Dear editor,
A 75-year-old woman with past medical history significant for hyperlipidemia, glaucoma, and breast cancer status-post lumpectomy and radiation in the distant past presented with 72 h of declining mental status. Her symptoms were notable for progressively worsening hypersomnia that resulted in her husband needing to wake her after 14 h of sleep on the day of presentation. Additionally, the patient displayed apraxia with inability to dress herself and had an episode of fecal incontinence.
At baseline, the patient was reported to be a high-functioning individual, who was able to accomplish her activities of daily living independently. She resided in Martha’s Vineyard with her husband and had traveled to Boston 1 day prior to symptom onset. On presentation, the patient was afebrile and hemodynamically stable. Her physical exam, including an extensive neurological exam, revealed that she was oriented to self, but not to place or time. She also exhibited short-term memory loss evidenced by her inability to recall information provided to her just minutes prior. The rest of the exam was unremarkable.
Basic laboratory parameters on presentation were non-revealing, apart from a mild hyponatremia with a sodium of 131. Cerebrospinal fluid (CSF) evaluation revealed lymphocytic pleocytosis and normal levels of protein and glucose (Table 1).
Table 1.
Results of CSF analysis
| CSF | Tube 1 | Tube 3 | Reference range |
|---|---|---|---|
| Color | Colorless | Colorless | Coloreless |
| Character | Clear | Clear | Clear |
| Xanthochromia | Absent | Absent | Absent |
| WBC | 41 | 69 | 0–5 10*3/uL |
| Differential | 6% PMN, 84% LY, 10% MONO | 3% PMN, 88% LY, 9% MONO | |
| RBC | 8 | 3 | 0–5 cells/CCM |
| Protein | 49 | 12–60 mg/dL | |
| Glucose | 60 | 40–70 mg/dL |
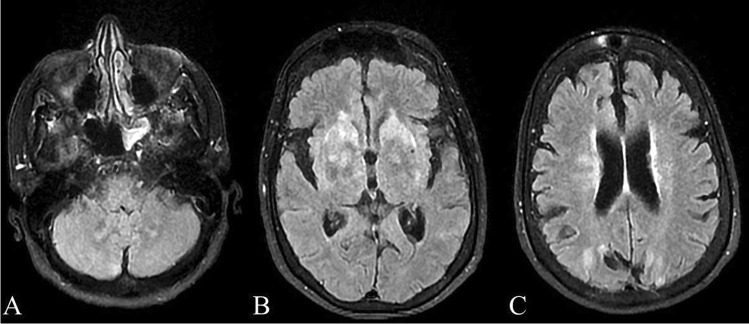
Imaging studies at presentation, including chest X-ray, head CT, and brain MRI, did not reveal any significant findings. However, a follow up MRI 1 week later revealed increased T2 signal present within the basal ganglia including the caudate nuclei, globus pallidus, and putamen bilaterally, as well as subcortical T2 hyperintensity in the bilateral posterior parietal lobes and in the cerebellum (Fig. 1).
Fig. 1.
Findings on MRI, T2 FLAIR sequence, 1 week after presentation. Increased signal can be appreciated bilaterally in the cerebellum (A), basal ganglia (B), including the caudate nuclei, globus pallidus, and putamen, and posterior parietal lobes (C)
A wide range of microbiology and immunology investigations were carried out (Table 2), including titers for VZV, babesiosis, tularemia, CSF VDRL, eastern equine encephalitis virus, West Nile virus, enterovirus, and CSF cryptococcal antigen, but all were negative. Serum HSV 1&2 IgG were positive, but both serum IgM and CSF PCR were negative. A tick-borne disease panel was notable for past infection (IgG only) with Borrelia burgdorferi and Anaplasma spp. EBV antibody titers were positive, but serum EBV PCR was negative. EBV convalescent titers obtained 21 days later showed a change in titers but given that the IgM titer did not change by a fourfold and the PCR was negative, it was unlikely that this represented an acute EBV infection. Finally, an autoimmune encephalitis panel did not reveal any findings.
Table 2.
