Abstract
Objective:
Currently, there is debate in the eating disorders field regarding how to define atypical anorexia (AAN), how prevalent it is in community and clinical settings, and how AAN rates compare with low-weight AN. This systematic review assesses AAN literature from 2007 to 2020, to investigate: (a) the demographic characteristics of AAN studies, (b) the prevalence of AAN compared with AN, (c) the range of operational definitions of AAN and the implications of these definitions, and (d) the proportion of patients with AAN and AN represented in consecutive admission and referral samples.
Method:
PsychINFO, CINAHL, PubMed, Greylit.org , and ProQuest databases were searched according to methods for Preferred Reporting Items for Systematic Reviews and Meta-Analyses systematic reviews, yielding 3,184 potential articles. Seventy-five eligible studies were coded for sixty-one variables.
Results:
Clinical samples predominantly included younger, female, white samples with limited diversity. In epidemiological designs, AAN was typically as common or more common than AN, and AAN rates varied significantly based on the population studied and operational definitions. In consecutive clinical samples, AAN was frequently less represented.
Discussion:
Although AAN appears to occur more frequently than AN in communities, fewer patients with AAN are being referred and admitted to eating disorder specific care, particularly in the United States. Given the significant medical and psychosocial consequences of AAN, and the importance of early intervention, this represents a crucial treatment gap. Additionally, results suggest the need for fine-tuning diagnostic definitions, greater diversity in AAN studies, and increased screening and referral for this vulnerable population.
Keywords: atypical anorexia nervosa, eating disorder not otherwise specified, higher weight eating disorder, other specified feeding and eating disorder, prevalence
Resumen
Objetivo:
Actualmente, hay debate en el campo de los trastornos alimenticios sobre cómo definir la anorexia atípica (ANA), cuán prevalente es en entornos comunitarios y clínicos, y cómo las tasas de ANA se comparan con AN de bajo peso. Esta revisión sistemática evalúa la literatura de ANA de 2007 a 2020, para investigar: 1) las características demográficas de los estudios de ANA, 2) la prevalencia de ANA en comparación con AN, 3) el rango de definiciones operativas de ANA y las implicaciones de estas definiciones, y 4) la proporción de pacientes con ANA y AN representados en muestras consecutivas de admisión y derivación.
Método:
Las bases de datos de PsychINFO, CINAHL, PubMed, Greylit.orgy y ProQuest fueron buscados de acuerdo con los métodos preferidos para el reporte de ítems para Revisiones Sistemáticas y Metanálisis de Revisiones Sistemáticas, dando lugar a 3184 artículos potenciales. Setenta y cinco estudios elegibles fueron codificados para 61 variables.
Resultados:
Las muestras clínicas incluían predominantemente muestras de femeninas, más jóvenes, y blancas con diversidad limitada. En los diseños epidemiológicos, la ANA era típicamente tan común o más común que AN, y las tasas de ANA variaban significativamente en función de la población estudiada y las definiciones operacionales. En muestras clínicas consecutivas, la ANA fue frecuentemente menos representada.
Discusión:
Aunque ANA parece ocurrir con más frecuencia que AN en las comunidades, menos pacientes con ANA están siendo referidos y admitidos a la atención específica del trastorno alimentario, particularmente en los Estados Unidos. Dadas las importantes consecuencias médicas y psicosociales de ANA, y la importancia de la intervención temprana, esto representa una brecha de tratamiento crucial. Además, los resultados sugieren la necesidad de ajustar las definiciones diagnósticas, una mayor diversidad en los estudios de ANA y un mayor cribado y referencia a tratamiento para esta población vulnerable.
The growth of anorexia nervosa (AN) research has led to more complex and nuanced conceptualizations of restrictive eating disorders (EDs). In particular, though AN diagnosis requires individuals to present at “significantly low weight,” physiological symptoms of malnutrition can occur within underweight, normal weight, and higher-weight bodies (Peebles, Hardy, Wilson, & Lock, 2010; Sawyer, Whitelaw, Grange, Yeo, & Hughes, 2016). Thus, some suggest that to better capture the diversity of patients with restrictive EDs, we must “move beyond ‘skinniness’” because the “face of AN is changing” (Garber, 2018, p. 669). Garber asserts that the large and growing number of patients with atypical AN (AAN) highlights important demographic shifts to which we must attend to ensure researchers keep pace with increasing ED patient diversity.
The Diagnostic and Statistical Manual of Mental Disorders (DSM-5; 2013) denotes AAN as the first of five clinical examples of Other Specified Feeding and EDs (OSFED). According to DSM-5 criteria, patients who meet criteria for AN, and have lost significant weight, yet are still within or above the normal body mass index (BMI) range, may be classified as AAN. These patients could include those whose bodies have resisted weight-loss despite caloric deprivation or those whose larger bodies have lost significant weight but have not reached BMIs considered significantly low.
While the DSM-5 recognizes that natural body variation (e.g., body build, weight history, physiological disturbances, developmental stage) precludes one universal standard for “significantly low weight,” it also posits a BMI <18.5 to guide clinicians in assessment. However, due to this diagnostic flexibility (and to iterative changes in AN criteria), operational differences in AN/AAN diagnostic practices persist (Stice, Marti, & Rohde, 2013; Thomas et al., 2010). Multiple thresholds for “low weight” and “significant weight loss” have been proposed (Forney, Brown, Holland-Carter, Kennedy, & Keel, 2017), with little consensus in the field. These debates have led some researchers to propose shedding the weight criteria for AN altogether, in favor of considering each individual’s age, gender, illness and weight trajectory, bone, and muscle mass, and to ensure that treatment remains as inclusive as possible (Phillipou & Beilharz, 2019).
What has come to be called “AAN” or “higher-weight AN” has gone by other names historically [e.g., “ED Not Otherwise Specified (EDNOS)-AN”, “subthreshold AN,” and “EDNOS-weight”]. Furthermore, in the DSM-IV-TR, AAN individuals were only partially captured in the residual EDNOS category (the second example presentation), which described individuals who met all criteria for AN, but whose weight was within the normal range (American Pyschiatric Association, 2000). By including samples of restricting patients in higher-weight bodies, DSM-5 conceptualization of AAN has expanded, with more patients meeting criteria for AAN in the DSM-5 than would have met criteria for EDNOS-AN in the DSM-IV.
Further complicating AAN nosology, due to being grouped within the residual ED diagnostic category, many AAN samples have been examined heterogeneously (e.g., combined with sub-threshold bulimia nervosa and/or binge ED), rather than in homogenous AAN groups. Thus, these methodological choices (along with diverging operational definitions) may obscure the characteristics of AAN, while making it more challenging to identify AAN samples in the literature.
Perhaps due to these issues of evolving terminology and residual case grouping, we are not aware of any systematic reviews that focus on the topic of higher-weight (i.e., atypical) AN. This represents an important knowledge gap in ED research, as AAN represents a large and growing portion of OSFED cases (Hay et al., 2017; Whitelaw, Gilbertson, Lee, & Sawyer, 2014), with some reporting a nearly five-fold increase in AAN patients presenting for care in the last decade alone (Whitelaw et al., 2014). Further, while researchers and clinicians have begun to assess various treatment interventions and medical protocols for AAN (e.g., Peebles et al., 2017; Swenne, Parling, & Salonen Ros, 2017), as a field we know very little about the scope of AAN, how often it occurs, and how frequently affected individuals access care.
While some research has reviewed EDNOS as a whole (Thomas, Vartanian, & Brownell, 2009), we are not aware of any studies that examine AAN samples specifically. To our knowledge, this is the first review to collate and synthesize data on AAN in an effort to better assess the scope of disease burden. Understanding the burden posed by AAN, requires locating individuals with AAN. It is critical to discover how prevalent AAN is in the population, and to clarify how AAN is operationalized differentially, and the potential impacts of those definitions. Understanding this heterogeneity will inform future research on AAN. Further, given the “changing face of AN” (Garber, 2018) and the changing demographic profile of ED patients (Mitchison et al., 2020), discovering which populations have been examined will inform the field regarding increasing inclusive AAN representation. Finally, it is also important to examine how many ED patients receiving care have AAN, as potential differences in prevalence rates of AAN in community and treatment settings could elucidate gaps in our treatment landscape. Understanding the degree to which AAN is present and untreated in our communities is important as we seek to increase the speed at which ED individuals are identified and referred for care.
1 |. THE CURRENT STUDY
To assess the scope of AAN, we posed the following aims: (a) describe the demographic characteristics (age, race, ethnicity, gender, socioeconomic status, and sexual orientation) of participants in studies of AAN, (b) describe the prevalence of AAN compared with AN in epidemiological studies, (c) qualitatively characterize how AAN is operationalized, the range of definitions used, where definitions diverge, and the potential implications of these different definitional thresholds, and (d) describe the proportion of patients with AAN and AN represented in studies using consecutive admissions and referral samples to better understand treatment need.
2 |. METHODS
The current systematic review followed the Preferred Reporting Items for Systematic Reviews and Metanalyses (PRISMA) statement (Moher, Liberati, Tetzlaff, & Altman, 2009). An adapted PRISMA Review Checklist can be found in the Table S1.
2.1 |. Eligibility criteria
All English language articles between January 1, 2007 and September 5, 2020, containing primary data (or secondary data analysis) involving a group appearing to meet criteria for DSM-5 AAN were evaluated. The arbitrary cutoff of 2007 was included after a review of all literature, in an effort to balance completing a comprehensive review of AAN literature with the need for more homogenous operational definitions. Additionally, the APA first established a DSM-5 task force for EDs in 2007 (Yan, 2007), representing a symbolic shift, as the field began transitioning toward new diagnostic frameworks.
For inclusion, potential articles had to include a sample which met all of the following four DSM-5 AAN criteria: (a) energy restriction, (b) fear of weight gain/behavior interfering with weight gain, (c) disturbance in self-perceived weight/shape or undue influence of weight/shape, and (d) BMI >18.5. Articles utilizing DSM-IV and DSM-III diagnostic criteria were eligible for inclusion, if the corresponding DSM-5 criteria capturing food restriction, fear of weight gain, and body image disturbance were met. Studies including subgroups meeting those four criteria (whether or not they were called “AAN”) were included in the analysis. Patient groups failing to meet clinical levels of distress (e.g., “subclinical” groups or prodromes) were excluded.
To assess AAN prevalence, we examined samples which produced prevalence rates for a given population. Eligible designs included random samples (e.g., Forney et al., 2017), samples capturing all individuals within a given catchment area (e.g., Hammerle, Huss, Ernst, & Bürger, 2016; Isomaa, Isomaa, Marttunen, & Kaltiala-Heino, 2010), twin studies (e.g., Mustelin, Lehtokari, & Keski-Rahkonen, 2016), cohort studies (e.g., Micali et al., 2017), community-based samples (e.g., Zimmerman, Francione-Witt, Chelminski, Young, & Tortolani, 2008), and large research samples (e.g., Mitchison et al., 2020). To address the proportion of AN and AAN in treatment samples, we included all articles which utilized consecutive referrals or admissions to treatment, or consecutive chart review. In cases of unclear eligibility, authors were contacted to confirm.
Weight criteria for AAN is complex, evolving with various DSM editions, and are often measured differently across studies and populations (e.g., adolescents v. adults). In this article, we defined AAN to include those with BMI >18.5, BMI percentile >10, percent median BMI (%mBMI) >90, percent-expected-body-weight/height >90, following criteria outlined by Kandemir et al. (2017). AAN samples limited to 18.5 < BMI < 19.0 were excluded, as these AAN samples could also be classified as AN, given flexibility within AN low-weight criteria. We decided to eliminate studies limited to this small clinical range (18.5 < BMI < 19.0) to better differentiate AAN samples from AN. Additionally, we included samples from the DSM-IV era, provided that some patients in that AAN sample had BMI >19.0, and no patients in the AAN sample had BMI <17.5.
2.2 |. Information sources
The PsychINFO, CINAHL, and PubMed data bases were searched, with the final literature search completed through September 5, 2020. Grey and unpublished literature were searched using the ProQuest and Greylit.org databases, in addition to soliciting unpublished data from a relevant listserv. Articles were also added through back-citations and study authors directly contacting the researchers.
2.3 |. Search
AAN has not yet been indexed with Medical Subject Headings (MeSH terms), thus necessitating a manual search for all related names of the disorder. Syntax was developed iteratively over the course of 3 months, in collaboration with consults to national ED experts and health science librarians. All searches, trial syntax, and results were documented. Ultimately, the following syntax was used:
(“partial anorexia”) or (“partial syndrome” and “anorexia”) or (“subthreshold” and “anorex* ”) or (“anorexia spectrum”) or (“DSM-5” and “anorexia”) or (“prodrom* ” and “anorex* ”) or (“higher-weight” and “anorex* ”) or (“sub-clinical” and “anorex* ”) or (“atypical” and “anorexia”) or (“higher-weight” and “restrictive eating disorder* ”) or (“subclinical” and “anorex* ”) or (“sub-threshold” and “anorex* ”) or (“subsyndromal anorex* ”) or (“higher-weight anorex* ”) or (“EDNOS-wt”) or (“weight suppression” and “anorexia”) or (“weight suppression” and “restrictive eating disorder”)
2.4 |. Study selection
A PRISMA (Moher et al., 2009) chart detailing the screening process and reasons for exclusions is found in Figure 1. During prescreening, articles were first screened for eligible years, language, and format, and duplicates were removed. Following this, we removed clearly unrelated articles in the title search. Full texts of the remaining articles (n = 1,041) were then assessed. Studies with unclear eligibility were discussed by authors EH and JM until consensus was reached to either exclude, include, or request further information from study authors. Pursuant to this, 28 study authors were contacted regarding eligibility (6 eligible, 9 no response, and 13 ineligible).
FIGURE 1.
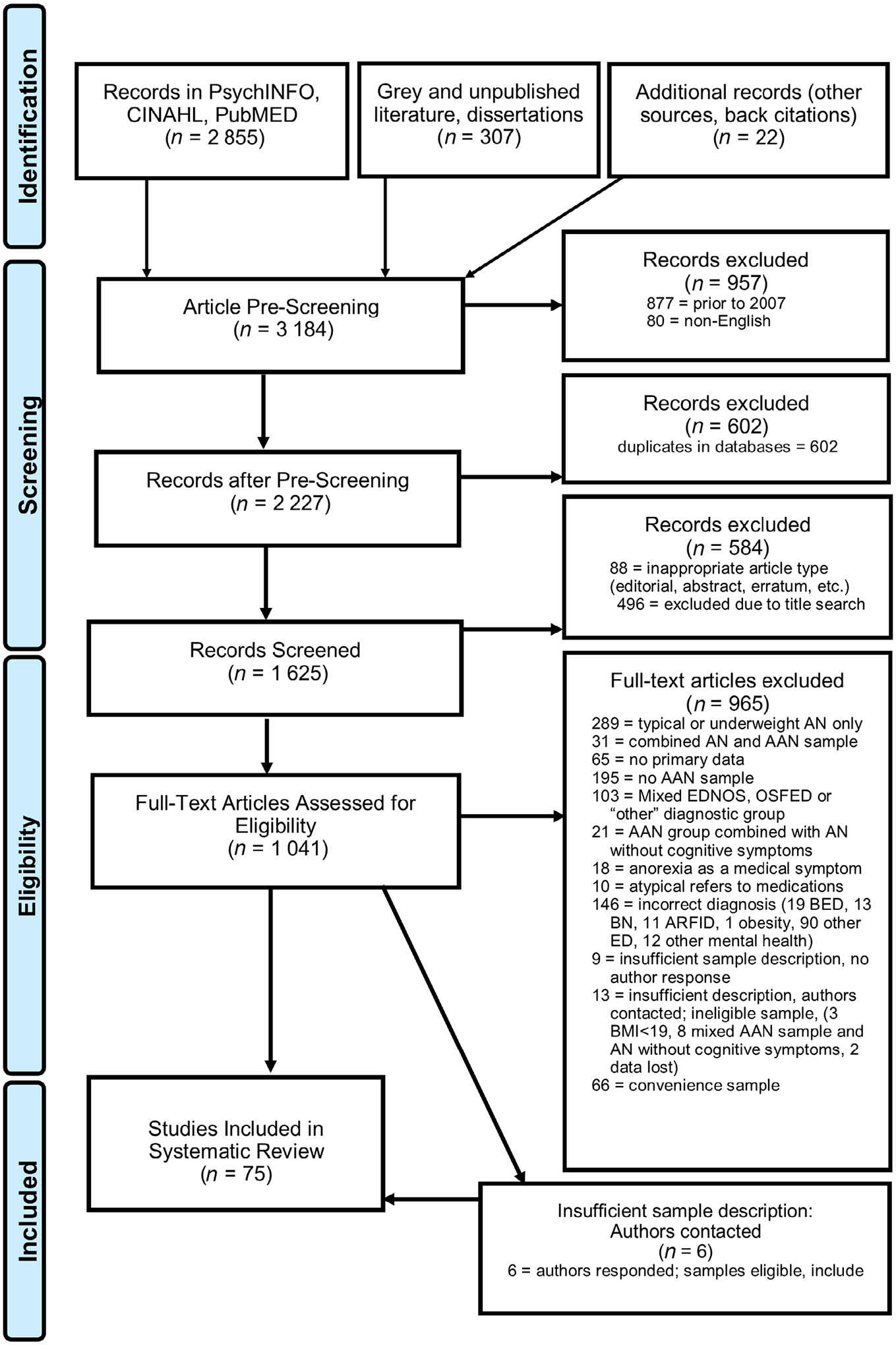
PRISMA flow chart. AAN, atypical anorexia nervosa; AN, anorexia nervosa; ARFID, avoidant/restrictive food intake disorder; BED, binge-eating disorder; BMI, body mass index; BN, bulimia nervosa; EDNOS, eating disorder not otherwise specified; OSFED, other specified feeding or eating disorder; PRISMA, Preferred Reported Items for Systematic Reviews and Meta-Analyses
2.5 |. Data collection process, data items, and summary measures
Complete data items are located in the Table S2. Sixty-one variables were coded by author EH into five topic matrices: Study Identification (e.g., authors, title, country of data collection), Sample Characteristics (e.g., mean age, percent female), Methods (e.g., design, data analysis, measures), Findings (e.g., lifetime prevalence rate, point prevalence rate), and Implications (e.g., main argument, implications). The following summary measures were used for each of the aims—Aim 1: percent female, gender, percent white race, percent Hispanic ethnicity, full racial and ethnic characteristics, socioeconomic characteristics, sexual orientation, mean age, and age range of the sample; Aim 2: lifetime prevalence, point prevalence, and 12-month prevalence (for each gender and total); Aim 3: operational definition of AN used, diagnostic reference for sample, and Aim 4: percent of consecutive samples diagnosed with AN and AAN.
