Abstract
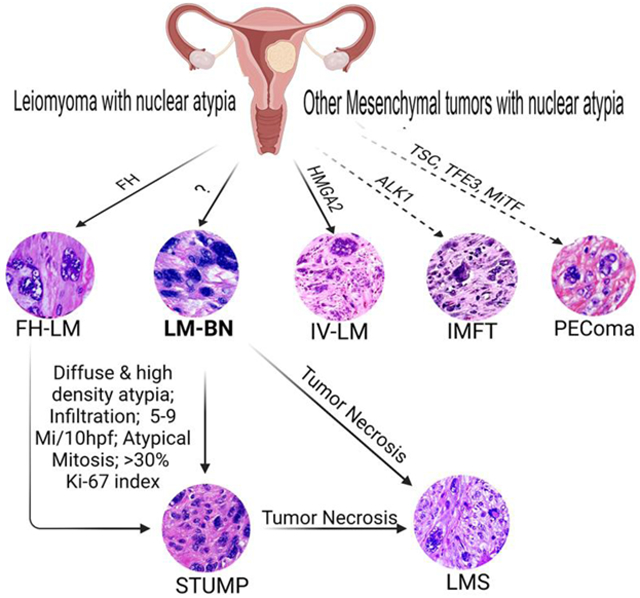
Leiomyoma with nuclear atypia describes a group of uterine smooth muscle tumors with a wide range of histologic and clinical presentations and remarkable nuclear atypia. These include fumarate hydratase-deficient leiomyoma (FH-LM), intravenous leiomyomatosis (IV-LM), and leiomyoma with bizarre nuclei (LM-BN). Other uterine mesenchymal tumors, such as perivascular epithelioid tumor (PEComa) and inflammatory myofibroblastic tumors (IMFT) are the mimickers of leiomyoma with nuclear atypia. LM-BN is the primary tumor model with a long history in gynecologic pathology, but the histogenesis of LM-BN remains largely unknown. Differentiating LM-BN from other benign variants, tumors with uncertain malignant potential (STUMP), or fully malignant leiomyosarcoma (LMS) can be diagnostically challenging. Recent progress has improved the diagnosis of many types of leiomyoma with nuclear atypia based on their specific histology and molecular alterations. LM-BN is now a diagnosis of exclusion. In this article, I review the history of leiomyoma with nuclear atypia and compare the clinical, histologic, and molecular features of LM-BN with those of its mimics. In particular, I highlight the current progress made in molecular genetics and pitfalls in the diagnosis of different myogenic tumors with nuclear atypia.
Keywords: Fumarate hydratase-deficient leiomyoma, gene mutation, intravenous leiomyomatosis, leiomyoma with bizarre nuclei, leiomyosarcoma, nuclear atypia, PEComa
Graphical Abstract
Introduction
Uterine smooth muscle tumors (USMT) include a wide spectrum of benign, atypical/uncertain malignant potential, and fully malignant tumor types based on their clinical presentation, histology, and molecular characteristics. Each tumor type has variants with distinct cytohistologic and molecular profiles. Leiomyomas and their variants account for the most common gynecologic USMT type in reproductive-age women. Leiomyosarcomas (LMS) are a rare malignancy seen in 1 in 200 to 800 hysterectomies for USMTs1. LMS are clinically aggressive with high recurrence and an overall poor prognosis2. Therefore, correct diagnosis of LMS by excluding benign mimics is critical for patient care. Recent advances in next-generation sequencing (NGS) have provided unprecedented tools to identify driver gene mutations and genomic alterations in many USMT types, allowing further tumor classification and more accurate diagnosis, as well as greater insight into the tumorigenesis of these entities. For example, many leiomyomas and their variants are driven by MED12 and HMGA2 mutations/alterations, while a small fraction of leiomyomas are caused by alterations in FH and COL5/63.
Leiomyoma with nuclear atypia is commonly encountered in our daily practice and often presents challenges to reaching a definitive diagnosis. Leiomyoma with nuclear atypia consists of a group of USMTs with a wide range of histologic and clinical presentations and remarkable nuclear atypia. In the past, pathologists relied on subtle differences in cytohistologic features and presentations of nuclear atypia to diagnose leiomyoma variants4, but the diagnostic accuracy of this approach was questionable. Variants of leiomyoma with nuclear atypia include fumarate hydratase deficient leiomyoma (FH-LM), intravenous leiomyomatosis (IV-LM), and leiomyoma with bizarre nuclei (LM-BN): differentiating these variants from other tumors with uncertain malignant potential or fully malignant LMS is challenging. Other uterine mesenchymal tumors, such as perivascular epithelioid tumor (PEComa) and inflammatory myofibroblastic tumors (IMFT) are also the tumor types for differential diagnosis. In practice, histologic evaluation remains the gold standard for differential diagnosis, based on specific patterns of nuclear atypia such as different cellularity, density, and atypical mitosis. More recently, the detection of biallelic alterations in fumarate hydratase made it possible to differentiate between FH-LM and LM-BN5,6. A lack of specific biomarkers and some overlapping histologic features between LM-BN and LMS can make diagnosis challenging, and investigation of molecular and genetic alterations specific to these diseases is ongoing.
This review presents the historical, pathological, clinical, and molecular aspects of leiomyoma with nuclear atypia and its variants. It also summarizes the most recent progress made in differential diagnosis, specifically, the discovery of genetic alterations and their role in the classification of leiomyoma with nuclear atypia. LM-BN is presented as the primary tumor type and is discussed in relation to other benign and malignant USMTs.
History of leiomyoma with nuclear atypia
Leiomyoma with nuclear atypia was recognized a century ago. The first report was in 1909, when Kelly and Cullen7 described uterine myomata with macroscopically unremarkable but histologically contained areas suggestive of “sarcomatous degeneration including the presence of giant cells, with one or several nuclei and large nucleoli.” They considered this a benign change and named it “sarcomatous degeneration.” In 1920, Evans8 provided a detailed description of atypical smooth muscle tumors in his paper, “Malignant myomata and related tumors of the uterus.” He reported that some smooth muscle tumors had “giant cells with large irregular, hyperchromatic, and usually multiple nuclei with minimal or absent mitoses in a background of fibrosis and hyalinization” (now recognized as a hallmark of LM-BN) and noted that these patients had no recurrences, some of them with long follow-up periods. He hypothesized that these tumors were benign and that the atypical nuclear changes were degenerative. Later, Novak and Anderson9 specifically referred to the atypical multinucleated cells as a “symplastic” change.
In 1961, Przybora10 examined 1195 USMTs and found 15 atypical tumors referred to as “leiomyosarcoma in situ,” due to their enlarged nuclear size and chromatin condensed with large vacuoles, with rare mitosis. Patients with leiomyosarcoma in situ were on average 7 years younger than those with LMS. Christopherson et al11 were the first (1972) to use the term “bizarre leiomyoma” in their study of 17 cases, which showed areas of giant and abnormal cells with <5 mitoses/10 high-power fields (HPF). They concluded that bizarre leiomyomas were benign based on long-term follow-up without recurrences or metastases. Clement et al12 described these tumors as “leiomyoma with bizarre nuclei.” In a study of 16 patients with IV-LM, they found two cases with these “bizarre nuclei,” characterized by large and pleomorphic nuclei more than 100 μm in diameter in a background of hyaline to fibrilla cytoplasm and intranuclear cytoplasmic inclusion. Later, Downes and Hart (1997)13 provided an in-depth histologic analysis and follow-up of 24 patients with leiomyoma with bizarre nuclei.
The term “atypical leiomyoma” was used by Hendrickson and Kempson (1979)14 to refer to tumors with high nuclear pleomorphism with none or 1 mitosis/10 HPF. The same term was used in a 1988 study of 46 problematic USMT by Evans et al, who reported three atypical leiomyomas15. In 1994, Bell et al thoroughly investigated 213 problematic USMTs based on histology, clinical, and follow-up data16. In that study, atypical leiomyoma was further divided into three subtypes: 1) atypical leiomyoma (focal or multifocal nuclear atypia with <5 mitoses/10 HPF, no tumor necrosis); 2) atypical leiomyoma with limited experience (focal and multifocal nuclear atypia with up to 15 mitoses/10 HPF, no tumor necrosis); and 3) atypical leiomyoma with low risk of recurrence (diffuse cytologic atypia with <10 mitoses/10 HPF, no tumor necrosis, 1/46 died from disease). Based on these early studies, atypical leiomyoma came to be defined and recognized as a USMT with high and pleomorphic nuclear atypia, no more than 9 mitoses/10 HPF, and no tumor necrosis17.
For decades, leiomyoma with nuclear atypia had been referred to by many different names in the literature and in pathology reports, including “atypical,” “pleomorphic,” “degenerative,” “bizarre,” and “symplastic” leiomyoma, which appeared as synonyms in the WHO classification in 2003. It wasn’t until 2014 that the terminology “leiomyoma with bizarre nuclei” (LM-BN) was adopted by WHO, and “atypical leiomyoma” and other names were no longer recommended (Table 1). LM-BN presents with histologic heterogeneity and remarkable nuclear atypia, but shares characteristics of other smooth muscle or mesenchymal tumors, with occasional recurrent and rare reports of malignant transformation18. LM-BN has molecular characteristics that overlap with those of malignant LMS. A search of PubMed for publications in the last 40 years using the search term “atypical leiomyoma” identified more than 300 publications, whereas the search term “leiomyoma with bizarre nuclei” yielded approximately 30 papers, underscoring that the pathology and medical research of this tumor entity deserves additional attention and investigation.
Table 1.
History of Leiomyoma with Bizarre Nuclei (LM-BN) Pathology
| Year | Terminology Used | Major Findings | References |
|---|---|---|---|
| 1909 | Sarcomatous degeneration | “Sarcomatous degeneration” including giant cells, several nuclei, and large nucleoli | Kelly and Cullen7 |
| 1920 | Malignant myomata | Giant cells with large irregular, hyperchromatic, and usually multiple nuclei with minimal or absent mitoses in a background of fibrosis and hyalinization | Evans8 |
| 1937 | Symplastic | Atypical multinucleated cells | Novak and Anderson9 |
| 1961 | Leiomyosarcoma in situ | Atypical cells with larger nuclear, chromatin condensed with large vacuoles, rare mitosis | Przybora10 |
| 1972 | Bizarre leiomyomas | Tumors with areas of giant and bizarre cells with<5 mitoses/10 HPF, and none developed recurrences or metastases | Christopherson et al11 |
| 1979 | Atypical leiomyoma | High nuclear pleomorphism with none or 1 mitosis/10 HPF | Hendrickson and Kempson14 |
| 1988 | Leiomyoma with bizarre nuclei | Bizarre nuclei, large and pleomorphic nuclei measured >100 μm | Clement et al12 |
| 1994 | Atypical leiomyoma | Focal or multifocal nuclear atypia with <9 mitoses/10 HPF and no tumor necrosis | Bell et al16 |
| 1997 | Leiomyoma with bizarre nuclei | Bizarre giant cells with <7 mitoses/10 HPF and no tumor necrosis | Downes & Hart13 |
| 2016 | Atypical leiomyoma type I and II | Type I associated with fumarate hydratase alteration with round or oval nuclei, distinct nuclear membranes, prominent nucleoli with perinucleolar halos Type II defined as LM-BN with elongated or spindled nuclei, irregular nuclear membranes, pinpoint or no nucleoli, and dark smudgy chromatin |
Ubago et al4,5 |
| 2020 | Leiomyoma with bizarre nuclei | Bizarre cells with eosinophilic or globular cytoplasm, smudged chromatin, and nuclear pseudoinclusion with <5 mitoses/10 HPF | WHO 2020 |
HPF, high-power fields; WHO, World Health Organization.
