Abstract
Background:
Hyperbaric oxygen therapy (HBOT) improves short-term outcomes for ulcerative colitis (UC) patients hospitalized for acute flares. Longer-term impacts and cost-effectiveness are unknown.
Methods:
We compared disease outcomes and cost-effectiveness of HBOT in addition to standard care versus standard of care for UC patients hospitalized for acute flares using a microsimulation model. Published literature was used for transition probabilities, costs, and quality-adjusted life year (QALY) estimates. We modeled 100,000 individuals in each group over a 5-year horizon, and compared rates of re-hospitalization, rescue medical therapy, colectomy, death, and cost-effectiveness at a willingness-to-pay of $100,000/QALY. Probabilistic sensitivity analyses were performed with 500 samples and 250 trials, in addition to multiple microsimulation sensitivity analyses.
Results:
The use of HBOT at the time of index hospitalization for an acute UC flare is projected to reduce the risk of re-hospitalization, inpatient rescue medical therapy, and inpatient emergent colectomy by over 60% (p < 0.001), and mortality by over 30% (p < 0.001), during a 5-year horizon. The HBOT strategy cost more ($5,600 incremental cost) but also yielded higher QALYs (0.13 incremental yield), resulting in this strategy being cost-effective ($43,000/QALY). Results were sensitivity to HBOT costs and rates of endoscopic improvement with HBOT. Probabilistic sensitivity analyses observed HBOT to be more cost-effective than standard of care in 95% of iterations.
Conclusion:
The use of HBOT to optimize response to steroids during the index hospitalization for an acute UC flare is cost-effective and is projected to result in significant reductions in disease-related complications in the long-term.
Keywords: hospitalization, cost-effectiveness, natural history
INTRODUCTION
It is estimated that nearly one-third of ulcerative colitis (UC) patients will require hospitalization for an acute flare within the first year of diagnosis, and approximately half will require hospitalization at some point in their disease course.1 The annual burden of UC related hospitalizations in the United States is estimated to be over 30,000 with significant increases in hospitalization rates over time.2 Once hospitalized, approximately half of these patients will fail to respond to intravenous steroids and require progression to second line rescue therapy.3-6 In-hospital rescue colectomy is associated with up to 5% mortality when done emergently in the hospital and carries significant longer-term morbidity.7,8 In-hospital rescue medical therapy with infliximab or cyclosporine will rescue up to half of patients who fail intravenous steroids, however, these patients remain at significant risk for re-hospitalization and colectomy for at least 1-year after discharge.9 Even among immunosuppressive naïve UC patients responding to intravenous steroids during hospitalization, an increased risk of colectomy remains at 1-year after discharge.10 Thus novel therapies are needed for hospitalized UC patients that improve both short-term outcomes and longer term outcomes after discharge.
Hyperbaric oxygen therapy (HBOT) results in increased tissue oxygenation, with multiple anti-inflammatory effects targeting mechanisms central to the pathogenesis of UC.11 We previously demonstrated in a phase 2A sham-controlled trial that when used for UC patients hospitalized for acute flares, HBOT resulted in improved disease remission and reduced risk of in-hospital progression to 2nd line therapy.12 In a follow-up phase 2B dose-finding trial we confirmed treatment effectiveness and observed a low rate of progression to 2nd line medical therapy and 3-month re-hospitalization rate.13 These trials provide early evidence for the potential therapeutic benefit of HBOT in this high-risk population, however, it is yet to be determined what the longer-term impacts of HBOT are on the natural history of the disease. Prior work has demonstrated that the single strongest predictor of long-term outcomes for hospitalized UC patients is their initial response to medical therapy in the hospital.14-17 Therefore, it could be hypothesized that by improving in-hospital response to steroids and avoidance of second line medical therapy, HBOT has the potential to influence the natural history of disease in the longer-term for these high-risk patients. We aimed to explore this potential through a microsimulation model.
METHODS
Microsimulation Model
We built an individual-based state-transition microsimulation (first order Monte Carlo) model to simulate the disease course of UC patients hospitalized for an acute flare. We modeled 100,000 UC patients treated with HBOT in combination with intravenous steroids during the first 5 days of the index hospitalization versus 100,000 UC patients treated with intravenous steroids alone for the first 5 days of the index hospitalization. For all subsequent hospitalizations patients in both groups were treated intravenous steroids alone for the first 5 days of hospitalization. At day 5, non-responders progressed on to 2nd line rescue therapy and responders were discharged on oral prednisone tapers with outpatient biologic and/or small molecule therapy for persistent or recurrent disease. The 5 day period for assessing response to intravenous steroids was chosen based on current and prior guidelines and consensus statements.18-21
We incorporated costs of all aspects of care, in addition to quality of life, disease progression and regression, and survival. We chose to model a 5-year time horizon to allow for sufficient time to observe the projected impact of HBOT on the natural history of the disease, and we chose a 3-month cycle length given the highest risk for re-hospitalization is within the first 3 months and this cycle length mirrors currently recommended disease monitoring approaches within treat-to-target monitoring strategies.22 The microsimulation model allowed for the ability to track individual simulated patients’ natural disease course with subsequent modifications of downstream probabilities, health outcomes, and complications. The microsimulation model was constructed with TreeAge Pro 2020 (TreeAge Software, Williamstown, MA).
Disease States and Base-case Assumptions
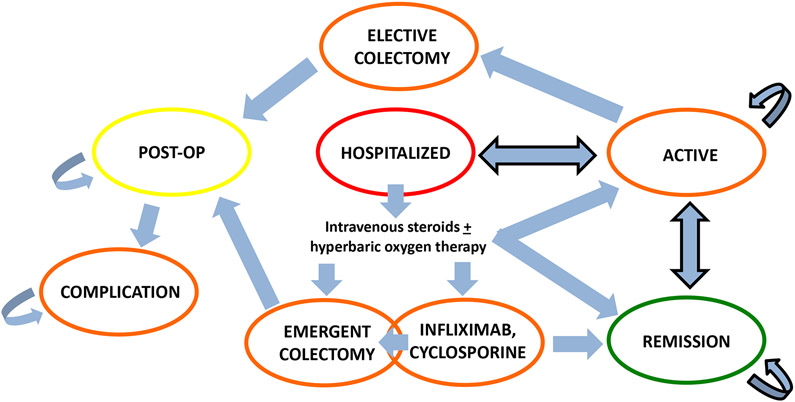
Figure 1 depicts the state-transition diagram. All patients began in the hospitalization state and could transition from there to alternative disease states. All patients could enter the death disease state either through complications from colectomy or natural causes from any disease state. The base-case was a 35-year old immunosuppressive naïve UC male requiring his first lifetime hospitalization for an acute flare (full Mayo 6-12, endoscopic sub-score and rectal bleeding sub-score both 2-3). We obtained health utility estimates for disease states from the literature as outlined in Table 11,3-5,7,9,12,13,22-34. In our microsimulation model, patients would begin a biologic or small molecule inhibitor if they failed intravenous steroids during the index hospitalization or if they entered into the active disease state (Mayo endoscopic sub-score 2 or 3) in the outpatient setting. We assumed that patients would not electively start biologic therapy after hospitalization to prevent disease relapse if in remission (Mayo endoscopic sub-score 0 or 1). We assumed that no patients had complications from their acute flare requiring emergent colectomy upon hospitalization (i.e. toxic megacolon), and that the majority of risk for re-hospitalization and colectomy was within the first year after discharge from the index hospitalization. We took into consideration whether patients would be incrementally more likely to choose colectomy in the outpatient setting with each successive disease flare versus assuming that patients would attempt available medical therapy prior to considering elective colectomy.
