Graphical abstract
Abbreviations: UAE, ultrasonic-assisted extraction; DES, deep eutectic solvent; P. scandens, Paederia scandens (Lour.); Merr. ABTS+, 2,2′-azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid radical; CE, catechin equivalent; CV, coefficient of variation; DES-UAE, ultrasonic-assisted extraction coupled with deep eutectic solvents; DW, dry weight; EtOH-UAE, ultrasonic-assisted extraction coupled with 80% ethanol; Fe(II)E, FeSO4 equivalent; FRAP, ferric-reducing antioxidant power; MetOH-UAE, ultrasonic-assisted extraction coupled with 70% methanol; MS, mass spectra; NMR, Nuclear magnetic resonance; TE, Trolox equivalent; TFC, total flavonoid content; 3D, three-dimensional; UHPLC-MS, ultra-high-performance liquid chromatography-mass spectrometry; W-UAE, ultrasonic-assisted extraction coupled with ultrapure water
Keywords: Ultrasonic-assisted extraction, Deep eutectic solvents, Paederia scandens (Lour.) Merr., Polyphenol, Antioxidant capacity
Highlights
-
•
UAE with deep eutectic solvent (DES) extracted polyphenols of P. scandens.
-
•
Chcl-EG afforded the highest TFC among 16 DESs coupled with UAE.
-
•
Optimization of Chcl-EG-UAE was studied by two-level factorial experiment and RSM.
-
•
Chcl-EG-UAE had the highest extractability compared with other methods.
-
•
30 polyphenols extracted by Chcl-EG-UAE were identified and quantified by UHPLC-MS.
Abstract
Ultrasonic-assisted extraction (UAE) coupled with deep eutectic solvent (DES) is a novel, efficient and green extraction method for phytochemicals. In this study, the effects of 16 DESs coupled with UAE on the extraction rate of polyphenols from Paederia scandens (Lour.) Merr. (P. scandens), an edible and medicinal herb, were investigated. DES synthesised with choline chloride and ethylene glycol at a 1:2 M ratio resulted in the highest extractability. Moreover, the effects of extraction parameters were investigated by using a two-level factorial experiment followed by response surface methodology The optimal parameters (water content in DES of 49.2%, the actual ultrasonic power of 72.4 W, and ultrasonic time of 9.7 min) resulted in the optimal total flavonoid content (TFC) (27.04 mg CE/g DW), ferric-reducing antioxidant power (FRAP) value (373.27 μmol Fe(Ⅱ)E/g DW) and 2,2′-azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid radical (ABTS+) value (48.64 μmol TE/g DW), closely matching the experimental results. Furthermore, a comparison study demonstrated that DES-UAE afforded the higher TFC and FRAP value than traditional extraction methods. 36 individual polyphenolic compounds were identified and quantified by ultra-high-performance liquid chromatography-mass spectrometry (UHPLC-MS) in P. scandens extracts, and of which 30 were found in the extracts obtained by DES-UAE. Additionally, DES-UAE afforded the highest sum of individual polyphenolic compound content. These results revealed that DES-UAE enhanced the extraction efficiency for polyphenols and provided a scientific basis for further processing and utilization of P. scandens.
1. Introduction
Polyphenols, widely distributed in plants, are a very diverse and multifunctional group of phytochemicals, containing flavonoids, phenolic acids, anthocyanins, procyanidins, and stilbenes. Accumulating studies have shown that polyphenols have natural antioxidant properties and play an important role in preventing food oxidation and exerting health-promoting effects [1], [2]. For these reasons, polyphenols or polyphenolic extracts from herbs, fruits, and vegetables have been wildly used in food, medicine, feed stuff and other fields [3], [4].
Paederia scandens (Lour.) Merr. (P. scandens) (“Jishiteng” in Chinese), an edible herb primarily grown in southern China, Vietnam, Japan and India, has been used as a Chinese traditional medicine for treating dyspepsia, jaundice, aches and dysentery, as well as used in many local traditional foods for centuries [5], [6]. The medical injection of P. scandens made in China has been used for relieving pain [7]. Recent studies have reported that P. scandens shows anti-arthritis [6], antioxidant [8], antifungal [8], antinociceptive [9], uric-acid-lowering [10], anti-inflammatory, immunomodulatory [11], and anticonvulsant and sedative properties [12]. The plants belonging to Paederia scandens (Lour.) Merr. or Paederia, such as Paederia scandens (Lour.) Merr. var. Tomentosa and Paederia foetida, were reported to exert hepatoprotective effects [13], therapeutic effects on adjuvant-induced arthritis [14], antifungal, antioxidant and analgesic effects [15], [7]. Some phytochemicals, including iridoid glycosides [11], [16], volatile oil [16], and phenolic acids [8], [16], have been identified in P. scandens. Moreover, flavonoids have been identified in Paederia scandens var. Mairei and Paederia chinensis Hance [17], [18], which may be primarily responsible for their pharmacological properties. Among these phytochemicals, iridoid glycosides have been most commonly studied, while the other compounds have been explored sporadically. Due to the biological activities and health-promoting effects of polyphenols from plants, polyphenols from P. scandens have attracted considerable attention. Quercetin and kaempferol were identified from the fruits of Paederia chinensis Hance [18]. Ishikura et al. [17] isolated and identified 13 flavonoids by 1H and 13C nuclear magnetic resonance (NMR) from leaves and stems of P. scandens var. Mairei. Bordoloi et al. [8] identified 6 phenolic acids from the ethanolic extract of P. scandens using high-performance liquid chromatographic method with diode-array detection (HPLC-DAD) and found the potent antioxidant and antifungal acitivties of the ethanolic extract. Cai et al. [19] also found that leaves and stems of P. scandens were rich in anthocyanin, with potent antioxidant capacity. These studies also revealed that potent biological properties of P. scandens extracts were closely correlated with polyphenol profiles (flavonoids, phenolic acids, and anthocyanin). The extraction methods played a key role in the analysis of polyphenol profiles of the extracts [20], [21], [22], [23]. Therefore, a high-efficiency method for extracting polyphenols from P. scandens is urgently needed.
DES is considered as a class of novel, efficient and green solvent, with the advantages of easy synthesis and a wide polarity range [24], [25]. DES is formed with hydrogen-bond acceptors and hydrogen-bond donors via hydrogen-bond interactions [26]. Recently, DES has been used to extract bioactives, such as flavonoids and phenolic acids, from plants [20], [23], [24]. DES has shown higher extraction efficiency for polyphenols from plants as compared to traditional solvents [22], [23]. UAE has been widely used for extracting protein, oil, and bioactives, including polyphenols, from dairy products, oilseeds, grains, vegetables and fruits [27], [28]. UAE can also improve the extraction efficiency of bioactive compounds by disrupting the cell-wall structure of plants using acoustic cavitation [27], [22]. DES-UAE significantly improved the extraction efficiency of polyphenols from ginger and Moringa oleifera L. leaves, as compared to UAE with organic solvents [22], [23], [29]. To the best of our knowledge, there is no study focusing on extraction of polyphenols from P. scandens using DES-UAE.
The present study screened the best DES to extract polyphenols from P. scandens, then optimized DES-UAE parameters to improve TFC and antioxidant capacity using a two-level factorial experiment, followed by response surface methodology. We also evaluated and compared the TFC, antioxidant capacity, and polyphenol profiles (identified and quantified by UHPLC-MS) of extracts obtained by DES-UAE and UAE coupled with traditional solvents.
