KEY POINTS
Spontaneous coronary artery dissection (SCAD) is an increasingly recognized cause of myocardial infarction (MI) representing 35% of acute coronary syndromes in women aged 50 years or younger and is the most frequent cause of MI in peripartum patients.
The diagnosis is made at coronary angiography through the recognition of angiographic patterns of dissection, and coronary artery tortuosity is often present.
Most patients can be treated conservatively (without stent implantation) and vessel healing has been shown angiographically in most patients within 4–8 weeks after admission to hospital.
Many patients continue to have chest pain syndromes after the initial SCAD event.
Evidence-based treatment guidance is limited; however, single antiplatelet therapy, β blockade, avoidance of high-intensity exercise and vascular screening for fibromuscular dysplasia and other associated vascular abnormalities are generally accepted approaches.
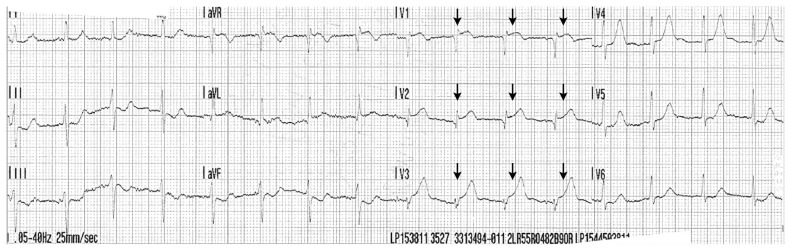
A 50-year-old woman had sudden-onset retrosternal chest pain. Paramedics were called, and her electrocardiogram (ECG) showed ST-elevation in the anterior precordial leads with associated Q-waves compatible with acute myocardial infarction (MI) (Figure 1). Forty minutes after the onset of chest pain, she suffered a witnessed ventricular fibrillation cardiac arrest. She received 1 minute of chest compressions and was defibrillated once, with return of spontaneous circulation. She was transferred to the nearest hospital with percutaneous coronary intervention (PCI) capability for urgent coronary angiography.
Figure 1:
A 50-year-old woman presented with ST elevation myocardial infarction (MI) secondary to spontaneous coronary artery dissection. Electrocardiogram from initial presentation showing ST elevation in the anterior precordial leads with associated Q-waves compatible with acute MI (arrows).
The patient’s medical history included hypertension, smoking, chronic obstructive pulmonary disease, hysterectomy, depression and occasional pulsatile tinnitus. She did not have a history of dyslipidemia or diabetes.
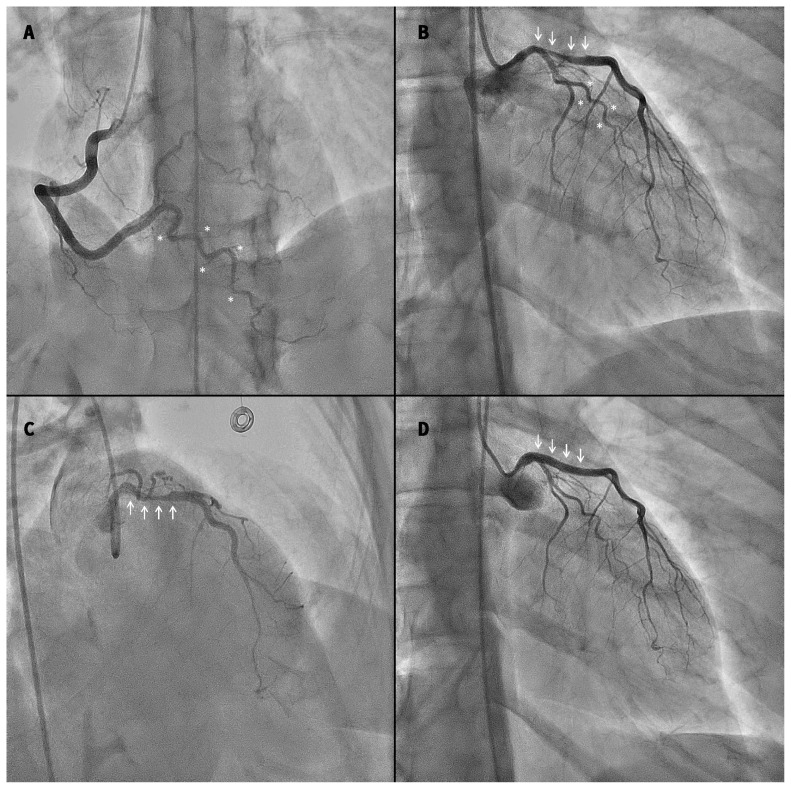
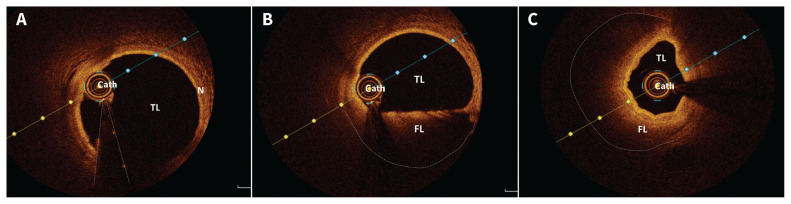
