Abstract
Cerium oxide nanoparticles have significantly improved catalytic properties and are of increasing interest in the nanoparticle research field hence the current trends in cerium oxide nanoparticles are reviewed here. Unlike previous reviews which have focused primarily on the biosynthesis of cerium oxide nanoparticles, their properties, and applications, this review will focus on the unique physical, chemical, and biological properties of cerium oxide nanoparticles, the role of oxygen vacancies or defects in the lattice structure, the ratio of oxidation states in determining their catalytic properties and applications in biosensing, drug or gene delivery, etc. have been discussed. Furthermore, the limitations of the bare form of cerium oxide nanoparticles and the advances in the field of surface coating by different ligands to overcome the issues of bare nanoparticles have been discussed. The review concludes with a discussion on the environmental aspects and toxicity of cerium oxide nanoparticles and their potential future in practical applications.
Keywords: Cerium oxide nanoparticles, SOD, Catalase, Biomedical applications, Surface coatings
Introduction
Over the past decades, advancement in the field of nanotechnology seeks to bring a revolution in the area of research. Nanotechnology is an integrative scientific field that brings together biology, medicine, biotechnology, molecular engineering, and physical sciences under one roof (Rangasamy 2011). Nanotechnology deals with the materials having at least one dimension in the nanoscale (1–100 nm) and comprises developing or modifying structures of that size. These materials are termed nanoparticles or nanomaterials or nanostructures (Woldeamanuel et al. 2021). Nanoparticles (NPs) have gained salience because of their unique and tunable properties, such as electrical and thermal conductivity, light absorption, melting point, magnetic properties, catalytic activity, wettability, etc. resulting in their ameliorated performance as compared to their bulk counterparts (Jeevanandam et al. 2018). Depending on the method of synthesis, precursor, solvent, and physicochemical properties, NPs can be divided into different categories. However, broadly, NPs are of three types: organic (polymers and liposomes), inorganic (ceramics, metals, metal oxides, and quantum dots), and carbon-based NPs (Fullerenes and carbon nanotubes) (Mauricio et al. 2018). Among them, metal oxide NPs (MONPs) is emerging as an area of acute scientific research because of their unique antioxidant and catalytic properties, high chemical stability, tunable size, large surface area, and biocompatibility. Several MONPs have been reported till now, in which iron oxide, titanium oxide, zirconium oxide, and cerium oxide NPs are the ones most widely studied (Chavali and Nikolova 2019). MONPs have been reported for various biomedical applications, such as in medical implants, anti-inflammatory, antioxidants, anticancer and antimicrobial agents, biosensing, imaging, drug delivery, and recently as nanozymes (Celardo et al. 2011; Zhang et al. 2013b; Wang et al. 2015; Yadav and Singh 2021b; Singh 2016).
Cerium is one of the most reactive and copious rare elements of the lanthanide series of the periodic table. Due to its electropositive nature, it exists in two oxidation states: i.e., Ce3+ (trivalent) and Ce4+ (tetravalent). Cerium is more stable in its Ce4+ state, because its electronic configuration is [Xe]4f0 than the Ce3+ state, which has the electronic configuration of [Xe]4f1. When cerium combines with oxygen during NP formulation, it attains a fluorite crystalline (FCC) structure (Xu and Qu 2014). Cerium oxide NPs (CeNPs) have been formulated as riveting nanomaterials with excellent biomedical applications. Two types of cerium oxides are possible: (1) Cerium dioxide (CeO2) and Cerium sesquioxide (Ce2O3) but CeO2 is the more stable form and hence utilized more as compared to Ce2O3. Both oxidation states (3+ and 4+) co-exist on the surface of CeNPs and it can switch between these two states depending on the environment. This shuttling among oxidation states is causative of the antioxidant and catalytic properties of CeNPs (Younis et al. 2016). CeNPs have been explored for various applications, such as catalysis, fuel cells, gas sensors, ultraviolet (UV) absorbers, energy storage devices, optical devices, sensing, medicine, imaging, nanozymes, antioxidants, free radical scavengers, etc. (Nadeem et al. 2020; Walkey et al. 2015; Charbgoo et al. 2017; Shcherbakov et al. 2020; Wason and Zhao 2013).
Free radicals are highly reactive and unstable products, which are brought forth as a by-product in our body via normal cellular reactions (oxidation metabolism). They are highly reactive and unstable as they comprise one or more valance electrons in their outermost shell. They tend to acquire electrons from other compounds to get stability (Alkadi 2020). As an ensue, the attacked compound or molecule itself gets converted into a free radical and this chain reaction can damage cells and their organelles (Ifeanyi 2018). Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) are two basic types of free radicals ubiquitous in living systems. ROS and RNS play a dual role (both beneficial and deleterious effects) in the body. A lower level of free radicals is beneficial for the living system as they have a role in the immune system, redox regulation; cellular signaling pathways, etc. However, when the level of free radicals exceeds normal values, then they cause potential harm to cellular molecules, such as lipids, nucleic acids, and proteins (Fig. 1). To manage the level of free radicals in the body, antioxidants are present. When there is an imbalance between the free radical generation and the number of antioxidants in the body, then a arises known as oxidative/nitrosative stress (Sharma et al. 2018). Oxidative stress may ensue in many disorders, such as cancer, diabetes, macular degeneration, inflammatory disorders, rheumatoid arthritis, etc. (Rani et al. 2016). CeNPs have the latent potential to scavenge free radicals and defend cells from oxidative stress and related disorders (Yadav and Singh 2021a; Lord et al. 2021).
Fig. 1.
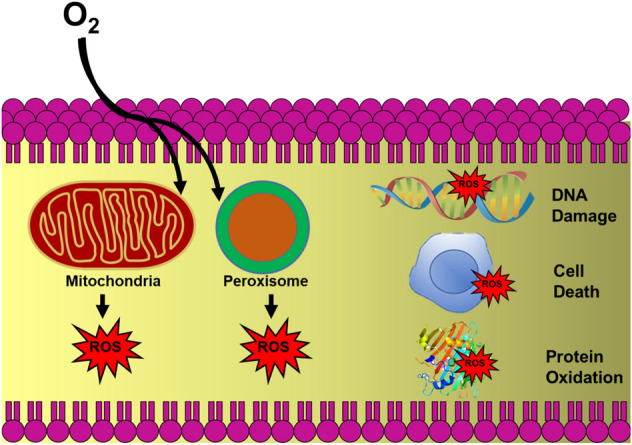
Schematic showing generation of ROS inside cells during normal cellular metabolism and the damage caused by ROS inside the cell
In this review, the physicochemical and biological properties of CeNPs, biomedical applications, and surface coating of CeNPs are discussed in detail. Prior reviews have focused on the synthesis and applications of CeNPs but here, the surface coatings of CeNPs along with their properties and biomedical applications are described. CeNPs exhibit various catalytic properties but still, their use is limited because of limitations faced by bare CeNPs. The need for surface coating and the impact of ligand coating on the activities of CeNPs are discussed. This review accentuates the status of CeNPs and concludes with recommendations for prospects, which could project the research towards commercial applications of CeNPs.
Properties of cerium oxide nanoparticles
Electronic and crystal structure
CeO2 is the most stable form of CeNPs that has FCC lattice structure with electronic configuration [Xe] 4f15d16s2. CeNPs comprise eight oxygen (cubic oxygen sub-lattice) atoms bonded with cerium atoms (at alternate cube centers). Figure 2 illustrates the crystal structure of CeNPs having four coordinated oxygen atoms (shown in green big balls) and eight coordinated cerium atoms (shown in red small balls; Younis et al. 2016).
Fig. 2.
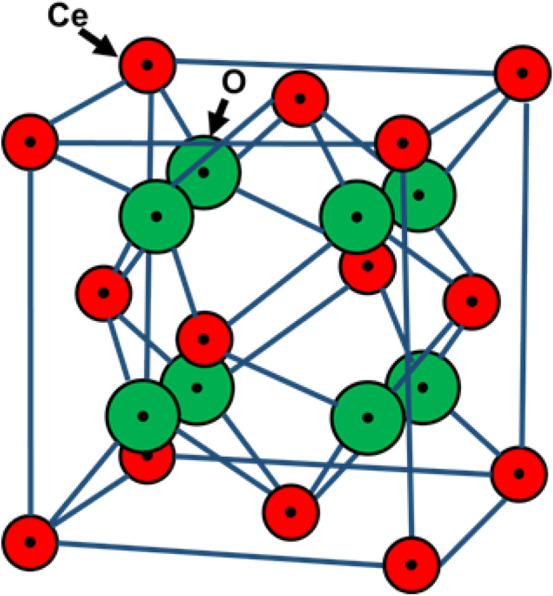
Schematic showing fluorite lattice structure of cerium oxide nanoparticles
Surface defects/oxygen vacancies
In crystalline structures, the atoms are arranged symmetrically and imperfections/defects are generated when an atom is ejected from its lattice position which breaks the symmetry of the crystal structure. In CeNPs, intrinsic and extrinsic defects may occur depending on the surrounding conditions. Intrinsic defects occur due to redox reactions occurring between the CeNPs surface and surroundings. The lack of one or more oxygen atoms from the lattice surface results in oxygen vacancies or defects on the lattice surface (Aleksandrov et al. 2016; Kullgren et al. 2012). Extrinsic defects may also occur in CeNPs by the intromission of any dopant or impurity. However, defects due to oxygen vacancies are more stable and dominant in CeNPs. The transition between two oxidation states (Ce3+ and Ce4+) is coupled mainly to oxygen defects on the CeNPs lattice. The procedure of oxygen vacancy formation during interconversion of oxidation states is shown in the following equation:
| 1 |
During this procedure, when one oxygen atom leaves the lattice surface of CeNPs as per Eq. 1, then two Ce4+ atoms get converted into Ce3+ to contend the charge. Figure 3 illustrates the mechanism of charge distribution during the oxygen vacancy formation of CeNPs.
Fig. 3.
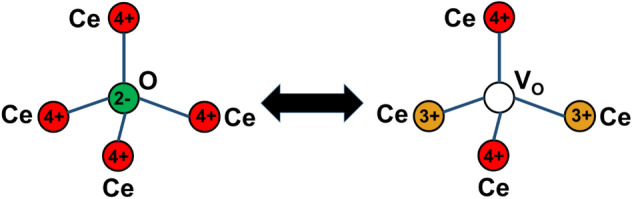
Schematic showing charge transfer during oxygen vacancy formation on the surface of cerium oxide nanoparticles
Size and doping effect
The size of CeNPs plays a vital role in ascertaining the reactivity, oxygen vacancy formation, and electrical properties (conductivity) of CeNPs. A decrease in the size of CeNPs leads to the formation of more oxygen vacancies (Singh et al. 2020). On the other hand, doping introduces extrinsic defects on the surface of CeNPs. Paier et al. reported that when CeNPs were doped with lanthanum (La), the oxygen vacancies increased on the surface along with the surface area (Paier et al. 2013).
Catalytic properties
CeNPs have been reported for various catalytic activities (Krishna et al. 1998; Ami and Suzuki 1998; Logothetidis et al. 2004; Marabelli and Wachter 1987). CeNPs have the potential to manage their catalytic behavior in harsh conditions also. CeNPs can behave like various enzymes and exhibit excellent antioxidant properties (Dhall and Self 2018). The ratio of the oxidation states on the surface ascertains the antioxidant properties of the CeNPs. The oxygen vacancies present on the surface permit the interconversion of these two oxidation states of CeNPs (Karakoti et al. 2009). CeNPs have been reported for exhibiting various enzyme-like activities (Fig. 4), such as superoxide dismutase (SOD), phosphatase, catalase, phosphotriesterase, peroxidase, oxidase, etc. (Dhall et al. 2017; Yadav et al. 2019). The major catalytic activities of CeNPs are as discussed below
Fig. 4.