Microbiology and immunology tests
| Test | Admission | Convalescent | Reference range |
|---|---|---|---|
| EBV VCA IgG | 340 | > 750 | < 22.00 U/mL |
| EBV VCA IgM | 82.7 | 68.7 | < 44.00 U/mL |
| EBV-Na Ab | 289 | > 600 | < 22.00 U/mL |
| EBV DNA PCR, serum | < 200 copies/ml | < 200 copies/ml | |
| Monospot | Negative | ||
| VZV IgM | < 0.9 | < 0.9 | |
| VZV IgG | > 4000 | > 165 index | |
| HSV 1 IgG | 24.70 High | < 0.90 index | |
| HSV 1 IgM | Negative | ||
| HSV1 CSF PCR | Negative | ||
| HSV 2 IgG | 7.07 High | < 0.90 index | |
| HSV 2 IgM | Negative | ||
| HSV2 CSF PCR | Negative | ||
| CMV IgM | < 30.00 | < 30.00 AU/mL | |
| CMV IgG | < 0.60 | < 0.60 U/mL | |
| CMV DNA, real time PCR | < 200 copies/ml | < 200 IU/mL | |
| CMV DNA, QN PCR | < 2.30 | < 2.30 log IU/mL | |
| HIV Ag/Ab, 4th generation | Non reactive | ||
| VDRL CSF | Negative | ||
| Cryptococcus antigen, CSF | Negative | ||
| Enterovirus CSF PCR | Negative | ||
| R. rickettsii IgM | Not detected | ||
| R. rickettsii IgG | Not detected | ||
| RSMF IgM | Not detected | ||
| RSMF IgG | Not detected | ||
| E. chaffeensis IgG | < 1:64 | < 1:64 | |
| E. chaffeensis IgM | < 1:20 | < 1:20 | |
| E. chaffeensis DNA | Not detected | ||
| A. phagocytophilum IgG | 1:64 (High) | < 1:64 | |
| A. phagocytophilum IgM | < 1:20 | < 1:20 | |
| A. phagocytophilium DNA | Not detected | ||
| B. microti DNA | Not detected | ||
| B. miyamotoi DNA | Not detected | ||
| Lyme by PCR, whole Blood | Not detected | ||
| Lyme disease Abs IgG | Positive (7/10 antibodies reactive) | ≤ 4/10 reactive against borrelial proteins | |
| Lyme disease Abs IgM | Negative (1/3 antibodies reactive) | ≤ 1/3 reactive against borrelial proteins | |
| F. tularencis Ab | < 1:20 | < 1:20 | |
| M. pneumoniae IgM | 59 | < 770 U/ml | |
| M. pneumoniae IgG | 2.92 | < 0.9 | |
| S. pneumoniae urinary antigen | Negative | ||
| L. pneumoniae urinary antigen | Negative | ||
| Group A Streptococcus antigen, throat swab | Negative | ||
| SARS-CoV-2 RNA | Negative | Negative | |
| HCV Ab | Negative | ||
| HBsAg | Negative | ||
| HBsAb | Negative | ||
| HBcAb | Negative | ||
| Eastern Equine Encephalitis virus IgG | < 1:16 | < 1:16 | |
| Eastern Equine Encephalitis virus IgM | < 1:16 | < 1:16 | |
| West Nile IgG | < 1.3 | < 1.3 index | |
| West Nile IgM | < 0.9 | < 0.9 index | |
| Powassan virus IgM, CSF | Positive | ||
| Inflammatory and autoimmune markers | |||
| CRP | < 5 | < 10 mg/L | |
| ESR | 79 | 18 | 0–36 mm/hr |
| Rheumatoid factor | 20.8 | < 12 IU/ml | |
| ANA | Negative | ||
| ANCA | Negative | ||
| C3 complement | 121 | 83–193 mg/dL | |
| Complement total (CH50) | 37 | 31–60 U/mL | |
The patient’s presentation, which consisted of subacute onset of confusion, lymphocytic pleocytosis in the CSF, and findings of inflammation in the basal ganglia, parietal lobes, and cerebellum on MRI were suggestive of encephalitis. The differential diagnosis included autoimmune encephalitis, acute disseminated encephalomyelitis (ADEM), viral encephalitis, and tick-borne associated encephalitis. Given that the patient resided in Martha’s Vineyard, an island off the coast of Massachusetts, USA, which suffers from a heavy burden of tick-borne infections, such as Lyme disease, West Nile Virus, and Anaplasma encephalitis were evaluated for.
Laboratory testing was negative for common viral causes of encephalitis, autoimmune encephalitis, and acute tick-borne illnesses, including West Nile Virus; notably, patient had remote past infection for Anaplasmosis and Lyme disease.
Specifically, the involvement of the basal ganglia made Powassan virus encephalitis a possible diagnosis, as this finding has been associated with infections by Flaviviridae [1]. Therefore, after 9 days of hospitalization, a CSF sample was submitted to the CDC for antibody titers against Powassan virus.
Based on the initial CSF finding of lymphocytic pleocytosis, the patient was started on an empiric 10-day acyclovir course; her CSF PCR for HSV 1 & 2 resulted as negative. She completed a course of ceftriaxone for empiric coverage of central nervous system bacterial infections and received a 28-day course of doxycycline, in the event that she had Lyme meningitis. She also received a 6-day course of Prednisone 60 mg daily with tapering, which was associated with a slight improvement of her symptoms — she could ambulate to the bathroom, was more conversant, and had a slight improvement in her short-term memory. She subsequently also received a 4-day course of IVIG, without any further improvement of her symptoms.
The patient’s neurological function improved slightly, as she was more conversant after corticosteroid administration, but she remained oriented only to self. She was discharged after a total of 33 days of hospitalization. Two weeks post-discharge, the CDC reported that her CSF was positive for Powassan virus IgM antibodies. On follow-up examination 7 weeks post-discharge, her cognitive function remained impaired, which was supported by her scoring 13/30 on a Mini Mental State Exam, but she was otherwise physically doing well. Unfortunately, 4 months later, she continues to be cognitively impaired and her MRI remains unchanged.
