Abstract
Identifying Nocardia species in routine tests is often difficult owing to its culture time. We report a case of nocardiosis characterized by multiple abscesses of disseminated disease. A man in his 50 's presented with inflammation on the left buttock and right lower leg. Nocardia farcinica was isolated from the abscess on his buttock and confirmed using matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF MS). Radiological assessment revealed multiple abscesses in his brain, left lung, and right thigh. He was successfully treated with antibiotics. Accurate species identification by MALDI-TOF MS aids in the optimal treatment of nocardiosis.
Keywords: Nocardia farcinica, MALDI-TOF MS, Multiple abscess
Introduction
Infections caused by Nocardia species (spp.) are opportunistic infections that occur mainly in immunocompromised patients. Because different species have different properties and drug susceptibility, species identification is important to determine the treatment strategy. However, it is often difficult to identify Nocardia spp in routine bacteriological analysis because of the time required for culture. In this report, we describe a case in which the rapid identification by matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF MS) was useful in the early diagnosis of multiple abscesses caused by N. farcinica.
Case report
A 54-year-old male who is on immunosuppressive drugs, presented with swelling and pain in the left buttock and edema in both lower limbs. For two weeks, he had been aware of pain in the left buttock when seated and was treated with analgesics at another hospital. He was admitted to our hospital for further investigation and treatment because the pain in the buttock had worsened, and he had developed edema in the lower leg.
He was taking 30 mg of prednisolone, 50 mg of azathioprine, and 40 mg of furosemide daily for the treatment of eosinophilic gastroenteritis and associated hypoalbuminemia, for which he had been attending the gastroenterology department of our hospital.
There was no fever and respiratory or gastrointestinal symptoms. He complained of redness, swelling, and a heat sensation on the left buttock. He also had indenting edema in both lower limbs. Redness, swelling, and heat extended from the back of the right thigh to the ankle, and blistering with bleeding occurred on the back of the both lower leg.
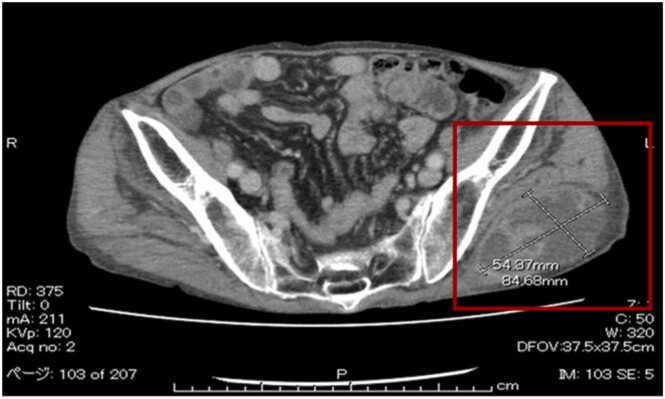
Blood tests showed an elevated inflammatory response: white blood cell count = 15,900/µL, C-reactive protein = 1.91 mg/dL, and hypoalbuminemia (Albumin = 1.9 g/dL). A computed tomography (CT) of the abdomen and pelvis detected a mass lesion, the size of a child's head and covered by a capsule, in the left gluteal region (Fig. 1).
Fig. 1.
Pelvic computed tomography: Left hip shows a mass lesion the size of a child's head covered by a capsule.
Antimicrobial chemotherapy with cefazolin (2 g×3/day) was initiated on day-1 for suspected cellulitis on the right lower leg. The edema in both lower legs was thought to be an exacerbation of hypoalbuminemia, and albumin preparations and diuretics were added to the treatment.
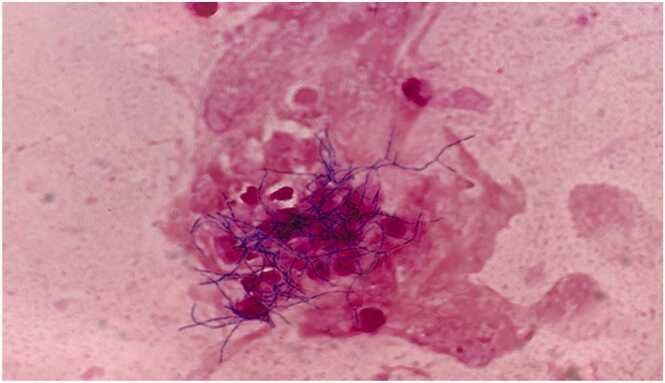
The next day (day-2), an echocardiography-guided puncture was performed on the mass lesion of the left buttock, and the aspirate (abscess contents) was submitted for microbiological analyses. The Gram stain of the aspirate showed branched gram-positive rods (Fig. 2) and, suspecting actinomycosis, we changed the antimicrobial agent from cefazolin to meropenem (0.5 g×3/day). The aspirate was cultured onto sheep blood agar medium (Pourmedia M58, blood agar, Eiken Chemical Co., Ltd.,) and Brucella HK medium (RS) (Kyokuto Pharmaceutical Co., Ltd.,) and incubated at 35 °C in 5% carbon dioxide and anaerobic conditions, respectively.