Figure 1: Disease State Transitions.
Death can occur at any time through natural causes in addition to occurring after colectomy. Disease states are color-coded based on health utility. Active disease state: Mayo endoscopic sub-score 2 or 3; Remission disease state: Mayo endoscopic sub-score 0 or 1.
Table 1:
| Variable | Baseline Estimate |
Range | Source | Key Assumptions |
|---|---|---|---|---|
| Disease state transition probabilities | ||||
| Biologic and small molecule inhibitor treatment response and relapse patterns in outpatient setting | Dulai PS et al.22 | See prior microsimulation publication for key assumptions, baseline estimates, and ranges/distributions regarding outpatient disease management and course | ||
| 3-month re-hospitalization among hospitalized patients treated with HBOT | 10% | 5-20% | Dulai PS et al.12,13 | 10% reduction in health utility with hospitalization |
| 3-month re-hospitalization among hospitalized patients not treated with HBOT | 30% | 20-40% | Nguyen N et al.55 | |
| Hospitalization for disease worsening in outpatient setting | 15% over 1 year | 5-30% | Mao EJ et al.23 Perera S YS et al.24 Fumery M et al.1 |
|
| Non-response to intravenous steroids in combination with HBOT and requirement for 2nd line rescue therapy when hospitalized for acute flare | 15% | 10-30% | Dulai PS et al.12,13 | All patients willing to undergo rescue medical therapy for non-response to steroids. After 2 in-hospital medical rescue cycles (infliximab and cyclosporine), patients will need emergent colectomy on subsequent hospitalization for non-response to steroids |
| 3-month endoscopic healing for HBOT treated UC patients | 30% | 10-50% | Dulai PS et al.12,13 | |
| Non-response to intravenous steroids alone and requirement for 2nd line rescue therapy when hospitalized for acute flare | 50% | 25-85% | Dulai PS et al.5 Thomas MG et al.3 |
|
| Emergent colectomy in-hospital | 0% 25% rate of failed rescue medical therapy |
0-100% 10-50% |
Estimated Williams JG et al.4 Narula N et al.9 |
|
| Elective colectomy in outpatient setting | 0% | 0-100% | Estimated | Assumed no patients will choose elective colectomy as first line therapy. Probability of choosing elective colectomy incremental with each cycle of active disease state, with 100% probability after failing dose optimization of all biologics and small molecule inhibitors |
| Risk of death with colectomy | 1% for elective 5% for emergent |
0-2% 3-8% |
Dulai PS et al.5 Peyrin-Biroulet L et al.26 Singh S et al.7 |
Terminal state |
| Post-operative complications | 49% over 5 years | 9-65% | Peyrin-Biroulet L et al.26 Lindsay J. et al.27 |
Cost of complication assigned once but health utility remains throughout remainder of model cycles |
| Health Utilities | ||||
| Mucosal Inflammation (MES 2 or 3) | 0.42 | 0.40-0.44 | Archer R et al.28 Stawowczyk E et al.29 |
|
| Endoscopic improvement (MES 0 or 1) | 0.88 | 0.86-0.88 | Archer R et al.28 Stawowczyk E et al.29 |
|
| Post-op without complication | 0.61 | 0.42-0.70 | Archer R et al.28 Stawowczyk E et al.29 |
|
| Post-op with complication | 0.42 | 0.32-0.61 | Archer R et al.28 Stawowczyk E et al.29 |
|
| Costs | ||||
| HBOT | $2,700 | $1,000-5,000 | Medicare rate | 5 HBOT sessions per phase 2A and 2B trial protocols |
| Hospitalization | $4,124a | $2,000-8,000 | Coward S et al.30 | This cost was not inclusive of need for rescue medical or surgical therapy |
| Emergent In-hospital Colectomy | $17,773a | $10,000-$30,000 | Coward S et al.30 | |
| Elective outpatient Colectomy | $10,737a | $5,000-$15,000 | Coward S et al.30 | |
| Post-op Complication | $34,174 | $20,000-$50,000 | Peyrin-Biroulet L et al.26 | Cost assigned once for average estimated total cost of post-op complications |
| Cost of Biologic, small molecule inhibitor, and in-hospital rescue medical therapy | Infliximab: $35,473 per year Adalimumab: $75,517 per year Vedolizumab: $48,781 per year Ustekinumab: $115,863 per year Tofacitinib: $49,830 per year Inpatient Infliximab: $6,000 per dose Inpatient cyclosporine: $1,000 |
Archer R et al.28 Stawowczyk E et al.29 Yokomizo L et al.31 Wilson M. et al.32 Milev S et al.33 Chaudary M et al.34 |
Sequencing took into consideration prior network meta-analyses, phase 3 clinical trial effectiveness data, and post-hoc observations regarding effectiveness in reducing risk of colectomy. Infliximab was considered first line therapy in this cohort given superiorirty to other therapies in network meta-analyses and evidence of preventing colectomy in post-hoc analyses of ACT. | |
Costs were obtained from the literature and adjusted to 2019 dollars.
