultured PLDC by 3.5-fold (Fig. 7A, C).
FIG. 7.
Inhibition of autophagy by Mefloquine (MQ) and verteporfin (VP) reestablishes PLDC cilia and ciliary localization of ARL13B, ARL3, and TGR5. (A, C) MQ and VP increased the length of cilia (red) in cultured PLDC by 3-fold, and (B, D) in PCK rats by 2-fold. In PCK rats treated with DMSO (control), Arl13b (magenta) and TGR5 (red) (E) and Arl3 (magenta) and TGR5 (red) (F) are depleted in cholangiocyte cilia (green, acetylated α-tubulin). In response to treatment with MQ and VP, ciliary localization of Arl13b, Arl3, and TGR5 was reestablished. Scale bar, 5 μm. Nuclei (blue, DAPI). Data are presented as Mean±SD. n=10 cilia for each experimental condition; ****p<0.0001.
In PCK rats treated with MQ and VP, we assessed the length of cholangiocyte cilia (Fig. 7B, D) and ciliary localization of Arl13b, Arl3 and TGR5 (Fig. 7E, F). Both, MQ and VP, were well tolerated by PCK rats without effects on serum biochemistry (Supporting Information, Figure 1). In response to treatment with MQ and VP, the length of cholangiocyte cilia was increased by 2.5-fold (Fig. 7B, D), and ciliary localization of Arl13b, Arl3 and TGR5 was reestablished (Fig. 7E, F), whereas the total levels of these three proteins was not affected by the drugs (Supporting Information, Figure 2)
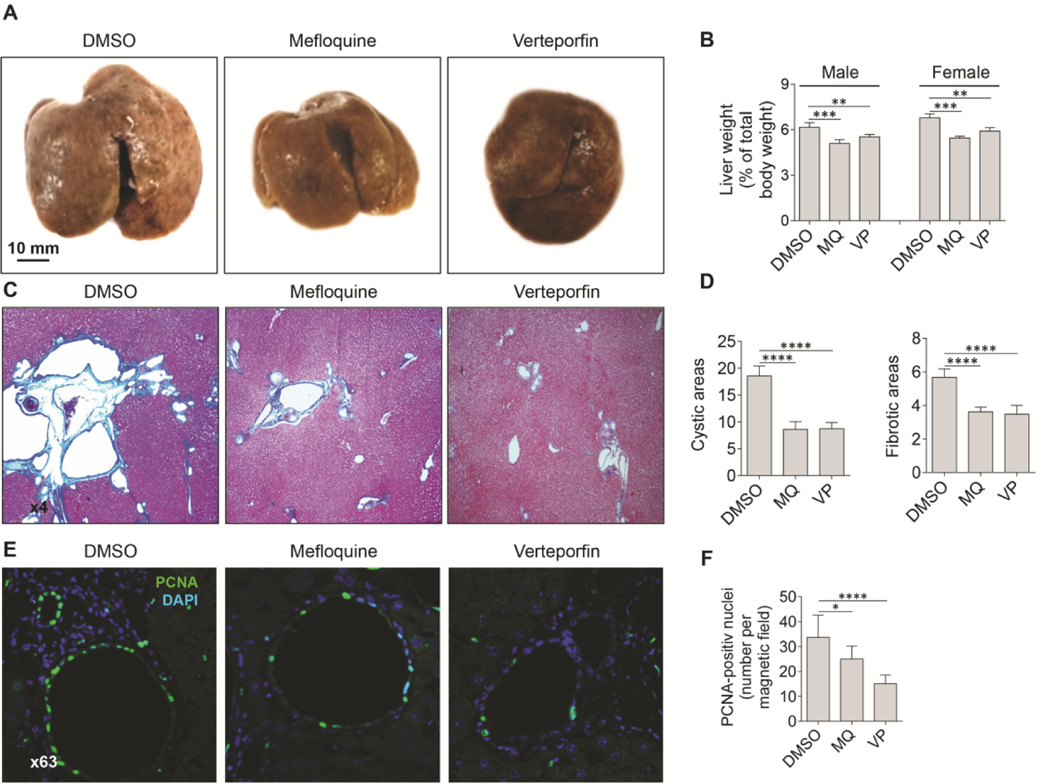
MQ and VP, were also beneficial for the progression of PLD in PCK rats (Fig. 8). Both drugs reduced the liver weight of male and female PCK rats by 9–12% (Fig. 8A, B). In response to MQ and VP, hepatic cystic areas were reduced by 45%, and fibrotic areas were reduced by 35% (Fig. 8C, D). The positive effects of MQ and VP on PLD progression in PCK rats was associated with inhibition of cholangiocyte proliferation by 15–30% (Fig. 8E, F).