2.6 |. Synthesis of results
We used Garrard’s matrix method to synthesize data (Garrard, 2014). In brief, this method involves reading articles chronologically, and analyzing the literature using a matrix of variables to compare studies. Variables (matrix columns) are compared across studies (matrix rows), and analyzed for themes, convergences of findings, and divergences of findings. For quantitative findings, data (e.g., prevalence rates, percentages of samples) were extracted and tabulated and descriptive statistics were calculated. For qualitative results, operational definitions were reviewed for common themes and places of divergence and convergence; additionally, each definition was reviewed individually for implications of which groups may be systematically excluded.
2.7 |. Risk of bias in individual studies and across studies
To compare study quality systematically across study designs and methodologies, this review utilized the Quality Assessment Scale (QAS; Kmet, Lee, & Cook, 2004) scored by author EH. The QAS is a validated tool (Kmet et al., 2004), cited in over 1,000 studies, that has been utilized for quality assessment within multiple research designs, including prevalence studies (Migliavaca, Stein, Colpani, Munn, & Falavigna, 2020). The QAS compares articles on 14 elements, including the research question, study design, comparison groups, random assignment and blinding, outcome measures, sample size, analytic methods, variance reporting, confounding variables, results reporting, and conclusions (Kmet et al., 2004). Elements were assessed as present (2), absent (0), partially present (1), or “n/a.” Summary scores were calculated by taking the mean of all items, and scaling scores from 0 to 1.0, with a score of 0 indicating the absence of all criteria, and a score of 1.0 indicating the presence of all criteria. Articles were scored accordingly, and reasons for low scores recorded, with the researcher reviewing scores and reasons for lower scores after every 10 articles scored. All epidemiological studies were scored for quality, and all studies were included in narrative analysis, regardless of QAS score. Additionally, to reduce risk of sample over-representation, studies utilizing overlapping samples (Ernst, Bürger, & Hammerle, 2017; Fairweather-Schmidt & Wade, 2014; Hammerle et al., 2016; Machado, Gonçalves, & Hoek, 2013; Machado, Machado, Gonçalves, & Hoek, 2007; Wade & O’Shea, 2015) were combined when possible, such that the sample was only counted once toward meta-analysis (e.g., weighted forest plots). Studies using partially overlapping samples (e.g., sampling different groups from a larger sample) were noted to have overlapping samples for transparency, and each study was coded separately so full demographics for each sample could be ascertained.
3 |. RESULTS
3.1 |. Study selection
The search strategy yielded 3,184 articles for possible inclusion (See Figure 1 for PRISMA Flow Diagram). Prescreening eliminated 2,143 articles based on year, article type, duplicates, and/or title search (see Figure 1 for full details). One thousand and forty-one full text articles were assessed for eligibility, of which nine hundred and sixty-five were excluded due to exclusion criteria fully described in Figure 1. An additional 28 studies contained research methods with unclear eligibility; these 28 authors were contacted to clarify eligibility. Nine authors failed to respond and were excluded, 13 studies were deemed ineligible based on author response, and an additional 6 studies met inclusion criteria with author clarification. Final sample for this systematic review included 75 articles; of these, 17 utilized community-based epidemiological samples, and 58 utilized consecutive clinical admissions or referrals.
3.2 |. Risk of bias
Epidemiological studies were scored for quality using the QAS (Kmet et al., 2004) with summary scores ranging from .64 to .91 (M = .84). Four epidemiological studies (29%) utilized two-stage designs; the remaining 10 studies (64%) relied on a single time point measurement to determine diagnosis. Two studies utilized cohort designs and measured diagnoses over three consecutive waves (Allen, Byrne, Oddy, & Crosby, 2013; Wade & O’Shea, 2015). Seven utilized school-based or university-based samples, three studies utilized twin cohorts, two utilized population-based designs, two utilized birth cohort samples, and one used an outpatient psychiatric patient sample. Sample size was commonly an issue, such that even in larger population-based designs, sample sizes often precluded intended subgroup analyses.
3.3 |. Aim 1: Study demographic characteristics
Demographic characteristics of included epidemiological studies are shown in Table 1; demographic characteristics of studies utilizing consecutive admissions and referrals are shown in Table 2. As four studies had total overlap, two of these studies (one from each pair) were not counted in results percentages. Nearly all studies (97%, n = 73) defined gender in a binary fashion (e.g., males/females, boys/girls), with one study including a third gender category (“other”; Mitchison, Hay, Slewa-Younan, & Mond, 2014) and one study including nonbinary and “decline to disclose” options (Flatt, 2020). Only one named gender identity samples such as nonbinary, transgender, or gender non-conforming individuals (Flatt, 2020). While two studies (3%) included only male participants, 38% (n = 28) of studies were limited to females. Overwhelmingly, study populations were female, with nearly two thirds of studies (64%, n = 47) having females making up at least 90% of the sample. Studies were mostly focused on adolescent populations, with 81% (n = 59) of studies including adolescents aged 13–19 years. Studies with children showed moderate representation with 36% (n = 26) including children aged 12 and under. Fifty-five percent (n = 40) of studies included young adult samples between the ages of 20–30 years. By contrast, mid-adults and older adults were least represented with only 33% (n = 24) of samples including individuals over age 31. A minority of studies reported race as a demographic variable (36%, n = 26), and all but nine of these studies reported study populations with at least 85% of the sample being of white race. Only 17 studies (23%) included a full racial breakdown of their sample; 14 (19%) studies included measures of Hispanic ethnicity. Only three studies (4%) included measures of sexual orientation; one (1%) included a measure of disability status. For full racial, ethnicity, socioeconomic status, and other demographics (see Tables 1 and 2).
TABLE 1.
Demographic characteristics of epidemiological studies
| Age in years | Race and ethnicity demographics | Other demographic information | % sample | Quality Summary score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Article Author, year | N Sample N | Sex % female | Mean (SD)a; range | % white | Racial, ethnicity, and nationality details | SES, education, etc. | AAN | AN | |
| Machado et al. (2007) | 2,028 | 100% | 16.2 (1.3); 16.1 (1.2); 12–23 | NR | Study conducted in Portugal. | 21% high SES, 22% medium high SES, 32% medium SES, 24% medium low SES, 1% low SES. 9th-12th graders from 11 public schools. | 0.2% | 0.4% | 0.82 |
| Zimmerman et al. (2008) | 2,500 | 60.6% | 38.3 (12.8); NR | 87.6% | Study conducted in US. | Community-based outpatient clinic patients. 90.2% graduated high school; 41.6% married; and 31.0% single. | 0.4% | 0.0% | 0.77 |
| Isomaa et al. (2010) | 606 | 47.5% | 15.4 (0.4); 15 | NR | Study conducted in Finland. | Sampled rural and urban areas. All 15-year-old pupils in 9th grade in Jakobstad region. Some schools spoke Finnish, others Swedish. | 1.8% | 0.7% | 0.91 |
| Allen et al. (2013) | 1,383 | 51% | 14.0 (0.2); 16.9 (0.2); 20.0 (0.4); 14–20 | NR | Born in Australia. | Single-parent families and lower income families were statistically less represented. | m: 0.3, 0.0, 0.3%; f: 0.9, 0.0, 0.1% | m: 0.0, 0.0, 0.0%; f: 0.3,b, 0.6% | 0.86 |
| Machado et al. (2013) | 3,048 | 100% | 16.2 (1.3); 21.8 (4.1); 12–58 | NR | Study conducted in Portugal. | Students in high school and university. | 0.40% | 0.7% | 0.82 |
| Stice et al. (2013) | 496 | 100% | 13; 12–23 | 68% | Study conducted in US. 2% Asian/Pacific Islanders, 7% African Americans, 68% Caucasians, 18% Hispanic, 1% Native Americans, 4% other or mixed racial heritage. | Average parental education: 29% high school graduate or less, 23% some college, 33% college graduate, 15% graduate degree. All in 7th or 8th grade. | 2.8% | 0.8% | 0.91 |
| Fairweather-Schmidt and Wade (2014); including Wade and O’Shea (2015) | 699 | 100% | 14.0 (NR); 15.1 (NR); 16.9 (NR); 12.7–19.8 | 100% | Study conducted in Australia. Sample was Caucasian. | SES index for areas: 101.14 (standard mean: 100). | 1.9% AAN; 4.7% RED | 2.0% | 0.87 |
| Hammerle et al. (2016); including Ernst et al. (2017) | 1,654 | 52.8% | 13.4 (0.8); NR | NR | Sample from a single region in Germany. | All types of secondary schools in Rhineland-Palatinate. 55.6% 7th grade; 44.4% 8th grade. | 3.6% | 0.3% | 0.87 |
| Mustelin et al. (2016) | 2,825 | 100% | 24.4 (0.9); 22–27 | NR | Born in Finland. | NR | 0.2% | 0.78 | |
| Forney et al. (2017) | 2,464 | 67.8% | f: 35.3 (11.8); m: 32.4 (12.3); NR | 67% | Study conducted in US. 67.0% non-Hispanic Caucasian, 17.4% Asian, 6.7% Hispanic, 6.3% African American, 0.6% American Indian/Alaska native, 0.4% native Hawaiian/other Pacific islander, 1.8% “other” or did not answer. | Attending a private university. | 2–13% | 0.82 | |
| Micali et al. (2017) | 5,658 | 100% | 47.8 (4.5); “midlife” | 97.9% | Study conducted in United Kingdom. | Women with higher education were overrepresented. Participants birthed babies. | 1.7% | 3.6% | 0.78 |
| Hay et al. (2017) | 5,737 | 49.8% | 43.5 (NR); 15+ | NR | Study conducted in Australia. | Median income for people with AN-broad was $50–60,000. | 2.5% | 0.5% | 0.91 |
| Castelao-Naval et al. (2019) | 422 | 60.0% | 24.5 (6.6); 18–75 | NR | Study conducted in Spain. | University students with these majors: 56.4% nursing, 32.3% physiotherapy, 7.1% podiatry + physiotherapy, 4.3% optometry. | 12.8% risk of AN | 0.64 | |
| Mitchison et al. (2020) | 5,191 | 48.4%c | 14.9 (NR); 11–19 | NR | 88.2% born in Australia. | Lower SES students were statistically less represented. 41.7% grades 7–8; 39.7% grades 9–10; 18.6% grades 11–12. | 2.9% | 0.7% | 0.91 |
| Silén et al. (2020) | 1,347 | 52.6% | 22.4 (0.7); 21–26 | NR | Born in Finland. | NR | 1.2% | 3.4% | 0.86 |
Abbreviations: AN, anorexia nervosa; AAN, atypical anorexia nervosa; f, female; m, male; NR, not reported; PD, purging disorder; RED, restrictive eating disorder; SES, socioeconomic status; US, United States.
Multiple waves or studies separated by semi-colons.
Age group not explicitly stated.
49.2% male, and 2.4% “other” gender.
TABLE 2.