Modern pathology for leiomyoma with nuclear atypia
Leiomyoma with nuclear atypia refers to benign smooth muscle tumors with nuclear atypia. As the primary tumor type, LM-BN is characterized by a large, irregular nuclear contour containing dark and smudged chromatin with degenerative changes, often with multinucleated cells and pseudo-nuclear inclusion17. Such nuclear features are shared by other uterine tumor variants, however. Since the cause and tumorigenesis of LM-BN is largely unknown, LM-BN may be considered as a diagnostic exclusion.
Leiomyoma with bizarre nuclei (LM-BN)
LM-BN is a common incidental finding in solitary or multiple leiomyoma. LM-BN may appear as various colors (tan, pink, white, yellow, and brown) and consistencies (slightly soft and less bulging due to its cellular nature) (Figure 1). Grossly, these tumors are well-circumscribed and occasionally have areas of ischemic necrosis. Tumor size ranges widely, from 0.7 to 20 cm with mean tumor size of 7 cm4.
Figure 1.
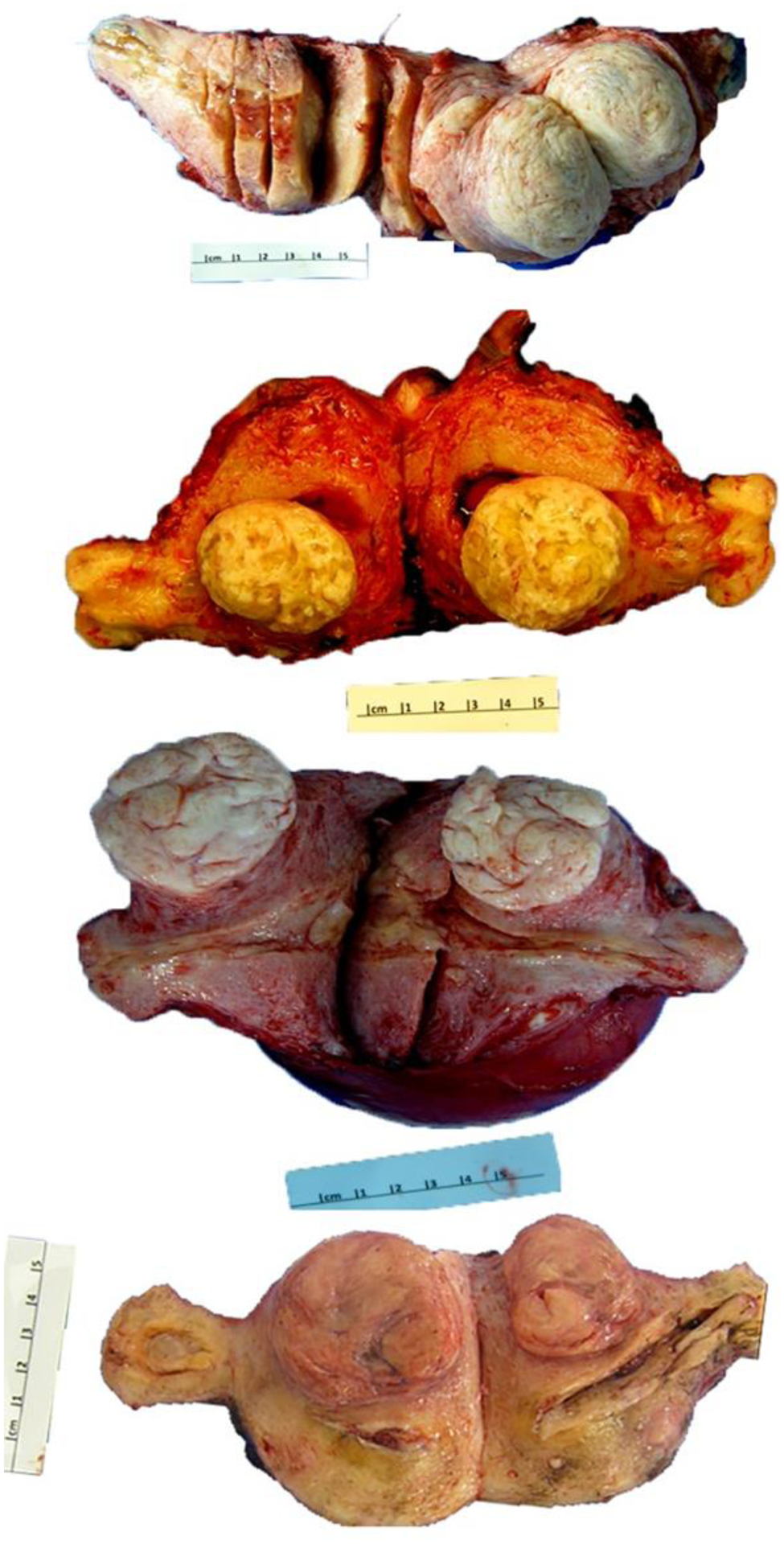
Photomacrographs of leiomyoma with bizarre nuclei in 4 examples.
Nuclear atypia can be readily detected at low magnification (4x objective lenses). Bizarre nuclei are characterized by a large nuclear size ranges from 10 to 100 μm, or 3- to 10-fold larger than reference smooth muscle cells; an irregular nuclear shape with elongated/spindle fusiform or round/oval shape with an irregular nuclear membrane; and hyperchromatic, dark, degenerative/smudgy, coarse, clumped chromatin with pseudonuclear inclusion or vacuoles (Figure 2). Multinucleation or pseudo-lobular nucleation are frequently seen. Nucleoli are usually small or inconspicuous, but eosinophilic giant nucleoli are occasionally seen. The latter should be carefully evaluated to exclude FH-LM, epithelioid LMS, or PEComa4. By carefully comparing nuclear features, we were able to differentiate most LM-BN from FH-LM4,5. The cytoplasm is usually eosinophilic with indistinct cell border, surrounded by hyaline extracellular matrix (Figure 2). Some LM-BN show cellular and spindle cell arrangement with fibrillated cytoplasm reminiscent of LMS.
Figure 2.
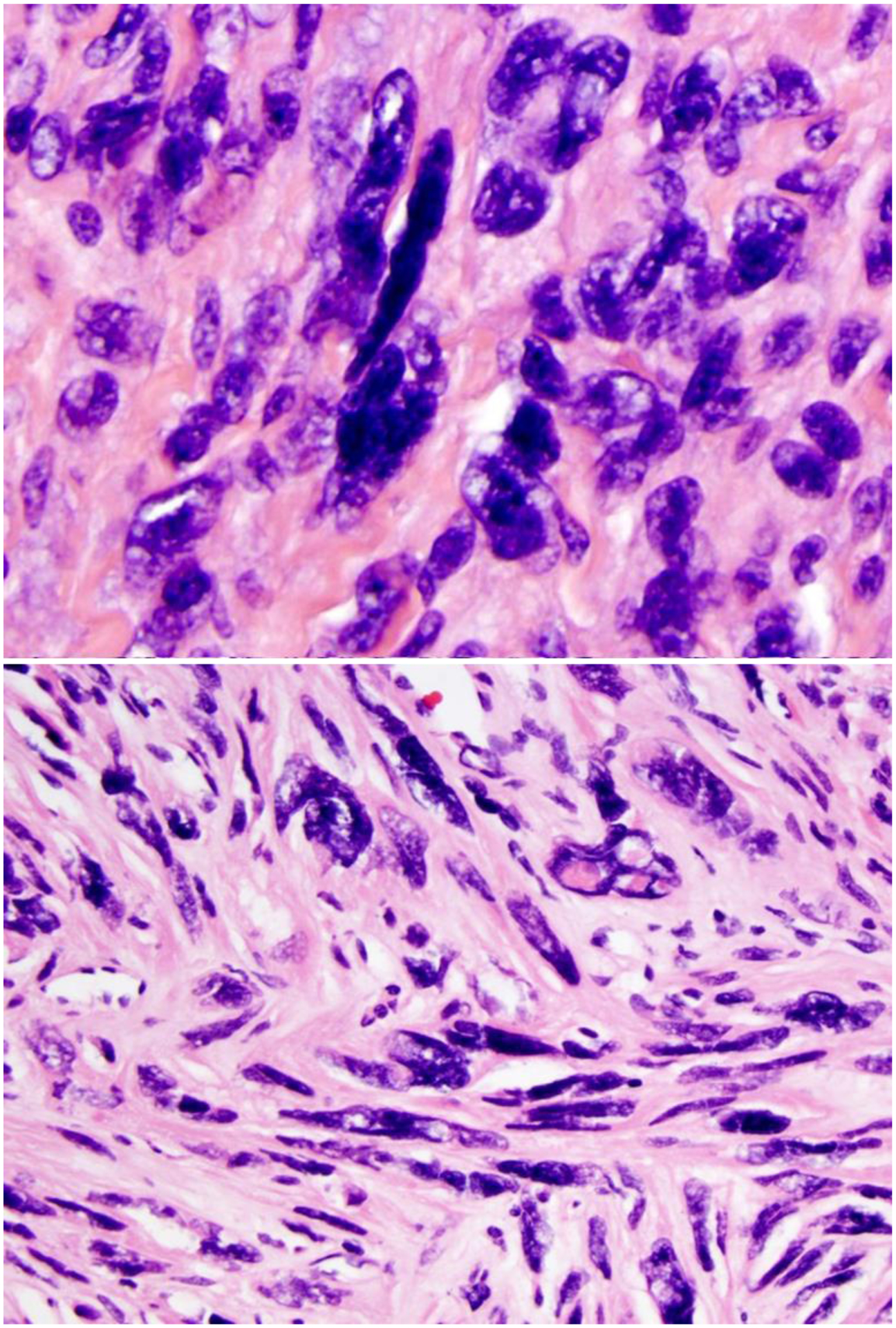
Photomicrographs of leiomyoma with bizarre nuclei.
LM-BN coexist with or inside of typical leiomyomas, and rarely as a solitary presentation4. The density, cellularity, and focality of nuclear atypia vary widely from case to case. The density of nuclear atypia is defined as the percentage of large and bizarre nuclei in a tumor. Density thresholds are arbitrarily defined as low (<10%), intermediate (10–50%), and high (>50%)4. The frequency of density of nuclear atypia in LM-BN is equally divided amongst low, intermediate, and high (Table 2). Increased cellularity or hypercellular LM-BN is another common finding. According to five large case series, 21–47% of LM-BN were hypercellular (Table 2) and this was often associated with hypercellular leiomyoma (Figure 3)4,13,16,19,20. The focality of nuclear atypia in LM-BN is defined as low (1–3 foci, <10% of tumor cells), intermediate (>3 foci, <50% of tumor volume), and high (>50% tumor volume) density of nuclear atypia (Figure 4). Overall, 10% of LM-BN have focal, 30% multifocal, and 60% diffuse patterns of nuclear atypia4,13,19,20. The low frequency of focal nuclear atypia (10% of tumors) may be due to a lower detection rate or under-sampling. How best to define leiomyomas with rare and scattered nuclear atypicality remains controversial. A high density and diffuse pattern of nuclear atypia is always a concern and careful evaluation of the entire lesion is necessary in these cases. Based on the available data, hypercellular and diffuse nuclear atypia LM-BN has been associated with recurrence and is often reclassified to smooth muscle tumor of uncertain malignant potential STUMP in younger patients21,22. Vessels showing a hyaline change is common, while dilated vessels (staghorn) are much less common in LM-BN than in other USMTs4,13,20.