FIG. 8.
Mefloquine and verteporfin are beneficial for PLD progression in PCK rats. (A) Representative images of control (DMSO) and MQ- and VP-treated livers of PCK rats. (B) In both, males, and females, treated with MQ and VP, the liver weight was decreased and (C, D) cystic (H&E staining) and fibrotic areas (trichrome staining) were reduced. (E, F) Both, MQ and VP inhibited cholangiocyte proliferation as assessed by the number of the PCNA positive nuclei (green). Scale bar 10 mm. Nuclei (blue, DAPI). Mean±SD, n=8 livers and liver lobs for each condition, *p<0.05; **p<0.001; ****p<0.0001.
DISCUSSION
The key findings of this work relate to the intersection in PLDC between enhanced autophagy, morphologically and functionally abnormal primary cilia, and progression of hepatic cystogenesis. In PLD cholangiocytes: i) ciliogenesis is impaired; ii) autophagy is enhanced when ciliogenesis is impaired; iii) ciliogenic ARL proteins are depleted in cilia and ARL13B is present in autophagosomes; iv) HDAC6 is overexpressed, localized to cilia, and ubiquitinates ARL3 and ARL13B initiating the autophagic degradation of ARL proteins; v) depletion of ARL3 and ARL13B impacts ciliary localization of a G-protein coupled bile acid receptor, TGR5; and vi) pharmacologic inhibition of autophagy by mefloquine and verteporfin in in vitro and in vivo models of PLD reestablishes cholangiocyte cilia, ciliary localization of ARL3, ARL13B and TGR5, and reduces hepatic cystogenesis.
The mechanisms of hepatic cystogenesis consist of primary (mutations in PLD-causative genes), secondary (initiation of cyst formation) and tertiary (progression of hepatic cystogenesis) molecular and cellular events in cholangiocytes.(2, 3, 7, 37, 38) To date, twelve PLD-causative genes have been discovered; nevertheless, despite many advances in genetics, it remains unknown how mutations in PLD-causative genes initiate hepatic cyst formation.(7, 37) In contrast, the mechanisms of the progression of hepatic cystogenesis are partially clarified. Limited data generated primarily by us show that these mechanisms are primarily linked to 30 dysregulated pathways in PLD cholangiocytes including alterations in autophagy that result from overexpression of ATG (autophagy related genes), cilia-mediated intracellular cAMP signaling and cholangiocyte proliferation that are considered current targets for therapeutic interventions in PLD.(7, 39)
However, off-label use of synthetic somatostatin analogs that reduce the cAMP levels and cholangiocyte proliferation is the only standard-of-care drug treatment for PLD. But the drugs are expensive, changes in liver volume are modest, and ~15% of patients are non-responders.(40, 41) Thus, continued search for new PLD therapies is necessary. Because PLD belongs to the cholangiociliopathies that result from defects in the structure and sensory/signaling functions of cholangiocyte primary cilia, pharmacological approaches to reduce cilia-mediated hepatic cystogenesis via reestablishment of the morphology and functions of cholangiocyte cilia (i.e., ciliotherapy) appear promising.
