Abstract
Medical robots are invaluable players in non-pharmaceutical treatment of disabilities. Particularly, using prosthetic and rehabilitation devices with human-machine interfaces can greatly improve the quality of life for impaired patients. In recent years, flexible electronic interfaces and soft robotics have attracted tremendous attention in this field due to their high biocompatibility, functionality, conformability, and low-cost. Flexible human-machine interfaces on soft robotics would make a promising alternative to conventional rigid devices, which could potentially revolutionize the paradigm and future direction of medical robotics in terms of rehabilitation feedback and user experience. In this review, the fundamental components of the materials, structures, and mechanisms in flexible human-machine interfaces are summarized by recent and renown applications in five primary areas: physical and chemical sensing, physiological recording, information processing and communication, soft robotic actuation, and feedback stimulation. This review further concludes by discussing the outlook and current challenges of these technologies as a human-machine interface in medical robotics.
Keywords: flexible electronics, medical robotics, machine learning, human-machine interaction, prosthetics, rehabilitation
Flexible electronics and devices could potentially revolutionize the paradigm and future direction of medical robotics. Herein, the materials, structures, and mechanisms in flexible human-machine interfaces used in prosthetic and rehabilitation robots are summarized in five primary areas: sensing, recording, communication, actuation, and stimulation. The current challenges and outlook of these technologies in medical robotics are discussed.
1. Introduction
With a growing population and the subsequent rise of age-associated diseases, the development and integration of novel sensors and materials into medical robotics have the potential to prolong life expectancy and enrich the quality of life for the next generation.[1–5] The motivation for the advancement of medical robotics stems from the United Nations’ (UN) report that 46% of seniors (defined as 60 years and older) have some form of disability in comparison to 15% of the general public.[6] The higher ratio of disabled persons in the senior population is due to the greater frequency of age-associated diseases such as stroke, Parkinson’s, and Alzheimer’s disease that can lead to physical and cognitive impairment.[3,5,7] According to the Center for Disease Control (CDC), of disabled Americans, 13.7% of the population has a mobility impairment and 10.8% has a cognitive disability that can be alleviated with medical-assisted robotics and novel therapeutics.[3,8,9]
Despite the prevalence of cognitive and physical disabilities, the application of robotics for medical therapeutics only coincided with the paramount shift in medical ideology in the early 1980s on the neuroplasticity of the brain after injury.[10–13] In the late 20th century, researchers found that over long periods of time guided exercises had a significant improvement in restoring lost brain function and mobility.[14] These remedial motions were integrated into a wheelchair-adaptable therapeutic for stroke patients in 1989 with MANUS: the first medical robot designed specifically for rehabilitation.[3,12,15,16] Later in the 1990s, the KAIST KARES wheelchair further integrated torque sensors and vision-based servoing to allow for guided user navigation.[17,18] The field has continued to grow well into the 21rst century, with the keyword “rehabilitation robotics” in PubMed’s annual academic articles skyrocketing from 191 to 772 papers in 2010 and 2020 respectively.
The progression of feedback sensors, modern designs, and novel materials similarly generated broader user-acceptance of medical prosthetics.[19,20] Up until the sixteenth century, prosthetic appendages were mainly used as a form of aesthetic with minimal added functionality past holding and gripping tightly onto objects.[21] It was not until the early 1500s – when people could fabricate prosthetic limbs using complex moveable metal (iron) designs to replace or augment the fixed fabric, copper, and wooden schemes – that mechanical prosthetics were given broader mobility, stability, and functionally.[22] The innovation in prosthetic fabrication in the 21rst century has further advanced through the development of three dimensional (3D) printable soft materials and electronic skin sensors for flexible lightweight designs that enhance user-communication with their environment.
Despite the recent improvements to the material, design, and functionality of medical robotics, as well a $6.39 billion dollars market,[23] up to 40% of limb impairment patients still option out of using artificial replacements.[19] The common issues cited are the unnatural feel, poor functionality, and heavy weight of the device.[19] At its core, the inherent limitation of wearable robotics is that the electronics are not recognized as an extension of the human body, resulting in a high cognitive effort for the user to control. Additional shortcomings include their limited environmental feedback, power supply, and failure to autonomously respond appropriately to external (possibly dangerous) stimuli.[24] For many prostheses, the same sensory information can be acquired through the users’ stump.[19] Recent advancements in soft robotics and electronic skin (e-skin) may offer a way to bridge this communication gap, allowing the user to have broader functionality in their medical device for an enhanced user-experience.[25]
Initially proposed in science fiction movies, the first tangible application of e-skin for prosthetics occurred in 1974 with minor sensory feedback incorporated onto a robotic arm.[26] Now the base of many wearable devices, e-skin has been shown to outperformed human skin in capturing thermal, humidity, physiological, various chemical, and tactile sensations while maintaining a high spatiotemporal resolution under varying degrees of strain. Unfortunately, recording these multimodal chemical, temperature, and pressure signals can drain power and electrically interfere with the accuracy of sensor readings. It is therefore imperative to reduce the sensor density and improve the efficiency of data collection by maximizing the environmental information extracted through novel signal processing and machine learning (ML) techniques.[27] To make the replacement from current rigid electronics, e-skin requires a low mechanical modulus (high stretchability),[28–30] aesthetic appearance,[31] low-cost,[32] lightweight design,[29] large-scale fabrication techniques,[32] self-healing properties,[30] and be thermally stable.[29] With these functions, e-skin can provide the user with a more accurate understanding of their dynamic environment as well as update medical staff on the progress of their patient.
The combination of soft robotics with e-skin can additionally provide the user with a safe, light, and low complexity mode of actuation without the high cognitive strain associated with its rigid and heavy counterpart.[33–35] In particular, when e-skin tactile sensing communicates harmful external stimuli to the user, soft robotics can utilize its flexibility and high degree of freedom to efficiently move away and adapt to the scenario.[19,36] Together, e-skin and soft robotics can therefore alleviate problems such as limited functionality,[37] heavy weight,[19] and poor aesthetic design.[31] Unfortunately, the tradeoff to such mobility is structural degradation of the robot over long periods of duress. To replace the current metal designs, soft robotic materials therefore require the ability to withstand rapid exposure to extreme temperatures and toxic chemicals as well as continuous deformations and elongations.[38] Through dual sensory communication and flexible robotic actuation, e-skin and soft robotics devices can restore the user’s lost sensory awareness in the extremity, allowing the patient to see the limb as an extension of their body rather than a lifeless mechanical appendage.
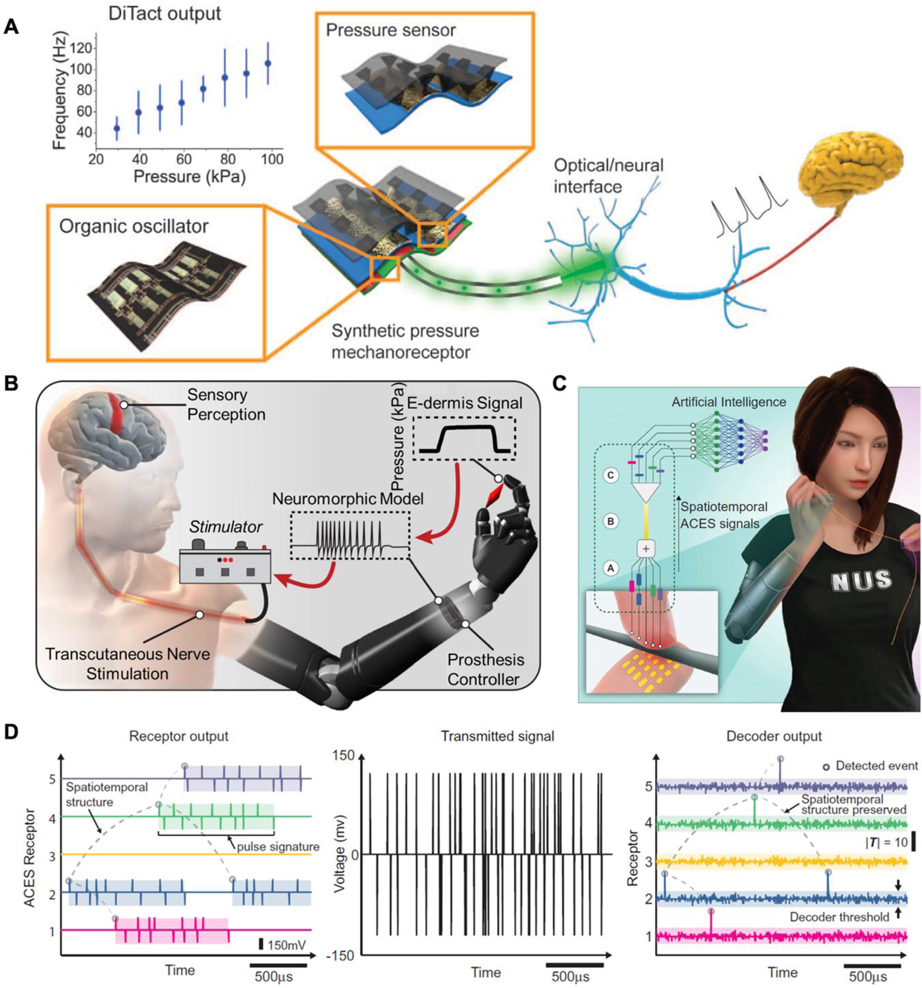
This review investigates the recent progressions made in flexible electronics and soft devices that tackle the current user-problems associated human-machine interaction (HMI) in medical robotics. The article breaks down HMI devices for medical robotic applications into five major categories: sensing, recording, communication, actuation, and stimulation (Figure 1). The first component, materials, provides a broad overview of the different compounds and structures that are currently being investigated for various medical robotic applications. The subsequent sensing section reviews mechanical, temperature, and chemical sensors that have been applied to e-skin. The paper provides specific attention to bioelectrical signal recording in the following section, analyzing the different techniques used to invasively and non-invasively detect electrophysiological information. After data collection methodologies have been discussed, the review will compare the various algorithms and techniques used to process and communicate information to the user. The review will then discuss soft robotics and actuators (thermal and mechanical) for human-like lightweight motion. Finally, the paper will end on robotic stimulation, in which various electrical and burgeoning optogenetic stimulations as well as noninvasive virtual reality (VR) and artificial reality (AR) applications are assessed. Each area of the review has been further supplemented with recent research studies, indicating the stage and future application of each methodology. In the final section, the challenges of flexible electronics and devices are summarized, noting their potential future directions within the scientific community.
Figure 1.
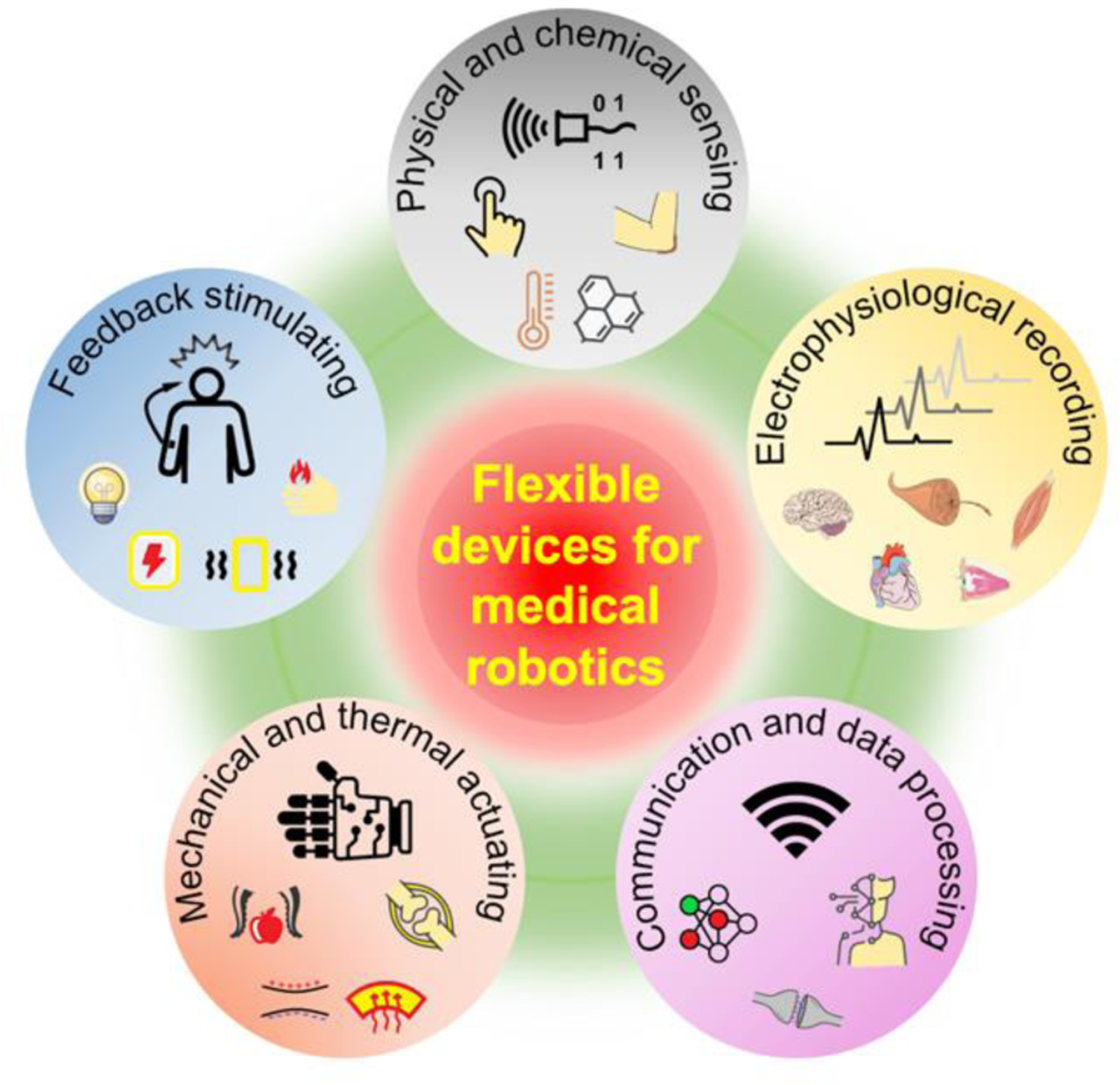
Representative applications of flexible electronics in HMIs for medical robotics.
2. Materials and Structures
The incorporated materials (Figure 2) and structural designs (Figure 3) in flexible devices directly impact their various properties. One of the most important properties for medical applications is biocompatibility, which includes the safety, comfort, and normal functionality of the device in vivo or on the skin. In the following subsection, various flexible materials and structural designs in HMI devices are introduced, alongside their desired properties, with specific attention given to their biocompatibility and functionality in various medical robotics.
Figure 2.
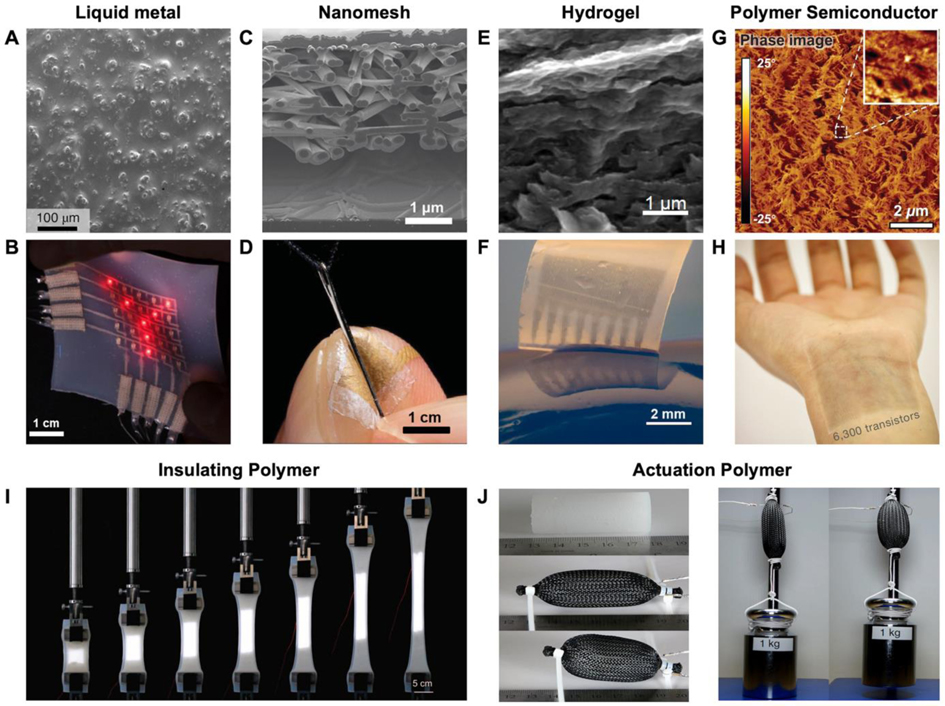
Materials for soft electronic devices. A) Scanning electron microscopy (SEM) image of the surface on a biphasic gallium–indium (bGaIn) alloy film. B) Photograph of a multilayer LED display based on the bGaIn interconnects. A,B) Reproduced with permission.[40] Copyright 2021, Springer Nature. C) Cross-sectional SEM image of a gold nanomesh-based pressure sensor. D) The ultrathin pressure sensor attached conformally to the index finger. C,D) Reproduced with permission.[44] Copyright 2020, American Association for the Advancement of Science (AAAS). E) SEM image of interconnected poly(3,4-ethylenedioxythiophene) (PEDOT) polymer networks in the electrically conductive hydrogels (ECH). F) A micropatterned ECH elastic electrode array. E,F) Reproduced with permission.[58] Copyright 2019, Springer Nature. G) Atomic force microscope (AFM) phase image of a semi-conductive polymer under 100% strain. G) Reproduced with permission.[41] Copyright 2017, AAAS. H) A large-scale array of intrinsically stretchable transistors using the polymer as the semiconductor layer. H) Reproduced with permission.[42] Copyright 2018, Springer Nature. I) A hyper-elastic light-emitting capacitor encapsulated in insulating polymer (Ecoflex 00–30) under uniaxial stretching. I) Reproduced with permission.[66] Copyright 2016, American Association for the Advancement of Science. J) McKibben-type artificial muscles (soft polymer composite material inside braided mesh sleeving) and corresponding actuation. J) Reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International license.[65] Copyright 2017, The Authors, published by Springer Nature.
Figure 3.
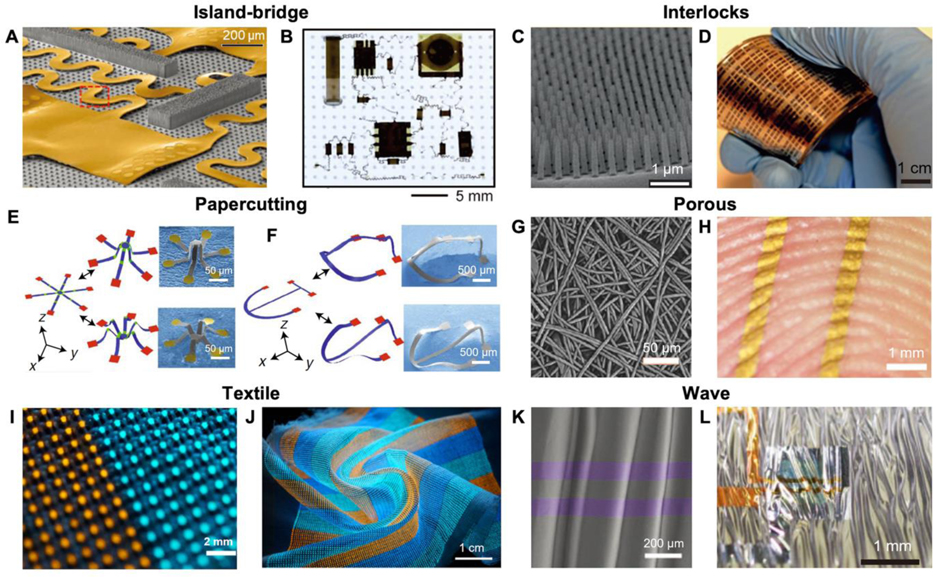
Structures of soft electronic devices. A) Angled-view SEM of serpentine bridging interconnect networks. B) Optical image of the island-bridge device at equal-biaxial 50% strains. A,B) Reproduced with permission.[43] Copyright 2014, AAAS. C) SEM image of a dense array of reversible interlocking of Pt-coated polymer nanofibers. D) Photograph showing a nanofibers-based strain gauge with interlocking structure. C,D) Reproduced with permission.[61] Copyright 2012, Springer Nature. E) Papercutting inspired 3D microelectronic devices formed of SU8+Silicon in micrometers scale. F) 3D microelectronic devices in tens of micrometers scale using silicon. E,F) Reproduced with permission.[70] Copyright 2018, Springer Nature. G) SEM image of a nanomesh-based conductor with porous structure. H) A photo of a conductor attached conformally to a fingertip. G,H) Reproduced with permission.[73] Copyright 2017, AAAS. I) Photograph of a soft multicolor display in form of textile. J) The display under complex deformations, including bending and twisting. I,J) Reproduced with permission.[47] Copyright 2021, Springer Nature. K) SEM image of ultrathin transistors on an elastomer with waves formed by pre-stretching. L) Stretchable transistors on the elastomer with waves. K,L) Reproduced with permission.[48] Copyright 2013, Springer Nature.
2.1. Mechanical biocompatibility
From a mechanical perspective, biocompatible devices for medical applications must be flexible, elastic, and compliant enough to adapt to any target tissues’ complex morphology. Proper conformability can reduce damage to the device and body during tissue displacement and motion artifacts while also improving patient comfort and outcome.[39] HMI materials can be broadly classified into two categories: inherently elastic and intrinsically non-stretchable components. The former, such as liquid metals[40] (Figure 2A and 2B) and conductive or semiconductive polymers,[41,42] directly rely on their mechanical properties for flexibility without further modification (Figure 2G and 2H). The latter (rigid materials), on the other hand, gain elasticity through flexible structural designs. A common method for incorporating rigid components into flexible devices is to use thin strips of the material. Bending strains decrease linearly with thickness, making inherently inelastic materials flexible when fabricated into ultrathin films or wires (on the nanometers/micrometers scale). Typical examples are metallic films on flexible circuits,[43] or nanomesh electrodes (Figure 2C,D) in flexible sensors.[44] Another way to obtain flexibility is through structural designs, like serpentine (Figure 3A and 3B),[45] kirigami,[46] fabric (Figure 3I and 3J),[47] and waves (Figure 3K and 3L).[48] Such structures can not only endow a degree of flexibility and stretchability to rigid devices, but also further enhance the elasticity of inherently soft devices.[49] Upon gaining flexibility through materials and structures, HMI devices must be further processed into the appropriate biocompatible morphology suitable for different tissues (similar material to tissues).
2.2. Chemical biocompatibility
The chemical composition of flexible devices should be adaptable to various biochemical environments (biofluids like sweat and interstitial fluid) to ensure user safety and prevent device failure, especially in biological fluids containing various erosive ions and attacking immune cells.[50] For implantable devices, noble metals such as platinum (Pt) and gold (Au) play an important role in neural interface electrodes due to their chemical stability in physiological environments.[51] Another commonly used biocompatible material is hydrogels (see Figure 2E and 2F). As a material whose features resemble water-rich tissue in the human body, hydrogels can greatly reduce the inflammatory response of foreign objects, regulate cell or protein attachment, and prevent device failure in vivo,[52] which is important for implants.[53,54] Coating hydrogels on electronics[55] or mixing conductive materials such as carbon nanotubes (CNTs),[56] ionic liquids,[57] or conductive polymers[58] into the gel can help maintain biocompatibility while also preserving the electronic properties. For devices that are not chemically biocompatible, the last commonly used technique is to completely seal the device in biocompatible material, like polyimide (PI) or polydimethylsiloxane (PDMS).[59]
2.3. Functionality and performance
The functionality and performance of many medical robotics can be evaluated by the device’s sensitivity, electrode interface impedance, actuation capability, or stimulator charge injection capability, all of which are dependent on the electrical properties of the material and structural designs. As an example, conductive polymers and hydrogels have considerable charge storage and injection capabilities. Because of this, they are commonly used in electrostimulation electrodes,[58] electrophysiological recording electrodes, and nano-percolation networks that maintain statistical stability of impedance under deformation conditions.[44,60] In terms of structural design, such as mechanical sensor microstructure, to improve the functionality and sensitivity of the device (Figure 3C and 3D),[61] the combination of electroactive polymer (EAP) actuator and hydraulic structure can improve actuation capability[62,63] as the structural design of a pneumatic chamber will achieve the pre-defined actuating motion (Figure 2I and 2J).[64–66] In addition, self-healing can be achieved by leveraging the bonding properties of the relevant materials,[67] while biodegradability is mainly achieved by using inherently unstable materials.[68,69] Notably, 3D flexible electronics maintain conductivity while gaining an extra dimension and thus more sophisticated functions that 2D electronics do not offer (Figure 3E and 3F).[70–72]
2.4. Comfort and convenience
For wearable devices, biocompatibility also includes comfort and convenience, as the user needs to wear the device for long periods of time. Generally, porous structures have a high stretchability and breathability (See Figure 3G and 3H), which can facilitate conformal and comfortable attachment to the skin.[73] Moreover, textiles, the most common structure in our clothes, are also naturally porous due to the space between the fibers and yarns.[74] Due to their breathability, these aforementioned 2D porous materials facilitate the outflow and evaporation of sweat and are not only comfortable to wear but also do not cause much skin irritation.[75] In addition to breathability, lightweight is also an attractive feature of flexible devices. Unlike rigid devices based on bulky metal and silicon materials, flexible devices made of plastic and rubber are usually lighter. For example, using flexible lightweight materials, a prosthetic system weighing 300 grams was developed that mimics a commercially available prosthetic device that weighs more than 400 grams.[76] The benefit of lightweight devices is that they provide a more labor-saving feeling for better user comfort. Furthermore, the transparency based on polymers, or nanomesh materials, also contributes to greater aesthetic properties of flexible devices.[77,78]
2.5. Discussion
Collectively, research into materials and structures is fundamentally driving the growth of flexible electronics. It is important to note that most of the materials and structures discussed are versatile and can have multiple roles in prosthetics and rehabilitation robotics such as sensing and recording, computing and storage, as well as actuation and stimulation. The current challenges and potential future directions of materials and structures in flexible electronics include: 1) Although most studies mention durability and functionality, for many devices this is just a theoretical prediction or proof of concept. 2) Compared to rigid devices, the manufacturing precision and integration density of flexible devices are still in their infancy. This places a high requirement on the machinability of materials and structures to ensure flexibility. 3) The manufacturing cost of materials and structures is the key in determining the device’s price. Many materials and structures are already costly for proof of concept in the lab. For mass production, low-cost solutions need to be continuously explored.
3. Sensing
In the human body, the skin protects internal tissue from damage[79] while transmitting abundant external information to the brain through various high-density subcutaneous receptors.[80] In this way, the skin acts as a platform for the embodiment and extraction of internal and external sensations, such as physical (pressure and stretching),[81,82] chemical (perspiration),[83] and physiological (respiration and pulse)[84,85] information.
In medical robotics, e-skin presents itself as an optimal replacement for human skin to collect external and internal information from a patient. In the past decade, various flexible e-skin sensors that mimic the functions and features of human skin have already been integrated onto medical robotics to sense external information[86] for rehabilitation applications.[87] In rehabilitation, the absence of supervision may lead to incorrect patient posture, which reduces the efficiency of rehabilitation and can even aggravate a disability.[88] Collecting information from the patient during rehabilitation, specifically strain[89] and pressure,[90] can inform the therapist in real-time about the accuracy of the movement as well as the physiological status. At the current stage, e-skin can mimic or surpass human skin performance in mechanical and thermal sensing (as shown in Table 1) and functionalities in terms of various chemical,[91] physiological, and proximity sensing[92] to deliver accurate and diversified information to users. In such scenarios, e-skin sensing can achieve autonomous electronic rehabilitation by monitoring the patient in real-time, providing a promising solution for the development of personalized rehabilitation science.[93,94]
Table 1.
Comparison of key sensing performance of natural skin and artificial skin.
| Limit of detection | Sensitivity pressure | Spatial resolution | Response time | Sensitivity (temperature) | Mechanical property | Refs. |
|---|---|---|---|---|---|---|
| 1 mN[86] | 0.078–0.018 kPa[95] | 1 mm[96] | 15 ms[86] | 20 mk[97] | Stretchable ~30%[95] | Human skin |
| 0.08 Pa | >220 kPa−1 | 50 μm | 9 ms | NA | Flexible | Bai et al.[98] |
| NA | 0.01 kPa−1 | 0.1 mm | 15 ms | NA | Flexible | Yan et al.[99] |
| 1 ug ~ 1.25 Pa | 4.4 kPa−1 | 5 mm | NA | NA | Flexible | Wu et al.[100] |
| 0.3 Pa | >5000 kPa−1 | 1000 DPIa | <1 ms | NA | Flexible | Lee et al.[101] |
| <0.5 Pa | 192 kPa−1 | 0.8 cm | 10 ms | NA | Flexible | Zang et al.[102] |
| NA | NA | 5 mm | NA | 20 mk | Flexible | Yokota et al.[103] |
| 7.3±1.2 Pa | >1.25 MPa−1 | 2 mm | NA | 2410 ppm °C−1 | Stretchable ~800% | Hua et al.[104] |
| 10 Pa | ~1.78×10−3 kPa−1 | 318 CPIb | 32 ms | 3×10−4 °C −1 | Flexible | An et al.[105] |
| NA | 0.41% kPa−1 or 0.075% kPa−1 | 0.5 mm | NA | ~0.01 °C −1 | Stretchable ~20% | Kim et al.[106] |
| 100 Pa | 28.9 kPa−1 | 1 mm | 40 ms | <0.1 K | Flexible | Zhang et al.[107] |
DPI, dots per inch
CPI, capacitors per inch.
3.1. Pressure sensing
There are many e-skin pressure sensing mechanisms that have been widely and reliably adopted in medical robotics.[108–110] Several of these exemplary principles (such as piezoresistive, piezocapacitive, piezoelectric, piezoionic, and more) are discussed below alongside their applications in prosthetic sensing and rehabilitation health monitoring.
3.1.1. Pressure sensing mechanisms
Conventional static force-sensing mechanisms in e-skins mainly consist of resistive and capacitive sensing. Due to its simple mechanism and convenient data collection strategy, resistive pressure sensing has been extensively applied using various recording mechanisms.[111] Bulky piezoresistive e-skins, like sponge-based sensors, rely primarily on pressure-induced changes in the number of conductive pathways[112] or in the shape of the sensing material.[113] Meanwhile, another resistive sensor may analyze the changes in the contact resistance, like the quantum tunneling effect (Figure 4A).[114,115] This type is much more sensitive and thinner than the bulky option. An e-skin based on bilayer microdome arrays can use microstructures to maximize the changes in surface contact resistance based on the tunneling effect (Figure 4B).[115] Nevertheless, some drawbacks, like large hysteresis,[116] large confounding temperature sensitivity,[117] and varying pressure sensitivity[118] have limited the performance of resistive sensors. Compared with resistive sensors, capacitive sensors have excellent linearity with lower power consumption.[119] For piezocapacitive e-skins, capacitance changes are mainly based on the deformation of the dielectric layer. Normal pressure and tangential strain can be measured through a capacitive sensor as the dielectric layer can be deformed under tensile and external pressure (Figure 4C). As demonstrated in Figure 4D,[120] a capacitive pressure sensing array with cross-arranged electrodes was fabricated using CNTs as the electrode material and PDMS as the dielectric layer.
Figure 4.
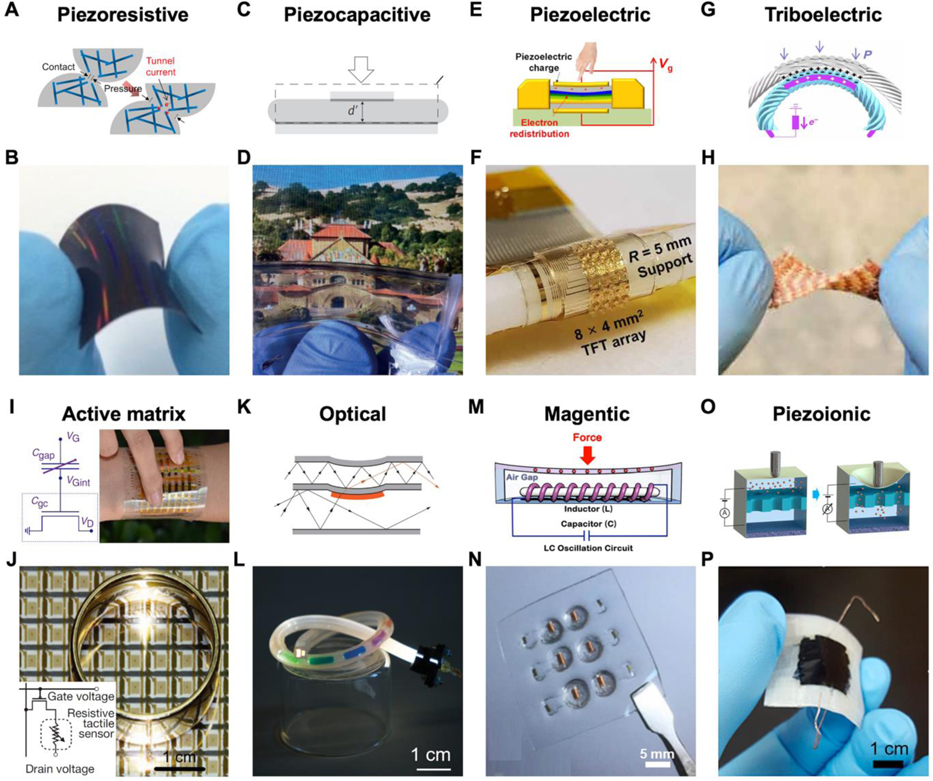
Sensing mechanisms and examples of flexible pressure sensors. A) A piezoresistive mechanism. B) A giant tunneling effect-based piezoresistive pressure sensor. A,B) Reproduced with permission.[115] Copyright 2014, American Chemical Society. C) Piezocapacitive mechanism. D) Transparent piezocapacitive sensors. C,D) Reproduced with permission.[120] Copyright 2011, Springer Nature. E) A piezoelectric mechanism. F) Scalable tactile sensor arrays with piezoelectric materials in active matrixes. E,F) Adapted with permission.[124] Copyright 2020, AAAS. Reprinted/adapted from ref.[124]. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC). G) Triboelectric sensing mechanism. H) Triboelectric mechanism-based textile for pressure sensing. G,H) Adapted with permission.[127] Copyright 2020, American Association for the Advancement of Science (AAAS). Reprinted/adapted from ref.[127]. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC). I) Piezocapacitive active matrix. Reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International license.[102] Copyright 2015, The Authors, published by Springer Nature. J) Piezoresistive active matrix. Reproduced with permission.[48] Copyright 2013, Springer Nature. K) Optic pressure sensing mechanism. L) A stretchable multifunctional fiber-optic sensor. K,L) Reproduced with permission.[136] Copyright 2020, AAAS. M) A magnetic mechanism. N) A tactile sensor based on GMI material. M,N) Reproduced with permission.[100] Copyright 2018, AAAS. O) Piezoionic mechanism. P) A patchable pressure sensor mimicking ion-channel-engaged sensory organs. O,P) Reproduced with permission.[145] Copyright 2016, American Chemical Society.
