Abstract
l-lactate acts as a signaling molecule in bovine granulosa cells (GCs). The initiated alterations depend on the transport of l-lactate into the cells via monocarboxylate transporters. In the present study, we further elucidated the intracellular actions of l-lactate and tested whether the PKA signaling pathway is involved. Therefore, we treated cultured bovine GCs with l-lactate and PKA inhibitors H-89 and KT5720, and with an activator of PKA, 6-Bnz-cAMP. l-lactate treatment resulted in decreased estradiol production and downregulation of CYP19A1, FSHR, and LHCGR as well as in the upregulation of the markers of early luteinization PTX3, RGS2, and VNN2. These specific l-lactate effects were almost completely abolished by pre-treatment of the GCs with both inhibitors of PKA signaling. In addition, also the l-lactate-induced upregulation of LDHA and of the monocarboxylate transporters SLC16A1 and SLC16A7 was abolished after PKA inhibition. An activation of the PKA with 6-Bnz-cAMP revealed similar effects on the gene expression like l-lactate alone. In summary, the presented data demonstrate that l-lactate-induced effects on GCs are mediated via PKA signaling thus supporting the role of l-lactate as signaling molecule during the folliculo-luteal transition.
Supplementary information
The online version contains supplementary material available at 10.1007/s00441-021-03569-7.
Keywords: Tissue culture, Luteinization, Ovary, Gene expression, Steroid hormones
Introduction
The folliculo-luteal transition of the ovarian follicle is triggered by LH (luteinizing hormone) and leads to profound morphological and physiological changes (Adams et al. 2008; Bao and Garverick 1998). This reorganization is initiated by a precise regulation of gene expression, in particular of genes involved in steroidogenesis (Christenson et al. 2013; Lussier et al. 2017). The key enzyme of estradiol synthesis, aromatase, encoded by CYP19A1, is massively downregulated along with the gonadotropin receptors FSHR and LHCGR in the granulosa cell (GC) layer. Other genes are known to be remarkably upregulated, among them RGS2 (regulator of G protein signaling 2), VNN2 (vanin 2), and PTX3 (pentraxin 3). Therefore, these genes can be considered as marker genes for an LH-induced transformation of bovine GCs. As GCs are the major source of estrogen production and proved to be primarily targeted by LH (Christenson et al. 2013), we established a bovine GC culture model to study effects on steroidogenesis (Baufeld and Vanselow 2013, 2018b). Previously, we could show that l-lactate is able to induce a specific differentiation process in cultured bovine GCs that is dependent upon l-lactate transport into the cells via specific monocarboxylate transporters (Baufeld and Vanselow 2018a). Studies in other cell types also support the role of l-lactate as signaling molecule affecting the expression of genes (Constant et al. 2000; Yang et al. 2014). In an mRNA microarray approach, we could confirm that l-lactate led to genome-wide alterations that might initiate differentiation of GCs and gathered first hints of “cAMP-mediated signaling” in these cells (Baufeld et al. 2019). Consequently, here, we investigated whether the PKA signaling cascade might be involved. This pathway is known as a main component involved in the regulation of the LH- and FSH (follicle-stimulating hormone)-induced changes in the follicle (Escamilla-Hernandez et al. 2008; Richards and Pangas 2010). Therefore, we analyzed the effects of PKA inhibitors and of an activator of PKA on l-lactate-treated GCs using steroid hormone production and transcriptional changes of marker genes as readout.
