The emergence of pathogenic bacteria resistant to most, if not all, currently available antimicrobial agents has become a critical problem in modern medicine, particularly because of the concomitant increase in immunosuppressed patients. The concern that humankind is reentering the “preantibiotics” era has become very real, and the development of alternative antiinfection modalities has become one of the highest priorities of modern medicine and biotechnology.
Prior to the discovery and widespread use of antibiotics, it was suggested that bacterial infections could be prevented and/or treated by the administration of bacteriophages. Although the early clinical studies with bacteriophages were not vigorously pursued in the United States and Western Europe, phages continued to be utilized in the former Soviet Union and Eastern Europe. The results of these studies were extensively published in non-English (primarily Russian, Georgian, and Polish) journals and, therefore, were not readily available to the western scientific community. In this minireview, we briefly describe the history of bacteriophage discovery and the early clinical studies with phages and we review the recent literature emphasizing research conducted in Poland and the former Soviet Union. We also discuss the reasons that the clinical use of bacteriophages failed to take root in the West, and we share our thoughts about future prospects for phage therapy research.
DISCOVERY OF BACTERIOPHAGES AND EARLY PHAGE THERAPY RESEARCH
Discovery of bacteriophages.
Bacteriophages or phages are bacterial viruses that invade bacterial cells and, in the case of lytic phages, disrupt bacterial metabolism and cause the bacterium to lyse. The history of bacteriophage discovery has been the subject of lengthy debates, including a controversy over claims for priority. Ernest Hankin, a British bacteriologist, reported in 1896 (21) on the presence of marked antibacterial activity (against Vibrio cholerae) which he observed in the waters of the Ganges and Jumna rivers in India, and he suggested that an unidentified substance (which passed through fine porcelain filters and was heat labile) was responsible for this phenomenon and for limiting the spread of cholera epidemics. Two years later, the Russian bacteriologist Gamaleya observed a similar phenomenon while working with Bacillus subtilis (48), and the observations of several other investigators are also thought to have been related to the bacteriophage phenomenon (72). However, none of these investigators further explored their findings until Frederick Twort, a medically trained bacteriologist from England, reintroduced the subject almost 20 years after Hankin's observation by reporting a similar phenomenon and advancing the hypothesis that it may have been due to, among other possibilities, a virus (70). However, for various reasons—including financial difficulties (68, 70)—Twort did not pursue this finding, and it was another 2 years before bacteriophages were “officially” discovered by Felix d'Herelle, a French-Canadian microbiologist at the Institut Pasteur in Paris.
The discovery or rediscovery of bacteriophages by d'Herelle is frequently associated with an outbreak of severe hemorrhagic dysentery among French troops stationed at Maisons-Laffitte (on the outskirts of Paris) in July-August 1915, although d'Herelle apparently first observed the bacteriophage phenomenon in 1910 while studying microbiologic means of controlling an epizootic of locusts in Mexico. Several soldiers were hospitalized, and d'Herelle was assigned to conduct an investigation of the outbreak. During these studies, he made bacterium-free filtrates of the patients' fecal samples and mixed and incubated them with Shigella strains isolated from the patients. A portion of the mixtures was inoculated into experimental animals (as part of d'Herelle's studies on developing a vaccine against bacterial dysentery), and a portion was spread on agar medium in order to observe the growth of the bacteria. It was on these agar cultures that d'Herelle observed the appearance of small, clear areas, which he initially called taches, then taches vierges, and, later, plaques (68). D'Herelle's findings were presented during the September 1917 meeting of the Academy of Sciences, and they were subsequently published (18) in the meeting's proceedings. In contrast to Hankin and Twort, d'Herelle had little doubt about the nature of the phenomenon, and he proposed that it was caused by a virus capable of parasitizing bacteria. The name “bacteriophage” was also proposed by d'Herelle, who, according to his recollections (68), decided on this name together with his wife Marie on 18 October 1916—the day before their youngest daughter's birthday (d'Herelle apparently first isolated bacteriophages in the summer of 1916, approximately 1 year after the Maisons-Laffitte outbreak). The name was formed from “bacteria” and “phagein” (to eat or devour, in Greek), and was meant to imply that phages “eat” or “devour” bacteria.
D'Herelle, who considered himself to be the discoverer of bacteriophages, was made aware (12, 71) of the prior discovery of Twort but maintained that the phenomenon described by Twort was distinct from his discovery. In the meantime, in contrast to Twort, d'Herelle actively pursued studies of bacteriophages and strongly promoted the idea that phages were live viruses—and not “enzymes” as many of his fellow researchers thought. The priority dispute ceased eventually, and many scientists accepted the independent discovery of bacteriophages and simply referred to it as the “Twort-d'Herelle phenomenon” and, later, the “bacteriophage phenomenon.”
Early studies of phage therapy.
Not long after his discovery, d'Herelle used phages to treat dysentery, in what was probably the first attempt to use bacteriophages therapeutically. The studies were conducted at the Hôpital des Enfants-Malades in Paris in 1919 (68) under the clinical supervision of Professor Victor-Henri Hutinel, the hospital's Chief of Pediatrics. The phage preparation was ingested by d'Herelle, Hutinel, and several hospital interns in order to confirm its safety before administering it the next day to a 12-year-old boy with severe dysentery. The patient's symptoms ceased after a single administration of d'Herelle's antidysentery phage, and the boy fully recovered within a few days. The efficacy of the phage preparation was “confirmed” shortly afterwards, when three additional patients having bacterial dysentery and treated with one dose of the preparation started to recover within 24 h of treatment. However, the results of these studies were not immediately published and, therefore, the first reported application of phages to treat infectious diseases of humans came in 1921 from Richard Bruynoghe and Joseph Maisin (13), who used bacteriophages to treat staphylococcal skin disease. The bacteriophages were injected into and around surgically opened lesions, and the authors reported regression of the infections within 24 to 48 h. Several similarly promising studies followed (44, 49, 66), and encouraged by these early results, d'Herelle and others continued studies of the therapeutic use of phages (e.g., d'Herelle used various phage preparations to treat thousands of people having cholera and/or bubonic plague in India [68]). In addition, several companies began active commerical production of phages against various bacterial pathogens.
COMMERCIAL PRODUCTION OF PHAGES
D'Herelle's commercial laboratory in Paris produced at least five phage preparations against various bacterial infections. The preparations were called Bacté-coli-phage, Bacté-rhino-phage, Bacté-intesti-phage, Bacté-pyo-phage, and Bacté-staphy-phage, and they were marketed by what later became the large French company L'Oréal (68). Therapeutic phages were also produced in the United States. In the 1940s, the Eli Lilly Company (Indianapolis, Ind.) produced seven phage products for human use, including preparations targeted against staphylococci, streptococci, Escherichia coli, and other bacterial pathogens. These preparations consisted of phage-lysed, bacteriologically sterile broth cultures of the targeted bacteria (e.g., Colo-lysate, Ento-lysate, Neiso-lysate, and Staphylo-lysate) or the same preparations in a water-soluble jelly base (e.g., Colo-jel, Ento-jel, and Staphylo-jel). They were used to treat various infections, including abscesses, suppurating wounds, vaginitis, acute and chronic infections of the upper respiratory tract, and mastoid infections. However, the efficacy of phage preparations was controversial (20, 26), and with the advent of antibiotics, commercial production of therapeutic phages ceased in most of the Western world. Nevertheless, phages continued to be used therapeutically—together with or instead of antibiotics—in Eastern Europe and in the former Soviet Union. Several institutions in these countries were actively involved in therapeutic phage research and production, with activities centered at the Eliava Institute of Bacteriophage, Microbiology, and Virology (EIBMV) of the Georgian Academy of Sciences, Tbilisi, Georgia, and the Hirszfeld Institute of Immunology and Experimental Therapy (HIIET) of the Polish Academy of Sciences, Wroclaw, Poland.
EIBMV.
