Abstract
Cyclodextrin metal–organic framework by ultrasound-assisted rapid synthesis for caffeic acid (CA) loading and antibacterial application (U-CD-MOF) was successfully studied and this method shortened the preparation time to a few minutes. It was found that the ultrasonic power, reaction time and temperature would affect the morphology and size of the obtained crystal. Under the optimal conditions, U-CD-MOF had a cubic structure with uniform size of 8.60 ± 1.95 μm. U-CD-MOF was used to load the antibacterial natural product CA to form the composite (CA@U-CD-MOF) and the loading rate of CA@U-CD-MOF to CA could reach 19.63 ± 2.53%, which was more than twice that of γ-CD. Various techniques were applied to characterize the synthesized crystal, including Powder X-ray diffraction (PXRD), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), thermogravimetric analysis (TGA), and N2 adsorption. In addition, antibacterial tests were performed on the obtained crystal. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of CA@U-CD-MOF for Escherichia coli O157: H7 (E. coli O157: H7) were both 25 mg·mL−1, and the MIC for Staphylococcus aureus (S. aureus). was 25 mg·mL−1. The sustained release behavior of CA@U-CD-MOF to CA in ethanol fitted well to Higuchi model and the loading of CA was supported by molecular docking results. In general, U-CD-MOF was successfully achieved by ultrasound-assisted rapid synthesis and the obtained crystal was further evaluated for potential antibacterial application.
Keywords: Cyclodextrin metal–organic framework, Ultrasound-assisted, Caffeic acid, Antibacterial
1. Introduction
Metal-organic frameworks (MOFs) are considered to be a new type of porous material in which organic ligands and metal ions can self-assemble to form a network structure [1]. For early research, MOFs are mainly used in the catalysis, separation and storage of gas mixtures [2], [3]. The applications of MOF are now being expanded to include energy storage devices, sensor detection, adsorption of harmful substances, new catalysis, antibacterial and biomedical applications [4], [5], [6], [7]. In addition, the high porosity and large specific surface area of MOFs are suitable for the delivery of drugs, plant nature products, antibiotics and other biological active ingredient as a carrier [8].
The rapid synthesis of MOFs is important to their widely applications. Thus, many methods have been directed to shorten the synthesis time such as ultrasound [9], microwave [10], mechanochemical [11]. The method of preparing MOFs based on ultrasound has been proved to be a simple and effective method [12], [13], [14] that can shorten the reaction time, and a series of MOFs have been reported with ultrasound [15], [16], [17].
The development of MOFs has been extended to the food industry, mainly in the form of carriers for food packaging, antibacterial, etc. [18]. Considering the applications in the food industry, MOFs with good biocompatibility and non-toxic property are given priority. Recently, a concept of “edible” MOF named cyclodextrin metal organic framework (CD-MOF) has been reported [19]. CD-MOF is synthesized by using γ-CD as an organic ligand, and low-toxic alkali metal potassium ions (K+) as metal ions. CD-MOF can be further synthesized by using food-grade potassium benzoate, food-grade cyclodextrin, and ethanol obtained from grain distillation additionally. CD-MOF has spherical voids with a diameter of 1.7 nm and a pore size of 0.78 nm, which is body-centered cubic extended structures [20]. Therefore, CD-MOF is very suitable for application in the food industry that has loaded many popular natural substances, such as polyphenols [21], flavoring agents [22], lipids [23], etc. In addition, the antimicrobial activity is one of the most relevant biological activities that has been associated with materials in in food or food contact materials [24], CD-MOF can load sulfadiazine [25], curcumin [26], epigallocatechin gallate [27] and other natural phytochemicals to achieve antibacterial and anti-inflammatory applications based on its excellent cavity.
The preparation time of CD-MOF in the early research was relatively able to produce cubic crystals by vapor diffusion method at room temperature over 7 d [19]. Subsequently, a method using cetyltrimethylammonium bromide (CTAB) to improve the vapor diffusion method has been reported to produce CD-MOF which could shorten the prepare time of CD-MOF to 26–32 h [28]. In addition, the modified vapor diffusion method has been reported, and CD-MOF can be obtained within 6–24 h [29]. Recently, it has been reported that the improved vapor diffusion method using short-chain starch granules could obtain CD-MOF within 6 h [30]. Nonetheless the vapor diffusion method is difficult to be used to manufacture CD-MOF for large-scale production and future industrial use. Microwave-assisted method for preparation of CD-MOF was a potential application method [31], however the conditions that require high-temperature heating may limit the popularization. The rapid synthesis of CD-MOF is a crucial factor in determining whether it can be practically applied to the food industry.
In this study, we report a methos for the rapid synthesis of CD-MOF by ultrasound-assisted. We could prepare the crystal with uniform size and morphology by optimizing the ultrasound power, reaction time and reaction temperature in the preparation process. Caffeic acid (CA) was chosen as natural antibacterial product model to study the loading efficiency of the crystal and the antibacterial ability of the obtained CD-MOF composite was investigated.
2. Material and methods
2.1. Materials
CA (99%, CAS number 331–39-5) was purchased from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). CTAB (CAS number 57–09-0), Polyethylene glycol 8000 (PEG 8000, CAS number 25322–68-3) was purchased from Shanghai Aladdin Bio-Chem Technology Co. Ltd. (Shanghai, China). The γ-CD (98%, CAS number 7664–93-9) and potassium hydroxide (KOH, 90%, CAS number 7664–93-9) were purchased from Shang Macklin Biochemical Co. Ltd. (Shanghai, China). Ethanol (99.7%, CAS number 64–17-5), methanol (MeOH, 99.5%, CAS number 67–56-1) and dichloromethane (DCM, 99.5%, CAS number 75–09-2) were obtained from Sinopharm Chemical Reagents Co. Ltd. (Shanghai, China). Nutrient broth (NB), Plate count agar, Staphylococcus aureus (ATCC 25923), Escherichia coli O157:H7 (ATCC 35150) were purchased from Qingdao Hope Bio-technology Co., Ltd. (Qingdao, China). Experimental ultra-pure water was processed by Smart-N system (Heal Force Biotech, Hk).
