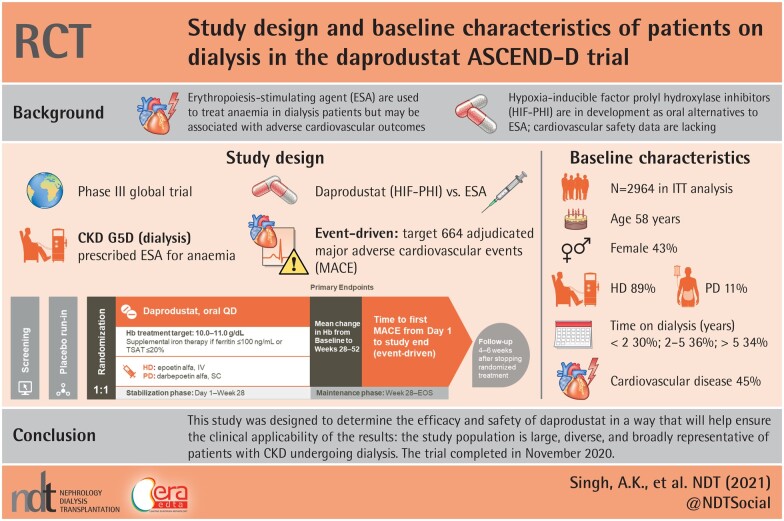
Abstract
Background
The Anemia Studies in chronic kidney disease (CKD): Erythropoiesis via a Novel prolyl hydroxylase inhibitor Daprodustat-Dialysis (ASCEND-D) trial will test the hypothesis that daprodustat is noninferior to comparator epoetin alfa or darbepoetin alfa for two co-primary endpoints: hemoglobin (Hb) efficacy and cardiovascular (CV) safety.
Methods
We report the trial design, key demographic, clinical and laboratory findings, and baseline therapies of 2964 patients randomized in the open-label (sponsor-blinded) active-controlled, parallel-group, randomized ASCEND-D clinical trial. We also compare baseline characteristics of ASCEND-D patients with patients who are on dialysis (CKD G5D) enrolled in other large CV outcome trials (CVOTs) and in the most relevant registries.
Results
The median age of patients was 58 years, 43% were female; 67% were White and 16% were Black. The median Hb at baseline was 10.4 g/dL. Among randomized patients, 89% were receiving hemodialysis and 11% peritoneal dialysis. Among key comorbidities, 42% reported a history of diabetes mellitus and 45% a history of CV disease. Median blood pressure was 134/74 mmHg. The median weekly dose of epoetin was 5751 units. Intravenous and oral iron uses were noted in 64 and 11% of patients, respectively. Baseline demographics were similar to patients with CKD G5D enrolled in other CVOTs and renal patient registries.
Conclusions
ASCEND-D will evaluate the efficacy and safety of daprodustat compared with epoetin alfa or darbepoetin alfa in the treatment of patients with anemia with CKD G5D.
This trial is registered with ClinicalTrials.gov: NCT02879305. EudraCT Number: 2016-000541-31; Sponsor Protocol Number: 200807.
Keywords: anemia, baseline data, daprodustat, dialysis, recombinant human erythropoietin
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS
What is already known about this subject?
anemia is a common complication in patients with chronic kidney disease (CKD); untreated, it is ubiquitous in patients with CKD who are on dialysis;
treatment of anemia with erythropoiesis-stimulating agents (ESAs) successfully corrects hemoglobin (Hb) levels; however, ESAs can be associated with adverse cardiovascular (CV) outcomes; and
this large study is needed to evaluate whether daprodustat—a hypoxia-inducible factor prolyl hydroxylase inhibitor—is noninferior to comparator epoetin alfa or darbepoetin alfa for two co-primary endpoints: Hb efficacy and CV safety in patients with CKD who are on dialysis.
What this study adds?
this is one of the largest anemia studies in dialysis patients (n = 2964), being performed in 35 countries across Europe, North America, Latin America and Asia Pacific. Baseline characteristics were similar to patients enrolled in other large CV outcome trials and relevant patient registries, thus supporting the generalizability of this study population;
a high proportion of the study population has a history of CV disease and/or diabetes mellitus; however, on average, control of diabetes and blood pressure were consistent with Kidney Disease: Improving Global Outcomes guidelines or local equivalents; and
standardizing the doses of randomized treatment, along with utilizing the same dose adjustment algorithm, iron management criteria and anemia rescue algorithm allow for a more unbiased comparison between the groups.
What impact this may have on practice or policy?
this study was designed to determine the efficacy and safety of daprodustat in a way that will help ensure the clinical applicability of the results: the study population is large, diverse and broadly representative of patients with CKD undergoing dialysis; and
if daprodustat is noninferior to ESAs, it may provide an alternative oral dosing regimen to existing treatment options, which may be preferable among certain patients.
INTRODUCTION
Anemia is ubiquitous among patients with chronic kidney disease (CKD) who are on dialysis (CKD G5D) [1]. The introduction of recombinant human erythropoietin (rhEPO) treatment in 1989 was one of the most important advances in the treatment of patients on dialysis and other patients with CKD. In the past, severe anemia was common, diminishing patients’ quality of life and resulting in the need for frequent blood transfusions [2]. Treatment with rhEPO and its analogs [erythropoiesis-stimulating agents (ESAs)] to partially correct anemia has improved patients’ lives and substantially reduced requirements for blood transfusion. However, several randomized trials have demonstrated either no benefit or even harm in relation to cardiovascular (CV) and other outcomes when treatment with rhEPO and its analogs were used to normalize hemoglobin (Hb) in patients with CKD [3–6]. Indeed, post hoc analysis have suggested that exposure to high doses of exogenous rhEPO may present a possible increase in CV and mortality risk in these patients [7–9].
The emergence of newer compounds termed hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs) to stimulate erythropoiesis through the inhibition of HIF-prolyl hydroxylase (PHD) enzymes PHD1, PHD2 and PHD3 may represent an alternative treatment strategy [10]. Recently approved in China and Japan, these agents are currently in development for the rest of the world [11–13]. PHD inhibition leads to stabilization of HIF-α transcription factors and expression of HIF-responsive genes involved in adaptation to hypoxia, including EPO and genes that regulate iron uptake, mobilization and transport, as well as resulting in decreased hepcidin production [14, 15]. Given the safety concerns with rhEPO and its analogs and challenges associated with parenteral therapies in some CKD populations, HIF-PHIs such as daprodustat (previously GSK1278863) are being developed to treat anemia of CKD.
In prior clinical trials of up to 52 weeks in Japan, daprodustat increased Hb to target goals in patients with anemia as effectively as darbepoetin alfa [16]. However, unlike rhEPO therapy, daprodustat increased Hb without raising plasma EPO to supraphysiologic levels [17, 18]. Across the trials published to date, daprodustat appears generally well tolerated with the more frequently reported adverse events being common events characteristic of the target populations [16–19]. As an oral alternative to the parenterally administered rhEPOs, daprodustat may also prove to be more convenient to nondialysis and peritoneal dialysis (PD) patients, as it is more easily delivered, stored and administered.
Here we describe the essential design elements and baseline characteristics of patients randomized in the Anemia Studies in CKD: Erythropoiesis via a Novel PHI Daprodustat-Dialysis (ASCEND-D) trial.
MATERIALS AND METHODS
Study design
ASCEND-D is a global, randomized, open-label (sponsor-blind), parallel-group, active-controlled, event-driven Phase 3 trial comparing the efficacy and safety of daprodustat in patients with CKD G5D being treated with an ESA for anemia (ClinicalTrials.gov: NCT02879305; EudraCT Number: 2016-000541-31). The study was approved by the ethics committee at every participating institution and was conducted according to the recommendations of Good Clinical Practice and the declaration of Helsinki. All patients provided written informed consent.