Normalized to US cost at conversion rate of 0.75 Canadian to US dollar. All estimates normalized to 3-month cycle length. Cost of rescue medical therapy for non-response to intravenous steroids equivalent to outpatient biologic therapy. Baseline risk of mortality incorporated using Social Security Life Tables. When not available in the literature, standard deviations for probabilities, costs, and utilities were assumed to be 20% of the mean. HBOT: hyperbaric oxygen therapy; MES: Mayo endoscopic sub-score
Biologic and Small Molecule Inhibitor Sequencing
Effectiveness estimates for inpatient and outpatient use of biologics (infliximab, adalimumab, vedolizumab, ustekinumab), cyclosporine, and tofacitinib were obtained from published literature taking into consideration results from the original CySIF and CONSTRUCT trials,4,25 a network meta-analyses for advanced therapies in UC,35 societal guidelines and position statements,20,36 and phase 3 trials and post-hoc analyses specifically focusing on impact of therapy on avoidance of hospitalization and/or colectomy.23 Based on the totality of evidence, infliximab was considered first line medical rescue therapy in the inpatient setting and first line biologic therapy in the outpatient setting. In the inpatient setting, patients could have a trial of cyclosporine during the subsequent hospitalization but sequential infliximab-cyclosporine therapy during a single hospitalization was not attempted due to the increased risk for adverse events with this salvage therapy approach.37,38 Accelerated inpatient infliximab dosing was not incorporated due to uncertainties in outcomes and optimal dosing strategies for this approach.39 In the outpatient setting, patients would progress through adalimumab, ustekinumab, tofacitinib and vedolizumab, with attempts at dose optimization for all therapies prior to switching agents.
Model Transition Probabilities and Costs
Probabilities in transitions between disease states and disease outcomes, as well as distributions and costs were obtained from the literature as outlined in Table 1. All transition probabilities were adjusted for the 5-year horizon and 3-month cycle length. Tracker variables were used to modify an individual patients’ life cycle within the microsimulation model, and also to quantify key disease outcomes and projections. These tracker variables follow each of the simulated patients throughout their independent life cycle within the microsimulation model, and they allowed for the ability to modify the inpatient rescue medical or surgical therapy used based on prior exposures (i.e. cyclosporine only after failing infliximab previously in the outpatient or inpatient setting, and rescue in-hospital surgery only after failing both cyclosporine and infliximab) and modify the outpatient biologic or small molecule inhibitor therapy treatment regimen, dosing regimen, and associated costs, based on prior disease flares and treatment response patterns.
Outcome Measures and Analyses
Effectiveness was measured through objective quantification of health outcomes and also through quality-adjusted life-years (QALYs).40 Key health outcomes of interest were rates of re-hospitalization, in-hospital medical rescue therapy, need for emergent colectomy, and death. Our analysis was conducted from a healthcare payer perspective which incorporated all costs and utilities associated with interventions regardless of who incurs them. An annual 3% discount rate was applied for all costs and QALYs, and the incremental cost-effectiveness ratio (ICER), defined as the incremental cost between the two treatment strategies divided by the incremental effectiveness (incremental QALYs), was calculated to determine cost-effectiveness. A willingness-to-pay threshold of $100,000/QALY was used as our benchmark for considering HBOT to be cost-effective.
Validation of our microsimulation model was done against the published literature for expected rates of outcomes over a 5-year horizon.1,41-52 These studies used for validation of our microsimulation model were specifically kept separate from the studies used for model input to guide model estimates, thereby allowing for independent validation between the outcomes observed in the built model and expected outcomes based on published estimates. For the CySIF trial specifically we used the original trial to inform the model input,25 but the post-hoc long-term follow-up studies were used independently to validate our models ability to accurately project longer term outcomes.41
Multiple one-way deterministic sensitivity analyses were performed, with subsequent 2- and 3-way microsimulation sensitivity analyses for parameters identified to influence cost-effectiveness. An exploratory sensitivity analysis was also performed comparing HBOT during the first 5 days of hospitalization to the use of infliximab on day 1 of hospitalization. In this exploratory analysis HBOT and infliximab were given in combination with intravenous steroids. Model inputs for this exploratory sensitivity analysis were obtained from published literature for outpatient and inpatient infliximab use, with multiple deterministic sensitivity analyses performed to assess thresholds of cost-effectiveness. Finally, a probabilistic sensitivity analysis (second order Monte Carlo simulation) was also performed to determine the impact of uncertainty in all model inputs (transition probabilities, costs, and health utilities) using a Monte Carlo microsimulation with 500 samples and 250 trials. Within this probabilistic sensitivity analysis all probabilities, costs, and health utilities are randomly set within the distribution patterns obtained from the primary cohorts used for model input prior to running the microsimulation model of 100,000 individuals in each strategy. This allows for an assessment of how variability in combinations of these estimates may impact observed cost-effectiveness (i.e. the joint effect of parameter uncertainty).53
RESULTS
Effectiveness in Standard of Care Strategy Group and Comparison to Expected Population Estimates
Our standard of care strategy group microsimulation model demonstrated good validity when compared to published literature for expected 5-year outcomes in UC patients hospitalized for an acute flare. The in-hospital colectomy rate in our model for this group was 19% which is in accordance with the literature suggesting rates to be approximately 20% at the population level,52 and anticipated rate of being less than 30% in experienced centers.42 In our model we assumed patients would be increasing more likely to choose colectomy over medical therapy over time. When assuming that patients would not electively choose colectomy prior to attempts at medical therapy, the 5-year outpatient colectomy rate in our model for this group was 37% which is consistent with long-term follow-up data from the CySIF trial and ENEIDA registry where 5-year cumulative rates for colectomy were 35-38%,41,43 and estimates from other cohort studies for UC patients requiring hospitalization for an acute flare.44-47 The 5-year re-hospitalization rate in our model for this group was 53%, which is consistent with expected 5-year cumulative estimates of approximately 50% for re-hospitalization from population based cohort studies,1 and 55% from cohort studies specifically for UC patients requiring hospitalization for an acute flare.48 The mortality rate of 1.8% in our model for this group is in keeping with the ENEIDA registry,43,49 and expected mortality rate in this population.42,50,51
Comparative Effectiveness and Cost-Effectiveness of Hyperbaric Oxygen Therapy to Optimize Response to Intravenous Steroids for Hospitalized UC Patients
The use of HBOT in addition to intravenous steroids during the index hospitalization for an acute UC flare was projected to significantly improve all health outcomes, including need for re-hospitalization, rescue medical therapy, emergent colectomy, and mortality. (Table 2) The HBOT treated UC patient group had higher average costs ($5,600) compared to the group treated with intravenous steroids alone, but they also had higher QALYs (0.13 QALYs), equating to an ICER of $43,000 per additional QALY. This is below the threshold of $100,000/QALY, making HBOT a cost-effective treatment option to optimize response to intravenous steroids in UC patients hospitalized for an acute flare.
