Abstract
Background
Patients with severe Coronavirus Disease 2019 (COVID-19) are treated with corticosteroids.
Aim
We aimed to evaluate the role of corticosteroid treatment in candidemia development during the COVID-19 pandemic.
Methods
This retrospective study was conducted in a Greek ICU, from 2010 to August 2021, encompassing a pre-pandemic and a pandemic period (pandemic period: April 2020 to August 2021). All adult patients with candidemia were included.
Results
During the study period, 3,572 patients were admitted to the ICU, 339 patients during the pandemic period, of whom 196 were SARS-CoV-2-positive. In total, 281 candidemia episodes were observed in 239 patients, 114 in the pandemic period. The majority of candidemias in both periods were catheter-related (161; 50.4%). The incidence of candidemia in the pre-pandemic period was 5.2 episodes per 100 admissions, while in the pandemic period was 33.6 (p < 0.001). In the pandemic period, the incidence among COVID-19 patients was 38.8 episodes per 100 admissions, while in patients without COVID-19 incidence was 26.6 (p = 0.019). Corticosteroid administration in both periods was not associated with increased candidemia incidence.
Conclusions
A significant increase of candidemia incidence was observed during the pandemic period in patients with and without COVID-19. This increase cannot be solely attributed to immunosuppression (corticosteroids, tocilizumab) of severe COVID-19 patients, but also to increased workload of medical and nursing staff.
Keywords: Candidemia, COVID-19, SARS-CoV-2, Critically-ill patients, ICU, Corticosteroids
Introduction
Candida species are a significant cause of healthcare infections especially among patients hospitalized at Intensive Care Units (ICU).1 Several explanations exist for such an increased incidence of candidemia among critically ill patients, such as presence of intravascular lines, prolonged antimicrobial treatment, comorbidities, and immunosuppression, including corticosteroid treatment.2, 3, 4 An association between corticosteroids and increased incidence of candidemia is already established.4
Since the beginning of the Coronavirus Disease 2019 (COVID-19) pandemic, a significant proportion of patients developed severe respiratory insufficiency requiring ICU hospitalization; corticosteroids are increasingly prescribed for managing COVID-19 in patients with respiratory insufficiency due to their beneficial effects.5 Therefore, as previously shown, patients hospitalized at ICU and treated with corticosteroids for severe COVID-19 are at high risk of invasive fungal infections, including pulmonary aspergillosis and candidemia.3, 4, 5, 6
In the present study, we evaluated the epidemiological and clinical characteristics of patients with candidemia during the COVID-19 pandemic and the role of corticosteroid treatment in the development of such infection.
Material and methods
The retrospective study was conducted in the ICU of the University General Hospital of Patras, Greece from January 2010 to August 2021. From January 2010 to April 2020, the ICU comprised 13 beds. During the pandemic period (April 2020 to August 2021), the number of ICU beds gradually increased to 33 beds. The study was approved by the Ethics Committee of our institution that waived the need for informed consent (HEC n° 3324).
All adult patients admitted to the ICU with at least one positive blood culture for Candida spp. were included in the study, during the pre-pandemic (2010 to March 2020) and the pandemic period. Patients in both periods were subdivided into two groups depending on whether they had received corticosteroid treatment (for > 3 days). Patients in the second period were subsequently categorized according to the result of SARS-CoV-2 PCR. Data regarding demographic characteristics, chronic illnesses, length of hospitalization, prior surgery, administration of antimicrobial and antifungal were collected from ICU's computerized database and patients’ chart reviews.
Two blood culture sets (one from peripheral site and one from the central venous line) were obtained from ICU patients with fever (≥38.0 °C) or clinical presentation suggestive of infection and sent off to the clinical laboratory of the Microbiology Department. In case of blood culture positivity (Bact/Alert 3D, Biomerieux, Marcy l'Etoile, France) they were further inoculated on Sabouraud dextrose agar. Plates were incubated at 37 °C for 96 h before assessed as negative. Yeasts from positive cultures were identified to species level using the API 20C AUX System (bioMerieux) or Vitek 2 YST card (bioMerieux).