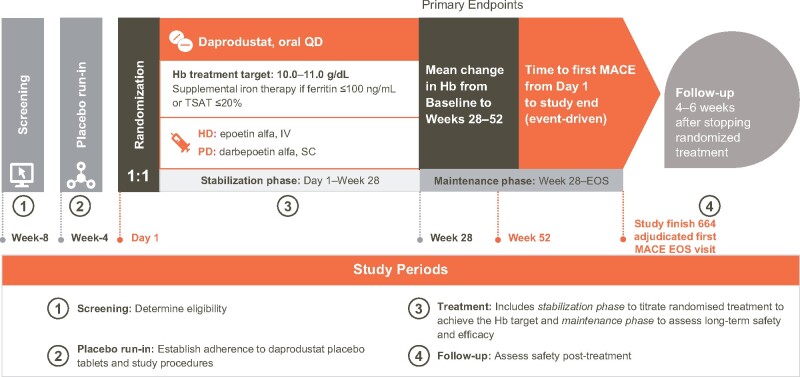
ASCEND-D consists of four periods: a screening period, a placebo run-in period, a treatment period and a follow-up period (Figure 1). The 4-week screening period permitted eligibility determination based on laboratory assessments to be confirmed, while the 4-week run-in period was used to establish adherence to daprodustat placebo tablets and study procedures. Prior ESAs were continued during the screening and run-in periods. Subjects were randomized to daprodustat or rhEPO control [intravenous (IV) epoetin alfa for hemodialysis (HD) patients and subcutaneous darbepoetin alfa for PD patients]. Thereafter, the treatment period was divided into a stabilization phase from Day 1 to Week 28, and a maintenance phase (MP) from Week 28 to the end of study (EOS) visit, with dose titration to achieve the pre-specified Hb target range (10–11 g/dL). The follow-up period consisted of a visit 4–6 weeks after stopping randomized treatment, only for those patients who continued randomized treatment until the EOS visit.
FIGURE 1.
ASCEND-D study design. Serum and plasma samples are collected at baseline, Week 28 and Week 52 for future analysis of biomarkers of CV risk and iron metabolism.
Patients attended routine follow-up at least every 4 weeks during Year 1 of the study and at least every 12 weeks thereafter. Patients who permanently discontinued randomized treatment prior to the EOS were followed at 12-weekly intervals off-treatment until the EOS visit. Serum and plasma samples were collected at baseline, Week 28 and Week 52 for future analysis of biomarkers and iron metabolism.
Eligibility criteria
Eligibility was determined at Week 8, with select criteria confirmed at Day 1 (randomization). Eligible patients were adults, treated with an approved ESA for ≥6 weeks before screening, had a screening Hb of 8–12 g/dL, on a consistent mode of dialysis for >90 days before screening, demonstrated adherence to daprodustat placebo tablets during the run-in period, and able to provide informed consent. The key inclusion and exclusion criteria are provided in Table 1 and complete entry criteria are outlined in Supplementary data, Table S1.
Table 1.
Key inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
Age: 18 to ≤99 years of age ESAs: Use of any approved ESA for at ≥6 weeks before screening and between screening and randomization Hb concentration: On Week 8:
On randomization (Day 1):
Dialysis: On dialysis >90 days before screeningc Frequency of dialysis: HD ≥2 times/week and PD ≥5 times/week. Home HD ≥2 times/week Compliance with placebo [randomization (Day 1) only]: ≥80% and ≤120% compliance with placebo during run-in period |
Kidney transplant: Planned living kidney transplant within 52 weeks after study start (Day 1) Iron: Ferritin ≤100 ng/mL (≤100 μg/L), TSAT ≤20%, at screening Evidence of nonrenal anemia: Aplasias, untreated pernicious anemia, thalassemia major, sickle cell disease or myelodysplastic syndrome, GI bleeding CV comorbidities: MI or acute coronary syndrome or stroke or TIA ≤4 weeks of screening, NYHA Class IV heart failure, uncontrolled hypertension (contraindicating rhEPO use) Liver disease (any one of the following):
Malignancy: History of malignancy within the 2 years before screening through to randomization (Day 1) or currently receiving treatment for cancer, or complex kidney cyst Females only: Pregnancy (as confirmed by a positive serum human chorionic gonadotrophin test), breastfeeding or subject is of reproductive potential and does not agree to follow one of the contraceptive options listed in the List of Highly Effective Methods for Avoiding Pregnancy Other conditions: Any other condition, clinical or laboratory abnormality, or examination finding that the investigator considers would put the subject at unacceptable risk, which may affect study compliance (e.g. intolerance to rhEPO) or prevent understanding of the aims or investigational procedures or possible consequences of the study |
Determined using HemoCue, a point of care test.
Minimum ESA dose: epoetins (including biosimilars): 1500 U/week IV or 1000 U/week subcutaneous; darbepoetin alfa: 20 μg/4 weeks subcutaneous/IV; methoxy PEG-epoetin: 30 µg/month subcutaneous/IV.
Patients receiving PD were restricted to <15% of the overall study population.
Ophthalmological exclusions were not included given completed studies with daprodustat did not identify any clinically meaningful changes in proliferative retinopathy, macular edema or choroidal neovascularization with daprodustat [16, 18]. ULN, upper limit of the normal range; TSAT, transferrin saturation; GI, gastrointestinal.
Study treatments and management strategies
Daprodustat and rhEPO dosing strategies and iron treatment for managing Hb are detailed in Table 2. A rescue algorithm was in place to minimize the risk of patients having an inadequate Hb response for an extended period and to enable consistency in the application of rescue therapy across the study (Table 3).
Table 2.
Study treatments and management strategies
| Study Treatment | Initiation | Protocol-specified dose adjustment algorithma |
|---|---|---|
| Daprodustat |
Starting dose 4–12 mg based on prior ESA dose at randomization Nine dose steps available (1, 2, 4, 6, 8, 10, 12, 16 and 24 mg) |
Dose adjustments (i.e. increase, decrease, maintain or withhold if Hb ≥12 g/dL) are implemented by the IRT system to maintain Hb concentrations within the range of 10–11 g/dLb Hb value measured at least every 4 weeks (Day 1 through Week 52) or at least every 12 weeks (post-Week 52 until the end of treatment) From Week 52 onward, additional 4-weekly study visits to check Hb and dispense randomized treatment are required if: Hb is outside the target range; dose has changed; a moderate CYP2C8 inhibitor has been started/stopped/changed; patient has changed from HD to PD; per investigator discretion to allow for an early dose adjustment |
| rhEPO |
Starting dose based on patients’ prior ESA dose (converted to the study ESA type) and Hb at the time of randomization Pre-defined dose-stepsc: IV epoetin alfa—stepwise increases or decreases in weekly dose from 20% to 33% for most steps (when patients were receiving from 1500 to 60 000 U IV as a total weekly dose; doses ≤10 000 U are administered once a week; doses >10 000 U are administered 3 times a week); darbepoetin alfa—stepwise increases or decreases in weekly dose from 20% to 33% for most steps (20–400 µg as a total 4-weekly dose; doses ≤150 µg are administered every 4 weeks; 200 and 300 µg are divided and administered every 2 weeks; 400 µg is divided and administered once a week) |
|
| Iron |
Started if TSAT is ≤20% and/or ferritin is ≤100 ng/mL Type of iron, dose and route is determined by the investigator based on local clinical practice and the patient’s iron status |
Iron must be stopped if values of ferritin >800 ng/mL and TSAT >20% or if TSAT >40% are present Investigators are to be guided by local/regional guidelines and may stop administration of iron at a lower ferritin or TSAT level if clinically indicated; the framework for starting and stopping iron is based on a review of global and regional iron guidelines, as well as input from the ASCEND SCs |
|
The Hb and Iron sub-committee of the SC is monitoring blinded patient Hb and iron data during the trial Assessment of the quality of clinical care provided to patients was monitored by the Standard of Care sub-committee of the SC. | ||
During the trial, overrides of the dose adjustment algorithm for exceptional circumstances associated with a safety concern are permitted if approved by the sponsor.