Table 2:
Predicted 5-year outcomes and cost-effectiveness when using hyperbaric oxygen therapy to optimize response to intravenous steroids at the first hospitalization for an acute ulcerative colitis flare
| HBOT + IVS (n=100,000) |
IVS Alone (n=100,000) |
Percentage reduction with HBOT + IVS vs IVS alone |
P value for outcomes, ICER for cost- effectiveness |
|
|---|---|---|---|---|
| Re-hospitalization over 5 years (95% Confidence Intervals) | 19,775 (19,232 – 20,406) | 52,509 (45,980 – 59,436) | 62% | p < 0.001 |
| Total number of in-hospital medical rescue cycles; inclusive of index hospitalization (95% Confidence Intervals) | 29,170 (16,417 – 42,208) | 71,734 (18,277 – 119,427) | 59% | p < 0.001 |
| In-hospital Emergent Colectomy (95% Confidence Intervals) | 7,307 (4,139 – 9,728) | 18,789 (4,560 – 33,573) | 61% | p < 0.001 |
| Death over 5 years (95% Confidence Intervals) | 1,263 (360 – 2,074) | 1,833 (554 – 3,031) | 31% | p < 0.001 |
| Cost Differential (95% Confidence Intervals) | $5,600 more with HBOT strategy (−$4,000 to $15,900 incremental cost) | $43,000/QALY (−$47,500 to $107,700 per QALY) | ||
| Quality Effectiveness (95% Confidence Intervals) | 0.13 QALY more with HBOT strategy (0.08 to 0.17 incremental QALY) | |||
IVS: intravenous steroids; HBOT: hyperbaric oxygen therapy; ICER: incremental cost-effectiveness ratio
Results were obtained from second order Monte Carlo simulations (probabilistic sensitivity analyses) and are reported for 100,000 simulated UC patients per treatment strategy
ICER < $100,000 considered cost-effective
95% confidence intervals obtained from probabilistic sensitivity analysis
Sensitivity Analyses
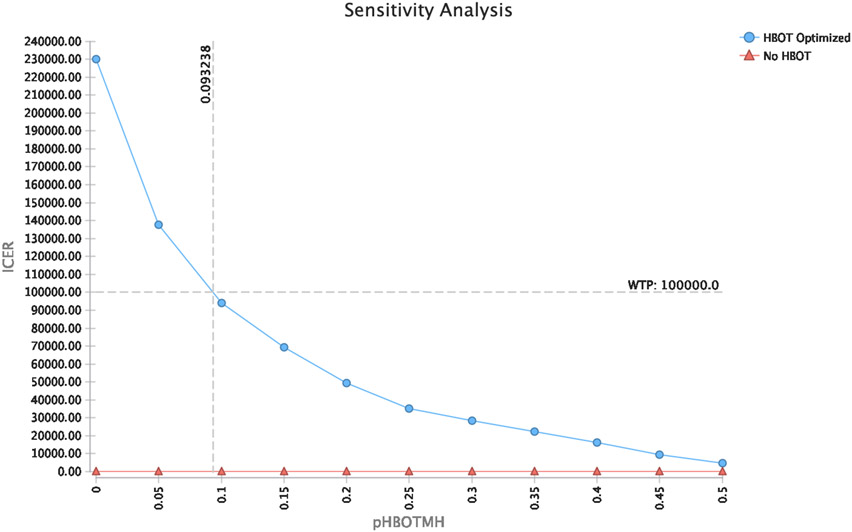
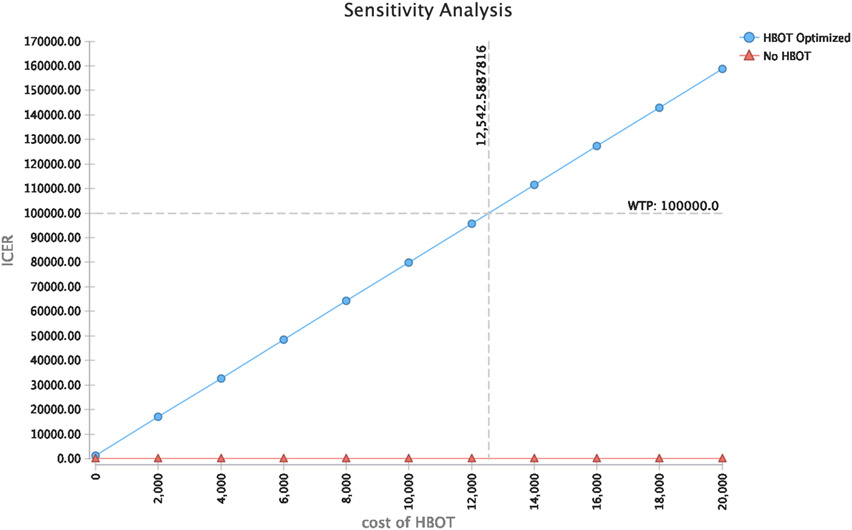
Cost-effectiveness results were sensitive to HBOT treatment costs and probability for achieving endoscopic improvements in disease activity (Mayo endoscopic sub-score 0 or 1) within 3 months of HBOT. (Figures 2 and 3) HBOT was cost-effective if costs of HBOT treatments were less than $12,500 (i.e. less than $2,500 per HBOT session). This is less than the cost of 1,200 mg of infliximab or an accelerated rescue dose of 10 mg/kg for a 70kg adult male after considering infusion and administration costs.54 HBOT was cost-effective if rates of endoscopic improvement at 3 months with HBOT were greater than 10%.
Figure 2: Sensitivity analysis of cost-effectiveness at different rates of endoscopic improvement with hyperbaric oxygen therapy.
Hyperbaric oxygen therapy is cost-effective at a willingness to pay threshold of $100,000 when the rate of achieving endoscopic improvements in disease activity by 3 months with hyperbaric oxygen therapy is over 10%. ICER: Incremental cost-effectiveness ratio; Effect: Quality Adjusted Life Years (QALY); Avg. CE: Average Cost-effectiveness; HBOT: Hyperbaric oxygen therapy; WTP: willingness to pay; pHBOTMH: probability of achieving endoscopic improvements in activity with HBOT (Mayo endoscopic sub-score 0 or 1).
Figure 3: Sensitivity analysis of cost-effectiveness at different costs of hyperbaric oxygen therapy.