SPSS version 23.0 (SPSS, Chicago, IL) software was used for data analysis. Categorical variables were analyzed using the Fisher's exact test and continuous variables using Mann-Whitney U; p < 0.05 was considered statistically significant.
Results
In total, 3572 patients were admitted to the ICU during the study, 339 during the pandemic period, of whom 196 were SARS-CoV-2-positive. Overall, 281 candidemia episodes were documented in 239 patients, 114 during the pandemic and 167 in the pre-pandemic period.
The incidence of candidemia in the pre-pandemic period was 5.2 episodes per 100 admissions (3.7 in patients without vs. 6.2 in those with corticosteroids; p = 0.003), while in the pandemic period it was 33.6 episodes per 100 admissions (Table 1). In the pandemic period, the incidence among COVID-19 patients was 38.8 episodes per 100 admissions (21.4 in patients without vs. 40.1 in patients with corticosteroids; p = 0.167), while in patients without COVID-19 the incidence was 26.6 episodes per admission (24.7 in patients without vs. 29.3 in patients with corticosteroids; p = 0.543); the difference between COVID-19 and non-COVID-19 patients was statistically significant (p = 0.019)
Table 1.
Candidemia episodes per admission according to period of hospitalization, COVID-19, and corticosteroid administration.
| Pre-pandemic period (January 2010‒March 2020) |
Pandemic period (April 2020‒August 2021) |
pa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Without corticosteroids | With corticosteroids | All patients | Without COVID-19 |
COVID-19 |
All patients | ||||||
| Without corticosteroids | With corticosteroids | All patients | Without corticosteroids | With corticosteroids | All patients | ||||||
| ICU admissions | 1337 | 1896 | 3233 | 85 | 58 | 143 | 14 | 182 | 196 | 339 | |
| Candidemia episodes | 50 | 117 | 167 | 21 | 17 | 38 | 3 | 73 | 76 | 114 | |
| Candidemia episodes per 100 admissions | 3.7 | 6.2 | 5.2 | 24.7 | 29.3 | 26.6 | 21.4 | 40.1 | 38.8 | 33.6 | <0.001 |
| Candidemia episodes per 1000 patient-days | 2.6 | 6.6 | 4.5 | 13.0 | 14.9 | 13.8 | 17.4 | 19.2 | 19.1 | 16.9 | <0.001 |
| ICU mortality | 20.1% | 43.0% | 30.0% | 32.9% | 43.1% | 37.1% | 42.9% | 59.3% | 58.2% | 49.2% | <0.001 |
Comparison between pandemic and pre-pandemic period.
Candida non-albicans species predominated in both periods (206 isolates; 73.0%) with C. parapsilosis being the most commonly isolated (152 isolates; 53.9%) followed by C. glabrata (Nakaseomyces glabrataa; 24; 8.5%), C. tropicalis (23; 8.2%), C. lusitaniae (Clavispora lusitaniae; 3; 1.1%), C. krusei (Pichia kudriavzevii; 3; 1.1%) and C. guilliermondii (Meyerozyma guilliermondii; 1; 0.4%).The majority of candidemias in both periods represented catheter-related (161; 50.4%), followed by unknown origin candidemia (111; 39.4%).
Univariate analyses of patients’ characteristics according to the period of hospitalization and SARS-CoV-2 infection are shown in Table 2. The comparison of pandemic patients with and without SARS-CoV-2 infection showed that those with SARS-CoV-2 infection had higher rates of overweight or obesity, prior corticosteroid or tocilizumab administration and lower rates of prior antifungal administration. The same factors were identified in the comparison of patients with SARS-CoV-2 infection and patients in the pre-pandemic period.
Table 2.