Based on the HemoCue Hb value.
Complete details of rhEPO dose steps (dose and frequency) are outlined in Supplementary data, Table S5.
IRT, Interactive Response Technology.
Table 3.
Rescue algorithm for anemia management
| Evaluate subject for rescue if: HemoCue Hb remains <9 g/dL (at a scheduled study visit, Week 4 onwards) despite threea consecutive dose increases above the startingb or post-rescuec dose (where HemoCue Hb is <9 g/dL before each dose increase) OR HemoCue Hb is <7.5 g/dL despite a dose increase at the prior study visit | |
| Step 1: Initial intervention |
While continuing randomized treatment (increase dose if HemoCue Hb <7.5 g/dL; otherwise maintain current dose), intervene with one or more of the following as dictated by clinical comorbidities: Single course of IV iron up to 1000 mg (in addition to the iron management criteria) Transfusion of up to two units of PRBC if clinically indicated Allow additional 4 weeks on randomized treatment (Note: this is a required choice; can be combined with either or both of the above) |
| Step 2: Rescue |
Check HemoCue Hb 4 ± 1 weeks from last study visit; earlier checks of Hb may be obtained to advise further intervention as clinically indicated Randomized treatment should be permanently discontinued, and the subject should be rescued according to local clinical practice if either: HemoCue Hb remains <9 g/dL despite initial intervention based on the average of two HemoCue Hb valuesd OR more than two units of PRBC were needed for transfusion (and was not related to acute bleeding) |
Two consecutive dose increases if starting/post-rescue dose is daprodustat 12 mg, epoetin alfa 42 000 U per week or darbepoetin alfa 200 µg over 4 weeks; one dose increase if starting/post-rescue dose is daprodustat 16 mg, epoetin alfa 48 000 U per week or darbepoetin alfa 300 µg over 4 weeks; and no prior dose increase if starting/post-rescue dose is daprodustat 24 mg, epoetin alfa 60 000 U per week or darbepoetin alfa 400 µg over 4 weeks (top dose).
For patients who have switched from HD to PD who are randomized to rhEPO, the baseline dose for the purposes of the rescue algorithm is the new darbepoetin alfa dose.
For patients who previously were evaluated for rescue and who can continue in the trial, ‘post-rescue’ dose is the dose of randomized treatment that a subject is receiving at the study visit after initial intervention.
Repeat HemoCue Hb at the same study visit to confirm Hb (using the same sample); take average of two values.
PRBC, packed red blood cells.
Objectives and endpoints
This trial was developed in consultation with the USA and European regulatory agencies. The co-primary noninferiority (NI) objectives of the trial are to compare Hb efficacy and CV safety among patients receiving daprodustat versus those receiving rhEPO. The NI Hb efficacy objective will be assessed with the co-primary endpoint of mean change in Hb between baseline and the evaluation period (EP; average over Weeks 28–52). An external, independent and blinded endpoints committee (Duke Clinical Research Institute) will adjudicate events used to assess the NI CV safety objective with the co-primary endpoint of time to first adjudicated major adverse CV event [MACE; the composite of all-cause mortality, nonfatal myocardial infarction (MI) and nonfatal stroke]. Principal secondary superiority endpoints, including superiority assessment of MACE, and other secondary endpoints are listed in Table 4.
Table 4.
Primary and secondary objectives and endpoints
| Objectives | Endpoints |
|---|---|
| Co-primary objectives | Co-primary endpoints (tested in parallel for NI) |
| To compare daprodustat with rhEPO for CV safety (NI) | Time to first occurrence of adjudicated MACE (composite of all-cause mortality, nonfatal MI and nonfatal stroke) |
| To compare daprodustat with rhEPO for Hb efficacy (NI) | Mean change in Hb between baseline and EP (mean over Weeks 28–52) |
| Principal secondary objectives | Principal secondary endpoints (tested for superiority, adjusted for multiplicity) |
| To compare daprodustat with rhEPO on CV safety endpoints | Time to first occurrence of adjudicated: MACE; MACE or a thromboembolic event (vascular access thrombosis, symptomatic deep vein thrombosis or symptomatic pulmonary embolism); MACE or a hospitalization for heart failure |
| To compare daprodustat with rhEPO on the use of IV iron | Average monthly IV iron dose (mg)/subject to Week 52 |
| Secondary objectives | Secondary endpoints (tested for superiority,a no multiplicity adjustment) |
| To compare daprodustat with rhEPO on additional CV safety endpoints | All-cause mortality, CV mortality, fatal or nonfatal MI, fatal or nonfatal strokeb; MACE or hospitalization for heart failureb (recurrent events analysis); CV mortality or nonfatal MIb; all-cause hospitalization; all-cause hospital re-admission within 30 days; MACE or hospitalization for heart failure or thromboembolic eventsb; hospitalization for heart failureb; thromboembolic eventsb |
| To compare daprodustat with rhEPO on Hb variability | Hb change from baseline to Week 52a; n (%) responders, defined as mean Hb within the Hb analysis range 10–11.5 g/dL during EPb; percentage of time Hb in analysis range (10–11.5 g/dL) during the EP (Weeks 28–52) and during the maintenance phase (MP ; Week 28 to end of trial) (NI analysis that will use a margin of 15% less time in range)a |
| To compare daprodustat with rhEPO on BP | Change from baseline in SBP, DBP and MAP at Week 52 and at end of treatment; number of BP exacerbation events per 100 patient years; n (%) with at least one BP exacerbation event during study |
| To compare daprodustat with rhEPO on the time to rescue (defined as permanently stopping randomized treatment due to meeting rescue criteria) | Time to stopping randomized treatment due to meeting rescue criteria |
| To compare daprodustat with rhEPO on HRQoL and utility score | Mean change in SF-36 HRQoL scores PCS, MCS and 8 health domains between baseline and Weeks 8, 12, 28 and 52, of particular interest are the changes from baseline in the vitality and physical functioning domains at Weeks 28 and 52; change from baseline in Health Utility (EQ-5D-5L) score at Week 52; change from baseline in EQ VAS at Week 52 |
| To compare daprodustat with rhEPO on the symptom severity and change | Change from baseline at Weeks 8,12, 28 and 52 in PGI-S |
Conversion factors from g/dL to g/L is 10 and from g/dL to mmol/L is 0.6206 (e.g. Hb of 10–11 g/dL is equivalent to 100–110 g/L or 6.2–6.8 mmol/L).
Hb change from baseline to Week 52 is tested for NI, using the −0.75 g/dL margin used in the co-primary analysis. Percentage time in range is tested first for NI, then for superiority. Events adjudicated.
To account for within-subject variability, 0.5 g/dL was added to the upper end of the target range to create a defined analysis range of 10.0–11.5 g/dL.
DBP, diastolic BP; EQ-5D-5L, EuroQoL 5-dimension 5-level; EQ VAS, EuroQoL visual analog scale; HRQoL, health-related quality of life; MAP, mean arterial pressure; MCS, Mental Component Score; PCS, Physical Component Score; PGI-S, patient global impression of severity; SBP, systolic BP; SF-36, Short Form-36 item.