2.2. The preparation of U-CD-MOF
As shown in Figure S1, the mixed solution (Figure S1B) was obtained by γ-CD (648 mg) and KOH (256 mg) in ultra-pure water (20 mL). The mixed solution was filtered with a 0.45 μm filter membrane and stirred at room temperature. MeOH (12 mL) and the above solution were added in the tube to form a white solution. Then transfer the tube to a water bath at 60 °C to stand 10 min, a clear and transparent solution was obtained. The probe (diameter 6 mm) of an ultrasonic generator (JY92-IIDN, SCIENTZ, Inc) was inserted under the liquid surface in the tube. The clear and transparent solution was ultrasonically processed at a frequency of 20 kHz and a power of 540 W, reaction under intermittent action for 10 min, the intermittent ultrasonic action mode is on for 2 s, off for 2 s, while PEG-8000 (256 mg) was quickly added after the start of the ultrasonic treatment to trigger the deposition of crystalline materials. In addition, the rapid synthesis and the regular morphology of U-CD-MOF crystal are inseparable from PEG-8000 as the excipient which was used as the size modulator for controlling the size and morphology of MOF crystals in aqueous systems [31]. The crude product was obtained after the reaction. The crude product was allowed stand for 1 h to obtain the white precipitate. The precipitate was centrifuged at 5000 rpm for 5 min and washed with MeOH for 3 times, then the precipitate was dispersed in MeOH and dried at 50 °C under vacuum for 12 h (U-CD-MOF, sample S1). Different processing parameters such as ultrasonic power, reaction time and temperature were used to obtain CD-MOF with uniform morphology and size. The samples prepared under different conditions were named as S1 to S16, the specific information was summarized in Table S1. In contrast, CD-MOF prepared by modified vapor diffusion method was also studied (Figure S1A) [29].
2.3. The preparation of CA@U-CD-MOF and CA@γ-CD
U-CD-MOF was evenly dispersed in DCM for 3 d to activate, then U-CD-MOF (5 mg) was mixed with CA ethanol solution (5 mL, 8 mg·mL−1). The suspension was shaken (300 rpm) and incubated for 12 h. The mixture then was centrifuged at 5000 rpm to obtain the precipitate. The preparation optimization conditions of CA@U-CD-MOF by single factor experiment were as follows: optimized the molar ratio of U-CD-MOF (γ-CD) to CA (2:1, 1:1, 1:2 1:4, 1:8, 1:16, 1:32, 1:64, 1:128), and γ-CD concentration could be obtained by the empirical formula [(C48H80O40) (KOH)2]n of CD-MOF [19], the reaction time (10, 30, 60, 180, 360, 720, 900 min) and temperature (20, 30, 40, 50, 60 °C), respectively. The precipitate was washed with MeOH and dried under vacuum at 50 °C to prepare CA-loaded U-CD-MOF (CA@U-CD-MOF). The same steps could be used to obtain the CA-loaded γ-CD (CA@γ-CD).
2.4. The characterization of U-CD-MOF and CA@U-CD-MOF
All synthesized CD-MOF samples were characterized by using the following instruments: Scanning Electron Microscope (SEM) (Gemini SEM 360, ZEISS, German) for MOF crystals and the samples were fixed on the conductive glue and spray gold before the measurement, and using image J to calculate the average size of each sample by 300 particles. Fourier Transform Infrared Spectroscopy (FTIR) (Nicolet IS50, Thermofisher, USA) spectra for MOF crystals, MOF crystals were fully grounded and then mixed with potassium bromide to obtain a transparent round sheet by pressing for testing, and the wave number range was 500–4000 cm−1 with a resolution of 4 cm−1. Thermogravimetric Analysis (TGA) (STARe System TGA2, Mettler Toledo, Switzerland) for MOF crystals, the test was carried out in a temperature range of 30 to 500 °C under a heating rate of 10 under constant nitrogen purging of 50, and the sample was placed into Tzero aluminum pan. The powder X-ray diffraction (PXRD) (D8 Advance, Bruker, German) for MOF crystals and the experimental conditions: The diffraction source was Cu Kɑ at 30 kV and 40 mA. The diffraction intensity was recorded in the range of 2° to 50°at a scanning rate of 5°/min. Simulated powder diffraction patterns of CD-MOF which was obtained from the report [19] were calculated using materials studio 2017 software (Cambridge Crystallographic Data Centre). The specific surface area and pore volume distribution were measured by Nitrogen adsorption–desorption isotherm using a porosimeter with a liquid nitrogen bath (AUTOSORB-1-C, Quantachrome, USA), before the test, the samples were fully soaked in an appropriate amount of MeOH for 3 d to achieve activation and then dried under vacuum at room temperature, the samples were dried at 70 °C for 24 h to remove residual solvent.
2.5. The determination of CA standard curve equation
CA content could be calculated by measuring the absorbance of λmax at 320 nm by Ultraviolet–visible spectrophotometer (uv2600, Shimadzu, Japan). The amount of CA standard was dissolved in 75% ethanol solution to prepare a certain mass concentration as the reference substance solution: 6, 8, 10, 12, 14 μg·mL−1. The standard curve equation and UV spectrum was shown in Figure S4. The loading rate of CA from CA@U-CD-MOF was calculated in the composite material according to the following formula:
| (1) |
where was the loading rate (%), was the content of CA in the CA@U-CD-MOF (mg), and was the content (mg) of CA@U-CD-MOF.
2.6. Release study of CA@U-CD-MOF
CA@U-CD-MOF (50 mg) was suspended in 500 mL of water, phosphate buffered saline (PBS) and ethanol, and maintained at room temperature with a shaking rate of 50 rpm. Aliquots of 200 μL were withdrawn at predefined time intervals and the same volume of fresh solvent was added and cumulative release percentage was calculated as following formula:
| (2) |
where was the cumulative release percentage (%), was the content (mg) of release CA, and as the content (mg) of total CA.
2.7. Antibacterial activity
To determine the antibacterial activity of MOF crystals, S. aureus and E. coli O157:H7 were used as representative Gram-positive and Gram-negative bacteria, respectively. These strains were prepared by culturing in NB medium at 37 °C with continuous shaking (180 rpm·min−1) for 18 h. After obtaining the bacterial concentration by the plate counting method, the bacterial concentration was adjusted to 1 × 106 cfu/mL with sterile water. 100 µL of culture representing ∼ 1 × 106 cells was added into 96-well plates containing 100 µL of NB supplemented with appropriate concentrations of CD-MOF (0 to 200 mg/mL), CA (0 to 200 mg/mL) and CA@CD-MOF (0 to 200 mg/mL). And NB was used only as a negative control and without MOF crystals as a positive control. The plate was incubated in a culture temperature at 37 °C for 24 h and using the plate counting method to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). Herein, we defined the MIC for MOF crystals as the lowest minimum concentration of MOF crystals that inhibited complete the growth of bacteria and the MBC for MOF crystals as the lowest minimum concentration of MOF crystals that 99.9% kill from initial bacteria.