Piezoelectric and triboelectric have their unique advantages in dynamic force measurements, i.e. high-frequency measurement, and self-power supply.[121–123] As an example of piezoelectric sensors, a dual-gated ZnO-based thin-film transistor (TFT) was fabricated (Figure 4E)[124] where external pressure causes noticeable voltage changes in the flat-band of the device (Figure 4F). In practice, this sensing mechanism is well-suited for HMI’s high-frequency environment, such as the detection of surface textures by robotic hands (high-frequency vibrations) and [125] wearable pulse monitors.[126] A representative example is shown in Figure 4G and 4H, where an all-textile triboelectric generator (TENG)-based machine washable sensor array was woven into different parts of a vest to enable measurement of pulse and respiratory waves.[127] Nonetheless, the major drawbacks of these two sensing mechanisms are the unreliable static sensing performances and drift in long-term measurements.[108]
Achieving a high integration density of sensing arrays is dependent on the fabrication process, materials, and the data acquisition method.[128] Meanwhile, the high integration density of the electronics easily leads to crosstalk and interference of the signals. An attractive approach to address this issue is to have transistors that maintain the sensing unit state integrated around the device to control and amplify the signal, namely the “active matrix”.[129] The previously mentioned resistive, capacitive, and piezoelectric cells are all capable of being integrated into active matrices. As shown in Figure 4I, capacitive integration uses primarily pressure-sensitive capacitors directly as gate capacitors, where the change in capacitance is translated into a variation in drain current in the field-effect transistor (FET)-based device.[102,130–132] On the other hand, piezoresistive sensors can be connected to the source or drain of the FET, where the drain current of the FET varies with the applied pressure (Figure 4J).[48,77,133–135]
Other pressure sensing mechanisms, like optical,[136–138] magnetic,[99,139] and piezoionic,[140–142] are emerging and accelerating the growth of the pressure-sensitive e-skin field. Optical pressure sensors rely on the differences in optical signal between the electromagnetic emitter and receiver, where an external force can deform the light guide (transmission medium) and alter the optical signal received (Figure 4K). By exploiting this principle, Figure 4L demonstrates a stretchable distributed fiber-optic sensor that can simultaneously monitor real-time deformations such as bending and pressing.[136] In addition to optical methods, magnetic sensors are also suitable for high-resolution pressure perception.[143] A pressure sensor was recently developed using soft materials that contain magnetic particles, where the external force applied changes the uniformly distributed magnetic particles and changes the internal magnetic field in the device.[99] As another example, Figure 4M and 4N illustrate a GMI-based pressure sensor with a high sensitivity to force stimuli.[100] Some innovative e-skins based on the piezoionic mechanism have been proposed.[140,144] The mechanical deformation of the e-skin can be indicated by a change in voltage or current due to the redistribution of ions with different mobility (Figure 4O). As demonstrated in Figure 4P, e-skins can utilize the piezoionic principle to measure the applied force by detecting the current between the nanopore membrane.[145]
3.1.2. Pressure sensing in prosthetics
Most existing commercial prostheses lack sensory capabilities and prevent amputees from actively and passively perceiving external information, which dramatically diminishing the user's ownership of the prosthesis. Flexible pressure-sensitive e-skins can be integrated on prostheses to provide sensory functions by transducing mechanical information to electrical signals. Passively detecting various external signals and actively sensing the state of the grip grant amputees the ability to perceive environmental stimuli and self-position their limbs, thus bringing revolutionary functionality to prosthetics.[146]
Physical integration of the e-skin with the prosthetic is the first and most basic step in establishing the human-machine interface. However, integrating e-skins may limit their sensing performance. One fundamental problem lies in the mechanical attachment of the e-skin to the complaint 3D shapes of artificial limbs, which leads to strain interference in sensor performance. Assorted tactics have been reported to mitigate bending-induced parameter variations from materials and structural design aspects.[48,77] From functional, durable, and aesthetic perspectives, conformal integration should be the first consideration, where most e-skins are attached directly and seamlessly to the prostheses’ surface using adhesives.[139,147] Others like the fabric e-skin can be conformally attached to the surface of the robotic arm using a computer-aided design and weaving technology. (Figure 5A).[148] In an alternative integration example, mold methods can transform the complex geometry of prostheses into relatively regular shapes that are smoother for e-skin installation.[112,149] Mentioning precision and quickness, fabricating flexible e-skins on robotic surfaces through optical scanning to model and 3D printing is another potential method for personalized integration.[150,151] Not as sophisticated as the above solutions, direct sprayed-on or coated integration are simple methods. As a model case, an e-skin named “Electrick” fabricated by simple coating technologies was reported. After spraying conductive materials and integrating electrodes onto the object, electric field tomography was performed to detect the shunting current between the user’s finger and sensors for touch sensing.[152] Researchers have also reported options for prostheses with flexible sensors embedded in the manufacturing process,[153,154] which solves the problem of surface mounting, but also presents a replacement challenge.
Figure 5.
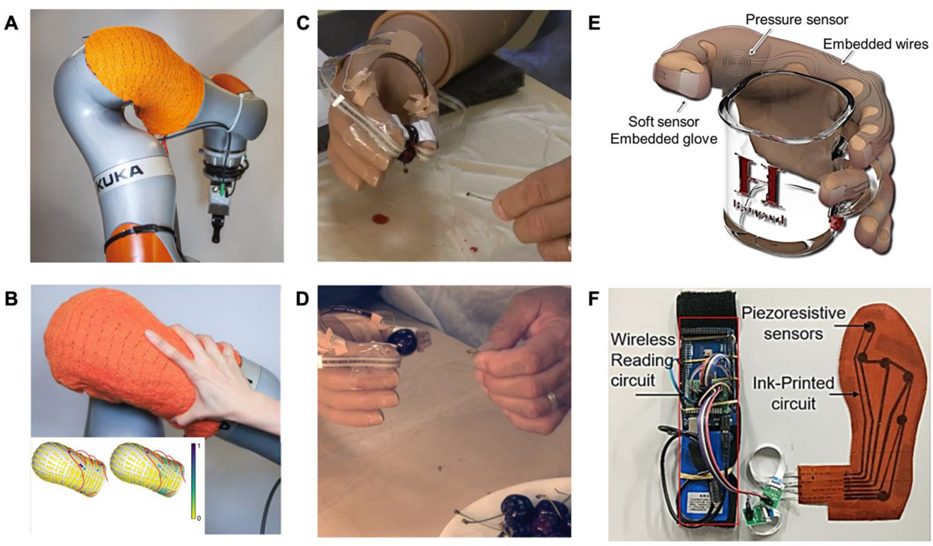
Applications of flexible pressure sensors in medical robotics. A) Pressure-sensitive textiles integrated on a robotic arm. B) Demonstration of human touch on the arm and corresponding pressure mapping. A,B) Reproduced with permission.[148] Copyright 2021, Springer Nature. C) A cherries’ stem pulling experiment for illustrating the importance of tactile sensing ability in active prosthetic control. Without tactile feedback, the subject removed the stem but crushed the cherry attribute to unstable force control. D) Subjects wearing an artificial hand with tactile sensing ability removed the stem without damaging the cherry. C,D) Reproduced with permission.[157] Copyright 2014, AAAS. E) A flexible pressure sensor for detecting grasping force. Reproduced with permission.[162] Copyright 2014, IEEE. F) Smart insoles to monitor plantar pressure. Reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International license.[163] Copyright 2019, The Authors, published by MDPI.
Daily interactions between amputees and the environment in the form of touch and collisions require passive pressure sensing. For example, coverage of piezoresistive textiles on a robotic arm can provide a spatial map of pressure distributions (Figure 5B).[148] Compared with passive perception, active sensing refers to amputees detecting unstructured environments with prosthetics subjectively, such as identifying the stiffnesses and texture of objects, user-interaction with people, and grasping.[155] As an example, parallel ridges were fabricated on the surface of a poly(vinylidene difluoride) (PVDF)-based piezoelectric sensor to detect texture-induced vibrations.[156] Furthermore, adding e-skins on prosthetics creates a closed-loop sensory feedback control, which allows for a more accurate, facile, and compliant touch and grasp. In an example, sensory feedback control was demonstrated by pulling the stem from a cherry (without crushing it) using an artificial limb, which requires facile control through relayed feedback of the grasping force (Figure 5C and 5D).[157] Collectively, with the assistance of artificial skin, active and passive interactions make up the prosthetic pressure perception.
3.1.3. Pressure sensing in rehabilitation
Pressure monitoring is essential for rehabilitation, especially for extremities recovery and small pressure capture in health monitoring (heartbeats, pulse, etc.). Eg. fingertip and palm pressure feedback can inform the user about their gripping force for stroke patients to regain accurate grasping ability.[44,158–161] A liquid metal-based pressure-sensitive glove was used to train a subject to relearn a specific manual skill by providing real-time pressure monitoring (Figure 5E).[162] In another application, the medical importance of plantar pressure monitoring in patients with lower extremity disabilities are tremendous. Through analyzing the pressure concentration points on the foot, the gait phase can be identified for real-time correction of improper movements in rehabilitation.[163] Additionally, the imitation of human gait also facilitates the adaptability and compliance of bionic prosthesis to the human body.[164,165] In one example, a pressure-sensitive insole with an inkjet printing circuit and sensing units made of CNT embedded in PDMS was fabricated for detecting plantar pressure. These pressure data can be used to train ML algorithms to classify human gait phases (Figure 5F).[163] In rehabilitation, micro-vibrational physiological signals, such as a heartbeat[166,167] and pulse,[168,169] can provide information on the body's status and load, allowing for the immediate adjustment of the rehabilitation intensity and planning. Moreover, pressure monitoring of bedridden patients or prosthetic sockets can also effectively prevent skin ulceration due to prolonged pressure.[170,171]
3.2. Strain sensing
The mechanism for strain and pressure sensing overlap in many ways. However, there are still many differences in applications that are worthy of further consideration and discussion, especially for the detection of human and prosthetic postures.
3.2.1. Strain sensing mechanisms
The mechanisms of pressure sensors mentioned above, like resistive, capacitive, and optical, etc.,[172] can also be applied to flexible strain sensors. It is worth noting that some microscopic mechanisms in strain sensing, like misplace,[173,174] disconnection,[175] and crack propagation[168,176] are not available in pressure sensing. The most widely accepted capacitive structure for strain sensors is the “sandwich” structure. Inherent stretchable polymers are attractive materials for dielectric layers due to their stretchability and permittivity. Nanomaterials[177,178] and liquid metals[179] can be used as electrodes owing to their stable conductivity under tensile strain. Piezoelectric and triboelectric strain sensors are two other major types of stretchable strain sensors.[180–182] Piezoelectric materials, like BaTiO3,[183] zinc oxide (ZnO),[184] PVDF,[185,186] can be utilized to fabricate strain sensors by converting strain readings into voltage signals. Similar to the mechanism behind pressure sensing, flexible waveguides[136,153] and magnetic materials[187,188] have also been investigated for wearable strain sensing applications.
3.2.2. Strain sensing in prosthetics
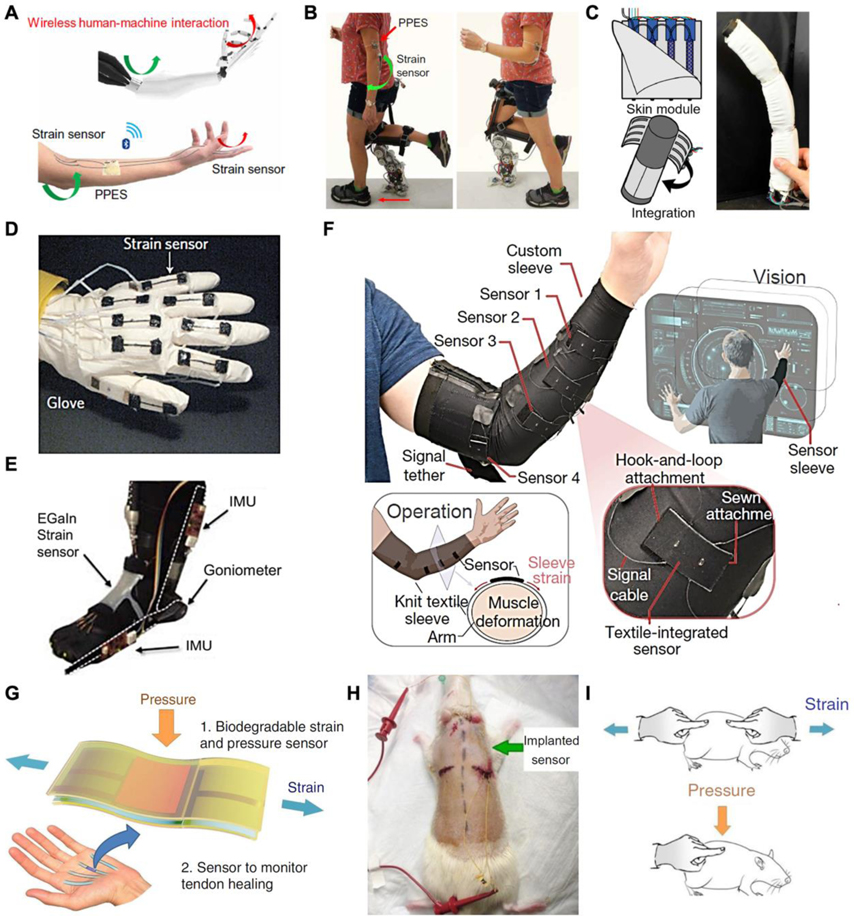
Flexible strain gauges are commonly used in prosthetic control and proprioception. Prosthetics can be controlled by neural electrophysiological signals (will be further discussed in section 4) and mechanical signals such as gestures[189,190] or body movements.[191] By using simple and accurate signal gathered from non-invasive and visible sensors, gesture control strategy has become an intuitive and reliable control method for prosthetics.[146] Flexible strain sensors prove to be promising alternatives to monitor these mechanical signals owing to their stretchability, durability, and lightweight nature. As shown in Figure 6A, a biofuel powered strain sensor was integrated onto a human elbow to capture strain signals. By utilizing the strain signal, the subject successfully controlled the prosthetic to assist walking in real-world environments (Figure 6B).[192] Tiny stretching of the skin, such as muscle contractions and relaxation (i.e. mechanomyography) can also be detected by flexible strain gauges[193–195] or pressure-sensitive units[196] for prosthetic control.
Figure 6.
Applications of flexible strain sensors in medical robotics. A) A perspiration-powered integrated e-skin (PPES) and strain sensor. B) The use of PPES and strain sensor for prosthesis control. A,B) Reproduced with permission.[192] Copyright 2020, AAAS. C) A robot skin with integrated strain sensors and actuators that can turn objects into soft robots. Reproduced with permission.[200] Copyright 2018, AAAS. D) A data glove using stretchable strain sensors. Reproduced with permission.[175] Copyright 2011, Springer Nature. E) A liquid metal-based strain sensor for detecting ankle angles. Reproduced with permission.[201] Copyright 2011, IEEE. F) Demonstration of a textile-based strain sensor-integrated sleeve to detect hand motion. Reproduced with permission.[194] Copyright 2020, Springer Nature. G) An implantable strain sensor can be attached to the tendon for real-time healing assessment. H) The strain sensor implanted subcutaneously on the back of a rat. I) External strain and pressure applied on the sensor. G-I) Reproduced with permission.[204] Copyright 2018, Springer Nature.
Besides pressure sensing, proprioception is another crucial perception for prosthetics, which allows the amputee to directly feel the posture of the device. In particular, flexible strain sensors can sense the stretching and relaxation of prosthetics like the human body's mechanoreceptors located on the muscles, skin, and tendons.[197] Commercial prosthetics are mostly driven by motors, where the angle encoder inside the device can accurately reflect the movements. Soft actuators lack this ability because of their continuous mode of motion, making the application of flexible strain sensors on soft actuators extremely attractive.[37,198,199] A noteworthy example to detect bending angles, an essential part of its self-perception, is flexible capacitive sensors alongside soft actuators. This hybrid robotic skin can turn inanimate objects into soft robots by estimating their current position. As shown in Figure 6C, to demonstrate the proprioceptive ability, the bend angle of a foam integrated with the robotic skin was calculated from the dimension of the device and the electrical signals.[200] Therefore, the combination of soft actuators and flexible sensors is a promising direction for future soft robotic systems.
3.2.3. Strain sensing in rehabilitation
For rehabilitation, the rotation of the joints, the contraction and relaxation of the muscles, and the natural breath cause strain on the human skin and are essential information to record. A healthy hand allows for dexterous manipulation. In terms of rehabilitation, the angle of the hand joints monitored by the sensors can be used as a basis for measuring movements, which provides rich data for hand rehabilitation. As shown in Figure 6D, a stable flexible strain gauge based on the “island and gap” structure of aligned CNT thin films was recently demonstrated.[175] Using this strain gauge for hand rehabilitation, a data glove can be fabricated to accurately detect the motion of each finger individually. In gait rehabilitation, ankle angles are essential physical information for orthotic devices to address pathological gaits. Figure 6E presents an EGaIn based-strain sensor that can be applied on top of the ankle to measure the joint’s angle to provide feedback on the foot’s motion during rehabilitation.[201] Unlike large strain measurements of joint angles, measuring the strain from small muscle deformations requires a high sensitivity. Figure 6F demonstrates an ultra-sensitive and resilient strain-mediated contact in anisotropically resistive structures based on a compliant strain gauge. To verify the effectiveness of this sensor, researchers fabricated a textile-based sensor that was integrated into the sleeve to detect small muscle deformations and classify hand and wrist movements.[194] On the skin, respiratory waves and pulses can also contribute to slight stretches, which can be captured by sensitive strainers for health monitoring during rehabilitation.[94,202,203]
In addition to monitoring external strain on the human skin, recently in vivo strain sensing has been investigated to monitor inner tissue, e.g. tendons and muscle recovery, by continuously providing real-time and long-term information for rehabilitation surveillance.[179,204] As shown in Figure 6G, an implantable capacitive multifunctional sensor was designed to measure pressure and strain signals under the skin. After being implanted on the back of a rat (Figure 6H), the in vivo sensor can accurately and stably detect strain and pressure signals applied on the implanted region (Figure 6I). This device was fabricated with biodegradable materials, which avoids the need for surgical extraction.[204] The design of these strainers provide a new paradigm for in vivo biomechanical measurements.
3.3. Thermal sensing
As temperature is another dimension of physical information beyond mechanical perception, mimicking thermoreceptors on the human skin is vital for the sensory function of prostheses. Currently, some temperature-sensitive e-skins can outperform the human skin in terms of sensitivity, accuracy, and detection range.[205,206]
3.3.1. Thermal sensing mechanisms
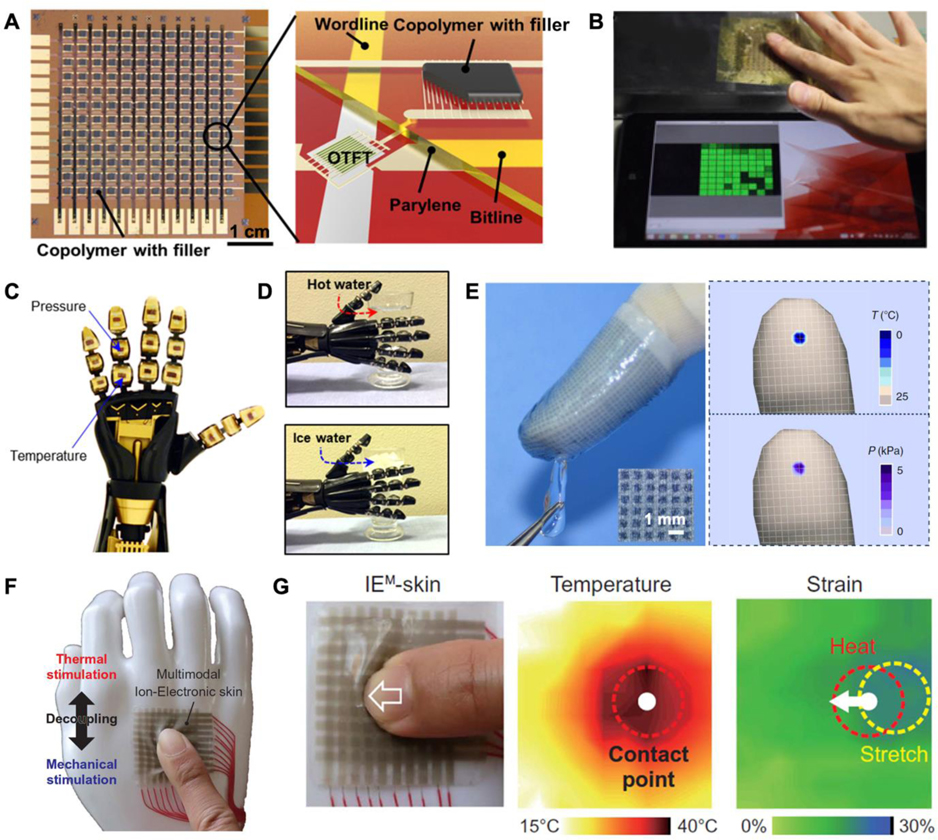
Resistive metallic temperature sensors[207] and thermistors[208] are the most commonly used sensors in flexible thermal electronics. The mechanism of resistive metallic temperature detectors relies on the linearity between resistance and temperature found in metals.[209] Thin metal films, like Au,[210] Pt[211] are widely employed in constructing flexible temperature detectors due to their good linearity and proven success in their microfabrication technologies. Unfortunately, the sensitivity of many metal materials can be a significant drawback for temperature sensing applications. Thermistors are another common class of resistive temperature sensor and can be categorized into nonlinear positive temperature coefficient (PTC) and negative temperature coefficient (NTC) sensors. In the PTC type, fluctuations in the temperature alter the specific volume of the material, which in turn affect its resistance and current measured. Conductive nanomaterial-filled polymers[212,213] are a promising candidate for flexible PTC temperature sensors, where the volume changes as the material progresses through the melting point of crystalline regions, thus affecting the resistance.[214] For measuring spatial temperature gradients, an organic active matrix with polymer PTC sensor pads was fabricated on a PI substrate, which exhibits a bending insensitivity and high spatial resolution (Figure 7A and 7B).[103] The negative temperature coefficient (NTC) thermistor has a clear advantage over other temperature sensors in that it exhibits a much simpler structure while presenting equivalently high temperature sensitivity.[215]
Figure 7.
Temperature sensors and applications in medical robotics. A) A PTC temperature sensing array. B) Temperature mapping after touching the sensing sheet. A,B) Reproduced with permission.[103] Copyright 2015, The Authors, published by National Academy of Sciences. C) A prosthetic hand integrated with pressure and temperature sensors on different regions. D) The robotic hand touching and identifying hot and cold cups. C,D) Adapted with permission.[222] Copyright 2017, AAAS. Reprinted/adapted from ref. [222]. The Authors, some rights reserved; exclusive licensee AAAS. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC). E) An e-finger assembled with flexible dual-parameter temperature–pressure sensors touching an ice cube and corresponding photograph temperature and pressure mappings. E) Reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International license.[107] Copyright 2015, The Authors, published by Springer Nature. F) A multimodal ion-electronic skin attached to a dummy hand and its temperature/strain sensor responses under a weak unidirectional shear. G) Photo of finger press and corresponding schematic of temperature and strain variation. F,G) Reproduced with permission.[230] Copyright 2020, AAAS.
In addition to resistive temperature sensors, thermocouples and PN junction sensors are also two common thermosensing mechanisms. Thermocouples can generate a potential difference under different temperature between different materials based on the thermoelectric (Seebeck) phenomenon.[216] Whereas the forward voltage in PN junction temperature sensors can vary with temperature, converting the heat into electrical signals in the diodes and transistors. The advantage of this method is its small size, fast response time, and good linearity.[106,217] Moreover, some optical temperature sensors use infrared (IR) thermography[218] and colorimetric techniques with thermochromic liquid crystals that can be used to record the temperature more intuitively[219] and have already been applied to measure the temperature of the human body. Some remarkable designs based on biomaterials and structures have superior temperature response properties, offering another method for thermal perception.[97]
3.3.2. Thermal sensing applications
Thermal perception for prosthetics can complement pressure sensing for extracting more tactile information from the environment, which not only informs the amputee about the environment thermographically, but can also prevents the user from danger or potential device failure due to extreme heat or cool.[220,221] Figure 7C shows an intrinsically stretchable rubbery electronic, which can be used to fabricate thermistors.[222] Researchers demonstrated the sensor’s functions by using a robotic hand equipped with e-skin to grab and measure the temperature of different cups. (Figure 7D) One important lesson learned from studying the natural perception of human skin is that the identification of materials is inseparable from accurate temperature perception. As a heat source and sensor, the human hand can effectively perceive the heat dissipation ability for fluid flow rate sensing[223] and identifying the thermal characteristics of surface materials.[224] One such example used an artificial fingertip with e-skin to acquire information about the thermal properties and surface texture of different materials. Using machine learning, the combination of vibrational and thermal information was used to identify the group (e.g. wood, metal, plastic) and type (e.g. aluminum, copper, pine, etc.) of the material.[224] In medical rehabilitation, temperature sensing is commonly used to monitor the body, which can be a reference for health.[225,226]
In the field of medical rehabilitation, monitoring body temperature at different locations can reflect the body's state (both physiological and psychological) in real time. For example, the potential fatigue and psychological tension during the rehabilitation process can cause temperature variations due to sweat evaporation.[226] In addition, some disease-induced disabilities have distinct thermal characteristics (either high or low) at the extremities due to abnormal blood circulation and metabolism state. For example, the foot temperature of some diabetic patients can be about 5 degrees Celsius higher than that of healthy people. Long-term monitoring of body temperature by using flexible temperature sensors is convenient and can effectively prevent medical abnormalities such as foot ulcers.[227]
3.3.3. Multifunctional simultaneous sensing
Simultaneous detection of thermal and mechanical signals is challenging due to the coupling problem; however, it is possible. Five mainstream existing multisensory (mechanical and thermal) detection modes have been developed. The most conventional multisensory model is for temperature and pressure sensors to be placed on different parts of a prosthesis (Figure 7C and 7D).[222] Integrating the two sensors into one substrate would provide a higher spatial resolution.[104,105] Nonetheless, both types mentioned above cannot intrinsically measure two signals simultaneously at one point, resulting in a waste of valuable space at key locations (like fingertips). The development of novel dual-parameter sensors that transduce different stimuli into separate signals can minimize signal interference, allowing the detection of both temperature and pressure at a single point without decoupling analysis.[107,228,229] In Figure 7E, by taking advantage of independent thermoelectric and piezoresistive effects, a dual parameter device can simultaneously transduces temperature and pressure stimuli into separate electrical signals.[107] Another potential solution is to measure pressure and temperature in a single parameter, which is more efficient data collection. The first stretchable e-skin that decoupled temperature and strain in a single parameter is shown in Figure 7F.[230] The e-skin has a simple electrode-electrolyte-electrode structure, where two variables (temperature and strain) are derived from analyzing the ion relaxation dynamics: the charge relaxation time of the capacitance as a strain-insensitive feature to measure absolute temperature, and the normalized capacitance as a temperature-insensitive extrinsic feature to measure strain. In the demonstration, it can provide real-time thermal information, force directions, and strain graphics in various tactile motions (shear, pinch, spread, torsion; Figure 7G). Another promising multimodal detection method based on machine learning is the “cross-reactive” sensor matrix. Instead of responding to certain specific stimuli like conventional “lock and key” sensors, this sensor has the ability to respond to a wide range of stimuli. Utilizing machine learning methods to directly analyze the coupled multimodal data, these devices can achieve a certain degree of decoupling. This approach greatly reduces the complexity of the sensor mechanism and structure.[231] These dual-monitoring devices greatly enriches the external information that can be acquired by prosthetic haptics.
3.4. Chemical sensing
Although physical sensing still dominates the field, the past decade has seen an exponential growth in the exploration of molecular detection, which provides another dimension for human understanding of their own health condition and external environment.
3.4.1. Chemical sensing mechanisms
For chemical detection, the mainstream detection routes are electrochemical and optical detection. Electrochemical methods are attractive due to their high sensitivity, low response time, and long-term stability.[232] Electrochemistry has several main regimes for chemical detection: the amperometric approach (including voltammetry and chronoamperometry)[91] based on redox reactions, the potentiometric approach (based on the Nernst equation),[91,233] and electrochemical impedance spectroscopy (EIS) based on analyzing surface properties changes caused by affinity binding.[234] In addition to conventional electrochemical biosensing techniques, the transistor-based approach, including field-effect transistors (FET) and organic electrochemical transistors (OECT) also show great promise owing to their ability for in situ amplification of the detected signal.[235,236] For optical methods there are also two prevailing techniques. One is based on the colorimetric method, whose principle and structure are simple, but more difficult to reach high sensitivity. In recent years, with the popularity of smartphone cameras, the quantitative measurement of color is highly attractive for in-home monitoring of optical sensors.[237] The last method is the fluorescence approach, where the wavelength of the light emitted is larger than the wavelength received, which is more sensitive and suitable for trace substance measurement.[238]
3.4.2. Chemical sensing applications
Chemical sensors for prosthetic and robotic applications have been less investigated than mechanical and thermal sensors. From a bionic perspective, human skin does not have accurate chemical sensing capabilities, but rather uses mechanoreceptors to sense chemical contact and nociceptors to perceive chemical damage. However, there are enormous quantities and types of chemicals in the environment (e.g., gases, food, toxins, etc.), where rapid and accurate identification of these substances could provide the user with essential information about the environment. Thus, adding chemical sensing capability to prostheses can prevent users from being exposed to harmful chemicals, such as organophosphate pesticide residues in agricultural products, and subsequently reduce the potential risk of harm.[239] Moreover, some researchers have also proposed the employment of chemosensing in daily diets.[91] Furthermore, the chemical and biomolecular information collected by the e-skin from the human body could greatly benefit the design of next-generation HMI toward personalized robotic rehabilitation.
Although chemical sensing is not sufficiently developed for prosthesis, in the field of health monitoring, sweat chemical sensing (including electrochemical and optical modalities) is well-studied and highly relevant to rehabilitation exercises.[240] Sweat detection can respond in real-time to changes in the volume and rate of sweat loss,[241,242] as well as the concentration of various ions and metabolites in the body.[45,243–245] In this regard, sweat can potentially communicate to the user about health and rehabilitation progress, informing the user when to rehydrate and replenish electrolytes. Moreover, some diseases of the locomotor system provoke abnormal elevations of metabolites and biomarkers, such as L-dopa in Parkinson’s disease[246–248] uric acid in gout,[45] which are very important evaluation factors. In addition to monitoring patient fatigue, sweat sensing can also be used for psychological applications by monitoring the level of stress hormones such as cortisol and norepinephrine.[249,250] In this promising field, many in-situ, multi-channel devices are emerging.[251–253] Recently, a fully integrated wearable sensor array for multiplexed in-situ perspiration analysis was reported, where the sodium, potassium, lactate, and glucose content of sweat can be measured simultaneously by electrochemical methods. Conventional commercially available integrated-circuit components (more than ten chips) can be applied on a FPCB to serve as data processing and transmission.[83] Integrating in-situ sensing, on-site processing, and data transmission together, chemical sensing platforms can achieve the goal of continuous, real-time sensing of ions and metabolites, which is crucial to obtain more comprehensive knowledge about a wearer’s well-being.
3.5. Other Sensing Techniques
While the skin has receptors for sensing mechanical and thermal information, there are no specific receptors that sense humidity. Rather, the human brain is able to "perceive" wetness indirectly by analyzing mechanical and thermal information (normal pressure and tangential adhesion between the liquid and the skin as well as heat conduction of the liquid).[254,255] Such mechanisms, which may require intelligent cross-sensor algorithms, are not available in the current e-skin. Existing flexible humidity sensors are mainly fabricated by accommodating different transducing materials, such as graphene, CNT, and MoS2, on flexible substrates. The change in the resistive or dielectric properties of these materials as they absorb moisture is used to reflect the humidity in the air.[256–258] Due to new sensing mechanisms, e-skin can exhibit greater functionality beyond the human skin, such as proximity[259,260] and magnetic field sensing,[261,262] which can be seen as complements to the natural skin capabilities, allowing HMI devices to obtain richer information about the external environment.
Other mechanisms of flexible health monitoring in rehabilitation, like optoelectronic[263,264] and ultrasound[265,266] devices, can also be applied to heartbeat, pulse oximetry, blood pressure, and other on-body measurements. As an example, organic flexible LEDs and photodiodes are driving the application of photoplethysmogram (PPG) in the wearable sector. PPG uses optical differences between the vascular and other tissues or oxygenated and deoxygenated hemoglobin to measure heart rate,[263] blood oxygen,[267] and other in-depth cardiovascular information.[268]
3.6. Discussion
In this section, the mechanisms and applications behind pressure, strain, temperature, and chemical sensors are discussed for human-machine interfaces in medical robotics. In many cases, the sensing capabilities of each have surpassed human skin in terms of function and performance, which is crucial for the development of artificial limbs with the same or better sensory function as human limbs. This allows the users to better perceive the information around them. Providing real-time monitoring of human motions and physiological signatures provides rich data for the field of disability rehabilitation, which is conducive to the formulation of personalized rehabilitation strategies to achieve better rehabilitation results.
Despite the rapid expansion of all these flexible sensors in the last decade, there are still many challenges to be investigated. First, flexible sensors attached to prostheses and human skin generally require compliant flexible characteristics, which places a limit on the thickness of e-skins. Normally, ultra-thin e-skins have better mechanical compliance, but their mechanical strength decreases significantly with their thickness. How to endow flexible sensors ultra-low thickness and high strength through material selection and structural design will directly determine the performance and durability of these interfaces. Secondly, fabrication of large area e-skins is challenging. Most of the existing research on flexible sensors are miniaturized proof-of-concept. Large-area fabrication must still comply with conformal design from 2D to 3D, signal crosstalk handling, the self-healing requirement, as well as the low preparation costs (time and money). Thirdly, the principles of bionics have not been fully developed and applied to electronic skin, like moisture detection. Lastly, further sensing capabilities beyond human skin still need to be explored, especially in chemical detection, as reliable chemical sensing opens up another dimension for the human to perceive the external environment.
4. Electrophysiological recording
Electrophysiological signals carry a wealth of information about the human body. One prominent parameter carried through the nervous system is movement intention. Motor intention originates from the primary motor cortex of the brain and is transmitted from the central nervous system to the peripheral nervous system in the form of electrophysiological signals, which are translated into mechanical contractions of the muscles.[269] Such bioelectrical signals propagating through the nervous system can be detected both in vivo and on the skin’s surface (using electrodes). Subcutaneous invasive electrophysiological signals can be derived through brain interfaces,[270–272] using electrocorticography (ECoG)[273,274] on the cortical surface as well as local field potentials (LFP)[275,276] extracted from electrodes inserted into the cortex. Invasive recording techniques routinely use peripheral in vivo tissues with electroneurography (ENG)[277,278] and electromyography (EMG),[279,280] which can record peripheral nerve activity and muscle electrical signals respectively. Non-invasive electrophysiological recording is also widely accepted and can be extracted on the skin’s surface. Common wearable techniques include electroencephalography (EEG),[281,282] surface electromyography (sEMG),[283,284] and electrooculography (EOG),[285] which capture the electrical activity of the brain, muscles, and eye movements respectively. Utilizing these electrophysiological signals to capture and decode the movement intention can provide a natural way to control prosthetics. Thus, the vast number of studies on different interface locations and various probing devices to record electrophysiological signals have established the foundation of user-communication with prosthetics.[24,286,287] Another popular detection technique for electrophysiological signals captured via invasive and wearable devices is electrocardiogram (ECG),[288,289] which cannot be used for prosthetic control, but does provide auxiliary information for disability rehabilitation monitoring.