Schematic showing catalytic properties of cerium oxide nanoparticles
Superoxide dismutases (SOD) are the enzymes that lead to the dismutation of harmful superoxide free radicals () into hydrogen peroxide (H2O2) and molecular oxygen (O2) (Zelko et al. 2002). The reaction catalyzed by SOD enzymes is shown in the following equation:
| 2 |
The SOD-like activity of CeNPs was first reported in 2007 by Korsvik et al. (2007). The dismutation of into H2O2 and O2 was detected following the reduction of cytochrome C. They reported that CeNPs with a higher Ce3+/4+ ratio on the surface exhibit excellent SOD-like activity. A study by Deshpande et al. showed that smaller size and a high surface-to-volume ratio ensued in a higher Ce3+/4+ ratio (Deshpande et al. 2005). The SOD-like activity of CeNPs has also been reported to be associated with redox coupling between oxidation states Ce4+ and Ce3+ (Li et al. 2015). The unique property of CeNPs to auto regenerate its surface increases its radical scavenging potential multi-folds. Reports suggest that SOD-like activity does not compromise after surface coating, but in the presence of high ionic strength buffers (such as phosphate buffer saline), the SOD-like activity does get compromised (Singh et al. 2011).
Catalases are the enzymes that degrade H2O2 into molecular O2 and water (H2O) (Góth et al. 2004). The reaction catalysed by catalases is as shown in the following equation:
| 3 |
The catalase-like activity of CeNPs was first reported by Pirmohamed et al. (2010). CeNPs with a higher Ce4+/3+ratio on their surface exhibits good catalase-like activity. Singh et al. demonstrated that the catalase-like activity of CeNPs is not affected by ionic buffers, phosphate ions, or cell culture medium (Singh and Singh 2019). However, surface coating resulted in alteration in the catalase-like activity of CeNPs, e.g., polymer coating to CeNPs resulted in inhibition of catalase-like activity (Baldim et al. 2019), binding of Keggin ions resulted in enhancement of the catalase-like activity of CeNPs (Yadav and Singh 2021b), etc. Based on the catalase-like activity of CeNPs, Singh et al. established the ROS scavenging ability of CeNPs in human hepatocytes (WRL-68). In the study, the authors inhibited the natural catalase enzyme in the cells using 3-Amino-1,2,4-Triazole (3-AT). Inhibition of catalase enzyme resulted in ROS generation inside the cells and hence cell death. However, cells pre-incubated with CeNPs showed ROS scavenging by degrading H2O2 in the cells (Singh and Singh 2019).
Oxidases are the oxidoreductases enzymes that catalyze oxidation reactions, where molecular oxygen acts as an electron acceptor (Komkova et al. 2021). The reaction catalyzed by oxidases is shown in the following equation:
| 4 |
Asati et al. first reported the oxidase-like activity of CeNPs. They demonstrated that oxidase-like activity of CeNPs is related to pH and maximum activity was observed at acidic pH (pH = 4) (Asati et al. 2009a). Furthermore, they utilized dextran-coated CeNPs (conjugated with folate) to design an immunoassay to detect the folate-expressing cells. Surface coatings can alter the oxidase-like activity of CeNPs. Yang et al. showed that capping of CeNPs with fluoride could amend the oxidase-like activity of CeNPs (Yang et al. 2018). On the other hand, polymer binding inhibited the oxidase-like activity of CeNPs (Baldim et al. 2019).
Peroxidases are the enzymes that catalyze the degradation of H2O2 and generate hydroxyl radicals(Komkova et al. 2021). The reaction catalyzed by peroxidases is as shown in in the following equation:
| 5 |
Asati et al. reported the peroxidase-like activity of CeNPs in 2011 (Asati et al. 2011). CeNPs can oxidize the substrate 3,3ʹ,5,5 ʹ -tetramethylbenzidine (TMB) in the presence of H2O2, similar to natural peroxidase enzymes. Based on the peroxidase-like activity of CeNPs, they developed a glucose-sensing method. Surface coatings have a great impact on the peroxidase-like activity of CeNPs. Baldim et al. showed that the peroxidase-like activity of CeNPs gets heightened greatly after polymer coating. Their mechanistic studies suggested that the improved peroxidase-like activity of CeNPs was due to an increase in hydroxyl radical generation in the reaction (Baldim et al. 2019).
Phosphatases are enzymes, that result in the hydrolysis of esterified phosphoric acid and remove a phosphate group from the substrate. Alkaline phosphatases are the most common phosphatases in the living system (Kay 1930). Kuchma et al. reported that CeNPs also exhibit phosphatase-like activity. They showed that CeNPs could interact with the phosphate ester bond of p-nitrophenyl phosphate (pNPP) resulting in the removal of a phosphate group (Kuchma et al. 2010). The reaction catalyzed by phosphatases is shown in the following equation:
| 6 |
In a study by Dhall et al. the mechanism of phosphatase-like activity of CeNPs was studied and they reported two potential inhibitors of the activity (Dhall et al. 2017). Their results showed that CeNPs could hydrolyze the substrate pNPP into p-nitrophenol and release a phosphate group. Furthermore, they affirmed their results with another substrate [4-methylumbelliferyl phosphate (MUP) and 2-amino-6-mercapto-7-methyl purine riboside (MESG)]. They utilized four anionic inhibitors (molybdate, tungstate, sulfate, and selenite) to check the inhibition of the activity and their results revealed that molybdate and tungstate were potent inhibitors of the phosphatase-like activity of CeNPs.
Biomedical applications of cerium oxide nanoparticles
CeNPs are one of the most promising potential MONPs, which appealed to the attention of scientists from various fields. They have enormous applications in the field of environment, agriculture, and biomedicine. The biomedical applications of CeNPs are discussed below in this section.
Anticancer
CeNPs have profound anticancer properties which include cytoprotecting normal cells from ROS by scavenging free radicals and the generation of ROS inside cancer cells (Pešić et al. 2015). In cancer cells, the rate of glycolysis and lactic acid formation is swift, due to which cells have an acidic pH. At lower pH, CeNPs lose antioxidant activity and behave as a pro-oxidant which releases ROS and damages cell organelles (Rajeshkumar and Naik 2018; Gao et al. 2014). In 2006, the anticancer property of CeNPs was studied by Lin et al. in A549 cells (human lung cancer cells; Lin et al. 2006). They showed the time and concentration-dependent cytotoxicity of CeNPs in A549 cells. Treatment of CeNPs (3.5–23.3 μg/ml) to cells results in ROS generation in cells and oxidative stress in cells is reflected by a reduction in glutathione and α-tocopherol levels. On the other hand, Renu et al. demonstrated that CeNPs have toxicity toward PC3 (prostate cancer cells) at 5 mg/ml but are non-toxic to L929 cells (mouse fibroblast cells). They synthesized CeNPs (3+) by hydrolysis method with higher Ce3+/4+ ratio and CeNPs (4+) by hydrothermal method with higher Ce4+/3+ ratios. Their results showed that CeNPs (4+) have higher toxicity toward PC3 cells than CeNPs (3+) due to their higher cellular internalization (Renu et al. 2012). The mechanism of anticancer activity of CeNPs is shown in Fig. 5.
Fig. 5.
Schematic showing anticancer activity of cerium oxide nanoparticles. In normal cells, CeNPs scavenge ROS and exhibit its antioxidant activity, whereas in cancer cells they lose their antioxidant activity due to acidic pH and start generating ROS
Alili et al. demonstrated that polymer-coated CeNPs were nontoxic to stromal cells and showed cytotoxically, pro-apoptotic, and anti-invasive ability toward melanoma cells (Alili et al. 2013). Furthermore, they first reported the in vivo anticancer activity of CeNPs in immuno-deficient nude mice. They observed a substantial decrease in tumor weight and volume after treatment of CeNPs in mice. In another in vivo study by Hijaz et al., it was shown that folic acid-coated CeNPs have inhibitory effects on ovarian cancer cells (Hijaz et al. 2016). They coated the surface of CeNPs with folic acid to improve the specificity of CeNPs towards target ovarian cancer cells. In vitro studies performed by them on A2780 and C200 (ovarian cancer cells) showed that folic acid coating onto CeNPs improved the cellular internalization of CeNPs and inhibited cell proliferation. Furthermore, they performed in vivo studies on A2780 generated mouse model and found that folic acid-coated CeNPs (0.1 mg/kg body weight) decreased the tumor load significantly by inhibiting cell proliferation and angiogenesis. A study performed by Jana et al. showed that CeNPs exhibit significant cytotoxicity toward colon cancer cells in humans (Jana et al. 2014). They demonstrated that CeNPs exhibit time and dose-dependent cytotoxicity towards HCT 15 cells (human colorectal adenocarcinoma-derived cells) by depolarizing the mitochondrial membrane. HCT 15 cells were treated with a concentration range (10–100 µM) of CeNPs for 24 h and the results showed dose-dependent cytotoxicity in cells. Furthermore, cells were exposed to a 10 µM concentration of CeNPs for 24, 48, and 72 h and time-dependent cytotoxic behavior of CeNPs was observed. Nourmohammadi et al. demonstrated the anticancer property of CeNPs against WEHI 164 (mouse fibrosarcoma tumor cells) (Nourmohammadi et al. 2019). They showed that CeNPs increased the ROS levels and induced apoptosis in WEHI 164 cells in a concentration-dependent manner (from concentration ≥ 15.63–500 µg/ml). Table 1 summarizes the anticancer ability of CeNPs studied in various types of cancer cells.
Table 1.
Anticancer activities of cerium oxide nanoparticles
| S No | Size of CeNPs | Type of CeNPs | Type of cancer cell | Mechanism of action | Reference |
|---|---|---|---|---|---|
| 1 | 4 nm | Bare |
DLD1-TxR (adenocarcinoma), NCI-H460 (Lung carcinoma) |
Changes in intracellular redox status caused ROS inside cells and led to cell death | Pešić et al. (2015) |
| 2 | 20 nm | Bare |
A549 (Lung cancer) |
Treatment of CeNPs (3.5–23.3 μg/ml) resulted in lipid peroxidation and cell membrane damage | Lin et al. (2006) |
| 3 | 100 nm | Bare | PC3 (Prostate cancer) | CeNPs got accumulated in lysosomes of cancer cells and led to ROS-induced cell damage | Renu et al. (2012) |
| 4 | 5 nm | Dextran-coated |
A 375 (Human melanoma) |
Improved cellular uptake of NPs and ROS induced cell death | Alili et al. (2013) |
| 5 | 10 nm | Folic acid-coated | A2780 and C200 (Ovarian cancer) | Improved cellular internalization of CeNPs and inhibition of cell proliferation and cell death by caspase 7/3 activation | Hijaz et al. (2016) |
| 6 | 3–5 nm | Bare | HCT 15 cells (Human colorectal adenocarcinoma-derived cells) | Depolarization of the mitochondrial membrane resulted in cell death | Jana et al. (2014) |
| 7 | 30 nm | Bare | WEHI 164 (Mouse fibrosarcoma tumor) | CeNPs increased the Bax expression in cancer cells and showed ROS-induced apoptosis | Nourmohammadi et al. (2019) |
| 8 | >25 nm | Bare | IMR 32 (Human neuroblastoma) | ROS-induced oxidative stress and genotoxicity induced by CeNPs resulted in cell death | Kumari et al. (2014) |
The type of cancer cells and mechanism of anticancer action are described in the table
Antioxidant
Antioxidants scavenge the ROS/RNS generated inside cells and protect cells from oxidative stress. CeNPs exhibit the potential to behave like antioxidants and scavenge free radicals. The antioxidant property of CeNPs is linked to redox shuttling between oxidation states (3+ and 4+) as is shown in Fig. 6.