2.8. Molecular docking of CA and CD-MOF
The crystal structure of CD-MOF was chosen from previous study [19]. The non-periodic structure expanded by CD-MOF was used. The K+ ion which did not affect the docking results was deleted and H2O was used to replace the OH− ion. The resulting model of CD-MOF was composed of twelve molecules which included six hydrophobic cavities and one hydrophilic cavity and used to the docking model [26]. The structure of CA molecule was built in the Materials Visualizer module in Materials Studio 2017 (MS, Accelrys Inc.). The Forcite module in MS was employed for energy minimization and molecular dynamics (MD) simulation. The docking tools Auto Dock Vina 1.1.2 was used to simulate the molecular docking process [31] and the explicated method was depicted in Supporting Information.
3. Results and discussion
3.1. Effects of parameters on U-CD-MOF crystal assembly
As shown Fig. 1, the parameters of ultrasound power, reaction time and temperature were important to the formation of U-CD-MOF crystal in the ultrasound-assisted preparation. Different ultrasound power from 90 to 720 W were considered for the optimization. The SEM images of the crystals obtained under different ultrasound power as shown in Fig. 1A. The morphology of the samples had no obvious changed under the ultrasound power of 720 (S2), 630 (S3), 450 (S4), 360 (S5), 270 (S6), 180 (S7), 90 (S8) W, and their particle size were all between 2 and 3 μm. Interestingly, the morphology of the sample obtained under the ultrasonic power of 540 W had a typical uniform cubic state and the size was about 8 μm, which showed obviously difference compared to other groups. Fig. 1B showed that the particle size and the coefficient of variation (CV, the ratio of the standard deviation of the particle size to the average value) obtained under different ultrasonic power. It could be seen that the crystal prepared under the condition of 540 W had the smallest CV indicated that the particle size distribution of samples prepared under this condition was more uniform. A similar phenomenon was also found that narrower particle size distribution could be obtained under a specific ultrasonic power of some other samples [32].
Fig. 1.
SEM morphology images of U-CD-MOF crystals obtained after different ultrasonic power of 90, 180, 270,360, 450, 540, 630, 720 W (A) and particle size with coefficient of variation (B); SEM morphology images of U-CD-MOF crystals obtained after different ultrasound reaction time of 1, 3, 5,10, 15 min (C) and particle size with coefficient of variation (D); SEM morphology images of U-CD-MOF crystals obtained after different ultrasound treat temperature of 30, 40, 50,60, 70 °C (E) and particle size with coefficient of variation (F).
The reaction time had been optimized in different reaction time ranges from 1 to 15 min. The SEM images of the crystals obtained under different reaction time as shown in Fig. 1C. The morphology of the samples had no obvious changed under the reaction time of 1 (S9), 3 (S10), 5 (S11), 15 (S12) min, and particle size of S9-11 were all between 2 and 3 μm. Interestingly, the particle size increased while the reaction time increased to 10 min, which may due to the increase in reaction time that caused the nucleation of U-CD-MOF crystals to increase and accelerate the aggregation [33]. When the reaction time continued to increase to 15 min, the particle size gradually decreased which may due to more cavitation bubbles which were generated in the solution, and increased the collision of particles and reduces the particle size [34]. Fig. 1D showed that particle size and CV obtained under different reaction time and the sample obtained with reaction time of 10 min was more uniform.
The temperature effect on the size and morphology of U-CD-MOF were also investigated additionally. SEM images of samples obtained at different temperatures of 30 (S13), 40 (S14), 50 (S15), 70 °C (S16) as shown in Fig. 1E. An obvious influence of temperature on the morphology of sample was recorded. At lower temperature (30 °C), the mixed mother solution was still white after ultrasound treatment and the particle size of S13 sample obtained this condition was less than 1 μm.
This observation could be attributed to the during the crystallization process, lower temperature leaded to the dense distribution of nucleation sites, so MeOH as an antisolvent may make the mother solution recrystallize at low temperature to form fine crystals. While PEG-8000 as a size adjuster required the solution to obtain the ability to adjust the size of crystals in a non-supersaturated condition, that is, could adjust the size of the newly obtained crystals at high temperature [31]. In addition, it could be seen in Fig. 1F that as the temperature increased, the particle size of the samples also increased, this phenomenon may be attributed to the fact that the nucleation rate was inversely proportional to the temperature during the crystallization process. The smaller number of crystal nuclei formed at high temperature would further lead to the growth of crystals and produce larger crystal particles [34].
The PXRD was further used to analyze samples with different preparation process conditions. As shown in Figure S2, the patterns of samples prepared with different ultrasonic power (A) had the same characteristic diffraction peak intensity, and the characteristic peaks were obvious which indicating the obtained U-CD-MOF crystal structure was complete. Similar results could be observed in the patterns of samples prepared at different reaction times (B). The patterns of samples prepared at different temperature (C) showed different results that the characteristic peaks of the sample prepared under low temperature conditions (30 °C) were weaker, indicating that the crystal structure was relatively poor, and crystal structure was incomplete, meanwhile the characteristic diffraction peaks of the samples prepared under the condition of 60 and 70 °C were consistent and had good crystallinity [31]. The PXRD results showed that the ultrasonic power and the reaction time were not the main factors that affecting the structure of the prepared U-CD-MOF crystal, and the low temperature had a greater effect on the structure of the U-CD-MOF crystal.
3.2. Characterization of U-CD-MOF
As shown in Figure S3, comparing the U-CD-MOF prepared in this study with the CD-MOF obtained by the vapor diffusion method. revealed that CD-MOF crystal obtained by the vapor diffusion method (A) presented a representative cubic structure with relatively uniform size and morphology. The U-CD-MOF crystal (B) obtained by the ultrasound-assisted in this study also presented a typical cubic structure with uniform size and morphology.
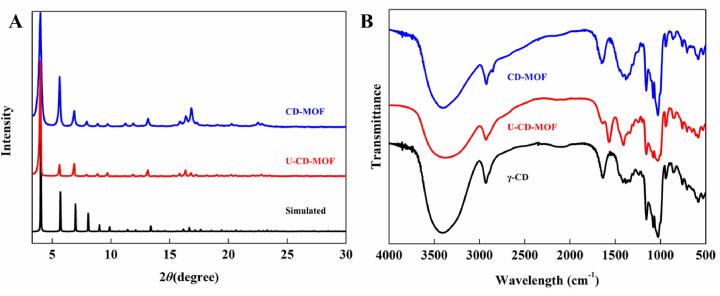
The PXRD patterns of CD-MOF prepared by the vapor diffusion method and U-CD-MOF were shown in Fig. 2A, both crystals had similar diffraction peaks at 4°, 5.7°, 6.9°, 13.4° and 16.7°, which were consistent with the calculation and simulation result [19]. The main peak position of the crystals had obvious characteristic crystalline peaks, which was consistent with the previous studies [20], suggesting that the ultrasound-assisted method could successfully prepare CD-MOF.
Fig. 2.