Table 2.
Histologic Features in Leiomyoma with Bizarre Nuclei (LM-BN)
| Study | Bell et al, 199416 | Downes & Hart, 199713 | Ly et al, 201419 | Croce et al, 201420 | Ubango et al, 20164,5 | Bennett et al 201757 |
|---|---|---|---|---|---|---|
| Cases (No.) | 56 | 24 | 51 | 59 | 60 | 31 |
| Age range, years (mean) | 40.0 | 40.7 | 42.5 | 45.0 | 40.5 | 46.7 |
| Tumor size, cm (mean) | 8.0 | 4.2 | 6.8 | 7.3 | 7.6 | 9.0 |
| Focal nuclear atypia | NA | 13% | 12% | 25% | 13% | 39% |
| Distribution | ||||||
| Multifocal | NA | 37% | 29% | 44% | 25% | NA |
| Diffuse | NA | 50% | 59% | 31% | 62% | 61% |
| Nuclear atypia density | ||||||
| Low | 0 | 33% | NA | 47% | 5% | NA |
| Med | 45% | 25% | NA | 32% | 62% | NA |
| High | 55% | 42% | NA | 20% | 33% | NA |
| Mitoses/10 HPF | ||||||
| ≤1 | 21% | 13% | 73% | 63% | 8% | NA |
| 1–5 | 61% | 83% | 25% | 32% | 82% | NA |
| 6–9 | NA | 4% | NA | 5% | 10% | NA |
| Tumor cellularity | ||||||
| Low | 11% | 21% | NA | 2% | 5% | 13% |
| Med | 55% | 58% | NA | 53% | 48% | NA |
| High | 34% | 21% | 6% | 45% | 47% | 87% |
| Staghorn vessels | NA | 21% | NA | 34% | 63% | 65% |
| Prominent nucleoli | NA | NA | NA | 32% | 47% | 71% |
| Eosinophilic globules | NA | NA | NA | 64% | 48% | 84% |
| Growth pattern | ||||||
| Pushing | 70% | 96% | 100% | 98% | 92% | 100% |
| Infiltrating | 30% | 4% | 2% | 8 % | ||
| FH alteration | NA | NA | NA | NA | 54% | 55% |
FH, fumarate hydratase; HPF, high-power fields; NA, not available.
Figure 3.
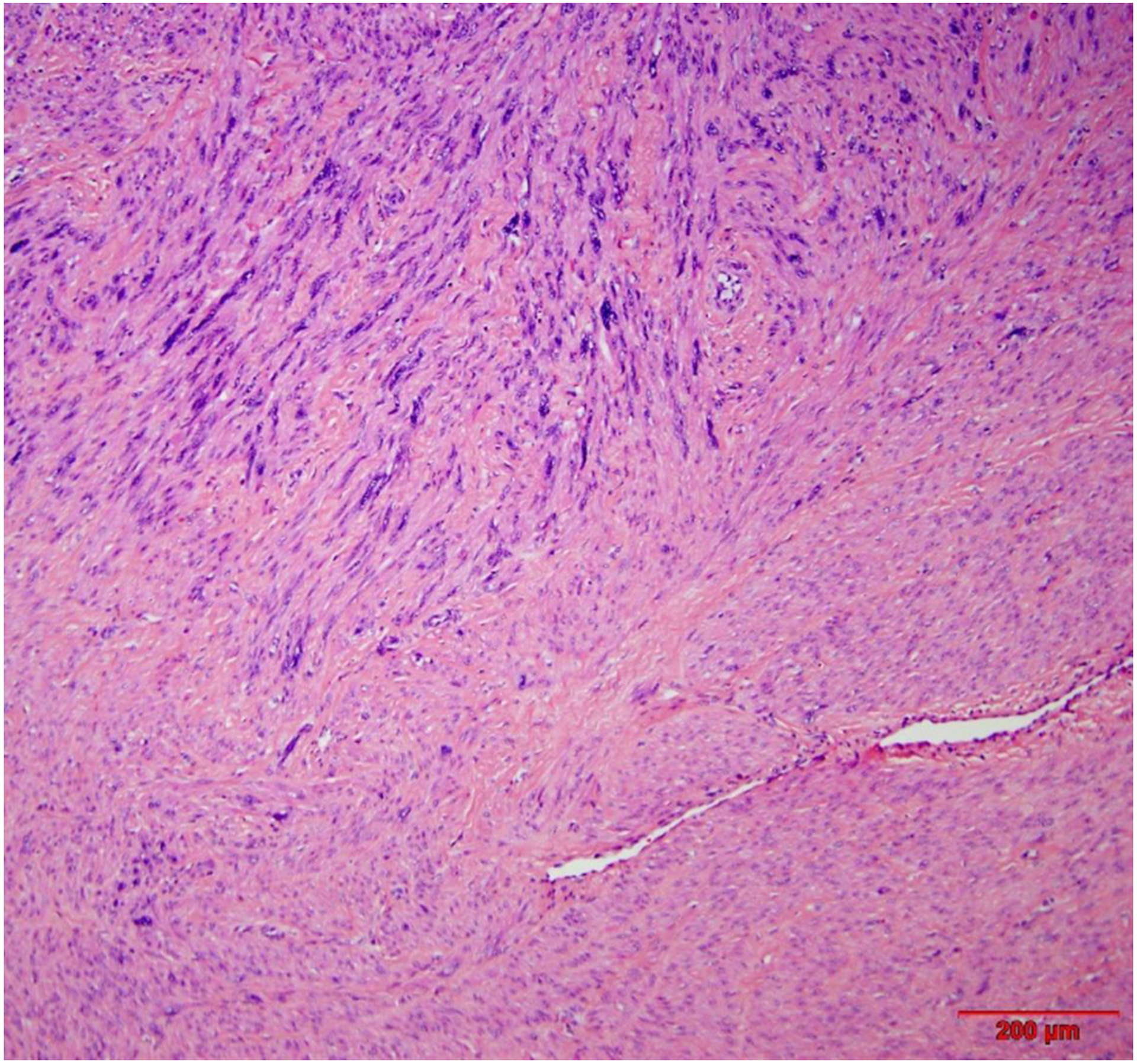
Intermediate power view of leiomyoma with bizarre nuclei shows area of usual type leiomyoma (right low corner).
Figure 4.
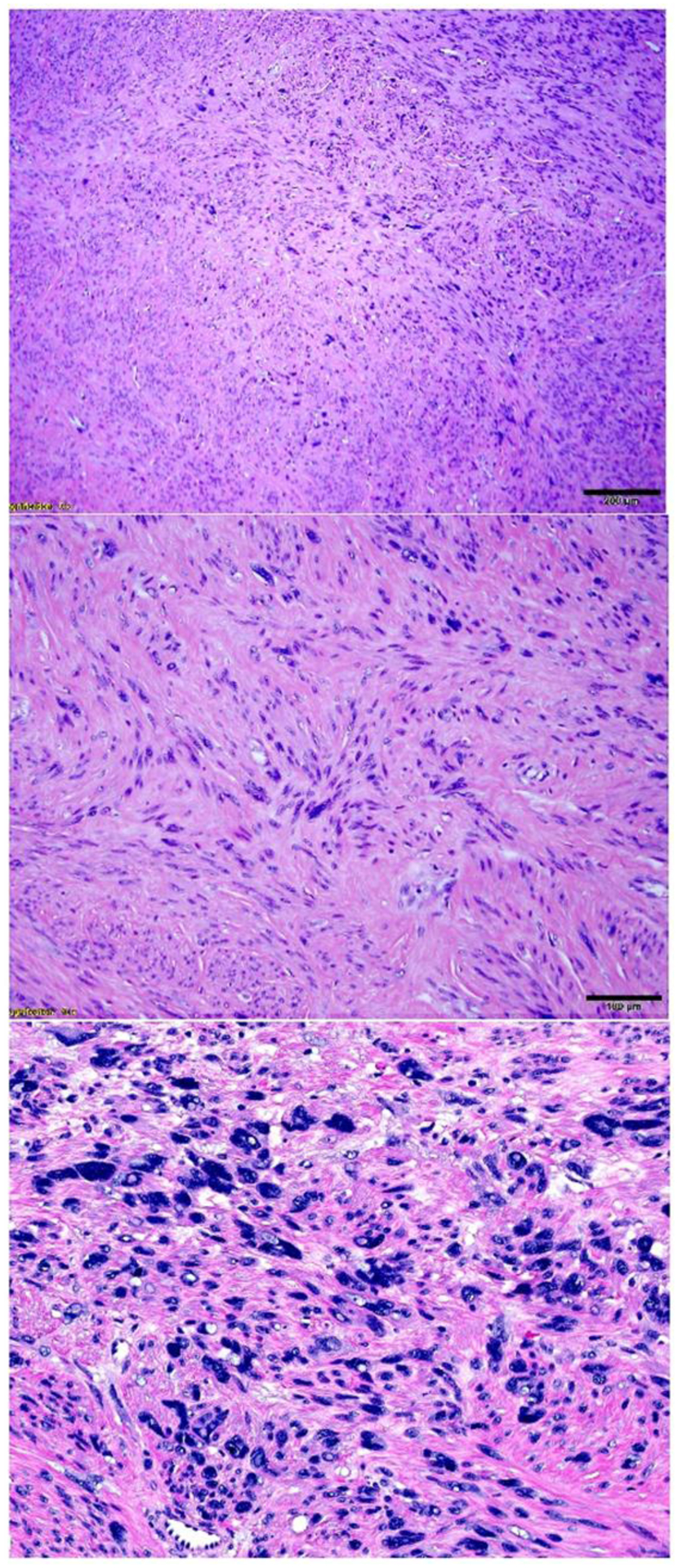
Intermediate power view of leiomyoma with bizarre nuclei shows low (A), intermediate (B) and high (C) density of nuclear atypia.