The Eliava Institute (http://www.geocities.com /hotsprings/spa/5386) was founded in 1923 by Giorgi Eliava, a prominent Georgian bacteriologist, together with Felix d'Herelle. D'Herelle spent several months in Georgia collaborating with Eliava and other Georgian colleagues, and he intended to move to Tbilisi permanently (a cottage built for his use still stands on the Institute's grounds). However, in 1937 Eliava was arrested by Stalin's NKVD (the predecessor of the KGB), pronounced a “People's Enemy,” and executed. Frustrated and disillusioned, d'Herelle never returned to Georgia. Nonetheless, the Institute survived and later became one of the largest facilities in the world engaged in the development of therapeutic phage preparations. The Institute, during its best times, employed approximately 1,200 researchers and support personnel and produced phage preparations (often several tons a day) against a dozen bacterial pathogens, including staphylococci, Pseudomonas, Proteus, and many enteric pathogens. Most of the Soviet studies reviewed in this article involved phages developed and produced at the EIBMV.
HIIET.
The Hirszfeld Institute (http://surfer.iitd.pan.wroc.pl/index1.htm) was founded in 1952, and its staff has been actively involved in phage therapy research since 1957, when therapeutic phages were used to treat Shigella infections (B. Weber-Dabrowska, personal communication). The bacteriophage laboratory of the Institute was instrumental in developing and producing phages for the treatment of septicemia, furunculosis, and pulmonary and urinary tract infections and for the prophylaxis or treatment of postoperative and postraumatic infections. In many cases, phages were used against multidrug-resistant bacteria that were refractory to conventional treatment with antibiotics. The most detailed studies published in English on the use of phages in clinical settings have come from this institute (52–58).
PRECLINICAL STUDIES IN ANIMALS
One of the best-known series of recent studies on the use of phages in veterinary medicine came from the laboratory of William Smith and his colleagues (59–62) at the Institute for Animal Disease Research in Houghton, Cambridgeshire, Great Britain. In one of their early papers (59), the authors reported the successful use of phages to treat experimental E. coli infections in mice. During subsequent studies (60–62), the authors found that a single dose of specific E. coli phage reduced, by many orders of magnitude, the number of target bacteria in the alimentary tract of calves, lambs, and piglets infected with a diarrhea-causing E. coli strain. The treatment also stopped the associated fluid loss, and all animals treated with phages survived the bacterial infection. These studies were reviewed by other authors (5, 8, 14) and were evaluated using mathematical models and statistical analyses (31). Also, the success of these studies rekindled interest in phage therapy in the West and prompted other researchers to investigate the effect of phages on antibiotic-resistant bacteria capable of causing human infections. For example, Soothill et al. (63–65) reported the utility of phages in preventing and treating experimental disease in mice and guinea pigs infected with Pseudomonas aeruginosa and Acinetobacter, and they suggested that phages might be efficacious in preventing infections of skin grafts used to treat burn patients. However, it is unclear whether any of these “preclinical” studies were used as the basis for subsequent human clinical trials. In fact, although many human trials probably were preceded by at least some preliminary testing with laboratory animals, there are only a very limited number of publications in which such an approach can be traced. One example is recent studies (10, 11) evaluating the efficacy of bacteriophages for the treatment of infections caused by Klebsiella ozaenae, Klebsiella rhinoscleromatis scleromatis and Klebsiella pneumoniae. The phage preparation was reported (10) to be (i) efficacious in treating experimental infections of mice and (ii) nontoxic in mice and guinea pigs; i.e., gross and histological changes were not observed after intravenous (i.v.), intranasal, and intraperitoneal administration, even after a dose approximately 3,500-fold higher (estimated by body weight) than the human dose was given to mice during acute toxicity studies. In addition, the authors delineated the optimal phage concentration and admininstration route and reported other pertinent details which they considered to be important in designing subsequent human volunteer trials. They subsequently (11) used the results of their preclinical studies to evaluate the safety and efficacy of the phages in treating 109 patients having Klebsiella infections. The phage preparation was reported to be both effective (marked clinical improvements with associated bacteriological clearance) in treating Klebsiella infections and nontoxic for the patients.
PROPHYLAXIS AND TREATMENT OF BACTERIAL INFECTIONS IN HUMANS
The international literature contains several hundred reports on phage therapy in humans, with the majority of recent publications coming from researchers in Eastern Europe and the former Soviet Union and only a few reports (1, 30, 73) published in other countries. In the English language literature, several reviews of phage therapy have recently been published (3, 8, 14). In addition, comprehensive information about the discovery of bacteriophages and the history of phage therapy has been published recently by Yale University Press (68) and included in a web page (http://www.evergreen.edu/user/t4/phagetherapy/phagethea.html). Clearly, it would be impossible to summarize all of these publications in this minireview; therefore, we have focused our minireview primarily on papers published in the non-English literature not widely accessible to the international scientific community. Overall, we have reviewed over a hundred phage therapy publications available in the Georgian, Russian, and English literature, including Ph.D. theses and meeting presentations from the former Soviet Union. However, theses and meeting presentations (all speaking in favor of phage therapy) are not discussed here, and we have focused primarily on reports published in peer-reviewed journals. Some of the major human phage therapy studies from Poland and the former Soviet Union are summarized in Table 1.
TABLE 1.
Some of the major human phage therapy studies performed in Poland and the former Soviet Union
| Reference(s) | Infection(s) | Etiologic agent(s) | Comments |
|---|---|---|---|
| Babalova et al. (7) | Bacterial dysentery | Shigella | Shigella phages were successfully used for prophylaxis of bacterial dysentery. |
| Bogovazova et al. (11) | Infections of skin and nasal mucosa | K. ozaenae, K. rhinoscleromatis, and K. pneumoniae | Adapted phages were reported to be effective in treating Klebsiella infections in all of the 109 patients. |
| Cislo et al. (17) | Suppurative skin infections | Pseudomonas, Staphylococcus, Klebsiella, Proteus, and E. coli | Thirty-one patients having chronically infected skin ulcers were treated orally and locally with phages. The success rate was 74%. |
| Ioseliani et al. (22) | Lung and pleural infections | Staphylococcus, Streptococcus, E. coli, and Proteus | Phages were successfully used together with antibiotics to treat lung and pleural infections in 45 patients. |
| Kochetkova et al. (25) | Postoperative wound infections in cancer patients | Staphylococcus and Pseudomonas | A total of 131 cancer patients having postsurgical wound infections participated in the study. Of these, 65 patients received phages and the rest received antibiotics. Phage treatment was successful in 82% of the cases, and antibiotic treatment was successful in 61% of the cases. |
| Kucharewicz-Krukowska and Slopek (27) | Various infections | Staphylococcus, Klebsiella, E. coli, Pseudomonas, and Proteus | Immunogenicity of therapeutic phages was analyzed in 57 patients. The authors concluded that the phages' immunogenicity did not impede therapy. |
| Kwarcinski et al. (29) | Recurrent subphrenic abscess | E. coli | Recurrent subphrenic abscess (after stomach resection) caused by an antibiotic-resistant strain of E. coli was successfully treated with phages. |
| Litvinova et al. (32) | Intestinal dysbacteriosis | E. coli and Proteus | Phages were successfully used together with bifidobacteria to treat antibiotic-associated dysbacteriosis in 500 low-birth-weight infants. |
| Meladze et al. (33) | Lung and pleural infections | Staphylococcus | Phages were used to treat 223 patients having lung and pleural infections, and the results were compared to 117 cases where antibiotics were used. Full recovery was observed in 82% of the patients in the phage-treated group, as opposed to 64% of the patients in the antibiotic-treated group. |
| Miliutina and Vorotyntseva (35) | Bacterial dysentery and salmonellosis | Shigella and Salmonella | The effectiveness of treating salmonellosis using phages and a combination of phages and antibiotics was examined. The combination of phages and antibiotics was reported to be effective in treating cases where antibiotics alone were ineffective. |
| Perepanova et al. (40) | Inflammatory urologic diseases | Staphylococcus, E. coli, and Proteus | Adapted phages were used to treat acute and chronic urogenital inflammation in 46 patients. The efficacy of phage treatment was 92% (marked clinical improvements) and 84% (bacteriological clearance). |
| Sakandelidze and Meipariani (45) | Peritonitis, osteomyelitis, lung abscesses, and postsurgical wound infections | Staphylococcus, Streptococcus, and Proteus | Phages administered subcutaneously or through surgical drains in 236 patients having antibiotic-resistant infections eliminated the infections in 92% of the patients. |
| Sakandelidze (46) | Infectious allergoses (rhinitis, pharyngitis, dermatitis, and conjunctivitis) | Staphylococcus, Streptococcus, E. coli, Proteus, enterococci, and P. aeruginosa | A total of 1,380 patients having infectious allergoses were treated with phages (360 patients), antibiotics (404 patients), or a combination of phages and antibiotics (576 patients). Clinical improvement was observed in 86, 48 and 83% of the cases, respectively. |
| Slopek et al. (52–58) | Gastrointestinal tract, skin, head, and neck infections | Staphylococcus, Pseudomonas, E. coli, Klebsiella, and Salmonella | A total of 550 patients were treated with phages. The overall success rate of phage treatment was 92%. |
| Stroj et al. (67) | Cerebrospinal meningitis | K. pneumoniae | Orally administered phages were used successfully to treat meningitis in a newborn (after antibiotic therapy failed). |
| Tolkacheva et al. (69) | Bacterial dysentery | E. coli and Proteus | Phages were used together with bifidobacteria to treat bacterial dysentery in 59 immunosuppressed leukemia patients. The superiority of treatment with phage-bifidobacteria over antibiotics was reported. |
| Weber-Dabrowska et al. (74) | Suppurative infections | Staphylococcus and various gram-negative bacteria | Orally administered phages were used to successfully treat 56 patients, and the phages were found to reach the patients' blood and urine. |
| Zhukov-Verezhnikov et al. (77) | Suppurative surgical infections | Staphylococcus, Streptococcus, E. coli, and Proteus | The superiority of adapted phages (phages selected against bacterial strains isolated from individual patients) over commercial phage preparations was reported in treating 60 patients having suppurative infections. |
Polish papers.
