Abstract
Several new quinolones that exhibit enhanced in vitro activity against Streptococcus pneumoniae have been developed. Using a dynamic in vitro model, we generated time-kill data for ciprofloxacin, clinafloxacin, grepafloxacin, levofloxacin, moxifloxacin, and trovafloxacin against three isolates of quinolone-susceptible S. pneumoniae. Three pharmacokinetic profiles were simulated for each of the study agents (0.1, 1, and 10 times the area under the concentration-time curve [AUC]). Target 24-h AUCs were based upon human pharmacokinetic data resulting from the maximal daily doses of each agent. Ciprofloxacin was the least active agent against all three isolates. With regimens that simulated the human 24-h AUC, ciprofloxacin resulted in an initial, modest decline in the numbers of CFU per milliliter; however, by 48 h the numbers of CFU per milliliter returned to or exceeded the starting inoculum. At the AUC, levofloxacin resulted in variable bacteriostatic and bactericidal activities against the isolates. The remaining agents yielded bactericidal (99.9% reduction) activity by 48 h with regimens that simulated the AUC. At 0.1 time the AUC ciprofloxacin and levofloxacin produced no inhibitory effect, grepafloxacin exhibited bacteriostatic activity, trovafloxacin had mixed static and cidal activities, and clinafloxacin and moxifloxacin caused significant reductions in the numbers of CFU per milliliter by 48 h. All six agents produced cidal activity at 10 times the AUC. In this dynamic in vitro model of infection, the quinolones demonstrated various degrees of activity against S. pneumoniae. The rank order of activity, with respect to bactericidal effect, was ciprofloxacin (least active) ≪ levofloxacin < grepafloxacin, trovafloxacin < clinafloxacin and moxifloxacin (most active). The rank order of the agents with respect to the selection of resistance was ciprofloxacin (most likely) > grepafloxacin, moxifloxacin, and trovafloxacin > levofloxacin > clinafloxacin.
The frequency of isolation of penicillin-nonsusceptible strains of Streptococcus pneumoniae increased dramatically during the 1990s (1, 5, 21). Additionally, cross-resistance of these isolates to other classes of antimicrobials such as the cephalosporins, trimethoprim-sulfamethoxazole, macrolides, chloramphenicol, and tetracyclines is extremely common (6). As a result, selection of antimicrobials for the treatment of pneumococcal infections, especially selection of empiric therapy, has become more complicated. The resistance of pneumococci to fluoroquinolones occurs infrequently, even among isolates exhibiting high-level resistance to penicillin (2, 20). Over the past few years, a variety of new fluoroquinolones with enhanced activity against gram-positive pathogens including S. pneumoniae have been developed.
Experience with ciprofloxacin in the treatment of pneumococcal infections has left many clinicians wary of using quinolones for the management of pneumococcal respiratory tract infections. These individuals cite reports of treatment failures that have resulted in breakthrough bacteremia among patients receiving ciprofloxacin therapy (3, 9, 10, 12, 18); E. Perez-Trallero, J. M. Garcia-Arenzana, J. A. Jimenez, and A. Peris, Letter, Eur. J. Clin. Microbiol. Infect. Dis. 9:905–906, 1990). Proponents of the newer quinolones, however, argue that these treatment failures were not the result of emergence of resistance but were the result of suboptimal dosing of the drug and failure to achieve optimal pharmacodynamic conditions. The superior potencies of the newer quinolones generally result in improved pharmacodynamic parameters and are therefore thought to lessen the likelihood of treatment failure and reduce the emergence of quinolone-resistant isolates (7, 14, 15).
In vitro dynamic models are tools used to evaluate the killing kinetics of antimicrobials under controlled conditions that allow the simulation of human pharmacokinetic parameters. Although the data generated via these models are extremely useful, the models are time-consuming and costly to run. As a result, investigators frequently test only a limited number of antimicrobials and isolates. Additionally, variability among test conditions and models may make comparison of the data generated by various investigators difficult to compare. The goal of the current study was to describe and compare the killing dynamics of six fluoroquinolones (ciprofloxacin, clinafloxacin, grepafloxacin, levofloxacin, moxifloxacin, and trovafloxacin) under three different simulated pharmacokinetic profiles against three clinical isolates of S. pneumoniae by using a dynamic in vitro model of infection.