There are two broad categories for electrophysiology recording - implantable (also known as sensing neural interfaces) and non-invasive (also known as wearable electrodes) recording.[290–292]. Of the two, non-invasive sensors are more extensively researched and is generally preferred by many patients. Compared to commercially available rigid electrodes based on silicon wafers and rigid packaging, flexible electrodes are also more biocompatible (details described in section 2) and can adapt to the complex tissue geometries of the human body,[293–295] such as sulci in the cerebral cortex,[296,297] bundled peripheral nerves,[298] and stretching motions on the skin.[299] In this section, we will mainly review flexible implantable and epidermal electrophysiological recorders for medical robotics.
4.1. Invasive modalities
Implantable electrodes that are embedded under the skin or inserted into the target tissue, especially around deep or delicate tissues, are more accurate than non-invasive electrodes as they obtain substantially more biological signals. Various electrodes, with different materials, shapes, and characteristics, can be places on or in the human brain, peripheral nerves, and muscles to record ECoG,[300] LFP,[301] ENG[302] and EMG[303] respectively.
4.1.1. Sensing neural interfaces
Biocompatibility is the determining factor for the safety and stability of implants in long-term chronic operation (see section 2).[304] Apart from the biocompatibility, the requirements of implantable sensing neural interfaces for good performance lie primarily in the device impedance, and hence conductivity and capacitance. As impedance adds noise to the signal, lower impedance electrodes are expected to have a higher signal-to-noise ratio (SNR) overall. Moreover, low electrode impedance combined with the distributed capacitance between the electrodes and the recording amplifier will enhance the high-frequency response performance of the electrode.[305]
Materials underlying the biocompatibility and electrical properties of the implantable devices are constantly developing. Traditional commercial implantable neural interfaces (e.g., Utah electrodes[306] and Michigan electrodes[307]) typically contain noble metals[308] (high conductivity and chemical stability), and silicon-based materials[309] (ideally suited to existing microfabrication techniques) as electrodes. With further evolution of processing technology, these materials can be fabricated into ultra-thin and ultra-fine forms, thus enabling high-density integration, as well as endowing some flexibility.[310,311] Recent advancements in soft and nanomaterials have opened up more options for flexible recording electrodes, like conductive polymers (e.g., PEDOT)[302,312] and nanomaterial composites (metal-based, CNT, graphene)[313–317]. Apart from the conductive functional materials, the insulative packaging materials are also a critical part of sensing recorders. Many popular insulating soft materials have been used for packaging sensing electrodes,[318] such as PI,[319,320] PDMS,[321,322] hydrogel,[323,324] etc., as they have suitable mechanical, dielectric and biological properties.
Recently, there has been a focus on wireless transmission of data and energy for flexible implants, which can reduce messy wires and improve user mobility and social interaction.[325] Moreover, given the damage of the surgery to the human body, fully implantable non-removable devices should be operational for a long period of time (ideally lasting a full human lifetime) to avoid the surgery associated with frequent battery replacement. Recent advances in wireless charging and self-powered technologies have been established to limit the number of times a user must undergo replacement surgery.[326] Specifically, recent studies have demonstrated the feasibility of piezoelectric,[327] near field communication (NFC),[328] and ultrasonic technologies[329] in power supply and data transmission.
4.1.2. ECoG and LFP
As invasive brain monitoring electrodes, ECoG and LFPs play an important role in examining motor intention and elucidating the fundamental pathogenesis of various neurological disorders, such as epilepsy and Parkinson’s disease.[330,331] ECoG and LFPs can be delineated depending if they are inserted into the cerebral cortex. LFP recorders mostly use microelectrodes to penetrate directly into the cerebral cortex, which capture more accurate and deeper brain signals. In contrast, ECoG electrodes are generally placed on the surface of the cerebral cortex and can collect signals without penetrating the tissue, with a larger recording area with relatively less damage to the brain.[270] Harvesting these brain signals as an information source bring three distinct advantages to HMI. Firstly, the brain acts as the source of motor intention, which is essential to reduce the delay time between conscious action and robotic actuation.[332] Moreover, the high spatial and temporal resolution of ECoG and LFPs can provide finer and more accurate control signals for prostheses as the neuronal areas can be recorded independently at a higher density.[333,334] Beyond these, as the recorders directly interface with the brain, they circumvent the communication and control channels in peripheral nerves and muscles, which is of great significance for patients with damaged peripheral nervous systems or other severe spinal cord injuries.[335]
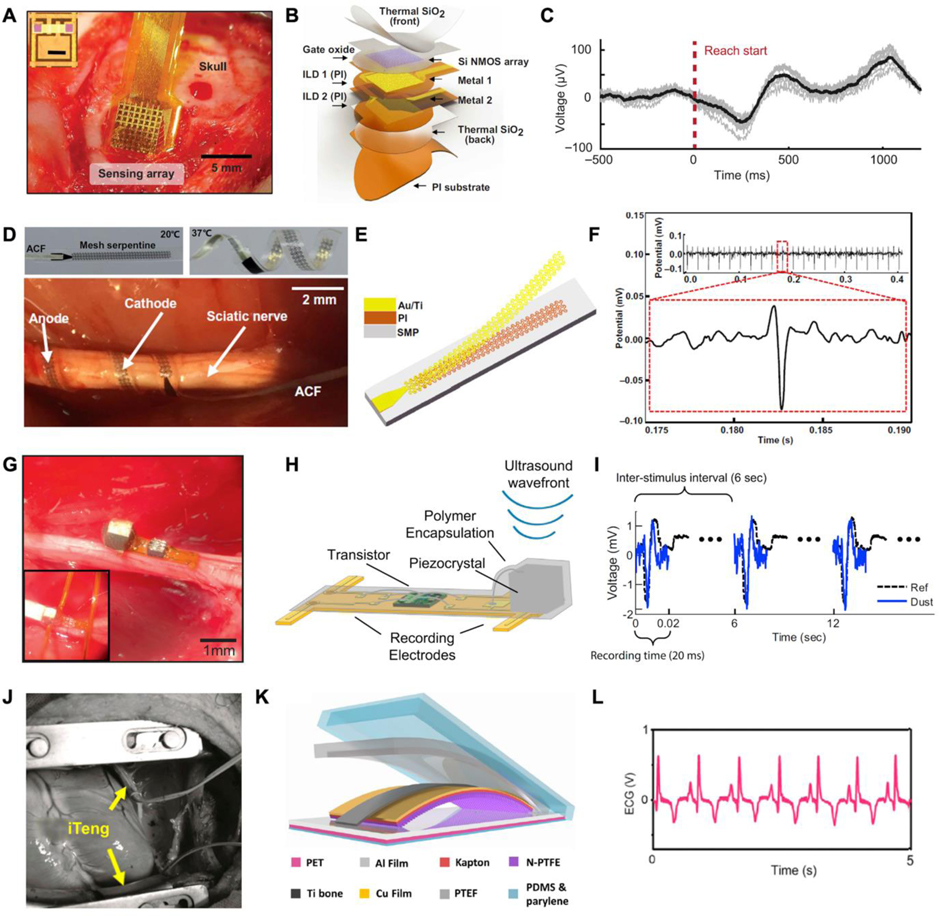
From the perspective of information richness, it is apparent that a one-channel recording neural interface is not the best choice, especially for brain-machine interfaces (BMIs). Thus, neural interfaces are rapidly evolving in two conceptually different,[304] but complementary,[336] directions (high integration[337] and flexibility[290]). Flexible electrode array is an attractive approach to combine high density and flexibility, increasing data collection. Conventional BMIs with probe-like morphology for LFP recordings are generally small and rigid.[338] As materials and manufacturing processes evolve, more micro-wires are becoming flexible alternatives to microneedle arrays, which are able to remain relatively stationary as the brain moves, improving signal stability while reducing tissue damage.[339–342] An exemplary case is a flexible filamentary bioinspired neuron-like electronic, which consists of a polymer-metal-polymer structures. The bending stiffness of this implant is comparable to that of a neuron’s axon, enabling biocompatible high-resolution LFP recording.[310] Referring to another brain (ECoG) recording, ultrathin film electrode arrays are a promising and widely adopted option for electrodes used on the surface of the cortex, owing to its high recording density in a non-penetrating fashion.[296,343–346] With this format, a multiplexed neural interface with capacitive electrodes incorporates high spatial resolution with long-term temporal mapping capabilities on a thin PI substrate (Figure 8A). In this device, the thermally grown silicon dioxide (t-SiO2) serves as a biofluid barrier as well as a dielectric medium, providing both encapsulation and capacitive functions (Figure 8B). To verify the feasibility of the device, the arrays were implanted over sensorimotor cortices in monkeys, which presents a stable long-term recording. (Figure 8C).[347] As another representative type of flexible structures, the mesh structure also distinguishes it for both inserted and superficial neural implants owing to its stretchability and adaptability.[301,348–350]
Figure 8.
Implantable electrophysiological recorders. A) A neural interface was implanted epidurally over rat’s cortex to record μECoG. B) Exploded view of the high-definition neural interface for μECoG mapping. C) Corresponding recorded μECoG signals. A-C) Reproduced with permission.[347] Copyright 2020, American Association for the Advancement of Science. D) Bipolar twining electrodes integrated on the sciatic nerve for ENG recording. E) Layout of the twining electrode. F) Recorded ENG signal evoked by the shaking of the anesthetized rabbit’s leg. D-F) Reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International license.[358] Copyright 2019, The Authors, published by AAAS. G) Optical image of an ultrasonic neural dust. H) Exploded view of the key components of the EMG recording system. I) EMG signals recorded by the neural dust. G-I) Reproduced with permission.[372] Copyright 2016, Elsevier. J) Photographs of a TENG-powered ECG recording system conformed around a pig heart. K) Exploded view of the ECG recording system. L) The filtered ECG recorded in vivo. J-L) Reproduced with permission.[382] Copyright 2016, American Chemical Society.
4.1.3. ENG
Electrical activity recorded from efferent axons in peripheral nerves provide another alternative for monitoring motor intention. In contrast to the neurons hidden in the cortex, peripheral neurons have a cable-like morphology. Various adapted peripheral electrodes have been proposed, such as implants around the nerve (cuff electrodes[351] and flat interface nerve electrodes (FINE)[352]), longitudinally through the nerves (wire[353] and longitudinal interfascicular electrodes (LIFE)[354]), and through the nerves via shafts (such as transverse interfascicular multichannel electrodes (TIME)[355]). To accommodate peripheral nerve movement and deformation, most of the above electrodes are moderately flexible. For these cable-like peripheral nerves, the flexible cuff electrodes (both spiral[356,357] and helical[358,359] shaped) are the most common ones that must be mentioned.[360] In this type, spiral means that the ability to circumscribe the nerve, which somewhat limits the variation in nerve diameter (nerve growth), unless the electrodes are stretchable spiral cuffs.[302,361] For helical electrodes, self-morphology is an attractive property.[362] As shown in Figure 8D, an electrode inspired by climbing twining plants for neural recording and stimulation was fabricated.[358] This device mainly consists of an array of serpentine electrodes (Au, 200 nm thick) and PI strips (2 μm thick) on matching substrates of shape memory polymer (SMP; ~100 μm thick; Figure 8E) that enabling devices wrapped on nerves under body temperature. In vivo Vagus nerve stimulation was carried out on a rabbit to demonstrate the validity of the device (Figure 8F). Nevertheless, ENG signals have a low signal-to-noise ratio and limited stability.[363]
4.1.4. EMG
The exploration of electromyography for prosthesis control is based on the assumption that the user’s intentions can be extracted from the activation of the remnant muscles.[364,365] In contrast to surface EMG, implantable EMG has many advantages, such as higher signal quality, less movement artifacts, and the ability to record small and deep muscle activity.[364] Generally, two methods, penetrating or surficial (those between the epimysium and the skin), undertake the EMG recording.[366] The penetrating electrodes are mostly rigid enough to maintain their structural integrity in the muscle.[367,368] In contrast, surface electrodes generally are more flexible,[364,369] whose morphology in the limited space between this epimysium and the skin is either 1D or 2D thin electrode layers[370,371] or very tiny electrodes (millimeter or even sub-millimeter level).[369,372] As a representative case of tiny wireless electrodes, millimeter neural interface (“neuron dust”) provides a promising and effective solution to record electrical activities of various neural tissue.[54,373,374] In one representative example, an ultrasonic backscattering concept-based neural dust demonstrated stable wireless ENG and EMG recording from the sciatic nerve and the gastrocnemius muscle respectively (Figure 8G). In the neural dust, a piezoelectric crystal receiving ultrasonic pulses acts as a wireless power supply and data transmitter, a single custom transistor serves as a data transducer, and a pair of recording electrodes can be integrated on a PI substrate of size 0.8×3×1 mm (Figure 8H and 8I).[372] The microscopic size of these “dust” also opens up the possibility of injectable implantation, which greatly reduces the difficulty and expense of implantation.
4.1.5. ECG
Studies toward ECG recording, an important physiological indicator for patient health, also facilitate the development of rehabilitation. In fact, the heartbeat is an autonomic response without intend control, thus, ECG signals cannot be applied to control prosthetics. In the disability rehabilitation, monitoring ECG signals still provides useful information for analyzing the relationship between rehabilitation intensity and body load. Also, ECG monitoring provides immediate alerts such as arrhythmia, bradycardia, tachycardia, heart failure, etc.[288] Unlike static neural implants mentioned above, in vivo ECG recording equipment on the surface of the heart is subjected to dynamic movement.[375–378] Consequently, leveraging this biomechanical beating to energize the implant is a unique feature of some ECG monitors compared to the other aforementioned electrophysiological recorders.[379] As implants, flexible self-powered ECG devices have been explored through piezoelectric,[380,381] triboelectric[382,383], and photovoltaic[384] methods. For example, Figure 8J demonstrates a TENG-powered ECG recording system conformed around a pig heart. Owing to the triboelectric material, which couples the contact electrification and electrostatic induction mechanism in the heartbeat (Figure 8K), and the structure, the device successfully performed real-time wireless cardiac monitoring (Figure 8L).[382] This self-powered approach holds the promise of true freedom of movement for rehabilitators. However, both invasive and wearable ECG measurements require electrodes to be placed directly on the surface of human tissues, which can cause discomfort to the user. In recent years, the rapid emergence of PPG is a potential alternative monitoring solution for ECG, as it can obtain superficial vascular information through infrared optical signals, while being placed comfortably around the finger, ear, or forehead in a non-contact way.[268]
4.2. Non-invasive methods
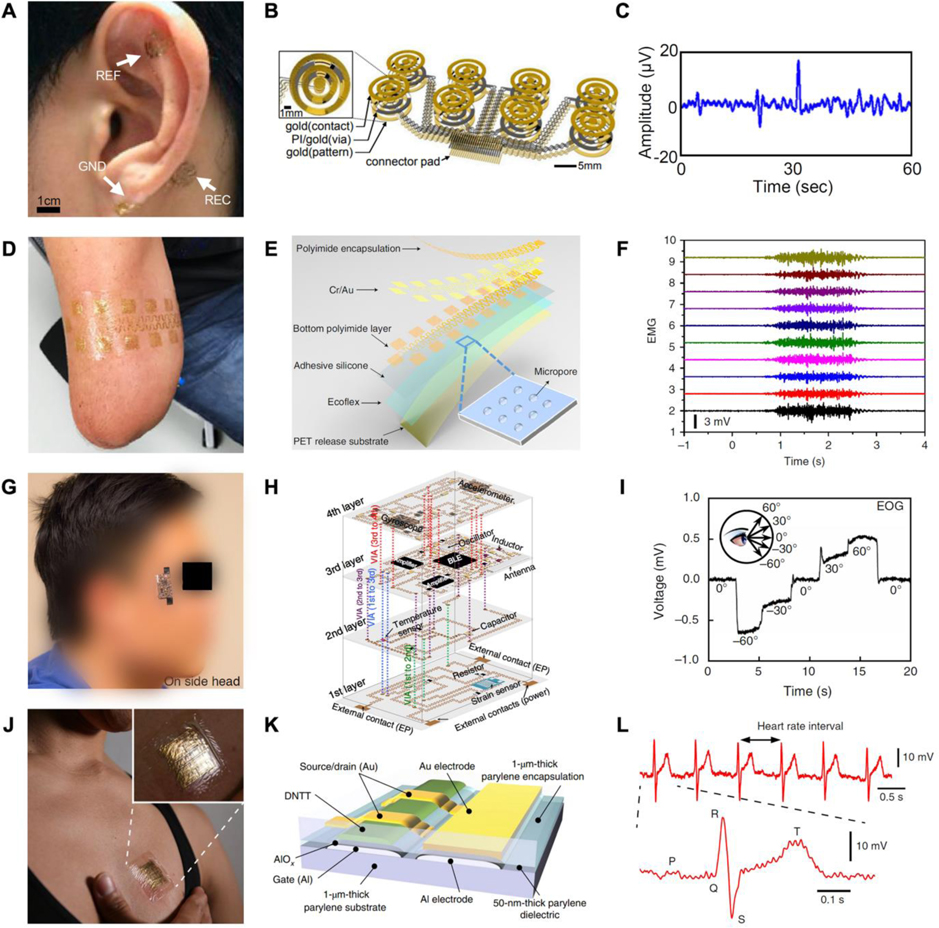
In recent years, the emergence of designs and technologies resembling ultra-thin epidermal electrodes,[299,385,386] multichannel large-area electrodes,[387,388] and flexible hybrid integrated systems in the field of flexible electronics[389,390] has brought unlimited possibilities for non-invasive physiological electrical recording in the field of medical robotics.
4.2.1. Skin surface electrophysiology
Non-invasive electrophysiological recording techniques are more common than invasive procedures as their convenience and safety can relieve users from the risk of surgery. Compared with neural implants, epidermal electrodes are suitable for large area and long-term recording. Vast non-invasive electrophysiological signals are widely investigated and used for prosthetic controlling, including EEG,[43,287,391] sEMG,[392–395] and EOG,[396–398], in academic laboratories and commercial products. As the most common brain signal in clinical applications, EEG has been widely utilized in prosthetics and other HMI applications.[285,399] The richness of EEG signal is an advantage, but it also brings the complexity of decoding. In the context of non-invasive interfaces for controlling mechanical hands, a concrete possibility arises from forearm surface EMG: a technique by which muscle activation potentials are gathered by electrodes placed on the patient’s forearm skin. These potentials can be used to track which muscles the patient is willing to activate and with what force. Surface EMG is therefore, in principle, a low-cost and specific way of detecting what the patient wants the prosthetic to do.[400,401] EOG is a technique that measures the resting potential of the retina by using two electrodes placed on the subjecťs face. In this case, eye movements can be detected by calibrating the potentials in a simple and stable way.[402,403] Lastly, extracorporeal monitoring is the most popular form of ECG monitoring, which provides a real-time response and long-term monitoring to rehabilitative exercise load and assesses exercise capacity.[404]
4.2.2. Epidermal electrodes
Modern commercial clinical electrodes mainly rely on bulky metals (Ag/AgCl), interfaced with conductive gels, which are uncomfortable to wear, irritates the skin, and tends to dry out. This makes them unsuitable for daily, simple, long-term recording. In contrast, flexible dry epidermal electrodes based on conductive materials and stretchable structures overcame these drawbacks. Due to its low Young's modulus and excellent stretchability, the flexible electrode can form a relatively stable combination with the skin, which can effectively reduce the contact impedance in measurement and increase the SNR (by reducing movement artifacts).[93] Moreover, by virtue of the lightweight and ultrathin structure, the flexible epidermal electrodes can achieve aesthetics, comfortability, even imperceptibility for daily long-term electrophysiological recordings.
The need to provide mechanical compliance on the human skin, combined with the demand on low electrical impedance, has brought forth a wave in development of materials and structures for epidermal electrodes. The stretchability and conductivity of epidermal electrodes often comes from metal[405,406] or intrinsically stretchable conductive materials (like conductive polymers,[386] liquid metal[407] or percolation networks of graphene[408] and nanomaterials[409]) electrodes with delicate structures (e.g., serpentine flexure[299,410]). In addition to mechanical and electrical performance, two noteworthy challenges remain for the comfort and longevity of epidermal electrodes: adhesion and permeability. From the perspective of adhesion, interface microstructures, glue and chemical treatment of the interface,[411,412] have been proposed for intimate attachment on skin. In permeability issue, the accumulation of sweat can lead to adhesion off delamination increasing motion artifacts and contact impedance, irritating the skin, or directly causing device failure. Gas permeability enables sweat to evaporate with the flow of air, while liquid permeability enables sweat to be discharged directly outside the device. To improve permeability, a number of structures have been explored, including textiles, meshes, and porous architectures.[73,75,413]
Researchers have developed various versatile epidermal electrodes with high performance that can universally measure EEG, EMG, EOG, and ECG through placing electrodes on different areas of the skin.[25,414] One of the advantages of flexible over rigid electrodes is that they are suitable for mounting to complicated morphologies on the skin surfaces where space is limited. For example, in the case of EEG measurements, the auricle and mastoid (around the ear) can serve as effective points for the flexible reference/grounding electrode and the recording electrode respectively because the auricle is electrically isolated from the scalp and the mastoid is on the scalp (Figure 9A). To install the electrodes on the complex topography of the auricle, an epidermal electrode, consisting of filamentary serpentine traces (300-nm-thick and 30-μm-wide patterns of Au with 1.2-μm-thick layers of PI above and below) and elastomer (Ecoflex) was designed (Figure 9B and 9C).[391] The feasibility of epidermal electrodes with single-channel recording electrophysiological signals has been proved in many studies. The spatial mapping ability limited in a small region and single signal channel greatly hinders the information density of these devices. Therefore, multichannel, large area, even body-scale epidermal systems are of interest to researchers.[415] As demonstrated in Figure 9D, a high-density epidermal sensing array consisting of 17 mesh electrodes is integrated on the residual limbs to record EMG signals for prosthetic applications. The fractal mesh-shaped electrodes are made of Au/Cr by conventional microfabrication techniques. The PI-coated encapsulated interconnects provide electrical insulation and mechanical strain isolation, and additionally the microporous soft silicone layer provides a highly permeable interface with the skin (Figures 9E and 9F). This large area electrode has demonstrated the feasibility of mounting on the head, back, and stump for different electrophysiological recordings.[388] In recording system, signal processing and transmission can bridge raw data from epidermal electrophysiological recording systems with user-friendly output devices (cellphones, smartwatches). In this signal loop, on-site signal processing is beneficial to reduce the loss of sensing information and filter noise as well as improve the transmission efficiency and enable the system to be fully portable.[416] In order to ensure the connection stability of electrical signals and the computing ability of processing circuits, commercial silicon-based chips, FPCB technologies,[417] and stretchable hybrid circuits are exploited to incorporate the critical signal conditioning, processing, and wireless transmission functionalities using readily available integrated-circuit components. A high integrated 3D stretchable electrophysiological recording system can be attached on the side of the face to record EOG (Figure 9G). As shown in Figure 9H and 9I, benefitting from the serpentine structure of Cu interconnections and stretchable substrates, the device can be mounted on human skin conformally and maintain stable electrical performance under stretching. Moreover, the multilayers structures greatly miniaturize the devices, which enhances space utilization.[418] Although such flexible hybrid electrophysiological systems have been reported, their comfortability to practical healthcare and medicine is limited due to the rigid components. Recently, a fully ultra-flexible organic differential amplifier that can record weak human physiological potentials with high signal integrity was reported (Figure 9J). By the design of the OFTF structure (Figure 9K) and circuit strategy for a differential amplifier, the device successfully realizes the acquisition and processing of ECG signal with fully flexible circuit (Figure 9L).[419]
Figure 9.
Non-invasive electrophysiological recorders. A) Optical image of an integrated set of electrodes on the auricle and mastoid to record EEG. B) Schematic illustration of the flexible EEG electrodes. C) Typical raw EEG output signals (Including eye closure at 30 seconds). A-C) Reproduced with permission.[391] Copyright 2015, The Authors, published by National Academy of Sciences. D) Photographs of multichannel epidermal electrodes on residue limbs for surface EMG recording. E) Exploded view of the large-area epidermal electrodes. F) EMG recordings results of different channels. D-F) Adapted with permission.[388] Copyright 2019, Springer Nature. G) Camera image of a stretchable three-dimensional integrated system on the side head of subjects to record EOG signal. G) Exploded view of the key components of the EOG recording system. I) Corresponding recorded EOG signals versus different gazing angles. G-I) Reproduced with permission.[418] Copyright 2018, Springer Nature. J) Picture of a fully flexible electrophysical-recording system attached to the human chest to measure ECG. K) Cross-sectional diagram of an ultrathin differential amplifier circuit. L) The differential output ECG signal amplified by the ultra-flexible circuit. J-L) Reproduced with permission.[419] Copyright 2019, Springer Nature.
4.3. Discussion
In this section, the classification of electrophysiological signals, and medical robotic applications are the focus of the description. Different electrophysiological signals have different application scenarios and unique advantages in HMI systems. In order to obtain these physiological signals, the development of flexible implantable and epidermal electrodes has been instrumental, where the choice of materials and the design of stretchable structures are the priorities of research for both. However, implantable electrodes are more specialized and require special structures to adapt to in vivo tissues, while in contrast, electrical recordings from epidermal electrodes tend to be universal. Both implantable and epithelial electrodes are on the fast track and they face both shared and specific challenges waiting to be addressed.
A common feature of implants and epidermis is the pursuit of ideal materials (including functional materials, encapsulation materials). In terms of functional materials, the key issue is how to achieve the most fidelity electrical measurements according to the equivalent circuit between tissue and electrode, which is closely related to the device interface impedance. Another key is the consideration of encapsulation. The focus of encapsulation materials for implantable devices is biocompatibility and hermeticity. A proper encapsulation ensures, on the one hand, that rejection by the body is minimized and, on the other hand, that the body fluid environment does not erode the device. In this regard, natural biomaterials may be a breakthrough. For epidermal electronic packaging is hoped that it has high strength, high elasticity, lightweight, and breathability. The bond of flexible electronics on internal and external human tissues is another key problem. The floating movement of the device in the body environment will directly lead to the failure of the record, and even cause infection, resulting in life danger. How to stabilize the device to the surface of the tissue without affecting the electrical function requires highly on the design of interface materials and structures.[420] At the same time, the fixation of the device should also consider the replacement problem. The adhesion of epidermal electrons also needs consideration of irritation to the skin and the effect on impedance to the interface.
5. Communication
The primary function of the somatosensory nervous system is to act as a communication pathway between environmental bodies and our perception of the world.[421] Specifically, the ability to record and distinguish each sensation allows people to assess possible dangers as well as experience the space they are inside.[421] This process begins with signal transduction of sensory information, discrimination of important signals (neural decoding), and comprehension of the event (neural encoding).[422] The given transduction pathway occurs via triggered mechanoreceptors, nociceptors, thermoreceptors, and proprioceptors, which respectively provide the sensation of touch, pain, temperature, and motion.[423,424] Upon receiving their respective stimuli, these receptors destabilize afferent neurons, which at a certain voltage threshold can convert the physical sensation into an electrical pulse (a spike). The spikes then collectively travel through the nervous system and carry information up into the brain where it is subsequently processed and interpreted for conscious thought.[423]
The most prominent communication pathway in our body, by size alone, is the skin.[421] The sensation of touch on the skin provides users the ability to distinguish pain, hold onto objects, and discriminate different materials.[421] Inside the palm, the perception of touch is specifically received via tactile afferent neurons located in the glabrous portion of the hand, primed with millisecond-level discrimination of objects.[425] The time it takes to recognize an event is dependent on the processing speed from each contributing neuronal cell as well as the density of cells present in the contact area.[425] The signals received by the brain can thereby be processed based on the amplitude, number, frequency, location, and type of receptors triggered.
5.1. Sensory relay in medical robotics
Unfortunately, the transduction of external signals into the brain using prosthetics and rehabilitation robotics cannot follow the same communication pathway due to the lack of interface between the mechanical parts and the human body. While in vitro and on-body artificial sensory neurons can communicate to batches of afferent cells using optical methods, they currently lack the ability to selectively connect with individual neurons (Figure 10A).[426,427] Despite the biological benefit of direct neuron communication, without single cell precision, artificial sensory neurons cannot communicate an accurate spatiotemporal relay of each signal to the user, which in turn makes it harder to discriminate environmental stimuli in many cases. The problem with inaccurate communication channels in medical robotics is that it goes against visual cues from the user leading to cognitive confusion,[428] which is the number one reason why 40% of amputees reject wearing a prosthetic device.[19]
Figure 10.
Informational transform in HMI. A) A power-efficient skin-inspired mechanoreceptor transducing pressure signals into digital frequency signals directly for optogenetic stimulation. Reproduced with permission.[427] Copyright 2015, AAAS. B) A neuromorphic interface producing receptor-like spiking neural activity for tactile stimuli feedback. Reproduced with permission.[430] Copyright 2018, AAAS. C) Illustration of artificial receptors asynchronously transducing tactile events into pulse signatures. D) Artificial receptors generate tactile events with spatiotemporal structures (dashed lines) that encode the stimulation sequence. C,D) Reproduced with permission.[433] Copyright 2019, AAAS.
The alternative to installing a direct (invasive) communication line into the nervous system is to redirect the signals onto active tissue on the surface of the skin based on the type of sensory information provided. While this technique is simpler to perform experimentally than invasive methods, there are nuances in adapting the stimulation modality to the user while minimizing cognitive confusion.[19,428] Specifically, users prefer a modality-matched feedback system where vibrations are felt as vibrations and temperature is felt as heat.[19] The most common indirect communication methods currently on the market are electrocutaneous, vibrotactile, skin stretching, and auditory cues.[429] For auditory stimulation, researchers found a reduced cognitive effort in interpreting different hand gestures using sound cues in contrast to relying on vision alone.[428] Hand amputees have been shown to differentiate painful (noxious) and safe (innocuous) tactile sensations using pulse width and frequency modulated transcutaneous electrical nerve stimulation (a shock) on their residual limb (Figure 10B).[430]
There are many tradeoffs to investigate when developing and utilizing these wearable feedback electrodes as a communication interface. In electrocutaneous stimulation for grip intensity, amplitude modulation is the most important parameter for accurate association and comprehension of the received stimuli; however, amplitude modulation is also known to be susceptible to sensation adaptations.[431] In contrast, while pulse frequency modulation is harder for users to associate with grip intensity, it has been shown to be reliably discriminated over a longer period of time.[431] Long-term comprehension of a signal must therefore be evaluated separately from short term accuracy. Additional electrode parameters to optimize are the surface area, material, and adhesion of the stimulation device. While a larger contact area with the user’s skin allows for a higher signal discrimination, in practice most devices are limited by the available surface area on the extremity (most notably when on a finger).[429] Therefore, it is important to optimize these design tradeoffs not only based on the initial signal being relayed, but also on the personal situation of the patient. The continual advancement of novel relay modalities provides an interface for transmitting different external signals to the user, allowing them to interact with their physical reality.
5.2. Time delay in feedback sensors
Regardless of the feedback modality chosen, signals should be transmitted to the user relatively fast for the patient to have enough time to decode, recognize, and respond to the sensation. In abled-bodied patients, the latency period for tactile sensations to reach the cuneate nucleus is 14–28 milliseconds. Any delay beyond 300 milliseconds will further result in a user-perceived lag.[432] Unfortunately, for multiple signals, this poses a problem as electronics are limited by the amount of data they can process simultaneously. Furthermore, processing multiple signals in parallel can result in electrical interference between the sensors due to a small current induced from an adjacent wire’s magnetic field. Transistor switches can minimize the magnetic overlap by sending signals asynchronously, but this additionally adds to the processing delay time. Recently, an asynchronously coded e-skin (ACES) was developed that can simultaneously transmit information from 10,000 sensors using only a single wire with a latency period of 1 millisecond (Figure 10C and 10D).[433] Further research into processing data without electrical interference while minimizing the time delay can help maximize the amount of data that medical robots can extract from their environment.
Adding onto the time delay in signal processing, most electronics use von Neumann architectures (first introduced in 1945), which separates the memory and central processing units (CPU; executes commands) in a device, allowing only a small bandwidth of communication between the two components. Practically, this means that every time the computer looks up or stores variables, data, and files, the program being executed by the CPU’s thread will have to stop and wait as the memory is being accessed. The limited communication line between the two units leads to the well-known von Neumann bottleneck: the inherent idle time in receiving information from computer memory due to the small transfer rate between the CPU and stored memory.[434] Researchers have been trying to increase this bandwidth (the pathway between the two systems) by increasing the number of transistors on a chip (following Moore’s law); however, the ability to compact more transistors together without losing functionality due to massive heat generation is beginning to slow down as processing nodes decrease past 2–3 nm.[435–437]
Another approach researchers have taken to mitigate the von Neumann bottleneck is to safely parallelizing data flow between the CPU and memory – like how the brain accesses information using multiple neurons at the same time without any interference.[438] By parallelizing the CPU’s data access, one can transfer more information (using the same transfer rate per bandwidth) in the same amount of time. Recent studies have investigated this neuromorphic model using artificial intelligence to create non-von Neumann architectures.[439] One such promising artificial intelligent model is the Spiking Neural Network (SNN) design. In SNNs, data is only sent when a given node receives an input voltage (a spike) above some threshold, allowing multiple nodes to be activated at the same time.[439] In literature, SNNs have been used in dynamic neuromorphic asynchronous processors with an insignificant time delay, outperforming other modern von Neumann architectures in accessing stored data.[439] Further research into the integration of ML in computer architectures can enhance data utilization and parallelization, fundamentally changing the speed at which computers transmit information.
5.3. Artificial sensory neurons
Another promising neuromorphic design for communication is artificial sensory neurons, which have already been applied to measure tactile, noxious, and mechanical stimuli.[440] The general schematic for an artificial sensory neuron consists of three portions: an initial sensor that receives environmental stimuli (such as a pressure sensor), a transducer that converts the signal into a voltage pulse (like in neurons), and a transistor that integrates and transmits the signals to the user. Artificial sensory neurons are commonly used for muscle stimulation, and were recently applied to create a reflex arc inside a cockroach’s prosthetic limb.[441] Artificial sensory neurons have been used to enhance user performance while reading braille by producing auditory cues when different letters are recognized.[441] Integrating artificial intelligence into these neurons, researchers further developed a neuromorphic tactile processing system (NeuTap) that acquires tactile sensory information with a 0.4% accuracy for spatiotemporal features.[442]
By mimicking their biological counterpart, artificial sensory neurons act as a natural interface for communicating external stimuli into the body. Non-invasive communication in artificial sensory neurons generally involves optical stimulation of the cells. The advantage of optical transduction is that the light is low energy (visible or infrared) and the device is robust and flexible. In the future, the neuromorphic, flexible, and biocompatible design of artificial sensory neurons will allow researchers to possibly integrate the sensors directly into the central nervous system, opening the door for possible single neuron communication.
5.4. Artificial intelligence in medical robotics
Artificial intelligence (AI), specifically machine learning (ML), in data communication can enhance the accuracy and reduce the volume of sensory information needed, uncovering hidden – possibly overlapping – features present in the data. In machine learning, there are three general subcategories of algorithms used to model a new system: supervised learning, unsupervised learning, and reinforcement learning. For the purposes of stimuli identification and feature analysis in HMI devices, one should train the algorithm with real data, limiting the number of mistakes made in the process by performing supervised learning. This section will therefore only focus on supervised learning modules, the most common being convolutional neural networks (CNN), support vector machines (SVM), k-nearest neighbors (KNN), and artificial neural networks (ANN).