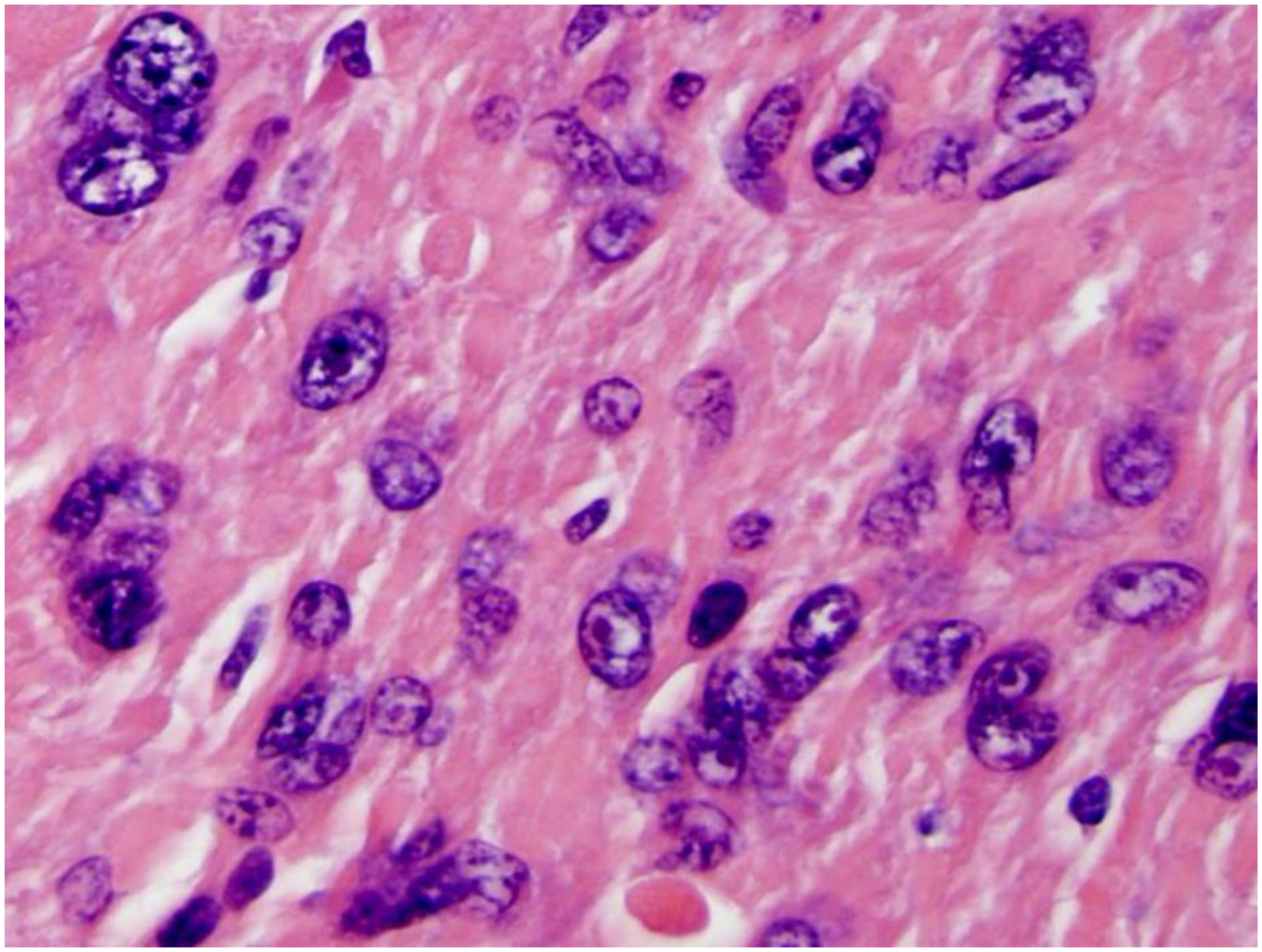
The mitotic index of LM-BN was previously defined as <10 mitoses/10 HPF17. Studies suggest no difference in the clinical course of LM-BN with 6–9 mitoses/10 HPF compared to tumors with lower mitotic indices4,13,20. Stanford investigators and others described LM-BN with 6–9 mitoses/10 HPF as “atypical leiomyomas with low risk of recurrence” that may recur with 2–9 years follow up23–25. Fortunately, studies suggest that only 4–10% of LM-BN have a mitotic index of 6–9 mitoses/10 HPF4,13,16,19,20. LM-BN with diffuse nuclear atypia in young patients with 6–9 mitoses/10 HPF are always a concern; this group of tumors is diagnosed as STUMP in the 2020 WHO tumor classification26. As both nuclear pyknosis and karyorhexis are common findings in LM-BN, the degenerating nuclei undergoing apoptosis may have an appearance that mimics mitosis, so-called “pseudoatypical mitosis”27. In difficult cases, mitotic counts in many sections and multiple 10 HPFs should be considered. In uncertain cases, immunostaining for Ki-67 or PHH3 may aid in differential diagnosis. The presence of atypical mitoses (Figure 5) and a high Ki-67 index (>30% of tumor cells) are always worrisome features. Other features may complicate the differential diagnosis of LM-BN from STUMP, including the presence of an infiltrating border. A tumor-infiltrating growth pattern is seen in up to 8% of LM-BN based on four of five studies reviewed4,13,16,19,20. The role of infiltration in LM-BN remains undetermined.
Figure 5.
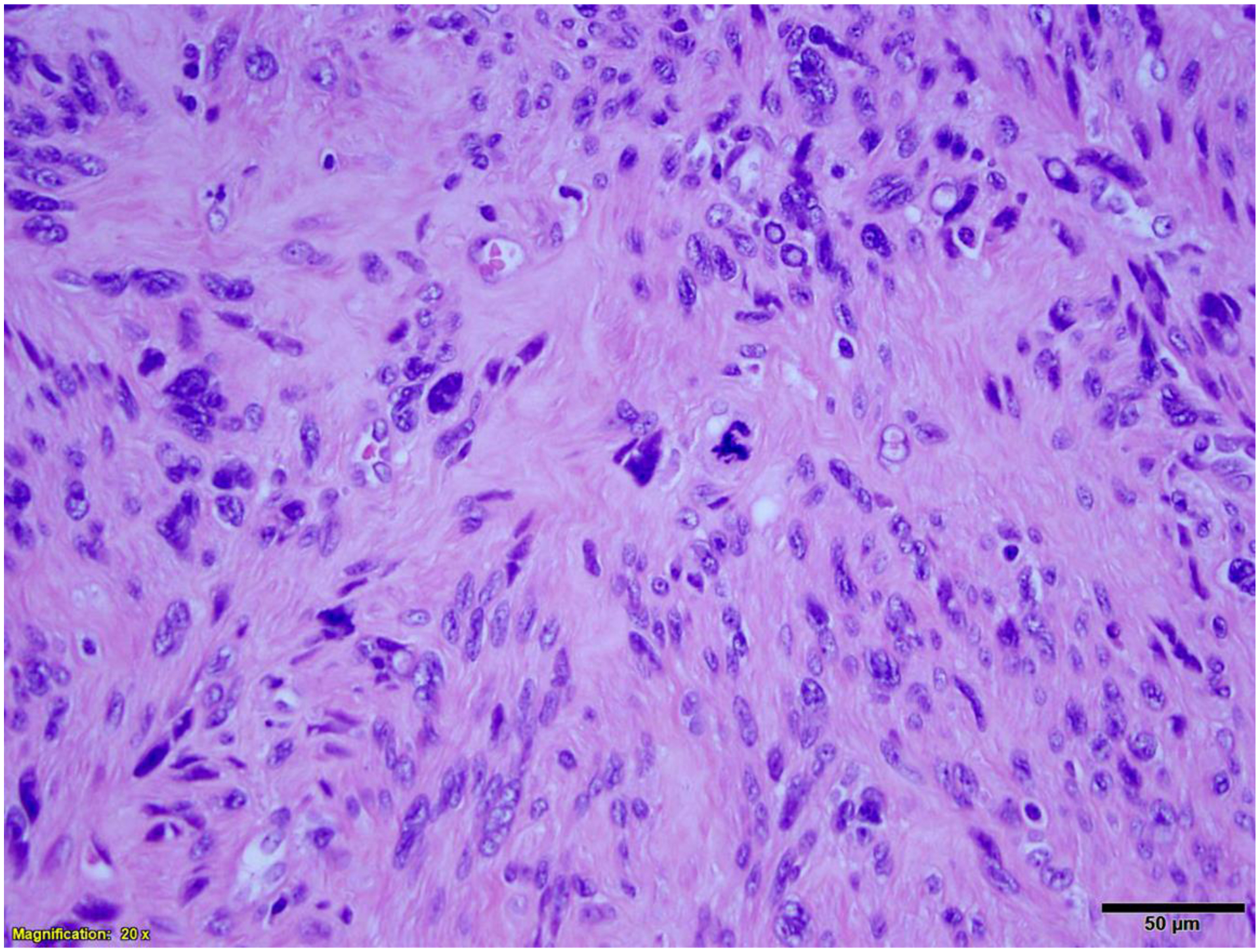
Leiomyoma with bizarre nuclei and atypical mitosis (arrowhead).
Despite careful evaluation of the histologic features mentioned above, many LM-BN and other benign variants of USMTs can be misinterpreted as LMS. For example, one study found that 10 cases (17%) of 59 LM-BN were originally diagnosed as LMS20. Another study reported that as many as 29% of cases (168/419) were misinterpreted as LMS28. Both conventional and epithelioid LMS can show remarkable nuclear atypia presenting as spindle-shaped or rounded nuclei. LMS is often histologically heterogeneous, mixed with well-differentiated (leiomyoma-like), atypical (bizarre leiomyoma-like), and frankly malignant, high-grade components. Mixed hyper-, normo- and hypocelluarity is common in LMS29. Such a wide range of histologic features creates a significant diagnostic challenge.
Histologic evaluation remains the key method in diagnosis of LM-BN23 and no reliable biomarkers can be used to clearly separate it from LMS or other diagnostically challenging cases. Molecular studies reveal that LM-BN may harbor some changes that are commonly seen in LMS30,31. Ünver et al32 detected by immunohistochemistry cell cycle regulators including p16 and p21 in their study of 14 LM-BN and 21 LMS cases. Chen and Yang33 found a significant overlap in p16 staining when comparing LMS with LM-BN, with up to 60% of LM-BN retaining elevated p16 protein in tumor cells. Approximately 37% of LM-BN tumors are diffusely immune-positive for p53, and 10–15% of LM-BN harbor MED12 mutations or overexpress HMGA23,30. In questionable cases, a panel of markers can be considered, including ER, PR, p16, p53, and Ki-67. In our study, diffuse immunoreactivity for ER and PR were seen in 74.1% and 96.0% LM-BN4. Approximately 50% of LM-BN showed diffuse immunoreactivity for p16 and 20–30% of cases were strong and diffusely positive for p534. The Ki-67 index varies widely among LM-BN cases, ranging from 0 to 30%20,34, with most cases showing a Ki-67 index of <10%. LMS is characterized by genomic instability—as evidenced by pervasive, seemingly random karyotypic abnormalities35,36. Global copy number alterations are characteristic of this tumor type; the most frequently reported regions of chromosomal losses are 1p36.32, 4q35.1, 13q14, and17p13, and the most frequent gains are in 1q21,17p12, and 19q1335,37–40.
The presence of similar molecular changes in LM-BN and LMS30,31 raises the question of whether these two tumor types may share a common pathogenic pathway or represent different stages of tumor progression, at least in some cases. The immunohistochemical profiles and genetic aberrations of the examined cases suggest that LMS could arise from preexisting leiomyoma-like areas that often have a symplastic or cellular morphology41. Liegl-Atzwanger et al31 compared 13 cases of LM-BN and 14 cases of LMS using array-CGH, and found that both LM-BN and LMS had sizeable unbalanced genomic alterations of mostly deletions and rare gains. They reported that it was impossible to separate the LM-BN from LMS by evaluating only copy number variations (CNV). When comparing global CNV patterns among FH-LM, LM-BN, and LMS, widespread genomic CNVs involving nearly all chromosomes were seen in both LM-BN and LMS42. Specifically, 37 common CNV peaks including 8 gains and 29 losses were shared by LM-BN and LMS. These 37 significant CNV foci demonstrated overlapping genomic copy number changes in the two tumor types based on PCA analysis. These differences support the idea that LM-BN and LMS are molecularly related. In contrast, a frequent loss of 1q43–44 was seen only in FH-LM. This region contains FH gene, which is the main driver gene for FH-LM. Thus, FH-LM can be readily distinguished from LM-BN and LMS based on its gene mutation and genomic CNV patterns6,42.