Randomization and stratification
Patients were stratified by dialysis type {HD [including hemodiafiltration (HDF) and hemofiltration (HF)] or PD}, by region, and by participation in the ambulatory blood pressure (BP) monitoring sub-study. Following stratification, patients were randomized 1:1 to receive oral daprodustat or rhEPO control. A central randomization approach was used to protect against selection bias due to the open-label design.
Statistical analysis
A sample size of 3000 was planned for this event-driven trial based on the co-primary CV safety objective and an event target of 945 adjudicated first MACE. This includes on- and off-treatment MACE in the intent-to-treat (ITT) population. This event count provides ∼90% power to establish NI with a NI margin hazard ratio of 1.20 for daprodustat compared with rhEPO, assuming a true underlying 3% lower relative risk of MACE in favor of daprodustat (i.e. a true underlying hazard ratio of 0.97), and 80% power for NI under the assumption that the true underlying risk of MACE is the same in both groups (i.e. a true underlying hazard ratio of 1.00). The study completed randomization in August 2018. In August 2020, prior to study unblinding and after discussion with the regulatory authorities, as well as approval with the external steering committees (SCs) and the Independent Data Monitoring Committee (IDMC), the MACE NI margin was changed to 1.25, reducing the event target to 664 while maintaining ∼90% power. The rationale for the NI margin change was to accelerate study closeout in consideration of the coronavirus disease 2019 (COVID-19) pandemic and to align with the NI margin used in other HIF-PHI clinical studies [20]. There are no identified risks to subject safety or data integrity with these changes.
The planned study size provides ˃99% power for the Hb NI test with a NI margin of −0.75 g/dL for the (daprodustat – rhEPO) Hb difference. This includes on and off-treatment Hb values in the ITT population. Multiple imputation will be used to impute missing Hb values. The co-primary endpoints will be tested in parallel for NI at the one-sided 2.5% level, and NI will need to be established for both co-primary endpoints to proceed to evaluate the principal secondary endpoints for superiority. Statistical testing for the principal secondary endpoints will be adjusted for multiplicity using the Holm–Bonferroni for multiplicity adjustment [21].
Descriptive statistics in the form of number and percentage of patients or median and 25th and 75th percentiles (P25 and P75) are provided for baseline variables. Baseline values are presented for the ITT population, overall and by CV disease (CVD) history, defined as having a history of at least one of the following: angina pectoris, MI, stroke, transient ischemic attack (TIA), coronary artery disease, heart failure, atrial fibrillation, cardiac arrest or valvular heart disease.
Study oversight
ASCEND-D was developed in collaboration with the Executive SC (ESC) and SC. The ESC provides academic and scientific leadership and ensures that conduct of this study as well the other the Proactive IV Iron Therapy in HD Patients (PIVOTAL) studies in the ASCEND program conform to protocols. The SC provides scientific, medical and operational advice to the ESC. Members of these committees comprised Hb and iron, standard of care and regional recruitment and retention sub-committees to review in-stream, blinded, aggregate data on an ongoing basis to identify potential issues and to escalate to the SC and ESC as required. An IDMC reviews safety and efficacy data as defined in the protocols and makes recommendations for additions or adjustments, as well as evaluating the co-primary MACE endpoint at a planned interim analysis to assess for futility of achieving NI at study completion. An external, independent and blinded Clinical Events Classification (CEC) group, led by the Duke Clinical Research Institute, in collaboration with George Clinical, was in charge of adjudicating pre-defined events (all-cause mortality, MI, stroke, hospitalization for heart failure and thromboembolic events). Committee members and their respective affiliations, along with the CEC Primary Investigator, are presented in Supplementary data, Table S2.
Comparison with other large CV outcome trials and relevant registries
To assess generalizability, we compared baseline characteristics of ASCEND-D patients with those enrolled in two other large, randomized, controlled, trials, the INNO2VATE prevalent trial [20] and the PIVOTAL trial [22, 23], which evaluated anemia treatment in maintenance dialysis patients. A comparison of the ASCEND-D population was also made with more contemporaneous registry data sets with sufficient patient information to allow meaningful comparison, i.e. Dialysis Outcomes and Practice Patterns Study (DOPPS), US Renal Data System (USRDS) [24, 25].
RESULTS
ASCEND-D is being conducted in 431 centers in 35 countries. The country-level patient distribution is listed in Supplementary data, Table S3. In total, 44% of patients originated in Europe Middle East Africa (EMEA), 29% in North America (NA; predominantly USA), 14% in Latin America (LA) and 13% in the Asia Pacific (APAC) region.
Screening, run-in and randomization
A total of 5408 patients were screened, including patients who were re-screened and 2444 (45%) who did not meet entry criteria and were not randomized. The reasons for screen failure are listed in Supplementary data, Table S4. A total of 2964 patients were randomized. One additional patient was randomized but had not provided valid informed consent so was removed from the randomized count.
Demographic characteristics
Baseline characteristics are summarized in Table 5. The ITT cohort has a median age of 58 years with 43% being female. Eighty-nine percent of patients were treated with HD and 11% with PD.
Table 5.
Baseline characteristics of the overall ITT population and by CVD history
| ITT population | CVD |
||
|---|---|---|---|
| Yes | No | ||
| (N = 2964) | (n = 1320) | (n = 1644) | |
| Age, years | 58.0 (47.0–68.0) | 63.0 (54.0–71.0) | 54.0 (43.0–64.0) |
| Women, % | 43 | 40 | 45 |
| Race, % | |||
| White | 67 | 69 | 65 |
| Black | 16 | 17 | 15 |
| Asian | 12 | 10 | 14 |
| American Indian or Alaska Native | 2 | 1 | 2 |
| Native Hawaiian or other Pacific Islander | 2 | 2 | 1 |
| Multiple | 2 | <1 | 4 |
| Time since initiation of dialysis at screening (%) | |||
| 0 to <2 years | 30 | 30 | 31 |
| 2 to <5 years | 36 | 35 | 36 |
| ≥5 years | 34 | 34 | 33 |
| Dialysis modality at randomization (%) | |||
| HD | 89 | 91 | 86 |
| HD—conventional | 85 | 88 | 82 |
| HDF/HF | 4 | 3 | 4 |
| PD | 11 | 9 | 14 |
| Missing | <1 | – | <1 |
| Dialysis access type used at randomization (%) | |||
| AVF | 69 | 71 | 67 |
| AVG | 9 | 9 | 8 |
| CVC—tunneled | 9 | 10 | 7 |
| CVC—nontunneled | 1 | <1 | 2 |
| Peritoneal catheter | 11 | 8 | 13 |