Demographic characteristics of consecutive admission and referral studies
| Age in years | Racial characteristics | Other demographic characteristics | % sample | |||||
|---|---|---|---|---|---|---|---|---|
| Article Author, year | N Sample Na | Sex % femaleb | Mean (SD)b; range | % white | Race, ethnicity, and nationality characteristics | Socioeconomic status, education, sexual orientation, etc. | AAN | AN |
| Rockert, Kaplan, and Olmsted (2007) | 1,449 | 97% | 28.5 (8.9); 15–70 | NR | NR | NR | 11.8% + 10.0% PD | 23.5% |
| Eddy, Doyle, Hoste, Herzog, and Le Grange (2008) | 281 | 92.2% | 16 (2.0); 12–19 | 87.9% | 74.7% non-Hispanic white, 13.2% Hispanic white, 7.5% African American, 1.1% Asian, 2.8% other, 1.8% none. | NR | 10.7% | 20.3% |
| Santonastaso et al. (2009) | 2,117 | 100% | 21.2 (5.0) AAN; NR | NR | NR | NR | 4.0% | 20.9% |
| Ricca et al. (2010) | 103 | 100% | 29.9 (8.9); 16–45 | NR | NR | Mean of 13.53 years education for AN and 14.00 years education for subthreshold AN. | 34.0% | 51.5% |
| Thomas et al. (2010) | 76 | 100% | 18.7 (1.5); 16–23 | 97% | 97.0% non-Latino white, 1.5% Latino, 1.5% multiracial. | NR | NR | 32.9% |
| Peebles et al. (2010) | 1,310 | 100% | 15.4 (2.0); 8–19 | 75.3% | 75.3% white, 8.2% Asian, 7.5% Hispanic, 1.1% black, 0.2% Pacific islander, 7.5% other. | NR | 3.5% | 25.2% |
| Sysko and Walsh (2011) | 247 | 95.1% | 25.2–35.7 (NR); NR | NR | NR | NR | 2.0% | 35.0% |
| Bayes and Madden (2011) | 10 | 0% | 12.8 median; 14–18 | NR | NR | 80% metropolitan regions, 10% rural, 10% interstate. 100% lived at home with parents. 40% attended primary school, 60% attending high school. 70% lived with both parents; 30% lived with mothers (divorced). | 40.0% | 30.0% |
| Fairburn and Cooper (2011) | 167 | NR | NR (NR); NR | NR | NR | NR | 6.6% | 28.7% |
| Schreyer (2012) | 173 | 100% | 28.1 (11.6); NR | 89.00% | 89.0% non-Hispanic white, 4.0% African American, 1.2$ Asian American, 2.9% Hispanic, 2.3% other race. | NR | 28.30% | 45.70% |
| Weingeroff (2012) | 290 | 78% | 27.6 (6.9); NR | 89% | NR | Mean socioeconomic score of 2.6 on the Hollinghead-Redlich scale; 41% received social security disability insurance. | 36% | 21% |
| Birgegård, Norring, and Clinton (2012) | 2,584 | 96.8% | 23 (8.3); 10–67 | NR | NR | NR | 28.0% | 22.0% |
| Sysko et al. (2012) | 70; 55 | 91.4% | 31.3 (11.1); 18–73, 18–64 | NR | NR | NR | 4.3% | 32.9% |
| Ornstein et al. (2013) | 215 | 88.6% | 15.4 (3.3); 8–21 | NR | NR | NR | 22.3% | 40.0% |
| Ekeroth, Clinton, Norring, and Birgegård (2013) | 2,233 | 100% | 25.7 (7.8); 18–67 | NR | NR | NR | 13.9% AAN; 8.2% PD | 29.5% |
| Conceição et al. (2013) | 12 | 100% | 46.8 (16.6); 23–69 | NR | NR | NR | 58.3% | 25.0% |
| Kachani, Barroso, Brasiliano, Hochgraf, and Cordás (2014) | 85 | 100% | 28.2 (NR); 18–55 | 0% | Brazilian. | 3.5% primary education, 35.3% secondary education, 30.6% some university, 24.7% university degree. 25.9% student, 36.5% employed, 8.2% housewife, 29.4% unemployed. 78.8% no children, 21.2% 1+ children; 90.6% heterosexual, 5.0% homosexual, 3.5% bisexual. | 31.8% | 68.2% |
| Forman et al. (2014) | 700 | 86.3% | 15.3 (2.4); 9–21 | 87.6 | NR | NR | 33.9% | 53.6% |
| Whitelaw et al. (2014) | 99 | 87% | 15.2 (1.5); 12–19 | NR | NR | NR | 26.3% | 73.7% |
| Caudle, Pang, Mancuso, Castle, and Newton (2015) | 285 | 94.3% | 26 (NR); 17–78 | Majority | NR | NR | 6.7% | 61.8% |
| Damiano, Reece, Reid, Atkins, and Patton (2015) | 39 | 100% | 15.6 (1.5); 13–18 | NR | 97.4% born in Australia, 2.6% born in Croatia. | English primary language spoken at home for 94.9%, 2.6% spoke Cantonese, 2.6% spoke Serbian. | 74.4% | 25.6% |
| Kass et al. (2015) | 515 | 92.2% | 15.4 (2.0); 7–18 | 89.9% | 12.6% Hispanic. | Family income in US dollars: $117,200. | 21.6% | 38.3% |
| Fichter, Quadflieg, Gierk, Voderholzer, and Heuser (2015) | 605; 408; 131 | 97.9%; 96.8%; 100% | 24.9 (10.4); NR | NR | NR | 10.1% <9 years education, 67.9% 10–13 years education, 12.7% 14+ years education, 9.3% still in professional training or study. 87.9% never married, 9.9% married, 2.2% divorced, separated, or widowed; 34.3% in relationship. 54.7% live with parents/relatives, 16.7% live alone, 14.7 live with partner, 13.9% other. | 7.2% | 50.2% |
| Redgrave et al. (2015) | 361 | 91.7% | 28.46 (NR); 11–78 | 92.8% | NR | 22.1% | 77.9% | |
| Monge et al. (2015) | 635; 359 | 86.1% | 15.3 (2.4); 9–21 | 89.5% | NR | NR | 35.6% | 50.6% |
| Welch, Ghaderi, and Swenne (2015) | 664 | 91.3% | m: 14.9 (1.6); f: 15.2 (1.7); 13–19 | NR | NR | NR | 86.1% | 8.7% |
| Silén et al. (2015) | 47 | 91.4% | 14.6 (1.2); NR | NR | NR | NR | 2.1% | 97.9% |
| Thomas et al. (2015) | 150 | 100% | 18.1 (2.6); 13–27 | 94% | 1.3% American Indian/Alaska Native, 2.7% Black/African American, 4.0% Asian, 94.0% White. 4.0% Hispanic/Latino. | 47.3% some high school, 12.7% high school graduate, 34.7% some college, 4.0% degree. 88.0% heterosexual, 4.7% homosexual, 5.3% bisexual, 0.7% other. | 4.7–15.3% | 46.0–46.7% |
| Hughes, Le Grange, Court, and Sawyer (2017) | 286 (42) | 88% | 15.4 (1.4); 12–18 | NR | NR | 67% intact family. | 14.7% | NR |
| Sawyer et al. (2016) | 256 | 88% | 15.5 (1.4); 14–16 | NR | NR | NR | 16.4% | 46.1% |
| Makhzoumi et al. (2017) | 211 | 100% | 28.5 (12.6); NR | 88.80% | NR | NR | 15.0% | 85.0% |
| Swenne (2016) | 275 | 100% | 13.4–18.8 (1.3–1.4); <18 | NR | NR | NR | 86.9% | 9.8% |
| Koutek, Kocourkova, and Dudova (2016) | 47 | 100% | 15.5 (NR); 10.25–18 | NR | NR | 45% parents divorced. 47% both biological parents; 36% one biological parent; 13% one biological parent and partner; 4% other family typology. | 17.0% | 77.0% |
| Smith et al. (2016) | 129 | 94.6% | 15.8 (2.4); 10–22 | 89.1% | 89.1% non-Hispanic Caucasian, 3.9% Asian American, 1.6% African American, 1.6% Hispanic/Latino, 0.8% American Indian/Alaskan, 0.8% multiracial, 2.3% did not answer. | NR | 12.4% | 87.6% |
| Schreyer et al. (2016) | 202 | 94.60% | 31.8 (12.6); NR | 89% | NR | NR | 13.40% | 80.10% |
| Månsson, Parling, and Swenne (2016) | 47 | 98% | 15.1 (1.5); 11.6–17.6 | NR | 94% both parents born in Sweden. | 68% living with both biological parents. | 78.7% | 19.1% |
| Darrow, Accurso, Nauman, Goldschmidt, and Le Grange (2017) | 123 | 92.7% | 15.7 (1.9); 10–18 | 86.2% | 86.2% White, 6.5% Black/African American, 2.4% Asian, 4.9% mixed race. 20.3% Hispanic | NR | 12.2% | 18.7% |
| Nakai et al. (2017) | 304 | 96.4% | 25.2 (7.6); 16–45 | 0% | All of Japanese ethnicity. | NR | 2.3% | 35.2% |
| Bacopoulou et al. (2017) | 37 | 100% | 14.7 (NR); 12–20 | NR | NR | NR | 13.5% | 48.6% |
| Swenne et al. (2017) | 341 (201) | 93.5% | 15 (1.7); 9.4–17.8 | NR | NR | NR | 84.8% | 12.0% |
| Swenne and Ros (2017) | 339 | 100% | 15 (1.7); 10–17 | NR | NR | NR | 84.7% | 15.3% |
| Peebles et al. (2017) | 215 | 88% | 15.3 (NR); 5.8–23.2 | 86.0% | 86% White, 5% Black or African American, 2% Asian, 7% Other. 2% Hispanic or Latino; 98% not Hispanic or Latino. | NR | 18.0% | 64.0% |
| Kennedy et al. (2017) | 522 | 88% | 15.6 (2.3); NR | 81.4% | NR | NR | 39.5% | 60.5% |
| Nagata et al. (2017) | 1,083 | 88.7% | 15.6 (NR); 10–19 | NR | NR | NR | 7.8% | 71.4% |
| Matthews, Lenz, Peugh, Copps, and Peterson (2018) | 51 dyads | 76%; 92.1% | 14.9 (1.41); NR | NR | NR | NR | 33.30% | 66.70% |
| Wilkes (2018) | 34 | 100% | 35.7 (13.73); 18–75 | 82% | 82% white, 6% black, 11% more than one race. 0% Hispanic. | Highest education: 6% high school, 27% some college, 9% 2-year college, 29% 4-year college, 9% some grad school, 21% completed grad school. Work: 3% wage earner, 3% retired, 9% homemaker, 21% student, 12% part time, 35% full time, 18% unemployed. Family annual income in thousands: 24% less than $50, 35% $50–$80, 15% $80–$100, 12% $100–$150, 15% more than $150. | 11.80% | 14.70% |
| Nakai et al. (2018) | 1,363 | 98.2% | 22.0 (5.6); 15–51 | 0.0% | All of Japanese ethnicity. | NR | 2.5% | 40.2% |
| Riesco et al. (2018) | 176 | 100% | 25.2 (7.6); NR | NR | 93.9% Spanish. 6.1% immigrant. | 40.2% primary education, 43.9% secondary education, 27.0% university; 28.0% unemployed, 72.0% employed. 82.9% single, 8.5% married, 8.5% divorced. | 46.6% | NA |
| Whitelaw, Lee, Gilbertson, and Sawyer (2018) | 171 | 84.0% | 15.4 (1.4); 12–19 | NR | NR | NR | 31.0% | 69.0% |
| Lebow et al. (2019) | 153 | 88.9% | 14.7 (2.3); <18 | 86.3% | 86.3% non-Hispanic White, 13.7% Hispanic Whites, 2.0% Black or African American, 1.3% Asian, 1.3% >1 race/ethnicity. | NR | 45.1% | 54.9% |
| Monteleone et al. (2020) | 52 | 100% | 23.2 (5.4); 18+ | NR | NR | NR | 23.1% | 32.7% |
| Nagata et al. (2019) | 286 | 93% | 15.3 (2.0); 9–20 | NR | NR | NR | 8.0% | 92.0% |
| Schorr et al. (2019) | 103 | 0% | 26.2 (6.0); 18–63 | 100.0% | NR | NR | 17.5% | 25.2% |
| Andrés-Pepiñá et al. (2020) | 38 | 100% | 37.0 (4.0); NR | NR | NR | NR | 10.5% | 89.5% |
| Zanna et al. (2020) | 346 | 83.0% | 15.8 (2.5); <18 | NR | NR | NR | 17.1% | 55.8% |
| Flatt (2020) | 24,043 | 90.20% | NR (NR); 13–45 | 81.70% | 81.7% white; 3.9% Asian; 2.5% black; 0.8% American Indian/Alaska native; 0.3% native Hawaiian/Pacific Islander; 6.1% more than one race; 4.6% other race. 10.9% Hispanic. | 25.3% identified as LGBTQ. 3.8% lived with a disability. Annual income in thousands: 20.2% less than $20; 15.9% $20–$40; 15.1% $40–$60; 13.4% $60–$80; 10.0% $80–$100; 15.5% $100–$150; 11.8% > $150. | 3.60% | 5.60% |
| Smith (2020) | 223 | 100% | NR (NR); 14+ | NR | NR | NR | 6.30% | 39.90% |
| Monteleone et al. (2020) | 42 | 100% | 24.1 (4.1); 18+ | NR | NR | NR | 9.5% | 40.5% |
Abbreviations: AN, anorexia nervosa; AAN, atypical anorexia nervosa; m, male; f, female; LGBTQ, lesbian, gay, bisexual, transgender, questioning; NR, not reported.
(Parentheses) denote study sample if different from total N of admissions.
Multiple waves or studies separated by semi-colons.
3.4 |. Aim 2: Prevalence in epidemiological samples
Seventeen studies (with three overlapping samples) utilized epidemiological methods to identify cases of AAN in communities, using cross-sectional (Castelao-Naval et al., 2019; Ernst et al., 2017; Forney et al., 2017; Hammerle et al., 2016; Isomaa et al., 2010; Machado et al., 2007; Machado et al., 2013; Micali et al., 2017; Mitchison et al., 2020; Zimmerman et al., 2008), longitudinal (Allen et al., 2013; Stice et al., 2013), and twin study methods (Fairweather-Schmidt & Wade, 2014; Mustelin et al., 2016; Silén et al., 2020; Wade & O’Shea, 2015). Among these three included samples from the United States (Forney et al., 2017; Stice et al., 2013; Zimmerman et al., 2008), while others used data from Portugal (Machado et al., 2013), Finland (Isomaa et al., 2010; Mustelin et al., 2016; Silén et al., 2020), Australia (Allen et al., 2013; Fairweather-Schmidt & Wade, 2014; Hay et al., 2017), Germany (Hammerle et al., 2016), Spain (Castelao-Naval et al., 2019) and the United Kingdom (Micali et al., 2017). The majority surveyed adolescent and young adult populations under age 30 (Allen et al., 2013; Castelao-Naval et al., 2019; Ernst et al., 2017; Fairweather-Schmidt & Wade, 2014; Isomaa et al., 2010; Machado et al., 2013; Mitchison et al., 2020; Mustelin et al., 2016; Silén et al., 2020; Stice et al., 2013), and six surveyed adults over age 31 (Castelao-Naval et al., 2019; Forney et al., 2017; Hay et al., 2017; Machado et al., 2013; Micali et al., 2017; Zimmerman et al., 2008). Prevalence data for these epidemiological studies are summarized in Table 3.
TABLE 3.
Prevalence data in epidemiological studies
| Study | Study population | Point prevalence of AANa | Point prevalence of ANa | Lifetime prevalenceb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | First author | Operationalized AAN definition | Not included in AAN | N; country; sample | Female | Male | Total | Female | Male | Total | Female AAN | Female AN |
| 2007 | Machadoc | “AN normal weight:” all symptoms of AN except that BMI > 17.5. | AAN with BMI > 24 | 2028; Portugal; epi sample of female high school students. | 0.15% observed (0.00–0.32) 0.20% extrapolated (0.00–0.39) | 0.39% observed (0.12–0.67), 0.52% extrapolated (0.21–0.84) | ||||||
| 2008 | Zimmerman | “Subthreshold AN”: Meet all DSM-IV AN criteria except amenorrhea and weight > 15% of IBW. | None identified. | 2,500: United States; community psychiatric outpatients. | 0.4% (no CI) | 0% (no CI) | ||||||
| 2010 | Isomaa | “AN-NOS”: Weight phobia, and fear of weight gain, and BMI > 17.5. | None identified. | 606; Finland; All ninth graders. | 1.8% (no CI) | 0.7% (no CI) | 4.9% (no CI) | 1.8% (no CI) | ||||
| 2013 | Machadoc | AN-BSD: AN-BSD with significant weight loss at or above minimally acceptable body weight. | None identified; unclear how significant WL was defined. | 3,048; Portugal; epi sample of female high school and university students. | 0.36% (no CI) | 0.69% (no CI) | ||||||
| 2013 | Allen | Marked fear of weight gain and body image disturbance, and loss of at least 18–20% IBW over a 3–4 years. | Those <18–20% WL; those who regained WL within the 3–4 year period. | 1,383; Australia; cohort of adolescents in birth cohort. | 0.9% age 14; 0.0% age 17; 0.1% age 20 (no CI) | 0.3% age 14; 0.0% age 17; 0.3% age 20 (no CI) | 0.3% age 14; unclear age 17; 0.6% age 20; (unclear CI) | 0.0% age 14; 0.0% age 17; 0.0% age 20 (unclear CI) | ||||
| 2013 | Stice | “Atypical AN”: WL > 10%, definite fear of weight gain >75% of days for 3+ months, weight/shape one of the main aspects of self-evaluation; 85%+ of mBMI. | Those with less than 10% reduction in weight. | 496; United States; community sample from urban schools. | 2.8% by age 20 (1.3–4.3) | .8% by age 20 (0.2–1.4) | ||||||
| 2014 | Fairweather-Schmidt; also including Wade, 2015c | “Atypical AN”: Meets all criteria for AN except BMI <18.5. Among adolescents, loss of >1.3 BMI points. Also, RED: Restrict/exercise compulsively without significant WL. | Trumping AN > BN > BED > AAN > PD > SubBN; RED separate from AAN. | 699; Australia; adolescent female twins. | 1.1% wave 1, 1.0% wave 2,1.0% wave 3, 1.9% total AAN; 4.7% RED (no CIs) | 0.3% wave 1, 1.0% wave 2, 1.8% wave 3, 2.0% total (no CIs) | ||||||
| 2016 | Hammerle; also including Ernst, 2017c | “OSFED-AN:” DSM-5 AN criteria B and C met and BMI between 10th and 50th percentile. | OSFED-AN excludes AAN > 50th percentile | 1,654; Germany; all 7th and 8th graders. | 3.6% (2.7–4.5) | 0.3% (0.1–0.7) | ||||||
| 2016 | Mustelin | “DSM-5 AAN” with no history of any threshold disorder in lifetime. | AAN with history of threshold diagnosis. | 2,825; Finland; female adult twins, 1975–1979. | 0.2% (no CI for AAN) | |||||||
| 2017 | Forney | “DSM-5 AAN” with cutoff of 18.5 and 5% WL, 10% WL, and 15% WL. | Those with WL below 5%. | 2,464; United States; random sample of private university students. | 13% with 5% WL, 6.8% with 10% WL, 2.8% with 15% WL (no CIs) | 6.0% with 5% WL, 2.8% with 10% WL, 2.0% with 15% WL (no CIs) | ||||||
| 2017 | Micali | “Atypical AN”: Endorses DSM-5 criteria A, B, C for AN and BMI > 18.5 | BED > BN > AN, and full diagnosis >OSFED in same time frame. | 5,658; United Kingdom; population study of birthing women. | .35% 12-month prevalence (0.20–0.63) | .23% 12-month prevalence (0.16–0.47) | 1.7% (1.22–2.39) | 3.64% (2.81–4.72) | ||||
| 2017 | Hay | “Atypical AN”: BMI 18.5+, weekly strict dieting or fasting, overvaluation of weight and shape at least a 4/6. | None identified. | 5,737; Australia; population survey of all over age 15. | 2.5% (2.0–3.1) | 0.5% (0.3–0.5) | ||||||
| 2019 | Castelao-Naval | All the criteria for AN are met, except that the individual’s weight is above the normal range or within the normal range. | Unclear; two conflicting definitions provided. | 422; Spain; sample of health science university students. | 19.4% (no CI) “Risk of AAN” | 1.9% (no CI) “Risk of AAN” | 12.8% (no CI) “risk of AAN” | |||||
| 2019 | Mitchisond | Current BMI%ile >10; AND lost weight in the past 4 weeks; AND persistent extreme weight control behavior OR fear of weight gain OR felt fat over the past 4 weeks; AND extreme weight/shape concerns over the past 4 weeks; AND not meeting criteria for AN/BN/BED. | Adolescents who are weight suppressed and not currently losing weight. | 5,191; Australia; EveryBODY study of adolescents from various schools. | 4.8% (no CI) | 1.2% (no CI) | 2.9% (no CI), 2.2 stringent criteria, 2.8 lenient criteria | 1.3% (no CI) | 0.0% (no CI) | 0.7% (no CI), 0.5 stringent criteria, 0.6 lenient criteria | ||
| 2020 | Silén | All criteria for AN met, except despite weight loss, minimum BMI > 18.5. | Those with lifetime diagnoses of AN, BN, BED. | 1,347; Finland; all adult twins born 1983–1987. | 2.1% (1.3–3.5)b | 6.2% (4.6–8.3)b | ||||||
Abbreviations: AAN, atypical AN; AN, anorexia nervosa; AN-BSD, AN and behaviorally similar disorders; BMI, Body Mass Index, BED, binge ED; BN, bulimia nervosa; CI, confidence interval; ED, eating disorder; epi, epidemiological; IBW, Ideal Body weight; NOS, not otherwise specified; OSFED, Other Specified Feeding and ED; PD, purging disorder; RED, restrictive ED; subBN, subthreshold BN; WL, weight loss.