LM-BN is usually identified incidentally after myomectomy or hysterectomy for leiomyoma. Abnormal uterine bleeding, pelvic pain, or abdominal bloating may present similarly for leiomyoma and LMS. LM-BN is a rare tumor type. A mean age of LM-BN at diagnosis is 42.5 to 49.8 years of age, about 10–15 years younger than patients with LMS. For those patients with LM-BN who have undergone myomectomy, further hysterectomy and close follow-up are the treatment options, as its recurrence rate is low (Table 3). Downes and Hart43 followed up 24 cases of LM-BN for a mean of 11.2 years (range: 1–18.9 years), the longest follow-up period in the published data. They found there no recurrence or metastasis. Ly and colleagues44 also investigated the clinical features of 51 LM-BN with an average follow-up period of 42 months (range: 0.3–121.8 months). They reported recurrence of LM-BN after hysterectomy in 1 patient and after myomectomy in 3 additional patients. Together, these studies indicate LM-BN tends to have a benign clinical course with occasional recurrence but no disease-related death.
Table 3.
Clinical Outcome of Leiomyoma with Bizarre Nuclei (LM-BN)
| Study (year) | Patients (n) | Mean age (year) | Mean tumor size (cm) | Surgery type (n) | Follow-up (months) | Recurrence (%) | |
|---|---|---|---|---|---|---|---|
| Hysterectomy | Myomectomy | ||||||
| Downes & Hart, 199713 | 24 | 40.7 | 4.2 | 18 | 6 | 135 (12–227) | 8.7 |
| Bell et al, 199416 | 55 | 40 | 8.0 | 34 | 21 | 24–116 | 6.5 |
| Ly et al, 201319 | 51 | 42.5 | 6.8 | 34 | 17 | 42 (0.3–121.8) | 1.9 |
| Zhang et al, 201430 | 42 | 46.9 | 7.4 | 23 | 19 | 90 (13–234) | 4.8 |
| Croce et al, 201420 | 59 | 45 | 7.3 | 42 | 17 | 72 (12–156) | 0 |
| Liegl-Atzwanger et al, 201631 | 13 | 48 | 7 | 11 | 2 | 66 (14–105) | 0 |
| Ubago et al, 20164 | 60 | 43.7 | 6.6 | 42 | 28 | 114 (37–258) | 7 |
| Bennett et al, 20176 | 31 | 46.7 | 9 | 23 | 7 | 7.4 (2.5–22yrs) | 0 |
| Kefeli et al, 201818 | 30 | 49.8 | 6.1 | 22 | 8 | 58.1 (3–122) | 0 |
| Gregova et al, 201952 | 108 | 43 | NA | 60 | 45 | 19 years | 0 |
NA, not available.
The current recommendation is that LM-BN be managed in a conservative manner, as many patients are of reproductive age, with appropriate follow-up, especially for tumors that are large and contain high density and diffuse nuclear atypia18,20. Imaging studies should be carried out at least once a year; pelvic ultrasound, computed tomography, or magnetic resonance imaging may be used to detect any new lesions. For recurrent LM-BN, hysterectomy is the treatment of choice for women who have completed their families. For those who wish to preserve fertility, successful pregnancy after myomectomy has been described, but women should be informed of the likelihood of recurrence, and be followed up vigilantly with imaging studies.
Fumarate hydratase deficiency leiomyoma (FH-LM)
FH-LM is a rare variant of USMTs, found in <1% (12/1152) of unselected uterine leiomyoma45. Most sporadic FH-LM are caused by somatic biallelic inactivation of FH5. In contrast, FH germline mutations can been found in 85% (89/105) of patients with hereditary leiomyomatosis and renal cell carcinoma (HLRCC)46. Patients with HLRCC tend to be younger than those with uterine leiomyoma. One study found that 2.57% (5/194) of leiomyoma in patients younger than 40 years of age carry FH mutations47. The presence of an FH germline mutation should prompt evaluation for HLRCC or Reed syndrome48. Awareness of this specific type of tumor, particularly in younger patients, is important for identifying potential germline mutation49.
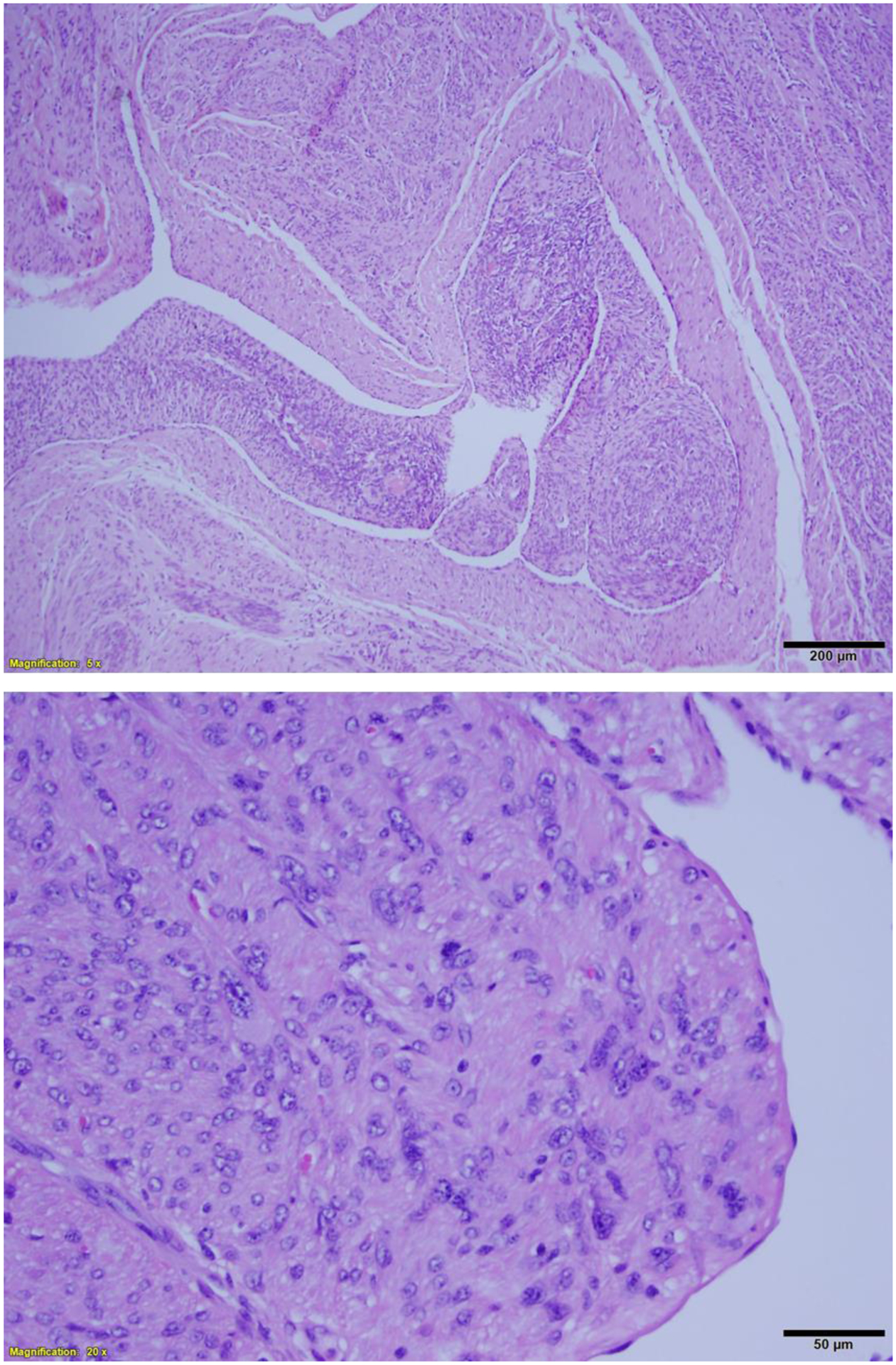
FH-LM shows remarkable nuclear atypia, presenting in focal or diffuse patterns (Figure 6). FH-LM could be readily separated from LM-BN based on characteristic differences in nuclear features and tumor growth patterns4,6,50–52. Typically, FH-LM shows round or oval nuclei and both large and small nuclei, distinct and smooth nuclear membranes, and prominent eosinophilic macronucleoli with perinucleolar halos. FH-LM tumors show a “neurilemmoma-like” growth pattern, with short fascicles, storiform growth, cells with fibrillary cytoplasm, intra and extracellular eosinophilic globules, and staghorn branching vessels (Figure 6)4,50. Microscopic examination reveals areas of hypercellular and less cellular tumor cell proliferation with varied density of nuclear atypia. In hypercellular areas, tumor cells are disorganized with remarkable nuclear atypia characterized by round oval large nuclei with prominent nucleoli and paranucleoli hallo (Figure 7). Mitotic activity is generally low, ranging from 1–4 mitoses/10 HPF.
Figure 6.
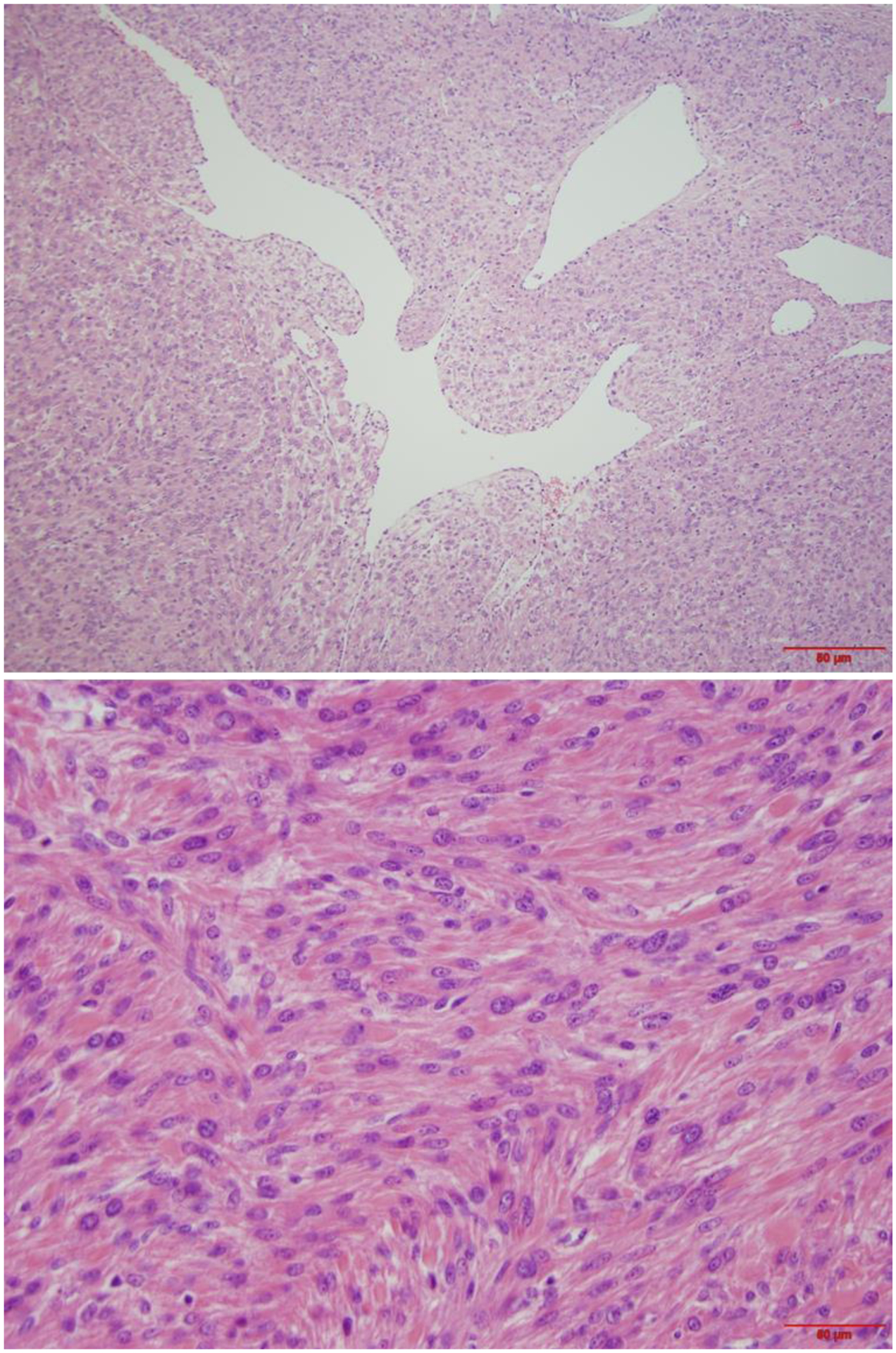
Leiomyoma with fumarate hydratase alteration. A. Low power field shows dilated and staghorn like vessels; B. intermediate power field demonstrates tumor cell arrangement similar to peripheral nerve sheath tumor.