(This study was presented at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, 26 to 29 September 1999, San Francisco, Calif.).
MATERIALS AND METHODS
Antimicrobial agents.
An analytical-grade powder of each quinolone was obtained from the respective manufacturer: ciprofloxacin, Bayer Corporation (West Haven, Conn.); clinafloxacin, Parke-Davis (Morris Plains, N.J.); grepafloxacin, GlaxoWellcome (Research Triangle Park, N.C.); levofloxacin, Ortho-McNeil Pharmaceutical (Raritan, N.J.); moxifloxacin, Bayer Corporation; and trovafloxacin, Pfizer Pharmaceutical (New York, N.Y.). Aqueous stock solutions were prepared for each agent and stored in unit-of-use aliquots at −80°C. Dimethyl sulfoxide (9% [vol/vol]) was used to aid the solubilization of trovafloxacin.
Study isolates.
Three clinical strains of S. pneumoniae strains (R1, R11, and R20) were selected for testing. Strains R1 and R11 were resistant to penicillin (MICs, 2 and 8 μg/ml, respectively). All isolates were macrolide susceptible. The MIC of each of the quinolones for the test isolates was determined by broth microdilution techniques (19). MICs were determined prior to the initiation of the time-kill experiments and each day the isolate was used in the model.
Time-kill model.
A one-compartment glass model consisting of a central culture compartment, medium and waste reservoirs, and a peristaltic pump was used. Mueller-Hinton broth supplemented with 5% lysed horse blood (PML Microbiologicals, Wilsonville, Oreg.) served as the culture medium. Prior to the initiation of the time-kill experiments, the pharmacokinetics of each quinolone in the model were verified. Each of the study agents was examined under three simulated pharmacokinetic profiles: (i) human area under the concentration-time curve (AUC) from time zero to 24 h (AUC0–24), (ii) 0.1 time the human AUC0–24, and (iii) 10 times the human AUC0–24. The target pharmacokinetic parameters for each agent are provided in Table 1.
TABLE 1.
Target and simulated pharmacokinetic parameters
| Fluoroquinolone and AUC | Dosing regimena | Target AUC0–24 (μg · h/ml) | Simulated AUC0–24 (μg · h/ml) | Simulated peak concn (μg/ml) | Target t1/2 (h) | Simulated t1/2 (h) |
|---|---|---|---|---|---|---|
| Ciprofloxacin | 750 mg BID | 40 | 28 | 2.0 | 4.5 | 4.8 |
| 0.1 × | 4.0 | 3.7 | 0.25 | |||
| 10 × | 400 | 406 | 19 | |||
| Clinafloxacin | 400 mg BID | 47 | 54 | 3.2 | 7.0 | 6.5 |
| 0.1 × | 4.7 | 2.4 | 0.5 | |||
| 10 × | 470 | 430 | 23.6 | |||
| Grepafloxacin | 600 mg QD | 28 | 51 | 2.3 | 15.5 | 15.0 |
| 0.1 × | 2.8 | 2.0 | 0.14 | |||
| 10 × | 280 | 540 | 13.7 | |||
| Levofloxacin | 500 mg QD | 48 | 42 | 4.8 | 7.5 | 7.0 |
| 0.1 × | 4.8 | 4.7 | 0.5 | |||
| 10 × | 480 | 370 | 32.4 | |||
| Moxifloxacin | 400 mg QD | 48 | 53 | 3.0 | 12 | 14.3 |
| 0.1 × | 4.8 | 6.4 | 0.34 | |||
| 10 × | 480 | 653 | 29 | |||
| Trovafloxacin | 200 mg QD | 35 | 48 | 4.3 | 12 | 13.8 |
| 0.1 × | 3.5 | Not calculatedb | ||||
| 10 × | 350 | 268 | 33 |
BID, twice a day; QD, once a day.
An AUC for the 0.1 time the AUC regimen that simulated could not be calculated because the concentration of trovafloxacin in many of the samples fell below the limit of detection.