Convolutional neural networks are used for extracting information from photos by interpreting (classifying) different features inside an image.[443] For sensory data in medicine, a common application of CNNs is to diagnose patients from an EEG, EMG, or ECG recording. During classification, CNNs extract quantifiable information from these images (which are represented by a matrix of numbers) by first downsizing or sectioning off key areas of the photo into smaller matrices,[443] where each color pixel is represented by three integer RGB values ranging from 0 to 255. Down-sampling is achieved by convolving (sliding) different matrix filters across the photo and remapping the data into a new array that can be used to identify different features inside the image.[443] As a simple example, the convolution of a vertical edge detection filter is shown below:
By searching for the non-zero values, it is now clear from the new array on the right that there is a vertical line feature in the middle of the image matrix on the left, which can be further sent off for processing and labeling inside the neural network. In practice, CNNs use multiple filters to extract different key features within a photo. For medical robotics, CNNs can be applied to textile-based tactile sensors to classify human poses, motions, and other user interactions (Figure 11A–11C).[160,444] CNNs have also been widely used to analyze and identify tactile maps generated from grabbing onto objects as well as quantifying their weight (Figure 11D and 11E).[148] CNNs have additionally been shown to detect Alzheimer’s disease in MRI images.[445] It is worth noting that similar analysis can be performed using artificial neural networks, just by extracting the key features first (without using a matrix filter). By first preprocessing EMG data, ANNs were shown to classify 17 hand gestures from 6 EMG electrodes with an accuracy of 83%,[446] as well as 5 hand gestures from 8 EMG electrodes with an accuracy of 98.7%.[447] The benefit of CNNs over other ML algorithms (such as ANNs), however, is that there is little preprocessing required on (most) imaging data as the information will inevitably be compressed later inside the network.
Figure 11.
Machine learning for tactile sensing in HMI. A) The flow figure of a scalable tactile glove as a platform to learn from human grasps. B) The design of the tactile glove architecture and the calibration results of the force sensors. C) Optimization of the ML algorithm for the recognition of commonly grabbed objects. A-C) Reproduced with permission.[160] Copyright 2019, Springer Nature. D) Example images and tactile frames of some words pressed on a pressure-sensitive vest. E) The relationship between classification accuracy and sensor resolution in confusion matrix of ML algorithm. D,E) Reproduced with permission.[148] Copyright 2021, Springer Nature.
Unlike convolutional neural networks, the support vector machine and k-nearest neighbor algorithms classify data by first grouping the points into different subcategories. Support vector machines segment the space by defining a boundary plane to maximally separate the greatest number of similar points (specifically focusing on the edge points at the boundary). Meanwhile, the k-nearest neighbors algorithm classifies incoming data by minimizing the (weighted) distance between each testing group’s points (i.e., each point is classified with the closest group’s label while minimizing the total error). If the input points (the features) cannot be well separated into different groups, one can further apply a kernel function to remap the data into a higher dimension where it is easier for boundary lines to section off the information. In medical robotics, the KNN algorithm has been shown to outperform other ML techniques (including SVMs) for proprioception sensing when training a soft robotic hand to recognize its bending and twisting angles.[199] Meanwhile, SVMs have been successfully used by soft robotics in tactile sensing to identify objects with an accuracy of 98.1%.[448] The drawback to using these algorithms is that the input features to the ML models might not be readily available. For example, EEG, EMG, and ECG data outputs a semi-continuous flow of points, most of which are not relevant or hidden by noise. Much of the data needs preprocessing to first extract useful features in the correct format. In practice, the final accuracy of these models is highly dependent on the feature extraction and preprocessing methods chosen, as clean data (good features) yields a high accuracy. Therefore, research into different pre-processing techniques for ML applications is imperative to demonstrate the success and applicability of SVM and KNN algorithms in medical robotics.
With an abundance of ML techniques available, it can be daunting picking which algorithm will benefit a given dataset the most. Unfortunately, there is no formal proof that any ML algorithm will enhance the classification accuracy of medical data above randomly guessing. It is rather in practice that one sees the learning capabilities that ML offers. The inability to formally postulate whether ML will enhance the accuracy in a dataset beforehand partly stems from the possibility of bad and misused data present in the input samples. Furthermore, human sweat, slight deformations in sensors positions, and electronic degradation can adjust on-body readings that no longer match the feature-space mapped out by the model. This results in the natural decline of the ML accuracy over time.
Another issue commonly overlooked in ML applications is sampling enough data points across all gender, race, age, and sexuality.[449] One recent and prominent example of misapplying ML across a population is Amazon’s facial recognition software (Rekognition) that was launched in 2016 and sold to several government agencies. Despite performing well in training groups, in practice, Rekognition was found to preferentially label innocent people in minority groups as potential criminals.[450] Such prejudice is actually quite common in many other AI-based facial recognition platforms used in industry, despite racial bias in ML being well-documented in the literature. In practice, when trained across the whole population, ML models preferentially mislabel people from minority groups, but when applied to each individual subgroup the models maintains their high accuracy.[451] Recognizing that there is a racial, gender, sexuality, and age component in medical data reaffirms the need to account for such factors in ML applications in medical robotics to prevent the algorithm from finding a local optimum that overfits the majority of the population while underperforming on minority groups.[449,451]
5.5. Discussion
Communicating external sensory information to a patient is an important step in establishing a connection between the user and their phantom limb. There are two main routes researchers can take in relaying sensory feedback information: invasive contact with the nervous system and external stimulating the user’s limb. When stimulating the user in response to an external impulse, matching the feedback modality of the stimulus (when possible) results in a lower cognitive effort for the user to recognize and react to the event. When multiple signals are provided to the patient at once, minimizing the idle time between data processing and user relay is important to maintain spatiotemporal information about the signal as well as match the expectation from visual cues. ML and other neuromorphic architectures offer a way to speed up this processing time, which is currently limited by electronic interference and the von Neumann bottleneck. Through advancing communication channels in rehabilitation robotics and prosthetics, medical robotics can be fully integrated into the user’s daily routine, allowing for higher user acceptance of many therapeutic technologies.
6. Actuation
The inherently soft and stretchable skeletal muscles, including the muscle bellies and tendons, are the natural mechanical actuators in the human body.[452] Through the leverage of joints, changes in length and tension of the muscles are translated into rotational movements and forces.[453] Take the human hand for example. The human hand can achieve various dexterous gestures with almost 23 degrees of freedoms (DOFs).[454] In contrast, most commercial prostheses have only about 4 DOFs[455,456] due to the complexity of computing control signals for multiple rigid motors. Although motor-driven prostheses are precise and adaptable, their low mechanical compliance, high stiffness, and heavy weight are substantially different to natural human limbs, which may lead to physiological and psychological rejection of prostheses by amputees.[457] Despite these issues, prosthetic actuation has repeatedly been shown to enhance rehabilitation recovery through assisted motion actuation[458] and heat therapy[203] after paralysis, muscle injury, or loss of limb.[459]
Soft robotics allow for flexible artificial actuation and can act as the base for flexible sensing electronics (as discussed above) for prosthetic and rehabilitation applications. Currently, various soft mechanical[76,460] and thermal actuators[106,461] have been extensively adopted as artificial muscles and heat sources in prosthetic actuation. The most attractive features of soft actuators over rigid prosthetics are their high biocompatibility and biomimicry[38] (on top of their mechanical compliance, lightweight, silence, high power-to-weight ratio, and safety), which can naturally conform to and imitate the human muscle. The main issue with the current rigid and metal-based medical devices is that they carry a risk of causing irritation or damage to the human body, which can negatively affect rehabilitation. Soft-robotic systems therefore show great potential in medical and wearable applications.[462–464] In this section, soft mechanical and thermal actuators alongside their HMI applications are discussed in detail.
6.1. Motion actuators
Developing artificial muscles that have a high-power density and fast response times, like natural muscles, have been a hot area of actuator research. Researchers have focused tremendous efforts on the development of soft actuators on human scales, such as pneumatics, tendon-like, and EAP soft actuators, which are expected to revolutionize the potential directions and applications of robotics,[64,465] prosthetics,[466,467] and rehabilitation.[38,468]
6.1.1. Pneumatic actuators
Soft pneumatic actuators can be deformed in a controlled manner through inflation or evacuation of special structures[469] or external binding modes (fiber shells).[470] Broadly speaking, there are two types of pneumatic actuators: PAM-pneumatic artificial muscles (known as McKibben actuators) for linear actuation[471–473] and FEA- fluidic elastomer actuators, which are composed of low durometer rubber and driven by relatively low air pressure (3–8 psi) for bending.[474–476] In contrast to the rigid robotic hands with limited DOFs, pneumatic soft hands can achieve continual deformation with a simple input signal, resulting in self-adaptation that is comparable to the high DOFs in human hands.[477,478] As a representative case, Figure 12A illustrates a fiber-reinforced FEA robotic hand with optical multifunctional sensors embedded inside for strain and pressure sensing. In the functional demonstration, the soft prosthetic can not only perform dexterous operations in a self-adaptive way by controlling the inner air pressure, but it also achieves a variety of tactile sensing functions through simple artificial nerve innervation. Due to its sensing and actuating abilities, the soft hand has been successfully applied in grasping and HMI scenarios (Figure 12B and 12C).[153] Bionic elements can also be added to the pneumatic hand design to achieve a more realistic gripping effect, such as flexible joints and hard finger bones, which can be achieved through FEA and plastic respectively.[76] Unfortunately, the softness of the pneumatic prosthesis brings uncertainty in modeling and control. In addition, issues like slow actuation also limit pneumatic applications.
Figure 12.
Soft actuators simulating human limbs. A) Schematic of an optoelectronically innervated soft pneumatic prosthetic hand. B) The soft pneumatic hand holding a coffee mug. C) A hand shaking using the soft pneumatic hand. A-C) Reproduced with permission.[153] Copyright 2016, AAAS. D) Schematic of a tendon-driven prosthetic hand. E) Time-lapse image of the tendon-driven hand catching a thrown ball. F) Demonstration of the tendon-driven hand crushing an aluminum can. D-F) Reproduced with permission.[460] Copyright 2018, AAAS. G) Schematic of an EAP based artificial muscle. H) Lifting heavy objects by using the artificial muscle. I) A self-sensing planar HASEL actuator powering a robotic arm. G-I) Reproduced with permission.[62] Copyright 2018, AAAS.
6.1.2. Tendon driven actuators
Tendon driven actuators have been used in robotics for a long time. The name is a generic term, because the “tendon” can be powered by many sources, such as shape memory alloys (SMAs),[479] SMPs,[480] and thermo-responsive polymers[481]. Each power source has its own advantages, but, unfortunately, they are all too slow for prosthetic actuation. In this section, we will therefore only discuss actuators that can be applied to medical robotics, exclusively to the most widely implemented motor-based tendon driven devices. In these devices, cables in the forms of variable length tendons are embedded in soft or rigid skeletons to provide a controlled deformation.[482,483] Compared with pneumatic actuating methods, tendon driven grippers have a higher grasping speed and strength.[484,485] As an example, Figure 12D shows a low-cost, 3D-printed tendon-driven prosthetic hand, which optimizes the relationship between grasping speed and force by using a continuously variable transmission (CVT) system. In no-load, CVTs have a large radius and fast spool drive (Figure 12E), but also apply a small total force because the moment arm is large. At high loads, the exact opposite is true (Figure 12F), where the soft hand achieves an ideal self-adaptability for grasping (there is a trade-off between strength and dexterity).[460] It is worth noting, most single tendon drive does not have the ability to actuate bidirectionally (no "push" and self-recoverability), so multiple cables are required to recover structures,[486,487] similar to how human agonist and antagonistic muscles move.
6.1.3. EAP actuators
In contrast to soft pneumatic actuators that demand gas supplements and tendon driven actuators that require motors, EAP actuators are activated directly through electrical signals.[488,489] However, the actuation stress, strain, and efficiency of EAPs (aforementioned in section 2.1.4) are problematic in applications to prosthetics, especially for ionic polymer actuators.[490] Likewise, dielectric elastomer actuators (DEAs) at human scale can only generate relatively low force (below 100 mN) but require a high working voltage (around 1000 kV) to operate.[63] In order to amplify the force output, a lot of studies have focused on the structural design of EAPs, like parallel connections[491] and hydraulic amplification.[62,492] For example, a muscle-mimetic soft EAP transducer is shown in Figure 12G. Combining the actuating ability of dielectric elastomer actuators and all–soft matter hydraulic architectures, the output force of hydraulically amplified self-healing electrostatic (HASEL) actuators can be greatly amplified. To verify the linear actuating ability of a single-unit planar HASEL actuator, a 4kg object lifting experiment at 69% linear actuation strain was exhibited (Figure 12H). In this design, the combination of high actuation strain and the ability to scale up to a large actuation force is critical for the development of high-performance actuators for human-scale artificial muscles. The combination of two planes of HASEL actuators with simultaneous capacitance measurements can be used to reflect the actuation state of the robotic arm (Figure 12I).[62] The actuation of the EAP is silent, which is more analogous to human muscles than other actuation modalities.
6.1.4. Motion therapy
The field of soft wearable robotics offers an opportunity for rehabilitation, especially in motion therapy. Although various soft actuators have been proven to be effective as artificial muscles, some of their features constrain their deployment in wearable rehabilitation applications, such as their heavy weight, slow actuation, dangers associated with high operating voltages, and thermal irritation of thermally activated polymers. Because of this, pneumatic and tendon-actuation are currently the most suitable forms of wearable rehabilitation in soft robots. These two actuation methods can be applied to gloves, exoskeletons, and foot orthoses, providing a new paradigm for rehabilitation devices.[459]
A soft robo’s ability to adapt to curved and irregular surfaces is crucial in hand rehabilitation for people with grasping pathologies. For example, an elastomeric bladder-based robotic FEA glove for safely distributing forces along the length of the finger can provide active flexion and passive extension of the fingers. To produce specific bending, anisotropic fiber reinforcements are applied to these bladders (Figure 13A).[394] Partial hand paralysis is one of the most common complications in stroke patients, and mirror therapy is a promising method for hand rehabilitation in this situation. Figure 13B introduces a pair of gloves, i.e., a sensory glove and a motor glove, which are used to measure the gripping force and bending angle in a normal hand and guide the affected hand with enough driving force to perform training tasks in the same way, respectively.[158] Upper and lower limb rehabilitation is a systematic work. Cable-like actuators are more suitable to mimic natural muscles in this complex scenario owing to their simple structures. For instance, a soft bionic ergonomic exoskeleton robot with 7 DOFs is shown in Figure 13C to assist upper-body motions and to limit stroke complications in a natural way.[493] Some diseases, like stroke, poliomyelitis, will induce hemiparetic gait and inhibit movement. Recent years have seen the development of powered exoskeletal devices designed to enable walking. As an example, a lightweight, soft wearable robot (exosuit) that interfaces to the paretic limb of a stroke patient via garment-like, functional textile anchors were reported. Exosuits produce gait-restorative joint torques by transmitting mechanical power from waist-mounted body-worn or off-board actuators to the wearer through the interaction of textile anchors and cable-based transmission (Figure 13D).[494] PAM can also serve as tendon-like actuators for rehabilitation devices. Figure 13E shows the design and control of a wearable robotic device powered by pneumatic artificial muscles in ankle-foot rehabilitation which provides active assistance without restricting natural DOFs at the ankle joint.[495] Dedicated tendon driven actuating systems are suitable for bi-directional actuation in foot rehabilitation. For example, a soft wearable 3D printed robotic ankle-foot orthosis with a bi-directional tendon-driven actuator was proposed. Through the integration of the device with the gait sensing module, the system has the potential to improve hemiplegic gait after stroke (Figure 13F).[496] While these rehabilitation strategies based on soft devices look promising, the portability issue still overshadows their development. Pneumatic devices are complicated by air tubes, pumps, and cable actuators with motors as the power source. All of these challenges are also faced by heavy and rigid devices, which are the current challenges for fully integrated wearable designs.
Figure 13.
Soft actuators for disability rehabilitation. A) A pneumatic soft actuating glove for hand rehabilitation. Reproduced with permission.[394] Copyright 2016, IEEE. B) Motor glove with animation of tendon design for hand rehabilitation. Reproduced with permission.[158] Copyright 2021, IEEE. C) Bio-inspired soft exoskeleton for upper limb rehabilitation to reduce stroke-induced complications. Reproduced with permission.[493] Copyright 2021, IOP Publishing. D) Soft robotic exosuit for lower limb rehabilitation improves walking in patients after stroke. Reproduced with permission.[494] Copyright 2017, AAAS. E) Pneumatic soft wearable robotic device for ankle–foot rehabilitation. Reproduced with permission.[495] Copyright 2014, IOP Publishing. F) Soft wearable tendon-driven robotic ankle-foot-orthosis for post-stroke patients. Reproduced with permission.[496] Copyright 2019, IEEE.
6.2. Thermal actuators
Intimate conformal contact with the skin promoting heat transfer between heaters and skin. In comparison, flexible heaters perform better than rigid heaters, mainly due to the difference in conformability between the two and the skin contact interface. Most flexible thermoactuators are based on the Joule effect.[413,497,498] Recently, the Peltier cooling effect has also been used for the thermal cooling in flexible devices (also known as semiconductors coolers).[499–501] Thermal actuators provide an additional dimension beyond mechanical and electronic for HMI. Specifically, these skin-like thermal actuators can be used for prosthetic applications,[106,502] AR/VR feedback thermal stimulators,[191,503,504] and thermal therapy in rehabilitation.[505–507]
6.2.1. Thermal principle
Thermodynamics are no more than heating and cooling, and the electrothermal mechanism of these two modes are almost constant, i.e., the Joule thermal effect and the Peltier cooling effect. The Joule heating is the thermal effect of electric current that is commonly found in resistors. Various soft conductive materials such as thin metal,[106] conductive polymers,[508] nanomaterials,[509] etc. can be thermally actuated in the form of resistors. Under a constant voltage, materials with high electrical and thermal conductivity tend to achieve a fast-thermal response. For cooling, the Peltier effect means that when there is a current through a circuit composed of different conductors, heat absorption and exothermic phenomena occur at the junctions of different conductors with different current directions, which are usually used in n-p semiconductors. Taking advantage of this heat absorption phenomenon, the cooling surface temperature can be effectively reduced. However, compared to rigid thermoelectric coolers, the performance of all flexible Peltier cooling devices is far from adequate for HMI use.[510] Most "flexible" devices that have been developed are partially deformable by using small-sized rigid materials embedded in soft substrates.[511]
6.2.2. Thermal applications in HMI
The human skin not only has the function of sensory perception, but also has the thermal actuating capability.[512] Endowing e-skins with "temperature" can help amputees interact with others and improve people's acceptance of prostheses in social touch situations.[502] As shown in Figure 14A, an e-skin with stretchable metal-based heaters is warmed to ~36.5°C to mimic body temperature on artificial prosthetics. Various sensors and Au electroresistive heaters are connected with serpentine networks and encapsulated in stretchable silicone to mimic the human skin functions. (Figure 14B). After touching a baby doll using a robotic hand with the e-skin, the heat transfer to the baby doll is then captured with an infrared camera, which is similar to that of human natural hand (Figure 14C).[106] Besides, the flexible epidermal heater can directly feedback the thermal information perceived by the sensors on prosthesis from the outside world back to the skin of the residual limb accurately, which can also be referred to as the AR/VR in the thermal dimension. As an example, a pliable Ag-Au nanocomposite elastomer has been proposed. Thermal actuating patch based on this material show small changes in resistance and stable heating properties during deformation. The softness of the patch ensures a firm contact with the skin and reliable heat transfer even when the wrist is flexed or extended.[503] However, most thermal actuators are based on Joule heating, whose function is to generate a higher temperature than original state. In most cases, both heating and cooling are needed in thermal feedback stimulation. Peltier cooling is a promising solution for the challenge. Figure 14D and 14E show a highly stretchable thermo-haptic device to provide an artificial hot and cold thermal sensation to skin for wearable cutaneous VR applications. The device is based upon a thermally conductive elastomer backbone and thermoelectric materials connected with stretchable interconnecting electrode, which enables heat transfer under maximum stretching over 230%.[501] Heat therapy is another promising application of thermal actuators for rehabilitation. A recent demonstration is a multifunctional device for the diagnosis and therapy of movement disorders, which is shown in Figure 14F. In this platform, heaters can degrade the physical bonding between the nanoparticles and the drugs, and pharmacological agents loaded in the nanoparticles are thus diffused transdermally (Figure 14G).[513] Flexible heaters, like a graphene-AgNP-based heater, can also be combined with traditional Chinese medicine to treat arthritis.[514]
Figure 14.
Thermal actuators for medical robotics. A) Photograph of a smart artificial skin integrated with sensors and actuators covering a prosthetic hand. B) An exploded view of the artificial skin comprised of six stacked layers and microscopic image of electroresistive heater. C) Images of the prosthetic limb caring a baby doll and IR camera images of temperature mapping in this condition. A-C) Reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International license.[106] Copyright 2014, The Authors, published by Springer Nature. D) Simplified illustration and exploded view of a skin-like thermo-haptic device. E) Localized cooling and heating of hand-shaped PDMS with the device placed beneath PDMS. D,E) Reproduced with permission.[501] Copyright 2020, Wiley-VCH. F) Image of a multifunctional wearable devices for diagnosis and heat therapy of movement disorders. G) Temperature distribution measurement of the heater on the skin patch. F,G) Reproduced with permission.[513] Copyright 2014, Springer Nature.
6.3. Discussion
Mechanical and thermal actuations are the two dominant types of actuation both in human body and soft artificial devices. In particular, pneumatic-based, tendon-based actuation, and some high-power EAPs have been frequently used for broad mechanical actuation applications, while Joule thermal effect-based thermal resistors and Peltier effect-based semiconductor coolers are the classical players in thermal actuation. These soft actuators are more compatible with the human body, making them promising in many fields, ranging from bionic prosthetics to rehabilitation HMIs.
For the future orientation of soft actuators, the following aspects can be contemplated. In many cases, multiple soft actuators need to operate in concert to achieve complex actuating tasks. Here, the drawbacks of large misalignment of these actuators can be superimposed, resulting in actual results that deviate significantly from the preset task. This requires either modeling the actuator control or using a combination of flexible sensors and actuators to achieve closed-loop control. Moreover, unlike other static devices, mechanical soft actuators are subject to long-term dynamic changes that are highly susceptible to fatigue failure of the device. Moreover, most soft materials are prone to oxidation, which reduces durability of soft actuators. Therefore, Research on the durability of device materials needs to be explored in depth. In the next challenge, although, the response speed of thermal actuators can be improved by material, structure, power, etc. However, the recovery from high to low temperature is usually a slow process. Such thermal inertia is a matter of concern. Last but not least, the intimate combination of soft actuators and flexible sensors is also a new direction for future soft system development, or the development of smart materials with both actuation and sensing characteristics can also eliminate the heterogeneous problems in integration.
7. Stimulation
Stimulation holds tremendous promise for prosthetic information feedback[86,515] and rehabilitation.[329,516,517] From the perspective of haptic restoration, stimulation is the user-input portion of the bidirectional information interface between the human body and prosthesis. Specifically, stimulation modalities can transmit biopotential electrical, optical, or mechanical signals to the body for sensation. Many studies have shown that feedback stimulation has the potential to not only regain tactile and thermal sensation in amputees,[106,518] but also improve their accuracy and confidence in manipulating the prosthesis, such as grasping objects.[157,455] Moreover, some subjects wearing prostheses with sensory feedback reported that stimulation eliminates phantom pains in the artificial limbs.[157,519] In rehabilitation therapy, stimulations have also been prescribed to treat disabilities caused by neurological disorders through neural modulation, such as deep brain stimulation for paralysis and Parkinson's disease.[305,520] Collectively, tactile and proprioceptive restoration as well as motor rehabilitation can be achieved by stimulating the corresponding sensory and motor nerves.
Existing literature has documented several types of stimulation methods, such as electrostimulation, optogenetic stimulation, and vibrostimulation. These flexible stimulators are highly biocompatible, lightweight, safe, and portable in the form of implants or wearable devices. Thus, they have garnered the attention of academia, industry, and users. In this section, we primarily review these flexible implantable and non-invasive stimulators for prosthetic and rehabilitation applications.
7.1. Implantable stimulators
In recent years, profiting from the academic interest and deep exploration in neuroscience, flexible implantable stimulators with various mechanisms (electrical,[521–523] optogenetic,[524,525] ultrasonic,[526,527] magnetic[528]) are being developed as non-pharmacological treatment options. These flexible stimulators function by releasing (electrical and optical) signals into the brain,[272] spinal cord,[522] and periphery nerves[529] to achieve neural modulation for associated sensory perception and motor rehabilitation.
7.1.1. Electrical stimulators
Electrical signals are the predominant form of nerve excitation propagating in the human body. Therefore, implantable electrical stimulators are widely deployed because of signal compatibility. Compared to non-invasive electrical stimulators, implantable electrodes can be precisely inserted or attached to specific sensory afferent and motor efferent nerves, allowing sensory feedback and modulation therapy to be more natural and effective.
As a basic architecture, soft implants for electrical stimulation typically consist of flexible substrates and active electrodes. The former is mechanically stable and compatible with the neural tissue and serves to carry and protect the electrode. Common substrate materials include PI and hydrogel, which can remain chemically inert in vivo while also providing sufficient dielectric strength to prevent discharge breakdown. The latter is embedded in the substrate as a pivotal component of electrical stimulation, delivering current to the target tissue.[515] As a general rule, electrical stimulators can be classified by their mechanism of operation as either capacitive or faradic. The capacitive type relies mainly on the double-layer charge at the electrode-electrolyte interfaces to inject charge, while the faradic type is based on the redox reactions and ions movement at faradaic electrodes which provides high levels of charge for stimulation.[305]
Electrode material is a decisive factor in the electrical performance of implantable stimulation electrodes. Traditionally, capacitive and Faraday electrical stimulation occurs simultaneously on the surface of metal stimulating electrodes (Au and Pt).[530,531] Generally, nitrides and oxides of titanium (TiN and TiO2) are popular options for capacitive electrical stimulators due to their chemical inertness and high electrochemical safety limitation. Faradic electrodes based on oxides of iridium (IrOx) tend to have a higher charge capacity compared to titanium-derived electrodes due to their excellent electrochemical properties.[532,533] In the recent decade, various nanomaterials[301,345,534,535] and conductive polymers[536–538] have also made their way into implantable stimulation electrodes, which have higher charge injection capacity (1~15 mC cm−2) compared to conventional materials (Pt 0.05~0.15 mC cm−2, TiN 0.9 mC cm−2).[39] Nanomaterials can constitute percolation networks that can adapt to the deformation of neural tissue while maintaining high electrical performance. On the other hand, the considerable surface area of nanomaterials can enhance charge injection capabilities. The intrinsic mechanical elasticity, favorable charge storage and injection capacity of conductive polymers provide an unparalleled option for faradic electrical stimulators. In addition, the combination of hydrogels and conductive polymers is a hot area of research since the former has mechanical properties similar to those of nerves and can provide a hydrophilic interface that reduces the adhesion of non-specific proteins.[58,539–542]
The cortex stimulator refers to the well-known stimulating brain-machine interface (BMI). There are two main categories of stimulating BMIs. The first is penetrating microelectrodes with low-charge/phase thresholds, high-charge density thresholds and smaller surface area, which are more suitable for stimulating specific functional areas in a selective and high spatial resolution way.[543,544] Although the second type, thin film flexible electrical stimulators, are available in non-inserted way on the cortical surface to reduce damage to brain tissue, the array density of such devices is much smaller than the first, and they are more suitable for stimulation of relatively large brain areas.[545,546] For example, by using commercial 2D stimulation electrodes, which consist of a silicone sheet (4 cm × 4 cm in size and 1 mm thick) and 60 electrodes facing the brain (Figure 15A), researchers have found that sensory intensity, type of sensation, and evocation of sensation from a range of locations from the fingers to the upper arm can be modulated by electrical stimulation (Figure 15B).[546] The stimulation in the brain is always the most direct, but also the most ethically constrained technology, which requires a high level of assurance for safety.
Figure 15.
Invasive stimulations. A) An electrode array on the surface of somatosensory cortex. B) Different functional cortex location on brain surface. A,B) Reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International license.[546] Copyright 2017, The Authors, published by PLOS publishing. C) A spinal electronic dura as the long-term multimodal neural interface. D) Illustration of the e-dura implant inserted in the spinal subdural space. C,D) Reproduced with permission.[548] Copyright 2015, AAAS. E) An electrical stimulator for the regeneration of peripheral nerve. F) The electrical stimulation is activated by a transmitting coil. E,F) Reproduced with permission.[303] Copyright 2018, Springer Nature. G) A multifunctional system with optogenetic stimulation function. H) Process of the device injection into the brain. G,H) Reproduced with permission.[566] Copyright 2013, AAAS. I) A wireless optoelectronic system. J) The anatomy of the system on the spinal cord. I,J) Reproduced with permission.[569] Copyright 2017, Wolters Kluwer Health. K) Light delivery using wirelessly powered and fully internal implants. L) The implant allows for wireless optogenetic stimulation of peripheral nerve endings. K,L) Reproduced with permission.[574] Copyright 2015, Springer Nature.
Afferents and efferent projections from the spinal grey matter also carry somatosensory and motorial information.[515] With the aid of medical imaging and genetic labelling technologies, epidural and subdural electrical stimulators could be integrated on spinal cord for sensory feedback and motor function modulation in forms of linear-type probes and paddle-type probes.[86] The former is less invasiveness and easier to implant. But the latter one have a better stimulation effect.[547] In an example, Figure 15C shows a paddle like neural implants named “e-dura”, which can have long-term biological integration in the central nervous system of spinal cord.[548] This spinal location allows for multitype stimulations, like electrical modulation and local drug application to alleviate the neurological defects for a long time. Finally, this e-dura was effectively exploited to restore locomotion of a rat after spinal cord injury (Figure 15D). Furthermore, stimulation of the spine can suppress pain and restore movement in paralyzed patients.[549]
The common structures of peripheral nerve electrical stimulators are similar to that of the electrophysiological recorders mentioned in section 4.1.3. They can be generally classified into two categories, the penetrating neural interfaces (TIME, LIFE, Utah) and the wrapping type (FINE, Cuff).[357,455,550,551] Most of these neural interfaces can be somehow flexible to improve mechanical compliance for adapting to the morphology of the peripheral nerve and improving biocompatibility. As an example of TIME, a PI-Pt-IrOx stack was inserted into the peripheral nerve in a transverse manner with the aid of a pre-attached needle.[552] LIFEs tend to be wire-shaped and flexible to accommodate the radial bending of cable-like nerves.[553] Some Utah electrodes also use flexible substrates to improve their adaptability to complex surface.[544] The wraparound peripheral nerve interface can reduce foreign body reactions caused by insertion.[58,302,551,554] As an example, Figure 15E demonstrates a wireless bioresorbable electronic system for peripheral neurons modulations. Utilizing a deposited layer of Mg embedded in insulting polymer to deliver electrical stimuli from the receiver antenna to the tissue, the system successfully enhanced neuroregeneration and functional recovery in rodent models (Figure 15F).[303] Of the three implantable electrical stimulation protocols mentioned above, peripheral nerve stimulation is currently the most feasible and convenient way to restore the sense of touch in humans.
7.1.2. Optogenetic stimulators
Optogenetic stimulation is based on opsins that are sensitive to light signals and only expressed in certain types of cells. By genetically engineering these proteins into target neurons and controlling the light around them, these neurons can be activated or inhibited, which is particularly important for sensory and motor recovery and rehabilitation.[555] Fortunately, optogenetic stimulation does not lead to muscle fatigue as prolonged and irregular electrical stimulation does. Furthermore, optogenetic stimulation can excite nerve cells and inhibit them, whereas electrical stimulation only excites nerves.[556] Many studies have delved into the relationship between optogenetic stimulation and neurological disorders and control, such as Parkinson's,[557] Alzheimer's,[558] spinal cord injuries,[559] and somatosensory nociception,[560,561] which have indistinguishable relationship with prosthetic control, feedback, and human movement rehabilitation. In spite of its appealing advantages and functions, optogenetics is still mainly exist in theoretical and animal experiments, without few practical applications to humans, currently.
Light delivery has proven to be a vexing challenge in optogenetic applications, which typically require implantable optical stimulators. With the advances of materials science and manufacturing technologies, flexible light-emitting diodes (LED) [562] and microscale, inorganic LED (μ-ILED)[563] with biocompatibility have been reported to facilitate the progress in optogenetic stimulation for central and peripheral nervous system. For instance, flexible deep brain needles or wires offers the most widely adopted carrier for optogenetic localization of cellular-scale stimulation in brain.[342,563–568] Figure 15G shows a multifunctional system consisting of μ-ILEDs and photodetector for optogenetic stimulation. This radio frequency-based optogenetics device and its successful operation for up to four weeks represent a novel stride into closed-loop wireless optogenetic modulation for expediting the adoption of optogenetics technologies in clinical applications (Figure 15H).[566] Optogenetics was initially applied in rodent models to control neural circuits in the brain, but recent research efforts have shown great promise in modulating the activity of the spinal cord and peripheral nervous system.[556] However, the application of optogenetics to the spinal cord is somewhat limited by the complex structure, dynamic mechanical characteristics, anchorless nature and low redundancy of the spinal cord that render stable insertion of luminescent sites to target regions of the spinal cord almost impossible. Thus, optical stimulators for spinal cord are generally flexible enough to be wraparound or wire-like.[556,569–571] For example, a fully implantable, battery-free wireless optoelectronic devices for spinal optogenetics is illustrated in Figure 15I. [569] This device design avoids the tethered operation in traditional optic fiber implants. Based on NFC data and power transmission, the device could be implanted over the mouse spinal cord in a minimally invasive way without impeding movement of the mouse (Figure 15J). Additionally, for fully implantable optogenetic stimulators, the mass and size of the devices have a nonnegligible effect on the animal’s freedom of movement and behavior by preventing animals from entering small enclosures or engaging in normal social interactions with others. Therefore, miniaturization or cuff shape is still the mainstream design of peripheral nerve optical stimulators[572–575]. Figure 15K and 15L demonstrate a wireless optogenetic devices weigh 20–50 mg, which is two orders of magnitude smaller and lighter than previously reported wireless optogenetic systems.[574] In addition to the above mentioned types, there are alternative stimulation modalities in optical stimulation, such as array-based optical brain stimulators[576] and transcutaneous stimulation,[577] yet some of these are relatively restricted in their selectivity for specific nerves.
7.2. Non-invasive stimulators
Emerging noninvasive tactile stimulation methods, like transdermal electrical, mechanical stimulation, thermal feedback (introduced in section 6.2.2), provide alternative solutions to stimulate the human body for prosthetic and rehabilitation applications. These methods also can serve as multidimensional, multisensory complements to VR/AR in HMI for a more immersive and realistic experience.
Compared with implantable stimulators, noninvasive stimulators needn’t any surgical intervention, which greatly enhances the convenience and safety of stimulation. By mimicking the mechanical properties (modulus, thickness) of human skin, most flexible wearable devices are becoming more comfortable and conformable than rigid stimulators to attach on human skin directly and evoke the sensory and motor function.