| Other | 1 | <1 | 2 |
| Missing | <1 | <1 | <1 |
| Baseline dialysis adequacy | |||
| Kt/V urea for HD patients | 1.50 (1.31–1.72) | 1.50 (1.31–1.70) | 1.50 (1.31–1.73) |
| URR for HD patients (%) | 72 (66–77) | 72 (67–78) | 72 (66–77) |
| Kt/V urea for PD patients | 1.96 (1.70–2.22) | 1.92 (1.74–2.17) | 1.97 (1.68–2.26) |
| Baseline post-dialysis weight (kg) | 74.7 (63.0–88.5) | 76.5 (64.0–90.7) | 73.0 (62.0–86.5) |
| Baseline estimated dry weight (kg) | 74.5 (62.7–88.0) | 76.1 (64.0–90.5) | 73.0 (61.8–86.0) |
| Baseline post-dialysis BMI (kg/m2) | 26.8 (23.1–31.3) | 27.3 (23.4–31.8) | 26.3 (22.8–30.9) |
| CVD history (%)a,b | 45 | 100 | – |
| Coronary artery disease | 23 | 51 | – |
| Heart failure | 17 | 39 | – |
| Valvular heart disease | 11 | 26 | – |
| Angina pectoris | 10 | 22 | – |
| Atrial fibrillation | 9 | 20 | – |
| MI | 9 | 19 | – |
| Stroke | 7 | 15 | – |
| TIA | 4 | 10 | – |
| Cardiac arrest | 1 | 3 | – |
| Thromboembolic events, %c | 17 | 21 | 14 |
| Diabetes, % | 42 | 49 | 35 |
| Cancer, % | 5 | 6 | 4 |
| Smoking status | |||
| Current smoker, % | 9 | 9 | 9 |
| Former smoker, % | 21 | 26 | 17 |
| Baseline post-dialysis BP, mmHg | |||
| SBP | 134.0 (120.0–150.0) | 134.0 (120.0–150.0) | 134.0 (120.0–150.0) |
| DBP | 74.0 (65.0–82.0) | 71.0 (62.0–80.0) | 76.0 (67.0–83.3) |
| MAP | 93.7 (84.0–103.3) | 92.6 (83.3–102.0) | 95.3 (85.6–104.7) |
| Baseline laboratory values | |||
| hsCRP, mg/L | 4.0 (1.5–10.4) | 4.5 (1.7–12.2) | 3.6 (1.4–9.3) |
| Albumin, g/dL | 3.90 (3.60–4.10) | 3.90 (3.60–4.10) | 3.90 (3.70–4.10) |
| Hb (g/dL) | 10.40 (9.70–11.10) | 10.40 (9.80–11.00) | 10.45 (9.70–11.10) |
| <10 g/dL (%) | 32 | 31 | 32 |
| 10–11 g/dL (%) | 43 | 44 | 41 |
| >11 g/dL (%) | 26 | 25 | 27 |
| Hb A1c (%) (in patients with diabetes) | 6.40 (5.40–7.70) | 6.50 (5.60–7.80) | 6.30 (5.30–7.50) |
| White blood cells (×109/L) | 6.30 (5.10–7.60) | 6.40 (5.20–7.60) | 6.20 (5.10–7.60) |
| Platelets (×109/L) | 194.0 (157.0–238.0) | 190.0 (153.0–234.0) | 198.0 (161.0–242.0) |
| TSAT (%) | 33.0 (26.0–41.0) | 32.0 (25.0–41.0) | 33.0 (26.0–42.0) |
| Ferritin, µg/L | 595.0 (343.5–961.5) | 627.5 (367.0–990.5) | 578.0 (331.0–932.0) |
| Hepcidin, µg/L | 178.5 (110.9–257.5) | 179.5 (111.3–259.3) | 177.9 (110.9–256.1) |
| Total cholesterol, mg/dL | 152.5 (125.5–183.4) | 148.6 (121.6–179.5) | 154.4 (129.3–185.3) |
| Low-density lipoprotein cholesterol | 81.1 (61.0–103.1) | 79.9 (57.9–102.3) | 83.0 (62.9–103.9) |
| High-density lipoprotein cholesterol | 40.5 (32.8–52.1) | 40.5 (32.8–50.2) | 40.5 (32.8–52.1) |
| Medications, % | |||
| Diabetes medications | 30 | 37 | 25 |
| Insulin | 23 | 28 | 19 |
| ACEi or ARB | 46 | 46 | 46 |
| Beta-blocker | 54 | 65 | 45 |
| Statin | 41 | 51 | 32 |
| Aspirin | 34 | 48 | 24 |
| Vitamin K antagonist | 5 | 9 | 2 |
| Phosphate bindersa | 76 | 77 | 75 |
| Iron-based | 5 | 5 | 4 |
| Calcium-based | 48 | 47 | 49 |
| Noncalcium and noniron based | 35 | 36 | 34 |
| Vitamin D | 58 | 61 | 55 |
| Calcimimetics | 18 | 20 | 16 |
| Oral irond | 11 | 11 | 12 |
| IV iron | 64 | 65 | 63 |
| Standardized IV iron dose iron (mg/month) | 194 (100–272) | 190 (100–260) | 200 (100–272) |
| Prior ESA use (%) | >99 | >99 | >99 |
| Prior ESA type at randomization, % | |||
| Darbepoetin alfa only | 20 | 21 | 19 |
| Epoetin only | 68 | 66 | 69 |
| Methoxy PEG-epoetin beta only | 11 | 12 | 10 |
| Multiple | 1 | 1 | 2 |
| Missing | <1 | <1 | <1 |
| Standardized prior ESA dose, U/weeke | 5751 (3155–9694) | 5500 (3018–9166) | 5886 (3371–10268) |
| Baseline ERI, U/kg/wk/g/Lf | 0.74 (0.41–1.31) | 0.68 (0.40–1.20) | 0.78 (0.43–1.38) |
Results are based on the in-stream database as of 20 April 2020. Until the time of database lock, data entered into the electronic case report form may be updated by investigator site staff. Therefore, final data may change with continued data updates.
Continuous variables are expressed as median (P25 and P75). All baseline laboratory tests were performed by central laboratory except for Hb, which uses central laboratory values if available, or a point of care HemoCue value if the central laboratory value is missing. If Kt/V urea values were not available, URR values were recorded. Hb A1C was only collected for patients with diabetes. Standardized IV iron doses are provided only for patients using IV iron at baseline.
Subjects may be counted in multiple rows.
CVD in ASCEND-D was defined as angina pectoris, MI, stroke, coronary artery disease, TIA, heart failure, atrial fibrillation, cardiac arrest and valvular heart disease.
Thromboembolic events include pulmonary embolism, deep vein thrombosis, retinal vein occlusion, AVG thrombosis, AVF thrombosis and CVC thrombosis.
Includes ferric citrate.
See Supplementary data, Table S6 for ESA dose conversion details.
ERI is defined as the standardized prior ESA dose (U/week) divided by the screening estimated dry weight (kg), then divided by the Hb (g/L) achieved at randomization.
BMI, body mass index; ERI, erythropoietin resistance index; hsCRP, high-sensitivity C-reactive protein; URR, urea reduction ratio.
Clinical characteristics
Forty-five percent of patients reported a history of CVD (Table 5). A history of stroke was reported by 7% and TIA by 4%. Among patients with CVD, 51% had a history of coronary artery disease, 22% angina pectoris and 19% MI. More patients with CVD had diabetes mellitus than patients without CVD (49% versus 35%, respectively). Likewise, use of beta-blockers, statins, vitamin K antagonists and aspirin was higher among patients with a history of CVD. Patients with and without reported CVD had similar BPs; ∼46% were taking angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor II blockers (ARB).
A functioning arterio-venous fistula (AVF) was present in ∼69% of patients; 9% had an AV graft (AVG); a tunneled or nontunneled central venous catheter (CVC) was present in 9% and 1%, respectively.
ASCEND-D compared with other large CVOTs
Patients enrolled in ASCEND-D generally had similar demographic characteristics as patients in the other CVOTs (Table 6). The ASCEND-D and the INNO2VATE prevalent trials, the latter investigating another HIF-PHI, vadadustat, were of similar trial design utilizing an rhEPO active control, while PIVOTAL investigated high versus low-dose IV iron. Both INNO2VATE and ASCEND-D were global trials; however, ASCEND-D included more patients in EMEA and less in NA than INNO2VATE; in contrast, PIVOTAL was conducted in the UK. The HIF-PHI patient populations were of similar racial composition, while PIVOTAL overwhelmingly enrolled white patients. There were similar rates for CVD history (utilizing similar definitions) for both the ASCEND-D and the INNO2VATE trials [not reported (NR) for PIVOTAL], while diabetes history was similar for all trials. ASCEND-D had higher rates of hypertension than PIVOTAL and INNO2VATE and higher rates of heart failure than PIVOTAL (NR for INNO2VATE).