Figure 7.
High power view of nuclear features of leiomyoma with fumarate hydratase alteration.
Performing immunohistochemistry for 2SC or FH can be a reliable screening ancillary test to confirm diagnosis. FH immunopositivity appears as strong and diffuse staining (dot-like and granular) in the cytoplasm and mitochondria (Figure 8)5. FH-positive staining can be detected in normal myometrium and tumors without FH alteration, and is complete loss of FH expression in FH-LM. However. 2SC immunopositivity appears as a strong and diffuse (block-like) cytoplasmic and nuclear staining5 and can be seen in tumors with biallelic inactivation of FH (Figure 8). Approximately one-third of FH-LM cases have detectable FH gene mutations5. The FH gene is located on chromosome 1q34 and this region is frequently deleted in sporadic FH-LM6,42.
Figure 8.
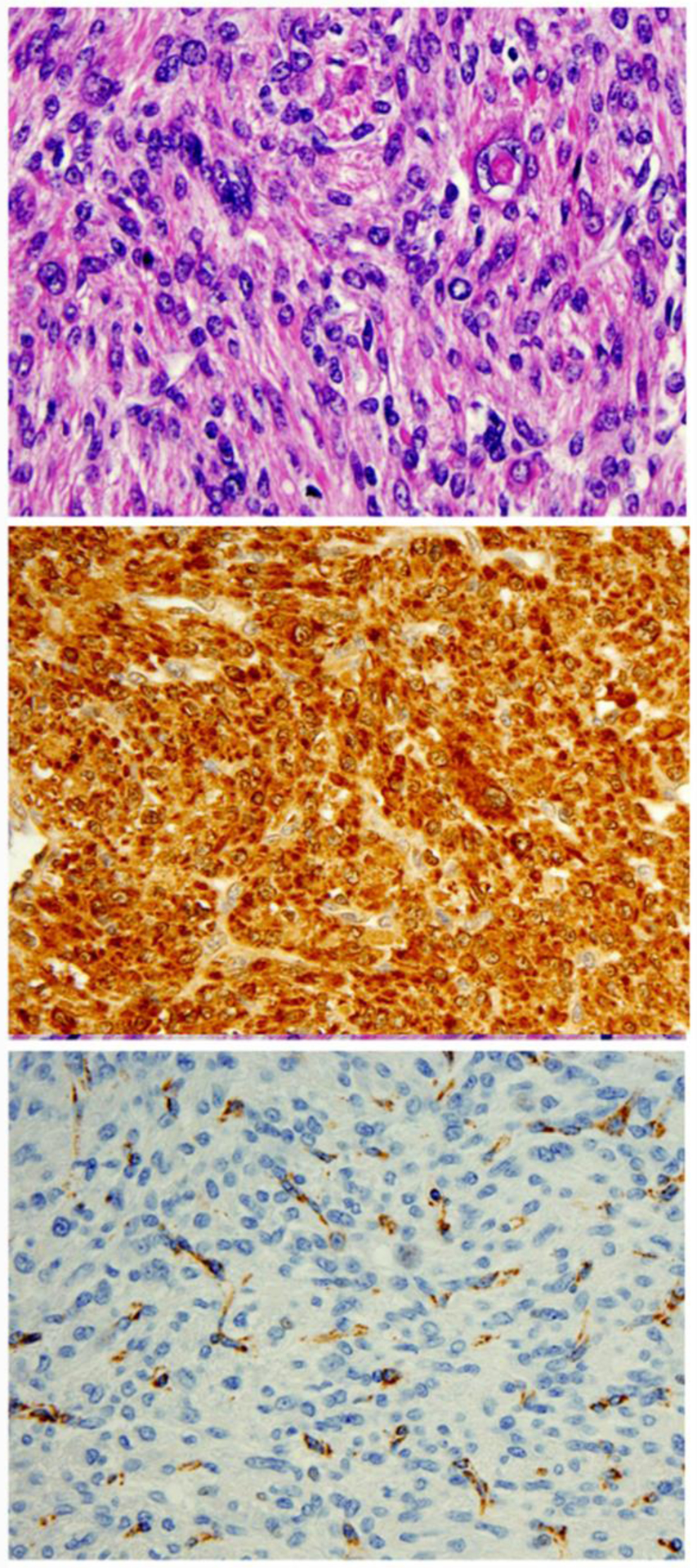
Immunohistochemistry analysis of 2SC (B) and FH (C) in leiomyoma with fumarate hydratase alteration.
Sporadic FH-LM is benign in general, and true malignant transformation is extremely rare45. Somatic or germline FH-LM with high density and diffuse nuclear atypia, increased mitoses of up 9/10 HPF, and/or infiltrating borders may be considered as STUMP and some recurrence has been reported52.
Intravenous leiomyomatosis with nuclear atypia (IV-LM)
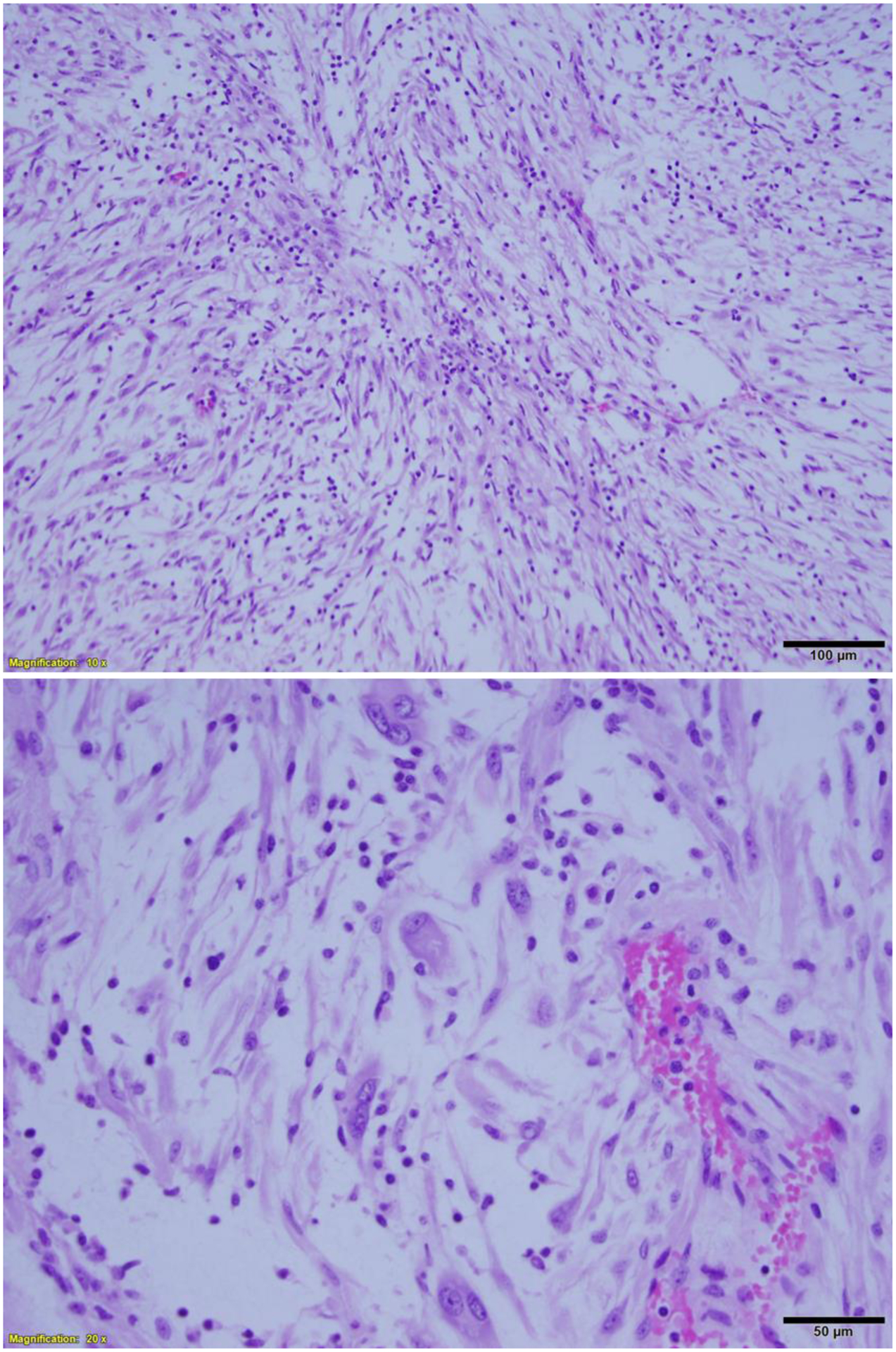
IV-LM occurs most often in perimenopausal women53. Patients typically present with pelvic pain and menorrhagia and may show symptoms of congestive heart failure when there is extensive intravascular involvement. Grossly, tumors consist of multiple intravascular masses involving and extending along uterine and extrauterine veins53. Tumors originate in the myometrium and form “worm-like plugs” within vessels; they are composed of tumor cells similar to usual type leiomyoma. In addition, IV-LM often has an organized corded and/or perivascular tumor arrangement and may have areas of cytological atypia (Figure 9). Nuclear atypia is characterized by large and irregular nuclear contour with dark and hyperchromatic nuclei (Figure 9)12. Such cytohistologic features are quite similar to those of LM-BN; however, recent several molecular studies reported that IV-LM is commonly associated with HMGA2 overexpression53,54 and has specific molecular changes distinct from LM-BN42. Though patients with IV-LM may present with severe symptoms, risk of local recurrence or metastasis is generally low53,55.
Figure 9.
Intravenous leiomyomatosis (A) with areas of nuclear atypia (B).