Multiple waves or studies separated by semi-colons.
Only one study featured male and total lifetime prevalence rates (Silén et al., 2020). Within this study, AAN lifetime male prevalence was 0.16% (0.02–1.1), and total lifetime prevalence for AAN (male and female) was 1.2% (0.7–2.0). Lifetime male prevalence for AN was 0.3% (.08–1.3), and total lifetime prevalence for AN was 3.4% (2.5–4.6).
This denotes studies with overlapping samples: Machado et al. (2007) and Machado et al. (2013) included overlapping samples so only Machado et al. (2013) is utilized in analysis [Machado et al., 2007 included here for comprehensiveness]. Similarly, Ernst et al. (2017) and Hammerle et al. (2016), and Fairweather-Schmidt and Wade (2014) and Wade and O’Shea (2015) also overlap; only Fairweather-Schmidt and Wade (2014) and Hammerle et al. (2016) are included in analysis.
“Other” gender individuals had a prevalence rate of AAN of 7.5 and 3.0% for AN.
3.4.1 |. Point and 12-month prevalence
Figure 2 shows a forest plot of all female point prevalence rates, weighted by sample size. As shown in Figure 2, point prevalence for AAN ranged greatly, from 0.15–13.0%. With few exceptions (Machado et al., 2013), point prevalence of AAN met or exceeded those of AN in studies which measured prevalence of both disorders (Allen et al., 2013; Fairweather-Schmidt & Wade, 2014; Hammerle et al., 2016; Isomaa et al., 2010; Micali et al., 2017; Mitchison et al., 2020; Zimmerman et al., 2008). Additionally, within the forest plot, the studies clearly reflect the broadening of DSM-5 AAN criteria, as studies following 2013 (with the advent of DSM-5) reported markedly higher prevalence rates than had been previously reported under DSM-IV criteria for EDNOS-AN. Often studies with less restrictive diagnostic criteria [e. -g., lower percentage weight loss requirements, no upper limit on BMI, AAN trumping purging disorder (PD) in diagnostic hierarchy] resulted in higher point prevalence rates (Fairweather-Schmidt & Wade, 2014; Forney et al., 2017; Hay et al., 2017) than those with narrower criteria, such as those relying on DSM-IV-TR which required that samples have amenorrhea and normal BMI (Isomaa et al., 2010; Zimmerman et al., 2008) or less conventional diagnostic hierarchy practices (Micali et al., 2017). Some studies also operationalized diagnostic hierarchy in less common ways, as in the case of Micali and colleagues, who reported operationalizing diagnostic trumping in the reverse order of more common practices, reporting that “BED trumped BN, and BN trumped AN” in order to align with the researchers’ previously published work (Micali et al., 2017, p. 4). Finally, while only one epidemiological study included a gender category beyond binary genders (Mitchison et al., 2020), those who identified as “other gender” had prevalence of AAN at 7.5% and AN at 3.0%, more than double the rates of other genders.
FIGURE 2.
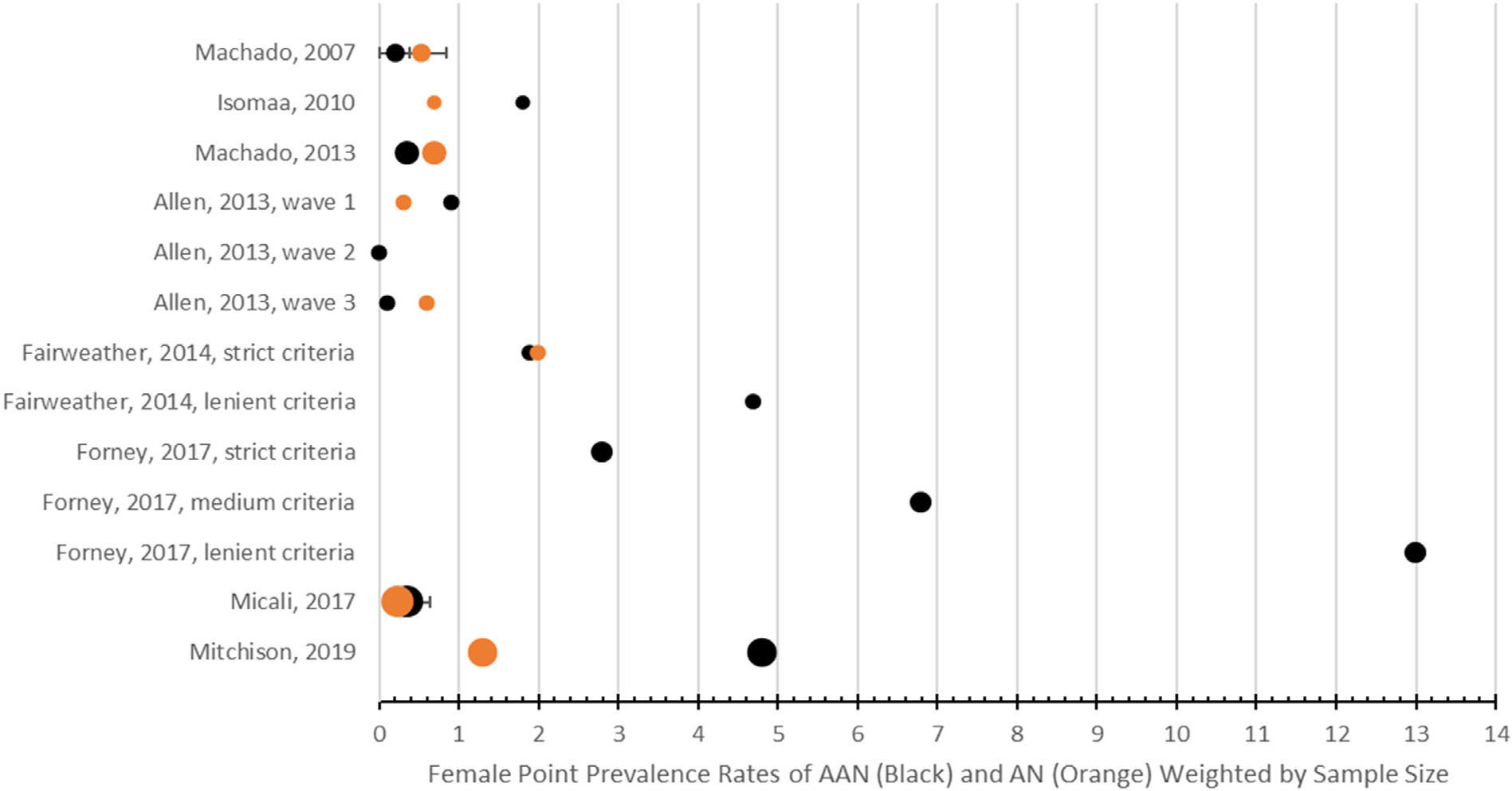
Forest plot of female point prevalence rates of AN and AAN with 95% confidence intervals. Black: prevalence rates of AAN. Orange: prevalence rates of AN. AAN, atypical anorexia nervosa; AN, anorexia nervosa
3.4.2 |. Lifetime prevalence
Figure 3 shows a forest plot of female lifetime prevalence rates for AN and AAN, with 95% confidence intervals, weighted by sample size. Lifetime prevalence for AAN ranged from 0.2–4.9%. In half the studies comparing AAN to AN, lifetime prevalence rates of AAN were double or triple that of AN (Isomaa et al., 2010; Stice et al., 2013). Both studies which found AN lifetime prevalence exceeding AAN (Micali et al., 2017; Silén et al., 2020) permitted one lifetime ED diagnosis per individual; as such, threshold AN trumped AAN. Thus, persons who experienced both AN and AAN were classified as AN, or who met criteria for both AAN and another threshold diagnosis in their lifetime (e.g., BN or BED), were not classified as AAN.
FIGURE 3.
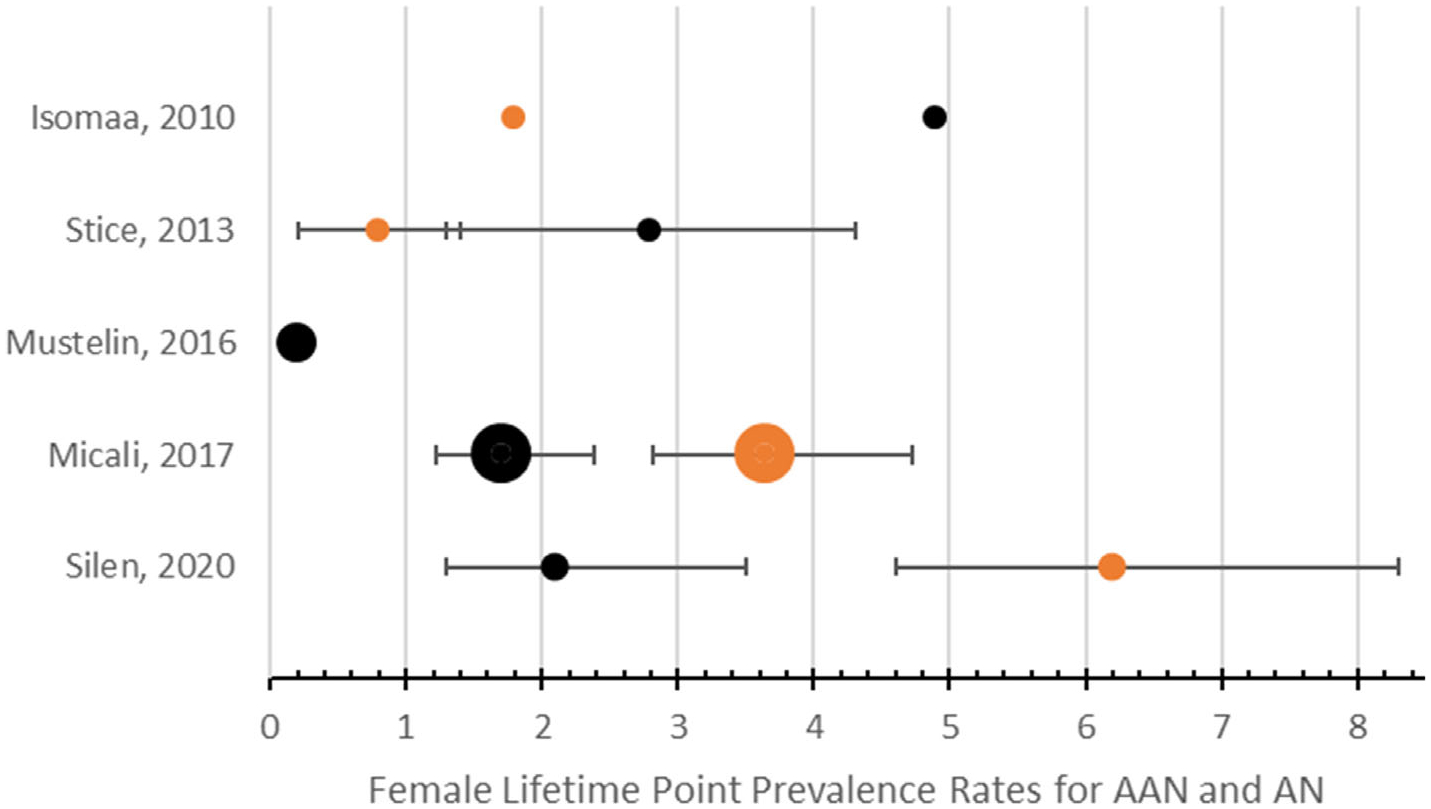
Forest plot of female lifetime prevalence rates of AN and AAN with 95% confidence intervals. Black: prevalence rates of AAN. Orange: prevalence rates of AN. AAN, atypical anorexia nervosa; AN, anorexia nervosa
3.5 |. Aim 3: Characterizing atypical anorexia nervosa operational definitions, convergences, divergences, and implications
Operational definitions of AAN are shown in Table 4. A qualitative review of these definitions shows that while there is convergence around the language typically used to operationalize AAN (e.g., “individual meets criteria for AN, except despite weight loss, individual’s weight is x…”), several areas of divergence in operational definitions include: (a) the presence of amenorrhea (e.g., Månsson et al., 2016; Ricca et al., 2010), (b) the prohibition of purging and compensatory behaviors (e.g., Bacopoulou et al., 2017; Rockert et al., 2007), (c) limitations on BMI range (e.g., Whitelaw et al., 2014), (d) amount of weight loss considered “significant” (e.g., Forney et al., 2017), (e) severity of cognitive symptoms (e.g., Stice et al., 2013), (f) history of full-threshold ED diagnosis (e.g., Silén et al., 2020), and (g) trumping order of OSFED presentations (e.g., Ekeroth et al., 2013; Micali et al., 2017). Operational definitions requiring amenorrhea or normal body weight largely (but not exclusively) occurred in earlier studies referencing DSM-IV-TR criteria.
TABLE 4.