The PXRD patterns of synthesized CD-MOF by vapor diffusion method (blue), U-CD-MOF (red) and calculated pattern (black) (A); The FT-IR patterns of synthesized CD-MOF by vapor diffusion method (blue), U-CD-MOF (red) and γ-CD (black) (B).
The FT-IR patterns of γ-CD, CD-MOF prepared by the vapor diffusion method and U-CD-MOF were shown in Fig. 2B. The FT-IR spectra of γ-CD, CD-MOF and U-CD-MOF had similar characteristic peaks: peak at 3000–3500 cm−1 accounting for –OH stretching vibration; peaks approximately 1629 and 1409 cm−1 corresponding to a hydrate water vibration and –OH plane bending vibration, respectively [35].A new peak at 1566 cm−1 was observed in both CD-MOF and U-CD-MOF, but not found in γ-CD, which is similar to that reported previously [36].
3.3. Optimization of CA@U-CD-MOF preparation process
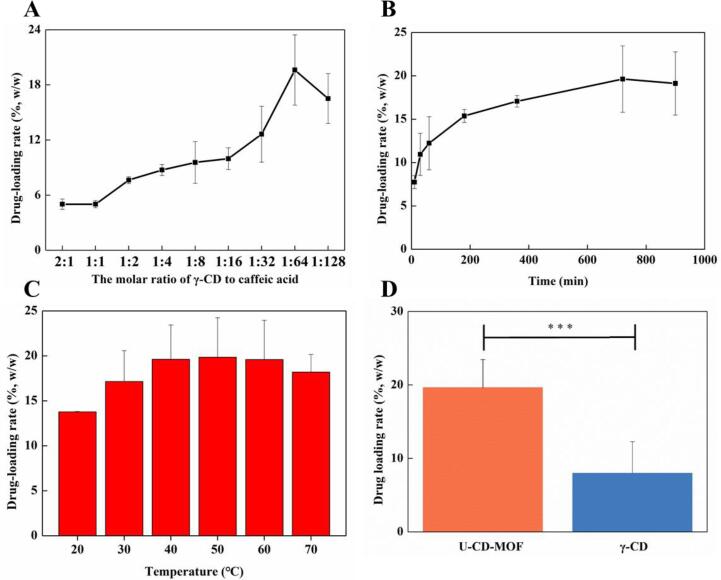
Fig. 3A showed that when the molar ratio of U-CD-MOF (γ-CD) to CA was 2:1 and 1:1, respectively, the loading rate of CA was about 5%. As the amount of CA increased, that is, the molar ratio of U-CD-MOF (γ-CD) to CA increased, and the loading rate of CA increased accordingly and reached the maximum when the molar ratio was 1:64 (19.63 ± 3.82%), as CA further increased the loading rate of CA decreased (16.52 ± 2.72%). This situation may be explained that when the amount of CA added in the early stage, there were still lots of cavities in U-CD-MOF that could be used for adsorption. Therefore, as the amount of CA increased, the loading rate also increased, and when the CA molecules gradually occupied the cavities, which caused the adsorption capacity to reach saturation [37]. In addition, excessive CA may accumulate and reduce the effective adsorption area of the U-CD-MOF thereby affecting the loading rate [38].
Fig. 3.
Effect of the molar ratio of U-CD-MOF (γ-CD) to CA (A), reaction time (B) and temperature (C) on the loading rate. Comparison of U-CD-MOF prepared with equimolar amount of γ-CD and γ-CD on CA loading rate (D), ***, indicating the significant difference between U-CD-MOF and γ-CD groups: ***, P less than 0.001.
When the reaction time was the shortest (10 min), the loading rate of CA was the lowest (7.74 ± 0.75%). As the incubation time continues to increase to 720 min, the loading rate of U-CD-MOF to CA gradually increased and tended to be stable, and the adsorption process of the system tended to be balanced (Fig. 3B). When the temperature was the lowest (20 °C), the loading rate of CA was the lowest (13.78 ± 0.05%). As the incubation temperature continued to increase, the loading rate of CA gradually increased and became stable at 40–60 °C. This may be explained that the surface of the carrier may be activated at higher temperature, exposing more active sites and increasing the load capacity [39].
As shown in Fig. 3D, under the same conditions, the loading rate of CA relative to γ-CD was only 7.99 ± 4.23%, while the loading rate of U-CD-MOF to CA was as high as 19.63 ± 3.82%, which increased 2 times more. The increase in loading rate may explain that the formation of clathrates between γ-CD and CA, as one γ-CD molecule could hold one CA molecule, while compared with γ-CD, CD-MOF not only retained the internal cavity of γ-CD, but also formed a large internal cavity (with a diameter of 1.7 nm) by coordinating with potassium ions, and could also accommodate additional CA molecules that increased the loading rate [40].
3.4. Characterization of CA@U-CD-MOF
The PXRD pattern of U-CD-MOF, CA, CA and U-CD-MOF physical mixture and CA@U-CD-MOF were shown in Fig. 4A. CA had obvious characteristic diffraction peaks at 14.1°, 15.9°, 17.5°, 24.4°, 25.7° and 27°, of which 27° was the strongest, showing that CA was a complete crystal structure and has a good crystal form. CA and U-CD-MOF physical mixture (20%, w/w) showed characteristic peaks belonging to CD-MOF at 4°, 5.7°, 6.9°, 13.4° and 16.7°, meanwhile the characteristic peaks of caffeic acid also appeared at 14.1°, 15.9°, 17.5°, 24.4°, 25.7°, and 27°, indicating that the pure physical mixture of CA and U-CD-MOF maintained CA and U-CD-MOF in good crystal characteristics, and there was no compound formation of CA and U-CD-MOF. However, CA@U-CD-MOF had characteristic peaks of U-CD-MOF at 4°, 5.7°, 6.9°, 13.4° and 16.7°, and the peak shape intensity was basically the same. The crystal characteristic peak intensity of CA@U-CD-MOF changed slightly, which may be account for the change of the spatial structure of the CA. At the same time the characteristic peak of CA almost completely disappeared, indicating that during the loading process, due to the interaction of U-CD-MOF and CA, CA changed from a crystalline state to an amorphous state and completely entered the cavity of the U-CD-MOF [40].
Fig. 4.
PXRD patterns (A) FT-IR patterns (B) and of U-CD-MOF (black), CA (red), CA and U-CD-MOF physical mixture (pink), CA@U-CD-MOF (blue) crystals (A).