Time-kill analysis was performed with each of the pneumococcal isolates against each of the fluoroquinolone agents under all three simulated pharmacokinetic conditions. Prior to use, the test isolate was subcultured twice on blood agar plates (Remel, Lexana, Kans.). Immediately prior to the initiation of the study, a standardized bacterial suspension was prepared by suspending colonies from a 24-h culture plate in normal saline and adjusting the suspensions to a 3.0 McFarland turbidity standard. An appropriate volume of the standardized suspension was then introduced into the central compartment of the model. This resulted in a starting inoculum of approximately 5 × 105 to 1 × 106 CFU/ml. The central compartment was then placed in a water bath on a magnetic stirring plate. At time zero, an appropriate amount of quinolone was added to the central compartment. Four models were run simultaneously: (i) control (no drug), (ii) the human AUC0–24, (iii) 0.1 time the human AUC0–24, and (iv) 10 times the human AUC0–24. A peristaltic pump was then programmed and activated. The model was allowed to run for 48 h. Additional doses of drug were added to the central compartment when appropriate to simulate the dosing regimens of the test agents (times 12, 24, and 36 h for ciprofloxacin and clinafloxacin and time 24 h for the remainder of the quinolones). The temperature of the central compartment was maintained at 37°C throughout the study period. At predetermined time points over the 48-h study period, samples of the culture medium were removed from the central compartment and serially diluted as appropriate, and 100 μl was plated on to blood agar plates. Determinations of viable colony counts were performed following incubation of the culture plates in the presence of CO2 at 37°C for 24 h. Additionally, at 24 and 48 h, 100 μl was removed from the central compartment and plated without dilution onto agar plates containing the fluoroquinolone being tested at concentrations equal to two and four times the preexperiment MIC. If growth was noted on drug-containing agar, colonies were selected and MICs were redetermined for all of the quinolones. All experiments were performed in duplicate.
According to the sampling methods used, no antibiotic carryover was noted over the range of concentrations over which samples were removed from the central compartment and plated directly without dilution (13). The lower limit of accurately and reproducibly countable bacteria obtained with a 100-μl sampling volume was determined to be 30 CFU/ml (13). The variability associated with sampling techniques was determined to range from 12 to 20% for the various isolates.
Drug analysis.
Samples removed from the model for drug concentration determination were analyzed by microbiological assay methods with Klebsiella pneumoniae ATCC 10031 as the indicator organism. Standard curves were constructed for each quinolone over a concentration range of 0.125 to 25 μg/ml. Briefly, antibiotic medium 5 (Difco, Detroit, Mich.) agar plates (150 mm) were prepared. Immediately prior to use, the entire surface of the culture plate was swabbed with a 0.5 McFarland suspension of K. pneumoniae ATCC 10031. Upon drying, 6-mm sterile paper disks were aseptically placed on the surface of the plate. Twenty microliters of the antibiotic standard or unknown sample was placed on each paper disk. Each standard or sample was placed on three separate disks (in triplicate). The plates were then incubated at 37°C for 16 to 18 h. Following incubation, zone size diameters were determined and recorded. These procedures resulted in standard curves with correlation coefficients (r2) of >0.97. Intra- and interday coefficients of variation (CVs) were ≤10%.
Data analysis.
For each quinolone regimen, a concentration-versus-time profile was constructed. Drug concentration data were fitted by using WinNonlin (Scientific Consulting, Inc.), and the AUC0–24, half-life (t1/2), maximum concentration of drug, and minimum concentration of drug were calculated.
Time-kill data from duplicate runs were averaged and plotted as a function of time. The rate of killing observed with each quinolone was compared with the medium flow rate to determine whether the reduction in the numbers of CFU was due to the killing effect of the drug or a dilution effect. If the rate of reduction was less than that which would have been predicted by flow rate alone, the reduction was attributed to the effect of the drug. However, if the reduction in the numbers of CFU was greater than or equal to that predicted by the flow rate, a mathematical model that allowed correction for this dilutional effect was used (11). The changes in the log number of CFU per milliliter from the starting inocula at times of 24 and 48 h were determined for each regimen and isolate. Bacteriostatic or inhibitory was defined as a <99.9% reduction in the numbers of CFU per milliliter versus the starting inoculum. Bactericidal was defined as a ≥99.9% reduction in the numbers of CFU per milliliter versus the starting inoculum. The presence or absence of regrowth over the study period was noted. The AUC:MIC and peak:MIC were calculated for each regimen and isolate by using calculated pharmacokinetic values. Composite parameter-response curves were then constructed by plotting AUC:MIC or peak:MIC as a function of the change in the log10 numbers of CFU per milliliter at 24 h from the starting inoculum. Data were then fitted with SigmaPlot (SPSS, Inc.) by using a four-parameter Hill equation: f = y0 + a(xb)/(cb + xb), where f is the observed effect (change in numbers of CFU per milliliter), y0 is the minimal change in the numbers of CFU per milliliter noted, a is the difference between the minimal and maximal changes in the numbers of CFU per milliliter, x is the pharmacodynamic parameter of interest, c is equal to 50% of x, and b is a constant. The 50, 90, and 99% effective ratio (ERs; ER50, ER90, and ER99, respectively) were calculated for each plot.