Materials and methods
Isolation of GCs and cell culture
Ovaries were obtained from a local slaughterhouse, collected in 1 × PBS supplemented with 100 IU penicillin, 0.1 mg/ml streptomycin, and 0.5 µg/µl amphotericin. After washing in 1 × PBS (with antibiotics), GCs were aspirated from follicles (< 6 mm) (Nimz et al. 2009). Cells obtained from 15 to 30 follicles per ovary were pooled in 1 × PBS. Per cell preparation, 20 to 40 ovaries from different cows with undefined cyclicity status were included. Determination of living cells was conducted with the trypan blue exclusion method. GCs were cryo-preserved as described previously (Baufeld and Vanselow 2018a). All experiments were performed at least 3 times with different cell preparations. GCs were cultured according to our established E2-active GC culture model that allows the analysis of early folliculo-luteal differentiation (Baufeld and Vanselow 2013, 2018b). Cryo-preserved GCs were thawed, immediately centrifuged (3 min, 500 × g) and re-suspended in culture media. Cells were cultured (1.2–1.3 × 105 cells/well) serum-free in α-MEM containing l-glutamin (2 mM), sodium bicarbonate (0.084%), BSA (0.1%), HEPES (20 mM), sodium selenite (4 ng/ml), transferrin (5 μg/ml), insulin (10 ng/ml), non-essential amino acids (1 mM), penicillin (100 IU/ml), and streptomycin (0.1 mg/ml), and additionally supplemented with FSH (20 ng/ml), R3 IGF-1 (50 ng/ml), and androstenedione (2 μM). Further, GCs were treated with sodium l-lactate (30 mM) or vehicle control (sodium chloride, 30 mM). Sodium chloride was shown previously to be an appropriate control as no changes in the pH were observed (Baufeld and Vanselow 2018a). Inhibitor studies with H-89 and KT5720, two different protein kinase A (PKA) inhibitors (Kurowska et al. 2020; Shrestha and Meidan 2018), were performed in a pre-treatment approach. GCs were initially treated for 48 h with 15 µM H-89 and 200 nM KT5720, respectively, and thereafter supplemented with l-lactate. In another setup of experiments, PKA was activated with the cAMP analogon, 6-Bnz-cAMP. For this, GCs were stimulated 24 h before the end of the culture with 1 mM 6-Bnz-cAMP. Untreated GCs served as suitable control.
All reagents were purchased from Merck Millipore (Berlin, Germany) if not stated otherwise. Granulosa cells were cultured in 24-well collagen R coated plates (0.02%; Serva, Heidelberg, Germany) for 8 days at 37 °C and 5% CO2 with media exchange of two thirds every second day. The long duration of cell culture is necessary for the GCs to recover from isolation and plating stress and to resume E2 production and thus develop an estradiol-active status in vitro (Baufeld and Vanselow 2013; Yenuganti and Vanselow 2017).
RNA isolation, cDNA synthesis, qPCR, and hormone measurement
Total RNA was isolated with the NucleoSpin RNA Kit (Macherey–Nagel, Düren, Germany) following the manufacturer’s instructions. Subsequently, RNA concentration was measured in a NanoDrop 1000 Spectrophotometer (Thermo Scientific, Bonn, Germany). Synthesis of cDNA was performed (SensiFAST cDNA Synthesis Kit, Bioline, Luckenwalde, Germany) from 170 to 250 ng RNA. Quantitative Real-Time PCR was executed with SensiFAST SYBR No-ROX (Bioline) and gene-specific primers (Supplementary Table 1) as described before (Baufeld and Vanselow 2018a). Amplification of correct PCR products was confirmed by gel electrophoresis (3% agarose, ROTI GelStain (Roth)). External standards were used for absolute quantification. Standards were freshly prepared, and dilutions of five different concentrations (5 × 10−12–5 × 10−16 g DNA/reaction) were co-amplified. Transcript abundance was normalized to the reference gene RPLP0, showing stable expression in different culture conditions.
Estradiol (E2) and progesterone (P4) concentrations were measured with a competitive 3H-radioimmunoassay (RIA). The tracer [2,4,6,7-3H]estradiol-17β (GE Healthcare, Freiburg, Germany) was used for E2 detection and [1,2,6,7-3H(N)]progesterone (PerkinElmer, Boston, USA) for P4 detection. The measurement was executed in a liquid scintillation counter with an integrated RIA calculation program (TriCarb 2900 TR, PerkinElmer). Intra- and interassay coefficient of variations (CVs) were 6.9% and 9.9% for P4, whereas intra- and interassay CVs were 7.6% and 9.8%, respectively.
Statistics
Each experiment was performed in triplicates from different GC preparations. For statistical analysis, SigmaPlot 11.0 Statistical Analysis System (Jandel Scientific, San Rafael, CA, USA) was used. Untransformed data were used for statistical analysis of qPCR and hormone measurement. The student’s t test or Mann–Whitney test depending on fulfillment of t test criteria was applied. Multiple comparisons were performed using one-way ANOVA with an appropriate post hoc test. The level of significance was set at P < 0.05. The data are shown as log2-transformed values relative to the respective control.