Inflammatory myofibroblastic tumor (IMFT)
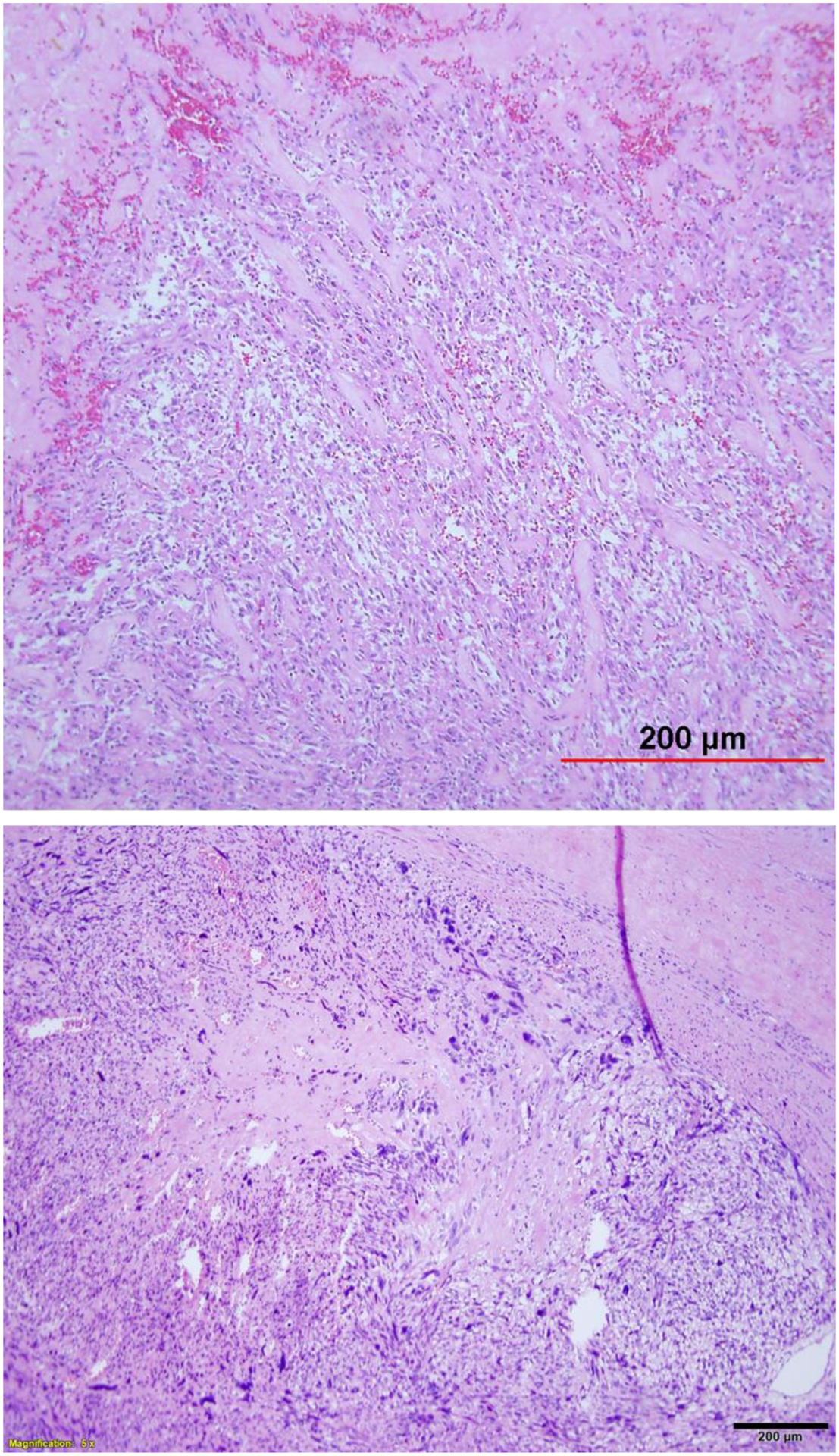
Uterine IMFT is a rare tumor type first reported by Gilks et al in the 198756. IMFT has intermediate biological potential and may be locally aggressive57. Awareness of this tumor type and careful evaluation of histologic features, in conjunction with immunostaining, is necessary for a definitive diagnosis. Grossly, IMFT appears in a well-circumscribed to focally irregular and even infiltrative pattern. As described by Parra-Herran et al57, IMFT can present with myxoid (common) and fascicular (less common) patterns. The myxoid pattern consists of an edematous, myxoid background containing uniformly distributed, plump myofibroblastic cells admixed with inflammatory infiltrate57. Cellular bundles display a tissue culture-like appearance, similar to nodular fasciitis (Figure 10). The majority of cells are spindled with fusiform nuclei and open, even chromatin. Occasional cells with an epithelioid appearance may be present57. IMFT with severe nuclear atypia (Figure 10) has been reported in 15% of tumors showing large and ganglion-like giant cells58. Overall, tumor cells show mostly mild atypia with a low rate of mitoses, and tumor necrosis is rarely seen57. IFMTs demonstrate cytoplasmic immunoreactivity for ALK. Immunointensity varies from case to case. Most cases show strong ALK positivity; however, some tumors are only moderately to weakly positive, with more intense staining observed in myxoid versus fascicular areas. IMFT, as its name implies, originates in myofibroblasts and can be recognized with proper histology and immunohistochemistry analyses.
Figure 10.
Inflammatory myofibroblastic tumor (A) with areas of nuclear atypia (B).
PEComa
Uterine PEComas are rare tumors with features similar to smooth muscle tumors. According to a large series by Bennett et al59, most tumors arise from the uterine corpus and less commonly the cervix. Histologically, uterine PEComas are characterized by spindled to epithelioid cells with a clear to eosinophilic granular cytoplasm in a nested, trabecular, or sheet-like pattern. Uncommonly, tumor cells are organized as fascicles, single cells, or in a pseudoalveolar pattern. Individual tumor cells can show moderate to severe nuclear atypia with occasional macronucleoli and multinucleated cells (Figure 11). Reported mitotic figures vary; they ranged from 0–36 mitoses/10 HPF in the series by Bennett et al59. Delicate, capillary-like vasculature is most common, although thick-walled and staghorn vessels have been reported59. Stromal hyalinization (Figure 11), necrosis, and myometrial invasion are also common. Tumors with diffuse hyalinization can be categorized as sclerosing PEComa. Nuclear atypia and growth patterns in PEComa can be similar to those seen in FH-LM or LM-BN. Key histologic findings favoring a uterine PEComa diagnosis include predominance of delicate, capillary-like vasculature versus mostly thick-walled vessels in leiomyomas59, characteristic tumor cells organized around vessel walls and cells with a eosinophilic to clear, granular cytoplasm. It is imperative that all questionable cases be subjected to ancillary immunohistochemical testing. PEComas are most commonly positive for HMB-45 and smooth muscle markers such as SMA, desmin, and h-caldesmon. Most tumors are also positive for Melan-A and MITF with varying intensity59. Genetic analysis has demonstrated some PEComas associated with TSC1 and TSC2 mutations, and TFE3 and RAD51B rearrangements can be detected in rare tumors by FISH analysis59. Cathepsin K has been shown to be diffusely positive in uterine PEComas, even when melanocytic markers are negative60. Current criteria for malignant PEComas include at least 3 of the following features: size ≥5 cm, high-grade atypia, >1 mitosis/50 HPF, necrosis, and lymphovascular invasion60. However, based on a recent large case series study, these criteria may not match clinical outcomes59.
Figure 11.
Perivascular epithelioid tumor (PEComa) of epithelioid (A) and spindle cell (B) variants with nuclear atypia.
Conclusion
USMT is the most common reproductive system neoplasm in women, with approximately 600,000 hysterectomies and myomectomies for leiomyoma performed in the United States each year. Leiomyomas with nuclear atypia are rarely encountered, but commonly present a diagnostic dilemma when distinguishing between leiomyoma variants, LMS, and other mesenchymal tumors. Histologic evaluation alone may not be sufficient for definitive diagnosis. Newly established ancillary immunohistochemical and molecular tests can be valuable tools that facilitate differential diagnosis. Critical questions remain regarding the relationship between LM-BN and LMS. Emerging data show that these tumors are DNA unstable and share many molecular and biomarker alterations. Currently, no direct evidence has demonstrated a tumorigenic relationship between LMS and LM-BN. Future studies will focus on the mechanisms behind the observed genomic instability in LM-BN and LMS and uncover the true biologic changes leading to DNA instability and nuclear atypia in these tumors.
Highlights:
Leiomyomas with nuclear atypia are a group of rare tumors with distinct histology
Diagnosis of leiomyoma with bizarre nuclei (LM-BN) is challenging
LM-BN has a wide range of features that overlap with other tumor types
Advances in histologic, clinical, and molecular analysis have improved diagnosis
Several diagnostic pitfalls persist for leiomyoma with nuclear atypia
Acknowledgement:
This work was partially supported by National Institutes of Health (R01CA254367) and the Diana’s Fibroid Foundation.