Fig. 2.
Gram-stained image of left buttock puncture fluid.
Although there was no growth of bacteria on the anaerobic culture medium, micro-colonies grew on the sheep blood agar after 18 h of incubation. The MALDI Biotyper (Bruker Japan) identified the top 10 isolates as N. farcinica (Score Value 2.216).
On day-3, N. farcinica was identified from the culture of the specimen. Drug susceptibility testing was conducted using the Etest method, and the results were determined according to the breakpoints described by the Clinical and Laboratory Standards Institute (CLSI) M24-A3 (Table 1). The results showed that the isolate was sensitive to imipenem, amikacin, levofloxacin, and sulfamethoxazole-trimethoprim. The antimicrobial agent was changed to sulfamethoxazole-trimethoprim 11.9 mg/kg/day.
Table 1.
Results of susceptibility testing.
| Antimicrobial agent | MIC (µg/mL) | Category |
|---|---|---|
| Ceftriaxone | 64 | R |
| Imipenem | 2 | S |
| Amikacin | 1 | S |
| Gentamicin | 32 | R |
| Minocycline | 4 | I |
| Levofloxacin | 0.5 | S |
| Sulfamethoxazole - Trimethoprim | 2.38/0.12 | S |
S: susceptible,I: intermediate,R: resistant.
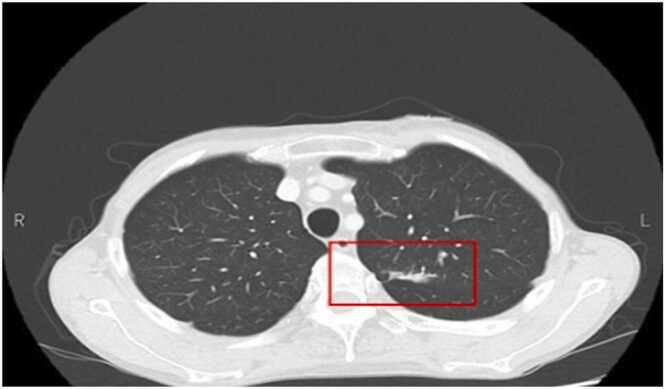
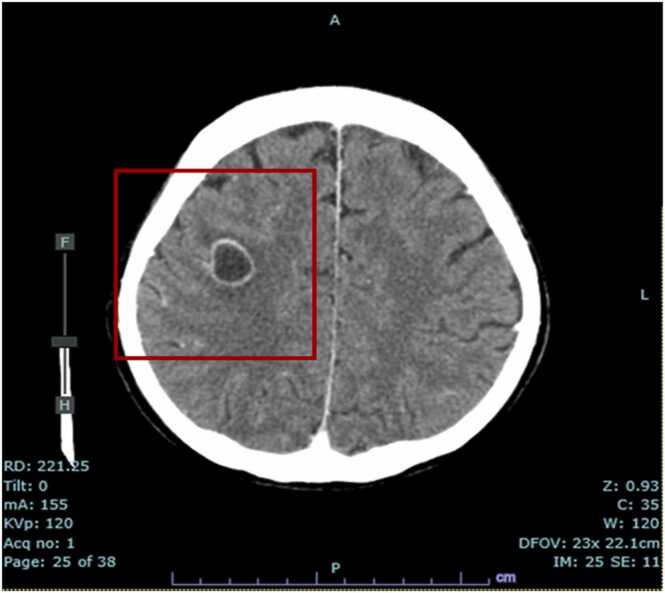
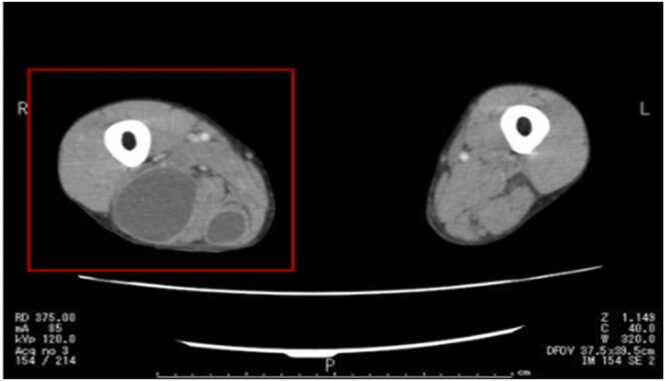
On day-5 of hospitalization, incision and drainage was performed under local anesthesia, and the same species was detected from the pus specimen. By day-7, the edema of both lower legs and the pain in the buttocks had subsided. We suspected the possibility of disseminated lesions based on the results of the bacterial species identification and performed cerebral, chest, pelvic, and leg CT scans and magnetic resonance imaging, by which multiple abscesses were detected in the parenchyma, left lung, and right thigh (Fig. 3, Fig. 4, Fig. 5).