Fig. 6.

Schematic showing antioxidant activity of cerium oxide nanoparticles during its redox switching mechanism
Kim et al. demonstrated that levan-coated CeNPs exhibit improved antioxidant properties as compared to bare CeNPs. The ROS level decreased after treatment of levan-coated CeNPs to H2O2 stimulated mouse fibroblast NIH3T3 cells and HEK293T cells. The ROS levels of cells were quantified by incubating cells with H2O2 followed by treatment of CeNPs (100 µg/ml). CeNPs treated cells showed reduced cellular ROS in presence of H2O2 (Kim and Chung 2016). Another study, Chen et al. showed the antioxidant property of CeNPs in endothelial cells. CeNPs scavenged H2O2 and decreased the overproduction of ROS, which lead to a decrease in cellular death. CeNPs showed a protective effect against damage induced by H2O2 (1 mM) in a concentration-dependent manner (5–40 µg/ml) (Chen et al. 2013). In a separate study by Das et al., the antioxidant property of CeNPs was shown in a serum-free cell culture model of an adult rat spinal cord. Their study unveiled that cell survival was higher in CeNPs treated cells as compared to untreated control cells. They concluded that CeNPs acts as an antioxidant scavenger and protect rat spinal cord neurons from damage (Das et al. 2007). Furthermore, a study by Colon et al. showed that CeNPs provide radioprotection to the gastrointestinal epithelium. In their study, they pre-treated the CRL 1541 (human colon) cells with CeNPs for 24 h and then exposed them to harmful radiations. Results showed that CeNPs act as antioxidants and prevented the colon cells from radiation-induced cell damage by increasing the production of superoxide dismutase (Colon et al. 2010). Singh et al. studied the antioxidant property of CeNPs against 3-AT induced oxidative stress in WRL-68 (human liver cells). They exposed WRL cells to 3-AT to inhibit the action of the catalase enzyme, which resulted in an accumulation of H2O2 in the cells. CeNPs treated cells showed protection against H2O2 induced ROS inside the cells. Treatment of 80, 100, and 150 mM 3-AT resulted in the reduction of cell viability to 82, 80, and 76%, whereas cells pre-incubated with CeNPs (150 µM), followed by 3-AT treatment showed cellular improved viability as 91, 85, 79% (Singh and Singh 2019). Yadav et al. showed the antioxidant property of rod-shaped CeNPs in WRL-68 cells. They showed that rod-shaped CeNPs have a better cellular internalization in the cells and provide protection against ROS (Yadav and Singh 2021c). Rubio et al. showed the antioxidant property of CeNPs in pulmonary-like cell systems against KBrO3 induced oxidative stress(Rubio et al. 2016). Other studies based on the antioxidant property of CeNPs have also been reported in different cell lines (Perez et al. 2008; Ranjbar et al. 2018; Ciofani et al. 2014; Pagliari et al. 2012; Marino et al. 2017). Table 2 summarizes the antioxidant property of CeNPs.
Table 2.
Antioxidant activities of cerium oxide nanoparticles
| S No | Size of CeNPs |
Type of CeNPs | Type of cell | Type of free radical | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|
| 1 | 40 nm | Levan coated | NIH3T3 (mouse fibroblast cells) | Hydrogen peroxide | Inhibition of H2O2 induced ROS | Kim and Chung (2016) |
| 2 | 20 nm | Bare | Endothelial cells | Hydrogen peroxide | Inhibition of ROS by mimicking SOD and catalase enzymes | Chen et al. (2013) |
| 3 | 3–5 nm | Bare | Adult rat spinal cord cells | Hydrogen peroxide | Neuroprotection to rat spinal cord neurons by scavenging ROS during redox switching | Das et al. (2007) |
| 4 | 3–5 nm | Bare | CRL 1541 (human colon cells) | Superoxide free radical | Protection from cell damage (caused by radiation) by improving the generation of SOD 2 | Colon et al. (2010) |
| 5 | ~1.88 nm | Bare | WRL-68 (human liver cells) | Hydrogen peroxide | Protection from oxidative stress induced by 3-AT | Singh and Singh (2019) |
| 6 | 48 nm | Bare | WRL-68 (human liver cells) | Hydrogen peroxide | Protection from oxidative stress induced by 3-AT | Yadav and Singh (2021c) |
| 7 | < 25 nm | Bare | BEAS-2B (human epithelial lung cells) | Superoxide free radical | Protection from KBrO3 induced oxidative stress by scavenging ROS | Rubio et al. (2016) |
| 8 | 4 nm | Dextran coated | Cardiomyocytes and human dermal fibroblasts | Hydrogen peroxide | Protection from H2O2 induced oxidative stress | Perez et al. (2008) |
| 9 | 30 nm | Bare | Brain tissues of rats | Superoxide free radical | Protection against Paraquat induced neuronal oxidative stress and apoptosis | Ranjbar et al. (2018) |
| 10 | 5–8 nm | Bare | Cardiac progenitor cells | Hydrogen peroxide | Protection against H2O2 induced oxidative stress | Pagliari et al. (2012) |
| 11 | 5–80 nm | Bare | Neuron-like PC12 cells | Superoxide free radical | Neuroprotection to cells by regulating the genes involved in cellular defense | Ciofani et al. (2014) |
| 12 | >5 nm | Gelatin coated | Neuron-like SH-SY5Y cells | Hydrogen peroxide |
Protection to cells by neurite development and alignment |
Marino et al. (2017) |
Types of free radicals scavenged and mechanism of ROS scavenging by CeNPs are summarized in the table
Antibacterial
CeNPs have been investigated for their antibacterial properties against both gram-positive and gram-negative bacteria(Zhang et al. 2019). There are two possible ways of bacterial cell death by CeNPs: direct contact or by indirect contact. In direct contact CeNPs get adsorbed directly into the bacterial cell and lead to cell wall damage followed by damage to cellular organelles by ROS generation inside the cell and in indirect contact CeNPs interact with the surroundings of bacteria and generate ROS, Ce3+ sites react with the generated ROS and further produce radicals and anions by the oxidative reaction. This reaction impairs membrane integrity and leads to bacterial cell death (Thakur et al. 2019). Figure 7 shows two mechanisms of CeNPs interaction with bacterial cells.
Fig. 7.

Schematic showing methods of interaction of cerium oxide nanoparticles with bacterial cells
Thill et al. investigated the antibacterial property of CeNPs against Escherichia coli (gram-negative bacteria) in 2006 (Thill et al. 2006). They demonstrated that positively charged CeNPs showed a great electrostatic interaction with bacterial membrane. Adsorption of a large amount of CeNPs on bacterial surfaces proved fatal to E. coli. Dar et al. also showed the antibacterial property of CeNPs against E. coli (Dar et al. 2017). Similar studies showing antibacterial activity of CeNPs against E. coli. have been performed by other researchers also. CeNPs showed excellent antibacterial activity against E. coli. After treatment of 10 mg/ml CeNPs for 10 min, only 6.8% of cells were viable, whereas after 20 min all cells lost viability (Kartsonakis et al. 2008; Kuang et al. 2011). Kannan et al. studied the antibacterial property of CeNPs on both; gram-positive (Staphylococcus aureus) and gram-negative (E. coli) bacteria (Kannan and Sundrarajan 2014). In a similar study by Arumugam et al. (2015), it was shown that gram-positive bacteria are more susceptible to CeNPs than gram-negative. Senthilkumar studied the antibacterial property of CeNPs against E. coli and Bacillus subtilis and reported that CeNPs interacted directly on the surface of bacteria and resulted in cell membrane disruption (Senthilkumar et al. 2017). Table 3 summarizes the antibacterial activities of CeNPs.
Table 3.
Antibacterial activities shown by cerium oxide nanoparticles
| S No | Size of CeNPs | Type of bacteria | Name of bacteria | Mechanism of action | Reference |
|---|---|---|---|---|---|
| 1 | 7 nm | Gram-negative | Escherichia coli | ROS generation in a cell due to adsorption of a large number of CeNPs on bacterial membrane | Thill et al. (2006) |
| 2 | 3.5–6.5 nm | Gram-negative | Escherichia coli | CeNPs showed concentration and size-dependent antibacterial activity to HB101 K-12 strain of E. coli by ROS generation | Dar et al. (2017) |
| 3 | 140 nm | Gram-negative | Escherichia coli | The semiconductor properties of polymers improved the antibacterial activity of polymer-coated CeNPs | Kartsonakis et al. (2008) |
| 4 | 7 nm | Gram-negative | Escherichia coli | Intracellular ROS generation inside cells due to direct contact of CeNPs with E. coli was responsible for the obtained antibacterial property | Kuang et al. (2011) |
| 5 | 10 nm | Gram-negative | Escherichia coli | CeNPs resulted in maximum antibacterial activity at pH 6 and activity started decreasing at alkaline pH | Shah et al. (2012) |
| 6 | 25–50 nm | Gram negative | Escherichia coli | The redox potential of CeNPs increased ROS generation and hence oxidative stress inside E. coli cells | Li et al. (2012) |
| 7 | 100 nm | Gram negative | Escherichia coli | CeNPs combined with three different non-ionic surfactants showed improved toxicity to bacterial cells as compared to bare CeNPs (almost 20 times more) | Cuahtecontzi-Delint et al. (2013) |
| 8 | 25–30 nm | Gram positive and gram-negative | Staphylococcus aureus and Escherichia coli | Inactivation of cellular proteins resulted in antibacterial action | Kannan and Sundrarajan (2014) |
| 9 | 5 nm | Gram positive and gram-negative | Staphylococcus aureus and Escherichia coli | ROS generation due to uneven ridges and oxygen defects in CeNPs | Arumugam et al. (2015) |
| 10 | 3.61–24.4 nm | Gram positive and gram-negative | Bacillus subtilis and Escherichia coli | Disruption of cell membrane resulted in antibacterial activity of CeNPs | Senthilkumar et al. (2017) |
The types of bacteria and mechanism of action in antibacterial activity of CeNPs are mentioned in the table
Drug/gene delivery
CeNPs have been investigated for drug/gene delivery application. CeNPs are good anticancer agents and can be used as a vector for gene/drug delivery. Hence, CeNPs render a synergistic effect against cancer cells. In a study by Patil et al. (2007), it was shown that CeNPs could be utilized as a potential drug delivery device. They demonstrated that CeNPs conjugated with carboxybenzene sulfonamide (an inhibitor of the human carbonic anhydrase enzyme) can be used for the treatment of glaucoma. Sulthana et al. demonstrated that polyacrylic acid (PAA-coated CeNPs) can be utilized as a drug carrier for the treatment of non-small-cell lung cancer (NSCLC). They loaded two drugs (doxorubicin and ganetespib) with folate conjugated CeNPs to target NSCLC. They reported that double drugs loaded CeNPs caused more than 80% of death in NSCLC, whereas single drug-loaded CeNPs results in 40% of cell death in 48 h (Sulthana et al. 2017). In a separate study by Das et al., doxorubicin-loaded CeNPs were used against ovarian cancer cells. They reported that doxorubicin-loaded CeNPs showed good drug loading content (22.41%) and higher cellular uptake and retention of the drug as compared to free drugs. Furthermore, they demonstrated that drug-loaded CeNPs exhibited higher levels of apoptosis and cell proliferation inhibition as compared to the free drug (Das et al. 2017). Li et al. developed a CeNPs based delivery system by binding chlorin e6 (Ce6) and folic acid (FA) on polymer (polyethyleneimine–polyethylene glycol) coated CeNPs to target breast cancer cells. They reported a photodynamic therapy for the treatment of drug-resistant MCF-7/ADR (human breast cancer cells). Upon near-infrared (NIR) irradiation, polymer conjugated drug-loaded CeNPs generate ROS which resulted in the reduction of P-glycoprotein expression, and lysosomal membrane permeability, and causes cytotoxicity towards MCF-7/ADR (Li et al. 2016). Kalashnikova et al. reported about dextran-coated CeNPs loaded with curcumin as an anticancer agent against neuroblastoma cells. They demonstrated that curcumin-loaded CeNPs were toxic to neuroblastoma cells, while normal cells were not harmed by them (Kalashnikova et al. 2017). Figure 8 shows the mechanism of gene delivery by CeNPs.