RESULTS
Susceptibility data.
All of the isolates were quinolone susceptible on the basis of the ciprofloxacin and levofloxacin MICs. Also, these isolates were found to lack mutations in the quinolone resistance-determining regions of parC and gyrA. The median MICs of the quinolones for each of the pneumococcal isolates are presented in Table 2.
TABLE 2.
Median fluoroquinolone MICs
| Fluoroquinolone | MIC (μg/ml)
|
||
|---|---|---|---|
| S. pneumoniae R1 | S. pneumoniae R11 | S. pneumoniae R20 | |
| Ciprofloxacin | 1.0 | 1.0 | 1.0 |
| Clinafloxacin | 0.06 | 0.03 | 0.03 |
| Grepafloxacin | 0.125 | 0.125 | 0.125 |
| Levofloxacin | 1.0 | 0.5 | 0.5 |
| Moxifloxacin | 0.03 | 0.03 | 0.015 |
| Trovafloxacin | 0.06 | 0.03 | 0.03 |
Time-kill data.
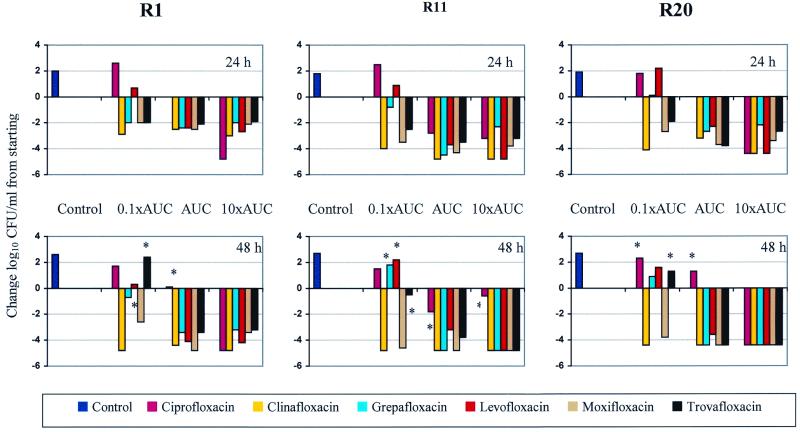
All of the test strains of S. pneumoniae remained viable in the model over the 48-h study period. At the 48-h time point each of the three strains exhibited >2 log10 CFU/ml of growth. A summary of the change in the log10 number of CFU per milliliter at times of 24 and 48 h versus the starting inoculum are presented in Fig. 1. All regimens resulted in colony count reductions that were slower than those that would have been predicted by a dilutional effect alone. Therefore, no mathematical corrections were used. With regimens that simulated 0.1 time the AUC0–24, only clinafloxacin was consistently bactericidal against all three isolates of S. pneumoniae tested. Moxifloxacin exhibited bactericidal activity against two of the three isolates when it was tested under conditions that simulated 0.1 time the AUC0–24. For the remaining four quinolones, slight growth or a reduction in the numbers of CFU per milliliter followed by subsequent regrowth was observed following exposure to 0.1 time the AUC0–24. With regimens that simulated the observed human AUC0–24, all of the agents except ciprofloxacin reached the bactericidal endpoint by 48 h. In contrast, ciprofloxacin resulted in growth over the starting inoculum for two of the three isolates and a <2-log10 CFU/ml reduction for the third isolate. At 10 times the simulated human AUC0–24, all of the agents with the exception of ciprofloxacin against isolate R11 caused 3-log10 reductions in the numbers of CFU of the test isolates per milliliter by 48 h.
FIG. 1.