Fig. 3.
Chest computed tomography: Mass shadows are seen in the left lung field.
Fig. 4.
Cerebral computed tomography: Nodule with ring-like enhancing effect in the subcortical white matter of the right frontal lobe, surrounded by an extended hypoabsorptive area that may be edematous changes.
Fig. 5.
Pelvic leg computed tomography: Showing abscess in right thigh.
On day-13, the antimicrobial agent was changed to imipenem/cilastatin (0.5 g×3/day) because of hyponatremia and nausea caused by sulfamethoxazole-trimethoprim. No new lesions developed thereafter, and the patient was discharged on day-42 and continued outpatient treatment with moxifloxacin (400 mg/day) without a relapse.
Discussion
Nocardia spp. are widespread in nature, especially in soil, and are rarely endemic to humans. However, they can cause opportunistic human infections in compromised hosts. The most common routes of human infection are invasion through the respiratory tract to the lungs or through wounds in the skin; these frequently result in disseminated disease such as bacteremia, osteomyelitis, or brain abscesses [1].
In a retrospective study of 1050 patients with Nocardia spp. infection, 64% had immunocompromised conditions owing to corticosteroid use, malignancy, and HIV infection. The most common sites of infection were the lungs and skin, with an incidence of 39% and 32%, respectively. Multiple-site infections were often seen (336 cases, 32%), and central nervous system lesions, including brain abscesses, were found in 44% (148 cases) with multiple-site lesions [2].
In Japan, Nocardia spp. have been detected with the following frequency: N. farcinica (25%), N. cyriacigeorgica (18%), N. brasiliensis (9%), N. nova (8%), and N. otitidiscaviarum (7%) [3]. N. farcinica, in particular, has a high affinity for the brain and is prone to forming brain abscesses [4]. The mortality rate due to brain abscesses caused by Nocardia spp was reported to be 31%, which is higher than that of other pathogens (<10%). Therefore, early identification of the species is crucial in the treatment of nocardiosis [5].
Nocardia spp. differ in antimicrobial susceptibility and virulence; thus, genus-level identification is insufficient, and species-level identification is necessary for the appropriate selection of antibiotics [6]. However, it can take ≥ 3 days for Nocardia to form colonies on laboratory agar, and it is often difficult to identify the specific species by biochemical characterization. MALDI-TOF MS is a rapid and accurate method for the identification of microbial species. In an evaluation of 60 strains of the genus Nocardia using the MALDI Biotyper, the identification agreement with 16 S rRNA sequencing was 100% for N. farcinica and 73% for other Nocardia species when the score cutoff value was set at 1.8. Even when candidates below the cutoff value were included, the name of the identified species was provided in all cases [7].
In this case, the use of MALDI-TOF MS enabled us to identify the causative organism as N. farcinica within one day of culture, from micro-colonies that grew on the blood agar. Although there were no symptoms of central nervous system involvement at an early stage, we suspected Nocardia disseminated disease and performed a systemic radiological assessment, which led to the early detection of multiple abscesses.
The first-line antimicrobial agent for nocardiosis is sulfamethoxazole-trimethoprim, which requires antimicrobial therapy for at least 2–3 months and> 1 year in severe cases. In this case, hyponatremia was observed as a side effect of sulfamethoxazole-trimethoprim, and the antimicrobial agent had to be changed. In such cases, drug susceptibility testing is essential to determine which antibiotics would be effective in eliminating the pathogen. The CLSI recommends the trace broth microdilution method for antimicrobial susceptibility testing [8], but the results may vary depending on the facility and the assessor; thus, the disc diffusion method is considered to have better reproducibility [9]. Two studies reported good concordance rates of 96.2% and 96.6% respectively, when comparing the Etest, with the broth microdilution method [10], [11]. We used the Etest for the drug susceptibility testing of Nocardia farcinica in the current study because of the associated simplicity and flexibility in selecting the appropriate drug for treatment. Among the drugs showing efficacy were imipenem, amikacin, levofloxacin, sulfamethoxazole-trimethoprim, carbapenem, and fluoroquinolone. Moxifloxacin and linezolid have been used effectively in the treatment of brain abscesses caused by N. farcinica [12], [13]. We chose imipenem and successfully controlled the infection, followed by moxifloxacin after the patient was discharged to prevent a relapse. The investigation of further cases are needed to select optimal second-line antibiotics for nocardiosis, especially for deep-seated abscesses.