Table of operational definitions
| First author, year | Operationalization of AAN | Diagnostic criteria | Comments on operationalization |
|---|---|---|---|
| Rockert et al. (2007) | “Normal weight restrictors” group who had no bingeing or compensation behaviors. | DSM-IV | There was a separate group for those with fasting and purging (BMI > 18.5, no OBE, purging) may approximate AAN, purging type. |
| Machado et al. (2007) | Met all criteria of AN except that current weight is in the normal range, above 17.5. | DSM-IV | Separate group for normal weight individuals with inappropriate compensatory behaviors after eating small amounts of food. |
| Zimmerman et al. (2008) | Subthreshold AN who do not meet AN criteria due to weight above 85% IBW. | DSM-IV | Sample above 85% IBW and amenorrheic. |
| Eddy et al. (2008) | Normal and higher-weight restrictors were captured in the “other EDNOS” category and were not statistically different from AN in excessive exercise or fasting. | DSM-IV-TR | This group captured normal weight AAN only; higher-weight participants were excluded. |
| Santonastaso et al. (2009) | AAN met all criteria for AN except despite a significant weight loss, the individual’s weight is in the normal range. | DSM-IV | This group captured normal weight AAN only; higher-weight participants were excluded. |
| Isomaa et al. (2010) | AN not otherwise specified was diagnosed if the subject experienced weight phobia, and fear of gaining weight, but had BMI over 17.5. | DSM-IV | Individuals between 17.5 and 18.5 would be classified as AAN in DSM-5. |
| Ricca et al. (2010) | Group met all DSM-IV AN criteria except the weight criterion. | DSM-IV | All AAN had amenorrhea; may be a more severe sample. |
| Thomas et al. (2010) | Study examined patients with discordant diagnoses, demonstrating the lack of clarity of some AAN criteria. | DSM-IV | Discordant diagnoses occurred between EDNOS-AN and AN, and between EDNOS AN and BN. |
| Peebles et al. (2010) | Met all symptoms of DSM-IV AN except weight and had lost at least 25% of their body weight. | DSM-IV | This study utilized one of the highest weight suppression requirements (25%). |
| Bayes and Madden (2011) | AAN met all criteria for DSM-IV AN except weight not below 85% IBW. | DSM-IV-TR | Individuals between 86%−90% IBW would be classified as AAN in DSM-5. |
| Fairburn and Cooper (2011) | “Restrained ED:” restriction dominant feature of EDNOS. | DSM-IV, DSM-5 | This group appears to capture EDNOS with primarily AN behaviors; appears more lenient. |
| Schreyer (2012) | EDNOS subthreshold AN-restricting and subthreshold AN-binge/purge. | DSM-IV-TR | Per conversation with author, menstrual status was not part of diagnostic criteria for anorexia, and EDNOS-AN subtypes likely all met DSM-5 AAN criteria. |
| Weingeroff (2012) | Subthreshold AN with severe food restriction, clinically significant and interfering preoccupation with weight and shape, fear of weight gain, loss of at least 15% of their body weight recently, and body weight greater than 85% ideal body weight. | DSM-III-R | This group appears to closely match DSM-5 AAN. |
| Birgegård et al. (2012) | Met all criteria for AN except BMI cutoff; additionally no purging and normal weight. | DSM-IV, DSM-5 | This group would miss AAN with purging or AAN above normal weight. |
| Sysko et al. (2012) | AN-BSD with significant weight loss at or above minimally acceptable weight. | DSM-IV, DSM-5 | This group appears to closely match DSM-5 AAN. |
| Ornstein et al. (2013) | AAN according to proposed DSM-5 diagnostic criteria as of November 23, 2010. | DSM-IV, DSM-5 | This group appears to closely match DSM-5 AAN. |
| Conceição et al. (2013) | DSM-5 AAN; limited to restricting subtype (no purging). | DSM-IV, DSM-5 | This group appears to closely match DSM-5 AAN; however, only restricting subtype was included. |
| Ekeroth et al. (2013) | DSM-5 AAN without any purging over BMI > 18.5 (appears to only include normal weight individuals per column R of A15). Trumping PD > AAN. | DSM-5 | This group appears to miss AAN with purging or AAN above normal weight. Nearly all PD also met criteria for AAN. |
| Stice et al. (2013) | AAN required at least a 10% reduction in weight, definite fear of weight gain more than 75% of the days for at least 3 months, weight and shape were one of the main aspects of self-evaluation; used 85% of median expected BMI as AN cutoff. | DSM-5 | Very specific operational definition, specifying weight loss threshold (10%) with markers of clinical severity. |
| Allen et al.(2013) | AAN: Participants had lost considerable weight (weight loss of at least two standard deviations more than sample mean, 18–20%) over the preceding 3 to 4 years without yet being markedly underweight and endorsed marked fear of weight gain and body image disturbance. | DSM-IV-TR, DSM-5 | Utilized specific clinical cutoffs for fasting and overvaluation, and high weight suppression requirement (18–20%). |
| Machado et al. (2013) | AN-BSD with significant weight loss at or above minimally acceptable body weight. | DSM-IV, DSM-5, BC for EDs | This group appears to closely match DSM-5 AAN. |
| Kachani et al. (2014) | This study did not exclude patients from AN based on weight. Personal communication with author clarified that 14/44 AN patients had BMIs between 19–26.4. | DSM-IV-TR | This group appears to closely match DSM-5 AAN with amenorrhea. |
| Fairweather-Schmidt and Wade (2014) | AAN met all criteria for AN except BMI is above 18.5, or individuals who despite significant weight loss, have weight within or above the normal range. Among adolescents significant weight loss was defined as losing 1.3 BMI points. Diagnostic trumping AN>BN > BED>AAN > PD > SUBBN. |
DSM-5 | Defined significant weight loss as 1.3 BMI points and AAN trumped PD. |
| Forman et al. (2014) | Met all of the criteria for AN, except that, despite significant weight loss, the individual’s weight is within or above the normal range. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Whitelaw et al. (2014) | “EDNOS-wt:” met all criteria for AN except weight not less than 85% of median BMI. | DSM-IV | This group captured normal weight AAN with amenorrhea. |
| Wade, Bergin, Martin, Gillespie, and Fairburn (2006) | DSM-5 AAN; also looked at separate group of girls (UFED) who restricted and/or exercised compulsively (RED group) for weight loss but had not experienced a significant enough weight loss and were higher-weight on the whole. | DSM-5 | It was unclear to what extent the RED group was weight suppressed, and therefore unclear whether they would meet AAN criteria. |
| Thomas et al. (2015) | “DSM-5 AAN” | DSM-IV, DSM-5 | This group appears to closely match DSM-5 AAN. |
| Caudle et al. (2015) | The authors note that a small subgroup of AN patients were actually AAN (with rapid weight loss or high premorbid weight), with BMI over 18.5. The authors note that the term atypical would be an alternate way of describing these individuals. They included these higher-weight individuals in the AN group due to similarities with the rest of the AN group in every other respect. | DSM-5, DSM-IV | This group appears to closely match DSM-5 AAN. |
| Damiano et al. (2015) | “Subthreshold AN” were within a healthy weight range according to BMI z-score categories, adjusted for age and gender growth standards, but had lost a significant amount of weight, meeting the criteria for subthreshold AN. | DSM-IV-TR | This group appears to closely match DSM-5 AAN. |
| Monge et al. (2015) | Utilized proposed DSM-5 criteria for AAN. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Kass et al. (2015) | DSM-5 AAN. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Fichter et al. (2015) | DSM-5 AAN. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Redgrave et al. (2015) | Subthreshold AN (amenorrhea not required for full AN) where patients had fear of fatness and/or body image distortion, and were above AN cutoff. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Damiano et al. (2015) | Subthreshold AN was diagnosed if participants had lost a significant amount of weight but were still within the healthy weight range. | DSM-IV-TR | This group was amenorrheic and did not include those above normal BMI. |
| Welch et al. (2015) | DSM-5 AAN. | DSM-5, DSM-IV | Applied DSM-5 criteria retrospectively. This group appears to closely match DSM-5 AAN. |
| Silén et al. (2015) | AAN according to ICD-10 (which included those without amenorrhea, those with higher-weight, those without fear of fat, and those with at least two symptoms missing). A separate count was included for those who met AN criteria except for weight cutoff. | ICD-10; DSM-5 | This group appears to closely match DSM-5 AAN. |
| Schreyer et al. (2016) | Subthreshold AN-restricting and subthreshold AN-binge/purge. | DSM-IV-TR | Per conversation with author subthreshold-AN subtypes met DSM-5 AAN criteria; there is a chance some had a previous diagnosis of AN. |
| Koutek et al. (2016) | “Atypical anorexia nervosa.” | Unknown | This group appeared to be DSM-5 AAN. |
| Smith et al. (2016) | Cutoff for atypical AN was defined as % mBMI>89%, and as BMI >17.5. | DSM5 | This group appears to closely match DSM-5 AAN. |
| Månsson et al. (2016) | Restrictive EDNOS not meeting weight criteria for DSM-IV AN. | DSM-IV | This group appears to be amenorrheic and uses DSM-IV weight cutoff. |
| Hammerle et al. (2016) | OSFED-AN: AN criteria B and C must be met according to DSM-5 and body weight below 50th percentile BMI (and above 10th %ile). | DSM-5, ICD-10 | This group appears to closely match DSM-5 AAN. |
| Hughes et al. (2017) | DSM-5 AAN with loss of at least 10% body weight, BMI > 90% mBMI and not less than 90% in last year. Trumping: AAN > PD | DSM-5 | Defined significant weight loss as 10%. |
| Mustelin et al. (2016) | DSM-5 AAN with no history of any threshold diagnosis in lifetime. | DSM-5 | This group excludes those with AAN with past threshold ED diagnosis. |
| Sawyer et al. (2016) | DSM-5 AAN wherein all of the criteria for AN are met, except that despite significant weight loss, the individual’s weight is within or above the normal range. Weight less than or equal to 90% mBMI with at least 10% weight loss. | DSM-5 | Defined significant weight loss as 10%. |
| Swenne (2016) | EDNOS (restrictive type): Author clarified that this was an AAN group which also featured restrictors who had not lost what might be considered “significant weight” but had all other symptoms of AN. | DSM-5, DSM-IV | Applied DSM-5 criteria retrospectively. This group utilized lenient definitions of “significant weight loss.” |
| Bacopoulou et al. (2017) | AAN, restricting type by DSM-5 criteria. | DSM-5 | This group excludes those with purging. |
| Swenne et al. (2017) | Restrictive ED (OSFEDr) who did not fulfill weight criteria for AN. BMI SDS < −2.0 was used as the weight criterion for AN. | DSM-IV, DSM-5 | Applied DSM-5 criteria retrospectively. |
| Swenne and Ros (2017) | Restrictive ED that did not fulfill weight criteria for AN according to DSM-IV. | DSM-IV | Appears to be amenorrheic sample above BMI 17.5. |
| Forney et al. (2017) | DSM-5 AAN with BMI > 18.5. This study tested three weight suppression standards and compared prevalence: 5%, 10%, and 15% weight loss. | DSM-5 | This group appears to closely match DSM-5 AAN and tested the implications of multiple weight suppression definitions. |
| Nagata et al. (2017) | DSM-5 AAN. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Micali et al. (2017) | Endorsed DSM-5 criteria A, B, C for AN and BMI > 18.5. Trumping: BED>BN > AN and full diagnosis>OSFED in same time frame. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Kennedy et al. (2017) | Criteria for AAN per DSM-5. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Nakai et al. (2017) | AAN DSM-5 with BMI cutoff of 18.5. | DSM-5, DSM-IV | This group appears to closely match DSM-5 AAN. |
| Peebles et al. (2017) | DSM-5 AAN. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Hay et al. (2017) | BMI at or above 18.5 and weekly strict dieting or fasting, and overvaluation of weight and shape at least a 4/6. | DSM-5 | Utilized specific clinical cutoffs for fasting and overvaluation. |
| Ernst et al.(2017) | AN criteria B and C must be met according to DSM 5 and current body weight below 50th BMI percentile. | DSM-IV, DSM-5 | This group does not include AAN at higher weights. |
| Darrow et al. (2017) | DSM-5 AAN. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Riesco et al. (2018) | “OSFED AAN.” | Not specific | This group appears to closely match DSM-5 AAN. |
| Wilkes (2018) | OSFED atypical anorexia. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Whitelaw et al. (2018) | AAN: Met criteria for AN except weight is greater or equal to 85%mBMI. | DSM-IV | This group appears to be amenorrheic and weight range is unclear. |
| Lebow et al. (2019) | EDNOS above the 15th percentile BMI for age at presentation, but engaged primarily in restrictive eating, and were at low weight as defined in the context of developmental trajectory and physical health were reclassified as having AAN. | DSM-IV, DSM-5 | It is unclear if this group was amenorrheic due to use of DSM-IV and DSM-5 criteria (retroactively). |
| Matthews et al. (2018) | DSM-5 AAN. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Andrés-Pepiñá et al. (2020) | DSM-5 AAN. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Castelao-Naval et al. (2019) | States “DSM-5 AAN,” and then lists two different definitions on page 282. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Nakai et al. (2018) | DSM-5 AAN with cutoff of 18.5. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Nagata et al. (2019) | DSM AAN | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Schorr et al. (2019) | AAN: Psychological symptoms characteristic of AN and significant weight loss, but not currently low weight, with BMI equal to or greater than 18.5. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Mitchison et al. (2020) | AAN: Current BMI%ile > 10; AND lost weight in the past 4 weeks; AND persistent extreme weight control behavior (fasting/strict dieting/detox, self-induced vomiting, laxative misuse, driven exercise, or misuse of insulin or other drugs) OR fear of weight gain OR felt fat over the past 4 weeks; AND extreme weight/shape concerns over the past 4 weeks; AND not meeting criteria for AN or BN or BED. | DSM-5 | Very specific operational definition, specifying markers of clinical severity including recent weight loss; however, does not provide definition for how much weight needed to be lost recently. |
| Zanna et al. (2020) | DSM-5 AAN. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Monteleone et al. (2020) | DSM-5 AAN. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Flatt (2020) | Meeting all DSM-5 criteria for AN except not considered underweight. | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Smith (2020) | OSFED atypical anorexia | DSM-5 | This group appears to closely match DSM-5 AAN. |
| Silén et al. (2020) | DSM-5 AAN with no prior history of AN, BN, BED. | DSM-5 | This group excludes those with AAN with past threshold ED diagnosis. |
Abbreviations: AAN, atypical anorexia; AN, anorexia nervosa; AN-BSD, AN and behaviorally similar disorders; BC for EDs, Broad categories for the diagnosis of EDs; BED, binge-eating disorder; BMI, body mass index; BMI%ile, BMI percentile; BN, bulimia nervosa; DSM III-R, diagnostic and statistical manual version III revised; DSM IV, diagnostic and statistical manual version IV; DSM-IV-TR, diagnostic and statistical manual version IV text revised; DSM-5, DSM version 5; IBW, ideal body weight; mBMI, median BMI; PD, purging disorder; SDS, standard deviation score.
3.5.1 |. Weight cutoffs
The most common area of definition divergence involved what constitutes “significant weight loss.” Operational definitions included: 5% weight loss, 10% weight loss, 15% weight loss, 18–20% weight loss, 25% weight loss, loss of 1.3 BMI points, and no significant weight loss (Forney et al., 2017; Hughes et al., 2017; Peebles et al., 2010; Swenne, 2016). Generally, studies which employed more stringent weight criteria reported lower AAN prevalence than those without weight maximums. For example, when requiring participants to demonstrate “significant weight loss” per DSM-5 criteria, Wade and O’Shea (2015) found an overall prevalence rate of 1.9% for AAN. However, when they removed that requirement an additional 4.7% of participants were classified as AAN, and this group repeatedly demonstrated similar levels of impairment (e.g., ED severity, body dissatisfaction) compared with AAN and full-threshold ED diagnoses. Similarly, Forney et al. (2017) tested the impact of three separate weight loss criteria cut-offs (i.e., 5, 10, and 15% of body weight) on prevalence rates and levels of clinical impairment. Although prevalence rates were directly impacted by these changing criteria, (e.g., 13.0% of women qualified for AAN with 5% weight loss, but only 2.8% of women qualified for AAN with 15% of weight loss), all three diagnostic cut-off groups evidenced clinically meaningful symptom profiles (Forney et al., 2017, p. 959), which the authors argued justified AAN classification.
3.5.2 |. Diagnostic ambiguity and differentiation
Regarding ambiguity in AAN cases, several studies examining consecutive clinic referrals addressed reliability of diagnoses (e.g., Sysko et al., 2012; Thomas et al., 2010; Thomas et al., 2015). Several reasons were identified for diagnostic ambiguity with AAN, two of which are detailed here. First, differentiating PD from AAN posed challenges, with the operationalized diagnostic hierarchy of non-threshold diagnoses largely determining rates of these disorders. For instance, Ekeroth et al. (2013) classified 13.9% of their sample as AAN. However, of those with PD, 180/184 patients also met criteria for AAN which would have added an additional 8.1% of the sample if AAN had trumped PD. Similarly, Birgegård et al. (2012) found that 21% of their sample classified as AAN when PD trumped AAN; however, 28% of the sample was classified as AAN when AAN trumped PD. Finally, Rockert et al. (2007) found equivalent levels of fasting between AAN and PD subgroups, though 11.8% of the sample was classified as AAN (termed “normal weight restrictors” with purging being a disqualifier) and 10.0% of the sample was classified as PD, further obfuscating the distinction between AAN and PD.
Second, as in the prevalence studies, the weight cutoff criteria added variability in study protocols. For instance, in a study of diagnostic reliability, Thomas et al. (2010) found that 7.9% of discordant diagnostic cases likely occurred because patients typically lose around one pound after their first night in an inpatient facility, either due to intentional fluid manipulation or due to normal bodily shifts. Thus, patients who hovered just above diagnostic weight cutoff for AN at admission (perhaps being classified with AAN, bulimia nervosa, or PD), then met AN criteria the following morning (Thomas et al., 2010). These studies concluded that cases close to the BMI cutoff for AN (or “cusp-diagnoses”) pose additional diagnostic challenges. Additionally, some studies suggested different cutoffs for AN according to their unique study population, such as bariatric surgery populations (Conceição et al., 2013).
Relatedly, we also examined all studies to determine what proportion of study participants fell into this “cusp-diagnosis” with 18.5 > BMI > 19.0. While inclusion criteria required that some participantshave BMI >19.0 (to more clearly delineate between AN and AAN), understanding what proportion of AAN patients fell within the cusp, lending themselves to either diagnosis at the discretion of the clinician (American Psychiatric Association, 2013). In total, six studies (8.2%; mostly occurring near the transition to DSM-5) described this diagnostic-cusp group to inform the debate regarding weight criteria when moving from DSM-IV to DSM-5. Study methodologies and rates of these cusp-groups varied greatly: Eddy et al. (2008) reported 5% (n = 15) of their sample fell within the cusp of 86–90% ideal body weight (IBW), Zimmerman et al. (2008) had one participant (0.04%) with an underweight BMI (18.2), and Peebleset al. (2010) reported 9.2% (n = 121) with BMI 85–90% IBW Conceição et al. (2013) reported none of their bariatric sample fell within this cusp. Thomas et al. (2010) reported that 7.9% of patients’ BMIs shifted from greater than 85%IBW to less than 85% IBW after 24-hr of care, and Forman et al. (2014) further illustrates this diagnostic overlap in the diagnostic-cusp group explaining that 27.3% of individuals assigned an AAN diagnosis were less than 90% mBMI, while 13.7% of patients diagnosed with AN had BMI ≥90%mBMI.