The most detailed English language reports on phage therapy in humans were by Slopek et al., who published a series of six papers (52–57) on the effectiveness of phages against infections caused by several bacterial pathogens, including multidrug-resistant mutants. Their seventh paper (58) summarized the results of all these studies, and it is discussed in some detail here. Five hundred fifty patients having bacterial septicemia and ranging in age from 1 week to 86 years were treated at a total of 10 clinical departments and hospitals located in three different cities. Antibiotic treatment (no information was given about the specific antibiotics used) was reported to be ineffective in 518 of the patients, leading to the decision to use phage therapy. The etiologic agents in the studies of Slopek et al. (52–58) were staphylococci, Pseudomonas, Escherichia, Klebsiella, and Salmonella, and treatment was initiated after isolating the etiologic agents and selecting specific, highly potent phages from a collection of more than 250 lytic phages. Phages were administered as follows: (i) orally, three times a day before eating and after neutralizing gastric acid by oral administration of baking soda or bicarbonated mineral water a few minutes prior to phage administration; (ii) locally, by applying moist, phage-containing dressings directly on wounds and/or pleural and peritoneal cavities; and (iii) by applying a few drops of phage suspension to the eye, middle ear, or nasal mucosa. During the course of phage treatment, the etiologic agents were continuously monitored for phage susceptibility, and if phage resistance developed, phages were replaced with different bacteriophages lytic against the newly emerged, phage-resistant bacterial mutants. The duration of treatment was 1 to 16 weeks, and in some cases phages were applied for up to 14 days after negative cultures were obtained. The rates of success (marked to complete recovery in conjunction with negative cultures) ranged from 75 to 100% (92% overall) and were even higher (94%) with the 518 patients for whom antibiotic therapy was ineffective. Control groups without phage treatment were not included in the study.
In other publications from Poland (Table 1), phages were reported to be effective in treating cerebrospinal meningitis in a newborn (67), skin infections caused by Pseudomonas, Staphylococcus, Klebsiella, Proteus, and E. coli (17), recurrent subphrenic and subhepatic abscesses (29), and various chronic bacterial diseases (23). In addition to being effective in the treatment of long-term suppurative infections, phage therapy was found, in a recent study (75), to normalize tumor necrosis factor alpha (TNF-α) levels in serum and the production of TNF-α and interleukin-6 by blood cell cultures.
Soviet papers.
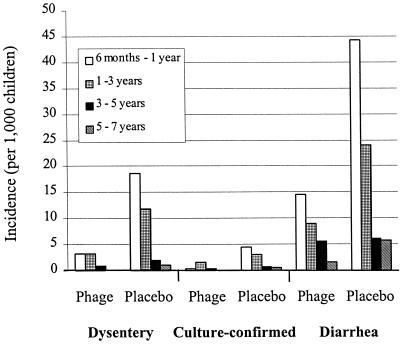
One of the most, if not the most, extensive studies evaluating the utility of therapeutic phages for prophylaxis of infectious diseases was conducted in Tbilisi, Georgia, during 1963 and 1964 (7) and involved phages against bacterial dysentery. A total of 30,769 children (6 months to 7 years old) were included in the study. Of these, children on one side of the streets (17,044 children) were given Shigella phages orally (once every 7 days), and the children on the other side of the streets (13,725) did not receive phages. The children in both groups were visited on a once-a-week basis to administer phages and monitor their overall status. Fecal samples from all children having gastrointestinal disorders were tested for the presence of Shigella spp. and other, unspecified diarrhea-causing bacteria. Based on clinical diagnosis, the incidence of dysentery was 3.8-fold higher in the placebo group than in the phage-treated group (6.7 and 1.76 per 1,000 children, respectively) during the 109-day study period; based on the culture-confirmed cases, the incidence of dysentery was 2.6-fold higher in the placebo group than in the phage-treated group (1.82 and 0.7, respectively) (Fig. 1). The phage effectiveness index (disease incidence per 1,000 children in the placebo group divided by the corresponding number in the phage-treated group) was highest in children between 6 months and 1 year of age and was lowest in children 5 to 7 years of age. An interesting outcome of the study was that there was an overall reduction (2.3-fold) in diarrheal diseases of unknown origin among children treated with phages compared to the children in the placebo group. This may have been observed because some dysentery cases were not diagnosed as such (but were prevented with the Shigella phage preparation) or because the phage preparation, although developed specifically against Shigella species, was also active against some additional gastrointestinal pathogens.
FIG. 1.
The incidence of clinical dysentery, culture-confirmed dysentery, and diarrheal disease of undetermined etiology in phage-treated and phage-untreated (placebo) children 6 months to 7 years of age (the data are from reference 7).
Many similar clinical studies, albeit conducted on a smaller scale, have yielded similar results (Table 1). To give but a few examples, phages have been reported to be effective in treating staphylococcal lung infections (22, 33), P. aeruginosa infections in cystic fibrosis patients (50), eye infections (43), neonatal sepsis (38), urinary tract infections (40), and surgical wound infections (39, 41). However, as with the Polish studies, controls were not included in the majority of these trials or controls were used but information needed for rigorous evaluation of the authors' conclusions was not provided. For example, a study which was meant to be a double-blind trial evaluating the efficacy of bacteriophages for prophylaxis and/or treatment of bacterial dysentery was conducted in 1982-1983 and included soldiers of the Red Army stationed in four distinct geographic regions of the former Soviet Union (6). The study was conducted so that all information about the patients and preparations given to them was coded (i.e., the study was performed in a double-blinded manner), and the authors reported that the incidence of dysentery in the phage-treated groups was approximately 10-fold less than in the control group (P < 0.0001). However, information was not presented concerning the number of patients enrolled in each arm of the study and the methods used to evaluate the results. Thus, it is impossible to evaluate rigorously the efficacy of the phage treatment used in the study.