7.2.1. Mechanical stimulators
Perception of the residual limb can be elicited by mechanical stimulation, such as vibration and static force stimulation. From a physiological point of view, the type of perception evoked by the feedback depends mainly on the different mechanical stimulation parameters. E.g. for force stimuli, the location of the stimulus within the array, the magnitude of the force, and even the direction of the stimulus force are all relevant factors. Whereas, the frequency and amplitude of the vibration are keys to generate different types of sensory information in the vibration stimulus.[360] For arraying stimulators, the feedback stimulation can be achieved with continuous temporal and spatial variation.[578]
Several mechanisms of micromechanical stimulation have been reported, common ones such as forces generated by fluids (hydraulic, pneumatic),[579,580] heat-induced deformation,[581–583] etc. These mechanisms are able to output significantly larger forces and deformation for static force stimulation. The study in feedback of arraying vectoral forces with these mechanisms is an area of interest. For example, a matrix composed of 15 SMA-based plasters can be attached to human skin, and the user can perceive different touch patterns through the tangential and shear forces generated by SMAs and changes in the location of working units.[584] Nonetheless, the low response speed and hysteresis of fluidic and heat-induced deformation stimulators limit both mechanisms to stimulate the skin in the form of high frequency vibrations.[585]
When it comes to the high bandwidth vibro-stimulation, it is necessary to mention DEAs,[586–591] which naturally have the advantages like an extremely wide range of stimulation frequencies and easy integration at high spatial density. However, there are still some drawbacks that hinder its adoption, such as limited actuation force and amplitude, and the need for high operating voltage (more 1kV). To solve the issue of insufficient stimulation force, multi-layer stacking structure,[592] or hydraulic pressure to amplify the force have been proposed.[63,593,594] In this high frequency vibration stimulation, piezoelectric stimulators based on the inverse piezoelectric effect are also a good alternative.[595–597] In addition to the stimulation principles mentioned above, electromagnetic stimulator can balance the frequency range and the magnitude of the stimulation force, whose mechanism is the based on the force applied to the conductor in a magnetic field. For example, Figure 16A shows a coin-sized (18 mm) electromagnetic exciter with a milliampere operating current, a Young's modulus of 130 kPa, close to the skin modulus, and an output amplitude of 300 μm (Figure 16B and 16C).[598] However, the need for coils and permanent magnets, which are usually rigid, always makes such stimulators occupy a large volume.
Figure 16.
Non-invasive feedback stimulation. A) Exploded-view schematic of wireless mechanical haptic AR/VR device. B) Prosthetics application of the device. C) The device produces a haptic pattern of sensation. A-C) Reproduced with permission.[598] Copyright 2019, Springer Nature. D) Schematic of a self-powered electro-tactile system based on transdermal electrical stimulation. E) HMI application of the TENG-powered electrical stimulator. F) The generated random array and the identification recurrence results of the subjects. D-F) Adapted with permission.[604] Copyright 2021, AAAS. Reprinted/adapted from ref.[604]. The Authors, some rights reserved; exclusive licensee AAAS. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
7.2.2. Electrical stimulators
Flexible epidermal electrotactile display is another haptic interface that provides stimulation by applying an electric current through the skin surface.[430] It has the potential to replace mechanical haptic displays owing to several advantages, such as small and thin size, lightweight and rapid responsiveness. Electrical stimulation roughly includes two types, one with voltage control, which is capable of reducing skin burns feeling during stimulation, and the other with current control, which is relatively insensitive to differences in the feature of the skin interface.[360,599,600] In addition to haptic feedback, electrical stimulation is also attractive pain relief and sensory-motor control for prosthetics and orthotics.[599,601]
Integrating epidermal electrodes, which provide opportunities in intimate, long-term interfaces to the human body,[602] on amputees’ remaining extremities has been tested in some studies.[603] A representative example is that subjects wearing a PI/Au-based stimulating and sensing hybrid platform attempted to control a robot hand to grip a bottle both with and without electro-tactile stimulation. The result illustrates that subjects with force feedback were able to stop grasping at any desired level of gentle touch, while those without force feedback cannot. Moreover, in this demonstration, the facts that the parameters of the applied current on the skin determines the tactile stimulation comfort, and stimulation with a large current will induce muscle contractions were also verified.[599] Conventional electrical stimulators cannot work without bulky power sources, which greatly confined their portability. TENGs provide a potential solution to achieve self-powered, wireless electrical stimulation. Figure 16D presents a noninvasive electrical stimulation system consisting of a TENG array for power supply and a ball-shaped electrode array for current simulation. Subjects wearing the system can identify different virtual spatial patterns by spatio-temporal varying electrical stimulation (Figure 16E and 16F).[604] Nevertheless, the variability and over time drift of skin impedance also present perplexing challenges for electrotactile stimulation at the same time.[598]
7.3. Discussion
This subsection focuses on the process of transferring external information into the human body in the form of various stimulus in HMI, and summarizes the prevailing implantable electrostimulation, optogenetic stimulation devices and wearable electrostimulation along with mechanical stimulation devices in this field. These flexible devices serve as interfaces that establish a solid foundation for the human body to obtain external information or to receive therapeutic treatment.
Nonetheless there are still some distinct challenges in this area that are waiting to be addressed. The first problem is energy supply. One of the differences between stimulators and sensors is that stimulators consume much more energy than sensors. Currently well-studied energy supply schemes are ultrasound, NFC, piezoelectric, etc. Improving the efficiency of these existing energy supply methods and exploring new ones will facilitate the promotion of stimulators. Next challenge is stimulation density, due to structural, material, or fabrication limitations, the spatial density of many stimulation arrays is limited, for example, high-density implantable electrical stimulators can easily exceed cell safety limits while achieving stimulation margins. Multifunctional integration should also be attached attention. Various implantable or wearable stimulators have unique advantages, and multimodal stimulation is a potentially advantageous pooling option. Finally, the potential development of flexible devices for many stimulation mechanisms is still not receiving community attention, such as acoustic stimulation, and these flexible devices can also potentially be explored as interfaces for HMI.
8. Systematic integration
Sensors, recorders, artificial neurons, actuators, and stimulators are all devices that are based on soft technologies that acquire and process information from external environments and the human body. Each of these systems is thereby subjected to human movement, requiring dexterity as the devices change shape. In a system, they can work independently, but, ideally, each part can form a closed-loop system that is interlinked to perform more precise and complex tasks.[76,153] Currently, some integrated work can enhance space utilization,[599] reduce manufacturing costs,[155] and achieve in-situ information sensing, processing, and actuating simultaneously for more accurate therapy.[548,605]
A flexible prosthetic system has a representative flow of information with various integrated components. For example, the electrophysiological signals recorded by a flexible recorder are processed by machine learning algorithms to decode movement intentions, which can be used to control a soft robotic hand. With the aid of soft robotic hand motions, flexible e-skins can then acquire various signals from the exterior environment. Such stimuli can be further converted into electrophysiological signals through artificial synapses, which are then fed back into the body through flexible stimulators. All components must have good flexibility, biocompatibility, and biosimilarity. As an exemplary case, a soft EMG controlled pneumatic neuroprosthetic with ionic capacitive sensors and flexible stimulators was developed to reduce manufacturing costs and achieve lightweight actuation. The on-body test of this system showed excellent results: after 15 minutes of training, the amputee was able to use the flexible prosthetic system to perform a range of precise gripping and social tasks, demonstrating tremendous potential for future applications.[76]
For medical rehabilitation, system integration is also critical to achieve precise treatment and assess rehabilitation efficacy. For example, implantable systems for motion-related epilepsy rely on real-time acquisition and analysis of pre-seizure brain electrical signals and perform compensatory electrical stimulation to eliminate abnormal brain waves and stop the epilepsy midway.[606] In addition to the neurological aspects of motor therapy, flexible system integration can also be applied to the limb, such as " mirror" therapy for the rehabilitation of stroke patients, which is based on monitoring the healthy limb and mirroring the actuation to the hemiplegic limb. This combination therapy can achieve independent rehabilitation without a guide.[158] The evaluation of optogenetic stimulation relies mainly on real-time recording of the in situ electrophysiological signals. Consequently, many integrated flexible stimulation systems can also perform electrophysiological recording.[52]
9. Prospects and Outlooks
In today’s digital era, numerous man-made digital mechatronics products are beginning to permeate into natural analogous human life, and the boundaries between the two are gradually blurring. However, there is no denying the legitimate fact that the interaction between them should always be human-centered, that is to say, to seek the maximum functionality and performance of the device under the premise of biocompatibility. Because of favorable biocompatibility, high bionic, low-cost, and safety features, soft electronics and devices, a field that has emerged in recent decades, can gradually shoulder the role of bridging the gap between the human and machines. This is especially true in medical robotics for people with disabilities, such as prosthetic engineering, rehabilitation science and other related fields. Soft devices involve extremely comprehensive technologies, including flexible sensing, soft actuation, brain-machine interface and other relevant domains. These devices based on new materials and structures integrate the information flow from the human body with that from the external environment, and use ML methods for deep processing to control machines for achieving target actuation effects or feedback to humans themselves in a biocompatible form. Human expect machines in a broad term to be friendly for coexisting with human, and to make a difference for a better living. Although the attributes of these devices are very diverse, they also have common problems with corresponding solutions as human-machine interfaces, which are proposed and discussed in the following.
Biocompatibility always comes first in human-machine interfaces for medical robotics. Assuring that materials are not toxic to humans is the most elemental principle of biocompatibility. While many conceptual devices based on various materials have been validated in academic laboratories. However, the long-term impact of these materials on the human body remains an uncertainty limiting the actual long-term commercial application of the devices in HMI. An example is the discussion of the safety of nanomaterials.[607,608] Long-term validation experiments in animals with toxicological analysis are potential toxicity assessment solutions. In addition, biocompatibility encompasses the fields of biomechanics, biochemistry, and immunology, which require materials that are mechanically compliant, chemically inert, etc. Therefore, in-depth exploration of materials and elastic structures is a major direction to improve biocompatibility.
Performance is the criterion for device functionality. In sensors, the balance and optimization of sensitivity, linearity, response time, limit of detection, measuring range, hysteresis and other properties are constantly pursued by the academic community. While for neural interfaces, impedance in recording and charge injection capability in stimulation are key properties. Actuation range, amplitude, frequency, response time are the criteria that determine the performance of soft actuators. The multiple performance of the device often has an implicated relationship, the optimization of one performance may bring another performance limitation (such as sensitivity vs. range,[112] accuracy vs. softness[35] etc.), how to balance and optimize the device parameters according to the performance requirements of the application is a point of consideration for personalized device design.
Interface issues for signal relay are a major challenge in HMI robotics, specifically for mechanical and electrical components. There are two main types of interfaces: within the device itself and between biological and mechanical components. For both of these examples, interface problems mainly arise in heterogeneous integration of different materials, such as for material bonding and embedding, which can be addressed by micromechanics, chemical bonding links, etc.[609] Specifically for human-device interfaces, there are also macro interface problems one must consider. For example, the large conformal attachment of prosthetic skin,[149] sweat trap between the wearable sensor and the skin,[75] and fixation of neural implants.[420] By addressing these key issues, one can affirm proper device function.
Sensor design should be forward-thinking, using intelligent algorithms to inform which sensors are required for proper data analysis. The reason why humans are specialized in perception and movement is not only due to the excellent performance of receptors and musculoskeletons, but also because the brain is skilled in processing this raw information. There have been many outstanding examples of exploiting machine learning in the field of sensors and actuators, such as extracting motion intent from electrophysiological signals,[287,610] using haptics to identify object categories,[160,611] decoupling multimodal signals from cross-reactive sensor arrays,[231,612] and controlling soft robots[613,614]. However, most of the hardware research is still delinked from intelligent algorithms. Machine learning enhanced hardware design can help limit the number of sensors and electronics needed to collect data.
Maintaining proper power supply is another important issue in HMI devices. Traditional tethered and battery power suffer from motion restrictions and sophisticated recharging processes respectively, which are obstacles to the application of both wearable and implantable devices. Researchers are currently investigating wireless-powered and self-powered devices, which is of upmost important for invasive devices that currently require surgery for battery replacement. For wireless energy, NFCs[568] and ultrasonic[372] are good powering methods, but still need the energy transmitter, as well as receiving energy at a certain range; biofuel cells,[615] piezoelectric,[122] triboelectric,[127] solar[616] and other self-powered methods are prominent representatives of power supply for low-energy devices, truly enabling the user to move freely. However, for soft devices with high energy consumption, such as soft prostheses, the energy supply method still needs to be further explored.
The fabrication of many flexible devices and electronics is still based on manual work, which increases the uncertainty of the device performance as well as the difficulty of mass production. To eliminate these issues, some potential mass-processing technologies are emerging, such as laser-based processing,[249,617] roll-to-roll printing,[618,619] inkjet printing,[620] and other technologies for flexible electronics that offer excellent and consistent performance while ensuring fast, low-cost manufacturing. Therefore, tailoring the fabrication technique to such mass-producible platform or exploring new processing technologies can boost the market application of these low-cost soft devices.
System integration is the ultimate goal for biomimetic prosthetics and is a closed loop where all the above-mentioned device types are connected.[621] Specifically, flexible electrophysiological recorders capture the movement intent from human body, decodes it, and forms a prosthetic control signal. Under the guidance of the signal, the soft robots (prosthesis) moves and perceives external information using the e-skin mounted on the actuator. In turn, this information is stimulated back to the body in the form of biocompatible signals through the flexible stimulator, constituting a complete closed-loop. Among these, the physical integration of sensors and actuators, the communication and transformation of in vivo signal-electrical signal-physical information are potentially challenges for the deepening of scientific research. Promisingly, some prospective systematic works have been reported.[76,153]
Acknowledgements
Wenzheng Heng and Samuel Solomon contributed equally to this work. This project was supported by the National Institutes of Health grant R01HL155815, Office of Naval Research grant N00014–21-1–2483 and N00014–21-1–2845, the Translational Research Institute for Space Health through NASA NNX16AO69A, the Tobacco-Related Disease Research Program grant R01RG3746, and Carver Mead New Adventures Fund at California Institute of Technology.
Biography
Wenzheng Heng received his B.S. degree in mechatronics engineering from Zhejiang University, Hangzhou, China, in 2020. He joined Dr. Wei Gao’s research group in 2020 and is currently pursuing his Ph.D. degree in medical engineering at Caltech. His research interests include wearable devices for medical and robotic applications, flexible electronics, and human-machine interfaces.

Samuel Solomon received his BS in chemistry-biology and physics from the Massachusetts Institute of Technology. Currently, he is pursuing his PhD in medical engineering under the supervision of Dr. Wei Gao at the California Institute of Technology. His main research interests include wearable electronics, machine learning, and aeromedical technology.

Wei Gao is currently an assistant professor of medical engineering at the California Institute of Technology. He received his Ph.D. in chemical engineering from the University of California, San Diego in 2014. He worked as a postdoctoral fellow in electrical engineering and computer sciences at the University of California, Berkeley between 2014 and 2017. His current research interests include wearable biosensors, robotics, flexible electronics, and nanomedicine.

Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Broadbent E, Stafford R, MacDonald B, Int. J. Soc. Robot 2009, 1, 319. [Google Scholar]
- [2].Flandorfer P, J Popul Res (Canberra) 2012, 2012. [Google Scholar]
- [3].Krebs H, Dipietro L, Levy-Tzedek S, Fasoli S, Rykman-Berland A, Zipse J, Fawcett J, Stein J, Poizner H, Lo A, Volpe B, Hogan N, IEEE Eng. Med. Biol. Mag. 2008, 27, 61.19004697 [Google Scholar]
- [4].Tijsen LM, Derksen EW, Achterberg WP, Buijck BI, Clin Interv Aging 2019, 14, 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jaul E, Barron J, Front. Public Health 2017, 5, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].United Nations, Ageing and disability, https://www.un.org/development/desa/disabilities/disability-and-ageing.html, accessed: 2016.
- [7].Coco DL, Lopez G, Corrao S, Vasc Health Risk Manag 2016, 12, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Laut J, Porfiri M, Raghavan P, Curr Phys Med Rehabil Rep 2016, 4, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].CDC, Disability Impacts All of Us, https://www.cdc.gov/ncbddd/disabilityandhealth/infographic-disability-impacts-all.html, accessed: 2019.
- [10].Winship IR, Murphy TH, Neuroscientist 2009, 15, 507. [DOI] [PubMed] [Google Scholar]
- [11].Jenkins WM, Merzenich MM, in Progress in Brain Research (Eds.: Seil FJ, Herbert E, Carlson BM), Elsevier, 1987, pp. 249–266. [DOI] [PubMed] [Google Scholar]
- [12].Krebs HI, Volpe BT, in Handbook of Clinical Neurology (Eds.: Barnes MP, Good DC), Elsevier, 2013, pp. 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rothwell JC, Folia Phoniatr Logop 2010, 62, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ashley MJ, Cerebrum (N.Y.N.Y., Online) 2012, 2012, 8. [PMC free article] [PubMed] [Google Scholar]
- [15].Hogan N, Krebs HI, Charnnarong J, Srikrishna P, Sharon A, in Telemanipulator Technology, International Society for Optics and Photonics, 1993, pp. 28–34. [Google Scholar]
- [16].van Vliet P, Wing AM, Phys Ther 1991, 71, 39. [DOI] [PubMed] [Google Scholar]
- [17].Van der Loos HFM, Reinkensmeyer DJ, Guglielmelli E, in Springer Handbook of Robotics (Eds.: Siciliano B, Khatib O), Springer International Publishing, Cham, 2016, pp. 1685–1728. [Google Scholar]
- [18].Song W-K, Lee H-Y, Kim J-S, Yoon Y-S, Bien Z, in Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Vol. 20 Biomedical Engineering Towards the Year 2000 and Beyond (Cat. No. 98CH36286), IEEE, 1998, pp. 2682–2685. [Google Scholar]
- [19].Stephens-Fripp B, Alici G, Mutlu R, IEEE Access 2018, 6, 6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bates TJ, Fergason JR, Pierrie SN, Curr. Rev. Musculoskelet. Med. 2020, 13, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ring ME, Plast. Reconstr. Surg. 1991, 87, 174. [DOI] [PubMed] [Google Scholar]
- [22].Zuo KJ, Olson JL, Plast Surg (Oakv) 2014, 22, 44. [PMC free article] [PubMed] [Google Scholar]
- [23].Grand View Research “Prosthetics & Orthotics Market Size Report, 2021–2028”, https://www.grandviewresearch.com/industry-analysis/prosthetics-orthotics-market, accessed: 2020.
- [24].Tucker MR, Olivier J, Pagel A, Bleuler H, Bouri M, Lambercy O, del R. Millán J, Riener R, Vallery H, Gassert R, J. Neuroeng. Rehabilitation 2015, 12, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yang JC, Mun J, Kwon SY, Park S, Bao Z, Park S, Adv. Mater. 2019, 31, 1904765. [DOI] [PubMed] [Google Scholar]
- [26].Clippinger FW, Avery R, Titus BR, Bull. prosthet. res 1974, 10, 247. [PubMed] [Google Scholar]
- [27].Mohr DC, Zhang M, Schueller SM, Annu Rev Clin Psychol 2017, 13, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Herbert R, Kim J-H, Kim Y, Lee H, Yeo W-H, Materials 2018, 11, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Trung TQ, Lee N-E, Adv. Mater. 2017, 29, 1603167. [DOI] [PubMed] [Google Scholar]
- [30].Benight SJ, Wang C, Tok JBH, Bao Z, Prog. Polym. Sci. 2013, 38, 1961. [Google Scholar]
- [31].Cruz RLJ, Ross MT, Powell SK, Woodruff MA, Front. Bioeng. Biotechnol. 2020, 8, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang M, Luo Y, Wang T, Wan C, Pan L, Pan S, He K, Neo A, Chen X, Adv. Mater. 2021, 33, 2003014. [DOI] [PubMed] [Google Scholar]
- [33].Kim S, Laschi C, Trimmer B, Trends Biotechnol. 2013, 31, 287. [DOI] [PubMed] [Google Scholar]
- [34].Whitesides GM, Angew. Chem. Int. Ed. 2018, 57, 4258. [DOI] [PubMed] [Google Scholar]
- [35].Rus D, Tolley MT, Nature 2015, 521, 467. [DOI] [PubMed] [Google Scholar]
- [36].Dahiya R, Yogeswaran N, Liu F, Manjakkal L, Burdet E, Hayward V, Jorntell H, Proc IEEE Inst Electr Electron Eng 2019, 107, 2016. [Google Scholar]
- [37].Shih B, Shah D, Li J, Thuruthel TG, Park Y-L, Iida F, Bao Z, Kramer-Bottiglio R, Tolley MT, Sci. Robot. 2020, 5, eaaz9239. [DOI] [PubMed] [Google Scholar]
- [38].Cianchetti M, Laschi C, Menciassi A, Dario P, Nat. Rev. Mater. 2018, 3, 143. [Google Scholar]
- [39].Chen R, Canales A, Anikeeva P, Nat. Rev. Mater. 2017, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liu S, Shah DS, Kramer-Bottiglio R, Nat. Mater. 2021, 20, 851. [DOI] [PubMed] [Google Scholar]
- [41].Xu J, Wang S, Wang G-JN, Zhu C, Luo S, Jin L, Gu X, Chen S, Feig VR, To JWF, Rondeau-Gagné S, Park J, Schroeder BC, Lu C, Oh JY, Wang Y, Kim Y-H, Yan H, Sinclair R, Zhou D, Xue G, Murmann B, Linder C, Cai W, Tok JB-H, Chung JW, Bao Z, Science 2017, 355, 59. [DOI] [PubMed] [Google Scholar]
- [42].Wang S, Xu J, Wang W, Wang G-JN, Rastak R, Molina-Lopez F, Chung JW, Niu S, Feig VR, Lopez J, Lei T, Kwon S-K, Kim Y, Foudeh AM, Ehrlich A, Gasperini A, Yun Y, Murmann B, Tok JB-H, Bao Z, Nature 2018, 555, 83. [DOI] [PubMed] [Google Scholar]
- [43].Xu S, Zhang Y, Jia L, Mathewson KE, Jang K-I, Kim J, Fu H, Huang X, Chava P, Wang R, Bhole S, Wang L, Na YJ, Guan Y, Flavin M, Han Z, Huang Y, Rogers JA, Science 2014, 344, 70. [DOI] [PubMed] [Google Scholar]
- [44].Lee S, Franklin S, Hassani FA, Yokota T, Nayeem MOG, Wang Y, Leib R, Cheng G, Franklin DW, Someya T, Science 2020, 370, 966. [DOI] [PubMed] [Google Scholar]
- [45].Yang Y, Song Y, Bo X, Min J, Pak OS, Zhu L, Wang M, Tu J, Kogan A, Zhang H, Hsiai TK, Li Z, Gao W, Nat. Biotechnol. 2020, 38, 217. [DOI] [PubMed] [Google Scholar]
- [46].Xu K, Lu Y, Honda S, Arie T, Akita S, Takei K, J. Mater. Chem. C 2019, 7, 9609. [Google Scholar]
- [47].Shi X, Zuo Y, Zhai P, Shen J, Yang Y, Gao Z, Liao M, Wu J, Wang J, Xu X, Tong Q, Zhang B, Wang B, Sun X, Zhang L, Pei Q, Jin D, Chen P, Peng H, Nature 2021, 591, 240. [DOI] [PubMed] [Google Scholar]
- [48].Kaltenbrunner M, Sekitani T, Reeder J, Yokota T, Kuribara K, Tokuhara T, Drack M, Schwödiauer R, Graz I, Bauer-Gogonea S, Bauer S, Someya T, Nature 2013, 499, 458. [DOI] [PubMed] [Google Scholar]
- [49].Wang C, Wang C, Huang Z, Xu S, Adv. Mater. 2018, 30, 1801368. [DOI] [PubMed] [Google Scholar]
- [50].Feiner R, Dvir T, Nat. Rev. Mater. 2018, 3, 17076. [Google Scholar]
- [51].Won SM, Song E, Zhao J, Li J, Rivnay J, Rogers JA, Adv. Mater. 2018, 30, 1800534. [DOI] [PubMed] [Google Scholar]
- [52].Park S, Yuk H, Zhao R, Yim YS, Woldeghebriel EW, Kang J, Canales A, Fink Y, Choi GB, Zhao X, Anikeeva P, Nat. Commun. 2021, 12, 3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lin S, Yuk H, Zhang T, Parada GA, Koo H, Yu C, Zhao X, Adv. Mater. 2016, 28, 4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rivnay J, Wang H, Fenno L, Deisseroth K, Malliaras GG, Sci. Adv. 2017, 3, e1601649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wirthl D, Pichler R, Drack M, Kettlguber G, Moser R, Gerstmayr R, Hartmann F, Bradt E, Kaltseis R, Siket CM, Schausberger SE, Hild S, Bauer S, Kaltenbrunner M, Sci. Adv. 2017, 3, e1700053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, bok Kim S, Nikkhah M, Khabiry M, Azize M, Kong J, Wan K, Palacios T, Dokmeci MR, Bae H, (Shirley) Tang X, A. Khademhosseini, ACS Nano 2013, 7, 2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Keplinger C, Sun J-Y, Foo CC, Rothemund P, Whitesides GM, Suo Z, Science 2013, 341, 984. [DOI] [PubMed] [Google Scholar]
- [58].Liu Y, Liu J, Chen S, Lei T, Kim Y, Niu S, Wang H, Wang X, Foudeh AM, Tok JB-H, Bao Z, Nat. Biomed. Eng. 2019, 3, 58. [DOI] [PubMed] [Google Scholar]
- [59].Song E, Li J, Won SM, Bai W, Rogers JA, Nat. Mater. 2020, 19, 590. [DOI] [PubMed] [Google Scholar]
- [60].Zhou W, Yao S, Wang H, Du Q, Ma Y, Zhu Y, ACS Nano 2020, 14, 5798. [DOI] [PubMed] [Google Scholar]
- [61].Pang C, Lee G-Y, Kim T, Kim SM, Kim HN, Ahn S-H, Suh K-Y, Nat. Mater. 2012, 11, 795. [DOI] [PubMed] [Google Scholar]
- [62].Acome E, Mitchell SK, Morrissey TG, Emmett MB, Benjamin C, King M, Radakovitz M, Keplinger C, Science 2018, 359, 61. [DOI] [PubMed] [Google Scholar]
- [63].Leroy E, Hinchet R, Shea H, Adv. Mater. 2020, 32, 2002564. [DOI] [PubMed] [Google Scholar]
- [64].Jiao Z, Zhang C, Wang W, Pan M, Yang H, Zou J, Adv. Sci. 2019, 6, 1901371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Miriyev A, Stack K, Lipson H, Nat. Commun. 2017, 8, 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Larson C, Peele B, Li S, Robinson S, Totaro M, Beccai L, Mazzolai B, Shepherd R, Science 2016, 351, 1071. [DOI] [PubMed] [Google Scholar]
- [67].Kang J, Tok JB-H, Bao Z, Nat. Electron. 2019, 2, 144. [Google Scholar]
- [68].Kang S-K, Murphy RK, Hwang S-W, Lee SM, Harburg DV, Krueger NA, Shin J, Gamble P, Cheng H, Yu S, Nature 2016, 530, 71. [DOI] [PubMed] [Google Scholar]
- [69].Wu X, Zhou J, Huang J, Macromol Rapid Commun 2018, 39, 1800084. [DOI] [PubMed] [Google Scholar]
- [70].Fu H, Nan K, Bai W, Huang W, Bai K, Lu L, Zhou C, Liu Y, Liu F, Wang J, Han M, Yan Z, Luan H, Zhang Y, Zhang Y, Zhao J, Cheng X, Li M, Lee JW, Liu Y, Fang D, Li X, Huang Y, Zhang Y, Rogers JA, Nat. Mater. 2018, 17, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kim BH, Li K, Kim J-T, Park Y, Jang H, Wang X, Xie Z, Won SM, Yoon H-J, Lee G, Nature 2021, 597, 503. [DOI] [PubMed] [Google Scholar]
- [72].Miao L, Song Y, Ren Z, Xu C, Wan J, Wang H, Guo H, Xiang Z, Han M, Zhang H, Adv. Mater. 2021, 33, 2102691. [DOI] [PubMed] [Google Scholar]
- [73].Miyamoto A, Lee S, Cooray NF, Lee S, Mori M, Matsuhisa N, Jin H, Yoda L, Yokota T, Itoh A, Sekino M, Kawasaki H, Ebihara T, Amagai M, Someya T, Nat. Nanotech. 2017, 12, 907. [DOI] [PubMed] [Google Scholar]
- [74].Li Z, Zhu M, Shen J, Qiu Q, Yu J, Ding B, Adv. Funct. Mater. 2020, 30, 1908411. [Google Scholar]
- [75].Yeon H, Lee H, Kim Y, Lee D, Lee Y, Lee J-S, Shin J, Choi C, Kang J-H, Suh JM, Kim H, Kum HS, Lee J, Kim D, Ko K, Ma BS, Lin P, Han S, Kim S, Bae S-H, Kim T-S, Park M-C, Joo Y-C, Kim E, Han J, Kim J, Sci. Adv. 2021, 7, eabg8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gu G, Zhang N, Xu H, Lin S, Yu Y, Chai G, Ge L, Yang H, Shao Q, Sheng X, Nat. Biomed. Eng. 2021, 1. [DOI] [PubMed] [Google Scholar]
- [77].Lee S, Reuveny A, Reeder J, Lee S, Jin H, Liu Q, Yokota T, Sekitani T, Isoyama T, Abe Y, Suo Z, Someya T, Nat. Nanotech. 2016, 11, 472. [DOI] [PubMed] [Google Scholar]