Upon arrival at hospital, the patient had ongoing mild chest pain, with persistent infarct pattern on ECG. Her blood pressure was 113/75 mm Hg, and heart rate was 112 beats/min. Coronary angiography showed smooth-appearing coronary arteries with tortuosity noted in all vessels (Figure 2A). An unusual reduction in vessel calibre was noted in the distal left main artery and proximal left anterior descending artery (LAD) over a 20- to 25-mm segment (Figure 2B and 2C). The interventional cardiologist was concerned about the possibility of spontaneous coronary artery dissection (SCAD) rather than traditional atherosclerosis with plaque rupture. Optical coherence tomography (OCT) confirmed SCAD (Figure 3). These images showed intramural hematoma with both true and false lumens starting from the distal left main artery and extending about 20 mm into the proximal LAD, causing 50%–80% narrowing. Because brisk flow was noted in the coronary artery, and the patient was minimally symptomatic and hemodynamically stable, she was managed conservatively without PCI. Echocardiography showed a left ventricular ejection fraction (LVEF) of 35%–40% with apical akinesis, and no left ventricular thrombus. An exercise stress test was negative for ischemia. The patient’s discharge medications were acetylsalicylic acid (ASA), metoprolol, perindopril and inhalers.
Figure 2:
(A) Coronary angiography at initial presentation showing the dominant right coronary artery in the anterior–posterior cranial projection, revealing no luminal irregularities. Tortuosity of the posterior interventricular branch is noted (asterisks). (B) Coronary angiography of the left coronary artery in the right anterior oblique (RAO)–caudal projection at initial presentation. Arrows indicate luminal narrowing of the distal left main artery extending 20 mm into the proximal left anterior descending (LAD) artery. Asterisks indicate tortuosity of the left circumflex artery. (C) Coronary angiography of the left coronary artery in the RAO–cranial projection at initial presentation. Arrows indicate luminal narrowing of the distal left main and proximal LAD coronary arteries. (D) Repeat coronary angiography of the left coronary artery in the RAO–caudal projection, performed 2 weeks after initial presentation for recurrent chest pain. Arrows show angiographic resolution of the left main and proximal LAD artery dissection.
Figure 3:
Optical coherence tomography (OCT) images of (A) the normal distal left anterior descending (LAD) artery, revealing the true lumen (TL), normal coronary artery wall (N) and OCT imaging catheter (cath.); (B) the dissected proximal LAD artery with both TL and false lumen (FL) and the OCT imaging catheter (cath.) — notably, the FL contains the intramural hematoma; and (C) the dissected proximal LAD artery, showing both the TL and the FL that is causing 50%–80% narrowing of the TL by extrinsic compression over this segment.