Univariate analyses of patients’ characteristics according to period of hospitalization and COVID-19 infection.
| Characteristics | Pandemic period |
Pre-pandemic period |
||||
|---|---|---|---|---|---|---|
| Without COVID-19 (n = 38) | With COVID-19 (n = 77) | pa | All patients (n = 167) | pb | pc | |
| Demographics | ||||||
| Age (years) | 63.5 (55.0‒72.0) | 68.0 (59.0‒77.0) | 0.063 | 58.0 (45.5‒71.0) | 0.081 | <0.001 |
| Male sex | 24 (63.2%) | 48 (62.3%) | 1.000 | 103 (61.7%) | 1.000 | 1.000 |
| Comorbidities | ||||||
| Diabetes mellitus | 8 (21.1%) | 22 (28.6%) | 0.500 | 26 (15.6%) | 0.468 | 0.024 |
| Chronic obstructive pulmonary disease | 3 (7.9%) | 12 (15.6%) | 0.379 | 17 (10.2%) | 1.000 | 0.287 |
| Chronic heart failure | 1 (2.6%) | 1 (1.3%) | 1.000 | 7 (4.2%) | 1.000 | 0.441 |
| Chronic kidney disease | 0 (0%) | 5 (6.5%) | 0.169 | 9 (5.6%) | 0.215 | 0.770 |
| Malignancy | 4 (10.5%) | 73 (9.1%) | 0.217 | 23 (13.8%) | 0.792 | 0.024 |
| Immunosuppressive therapy (before admission) | 1 (2.6%) | 6 (7.8%) | 0.422 | 13 (7.8%) | 0.475 | 1.000 |
| Obesity | 9 (23.7%) | 30 (39.0%) | 0.143 | 38 (22.8%) | 1.000 | 0.014 |
| Overweight or obese | 25 (65.8%) | 67 (87.0%) | 0.012 | 111 (66.5%) | 1.000 | 0.001 |
| Charlson Co-morbidity Index | 3.0 (1.0‒7.0) | 4.0 (2.0‒7.0) | 0.107 | 3.0 (1.0‒5.0) | 0.686 | 0.003 |
| Hospitalization data | ||||||
| Corticosteroid administration | 17 (44.7%) | 73 (94.8%) | <0.001 | 117 (70.1%) | 0.004 | <0.001 |
| Tocilizumab | 0 (0%) | 62 (80.5%) | <0.001 | 0 (0%) | ‒ | <0.001 |
| Days at risk (from ICU admission to first episode of candidemia)d | 21.5 (12.8‒39.0) | 13.0 (6.0‒18.5) | <0.001 | 12.0 (6.0‒25.0) | 0.003 | 0.426 |
| Prior hospitalization | 15 (39.5%) | 54 (70.1%) | 0.002 | 51 (30.5%) | 0.337 | <0.001 |
| Days at risk (from hospital admission to first episode of candidemia) d | 30.0 (16.0‒49.0) | 18.0 (12.0‒26.0) | <0.001 | 20.0 (10.0‒33.0) | 0.005 | 0.756 |
| Prior surgery (within a month form candidemia) | 17 (44.7%) | 6 (7.8%) | <0.001 | 71 (42.5%) | 0.857 | <0.001 |
| Prior antimicrobial administration (within a month form candidemia) | 38 (100%) | 77 (100%) | ‒ | 167 (100%) | ‒ | ‒ |
| Prior antifungal administration (within a month form candidemia) | 26 (68.4%) | 31 (40.3%) | 0.006 | 101 (60.5%) | 0.460 | 0.004 |
| Azoles | 4 (10.5%) | 1 (1.3%) | 26 (15.6%) | |||
| Fluconazole | 4 (10.5%) | 0 (0%) | 25 (15.0%) | |||