In order to study the interaction between CA and U-CD-MOF, the FT-IR patterns of U-CD-MOF, CA, CA and U-CD-MOF physical mixture and CA@U-CD-MOF were recorded as shown in Fig. 4B. The characteristic functional group peaks of U-CD-MOF included the stretching vibration of the –OH hydroxyl group at about 3000–3600 cm−1, the stretching vibration of –CH- and –CH2- at about 2800–3000 cm−1, the bending vibration of C–H and CH2 at about 1300–1500 cm−1, these characteristic peaks were consistent with previous reports on CD-MOF [41]. The characteristic functional group of CA had the –OH and –COOH stretching vibration of the benzene ring at 3430 and 3236 cm−1, the C = O stretching vibration at 1650 cm−1, and the bending vibration of the –OH on the benzene ring at 1280 cm−1, the stretching vibration of benzene ring C = C at 1620 and 1450 cm−1 [42]. The FT-IR patterns of CA and U-CD-MOF physical mixture could clearly observe the characteristic peak of CA but the overall peak intensity was weakened, and the absorption peaks at 3430 and 3236 cm−1 were relatively weak which was caused by the overlap of the characteristic peak of U-CD-MOF, indicating that pure CA and U-CD-MOF physical mixture would not create new chemical bonds between CA and U-CD-MOF. The FT-IR patterns of CA@U-CD-MOF retained the characteristic peak of U-CD-MOF, while the main characteristic peak of CA did not appear (3236, 1650, 1450 cm−1), however, CA@U-CD-MOF had the characteristic peak of CA at 1280 cm−1 compared to U-CD-MOF, which could be explained that most of CA which was in CA@CD-MOF entered the internal cavity of U-CD-MOF, causing the characteristic peak of CA to be covered up, CA accumulated in the cavity of CD-MOF due to the high load, which destroyed the spatial structure of U-CD-MOF, and exposed some CA molecules on the surface of U-CD-MOF [40].
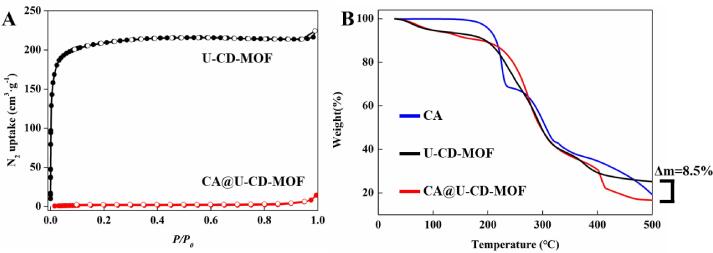
Nitrogen gas adsorption was used to measure the porosity of U-CD-MOF and CA@U-CD-MOF. Barret-Joyner-Halend (BJH) and density functional theory (DFT) models were used to calculate the pore volume distribution (Table 1), while Brunauer-Emmett-Teller (BET) and Langmuir theoretical models were used to predict the specific surface area, respectively. The isotherms of U-CD-MOF showed a type Ⅰ shape, indicating the existence of micropores (Fig. 5A) [19] and the calculated BET surface area was 836.40 m2·g−1 for U-CD-MOF, which was similar to previous report [40], [43]. The BET surface area of CA @U-CD-MOF reduced to 6.42 m2·g−1 suggesting that with the increment of CA, the pores of U-CD-MOF were reduced. The DFT pore volume of CA @U-CD-MOF decreased from 0.22 cm3·g−1 to 0.009 cm3·g−1, which was similar to previous report that due to CA molecules entered the cavity of U-CD-MOF and filled the pores of U-CD-MOF [26], this result was consistent with results of FTIR and PXRD.
Table 1.
The surface area and pore volume of U-CD-MOF and CA@U-CD-MOF.
| Sample | Surface Area (m2·g−1) | Pore volume (cm3·g−1) | ||
|---|---|---|---|---|
| Langmuir | BET | BJH | DFT | |
| U-CD-MOF | 925.28 | 836.34 | 0.64 | 0.22 |
| CA@U-CD-MOF | 7.38 | 6.42 | 0.024 | 0.009 |
Fig. 5.
N2 adsorption/desorption isotherms of U-CD-MOF (black) and CA@U-CD-MOF (red) (A). TGA spectra of CA (blue), U-CD-MOF (black) and CA@U-CD-MOF (red) (B).
TGA was chosen to evaluate the thermal stability of CA, U-CD-MOF and CA@U-CD-MOF as shown in Fig. 5B, CA had good stability at 100 °C, but the subsequent curve showed that thermal decomposition occurs during the entire process [44]. U-CD-MOF was thermally degraded at about 170 °C and entered the stage of quality loss meanwhile CA@CD-MOF also had a similar curve. CA@U-CD-MOF and U-CD-MOF had a weight difference of 8.5% at about 500 °C, which may be caused by the thermal decomposition loss of the loaded CA [45]. In addition, compared with the rapid mass loss of CA around 200℃, CA@CD-MOF had a slower curve which indicated that the CA in the cavity of U-CD-MOF were protected and the thermal stability of CA was improved.
3.5. Release study
It could be seen that CA@U-CD-MOF exhibited a burst release of CA in water and PBS (Fig. 6A), and the release amount could be close to 100% in about 1 min which was caused by the excellent hydrophilicity of CD-MOF [46].
Fig. 6.
The release curve of CA@CD-MOF in water and PBS (A), ethanol (B); Fitting equation: the Zero-order equations (C), First order equation (D), Higuchi equation (E).
When CA@U-CD-MOF was placed in an ethanol (Fig. 6B), the cumulative release rate reached 52.16 ± 0.33% at 90 min, then the cumulative release rate reached 66.16 ± 0.42% at 2160 min, indicating that the CA@U-CD-MOF could achieve a sustained release effect in ethanol. Fitting the process of CA@U-CD-MOF releasing CA in ethanol with three release models: Zero-order model (C), First-order model (D) and Higuchi model (E). Using the equation fitting correlation coefficient R2 to judge best fit model, the results showed that the order of the best fitting model was Higuchi model > Zero-order model > First-order model (Table 2), and the possible underlying mechanism of CA@CD-MOF is the diffusion process [47].
Table 2.
Kinetics model fitting results of CA@U-CD-MOF releasing CA in ethanol.
| Equation | Zero-order model | First-order model | Higuchi model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q (%) = kt + k0 | Q (%) = k (1-e- k0t) | Q (%) = kt1/2 + k0 | ||||||||
| Release conditions | k | k0 | R2 | k | k0 | R2 | k | k0 | R2 | |
| CA | 0.9617 | 33.70 | 0.9282 | 0.1493 | 59.37 | 0.1729 | 0.3925 | 48.53 | 0.9500 |
3.6. Antibacterial study
E. coli O157:H7 and S. aureus were used to test the antibacterial ability of the prepared MOF crystals. The MIC and MBC observed were showed in Table 3. U-CD-MOF had no antibacterial effect at the concentration of 200 mg·mL−1, but while CA@U-CD-MOF showed MIC at 25 mg·mL−1 in E. coli O157:H7 and 25 mg·mL−1 in S. aureus. Furthermore, the MBC for CA@U-CD-MOF was 25 mg·mL−1 in E. coli O157:H7 and no value could be observed in the measurement range in S. aureus. The result indicated that the antibacterial activity of CA@U-CD-MOF was more obvious in E. coli O157:H7 than S. aureus. It may be caused by the difference in the cell walls of E. coli O157:H7 and S. aureus [48]. Considering the loading rate of CA in U-CD-MOF and the antibacterial effect of CA alone, we speculated that U-CD-MOF may have a certain influence on the antibacterial function of CA.