Table 6.
Comparison of ASCEND-D baseline characteristics with characteristics of patients enrolled in large CVOTs in a dialysis population
| ASCEND-D (n = 2964) | INNO2VATE prevalent trial (n = 3554) [16] | PIVOTAL (n = 2141) [22, 23] | |
|---|---|---|---|
| Design | |||
| Population | Dialysis (>90 days) with anemia of CKD | Dialysis (≥12 weeks) with anemia of CKD | HD (≤1 year) treated with ESA and ferritin <400 μg/L and TSAT <30% |
| Blinding | Open-label (sponsor-blind) | Open-label (sponsor-blind) | Open-label |
| Intervention | Daprodustat | Vadadustat | IV Iron |
| Control | Active-controlled (rhEPO)-HD, darbepoetin alfa-PD | Active-controlled (darbepoetin alfa) | Active controlled |
| Location | 44% EMEA; 29% NA (predominantly USA); 14% LA; 13% APAC | 61% USA; 11% Europe; 27% rest of regions | UK |
| Demographics | |||
| Age, years | 58 | 58 | 65 |
| Women, % | 43 | 44 | 35 |
| BMI, kg/m2 | 26.8 | 28.6 | 28 |
| Race, % | |||
| White | 67 | 63 | 79 |
| Black | 16 | 25 | 9 |
| Asian | 12 | 5 | 9 |
| Other | 6 | 5 | 3 |
| History, % | |||
| CVD | 45 | 50 | NR |
| Diabetes | 42 | 45 | 44 |
| Heart failure | 17 | NR | 4 |
| Hypertension | 92 | 51 | 73 |
| MI | 9 | NR | 9 |
| Stroke | 7 | NR | 8 |
| BP, mmHg | |||
| SBP | 134 | 143 | 144 |
| DBP | 74 | 76 | 73 |
| Hb, g/dL | 10.4 | 10.2 | 10.6 |
| Concomitant medications, % | |||
| ACEi/ARB | 46 | 20 (ACEi), 23 (ARB) | 27.8 |
| Antiplatelet therapy | 34 (aspirin) | 37 (aspirin) | 45.4 |
| Phosphate binders | 76 | NR | 38.4 |
| Statin | 41 | 42 | 59.7 (lipid-lowering) |
| ESA use, % | >99 | 100a | 100 |
| ESA dose (standardized to epoetin, Units per kg/week) | 5751 (77 U/kg/week)2 | 114 U/kg/week | 8000 (100 U/kg/week)b |
| IV iron (%) | 64 | 16.2 | NR |
Continuous variables are expressed as medians (ASCEND-D and PIVOTAL) and means (INNO2VATE). CVD definition varies by study (ASCEND-D: angina pectoris, MI, stroke, coronary artery disease, TIA, heart failure, atrial fibrillation, cardiac arrest and valvular heart disease; INNO2VATE prevalent trial: coronary artery disease, MI, stroke and heart failure).
BMI, body mass index.
Assumption based on eligibility criteria.
ESA dose standardized to epoetin units per kg/week calculated using baseline weight.
Both INNO2VATE and PIVOTAL had slightly higher baseline BP measures than ASCEND-D (Table 6) but similar Hb levels. Concomitant medications were similar for the HIF-PHI trials; however, PIVOTAL reported lower ACEi/ARB use and higher antiplatelet therapy and lipid-lowering use. Interestingly, prior ESA dose was lower in ASCEND-D than in the other CVOTs, while the proportion of subjects using IV iron was higher in ASCEND-D than in INNO2VATE (NR in PIVOTAL).
ASCEND-D compared with registry data sets
To assess generalizability, we compared demographic and clinical characteristics of patients enrolled in the ASCEND-D study with several contemporaneous, real-world, global registry datasets, including DOPPS [24, 26] and USRDS [25] (Table 7), with DOPPS including patients from the USA and Europe. Other global registries were explored but excluded from comparison due to the sparsity of the pertinent data. Data from DOPPS and USRDS were generally similar to the ASCEND-D population. Notable differences included race where a larger Black population was reported in the USRDS than ASCEND-D, which only comprised 29% patients from the USA. Hb levels were higher in the global DOPPS dataset than ASCEND-D where subjects were dosed to achieve Hb concentrations within the range of 10–11 g/dL; interestingly, higher ESA doses were seen in the US datasets relative to ASCEND-D.
Table 7.
Comparison of ASCEND-D baseline characteristics with characteristics of patients on HD registered on global databases
| ASCEND-D | DOPPS [26] | DOPPS Practice Monitor February 2020 [24] | USRDS [25] | |
|---|---|---|---|---|
| Population | Dialysis (>90 days) with anemia of CKD | Patients on HD with ESRD who survived ≥12 months after enrollment in DOPPs (2005–15) | Patients on HD; DOPPS 7: ∼35 facilities randomly selected utilizing the Visonex EHR software (Green Bay, WI)a | Prevalent ESRD [2018 data] |
| Region/countries | NA, EMEA, APAC, LA (see Supplementary data, Table S3 for country-level patient distribution) | Europeb, Canada and USA | USA | USA |
| Demographics | ||||
| Age, years | 58 | 63.6 | 63 | 60 |
| Women, % | 43 | 43 | 41 | 42 |
| BMI, kg/m2 | 26.8 | 27.9 | 28.5 | NR |
| Race, % | ||||
| White | 67 | NR | NR | 62 |
| Black or African American | 16 | 36 USA; NR non-USA | 36 | 30 |
| Asian | 12 | NR | NR | 5 |
| Other | 6 | NR | NR | 3 |
| Hb, g/dL | 10.4 | 11.3 | 10.7 | HD: 10.7 |
| PD: 10.9 | ||||
| ESA dose standardized to epoetin | 5751 (median) (U/week) | NR | 10 271 (U/week) | 9784 U/w epoetin alfa (HD) |
| 145 µg/month darbepoetin (HD) | ||||
| 146 µg/month Mircera (HD) | ||||
| 9019 U/w epoetin alfa (PD) | ||||
| 144 µg/month darbepoetin (PD) | ||||
| 145 µg/month Mircera (PD) | ||||
| TSAT, % | 33 | NR | 29.5 | HD: ≥20 in 82.0% |
| PD: ≥20 in 86.1% | ||||
| Ferritin, µg/L | 595.0 | NR | 829.0 | HD: >200 in 93.8% |
| PD: >200 in 86.0% | ||||
| Kt/V urea for HD patients | 1.5 | 1.6 | 1.62 | HD: ≥1.2 (96.9%) |
| PD: Weekly ≥1.7 (94.7%) | ||||
| Dialysis access type (%) | At randomization | NR | 66 | |
| AVF | 69 | NR | 65 | 17 |
| AVG | 9 | NR | 18 | 18 (catheter, NS) |
| CVC | 10 | NR | 17 (catheter, NS) | NR |
| Peritoneal catheter | 11 | NR | NR | NR |
| Other | 1 | NR | NR | NR |
| Missing | <1 | NR | ||
Continuous variables are expressed as medians (ASCEND-D) and means (DOPPS, DOPPS Practice Monitor and USRDS). ESRD, end-stage renal disease; NS, not specified.
Selection from among each of the two largest dialysis organizations, and ∼100 small and medium-chain, independent and hospital-based facilities.
Belgium, France, Germany, Italy, Spain, Sweden and UK.