2. Materials and methods
2.1. Materials and chemicals
The fresh aerial parts of P. scandens were purchased from the Dongmen Market (Haikou, Hainan Province, China) in August 2020 and identified by Dr. Qiang Liu from the Department of Pharmacognosy, Hainan Medical University (Haikou, China). After washing with tap water, plants were dried at 50 ℃ for 8 h in an electric thermostat blast drying oven (DHG-9140A, Shanghai Yiheng Scientific Instrument Co. Ltd, Shanghai, China), crushed through a 60 mm mesh, packed and stored at − 20℃.
Apigenin, acacetin, diosmetin, hesperidin, rutin and quercitrin for UHPLC-MS were purchased from Qiyun Biotechnology (Guangzhou, China). Folin–Ciocalteu reagent, as well as the remaining polyphenol standards for UHPLC-MS, was purchased from Sigma Chemical Co., Ltd. (Shanghai, China). All chemicals used for DES synthesis were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Total antioxidant capacity assay kits employing ABTS+ scavenging capacity method and FRAP method were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China).
2.2. Preparation of DESs
According to previously reported methods, 16 kinds of DESs were synthesized by mixing the hydrogen-bond acceptor and hydrogen-bond donor at the appropriate molar ratio with continuous stirring at 1500 rpm at 80 ℃, forming a transparent and uniform liquid [24], [22]. Chemicals and their molar ratios, as well as the abbreviations of the DESs, are shown in Table 1.
Table 1.
List of DESs.
| Solvent abbreviation | Combination | Molar ratio |
|---|---|---|
| ChCl-MA | Choline chloride-Malic acid | 1:1 |
| ChCl-Gly | Choline chloride-Glycerol | 1:2 |
| ChCl-OA | Choline chloride-Oxalic acid | 1:1 |
| ChCl-Xyl | Choline chloride-Xylitol | 1:1 |
| Pro-Gly-1 | L-Proline- Glycerol | 2:5 |
| ChCl-Lev | Choline chloride-Levulinic acid | 1:2 |
| ChCl-EG | Choline chloride-Ethylene glycol | 1:2 |
| ChCl-Glu | Choline chloride-Glucose | 5:2 |
| ChCl-TrG | Choline chloride-Triglycol | 1:4 |
| Bet-Lev | Betaine-Levulinic acid | 1:2 |
| Bet-Gly | Betaine-Glycerol | 1:1 |
| Pro-EG | L-Proline-Ethylene glycol | 1:2 |
| Pro-Lev | L-Proline-Levulinic acid | 1:2 |
| Pro-Gly-2 | L-Proline-Glycerol | 1:2 |
| Pro-LA | L-Proline-Lactic acid | 1:2 |
| CA-Gly | Citric acid-Glycerol | 1:2 |
2.3. Determination of TFC
TFC was determined using the AlCl3-NaNO2 colorimetric method at 510 nm as described previously [30]. Briefly, the extract or a standard solution (250 μL) was mixed with deionized water (1.25 mL) and NaNO2 solution (5% [w/w], 75 μL) sequentially. After 6 min, 10% (w/w) AlCl3·6H2O solution (150 μL) was added, then 5 min later NaOH solution (1 M, 0.5 mL) was added followed by adjusting the total volume to 3.0 mL with deionised water. TFC was calculated by comparing the calibration curve of catechin and expressed as mg catechin equivalent per g of dry weight of plant powder (mg CE/g DW).
2.4. Antioxidant capacity
Antioxidant capacity was determined by commercial kits (FRAP and ABTS+ scavenging capacity assays) following the manufacturer’s protocols. For FRAP assay, the extracts or FeSO4 solutions ranging from 0.15 to 1.5 mmol (5 μL) was mixed with 180 μL of FRAP working solution and kept for 5 min at 37℃. The absorbance of the mixed solution was recorded at 593 nm. For ABTS+ scavenging capacity assay, the extracts or Trolox solutions ranging from 0.15 to 1.5 mmol (10 μL) was mixed with 200 μL of ABTS+ working solution and kept for 6 min at 37℃. The absorbance was recorded at 734 nm. Values were calculated by comparing standard curves of FeSO4 and Trolox, and expressed as μmol FeSO4 equivalent per g of dry plant powder (μmol Fe(II)E/g DW) and μmol Trolox equivalent per g of dry weight of plant powder (μmol TE/g DW), respectively.
2.5. Optimization of extraction experiments
2.5.1. Screening of DESs
P. scandens powder (0.5 g) was added in the prepared DESs (5 mL, 40% water, v/v) in a centrifuge tube and then the centrifuge tube was put into a bath-type ultrasonic instrument (SB25-12DTD, Ningbo Xinzhi Biotechnology Co. Ltd) with a temperature-controlled system under an ultrasonic frequency of 40 KHz. The mixture was extracted at the actual ultrasonic power 77.0 W (the set ultrasonic power 320 W) at 40 °C for 30 min and centrifuged for 10 min at 13,000 rpm to obtain the supernatant. TFC of the supernatant was used to evaluate extraction efficiency of different DESs.
A calorimetric assay was used to measure the actual ultrasonic power [31]. Briefly, 15 L water was poured into the bath system. The ultrasonic process lasted for 30 min at the ultrasonic power set at 240 W, 300 W, 320 W or 360 W. The temperature was measured in the centrifuge tube before and after the ultrasonic process using thermometer. The temperature was collected at fifteen sites (four corners and the center at upper layer, middle layer, and bottom layer of water in the centrifuge tube from triplicate experiments. The actual ultrasonic power was calculated according to equation: the actual ultrasonic power (W) = m*Cw*(dT/dt), where m was the mass (kg) of the water submitted to the ultrasonic processing, Cw was the specific heat of the water at constant pressure, and dT/dt was the rate of temperature rise during the process time (°C/s) [31]. The actual ultrasonic power for 240 W, 300 W, 320 W and 360 W was 57.7 W, 72.2 W, 77.0 W, and 86.6 W, respectively. In order to increase the accuracy, the actual ultrasonic power was used.
2.5.2. Two-level factorial experiment
A two-level factorial experiment was performed using Minitab v.17 (Philadelphia, PA, USA) to ascertain the effect of five factors (water content in DES [A], liquid–solid ratio [B], the actual ultrasonic power [C], ultrasonic time [D], and ultrasonic temperature [E]) and their interactive effects on TFC of P. scandens extracts (Table 2, Table 3), where TFC was the response. A normal plot of the standardized effects relied on position of effect points relative to the standard line, while a pareto chart of the standardized effects evaluated the significance of the primary or interactive effects according to the column magnitude in contrast to other columns [29]. The key factors with a remarkable influence on TFC of P. scandens were selected to conduct the response surface methodology.
Table 2.
Factors and levels of two-level factorial experiment.
| Independent variable | Units | Experimental value | |
|---|---|---|---|
| Low (−1) | High (+1) | ||
| A: Water contents in DES | % | 20 | 50 |
| B: Liquid-solid ratio | mL/g | 10 | 40 |
| C: The actual ultrasonic power | W | 57.7 | 86.6 |
| D: Ultrasonic time | min | 10 | 40 |
| E: Ultrasonic temperature | ℃ | 30 | 60 |
Table 3.