Cholangiocyte cilia in patients with ADPKD-associated PLD, and in PCK rats, an animal model of ADPKD-associated PLD, are malformed, shortened and depleted of proteins that provide sensory and signaling functions of these organelles.(11, 12) Ciliary abnormalities lead to a cilia-mediated increase in cAMP signaling, cholangiocyte proliferation, and eventually to progression of hepatic cyst growth.(6) The mechanisms of pathological transformation of primary cilia in PLDC remain unknown. Recent studies suggest that structural and functional properties of primary cilia in different cell types are facilitated by autophagy via degradation of selective proteins involved in ciliogenesis.(13–15)
Based on our previous observations(11, 12, 21) that PLDC are characterized by abnormal primary cilia and increased autophagy, we hypothesized that autophagy is involved in formation of defective PLDC cilia via depletion of ARL3 and ARL13B that are required for ciliogenesis and targeting of GPCRs to the ciliary membrane.(22–26) As we predicted, depletion of ARL proteins resulted in morphological abnormalities of PLDC cilia and misplacement of a GPCR bile acid receptor, TGR5, to the apical plasma membrane, which, as we previously demonstrated, (31) leads to abnormal cAMP signaling, enhanced cholangiocyte proliferation and hepatic cystogenesis.
We have previously described in detail the intersection between abnormalities of PLDC ciliary and enhanced hepatic cystogenesis.(11, 12) In this study, we focused on the mechanisms involved. We found that in cholangiocytes of human ADPKD-associated PLD and PCK rats, autophagy is enhanced during impaired ciliogenesis leading to depletion of ARL3 and ARL13B and enhanced hepatic cystogenesis.
It has been previously proposed that ciliary localization of ARL3 and ARL13B is regulated by HDAC6 via deacetylation of a putative adaptor protein in the IFT complex affecting ARL13B-ARL3-mediated ciliogenesis and ciliary signaling functions.(24) Our results suggest that in PLDC, HDAC6, which functions not only as deacetylase but also as ubiquitinase, is overexpressed, localized to cilia, and initiates autophagy-facilitated depletion of ARL3 and ARL13B. Depletion of ARL3 and ARL13B in PLDC cilia not only disturbs the morphology of these organelles, but also prevents ciliary localization of a G protein-coupled bile acid receptor, TGR5, which plays an important role in PLD progression.(31)
The presence of ARL13B in autophagosomes of PLDC suggests that a specific type of selective autophagy, previously termed “ciliophagy,” might contribute to PLD progression. (42). Our data showing that HDAC6 is overexpressed and localized to PLDC cilia, ubiquitinates ARL3 and ARL13B, and presumably targets ubiquitinated proteins to autophagosomes, support the idea that ciliophagy of selective ciliary proteins takes place in PLDC. It is likely that ciliary proteins, ARL3 and ARL13B, ubiquitinated by HDAC6, are delivered to autophagosomes by the specific autophagy receptors (SARs) of the ubiquitin-binding p62/SQSTM1-like receptor group.(43, 44) This suggestion is supported by our previously published work in which we demonstrated that the level of p62 (i.e., one of SARs) is significantly reduced in PCK rat cholangiocytes compared to NRC.(21)
Since alterations in autophagy contribute to abnormalities in PLDC cilia and cilia-mediated progression of hepatic cystogenesis, we focused on autophagy as a potential therapeutic target for restoration of cholangiocyte cilia, ciliary localization of ARL3, ARL13B, and TGR5, and PLD improvement. We repurposed FDA-approved drugs, mefloquine and verteporfin, as autophagy inhibitors,(45–47) and demonstrated in in vitro and in vivo models of PLD that these drugs reestablished the length of PLD cholangiocyte cilia, the ciliary localization of ARL3, ARL13B, and TGR5, and reduced hepatic cystogenesis.