Summary of change in the log10 number of CFU per milliliter at time 24 and 48 h versus the starting inoculum. ∗, regrowth.
Table 3 summarizes the data regarding observed the changes in the MICs of each of the quinolones at 24 and 48 h following drug exposure. With the exception of clinafloxacin, all of the quinolones demonstrated the ability to select for resistant isolates. This effect was most pronounced with regimens that simulated exposure to 0.1 time the AUC0–24. Exposure to ciprofloxacin regimens resulted in the emergence by 48 h of isolates for which MICs were from four- to eightfold higher than those for the preexposure isolate. This phenomenon occurred following exposure to regimens that simulated both 0.1 and 1 time the AUC0–24 for ciprofloxacin. For isolates for which ciprofloxacin-induced changes in the MICs were demonstrated, similar magnitudes of increases in MICs of all other quinolones were also detected (data not shown).
TABLE 3.
Median MICs for isolates recovered at 24 and 48 h following drug exposure
| Drug and AUC | MIC (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
S. pneumoniae R1
|
S. pneumoniae R11
|
S. pneumoniae R20
|
|||||||
| 0 h | 24 h | 48 h | 0 h | 24 h | 48 h | 0 h | 24 h | 48 h | |
| Ciprofloxacin | 1.0 | 1.0 | 1.0 | ||||||
| 0.1 × | 4.0 | 4.0 | 2.0 | 4.0 | —a | 8.0 | |||
| 1 × | 4.0 | 6.0 | 2.0 | 4.0 | 6.0 | 8.0 | |||
| 10 × | — | — | — | — | — | — | |||
| Clinafloxacin | 0.06 | 0.03 | 0.03 | ||||||
| 0.1 × | — | — | — | — | — | — | |||
| 1 × | — | — | — | — | — | — | |||
| 10 × | — | — | — | — | — | — | |||
| Grepafloxacin | 0.125 | 0.125 | 0.125 | ||||||
| 0.1 × | 0.25 | 4.0 | 0.5 | 1.0 | 0.5 | 1.25 | |||
| 1 × | — | — | — | — | — | — | |||
| 10 × | — | — | — | — | — | — | |||
| Levofloxacin | 1.0 | 0.5 | 0.5 | ||||||
| 0.1 × | — | 2.0 | — | 1.0 | — | — | |||
| 1 × | — | — | — | — | — | — | |||
| 10 × | — | — | — | — | — | — | |||
| Moxifloxacin | 0.03 | 0.03 | 0.015 | ||||||
| 0.1 × | 0.12 | 0.25 | 0.06 | — | 0.12 | 0.185 | |||
| 1 × | 0.12 | — | — | — | 0.12 | — | |||
| 10 × | 0.12 | — | 0.06 | — | 0.12 | — | |||
| Trovafloxacin | 0.06 | 0.03 | 0.03 | ||||||
| 0.1 × | 0.25 | 0.375 | 0.25 | 0.25 | 0.25 | 0.12 | |||
| 1 × | — | — | — | — | — | — | |||
| 10 × | — | — | — | — | — | — | |||
—, no organisms recovered on quinolone-containing agar.
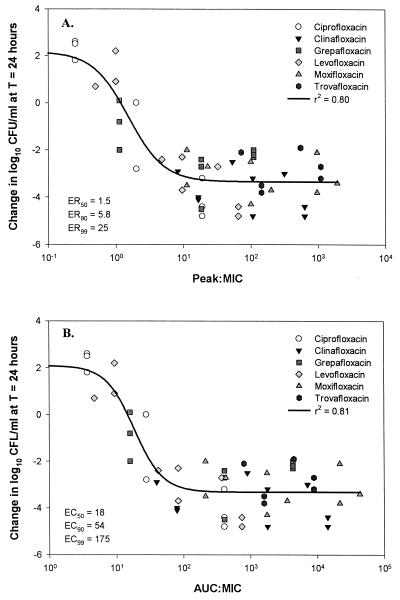
Composite plots of AUC:MIC and peak:MIC versus the change in the log10 numbers of CFU per milliliter for the fluoroquinolones against the three test isolates are presented in Fig. 2. Good correlations were noted between both AUC:MIC (r2 = 0.81) and peak:MIC (r2 = 0.80) and the observed reduction in the numbers of viable colonies at 24 h. The ratios (AUC:MIC and peak:MIC) that produced 50, 90, and 99% of the maximal effect were calculated. For the AUC:MIC these ratios were 18, 54, and 175 for ER50, ER90, and ER99, respectively (standard error of the estimate, 0.97). Values for peak:MIC were determined to be 1.5, 5.8, and 25, respectively (standard error of the estimate, 0.98). The standard error is a measure of the actual variability about the regression plane of the population. The underlying population generally falls within 2 standard errors of the calculated value.