Table 3.
MIC and MBC determinations of the U-CD-MOF, CA and CA@U-CD-MOF.
| Sample | E. coli O157:H7 | S. aureus | ||
|---|---|---|---|---|
| MIC (mg·mL−1) | MBC (mg·mL−1) | MIC (mg·mL−1) | MBC (mg·mL−1) | |
| U-CD-MOF | >200 | >200 | >200 | >200 |
| CA | 2 | 2 | 4 | 4 |
| CA@U-CD-MOF | 25 | 25 | 25 | >25 |
3.7. Molecular docking
The molecular docking study of CA and CD-MOF was carried out by a docking tool-based approach to explain the mechanism of CA loading by CD-MOF. As shown in Fig. 7, the possible structure conformations of CA molecules distributed in CD-MOF were obtained by docking CA molecules into the CD-MOF model individually. When a small amount of CA was entered into CD-MOF model (CA molecules with molar ratios of 1:2 (A) to γ-CD unit into CD-MOF model), CA molecules tended to be distributed in the D-γ-CD molecular pair (hydrophobic cavity) in CD-MOF model, and the docking free energy was −6.3 kcal/ mol. When with the introduction of more CA molecules entered into the CD-MOF model (CA molecules with molar ratios of 1:1 (B) to γ-CD unit into CD-MOF model), CA molecules tended to be distributed in different hydrophobic cavities [26]. In addition, when all the hydrophobic cavities are occupied, that is, CA molecules with molar ratios of 2:1 (C) to γ-CD unit into CD-MOF model, the remaining CA molecules would enter the large hydrophilic cavity inside the CD-MOF model and tended to be disordered [40] and the free energy of molecular docking was −4.9 kcal/mol. By extracting the D-γ-CD molecule pair unit structure from CD-MOF model, the interaction between CA molecule and the D-γ-CD was further studied. The results showed that when a CA molecule entered the D-γ-CD molecule pair unit, it tended to be distributed in the two pores formed by γ-CD, and through its own –OH group and –OH on γ-CD ring to form a hydrogen bond (D). When two CA molecules entered the same D-γ-CD at the same time, the results showed that caffeic acid molecules tended to be distributed in the cavity of γ-CD.
Fig. 7.
Molecular docking simulations of caffeic acid molecules distributed in CD-MOF. Add CA molecules with molar ratios of 1:2 (A), 1:1 (B), 2:1 (C) to γ-CD unit into CD-MOF model. H-bonds between caffeic acid and D-γ-CD (1:1) (D); H-bonds between caffeic acid and D-γ-CD (2:1) (E).
4. Conclusions
In conclusion, we successfully demonstrated the ultrasound-assisted rapid synthesis of U-CD-MOF, which shortened the preparation time from several hours to several minutes. The morphology and size of the resulting crystal could be changed by changing the ultrasonic power, reaction time and temperature. It is worth noting that low temperature condition (30 °C) may damage the integrity of the crystal structure. U-CD-MOF was used to load antibacterial natural product CA to prepare CA@U-CD-MOF. The loading rate of CA could reach 19.63 ± 2.53%. The obtained crystals were characterized by PXRD, FTIR, nitrogen gas adsorption and it was speculated that CA was loaded in the cavity of U-CD-MOF. In addition, CA@γ-CD was prepared with γ-CD and it was found that the loading rate of U-CD-MOF to CA was more than twice that of γ-CD. The antibacterial test results showed that the MIC and MBC of CA@CD-MOF for E. coli O157:H7 were also 25 mg·mL−1, while the MIC for S. aureus was 25 mg·mL−1. The sustained release behavior of CA@U-CD-MOF to CA in ethanol fitted well to Higuchi model and the molecular docking results showed that the CA molecule was most likely to enter the D-γ-CD structure in CD-MOF to achieve loading.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Mofei Shen drafted the manuscript. Jianwei Zhou, Yunlei Xianyu, Jinsong Feng, Donghong Liu, Mohamed Elhadidy and Tian Ding critically revised the article. This study is supported by the National Natural Science Foundation of China (grant 31972166).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.106003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Umemura A., Diring S., Furukawa S., Uehara H., Tsuruoka T., Kitagawa S. Morphology Design of Porous Coordination Polymer Crystals by Coordination Modulation. J. Am. Chem. Soc. 2011;133:15506–15513. doi: 10.1021/ja204233q. [DOI] [PubMed] [Google Scholar]
- 2.Gimenez-Marques M., Hidalgo T., Serre C., Horcajada P. Nanostructured metal-organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016;307:342–360. [Google Scholar]
- 3.Horcajada P., Gref R., Baati T., Allan P.K., Maurin G., Couvreur P., Férey G., Morris R.E., Serre C. Metal-Organic Frameworks in Biomedicine. Chemical Reviews. 2012;112:1232–1268. doi: 10.1021/cr200256v. [DOI] [PubMed] [Google Scholar]
- 4.Liu J.W., Chen L.F., Cui H., Zhang J.Y., Zhang L., Su C.Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. CHEMICAL SOCIETY REVIEWS. 2014;43:6011–6061. doi: 10.1039/c4cs00094c. [DOI] [PubMed] [Google Scholar]
- 5.Campbell M.G., Dincă M. Metal-Organic Frameworks as Active Materials in Electronic Sensor Devices. Sensors (Basel) 2017;17:1108. doi: 10.3390/s17051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maya F., Palomino Cabello C., Frizzarin R.M., Estela J.M., Turnes Palomino G., Cerdà V. Magnetic solid-phase extraction using metal-organic frameworks (MOFs) and their derived carbons. TrAC Trends in Analytical Chemistry. 2017;90:142–152. [Google Scholar]
- 7.Chen H., Qiu C., Jiang Y., Liao X., Wu D., Shen M., Ding T. Silver nanoparticles on UiO-66 (Zr) metal-organic frameworks for water disinfection application. Food Science and Human Wellness. 2022;11:269–276. [Google Scholar]