Experimental design and results of two-level factorial experiment.
| Run | A: Water contents in DES (%) | B: Liquid-solid ratio (mL/g) | C: The actual ultrasonic power (W) | D: Ultrasonic time (min) | E: Ultrasonic temperature (℃) | TFC (mg CE/g DW) |
|---|---|---|---|---|---|---|
| 1 | 20 | 40 | 57.7 | 10 | 60 | 18.52 ± 1.27 |
| 2 | 50 | 10 | 57.7 | 10 | 60 | 19.21 ± 0.39 |
| 3 | 50 | 40 | 57.7 | 40 | 60 | 19.90 ± 1.73 |
| 4 | 20 | 10 | 57.7 | 40 | 60 | 17.82 ± 1.23 |
| 5 | 20 | 40 | 57.7 | 40 | 30 | 13.99 ± 1.14 |
| 6 | 50 | 10 | 57.7 | 40 | 30 | 19.63 ± 0.75 |
| 7 | 20 | 40 | 86.6 | 40 | 60 | 14.75 ± 0.10 |
| 8 | 50 | 10 | 86.6 | 40 | 60 | 20.09 ± 0.83 |
| 9 | 50 | 40 | 86.6 | 10 | 60 | 24.90 ± 1.08 |
| 10 | 20 | 10 | 86.6 | 10 | 60 | 21.41 ± 0.79 |
| 11 | 20 | 40 | 86.6 | 10 | 30 | 21.17 ± 0.26 |
| 12 | 50 | 10 | 86.6 | 10 | 30 | 21.42 ± 0.94 |
| 13 | 50 | 40 | 57.7 | 10 | 30 | 20.08 ± 1.39 |
| 14 | 20 | 10 | 57.7 | 10 | 30 | 17.69 ± 0.60 |
| 15 | 50 | 40 | 86.6 | 40 | 30 | 19.38 ± 0.52 |
| 16 | 20 | 10 | 86.6 | 40 | 30 | 18.36 ± 0.66 |
2.5.3. Response surface experiment
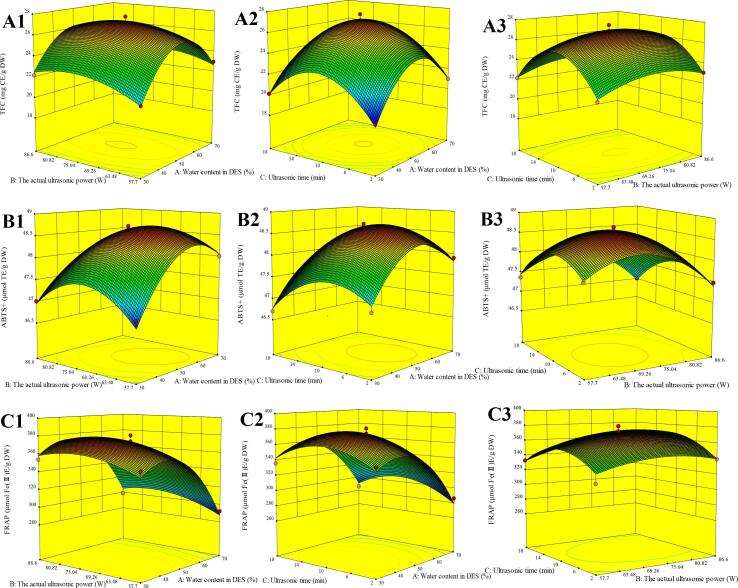
Based on the results of two-level factorial experiment, water content in DES (A, 30%, 50% and 70%), the actual ultrasonic power (B, 57.7, 72.2 and 86.6 W), and ultrasonic time (C, 2, 10 and 18 min) were considered as the main driving factors. The impact of these three independent variables on the three related responses (TFC, FRAP value and ABTS+ value) were evaluated at a constant liquid–solid ratio (20 mL/g) and ultrasonic temperature (40 °C) by performing the Box-Behnken design using Design-Expert v. 8.0.5 (Table 4, Table 5, Fig. 3). The verification experiments were conducted as DES-UAE in 2.6.
Table 4.
Box-Behnken design and resultant responses.
| Run | A: Water contentin DES (%) |
B: The actual ultrasonic power (W) |
C: Ultrasonic time (min) |
TFC (mg CE/g DW) | FRAP (μmol Fe(Ⅱ)E/g DW) | ABTS+ (μmol TE/g DW) | |||
|---|---|---|---|---|---|---|---|---|---|
| Actual value | Predicted value | Actual value | Predicted value | Actual value | Predicted value | ||||
| 1 | 70 | 72.2 | 18 | 19.42 ± 0.19 | 19.66 | 285.94 ± 9.40 | 290.96 | 47.56 ± 0.07 | 47.43 |
| 2 | 50 | 86.6 | 2 | 23.09 ± 0.11 | 23.02 | 339.04 ± 48.41 | 339.56 | 47.48 ± 0.04 | 47.40 |
| 3 | 50 | 57.7 | 18 | 22.16 ± 0.12 | 22.23 | 332.68 ± 4.96 | 332.16 | 47.36 ± 0.11 | 47.45 |
| 4 | 50 | 86.6 | 18 | 23.01 ± 0.12 | 22.77 | 365.85 ± 4.48 | 354.41 | 47.00 ± 0.02 | 46.98 |
| 5 | 70 | 57.7 | 10 | 22.97 ± 0.12 | 22.67 | 292.08 ± 6.66 | 287.58 | 48.00 ± 0.01 | 48.05 |
| 6 | 30 | 86.6 | 10 | 22.18 ± 0.21 | 22.48 | 353.87 ± 9.99 | 358.37 | 47.00 ± 0.11 | 46.95 |
| 7 | 50 | 57.7 | 2 | 22.08 ± 0.16 | 22.32 | 319.90 ± 3.35 | 331.34 | 47.83 ± 0.02 | 47.86 |
| 8 | 70 | 72.2 | 2 | 20.98 ± 0.33 | 21.04 | 286.06 ± 12.76 | 279.12 | 47.90 ± 0.10 | 47.83 |
| 9 | 50 | 72.2 | 10 | 27.59 ± 0.08 | 27.06 | 380.07 ± 18.24 | 372.33 | 48.60 ± 0.10 | 48.65 |
| 10 | 30 | 72.2 | 18 | 20.13 ± 0.04 | 20.06 | 335.58 ± 13.34 | 342.51 | 46.70 ± 0.16 | 46.77 |
| 11 | 30 | 57.7 | 10 | 21.11 ± 0.10 | 21.11 | 351.88 ± 21.03 | 345.46 | 46.97 ± 0.03 | 46.81 |
| 12 | 50 | 72.2 | 10 | 27.01 ± 0.08 | 27.06 | 363.64 ± 18.24 | 372.33 | 48.71 ± 0.10 | 48.65 |
| 13 | 30 | 72.2 | 2 | 19.26 ± 0.16 | 19.02 | 343.70 ± 34.56 | 338.68 | 47.08 ± 0.05 | 47.21 |
| 14 | 50 | 72.2 | 10 | 26.56 ± 0.08 | 27.06 | 373.29 ± 18.24 | 372.33 | 48.63 ± 0.10 | 48.65 |
| 15 | 70 | 86.6 | 10 | 22.53 ± 0.12 | 22.53 | 298.72 ± 10.26 | 305.14 | 46.82 ± 0.04 | 46.98 |
Table 5.