Fig. 8.
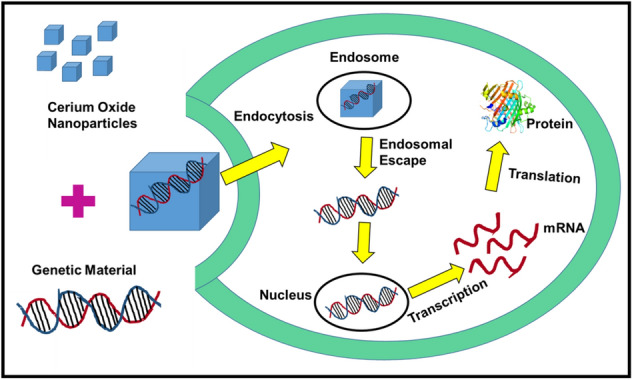
Schematic showing mechanism of gene delivery in cells by cerium oxide nanoparticles
Zhang et al. developed dithiol-polydopamine coated CeNPs nanorods as anticancer agents by loading doxorubicin against HepG2 (human liver cancer cells; Zhang et al. 2018). CeNPs have also been explored for their gene delivery application. In 2016, Das et al. reported that dimethyl dioctadecyl ammonium bromide (DODAB)–CeNPs could be utilized as non-viral gene delivery vectors for the transfection of plasmid DNA (pEGFPN1). The vector performance of DODAB–CeNPs was comparable with lipofectamine and better than calcium phosphate and DEAE-dextran for transfecting the small plasmids. The transfecting efficiency was observed in different cell lines [HEK293 (human embryonic kidney cells), MCF-7, and HepG2] (Das et al. 2016). Table 4 summarizes the drug/gene delivery application of CeNPs.
Table 4.
Drug/gene delivery application of cerium oxide nanoparticles
| S No | Size of CeNPs | Type of CeNPs | Name of drug/gene | Name of cell | Mechanism of action | Reference |
|---|---|---|---|---|---|---|
| 1 | 10–20 nm | Bare | Carboxybenzene sulfonamide | - | Inhibition of human carbonic anhydrase enzyme responsible for causing glaucoma | Patil et al. (2007) |
| 2 | 57 nm | Polyacrylic acid-coated | Doxorubicin and ganetespib | Non-small-cell lung cancer | The synergistic effect of drugs resulted in cell death in NSCLC cells by inhibiting Hsp90 (heat shock protein 90) | Sulthana et al. (2017) |
| 3 | 3–4 nm | Bare | Doxorubicin | A2780 | Inhibition of cell proliferation and apoptosis due to higher retention time of the drug | Das et al. (2017) |
| 4 | 3–5 nm | Polyethylenimine–polyethylene glycol coated | chlorin e6 (Ce6)/folic acid (FA) | MCF-7/ADR | CeNPs generate ROS after near-infrared irradiation, which resulted in cytotoxicity to MCF-7/ADR cells | Li et al. (2016) |
| 5 | 14 nm | Dextran coated | Curcumin | Neuroblastoma | Oxidative stress-induced stabilization of HIF-1α, and caspase-dependent apoptosis | Kalashnikova et al. (2017) |
| 6 | 12 nm | Polydopamine coated | Doxorubicin | HepG2 | The synergistic anticancer effect of drug and polydopamine coated CeNPs resulted in inhibition of HepG2 cells | Zhang et al. (2018) |
| 7 | 3–4 nm | Dimethyldioctadecylammonium bromide | Plasmid DNA (pEGFPN1) | HEK293, MCF-7, and HepG2 | The improved uptake of cells of nanovector/DNA complexes through caveolae and clathrin-mediated endocytosis resulted in the endosomal release which in turn supported the improved gene transfection efficiency | Das et al. (2016) |
The drugs/ genes delivered via CeNPs and their mechanism of action are mentioned in the table
Biosensor
Biosensors are analytical devices that convert any chemical or physical or biological signal into a quantifiable (optical or electrochemical) signal (Singh et al. 2020). CeNPs have been investigated for glucose, cholesterol, lactate, triglycerides, hypoxanthine, hydrogen peroxide, and other sensing applications (Nesakumar et al. 2013; Ansari et al. 2008; Charbgoo et al. 2017; Fallatah et al. 2019). Nesakumar et al. developed an electrochemical biosensor based on CeNPs for lactate sensing. They reported higher immobilization of lactate dehydrogenase on the surface of CeNPs due to their isoelectric points. The developed electrochemical biosensor has a linear response of 0.2–2 mM (Nesakumar et al. 2013). Ansari et al. reported the application of sol–gel-derived CeNPs in cholesterol biosensing. They demonstrated that the developed biosensor was cost-effective, more sensitive, and hence could be utilized in the fabrication of a potential biosensor for the diagnosis of coronary diseases (Ansari et al. 2008). Fallatah reported glucose sensing application of CeNPs. They fabricated CeNPs on various conducting surfaces, such as carbon cloth, carbon paper, and fluorine-doped tin oxide. Their results indicated that CeNPs fabricated on the carbon cloth had the best sensitivity in the range of 208–2290 μA/cm2 mM and the lowest detection limit of 1 nM(Fallatah et al. 2019). Saha et al. also developed CeNPs thin films for glucose sensing. They demonstrated that the developed biosensor showed good linearity of 25–300 mg/dL. The lower value of the Michaelis–Menten constant (1.01 mM) showed a high enzyme affinity of glucose oxidase to glucose (Saha et al. 2009). Solanki et al. immobilized lipase on the surface of CeNPs (sol–gel derived) and developed a triglyceride sensor. They reported that the developed biosensor showed a good linearity response and shelf life of 50–500 mg/dL and 32.8 mg/dL, respectively (Solanki et al. 2009). Zhang et al. reported the cholesterol sensing application of CeNPs. They deposited CeNPs on graphene and developed an electrogenerated chemiluminescence cholesterol biosensor. The developed cholesterol biosensor has linearity ranging from 12 to 7.2 mM and a detection limit of 4.0 μM (Zhang et al. 2013a). Mustafa et al. developed CeNPs based hypoxanthine biosensor for fish spoilage detection. The biosensor was developed by immobilizing xanthine oxidase and CeNPs on the surface of the silanized paper and the developed hypoxanthine biosensor showed a detection limit of 15–89 μM and the linearity ranging between 597 and 800 μM (Mustafa et al. 2021). Ansari et al. developed an H2O2 sensor based on horseradish peroxidase (HRP) immobilized CeNPs. They demonstrated that the developed biosensor showed Michaelis–Menten constant as 2.21 μM, and linearity ranging from 1.0–170 μM (Ansari et al. 2009). Table 5 summarizes the different biosensing applications of CeNPs.
Table 5.
Biosensing application of cerium oxide nanoparticles
| S. No | Size of CeNPs | Immobilizing enzyme | Method of immobilization | Biosensor | Observation | Reference |
|---|---|---|---|---|---|---|
| 1 | 30 nm | Nicotinamide adenine dinucleotide and lactate dehydrogenase | Electrodeposition | Lactate | Fabricated biosensor exhibits high sensitivity (571.19 µA mM−1) and linearity range of 0.2–2 mM | Nesakumar et al. (2013) |
| 2 | 34 nm | Cholesterol oxidase | Sol–gel | Cholesterol | The developed biosensor was cost-effective, more sensitive, and highly stable chemically with a linearity range of 0.2–2 mM | Ansari et al. (2008) |
| 3 | 90 nm | Glucose oxidase | Electrodeposition | Glucose | The developed sensor had good sensitivity ranging from 208 − 2290 μA/cm2 mM and the lowest detection limit of 1 nM | Fallatah et al. (2019) |
| 4 | 110 nm | Glucose oxidase | Pulsed laser deposition | Glucose | The developed biosensor showed good linearity of 25–300 mg/dL | Saha et al. (2009) |
| 5 | 35 nm | Lipase | Sol–gel | Triglyceride | They reported that the developed biosensor showed excellent linearity (50–500 mg/dL) and shelf life of 32.8 mg/dL | Solanki et al. (2009) |
| 6 | 90 nm | Cholesterol oxidase | Electrogenerated chemiluminescence | Cholesterol | The developed cholesterol biosensor had linearity of 12–7.2 mM and a detection limit of 4.0 μM | Zhang et al. (2013a) |
| 7 | 3.3 nm | Xanthine oxidase | Sol–gel | Hypoxanthine | The developed hypoxanthine biosensor had a detection limit of 15–89 μM and linearity ranging between 597 and 800 μM | Mustafa et al. (2021) |
| 8 | 40–45 nm | Horseradish peroxidase | Physisorption | Hydrogen Peroxide | The developed biosensor showed Michaelis–Menten constant as 2.21 μM, and linearity ranging from 1.0–170 μM | Ansari et al. (2009) |
The type of immobilizing enzyme, method of immobilization, type of developed biosensor with their characteristics are summarized in the table
Others
Apart from the above-cited applications, CeNPs have also been investigated for the treatment of diseases, as anti-inflammatory agents, and as bioscaffolds. Hirst et al. reported the anti-inflammatory properties of CeNPs in J774A.1 (murine macrophage cells). They reported that CeNPs were nontoxic to cells and reduced pro-inflammatory iNOS (nitric oxide synthetase) protein expression. Furthermore, their in vivo studies in the mice model suggested that CeNPs were well tolerated by mice and reduced the ROS level in a state of inflammation (Hirst et al. 2009). Due to their unique pharmacological properties, CeNPs have been used to treat oxidative stress-mediated neurodegenerative disorders (Naz et al. 2017; Estevez and Erlichman 2014).
Surface coating of cerium oxide nanoparticles
The application of CeNPs in various biological areas is described in the above sections but in brief, it has been used in different fields for various purposes. Despite having a wide range of applications, the use of CeNPs for treatment or therapeutic purposes is still restricted due to the limitations faced by the bare form of CeNPs; like loss of catalytic activity inside biological systems, formation of chemical or protein corona due to adsorption of proteins or biochemical on the surface, non-specific binding with other biomolecules due to highly reactive nature, etc. (Kumar et al. 2014).
Limitations of using cerium oxide nanoparticles
CeNPs exhibit unique properties, which makes them a suitable candidate for application in biomedicine, but still, their use is restricted due to the following limitations:
Aggregation: CeNPs due to their highly reactive surface, tend to aggregate or agglomerate. The size, dispersion medium and synthesis methods of CeNPs have a great impact on the agglomeration of CeNPs. Researchers have reported that in the presence of high ionic strength buffers, such as phosphate buffer saline (PBS) and cell culture medium, such as Dulbecco’s Modified Eagle Medium (DMEM), bare CeNPs form larger particles due to the aggregation within a few hours after mixing, which increases their toxicity and also results in loss of activity (Asati et al. 2010; Chanteau et al. 2009; Nanda 2016).
Formation of protein corona: CeNPs have reactive surfaces due to which proteins got adsorbed on their surface, resulting in protein corona formation. The protein corona formation determines the fate of uptake of CeNPs in cells and their clearance.