- 8.Shen M., Forghani F., Kong X., Liu D., Ye X., Chen S., Ding T. Antibacterial applications of metal–organic frameworks and their composites. Comprehensive Reviews in Food Science and Food Safety. 2020;19:1397–1419. doi: 10.1111/1541-4337.12515. [DOI] [PubMed] [Google Scholar]
- 9.Vaitsis C., Sourkouni G., Argirusis C. Metal Organic Frameworks (MOFs) and ultrasound: A review. Ultrason Sonochem. 2019;52:106–119. doi: 10.1016/j.ultsonch.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Khan N.A., Jhung S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015;285:11–23. [Google Scholar]
- 11.Pilloni M., Padella F., Ennas G., Lai S., Bellusci M., Rombi E., Sini F., Pentimalli M., Delitala C., Scano A., Cabras V., Ferino I. Liquid-assisted mechanochemical synthesis of an iron carboxylate Metal Organic Framework and its evaluation in diesel fuel desulfurization. Microporous and Mesoporous Materials. 2015;213:14–21. [Google Scholar]
- 12.Son W.J., Kim J., Kim J., Ahn W.S. Sonochemical synthesis of MOF-5. CHEMICAL COMMUNICATIONS. 2008:6336–6338. doi: 10.1039/b814740j. [DOI] [PubMed] [Google Scholar]
- 13.Li Z.-Q., Qiu L.-G., Wang W., Xu T., Wu Y., Jiang X. Fabrication of nanosheets of a fluorescent metal–organic framework [Zn(BDC)(H2O)]n (BDC=1,4-benzenedicarboxylate): Ultrasonic synthesis and sensing of ethylamine. Inorganic Chemistry Communications. 2008;11:1375–1377. [Google Scholar]
- 14.Li Z.-Q., Qiu L.-G., Xu T., Wu Y., Wang W., Wu Z.-Y., Jiang X. Ultrasonic synthesis of the microporous metal–organic framework Cu3(BTC)2 at ambient temperature and pressure: An efficient and environmentally friendly method. Materials Letters. 2009;63:78–80. [Google Scholar]
- 15.Nalesso S., Varlet G., Bussemaker M.J., Sear R.P., Hodnett M., Monteagudo-Oliván R., Sebastián V., Coronas J., Lee J. Sonocrystallisation of ZIF-8 in water with high excess of ligand: Effects of frequency, power and sonication time. Ultrason. Sonochem. 2021;76 doi: 10.1016/j.ultsonch.2021.105616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amaro-Gahete J., Klee R., Esquivel D., Ruiz J.R., Jiménez-Sanchidrián C., Romero-Salguero F.J. Fast ultrasound-assisted synthesis of highly crystalline MIL-88A particles and their application as ethylene adsorbents. Ultrason. Sonochem. 2019;50:59–66. doi: 10.1016/j.ultsonch.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Nirumand L., Farhadi S., Zabardasti A., Khataee A. Synthesis and sonocatalytic performance of a ternary magnetic MIL-101(Cr)/RGO/ZnFe2O4 nanocomposite for degradation of dye pollutants. Ultrason. Sonochem. 2018;42:647–658. doi: 10.1016/j.ultsonch.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 18.Magri A., Petriccione M., Gutiérrez T.J. Metal-organic frameworks for food applications: A review. Food Chemistry. 2021;354 doi: 10.1016/j.foodchem.2021.129533. [DOI] [PubMed] [Google Scholar]
- 19.Smaldone R.A., Forgan R.S., Furukawa H., Gassensmith J.J., Slawin A.M., Yaghi O.M., Stoddart J.F. Metal-organic frameworks from edible natural products. Angew Chem Int Ed Engl. 2010;49:8630–8634. doi: 10.1002/anie.201002343. [DOI] [PubMed] [Google Scholar]
- 20.Forgan R.S., Smaldone R.A., Gassensmith J.J., Furukawa H., Cordes D.B., Li Q., Wilmer C.E., Botros Y.Y., Snurr R.Q., Slawin A.M., Stoddart J.F. Nanoporous carbohydrate metal-organic frameworks. J Am Chem Soc. 2012;134:406–417. doi: 10.1021/ja208224f. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y., Li M.F., Wang L., Tan J., Li R., Jiang Z.T., Tang S.H., Li T.T. Improvement of the stabilities and antioxidant activities of polyphenols from the leaves of Chinese star anise (Illicium verum Hook. f.) using beta-cyclodextrin-based metal-organic frameworks. J Sci Food Agric. 2020 doi: 10.1002/jsfa.10642. [DOI] [PubMed] [Google Scholar]
- 22.M.P. Abucafy, B.L. Caetano, B.G. Chiari-Andreo, B. Fonseca-Santos, A.M. do Santos, M. Chorilli, L.A. Chiavacci, Supramolecular cyclodextrin-based metal-organic frameworks as efficient carrier for anti-inflammatory drugs, Eur J Pharm Biopharm, 127 (2018) 112-119. [DOI] [PubMed]
- 23.Blight B.A., Ahmad T.I., Shepherd H.J., Jennings C.S., Ferland L.I., Teat S.J., Rossman J.S. Sterol Uptake by an Alkali-beta-Cyclodextrin Metal-Organic Framework. CRYSTAL GROWTH & DESIGN. 2020;20:43–48. [Google Scholar]
- 24.Brandelli A. The interaction of nanostructured antimicrobials with biological systems: Cellular uptake, trafficking and potential toxicity. Food Science and Human Wellness. 2020;9(1):8–20. [Google Scholar]
- 25.Luo T., Shakya S., Mittal P., Ren X., Guo T., Bello M.G., Wu L., Li H., Zhu W., Regmi B., Zhang J. Co-delivery of superfine nano-silver and solubilized sulfadiazine for enhanced antibacterial functions. Int J Pharm. 2020;584 doi: 10.1016/j.ijpharm.2020.119407. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y., Zhao Y., Niu B., Luo Q., Zhang Y., Quan G., Pan X., Wu C. Cyclodextrin-based metal-organic frameworks for pulmonary delivery of curcumin with improved solubility and fine aerodynamic performance. Int J Pharm. 2020;588 doi: 10.1016/j.ijpharm.2020.119777. [DOI] [PubMed] [Google Scholar]
- 27.Ke F., Zhang M., Qin N., Zhao G., Chu J., Wan X. Synergistic antioxidant activity and anticancer effect of green tea catechin stabilized on nanoscale cyclodextrin-based metal–organic frameworks. Journal of Materials Science. 2019;54:10420–10429. [Google Scholar]
- 28.Furukawa Y., Ishiwata T., Sugikawa K., Kokado K., Sada K. Nano- and microsized cubic gel particles from cyclodextrin metal-organic frameworks. Angew Chem Int Ed Engl. 2012;51:10566–10569. doi: 10.1002/anie.201204919. [DOI] [PubMed] [Google Scholar]