In the majority of other studies, the effectiveness of phage therapy was not questioned and controls were used only to compare the effectiveness of new or modified phage preparations to that of prior phage preparations. For example, Zhukov-Verezhnikov et al. (77) compared the effectiveness of “adapted” bacteriophages (i.e., phages selected against bacterial strains isolated from individual patients) to that of commercially available phage preparations. The authors used phage preparations to treat 60 patients having suppurative surgical infections. Thirty patients were treated with phages specifically adapted to strains isolated from each patient, and an equal number of patients were treated with commercially available phage preparations targeted against staphylococci, streptococci, enteropathogenic E. coli, and Proteus. The adapted bacteriophages were reported to be five- to sixfold more effective in curing suppurative surgical infections than were the commercially available preparations, presumably because of their improved specificity.
Comparison of phages and antibiotics.
Lytic phages are similar to antibiotics in that they have remarkable antibacterial activity. However, therapeutic phages have some at least theoretical advantages over antibiotics (Table 2), and phages have been reported to be more effective than antibiotics in treating certain infections in humans (25, 33, 46) and experimentally infected animals (59). For example, in one study (33), Staphylococcus aureus phages were used to treat patients having purulent disease of the lungs and pleura. The patients were divided into two groups; the patients in group A (223 individuals) received phages, and the patients in group B (117 individuals) received antibiotics. Also, this clinical trial is one of the few studies using i.v. phage administration (48 patients in group A received phages by i.v. injection). The results were evaluated based on the following criteria: general condition of the patients, X-ray examination, reduction of purulence, and microbiological analysis of blood and sputum. No side effects were observed in any of the patients, including those who received phages intravenously. Overall, complete recovery was observed in 82% of the patients in the phage-treated group as opposed to 64% of the patients in the antibiotic-treated group. Interestingly, the percent recovery in the group receiving phages intravenously was even higher (95%) than the 82% recovery rate observed with all 223 phage-treated patients.
TABLE 2.
Comparison of the prophylactic and/or therapeutic use of phages and antibiotics
| Bacteriophages | Antibiotics | Comments |
|---|---|---|
| Very specific (i.e., usually affect only the targeted bacterial species); therefore, dysbiosis and chances of developing secondary infections are avoided (15). | Antibiotics target both pathogenic microorganisms and normal microflora. This affects the microbial balance in the patient, which may lead to serious secondary infections. | High specificity may be considered to be a disadvantage of phages because the disease-causing bacterium must be identified before phage therapy can be successfully initiated. Antibiotics have a higher probability of being effective than phages when the identity of the etiologic agent has not been determined. |
| Replicate at the site of infection and are thus available where they are most needed (59). | They are metabolized and eliminated from the body and do not necessarily concentrate at the site of infection. | The “exponential growth” of phages at the site of infection may require less frequent phage administration in order to achieve the optimal therapeutic effect. |
| No serious side effects have been described. | Multiple side effects, including intestinal disorders, allergies, and secondary infections (e.g., yeast infections) have been reported (76). | A few minor side effects reported (17, 58) for therapeutic phages may have been due to the liberation of endotoxins from bacteria lysed in vivo by the phages. Such effects also may be observed when antibiotics are used (42). |
| Phage-resistant bacteria remain susceptible to other phages having a similar target range. | Resistance to antibiotics is not limited to targeted bacteria. | Because of their more broad-spectrum activity, antibiotics select for many resistant bacterial species, not just for resistant mutants of the targeted bacteria (47). |
| Selecting new phages (e.g., against phage-resistant bacteria) is a relatively rapid process that can frequently be accomplished in days or weeks. | Developing a new antibiotic (e.g., against antibiotic-resistant bacteria) is a time-consuming process and may take several years (16, 51). | Evolutionary arguments support the idea that active phages can be selected against every antibiotic-resistant or phage-resistant bacterium by the ever-ongoing process of natural selection. |
BACTERIOPHAGES AS THERAPEUTIC AGENTS: MODE OF ACTION AND SAFETY PROFILE
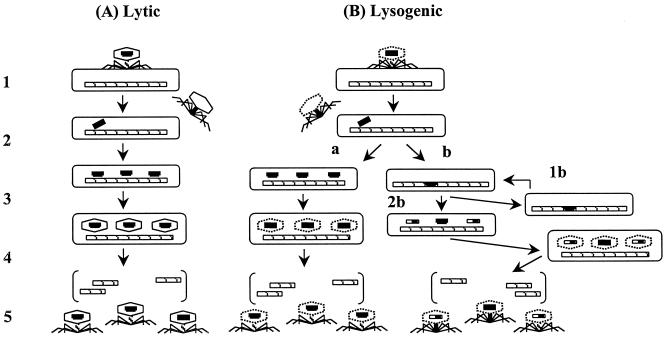
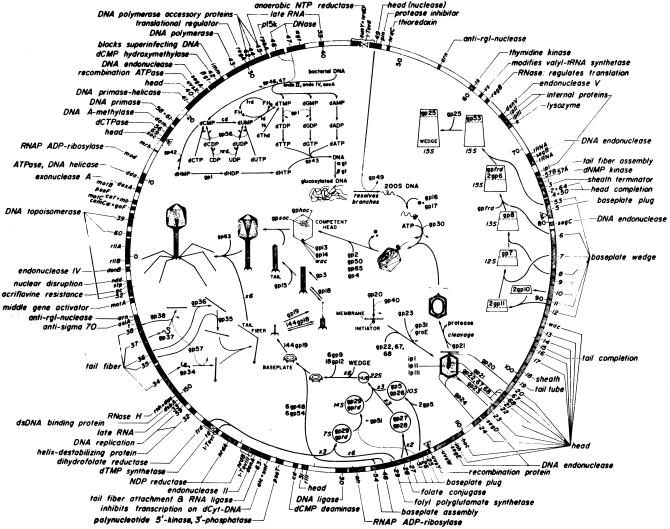
Mode of action. Despite the large number of publications on phage therapy, there are very few reports in which the pharmacokinetics of therapeutic phage preparations is delineated. The few publications available on the subject (10, 11) suggest that phages get into the bloodstream of laboratory animals (after a single oral dose) within 2 to 4 h and that they are found in the internal organs (liver, spleen, kidney, etc.) in approximately 10 h. Also, data concerning the persistence of administered phages indicate that phages can remain in the human body for relatively prolonged periods of time, i.e., up to several days (7). However, additional research is needed in order to obtain rigorous pharmacological data concerning lytic phages, including full-scale toxicological studies, before lytic phages can be used therapeutically in the West. As for their bactericidal activity, therapeutic phages were assumed to kill their target bacteria by replicating inside and lysing the host cell (i.e., via a lytic cycle). However, subsequent studies revealed that not all phages replicate similarly and that there are important differences in the replication cycles of lytic and lysogenic phages (Fig. 2). Furthermore, the recent delineation of the full sequence of the T4 phage (GenBank accession no. AF158101) and many years of elegant studies of the mechanism of T4 phage replication have shown that lysis of host bacteria by a lytic phage is a complex process consisting of a cascade of events involving several structural and regulatory genes (Fig. 3). Since T4 phage is a typical lytic phage, it is possible that many therapeutic phages act via a similar cascade; however, it is also possible that some therapeutic phages have some unique yet unidentified genes or mechanisms responsible for their ability to effectively lyse their target bacteria. For example, a group of authors from the EIBMV (2) identified and cloned an anti-Salmonella phage gene responsible, at least in part, for the phage's potent lethal activity against the Salmonella enterica serovar Typhimurium host strains. In another study (4), a unique mechanism has been described for protecting phage DNA from the restriction-modification defenses of an S. aureus host strain. Further elucidation of these and similar mechanisms is likely to yield information useful for genetically engineering optimally effective therapeutic phage preparations.
FIG. 2.