3.6 |. Aim 4: Rates of atypical anorexia nervosa in studies with consecutive referrals or admissions
Table 2 shows all studies utilizing consecutive referral or admission designs, the gender and ages of those samples, and the percentage of study participants categorized as AAN and AN within each study.
3.6.1 |. Rates of atypical anorexia nervosa in consecutive clinical referrals and admissions
A large proportion of referrals and admissions to ED treatment centers were comprised of AAN individuals. The current review identified 58 studies which tracked AAN individuals among consecutive referrals and admissions to ED specialty treatment centers. In these 58 studies, 28% (n = 16) showed AAN individuals comprising at least one-third of study participants; an additional 17% (n = 10) of studies showed AAN individuals comprising at least 20% of study participants. Overall, in 71% (n = 41) of studies of consecutive referrals and admissions to ED centers, AAN cases made up at least 10% of the study population reported. Additionally, multiple centers noted seeing referral and admission rates for AAN increase significantly; Whitelaw et al. (2014) reported a five-fold increase in the number of AAN cases over the years 2008–2014. Additionally, among studies that tracked sub-threshold ED presentations, AAN tended to be the most common presentation of any OSFED diagnosis, at times representing well over half the residual cases seen in clinical settings (Birgegård et al., 2012; Ekeroth et al., 2013; Nakai et al., 2017; Ornstein et al., 2013; Sawyer et al., 2016; Swenne & Ros, 2017; Sysko & Walsh, 2011).
3.7 |. Comparative rates of atypical anorexia nervosa and anorexia nervosa in consecutive clinical referrals and admissions
When considering rates of AAN compared with AN in consecutive referral/admission studies, AAN cases were generally less represented in clinical samples (compared with AN) than in epidemiological samples. As shown in Table 2, approximately 79% (n = 46) of studies reported more consecutively referred or admitted patients to have AN compared with AAN, with AN rates ranging from 1.5 to 17 times as common as AAN referrals and admissions. However, in 17% (n = 10) of studies, AAN exceeded AN referrals and admissions, and sometimes by a factor of three or more (Bayes & Madden, 2011; Damiano et al., 2015; Månsson et al., 2016; Swenne, 2016; Swenne et al., 2017; Swenne & Ros, 2017; Welch et al., 2015).
4 |. DISCUSSION
The advent of the DSM-5 has spurred increased inquiry into higher-weight restrictive EDs (Neumark-Sztainer, 2015). Despite growing interest in AAN, it is unclear how many people suffer with this disorder in the community, and in treatment settings, and how they compare to the proportion of individuals with AN. The recent classification of the disorder, variable terms used for AAN historically, and the diversity of operational definitions have made AAN difficult to study comprehensively. This review examined the literature to investigate (a) the demographic characteristics of AAN studies, (b) the prevalence rates of AAN in communities, (c) the range of operational definitions currently used in AAN literature, and (d) the proportion of ED clinical referrals and admits with this disorder. To our knowledge, this is the first literature review that specifically addresses these matters. Quantifying how many individuals suffer with AAN and how often they present in treatment settings is one of the first steps toward understanding the scope of the problem of AAN and getting these often-overlooked patients potentially life-saving treatment.
The first aim interrogated which populations and demographic groups are represented in literature on AAN by describing the demographic characteristics (age, race, gender, and sexual orientation) of participants in studies including AAN. In the 75 studies reviewed, most focused on adolescents (81%), with moderate representation of children and young adults. Middle and older adult populations (over age 30) were least represented in the literature, shown in only 33% of studies. This lower representation of adult samples is concerning given that one of the few epidemiological studies including adults found the mean age of AAN individuals to be 43.5 years (Hay et al., 2017). Thus, while many of the adolescent studies capturing AAN may essentially capture a prodrome of AN, it is also possible that patients may shift from AN to AAN later in life as bodies’ age and weight increases, or as individuals previously recovered from AN experience a reemergence of ED symptoms in later life transitions (Meeuwsen, Horgan, & Elia, 2010). Indeed, some studies have noted that large portions of AAN individuals had histories of underweight BMIs during earlier manifestations of their EDs (Schorr et al., 2017). Thus, epidemiological studies only examining adolescent populations may miss older age groups where AAN may be more prevalent. To capture AAN presentations more comprehensively, AAN researchers ought to recruit across the lifespan.
Additionally, only a minority of studies included male populations, and only two studies included gender minorities, despite higher rates of AAN within gender diverse and non-conforming populations (Mitchison et al., 2020). It was also common for studies to fail to report race as a demographic variable (or socioeconomic status), and the majority of studies reporting race surveyed samples where the vast majority of participants were white, further marginalizing the experiences of people of color. Taken in turn with the finding that EDs may be increasing most in populations previously thought to be least at risk for EDs (Mitchison et al., 2014), expanding sample diversity to include more males, sexual and gender minorities, and populations of color is imperative.
The second aim of this article was to review epidemiological studies to summarize prevalence findings. Though point prevalence varied greatly, rates of AAN generally met or exceeded those of AN. Similarly, in studies that examined lifetime prevalence, rates of AAN tended to exceed those of AN, except when only one lifetime ED diagnosis was permitted.
To better understand the disparate prevalence rates reported in the literature, the third aim utilized a brief qualitative review of all operational definitions utilized. Examination of definitions suggested that much of the variation in prevalence depended on how researchers operationalized definitions of various EDs, and what diagnostic hierarchy was adopted for each study. Additionally, the “diagnostic-cusp group” (BMIs between AN and AAN) emerged as a potentially important group, with some studies noting significant overlap in AN and AAN classifications, depending on how clinicians interpreted DSM-5 criteria, and the various operational definitions of “low weight” (Eddy et al., 2008; Forman et al., 2014; Peebles et al., 2010; Thomas et al., 2010). Gaining greater insight into the extent that these BMI groups may differ qualitatively and prognostically will inform future conceptualizations of anorexia nervosa-spectrum disorders. However, while diagnostic differences in how studies operationalized AAN weight criteria (APA, 2013) may contribute to the variance in prevalence rates found, these were not the only factors.
Similarly, diagnostic hierarchy decisions (e.g., whether AAN or PD was coded for those meeting both criteria) also impacted prevalence, such that AAN decreased when PD trumped AAN hierarchically, a finding that was also reflected in consecutive admission designs. Additionally, studies precluding diagnoses of AAN in the presence of lifetime full-threshold EDs similarly found lower AAN rates (Micali et al., 2017; Mustelin et al., 2016). It could be that, given the significant diagnostic crossover between EDs (Stice et al., 2013), studies that permitted one lifetime ED diagnosis found lower lifetime AAN compared with AN, as many with AAN have histories of full threshold EDs (Schorr et al., 2017). It is also possible that these operational decisions to count individuals with histories of both AAN and AN in the AN category could reflect an implicit assumption that AAN and AN exist on a spectrum, with low-weight AN being a potentially more severe end of that spectrum or AAN representing AN in a prodromal or partial-remission state.
An additional issue in operational definitions in both epidemiological and clinical studies with consecutive admissions involved whether or not AAN classification included those with purging behaviors. In the DSM-5, AN individuals are categorized into subtypes with purging and non-purging behaviors (AN-binge-purge subtype and AN-restrictive subtype, respectively); with significant portions of AN individuals engaging in purging behaviors (e.g., Micali et al. report over half of individuals with AN had binge-purge subtype). Multiple studies found significant overlap between AAN and PD (Birgegård et al., 2012; Ekeroth et al., 2013), which is unsurprising given the large proportion of AN patients who comprise a purging subtype (Godart et al., 2013; Micali et al., 2017). For example, in Stice et al.’s (2013) epidemiological study, the AAN group had an average of 9.8 compensatory behavior episodes per month. Thus, considering that significant proportions of those with AAN are classified as purging subtypes (Davenport, Rushford, Soon, & McDermott, 2015; Eddy et al., 2008), the distinction between a potential “AAN–purging subtype” and PD needs additional clarification. The overlaps in symptoms and behaviors between AAN and PD (Birgegård et al., 2012; Ekeroth et al., 2013; Rockert et al., 2007) suggest that the distinction between AAN and PD may be more of a subtype difference than a separate classification, with potential subtypes in AAN mirroring those in AN (i.e., restriction only, and restriction plus compensatory behaviors). This overlap in classification is exemplified in the study by Ekeroth et al. (2013), in which 180/184 patients with PD also met criteria for AAN.
The final aim of this article was to review clinical studies with consecutive admits and referrals to determine the proportion of patients with AAN in ED treatment settings. While epidemiological data elucidate the prevalence of AAN in communities, samples of consecutive referrals and admissions provide data on the proportion of ED patients with AAN referred to or receiving treatment. Generally, population-based studies in this review found AAN to be more common than AN (Ernst et al., 2017; Hammerle et al., 2016; Hay et al., 2017; Isomaa et al., 2010; Stice et al., 2013; Wade & O’Shea, 2015; Zimmerman et al., 2008). Though clinical findings were more mixed (with some finding AAN to be more common than AN in treatment settings, and many others finding the reverse), most clinical studies found fewer AAN patients referred and admitted to treatment compared with AN (Eddy et al., 2008; Peebles et al., 2010; Ricca et al., 2010; Santonastaso et al., 2009).
Similar to epidemiological studies, variation in referral and admission rates is likely partially attributed to differing operational definitions, including disparate weight cut-offs for AAN and varying diagnostic hierarchy rules between studies. However, the majority of clinical studies found AN to be more common in clinical settings, suggesting that AN patients’ typical visible emaciation may serve as an “overt indicator” (Thomas et al., 2015) to prompt more proactive screening and referral from providers. Discrepancies between population-based disease rates and clinical referral/admission data allude to potential difficulties AAN patients may experience in being screened, referred to, and accessing needed healthcare (Harrop, 2019; Sawyer et al., 2016).
It is also possible that the individuals with AAN in consecutive studies presented with lower clinical acuity or less distress, leading to less frequent referral or admission. While a full analysis of how these samples compared with each other clinically (e.g., severity of AAN compared with AN individuals) is beyond the scope of this review, some studies within this sample did compare the severity and impairment of those with AN and AAN. In general, studies examining medical consequences of AN and AAN reported similar medical concerns within these groups, with AAN patients consistently presenting at higher weights, but requiring similar care (Bayes & Madden, 2011; Conceição et al., 2013; Peebles et al., 2010; Peebles et al., 2017; Whitelaw et al., 2014). Additionally, multiple studies compared cognitive symptoms, ED behaviors, and clinical impairment, and found that AN and AAN samples did not differ in symptom presentation and severity (Caudle et al., 2015; Conceição et al., 2013; Eddy et al., 2008; Ekeroth et al., 2013; Fairweather-Schmidt & Wade, 2014; Forman et al., 2014; Ricca et al., 2010; Rockert et al., 2007; Ornstein et al., 2013; Santonastaso et al., 2009. In one study, AN had more emergency hospital admissions compared with AAN (Monge et al., 2015)., while other studies found AAN participants had more severe cognitive symptoms (Sawyer et al., 2016) or longer duration of illness (Forman et al., 2014; Rockert et al., 2007). While this is not a comprehensive overview (as not all studies compared AN and AAN samples), it suggests that for the studies considered in this review, AAN individuals presented with concerning medical and psychiatric impairment, often approximating that of AN samples, suggesting that delayed or missed referrals for care could negatively impact these patients.
It is interesting to note that in studies finding AAN more common than AN in clinical samples, all but two used samples of adolescents, suggesting that higher AAN rates may be due to some AAN cases presenting as prodromes of AN, with AAN adolescents identified and referred before progressing to greater weight loss and AN diagnosis. It is also possible that due to the perception of EDs as adolescent issues, adolescent providers may be more adept at screening and identifying ED cases that general practitioners may miss (Thomas et al., 2015).
Additionally, the national environments and corresponding healthcare systems may provide insight into why some studies found higher rates of AAN compared with AN in treatment samples. For example, all but one study reporting higher AAN rates (compared with AN) in treatment were conducted in Sweden and Australia, countries which employ universal healthcare systems, and have higher emphasis on ED prevention and early intervention to reduce healthcare costs (Swenne, 2016). As such, Swenne (2016) notes that more cases of AAN are likely identified due to broader definitions for screening and diagnosis, which reflect Sweden’s explicit efforts to limit treatment costs by intervening early and effectively with EDs. In fact, in these environments, Swenne states, there is a virtual “absence of treatment delay” for identified ED patients (i.e., all referred patients are seen within 2 weeks), which may prevent some AAN individuals from additional weight loss and potential AN diagnosis. This progression from AAN to AN has been noted in multiple case studies (Sim, Lebow, & Billings, 2013).
In contrast to the studies in Sweden and Australia, no studies from the US utilizing consecutive clinical samples, found higher rates of AAN individuals in treatment compared with AN, an important finding considering that epidemiological US samples did find higher rates of AAN compared with AN (Stice et al., 2013; Zimmerman et al., 2008). For instance, one US study reported data from two higher-weight adolescents with AAN who presented for care multiple times over a period of years with symptoms of starvation (e.g., significant weight loss, secondary amenorrhea, bradycardia, overuse injuries); despite this, years lapsed before their ED symptoms were identified and treated (Sim et al., 2013). In situations such as these, if adolescents fail to be identified early in the course of illness, AAN may be overlooked until the individual progresses to AN. This finding aligns with the current landscape of US healthcare services, in which higher “severity” of illness (with severity based on weight criteria in the DSM-5), may be required to qualify for insurance coverage for ED treatment, possibly explaining how epidemiological studies have identified higher rates of AAN individuals than appear to be reflected in US treatment contexts.
Lastly, the higher prevalence rates of AAN compared with AN in epidemiological studies combined with the lower referral rates of AAN compared with AN suggest that clinicians may be more adept at recognizing and referring adolescent presentations of AAN compared with adult presentations. Thomas et al. (2015) suggest that AAN may be more difficult to detect than other EDs due to a lack of “overt indicators” (e.g., emaciation, bingeing and purging), particularly in environments with culturally normative restrictive eating behaviors (e.g., dieting). In sum, the prevalence and consecutive admission data point to the overall concern that AAN individuals, who appear to exist in greater numbers in population-based contexts, are underserved in ED treatment, thus complicating AAN illness trajectories.
5 |. LIMITATIONS
This study has several important limitations. First, discussion of AAN was limited to literature after 2007. AAN was discussed in the literature prior to this date, though definitions were more heterogeneous; therefore, this review cannot be considered comprehensive of all AAN literature. Second, though using an established standardized quality assessment tool, this review utilized a single reviewer to assess quality of articles. Third, due to the limited number of studies addressing AAN prevalence, the different populations these studies surveyed (in regard to ages, countries, and genders), the different operational definitions employed, and the significant diversity of prevalence rates reported, this review was not able to draw firm conclusions about the rates of AAN. Larger prevalence studies, incorporating broader populations, and surveying people across the lifespan will be necessary to better understand the global burden of AAN. Finally, while we found that AAN appeared less represented in treatment samples, not all studies in this review reported on severity markers of AAN and AN. Thus, it is impossible to determine whether AAN individuals are less represented due to decreased clinical needs or due to AAN individuals going under-detected or under-referred.
6 |. CLINICAL IMPLICATIONS
When considering clinical implications, much of the difficulty defining AAN may stem from diverse interpretations of the DSM-5. Despite the DSM-5’s efforts to clearly delineate AN from AAN, confusion arises around DSM-5 criteria that appear contradictory. For example, when determining if a patient meets the weight criteria for AN (Criterion A), a guideline of a BMI <18.5 is proposed. Additionally, clinicians are encouraged to use clinical judgment, thinking of BMI <18.5 as a guideline, rather than rule: “In summary, in determining whether Criterion A is met, the clinician should consider available numerical guidelines, as well as the individual’s body build, weight history, and any physiological disturbances” (APA, 2013, p. 340), suggesting that factors beyond numerical BMI data should guide this determination. However, later in the DSM-5, when delineating OSFED, the DSM-5 states: “1. AAN: All of the criteria for AN are met, except that despite significant weight loss, the individual’s weight is within or above the normal range” (APA, 2013, p. 353). Thus, clinicians could either use clinical judgment to diagnose AN in individuals with BMI >18.5 with “significant” weight suppression and physiological complications of malnutrition or clinicians could rely on numerical BMI data to assign AAN. The DSM would benefit from greater clarity here, and looking forward to the DSM-6, clarifying whether clinicians are to rely on numerical BMI cutoffs or on other factors of severity or impairment (e.g., distress, weight-suppression, and physiological disturbance) will be necessary for the field to better conceptualize the AN and AAN diagnosis.