Nonspecific interaction: CeNPs also tend to bind with other biomolecules due to their reactive nature. The non-specific interaction results in loss of activity and changes in their morphology and stability.
Surface defects, charge, and oxidation state: The cellular localization and uptake of CeNPs greatly depend on the surface defects, charge, and ratio of oxidation states on the surface. Higher Ce3+/4+ ratios have been linked to the higher toxicity of CeNPs.
These limitations restrict the use of CeNPs in nanomedicine. Hence, there was a need for surface coating of CeNPs to overcome these limitations. Several endeavors have been made to coat the surface of CeNPs with different kinds of ligands for the improvement of their stability, biocompatibility, and catalytic activities.
Ligand coated cerium oxide nanoparticles
Asati et al. reported the oxidase-like activity of polymer-coated CeNPs (Asati et al. 2009b). They coated the surface of CeNPs with dextran and investigated the oxidase-like activity of dextran-coated CeNPs at acidic pH. Furthermore, on this basis of oxidase-like activity, they developed an immunoassay that outperformed the traditional enzyme-linked immunosorbent assay (ELISA). In traditional ELISA, HRP acts as a secondary antibody, which facilitates the oxidation of TMB after the addition of H2O2. In dextran-coated CeNPs based ELISA, TMB got oxidized directly due to oxidase-like activity of CeNPs without HRP and H2O2. Baldim et al. coated the surface of CeNPs with six different types of polymers and investigated the catalytic activities of CeNPs after polymer coating. Two polyacrylic acids (PAA) derived polymers and four polyethylene glycol (PEG) derived polymers were utilized to coat the surface of CeNPs and their results suggested that polymer coating has no impact on the SOD-like activity of CeNPs, and it improved peroxidase-like activity and decreased the catalase and oxidase-like activity of CeNPs (Baldim et al. 2020). Shah et al. showed the antibacterial activity of polymer-coated CeNPs. They reported that dextran-coated CeNPs were more stable and exhibited antibacterial properties against E. coli. Furthermore, they studied the impact of physical and chemical parameters on the antibacterial property of CeNPs and found that there was no impact of pH, aeration, and concentrations of salts and natural organic matters on the activity of dextran-coated CeNPs (Shah et al. 2012). Figure 9 shows the different kinds of ligands used to coat the surface of CeNPs.
Fig. 9.

Schematic showing ligands used for coating the surface of cerium oxide nanoparticles
Yokel et al. studied the effect of citric acid on the stability of CeNPs. Their results suggested that the binding of citrate improved the dissolution of CeNPs in an acidic environment, which may affect the fate of CeNPs inside cells (Yokel et al. 2019). CeNPs have also been coated with organophosphorus (organic compounds having phosphate group). Patel et al. reported the binding of phosphines on the surface of CeNPs via electrostatic interaction. They coated the surface of CeNPs with two different phosphines [triethyl phosphine (TEP) and 2,4,6 trimethoxyphenyl) phosphine (TTMPP)]. Their results suggested that the binding of phosphines resulted in the reversal of oxidation states and hence enzymatic activities of CeNPs (Patel et al. 2018). The binding of CeNPs has also been reported with polyoxometalates (metal-oxo crystals). Yadav et al. showed that the binding of phosphotungstic acid (PTA) and phosphomolybdic acid (PMA) changed the enzyme-like activities of CeNPs. The SOD, catalase, and peroxidase-like activities were found to be improved in presence of PTA, whereas the SOD-like activity of CeNPs was found to be decreased in presence of PMA (Yadav and Singh 2021b). Babu et al. demonstrated the effect of gold (Au) coating on the cytotoxicity and antibacterial properties of CeNPs. They studied the cytotoxicity of Au-coated CeNPs in RAW 264.7 (mice-derived macrophage cells) and A549 cells. Their results suggested that Au-coated CeNPs were nontoxic at 1–1000 µM concentrations towards RAW 264.7, whereas the highest cytotoxicity was observed in A549 cells as compared to bare AuNPs and CeNPs. Au-coated CeNPs showed excellent antibacterial properties against Bacillus subtilis, Staphylococcus aureus, Staphylococcus enteritidis, E. coli, and Lactiplantibacillus Plantarum, and proved themselves, potent antibacterial agents (Babu et al. 2014). Nethi et al. reported the silane [6-{2-[2-(2-methoxy-ethoxy)-ethoxy]-ethoxy} bi-hexyl) triethoxysilane] coating on the surface of CeNPs (Nethi et al. 2017). They showed that silanes-coated CeNPs exhibit improved angiogenic properties. In vivo studies performed by them suggested that coating of silanes on the surface of CeNPs induced cell proliferation in endothelial cells and blood vessels grow in a chick embryo. Hence, silane-coated CeNPs could be utilized in the treatment of angiogenesis-mediated disorders, such as cardiovascular, ocular disorders, etc. Apart from the various other assorted ligands such as peptides (Homayouni-Tabrizi et al. 2016), amino acids (Hartati et al. 2020), etc. also have been used to coat the surface of CeNPs to improve the biomedical applications of CeNPs. Table 6 summarizes the studies performed on the surface coating of CeNPs.
Table 6.
Ligands used in the surface coating of cerium oxide nanoparticles
| S. No | Ligand for surface coating | Nature of ligand | Type of interaction | Changes after surface coating | Reference |
|---|---|---|---|---|---|
| 1 | Dextran | Polymer | Physical adsorption | Improvement in the oxidase-like activity of CeNPs | Asati et al. (2009b) |
| 2 | Polyacrylic acid and polyethylene glycol | Polymer | Electrostatic interaction and Covalent binding | No impact on the SOD-, increase in peroxidase- and decrease in catalase and oxidase-like activity | Baldim et al. (2020) |
| 3 | Dextran | Polymer | Physical adsorption | Improvement in stability and antibacterial property of CeNPs | Shah et al. (2012) |
| 4 | Citric acid | Carboxylic acid | Electrostatic interaction | Improvement in the dissolution of CeNPs in an acidic environment | Yokel et al. (2019) |
| 5 | Triethyl phosphine and 2,4,6 trimethoxyphenyl) phosphine | Organophosphorus | Electrostatic interaction | Reversal of enzymatic activities | Patel et al. (2018) |
| 6 | Phosphotungstic acid and phosphomolybdic acid | Polyoxometalates | Electrostatic interaction | Improvement in the catalase, SOD, and peroxidase-like in presence of PTA, whereas SOD-like activity got compromised after interaction with PMA | Yadav and Singh (2021b) |
| 7 | Gold | Inorganic material | Electrostatic deposition | Improved antibacterial and anticancer properties of CeNPs | Babu et al. (2014) |
| 8 | 6-{2-[2-(2-methoxy-ethoxy)-ethoxy]-ethoxy} bi-hexyl)triethoxysilane | Silanes | Hydrogen bonding (siloxane binding) | Improved angiogenic properties of CeNPs | Nethi et al. (2017) |
| 9 | Brevinin-2R | Peptide | Covalent binding (peptide bond) | Improved anticancer activities of CeNPs | Homayouni-Tabrizi et al. (2016) |
| 10 | Anti HER-2 | Antibody | Covalent binding | Improved anticancer activities of CeNPs | Hartati et al. (2020) |
The nature of ligand, type of interaction among CeNPs and ligands, and the changes induced after surface coating are summarized in the table
Core–shell hybrids of cerium oxide nanoparticles
In biomedical applications, core–shell nanoparticles are widely used as they display relatively better enzymatic properties and also serve as multi enzymatic reaction mimics. Wu et al. recently showed that core–shell iron oxide and CeNPs showed effective scavenging of ROS and also developed them as magnetic resonance (MR) imaging contrast agents. They developed these core–shell NPs as theragnostic agents against ROS-induced inflammatory disorders as they had the potential for effective therapy as well as diagnosis. The innovative NPs consist of iron oxide nanoparticles as core (MR imaging agent) and CeNPs as shell (ROS scavenging agent; Wu et al. 2018). Another study by Bhagat et al. showed that gold core and CeNPs shell NPs developed as multienzyme mimics. Gold NPs and CeNPs both exhibit specific enzyme-like activities and the designing of core–shell NPs using these two NPs resulted in multi enzymatic activities in a single formulation. The developed NPs exhibit excellent SOD, catalase, and peroxidase-like activities (Bhagat et al. 2018). Izu et al. developed core–shell CeNPs using polymer. They developed a hybrid with CeNPs in core and polymer (poly(vinylpyrrolidone) as shell which improved the dispersibility and compatibility of NPs (Izu et al. 2011). Shah et al. also developed gold decorated CeNPs using phosphotungstic acid as the linker. CeNPs were in the core and gold NPs were arranged on CeNPs. The developed NPs showed excellent performance in catalytic reduction of 4-nitrophenol and also showed enhanced (twofold better) peroxidase-like activity (Shah et al. 2021).
Reports on the toxicity of cerium oxide nanoparticles
Recent research on the toxicity of CeNPs reported conflicting results: CeNPs have been reported as antioxidant agents (scavenge ROS; Yadav and Singh 2021c) and as pro-oxidants (ROS producing agents; Hussain et al. 2012) via different biological pathways. Some reports showed the protective effects of CeNPs against ROS overproduction by scavenging free radicals (Yadav and Singh 2021c). CeNPs have also been reported to improve the life span of brain cells and protect cells against mechanical trauma induced by free radicals (Rzigalinski et al. 2003). Because of their ROS scavenging property, CeNPs have also been reported to promote wound healing in an animal model by reducing oxidative stress (Davan et al. 2012). However, other studies reported the opposite nature of CeNPs and indicated the role of CeNPs in promoting oxidative stress and decreasing cellular viability. CeNPs have been shown to decrease the lifespan of Caenorhabditis elegans by ROS accumulation (Zhang et al. 2011). Also, CeNPs have been reported to cause liver damage in rats (Nalabotu et al. 2011) and showed cytotoxicity toward human lung epithelial cells (Park et al. 2008b). The reason for this antagonist behavior of CeNPs can be the particle size and Ce3+/4+ ratios. A report by Yokel et al. showed that a large surface area to volume ratio could be the major reason for increased Ce3+/4+ ratio and higher toxicity (Yokel et al. 2014). Still, more research is needed in this direction to understand the safe or toxic behavior of CeNPs.
Environmental prospects of cerium oxide nanoparticles
Apart from biomedical applications, CeNPs also have a wide range of environmental applications including polishing, as anti-corrosive agents, in solar cells, automotive exhaust treatment, water treatment, fuel oxidation, etc. When cerium is added to the diesel fuel along with a particulate filter, it results in a drastic decrease (approximate 90%) in particulate matter emission (Park et al. 2008a). As per US Environmental Protection Agency (EPA), particulate matters are human carcinogen as they contribute to the formation of ozone. Hence, to meet the standard limit of particulate matters, many efforts have been made including the use of cerium-based fuel additives. The use of cerium with a particulate filter decreased the particulate matter emission but some of the cerium escaped during emission and may accumulate on vegetables, sand, and water. This procedure is expected to increase the cerium concentration in the atmosphere. Cerium can be added to the environment via other sources like in landfills (from the electronic wastes), in water (from sewage water treatment and wastewater discharge from ceramic industries; Möller et al. 2003; Keller and Lazareva 2014). If CeNPs are not cleared from the system properly, they may create a problem for biological organisms and the environment. A fraction of CeNPs can escape adsorption to clearing sludge and avoid a clearance system (Limbach et al. 2008). As CeNPs are a new field of research, the chemistry and fate of CeNPs in the environment are not well studied. Much work is needed in this direction to reduce the hazards and toxicity of CeNPs in the environment.