Hyperbaric oxygen therapy is cost-effective at a willingness to pay threshold of $100,000 when the cost of 5 hyperbaric oxygen sessions is less than $12,500 (less than $2,500 per hyperbaric session). ICER: Incremental cost-effectiveness ratio; HBOT: Hyperbaric oxygen therapy; WTP: willingness to pay
In our base model, HBOT was used once during the index hospitalization for immunosuppressive naïve UC patients. We performed a sensitivity analysis where HBOT was used once only during the subsequent hospitalization, after patients had already been hospitalized previously for an acute flare and were already begun on outpatient biologic therapy (i.e. biologic exposed UC patients requiring re-hospitalization for an acute flare). In this model, the HBOT treatment strategy had less incremental cost than when it was used in the immunosuppressive naïve base model ($432 versus $5,600) and less incremental benefit (QALY 0.09 versus 0.13), but it was substantially more cost-effective in this scenario of being used for biologic exposed patients who required re-hospitalization for an acute flare as compared to immunosuppressive naïve patients requiring index hospitalization for an acute flare (ICER $5,000/QALY versus $43,000/QALY).
To address potential uncertainty with longer term horizons, a sensitivity analysis was performed varying the time horizon to 1, 2, 3, and 4 years. At 1, 2, and 3 years the HBOT strategy absolutely dominated standard of care (HBOT resulted in lower costs and higher QALY), and at 4 years HBOT was more cost-effective than standard of care ($32,200/QALY).
In an exploratory fashion, we modeled the cost-effectiveness of HBOT in combination with intravenous steroids during the first 5 days of hospitalization versus the up-front use of infliximab on day 1 of hospitalization in combination with intravenous steroids. When assuming sequential cyclosporine rescue therapy would not be used if up-front infliximab failed, the HBOT strategy was more cost-effective than up-front infliximab (ICER $80,700/QALY) due to a significantly lower rate of needing emergent colectomy in the hospital (7,347 versus 34,292, p < 0.001) and lower rate of mortality (1,235 versus 2,466, p < 0.001). If the rate of endoscopic improvement with up-front infliximab is over 35% then the HBOT strategy still results in higher QALYs but is no longer cost-effective, and if the rate of endoscopic improvement with up-front infliximab is over 75% then the up-front infliximab strategy results in higher QALYs and becomes more cost-effective. A 2-way sensitivity analysis for endoscopic improvement rates with HBOT and infliximab was performed and HBOT was more cost-effective than up-front infliximab if endoscopic improvement rates with HBOT were consistently above 35%, irrespective of infliximab endoscopic improvement rates. (Supplementary Figure 1)
Probabilistic Sensitivity Analysis
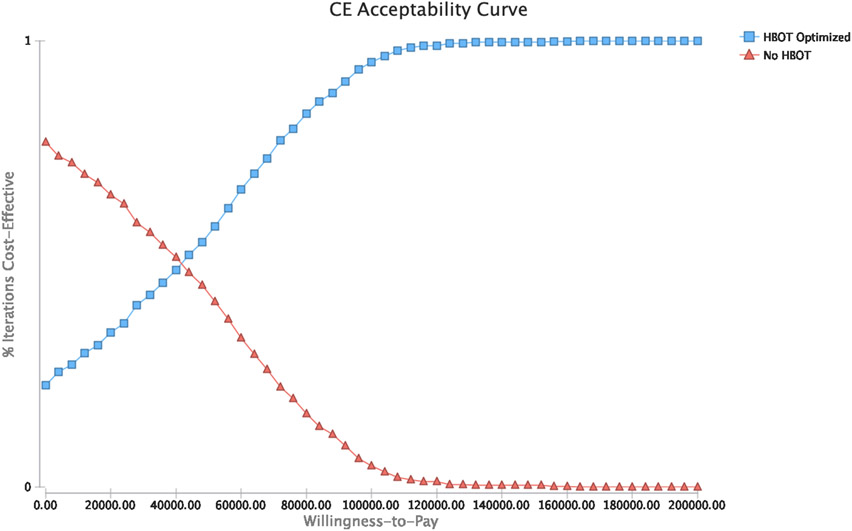
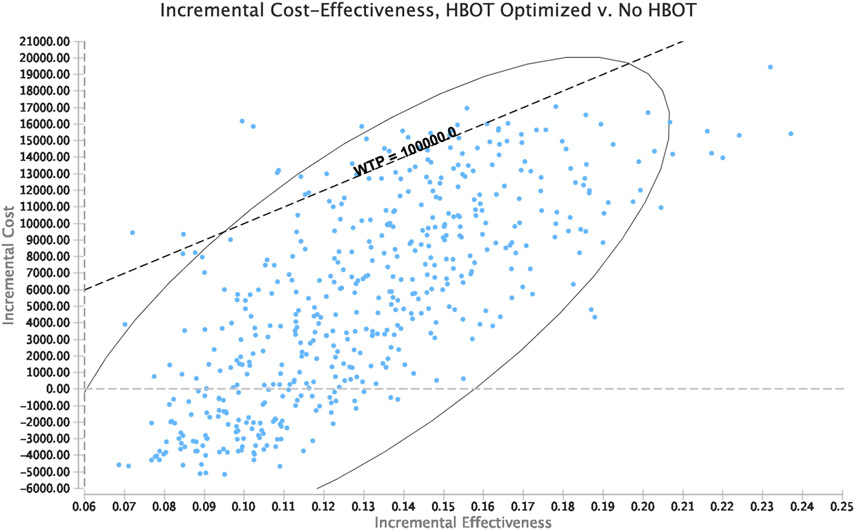
After introducing variability in all modeled probabilities, costs, and utilities, HBOT was observed to be cost-effective for optimizing response to intravenous steroids during the index hospitalization for an acute UC flare in 95% of iterations. (Figure 4)
Figure 4: Probabilistic Sensitivity Analysis.
Panel A: Acceptability curve; Panel B: ICER scatter plot for HBOT versus standard of care
HBOT: hyperbaric oxygen therapy; ICER: incremental cost-effectiveness ration; CE: Cost-effectiveness
DISCUSSION
The need for hospitalization and intravenous steroids for a patient with UC to manage an acute flare is associated with a poor prognosis and significant healthcare costs, morbidity, and mortality. Optimization of treatment response early in the hospitalization has the potential to influence patient well-being and healthcare resource utilization not just in the short- term, but also in the longer term. In this study we modeled the cost-effectiveness and long-term impact of using HBOT to optimize the initial response to intravenous steroids for UC patients requiring hospitalization for an acute flare. The use of HBOT in addition to intravenous steroids during the first 5 days of hospitalization for an acute flare was found to be cost-effective and projected to be associated with significant reductions in long-term risks for re-hospitalization, in-hospital medical rescue therapy, colectomy, and death compared to the use of intravenous steroids alone. These results were consistent across multiple sensitivity analyses and even more striking when HBOT was used for biologic exposed patients requiring hospitalization for acute flares. Finally, the use of HBOT in combination with intravenous steroids during the first 5 days of hospitalization was projected to be more cost-effective than the up-front use of infliximab in combination with intravenous steroids at the time of hospitalization. Taken together, these data further strengthen the support for integrating HBOT into current treatment algorithms for UC patients requiring hospitalization for an acute flare.