Mefloquine, commonly used to treat malaria and systemic lupus erythematosus, is a potent autophagy inhibitor that interrupts autophagic flux at the level of autophagosome-lysosome fusion. (46) Verteporfin, a drug that originally was used to treat age-related macular degeneration via photodynamic therapy, is active in its non-photoactivated form as an anti-cancer drug and autophagy inhibitor.(45, 47) Verteporfin inhibits multiple steps of autophagy including autophagic membrane nucleation, phagophore formation, autophagosome maturation, and autophagosome-lysosome fusion.(45, 47) Since our data show that inhibition of any steps of autophagy in PLDC leads to restoration of cilia and reduced hepatic cystogenesis, inhibition of autophagy could be considered a potent therapeutic approach in PLD, and potentially other cholangiociliopathies associated with enhanced autophagy.
The positive effect of mefloquine on autophagy-facilitated ciliogenesis has been recently reported in human retinal pigmented epithelial (RPE) cells.(48, 49) Mefloquine induced elongation of primary cilia in RPE cells presumably via inhibition of cilia disassembly; however the exact mechanisms remain unknown. In contrast, the potential ciliotherapeutic properties of verteporfin were unknown before our work.
In summary, our data are consistent with the concept that in PLDC, autophagy facilitates depletion of the ciliogenic proteins, ARL3 and ARL13B, which are ubiquitinated by HDAC6, resulting in malformed and shortened cilia deficient in the sensory/signaling G-protein coupled bile acid receptor, TGR5, and hepatic cystogenesis. Inhibition of autophagy by the repurposed FDA-approved drugs, mefloquine and verteporfin, results in reestablishment of PLDC cilia and reduced hepatic cystogenesis suggesting these drugs for potential consideration in PLD therapeutics.
Supplementary Material
Acknowledgments
Supported by DK24031 from the NIH, by the Mayo Clinic, by the Clinical and Optical Microscopy Cores of the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567), and by the Mayo Translational Polycystic Kidney Disease Center (P30DK090728).
Abbreviations:
- PLD
polycystic liver disease
- ADPKD
autosomal dominant polycystic kidney disease
- ARPKD
autosomal recessive polycystic kidney disease
- NHC
normal human cholangiocytes
- PLDC
polycystic liver disease cholangiocytes
- HDAC6
histone deacetylase 6
- GFP-LC3
Green Fluorescent Protein targeted to LC3
- Baf A1
Bafilomycin A1
- PLA
Proximity Ligation Assay
- MQ
mefloquine
- VP
verteporfin
Footnotes
Potential conflict of interest: Nothing to report.
Additional detailed Materials and Methods are described in the Supporting Information.
REFERENCES
Author names in bold designate shared co-first authoship.
- 1.Perugorria MJ, Masyuk TV, Marin JJ, Marzioni M, Bujanda L, LaRusso NF, Banales JM. Polycystic liver diseases: advanced insights into the molecular mechanisms. Nat Rev Gastroenterol Hepatol 2014;11:750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee-Law PY, van de Laarschot LFM, Banales JM, Drenth JPH. Genetics of polycystic liver diseases. Curr Opin Gastroenterol 2019;35:65–72. [DOI] [PubMed] [Google Scholar]
- 3.Masyuk TV, Masyuk AI, LaRusso NF. Polycystic liver disease: The interplay of genes causative for hepatic and renal cystogenesis. Hepatology 2018;67:2462–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikolajczyk AE, Te HS, Chapman AB. Gastrointestinal Manifestations of Autosomal-Dominant Polycystic Kidney Disease. Clin Gastroenterol Hepatol 2017;15:17–24. [DOI] [PubMed] [Google Scholar]