Replication cycles of lytic and lysogenic phages. (A) Lytic phages: step 1, attachment; step 2, injection of phage DNA into the bacterial host; step 3, shutoff of synthesis of host components, replication of phage DNA, and production of new capsids; step 4, assembly of phages; step 5, release of mature phages (lysis). (B) Lysogenic phages: steps 1 and 2 are similar to those of lytic phages (i.e., attachment and injection, respectively); starting with step 3, lysogenic phages can, among other possibilities, initiate a reproductive cycle similar to that of lytic phages (a) or integrate their DNA into the host bacterium's chromosome (lysogenization) (b). Lysogenized cells can replicate normally for many generations (1b) or at some point undergo lysogenic induction (2b) spontaneously or because of inducing agents such as radiation or carcinogens, during which time the integrated phage DNA is excised from the bacterial chromosome and may pick up fragments of bacterial DNA.
FIG. 3.
The genomic map of T4 phage. The full sequence of T4 phage has been determined, and several genes responsible for its lytic properties have been identified. For example, the genes encoding tail fibers (e.g., gp37) and baseplate wedges (e.g., gp12) are critical for phage-host cell recognition; the gp5 gene and possibly the gp25 gene encode lysozyme which weakens the bacterial cell wall and facilitates phage DNA injection into the cell; the ndd gene encodes the Ndd protein which disrupts the host nucleoid; the alc gene product is essential for inhibiting host cell transcription, etc. (from reference 28, with permission from the American Society for Microbiology).
Safety.
From a clinical standpoint, phages appear to be innocuous. During the long history of using phages as therapeutic agents in Eastern Europe and the former Soviet Union (and, before the antibiotic era, in the United States), phages have been administered to humans (i) orally, in tablet or liquid formulations (105 to 1011 PFU/dose), (ii) rectally, (iii) locally (skin, eye, ear, nasal mucosa, etc.), in tampons, rinses, and creams, (iv) as aerosols or intrapleural injections, and (v) intravenously, albeit to a lesser extent than the first four methods, and there have been virtually no reports of serious complications associated with their use (Table 2). In the United States, because of its apparent safety, phage phi X174 has been used to monitor humoral immune function in adenosine deaminase-deficient patients (36) and to determine the importance of cell surface-associated molecules in modulating the human immune response (37) (in the latter study, phages were intravenously injected into volunteers). Also, phages are extremely common in the environment (e.g., nonpolluted water has been reported [9] to contain ca. 2 × 108 bacteriophage per ml) and are regularly consumed in foods. However, it would be prudent to ensure further the safety of therapeutic phages before widely using them as therapeutic agents. For example, it would be important to ensure that they (i) do not carry out generalized transduction and (ii) possess gene sequences having significant homology with known major antibiotic resistance genes, genes for phage-encoded toxins, and genes for other bacterial virulence factors.
SPECIFIC PROBLEMS OF EARLY PHAGE THERAPY RESEARCH
Despite all the properties of lytic phages that would seem to favor their clinical use, they are not commonly used prophylactically or therapeutically throughout the world and their efficacy is still a matter of controversy. Many factors (some summarized in Table 3) have contributed to this situation.
TABLE 3.
Some of the problems with early therapeutic phage research and the ways they have been addressed in more recent studies or can be addressed in the future
| Problem | Comments | Solution and/or required approach |
|---|---|---|
| Narrow host range of phages | Because of the high specificity of phages, many negative results may have been obtained because of the failure to select phages lytic for the targeted bacterial species (19). | Determine the phage susceptibility of the etiologic agent before using phages therapeutically (40, 77); use polyvalent phage cocktails which lyse the majority of strains of the etiologic agent (15, 45, 58, 59). |
| Insufficient purity of phage preparations | Early therapeutic phages were in crude lysates of host bacteria, and they contained numerous contaminants (including endotoxins) that may have counteracted the effect of phages. | Ion-exchange chromatography, high-speed centrifugation, and other modern purification techniques should be used to obtain phage preparations of high purity (11). |
| Poor stability and/or viability of phage preparations | Some commercial phage preparations were supplemented with mercurials or oxidizing agents or were heat treated to ensure bacterial sterility (14). Many of these treatments also may have inactivated the phages, resulting in ineffective phage preparations. | Advanced purification techniques can be used to purify phages and to ensure that they are bacterium free. The viability and titer of phages should be determined before using them therapeutically. |
| Lack of understanding of the heterogeneity and mode of action of phages (i.e., lytic vs lysogenic phages) | Failure to differentiate between lytic and lysogenic phages may have resulted in some investigators using lysogenic phages, which are much less effective than lytic phages. | Carefully select for lytic phages. This is also critical for avoiding the possible horizontal transfer of bacterial toxin, antibiotic resistance, etc., genes by lysogenic phages (3) (Fig. 2). |
| Exaggerated claims of effectiveness of commercial phage preparations | One example of this would be the preparation called Enterophagos, which was marketed as being effective against herpes infections, urticaria, and eczema (8)—conditions against which phages could not possibly be effective. | Phage preparations should be accompanied by specific, scientifically supported information about their efficacy against specific bacterial pathogens, their possible side-effects, etc. |
| Failure to establish scientific proof of efficacy of phage treatment | Most clinical studies using therapeutic phages were conducted without placebo controls; also, when placebo controls were used, data were evaluated in a subjective manner questioned by many peers (20, 26). | Carefully controlled, double-blinded placebo studies with highly purified, lytic phages should be conducted, and results must be evaluated based on both clinical observations and scrupulous laboratory analysis. |
Failure to establish rigorous proof of efficacy.
One of the most important factors that have interfered with documenting the value of phage therapy has been the paucity of appropriately conducted, placebo-controlled studies. Ironically, d'Herelle caused substantial long-term harm to his idea of phages as therapeutic agents because, in his eagerness to transfer his laboratory studies to hospital and community settings, he performed clinical studies with phages without including placebo groups of patients. Starting with the first known use of phages in humans (the Enfants-Malades trials) and in all subsequent trials, d'Herelle administered phages to all sick patients. This failure to include placebo groups may be explained by the possibility that d'Herelle might have been reluctant to deprive anyone of therapy he believed could save his or her life. It could also have been due to the personal scientific style of d'Herelle, as he also failed to include placebo groups during his studies with chickens, when ethical considerations were not an issue (72). Similar failures were very common during the early history of phage therapy, and therefore the results frequently were controversial. To address this controversy, the Council on Pharmacy and Chemistry of the American Medical Association requested that a full review of the available literature on phage therapy be prepared for the Council's consideration. Consequently, Monroe Eaton and Stanhope Bayne-Jones reviewed more than 100 papers on bacteriophage therapy and in 1934 they published a detailed review of phage therapy (20). This report is one of the most detailed reviews of phage therapy ever published, and its conclusions were clearly not in favor of phage therapy. Among other conclusions, the authors stated that “d'Herelle's theory that the material is a living virus parasite of bacteria has not been proved. On the contrary, the facts appear to indicate that the material is inanimate, possibly an enzyme.” The authors further stated that “since it has not been shown conclusively that bacteriophage is a living organism, it is unwarranted to attribute its effect on cultures of bacteria or its possible therapeutic action to a vital property of the substance.” At the present time it is clear that the above conclusions of the report were incorrect. However, the report delivered a severe blow to the interest of Western scientists in evaluating the utility of phages for therapeutic purposes and it undoubtedly had a strong negative impact on the enthusiasm of funding agencies to support therapeutic phage research. In addition, 7 years after the Eaton-Bayne-Jones report, a second unfavorable report was published by Albert Krueger and Jane Scribner (26) as a sequel to the Eaton-Bayne-Jones report. The authors justified the need to write the second review because “much more information about both phage itself and its clinical utility has accumulated.” However, the authors' conclusions about the nature of phages also was incorrect since they stated “It is a protein of high molecular weight and appears to be formed from a precursor originating within the bacterium.” The authors further concluded that “it is equally evident that phage solutions possess no measurable degree of superiority over well known and accepted preparations.” Although the authors suggested that further evaluation of the therapeutic potential of phages might be warranted under thoroughly controlled conditions, their assessment (together with that of Eaton and Bayne-Jones) effectively stopped all major studies of phage therapy in the United States. In addition, a few years after the review was published, antibiotics became widely available, which further contributed to the decline of interest in phage therapy in the West. This was not affected by the continuing studies in the former Soviet Union and Eastern Europe since—as discussed above—many of these studies were not available to the international scientific community and/or were conducted in a manner which did not allow rigorous analysis of the author's conclusions.