- 29.Liu B., Li H., Xu X., Li X., Lv N., Singh V., Stoddart J.F., York P., Xu X., Gref R., Zhang J. Optimized synthesis and crystalline stability of γ-cyclodextrin metal-organic frameworks for drug adsorption. International Journal of Pharmaceutics. 2016;514:212–219. doi: 10.1016/j.ijpharm.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 30.Qiu C., Wang J., Zhang H., Qin Y., Xu X., Jin Z. Novel Approach with Controlled Nucleation and Growth for Green Synthesis of Size-Controlled Cyclodextrin-Based Metal-Organic Frameworks Based on Short-Chain Starch Nanoparticles. J Agric Food Chem. 2018;66:9785–9793. doi: 10.1021/acs.jafc.8b03144. [DOI] [PubMed] [Google Scholar]
- 31.Liu B., He Y., Han L., Singh V., Xu X., Guo T., Meng F., Xu X., York P., Liu Z., Zhang J. Microwave-Assisted Rapid Synthesis of γ-Cyclodextrin Metal-Organic Frameworks for Size Control and Efficient Drug Loading. Crystal Growth & Design. 2017;17:1654–1660. [Google Scholar]
- 32.Nii S., Takayanagi S. Growth and size control in anti-solvent crystallization of glycine with high frequency ultrasound. Ultrason. Sonochem. 2014;21:1182–1186. doi: 10.1016/j.ultsonch.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Taneja S., Shilpi S., Khatri K. Formulation and optimization of efavirenz nanosuspensions using the precipitation-ultrasonication technique for solubility enhancement. ARTIFICIAL CELLS NANOMEDICINE AND BIOTECHNOLOGY. 2016;44:978–984. doi: 10.3109/21691401.2015.1008505. [DOI] [PubMed] [Google Scholar]
- 34.Park M.W., Yeo S.D. Antisolvent Crystallization of Roxithromycin and the Effect of Ultrasound. SEPARATION SCIENCE AND TECHNOLOGY. 2010;45:1402–1410. [Google Scholar]
- 35.Zhang B., Huang J., Liu K., Zhou Z., Jiang L., Shen Y., Zhao D. Biocompatible Cyclodextrin-Based Metal-Organic Frameworks for Long-Term Sustained Release of Fragrances. Industrial & Engineering Chemistry Research. 2019;58:19767–19777. [Google Scholar]
- 36.He Y., Zhang W., Guo T., Zhang G., Qin W., Zhang L., Wang C., Zhu W., Yang M., Hu X., Singh V., Wu L., Gref R., Zhang J. Drug nanoclusters formed in confined nano-cages of CD-MOF: dramatic enhancement of solubility and bioavailability of azilsartan. Acta Pharm Sin B. 2019;9:97–106. doi: 10.1016/j.apsb.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unuabonah E.I., Adebowale K.O., Olu-Owolabi B.I., Yang L.Z., Kong L.X. Adsorption of Pb(II) and Cd(II) from aqueous solutions onto sodium tetraborate-modified Kaolinite clay: Equilibrium and thermodynamic studies. HYDROMETALLURGY. 2008;93:1–9. [Google Scholar]
- 38.Karthikeyan S., Balasubramanian R., Yer C.S.P. Evaluation of the marine algae Ulva fasciata and Sargassum sp for the biosorption of Cu(II) from aqueous solutions. BIORESOURCE TECHNOLOGY. 2007;98:452–455. doi: 10.1016/j.biortech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Wu S.L., Zhao X.D., Li Y.H., Du Q.J., Sun J.K., Wang Y.H., Wang X., Xia Y.Z., Wang Z.H., Xia L.H. Adsorption Properties of Doxorubicin Hydrochloride onto Graphene Oxide: Equilibrium. Kinetic and Thermodynamic Studies, MATERIALS. 2013;6:2026–2042. doi: 10.3390/ma6052026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J., Wu L., Guo T., Zhang G., Wang C., Li H., Li X., Singh V., Chen W., Gref R., Zhang J. A “Ship-in-a-Bottle” strategy to create folic acid nanoclusters inside the nanocages of gamma-cyclodextrin metal-organic frameworks. Int J Pharm. 2019;556:89–96. doi: 10.1016/j.ijpharm.2018.11.074. [DOI] [PubMed] [Google Scholar]
- 41.Hu Z., Li S., Wang S., Zhang B., Huang Q. Encapsulation of menthol into cyclodextrin metal-organic frameworks: Preparation, structure characterization and evaluation of complexing capacity. Food Chem. 2021;338 doi: 10.1016/j.foodchem.2020.127839. [DOI] [PubMed] [Google Scholar]
- 42.Jun L., Xingchi W., Ruyu B., Nianfeng Z., Juan K., Changhai J. Synthesis, characterization, and antioxidant activity of caffeic-acid-grafted corn starch. Starch/Staerke. 2018;70 [Google Scholar]
- 43.Li H., Zhu J., Wang C., Qin W., Hu X., Tong J., Yu L., Zhang G., Ren X., Li Z., Zhang J. Paeonol loaded cyclodextrin metal-organic framework particles for treatment of acute lung injury via inhalation. Int J Pharm. 2020;587 doi: 10.1016/j.ijpharm.2020.119649. [DOI] [PubMed] [Google Scholar]
- 44.Kumar N., Pruthi V., Goel N. Structural, thermal and quantum chemical studies of p-coumaric and caffeic acids. Journal of Molecular Structure. 2015;1085:242–248. [Google Scholar]
- 45.Shakya S., He Y., Ren X., Guo T., Maharjan A., Luo T., Wang T., Dhakhwa R., Regmi B., Li H., Gref R., Zhang J. Ultrafine Silver Nanoparticles Embedded in Cyclodextrin Metal-Organic Frameworks with GRGDS Functionalization to Promote Antibacterial and Wound Healing Application. Small. 2019;15(27) doi: 10.1002/smll.201901065. [DOI] [PubMed] [Google Scholar]
- 46.Singh V., Guo T., Xu H., Wu L., Gu J., Wu C., Gref R., Zhang J. Moisture resistant and biofriendly CD-MOF nanoparticles obtained via cholesterol shielding. Chem Commun (Camb) 2017;53:9246–9249. doi: 10.1039/c7cc03471g. [DOI] [PubMed] [Google Scholar]
- 47.Li Z., Peng Y., Xia X., Cao Z., Deng Y., Tang B. Sr/PTA Metal Organic Framework as A Drug Delivery System for Osteoarthritis Treatment. Scientific Reports. 2019;9:17570. doi: 10.1038/s41598-019-54147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrade M., Benfeito S., Soares P., Silva D.M.E., Loureiro J., Borges A., Borges F., Simoes M. Fine-tuning of the hydrophobicity of caffeic acid: studies on the antimicrobial activity against Staphylococcus aureus and Escherichia coli. RSC ADVANCES. 2015;5:53915–53925. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.