Results
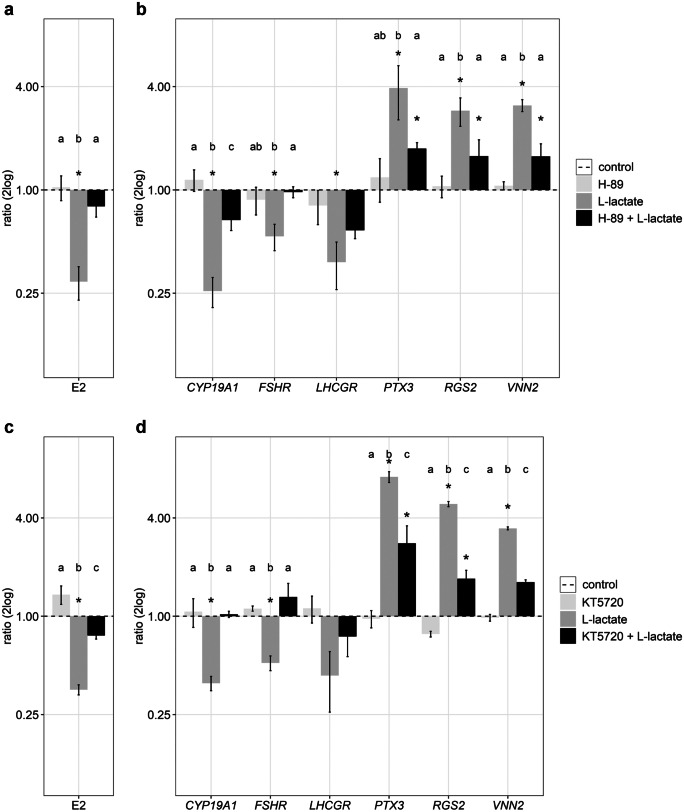
The treatment with l-lactate confirmed the previously described specific regulation of marker transcripts for luteinization, e.g., downregulation of CYP19A1, FSHR, and LHGCR, and upregulation of PTX3, RGS2, and VNN2 (Fig. 1, grey bars). This is in accordance with the observation of lower estradiol concentrations after l-lactate treatment. When the GCs were pre-treated with H-89, an established PKA inhibitor, E2 concentrations nearly reached control levels thus abolishing the l-lactate effect (Fig. 1a, black bars). In addition, effects on transcript abundances of CYP19A1 and of the other marker genes for luteinization were largely abolished even though in particular the upregulated transcripts (PTX3, RGS2, and VNN2) did not fully reach control levels (Fig. 1b, black bars). Treatment with another PKA inhibitor, KT5720, could confirm the partial or complete abolishment of the l-lactate effects on the concentration of estradiol or gene expression of marker genes (Fig. 1c and d). Treatment of GCs with the inhibitors H-89 and KT5720 alone did not result in significant changes compared to the vehicle control.
Fig. 1.
Effects of PKA inhibition in l-lactate-treated cells on hormone concentration and gene expression. GCs were cultured under 30 mM l-lactate treatment (dark grey bars) and after sole or additional pre-treatment (black bars) with 15 µM H-89 (a, b) and 200 nM KT5720 (c, d). E2 concentrations (a, c) and gene expression (b, d) were analyzed after 8 days compared to the vehicle control (NaCl, dashed line). Means and SEM are shown of n = 3 replicates. Student’s t-test or Mann–Whitney test was used comparing treatment to control (*) and ANOVA was used for multiple comparison between different treatments (letters), P < 0.05
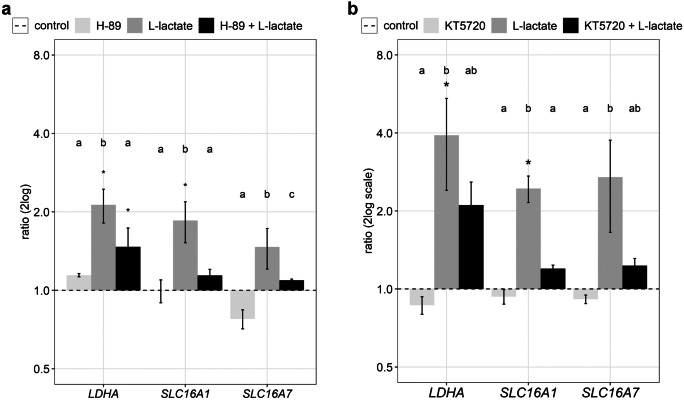
We also investigated effects of l-lactate and of PKA inhibitors on genes involved in lactate metabolism. Therefore, expression of LDHA, coding for the lactate dehydrogenase and of two known transporters for l-lactate, SLC16A1 and SLC16A7, was analyzed. As observed previously, l-lactate enhanced expression of LDHA and SLC16A1 (Fig. 2, grey bars). When the GCs were pre-treated with H-89, the l-lactate effects were significantly reduced (Fig. 2a, black bars). Inhibition of PKA with KT5720 again resulted in a reduction or near abolishment of the l-lactate effect.