ANOVA for response surface quadratic model.
| Source | df | Sum of squares | ||
|---|---|---|---|---|
| TFC | FRAP | ABTS+ | ||
| Model | 9 | 92.49** | 13527.79 ** | 6.35** |
| A- Water content | 1 | 1.30* | 6172.99** | 0.81** |
| B- The actual ultrasonic power | 1 | 0.77 ns | 464.15 ns | 0.43** |
| C- Ultrasonic time | 1 | 0.06 ns | 122.84 ns | 0.34* |
| AB | 1 | 0.57 ns | 5.42 ns | 0.37* |
| AC | 1 | 1.47* | 16.03 ns | 0.00 ns |
| BC | 1 | 0.01 ns | 49.20 ns | 0.00 ns |
| A2 | 1 | 51.89*** | 5157.44* | 2.24** |
| B2 | 1 | 4.56* | 432.56 ns | 1.65** |
| C2 | 1 | 41.70*** | 1809.79* | 1.15** |
| Residual | 5 | 0.96 | 667.80 | 0.13 |
| R2 | 0.9897 | 0.9530 | 0.9807 | |
| Adj R2 | – | 0.97 | 0.8683 | 0.9459 |
| Pre R2 | – | 0.91 | 0.3793 | 0.7056 |
| CV | – | 1.93 | 3.45 | 0.33 |
| Model (F-value) | – | 53.42 | 11.25 | 28.21 |
| Model (p-value) | – | 0.0002 | 0.0079 | 0.0009 |
| Lack of fit (F-value) | – | 0.54 | 2.60 | 11.44 |
| Lack of fit (p-value) | – | 0.7007 | 0.2898 | 0.0815 |
ns, not significant (p > 0.1);
*, difference is significant at 0.05 level (p < 0.05);
**, difference is significant at 0.01 level (p < 0.01);
***, difference is significant at 0.001 level (p < 0.001).
Fig. 3.
3D response surface curve showing the effects of independent variables on the TFC (A1-A3), ABTS+ (B1-B3) and FRAP (C1-C3). Mutual effects of water content in DES and the actual ultrasonic power on TFC (A1); Mutual effects of water content in DES and ultrasonic time on TFC (A2); Mutual effects of the actual ultrasonic power and ultrasonic time on TFC (A3); Mutual effects of water content in DES and the actual ultrasonic power on ABTS+ (B1); Mutual effects of water content in DES and ultrasonic time on ABTS+ (B2); Mutual effects of the actual ultrasonic power and ultrasonic time on ABTS+ (B3); Mutual effects of water content in DES and the actual ultrasonic power on FRAP (C1); Mutual effects of water content in DES and ultrasonic time on FRAP (C2); Mutual effects of the actual ultrasonic power and ultrasonic time on FRAP (C3).
2.6. Comparisons among DES-UAE and UAE coupled with traditional solvents
After mixing 0.5 g P. scandens powder with 10 mL of the above selected DES (choline chloride-ethylene glycol [ChCl-EG] with 49.2% of water content in it) (DES-UAE), ultrapure water (W-UAE), 80% ethanol (EtOH-UAE) or 70% methanol (MetOH-UAE), ultrasonic-assisted extraction was performed under the actual ultrasonic power 72.2 W (the set ultrasonic power 300 W) at 40 °C for 9.7 min. The supernatant was obtained by centrifugation at 13,000 rpm for 10 min. The traditional solvents used were chosen based on previous studies [27], [32], [33], [34], [35], which were efficient in polyphenolic extraction.
2.7. Identification and quantification of polyphenols by UHPLC-MS
UHPLC coupled with a Xevo triple quadrupole mass spectrometer system (Micromass Waters, Milford, MA, USA) was used for identification and quantification of polyphenols as per our previous methods, with some modifications [36]. An Acquity UHPLC BEH-C18 column (2.1 i.d. × 100 mm, 1.7 μm, Waters, USA) was used to separate polyphenols, with a gradient solvent system of 0.25% formic acid–water (elution A) and 0.25% formic acid–methanol (elution B) using the following parameters: 0–1 min, 5% B; 8 min, 25% B; 11 min, 60% B; 13 min, 100% B; 16 min, 100% B; and 16.1–20 min, 5% B. Polyphenols were identified using multiple reaction monitoring, and the basic structure of peaks were deduced by parent ions, fragment ions, and comparisons with published mass spectra (MS) data [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51]. Furthermore, polyphenols were assigned by comparing the respective standards using UHPLC-MS, and their quantification was achieved by comparing the calibration curve of respective standards expressed as μg per g of dry weight of plant powder (μg/g DW). MS was measured between m/z 50–1000 with the following constant parameters: cone voltage, 30 V; capillary voltage, 2.0 kV; drying gas (N2) flow, 1000 L/h; and drying gas temperature, 500 °C.
2.8. Statistical analysis
All data derived from experiments performed in triplicate were expressed as mean ± standard deviation. Statistical analysis was performed with IBM SPSS 24.0 using one-way ANOVA, followed by Duncan’s post hoc test, and p-value of < 0.05, was regarded as statistically significant.
3. Results and discussion
3.1. Selection of DES for the extraction of polyphenols from P. Scandens
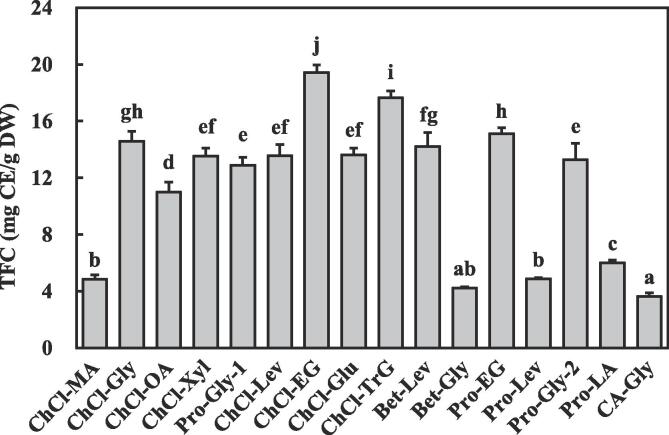
DESs are effective for cell wall dissolution due to their high hydrogen-bond basicity, allowing efficient intermolecular interactions between the DES and cellulose strands [24], [25]. Extraction efficiency is primarily related to DES polarity and viscosity, which are largely dependent on their constituents, the molar ratios of hydrogen-bond acceptors to hydrogen-bond donors and water content in DES [24], [27]. DESs are commonly based on sugars, alcohols, amines, amino acids, and organic acids [26]. Several studies have shown that the compositions of DES used to efficiently extract polyphenolic compounds are based on choline chloride as the hydrogen-bond acceptors and polyalcohols, organic acids, sugar, or amides as the hydrogen-bond donors [26], [27]. Therefore, in the present study, four types of hydrogen-bond acceptors (choline chloride, citric acid, betaine, and L-proline) and four types of hydrogen-bond donors (amide, acid, alcohol and glucose) were selected to prepare DESs. There was a significant difference among the TFC of P. scandens extracts obtained by the 16 DESs coupled with UAE (p < 0.05) (Fig. 1). The highest TFC of P. scandens extracts was obtained by ChCl-EG coupled with UAE (19.43 ± 0.53 mg CE/g DW), followed by ChCl-TrG coupled with UAE (17.65 ± 0.48 mg CE/g DW). Notably, CA-Gly and Bet-Gly coupled with UAE afforded the lowest TFC, which were 18.69% and 24.98% of the TFC obtained by ChCl-EG coupled with UAE. Different types of DESs were used to extract polyphenolic compounds and showed significantly different extraction efficiency [52]. Khezeli et al. [53] found that ChCl-EG (1:2) showed higher efficiency than ChCl-Gly (1:2), pure ethylene glycol and glycerol solvents in the extraction of ferulic, caffeic and cinnamic acids from seed oils. Cui et al. [54] found that 1,6-hexanediol-ChCl showed the highest efficiency in extracting genistin, genistein and apigenin from pigeon pea roots among 18 kinds of DESs. García et al. [20] found that there was an obvious difference in polyphenolic compounds extracted by different DESs from virgin olive oil. DES polarity and its approximation to the polarity of extracts are very critical for the extraction efficiency, due to the principle of “compounds are more likely to dissolve in solvents with similar polarity”[24], [27]. High viscosity of DESs results in low mass transfer and low compound diffusion [26]. For these reasons, in the present study, the highest TFC obtained by ChCl-EG coupled with UAE may be attributed to the high similarity between the polarities of ChCl-EG and polyphenols from P. scandens, along with suitable viscosity. Therefore, ChCl-EG was selected as the best green solvent for subsequent experiments.