FIG. 2.
Composite plots of peak:MIC (A) and AUC:MIC (B) versus change in the numbers of CFU per milliliter at 24 h. T, time.
DISCUSSION
In vitro systems capable of simulating human pharmacokinetic parameters are useful tools that provide insight into the killing kinetics of antimicrobial agents under conditions of constantly changing antimicrobial concentrations. In the current study, we examined the activities of six different fluoroquinolones, tested with three different simulated pharmacokinetic profiles, against three isolates of S. pneumoniae. In our model we simulated the total drug concentration profile of each of the agents. Although some investigators promote the use of a correction based on the percentage of protein binding, we are not sure that these corrections are appropriate on the basis of the dynamic nature of this binding (personal data). Instead, we opted to test several regimens that cover a range of simulated concentration profiles for each agent.
Other investigators have previously examined the activities of various quinolones against S. pneumoniae in dynamic in vitro models (14–16). Those studies evaluated the activities of various fluoroquinolone agents under pharmacokinetic conditions that simulate parameters likely to occur in humans following administration of a commonly used dose. In the current study, we not only simulated typical serum pharmacokinetic parameters but we also examined pharmacokinetic profiles that resulted in AUC0–24 values that were 1 log10 higher and lower than the standard AUC0–24 for serum (i.e., 0.1 and 10 times the AUC0–24). As a result, we were able to evaluate the antipneumococcal activities of the quinolones under extremes of drug exposure conditions, providing ourselves with more complete data with which to conduct a pharmacodynamic analysis.
Regimens that simulate 10 times the AUC0–24 exhibited the most rapid and complete activity against the test isolates. Additionally, under these conditions, none of the quinolones selected for strains for which MICs were elevated at 48 h. In contrast, under suboptimal exposure conditions, 0.1 time the AUC0–24, only clinafloxacin consistently exhibited bactericidal activity. Furthermore, at this level of exposure, isolates for which MICs were increased were recovered from runs with each of the quinolones except clinafloxacin. Of particular interest, resistant isolates were recovered from runs with ciprofloxacin following 24 and 48 h of exposure even when they were tested with regimens that simulate the AUC0–24. Similar findings were reported by Lacy and colleagues (14) with respect to the ability of ciprofloxacin to select for resistant strains of pneumococci following exposure to regimens that simulate exposure to AUC0–24. Also, even though we recovered isolates for which MICs were elevated following exposure to moxifloxacin under all simulated regimens, administration of the second dose of moxifloxacin was able to eradicate these bacteria. This finding lends support to the belief that the use of quinolones that demonstrate low MICs or low mutant protection concentrations (MPCs) might be beneficial in slowing the emergence of resistance (7). Even if selective pressures do result in the emergence of isolates for which MICs are two- to fourfold higher than those for the parent strain, these organisms still appear to be susceptible to the killing effect of the quinolone if the MICs or MPCs remain below achievable concentrations. It has been suggested that the use of compounds which possess a methoxy substitution at the C-8 position may result in enhanced killing of isolates that express low levels of quinolone resistance owing to their ability to stimulate the release of DNA breaks (22).
In an effort to examine the correlation between drug exposure and bactericidal activity, we constructed plots of AUC:MIC and peak:MIC versus the change in the log10 numbers of CFU per milliliter at 24 h. It should be noted that the characteristics of exposure-effect curves are dependent on the time point evaluated. For instance, a steeper curve might be expected if data from later times are examined because more of the regimens would have exerted the maximal effect. Analysis at such a time point would allow optimal evaluation of the magnitude of the effect. However, this analysis provides little insight into the relationship between exposure and the rate of activity because most of the regimens would already be at a point of maximal activity. Conversely, a shallower curve might be noted if an analysis had been undertaken with earlier data. At this time one might be able to characterize the relationship between drug exposure and the rate of activity. However, since few regimens would have yet to exert the maximal bactericidal effect, any pharmacodynamic analysis would not be complete with respect to extent of activity. We elected to evaluate antibacterial activity at 24 h because at this time point the various agents provided us with a relatively good spread of activity (i.e., change in the log10 numbers of CFU per milliliter). That is, we were able to garner information regarding the relationships between drug exposure and both the rate and extent of activity.