Fig. 2.
Effects of l-lactate and PKA inhibition on genes related to lactate metabolism. Cultured GCs were treated with 30 mM l-lactate (grey bars) and additionally pre-treated with a 15 µM H-89 (black bars) and b 200 nM KT5720 compared to the control (NaCl, dashed line). In addition, effects of both inhibitors alone (light grey bars) were checked. Means and SEM are shown of n = 3 replicates. Student’s t-test or Mann–Whitney test was used comparing treatment to control (*), and ANOVA was performed for multiple comparison between different treatments (letters), P < 0.05
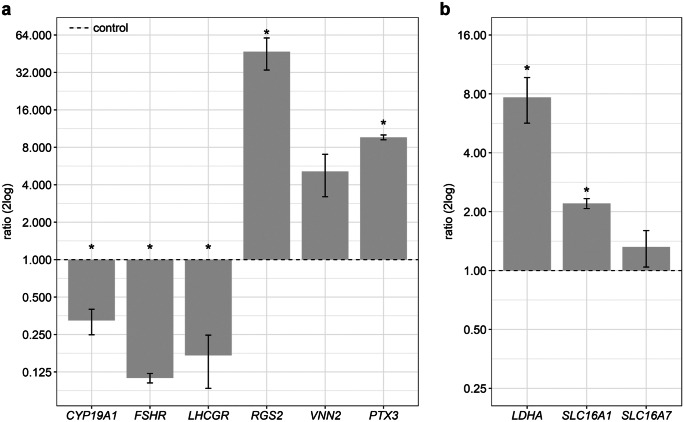
The activation of PKA with the cAMP analogon 6-Bnz-cAMP revealed a massive downregulation of CYP19A1, FSHR, and LHCGR compared to the untreated control (Fig. 3a). Other marker transcripts were highly upregulated, especially RGS2 showed a nearly 50-fold increase after PKA activation. The genes of the lactate metabolism, LDHA and SLC16A1, were upregulated probably due to the activation of PKA in culture (Fig. 3b).
Fig. 3.