The incremental cost-effectiveness ratio for HBOT in combination with intravenous steroids was $43,000 per additional QALY, which is cost-effective at even more conservative thresholds of $50,000/QALY. Recognizing that variability exists in routine practice and some uncertainty may exist in point estimates for model inputs, we performed a probabilistic sensitivity analysis to test the robustness of this observation where we introduced variability in all modeled probabilities, costs, and utilities. This is in contrast to deterministic sensitivity analyses where variability is introduced for only one variable at a time and the model is re-run repeatedly. Within this probabilistic sensitivity analysis, HBOT in combination with intravenous steroids was observed to be cost-effective compared to intravenous steroids alone in 95% of iterations at a willingness-to-pay threshold of $100,000/QALY, thereby confirming the robustness of our observation and cost-effectiveness of HBOT to optimize outcomes for UC patients requiring hospitalization for an acute. During deterministic sensitivity analyses, cost-effectiveness was observed to be sensitive to HBOT costs and the probability of achieving endoscopic improvements in disease activity with HBOT. The threshold cost identified for HBOT was $2,500 per HBOT session which is well beyond what could be anticipated for costs of this therapy in routine practice. The threshold rate of endoscopic improvement identified for HBOT was 10%, below which HBOT was no longer cost-effective. Within the phase 2A and 2B trials, the rates of endoscopic improvement with HBOT were over 30% suggesting this rate is likely achievable within routine practice. It is unclear if this rate of endoscopic improvement remains at 3 months when HBOT maintenance therapy is not used, and therefore further studies are needed to specifically evaluate 3-month endoscopic improvement rates with this strategy.
A notable observation within our microsimulation modeling was that the use of HBOT to optimize response to intravenous steroids was more cost-effective than using infliximab on day 1 of hospitalization for an acute UC flare. This observation was likely driven in part by 3 factors: 1) the incremental cost of infliximab relative to HBOT, 2) the ability to still use infliximab rescue for HBOT non-responders whereas inpatient medical rescue therapy is limited when using infliximab on day 1 of hospitalization, and 3) modeled estimates for response to up-front infliximab on day 1 of hospitalization. Data for the up-front use of infliximab is sparse and therefore wider estimates and uncertainty exists in this modeled comparison which relied heavily on outpatient estimates. If the rate of endoscopic improvement with up-front infliximab was above 35%, then HBOT was no longer cost-effective however it did still yield a higher QALY. In order for the up-front infliximab strategy to become more cost-effective with higher QALY the rate of endoscopic improvement with up-front infliximab would need to be above 75% which is unlikely in this severe population. Post-hoc analyses of the ACT trial have observed that severe disease is a predictor of decreased responsiveness to infliximab, and therefore rates of endoscopic improvement with up-front infliximab in this population of hospitalized UC patients is likely lower than would be expected in the outpatient setting and our model therefore likely accurately captured the anticipated cost-effectiveness of HBOT versus up-front infliximab.
Strengths of our work include the incorporation of multiple cost, disease, treatment, and utility components, and the use of microsimulation modeling which allows for incorporation of patient level simulations that take into consideration individual disease courses to modify downstream probabilities, utilities and outcomes. Confidence in our model can be taken from how closely our modeled standard of care (intravenous steroids alone followed by rescue infliximab, cyclosporine, and/or colectomy for non-response) comparator group resembled outcomes to be expected based on published literature and estimates. Given hospitalizations for acute flares represent a major driver of healthcare resource utilization and morbidity and mortality, our work is of particular importance for payors and population health management strategies. Given long-term comparator trials are difficult to perform, our methodology is of particular interest when considering the potential to compare the impact of other medical interventions on the natural history of disease. This analysis does carry some limitations, however, which are worth noting. The phase 2 HBOT trials were small and therefore imprecision exists in point estimates for modeling. This was overcome by introducing variability and ranges in potential estimates, but further larger trials and follow-up microcosting studies are needed to confirm our cost-effectiveness estimates. Detailed natural history studies for UC patients after their index hospitalization are also lacking in the United States at the population level. We were able to derive estimates for all probabilities from the literature, however, up-to-date population studies are needed to better inform our model particularly considering the progressive increase in UC related hospitalizations being observed.2 Finally, our analysis was from the vantage point of a healthcare payer and further modeling of alternative perspectives, particularly patient perspectives, will be needed.
In conclusion, prior phase 2 trials have demonstrated a short-term benefit for HBOT in the management of UC patients hospitalized for acute flares. In our current analysis we extend on this work through microsimulation modeling and observed that HBOT has the potential to significantly influence the longer-term natural history of the disease, and the use of HBOT to optimize response to intravenous steroids is cost-effective at the population level. A dedicated phase 3 trial is urgently needed to confirm the observations and treatment effects observed in earlier phase 2 trials in order to bring new strategies to this high risk cohort of patients whom have limited medical options.
Supplementary Material
Supplementary Figure 1: Two-way sensitivity analysis for rates of endoscopic improvement with hyperbaric oxygen therapy and infliximab when comparing hyperbaric oxygen therapy in combination with intravenous steroids versus up-front infliximab on day 1 plus steroids of hospitalization.
HBOT: Hyperbaric oxygen therapy; IFX: infliximab; pHBOTMH: probability of achieving endoscopic improvements (Mayo endoscopic sub-score 0 or 1) with HBOT; pIFXMH: probability of achieving endoscopic improvements with infliximab; WTP: willingness-to-pay
Acknowledgments:
Parambir S. Dulai is supported by an American Gastroenterology Association Research Scholar Award.
Footnotes
Disclosures: PSD reports consulting and research grants from Takeda, Janssen, Pfizer, Abbvie, Buhlmann, Polymedco. VJ reports consulting frees from Abbvie, Eli Lilly, GlaxoSmithKline, Arena pharmaceuticals, Genetech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials, Topivert, Celltrion; speakers fees from Takeda, Janssen, Shire, Ferring, Abbvie, Pfizer.