DISCUSSION
ASCEND-D was designed to include a broad population as representative of the overall dialysis population as possible, with the appropriate measures to enable valid efficacy and safety comparisons across treatment groups. Sites were selected to achieve a balance in recruitment across EMEA, NA and LA, and APAC. Entry criteria were developed to identify a stable, maintenance and adequately treated dialysis population. A placebo run-in period was established to confirm compliance with an oral medication and to minimize withdrawal of consent post-randomization seen in a prior daprodustat HD study [18]. Exclusions ensured events that could impact the safety analysis were not present, including anemia due to causes other than CKD, recent CV events or cancer and uncontrolled hypertension.
The Hb target range of 10–11 g/dL was selected to accommodate the varying anemia guidelines and ESA labeling worldwide. The selection of rhEPO control was based on pragmatic clinical dialysis practice. In earlier daprodustat clinical trials, investigators were responsible for managing rhEPO dosing in the control group, which led to higher Hb values than targeted [27]. Therefore, a decision was made to apply the same dose adjustment algorithm for both treatment groups, as well as to provide the study rhEPO and develop a standard set of dose steps that were aligned with rhEPO labeling. Similarly, iron management criteria and an anemia rescue algorithm have been developed, and used for both treatment groups. For the latter, only IV iron and/or transfusions were allowed, in addition to randomized treatment, as an early intervention to improve Hb before considering a patient to have met the rescue endpoint and to permanently discontinue randomized treatment. Standardizing the doses of randomized treatment, along with utilizing the same dose adjustment algorithm, iron management criteria and anemia rescue algorithm allow for a more unbiased comparison between the groups.
The baseline characteristics of dialysis patients recruited in ASCEND-D were similar to that of other large CVOTs of dialysis patients. The most relevant comparison is between ASCEND-D and the INNO2VATE prevalent trial, which are both investigating HIF-PHIs; trial design, demographic and clinical characteristics were similar. While INNO2VATE had a higher recruitment in the USA, ASCEND-D had a higher recruitment from EMEA. Comparisons with PIVOTAL [22] and other historical CVOTs in dialysis patients also indicate similar baseline characteristics [28–30], with the exception of a lower prior ESA dose and higher rate of IV iron usage for ASCEND-D. Utilization of a lower ESA dose in ASCEND-D likely reflects differences in US recruitment (29% ASCEND-D versus 61% INNO2VATE). In contrast to INNO2VATE, where only 16% of patients had baseline IV iron use, 64% of ASCEND-D patients had baseline IV iron use, comparable with DOPPS data [24].
Registry data provided an additional way to compare patient characteristics with ASCEND-D to determine generalizability. Because of its global nature, DOPPS is arguably a better comparator than country-based registries. ASCEND-D compares favorably with DOPPS with respect to demographic and clinical characteristics [26], thus supporting the generalizability of this study population, with the exception of a higher Hb in DOPPS given the practice pattern outside of the USA to treat to higher Hb targets than the pre-specified target in ASCEND-D, which was developed in line with worldwide ESA labeling. Comparisons with US datasets from DOPPS [26] and USRDS [25] demonstrated that baseline characteristics were generally similar, with only a few accountable differences (e.g. race and Hb level).
The main limitation of the ASCEND-D trial is the open-label design. Blinding dialysis patients to randomized treatment is challenging because the active comparator is either administered intravenously or subcutaneously, whereas daprodustat is an oral medication and would have introduced a number of complexities and potential limitations to the study, including limiting the generalizability of the study. Importantly, the adjudication of clinical outcomes is blinded to the treatment assignment minimizing the risk of ascertainment bias [31]. Likewise, the sponsor remained blind to treatment assignment throughout the trial. Although the study patient population is younger than the average age of dialysis patients, it is common for trials to recruit younger patients given that older patients are frailer and less likely to participate in trials. Additionally, the selection of rhEPO type, dose steps and frequency of administration were pre-specified in the comparator group and may differ from local ESA protocols. Likewise, a common dose adjustment algorithm across treatment groups was implemented, which may differ from local practice.
These limitations are balanced by other strengths. ASCEND-D is a prospective randomized CVOT with one of the largest numbers of patients recruited worldwide. Patients were recruited not only from academic centers but also from community practices. It is notable that the racial and ethnic composition of ASCEND-D, although similar to other large trials, is more diverse than often seen in trials enrolling dialysis patients. The standard of care for patients on dialysis (e.g. diabetes, BP control and dialysis adequacy) was consistent with Kidney Disease: Improving Global Outcomes guidelines or local equivalent [32]. Overall, the study population, including patients on PD, and prevalence of CVD appears typical of global patients undergoing dialysis, ideal for determining the safety and efficacy of daprodustat.
In conclusion, ASCEND-D enrolled 2964 patients who are broadly representative of patients with anemia of CKD on dialysis. The study will test the hypothesis that daprodustat is noninferior to comparator rhEPO for two co-primary endpoints, Hb efficacy and CV safety. Results from ASCEND-D, expected in late 2021, will inform on an alternative option to treat anemia in dialysis patients.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
Editorial support in the form of writing assistance, assembling figures, grammatical editing and referencing was provided by Jonathan Plumb, PhD, of Fishawack Indicia, part of Fishawack Health, and was funded by GlaxoSmithKline.
FUNDING
ASCEND-D is funded by GlaxoSmithKline.
AUTHORS’ CONTRIBUTIONS
A.K.S. contributed to the design, interpretation of data, supervision and management of the research, writing and critical review of this manuscript. All authors contributed to the design, interpretation of data, management of research, writing and critical review of this manuscript. All authors affirm that authorship is merited based on the International Committee of Medical Journal Editors authorship criteria. A.K.S. was the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
CONFLICT OF INTEREST STATEMENT
A.K.S. reports consultancy fees from GlaxoSmithKline and stock in Gilead. K.C. reports consultancy fees from GlaxoSmithKline. V.J. reports consultancy fees from GlaxoSmithKline. K.L.J. reports consultancy fees from GlaxoSmithKline. R.D.L. reports grants and personal fees from Bristol-Myers Squibb and Pfizer, personal fees from Boehringer Ingelheim and Bayer AG, and research grants from Amgen Inc., GlaxoSmithKline, Medtronic PLC and Sanofi Aventis. I.C.M.D. reports research grants, consultancy fees and honoraria from GlaxoSmithKline and Vifor Pharma. J.M.M. reports personal fees from Abbott, Hickma, Sun Pharmaceuticals and Servier, and that his employer received fees from Alnylam Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Cardurion, Cytokinetics, Dal-Cor, GlaxoSmithKline, Ionis, Novartis, Pfizer and Theracos. G.T.O. reports personal fees from Roche Mexico, Johnson & Johnson, Vifor and AbbVie. V.P. reports consultancy agreements with AbbVie, Bayer, Boehringer Ingelheim, Chinook, GlaxoSmithKline, Janssen, Pfizer, Astellas, AstraZeneca, Bayer, Baxter, Bristol-Myers Squibb, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Pharmalink, Relypsa, Retrophin, Roche, Sanofi, Servier and Vitae; research funding from Pfizer (supplied drug and seed funding for TESTING trial) and GlaxoSmithKline; honoraria from AbbVie, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Pfizer, Astellas, AstraZeneca, Bayer, Baxter, Bristol-Myers Squibb, Chinook, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Mitsubishi, Tanabe, Mundipharma, Novartis, Novo Nordisk, Pharmalink, Relypsa, Retrophin, Roche, Sanofi, Servier and Vitae; scientific advisor or membership: serving/served on steering committees for trials funded by AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Novo Nordisk and Retrophin; other interests/relationships reported include: Board Director: George Clinical, George Institute, Garvan Institute, Mindgardens Network, Childrens Cancer Institute and Victor Chang Cardiac Research Institute. S.S. reports grants and consultancy fees from Alnylam, AstraZeneca, Bayer, Bristol-Myers Squibb, Cytokinetics, Gilead, GlaxoSmithKline, Lilly, MyoKardia, Novartis, Respicardia, Sanofi Pasteur and Theracos, grants from Bellerophon, Celladon, Eidos, Ionis, Lone Star Heart, Mesoblast, NIH/NHLBI and Neurotronik, and consultancy fees from Akros, Amgen, Arena, Cardior, Cardurion, Corvia, Daiichi-Sankyo, Ironwood, Merck Sharp Dohme, Roche, Takeda, Quantum Genetics, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Dinaqor, Tremeau, CellProThera and Moderna. C.W. reports consultancy fees from Akebia, Astellas, AstraZeneca, Bayer, Chiesi, FMC Idorsia, Mundipharma, GlaxoSmithKline, Merck Sharp Dohme, Reata, Gilead, Tricida, Vifor, Lilly and Takeda, and grants and consultancy fees from Boehringer Ingelheim, Sanofi Genzyme and Shire. S.S.W. reports personal fees from Public Health Advocacy Institute, CVS, Roth Capital Partners, Kantum Pharma, Mallinckrodt, Wolters Kluewer, GE Health Care, GlaxoSmithKline, Mass Medical International, Barron and Budd (versus Fresenius), JNJ, Venbio, Strataca, Takeda, Cerus, Pfizer, Bunch and James, Harvard Clinical Research Institute (aka Baim), Oxidien, Sironax, Metro Biotechnology, Biomarin and Bain, and grants and personal fees from Allena Pharmaceuticals. D.C.W. reports honoraria and/or consultancy fees from AstraZeneca, Amgen, Astellas, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Jansen, Merck Sharp and Dohme, Mundipharma, Napp, Pharmacosmos, Reata, Tricida and Vifor Fresenius. A.W. reports personal fees from Roche, Bayer, Fresenius and Medice. A.R.C., A.B., A.M.M., B.C., L.K. and R.D. are employees of and stockholders in GlaxoSmithKline.