| Voriconazole | 0 (0%) | 1 (1.3%) | 2 (1.2%) | |||
| Echinocandins | 24 (63.2%) | 23 (29.9%) | 92 (55.1%) | |||
| Anidulafungin | 19 (50.0%) | 20 (26.0%) | 43 (25.7%) | |||
| Caspofungin | 2 (5.3%) | 2 (2.6%) | 31 (18.6%) | |||
| Micafungin | 5 (13.2%) | 2 (2.6%) | 25 (15.0%) | |||
| Liposomal-Amphotericin B | 2 (5.3%) | 11 (14.3%) | 10 (6.0%) | |||
| Infection data | ||||||
| Type of candidemia | ||||||
| Unknown origin | 14 (36.8%) | 35 (45.5%) | 0.427e | 62 (37.1%) | 1.000e | 0.260e |
| Catheter-related | 21 (55.3%) | 37 (48.1%) | 84 (50.3%) | |||
| Secondaryf | 3 (7.9%) | 5 (6.5%) | 21 (12.6%) | |||
| Candida spp | ||||||
| C. albicans | 3 (7.9%) | 25 (32.5%) | 0.005g | 48 (28.7%) | 0.004g | 0.552g |
| C. parapsilosis | 28 (73.7%) | 39 (50.5%) | 85 (50.9%) | |||
| C. glabrata (N. glabrataa) | 5 (13.2%) | 5 (6.5%) | 14 (8.4%) | |||
| C. tropicalis | 2 (5.3%) | 5 (6.5%) | 16 (9.6%) | |||
| Other speciesh | 0 (0%) | 3 (3.9%) | 4 (2.4%) | |||
| Outcome | ||||||
| 14-day mortality | 11 (28.9%) | 24 (31.2%) | 1.000 | 38 (22.8%) | 0.407 | 0.205 |
| ICU mortality | 19 (50.0%) | 51 (66.2%) | 0.107 | 93 (55.7%) | 0.590 | 0.126 |
Data are number (%) of patients or median (Q1–3).
Comparison of pandemic patients with and without SARS-CoV-2 infection.
Comparison of pandemic patients without SARS-CoV-2 infection and pre-pandemic patients.
Comparison of pandemic patients with SARS-CoV-2 infection and pre-pandemic patients.
Among 239 episodes of candidemia.
Unknown origin compared to known origin candidemia.
18 urinary tract infection, 9 intra-abdominal infection, 2 nosocomial meningitis.
C. albicans compared to non-albicans Candida.
Three C. lusitaniae (Clavispora lusitaniae), three C. krusei (Pichia kudriavzevii), one C. guilliermondii (Meyerozyma guilliermondii).
While ICU mortality in patients with COVID-19 was higher than that in patients without (58.2% vs. 37.1%; p < 0.001), no statistically significant difference was found for candidemia in aforementioned groups (66.2% vs. 50.0%; p = 0.107). ICU mortality (14-day and overall) was similar between periods.
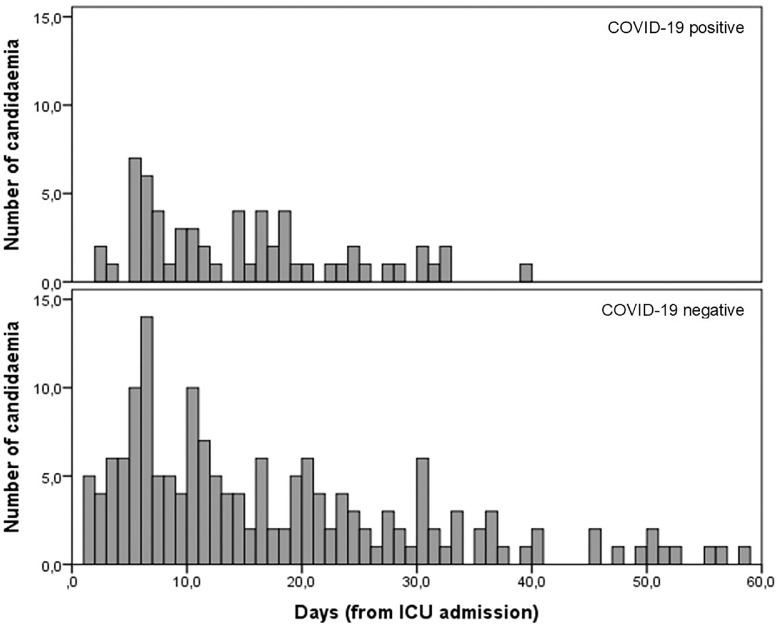
Median number of days since ICU admission until the first candidemia episode was similar (p = 0.101) between COVID-19 positive (13 days; 6‒18.5) and negative patients (combined pandemic and pre-pandemic periods; 15-days; 6‒29.5) (Fig. 1).