- [78].Shi L, Zhu T, Gao G, Zhang X, Wei W, Liu W, Ding S, Nat. Commun. 2018, 9, 2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Pang G, Yang G, Heng W, Ye Z, Huang X, Yang H-Y, Pang Z, IEEE Trans. Ind. Electron. 2021, 68, 3303. [Google Scholar]
- [80].Lumpkin EA, Caterina MJ, Nature 2007, 445, 858. [DOI] [PubMed] [Google Scholar]
- [81].Zhu P, Du H, Hou X, Lu P, Wang L, Huang J, Bai N, Wu Z, Fang NX, Guo CF, Nat. Commun. 2021, 12, 4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang Y, Lee S, Yokota T, Wang H, Jiang Z, Wang J, Koizumi M, Someya T, Sci. Adv. 2020, 6, eabb7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gao W, Emaminejad S, Nyein HYY, Challa S, Chen K, Peck A, Fahad HM, Ota H, Shiraki H, Kiriya D, Lien D-H, Brooks GA, Davis RW, Javey A, Nature 2016, 529, 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Xu K, Fujita Y, Lu Y, Honda S, Shiomi M, Arie T, Akita S, Takei K, Adv. Mater. 2021, 33, 2008701. [DOI] [PubMed] [Google Scholar]
- [85].Petritz A, Karner-Petritz E, Uemura T, Schäffner P, Araki T, Stadlober B, Sekitani T, Nat. Commun. 2021, 12, 2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Chortos A, Liu J, Bao Z, Nat. Mater. 2016, 15, 937. [DOI] [PubMed] [Google Scholar]
- [87].Ma Y, Zhang Y, Cai S, Han Z, Liu X, Wang F, Cao Y, Wang Z, Li H, Chen Y, Feng X, Adv. Mater. 2020, 32, 1902062. [DOI] [PubMed] [Google Scholar]
- [88].Hadjidj A, Bouabdallah A, Challal Y, in 2011 IEEE International Symposium on a World of Wireless, Mobile and Multimedia Networks, 2011, pp. 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Giorgino T, Tormene P, Maggioni G, Pistarini C, Quaglini S, IEEE trans. inf. technol. biomed. 2009, 13, 1012. [DOI] [PubMed] [Google Scholar]
- [90].Pappas IPI, Keller T, Mangold S, Popovic M, Dietz V, Morari M, IEEE Sens. J. 2004, 4, 268. [Google Scholar]
- [91].Ciui B, Martin A, Mishra RK, Nakagawa T, Dawkins TJ, Lyu M, Cristea C, Sandulescu R, Wang J, ACS Sens. 2018, 3, 2375. [DOI] [PubMed] [Google Scholar]
- [92].Yao S, Zhu Y, Nanoscale 2014, 6, 2345. [DOI] [PubMed] [Google Scholar]
- [93].Ha M, Lim S, Ko H, J. Mater. Chem. B 2018, 6, 4043. [DOI] [PubMed] [Google Scholar]
- [94].Niu S, Matsuhisa N, Beker L, Li J, Wang S, Wang J, Jiang Y, Yan X, Yun Y, Burnett W, Poon ASY, Tok JB-H, Chen X, Bao Z, Nat. Electron. 2019, 2, 361. [Google Scholar]
- [95].Chortos A, Bao Z, Mater. Today 2014, 17, 321. [Google Scholar]
- [96].Van Boven RW, Johnson KO, Neurology 1994, 44, 2361. [DOI] [PubMed] [Google Scholar]
- [97].Di Giacomo R, Bonanomi L, Costanza V, Maresca B, Daraio C, Sci. Robot. 2017, 2, eaai9251. [DOI] [PubMed] [Google Scholar]
- [98].Bai N, Wang L, Wang Q, Deng J, Wang Y, Lu P, Huang J, Li G, Zhang Y, Yang J, Xie K, Zhao X, Guo CF, Nat. Commun. 2020, 11, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Yan Y, Hu Z, Yang Z, Yuan W, Song C, Pan J, Shen Y, Sci. Robot. 2021, 6, eabc8801. [DOI] [PubMed] [Google Scholar]
- [100].Wu Y, Liu Y, Zhou Y, Man Q, Hu C, Asghar W, Li F, Yu Z, Shang J, Liu G, Sci. Robot. 2018, 3, eaat0429. [DOI] [PubMed] [Google Scholar]
- [101].Lee B, Oh J-Y, Cho H, Joo CW, Yoon H, Jeong S, Oh E, Byun J, Kim H, Lee S, Seo J, Park CW, Choi S, Park N-M, Kang S-Y, Hwang C-S, Ahn S-D, Lee J-I, Hong Y, Nat. Commun. 2020, 11, 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zang Y, Zhang F, Huang D, Gao X, Di C, Zhu D, Nat. Commun. 2015, 6, 6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yokota T, Inoue Y, Terakawa Y, Reeder J, Kaltenbrunner M, Ware T, Yang K, Mabuchi K, Murakawa T, Sekino M, Voit W, Sekitani T, Someya T, Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 14533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hua Q, Sun J, Liu H, Bao R, Yu R, Zhai J, Pan C, Wang ZL, Nat. Commun. 2018, 9, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].An BW, Heo S, Ji S, Bien F, Park J-U, Nat. Commun. 2018, 9, 2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kim J, Lee M, Shim HJ, Ghaffari R, Cho HR, Son D, Jung YH, Soh M, Choi C, Jung S, Chu K, Jeon D, Lee S-T, Kim JH, Choi SH, Hyeon T, Kim D-H, Nat. Commun. 2014, 5, 5747. [DOI] [PubMed] [Google Scholar]
- [107].Zhang F, Zang Y, Huang D, Di C, Zhu D, Nat. Commun. 2015, 6, 8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hammock ML, Chortos A, Tee BC-K, Tok JB-H, Bao Z, Adv. Mater. 2013, 25, 5997. [DOI] [PubMed] [Google Scholar]
- [109].Chun S, Son W, Kim H, Lim SK, Pang C, Choi C, Nano Lett. 2019, 19, 3305. [DOI] [PubMed] [Google Scholar]
- [110].Chun S, Kim J-S, Yoo Y, Choi Y, Jung SJ, Jang D, Lee G, Song K-I, Nam KS, Youn I, Son D, Pang C, Jeong Y, Jung H, Kim Y-J, Choi B-D, Kim J, Kim S-P, Park W, Park S, Nat Electron 2021, 4, 429. [Google Scholar]
- [111].Pan L, Chortos A, Yu G, Wang Y, Isaacson S, Allen R, Shi Y, Dauskardt R, Bao Z, Nat. Commun. 2014, 5, 3002. [DOI] [PubMed] [Google Scholar]
- [112].Heng W, Yang G, Pang G, Ye Z, Lv H, Du J, Zhao G, Pang Z, Adv. Intell. Syst. 2021, 3, 2000038. [Google Scholar]
- [113].Gao Y, Ota H, Schaler EW, Chen K, Zhao A, Gao W, Fahad HM, Leng Y, Zheng A, Xiong F, Zhang C, Tai L-C, Zhao P, Fearing RS, Javey A, Adv. Mater. 2017, 29, 1701985. [DOI] [PubMed] [Google Scholar]
- [114].Shi L, Li Z, Chen M, Qin Y, Jiang Y, Wu L, Nat. Commun. 2020, 11, 3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Park J, Lee Y, Hong J, Ha M, Jung Y-D, Lim H, Kim SY, Ko H, ACS Nano 2014, 8, 4689. [DOI] [PubMed] [Google Scholar]
- [116].Chiolerio A, Roppolo I, Bejtka K, Asvarov A, Pirri CF, RSC Adv. 2016, 6, 56661. [Google Scholar]
- [117].Wang L, Zhu R, Li G, ACS Appl. Mater. Interfaces 2020, 12, 1953. [DOI] [PubMed] [Google Scholar]
- [118].Jia J, Huang G, Deng J, Pan K, Nanoscale 2019, 11, 4258. [DOI] [PubMed] [Google Scholar]
- [119].Shintake J, Piskarev E, Jeong SH, Floreano D, Adv. Mater. Technol. 2018, 3, 1700284. [Google Scholar]
- [120].Lipomi DJ, Vosgueritchian M, Tee BC-K, Hellstrom SL, Lee JA, Fox CH, Bao Z, Nat. Nanotech. 2011, 6, 788. [DOI] [PubMed] [Google Scholar]
- [121].Fan F-R, Tian Z-Q, Lin Wang Z, Nano Energy 2012, 1, 328. [Google Scholar]
- [122].Wang ZL, Song J, Science 2006, 312, 242. [DOI] [PubMed] [Google Scholar]
- [123].Wang H, Han M, Song Y, Zhang H, Nano Energy 2021, 81, 105627. [Google Scholar]
- [124].Oh H, Yi G-C, Yip M, Dayeh SA, Sci. Adv. 2020, 6, eabd7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Yao H, Yang W, Cheng W, Tan YJ, See HH, Li S, Ali HPA, Lim BZH, Liu Z, Tee BCK, Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 25352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Dagdeviren C, Su Y, Joe P, Yona R, Liu Y, Kim Y-S, Huang Y, Damadoran AR, Xia J, Martin LW, Huang Y, Rogers JA, Nat. Commun. 2014, 5, 4496. [DOI] [PubMed] [Google Scholar]
- [127].Fan W, He Q, Meng K, Tan X, Zhou Z, Zhang G, Yang J, Wang ZL, Sci. Adv. 2020, 6, eaay2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Lamport ZA, Cavallari MR, Kam KA, McGinn CK, Yu C, Kymissis I, Adv. Funct. Mater. 2020, 30, 2004700. [Google Scholar]
- [129].Dai Y, Hu H, Wang M, Xu J, Wang S, Nat. Electron. 2021, 4, 17. [Google Scholar]
- [130].Schwartz G, Tee BC-K, Mei J, Appleton AL, Kim DH, Wang H, Bao Z, Nat. Commun. 2013, 4, 1859. [DOI] [PubMed] [Google Scholar]
- [131].Shin S-H, Ji S, Choi S, Pyo K-H, Wan An B, Park J, Kim J, Kim J-Y, Lee K-S, Kwon S-Y, Heo J, Park B-G, Park J-U, Nat. Commun. 2017, 8, 14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Huang Y-C, Liu Y, Ma C, Cheng H-C, He Q, Wu H, Wang C, Lin C-Y, Huang Y, Duan X, Nat. Electron. 2020, 3, 59. [Google Scholar]
- [133].Sekitani T, Zschieschang U, Klauk H, Someya T, Nat. Mater. 2010, 9, 1015. [DOI] [PubMed] [Google Scholar]
- [134].Wang Z, Guo S, Li H, Wang B, Sun Y, Xu Z, Chen X, Wu K, Zhang X, Xing F, Li L, Hu W, Adv. Mater. 2019, 31, 1805630. [DOI] [PubMed] [Google Scholar]
- [135].Wang C, Hwang D, Yu Z, Takei K, Park J, Chen T, Ma B, Javey A, Nat. Mater. 2013, 12, 899. [DOI] [PubMed] [Google Scholar]
- [136].Bai H, Li S, Barreiros J, Tu Y, Pollock CR, Shepherd RF, Science 2020, 370, 848. [DOI] [PubMed] [Google Scholar]
- [137].Kim T, Lee S, Hong T, Shin G, Kim T, Park Y-L, Sci. Robot. 2020, 5, eabc6878. [DOI] [PubMed] [Google Scholar]
- [138].Ramuz M, Tee BC-K, Tok JB-H, Bao Z, Adv. Mater. 2012, 24, 3223. [DOI] [PubMed] [Google Scholar]
- [139].Ge J, Wang X, Drack M, Volkov O, Liang M, Cañón Bermúdez GS, Illing R, Wang C, Zhou S, Fassbender J, Kaltenbrunner M, Makarov D, Nat. Commun. 2019, 10, 4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wu X, Ahmed M, Khan Y, Payne ME, Zhu J, Lu C, Evans JW, Arias AC, Sci. Adv. 2020, 6, eaba1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Amoli V, Kim JS, Jee E, Chung YS, Kim SY, Koo J, Choi H, Kim Y, Kim DH, Nat. Commun. 2019, 10, 4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Sun J-Y, Keplinger C, Whitesides GM, Suo Z, Adv. Mater. 2014, 26, 7608. [DOI] [PubMed] [Google Scholar]
- [143].Piacenza P, Sherman S, Ciocarlie M, IEEE Robot. Autom. Lett. 2018, 3, 1434. [Google Scholar]
- [144].Lee YR, Trung TQ, Hwang B-U, Lee N-E, Nat. Commun. 2020, 11, 2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Chun K-Y, Son YJ, Han C-S, ACS Nano 2016, 10, 4550. [DOI] [PubMed] [Google Scholar]
- [146].Farina D, Vujaklija I, Brånemark R, Bull AM, Dietl H, Graimann B, Hargrove LJ, Hoffmann K-P, Huang HH, Ingvarsson T, Nat. Biomed. Eng. 2021, 1. [DOI] [PubMed] [Google Scholar]
- [147].Boutry CM, Negre M, Jorda M, Vardoulis O, Chortos A, Khatib O, Bao Z, Sci. Robot. 2018, 3, eaau6914. [DOI] [PubMed] [Google Scholar]
- [148].Luo Y, Li Y, Sharma P, Shou W, Wu K, Foshey M, Li B, Palacios T, Torralba A, Matusik W, Nat. Electron. 2021, 4, 193. [Google Scholar]
- [149].Cheng G, Dean-Leon E, Bergner F, Rogelio Guadarrama Olvera J, Leboutet Q, Mittendorfer P, Proc IEEE Inst Electr Electron Eng 2019, 107, 2034. [Google Scholar]
- [150].Zhu Z, Park HS, McAlpine MC, Sci. Adv. 2020, 6, eaba5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Hensleigh R, Cui H, Xu Z, Massman J, Yao D, Berrigan J, Zheng X, Nat. Electron. 2020, 3, 216. [Google Scholar]
- [152].Zhang Y, Laput G, Harrison C, in Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems, ACM, Denver Colorado USA, 2017, pp. 1–14. [Google Scholar]
- [153].Zhao H, O’Brien K, Li S, Shepherd RF, Sci. Robot. 2016, 1, eaai7529. [DOI] [PubMed] [Google Scholar]
- [154].Tomar A, Tadesse Y, in Electroactive Polymer Actuators and Devices (EAPAD) 2016, International Society for Optics and Photonics, 2016, p. 979809. [Google Scholar]
- [155].Shen Z, Zhu X, Majidi C, Gu G, Adv. Mater. 2021, 2102069. [DOI] [PubMed] [Google Scholar]
- [156].Park J, Kim M, Lee Y, Lee HS, Ko H, Sci. Adv. 2015, 1, e1500661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler J, Tyler DJ, Sci Transl Med 2014, 6, 257ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Chen X, Gong L, Wei L, Yeh S-C, Da Xu L, Zheng L, Zou Z, IEEE Trans Industr Inform 2021, 17, 943. [Google Scholar]
- [159].Hughes J, Spielberg A, Chounlakone M, Chang G, Matusik W, Rus D, Adv. Intell. Syst. 2020, 2, 2000002. [Google Scholar]
- [160].Sundaram S, Kellnhofer P, Li Y, Zhu J-Y, Torralba A, Matusik W, Nature 2019, 569, 698. [DOI] [PubMed] [Google Scholar]
- [161].Zhu M, Sun Z, Zhang Z, Shi Q, He T, Liu H, Chen T, Lee C, Sci. Adv. 2020, 6, eaaz8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Hammond FL, Mengüç Y, Wood RJ, in 2014 IEEE/RSJ International Conference on Intelligent Robots and Systems, 2014, pp. 4000–4007. [Google Scholar]
- [163].Heng W, Pang G, Xu F, Huang X, Pang Z, Yang G, Sensors 2019, 19, 5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Petrini FM, Valle G, Bumbasirevic M, Barberi F, Bortolotti D, Cvancara P, Hiairrassary A, Mijovic P, Sverrisson AÖ, Pedrocchi A, Divoux J-L, Popovic I, Lechler K, Mijovic B, Guiraud D, Stieglitz T, Alexandersson A, Micera S, Lesic A, Raspopovic S, Sci Transl Med 2019, 11, eaav8939. [DOI] [PubMed] [Google Scholar]
- [165].Yang G, Pang G, Pang Z, Gu Y, Mäntysalo M, Yang H, IEEE Rev Biomed Eng 2019, 12, 34. [DOI] [PubMed] [Google Scholar]
- [166].Wang C, Yu Y, Yuan Y, Ren C, Liao Q, Wang J, Chai Z, Li Q, Li Z, Matter 2020, 2, 181. [Google Scholar]
- [167].Li Z, Wang ZL, Adv. Mater. 2011, 23, 84. [DOI] [PubMed] [Google Scholar]
- [168].Kang D, Pikhitsa PV, Choi YW, Lee C, Shin SS, Piao L, Park B, Suh K-Y, Kim T, Choi M, Nature 2014, 516, 222. [DOI] [PubMed] [Google Scholar]
- [169].Peng X, Dong K, Ye C, Jiang Y, Zhai S, Cheng R, Liu D, Gao X, Wang J, Wang ZL, Sci. Adv. 2020, 6, eaba9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Kwak JW, Han M, Xie Z, Chung HU, Lee JY, Avila R, Yohay J, Chen X, Liang C, Patel M, Jung I, Kim J, Namkoong M, Kwon K, Guo X, Ogle C, Grande D, Ryu D, Kim DH, Madhvapathy S, Liu C, Yang DS, Park Y, Caldwell R, Banks A, Xu S, Huang Y, Fatone S, Rogers JA, Sci Transl Med 2020, 12, eabc4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Oh YS, Kim J-H, Xie Z, Cho S, Han H, Jeon SW, Park M, Namkoong M, Avila R, Song Z, Lee S-U, Ko K, Lee J, Lee J-S, Min WG, Lee B-J, Choi M, Chung HU, Kim J, Han M, Koo J, Choi YS, Kwak SS, Kim SB, Kim J, Choi J, Kang C-M, Kim JU, Kwon K, Won SM, Baek JM, Lee Y, Kim SY, Lu W, Vazquez-Guardado A, Jeong H, Ryu H, Lee G, Kim K, Kim S, Kim MS, Choi J, Choi DY, Yang Q, Zhao H, Bai W, Jang H, Yu Y, Lim J, Guo X, Kim BH, Jeon S, Davies C, Banks A, Sung HJ, Huang Y, Park I, Rogers JA, Nat. Commun. 2021, 12, 5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Zhang C, Dong H, Zhang C, Fan Y, Yao J, Zhao YS, Sci. Adv. 2021, 7, eabh3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Cho C, Kang P, Taqieddin A, Jing Y, Yong K, Kim JM, Haque MF, Aluru NR, Nam S, Nat. Electron. 2021, 4, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Ma Y, Liu N, Li L, Hu X, Zou Z, Wang J, Luo S, Gao Y, Nat. Commun. 2017, 8, 1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Yamada T, Hayamizu Y, Yamamoto Y, Yomogida Y, Izadi-Najafabadi A, Futaba DN, Hata K, Nat. Nanotech. 2011, 6, 296. [DOI] [PubMed] [Google Scholar]
- [176].Han Z, Liu L, Zhang J, Han Q, Wang K, Song H, Wang Z, Jiao Z, Niu S, Ren L, Nanoscale 2018, 10, 15178. [DOI] [PubMed] [Google Scholar]
- [177].Cohen DJ, Mitra D, Peterson K, Maharbiz MM, Nano Lett. 2012, 12, 1821. [DOI] [PubMed] [Google Scholar]
- [178].Shin U-H, Jeong D-W, Park S-M, Kim S-H, Lee HW, Kim J-M, Carbon 2014, 80, 396. [Google Scholar]
- [179].Lee J, Ihle SJ, Pellegrino GS, Kim H, Yea J, Jeon C-Y, Son H-C, Jin C, Eberli D, Schmid F, Zambrano BL, Renz AF, Forró C, Choi H, Jang K-I, Küng R, Vörös J, Nat. Electron. 2021, 4, 291. [Google Scholar]
- [180].Zhang SL, Lai Y-C, He X, Liu R, Zi Y, Wang ZL, Adv. Funct. Mater. 2017, 27, 1606695. [Google Scholar]
- [181].Cui C, Wang X, Yi Z, Yang B, Wang X, Chen X, Liu J, Yang C, ACS Appl. Mater. Interfaces 2018, 10, 3652. [DOI] [PubMed] [Google Scholar]
- [182].Su Z, Wu H, Chen H, Guo H, Cheng X, Song Y, Chen X, Zhang H, Nano Energy 2017, 42, 129. [Google Scholar]
- [183].Dong G, Li S, Yao M, Zhou Z, Zhang Y-Q, Han X, Luo Z, Yao J, Peng B, Hu Z, Huang H, Jia T, Li J, Ren W, Ye Z-G, Ding X, Sun J, Nan C-W, Chen L-Q, Li J, Liu M, Science 2019, 366, 475. [DOI] [PubMed] [Google Scholar]
- [184].Dagdeviren C, Hwang S-W, Su Y, Kim S, Cheng H, Gur O, Haney R, Omenetto FG, Huang Y, Rogers JA, Small 2013, 9, 3398. [DOI] [PubMed] [Google Scholar]
- [185].Yu G-F, Yan X, Yu M, Jia M-Y, Pan W, He X-X, Han W-P, Zhang Z-M, Yu L-M, Long Y-Z, Nanoscale 2016, 8, 2944. [DOI] [PubMed] [Google Scholar]
- [186].Guo H, Wan J, Wu H, Wang H, Miao L, Song Y, Chen H, Han M, Zhang H, ACS Appl. Mater. Interfaces 2020, 12, 22357. [DOI] [PubMed] [Google Scholar]
- [187].Loong LM, Lee W, Qiu X, Yang P, Kawai H, Saeys M, Ahn J-H, Yang H, Adv. Mater. 2016, 28, 4983. [DOI] [PubMed] [Google Scholar]
- [188].Ota S, Ando A, Chiba D, Nat. Electron. 2018, 1, 124. [Google Scholar]
- [189].Zhang H, Han W, Xu K, Zhang Y, Lu Y, Nie Z, Du Y, Zhu J, Huang W, Nano Lett. 2020, 20, 3449. [DOI] [PubMed] [Google Scholar]
- [190].Tang L, Shang J, Jiang X, Sci. Adv. 2021, 7, eabe3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Sim K, Rao Z, Zou Z, Ershad F, Lei J, Thukral A, Chen J, Huang Q-A, Xiao J, Yu C, Sci. Adv. 2019, 5, eaav9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Yu Y, Nassar J, Xu C, Min J, Yang Y, Dai A, Doshi R, Huang A, Song Y, Gehlhar R, Ames AD, Gao W, Sci. Robot. 2020, 5, eaaz7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Pan C-T, Chang C-C, Yang Y-S, Yen C-K, Kao Y-H, Shiue Y-L, Sens. Actuator A Phys. 2020, 301, 111708. [Google Scholar]
- [194].Araromi OA, Graule MA, Dorsey KL, Castellanos S, Foster JR, Hsu W-H, Passy AE, Vlassak JJ, Weaver JC, Walsh CJ, Wood RJ, Nature 2020, 587, 219. [DOI] [PubMed] [Google Scholar]
- [195].Cai P, Wan C, Pan L, Matsuhisa N, He K, Cui Z, Zhang W, Li C, Wang J, Yu J, Wang M, Jiang Y, Chen G, Chen X, Nat. Commun. 2020, 11, 2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Jung P-G, Lim G, Kim S, Kong K, IEEE Trans Industr Inform 2015, 11, 485. [Google Scholar]
- [197].Proske U, Gandevia SC, Physiol. Rev. 2012, 92, 1651. [DOI] [PubMed] [Google Scholar]
- [198].Deimel R, Brock O, Int J Rob Res 2016, 35, 161. [Google Scholar]
- [199].Van Meerbeek IM, De Sa CM, Shepherd RF, Sci. Robot. 2018, 3, eaau2489. [DOI] [PubMed] [Google Scholar]
- [200].Booth JW, Shah D, Case JC, White EL, Yuen MC, Cyr-Choiniere O, Kramer-Bottiglio R, Sci. Robot. 2018, 3, eaat1853. [DOI] [PubMed] [Google Scholar]
- [201].Park Y-L, Chen B, Young D, Stirling L, Wood RJ, Goldfield E, Nagpal R, in 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems, 2011, pp. 4488–4495. [Google Scholar]
- [202].Yang Z, Pang Y, Han X, Yang Y, Ling J, Jian M, Zhang Y, Yang Y, Ren T-L, ACS Nano 2018, 12, 9134. [DOI] [PubMed] [Google Scholar]
- [203].Li Y-Q, Zhu W-B, Yu X-G, Huang P, Fu S-Y, Hu N, Liao K, ACS Appl. Mater. Interfaces 2016, 8, 33189. [DOI] [PubMed] [Google Scholar]
- [204].Boutry CM, Kaizawa Y, Schroeder BC, Chortos A, Legrand A, Wang Z, Chang J, Fox P, Bao Z, Nat. Electron. 2018, 1, 314. [Google Scholar]
- [205].Turkani VS, Maddipatla D, Narakathu BB, Bazuin BJ, Atashbar MZ, Sens. Actuator A Phys. 2018, 279, 1. [Google Scholar]
- [206].Mahmoud WE, Al-Bluwi SA, Sens. Actuator A Phys. 2020, 312, 112101. [Google Scholar]
- [207].Xu L, Gutbrod SR, Bonifas AP, Su Y, Sulkin MS, Lu N, Chung H-J, Jang K-I, Liu Z, Ying M, Lu C, Webb RC, Kim J-S, Laughner JI, Cheng H, Liu Y, Ameen A, Jeong J-W, Kim G-T, Huang Y, Efimov IR, Rogers JA, Nat. Commun. 2014, 5, 3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Park I-J, Jeong C-Y, Cho I-T, Lee J-H, Cho E-S, Kwon SJ, Kim B, Cheong W-S, Song S-H, Kwon H-I, Semicond Sci Technol 2012, 27, 105019. [Google Scholar]
- [209].Trung TQ, Lee N-E, Adv. Mater. 2016, 28, 4338. [DOI] [PubMed] [Google Scholar]
- [210].Webb RC, Bonifas AP, Behnaz A, Zhang Y, Yu KJ, Cheng H, Shi M, Bian Z, Liu Z, Kim Y-S, Yeo W-H, Park JS, Song J, Li Y, Huang Y, Gorbach AM, Rogers JA, Nat. Mater. 2013, 12, 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Kim D-H, Wang S, Keum H, Ghaffari R, Kim Y-S, Tao H, Panilaitis B, Li M, Kang Z, Omenetto F, Huang Y, Rogers JA, Small 2012, 8, 3263. [DOI] [PubMed] [Google Scholar]
- [212].Trung TQ, Ramasundaram S, Hong SW, Lee N-E, Adv. Funct. Mater. 2014, 24, 3438. [Google Scholar]
- [213].Harada S, Kanao K, Yamamoto Y, Arie T, Akita S, Takei K, ACS Nano 2014, 8, 12851. [DOI] [PubMed] [Google Scholar]
- [214].Luo Y, Wang G, Zhang B, Zhang Z, Eur. Polym. J. 1998, 34, 1221. [Google Scholar]
- [215].Shin J, Jeong B, Kim J, Nam VB, Yoon Y, Jung J, Hong S, Lee H, Eom H, Yeo J, Choi J, Lee D, Ko SH, Adv. Mater. 2020, 32, 1905527. [DOI] [PubMed] [Google Scholar]
- [216].Assumpcao D, Kumar S, Narasimhan V, Lee J, Choo H, Sci. Rep. 2018, 8, 13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Trung TQ, Ramasundaram S, Hwang B-U, Lee N-E, Adv. Mater. 2016, 28, 502. [DOI] [PubMed] [Google Scholar]
- [218].Ota H, Chao M, Gao Y, Wu E, Tai L-C, Chen K, Matsuoka Y, Iwai K, Fahad HM, Gao W, Nyein HYY, Lin L, Javey A, ACS Sens. 2017, 2, 990. [DOI] [PubMed] [Google Scholar]
- [219].Choi J, Bandodkar AJ, Reeder JT, Ray TR, Turnquist A, Kim SB, Nyberg N, Hourlier-Fargette A, Model JB, Aranyosi AJ, Xu S, Ghaffari R, Rogers JA, ACS Sens. 2019, 4, 379. [DOI] [PubMed] [Google Scholar]
- [220].Zhao S, Zhu R, Adv. Mater. 2017, 29, 1606151. [DOI] [PubMed] [Google Scholar]
- [221].Xu K, Lu Y, Yamaguchi T, Arie T, Akita S, Takei K, ACS Nano 2019, 13, 14348. [DOI] [PubMed] [Google Scholar]
- [222].Kim H-J, Sim K, Thukral A, Yu C, Sci. Adv. 2017, 3, e1701114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Sturm H, Lang W, Sens. Actuator A Phys. 2013, 195, 113. [Google Scholar]
- [224].Kerr E, McGinnity TM, Coleman S, Expert Syst. Appl. 2018, 94, 94. [Google Scholar]
- [225].Lin R, Kim H-J, Achavananthadith S, Kurt SA, Tan SCC, Yao H, Tee BCK, Lee JKW, Ho JS, Nat. Commun. 2020, 11, 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Lu Y, Fujita Y, Honda S, Yang S, Xuan Y, Xu K, Arie T, Akita S, Takei K, Adv. Healthc. Mater. 2021, 2100103. [DOI] [PubMed] [Google Scholar]
- [227].Salvo P, Calisi N, Melai B, Dini V, Paoletti C, Lomonaco T, Pucci A, Di Francesco F, Piaggesi A, Romanelli M, Int. J. Nanomedicine 2017, 12, 949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Wang Y, Wu H, Xu L, Zhang H, Yang Y, Wang ZL, Sci. Adv. 2020, 6, eabb9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Li G, Liu S, Wang L, Zhu R, Sci. Robot. 2020, 5, eabc8134. [DOI] [PubMed] [Google Scholar]
- [230].You I, Mackanic DG, Matsuhisa N, Kang J, Kwon J, Beker L, Mun J, Suh W, Kim TY, Tok JB-H, Bao Z, Jeong U, Science 2020, 370, 961. [DOI] [PubMed] [Google Scholar]
- [231].Lee JH, Heo JS, Kim Y-J, Eom J, Jung HJ, Kim J-W, Kim I, Park H-H, Mo HS, Kim Y-H, Park SK, Adv. Mater. 2020, 32, 2000969. [DOI] [PubMed] [Google Scholar]
- [232].Yang Y, Gao W, Chem. Soc. Rev. 2019, 48, 1465. [DOI] [PubMed] [Google Scholar]
- [233].Kim T, Kaur M, Kim WS, Adv. Mater. Technol. 2019, 4, 1900570. [Google Scholar]
- [234].Kinnamon D, Ghanta R, Lin K-C, Muthukumar S, Prasad S, Sci. Rep. 2017, 7, 13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Yang A, Yan F, ACS Appl. Electron. Mater. 2020, 3, 53. [Google Scholar]
- [236].Kim SH, Hong K, Xie W, Lee KH, Zhang S, Lodge TP, Frisbie CD, Adv. Mater. 2013, 25, 1822. [DOI] [PubMed] [Google Scholar]
- [237].Bandodkar AJ, Gutruf P, Choi J, Lee K, Sekine Y, Reeder JT, Jeang WJ, Aranyosi AJ, Lee SP, Model JB, Ghaffari R, Su C-J, Leshock JP, Ray T, Verrillo A, Thomas K, Krishnamurthi V, Han S, Kim J, Krishnan S, Hang T, Rogers JA, Sci. Adv. 2019, 5, eaav3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Sekine Y, Kim SB, Zhang Y, Bandodkar AJ, Xu S, Choi J, Irie M, Ray TR, Kohli P, Kozai N, Sugita T, Wu Y, Lee K, Lee K-T, Ghaffari R, Rogers JA, Lab Chip 2018, 18, 2178. [DOI] [PubMed] [Google Scholar]
- [239].Amit M, Mishra RK, Hoang Q, Galan AM, Wang J, Ng TN, Mater. Horizons 2019, 6, 604. [Google Scholar]
- [240].Bariya M, Nyein HYY, Javey A, Nat. Electron. 2018, 1, 160. [Google Scholar]
- [241].Reeder JT, Choi J, Xue Y, Gutruf P, Hanson J, Liu M, Ray T, Bandodkar AJ, Avila R, Xia W, Krishnan S, Xu S, Barnes K, Pahnke M, Ghaffari R, Huang Y, Rogers JA, Sci. Adv. 2019, 5, eaau6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Kwon K, Kim JU, Deng Y, Krishnan SR, Choi J, Jang H, Lee K, Su C-J, Yoo I, Wu Y, Lipschultz L, Kim J-H, Chung TS, Wu D, Park Y, Kim T, Ghaffari R, Lee S, Huang Y, Rogers JA, Nat. Electron. 2021, 4, 302. [Google Scholar]
- [243].Song Y, Min J, Yu Y, Wang H, Yang Y, Zhang H, Gao W, Sci. Adv. 2020, 6, eaay9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Baker LB, Model JB, Barnes KA, Anderson ML, Lee SP, Lee KA, Brown SD, Reimel AJ, Roberts TJ, Nuccio RP, Bonsignore JL, Ungaro CT, Carter JM, Li W, Seib MS, Reeder JT, Aranyosi AJ, Rogers JA, Ghaffari R, Sci. Adv. 2020, 6, eabe3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Sempionatto JR, Lin M, Yin L, Pei K, Sonsa-ard T, de Loyola Silva AN, Khorshed AA, Zhang F, Tostado N, Xu S, Nat. Biomed. Eng. 2021, 1. [DOI] [PubMed] [Google Scholar]
- [246].Lonini L, Dai A, Shawen N, Simuni T, Poon C, Shimanovich L, Daeschler M, Ghaffari R, Rogers JA, Jayaraman A, NPJ Digit. Med. 2018, 1, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Tai L-C, Liaw TS, Lin Y, Nyein HYY, Bariya M, Ji W, Hettick M, Zhao C, Zhao J, Hou L, Yuan Z, Fan Z, Javey A, Nano Lett. 2019, 19, 6346. [DOI] [PubMed] [Google Scholar]
- [248].Moon J-M, Teymourian H, De la Paz E, Sempionatto JR, Mahato K, Sonsa-ard T, Huang N, Longardner K, Litvan I, Wang J, Angew. Chem. Int. Ed. 2021, 60, 19074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Torrente-Rodríguez RM, Tu J, Yang Y, Min J, Wang M, Song Y, Yu Y, Xu C, Ye C, IsHak WW, Gao W, Matter 2020, 2, 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Tang W, Yin L, Sempionatto JR, Moon J-M, Teymourian H, Wang J, Adv. Mater. 2021, 33, 2008465. [DOI] [PubMed] [Google Scholar]
- [251].Yu Y, Nyein HYY, Gao W, Javey A, Adv. Mater. 2020, 32, 1902083. [DOI] [PubMed] [Google Scholar]
- [252].Bariya M, Li L, Ghattamaneni R, Ahn CH, Nyein HYY, Tai L-C, Javey A, Sci. Adv. 2020, 6, eabb8308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Emaminejad S, Gao W, Wu E, Davies ZA, Yin Yin Nyein H, Challa S, Ryan SP, Fahad HM, Chen K, Shahpar Z, Talebi S, Milla C, Javey A, Davis RW, Proc. Natl. Acad. Sci. U.S.A. 2017, 114, 4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Russell J, Vidal-Gadea AG, Makay A, Lanam C, Pierce-Shimomura JT, Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Tiest WMB, Kosters ND, Kappers AM, Daanen HA, Acta Psychol (Amst) 2012, 141, 159. [DOI] [PubMed] [Google Scholar]
- [256].Rivadeneyra A, Fernández-Salmerón J, Agudo M, López-Villanueva JA, Capitan-Vallvey LF, Palma AJ, Sens. Actuators B Chem. 2014, 195, 123. [Google Scholar]
- [257].Lim D-I, Cha J-R, Gong M-S, Sens. Actuators B Chem. 2013, 183, 574. [Google Scholar]
- [258].Miao L, Wan J, Song Y, Guo H, Chen H, Cheng X, Zhang H, ACS Appl. Mater. Interfaces 2019, 11, 39219. [DOI] [PubMed] [Google Scholar]
- [259].Sarwar MS, Dobashi Y, Preston C, Wyss JKM, Mirabbasi S, Madden JDW, Sci. Adv. 2017, 3, e1602200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Guo H, Wu H, Song Y, Miao L, Chen X, Chen H, Su Z, Han M, Zhang H, Nano Energy 2019, 58, 121. [Google Scholar]
- [261].Bermúdez GSC, Karnaushenko DD, Karnaushenko D, Lebanov A, Bischoff L, Kaltenbrunner M, Fassbender J, Schmidt OG, Makarov D, Sci. Adv. 2018, 4, eaao2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Almansouri AS, Alsharif NA, Khan MA, Swanepoel L, Kaidarova A, Salama KN, Kosel J, Adv. Mater. Technol. 2019, 4, 1900493. [Google Scholar]
- [263].Jinno H, Yokota T, Koizumi M, Yukita W, Saito M, Osaka I, Fukuda K, Someya T, Nat. Commun. 2021, 12, 2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Yokota T, Zalar P, Kaltenbrunner M, Jinno H, Matsuhisa N, Kitanosako H, Tachibana Y, Yukita W, Koizumi M, Someya T, Sci. Adv. 2016, 2, e1501856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Wang C, Qi B, Lin M, Zhang Z, Makihata M, Liu B, Zhou S, Huang Y, Hu H, Gu Y, Chen Y, Lei Y, Lee T, Chien S, Jang K-I, Kistler EB, Xu S, Nat. Biomed. Eng. 2021, 5, 749. [DOI] [PubMed] [Google Scholar]
- [266].Wang C, Li X, Hu H, Zhang L, Huang Z, Lin M, Zhang Z, Yin Z, Huang B, Gong H, Bhaskaran S, Gu Y, Makihata M, Guo Y, Lei Y, Chen Y, Wang C, Li Y, Zhang T, Chen Z, Pisano AP, Zhang L, Zhou Q, Xu S, Nat. Biomed. Eng. 2018, 2, 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Khan Y, Han D, Pierre A, Ting J, Wang X, Lochner CM, Bovo G, Yaacobi-Gross N, Newsome C, Wilson R, Arias AC, Proc. Natl. Acad. Sci. U.S.A. 2018, 115, E11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Ghamari M, Int. j. biosens. bioelectron. 2018, 4, DOI 10.15406/ijbsbe.2018.04.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].O’Doherty JE, Lebedev MA, Ifft PJ, Zhuang KZ, Shokur S, Bleuler H, Nicolelis MAL, Nature 2011, 479, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Buzsáki G, Anastassiou CA, Koch C, Nat. Rev. Neurosci. 2012, 13, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Thakor NV, Sci Transl Med 2013, 5, 210ps17. [DOI] [PubMed] [Google Scholar]
- [272].Bensmaia SJ, Miller LE, Nat. Rev. Neurosci. 2014, 15, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Shanechi MM, Nat. Neurosci. 2019, 22, 1554. [DOI] [PubMed] [Google Scholar]
- [274].Yanagisawa T, Hirata M, Saitoh Y, Goto T, Kishima H, Fukuma R, Yokoi H, Kamitani Y, Yoshimine T, J. Neurosurg 2011, 114, 1715. [DOI] [PubMed] [Google Scholar]
- [275].Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP, Nature 2012, 485, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP, Nature 2006, 442, 164. [DOI] [PubMed] [Google Scholar]
- [277].Irwin ZT, Schroeder KE, Vu PP, Tat DM, Bullard AJ, Woo SL, Sando IC, Urbanchek MG, Cederna PS, Chestek CA, J Neural Eng 2016, 13, 046007. [DOI] [PubMed] [Google Scholar]
- [278].Ortiz-Catalan M, Nat. Biomed. Eng. 2017, 1, 1. [Google Scholar]
- [279].Farina D, Vujaklija I, Sartori M, Kapelner T, Negro F, Jiang N, Bergmeister K, Andalib A, Principe J, Aszmann OC, Nat. Biomed. Eng. 2017, 1, 0025. [Google Scholar]
- [280].Aszmann OC, Roche AD, Salminger S, Paternostro-Sluga T, Herceg M, Sturma A, Hofer C, Farina D, Lancet 2015, 385, 2183. [DOI] [PubMed] [Google Scholar]
- [281].Edelman BJ, Meng J, Suma D, Zurn C, Nagarajan E, Baxter BS, Cline CC, He B, Sci. Robot. 2019, 4, eaaw6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Penaloza CI, Nishio S, Sci. Robot. 2018, 3, eaat1228. [DOI] [PubMed] [Google Scholar]
- [283].Furui A, Eto S, Nakagaki K, Shimada K, Nakamura G, Masuda A, Chin T, Tsuji T, Sci. Robot. 2019, 4, 31. [DOI] [PubMed] [Google Scholar]
- [284].Hahne JM, Schweisfurth MA, Koppe M, Farina D, Sci. Robot. 2018, 3, 19. [DOI] [PubMed] [Google Scholar]
- [285].Soekadar SR, Witkowski M, Gómez C, Opisso E, Medina J, Cortese M, Cempini M, Carrozza MC, Cohen LG, Birbaumer N, Vitiello N, Sci. Robot. 2016, 1, eaag3296. [DOI] [PubMed] [Google Scholar]
- [286].Hong K-S, Aziz N, Ghafoor U, J Neural Eng 2018, 15, 031004. [DOI] [PubMed] [Google Scholar]
- [287].Mahmood M, Mzurikwao D, Kim Y-S, Lee Y, Mishra S, Herbert R, Duarte A, Ang CS, Yeo W-H, Nat. Mach. Intell. 2019, 1, 412. [Google Scholar]
- [288].Guasch E, Mont L, Nat. Rev. Cardiol. 2017, 14, 88. [DOI] [PubMed] [Google Scholar]
- [289].Sheikh N, Sharma S, Nat. Rev. Cardiol. 2014, 11, 198. [DOI] [PubMed] [Google Scholar]
- [290].Jeong J-W, Shin G, Park SI, Yu KJ, Xu L, Rogers JA, Neuron 2015, 86, 175. [DOI] [PubMed] [Google Scholar]
- [291].Liu Y, Pharr M, Salvatore GA, ACS Nano 2017, 11, 9614. [DOI] [PubMed] [Google Scholar]
- [292].Obidin N, Tasnim F, Dagdeviren C, Adv. Mater. 2020, 32, 1901482. [DOI] [PubMed] [Google Scholar]
- [293].Cody PA, Eles JR, Lagenaur CF, Kozai TDY, Cui XT, Biomaterials 2018, 161, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Engineering Acoustics, Inc., C-2 vibrotactile tactor, https://www.eaiinfo.com/product/c2/, accessed: 2021.