References
- 1.Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural History of Adult Ulcerative Colitis in Population-based Cohorts: A Systematic Review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018;16(3):343–356 e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma C, Smith M, Guizzetti L, et al. Assessing National Trends and Disparities in Ambulatory, Emergency Department, and Inpatient Visits for Inflammatory Bowel Disease in the United States (2005-2016). Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas MG, Bayliss C, Bond S, et al. Trial summary and protocol for a phase II randomised placebo-controlled double-blinded trial of Interleukin 1 blockade in Acute Severe Colitis: the IASO trial. BMJ open. 2019;9(2):e023765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams JG, Alam MF, Alrubaiy L, et al. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. The lancet Gastroenterology & hepatology. 2016;1(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulai PS, Jairath V. Acute severe ulcerative colitis: latest evidence and therapeutic implications. Ther Adv Chronic Dis. 2018;9(2):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(1):103–110. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Al-Darmaki A, Frolkis AD, et al. Postoperative Mortality Among Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis of Population-Based Studies. Gastroenterology. 2015;149(4):928–937. [DOI] [PubMed] [Google Scholar]
- 8.de Silva S, Ma C, Proulx MC, et al. Postoperative complications and mortality following colectomy for ulcerative colitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9(11):972–980. [DOI] [PubMed] [Google Scholar]
- 9.Narula N, Marshall JK, Colombel JF, et al. Systematic Review and Meta-Analysis: Infliximab or Cyclosporine as Rescue Therapy in Patients With Severe Ulcerative Colitis Refractory to Steroids. Am J Gastroenterol. 2016;111(4):477–491. [DOI] [PubMed] [Google Scholar]
- 10.Vedamurthy A, Xu L, Luther J, et al. Long-Term Outcomes of Immunosuppression-Naïve Steroid Responders Following Hospitalization for Ulcerative Colitis. Dig Dis Sci. 2018;63(10):2740–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dulai PS, Gleeson MW, Taylor D, Holubar SD, Buckey JC, Siegel CA. Systematic review: The safety and efficacy of hyperbaric oxygen therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39(11):1266–1275. [DOI] [PubMed] [Google Scholar]
- 12.Dulai PS, Buckey JC Jr., Raffals LE, et al. Hyperbaric oxygen therapy is well tolerated and effective for ulcerative colitis patients hospitalized for moderate-severe flares: a phase 2A pilot multi-center, randomized, double-blind, sham-controlled trial. Am J Gastroenterol. 2018;113(10):1516–1523. [DOI] [PubMed] [Google Scholar]
- 13.Dulai PSea. A phase 2B randomized trial of hyperbaric oxygen therapy for ulcerative colitis patients hospitalized for moderate to severe flares. Alimentary pharmacology & therapeutics. 2020. [DOI] [PubMed] [Google Scholar]
- 14.Borren NZ, Khalili H, Luther J, Colizzo FP, Garber JJ, Ananthakrishnan AN. Second-Look Endoscopy in Hospitalized Severe Ulcerative Colitis: A Retrospective Cohort Study. Inflammatory bowel diseases. 2019;25(4):750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HS, Yang SK, Soh JS, et al. Short- and Long-Term Outcomes of Acute Severe Ulcerative Colitis in Korea: The 1999-2005 Cohort. Inflammatory bowel diseases. 2015;21(8):1825–1831. [DOI] [PubMed] [Google Scholar]
- 16.Jain S, Kedia S, Sethi T, et al. Predictors of long-term outcomes in patients with acute severe colitis: A northern Indian cohort study. J Gastroenterol Hepatol. 2018;33(3):615–622. [DOI] [PubMed] [Google Scholar]
- 17.Molnár T, Farkas K, Nyári T, Szepes Z, Nagy F, Wittmann T. Response to first intravenous steroid therapy determines the subsequent risk of colectomy in ulcerative colitis patients. J Gastrointestin Liver Dis. 2011;20(4):359–363. [PubMed] [Google Scholar]
- 18.Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology. 2020;158(5):1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114(3):384–413. [DOI] [PubMed] [Google Scholar]
- 20.Bitton A, Buie D, Enns R, et al. Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statements. Am J Gastroenterol. 2012;107(2):179–194; author reply 195. [DOI] [PubMed] [Google Scholar]
- 21.Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996;38(6):905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dulai PS, Sandborn WJ, Murphy J. Microsimulation Model to Determine the Cost Effectiveness of Treat to Target Strategies for Ulcerative Colitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2020. [DOI] [PubMed] [Google Scholar]
- 23.Mao EJ, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, Ananthakrishnan AN. Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn's disease and ulcerative colitis. Alimentary pharmacology & therapeutics. 2017;45(1):3–13. [DOI] [PubMed] [Google Scholar]
- 24.Perera S Y S, Stott-Miller M, Brady J. . Analysis of Healthcare Resource Utilization and Costs after the Initiation of Biologic Treatment in Patients with Ulcerative Colitis and Crohn’s Disease. . JHEOR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380(9857):1909–1915. [DOI] [PubMed] [Google Scholar]
- 26.Peyrin-Biroulet L, Germain A, Patel AS, Lindsay JO. Systematic review: outcomes and post-operative complications following colectomy for ulcerative colitis. Alimentary pharmacology & therapeutics. 2016;44(8):807–816. [DOI] [PubMed] [Google Scholar]
- 27.Lindsay JO, Bergman A, Patel AS, Alesso SM, Peyrin-Biroulet L. Systematic review: the financial burden of surgical complications in patients with ulcerative colitis. Alimentary pharmacology & therapeutics. 2015;41(11):1066–1078. [DOI] [PubMed] [Google Scholar]
- 28.Archer R, Tappenden P, Ren S, et al. Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262): clinical effectiveness systematic review and economic model. Health technology assessment (Winchester, England). 2016;20(39):1–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stawowczyk E, Kawalec P. A Systematic Review of the Cost-Effectiveness of Biologics for Ulcerative Colitis. PharmacoEconomics. 2018;36(4):419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coward S, Heitman SJ, Clement F, et al. Ulcerative colitis-associated hospitalization costs: a population-based study. Canadian journal of gastroenterology & hepatology. 2015;29(7):357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokomizo L, Limketkai B, Park KT. Cost-effectiveness of adalimumab, infliximab or vedolizumab as first-line biological therapy in moderate-to-severe ulcerative colitis. BMJ open gastroenterology. 2016;3(1):e000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson M, Lucas A, Cameron A, Luo M. Budget Impact of Adding Vedolizumab to a Health Plan Formulary as Another First-Line Biologic Option for Ulcerative Colitis and Crohn's Disease. American health & drug benefits. 2018;11(5):253–262. [PMC free article] [PubMed] [Google Scholar]