According to our pharmacodynamic analysis, both AUC:MIC and peak:MIC were significantly correlated (r2 > 0.80) with the reduction in the numbers of CFU per milliliter. With respect to peak:MIC, we calculated ER90 and ER99 to be approximately 5.8 and 25, respectively. These appear to be consistent across the quinolones. These ratios were similar to the peak:MIC of 12.2 prospectively determined to correlate with clinical and microbiological outcomes by Preston (17). With respect to AUC:MIC correlation, several investigators have proposed that ratios that range from 15:1 to >100:1 exhibit the best in vivo correlation with quinolone activity against pneumococci. When we calculated the ER50, or the ratio that results in a static effect, we found the ratio to be 18:1. The ratios that produced 90 and 99% of the maximal effect were calculated to be 54:1 and 175:1, respectively. Therefore, in the absence of host defense effects, it appears reasonable to target an AUC:MIC between 50 and 100 for the quinolones against S. pneumoniae to obtain near-maximal antibacterial effects. These data suggest that in vitro dynamic models are useful for the conduct of pharmacodynamic analyses and generate values that are clinically relevant.
We did attempt to correlate pharmacodynamic parameters with the emergence of resistance; however, we were unable to detect a good correlation between either ratio and the emergence of resistance. Despite this apparent lack of correlation, we did note that there appear to be differences with respect to the various agents regarding their ability to select for resistant strains. Clinafloxacin demonstrated the least selective effect. Levofloxacin appeared to be the next least likely agent to select for isolates for which MICs were elevated. Although isolates were recovered at 48 h following exposure to 0.1 time the AUC0–24 for two of the pneumococci, the observed increase in the MICs were only 1 dilution. For the remainder of the quinolones, when resistant strains were recovered, increases in the MICs were from 4 to 32 times higher than those for the preexposure isolates, with the median increase being 8-fold. This observation appears to suggest differences among the quinolones with respect to MPCs, affinity for the target site, or affinity for an efflux pump-mediated mechanism of resistance. Similar, discordant findings with regard to the ability of levofloxacin to select for pneumococcal isolates with reduced quinolone susceptibility have been reported by Fukuda and Hiramatsu (8) and Davies et al. (4). According to our data, we would rank the ability of the agents to select for isolates with reduced susceptibility as ciprofloxacin (most likely) > grepafloxacin, moxifloxacin, and trovafloxacin > levofloxacin > clinafloxacin.
In summary, the fluoroquinolones, with the exception of ciprofloxacin, appear to exhibit excellent activity against quinolone-susceptible isolates of S. pneumoniae. The activities of these agents appear to be optimized either when an AUC:MIC of between 50 and 100 is achieved or when a peak:MIC of >6 is reached. If the drug concentrations at the site of infection approximate or exceed those observed in the serum, all of the quinolones tested except ciprofloxacin would appear to be reasonable therapeutic selections. However, if lower drug concentrations at the site of infection are possible, then treatment failure and/or the selection of resistant pneumococci is likely.
ACKNOWLEDGMENT
This project was funded by a grant from Pfizer, Inc.