- [295].Afanasenkau D, Kalinina D, Lyakhovetskii V, Tondera C, Gorsky O, Moosavi S, Pavlova N, Merkulyeva N, Kalueff AV, Minev IR, Musienko P, Nat. Biomed. Eng. 2020, 4, 1010. [DOI] [PubMed] [Google Scholar]
- [296].Viventi J, Kim D-H, Vigeland L, Frechette ES, Blanco JA, Kim Y-S, Avrin AE, Tiruvadi VR, Hwang S-W, Vanleer AC, Wulsin DF, Davis K, Gelber CE, Palmer L, Van der Spiegel J, Wu J, Xiao J, Huang Y, Contreras D, Rogers JA, Litt B, Nat. Neurosci. 2011, 14, 1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Guan S, Wang J, Gu X, Zhao Y, Hou R, Fan H, Zou L, Gao L, Du M, Li C, Fang Y, Sci. Adv. 2019, 5, eaav2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Otchy TM, Michas C, Lee B, Gopalan K, Nerurkar V, Gleick J, Semu D, Darkwa L, Holinski BJ, Chew DJ, White AE, Gardner TJ, Nat. Commun. 2020, 11, 4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Kim D-H, Lu N, Ma R, Kim Y-S, Kim R-H, Wang S, Wu J, Won SM, Tao H, Islam A, Science 2011, 333, 838. [DOI] [PubMed] [Google Scholar]
- [300].Yu KJ, Kuzum D, Hwang S-W, Kim BH, Juul H, Kim NH, Won SM, Chiang K, Trumpis M, Richardson AG, Cheng H, Fang H, Thompson M, Bink H, Talos D, Seo KJ, Lee HN, Kang S-K, Kim J-H, Lee JY, Huang Y, Jensen FE, Dichter MA, Lucas TH, Viventi J, Litt B, Rogers JA, Nat. Mater. 2016, 15, 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Xie C, Liu J, Fu T-M, Dai X, Zhou W, Lieber CM, Nat. Mater. 2015, 14, 1286. [DOI] [PubMed] [Google Scholar]
- [302].Liu Y, Li J, Song S, Kang J, Tsao Y, Chen S, Mottini V, McConnell K, Xu W, Zheng Y-Q, Tok JB-H, George PM, Bao Z, Nat. Biotechnol. 2020, 38, 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Koo J, MacEwan MR, Kang S-K, Won SM, Stephen M, Gamble P, Xie Z, Yan Y, Chen Y-Y, Shin J, Birenbaum N, Chung S, Kim SB, Khalifeh J, Harburg DV, Bean K, Paskett M, Kim J, Zohny ZS, Lee SM, Zhang R, Luo K, Ji B, Banks A, Lee HM, Huang Y, Ray WZ, Rogers JA, Nat. Med. 2018, 24, 1830. [DOI] [PubMed] [Google Scholar]
- [304].Wellman SM, Eles JR, Ludwig KA, Seymour JP, Michelson NJ, McFadden WE, Vazquez AL, Kozai TDY, Adv. Funct. Mater. 2018, 28, 1701269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Cogan SF, Annu Rev Biomed Eng 2008, 10, 275. [DOI] [PubMed] [Google Scholar]
- [306].Maynard EM, Nordhausen CT, Normann RA, Electroencephalogr. clin. neurophysiol 1997, 102, 228. [DOI] [PubMed] [Google Scholar]
- [307].Wise KD, IEEE eng. med. biol. mag. 2005, 24, 22. [DOI] [PubMed] [Google Scholar]
- [308].Malagodi MS, Horch KW, Schoenberg AA, Ann Biomed Eng 1989, 17, 397. [DOI] [PubMed] [Google Scholar]
- [309].Najafi K, Ji J, Wise KD, IEEE. Trans. Biomed. Eng. 1990, 37, 1. [DOI] [PubMed] [Google Scholar]
- [310].Yang X, Zhou T, Zwang TJ, Hong G, Zhao Y, Viveros RD, Fu T-M, Gao T, Lieber CM, Nat. Mater. 2019, 18, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Li J, Song E, Chiang C-H, Yu KJ, Koo J, Du H, Zhong Y, Hill M, Wang C, Zhang J, Chen Y, Tian L, Zhong Y, Fang G, Viventi J, Rogers JA, Proc. Natl. Acad. Sci. U.S.A. 2018, 115, E9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Kozai TDY, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA, Kipke DR, Nat. Mater. 2012, 11, 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Tian B, Liu J, Dvir T, Jin L, Tsui JH, Qing Q, Suo Z, Langer R, Kohane DS, Lieber CM, Nat. Mater. 2012, 11, 986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Lienemann S, Zötterman J, Farnebo S, Tybrandt K, J Neural Eng 2021, 18, 045007. [DOI] [PubMed] [Google Scholar]
- [315].Lu L, Fu X, Liew Y, Zhang Y, Zhao S, Xu Z, Zhao J, Li D, Li Q, Stanley GB, Nano letters 2019, 19, 1577. [DOI] [PubMed] [Google Scholar]
- [316].Park D-W, Schendel AA, Mikael S, Brodnick SK, Richner TJ, Ness JP, Hayat MR, Atry F, Frye ST, Pashaie R, Nat. Commun. 2014, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Kuzum D, Takano H, Shim E, Reed JC, Juul H, Richardson AG, de Vries J, Bink H, Dichter MA, Lucas TH, Coulter DA, Cubukcu E, Litt B, Nat. Commun. 2014, 5, 5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Teo AJ, Mishra A, Park I, Kim Y-J, Park W-T, Yoon Y-J, ACS Biomater. Sci. Eng. 2016, 2, 454. [DOI] [PubMed] [Google Scholar]
- [319].van Daal RJ, Sun J-J, Ceyssens F, Michon F, Kraft M, Puers R, Kloosterman F, J Neural Eng 2020, 17, 016046. [DOI] [PubMed] [Google Scholar]
- [320].Rousche PJ, Pellinen DS, Pivin DP, Williams JC, Vetter RJ, Kipke DR, IEEE. Trans. Biomed. Eng. 2001, 48, 361. [DOI] [PubMed] [Google Scholar]
- [321].Delivopoulos E, Chew DJ, Minev IR, Fawcett JW, Lacour SP, Lab Chip 2012, 12, 2540. [DOI] [PubMed] [Google Scholar]
- [322].Guo L, Guvanasen GS, Liu X, Tuthill C, Nichols TR, DeWeerth SP, IEEE Trans Biomed Circuits Syst 2012, 7, 1. [DOI] [PubMed] [Google Scholar]
- [323].Lu Y, Wang D, Li T, Zhao X, Cao Y, Yang H, Duan YY, Biomaterials 2009, 30, 4143. [DOI] [PubMed] [Google Scholar]
- [324].Huang W-C, Ong XC, Kwon IS, Gopinath C, Fisher LE, Wu H, Fedder GK, Gaunt RA, Bettinger CJ, Adv. Funct. Mater. 2018, 28, 1801059. [Google Scholar]
- [325].Won SM, Cai L, Gutruf P, Rogers JA, Nat. Biomed. Eng. 2021, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Song Y, Mukasa D, Zhang H, Gao W, Acc. Mater. Res. 2021, 2, 184. [Google Scholar]
- [327].Dagdeviren C, Yang BD, Su Y, Tran PL, Joe P, Anderson E, Xia J, Doraiswamy V, Dehdashti B, Feng X, Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Kang S-K, Murphy RKJ, Hwang S-W, Lee SM, Harburg DV, Krueger NA, Shin J, Gamble P, Cheng H, Yu S, Liu Z, McCall JG, Stephen M, Ying H, Kim J, Park G, Webb RC, Lee CH, Chung S, Wie DS, Gujar AD, Vemulapalli B, Kim AH, Lee K-M, Cheng J, Huang Y, Lee SH, Braun PV, Ray WZ, Rogers JA, Nature 2016, 530, 71. [DOI] [PubMed] [Google Scholar]
- [329].Piech DK, Johnson BC, Shen K, Ghanbari MM, Li KY, Neely RM, Kay JE, Carmena JM, Maharbiz MM, Muller R, Nat. Biomed. Eng. 2020, 4, 207. [DOI] [PubMed] [Google Scholar]
- [330].Miller KJ, Schalk G, Fetz EE, den Nijs M, Ojemann JG, Rao RPN, Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Little S, Perrone R, Wilt R, de Hemptinne C, Yaroshinsky MS, Racine CA, Wang SS, Ostrem JL, Larson PS, Wang DD, Nat. Biotechnol. 2021, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Schwartz AB, Cui XT, Weber DJ, Moran DW, Neuron 2006, 52, 205. [DOI] [PubMed] [Google Scholar]
- [333].Hotson G, McMullen DP, Fifer MS, Johannes MS, Katyal KD, Para MP, Armiger R, Anderson WS, Thakor NV, Wester BA, Crone NE, J Neural Eng 2016, 13, 026017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Fifer MS, Acharya S, Benz HL, Mollazadeh M, Crone NE, Thakor NV, IEEE Pulse 2012, 3, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Yanagisawa T, Hirata M, Saitoh Y, Kishima H, Matsushita K, Goto T, Fukuma R, Yokoi H, Kamitani Y, Yoshimine T, Ann. Neurol. 2012, 71, 353. [DOI] [PubMed] [Google Scholar]
- [336].Khodagholy D, Gelinas JN, Zhao Z, Yeh M, Long M, Greenlee JD, Doyle W, Devinsky O, Buzsáki G, Sci. Adv. 2016, 2, e1601027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Jun JJ, Steinmetz NA, Siegle JH, Denman DJ, Bauza M, Barbarits B, Lee AK, Anastassiou CA, Andrei A, Aydın Ç, Barbic M, Blanche TJ, Bonin V, Couto J, Dutta B, Gratiy SL, Gutnisky DA, Häusser M, Karsh B, Ledochowitsch P, Lopez CM, Mitelut C, Musa S, Okun M, Pachitariu M, Putzeys J, Rich PD, Rossant C, Sun W, Svoboda K, Carandini M, Harris KD, Koch C, O’Keefe J, Harris TD, Nature 2017, 551, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Rousche PJ, Normann RA, Neurosci J Methods 1998, 82, 1. [DOI] [PubMed] [Google Scholar]
- [339].Massey TL, Santacruz SR, Hou JF, Pister KSJ, Carmena JM, Maharbiz MM, J Neural Eng 2019, 16, 016024. [DOI] [PubMed] [Google Scholar]
- [340].Nicolelis MAL, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, Wise SP, Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Musk E, J. Medical Internet Res 2019, 21, e16194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Canales A, Jia X, Froriep UP, Koppes RA, Tringides CM, Selvidge J, Lu C, Hou C, Wei L, Fink Y, Anikeeva P, Nat. Biotechnol. 2015, 33, 277. [DOI] [PubMed] [Google Scholar]
- [343].Khodagholy D, Gelinas JN, Thesen T, Doyle W, Devinsky O, Malliaras GG, Buzsáki G, Nat. Neurosci. 2015, 18, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Chiang C-H, Won SM, Orsborn AL, Yu KJ, Trumpis M, Bent B, Wang C, Xue Y, Min S, Woods V, Yu C, Kim BH, Kim SB, Huq R, Li J, Seo KJ, Vitale F, Richardson A, Fang H, Huang Y, Shepard K, Pesaran B, Rogers JA, Viventi J, Sci Transl Med 2020, 12, eaay4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Tybrandt K, Khodagholy D, Dielacher B, Stauffer F, Renz AF, Buzsáki G, Vörös J, Adv. Mater. 2018, 30, 1706520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Thunemann M, Lu Y, Liu X, Kılıç K, Desjardins M, Vandenberghe M, Sadegh S, Saisan PA, Cheng Q, Weldy KL, Lyu H, Djurovic S, Andreassen OA, Dale AM, Devor A, Kuzum D, Nat. Commun. 2018, 9, 2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Anderson HansE., Weir RichardF. ff., Neural Regen Res 2019, 14, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Kim D-H, Viventi J, Amsden JJ, Xiao J, Vigeland L, Kim Y-S, Blanco JA, Panilaitis B, Frechette ES, Contreras D, Kaplan DL, Omenetto FG, Huang Y, Hwang K-C, Zakin MR, Litt B, Rogers JA, Nat. Mater. 2010, 9, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Zhou T, Hong G, Fu T-M, Yang X, Schuhmann TG, Viveros RD, Lieber CM, Proc. Natl. Acad. Sci. U.S.A. 2017, 114, 5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Liu J, Fu T-M, Cheng Z, Hong G, Zhou T, Jin L, Duvvuri M, Jiang Z, Kruskal P, Xie C, Suo Z, Fang Y, Lieber CM, Nat. Nanotech. 2015, 10, 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Yu H, Xiong W, Zhang H, Wang W, Li Z, J Microelectromech Syst 2014, 23, 1025. [Google Scholar]
- [352].Dweiri YM, Stone MA, Tyler DJ, McCallum GA, Durand DM, J. Vis. Exp. 2016, 54388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].McNaughton TG, Horch KW, Neurosci J Methods 1996, 70, 103. [DOI] [PubMed] [Google Scholar]
- [354].Poppendieck W, Muceli S, Dideriksen J, Rocon E, Pons JL, Farina D, Hoffmann K-P, in 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2015, pp. 7135–7138. [DOI] [PubMed] [Google Scholar]
- [355].Boretius T, Yoshida K, Badia J, Harreby K, Kundu A, Navarro X, Jensen W, Stieglitz T, in 2012 4th IEEE RAS EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), 2012, pp. 282–287. [Google Scholar]
- [356].Wang L, Lu C, Yang S, Sun P, Wang Y, Guan Y, Liu S, Cheng D, Meng H, Wang Q, He J, Hou H, Li H, Lu W, Zhao Y, Wang J, Zhu Y, Li Y, Luo D, Li T, Chen H, Wang S, Sheng X, Xiong W, Wang X, Peng J, Yin L, Sci. Adv. 2020, 6, eabc6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Song K-I, Seo H, Seong D, Kim S, Yu KJ, Kim Y-C, Kim J, Kwon SJ, Han H-S, Youn I, Lee H, Son D, Nat. Commun. 2020, 11, 4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [358].Zhang Y, Zheng N, Cao Y, Wang F, Wang P, Ma Y, Lu B, Hou G, Fang Z, Liang Z, Yue M, Li Y, Chen Y, Fu J, Wu J, Xie T, Feng X, Sci. Adv. 2019, 5, eaaw1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [359].Xiang Z, Yen S-C, Sheshadri S, Wang J, Lee S, Liu Y-H, Liao L-D, Thakor NV, Lee C, Adv. Mater. 2016, 28, 4472. [DOI] [PubMed] [Google Scholar]
- [360].Ghafoor U, Kim S, Hong K-S, Front. Neurorobot. 2017, 11, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Maeng J, Rihani RT, Javed M, Rajput JS, Kim H, Bouton IG, Criss TA, Pancrazio JJ, Black BJ, Ware TH, J. Mater. Chem. B 2020, 8, 6286. [DOI] [PubMed] [Google Scholar]
- [362].Zhang M, Guo R, Chen K, Wang Y, Niu J, Guo Y, Zhang Y, Yin Z, Xia K, Zhou B, Wang H, He W, Liu J, Sitti M, Zhang Y, Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Micera S, Rossini PM, Rigosa J, Citi L, Carpaneto J, Raspopovic S, Tombini M, Cipriani C, Assenza G, Carrozza MC, Hoffmann K-P, Yoshida K, Navarro X, Dario P, Neuroeng J Rehabilitation 2011, 8, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Lewis S, Russold M, Dietl H, Ruff R, Audi JMC, Hoffmann K-P, Abu-Saleh L, Schroeder D, Krautschneider WH, Westendorff S, Gail A, Meiners T, Kaniusas E, IEEE Trans Instrum Meas 2013, 62, 1972. [Google Scholar]
- [365].Salminger S, Sturma A, Hofer C, Evangelista M, Perrin M, Bergmeister KD, Roche AD, Hasenoehrl T, Dietl H, Farina D, Aszmann OC, Sci. Robot. 2019, 4, eaaw6306. [DOI] [PubMed] [Google Scholar]
- [366].Farina Dario, Vujaklija Ivan, Rickard Brånemark, Bull Anthony MJ, Dietl Hans, Graimann Bernhard, Hargrove Levi J, Hoffmann Klaus-Peter, Huang He Helen, Ingvarsson Thorvaldur, Janusson Hilmar Bragi, Kristjánsson Kristleifur, Kuiken Todd, Micera Silvestro, Stieglitz Thomas, Sturma Agnes, Tyler Dustin, Aszmann Oskar C Nat. Biomed. Eng 2021, 1–13. [DOI] [PubMed] [Google Scholar]
- [367].Baker JJ, Scheme E, Englehart K, Hutchinson DT, Greger B, IEEE Trans. Neural Syst. Rehabilitation Eng. 2010, 18, 424. [DOI] [PubMed] [Google Scholar]
- [368].Merrill DR, Lockhart J, Troyk PR, Weir RF, Hankin DL, Artif Organs 2011, 35, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [369].McAvoy M, Tsosie JK, Vyas KN, Khan OF, Sadtler K, Langer R, Anderson DG, Theranostics 2019, 9, 7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Muceli S, Bergmeister KD, Hoffmann K-P, Aman M, Vukajlija I, Aszmann OC, Farina D, J Neural Eng 2018, 16, 016010. [DOI] [PubMed] [Google Scholar]
- [371].Ortiz-Catalan M, Håkansson B, Brånemark R, Sci Transl Med 2014, 6, 257re6. [DOI] [PubMed] [Google Scholar]
- [372].Seo D, Neely RM, Shen K, Singhal U, Alon E, Rabaey JM, Carmena JM, Maharbiz MM, Neuron 2016, 91, 529. [DOI] [PubMed] [Google Scholar]
- [373].Seo D, Carmena JM, Rabaey JM, Maharbiz MM, Alon E, Neurosci J Methods 2015, 244, 114. [DOI] [PubMed] [Google Scholar]
- [374].Patch K, Nat. Biotechnol. 2021, 39, 255. [DOI] [PubMed] [Google Scholar]
- [375].Sim K, Ershad F, Zhang Y, Yang P, Shim H, Rao Z, Lu Y, Thukral A, Elgalad A, Xi Y, Tian B, Taylor DA, Yu C, Nat. Electron. 2020, 3, 775. [Google Scholar]
- [376].Fang Y, Prominski A, Rotenberg MY, Meng L, Acarón Ledesma H, Lv Y, Yue J, Schaumann E, Jeong J, Yamamoto N, Jiang Y, Elbaz B, Wei W, Tian B, Nat. Nanotechnol. 2021, 16, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Fang H, Yu KJ, Gloschat C, Yang Z, Song E, Chiang C-H, Zhao J, Won SM, Xu S, Trumpis M, Zhong Y, Han SW, Xue Y, Xu D, Choi SW, Cauwenberghs G, Kay M, Huang Y, Viventi J, Efimov IR, Rogers JA, Nat. Biomed. Eng. 2017, 1, 0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [378].Tringides CM, Vachicouras N, de Lázaro I, Wang H, Trouillet A, Seo BR, Elosegui-Artola A, Fallegger F, Shin Y, Casiraghi C, Nat. Nanotechnol. 2021, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [379].Zheng Q, Tang Q, Wang ZL, Li Z, Nat. Rev. Cardiol. 2021, 18, 7. [DOI] [PubMed] [Google Scholar]
- [380].Lu B, Chen Y, Ou D, Chen H, Diao L, Zhang W, Zheng J, Ma W, Sun L, Feng X, Sci. Rep. 2015, 5, 16065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [381].Dagdeviren C, Yang BD, Su Y, Tran PL, Joe P, Anderson E, Xia J, Doraiswamy V, Dehdashti B, Feng X, Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [382].Zheng Q, Zhang H, Shi B, Xue X, Liu Z, Jin Y, Ma Y, Zou Y, Wang X, An Z, Tang W, Zhang W, Yang F, Liu Y, Lang X, Xu Z, Li Z, Wang ZL, ACS Nano 2016, 10, 6510. [DOI] [PubMed] [Google Scholar]
- [383].Ouyang H, Liu Z, Li N, Shi B, Zou Y, Xie F, Ma Y, Li Z, Li H, Zheng Q, Qu X, Fan Y, Wang ZL, Zhang H, Li Z, Nat. Commun. 2019, 10, 1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [384].Park S, Heo SW, Lee W, Inoue D, Jiang Z, Yu K, Jinno H, Hashizume D, Sekino M, Yokota T, Fukuda K, Tajima K, Someya T, Nature 2018, 561, 516. [DOI] [PubMed] [Google Scholar]
- [385].Zhao Y, Zhang S, Yu T, Zhang Y, Ye G, Cui H, He C, Jiang W, Zhai Y, Lu C, Gu X, Liu N, Nat. Commun. 2021, 12, 4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [386].Zhang L, Kumar KS, He H, Cai CJ, He X, Gao H, Yue S, Li C, Seet RC-S, Ren H, Ouyang J, Nat. Commun. 2020, 11, 4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [387].Wang Y, Yin L, Bai Y, Liu S, Wang L, Zhou Y, Hou C, Yang Z, Wu H, Ma J, Shen Y, Deng P, Zhang S, Duan T, Li Z, Ren J, Xiao L, Yin Z, Lu N, Huang Y, Sci. Adv. 2020, 6, eabd0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Tian L, Zimmerman B, Akhtar A, Yu KJ, Moore M, Wu J, Larsen RJ, Lee JW, Li J, Liu Y, Metzger B, Qu S, Guo X, Mathewson KE, Fan JA, Cornman J, Fatina M, Xie Z, Ma Y, Zhang J, Zhang Y, Dolcos F, Fabiani M, Gratton G, Bretl T, Hargrove LJ, Braun PV, Huang Y, Rogers JA, Nat. Biomed. Eng. 2019, 3, 194. [DOI] [PubMed] [Google Scholar]
- [389].Yamamoto Y, Harada S, Yamamoto D, Honda W, Arie T, Akita S, Takei K, Sci. Adv. 2016, 2, e1601473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [390].Liu Y, Norton JJS, Qazi R, Zou Z, Ammann KR, Liu H, Yan L, Tran PL, Jang K-I, Lee JW, Zhang D, Kilian KA, Jung SH, Bretl T, Xiao J, Slepian MJ, Huang Y, Jeong J-W, Rogers JA, Sci. Adv. 2016, 2, e1601185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [391].Norton JJS, Lee DS, Lee JW, Lee W, Kwon O, Won P, Jung S-Y, Cheng H, Jeong J-W, Akce A, Umunna S, Na I, Kwon YH, Wang X-Q, Liu Z, Paik U, Huang Y, Bretl T, Yeo W-H, Rogers JA, Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [392].Kireev D, Ameri SK, Nederveld A, Kampfe J, Jang H, Lu N, Akinwande D, Nat. Protoc. 2021, 16, 2395. [DOI] [PubMed] [Google Scholar]
- [393].Yang G, Deng J, Pang G, Zhang H, Li J, Deng B, Pang Z, Xu J, Jiang M, Liljeberg P, Xie H, Yang H, IEEE J. Transl. Eng. Health Med. 2018, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [394].Delph MA, Fischer SA, Gauthier PW, Luna CHM, Clancy EA, Fischer GS, in 2013 IEEE 13th International Conference on Rehabilitation Robotics (ICORR), IEEE, Seattle, WA, 2013, pp. 1–7. [DOI] [PubMed] [Google Scholar]
- [395].Won P, Park JJ, Lee T, Ha I, Han S, Choi M, Lee J, Hong S, Cho K-J, Ko SH, Nano Lett. 2019, 19, 6087. [DOI] [PubMed] [Google Scholar]
- [396].Pu X, Guo H, Chen J, Wang X, Xi Y, Hu C, Wang ZL, Sci. Adv. 2017, 3, e1700694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [397].Li J, Wang Y, Liu L, Xu S, Liu Y, Leng J, Cai S, Adv. Funct. Mater. 2019, 29, 1903762. [Google Scholar]
- [398].Mishra S, Kim Y-S, Intarasirisawat J, Kwon Y-T, Lee Y, Mahmood M, Lim H-R, Herbert R, Yu KJ, Ang CS, Yeo W-H, Sci. Adv. 2020, 6, eaay1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [399].Leeb R, Tonin L, Rohm M, Desideri L, Carlson T, del R. Millán J, Proc IEEE Inst Electr Electron Eng 2015, 103, 969. [Google Scholar]
- [400].Castellini C, van der Smagt P, Biol Cybern 2009, 100, 35. [DOI] [PubMed] [Google Scholar]
- [401].Ahmadizadeh C, Merhi L-K, Pousett B, Sangha S, Menon C, IEEE Robot Autom Mag 2017, 24, 102. [Google Scholar]
- [402].Ameri SK, Kim M, Kuang IA, Perera WK, Alshiekh M, Jeong H, Topcu U, Akinwande D, Lu N, NPJ 2D Mater. Appl. 2018, 2, 19. [Google Scholar]
- [403].Witkowski M, Cortese M, Cempini M, Mellinger J, Vitiello N, Soekadar SR, Neuroeng J Rehabilitation 2014, 11, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [404].Bayoumy K, Gaber M, Elshafeey A, Mhaimeed O, Dineen EH, Marvel FA, Martin SS, Muse ED, Turakhia MP, Tarakji KG, Elshazly MB, Nat. Rev. Cardiol. 2021, 18, 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [405].He K, Liu Z, Wan C, Jiang Y, Wang T, Wang M, Zhang F, Liu Y, Pan L, Xiao M, Yang H, Chen X, Adv. Mater. 2020, 32, 2001130. [DOI] [PubMed] [Google Scholar]
- [406].Jeong J, Kim MK, Cheng H, Yeo W, Huang X, Liu Y, Zhang Y, Huang Y, Rogers JA, Adv. Healthc. Mater. 2014, 3, 642. [DOI] [PubMed] [Google Scholar]
- [407].Guo C, Yu Y, Liu J, J. Mater. Chem. B 2014, 2, 5739. [DOI] [PubMed] [Google Scholar]
- [408].Li Z, Guo W, Huang Y, Zhu K, Yi H, Wu H, Carbon 2020, 164, 164. [Google Scholar]
- [409].Fang Y, Li Y, Wang X, Zhou Z, Zhang K, Zhou J, Hu B, Small 2020, 16, 2000450. [DOI] [PubMed] [Google Scholar]
- [410].Yang S, Chen Y-C, Nicolini L, Pasupathy P, Sacks J, Su B, Yang R, Sanchez D, Chang Y-F, Wang P, Schnyer D, Neikirk D, Lu N, Adv. Mater. 2015, 27, 6423. [DOI] [PubMed] [Google Scholar]
- [411].Tang J, Li J, Vlassak JJ, Suo Z, Soft Matter 2016, 12, 1093. [DOI] [PubMed] [Google Scholar]
- [412].Chen C-M, Chiang C-L, Lai C-L, Xie T, Yang S, Adv. Funct. Mater. 2013, 23, 3813. [Google Scholar]
- [413].Sun B, McCay RN, Goswami S, Xu Y, Zhang C, Ling Y, Lin J, Yan Z, Adv. Mater. 2018, 30, 1804327. [DOI] [PubMed] [Google Scholar]
- [414].Wu H, Yang G, Zhu K, Liu S, Guo W, Jiang Z, Li Z, Adv. Sci. 2021, 8, 2001938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [415].Guo W, Zheng P, Huang X, Zhuo H, Wu Y, Yin Z, Li Z, Wu H, ACS Appl. Mater. Interfaces 2019, 11, 8567. [DOI] [PubMed] [Google Scholar]
- [416].Chung HU, Kim BH, Lee JY, Lee J, Xie Z, Ibler EM, Lee K, Banks A, Jeong JY, Kim J, Ogle C, Grande D, Yu Y, Jang H, Assem P, Ryu D, Kwak JW, Namkoong M, Park JB, Lee Y, Kim DH, Ryu A, Jeong J, You K, Ji B, Liu Z, Huo Q, Feng X, Deng Y, Xu Y, Jang K-I, Kim J, Zhang Y, Ghaffari R, Rand CM, Schau M, Hamvas A, Weese-Mayer DE, Huang Y, Lee SM, Lee CH, Shanbhag NR, Paller AS, Xu S, Rogers JA, Science 2019, 363, eaau0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [417].Khan Y, Garg M, Gui Q, Schadt M, Gaikwad A, Han D, Yamamoto NAD, Hart P, Welte R, Wilson W, Czarnecki S, Poliks M, Jin Z, Ghose K, Egitto F, Turner J, Arias AC, Adv. Funct. Mater. 2016, 26, 8764. [Google Scholar]
- [418].Huang Z, Hao Y, Li Y, Hu H, Wang C, Nomoto A, Pan T, Gu Y, Chen Y, Zhang T, Li W, Lei Y, Kim N, Wang C, Zhang L, Ward JW, Maralani A, Li X, Durstock MF, Pisano A, Lin Y, Xu S, Nat. Electron. 2018, 1, 473. [Google Scholar]
- [419].Sugiyama M, Uemura T, Kondo M, Akiyama M, Namba N, Yoshimoto S, Noda Y, Araki T, Sekitani T, Nat. Electron. 2019, 2, 351. [Google Scholar]
- [420].Yang Q, Wei T, Yin RT, Wu M, Xu Y, Koo J, Choi YS, Xie Z, Chen SW, Kandela I, Yao S, Deng Y, Avila R, Liu T-L, Bai W, Yang Y, Han M, Zhang Q, Haney CR, Benjamin Lee K, Aras K, Wang T, Seo M-H, Luan H, Lee SM, Brikha A, Ghoreishi-Haack N, Tran L, Stepien I, Aird F, Waters EA, Yu X, Banks A, Trachiotis GD, Torkelson JM, Huang Y, Kozorovitskiy Y, Efimov IR, Rogers JA, Nat. Mater. 2021, DOI 10.1038/s41563-021-01051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [421].Dussor G, Koerber HR, Oaklander AL, Rice FL, Molliver DC, Brains res. rev. 2009, 60, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [422].Dimitrov AG, Miller JP, Network (N Y) 2001, 12, 441. [PubMed] [Google Scholar]
- [423].Hao J, Bonnet C, Amsalem M, Ruel J, Delmas P, Pflugers Arch. 2015, 467, 109. [DOI] [PubMed] [Google Scholar]
- [424].Arezzo JC, Schaumburg HH, Spencer PS, Environ. Health Perspect. 1982, 44, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [425].Ackerley R, Carlsson I, Wester H, Olausson H, Backlund Wasling H, Front. Behav. Neurosci. 2014, 8, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [426].Mosbacher Y, Khoyratee F, Goldin M, Kanner S, Malakai Y, Silva M, Grassia F, Simon YB, Cortes J, Barzilai A, Levi T, Bonifazi P, Sci. Rep. 2020, 10, 7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [427].Tee BC-K, Chortos A, Berndt A, Nguyen AK, Tom A, McGuire A, Lin ZC, Tien K, Bae W-G, Wang H, Mei P, Chou H-H, Cui B, Deisseroth K, Ng TN, Bao Z, Science 2015, 350, 313. [DOI] [PubMed] [Google Scholar]
- [428].Gonzalez J, Soma H, Sekine M, Yu W, Neuroeng J Rehabilitation 2012, 9, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [429].Antfolk C, D’Alonzo M, Rosén B, Lundborg G, Sebelius F, Cipriani C, Expert Rev Med Devices 2013, 10, 45. [DOI] [PubMed] [Google Scholar]
- [430].Osborn LE, Dragomir A, Betthauser JL, Hunt CL, Nguyen HH, Kaliki RR, Thakor NV, Sci. Robot. 2018, 3, eaat3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [431].Kaczmarek KA, Webster JG, Bach-y-Rita P, Tompkins WJ, IEEE Trans. Biomed. Eng. 1991, 38, 1. [DOI] [PubMed] [Google Scholar]
- [432].Hudgins B, Parker P, Scott RN, IEEE Trans. Biomed. Eng. 1993, 40, 82. [DOI] [PubMed] [Google Scholar]
- [433].Lee WW, Tan YJ, Yao H, Li S, See HH, Hon M, Ng KA, Xiong B, Ho JS, Tee BCK, Sci. Robot. 2019, 4, eaax2198. [DOI] [PubMed] [Google Scholar]
- [434].von Neumann J, IEEE Ann. Hist. Comput. 1993, 15, 27. [Google Scholar]
- [435].Chen W, Cai L, Wang K, Zhang X, Liu X, Du G, Microelectron Reliab 2019, 98, 63. [Google Scholar]
- [436].Ajayan J, Nirmal D, Tayal S, Bhattacharya S, Arivazhagan L, Fletcher AA, Murugapandiyan P, Ajitha D, Microelectronics J 2021, 105141. [Google Scholar]
- [437].Arenas C, Herrera G, Muñoz E, Munoz RC, Mater. Res. Express. 2021, 8, 015026. [Google Scholar]
- [438].Li H, Gao B, Chen Z, Zhao Y, Huang P, Ye H, Liu L, Liu X, Kang J, Sci. Rep. 2015, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [439].Moradi S, Qiao N, Stefanini F, Indiveri G, IEEE Trans Biomed Circuits Syst 2018, 12, 106. [DOI] [PubMed] [Google Scholar]
- [440].Wan C, Cai P, Wang M, Qian Y, Huang W, Chen X, Adv. Mater. 2020, 32, 1902434. [DOI] [PubMed] [Google Scholar]
- [441].Kim Y, Chortos A, Xu W, Liu Y, Oh JY, Son D, Kang J, Foudeh AM, Zhu C, Lee Y, Niu S, Liu J, Pfattner R, Bao Z, Lee T-W, Science 2018, 360, 998. [DOI] [PubMed] [Google Scholar]
- [442].Wan C, Chen G, Fu Y, Wang M, Matsuhisa N, Pan S, Pan L, Yang H, Wan Q, Zhu L, Chen X, Adv. Mater. 2018, 30, 1801291. [DOI] [PubMed] [Google Scholar]
- [443].Yiyue L, Yu F, Xianjun C, J Phys Conf Ser 2021, 1848, 012038. [Google Scholar]
- [444].Patel M, Kalani N, in IOP Conference Series: Materials Science and Engineering, IOP Publishing, 2021, p. 012008. [Google Scholar]
- [445].Ebrahimi A, Luo S, Initiative ADN, J Med Imaging (Bellingham) 2021, 8, 024503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [446].Jiralerspong T, Nakanishi E, Liu C, Ishikawa J, Appl. Sci. 2017, 7, 1163. [Google Scholar]
- [447].Zhang Z, Yang K, Qian J, Zhang L, Sensors 2019, 19, 3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [448].Jin T, Sun Z, Li L, Zhang Q, Zhu M, Zhang Z, Yuan G, Chen T, Tian Y, Hou X, Lee C, Nat. Commun. 2020, 11, 5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [449].Gianfrancesco MA, Tamang S, Yazdany J, Schmajuk G, JAMA Intern. Med. 2018, 178, 1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [450].Melton M, “Amazon Rekognition Falsely Matches 26 Lawmakers to Mugshots As California Bill To Block Moves Forward,” https://www.forbes.com/sites/monicamelton/2019/08/13/amazon-rekognition-falsely-matches-26-lawmakers-to-mugshots-as-california-bill-to-block-moves-forward/, accessed: 2021.