Between 2010 and 2019, there have been 181 documented cases of Powassan virus encephalitis in the USA. The vast majority have been identified in four states, namely Minnesota, Wisconsin, Massachusetts, and New York, which when combined, make up 74% of cases. Of these 181 cases, 166 had neuroinvasive disease (91.7%) and 21 (11.6%) resulted in death. Of note, death occurred only in patients with neuroinvasive disease [2].
Rates of Powassan virus infections have been increasing, likely due to the increased awareness for arboviral encephalitides secondary to the West Nile Virus outbreaks, as well as the territorial expansion of one of the vectors of Powassan virus, Ixodes scapularis [2, 3]. Indeed, 85% of Powassan virus infections reported by the CDC between 2010 and 2015 included patients with encephalitis or meningitis, while only 15% of patients had non-neuroinvasive disease, suggesting that patients without CNS involvement are less likely to be tested for Powassan virus [3].
The 2 variants of Powassan virus are transmitted by different vectors. Lineage I is transmitted by Ixodes cookei, while Lineage II is transmitted by I. scapularis, the tick vector for Borrelia burgdorferi, Babesia microti, and Anaplasma phagocytophilum [3]. However, whereas the transmission of other tick-borne diseases requires prolonged tick attachment (48 h for Lyme disease and 24 h for babesiosis), Powassan virus can be transmitted to a new host within 15 min. What sets it apart is that the virus is located in the salivary glands of the tick prior to the tick bite, allowing it to rapidly infect the new host [4].
Regarding its clinical presentation, after an incubation period of 8 to 34 days, the disease initially manifests with malaise, nausea, and sore throat. Patients who develop neuroinvasive disease go on to manifest high fever, headache, and disorientation 1 to 3 days later. Other findings include cranial nerve palsies, convulsions, obtundation, tremors, twitching, nystagmus, hallucinations, hemiparesis, quadriplegia, and respiratory failure [3, 5].
Imaging findings of Powassan virus neuroinvasive disease are most commonly appreciated using MRI with T2-weighted fluid-attenuated inversion recovery (T2/FLAIR) sequences. Similar to other arboviruses, such as West Nile Virus, Powassan virus has a predilection for the deep foci in the basal ganglia, thalamus and brainstem. In addition, it may affect the cerebellum and cortex [1, 3]. Pathology findings include necrotizing inflammation with a lymphocytic infiltrate primarily in the gray matter, as well as reactive gliosis [3].
The diagnosis of Powassan virus encephalitis is established through the identification of the virus, either directly through PCR in serum or CSF samples during the prodromal phase, or indirectly, either through identification of IgM neutralizing antibodies in the serum or CSF during the acute illness phase or through serum IgG in later phases [2, 3].
Neuroinvasive disease can have devastating long-term complications, including cognitive deficits, memory loss, hearing impairment, aphasia, muscle wasting, dysarthria, apnea, psychosis, and spastic hemiplegia, which develop in about 50% of patients, while approximately 10% of cases result in death. There is no specific treatment for Powassan virus disease; therefore, prevention of the disease through the avoidance of tick bites is of utmost importance [3, 5].
In conclusion, Powassan virus encephalitis is a severe tick-borne infection that can lead to devastating long-term complications or even death. The diagnosis requires a high degree of clinical suspicion and is based on epidemiologic evidence of tick exposure in patients with symptoms and signs of central nervous system infection. In general, the combination of findings of encephalitis, history of recent travel to an endemic region, and T2/FLAIR hyperintensities in the basal ganglia should suggest Powassan virus encephalitis as a probable cause. As treatment remains supportive, prevention of tick bites is critical in limiting occurrence of disease in the population.
Acknowledgements
The authors would like to thank Dr. Adam Basiago for selecting the pertinent MRI slides and Mr. Mehdi Bandali for his critical review of the manuscript.
Data availability
Available upon request.
Declarations
Ethical approval
This work is in accordance with the requirements of the authors’ institution regarding research involving human participants, as well as with the 1964 Helsinki Declaration and its later amendments.
Consent to participate
The participant as well as her family have consented to the publication of the case report to the journal.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Solomon T. Flavivirus encephalitis. N Engl J Med. 2004;351(4):370–378. doi: 10.1056/NEJMra030476. [DOI] [PubMed] [Google Scholar]
- 2.CDC. Statistics & maps | Powassan. https://www.cdc.gov/powassan/statistics.html. Accessed 23 Aug 2021
- 3.Kemenesi G, Bányai K. (2019) Tick-borne flaviviruses, with a focus on Powassan virus. Clin Microbiol Rev 32(1). 10.1128/CMR.00106-17 [DOI] [PMC free article] [PubMed]
- 4.Ebel GD, Kramer LD. Short report: duration of tick attachment required for transmission of Powassan virus by deer ticks. Am J Trop Med Hyg. 2004;71(3):268–271. doi: 10.4269/ajtmh.2004.71.3.0700268. [DOI] [PubMed] [Google Scholar]
- 5.Della-Giustina D, Duke C, Goldflam K. (2021) Underrecognized tickborne illnesses: Borrelia miyamotoi and Powassan virus. 10.1016/j.wem.2021.01.005 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon request.