In conclusion, MALDI-TOF MS was useful in rapidly identifying N. farcinica, leading to an early diagnosis of multiple abscesses caused by this organism and the administration of a successful treatment strategy, with the appropriate antibiotics.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
No identifiers utilized in this case report.
Acknowledgments
We would like to thank Editage for the English language editing of our manuscript.
Author contribution
Shota Yonetani: Study conception ando design, Acquisition of data, Analysis ando interpretation of data. Hiroaki Ohnishi: Critical revision. All authors contributed to the writing of the final manuscript.
Declaration of Competing Interest
None declared.
References
- 1.Lerner P.I. Nocardiosis. Clin Infect Dis. 1996;22:903. doi: 10.1093/clinids/22.6.891. [DOI] [PubMed] [Google Scholar]
- 2.Beaman B.L., Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994;7:213–264. doi: 10.1128/CMR.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toyokawa M., Ohana N., Ueda A., Imai M., Tanno D., Honda M., et al. Identification and antimicrobial susceptibility profiles of Nocardia species clinically isolated in Japan. Sci Rep. 2021;11:16742. doi: 10.1038/s41598-021-95870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathisen G.E., Johnson J.P. Brain abscess. Clin Infect Dis. 1997;25:79. doi: 10.1086/515541. [DOI] [PubMed] [Google Scholar]
- 5.Mamelak A.N., Obana W.G., Flaherty J.F., Rosenblum M.L. Nocardial brain abscess: treatment strategies and factors influencing outcome. Neurosurgery. 1994;35:622–631. doi: 10.1227/00006123-199410000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Zintgraff J., Prieto M., Peña M., Simoiz F., Rosenblit S., D’Alessandro D., et al. When reporting Nocardia spp. is not enough. Brain abscess caused by Nocardia farcinica. Access Microbiol. 2020;2 doi: 10.1099/acmi.0.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarbrough M.L., Lainhart W., Burnham C.D. Identification of Nocardia, Streptomyces, and Tsukamurella using MALDI-TOF MS with the Bruker biotyper. Diagn Microbiol Infect Dis. 2017;89:92–97. doi: 10.1016/j.diagmicrobio.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Fihman V., Berçot B., Mateo J., Losser M.R., Raskine L., Riahi J., et al. First successful treatment of Nocardia farcinica brain abscess with moxifloxacin. J Infect. 2006;52:e99–e102. doi: 10.1016/j.jinf.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Moylett E.H., Pacheco S.E., Brown-Elliott B.A., Perry T.R., Buescher E.S., Birmingham M.C., et al. Clinical experience with linezolid for the treatment of Nocardia infection. Clin Infect Dis. 2003;36:313–318. doi: 10.1086/345907. [DOI] [PubMed] [Google Scholar]
- 10.CLSI . Clinical and Laboratory Standards Institute; Wayne, Pennsylvania: 2003. Susceptibility testing of mycobacteria, nocardiae and other aerobic actinomycetes. CLSI document M24-A. [PubMed] [Google Scholar]
- 11.Conville P.S., Brown-Elliott B.A., Wallace R.J., Witebsky F.G., Koziol D., Hall G.S., et al. Multisite reproducibility of the broth microdilution method for susceptibility testing of Nocardia species. J Clin Microbiol. 2012;50:1270–1280. doi: 10.1128/JCM.00994-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biehle J.R., Cavalieri S.J., Saubolle M.A., Getsinger L.J. Comparative evaluation of the E test for susceptibility testing of Nocardia species. Diagn Microbiol Infect Dis. 1994;19:101–110. doi: 10.1016/0732-8893(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 13.Ambaye A., Kohner P.C., Wollan P.C., Roberts K.L., Roberts G.D., Cockerill F.R. Comparison of agar dilution, broth microdilution, disk diffusion, E-test, and Bactec radiometric methods for antimicrobial susceptibility testing of clinical isolates of the Nocardia asteroides complex. J Clin Microbiol. 1997;35:847–852. doi: 10.1128/jcm.35.4.847-852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]