Introduction
Nicotine, a weak base, is the primary driver of tobacco use. The proportion of protonated vs freebase nicotine depends on the pH of the product. The extent of nicotine absorption across membranes and nicotine-mediated harshness of inhaled aerosols, which may affect satisfaction and abuse liability for different tobacco products, depend on extent of protonation. Essential in FDA regulation is consideration of the impact of pH on the clinical pharmacology of nicotine in various tobacco products.
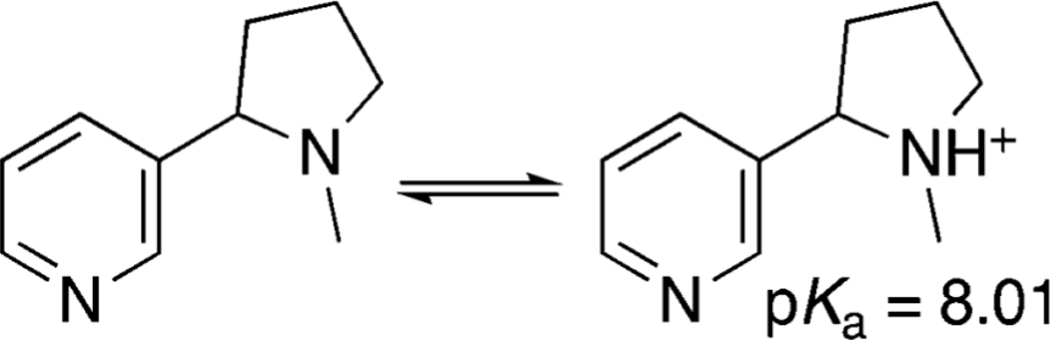
Nicotine is the primary driver of compulsive tobacco use1. Although not a major cause, nicotine may also contribute to some of the harmful effects of combusted tobacco use. Nicotine is a weak base, with a pKa of 8.0. At a pH of 8.0, 50% of nicotine is protonated and 50% unprotonated, the latter also called freebase nicotine (Fig 1). Nicotine is water soluble, but in its freebase form readily crosses lipid membranes and rapidly enters body tissues.
Figure 1.
Nicotine in tobacco products exists in two forms: nicotine freebase (left) and protonated nicotine (right). In water at 25 deg C, the pKa is 8.01 (Figure courtesy of Peyton Jacob III PhD)
Non-combusted nicotine products such a smokeless tobacco, nicotine gums and lozenges and nicotine patches are buffered to an alkaline pH to facilitate nicotine absorption through mucous membranes and the skin. Some combusted tobacco products like large and premium cigars and pipe tobacco deliver nicotine in smoke with a high pH, which facilitates buccal absorption of nicotine. At the same time at higher pH, the smoke is harsh and difficult to inhale, due to the impact of high concentrations of freebase nicotine on oropharyngeal and tracheal nicotinic cholinergic sensory receptors. Cigarette smoke typically has a pH of around 5.5–6.5, which makes the smoke less harsh and easier to inhale. Once cigarette smoke reaches the pulmonary alveoli, nicotine leaves the smoke and at the physiological pH of the lung is readily absorbed through the pulmonary capillaries and into the systemic circulation.
While it was previously reported that the pH of different commercial smokeless tobacco products differs, and that higher pH is associated with greater nicotine absorption and greater cardiovascular effects2, the Wilhelm study published in this issue of CPT is the first to study nicotine absorption and pharmacologic effects with experimental manipulation of the pH in a single commercial product – Copenhagen Long Cut3. The product pH was varied from 5.0 to 8.6, resulting in a variation in freebase nicotine from 0.1 to 79%. Participants placed a typical dose of 2 grams of tobacco between the lip and gum for 30 min, and blood levels and cardiovascular effects were measured over 240 minutes. Plasma nicotine AUC increased four-fold across the pH range of 5.0 to 8.6. Increases in heart rate and blood pressure were also greater at higher pH, but effect sizes were smaller than observed for blood nicotine AUC. Subjective effects, which are important in assessing abuse liability, were measured by protocol, but unfortunately were not presented in this paper.
One can assume that greater nicotine absorption with faster and higher peak blood nicotine levels would predict greater abuse liability. Other studies have reported greater subjective effects with higher pH smokeless tobacco products2. Some tobacco companies have marketed low pH smokeless tobacco products as starter products (presumably for nicotine-naïve youth), as well as progressively higher pH products that would promote and sustain dependence.
Of note is a highly addictive smokeless tobacco product called Iqmik, used by some Alaska Native Americans, which has a pH of 10.9, such that 99.9% of nicotine is in freebase form. Iqmik is home-made using commercial tobacco leaves mixed with ash from a fungus or tree wood to alkalinize the product. While the nicotine content of Iqmik and commercial smokeless tobacco products are similar, daily nicotine exposure among Alaska Native Americans was shown to be much higher for Iqmik users compared to both commercial smokeless tobacco users and cigarette smokers4.