Effects of PKA activation in cultured GCs. The cells were stimulated with 1 mM 6-Bnz-cAMP 24 h before the end of culture. The gene expression of a marker genes for LH-induced alterations and b genes related to the lactate metabolism were analyzed. Means and SEM are shown of n = 3 replicates. Asterisks indicate significant differences compared to the control (student’s t test, P < 0.05)
Discussion
PKA signaling is a major pathway mediating the FSH- and LH-dependent changes during folliculogenesis (Escamilla-Hernandez et al. 2008; Morris and Richards 1995; Murphy 2000). Involvement of PKA and PKC signaling in mediating l-lactate effects was also shown in skeletal muscle cells (Narumi et al. 2010). Therefore, in our present study, we aimed to elucidate if this functionally important pathway is also involved in the observed l-lactate effects in GCs. Application of the PKA inhibitors H-89 and KT5720 reduced or nearly abolished the l-lactate-induced effects on cultured bovine GCs. The direct activation of the PKA signaling pathways via the cAMP analogon 6-Bnz-cAMP in the cell culture model revealed a similar regulation as observed with l-lactate. This indicates that l-lactate effects are mediated via PKA signaling. Typically, the activation of PKA is known to be the result of G-protein coupled receptor activation. In neuronal cells and adipocytes, it was shown that l-lactate indeed acts on such a receptor, HCAR1 (Liu et al. 2009; Mosienko et al. 2015). HCAR1 is the only so far known G-protein coupled receptor that can bind l-lactate. The studies on HCAR1 in adipocytes reveal a decrease of cAMP concentration and inhibition of lipolysis (Cai et al. 2008; Liu et al. 2009). However, analysis of HCAR1 transcripts in the follicle leads to the suggestion that this receptor is not expressed in GCs but only in theca cells of the bovine follicle. Accordingly, the results further support our previous hypothesis that l-lactate uptake into cells is mandatory to facilitate a change in GCs’ gene expression profile. In our previous study, we observed an abolishment of the l-lactate effects by inhibiting the transporters for lactate (Baufeld and Vanselow 2018a). Moreover, the inhibition of PKA abolished the upregulation of l-lactate-related genes, especially of the transcripts encoding the monocarboxylate transporters, SLC16A1 and SLC16A7 (MCT1 and MCT2). Additionally, the presented data provide evidence that l-lactate induces effects comparable to that of a PKA activation. This further supports our hypothesis that the l-lactate effect is mediated via intracellular PKA signaling. Main transcription factors activated by PKA are CREB (cAMP response element-binding protein), CREM (cAMP-responsive modulator), and ATF1 (Sassone-Corsi 2012). The observed upregulation of transcripts due to l-lactate or PKA activator treatment is in line with the fact that CRE binding sites are present in the promotor region of the genes LDHA, RGS2, and VNN2 (Larabee et al. 2018; Perschbacher et al. 2020; Short et al. 1994). Within the CYP19A1 promoter, a CRE-like sequence (CLS) was found relevant to mediate the cAMP response (Hinshelwood et al. 1997; Michael et al. 1997). In the wake of the LH surge, the inducible cAMP early repressor (ICER) is expressed supporting the downregulation of CYP19A1 (Morales et al. 2003). Together with a subsequent and more detailed investigation of involved transcription factors, it would be also interesting to analyze the protein alterations of the lactate transporters to strengthen our hypothesis further.
Conclusions
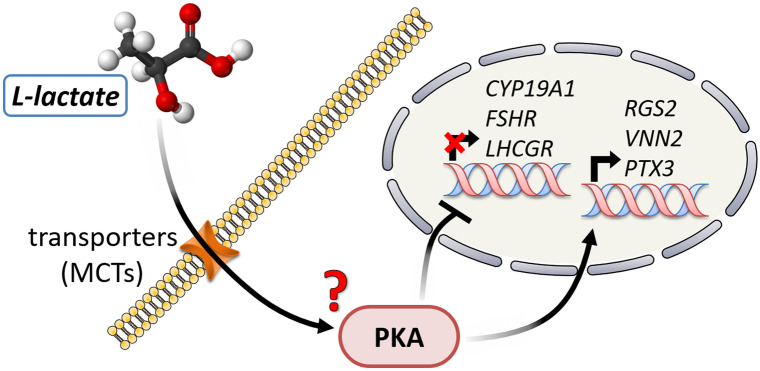
Taken together, these data extend our previous knowledge on the l-lactate-mediated differentiation of bovine GCs in culture. Besides the already demonstrated involvement of active transport of l-lactate into GCs, an interaction via PKA becomes evident (Fig. 4). In GCs, a possible way of l-lactate action might be the following: l-lactate enters the cells via specific transporters (MCTs). Subsequently, PKA is activated thus enhancing the action of the preovulatory LH surge.
Fig. 4.
Suggested signaling model of l-lactate action in bovine GCs. As a first essential step, l-lactate crosses the cell membrane via specific transporters (MCTs, see Baufeld and Vanselow 2018a, b). Specific effects on gene expression are then mediated by the PKA pathway thus supporting the LH initiated folliculo-luteal transition
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Veronica Schreiter, Maren Anders and Swanhild Rodewald for excellent technical support during the experiments.