The patient returned to hospital 2 weeks later with recurrent intermittent chest pain lasting 5–30 minutes. Her ECG showed precordial T-wave inversions similar to her predischarge ECG, and serum troponin levels were normal. Because of the critical location of the original SCAD, repeat angiography was performed. This examination showed normal coronary arteries, with complete angiographic resolution of the previously noted intramural hematoma and luminal compression of the left main artery and proximal LAD (Figure 2D), and improvement of LVEF to more than 50%.
Over the ensuing months, the patient continued to have intermittent episodes of chest pain and was seen in a regional SCAD clinic. Vascular screening was undertaken with 2 computed tomography angiograms of the head and neck, and the chest, abdomen and pelvis. She received a diagnosis of fibromuscular dysplasia (FMD) of both internal carotid arteries and coronary arteries. She was admitted to hospital again 6 and 8 months after the original presentation for recurrent chest pain, without ECG or cardiac marker evidence of infarction. Echocardiography performed 6 months post-MI showed normal left ventricular size and function (LVEF 62%). Coronary angiography at 8 months showed normal coronary arteries.
Discussion
Spontaneous coronary artery dissection is increasingly recognized as a nontraumatic, noniatrogenic, nonatherosclerotic cause of acute coronary syndromes (ACS). It represents about 4% of all ACS and 35% of ACS in women aged 50 years or younger.1,2 Spontaneous coronary artery dissection results from the development of a spontaneous intimal tear or bleed resulting in a hematoma within the tunica media, causing separation of the intima from the underlying vessel.1 The false lumen with intramural hematoma compresses the true lumen, resulting in ischemia.
Table 1 outlines the key differences in the demographics and management of SCAD compared with ACS from atherosclerosis. Spontaneous coronary artery dissection most commonly affects middle-aged women, with only 10%–15% of cases occurring in men.1,3 Patients with SCAD have lower rates of traditional cardiovascular risk factors.4 Risk factors for SCAD include acute emotional and physical stressors, pregnancy, connective tissue disorders (rarely) and FMD. Further research is required to understand its genetic determinants, as very few familial (F11R, TLN1) and sporadic (TSR1, PHACTR1, EDN1) gene mutations associated with SCAD have been identified.1
Table 1:
Key differences in the demographics, associated conditions and management of spontaneous coronary artery dissection compared with atherosclerotic acute coronary syndrome
| Characteristic | SCAD | Atherosclerotic ACS |
|---|---|---|
| Demographics | ||
| Sex | More frequent in women | More frequent in men |
| Hypertension | Less common | Common |
| Dyslipidemia | Less common | Common |
| Smoking | Less common | Common |
| Pregnancy | Common | Uncommon |
| Emotional or physical stress | Common | Uncommon |
| Migraines | Common | Uncommon |
| FMD | Common | Uncommon |
| Revascularization | ||
| PCI (balloon angioplasty with or without stenting) |
|
Recommended |
| Coronary artery bypass graft | When PCI is not possible | When PCI is not possible |
| Medical therapy | ||
| Antiplatelet agents |
|
Dual antiplatelet therapy for 1 year |
| β-blockers | Recommended | Recommended |
| ACE inhibitors | No clear benefit | Recommended |
| ARBs | No clear benefit | Recommended |
| Statins | No clear benefit | Recommended |
| Lifestyle | ||
| Diet | No restrictions | Healthy heart diet |
| Physical activity | Moderate intensity exercise | Exercise intensity as tolerated |
| Stress management | Recommended | Recommended |
| Smoking cessation | Recommended | Recommended |
| Additional screening | ||
| FMD screening | Computed tomography angiogram from head to pelvis recommended | Not required |
Note: ACE = angiotensin-converting-enzyme, ACS = acute coronary syndrome, ARB = angiotensin II receptor blocker, FMD = fibromuscular dysplasia, PCI = percutaneous coronary intervention, SCAD = spontaneous coronary artery dissection.