Recently nicotine pouch products, containing nicotine without other constituents of tobacco, have been marketed as a potentially safer way to consume nicotine compared to smoking or using smokeless tobacco. These products such as Zyn (Swedish Match), On! (Altria), Velo (RJ Reynolds) and Lyft (British American Tobacco) and others are sold in pouches with various levels of nicotine and many different flavors. Among U.S. marketed nicotine pouch products, pH ranges from 6.86 to 10.15. Total nicotine per pouch ranges from 1.3 to 6.1 mg, with freebase nicotine levels ranging from 0.2 to 6.1 mg. The wide range of pH and freebase nicotine suggest large differences in rate and extent of nicotine absorption, in abuse liability and also in the utility of replacing nicotine from cigarettes in those want to switch from tobacco to a less harmful nicotine product.
Perhaps the greatest impact of pH manipulation on the clinical pharmacology of nicotine delivery systems has been seen among electronic cigarette (e-cigarette) products. E-cigarettes are battery-powered devices that aerosolize a liquid containing propylene glycol and/or vegetable glycerin, nicotine and flavorings that is inhaled similarly to cigarette smoke6. E-cigarettes have the potential to provide nicotine by inhalation (with rapid delivery to the brain) in a non-combusted form to addicted cigarette smokers, thereby facilitating smoking cessation and harm reduction. However, the marketing of e-cigarettes has also raised concerns about a gateway to nicotine dependence among youth who are not cigarette smokers.
E-cigarettes have evolved over time with several generations of design, including cigarette-like devices, pen or tank style devices, advanced personalized vaporizers also called “mods” and “pod mod” devices that use replaceable cartridges (pods).
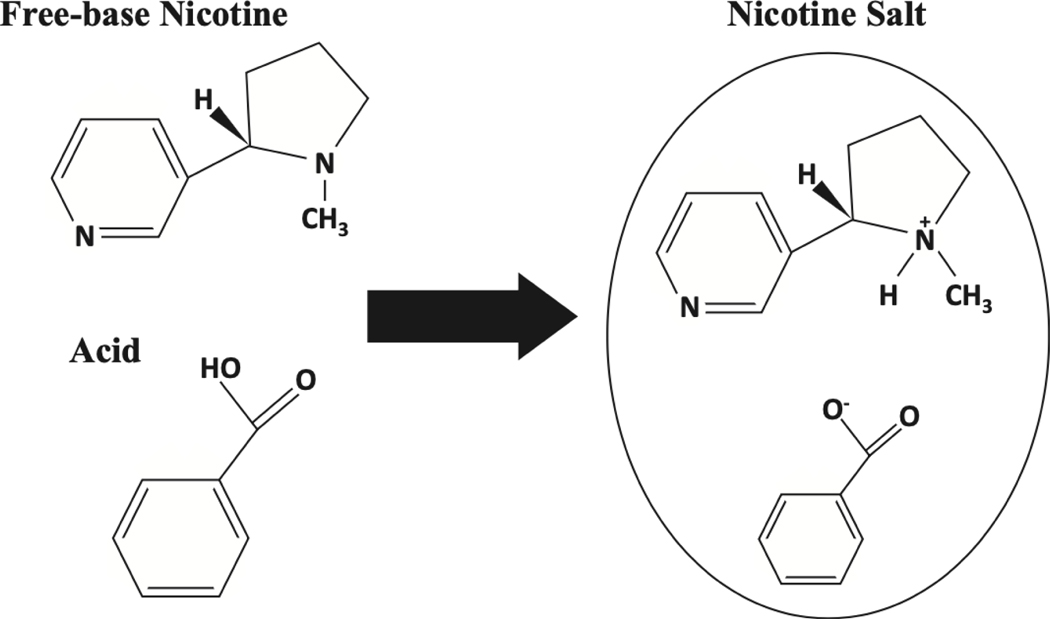
In the early generation of e-cigarettes, nicotine in the liquid was predominantly in the freebase form. Typical pH was in the 7–9 range. Typical concentrations of free base nicotine were 3 to 24 mg/ml, and the higher the nicotine concentration the higher the pH. With the introduction of pod system devices such as JUUL came nicotine salt-based liquids, using various salts with benzoic, lactic and levulinic the most common7 (Figure 2). Nicotine salt solutions with lower pH (for example 5.5 with JUUL) result in a high percentage of nicotine in the protonated form, which is much less irritating when inhaled, and allows for the use of much higher nicotine concentrations in the liquid (for example 59 mg/ml for JUUL). Now many e-cigarette and e-liquid manufacturers market products, including both pods and disposable e-cigarettes with nicotine salts in high concentrations (50 to 85 mg/ml) and with pH typically in the 5–6 range.
Figure 2.
Nicotine salt formation. Example of freebase nicotine combining with benzoic acid to form a solution of protonated nicotine and deprotonated benzoic acid (nicotine benzoate). Such nicotine salt solutions are found in many electronic cigarette liquids. (From Harvanko, A. M., Havel, C. M., Jacob, P. & Benowitz, N. L. Characterization of Nicotine Salts in 23 Electronic Cigarette Refill Liquids. Nicotine & Tobacco Research 22, 1239–1243 (2020); Copyright © The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco. All rights reserved.)