Additional problems.
Some additional problems with early phage research are summarized in Table 3. In addition to these problems, various hypotheses have been advanced to explain cases in which phage therapy was not effective. For example, Merril et al. (34) proposed that reticuloendothelial system clearance of phages from the patient may be a potential problem because it might reduce the number of phages to a level which is not sufficient to combat the infecting bacteria. To address this issue, the authors used a natural selection strategy (which they elegantly called the “serial passage” method) for selecting phages having an increased ability to remain in the circulation of mice. Elucidating the mechanisms responsible for this property of phages is likely to provide important information about the mechanisms of phage-host bacterial cell interaction. However, for practical purposes, the feasibility of routinely using the methodology in phage therapy is unclear; e.g., it may be cumbersome to “serially passage” every phage in a complex phage preparation through animals before further purifying and using it for therapeutic purposes. Moreover, the improved therapeutic value of “long lasting” phages has been questioned by some investigators (8), and it may be much simpler—if rapid clearance of phages is a problem in a particular setting—to repeatedly administer the same phage to the patient instead of serially passaging the phage beforehand.
The development of phage-neutralizing antibodies is another possible problem which may hamper phage effectiveness in lysing targeted bacteria in vivo. Indeed, the development of neutralizing antibodies after parenteral administration of phages has been well documented (27). However it is unclear how significant a problem this may be during phage therapy, especially when phages are administered orally and/or locally. In theory, the development of neutralizing antibodies should not be a significant obstacle during the initial treatment of acute infections, because the kinetics of phage action is much faster than is the host's production of neutralizing antibodies. Also, it is not clear how long the antibodies will remain in circulation. Thus, careful studies must be conducted to address the validity of this concern. For example, if administration of phages elicits only a brief, mild antibody response in the patient, phages given at a later time (e.g., to treat a recurring, acute infection) should not be affected. However, if phage-neutralizing antibodies are still present at the time the second course of treatment is necessary or if a rapid anamnestic immune response occurs before the phages exert their action, the value of repeated administration of increased doses of phages or of the administration of different phages having the same spectrum of activity but a different antigenic profile must be determined.
Another concern regarding the therapeutic use of lytic phages is that the development of phage resistance may hamper their effectiveness. Bacterial resistance to phages will unquestionably develop, although according to some authors (14) the rate of developing resistance to phages is approximately 10-fold lower than that to antibiotics. The rate of developing resistance against phages can be partially circumvented by using several phages in one preparation (much like using two or more antibiotics simultaneously). Most importantly, when resistance against a given phage occurs, it should be possible to select rapidly (in a few days or weeks) a new phage active against the phage-resistant bacteria.
It is also unclear how effective phages would be in treating diseases caused by intracellular pathogens (e.g., Salmonella species), where bacteria multiply primarily inside human cells and are inaccessible to phages. It is possible that phages will have only limited utility in treating infections caused by intracellular pathogens; however, phages have been reported (24) to be effective in preventing salmonellosis in children.
CONCLUSIONS
In summary, bacteriophages have several characteristics that make them potentially attractive therapeutic agents. They are (i) highly specific and very effective in lysing targeted pathogenic bacteria, (ii) safe, as underscored by their extensive clinical use in Eastern Europe and the former Soviet Union and the commercial sale of phages in the 1940s in the United States, and (iii) rapidly modifiable to combat the emergence of newly arising bacterial threats. In addition, a large number of publications, some of which are reviewed in this minireview, suggest that phages may be effective therapeutic agents in selected clinical settings. Granted, many of these studies do not meet the current rigorous standards for clinical trials and there still remain many important questions that must be addressed before lytic phages can be widely endorsed for therapeutic use. However, we think that there is a sufficient body of data—and a desperate enough need to find alternative treatment modalities against rapidly emerging, antibiotic-resistant bacteria—to warrant further studies in the field of phage therapy.
ACKNOWLEDGMENTS
We thank Arnold Kreger for his invaluable discussions and editorial comments; this minireview would not have been possible without his generous help. We gratefully acknowledge Elizabeth Kutter and Burton Guttman for their helpful comments and their permission to use the figure of the T4 genetic map, Beata Weber-Dabrowska for supplying information about phage research performed at the Hirszfeld Institute of Immunology and Experimental Therapy, and Amiran Meiphariani and Ramaz Katsarava for providing copies of some of the original Russian and Georgian articles on phage therapy.
Z.A. was supported in part by an International Training and Research in Emerging Infectious Diseases grant from the Fogarty International Center, National Institutes of Health. Additional support was provided by Intralytix, Inc. (a Maryland corporation working on the development of therapeutic phages), with which the authors have a financial relationship.
REFERENCES
- 1.Abdul-Hassan H S, El-Tahan E, Massoud B, Gomaa R. Bacteriophage therapy of pseudomonas burn wound sepsis. Ann Medit Burn Club. 1990;3:262–264. [Google Scholar]
- 2.Adamia R S, Matitashvili E A, Kvachadze L I, Korinteli V I, Matoyan D A, Kutateladze M I, Chanishvili T G. The virulent bacteriophage IRA of Salmonella typhimurium: cloning of phage genes which are potentially lethal for the host cell. J Basic Microbiol. 1990;10:707–716. doi: 10.1002/jobm.3620301002. [DOI] [PubMed] [Google Scholar]
- 3.Alisky J, Iczkowski K, Rapoport A, Troitsky N. Bacteriophages show promise as antimicrobial agents. J Infect. 1998;36:5–15. doi: 10.1016/s0163-4453(98)92874-2. [DOI] [PubMed] [Google Scholar]
- 4.Andriashvili I A, Kvachadze L I, Vashakidze R P, Adamia R S, Chanishvili T G. Molecular mechanisms of DNA protection from restriction endonucleases in Staphylococcus aureus cells. Mol Gen Mikrobiol Virusolol. 1986;8:43–45. [PubMed] [Google Scholar]
- 5.Anonymous. Phage therapy. Lancet. 1983;iii:1287–1288. [PubMed] [Google Scholar]
- 6.Anpilov L I, Prokudin A A. Preventive effectiveness of dried polyvalent Shigella bacteriophage in organized collective groups. Voenno-Med Zh. 1984;5:39–40. [PubMed] [Google Scholar]