Second, although prevalence rates varied between studies, epidemiological data were clear in identifying AAN comprising a considerable portion of ED diagnoses in the population. As such, AAN poses a burden of disease which necessitates treatment. Given the high rates of medical comorbidity associated with AAN (Peebles et al., 2017; Sawyer et al., 2016; Whitelaw et al., 2014), which are often commensurate with AN, it is imperative that these individuals receive specialized ED care. However, despite higher rates of prevalence in population studies, AAN appears to be undertreated in clinical settings, with more AN patients receiving referrals and treatment. A notable exception to this finding was that within studies conducted in countries with universal healthcare systems, rates of AAN patients admitted to treatment centers exceeded those of AN patients (Damiano et al., 2015; Månsson et al., 2016; Swenne, 2016; Welch et al., 2015). This suggests that healthcare systems with greater focus on prevention and early intervention strategies (and healthcare systems with more universal coverage) may increase the likelihood of screening and referring patients with AAN. Similarly, improving access to care and early screening, for those showing prodromes of AAN or AN, may ameliorate some of the disparities observed between AAN prevalence rates and treatment admissions. Such practices would require primary care clinicians to be versed in EDs to identify early warning signs and prodromal presentations, such as higher-weight patients presenting with overvaluation of weight and shape, dieting, or patients presenting with rapid weight loss.
7 |. RESEARCH IMPLICATIONS
Given the limited sample diversity represented across AAN studies, diversifying future samples in age, race, gender, sexual orientation, disability status, and BMI will add to the richness of AAN conceptualization. This is particularly needed considering the limited attention given to older AAN populations (over age 30), people of color, gender diverse or nonconforming patients, and those at the higher end of the weight spectrum. It is worth noting that the one study to include a gender non-conforming sample and report specific prevalence rates (Mitchison et al., 2020) found one of the highest point prevalence rates of AAN within this population, highlighting the need to address eating disturbances within trans and gender nonconforming population.
Second, greater consensus is needed regarding operationalizing AAN to promote cross-study comparison. A practical step to facilitating greater understanding of AAN involves eliminating skip patterns in surveys based on BMI to facilitate greater knowledge of ED behaviors across the weight spectrum. Similarly, eliminating weight limits for AAN samples (with a focus instead on meeting the cognitive and behavioral criteria) will aid in the identification of those with clinically significant EDs regardless of BMI. Additionally, moving toward more consistent trumping criteria for OSFED presentations (perhaps ones that mimic the threshold disorders) could facilitate more homogenous definitions.
Further, since AAN operational definitions differed most by the degree of weight loss required, and because there is not yet an evidence-based level of weight suppression in AAN shown to predict divergent outcomes, it may be prudent to abstain from specifying weight loss cutoffs. In every study that examined variable weight cut-offs, the authors concluded that despite using different operational cutoffs (e.g., 5% weight loss, 10% weight loss, or 15% weight loss; loss of 1.3 BMI points, or no specific weight loss requirement) each operationalized definition captured a group of clinically important cases, and lenient criteria was not necessarily associated with less severity (e.g., Forney et al., 2017; Wade & O’Shea, 2015). Thus, to move toward greater homogeneity in our definitions, the field may wish to consider whether a specific cutoff for significant weight loss is clinically useful and empirically supported.
Next, given the significant diagnostic crossover noted not only between AN and AAN, but also between AAN and other EDs, studies measuring lifetime ED diagnoses should account for multiple ED diagnoses in order to accurately capture prevalence rates of each disorder. Such crossover also suggests the need for longitudinal studies of diagnostic changes in patients over time to clarify ED progression, and the need for qualitative reports of these crossover experiences to determine how patients experience these transitions in diagnoses (e.g., perceiving this as a journey from one diagnosis to another, or simply experiencing this as the changing manifestation of the same disorder over time).
Finally, regarding research implications, Thomas et al. (2015) note that many population studies operationalize ED diagnoses in greater detail than is specified in the DSM-5, presumably in an effort to ensure diagnostic validity. However, such operational definitions may result in truncating potential diagnostic samples (such as BMI caps that necessarily limit the potential pool of patients), increasing sample homogeneity, and likely failing to capture the diversity of patients who may struggle with a given set of cognitive, behavioral, and physical symptoms. Previous authors have commented that the lack of standardized diagnostic hierarchy rules for subthreshold diagnoses contributes to the unreliability of specific OSFED diagnoses rates across studies (Thomas et al., 2015). Standardizing such practices could increase reliability across studies and clarify the nosology of various mixed presentations (e.g., classifying those with AAN and PD as AAN—purging type). As the field moves toward greater clarity and more consistent operational definitions, it would be useful to conduct meta-analyses of prevalence rates and then explore which characteristics predict these prevalence rates. Perhaps, with further investigation into ED classifications, we will discover that a transdiagnostic nosological framework, as proposed by several others (Wade et al., 2006) with a primary symptom subtyping will ultimately prove most useful for research that better understands ED risk factors, early identification, and treatments.
8 |. CONCLUSION
AAN presents as a significant psychiatric disorder, at least as common (if not more) than AN. However, fewer AAN individuals are being identified, diagnosed, and referred for care, representing a significant opportunity in the field. Improved screening and referral practices, in addition to improved access to care are needed to assure that those who suffer with AAN receive timely, appropriate care.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge Drs. Rico Catalano, Randal Beam, Paula Nurius, and Ryan Petros for their feedback on the manuscript. This publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number TL1 TR002318 and by a RSH Dissertation Grant through the Academy of Eating Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The work for this manuscript was completed on the land of the Coastal Salish, Duwamish, Arapaho, Cheyenne, and Lennie Lenape indigenous peoples.
Funding information
Academy of Eating Disorders, Grant/Award Number: RSH Dissertation Grant; National Institutes of Health, Grant/Award Number: TL1 TR002318
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
Note: References demarked with an asterisk were featured in the review.
- *.Allen K, Byrne SM, Oddy W, & Crosby RD (2013). DSM-IV-TR and DSM-5 eating disorders in adolescents: Prevalence, stability, and psychosocial correlates in a population-based sample of male and female adolescents. Journal of Abnormal Psychology, 122(3), 720–732. 10.1037/a0034004 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR (4th ed.). Washington, DC: APA. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed.). Arlington, VA: APA. [Google Scholar]
- *.Andrés-Pepiñá S, Plana MT, Flamarique I, Romero S, Borràs R, Julià L, … Castro-Fornieles J (2020). Long-term outcome and psychiatric comorbidity of adolescent-onset anorexia nervosa. Clinical Child Psychology and Psychiatry, 25(1), 33–44. 10.1177/1359104519827629 [DOI] [PubMed] [Google Scholar]
- *.Bacopoulou F, Lambrou GI, Rodanaki ME, Stergioti E, Efthymiou V, Deligeoroglou E, & Markantonis SL (2017). Serum kisspeptin concentrations are negatively correlated with body mass index in adolescents with anorexia nervosa and amenorrhea. Hormones, 16, 33–41. 10.14310/horm.2002.1717 [DOI] [PubMed] [Google Scholar]
- *.Bayes A, & Madden S (2011). Early onset eating disorders in male adolescents: A series of 10 inpatients. Australasian Psychiatry: Bulletin of Royal Australian and New Zealand College of Psychiatrists, 19(6), 526–532. 10.3109/10398562.2011.603328 [DOI] [PubMed] [Google Scholar]
- *.Birgegård A, Norring C, & Clinton D (2012). DSM-IV versus DSM-5: Implementation of proposed DSM-5 criteria in a large naturalistic database. International Journal of Eating Disorders, 45(3), 353–361. 10.1002/eat.20968 [DOI] [PubMed] [Google Scholar]
- *.Castelao-Naval O, Blanco-Fernández A, Meseguer-Barros CM, Thuissard-Vasallo IJ, Cerdá B, & Larrosa M (2019). Life style and risk of atypical eating disorders in university students: Reality versus perception. Enfermería Clínica (English Edition), 29(5), 280–290. [DOI] [PubMed] [Google Scholar]
- *.Caudle H, Pang C, Mancuso S, Castle D, & Newton R (2015). A retrospective study of the impact of DSM-5 on the diagnosis of eating disorders in Victoria, Australia. Journal of Eating Disorders, 3(1), 35–39. 10.1186/s40337-015-0072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Conceição E, Orcutt M, Mitchell J, Engel S, Lahaise K, Jorgensen M, … Wonderlich S (2013). Eating disorders after bariatric surgery: A case series. International Journal of Eating Disorders, 46(3), 274–279. 10.1002/eat.22074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Damiano SR, Reece JE, Reid S, Atkins L, & Patton G (2015). Empirically derived subtypes of full and subthreshold anorexia nervosa in adolescent females: Understanding general psychopathology and treatment implications. Eating Disorders, 23(3), 1–19. 10.1080/10640266.2014.1000106 [DOI] [PubMed] [Google Scholar]
- *.Darrow SM, Accurso EC, Nauman ER, Goldschmidt AB, & Le Grange D (2017). Exploring types of family environments in youth with eating disorders. European Eating Disorders Review, 25(5), 389–396. 10.1002/erv.2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport E, Rushford N, Soon S, & McDermott C (2015). Dysfunctional metacognition and drive for thinness in typical and atypical anorexia nervosa. Journal of Eating Disorders, 3(1), 1–9. 10.1186/s40337-015-0060-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Eddy KT, Doyle AC, Hoste RR, Herzog DB, & Le Grange D (2008). Eating disorder not otherwise specified in adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 47(2), 156–165. 10.1097/chi.0b013e31815cd9cf [DOI] [PubMed] [Google Scholar]
- *.Ekeroth K, Clinton D, Norring C, & Birgegård A (2013). Clinical characteristics and distinctiveness of DSM-5 eating disorder diagnoses: Findings from a large naturalistic clinical database. Journal of Eating Disorders, 1, 31–42. 10.1186/2050-2974-1-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ernst V, Bürger A, & Hammerle F (2017). Prevalence and severity of eating disorders: A comparison of DSM-IV and DSM-5 among German adolescents. International Journal of Eating Disorders, 50(11), 1255–1263. 10.1002/eat.22780 [DOI] [PubMed] [Google Scholar]
- *.Fairburn CG, & Cooper Z (2011). Eating disorders, DSM–5 and clinical reality. The British Journal of Psychiatry, 198(1), 8–10. 10.1192/bjp.bp.110.083881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Fairweather-Schmidt AK, & Wade TD (2014). DSM-5 eating disorders and other specified eating and feeding disorders: Is there a meaningful differentiation? International Journal of Eating Disorders, 47(5), 524–533. 10.1002/eat.22257 [DOI] [PubMed] [Google Scholar]
- *.Fichter MM, Quadflieg N, Gierk B, Voderholzer U, & Heuser J (2015). The Munich eating and feeding disorder questionnaire (Munich ED-quest) DSM-5/ICD-10: Validity, reliability, sensitivity to change and norms. European Eating Disorders Review, 23(3), 229–240. 10.1002/erv.2348 [DOI] [PubMed] [Google Scholar]
- *.Flatt Rachael E. (2020). Comparing eating disorder characteristics and treatment in self-identified athletes from the National Eating Disorders Association Online Screening Tool (Order No. 28000289). Retrieved from https://du.idm.oclc.org/login?url=https://www-proquest-com.du.idm.oclc.org/dissertations-theses/comparing-eating-disorder-characteristics/docview/2448984601/se-2?accountid=14608 [DOI] [PMC free article] [PubMed]
- *.Forman SF, McKenzie N, Hehn R, Monge MC, Kapphahn CJ, Mammel KA, … Rome ES (2014). Predictors of outcome at 1 year in adolescents with DSM-5 restrictive eating disorders: Report of the National Eating Disorders Quality Improvement Collaborative. Journal of Adolescent Health, 55(6), 750–756. 10.1016/j.jadohealth.2014.06.014 [DOI] [PubMed] [Google Scholar]
- *.Forney KJ, Brown TA, Holland-Carter LA, Kennedy GA, & Keel PK (2017). Defining “significant weight loss” in atypical anorexia nervosa. International Journal of Eating Disorders, 50(8), 952–962. 10.1002/eat.22717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A (2018). Moving beyond “skinniness”: Presentation weight is not sufficient to aassess malnutrition in patients with restrictive eating disorders across a range of body weights. Journal of Adolescent Health, 63, 669–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard J (2014). Health sciences literature review made easy: The matrix method (4th ed.). Burlington, MA: Jones & Bartlett Learning. 10.1093/ajhp/68.23.2302a [DOI] [Google Scholar]
- Godart NT, Legleye S, Huas C, Coté SM, Choquet M, Falissard B, & Touchette E (2013). Epidemiology of anorexia nervosa in a French community-based sample of 39,542 adolescents. Open Journal of Epidemiology, 3(2), 53–61. 10.4236/ojepi.2013.32009 [DOI] [Google Scholar]
- *.Hammerle F, Huss M, Ernst V, & Bürger A (2016). Thinking dimensional: Prevalence of DSM-5 early adolescent full syndrome, partial and subthreshold eating disorders in a cross-sectional survey in German schools. BMJ Open, 6(5), e010843. 10.1136/bmjopen-2015-010843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrop EN (2019). Typical-atypical interactions: One patient’s experience of weight bias in an inpatient eating disorder treatment setting. Women & Therapy, 42(1–2), 45–58. 10.1080/02703149.2018.1524068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hay P, Mitchison D, Collado AEL, Gonzalez-Chica DA, Stocks N, & Touyz S (2017). Burden and health-related quality of life of eating disorders, including avoidant/restrictive food intake disorder (ARFID), in the Australian population (report). Journal of Eating Disorders, 5, 1–10. 10.1186/s40337-017-0149-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hughes EK, Le Grange D, Court A, & Sawyer SM (2017). A case series of family-based treatment for adolescents with atypical anorexia nervosa. International Journal of Eating Disorders, 50(4), 424–432. 10.1002/eat.22662 [DOI] [PubMed] [Google Scholar]
- *.Isomaa A-L, Isomaa R, Marttunen M, & Kaltiala-Heino R (2010). Obesity and eating disturbances are common in 15-year-old adolescents: A two-step interview study. Nordic Journal of Psychiatry, 64(2), 123–129. 10.3109/08039480903265280 [DOI] [PubMed] [Google Scholar]
- *.Kachani AT, Barroso LP, Brasiliano S, Hochgraf PB, & Cordás TA (2014). Body checking and obsessive–compulsive symptoms in Brazilian outpatients with eating disorders. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity, 19(2), 177–182. 10.1007/s40519-014-0111-x [DOI] [PubMed] [Google Scholar]
- Kandemir N, Becker K, Slattery M, Tulsiani S, Singhal V, Thomas JJ, … Misra M (2017). Impact of low-weight severity and menstrual status on bone in adolescent girls with anorexia nervosa. International Journal of Eating Disorders, 50(4), 359–369. 10.1002/eat.22681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kass AE, Accurso EC, Goldschmidt AB, Anam S, Byrne CE, Kinasz K, … Le Grange D (2015). Picking and nibbling in children and adolescents with eating disorders. International Journal of Eating Disorders, 48(8), 1102–1105. 10.1002/eat.22444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kennedy GA, Forman SF, Woods ER, Hergenroeder AC, Mammel KA, Fisher MM, … Garber AK (2017). History of overweight/obesity as predictor of care received at 1-year follow-up in adolescents with anorexia nervosa or atypical anorexia nervosa. Journal of Adolescent Health, 60(6), 674–679. 10.1016/j.jadohealth.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmet LM, Lee RC, & Cook LS (2004). Standard quality assessment criteria for evaluating primary research papers from a variety of fields (Vol. 22). Edmonton: Alberta Heritage Foundation for Medical Research. [Google Scholar]