REFERENCES
- 1.Barry A L, Pfaller M A, Fuchs P C, Packer R R. In vitro activities of 12 orally administered antimicrobial agents against four species of bacterial respiratory pathogens from U.S. medical centers in 1992 and 1993. Antimicrob Agents Chemother. 1994;38:2419–2425. doi: 10.1128/aac.38.10.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen D K, McGeer A, De Azavedo J C, Low D E. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N Engl J Med. 1999;341:233–239. doi: 10.1056/NEJM199907223410403. [DOI] [PubMed] [Google Scholar]
- 3.Davies B I, Maesen F P, Baur C. Ciprofloxacin in the treatment of acute exacerbations of chronic bronchitis. Eur J Clin Microbiol. 1986;5:226–231. doi: 10.1007/BF02013995. [DOI] [PubMed] [Google Scholar]
- 4.Davies T A, Pankuch G A, Dewasse B E, Jacobs M R, Appelbaum P C. In vitro development of resistance to five quinolones and amoxicillin-clavulanate in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:1177–1182. doi: 10.1128/aac.43.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doern G V, Brueggemann A, Holley H P, Jr, Rauch A M. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–1213. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doern G V, Pfaller M A, Kugler K, Freeman J, Jones R N. Prevalence of antimicrobial resistance among respiratory tract isolates of Streptococcus pneumoniae in North America: 1997 results from the SENTRY antimicrobial surveillance program. Clin Infect Dis. 1998;27:764–770. doi: 10.1086/514953. [DOI] [PubMed] [Google Scholar]
- 7.Dong Y, Zhao X, Domagala J, Drlica K. Effect of fluoroquinolone concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:1756–1758. doi: 10.1128/aac.43.7.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuda H, Hiramatsu K. Primary targets of fluoroquinolones in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:410–412. doi: 10.1128/aac.43.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon J J, Kauffman C A. Superinfection with Streptococcus pneumoniae during therapy with ciprofloxacin. Am J Med. 1990;89:383–384. doi: 10.1016/0002-9343(90)90355-h. [DOI] [PubMed] [Google Scholar]
- 10.Hoogkamp-Korstanje J A, Klein S J. Ciprofloxacin in acute exacerbations of chronic bronchitis. J Antimicrob Chemother. 1986;18:407–413. doi: 10.1093/jac/18.3.407. [DOI] [PubMed] [Google Scholar]
- 11.Keil S, Wiedemann B. Mathematical corrections for bacterial loss in pharmacodynamic in vitro dilution models. Antimicrob Agents Chemother. 1995;39:1054–1058. doi: 10.1128/aac.39.5.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimbrough R C D, Gerecht W B, Husted F C, Wolfe J E. The failure of ciprofloxacin to prevent the progression of Streptococcus pneumoniae infections to meningitis. Mol Med. 1991;88:635–637. [PubMed] [Google Scholar]
- 13.Klepser M E, Ernst E J, Lewis R E, Ernst M E, Pfaller M A. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob Agents Chemother. 1998;42:1207–1212. doi: 10.1128/aac.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacy M K, Lu W, Xu X, Tessier P R, Nicolau D P, Quintiliani R, Nightingale C H. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin, and ampicillin against Streptococcus pneumoniae in an in vitro model of infection. Antimicrob Agents Chemother. 1999;43:672–677. doi: 10.1128/aac.43.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lister P D, Sanders C C. Pharmacodynamics of trovafloxacin, ofloxacin, and ciprofloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model. Antimicrob Agents Chemother. 1999;43:1118–1123. doi: 10.1128/aac.43.5.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacGowan A P, Bowker K E, Wootton M, Holt H A. Activity of moxifloxacin, administered once a day, against Streptococcus pneumoniae in an in vitro pharmacodynamic model of infection. Antimicrob Agents Chemother. 1999;43:1560–1564. doi: 10.1128/aac.43.7.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preston S L, Drusano G L, Berman A L, Fowler C L, Chow A T, Dornseif B, Reichl V, Natarajan J, Corrado M. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA. 1998;279:125–129. doi: 10.1001/jama.279.2.125. [DOI] [PubMed] [Google Scholar]
- 18.Righter J. Pneumococcal meningitis during intravenous ciprofloxacin therapy. Am J Med. 1990;88:548. doi: 10.1016/0002-9343(90)90442-g. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing, Eighth information supplement. M100–S8. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 20.Thornsberry C, Ogilvie P T, Holley H P, Jr, Sahm D F. Survey of susceptibilities of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis isolates to 26 antimicrobial agents: a prospective U.S. study. Antimicrob Agents Chemother. 1999;43:2612–2623. doi: 10.1128/aac.43.11.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornsberry C S, Brown D, Yee C, Bouchillon S K, Marler J K, Rich T. Increasing penicillin resistance in Streptococcus pneumoniae in the U.S. Infect Med. 1993;93(Suppl.):15–24. [Google Scholar]
- 22.Zhao X, Xu C, Domagala J, Drlica K. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc Natl Acad Sci USA. 1997;94:13991–13996. doi: 10.1073/pnas.94.25.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]