- 5.Strazzabosco M, Somlo S. Polycystic liver diseases: congenital disorders of cholangiocyte signaling. Gastroenterology 2011;140:1855–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masyuk T, Masyuk A, LaRusso N. Cholangiociliopathies: genetics, molecular mechanisms and potential therapies. Curr Opin Gastroenterol 2009;25:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masyuk TV, Masyuk AI, LaRusso NF. Polycystic Liver Disease: Advances in Understanding and Treatment. Annu Rev Pathol 2022.17:251–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masyuk AI, Masyuk TV, LaRusso NF. Cholangiocyte primary cilia in liver health and disease. Dev Dyn 2008;237:2007–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 2006;131:911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee SO, Splinter PL, et al. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol 2008;295:G725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masyuk TV, Huang BQ, Ward CJ, Masyuk AI, Yuan D, Splinter PL, Punyashthiti R, et al. Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology 2003;125:1303–1310. [DOI] [PubMed] [Google Scholar]
- 12.Masyuk TV, Lee SO, Radtke BN, Stroope AJ, Huang B, Banales JM, Masyuk AI, et al. Centrosomal abnormalities characterize human and rodent cystic cholangiocytes and are associated with Cdc25A overexpression. Am J Pathol 2014;184:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pampliega O, Orhon I, Patel B, Sridhar S, Diaz-Carretero A, Beau I, Codogno P, et al. Functional interaction between autophagy and ciliogenesis. Nature 2013;502:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Z, Lin MG, Stowe TR, Chen S, Zhu M, Stearns T, Franco B, et al. Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature 2013;502:254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orhon I, Dupont N, Pampliega O, Cuervo AM, Codogno P. Autophagy and regulation of cilia function and assembly. Cell Death Differ 2015;22:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maharjan Y, Lee JN, Kwak S, Lim H, Dutta RK, Liu ZQ, So HS, et al. Autophagy alteration prevents primary cilium disassembly in RPE1 cells. Biochem Biophys Res Commun 2018;500:242–248. [DOI] [PubMed] [Google Scholar]
- 17.Morleo M, Franco B. The Autophagy-Cilia Axis: An Intricate Relationship. Cells 2019;8, 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SM, Lim JS, Ramakrishina S, Kim SH, Kim WK, Lee J, Kang HC, et al. Brain Somatic Mutations in MTOR Disrupt Neuronal Ciliogenesis, Leading to Focal Cortical Dyslamination. Neuron 2018;99:83–97. [DOI] [PubMed] [Google Scholar]
- 19.Kaliszewski M, Knott AB, Bossy-Wetzel E. Primary cilia and autophagic dysfunction in Huntington’s disease. Cell Death Differ 2015;22:1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Yi S, Kang YE, Chang JY, Kim JT, Sul HJ, Kim JO, et al. Defective ciliogenesis in thyroid hurthle cell tumors is associated with increased autophagy. Oncotarget 2016;7:79117–79130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masyuk AI, Masyuk TV, Lorenzo Pisarello MJ, Ding JF, Loarca L, Huang BQ, LaRusso NF. Cholangiocyte autophagy contributes to hepatic cystogenesis in polycystic liver disease and represents a potential therapeutic target. Hepatology 2018;67:1088–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hor CH, Goh EL. Small GTPases in hedgehog signalling: emerging insights into the disease mechanisms of Rab23-mediated and Arl13b-mediated ciliopathies. Curr Opin Genet Dev 2019;56:61–68. [DOI] [PubMed] [Google Scholar]
- 23.Larkins CE, Aviles GD, East MP, Kahn RA, Caspary T. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell 2011;22:4694–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol 2010;189:1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Ling K, Hu J. The emerging role of Arf/Arl small GTPases in cilia and ciliopathies. J Cell Biochem 2012;113:2201–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell 2007;12:767–778. [DOI] [PubMed] [Google Scholar]
- 27.Bay SN, Long AB, Caspary T. Disruption of the ciliary GTPase Arl13b suppresses Sonic hedgehog overactivation and inhibits medulloblastoma formation. Proc Natl Acad Sci U S A 2018;115:1570–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrick JJ, Vogel P, Abuin A, Hampton B, Rice DS. ADP-ribosylation factor-like 3 is involved in kidney and photoreceptor development. Am J Pathol 2006;168:1288–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masyuk AI, Huang BQ, Radtke BN, Gajdos GB, Splinter PL, Masyuk TV, Gradilone SA, et al. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am J Physiol Gastrointest Liver Physiol 2013;304:G1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keitel V, Ullmer C, Haussinger D. The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem 2010;391:785–789. [DOI] [PubMed] [Google Scholar]