- 33.Milev S, DiBonaventura MD, Quon P, et al. An economic evaluation of tofacitinib for the treatment of moderately-to-severely active ulcerative colitis: modeling the cost of treatment strategies in the United States. Journal of medical economics. 2019;22(9):859–868. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhary MA, Fan T. Cost-Effectiveness of Infliximab for the Treatment of Acute Exacerbations of Ulcerative Colitis in the Netherlands. Biol Ther. 2013;3(1):45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. First- and Second-line Pharmacotherapies for Patients with Moderate to Severely Active Ulcerative Colitis: An Updated Network Meta-Analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019. [DOI] [PubMed] [Google Scholar]
- 37.Maser EA, Deconda D, Lichtiger S, Ullman T, Present DH, Kornbluth A. Cyclosporine and infliximab as rescue therapy for each other in patients with steroid-refractory ulcerative colitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2008;6(10):1112–1116. [DOI] [PubMed] [Google Scholar]
- 38.Narula N, Fine M, Colombel JF, Marshall JK, Reinisch W. Systematic Review: Sequential Rescue Therapy in Severe Ulcerative Colitis: Do the Benefits Outweigh the Risks? Inflammatory bowel diseases. 2015;21(7):1683–1694. [DOI] [PubMed] [Google Scholar]
- 39.Nalagatla N, Falloon K, Tran G, et al. Effect of Accelerated Infliximab Induction on Short- and Long-term Outcomes of Acute Severe Ulcerative Colitis: A Retrospective Multicenter Study and Meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2019;17(3):502–509.e501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. Jama. 2016;316(10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 41.Laharie D, Bourreille A, Branche J, et al. Long-term outcome of patients with steroid-refractory acute severe UC treated with ciclosporin or infliximab. Gut. 2018;67(2):237–243. [DOI] [PubMed] [Google Scholar]
- 42.Chen JH, Andrews JM, Kariyawasam V, et al. Review article: acute severe ulcerative colitis - evidence-based consensus statements. Alimentary pharmacology & therapeutics. 2016;44(2):127–144. [DOI] [PubMed] [Google Scholar]
- 43.Ordás I, Domènech E, Mañosa M, et al. Long-Term Efficacy and Safety of Cyclosporine in a Cohort of Steroid-Refractory Acute Severe Ulcerative Colitis Patients from the ENEIDA Registry (1989-2013): A Nationwide Multicenter Study. Am J Gastroenterol. 2017;112(11):1709–1718. [DOI] [PubMed] [Google Scholar]
- 44.Mocciaro F, Renna S, Orlando A, et al. Cyclosporine or infliximab as rescue therapy in severe refractory ulcerative colitis: early and long-term data from a retrospective observational study. Journal of Crohn's & colitis. 2012;6(6):681–686. [DOI] [PubMed] [Google Scholar]
- 45.Thorne K, Alrubaiy L, Akbari A, Samuel DG, Morrison-Rees S, Roberts SE. Colectomy rates in patients with ulcerative colitis following treatment with infliximab or ciclosporin: a systematic literature review. Eur J Gastroenterol Hepatol. 2016;28(4):369–382. [DOI] [PubMed] [Google Scholar]
- 46.Naves JE, Llaó J, Ruiz-Cerulla A, et al. Long-term comparative efficacy of cyclosporine- or infliximab-based strategies for the management of steroid-refractory ulcerative colitis attacks. Inflammatory bowel diseases. 2014;20(8):1375–1381. [DOI] [PubMed] [Google Scholar]
- 47.Szemes K, Soós A, Hegyi P, et al. Comparable Long-Term Outcomes of Cyclosporine and Infliximab in Patients With Steroid-Refractory Acute Severe Ulcerative Colitis: A Meta-Analysis. Front Med (Lausanne). 2019;6:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Golovics PA, Lakatos L, Mandel MD, et al. Does Hospitalization Predict the Disease Course in Ulcerative Colitis? Prevalence and Predictors of Hospitalization and Re-Hospitalization in Ulcerative Colitis in a Population-based Inception Cohort (2000-2012). J Gastrointestin Liver Dis. 2015;24(3):287–292. [DOI] [PubMed] [Google Scholar]
- 49.Ordás I, Domènech E, Mañosa M, et al. Post-operative morbidity and mortality of a cohort of steroid refractory acute severe ulcerative colitis: Nationwide multicenter study of the GETECCU ENEIDA Registry. Am J Gastroenterol. 2018;113(7):1009–1016. [DOI] [PubMed] [Google Scholar]
- 50.Ventham NT, Kennedy NA, Duffy A, et al. Comparison of mortality following hospitalisation for ulcerative colitis in Scotland between 1998-2000 and 2007-2009. Alimentary pharmacology & therapeutics. 2014;39(12):1387–1397. [DOI] [PubMed] [Google Scholar]
- 51.Opstelten JL, Vaartjes I, Bots ML, Oldenburg B. Mortality After First Hospital Admission for Inflammatory Bowel Disease: A Nationwide Registry Linkage Study. Inflammatory bowel diseases. 2019;25(10):1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allison J, Herrinton LJ, Liu L, Yu J, Lowder J. Natural history of severe ulcerative colitis in a community-based health plan. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2008;6(9):999–1003. [DOI] [PubMed] [Google Scholar]
- 53.Briggs AH, Goeree R, Blackhouse G, O'Brien BJ. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Making. 2002;22(4):290–308. [DOI] [PubMed] [Google Scholar]
- 54.Ollendorf DA, Lidsky L. Infliximab drug and infusion costs among patients with Crohn's disease in a commercially-insured setting. Am J Ther. 2006;13(6):502–506. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen NH, Koola J, Dulai PS, Prokop LJ, Sandborn WJ, Singh S. Rate of Risk Factors for and Interventions to Reduce Hospital Readmission in Patients With Inflammatory Bowel Diseases. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Two-way sensitivity analysis for rates of endoscopic improvement with hyperbaric oxygen therapy and infliximab when comparing hyperbaric oxygen therapy in combination with intravenous steroids versus up-front infliximab on day 1 plus steroids of hospitalization.
HBOT: Hyperbaric oxygen therapy; IFX: infliximab; pHBOTMH: probability of achieving endoscopic improvements (Mayo endoscopic sub-score 0 or 1) with HBOT; pIFXMH: probability of achieving endoscopic improvements with infliximab; WTP: willingness-to-pay