Outlook for the future—the path forward
CeNPs exhibit immense potential to scavenge ROS levels inside the cells and act as the theragnostic agent against various ROS-related disorders. Many factors determine its oxidative or anti-oxidative potential, such as pH of the system, surface defects, and coatings, synthesis procedures, surface oxidation state, etc. Core–shell hybrids improved their applications and properties but still, efforts are required to design single oxidation state CeNPs. A single oxidation state will allow CeNPs to show a particular enzymatic activity at one time and allow them to react with ROS in a biological system. In addition, exertions are needed to reduce the metal ion leaching to prevent the toxicity of CeNPs.
Conclusions and perspectives
CeNPs have been found to exhibit unique physical, chemical, and biological properties. The redox switching between two oxidation states (3+ and 4+) and oxygen defects present on the surface, made CeNPs a potential candidate for various applications. CeNPs have been reported for several biological applications, such as antibacterial agents, anticancer agents, antioxidants, in the treatment of diseases, drugs/gene delivery, etc. However, the bare form of CeNPs faces some limitations inside biological systems, such as aggregation or agglomeration in high ionic strength medium, protein or chemical corona formation due to adsorption of proteins or chemicals on the surface of CeNPs, non-specific binding with other biomolecules, etc. which increases their toxicity in the living systems. To overcome these issues, surface coating of various ligands has been applied on the surface of CeNPs. The surface coating with suitable, biocompatible, and bioactive molecules, not only stabilized the CeNPs but also improved their catalytic activities and in some cases also added some new properties to CeNPs. Attempts have been made to improve the biomedical applications of CeNPs using various kinds of ligands, which proved beneficial in some ways. Still, the toxicity mechanisms, tuning of catalytic activities, and redox behavior need systematic investigation. The research should be focused on the improvement and development of commercially active CeNPs by modifying their surface using suitable ligands.
Acknowledgements
The author is grateful to the Council of Scientific and Industrial Research, New Delhi for giving a senior research fellowship.
Declarations
Conflicts of interest
The author declares no conflicts of interest.
References
- Aleksandrov HA, Neyman KM, Hadjiivanov KI, Vayssilov GN. Can the state of platinum species be unambiguously determined by the stretching frequency of an adsorbed CO probe molecule? Phys Chem Chem Phys. 2016;18(32):22108–22121. doi: 10.1039/c6cp03988j. [DOI] [PubMed] [Google Scholar]
- Alili L, Sack M, von Montfort C, Giri S, Das S, Carroll KS, Zanger K, Seal S, Brenneisen P. Downregulation of tumor growth and invasion by redox-active nanoparticles. Antioxid Redox Signal. 2013;19(8):765–778. doi: 10.1089/ars.2012.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkadi H. A review on free radicals and antioxidants. Infect Disorders-Drug Targets. 2020;20(1):16–26. doi: 10.2174/1871526518666180628124323. [DOI] [PubMed] [Google Scholar]
- Ami T, Suzuki M. MOCVD growth of (100)-oriented CeO2 thin films on hydrogen-terminated Si (100) substrates. Mater Sci Eng, B. 1998;54(1–2):84–91. [Google Scholar]
- Ansari AA, Kaushik A, Solanki P, Malhotra B. Sol–gel derived nanoporous cerium oxide film for application to cholesterol biosensor. Electrochem Commun. 2008;10(9):1246–1249. [Google Scholar]
- Ansari AA, Solanki PR, Malhotra B. Hydrogen peroxide sensor based on horseradish peroxidase immobilized nanostructured cerium oxide film. J Biotechnol. 2009;142(2):179–184. doi: 10.1016/j.jbiotec.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Arumugam A, Karthikeyan C, Hameed A, Gopinath K, Gowri S, Karthika V. Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater Sci Eng C. 2015;49:408–415. doi: 10.1016/j.msec.2015.01.042. [DOI] [PubMed] [Google Scholar]
- Asati A, Santra S, Kaittanis C, Nath S, Perez JM. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew Chem Int Ed. 2009;48(13):2308–2312. doi: 10.1002/anie.200805279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asati A, Santra S, Kaittanis C, Nath S, Perez JM. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew Chem. 2009;121(13):2344–2348. doi: 10.1002/anie.200805279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asati A, Santra S, Kaittanis C, Perez JM. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano. 2010;4(9):5321–5331. doi: 10.1021/nn100816s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asati A, Kaittanis C, Santra S, Perez JM. pH-tunable oxidase-like activity of cerium oxide nanoparticles achieving sensitive fluorigenic detection of cancer biomarkers at neutral pH. Anal Chem. 2011;83(7):2547–2553. doi: 10.1021/ac102826k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu KS, Anandkumar M, Tsai T, Kao T, Inbaraj BS, Chen B. Cytotoxicity and antibacterial activity of gold-supported cerium oxide nanoparticles. Int J Nanomed. 2014;9:5515. doi: 10.2147/IJN.S70087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldim V, Bia N, Graillot A, Loubat C, Berret JF. Monophosphonic versus multiphosphonic acid based PEGylated polymers for functionalization and stabilization of metal (Ce, Fe, Ti, Al) oxide nanoparticles in biological media. Adv Mater Interfaces. 2019;6(7):1801814. [Google Scholar]
- Baldim V, Yadav N, Bia N, Graillot A, Loubat C, Singh S, Karakoti AS, Berret J-F. Polymer-coated cerium oxide nanoparticles as oxidoreductase-like catalysts. ACS Appl Mater Interfaces. 2020;12(37):42056–42066. doi: 10.1021/acsami.0c08778. [DOI] [PubMed] [Google Scholar]
- Bhagat S, Vallabani NS, Shutthanandan V, Bowden M, Karakoti AS, Singh S. Gold core/ceria shell-based redox active nanozyme mimicking the biological multienzyme complex phenomenon. J Colloid Interface Sci. 2018;513:831–842. doi: 10.1016/j.jcis.2017.11.064. [DOI] [PubMed] [Google Scholar]
- Celardo I, Pedersen JZ, Traversa E, Ghibelli L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale. 2011;3(4):1411–1420. doi: 10.1039/c0nr00875c. [DOI] [PubMed] [Google Scholar]
- Chanteau B, Fresnais J, Berret J-F. Electrosteric enhanced stability of functional sub-10 nm cerium and iron oxide particles in cell culture medium. Langmuir. 2009;25(16):9064–9070. doi: 10.1021/la900833v. [DOI] [PubMed] [Google Scholar]
- Charbgoo F, Ramezani M, Darroudi M. Bio-sensing applications of cerium oxide nanoparticles: advantages and disadvantages. Biosens Bioelectron. 2017;96:33–43. doi: 10.1016/j.bios.2017.04.037. [DOI] [PubMed] [Google Scholar]
- Chavali MS, Nikolova MP. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl Sci. 2019;1(6):1–30. [Google Scholar]
- Chen S, Hou Y, Cheng G, Zhang C, Wang S, Zhang J. Cerium oxide nanoparticles protect endothelial cells from apoptosis induced by oxidative stress. Biol Trace Elem Res. 2013;154(1):156–166. doi: 10.1007/s12011-013-9678-8. [DOI] [PubMed] [Google Scholar]
- Ciofani G, Genchi GG, Mazzolai B. Mattoli V (2014) Transcriptional profile of genes involved in oxidative stress and antioxidant defense in PC12 cells following treatment with cerium oxide nanoparticles. Biochim Biophys Acta (BBA) 1840;1:495–506. doi: 10.1016/j.bbagen.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Colon J, Hsieh N, Ferguson A, Kupelian P, Seal S, Jenkins DW, Baker CH. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomed Nanotechnol Biol Med. 2010;6(5):698–705. doi: 10.1016/j.nano.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Cuahtecontzi-Delint R, Mendez-Rojas MA, Bandala ER, Quiroz MA, Recillas S, Sanchez-Salas JL. Enhanced antibacterial activity of CeO2 nanoparticles by surfactants. Int J Chem Reactor Eng. 2013;11(2):781–785. [Google Scholar]
- Dar M, Gul R, Alfadda A, Karim M, Kim D-W, Cheung C, Almajid A, Alharthi N, Pulakat L. Size-dependent effect of nanoceria on their antibacterial activity towards Escherichia coli. Sci Adv Mater. 2017;9(7):1248–1253. [Google Scholar]
- Das M, Patil S, Bhargava N, Kang J-F, Riedel LM, Seal S, Hickman JJ. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28(10):1918–1925. doi: 10.1016/j.biomaterials.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Han JW, Choi Y-J, Song H, Cho S-G, Park C, Seo HG, Kim J-H. Cationic lipid-nanoceria hybrids, a novel nonviral vector-mediated gene delivery into mammalian cells: investigation of the cellular uptake mechanism. Sci Rep. 2016;6(1):1–13. doi: 10.1038/srep29197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Choi Y-J, Han JW, Reza AMMT, Kim J-H. Nanoceria-mediated delivery of doxorubicin enhances the anti-tumour efficiency in ovarian cancer cells via apoptosis. Sci Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-09876-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davan R, Prasad R, Jakka VS, Aparna R, Phani A, Jacob B, Salins PC, Raju D. Cerium oxide nanoparticles promotes wound healing activity in in-vivo animal model. J Bionanosci. 2012;6(2):78–83. [Google Scholar]
- Deshpande S, Patil S, Kuchibhatla SV, Seal S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl Phys Lett. 2005;87(13):133113. [Google Scholar]
- Dhall A, Self W. Cerium oxide nanoparticles: a brief review of their synthesis methods and biomedical applications. Antioxidants. 2018;7(8):97. doi: 10.3390/antiox7080097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhall A, Burns A, Dowding J, Das S, Seal S, Self W. Characterizing the phosphatase mimetic activity of cerium oxide nanoparticles and distinguishing its active site from that for catalase mimetic activity using anionic inhibitors. Environ Sci Nano. 2017;4(8):1742–1749. [Google Scholar]
- Estevez AY, Erlichman JS. The potential of cerium oxide nanoparticles (nanoceria) for neurodegenerative disease therapy. Nanomedicine. 2014;9(10):1437–1440. doi: 10.2217/nnm.14.87. [DOI] [PubMed] [Google Scholar]
- Fallatah A, Almomtan M, Padalkar S. Cerium oxide based glucose biosensors: influence of morphology and underlying substrate on biosensor performance. ACS Sustain Chem Eng. 2019;7(9):8083–8089. [Google Scholar]
- Gao Y, Chen K, Ma J-l, Gao F. Cerium oxide nanoparticles in cancer. Onco Targets Ther. 2014;7:835. doi: 10.2147/OTT.S62057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Góth L, Rass P, Páy A. Catalase enzyme mutations and their association with diseases. Mol Diagn. 2004;8(3):141–149. doi: 10.1007/BF03260057. [DOI] [PubMed] [Google Scholar]