- 7.Babalova E G, Katsitadze K T, Sakvarelidze L A, Imnaishvili N S, Sharashidze T G, Badashvili V A, Kiknadze G P, Meipariani A N, Gendzekhadze N D, Machavariani E V, Gogoberidze K L, Gozalov E I, Dekanosidze N G. Preventive value of dried dysentery bacteriophage. Zh Mikrobiol Epidemiol Immunobiol. 1968;2:143–145. [PubMed] [Google Scholar]
- 8.Barrow P A, Soothill J S. Bacteriophage therapy and prophylaxis: rediscovery and renewed assessment of potential. Trends Microbiol. 1997;7:268–271. doi: 10.1016/S0966-842X(97)01054-8. [DOI] [PubMed] [Google Scholar]
- 9.Bergh O, Borsheim K Y, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- 10.Bogovazova G G, Voroshilova N N, Bondarenko V M. The efficacy of Klebsiella pneumoniae bacteriophage in the therapy of experimental Klebsiella infection. Zh Mikrobiol Epidemiol Immunobiol. 1991;4:5–8. [PubMed] [Google Scholar]
- 11.Bogovazova G G, Voroshilova N N, Bondarenko V M, Gorbatkova G A, Afanas'eva E V, Kazakova T B, Smirnov V D, Mamleeva A G, Glukharev I A, Erastova E I, Krylov I A, Ovcherenko T M, Baturo A P, Yalsyk G V, Arefyeva N A. Immunobiological properties and therapeutic effectiveness of preparations from Klebsiella bacteriophages. Zh Mikrobiol Epidemiol Immunobiol. 1992;3:30–33. [PubMed] [Google Scholar]
- 12.Bordet J, Ciuca M. Remarques sur l'historique de recherches concernant la lyse microbienne transmissible. Compt Rend Soc Biol. 1921;84:745–747. [Google Scholar]
- 13.Bruynoghe R, Maisin J. Essais de thérapeutique au moyen du bacteriophage. C R Soc Biol. 1921;85:1120–1121. [Google Scholar]
- 14.Carlton R M. Phage therapy: past history and future prospects. Arch Immunol Ther Exp. 1999;5:267–274. [PubMed] [Google Scholar]
- 15.Chernomordik A B. Bacteriophages and their therapeutic-prophylactic use. Med Sestra. 1989;6:44–47. [PubMed] [Google Scholar]
- 16.Chopra I, Hodgson J, Metcalf B, Poste G. The search for antimicrobial agents effective against bacteria resistant to multiple antibiotics. Antimicrob Agents Chemother. 1997;41:497–503. doi: 10.1128/aac.41.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cislo M, Dabrowski M, Weber-Dabrowska B, Woyton A. Bacteriophage treatment of suppurative skin infections. Arch Immunol Ther Exp. 1987;2:175–183. [PubMed] [Google Scholar]
- 18.D'Herelle F. Sur un microbe invisible antagoniste des bacilles dysentériques. C R Acad Sci (Paris) 1917;165:373–375. [Google Scholar]
- 19.D'Herelle F. The bacteriophage and its clinical applications. Springfield, Ill: Charles C Thomas; 1930. [Google Scholar]
- 20.Eaton M D, Bayne-Jones S. Bacteriophage therapy. Review of the principles and results of the use of bacteriophage in the treatment of infections. JAMA. 1934;23:1769–1939. [Google Scholar]
- 21.Hankin E H. L'action bactericide des eaux de la Jumna et du Gange sur le vibrion du cholera. Ann Inst Pasteur. 1896;10:511. [Google Scholar]
- 22.Ioseliani G D, Meladze G D, Chkhetiia N S, Mebuke M G, Kiknadze N I. Use of bacteriophage and antibiotics for prevention of acute postoperative empyema in chronic suppurative lung diseases. Grudn Khir. 1980;6:63–67. [PubMed] [Google Scholar]
- 23.Kaczkowski H, Weber-Dabrowska B, Dabrowski M, Zdrojewicz Z, Cwioro F. Use of bacteriophages in the treatment of chronic bacterial diseases. Wiad Lek. 1990;43:136–141. [PubMed] [Google Scholar]
- 24.Kiknadze G P, Gadua M M, Tsereteli E V, Mchedlidze L S, Birkadze T V. Efficiency of preventive treatment by phage preparations of children's hospital salmonellosis. In: Kiknadze G P, editor. Intestinal infections. Tbilisi, Georgia: Sovetskaya Meditsina; 1986. pp. 41–44. [Google Scholar]
- 25.Kochetkova V A, Mamontov A S, Moskovtseva R L, Erastova E I, Trofimov E I, Popov M I, Dzhubalieva S K. Phagotherapy of postoperative suppurative-inflammatory complications in patients with neoplasms. Sov Med. 1989;6:23–26. [PubMed] [Google Scholar]
- 26.Krueger A P, Scribner E J. Bacteriophage therapy. II. The bacteriophage: its nature and its therapeutic use. JAMA. 1941;19:2160–2277. [Google Scholar]
- 27.Kucharewicz-Krukowska A, Slopek S. Immunogenic effect of bacteriophage in patients subjected to phage therapy. Arch Immunol Ther Exp. 1987;5:553–561. [PubMed] [Google Scholar]
- 28.Kutter E, Guttman B, Carlson K. The transition from host to phage metabolism after T4 infection. In: Karam J D, editor. Molecular biology of bacteriophage T4. Washington, D.C.: American Society for Microbiology; 1994. pp. 343–346. [Google Scholar]
- 29.Kwarcinski W, Lazarkiewicz B, Weber-Dabrowska B, Rudnicki J, Kaminski K, Sciebura M. Bacteriophage therapy in the treatment of recurrent subphrenic and subhepatic abscess with jejunal fistula after stomach resection. Pol Tyg Lek. 1994;49:535. [PubMed] [Google Scholar]
- 30.Lang G, Kehr P, Mathevon H, Clavert J M, Sejourne P, Pointu J. Bacteriophage therapy of septic complications of orthopaedic surgery. Rev Chir Orthop Reparatrice Appar Mot. 1979;1:33–37. [PubMed] [Google Scholar]
- 31.Levin B, Bull J J. Phage therapy revisited: the population biology of a bacterial infection and its treatment with bacteriophage and antibiotics. Am Naturalist. 1996;147:881–898. [Google Scholar]
- 32.Litvinova A M, Chtetsova V M, Kavtreva I G. Evaluation of efficacy of the use of E. coli-Proteus bacteriophage in intestinal dysbacteriosis in premature infants. Vopr Okhr Materin Det. 1978;9:42–44. [PubMed] [Google Scholar]
- 33.Meladze G D, Mebuke M G, Chkhetia N S, Kiknadze N I, Koguashvili G G, Timoshuk I I, Larionova N G, Vasadze G K. The efficacy of staphylococcal bacteriophage in treatment of purulent diseases of lungs and pleura. Grudn Khir. 1982;1:53–56. [PubMed] [Google Scholar]
- 34.Merril C R, Biswas B, Carlton R, Jensen N C, Creed G J, Zullo S, Adhya S. Long-circulating bacteriophage as antibacterial agents. Proc Natl Acad Sci USA. 1996;8:3188–3192. doi: 10.1073/pnas.93.8.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miliutina L N, Vorotyntseva N V. Current strategy and tactics of etiotropic therapy of acute intestinal infections in children. Antibiot Khimioter. 1993;1:46–53. [PubMed] [Google Scholar]
- 36.Ochs H D, Buckley R H, Kobayashi R H, Kobayashi A L, Sorensen R U, Douglas S D, Hamilton B L, Hershfield M S. Antibody responses to bacteriophage phi X174 in patients with adenosine deaminase deficiency. Blood. 1992;5:1163–1171. [PubMed] [Google Scholar]
- 37.Ochs H D, Nonoyama S, Zhu Q, Farrington M, Wedgewood R J. Regulation of antibody responses: the role of complement and adhesion molecules. Clin Immunol Immunopathol. 1993;67:S33–S40. [PubMed] [Google Scholar]
- 38.Pavlenishvili I, Tsertsvadze T. Bacteriophagotherapy and enterosorbtion in treatment of sepsis of newborns caused by gram-negative bacteria. Pren Neon Infect. 1993;11:104. [Google Scholar]
- 39.Peremitina L D, Berillo E A, Khvoles A G. Experience in the therapeutic use of bacteriophage preparations in suppurative surgical infections. Zh Mikrobiol Epidemiol Immunobiol. 1981;9:109–110. [PubMed] [Google Scholar]
- 40.Perepanova T S, Darbeeva O S, Kotliarova G A, Kondrat'eva E M, Maiskaia L M, Malysheva V F, Baiguzina F A, Grishkova N V. The efficacy of bacteriophage preparations in treating inflammatory urologic diseases. Urol Nefrol. 1995;5:14–17. [PubMed] [Google Scholar]
- 41.Pokrovskaya M P, Kaganova L C, Morosenko M A, Bulgakova A G, Skatsenko E E. Treatment of wounds with bacteriophages. 2nd ed. Moscow, USSR: State Publishing House “Medgiz,”; 1942. [Google Scholar]