Fig. 1.
Histogram of days since ICU admission until the first candidemia episode between COVID-19 positive and negative patients (combined pandemic and pre-pandemic period).
Discussion
In the present study, a significant increase of candidemia incidence was observed during the pandemic period in patients both positive and negative for COVID-19. Such an increase in incidence of candidemia among critically ill COVID-19 patients has been reported from different parts of the world.7, 8, 9,10,11
The link of immunosuppressive treatment (corticosteroids, tocilizumab) and candidemia development in COVID-19 patients was previously mentioned, but not been established.7,12 In the present study, although immunosuppressive treatment (corticosteroids, tocilizumab) was more common in COVID-19 patients, this could not explain the increased candidemia incidence in COVID-19 negative patients during the pandemic period. There are two possible explanations for this increase. First, to cover the needs for extended ICU capacity, the nurse workforce was increased by either hiring new nurses or allocating others from different departments, many of whom had no prior experience with critically ill patients or work experience overall. Even though rapid critical care training was implemented by the nurse leaders, this could not compensate for the lack of experience in a highly specialized environment.13 Second, femoral access for central venous catheter insertion was increasingly used during the pandemic, in both SARS-CoV-2-positive and negative patients. This practice that was previously associated with candidemia, originated from the increased workload of ICU specialists since the medical personnel was not increased.14
Candida non-albicans predominated before and during the pandemic period with C. parapsilosis being the most common in all groups.4 In contrast to other studies, the increased candidemia was not due to C. auris dissemination.15,16
ICU mortality (14-day and overall) was similar between periods and groups (with or without COVID-19). However, patients with COVID-19 had a higher mortality rate than those without; development of candidemia did not affect mortality in those patients. This finding contradicts two studies that have shown that candidemia in COVID-19 patients was associated with higher mortality than in patients without COVID-19.8,9 In the present study, the majority of candidemias in patients with and without COVID-19 were catheter-related or unknown origin (most of them being catheter-related); in all but five patients the central venous catheter was removed within 48 h from candidemia onset, leading to prompt and early source control.17
The timing of candidemia development was independent of COVID-19. Two previous studies presented different results related to the timing of candidemia development; one study showed that COVID-19 patients developed candidemia later than patients without COVID-19,9 and another reported that candidemia was developed earlier in COVID-19 patients.8
The present study has several limitations. First, it was a retrospective study with data from a single ICU. The percentage of patients with femoral central venous catheters was not calculated for the pandemic and pre-pandemic periods.
Conclusions
A significant increase of candidemia incidence in ICU patients was observed during the pandemic period independently of COVID-19. This increase cannot be solely attributed to the immunosuppression (corticosteroids, tocilizumab) of severe COVID-19 patients but also to increased workload of medical and nursing staff. The role immunosuppression to candidemia development in COVID-19 patients requires further evaluation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
CRediT authorship contribution statement