The pH of nicotine in e-liquids has implications both for product safety and addiction. Regular users of nicotine products tend to titrate their intake of nicotine to achieve desired levels in the body. A person using a nicotine salt product with high nicotine concentration is able to satisfy their nicotine need with small amounts of aerosol generated at relatively low temperature, which means lower exposure to oxidant chemicals and thermal degradation products8. The converse is the case for those who use low nicotine freebase liquids who are vaping higher temperature devices. Thus, use of high concentration nicotine salt liquids would be the safest way for cigarette smokers to transition from cigarettes to e-cigarettes for harm reduction.
On the other hand, the availability of easy to inhale high nicotine concentration salt liquids poses a concern for promoting nicotine addiction among youth. The use of e-cigarettes among youth has risen in recent years, with recent estimates of 11% of U.S. high school students using e-cigarettes in the past 30 days, and 28% of these using daily, suggesting addictive use. The adverse consequences of youth vaping may include development of long-lasting nicotine addiction and nicotine-induced delayed maturation of the adolescent brain.
The pH of cigars is also believed to influence the potential for adverse health effects as well as abuse liability. Large and premium cigar have higher pH, which may mean harsher smoke, lesser degrees of inhalation, and a less systemic exposure to smoke toxicants. Nicotine in alkaline cigar smoke is easily absorbed through the buccal mucosa which produces nicotine-related satisfaction, but absorption at a slower rate compared to inhaled nicotine, suggesting lower addiction potential. The consequence of high pH in large and premium cigars could be a lower risk of tobacco-induced disease compared to other cigars with lower smoke pH.
The FDA through the Center for Tobacco Products has regulatory authority not only over cigarettes and most cigars but also non-combusted nicotine products such as smokeless tobacco, oral nicotine products and e-cigarettes, including devices and liquids. The non-combusted tobacco products are likely to have utility in cigarette harm reduction by facilitating switching from combustible tobacco to less harmful forms of nicotine delivery. FDA regulation needs to address the important design characteristics of nicotine concentration and pH. For smokeless tobacco and oral nicotine products, higher pH may enhance nicotine satisfaction, but also increase abuse liability, particularly for youth. For e-cigarettes, lower pH and higher nicotine concentrations may mean less exposure to thermally generated toxicants and benefit smokers who want to switch, but may enhance abuse liability among youth. The European Union limits nicotine in e-liquids to 20 mg/ml, while at present there are no limits in the U.S.A. Some argue that a nicotine limit of 20 mg/ml is bad policy because it encourages the use of high power e-cigarettes that expose the user to more toxicants. A regulatory challenge to FDA is balancing the potential of non-combusted tobacco products to promote the tobacco endgame in adult cigarette smokers vs possible harm to youth9. Essential in such regulation is a consideration of the impact of pH on the clinical pharmacology of nicotine in various tobacco products.
Acknowledgments
Funding: Preparation of this commentary was supported by National Institutes of Health grants U54 HL147127, from the National Heart Lung and Blood Institute and by DA03924 from the National Institute on Drug Abuse.
Footnotes
Conflict of interest: Dr. Benowitz has been a consultant to Pfizer and Achieve Life Sciences, companies that market or are developing smoking cessation medications, and has been a paid expert witness in litigation against tobacco companies.
References
- 1.Benowitz NL Nicotine Addiction. N Engl J Med 362, 2295–2303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fant RV, Henningfield JE, Nelson RA & Pickworth WB Pharmacokinetics and pharmacodynamics of moist snuff in humans. Tobacco Control 8, 387–392 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilhelm J, Mishina E, Viray L, Paredes A. & Pickworth WB The pH of Smokeless Tobacco Determines Nicotine Buccal Absorption: Results of a Randomized Crossover Trial. Clin Pharma and Therapeutics cpt.2493 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Benowitz NL et al. Exposure to Nicotine and Carcinogens among Southwestern Alaskan Native Cigarette Smokers and Smokeless Tobacco Users. Cancer Epidemiol Biomarkers Prev 21, 934–942 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanfill S. et al. Characterization of Total and Unprotonated (Free) Nicotine Content of Nicotine Pouch Products. Nicotine & Tobacco Research 23, 1590–1596 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Benowitz NL, St. Helen G. & Liakoni E. Clinical Pharmacology of Electronic Nicotine Delivery Systems (ENDS): Implications for Benefits and Risks in the Promotion of the Combusted Tobacco Endgame. The Journal of Clinical Pharmacology 61, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvanko AM, Havel CM, Jacob P. & Benowitz NL Characterization of Nicotine Salts in 23 Electronic Cigarette Refill Liquids. Nicotine & Tobacco Research 22, 1239–1243 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kośmider L, Kimber CF, Kurek J, Corcoran O. & Dawkins LE Compensatory Puffing With Lower Nicotine Concentration E-liquids Increases Carbonyl Exposure in E-cigarette Aerosols. Nicotine & Tobacco Research 20, 998–1003 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Balfour DJK et al. Balancing Consideration of the Risks and Benefits of E-Cigarettes. Am J Public Health 111, 1661–1672 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]