Author contribution
AB and JV designed the experiments, analyzed the data, and wrote the paper. AB performed the experiments. Both authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by the FBN’s core budget.
Availability of data and materials
The datasets used and/or analyses are available from the corresponding author on reasonable request.
Declarations
Ethics approval
Not required for this study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams GP, Jaiswal R, Singh J, Malhi P. Progress in understanding ovarian follicular dynamics in cattle. Theriogenology. 2008;69:72–80. doi: 10.1016/j.theriogenology.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Bao B, Garverick HA. Expression of steroidogenic enzyme and gonadotropin receptor genes in bovine follicles during ovarian follicular waves: a review. J Anim Sci. 1998;76:1903–1921. doi: 10.2527/1998.7671903x. [DOI] [PubMed] [Google Scholar]
- Baufeld A, Koczan D, Vanselow J. L-lactate induces specific genome wide alterations of gene expression in cultured bovine granulosa cells. BMC Genomics. 2019;20:273. doi: 10.1186/s12864-019-5657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufeld A, Vanselow J. Increasing cell plating density mimics an early post-LH stage in cultured bovine granulosa cells. Cell Tissue Res. 2013;354:869–880. doi: 10.1007/s00441-013-1712-9. [DOI] [PubMed] [Google Scholar]
- Baufeld A, Vanselow J. Lactate promotes specific differentiation in bovine granulosa cells depending on lactate uptake thus mimicking an early post-LH stage. Reprod Biol Endocrinol. 2018;16:15. doi: 10.1186/s12958-018-0332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufeld A, Vanselow J (2018b) A tissue culture model of estrogen-producing primary bovine granulosa cells. J Vis Exp [DOI] [PMC free article] [PubMed]
- Cai TQ, Ren N, Jin L, Cheng K, Kash S, Chen R, Wright SD, Taggart AK, Waters MG. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem Biophys Res Commun. 2008;377:987–991. doi: 10.1016/j.bbrc.2008.10.088. [DOI] [PubMed] [Google Scholar]
- Christenson LK, Gunewardena S, Hong X, Spitschak M, Baufeld A, Vanselow J. Research resource: preovulatory LH surge effects on follicular theca and granulosa transcriptomes. Mol Endocrinol. 2013;27:1153–1171. doi: 10.1210/me.2013-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant JS, Feng JJ, Zabel DD, Yuan H, Suh DY, Scheuenstuhl H, Hunt TK, Hussain MZ. Lactate elicits vascular endothelial growth factor from macrophages: a possible alternative to hypoxia. Wound Repair Regen. 2000;8:353–360. doi: 10.1111/j.1524-475X.2000.00353.x. [DOI] [PubMed] [Google Scholar]
- Escamilla-Hernandez R, Little-Ihrig L, Orwig KE, Yue J, Chandran U, Zeleznik AJ. Constitutively active protein kinase A qualitatively mimics the effects of follicle-stimulating hormone on granulosa cell differentiation. Mol Endocrinol. 2008;22:1842–1852. doi: 10.1210/me.2008-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshelwood MM, Michael MD, Simpson AER. The 5-flanking region of the ovarian promoter of the bovine cyp19 gene contains a deletion in a cyclic adenosine 3,5-monophosphate-like responsive sequence. Endocrinology. 1997;138:3704–3710. doi: 10.1210/endo.138.9.5317. [DOI] [PubMed] [Google Scholar]
- Kurowska P, Mlyczyńska E, Dawid M, Dupont J, Rak A. Role of vaspin in porcine ovary: effect on signaling pathways and steroid synthesis via GRP78 receptor and protein kinase A†. Biol Reprod. 2020;102:1290–1305. doi: 10.1093/biolre/ioaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larabee JL, Hauck G, Ballard JD. Unique, intersecting, and overlapping roles of C/EBP β and CREB in cells of the innate immune system. Sci Rep. 2018;8:16931. doi: 10.1038/s41598-018-35184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, Sutton SW, Li X, Yun SJ, Mirzadegan T, Mazur C, Kamme F, Lovenberg TW. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J Biol Chem. 2009;284:2811–2822. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- Lussier JG, Diouf MN, Levesque V, Sirois J, Ndiaye K. Gene expression profiling of upregulated mRNAs in granulosa cells of bovine ovulatory follicles following stimulation with hCG. Reprod Biol Endocrinol. 2017;15:88. doi: 10.1186/s12958-017-0306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael MD, Michael LF, Simpson ER. A CRE-like sequence that binds CREB and contributes to cAMP-dependent regulation of the proximal promoter of the human aromatase P450 (CYP19) gene. Mol Cell Endocrinol. 1997;134:147–156. doi: 10.1016/S0303-7207(97)00178-0. [DOI] [PubMed] [Google Scholar]
- Morales V, Gonzalez-Robayna I, Hernandez I, Quintana J, Santana P, Ruiz de Galarreta CM, Fanjul LF. The inducible isoform of CREM (inducible cAMP early repressor, ICER) is a repressor of CYP19 rat ovarian promoter. J Endocrinol. 2003;179:417–425. doi: 10.1677/joe.0.1790417. [DOI] [PubMed] [Google Scholar]
- Morris JK, Richards JS. Luteinizing hormone induces prostaglandin endoperoxide synthase-2 and luteinization in vitro by A-kinase and C-kinase pathways. Endocrinology. 1995;136:1549–1558. doi: 10.1210/endo.136.4.7895665. [DOI] [PubMed] [Google Scholar]
- Mosienko V, Teschemacher AG, Kasparov S. Is L-lactate a novel signaling molecule in the brain? J Cereb Blood Flow Metab. 2015;35:1069–1075. doi: 10.1038/jcbfm.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BD. Models of luteinization. Biol Reprod. 2000;63:2–11. doi: 10.1095/biolreprod63.1.2. [DOI] [PubMed] [Google Scholar]
- Narumi K, Furugen A, Kobayashi M, Otake S, Itagaki S, Iseki K. Regulation of monocarboxylate transporter 1 in skeletal muscle cells by intracellular signaling pathways. Biol Pharm Bull. 2010;33:1568–1573. doi: 10.1248/bpb.33.1568. [DOI] [PubMed] [Google Scholar]
- Nimz M, Spitschak M, Schneider F, Fürbass R, Vanselow J. Down-regulation of genes encoding steroidogenic enzymes and hormone receptors in late preovulatory follicles of the cow coincides with an accumulation of intrafollicular steroids. Domest Anim Endocrinol. 2009;37:45–54. doi: 10.1016/j.domaniend.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Perschbacher KJ, Deng G, Sandgren JA, Walsh JW, Witcher PC, Sapouckey SA, Owens CE, Zhang SY, Scroggins SM, Pearson NA, Devor EJ, Sebag JA, Pierce GL, Fisher RA, Kwitek AE, Santillan DA, Gibson-Corley KN, Sigmund CD, Santillan MK, Grobe JL. Reduced mRNA expression of RGS2 (regulator of G protein signaling-2) in the placenta is associated with human preeclampsia and sufficient to cause features of the disorder in mice. Hypertension. 2020;75:569–579. doi: 10.1161/HYPERTENSIONAHA.119.14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120:963–972. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P (2012) The cyclic AMP pathway. Cold Spring Harb Perspect Biol 4 [DOI] [PMC free article] [PubMed]
- Short ML, Huang D, Milkowski DM, Short S, Kunstman K, Soong CJ, Chung KC, Jungmann RA. Analysis of the rat lactate dehydrogenase A subunit gene promoter/regulatory region. Biochem J. 1994;304(Pt 2):391–398. doi: 10.1042/bj3040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha K, Meidan R. The cAMP-EPAC pathway mediates PGE2-induced FGF2 in bovine granulosa cellS. Endocrinology. 2018;159:3482–3491. doi: 10.1210/en.2018-00527. [DOI] [PubMed] [Google Scholar]
- Yang J, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I, Magistretti PJ. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A. 2014;111:12228–12233. doi: 10.1073/pnas.1322912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenuganti VR, Vanselow J. Cultured bovine granulosa cells rapidly lose important features of their identity and functionality but partially recover under long-term culture conditions. Cell Tissue Res. 2017;368:397–403. doi: 10.1007/s00441-017-2571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyses are available from the corresponding author on reasonable request.