- Hartati YW, Letelay LK, Gaffar S, Wyantuti S, Bahti HH. Cerium oxide-monoclonal antibody bioconjugate for electrochemical immunosensing of HER2 as a breast cancer biomarker. Sens Bio-Sens Res. 2020;27:100316. [Google Scholar]
- Hijaz M, Das S, Mert I, Gupta A, Al-Wahab Z, Tebbe C, Dar S, Chhina J, Giri S, Munkarah A. Folic acid tagged nanoceria as a novel therapeutic agent in ovarian cancer. BMC Cancer. 2016;16(1):1–14. doi: 10.1186/s12885-016-2206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM. Anti-inflammatory properties of cerium oxide nanoparticles. Small. 2009;5(24):2848–2856. doi: 10.1002/smll.200901048. [DOI] [PubMed] [Google Scholar]
- Homayouni-Tabrizi M, Asoodeh A, Mashreghi M, Bazaz MR, Oskuee RK, Darroudi M. Attachment of a frog skin-derived peptide to functionalized cerium oxide nanoparticles. Int J Pept Res Ther. 2016;22(4):505–510. [Google Scholar]
- Hussain S, Al-Nsour F, Rice AB, Marshburn J, Yingling B, Ji Z, Zink JI, Walker NJ, Garantziotis S. Cerium dioxide nanoparticles induce apoptosis and autophagy in human peripheral blood monocytes. ACS Nano. 2012;6(7):5820–5829. doi: 10.1021/nn302235u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifeanyi OE. A review on free radicals and antioxidants. Int J Curr Res Med Sci. 2018;4(2):123–133. [Google Scholar]
- Izu N, Uchida T, Matsubara I, Itoh T, Shin W, Nishibori M. Formation mechanism of monodispersed spherical core–shell ceria/polymer hybrid nanoparticles. Mater Res Bull. 2011;46(8):1168–1176. [Google Scholar]
- Jana SK, Banerjee P, Das S, Seal S, Chaudhury K. Redox-active nanoceria depolarize mitochondrial membrane of human colon cancer cells. J Nanopart Res. 2014;16(6):1–9. [Google Scholar]
- Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018;9(1):1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalashnikova I, Mazar J, Neal CJ, Rosado AL, Das S, Westmoreland TJ, Seal S. Nanoparticle delivery of curcumin induces cellular hypoxia and ROS-mediated apoptosis via modulation of Bcl-2/Bax in human neuroblastoma. Nanoscale. 2017;9(29):10375–10387. doi: 10.1039/c7nr02770b. [DOI] [PubMed] [Google Scholar]
- Kannan S, Sundrarajan M. A green approach for the synthesis of a cerium oxide nanoparticle: characterization and antibacterial activity. Int J Nanosci. 2014;13(03):1450018. [Google Scholar]
- Karakoti AS, Singh S, Kumar A, Malinska M, Kuchibhatla SV, Wozniak K, Self WT, Seal S. PEGylated nanoceria as radical scavenger with tunable redox chemistry. J Am Chem Soc. 2009;131(40):14144–14145. doi: 10.1021/ja9051087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartsonakis IA, Liatsi P, Daniilidis I, Kordas G. Synthesis, characterization, and antibacterial action of hollow ceria nanospheres with/without a conductive polymer coating. J Am Ceram Soc. 2008;91(2):372–378. [Google Scholar]
- Kay H. Plasma phosphatase II. The enzyme in disease, particularly in bone disease. J Biol Chem. 1930;89(1):249–266. [Google Scholar]
- Keller AA, Lazareva A. Predicted releases of engineered nanomaterials: from global to regional to local. Environ Sci Technol Lett. 2014;1(1):65–70. [Google Scholar]
- Kim S-J, Chung BH. Antioxidant activity of levan coated cerium oxide nanoparticles. Carbohyd Polym. 2016;150:400–407. doi: 10.1016/j.carbpol.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Komkova MA, Andreeva KD, Zarochintsev AA, Karyakin AA. Nanozymes “Artificial Peroxidase”: enzyme oxidase mixtures for single-step fabrication of advanced electrochemical biosensors. ChemElectroChem. 2021;8(6):1117–1122. [Google Scholar]
- Korsvik C, Patil S, Seal S, Self WT. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun (Camb) 2007;10:1056–1058. doi: 10.1039/b615134e. [DOI] [PubMed] [Google Scholar]
- Krishna MG, Hartridge A, Bhattacharya A. Temperature and ionic size dependence of the properties of ceria based optionic thin films. Mater Sci Eng, B. 1998;55(1–2):14–20. [Google Scholar]
- Kuang Y, He X, Zhang Z, Li Y, Zhang H, Ma Y, Wu Z, Chai Z. Comparison study on the antibacterial activity of nano-or bulk-cerium oxide. J Nanosci Nanotechnol. 2011;11(5):4103–4108. doi: 10.1166/jnn.2011.3858. [DOI] [PubMed] [Google Scholar]
- Kuchma MH, Komanski CB, Colon J, Teblum A, Masunov AE, Alvarado B, Babu S, Seal S, Summy J, Baker CH. Phosphate ester hydrolysis of biologically relevant molecules by cerium oxide nanoparticles. Nanomed Nanotechnol Biol Med. 2010;6(6):738–744. doi: 10.1016/j.nano.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Kullgren J, Hermansson K, Castleton C. Many competing ceria (110) oxygen vacancy structures: from small to large supercells. J Chem Phys. 2012;137(4):044705. doi: 10.1063/1.4723867. [DOI] [PubMed] [Google Scholar]
- Kumar A, Das S, Munusamy P, Self W, Baer DR, Sayle DC, Seal S. Behavior of nanoceria in biologically-relevant environments. Environ Sci Nano. 2014;1(6):516–532. [Google Scholar]
- Kumari M, Singh SP, Chinde S, Rahman MF, Mahboob M, Grover P. Toxicity study of cerium oxide nanoparticles in human neuroblastoma cells. Int J Toxicol. 2014;33(2):86–97. doi: 10.1177/1091581814522305. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang W, Niu J, Chen Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano. 2012;6(6):5164–5173. doi: 10.1021/nn300934k. [DOI] [PubMed] [Google Scholar]
- Li Y, He X, Yin JJ, Ma Y, Zhang P, Li J, Ding Y, Zhang J, Zhao Y, Chai Z. Acquired superoxide-scavenging ability of ceria nanoparticles. Angew Chem. 2015;127(6):1852–1855. doi: 10.1002/anie.201410398. [DOI] [PubMed] [Google Scholar]
- Li H, Liu C, Zeng Y-P, Hao Y-H, Huang J-W, Yang Z-Y, Li R. Nanoceria-mediated drug delivery for targeted photodynamic therapy on drug-resistant breast cancer. ACS Appl Mater Interfaces. 2016;8(46):31510–31523. doi: 10.1021/acsami.6b07338. [DOI] [PubMed] [Google Scholar]
- Limbach LK, Bereiter R, Müller E, Krebs R, Gälli R, Stark WJ. Removal of oxide nanoparticles in a model wastewater treatment plant: influence of agglomeration and surfactants on clearing efficiency. Environ Sci Technol. 2008;42(15):5828–5833. doi: 10.1021/es800091f. [DOI] [PubMed] [Google Scholar]
- Lin W, Huang Y-w, Zhou X-D, Ma Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int J Toxicol. 2006;25(6):451–457. doi: 10.1080/10915810600959543. [DOI] [PubMed] [Google Scholar]
- Logothetidis S, Patsalas P, Evangelou E, Konofaos N, Tsiaoussis I, Frangis N. Dielectric properties and electronic transitions of porous and nanostructured cerium oxide films. Mater Sci Eng, B. 2004;109(1–3):69–73. [Google Scholar]
- Lord MS, Berret JF, Singh S, Vinu A, Karakoti AS (2021) Redox active cerium oxide nanoparticles: current status and burning issues. arXiv preprint arXiv:210606473 [DOI] [PubMed]
- Marabelli F, Wachter P. Covalent insulator CeO2: optical reflectivity measurements. Phys Rev B. 1987;36(2):1238. doi: 10.1103/physrevb.36.1238. [DOI] [PubMed] [Google Scholar]
- Marino A, Tonda-Turo C, De Pasquale D, Ruini F, Genchi G, Nitti S, Cappello V, Gemmi M, Mattoli V. Ciardelli G (2017) Gelatin/nanoceria nanocomposite fibers as antioxidant scaffolds for neuronal regeneration. Biochim Biophys Acta (BBA) 1861;2:386–395. doi: 10.1016/j.bbagen.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Mauricio M, Guerra-Ojeda S, Marchio P, Valles S, Aldasoro M, Escribano-Lopez I, Herance J, Rocha M, Vila J, Victor V. Nanoparticles in medicine: a focus on vascular oxidative stress. Oxidat Med Cell Longev. 2018;2018:6231482. doi: 10.1155/2018/6231482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller P, Morteani G, Dulski P. Anomalous gadolinium, cerium, and yttrium contents in the adige and isarco river waters and in the water of their tributaries (Provinces Trento and Bolzano/Bozen, NE Italy) Acta Hydrochim Hydrobiol. 2003;31(3):225–239. [Google Scholar]
- Mustafa F, Othman A, Andreescu S. Cerium oxide-based hypoxanthine biosensor for Fish spoilage monitoring. Sens Actuators, B Chem. 2021;332:129435. [Google Scholar]
- Nadeem M, Khan R, Afridi K, Nadhman A, Ullah S, Faisal S, Mabood ZU, Hano C, Abbasi BH. Green synthesis of cerium oxide nanoparticles (CeO2 NPs) and their antimicrobial applications: a review. Int J Nanomed. 2020;15:5951. doi: 10.2147/IJN.S255784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalabotu SK, Kolli MB, Triest WE, Ma JY, Manne ND, Katta A, Addagarla HS, Rice KM, Blough ER. Intratracheal instillation of cerium oxide nanoparticles induces hepatic toxicity in male Sprague-Dawley rats. Int J Nanomed. 2011;6:2327. doi: 10.2147/IJN.S25119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda HS. Surface modification of promising cerium oxide nanoparticles for nanomedicine applications. RSC Adv. 2016;6(113):111889–111894. [Google Scholar]
- Naz S, Beach J, Heckert B, Tummala T, Pashchenko O, Banerjee T, Santra S. Cerium oxide nanoparticles: a ‘radical’approach to neurodegenerative disease treatment. Nanomedicine. 2017;12(5):545–553. doi: 10.2217/nnm-2016-0399. [DOI] [PubMed] [Google Scholar]
- Nesakumar N, Sethuraman S, Krishnan UM, Rayappan JBB. Fabrication of lactate biosensor based on lactate dehydrogenase immobilized on cerium oxide nanoparticles. J Colloid Interface Sci. 2013;410:158–164. doi: 10.1016/j.jcis.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Nethi SK, Nanda HS, Steele TW, Patra CR. Functionalized nanoceria exhibit improved angiogenic properties. J Mater Chem B. 2017;5(47):9371–9383. doi: 10.1039/c7tb01957b. [DOI] [PubMed] [Google Scholar]
- Nourmohammadi E, Khoshdel-Sarkarizi H, Nedaeinia R, Sadeghnia HR, Hasanzadeh L, Darroudi M, Kazemi Oskuee R. Evaluation of anticancer effects of cerium oxide nanoparticles on mouse fibrosarcoma cell line. J Cell Physiol. 2019;234(4):4987–4996. doi: 10.1002/jcp.27303. [DOI] [PubMed] [Google Scholar]
- Pagliari F, Mandoli C, Forte G, Magnani E, Pagliari S, Nardone G, Licoccia S, Minieri M, Di Nardo P, Traversa E. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano. 2012;6(5):3767–3775. doi: 10.1021/nn2048069. [DOI] [PubMed] [Google Scholar]
- Paier J, Penschke C, Sauer J. Oxygen defects and surface chemistry of ceria: quantum chemical studies compared to experiment. Chem Rev. 2013;113(6):3949–3985. doi: 10.1021/cr3004949. [DOI] [PubMed] [Google Scholar]
- Park B, Donaldson K, Duffin R, Tran L, Kelly F, Mudway I, Morin J-P, Guest R, Jenkinson P, Samaras Z. Hazard and risk assessment of a nanoparticulate cerium oxide-based diesel fuel additive—a case study. Inhalation Toxicol. 2008;20(6):547–566. doi: 10.1080/08958370801915309. [DOI] [PubMed] [Google Scholar]