- 31.Masyuk TV, Masyuk AI, Lorenzo Pisarello M, Howard BN, Huang BQ, Lee PY, Fung X, et al. TGR5 contributes to hepatic cystogenesis in rodents with polycystic liver diseases through cyclic adenosine monophosphate/Galphas signaling. Hepatology 2017;66:1197–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masyuk TV, Radtke BN, Stroope AJ, Banales JM, Gradilone SA, Huang B, Masyuk AI, et al. Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology 2013;58:409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 2007;129:1351–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q, Hu J, Ling K. Molecular views of Arf-like small GTPases in cilia and ciliopathies. Exp Cell Res 2013;319:2316–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang M, Xiang S, Joo HY, Wang L, Williams KA, Liu W, Hu C, et al. HDAC6 deacetylates and ubiquitinates MSH2 to maintain proper levels of MutSalpha. Mol Cell 2014;55:31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grumati P, Dikic I. Ubiquitin signaling and autophagy. J Biol Chem 2018;293:5404–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boerrigter MM, Bongers E, Lugtenberg D, Nevens F, Drenth JPH. Polycystic liver disease genes: Practical considerations for genetic testing. Eur J Med Genet 2021;64:104–160. [DOI] [PubMed] [Google Scholar]
- 38.Perugorria MJ, Banales JM. Genetics: Novel causative genes for polycystic liver disease. Nat Rev Gastroenterol Hepatol 2017;14:391–392. [DOI] [PubMed] [Google Scholar]
- 39.Masyuk TV, Masyuk AI, LaRusso NF. Therapeutic Targets in Polycystic Liver Disease. Curr Drug Targets 2017;18:950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larusso NF, Masyuk TV, Hogan MC. Polycystic Liver Disease: The Benefits of Targeting cAMP. Clin Gastroenterol Hepatol 2016;14:1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Aerts RMM, Kolkman M, Kievit W, Gevers TJG, Nevens F, Drenth JPH. Drug holiday in patients with polycystic liver disease treated with somatostatin analogues. Therap Adv Gastroenterol 2018;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, et al. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest 2013;123:5212–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conway O, Akpinar HA, Rogov VV, Kirkin V. Selective Autophagy Receptors in Neuronal Health and Disease. J Mol Biol 2020;432:2483–2509. [DOI] [PubMed] [Google Scholar]
- 44.Kirkin V, Rogov VV. A Diversity of Selective Autophagy Receptors Determines the Specificity of the Autophagy Pathway. Mol Cell 2019;76:268–285. [DOI] [PubMed] [Google Scholar]
- 45.Donohue E, Tovey A, Vogl AW, Arns S, Sternberg E, Young RN, Roberge M. Inhibition of autophagosome formation by the benzoporphyrin derivative verteporfin. J Biol Chem 2011;286:7290–7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma N, Thomas S, Golden EB, Hofman FM, Chen TC, Petasis NA, Schonthal AH, et al. Inhibition of autophagy and induction of breast cancer cell death by mefloquine, an antimalarial agent. Cancer Lett 2012;326:143–154. [DOI] [PubMed] [Google Scholar]
- 47.Saini H, Sharma H, Mukherjee S, Chowdhury S, Chowdhury R. Verteporfin disrupts multiple steps of autophagy and regulates p53 to sensitize osteosarcoma cells. Cancer Cell Int 2021;21:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim ES, Shin JH, Park SJ, Jo YK, Kim JS, Kang IH, Nam JB, et al. Inhibition of autophagy suppresses sertraline-mediated primary ciliogenesis in retinal pigment epithelium cells. PLoS One 2015;10:e0118190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin JH, Bae DJ, Kim ES, Kim HB, Park SJ, Jo YK, Jo DS, et al. Autophagy Regulates Formation of Primary Cilia in Mefloquine-Treated Cells. Biomol Ther (Seoul) 2015;23:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.