Fig. 1.
Total flavonoid content (TFC) of P. scandens extracts obtained by 16 DESs coupled with UAE. Different letters indicate significant differences (p < 0.05). ChCl-MA, Choline chloride-Malic acid; ChCl-Gly; Choline chloride-Glycerol; ChCl-OA, Choline chloride-Oxalic acid; ChCl-Xyl, Choline chloride-Xylitol; Pro-Gly-1, L-Proline-Glycerol (2:5); ChCl-Lev, Choline chloride-Levulinic acid; ChCl-EG, Choline chloride-Ethylene glycol; ChCl-Glu, Choline chloride-Glucose; ChCl-TrG, Choline chloride-Triglycol; Bet-Lev, Betaine-Levulinic acid; Bet-Gly, Betaine-Glycerol; Pro-EG, L-Proline-Ethylene glycol; Pro-Lev, L-Proline-Levulinic acid; Pro-Gly-2, L-Proline-Glycerol (1:2); Pro-LA, L-Proline-Lactic acid; CA-Gly, Citric acid-Glycerol.
3.2. A two-level factorial experiment for selection of factors significantly affecting TFC
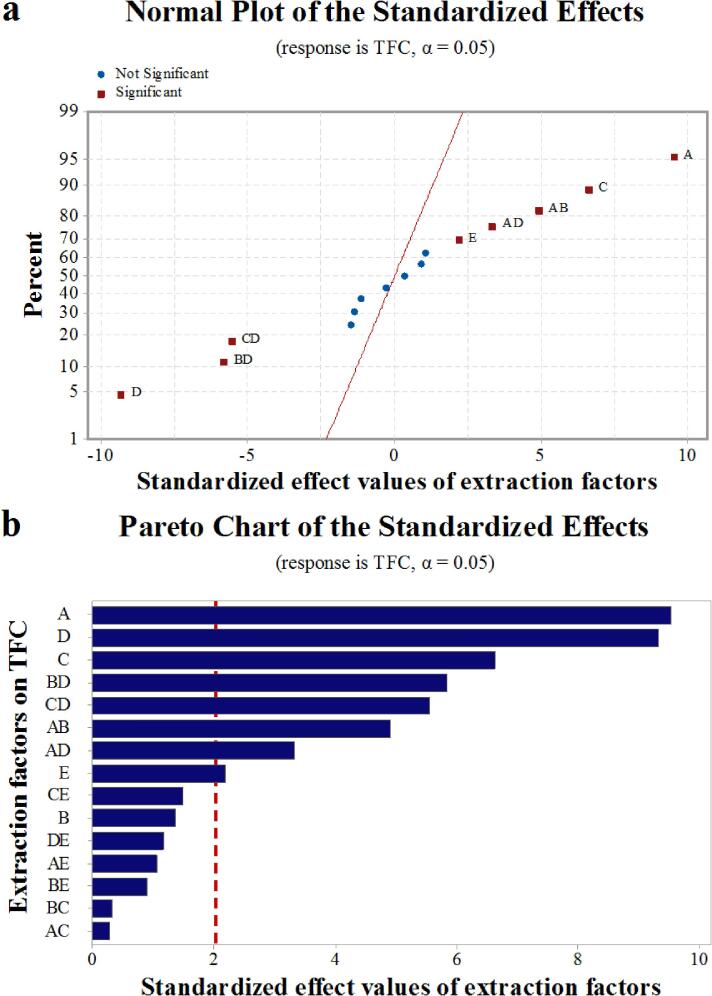
Water content in DES plays an important role in enhancing the extraction of bioactive substances from plants by altering DES polarity and lowing the viscosity [26], [27]. Moreover, liquid–solid ratio, ultrasonic time, the actual ultrasonic power, and ultrasonic temperature can largely influence extraction efficiency [22], [29]. In this study, the experimental model provided by a two-level factorial experiment (Table 2, Table 3) was used to generate the normal plot and pareto chart (Fig. 2) for highlighting the primary and interaction effects on TFC.
Fig. 2.
The normal plot (a) and the pareto chart (b) obtained from two-level factorial experiment showing the significance of the primary and interaction effects. Factor A, water content in DES; Factor B, liquid–solid ratio; Factor C, the actual ultrasonic power; Factor D, ultrasonic time; Factor E, ultrasonic temperature.
As shown in Fig. 2a, significant effects presented as single factors (water content in DES [A], the actual ultrasonic power [C], ultrasonic time [D], and ultrasonic temperature [E]), and interactive factors (AB, AD, CD, and BD). Moreover, the magnitudes of A, C, D, E, AB, AD, CD, and BD were all larger than the significant marker line, among which the top three significant factors were A, D and C in sequence (Fig. 2b). Consequently, water content in DES, the actual ultrasonic power, and ultrasonic time were chosen for the subsequent response surface experiment.
3.3. Response surface experiment
3.3.1. Model fitting
As shown in Table 4, TFC, FRAP values, and ABTS+ values ranged from 19.26 − 27.59 mg CE/g DW, 285.9–380.1 μmol Fe(II)E/g DW, and 46.7–48.7 μmol TE/g DW, respectively. As shown in Table 5, F-value and p-value revealed that the model was significant (p < 0.01), whereas the loss of fit of each model was insignificant (p > 0.05). For all responses, the R2 was higher than 0.95 and adjusted determination coefficients (adj. R2) were close to 0.9, revealing good model accuracy and a higher correlation between experimental and predicted values, which was confirmed by the low coefficient of variation (CV). Interaction among variables was presented using three-dimensional (3D) surface plots in Fig. 3.
3.3.2. Effect of variables on TFC
As shown in Table 5, the linear effect of water content in DES (A) and secondary effect of A2, B2 and C2 illustrated significant effects on TFC. The significant interaction between water content in DES (A) and ultrasonic time (C) on TFC was presented. The addition of water in DES varied DES polarity and viscosity [52], resulting in a change in extraction efficiency. Numerous studies found water content in DES was the main factor for DESs’ high extraction efficiency for various bioactive compounds including polyphenols [22], [23], [26], [27], [29], [52]. The relationship between TFC and these variables was expressed by the following equation (1):
YTFC = 27.06 + (0.40 × A) + (0.31 × B) – (0.087 × C) – (0.38 × AB) – (0.61 × AC) – (0.04 × BC) – (3.75 × A2) – (1.11 × B2) – (3.36 × C2) (1).
To clarify the interaction of the three variables on the TFC, 3D response surfaces (Fig. 3A1–A3) were applied. Fig. 3B showed the significant interaction between water content in DES (A) and ultrasonic time (C) on ABTS+, which raised with increasing water content in DES (A) up to approximately 49%, followed by a sharp reduction. DES polarity raised with increasing water content in DES. Extraction solvents with polarity close to polyphenolic compounds showed high extractability [24], [27], [52]. Moreover, an increase in water content in DES reduced the viscosity of DESs, increasing mass transfer and compound diffusion [25], [26]. Furthermore, ultrasonic treatment increased the extraction rate by disrupting the cell wall and facilitating solvent penetration through the plant tissue [24], [27]. However, ultrasonic treatment for longer time may result in polyphenol degradation [27], [28]. These results indicated high extraction efficiency of ChCl-EG coupled with UAE for polyphenols was mainly attributed to the similar polarity of ChCl-EG with polyphenols, the low viscosity of ChCl-EG that facilitated compound diffusion, and the suitable ultrasonic parameters that facilitated the solvent penetration.