Fibromuscular dysplasia is an arteriopathy that results in stenosis, aneurysms, dissection and arterial tortuosity. It most frequently involves the renal, carotid, vertebral and coronary arteries.1 Fibromuscular dysplasia has been identified in more than 60% of patients with SCAD.1,3 A recent review suggested that all patients undergo computed tomography angiography from head to pelvis, to screen for multivessel involvement.1
Most patients with SCAD present with chest pain. Some patients, like ours, have more severe presentations, such as ventricular arrhythmias (4%–14%), cardiogenic shock (2%) or sudden cardiac death.3 Serial cardiac biomarkers are typically elevated and ECG findings may be consistent with ST-elevation MI (25%–50%) or non–ST-elevation MI.3
Coronary angiography is used to diagnose SCAD. Angiographically, SCAD lesions have been described using the Yip–Saw classification.5 Spontaneous coronary artery dissection predominantly involves mid-to-distal coronary arteries, with the LAD artery most frequently affected (40%–70%) and the left main artery involved in 2% of cases.5 Single vessel involvement is most usual; however, multisegment involvement occurs in 25% of cases.5
Angiographically, SCAD can be mistaken for atherosclerotic coronary artery disease. In cases of diagnostic uncertainty, the use of intracoronary imaging such as OCT or intravascular ultrasonography can be used. As in our patient, the diagnosis of SCAD could be missed without OCT; therefore, maintaining a high clinical suspicion is important in patients with few traditional cardiac risk factors.
Acutely, the goal of management is to restore or preserve myocardial perfusion. Similar to our patient, spontaneous healing occurs in 95% of cases by 30 days after a SCAD event; however, 2%–8% of cases do not resolve with conservative therapy.6 In severe cases, revascularization may be considered if there is left main or severe proximal vessel dissection accompanied by ongoing ischemia or hemodynamic instability. Options for revascularization include PCI or coronary artery bypass graft (CABG). Previous retrospective reviews show that only 30% of PCI procedures have successful and durable results.1 Percutaneous coronary intervention in SCAD is associated with an increased risk of complications, including iatrogenic dissection, hematoma propagation, residual stenosis, repeat target-lesion revascularization due to stent thrombosis, in-stent restenosis and stent malapposition.7,8 In select cases, CABG may be considered if substantial myocardial territory is at risk and the lesion is not amenable to PCI. Patients who undergo CABG have increased early mortality rates (5%) owing to severe clinical presentations and more than two-thirds of grafts occlude in the long term because of competitive flow, as the SCAD lesion heals.9 Despite the risk of graft failure, CABG may be lifesaving for some patients.
No randomized controlled trial evidence exists to guide medical therapy for patients with SCAD. In patients who have undergone PCI, dual antiplatelet therapy is recommended for 1 year and guideline-directed medical therapy is recommended if left ventricular dysfunction or heart failure is present.1 In the absence of these clear indications, medical therapy remains controversial. In patients who have not undergone PCI, consensus on the use of single antiplatelet therapy with ASA versus dual antiplatelet therapy is lacking. Currently, many centres prefer single antiplatelet therapy. A single retrospective review reported lower rates of recurrent SCAD with the use of β-blockers and antihypertensive therapy to maintain normal blood pressure.5 Further research is required to guide medical therapy.
Rates of SCAD recurrence have been reported to range from 10% to 30% of patients within 3 years of their initial presentation, with an increased risk associated with severe coronary tortuosity, FMD, migraine headaches, hypertension and pregnancy.1 About 20% of patients have recurrence with pregnancy, with more than 70% of cases of recurrence occurring within the first week post-partum.1 As a result, patients are advised to avoid pregnancy after SCAD. Although SCAD is the most common cause of peripartum MI, SCAD has been noted across the age spectrum and among patients who are also nulliparous and postmenopausal; therefore, the relationship of SCAD and sex hormones is not certain.