- *.Koutek J, Kocourkova J, & Dudova I (2016). Suicidal behavior and self-harm in girls with eating disorders. Neuropsychiatric Disease and Treatment, 12, 787–793. 10.2147/NDT.S103015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lebow J, Sim L, Crosby RD, Goldschmidt AB, Le Grange D, & Accurso EC (2019). Weight gain trajectories during outpatient family-based treatment for adolescents with anorexia nervosa. International Journal of Eating Disorders, 52(1), 88–94. 10.1002/eat.23000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Machado PP, Machado BC, Gonçalves S, & Hoek HW (2007). The prevalence of eating disorders not otherwise specified. International Journal of Eating Disorders, 40(3), 212–217. 10.1002/eat.20358 [DOI] [PubMed] [Google Scholar]
- *.Machado PPP, Gonçalves S, & Hoek HW (2013). DSM-5 reduces the proportion of ednos cases: Evidence from community samples. International Journal of Eating Disorders, 46(1), 60–65. 10.1002/eat.22040 [DOI] [PubMed] [Google Scholar]
- *.Makhzoumi SH, Coughlin JW, Schreyer CC, Redgrave GW, Pitts SC, & Guarda AS (2017). Weight gain trajectories in hospital-based treatment of anorexia nervosa. International Journal of Eating Disorders, 50(3), 266–274. 10.1002/eat.22679 [DOI] [PubMed] [Google Scholar]
- *.Matthews A, Lenz KR, Peugh J, Copps EC, & Peterson CM (2018). Caregiver burden and illness perceptions in caregivers of medically hospitalized youth with anorexia nervosa. Eating Behaviors, 29, 14–18. 10.1016/j.eatbeh.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Meeuwsen S, Horgan GW, & Elia M (2010). The relationship between BMI and percent body fat, measured by bioelectrical impedance, in a large adult sample is curvilinear and influenced by age and sex. Clinical Nutrition, 29(5), 560–566. 10.1016/j.clnu.2009.12.011 [DOI] [PubMed] [Google Scholar]
- *.Månsson J, Parling T, & Swenne I (2016). Favorable effects of clearly defined interventions by parents at the start of treatment of adolescents with restrictive eating disorders. International Journal of Eating Disorders, 49, 92–97. 10.1002/eat.22379 [DOI] [PubMed] [Google Scholar]
- *.Micali N, Martini MG, Thomas JJ, Eddy KT, Kothari R, Russell E, … Treasure J (2017). Lifetime and 12-month prevalence of eating disorders amongst women in mid-life: A population-based study of diagnoses and risk factors.(clinical report). BMC Medicine, 15, 1394–1403. 10.1186/s12916-016-0766-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliavaca CB, Stein C, Colpani V, Munn Z, & Falavigna M (2020). Quality assessment of prevalence studies: A systematic review. Journal of Clinical Epidemiology, 127, 59–68. [DOI] [PubMed] [Google Scholar]
- Mitchison D, Hay P, Slewa-Younan S, & Mond J (2014). The changing demographic profile of eating disorder behaviors in the community. BMC Public Health, 14, 22–31. 10.1186/1471-2458-14-943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Mitchison D, Mond J, Bussey K, Griffiths S, Trompeter N, Lonergan A, … Hay P (2020). DSM-5 full syndrome, other specified, and unspecified eating disorders in Australian adolescents: Prevalence and clinical significance. Psychological Medicine, 50(6), 981–990. 10.1186/1471-2458-14-943 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ, 339(7716), B2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Monge MC, Forman SF, McKenzie NM, Rosen DS, Mammel KA, Callahan ST, … Romano ME (2015). Use of psycho-pharmacologic medications in adolescents with restrictive eating disorders: Analysis of data from the National Eating Disorder Quality Improvement Collaborative. Journal of Adolescent Health, 57, 66–72. 10.1016/j.jadohealth.2015.03.021 [DOI] [PubMed] [Google Scholar]
- *.Monteleone AM, Ruzzi V, Patriciello G, Cascino G, Pellegrino F, Vece A, … Maj M (2020). Emotional reactivity and eating disorder related attitudes in response to the trier social stress test: An experimental study in people with anorexia nervosa and with bulimia nervosa. Journal of Affective Disorders, 274, 23–30. 10.1016/j.jad.2020.05.051 [DOI] [PubMed] [Google Scholar]
- *.Mustelin L, Lehtokari VL, & Keski-Rahkonen A (2016). Other specified and unspecified feeding or eating disorders among women in the community. International Journal of Eating Disorders, 49(11), 1010–1017. 10.1002/eat.22586 [DOI] [PubMed] [Google Scholar]
- *.Nagata JM, Carlson JL, Golden NH, Long J, Murray SB, & Peebles R (2019). Comparisons of bone density and body composition among adolescents with anorexia nervosa and atypical anorexia nervosa. International Journal of Eating Disorders, 52(5), 591–596. 10.1002/eat.23048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Nagata JM, Carlson JL, Kao JM, Golden NH, Murray SB, & Peebles R (2017). Characterization and correlates of exercise among adolescents with anorexia nervosa and bulimia nervosa. International Journal of Eating Disorders, 50(12), 1394–1403. 10.1002/eat.22796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Nakai Y, Nin K, Noma S, Teramukai S, Fujikawa K, & Wonderlich SA (2017). The impact of DSM-5 on the diagnosis and severity indicator of eating disorders in a treatment-seeking sample. International Journal of Eating Disorders, 50(11), 1247–1254. 10.1002/eat.22777 [DOI] [PubMed] [Google Scholar]
- *.Nakai Y, Nin K, Noma SI, Teramukai S, Fujikawa K, & Wonderlich SA (2018). Changing profile of eating disorders between 1963 and 2004 in a Japanese sample. International Journal of Eating Disorders, 51(8), 953–958. 10.1002/eat.22935 [DOI] [PubMed] [Google Scholar]
- Neumark-Sztainer D (2015). Higher-weight status and restrictive eating disorders: An overlooked concern. Journal of Adolescent Health, 56, 1–2. 10.1016/j.jadohealth.2014.10.261 [DOI] [PubMed] [Google Scholar]
- *.Ornstein RM, Rosen DS, Mammel KA, Callahan ST, Forman S, Jay MS, … Walsh BT (2013). Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. Journal of Adolescent Health, 53(2), 303–305. 10.1016/j.jadohealth.2013.03.025 [DOI] [PubMed] [Google Scholar]
- *.Peebles R, Hardy KK, Wilson JL, & Lock JD (2010). Are diagnostic criteria for eating disorders markers of medical severity? Pediatrics, 125(5), e1193–e1203. 10.1542/peds.2008-1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Peebles R, Lesser A, Park CC, Heckert K, Timko CA, Lantzouni E, … Weaver L (2017). Outcomes of an inpatient medical nutritional rehabilitation protocol in children and adolescents with eating disorders. Journal of Eating Disorders, 5(7), 1–14. 10.1186/s40337-017-0134-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipou A, & Beilharz F (2019). Should we shed the weight criterion for anorexia nervosa. Australian & New Zealand Journal of Psychiatry, 53(6), 501–502. 10.1177/0004867418814958 [DOI] [PubMed] [Google Scholar]
- *.Redgrave GW, Coughlin JW, Schreyer CC, Martin LM, Leonpacher AK, Seide M, … Guarda AS (2015). Refeeding and weight restoration outcomes in anorexia nervosa: Challenging current guidelines. International Journal of Eating Disorders, 48(7), 866–873. 10.1002/eat.22390 [DOI] [PubMed] [Google Scholar]
- *.Ricca V, Castellini G, Lo Sauro C, Mannucci E, Ravaldi C, Rotella F, & Faravelli C (2010). Cognitive-behavioral therapy for threshold and subthreshold anorexia nervosa: A three-year follow-up study. Psychotherapy and Psychosomatics, 79(4), 238–248. 10.1159/000315129 [DOI] [PubMed] [Google Scholar]
- *.Riesco N, Agüera Z, Granero R, Jiménez-Murcia S, Menchón JM, & Fernández-Aranda F (2018). Other specified feeding or eating disorders (OSFED): Clinical heterogeneity and cognitive-behavioral therapy outcome. European Psychiatry, 54, 109–116. 10.1016/j.eurpsy.2018.08.001 [DOI] [PubMed] [Google Scholar]
- *.Rockert W, Kaplan AS, & Olmsted MP (2007). Eating disorder not otherwise specified: The view from a tertiary care treatment center. (clinical report). The International Journal of Eating Disorders, 40(7), S99–S103. 10.1002/eat.20482 [DOI] [PubMed] [Google Scholar]
- *.Santonastaso P, Bosello R, Schiavone P, Tenconi E, Degortes D, & Favaro A (2009). Typical and atypical restrictive anorexia nervosa: Weight history, body image, psychiatric symptoms, and response to outpatient treatment. International Journal of Eating Disorders, 42(5), 464–470. 10.1002/eat.20706 [DOI] [PubMed] [Google Scholar]
- *.Sawyer SM, Whitelaw M, Grange DL, Yeo M, & Hughes EK (2016). Physical and psychological morbidity in adolescents with atypical anorexia nervosa (report). Pediatrics, 137(4), e20154080. 10.1542/peds.2015-4080 [DOI] [PubMed] [Google Scholar]
- *.Schorr M, Drabkin A, Rothman MS, Meenaghan E, Lashen GT, Mascolo M, … Miller KK (2019). Bone mineral density and estimated hip strength in men with anorexia nervosa, atypical anorexia nervosa and avoidant/restrictive food intake disorder. Clinical Endocrinology, 90(6), 789–797. 10.1111/cen.13960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorr M, Thomas JJ, Eddy KT, Dichtel LE, Lawson EA, Meenaghan E, … Miller KK (2017). Bone density, body composition, and psychopathology of anorexia nervosa spectrum disorders in DSM-IV vs DSM-5. International Journal of Eating Disorders, 50(4), 343–351. 10.1002/eat.22603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreyer CC (2012). The effects of perceived coercion and readiness to change on treatment outcomes in patients with eating disorders (Order No. 3539487). Retrieved from https://du.idm.oclc.org/login?url=https://www-proquest-com.du.idm.oclc.org/dissertations-theses/effects-perceived-coercion-readiness-change-on/docview/1095591313/se-2?accoun
- *.Schreyer CC, Coughlin JW, Makhzoumi SH, Redgrave GW, Hansen JL, & Guarda AS (2016). Perceived coercion in inpatients with anorexia nervosa: Associations with illness severity and hospital course. International Journal of Eating Disorders, 49(4), 407–412. [DOI] [PubMed] [Google Scholar]
- *.Silén Y, Raevuori A, Jüriloo E, Tainio VM, Marttunen M, & Keski-Rahkonen A (2015). Typical versus atypical anorexia nervosa among adolescents: Clinical characteristics and implications for ICD-11. European Eating Disorders Review, 23(5), 345–351. 10.1002/erv.2370 [DOI] [PubMed] [Google Scholar]
- *.Silén Y, Sipilä PN, Raevuori A, Mustelin L, Marttunen M, Kaprio J, & Keski-Rahkonen A (2020). DSM-5 eating disorders among adolescents and young adults in Finland: A public health concern. International Journal of Eating Disorders, 53(5), 520–531. 10.1002/eat.23236 [DOI] [PubMed] [Google Scholar]
- Sim LA, Lebow J, & Billings M (2013). Eating disorders in adolescents with a history of obesity. (report). Pediatrics, 132(4), e1026–e1032. 10.1542/peds.2012-3940 [DOI] [PubMed] [Google Scholar]
- *.Smith M (2020). Treatment fidelity and diagnostic subtype as predictors of outcomes in intensive eating disorder treatment (Order No. 27740743). Retrieved from https://du.idm.oclc.org/login?url=https://www-proquest-com.du.idm.oclc.org/dissertations-theses/treatment-fidelity-diagnostic-subtype-as/docview/2387685506/se-2?accountid=14608
- *.Smith K, Lesser J, Brandenburg B, Lesser A, Cici J, Juenneman R, … Le Grange D (2016). Outcomes of an inpatient refeeding protocol in youth with anorexia nervosa and atypical anorexia nervosa at Children’s hospitals and clinics of Minnesota. Journal of Eating Disorders, 4, 35. 10.1186/s40337-016-0124-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Stice E, Marti CN, & Rohde P (2013). Prevalence, incidence, impairment, and course of the proposed DSM-5 eating disorder diagnoses in an 8-year prospective community study of young women. Journal of Abnormal Psychology, 122(2), 445–457. 10.1037/a0030679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Swenne I (2016). Influence of premorbid BMI on clinical characteristics at presentation of adolescent girls with eating disorders. (report). BMC Psychiatry, 16(82), 1–7. 10.1186/s12888-016-0788-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Swenne I, Parling T, & Salonen Ros H (2017). Family-based intervention in adolescent restrictive eating disorders: Early treatment response and low weight suppression is associated with favourable one-year outcome (report). BMC Psychiatry, 17, 1–10. 10.1186/s12888-017-1486-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Swenne I, & Ros HS (2017). Low weight gain at the start of a family-based intervention for adolescent girls with restrictive eating disorders predicted emergency hospital admission. Acta Paediatrica, 106(10), 1624–1629. 10.1111/apa.13974 [DOI] [PubMed] [Google Scholar]
- *.Sysko R, Roberto CA, Barnes RD, Grilo CM, Attia E, & Walsh BT (2012). Test–retest reliability of the proposed DSM-5 eating disorder diagnostic criteria. Psychiatry Research, 196(2–3), 302–308. 10.1016/j.psychres.2011.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sysko R, & Walsh BT (2011). Does the broad categories for the diagnosis of eating disorders (BCD-ED) scheme reduce the frequency of eating disorder not otherwise specified? International Journal of Eating Disorders, 44(7), 625–629. 10.1002/eat.20860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Thomas JJ, Delinsky SS, Germain SAS, Weigel TJ, Tangren CM, Levendusky PG, & Becker AE (2010). How do eating disorder specialist clinicians apply DSM-IV diagnostic criteria in routine clinical practice? Implications for enhancing clinical utility in DSM-5. Psychiatry Research, 178(3), 511–517. 10.1016/j.psychres.2010.05.021 [DOI] [PubMed] [Google Scholar]
- *.Thomas JJ, Eddy KT, Murray HB, Tromp MDP, Hartmann AS, Stone MT, … Becker AE (2015). The impact of revised DSM-5 criteria on the relative distribution and inter-rater reliability of eating disorder diagnoses in a residential treatment setting. Psychiatry Research, 229(1–2), 517–523. 10.1016/j.psychres.2015.06.017 [DOI] [PubMed] [Google Scholar]
- Thomas JJ, Vartanian LR, & Brownell KD (2009). The relationship between eating disorder not otherwise specified (EDNOS) and officially recognized eating disorders: Meta-analysis and implications for DSM. Psychological Bulletin, 135(3), 407. 10.1037/a0015326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TD, Bergin JL, Martin NG, Gillespie NA, & Fairburn CG (2006). A transdiagnostic approach to understanding eating disorders. The Journal of Nervous and Mental Disease, 194(7), 510–517. 10.1097/01.nmd.0000225067.42191.b0 [DOI] [PubMed] [Google Scholar]
- *.Wade TD, & O’Shea A (2015). DSM-5 unspecified feeding and eating disorders in adolescents: What do they look like and are they clinically significant? International Journal of Eating Disorders, 48(4), 367–374. 10.1002/eat.22303 [DOI] [PubMed] [Google Scholar]
- *.Weingeroff JL (2012). The course of eating disorder not otherwise specified and its subtypes in patients with borderline personality disorder (Order No. 3500655). Retrieved from https://du.idm.oclc.org/login?url=https://www-proquest-com.du.idm.oclc.org/dissertations-theses/course-eating-disorder-not-otherwise-specified/docview/927940080/se-2?accountid=14608
- *.Welch E, Ghaderi A, & Swenne I (2015). A comparison of clinical characteristics between adolescent males and females with eating disorders. BMC Psychiatry, 15(45), 1–7. 10.1186/s12888-015-0419-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Whitelaw M, Gilbertson H, Lee KJ, & Sawyer SM (2014). Restrictive eating disorders among adolescent inpatients. Pediatrics, 134(3), e758–e767. 10.1542/peds.2014-0070 [DOI] [PubMed] [Google Scholar]
- *.Whitelaw M, Lee KJ, Gilbertson H, & Sawyer SM (2018). Predictors of complications in anorexia nervosa and atypical anorexia nervosa: Degree of underweight or extent and recency of weight loss? Journal of Adolescent Health, 63(6), 717–723. 10.1016/j.jadohealth.2018.08.019 [DOI] [PubMed] [Google Scholar]
- *.Wilkes C (2018). Investigating the preliminary effectiveness, acceptability and feasibility of the first online intensive outpatient program for the treatment of eating disorders (Order No. 10750630). Retrieved from https://du.idm.oclc.org/login?url=https://www-proquest-com.du.idm.oclc.org/dissertations-theses/investigating-preliminary-effectiveness/docview/2234728223/se-2?accountid=14608
- Yan J (2007). APA announces DSM-V task force members. Psychiatric News, 42(16), 10–22. 10.1176/pn.42.16.0010 [DOI] [Google Scholar]
- *.Zanna V, Criscuolo M, Mereu A, Cinelli G, Marchetto C, Pasqualetti P, … Vicari S (2020). Restrictive eating disorders in children and adolescents: A comparison between clinical and psychopathological profiles. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity, 1–11. 10.1007/s40519-020-00962-z [DOI] [PubMed] [Google Scholar]
- *.Zimmerman M, Francione-Witt C, Chelminski I, Young D, & Tortolani C (2008). Problems applying the DSM-IV eating disorders diagnostic criteria in a general psychiatric outpatient practice. The Journal of Clinical Psychiatry, 69(3), 381–384. 10.4088/JCP.v69n0306 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