DATA AVAILABILITY STATEMENT
Anonymized individual patient data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
REFERENCES
- 1. Singh AK, Fishbane S.. The optimal hemoglobin in dialysis patients- a critical review. Semin Dial 2008; 21: 1–6 [DOI] [PubMed] [Google Scholar]
- 2. Patel TV, Singh AK.. Anemia in chronic kidney disease: new advances. Heart Fail Clin 2010; 6: 347–357 [DOI] [PubMed] [Google Scholar]
- 3. Besarab A, Bolton WK, Browne JK. et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339: 584–590 [DOI] [PubMed] [Google Scholar]
- 4. Singh AK, Szczech L, Tang KL. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 5. Drüeke TB, Locatelli F, Clyne N. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 [DOI] [PubMed] [Google Scholar]
- 6. Pfeffer MA, Burdmann EA, Chen CY. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 7. Szczech LA, Barnhart HX, Inrig JK. et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 2008; 74: 791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solomon SD, Uno H, Lewis EF. et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 2010; 363: 1146–1155 [DOI] [PubMed] [Google Scholar]
- 9. Kilpatrick RD, Critchlow CW, Fishbane S. et al. Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients. Clin J Am Soc Nephrol 2008; 3: 1077–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMahon GM, Singh AK.. Prolyl-hydroxylase inhibitors for the treatment of anemia in chronic kidney disease. Curr Opin Nephrol Hypertens 2019; 28: 600–606 [DOI] [PubMed] [Google Scholar]
- 11. Pergola PE, Spinowitz BS, Hartman CS. et al. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int 2016; 90: 1115–1122 [DOI] [PubMed] [Google Scholar]
- 12. Brigandi RA, Johnson B, Oei C. et al. ; PHI112844 Investigators. A novel hypoxia-inducible factor-prolyl hydroxylase inhibitor (GSK1278863) for anemia in CKD: a 28-day, phase 2A randomized trial. Am J Kidney Dis 2016; 67: 861–871 [DOI] [PubMed] [Google Scholar]
- 13. Provenzano R, Besarab A, Sun CH. et al. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin J Am Soc Nephrol 2016; 11: 982–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Q, Davidoff O, Niss K. et al. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J Clin Invest 2012; 122: 4635–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicolas G, Chauvet C, Viatte L. et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 2002; 110: 1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akizawa T, Nangaku M, Yonekawa T. et al. Efficacy and safety of daprodustat compared with darbepoetin alfa in Japanese hemodialysis patients with anemia: a randomized, double-blind, phase 3 trial. Clin J Am Soc Nephrol 2020; 15: 1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holdstock L, Meadowcroft AM, Maier R. et al. Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol 2016; 27: 1234–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meadowcroft AM, Cizman B, Holdstock L. et al. Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants on hemodialysis. Clin Kidney J 2019; 12: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akizawa T, Tsubakihara Y, Nangaku M. et al. Effects of daprodustat, a novel hypoxia-inducible factor prolyl hydroxylase inhibitor on anemia management in Japanese hemodialysis subjects. Am J Nephrol 2017; 45: 127–135 [DOI] [PubMed] [Google Scholar]
- 20. Eckardt KU, Agarwal R, Farag YM. et al. Global Phase 3 programme of vadadustat for treatment of anaemia of chronic kidney disease: rationale, study design and baseline characteristics of dialysis-dependent patients in the INNO2VATE trials. Nephrol Dial Transplant 2020; gfaa204. doi: 10.1093/ndt/gfaa204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6: 65–70 [Google Scholar]
- 22. Macdougall IC, White C, Anker SD. et al. ; on behalf of the PIVOTAL Trial investigators. Randomized trial comparing proactive, high-dose versus reactive, low-dose intravenous iron supplementation in hemodialysis (PIVOTAL): study design and baseline data. Am J Nephrol 2018; 48: 260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Macdougall IC, White C, Anker SD. et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med 2019; 380: 447–458 [DOI] [PubMed] [Google Scholar]
- 24. Arbor Research Collaborative for Health. DOPPS Practice Monitor. https://www.dopps.org/DPM/DPMSlideBrowser.aspx?type=ComGrp&id=7 (May 2020, date last accessed)
- 25. US Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020 [Google Scholar]
- 26. Douglas SF, Dluzniewski PJ, Kerry C. et al. Combinations of mineral and bone disorder markers and risk of death and hospitalizations in the international dialysis outcomes and practice patterns study. Clin Kidney J 2019; 13: 1056–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holdstock L, Cizman B, Meadowcroft AM. et al. Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants with chronic kidney disease. Clin Kidney J 2019; 12: 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chertow GM, Correa-Rotter R, Block GA. et al. Baseline characteristics of subjects enrolled in the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Nephrol Dial Transplant 2012; 27: 2872–2879 [DOI] [PubMed] [Google Scholar]
- 29. Wanner C, Krane V, März W. et al. ; Deutsche Diabetes-Dialyse-Studie (4D) Study Group. Randomized controlled trial on the efficacy and safety of atorvastatin in patients with type 2 diabetes on hemodialysis (4D study): demographic and baseline characteristics. Kidney Blood Press Res 2004; 27: 259–266 [DOI] [PubMed] [Google Scholar]
- 30. Fellström BC, Jardine AG, Schmieder RE. et al. ; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360: 1395–1407 [DOI] [PubMed] [Google Scholar]
- 31. Hansson L, Hedner T, Dahlöf B.. Prospective randomized open blinded end-point (PROBE) study. A novel design for intervention trials. Prospective Randomized Open Blinded End-Point. Blood Press 1992; 1: 113–119 [DOI] [PubMed] [Google Scholar]
- 32. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int 2012; 2: 279–335 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized individual patient data and study documents can be requested for further research from www.clinicalstudydatarequest.com.