3.3.3. Effect of variables on antioxidant capacity
FRAP value was significantly affected by A (p < 0.01), A2 (p < 0.05) and C2 (p < 0.05), among which water content in DES (A) showed a more significant effect on FRAP value. ABTS+ value was significantly affected by A, B, A2, B2, and C2 (p < 0.01), followed by C and AB (p < 0.05). However, the interaction of AC and BC had no significant effect on ABTS+ value (p > 0.05). The antioxidant capacity (FRAP and ABTS+) of P. scandens extracts was largely affected by water content in DES, which markedly changed DES polarity. DES polarity was primarily responsible for the composition and content of polyphenols in the extracts [20], [21], [22], [23]. Polyphenols are a large class of plant secondary metabolites with diverse structure and varying polarity [1]. Antioxidant capacity of extracts was closely associated with composition and content of polyphenols in the extracts [30], [22], [29].
Antioxidant capacity (FRAP and ABTS+) models were demonstrated by equations (2) and (3), and the 3D surface plots (Fig. 3B1-B3, C1-C3) were established according to equations (2) and (3).
YFRAP = 372.33 – (27.78 × A) + (7.62 × B) + (3.92 × C) + (1.16 × AB) + (2.00 × AC) + (3.51 × BC) – (37.37 × A2) – (10.82 × B2) – (22.24 × C2) (2).
YABTS+ = 48.65 + (0.32 × A) – (0.23 × B) – (0.21 × C) – (0.30 × AB) + (9.65 × 10−3 × AC) – (3.5 × 10−3 × BC) – (0.78 × A2) − (0.67 × B2) – (0.56 × C2) (3).
3.3.4. Verification of optimally predicted DES-UAE parameters
To substantiate the reliability of the response surface model design, experiments under the optimal parameters of DES-UAE predicted by the model were performed. Based on regression analysis of 3D surface plots and independent variables, the optimal extraction parameters for TFC were listed as follows: 49.2% of water content in DES, 72.4 W of the actual ultrasonic power (the set ultrasonic power 301.3 W), and 9.7 min of ultrasonic time. In order to adjust ultrasonic power easily, the verification experiments were performed under 72.2 W of the actual ultrasonic power (the set ultrasonic power 300 W), 49.2% of water content in DES, and 9.7 min of ultrasonic time. The experimental values determined were 27.09 ± 0.48 mg CE/g DW for TFC, 335.17 ± 4.32 μmol Fe(II)E/g DW for FRAP, and 48.91 ± 1.99 μmol TE/g DW for ABTS+, which accurately matched our predicted values with a low error (Table 6).
Table 6.
Comparative TFC and antioxidant capacities of P. scandens extracts obtained by DES-UAE and other methods.
| Extraction methods | TFC (mg CE/g DW) |
FRAP(μmol Fe(Ⅱ) E/g DW) |
ABTS+ (μmol TE/g DW) |
|---|---|---|---|
| DES-UAE (Predicted value) | 27.04 | 373.27 | 48.64 |
| DES-UAE (Experimental value) | 27.09 ± 0.48c | 335.17 ± 14.32c | 48.91 ± 1.99c |
| W-UAE | 17.95 ± 0.49b | 222.76 ± 11.99b | 46.30 ± 3.01bc |
| EtOH-UAE | 17.88 ± 0.41b | 186.89 ± 11.41a | 44.25 ± 0.16ab |
| MetOH-UAE | 13.54 ± 0.08a | 195.49 ± 2.42a | 43.83 ± 0.45ab |
Different letters in same column indicate significant differences (p < 0.05). UAE, ultrasonic-assisted extraction; DES-UAE, W-UAE, EtOH-UAE, MetOH-UAE: ultrasonic-assisted extraction coupled with deep eutectic solvent (DES-UAE), ultrapure water, 80% ethanol and 70% methanol, respectively.
3.4. Comparisons among DES-UAE and other methods
3.4.1. TFC and antioxidant capacity
A comparative study was conducted among DES-UAE and UAE coupled with traditional solvents to verify the high extractability of DES-UAE for polyphenols from P. scandens. As shown in Table 6, DES-UAE displayed significantly higher TFC and FRAP value than other methods (p < 0.05). The TFC and FRAP value obtained by DES-UAE were 1.51–2.02-fold and 1.37–1.79-fold of those obtained by other methods, respectively. Moreover, there was no significant difference between ABTS+ value obtained by DES-UAE and W-UAE (p > 0.05), whereas DES-UAE displayed significantly higher ABTS+ value than other methods (p < 0.05). These results indicated that DES-UAE was a high efficiency method compared with UAE coupled with traditional solvents (ultrapure water, 80% ethanol, and 70% methanol), which were usually used in the previous studies and showed a high efficiency for extracting polyphenols [27], [32], [33], [34], [35], [30], [36]. DES-UAE reportedly improved TFC and antioxidant capacity of Moringa oleifera L. leave extracts, compared with UAE coupled with traditional solvents [29], [22]. DES-UAE was a novel and high efficiency method for extracting polyphenols from P. scandens.
3.4.2. Identification of polyphenolic compounds by UHPLC-MS
Thirty-six polyphenols comprising of 5 benzoic acids, 5 hydroxycinnamic acids, 15 flavonols, 4 flavones, 1 isoflavone, 3 flavanones, 1 anthocyanidin, 1 procyanidin, and 1 stilbene were identified in P. scandens extracts, all of which were further confirmed with reference standards using UHPLC-MS. Besides these, many compounds still remain unknown (Table 7).
Table 7.
Identification of polyphenolic compositions in P. scandens by UHPLC-MS.