- [451].Klare BF, Burge MJ, Klontz JC, Bruegge RWV, Jain AK, IEEE Trans. Inf. Forensics Secur. 2012, 7, 1789. [Google Scholar]
- [452].Enoka RM, Neuromechanics of Human Movement, Human Kinetics, 2008. [Google Scholar]
- [453].Winters JM, Stark L, IEEE. Trans. Biomed. Eng. 1985, BME-32, 826. [DOI] [PubMed] [Google Scholar]
- [454].Baldi TL, Scheggi S, Meli L, Mohammadi M, Prattichizzo D, IEEE Trans Hum Mach Syst 2017, 47, 1066. [Google Scholar]
- [455].George JA, Kluger DT, Davis TS, Wendelken SM, Okorokova EV, He Q, Duncan CC, Hutchinson DT, Thumser ZC, Beckler DT, Marasco PD, Bensmaia SJ, Clark GA, Sci. Robot. 2019, 4, eaax2352. [DOI] [PubMed] [Google Scholar]
- [456].Lukyanenko P, Dewald HA, Lambrecht J, Kirsch RF, Tyler DJ, Williams MR, Neuroeng J Rehabilitation 2021, 18, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [457].Resnik L, Meucci MR, Lieberman-Klinger S, Fantini C, Kelty DL, Disla R, Sasson N, Arch Phys Med Rehabil 2012, 93, 710. [DOI] [PubMed] [Google Scholar]
- [458].Yap HK, Lim Jeong Hoon, Nasrallah F, Goh JCH, Yeow RCH, in 2015 IEEE International Conference on Robotics and Automation (ICRA), IEEE, Seattle, WA, USA, 2015, pp. 4967–4972. [Google Scholar]
- [459].Walsh C, Nat. Rev. Mater. 2018, 3, 78. [Google Scholar]
- [460].O’Brien KW, Xu PA, Levine DJ, Aubin CA, Yang H-J, Xiao MF, Wiesner LW, Shepherd RF, Sci. Robot. 2018, 3, eaau5543. [DOI] [PubMed] [Google Scholar]
- [461].Lau AHY, Chik GKK, Zhang Z, Leung TKW, Chan PKL, Adv. Intell. Syst. 2020, 2, 2000005. [Google Scholar]
- [462].Tefertiller C, Pharo B, Evans N, Winchester P, J Rehabil Res Dev 2011, 48, 387. [DOI] [PubMed] [Google Scholar]
- [463].Maeder-York P, Clites T, Boggs E, Neff R, Polygerinos P, Holland D, Stirling L, Galloway K, Wee C, Walsh C, J Med Device 2014, 8, 020933. [Google Scholar]
- [464].Asbeck AT, De Rossi SMM, Holt KG, Walsh CJ, Int J Rob Res 2015, 34, 744. [Google Scholar]
- [465].Markvicka EJ, Bartlett MD, Huang X, Majidi C, Nat. Mater. 2018, 17, 618. [DOI] [PubMed] [Google Scholar]
- [466].Zhao H, Jalving J, Huang R, Knepper R, Ruina A, Shepherd R, IEEE Robot Autom Mag 2016, 23, 55. [Google Scholar]
- [467].Mohammadi A, Lavranos J, Zhou H, Mutlu R, Alici G, Tan Y, Choong P, Oetomo D, PLoS One 2020, 15, e0232766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [468].Gordon KE, Sawicki GS, Ferris DP, J Biomech 2006, 39, 1832. [DOI] [PubMed] [Google Scholar]
- [469].Hu W, Alici G, Soft Robot. 2020, 7, 267. [DOI] [PubMed] [Google Scholar]
- [470].Connolly F, Walsh CJ, Bertoldi K, Proc. Natl. Acad. Sci. U.S.A. 2017, 114, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [471].Tondu B, Lopez P, IEEE Control Syst 2000, 20, 15. [Google Scholar]
- [472].Chou C-P, Hannaford B, IEEE trans. robot. autom. 1996, 12, 90. [Google Scholar]
- [473].Klute GK, Czerniecki JM, Hannaford B, in 1999 IEEE/ASME International Conference on Advanced Intelligent Mechatronics (Cat. No.99TH8399), 1999, pp. 221–226. [Google Scholar]
- [474].Marchese AD, Katzschmann RK, Rus D, Soft Robot. 2015, 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [475].Marchese AD, Komorowski K, Onal CD, Rus D, in 2014 IEEE International Conference on Robotics and Automation (ICRA), 2014, pp. 2189–2196. [Google Scholar]
- [476].Marchese AD, Rus D, Int J Rob Res 2016, 35, 840. [Google Scholar]
- [477].Morrow J, Shin H-S, Phillips-Grafflin C, Jang S-H, Torrey J, Larkins R, Dang S, Park Y-L, Berenson D, in 2016 IEEE International Conference on Robotics and Automation (ICRA), IEEE, Stockholm, Sweden, 2016, pp. 5024–5031. [Google Scholar]
- [478].Park Y-L, Santos J, Galloway KG, Goldfield EC, Wood RJ, in 2014 IEEE International Conference on Robotics and Automation (ICRA), 2014, pp. 4805–4810. [Google Scholar]
- [479].Rodrigue H, Wang W, Han M-W, Kim TJY, Ahn S-H, Soft Robot. 2017, 4, 3. [DOI] [PubMed] [Google Scholar]
- [480].Fan J, Li G, RSC Adv. 2017, 7, 1127. [Google Scholar]
- [481].Kanik M, Orguc S, Varnavides G, Kim J, Benavides T, Gonzalez D, Akintilo T, Tasan CC, Chandrakasan AP, Fink Y, Anikeeva P, Science 2019, 365, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [482].Seok S, Onal CD, Cho K-J, Wood RJ, Rus D, Kim S, IEEE ASME Trans Mechatron 2013, 18, 1485. [Google Scholar]
- [483].Calisti M, Giorelli M, Levy G, Mazzolai B, Hochner B, Laschi C, Dario P, Bioinspir Biomim 2011, 6, 036002. [DOI] [PubMed] [Google Scholar]
- [484].Dalley SA, Wiste TE, Withrow TJ, Goldfarb M, IEEE ASME Trans Mechatron 2009, 14, 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [485].Arjun A, Saharan L, Tadesse Y, in 2016 IEEE International Conference on Automation Science and Engineering (CASE), 2016, pp. 910–915. [Google Scholar]
- [486].Mohd Faudzi AA, Ooga J, Goto T, Takeichi M, Suzumori K, IEEE Robot. Autom. Lett. 2018, 3, 92. [Google Scholar]
- [487].In H, Kang BB, Sin M, Cho K-J, IEEE Robot Automat Mag 2015, 22, 97. [Google Scholar]
- [488].Mahato M, Tabassian R, Nguyen VH, Oh S, Nam S, Hwang W-J, Oh I-K, Nat. Commun. 2020, 11, 5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [489].Yin L-J, Zhao Y, Zhu J, Yang M, Zhao H, Pei J-Y, Zhong S-L, Dang Z-M, Nat. Commun. 2021, 12, 4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [490].Tiwari R, Garcia E, Smart Mater Struct 2011, 20, 083001. [Google Scholar]
- [491].Tang W, Lin Y, Zhang C, Liang Y, Wang J, Wang W, Ji C, Zhou M, Yang H, Zou J, Sci. Adv. 2021, 7, eabf8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [492].Zamanian AH, Porter DA, Krueger P, Richer E, American Society Of Mechanical Engineers Digital Collection, 2018. [Google Scholar]
- [493].Li N, Yang T, Yu P, Chang J, Zhao L, Zhao X, Elhajj IH, Xi N, Liu L, Bioinspir Biomim 2018, 13, 066001. [DOI] [PubMed] [Google Scholar]
- [494].Awad LN, Bae J, O’Donnell K, De Rossi SMM, Hendron K, Sloot LH, Kudzia P, Allen S, Holt KG, Ellis TD, Walsh CJ, Sci Transl Med 2017, 9, eaai9084. [DOI] [PubMed] [Google Scholar]
- [495].Park Y-L, Chen B, Pérez-Arancibia NO, Young D, Stirling L, Wood RJ, Goldfield EC, Nagpal R, Bioinspir Biomim 2014, 9, 016007. [DOI] [PubMed] [Google Scholar]
- [496].Kwon J, Park J-H, Ku S, Jeong Y, Paik N-J, Park Y-L, IEEE Robot. Autom. Lett. 2019, 4, 2547. [Google Scholar]
- [497].Yao S, Cui J, Cui Z, Zhu Y, Nanoscale 2017, 9, 3797. [DOI] [PubMed] [Google Scholar]
- [498].Lee H, Choi TK, Lee YB, Cho HR, Ghaffari R, Wang L, Choi HJ, Chung TD, Lu N, Hyeon T, Choi SH, Kim D-H, Nat. Nanotechnol. 2016, 11, 566. [DOI] [PubMed] [Google Scholar]
- [499].Ding J, Zhao W, Jin W, Di C, Zhu D, Adv. Funct. Mater. 2021, 31, 2010695. [Google Scholar]
- [500].Hu X, Gong X, Zhang M, Lu H, Xue Z, Mei Y, Chu PK, An Z, Di Z, Small 2020, 16, 1907170. [DOI] [PubMed] [Google Scholar]
- [501].Lee J, Sul H, Lee W, Pyun KR, Ha I, Kim D, Park H, Eom H, Yoon Y, Jung J, Lee D, Ko SH, Adv. Funct. Mater. 2020, 30, 1909171. [Google Scholar]
- [502].Cabibihan J-J, Jegadeesan R, Salehi S, Ge SS, in Social Robotics (Eds.: Ge SS, Li H, Cabibihan J-J, Tan YK), Springer, Berlin, Heidelberg, 2010, pp. 362–371. [Google Scholar]
- [503].Choi S, Han SI, Jung D, Hwang HJ, Lim C, Bae S, Park OK, Tschabrunn CM, Lee M, Bae SY, Yu JW, Ryu JH, Lee S-W, Park K, Kang PM, Lee WB, Nezafat R, Hyeon T, Kim D-H, Nat. Nanotechnol. 2018, 13, 1048. [DOI] [PubMed] [Google Scholar]
- [504].Webb RC, Pielak RM, Bastien P, Ayers J, Niittynen J, Kurniawan J, Manco M, Lin A, Cho NH, Malyrchuk V, Balooch G, Rogers JA, PLoS One 2015, 10, e0118131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [505].Choi MK, Park I, Kim DC, Joh E, Park OK, Kim J, Kim M, Choi C, Yang J, Cho KW, Hwang J-H, Nam J-M, Hyeon T, Kim JH, Kim D-H, Adv. Funct. Mater. 2015, 25, 7109. [Google Scholar]
- [506].Jang N-S, Kim K-H, Ha S-H, Jung S-H, Lee HM, Kim J-M, ACS Appl. Mater. Interfaces 2017, 9, 19612. [DOI] [PubMed] [Google Scholar]
- [507].Choi S, Park J, Hyun W, Kim J, Kim J, Lee YB, Song C, Hwang HJ, Kim JH, Hyeon T, Kim D-H, ACS Nano 2015, 9, 6626. [DOI] [PubMed] [Google Scholar]
- [508].Xu X, Zhou J, Chen J, Adv. Funct. Mater. 2020, 30, 1904704. [Google Scholar]
- [509].Jayathilaka WADM, Qi K, Qin Y, Chinnappan A, Serrano-García W, Baskar C, Wang H, He J, Cui S, Thomas SW, Ramakrishna S, Adv. Mater. 2019, 31, 1805921. [DOI] [PubMed] [Google Scholar]
- [510].Kim G-H, Shao L, Zhang K, Pipe KP, Nat. Mater. 2013, 12, 719. [DOI] [PubMed] [Google Scholar]
- [511].Hong S, Gu Y, Seo JK, Wang J, Liu P, Meng YS, Xu S, Chen R, Sci. Adv. 2019, 5, eaaw0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [512].Chang T-H, Li K, Yang H, Chen P-Y, Adv. Mater. 2018, 30, 1802418. [DOI] [PubMed] [Google Scholar]
- [513].Son D, Lee J, Qiao S, Ghaffari R, Kim J, Lee JE, Song C, Kim SJ, Lee DJ, Jun SW, Yang S, Park M, Shin J, Do K, Lee M, Kang K, Hwang CS, Lu N, Hyeon T, Kim D-H, Nat. Nanotechnol. 2014, 9, 397. [DOI] [PubMed] [Google Scholar]
- [514].Lin S-Y, Zhang T-Y, Lu Q, Wang D-Y, Yang Y, Wu X-M, Ren T-L, RSC Adv. 2017, 7, 27001. [Google Scholar]
- [515].Raspopovic S, Valle G, Petrini FM, Nat. Mater. 2021, 20, 925. [DOI] [PubMed] [Google Scholar]
- [516].Zheng H, Zhang Z, Jiang S, Yan B, Shi X, Xie Y, Huang X, Yu Z, Liu H, Weng S, Nurmikko A, Zhang Y, Peng H, Xu W, Zhang J, Nat. Commun. 2019, 10, 2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [517].Srinivasan SS, Maimon BE, Diaz M, Song H, Herr HM, Nat. Commun. 2018, 9, 5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [518].Petrini FM, Valle G, Strauss I, Granata G, Iorio RD, D’Anna E, Čvančara P, Mueller M, Carpaneto J, Clemente F, Controzzi M, Bisoni L, Carboni C, Barbaro M, Iodice F, Andreu D, Hiairrassary A, Divoux J-L, Cipriani C, Guiraud D, Raffo L, Fernandez E, Stieglitz T, Raspopovic S, Rossini PM, Micera S, Ann. Neurol. 2019, 85, 137. [DOI] [PubMed] [Google Scholar]
- [519].Petrini FM, Bumbasirevic M, Valle G, Ilic V, Mijović P, Čvančara P, Barberi F, Katic N, Bortolotti D, Andreu D, Lechler K, Lesic A, Mazic S, Mijović B, Guiraud D, Stieglitz T, Alexandersson A, Micera S, Raspopovic S, Nat. Med. 2019, 25, 1356. [DOI] [PubMed] [Google Scholar]
- [520].Kringelbach ML, Jenkinson N, Owen SLF, Aziz TZ, Nat. Rev. Neurosci. 2007, 8, 623. [DOI] [PubMed] [Google Scholar]
- [521].FitzGerald JJ, Lago N, Benmerah S, Serra J, Watling CP, Cameron RE, Tarte E, Lacour SP, McMahon SB, Fawcett JW, J Neural Eng 2012, 9, 016010. [DOI] [PubMed] [Google Scholar]
- [522].North RB, Proc IEEE Inst Electr Electron Eng 2008, 96, 1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [523].Lee S, Sheshadri S, Xiang Z, Delgado-Martinez I, Xue N, Sun T, Thakor NV, Yen S-C, Lee C, Sens. Actuators B Chem. 2017, 242, 1165. [Google Scholar]
- [524].Reddy JW, Kimukin I, Stewart LT, Ahmed Z, Barth AL, Towe E, Chamanzar M, Front. Neurosci. 2019, 13, 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [525].Bi X, Xie T, Fan B, Khan W, Guo Y, Li W, IEEE Trans Biomed Circuits Syst 2016, 10, 972. [DOI] [PubMed] [Google Scholar]
- [526].Pashaei V, Dehghanzadeh P, Enwia G, Bayat M, Majerus SJA, Mandal S, IEEE Trans Biomed Circuits Syst 2020, 14, 305. [DOI] [PubMed] [Google Scholar]
- [527].Lee J-H, Cho I-J, Ko K, Yoon E-S, Park H-H, Kim TS, Microsyst. Technol. 2017, 23, 2321. [Google Scholar]
- [528].Liu M, Tuovinen PH, Kawasaki Y, Yedeas MA, Saitoh Y, Sekino M, AIP Adv. 2019, 9, 035335. [Google Scholar]
- [529].Bettinger CJ, Bioelectron. med. 2018, 4, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [530].Cui H, Xie X, Xu S, Chan LLH, Hu Y, Biomed. Eng OnLine 2019, 18, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [531].Brummer SB, Turner MJ, IEEE. Trans. Biomed. Eng. 1977, 436. [DOI] [PubMed] [Google Scholar]
- [532].Cogan SF, Troyk PR, Ehrlich J, Plante TD, Detlefsen DE, IEEE. Trans. Biomed. Eng. 2006, 53, 327. [DOI] [PubMed] [Google Scholar]
- [533].Weiland JD, Anderson DJ, Humayun MS, IEEE. Trans. Biomed. Eng. 2002, 49, 1574. [DOI] [PubMed] [Google Scholar]
- [534].Hu L, Yuan W, Brochu P, Gruner G, Pei Q, Appl. Phys. Lett. 2009, 94, 161108. [Google Scholar]
- [535].McCallum GA, Sui X, Qiu C, Marmerstein J, Zheng Y, Eggers TE, Hu C, Dai L, Durand DM, Sci. Rep. 2017, 7, 11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [536].Venkatraman S, Hendricks J, King ZA, Sereno AJ, Richardson-Burns S, Martin D, Carmena JM, IEEE Trans. Neural Syst. Rehabilitation Eng. 2011, 19, 307. [DOI] [PubMed] [Google Scholar]
- [537].Guo L, Ma M, Zhang N, Langer R, Anderson DG, Adv. Mater. 2014, 26, 1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [538].Liu X, Yue Z, Higgins MJ, Wallace GG, Biomaterials 2011, 32, 7309. [DOI] [PubMed] [Google Scholar]
- [539].Sekine S, Ido Y, Miyake T, Nagamine K, Nishizawa M, J. Am. Chem. Soc. 2010, 132, 13174. [DOI] [PubMed] [Google Scholar]
- [540].Guiseppi-Elie A, Biomaterials 2010, 31, 2701. [DOI] [PubMed] [Google Scholar]
- [541].Sasaki M, Karikkineth BC, Nagamine K, Kaji H, Torimitsu K, Nishizawa M, Adv. Healthc. Mater. 2014, 3, 1919. [DOI] [PubMed] [Google Scholar]
- [542].Feig VR, Tran H, Lee M, Bao Z, Nat. Commun. 2018, 9, 2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [543].Gillis WF, Lissandrello CA, Shen J, Pearre BW, Mertiri A, Deku F, Cogan S, Holinski BJ, Chew DJ, White AE, Otchy TM, Gardner TJ, J Neural Eng 2018, 15, 016010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [544].Byun D, Cho SJ, Kim S, J Micromech Microeng 2013, 23, 125010. [Google Scholar]
- [545].Lu Y, Lyu H, Richardson AG, Lucas TH, Kuzum D, Sci. Rep. 2016, 6, 33526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [546].Hiremath SV, Tyler-Kabara EC, Wheeler JJ, Moran DW, Gaunt RA, Collinger JL, Foldes ST, Weber DJ, Chen W, Boninger ML, Wang W, PLoS One 2017, 12, e0176020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [547].Woodington BJ, Curto VF, Yu Y-L, Martínez-Domínguez H, Coles L, Malliaras GG, Proctor CM, Barone DG, Sci. Adv. 2021, 7, eabg7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [548].Minev IR, Musienko P, Hirsch A, Barraud Q, Wenger N, Moraud EM, Gandar J, Capogrosso M, Milekovic T, Asboth L, Torres RF, Vachicouras N, Liu Q, Pavlova N, Duis S, Larmagnac A, Voros J, Micera S, Suo Z, Courtine G, Lacour SP, Science 2015, 347, 159. [DOI] [PubMed] [Google Scholar]
- [549].Zhang M, Tang Z, Liu X, Van der Spiegel J, Nat. Electron. 2020, 3, 191. [Google Scholar]
- [550].Sheffler LR, Chae J, Muscle Nerve 2007, 35, 562. [DOI] [PubMed] [Google Scholar]
- [551].Choi YS, Hsueh Y-Y, Koo J, Yang Q, Avila R, Hu B, Xie Z, Lee G, Ning Z, Liu C, Xu Y, Lee YJ, Zhao W, Fang J, Deng Y, Lee SM, Vázquez-Guardado A, Stepien I, Yan Y, Song JW, Haney C, Oh YS, Liu W, Yoon H-J, Banks A, MacEwan MR, Ameer GA, Ray WZ, Huang Y, Xie T, Franz CK, Li S, Rogers JA, Nat. Commun. 2020, 11, 5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [552].Boretius T, Badia J, Pascual-Font A, Schuettler M, Navarro X, Yoshida K, Stieglitz T, Biosens. Bioelectron. 2010, 26, 62. [DOI] [PubMed] [Google Scholar]
- [553].Lago N, Yoshida K, Koch KP, Navarro X, IEEE Trans. Biomed. Eng. 2007, 54, 281. [DOI] [PubMed] [Google Scholar]
- [554].Lienemann S, Zötterman J, Farnebo S, Tybrandt K, J Neural Eng 2021, 18, 045007. [DOI] [PubMed] [Google Scholar]
- [555].Ward PJ, Clanton SL, English AW, Eur. J. Neurosci. 2018, 47, 294. [DOI] [PubMed] [Google Scholar]
- [556].Montgomery KL, Iyer SM, Christensen AJ, Deisseroth K, Delp SL, Sci Transl Med 2016, 8, 337rv5. [DOI] [PubMed] [Google Scholar]
- [557].Darlot F, Moro C, Massri NE, Chabrol C, Johnstone DM, Reinhart F, Agay D, Torres N, Bekha D, Auboiroux V, Costecalde T, Peoples CL, Anastascio HDT, Shaw VE, Stone J, Mitrofanis J, Benabid A-L, Ann. Neurol. 2016, 79, 59. [DOI] [PubMed] [Google Scholar]
- [558].Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, Adaikkan C, Canter RG, Rueda R, Brown EN, Boyden ES, Tsai L-H, Nature 2016, 540, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [559].Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J, J. Neurosci 2008, 28, 11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [560].Daou I, Tuttle AH, Longo G, Wieskopf JS, Bonin RP, Ase AR, Wood JN, De Koninck Y, Ribeiro-da-Silva A, Mogil JS, Seguela P, J. Neurosci 2013, 33, 18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [561].Prescott SA, Ma Q, De Koninck Y, Nat. Neurosci. 2014, 17, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [562].Kim D, Yokota T, Suzuki T, Lee S, Woo T, Yukita W, Koizumi M, Tachibana Y, Yawo H, Onodera H, Sekino M, Someya T, Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [563].Jeong J-W, McCall JG, Shin G, Zhang Y, Al-Hasani R, Kim M, Li S, Sim JY, Jang K-I, Shi Y, Hong DY, Liu Y, Schmitz GP, Xia L, He Z, Gamble P, Ray WZ, Huang Y, Bruchas MR, Rogers JA, Cell 2015, 162, 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [564].Kim CY, Ku MJ, Qazi R, Nam HJ, Park JW, Nam KS, Oh S, Kang I, Jang J-H, Kim WY, Kim J-H, Jeong J-W, Nat. Commun. 2021, 12, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [565].Park SI, Shin G, McCall JG, Al-Hasani R, Norris A, Xia L, Brenner DS, Noh KN, Bang SY, Bhatti DL, Jang K-I, Kang S-K, Mickle AD, Dussor G, Price TJ, Gereau RW, Bruchas MR, Rogers JA, Proc. Natl. Acad. Sci. U.S.A. 2016, 113, E8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [566].Kim T. -i., McCall JG, Jung YH, Huang X, Siuda ER, Li Y, Song J, Song YM, Pao HA, Kim R-H, Lu C, Lee SD, Song I-S, Shin G, Al-Hasani R, Kim S, Tan MP, Huang Y, Omenetto FG, Rogers JA, Bruchas MR, Science 2013, 340, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [567].Gutruf P, Krishnamurthi V, Vázquez-Guardado A, Xie Z, Banks A, Su C-J, Xu Y, Haney CR, Waters EA, Kandela I, Krishnan SR, Ray T, Leshock JP, Huang Y, Chanda D, Rogers JA, Nat. Electron. 2018, 1, 652. [Google Scholar]
- [568].Shin G, Gomez AM, Al-Hasani R, Jeong YR, Kim J, Xie Z, Banks A, Lee SM, Han SY, Yoo CJ, Lee J-L, Lee SH, Kurniawan J, Tureb J, Guo Z, Yoon J, Park S-I, Bang SY, Nam Y, Walicki MC, Samineni VK, Mickle AD, Lee K, Heo SY, McCall JG, Pan T, Wang L, Feng X, Kim T, Kim JK, Li Y, Huang Y, Gereau RW, Ha JS, Bruchas MR, Rogers JA, Neuron 2017, 93, 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [569].Samineni VK, Yoon J, Crawford KE, Jeong YR, McKenzie KC, Shin G, Xie Z, Sundaram SS, Li Y, Yang MY, Kim J, Wu D, Xue Y, Feng X, Huang Y, Mickle AD, Banks A, Ha JS, Golden JP, Rogers JA, Gereau RW, Pain 2017, 158, 2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [570].Wang Y, Xie K, Yue H, Chen X, Luo X, Liao Q, Liu M, Wang F, Shi P, Nanoscale 2020, 12, 2406. [DOI] [PubMed] [Google Scholar]
- [571].Lu C, Froriep UP, Koppes RA, Canales A, Caggiano V, Selvidge J, Bizzi E, Anikeeva P, Adv. Funct. Mater. 2014, 24, 6594. [Google Scholar]
- [572].Zhang Y, Mickle AD, Gutruf P, McIlvried LA, Guo H, Wu Y, Golden JP, Xue Y, Grajales-Reyes JG, Wang X, Krishnan S, Xie Y, Peng D, Su C-J, Zhang F, Reeder JT, Vogt SK, Huang Y, Rogers JA, Gereau RW, Sci. Adv. 2019, 5, eaaw5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [573].Park SI, Brenner DS, Shin G, Morgan CD, Copits BA, Chung HU, Pullen MY, Noh KN, Davidson S, Oh SJ, Yoon J, Jang K-I, Samineni VK, Norman M, Grajales-Reyes JG, Vogt SK, Sundaram SS, Wilson KM, Ha JS, Xu R, Pan T, Kim T, Huang Y, Montana MC, Golden JP, Bruchas MR, Gereau RW, Rogers JA, Nat. Biotechnol. 2015, 33, 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [574].Montgomery KL, Yeh AJ, Ho JS, Tsao V, Mohan Iyer S, Grosenick L, Ferenczi EA, Tanabe Y, Deisseroth K, Delp SL, Poon ASY, Nat. Methods 2015, 12, 969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [575].Towne C, Montgomery KL, Iyer SM, Deisseroth K, Delp SL, PLoS One 2013, 8, e72691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [576].Kwon KY, Sirowatka B, Weber A, Li W, IEEE Trans Biomed Circuits Syst 2013, 7, 593. [DOI] [PubMed] [Google Scholar]
- [577].Maimon BE, Zorzos AN, Bendell R, Harding A, Fahmi M, Srinivasan S, Calvaresi P, Herr HM, J Neural Eng 2017, 14, 034002. [DOI] [PubMed] [Google Scholar]
- [578].Novich SD, Eagleman DM, Exp Brain Res 2015, 233, 2777. [DOI] [PubMed] [Google Scholar]
- [579].Song K, Kim SH, Jin S, Kim S, Lee S, Kim J-S, Park J-M, Cha Y, Sci. Rep. 2019, 9, 8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [580].Sonar HA, Paik J, Front. Robot. AI 2016, 2, 38. [Google Scholar]
- [581].Richter A, Paschew G, Adv. Mater. 2009, 21, 979. [Google Scholar]
- [582].Besse N, Rosset S, Zarate JJ, Shea H, Adv. Mater. Technol. 2017, 2, 1700102. [Google Scholar]
- [583].Chossat J-B, Chen DKY, Park Y-L, Shull PB, IEEE Trans Haptics 2019, 12, 521. [DOI] [PubMed] [Google Scholar]
- [584].Muthukumarana S, Elvitigala DS, Forero Cortes JP, Matthies DJ, Nanayakkara S, in Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems, 2020, pp. 1–12. [Google Scholar]
- [585].Yang T-H, Kim JR, Jin H, Gil H, Koo J-H, Kim HJ, Adv. Funct. Mater. 2021, 2008831. [Google Scholar]
- [586].Zhu R, Wallrabe U, Wapler MC, Woias P, Mescheder U, Procedia Engineering 2016, 168, 1537. [Google Scholar]
- [587].Koo Ig Mo, Jung Kwangmok, Koo Ja Choon, Nam Jae-Do, Lee Young Kwan, Choi Hyouk Ryeol, IEEE Trans. Robot 2008, 24, 549. [Google Scholar]
- [588].Marette A, Poulin A, Besse N, Rosset S, Briand D, Shea H, Adv. Mater. 2017, 29, 1700880. [DOI] [PubMed] [Google Scholar]
- [589].Mun S, Yun S, Nam S, Park SK, Park S, Park BJ, Lim JM, Kyung K-U, IEEE Trans Haptics 2018, 11, 15. [DOI] [PubMed] [Google Scholar]
- [590].Hinchet R, Shea H, Adv. Mater. Technol. 2020, 5, 1900895. [Google Scholar]
- [591].Mullenbach J, Peshkin M, Colgate JE, IEEE Trans Haptics 2017, 10, 358. [DOI] [PubMed] [Google Scholar]
- [592].Zhao H, Hussain AM, Duduta M, Vogt DM, Wood RJ, Clarke DR, Adv. Funct. Mater. 2018, 28, 1804328. [Google Scholar]
- [593].Go G-T, Lee Y, Seo D-G, Pei M, Lee W, Yang H, Lee T-W, Adv. Intell. Syst. 2020, 2, 2000012. [Google Scholar]
- [594].Han AK, Ji S, Wang D, Cutkosky MR, IEEE Robot. Autom. Lett. 2020, 5, 4021. [Google Scholar]
- [595].Pacchierotti C, Sinclair S, Solazzi M, Frisoli A, Hayward V, Prattichizzo D, IEEE Trans Haptics 2017, 10, 580. [DOI] [PubMed] [Google Scholar]
- [596].Van Duong Q, Nguyen VP, Luu AT, Choi ST, Sci. Rep. 2019, 9, 13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [597].Dagdeviren C, Shi Y, Joe P, Ghaffari R, Balooch G, Usgaonkar K, Gur O, Tran PL, Crosby JR, Meyer M, Su Y, Chad Webb R, Tedesco AS, Slepian MJ, Huang Y, Rogers JA, Nat.Mater 2015, 14, 728. [DOI] [PubMed] [Google Scholar]
- [598].Yu X, Xie Z, Yu Y, Lee J, Vazquez-Guardado A, Luan H, Ruban J, Ning X, Akhtar A, Li D, Ji B, Liu Y, Sun R, Cao J, Huo Q, Zhong Y, Lee C, Kim S, Gutruf P, Zhang C, Xue Y, Guo Q, Chempakasseril A, Tian P, Lu W, Jeong J, Yu Y, Cornman J, Tan C, Kim B, Lee K, Feng X, Huang Y, Rogers JA, Nature 2019, 575, 473. [DOI] [PubMed] [Google Scholar]
- [599].Xu B, Akhtar A, Liu Y, Chen H, Yeo W-H, Park SI, Boyce B, Kim H, Yu J, Lai H-Y, Jung S, Zhou Y, Kim J, Cho S, Huang Y, Bretl T, Rogers JA, Adv. Mater. 2016, 28, 4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [600].Withana A, Groeger D, Steimle J, in Proceedings of the 31st Annual ACM Symposium on User Interface Software and Technology, Association for Computing Machinery, New York, NY, USA, 2018, pp. 365–378. [Google Scholar]
- [601].Deyo RA, Walsh NE, Martin DC, Schoenfeld LS, Ramamurthy S, Engl N J. Med. 1990, 322, 1627. [DOI] [PubMed] [Google Scholar]
- [602].Stephens-Fripp B, Sencadas V, Mutlu R, Alici G, Front. Bioeng. Biotechnol. 2018, 6, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [603].Weiss T, Front. Neurol. 2018, 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [604].Shi Y, Wang F, Tian J, Li S, Fu E, Nie J, Lei R, Ding Y, Chen X, Wang ZL, Sci. Adv. 2021, 7, eabe2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [605].Zou L, Tian H, Guan S, Ding J, Gao L, Wang J, Fang Y, Nat. Commun. 2021, 12, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [606].Vonck K, Boon P, Nat. Rev. Neurol. 2015, 11, 252. [DOI] [PubMed] [Google Scholar]
- [607].Sharifi S, Behzadi S, Laurent S, Forrest ML, Stroeve P, Mahmoudi M, Chem. Soc. Rev. 2012, 41, 2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [608].Valsami-Jones E, Lynch I, Science 2015, 350, 388. [DOI] [PubMed] [Google Scholar]
- [609].Jung D, Lim C, Shim HJ, Kim Y, Park C, Jung J, Han SI, Sunwoo S-H, Cho KW, Cha GD, Kim DC, Koo JH, Kim JH, Hyeon T, Kim D-H, Science 2021, 373, 1022. [DOI] [PubMed] [Google Scholar]
- [610].Kwon Y-T, Kim Y-S, Kwon S, Mahmood M, Lim H-R, Park S-W, Kang S-O, Choi JJ, Herbert R, Jang YC, Choa Y-H, Yeo W-H, Nat. Commun. 2020, 11, 3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [611].Yin H, Varava A, Kragic D, Sci. Robot. 2021, 6, eabd8803. [DOI] [PubMed] [Google Scholar]
- [612].Shan B, Broza YY, Li W, Wang Y, Wu S, Liu Z, Wang J, Gui S, Wang L, Zhang Z, Liu W, Zhou S, Jin W, Zhang Q, Hu D, Lin L, Zhang Q, Li W, Wang J, Liu H, Pan Y, Haick H, ACS Nano 2020, 14, 12125. [DOI] [PubMed] [Google Scholar]
- [613].Bern JM, Schnider Y, Banzet P, Kumar N, Coros S, in 2020 3rd IEEE International Conference on Soft Robotics (RoboSoft), 2020, pp. 417–423. [Google Scholar]
- [614].Chin K, Hellebrekers T, Majidi C, Adv. Intell. Syst. 2020, 2, 1900171. [Google Scholar]
- [615].Yin L, Moon J-M, Sempionatto JR, Lin M, Cao M, Trifonov A, Zhang F, Lou Z, Jeong J-M, Lee S-J, Xu S, Wang J, Joule 2021, 5, 1888. [Google Scholar]
- [616].Jinno H, Fukuda K, Xu X, Park S, Suzuki Y, Koizumi M, Yokota T, Osaka I, Takimiya K, Someya T, Nat. Energy 2017, 2, 780. [Google Scholar]
- [617].Wang M, Yang Y, Gao W, Trends in Chemistry 2021. [Google Scholar]
- [618].Cheng X, Song Y, Han M, Meng B, Su Z, Miao L, Zhang H, Sens. Actuator A Phys. 2016, 247, 206. [Google Scholar]
- [619].Bariya M, Shahpar Z, Park H, Sun J, Jung Y, Gao W, Nyein HYY, Liaw TS, Tai L-C, Ngo QP, Chao M, Zhao Y, Hettick M, Cho G, Javey A, ACS Nano 2018, 12, 6978. [DOI] [PubMed] [Google Scholar]
- [620].Nayak L, Mohanty S, Nayak SK, Ramadoss A, J. Mater. Chem. C 2019, 7, 8771. [Google Scholar]
- [621].Cheng G, Ehrlich SK, Lebedev M, Nicolelis MAL, Sci. Robot. 2020, 5, eabd1911. [DOI] [PubMed] [Google Scholar]