Matthaios Papadimitriou-Olivgeris: Conceptualization, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Fevronia Kolonitsiou: Methodology, Investigation, Writing – review & editing. Sotiria Kefala: Investigation, Writing – review & editing. Anastasia Spiliopoulou: Investigation, Data curation, Writing – review & editing. Diamanto Aretha: Methodology, Investigation, Writing – review & editing. Christina Bartzavali: Investigation, Data curation, Writing – review & editing. Argyro Siapika: Investigation, Writing – review & editing. Markos Marangos: Conceptualization, Resources, Writing – review & editing, Project administration. Fotini Fligou: Conceptualization, Resources, Writing – review & editing, Supervision.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Vincent J.L., Rello J., Marshall J., Silva E., Anzueto A., Martin C.D., et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 2.Muskett H., Shahin J., Eyres G., Harvey S., Rowan K., Harrison D. Risk factors for invasive fungal disease in critically ill adult patients: a systematic review. Crit Care. 2011;15:R287. doi: 10.1186/cc10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag. 2014;10:95–105. doi: 10.2147/TCRM.S40160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papadimitriou-Olivgeris M., Spiliopoulou A., Fligou F., Spiliopoulou I., Tanaseskou L., Karpetas G., et al. Risk factors and predictors of mortality of candidemia among critically ill patients: role of antifungal prophylaxis in its development and in selection of non-albicans species. Infection. 2017;45:651–657. doi: 10.1007/s15010-017-1050-z. [DOI] [PubMed] [Google Scholar]
- 5.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fekkar A., Neofytos D., Nguyen M.-.H., Clancy C.J., Kontoyiannis D.P., Lamoth F. COVID-19-associated pulmonary aspergillosis (CAPA): how big a problem is it? Clin Microbiol Infect. 2021;27:1376–1378. doi: 10.1016/j.cmi.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omrani A.S., Koleri J., Abid F.B., Daghfel J., Odaippurath T., Peediyakkal M.Z., et al. Clinical characteristics and risk factors for COVID-19-associated candidemia. Med Mycol. 2021;60:myab071. doi: 10.1093/mmy/myab071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayaaslan B., Eser F., Kaya Kalem A., Bilgic Z., Asilturk D., Hasanoglu I., et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses. 2021;64:1083–1091. doi: 10.1111/myc.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seagle E.E., Jackson B.R., Lockhart S.R., Georgacopoulos O., Nunnally N.S., Roland J., et al. The landscape of candidemia during the COVID-19 pandemic. Clin Infect Dis. 2021:ciab562. doi: 10.1093/cid/ciab562. [DOI] [PubMed] [Google Scholar]
- 10.Machado M., Estévez A., Sánchez-Carrillo C., Guinea J., Escribano P., Alonso R., et al. Incidence of candidemia is higher in COVID-19 versus non-COVID-19 patients, but not driven by intrahospital transmission. J Fungi (Basel) 2022;8:305. doi: 10.3390/jof8030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arastehfar A., Ünal N., Hoşbul T., Özarslan M.A., Karakoyun A.S., Polat F., et al. Candidemia among Coronavirus Disease 2019 patients in Turkey admitted to intensive care units: a retrospective multicenter study. Open Forum Infect Dis. 2022;9:ofac078. doi: 10.1093/ofid/ofac078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riche C.V.W., Cassol R., Pasqualotto A.C. Is the frequency of candidemia increasing in COVID-19 patients receiving corticosteroids? J Fungi (Basel) 2020;6:286. doi: 10.3390/jof6040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brickman D., Greenway A., Sobocinski K., Thai H., Turick A., Xuereb K., et al. Rapid critical care training of nurses in the surge response to the coronavirus pandemic. Am J Crit Care. 2020;29:e104–e107. doi: 10.4037/ajcc2020142. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald L., Baker C., Chenoweth C. Risk factors for candidemia in a children's hospital. Clin Infect Dis. 1998;26:642–645. doi: 10.1086/514580. [DOI] [PubMed] [Google Scholar]
- 15.Prestel C., Anderson E., Forsberg K., Lyman M., Perio M.A., Kuhar D., et al. Candida auris outbreak in a COVID-19 specialty care unit - Florida, July-August 2020. MMWR Morb Mortal Wkly Rep. 2021;70:56–57. doi: 10.15585/mmwr.mm7002e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moin S., Farooqi J., Rattani S., Nasir N., Zaka S., Jabeen K. C. auris and non-C. auris candidemia in hospitalized adult and pediatric COVID-19 patients; single center data from Pakistan. Med Mycol. 2021;59:1238–1242. doi: 10.1093/mmy/myab057. [DOI] [PubMed] [Google Scholar]
- 17.Horn D.L., Ostrosky-Zeichner L., Morris M.I., Ullmann A.J., Wu C., Buell D.N., et al. Factors related to survival and treatment success in invasive candidiasis or candidemia: a pooled analysis of two large, prospective, micafungin trials. Eur J Clin Microbiol Infect Dis. 2010;29:223–229. doi: 10.1007/s10096-009-0843-0. [DOI] [PubMed] [Google Scholar]