Abbreviations
- CNV
copy number variation
- FH-LM
fumarate hydratase-deficient leiomyoma
- HPF
high-power fields
- HLRCC
hereditary leiomyomatosis and renal cell carcinoma
- IHC
immunohistochemistry
- IMFT
inflammatory myofibroblastic tumor
- IV-LM
intravenous leiomyomatosis
- LM-BN
leiomyoma with bizarre nuclei
- LMS
leiomyosarcoma
- PEComa
perivascular epithelioid tumor
- STUMP
smooth muscle tumor of uncertain malignant potential
- TN
tumor necrosis
- USMT
uterine smooth muscle tumor
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI: Author has nothing to disclose
References
- 1.Leibsohn S, d’Ablaing G, Mishell DR Jr., Schlaerth JB. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. American journal of obstetrics and gynecology. 1990;162(4):968–974; discussion 974–966. [DOI] [PubMed] [Google Scholar]
- 2.Giuntoli RL 2nd, Metzinger DS, DiMarco CS, et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol. 2003;89(3):460–469. [DOI] [PubMed] [Google Scholar]
- 3.Makinen N, Kampjarvi K, Frizzell N, Butzow R, Vahteristo P. Characterization of MED12, HMGA2, and FH alterations reveals molecular variability in uterine smooth muscle tumors. Mol Cancer. 2017;16(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ubago JM, Zhang Q, Kim JJ, Kong B, Wei JJ. Two Subtypes of Atypical Leiomyoma: Clinical, Histologic, and Molecular Analysis. Am J Surg Pathol. 2016;40(7):923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q, Poropatich K, Ubago J, et al. Fumarate Hydratase Mutations and Alterations in Leiomyoma With Bizarre Nuclei. Int J Gynecol Pathol. 2018;37(5):421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett JA, Weigelt B, Chiang S, et al. Leiomyoma with bizarre nuclei: a morphological, immunohistochemical and molecular analysis of 31 cases. Mod Pathol. 2017;30(10):1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly HA, Cullen TS. Myomata of the uterus. Philadelphia: W. B. Saunders Company: Saunders; 1909. [Google Scholar]
- 8.Evans N Malignant Myomas and Related Tumors of the Uterus. Surg Gynecol Obstet. 1920;11:225–239. [Google Scholar]
- 9.Novak E, Anderson DF. Sarcoma of the uterus: Clinical and pathologic Study of Fifty-Nine Cases. Am J Surg Pathol. 1937;34(5):740–761. [Google Scholar]
- 10.Przybora LA. Leiomyosarcoma in situ of the uterus. Cancer. 1961;14(3):483–492. [DOI] [PubMed] [Google Scholar]
- 11.Christopherson WM, Williamson EO, Gray LA. Leiomyosarcoma of the uterus. Cancer. 1972;29(6):1512–1517. [DOI] [PubMed] [Google Scholar]
- 12.Clement PB, Young RH, Scully RE. Intravenous leiomyomatosis of the uterus. A clinicopathological analysis of 16 cases with unusual histologic features. Am J Surg Pathol. 1988;12(12):932–945. [PubMed] [Google Scholar]
- 13.Downes KA, Hart WR. Bizarre leiomyomas of the uterus: a comprehensive pathologic study of 24 cases with long-term follow-up. Am J Surg Pathol. 1997;21(11):1261–1270. [DOI] [PubMed] [Google Scholar]
- 14.Hendrickson MR, Kempson RL. Surgical pathology of the uterine corpus. Major Probl Pathol. 1979;12:1–580. [PubMed] [Google Scholar]
- 15.Evans HL, Chawla SP, Simpson C, Finn KP. Smooth muscle neoplasms of the uterus other than ordinary leiomyoma. A study of 46 cases, with emphasis on diagnostic criteria and prognostic factors. Cancer. 1988;62(10):2239–2247. [DOI] [PubMed] [Google Scholar]
- 16.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18(6):535–558. [PubMed] [Google Scholar]
- 17.Kurman RJ. Blaustein’s Pathology of the Female Genital Tract. Vol I. 5th ed: Spring; 2002. [Google Scholar]
- 18.Kefeli M, Caliskan S, Kurtoglu E, Yildiz L, Kokcu A. Leiomyoma With Bizarre Nuclei: Clinical and Pathologic Features of 30 Patients. Int J Gynecol Pathol. 2018;37(4):379–387. [DOI] [PubMed] [Google Scholar]
- 19.Ly A, Mills AM, McKenney JK, et al. Atypical leiomyomas of the uterus: a clinicopathologic study of 51 cases. Am J Surg Pathol. 2013;37(5):643–649. [DOI] [PubMed] [Google Scholar]
- 20.Croce S, Young RH, Oliva E. Uterine leiomyomas with bizarre nuclei: a clinicopathologic study of 59 cases. Am J Surg Pathol. 2014;38(10):1330–1339. [DOI] [PubMed] [Google Scholar]
- 21.Ip PP, Cheung AN, Clement PB. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases. Am J Surg Pathol. 2009;33(7):992–1005. [DOI] [PubMed] [Google Scholar]
- 22.Guntupalli SR, Ramirez PT, Anderson ML, Milam MR, Bodurka DC, Malpica A. Uterine smooth muscle tumor of uncertain malignant potential: a retrospective analysis. Gynecol Oncol. 2009;113(3):324–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. The American journal of surgical pathology. 1994;18(6):535–558. [PubMed] [Google Scholar]
- 24.Berretta R, Rolla M, Merisio C, Giordano G, Nardelli GB. Uterine smooth muscle tumor of uncertain malignant potential: a three-case report. International Journal of Gynecological Cancer Official Journal of the International Gynecological Cancer Society. 2010;18(5):1121–1126. [DOI] [PubMed] [Google Scholar]
- 25.Sung CO, Ahn G, Song SY, Choi YL, Bae DS. Atypical leiomyomas of the uterus with long-term follow-up after myomectomy with immunohistochemical analysis for p16INK4A, p53, Ki-67, estrogen receptors, and progesterone receptors. Int J Gynecol Pathol. 2009;28(6):529–534. [DOI] [PubMed] [Google Scholar]
- 26.Ip PPC, Tse KY, Tam KF. Uterine Smooth Muscle Tumors Other Than the Ordinary Leiomyomas and Leiomyosarcomas: A Review of Selected Variants With Emphasis on Recent Advances and Unusual Morphology That May Cause Concern for Malignancy. Advances in Anatomic Pathology. 2010;17(2):91–112. [DOI] [PubMed] [Google Scholar]
- 27.Ip PP, Cheung AN. Pathology of uterine leiomyosarcomas and smooth muscle tumours of uncertain malignant potential. Best Pract Res Clin Obstet Gynaecol. 2011;25(6):691–704. [DOI] [PubMed] [Google Scholar]
- 28.Abeler VM, Røyne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2010;54(3). [DOI] [PubMed] [Google Scholar]
- 29.Toledo G, Oliva E. Smooth muscle tumors of the uterus: a practical approach. Arch Pathol Lab Med. 2008;132(4):595–605. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Q, Ubago J, Li L, et al. Molecular analyses of 6 different types of uterine smooth muscle tumors: Emphasis in atypical leiomyoma. Cancer. 2014;120(20):3165–3177. [DOI] [PubMed] [Google Scholar]
- 31.Liegl-Atzwanger B, Heitzer E, Flicker K, et al. Exploring chromosomal abnormalities and genetic changes in uterine smooth muscle tumors. Mod Pathol. 2016;29(10):1262–1277. [DOI] [PubMed] [Google Scholar]
- 32.Unver NU, Acikalin MF, Oner U, Ciftci E, Ozalp SS, Colak E. Differential expression of P16 and P21 in benign and malignant uterine smooth muscle tumors. Archives of gynecology and obstetrics. 2011;284(2):483–490. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Yang B. Immunohistochemical analysis of p16, p53, and Ki-67 expression in uterine smooth muscle tumors. Int J Gynecol Pathol. 2008;27(3):326–332. [DOI] [PubMed] [Google Scholar]
- 34.Mills AM, Ly A, Balzer BL, et al. Cell cycle regulatory markers in uterine atypical leiomyoma and leiomyosarcoma: immunohistochemical study of 68 cases with clinical follow-up. Am J Surg Pathol. 2013;37(5):634–642. [DOI] [PubMed] [Google Scholar]
- 35.Lazar AJ, McLellan MD, Bailey MH, et al. Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. 2017;171(4):950–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salawu A, Ul-Hassan A, Hammond D, Fernando M, Reed M, Sisley K. High quality genomic copy number data from archival formalin-fixed paraffin-embedded leiomyosarcoma: optimisation of universal linkage system labelling. PLoS One. 2012;7(11):e50415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemming ML, Klega KS, Rhoades J, et al. Detection of Circulating Tumor DNA in Patients With Leiomyosarcoma With Progressive Disease. JCO Precis Oncol. 2019;2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chudasama P, Mughal SS, Sanders MA, et al. Integrative genomic and transcriptomic analysis of leiomyosarcoma. Nat Commun. 2018;9(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuppens T, Moisse M, Depreeuw J, et al. Integrated genome analysis of uterine leiomyosarcoma to identify novel driver genes and targetable pathways. Int J Cancer. 2018;142(6):1230–1243. [DOI] [PubMed] [Google Scholar]
- 40.Silveira SM, Villacis RA, Marchi FA, et al. Genomic signatures predict poor outcome in undifferentiated pleomorphic sarcomas and leiomyosarcomas. PLoS One. 2013;8(6):e67643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mittal KR, Chen F, Wei JJ, et al. Molecular and immunohistochemical evidence for the origin of uterine leiomyosarcomas from associated leiomyoma and symplastic leiomyoma-like areas. Mod Pathol. 2009;22(10):1303–1311. [DOI] [PubMed] [Google Scholar]
- 42.Gao T, Finkelman BS, Ban Y, et al. Integrated histologic and molecular analysis of uterine leiomyosarcoma and 2 benign variants with nuclear atypia. Cancer Sci. 2021;112(5):2046–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Downes KA, Hart WR. Bizarre Leiomyomas of the Uterus: A Comprehensive Pathologic Study of 24 Cases With Long-Term Follow-Up. The American journal of surgical pathology. 1997;21(11):1261–1270. [DOI] [PubMed] [Google Scholar]
- 44.Ly A, Mills AM, McKenney JK, et al. Atypical leiomyomas of the uterus: a clinicopathologic study of 51 cases. The American journal of surgical pathology. 2013;37(5):643–649. [DOI] [PubMed] [Google Scholar]
- 45.Harrison WJ, Andrici J, Maclean F, et al. Fumarate Hydratase-deficient Uterine Leiomyomas Occur in Both the Syndromic and Sporadic Settings. Am J Surg Pathol. 2016;40(5):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiuru M, Launonen V. Hereditary leiomyomatosis and renal cell cancer (HLRCC). Current molecular medicine. 2004;4(8):869–875. [DOI] [PubMed] [Google Scholar]
- 47.Joseph NM, Solomon DA, Frizzell N, Rabban JT, Zaloudek C, Garg K. Morphology and Immunohistochemistry for 2SC and FH Aid in Detection of Fumarate Hydratase Gene Aberrations in Uterine Leiomyomas From Young Patients. Am J Surg Pathol. 2015;39(11):1529–1539. [DOI] [PubMed] [Google Scholar]
- 48.Garcia Muret MP, Pujol RM, Alomar A, Calaf J, de Moragas JM. Familial leiomyomatosis cutis et uteri (Reed’s syndrome). Arch Dermatol Res. 1988;280 Suppl:S29–32. [PubMed] [Google Scholar]
- 49.Wei JJ. Atypical Leiomyoma With Features Suggesting of Fumarate Hydratase Mutation. Int J Gynecol Pathol. 2016;35(6):531–536. [DOI] [PubMed] [Google Scholar]
- 50.Reyes C, Karamurzin Y, Frizzell N, et al. Uterine smooth muscle tumors with features suggesting fumarate hydratase aberration: detailed morphologic analysis and correlation with S-(2-succino)-cysteine immunohistochemistry. Mod Pathol. 2014;27(7):1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miettinen M, Felisiak-Golabek A, Wasag B, et al. Fumarase-deficient Uterine Leiomyomas: An Immunohistochemical, Molecular Genetic, and Clinicopathologic Study of 86 Cases. Am J Surg Pathol. 2016;40(12):1661–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gregova M, Hojny J, Nemejcova K, et al. Leiomyoma with Bizarre Nuclei: a Study of 108 Cases Focusing on Clinicopathological Features, Morphology, and Fumarate Hydratase Alterations. Pathology oncology research : POR. 2020;26(3):1527–1537. [DOI] [PubMed] [Google Scholar]
- 53.Ordulu Z, Nucci MR, Dal Cin P, et al. Intravenous leiomyomatosis: an unusual intermediate between benign and malignant uterine smooth muscle tumors. Mod Pathol. 2016;29(5):500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quade BJ, Dal Cin P, Neskey DM, Weremowicz S, Morton CC. Intravenous leiomyomatosis: molecular and cytogenetic analysis of a case. Mod Pathol. 2002;15(3):351–356. [DOI] [PubMed] [Google Scholar]
- 55.Carr RJ, Hui P, Buza N. Intravenous leiomyomatosis revisited: an experience of 14 cases at a single medical center. Int J Gynecol Pathol. 2015;34(2):169–176. [DOI] [PubMed] [Google Scholar]
- 56.Gilks CB, Taylor GP, Clement PB. Inflammatory pseudotumor of the uterus. Int J Gynecol Pathol. 1987;6(3):275–286. [DOI] [PubMed] [Google Scholar]
- 57.Parra-Herran C, Quick CM, Howitt BE, Dal Cin P, Quade BJ, Nucci MR. Inflammatory myofibroblastic tumor of the uterus: clinical and pathologic review of 10 cases including a subset with aggressive clinical course. Am J Surg Pathol. 2015;39(2):157–168. [DOI] [PubMed] [Google Scholar]
- 58.Bennett JA, Nardi V, Rouzbahman M, Morales-Oyarvide V, Nielsen GP, Oliva E. Inflammatory myofibroblastic tumor of the uterus: a clinicopathological, immunohistochemical, and molecular analysis of 13 cases highlighting their broad morphologic spectrum. Mod Pathol. 2017;30(10):1489–1503. [DOI] [PubMed] [Google Scholar]
- 59.Bennett JA, Braga AC, Pinto A, et al. Uterine PEComas: A Morphologic, Immunohistochemical, and Molecular Analysis of 32 Tumors. Am J Surg Pathol. 2018;42(10):1370–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoolmeester JK, Howitt BE, Hirsch MS, Dal Cin P, Quade BJ, Nucci MR. Perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: clinicopathologic and immunohistochemical characterization of 16 cases. Am J Surg Pathol. 2014;38(2):176–188. [DOI] [PubMed] [Google Scholar]