Symptoms of SCAD have been associated with strenuous physical activity in 32% of patients, but the benefit of individualized, regular, moderate exercise likely outweighs the risks of recurrent SCAD with exercise. Patients should, however, avoid lifting heavy objects, extreme endurance training, elite competitive sports or activities that require prolonged straining.1
As highlighted by this case, recurrent chest pain, without ischemia, is common in patients with SCAD, particularly in the first year after SCAD. This may be related to SCAD vessel healing, with endothelial dysfunction or vasospasm and, less often, because of SCAD recurrence. Anxiety regarding recurrent SCAD is also a common symptom that may contribute to perceptions of chest discomfort. Anti-anginal therapy can be tried and, for most patients, symptoms will improve over time. We suggest an initial noninvasive approach with troponin, ECG and treadmill testing in stable patients; however, in cases of high clinical suspicion, repeat coronary angiography may be required to rule out recurrent SCAD.1
A retrospective review identified that 30%–40% of patients have symptoms of posttraumatic stress disorder (PTSD), anxiety and depression after their SCAD diagnosis.10 In these patients, anxiety diminished over time, but PTSD and depression were time independent. As a result, comprehensive long-term management, including cardiac rehabilitation, is important for patients after SCAD.
Conclusion
This case highlights SCAD as an important cause of ACS among younger patients. In cases of diagnostic uncertainty, the use of intracoronary imaging should be considered. Early recognition is essential as it has substantial implications for pharmacologic management and revascularization strategies. Conservative management is the mainstay of therapy, and most patients have a favourable prognosis. Systematic screening for FMD and other vascular abnormalities is suggested. The long-term management of patients with SCAD should include lifestyle modifications, maintenance of normal blood pressure, cardiac rehabilitation, contraception counselling and attention to mental health challenges encountered by many of these patients.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Footnotes
Competing interests: Mina Madan is supported by the University of Toronto Heart & Stroke Foundation Polo Chair in Cardiology. No other competing interests were declared.
This article has been peer reviewed.
For a first-person account of this condition, see www.cmaj.ca/lookup/doi/10.1503/cmaj.220516
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
References
- 1.Hayes SN, Tweet MS, Adlam D, et al. Spontaneous coronary artery dissection: JACC state-of-the-art review. J Am Coll Cardiol 2020;76:961–84. [DOI] [PubMed] [Google Scholar]
- 2.Nishiguchi T, Tanaka A, Ozaki Y, et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2016;5:263–70. [DOI] [PubMed] [Google Scholar]
- 3.Saw J, Starovoytov A, Humphries K, et al. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J 2019;40:1188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adlam D, García-Guimaraes M, Maas AHEM. Spontaneous coronary artery dissection: no longer a rare disease. Eur Heart J 2019;40:1198–201. [DOI] [PubMed] [Google Scholar]
- 5.Saw J, Humphries K, Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017;70:1148–58. [DOI] [PubMed] [Google Scholar]
- 6.Hassan S, Prakash R, Starovoytov A, et al. Natural history of spontaneous coronary artery dissection with spontaneous angiographic healing. JACC Cardiovasc Interv 2019;12:518–27. [DOI] [PubMed] [Google Scholar]
- 7.Prakash R, Starovoytov A, Heydari M, et al. Catheter-induced iatrogenic coronary artery dissection in patients with spontaneous coronary artery dissection. JACC Cardiovasc Interv 2016;9:1851–3. [DOI] [PubMed] [Google Scholar]
- 8.Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014;7:645–55. [DOI] [PubMed] [Google Scholar]
- 9.Tweet MS, Eleid MF, Best PJM, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014;7:777–86. [DOI] [PubMed] [Google Scholar]
- 10.Johnson AK, Hayes SN, Sawchuk C, et al. Analysis of posttraumatic stress disorder, depression, anxiety, and resiliency within the unique population of spontaneous coronary artery dissection survivors. J Am Heart Assoc 2020;9:e014372. [DOI] [PMC free article] [PubMed] [Google Scholar]