| Polyphenol sub-classes | Peak no. | λmax (nm) | Tentative assignment | Model | Parents ions |
Fragment ions | Reference |
|---|---|---|---|---|---|---|---|
| Benzoic acid and drivatives |
1 | 259,291 | vanillic acid | + | 169.0 | 125.0, 93.0 | [37] |
| 2 | 275 | vanillin | + | 153.0 | 138.0, 125.0, 93.0 | [40] | |
| 3 | 260,294 | 2-hydroxybenzoic acid | – | 137.0 | 93.0 | [38] | |
| 4 | 274,308 | protocatechualdehyde | – | 136.96 | 108.0, 81.0 | [39] | |
| 5 | 272 | ethyl gallate | – | 196.9 | 168.9, 124.1 | [37] | |
| Hydroxycinnamic acid and derivatives | 6 | 270,307 | p-coumaric acid | – | 163.1 | 119.0, 91.0 | [41], [37] |
| 7 | 299,323 | ferulic acid | – | 193.0 | 178.0, 149.0, 134.0 | [42], [41], [37] | |
| 8 | 299,323 | caffeic acid | – | 179.0 | 135.0, 79.0 | [42], [41], [37] | |
| 9 | 278,306 | trans-cinnamic acid | – | 146.95 | 118.9, 77.0, 40.1 | [38] | |
| 10 | 290,328 | rosmarinic acid | – | 358.96 | 196.96, 161.0 | [43] | |
| Flavonol | 11 | 280 | catechin | – | 289.07 | 244.9, 204.9, 137.1 | [38], [44] |
| 12 | 280 | epicatechin | – | 289.07 | 244.9, 204.9, 137.1 | [44] | |
| 13 | 270 | (-)-epigallocatechin | – | 304.98 | 179.0, 124.98 | [44] | |
| 14 | 270 | (-)-gallocatechin | – | 304.98 | 179.0, 124.98 | [44] | |
| 15 | 254,352 | myricitrin | – | 462.9 | 316.99 | [45] | |
| 16 | 255,347 | quercetin | – | 301.0 | 179.0, 151.0 | [43], [42], [38] | |
| 17 | 250,354 | hyperoside | – | 463.0 | 300.9, 270.9, 254.9 | [38] | |
| 18 | 255,355 | rutin | – | 609.0 | 301.1, 270.9, 178.7 | [41] | |
| 19 | 257,356 | quercitrin | – | 447.0 | 301.0, 179.0, 151.0 | [42], [41] | |
| 20 | 257,356 | isoquercitrin | – | 447.0 | 301.0, 179.0, 151.0 | [43], [41] | |
| 21 | 256,354 | guaiaverin | – | 432.9 | 270.9, 300.9 | [46] | |
| 22 | 266,348 | kaempferol 3-O-rutinoside | – | 592.9 | 254.9, 284.8 | [46] | |
| 23 | 264,346 | astragaline | – | 446.9 | 226.98, 254.9, 285.1 | [46] | |
| 24 | 254,343 | isorhamnetin-3-O- glucoside |
– | 476.9 | 313.96, 242.8 | [46] | |
| 25 | 254,354 | narcissin | + | 624.9 | 85.1, 316.9, 478.9 | [46] | |
| Flavone | 26 | 268,338 | apigenin | + | 271.0 | 227.0, 151.0 | [42] |
| 27 | 270,335 | acacetin | + | 285.0 | 153.0 | [49] | |
| 28 | 267,345 | diosmetin | + | 301.0 | 153.0, 111.0, 255.0, 257.0 |
[50] | |
| 29 | 252,348 | cynaroside | – | 446.9 | 107.0, 133.0, 284.8 | [46] | |
| Isoflavone | 30 | 260,327 | genistein | – | 268.9 | 108.7, 132.9, 159.0 | [41] |
| Flavanone |
31 | 283,327 | hesperidin | – | 609.0 | 301.0 | [41] |
| 32 | 270,350 | isovitexin | – | 430.9 | 280.9, 253.0 | [48] | |
| 33 | 270,334 | cosemetin | – | 430.9 | 268.9 | [47] | |
| Anthocyanidin | 34 | 276,530 | cyanidin | + | 286.9 | 109.1, 137.0 | [51] |
| Procyanidin | 35 | 280 | procyanidin B2 | – | 576.9 | 288.99, 406.9 | [46] |
| Stilbene | 36 | 282,308 | trans-piceid | + | 391.1 | 229.1, 135.0 | [37] |
| 37 | – | unknown | – | 469.3 | 417.3, 371.2, 315.3, 217.1 |
||
| 38 | – | unknown | – | 747.4 | 471.2, 419.3, 373.4 | ||
| 39 | – | unknown | – | 952.9 | 872.3, 619.1, 277.4 | ||
| 40 | – | unknown | – | 944.9 | 625.1, 485.3, 355.1, 299.4, 281.0 |
Peaks 1, 3, 4, and 5 at m/z 169.0, 137.0, 136.96, and 196.9 with their product ions 125.0 [M + H − CO2]+, 93.0 [M − H − CO2]−, 108.0 [M − H − CHO]−, and 168.9 [gallic acid − H]− were tentatively identified as vanillic acid, 2-hydroxybenzoic acid, protocatechualdehyde and ethyl gallate, respectively [37], [38], [39]. Vanillin (peak 2) was identified by its protonated ion [M + H]+ m/z 153.0, and product ions m/z 138.0 [M + H − CH3]+, m/z 125.0 [M + H − CO]+, and m/z 93.0 [M + H − CO − CH3OH]+, which was consistent with MS data from Flamini et al. [40]. Peaks 6–10, identified as p-coumaric acid, ferulic acid, caffeic acid, trans-cinnamic acid, and rosmarinic acid, showed precursor ions [M − H]− at m/z 163.1, 193.0, 179.0, 146.95, and 358.96 and their product ions at m/z 119.0 [M − H − CO2]−, 91.0 [M − H − CO2 − C2H4]− (peak 6), 149.0 [M − H − CO2]−, 178.0 [M − H − CH3]− (peak 7), 135.0 [M − H − CO2]− (peak 8), 118.9 [M − H − CO]− (peak 9), and 196.96 [dihydroxyphenyl-lactic acid − H]−, 161.0 [caffeic acid − H − H2O]− (peak 10), respectively [37], [41], [42], [38], [43]. Peaks 11 and 12 were observed as the same precursor ion [M − H]− at m/z 289.07, and the same product ions m/z 244.9 [M − H − CO2]− and 137.1 [M − H − 152]− (typical retro Diel-Alder fragmentation) with different retention time, and were distinguished as catechin and epicatechin [38], [44]. Peaks 13–14 produced the same precursor ion [M − H]− at m/z 304.98 and product ions at m/z 179.0 [M − H − C6H6O3]− and 124.98 [M − H − 152]− with different retention times, and were identified as (-)-epigallocatechin and (-)-gallocatechin [44]. Gallic, chlorogenic, vanillic, ferulic, p-coumaric, and caffeic acids have previously been identified by HPLC-DAD from the ethanolic extract of P. scandens by Bordoloi et al. [8], whereas 3-hydroxy-4-methoxybenzaldehyde, 2-hydroxybenzoic acid, 2,5-dihydroxybenzoic acid methyl ester, as well as 12 iridoid glycosides, were isolated and identified from 70% ethanol extract of P. scandens by MS [16].
Quercetin (peak 16) with deprotonated ions [M − H]– at m/z 301.0 and retro Diel-Alder fragments of flavon-3-ols (m/z 179.0, 151.0) were identified as quercetin, which was consistent with MS data from previous studies [38], [42], [43]. Flavonoid glycosides containing O-glycoside usually showed a loss of glycoside as the initial breakdown [55]. Myricitrin (peak 15), was authenticated by deprotonated molecule [M − H]– ion at m/z 462.9 and product ion m/z 316.99 (aglycone myricetin) for the loss of rhamnoside (−146 Th) [45]. Peaks 17–25 were respectively identified as hyperoside, rutin, quercitrin, isoquercitrin, guaiaverin, kaempferol 3-O-rutinoside, astragaline, isorhamnetin-3-O-glucoside, and narcissin by the loss of galactoside (−162 Th), rutinoside (−308 Th), rhamnoside (−146 Th), glucoside (−162 Th), or arabinoside (−132 Th) group [38], [41], [42], [43], [46]. Similarly, cynaroside (peak 29), hesperidin (peak 31), and cosemetin (peak 33) were distinguished by the loss of glycoside [46], [41], [47]. Quercetin and kaempferol were found in the fruits of Paederia chinensis Hance [18]. A previous study identified 13 flavonol-O-glycosides, including astragaline (kaempferol-3-O-glucoside), kaempferol-3-O-rutinoside, kaempferol-7-O-glucoside, isoquercitrin (quercetin-3-O-glucoside), kaempferol-3-O-rutinoside-7-O-glucoside, quercimeritrin (quercetin-7-O-glucoside), rutin (quercetin-3-O-rutinoside), quercetin-3-O-rutinoside-7-O-glucoside, paederinin (quercetin-3-O-rutinoside-7-O- xylosylglucoside), three unknown quercetin-O-glycosides and one unknown kaempferol-O-glycoside using 1H and 13C NMR from P. scandens var. Mairei. leaves and stems [17]. Flavonol-3-O-glycoside may be the characteristic compounds of Rubiaceae, including Paederia plants, as the 3-O-glycoside of quercetin and kaempferol found in the present study have also previously been reported in many plants of Rubiaceae [7], [56], including P. scandens. However, rutin and quercetin were not found in the ethanol maceration extract of P. scandens from India determined by HPLC-DAD [8].