- 42.Prins J M, Deventer S J, Kuijper E J, Speelman P. Clinical relevance of antibiotic-induced endotoxin release. Antimicrob Agents Chemother. 1994;38:1211–1218. doi: 10.1128/aac.38.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Proskurov V A. Use of staphylococcal bacteriophage for therapeutic and preventive purposes. Zh Mikrobiol Epidemiol Immunobiol. 1970;2:104–107. [PubMed] [Google Scholar]
- 44.Rice T B. Use of bacteriophage filtrates in treatment of suppurative conditions: report of 300 cases. Am J Med Sci. 1930;179:345–360. [Google Scholar]
- 45.Sakandelidze V M, Meipariani A N. Use of combined phages in suppurative-inflammatory diseases. Zh Mikrobiol Epidemiol Immunobiol. 1974;6:135–136. [PubMed] [Google Scholar]
- 46.Sakandelidze V M. The combined use of specific phages and antibiotics in different infectious allergoses. Vrach Delo. 1991;3:60–63. [PubMed] [Google Scholar]
- 47.Salyers A A, Amabile-Cuevas C F. Why are antibiotic resistance genes so resistant to elimination? Antimicrob Agents Chemother. 1997;41:2321–2325. doi: 10.1128/aac.41.11.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samsygina G A, Boni E G. Bacteriophages and phage therapy in pediatric practice. Pediatria. 1984;4:67–70. [PubMed] [Google Scholar]
- 49.Schless R A. Staphylococcus aureus meningitis: treatment with specific bacteriophage. Am J Dis Child. 1932;44:813–822. [Google Scholar]
- 50.Shabalova I A, Karpanov N I, Krylov V N, Sharibjanova T O, Akhverdijan V Z. Proceedings of IX International Cystic Fibrosis Congress. Zurich, Switzerland: International Cystic Fibrosis Association; 1995. Pseudomonas aeruginosa bacteriophage in treatment of P. aeruginosa infection in cystic fibrosis patients; p. 443. [Google Scholar]
- 51.Silver L L, Bostian K A. Discovery and development of new antibiotics: the problem of antibiotic resistance. Antimicrob Agents Chemother. 1993;37:377–383. doi: 10.1128/aac.37.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slopek S, Durlakowa I, Weber-Dabrowska B, Kucharewicz-Krukowska A, Dabrowski M, Bisikiewicz R. Results of bacteriophage treatment of suppurative bacterial infections. I. General evaluation of the results. Arch Immunol Ther Exp. 1983;31:267–291. [PubMed] [Google Scholar]
- 53.Slopek S, Durlakowa I, Weber-Dabrowska B, Kucharewicz-Krukowska A, Dabrowski M, Bisikiewicz R. Results of bacteriophage treatment of suppurative bacterial infections. II. Detailed evaluation of the results. Arch Immunol Ther Exp. 1983;31:293–327. [PubMed] [Google Scholar]
- 54.Slopek S, Durlakowa I, Weber-Dabrowska B, Dabrowski M, Kucharewicz-Krukowska A. Results of bacteriophage treatment of suppurative bacterial infections. III. Detailed evaluation of the results obtained in a further 150 cases. Arch Immunol Ther Exp. 1984;32:317–335. [PubMed] [Google Scholar]
- 55.Slopek S, Kucharewicz-Krukowska A, Weber-Dabrowska B, Dabrowski M. Results of bacteriophage treatment of suppurative bacterial infections. IV. Evaluation of the results obtained in 370 cases. Arch Immunol Ther Exp. 1985;33:219–240. [PubMed] [Google Scholar]
- 56.Slopek S, Kucharewicz-Krukowska A, Weber-Dabrowska B, Dabrowski M. Results of bacteriophage treatment of suppurative bacterial infections. V. Evaluation of the results obtained in children. Arch Immunol Ther Exp. 1985;33:241–259. [PubMed] [Google Scholar]
- 57.Slopek S, Kucharewicz-Krukowska A, Weber-Dabrowska B, Dabrowski M. Results of bacteriophage treatment of suppurative bacterial infections. VI. Analysis of treatment of suppurative staphylococcal infections. Arch Immunol Ther Exp. 1985;33:261–273. [PubMed] [Google Scholar]
- 58.Slopek S, Weber-Dabrowska B, Dabrowski M, Kucharewicz-Krukowska A. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981–1986. Arch Immunol Ther Exp. 1987;35:569–583. [PubMed] [Google Scholar]
- 59.Smith H W, Huggins M B. Successful treatment of experimental Escherichia coli infections in mice using phages: its general superiority over antibiotics. J Gen Microbiol. 1982;128:307–318. doi: 10.1099/00221287-128-2-307. [DOI] [PubMed] [Google Scholar]
- 60.Smith H W, Huggins M B. Effectiveness of phages in treating experimental E. coli diarrhoea in calves, piglets and lambs. J Gen Microbiol. 1983;129:2659–2675. doi: 10.1099/00221287-129-8-2659. [DOI] [PubMed] [Google Scholar]
- 61.Smith H W, Huggins M B. The control of experimental E. coli diarrhea in calves by means of bacteriophage. J Gen Microbiol. 1987;133:1111–1126. doi: 10.1099/00221287-133-5-1111. [DOI] [PubMed] [Google Scholar]
- 62.Smith H W, Huggins M B, Shaw K M. Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. J Gen Microbiol. 1987;133:1127–1135. doi: 10.1099/00221287-133-5-1127. [DOI] [PubMed] [Google Scholar]
- 63.Soothill J S, Lawrence J C, Ayliffe G A J. The efficacy of phages in the prevention of the destruction of pig skin in vitro by Pseudomonas aeruginosa. Med Sci Res. 1988;16:1287–1288. [Google Scholar]
- 64.Soothill J S. Treatment of experimental infections of mice by bacteriophage. J Med Microbiol. 1992;37:258–261. doi: 10.1099/00222615-37-4-258. [DOI] [PubMed] [Google Scholar]
- 65.Soothill J S. Bacteriophage prevents destruction of skin grafts by Pseudomonas aeruginosa. Burns. 1994;20:209–211. doi: 10.1016/0305-4179(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 66.Stout B F. Bacteriophage therapy. Texas State J Med. 1933;29:205–209. [Google Scholar]
- 67.Stroj L, Weber-Dabrowska B, Partyka K, Mulczyk M, Wojcik M. Successful treatment with bacteriophage in purulent cerebrospinal meningitis in a newborn. Neurol Neurochir Pol. 1999;3:693–698. [PubMed] [Google Scholar]
- 68.Summers W C. Felix d'Herelle and the origins of molecular biology. New Haven, Conn: Yale University Press; 1999. [Google Scholar]
- 69.Tolkacheva T V, Abakumov E M, Martynova V A, Golosova T V. Correction of intestinal dysbacteriosis with biological preparations in acute leukemia. Probl Gematol Pereliv Krovi. 1981;7:29–33. [PubMed] [Google Scholar]
- 70.Twort F W. An investigation on the nature of ultramicroscopic viruses. Lancet. 1915;ii:1241. [Google Scholar]
- 71.Twort F W. Researches on dysentery. Br J Exp Pathol. 1920;1:237–243. [Google Scholar]
- 72.Van Helvoort T. Bacteriological and physiological research styles in the early controversy on the nature of the bacteriophage phenomenon. Med Hist. 1992;3:243–270. doi: 10.1017/s0025727300055265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vieu J F, Guillermet F, Minck R, Nicolle P. Donnees actuelles sur les applications therapeutiques des bacteriophages. Bull Acad Natl Med. 1979;163:61. [PubMed] [Google Scholar]
- 74.Weber-Dabrowska B, Dabrowski M, Slopek S. Studies on bacteriophage penetration in patients subjected to phage therapy. Arch Immunol Ther Exp. 1987;35:563–568. [PubMed] [Google Scholar]
- 75.Weber-Dabrowska B, Zimecki M, Mulczyk M. Effective phage therapy is associated with normalization of cytokine production by blood cell cultures. Arch Immunol Ther Exp. 2000;48:31–37. [PubMed] [Google Scholar]
- 76.Yao J D C, Moellering R C., Jr . Antimicrobial agents. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 1474–1504. [Google Scholar]
- 77.Zhukov-Verezhnikov N N, Peremitina L D, Berillo E A, Komissarov V P, Bardymov V M, Khvoles A G, Ugryumov L B. A study of the therapeutic effect of bacteriophage agents in a complex treatment of suppurative surgical diseases. Sov Med. 1978;12:64–66. [PubMed] [Google Scholar]