- Park E-J, Choi J, Park Y-K, Park K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology. 2008;245(1–2):90–100. doi: 10.1016/j.tox.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Patel V, Singh M, Mayes EL, Martinez A, Shutthanandan V, Bansal V, Singh S, Karakoti AS. Ligand-mediated reversal of the oxidation state dependent ROS scavenging and enzyme mimicking activity of ceria nanoparticles. Chem Commun. 2018;54(99):13973–13976. doi: 10.1039/c8cc08355j. [DOI] [PubMed] [Google Scholar]
- Patil S, Reshetnikov S, Haldar MK, Seal S, Mallik S. Surface-derivatized nanoceria with human carbonic anhydrase II inhibitors and fluorophores: a potential drug delivery device. J Phys Chem C. 2007;111(24):8437–8442. [Google Scholar]
- Perez JM, Asati A, Nath S, Kaittanis C. Synthesis of biocompatible dextran-coated nanoceria with pH-dependent antioxidant properties. Small. 2008;4(5):552–556. doi: 10.1002/smll.200700824. [DOI] [PubMed] [Google Scholar]
- Pešić M, Podolski-Renić A, Stojković S, Matović B, Zmejkoski D, Kojić V, Bogdanović G, Pavićević A, Mojović M, Savić A. Anti-cancer effects of cerium oxide nanoparticles and its intracellular redox activity. Chem Biol Interact. 2015;232:85–93. doi: 10.1016/j.cbi.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS, King JE, Seal S, Self WT. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun. 2010;46(16):2736–2738. doi: 10.1039/b922024k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeshkumar S, Naik P. Synthesis and biomedical applications of cerium oxide nanoparticles–a review. Biotechnol Reports. 2018;17:1–5. doi: 10.1016/j.btre.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy M. Nano technology: a review. J Appl Pharm Sci. 2011;1(2):8–16. [Google Scholar]
- Rani V, Deep G, Singh RK, Palle K, Yadav UC. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193. doi: 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Ranjbar A, Asl SS, Firozian F, Dartoti HH, Seyedabadi S, Azandariani MT, Ganji M. Role of cerium oxide nanoparticles in a paraquat-induced model of oxidative stress: emergence of neuroprotective results in the brain. J Mol Neurosci. 2018;66(3):420–427. doi: 10.1007/s12031-018-1191-2. [DOI] [PubMed] [Google Scholar]
- Renu G, Rani V, Nair S, Subramanian K, Lakshmanan V-k. Development of cerium oxide nanoparticles and its cytotoxicity in prostate cancer cells. Adv Sci Lett. 2012;6(1):17–25. [Google Scholar]
- Rubio L, Annangi B, Vila L, Hernández A, Marcos R. Antioxidant and anti-genotoxic properties of cerium oxide nanoparticles in a pulmonary-like cell system. Arch Toxicol. 2016;90(2):269–278. doi: 10.1007/s00204-015-1468-y. [DOI] [PubMed] [Google Scholar]
- Rzigalinski BA, Bailey D, Chow L, Kuiry S, Patil S, Merchant S, Seal S. Cerium oxide nanoparticles increase the lifespan of cultured brain cells and protect against free radical and mechanical trauma. Faseb J. 2003;4:A606. [Google Scholar]
- Saha S, Arya SK, Singh S, Sreenivas K, Malhotra B, Gupta V. Nanoporous cerium oxide thin film for glucose biosensor. Biosens Bioelectron. 2009;24(7):2040–2045. doi: 10.1016/j.bios.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Senthilkumar R, Bhuvaneshwari V, Ranjithkumar R, Sathiyavimal S, Malayaman V, Chandarshekar B. Synthesis, characterization and antibacterial activity of hybrid chitosan-cerium oxide nanoparticles: as a bionanomaterials. Int J Biol Macromol. 2017;104:1746–1752. doi: 10.1016/j.ijbiomac.2017.03.139. [DOI] [PubMed] [Google Scholar]
- Shah V, Shah S, Shah H, Rispoli FJ, McDonnell KT, Workeneh S, Karakoti A, Kumar A, Seal S. Antibacterial activity of polymer coated cerium oxide nanoparticles. PLoS One. 2012;7(10):e47827. doi: 10.1371/journal.pone.0047827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah F, Yadav N, Singh S. Phosphotungstate-sandwiched between cerium oxide and gold nanoparticles exhibit enhanced catalytic reduction of 4-nitrophenol and peroxidase enzyme-like activity. Colloids Surf, B. 2021;198:111478. doi: 10.1016/j.colsurfb.2020.111478. [DOI] [PubMed] [Google Scholar]
- Sharma GN, Gupta G, Sharma P. A comprehensive review of free radicals, antioxidants, and their relationship with human ailments. Crit Rev Eukaryot Gene Expr. 2018;28(2):139–154. doi: 10.1615/CritRevEukaryotGeneExpr.2018022258. [DOI] [PubMed] [Google Scholar]
- Shcherbakov AB, Zholobak NM, Ivanov VK. Cerium Oxide (CeO2): synthesis, properties and applications. Amsterdam: Elsevier; 2020. Biological, biomedical and pharmaceutical applications of cerium oxide; pp. 279–358. [Google Scholar]
- Singh S. Cerium oxide based nanozymes: Redox phenomenon at biointerfaces. Biointerphases. 2016;11(4):04B202. doi: 10.1116/1.4966535. [DOI] [PubMed] [Google Scholar]
- Singh R, Singh S. Redox-dependent catalase mimetic cerium oxide-based nanozyme protect human hepatic cells from 3-AT induced acatalasemia. Colloids Surf, B. 2019;175:625–635. doi: 10.1016/j.colsurfb.2018.12.042. [DOI] [PubMed] [Google Scholar]
- Singh S, Dosani T, Karakoti AS, Kumar A, Seal S, Self WT. A phosphate-dependent shift in redox state of cerium oxide nanoparticles and its effects on catalytic properties. Biomaterials. 2011;32(28):6745–6753. doi: 10.1016/j.biomaterials.2011.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KR, Nayak V, Sarkar T, Singh RP. Cerium oxide nanoparticles: properties, biosynthesis and biomedical application. RSC Adv. 2020;10(45):27194–27214. doi: 10.1039/d0ra04736h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki PR, Dhand C, Kaushik A, Ansari AA, Sood K, Malhotra B. Nanostructured cerium oxide film for triglyceride sensor. Sens Actuators, B Chem. 2009;141(2):551–556. [Google Scholar]
- Sulthana S, Banerjee T, Kallu J, Vuppala SR, Heckert B, Naz S, Shelby T, Yambem O, Santra S. Combination therapy of NSCLC using Hsp90 inhibitor and doxorubicin carrying functional nanoceria. Mol Pharm. 2017;14(3):875–884. doi: 10.1021/acs.molpharmaceut.6b01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur N, Manna P, Das J. Synthesis and biomedical applications of nanoceria, a redox active nanoparticle. J Nanobiotechnol. 2019;17(1):1–27. doi: 10.1186/s12951-019-0516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thill A, Zeyons O, Spalla O, Chauvat F, Rose J, Auffan M, Flank AM. Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ Sci Technol. 2006;40(19):6151–6156. doi: 10.1021/es060999b. [DOI] [PubMed] [Google Scholar]
- Walkey C, Das S, Seal S, Erlichman J, Heckman K, Ghibelli L, Traversa E, McGinnis JF, Self WT. Catalytic properties and biomedical applications of cerium oxide nanoparticles. Environ Sci Nano. 2015;2(1):33–53. doi: 10.1039/C4EN00138A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed Nanotechnol Biol Med. 2015;11(2):313–327. doi: 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Wason MS, Zhao J. Cerium oxide nanoparticles: potential applications for cancer and other diseases. Am J Transl Res. 2013;5(2):126. [PMC free article] [PubMed] [Google Scholar]
- Woldeamanuel KM, Kurra FA, Roba YT. A review on nanotechnology and its application in modern veterinary science. Int J Nanomater Nanotechnol Nanomed. 2021;7(1):026–031. [Google Scholar]
- Wu Y, Yang Y, Zhao W, Xu ZP, Little PJ, Whittaker AK, Zhang R, Ta HT. Novel iron oxide–cerium oxide core–shell nanoparticles as a potential theranostic material for ROS related inflammatory diseases. J Mater Chem B. 2018;6(30):4937–4951. doi: 10.1039/c8tb00022k. [DOI] [PubMed] [Google Scholar]
- Xu C, Qu X. Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014;6(3):e90–e90. [Google Scholar]
- Yadav N, Singh S. Emerging trends in nanomedicine. Singapore: Springer; 2021. Nanoparticles catalyzing enzymatic reactions: recent developments and future prospects; pp. 51–80. [Google Scholar]
- Yadav N, Singh S. Polyoxometalate-mediated vacancy-engineered cerium oxide nanoparticles exhibiting controlled biological enzyme-mimicking activities. Inorg Chem. 2021;60(10):7475–7489. doi: 10.1021/acs.inorgchem.1c00766. [DOI] [PubMed] [Google Scholar]
- Yadav N, Singh S. SOD mimetic cerium oxide nanorods protect human hepatocytes from oxidative stress. Emerg Mater. 2021;4:1305–1317. [Google Scholar]
- Yadav N, Patel V, Singh S. Advances in spectroscopy: molecules to materials. Berlin: Springer; 2019. Cerium oxide-based Nanozymes in biology and medicine; pp. 193–213. [Google Scholar]
- Yang D, Fa M, Gao L, Zhao R, Luo Y, Yao X. The effect of DNA on the oxidase activity of nanoceria with different morphologies. Nanotechnology. 2018;29(38):385101. doi: 10.1088/1361-6528/aacf86. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Hussain S, Garantziotis S, Demokritou P, Castranova V, Cassee FR. The yin: an adverse health perspective of nanoceria: uptake, distribution, accumulation, and mechanisms of its toxicity. Environ Sci Nano. 2014;1(5):406–428. doi: 10.1039/C4EN00039K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokel RA, Hancock ML, Grulke EA, Unrine JM, Dozier AK, Graham UM. Carboxylic acids accelerate acidic environment-mediated nanoceria dissolution. Nanotoxicology. 2019;13(4):455–475. doi: 10.1080/17435390.2018.1553251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis A, Chu D, Li S. Cerium oxide nanostructures and their applications. Funct Nanomater. 2016;3:53–68. [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radical Biol Med. 2002;33(3):337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- Zhang H, He X, Zhang Z, Zhang P, Li Y, Ma Y, Kuang Y, Zhao Y, Chai Z. Nano-CeO2 exhibits adverse effects at environmental relevant concentrations. Environ Sci Technol. 2011;45(8):3725–3730. doi: 10.1021/es103309n. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yuan R, Chai Y, Wang C, Wu X. Cerium oxide–graphene as the matrix for cholesterol sensor. Anal Biochem. 2013;436(2):69–74. doi: 10.1016/j.ab.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Xu YD, Ma YY, Qiu LL, Wang Y, Kong JL, Xiong HM. Biodegradable ZnO@ polymer core–shell nanocarriers: pH-triggered release of doxorubicin in vitro. Angew Chem. 2013;125(15):4221–4225. doi: 10.1002/anie.201300431. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wu X, Hou C, Shang K, Yang K, Tian Z, Pei Z, Qu Y, Pei Y. Dual-responsive dithio-polydopamine coated porous CeO2 nanorods for targeted and synergistic drug delivery. Int J Nanomed. 2018;13:2161. doi: 10.2147/IJN.S152002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhang C, Zhai X, Luo F, Du Y, Yan C. Antibacterial mechanism and activity of cerium oxide nanoparticles. Sci China Mater. 2019;62(11):1727–1739. [Google Scholar]