Isovitexin (apigenin-6-C-glucoside) (peak 32) was characterized by the deprotonated ion [M − H]− at m/z 430.9 and product ions at m/z 280.9 corresponding to the fragment [M − H − 150]− and m/z 253.0 [M − H − 150 − CO]− as reported by Pereira et al. [48], which has a different fragmentation pathway with its isomer apigenin-7-O-glucoside (cosemetin). Apigenin (peak 26) was characterized by the protonated ion [M + H]+ at m/z 271.0 and product ions m/z 227.0 and 151.0 [42]. Acacetin (peak 27) and diosmetin (peak 28) were respectively observed as protonated ions [M + H]+ at m/z 285.0 and 301.0, with the same product ion at m/z 153.0 corresponding to the retro Diel-Alder cleavage of C-ring [49], [50]. Genistein (peak 30) was characterized by the parent ion [M − H]− at m/z 268.9 and product ion at m/z 132.9, consistent with MS data from Mattonai et al. [41]. Cyanidin (peak 34), a typical anthocyanidin, was identified by the deprotonated ion [M − H]− at m/z 286.9 and product ions corresponding to fragments [M − H − 150]− and [M − H − 150 − CO]− [51]. Cai et al. [19] found that leaves and stems of P. scandens were rich in anthocyanin. Procyanidin B2 (peak 35) was identified by the deprotonated ion [M − H]− at m/z 576.9 and product ion at m/z 288.99 corresponding to loss of the (epi)catechin entity [46]. Trans-piceid (peak 36) was identified by its loss of glucoside to form resveratrol and further assigned by comparison with retention time of trans-piceid standard [37].
3.4.3. Quantification of individual polypenolic compounds by UHPLC-MS
As shown in Table 8, the number and contents of individual polyphenolic compounds obtained by DES-UAE and other methods varied considerably. There were 30 individual polyphenolic compounds in the extracts obtained by DES-UAE and MetOH-UAE, followed by EtOH-UAE (29 compounds) and W-UAE (25 compounds).
Table 8.
Quantitation of individual polyphenolic compounds in P. scandens by UHPLC-MS.
| Compounds | Content (μg/g DW) | |||
|---|---|---|---|---|
| DES-UAE | W-UAE | EtOH-UAE | MetOH-UAE | |
| vanillic acid | 1.64 ± 0.11a | 2.01 ± 0.13b | ND | 1.80 ± 0.10ab |
| vanillin | 0.36 ± 0.02a | 0.42 ± 0.02b | 0.40 ± 0.02ab | 0.42 ± 0.01b |
| 2-hydroxybenzoic acid | 42.20 ± 1.58b | 44.26 ± 1.26b | 38.88 ± 0.79a | 39.58 ± 0.26a |
| protocatechualdehyde | 1.92 ± 0.18b | 2.98 ± 0.12c | 1.42 ± 0.05a | 2.10 ± 0.08b |
| ethyl gallate | ND | ND | 0.12 ± 0.01 | ND |
| p-coumaric acid | 4.78 ± 0.19a | 9.80 ± 0.23c | 5.46 ± 0.26b | 5.82 ± 0.22b |
| ferulic acid | 5.28 ± 0.28b | 0.86 ± 0.04a | 4.76 ± 0.37b | 7.40 ± 0.25c |
| caffeic acid | 2.56 ± 0.20b | 5.64 ± 0.21c | 1.70 ± 0.10a | 1.56 ± 0.08a |
| trans-cinnamic acid | 0.70 ± 0.04ab | 0.62 ± 0.04a | 0.72 ± 0.02b | 0.64 ± 0.02a |
| rosmarinic acid | 0.40 ± 0.02a | ND | 0.40 ± 0.02a | 0.40 ± 0.02a |
| Number of phenolic acids | 9 | 8 | 9 | 9 |
| Sum of individual phenolic acid content | 59.84 ± 1.50b | 66.58 ± 1.45c | 53.86 ± 1.25a | 59.72 ± 0.83b |
| catechin | 0.16 ± 0.01b | ND | 0.10 ± 0.01a | 0.16 ± 0.01b |
| epicatechin | 0.38 ± 0.01a | ND | 0.48 ± 0.02b | 0.50 ± 0.02b |
| (-)-epigallocatechin | ND | ND | ND | 0.36 ± 0.02 |
| (-)-gallocatechin | 0.10 ± 0.003c | 0.06 ± 0.002a | 0.08 ± 0.002b | 0.06 ± 0.002a |
| myricitrin | 0.70 ± 0.03a | ND | 0.88 ± 0.04b | 0.86 ± 0.04b |
| quercetin | 0.88 ± 0.03b | 0.06 ± 0.003a | 1.88 ± 0.16d | 1.04 ± 0.06c |
| hyperoside | 12.98 ± 0.55b | 10.96 ± 0.46a | 13.42 ± 0.52b | 13.38 ± 0.39b |
| rutin | 582.76 ± 13.69c | 472.66 ± 9.89b | 438.08 ± 11.23a | 427.68 ± 14.68a |
| quercitrin | 9.34 ± 0.34b | 3.62 ± 0.16a | 10.54 ± 0.42c | 10.22 ± 0.41c |
| isoquercitrin | 10.84 ± 0.35b | 8.90 ± 0.35a | 10.90 ± 0.41b | 11.04 ± 0.46b |
| guaiaverin | 56.48 ± 3.02b | 9.32 ± 0.41a | 54.62 ± 1.56b | 55.5 ± 2.59b |
| kaempferol 3-O-rutinoside | 108.00 ± 6.25a | 109.96 ± 5.65a | 101.82 ± 3.65a | 100.08 ± 5.26a |
| astragaline | 2.64 ± 0.12a | 2.48 ± 0.13a | 2.66 ± 0.15a | 2.58 ± 0.10a |
| isorhamnetin-3-O-glucoside | 0.22 ± 0.01b | 0.68 ± 0.03c | ND | 0.16 ± 0.01a |
| narcissin | 53.36 ± 1.58b | 49.46 ± 1.99a | 47.88 ± 1.56a | 49.34 ± 1.85a |
| apigenin | 0.34 ± 0.02c | 5.84 ± 0.20d | 0.26 ± 0.01b | 0.18 ± 0.01a |
| acacetin | 0.04 ± 0.002 | ND | ND | ND |
| diosmetin | 4.40 ± 0.14b | 41.00 ± 1.36c | 4.40 ± 0.16b | 3.34 ± 0.12a |
| cynaroside | 0.18 ± 0.01a | ND | 0.34 ± 0.02b | 0.72 ± 0.02c |
| genistein | ND | 0.24 ± 0.01 | ND | ND |
| hesperidin | ND | 0.90 ± 0.02 | ND | ND |
| isovitexin | ND | ND | 0.06 ± 0.002 | ND |
| cosemetin | 0.38 ± 0.01b | ND | 0.46 ± 0.01c | 0.16 ± 0.01a |
| cyanidin | 1.48 ± 0.07a | 11.30 ± 0.21d | 5.94 ± 0.15c | 1.92 ± 0.10b |
| Number of flavonoids | 20 | 16 | 19 | 20 |
| Sum of individual flavonoid content | 845.66 ± 24.35c | 727.44 ± 18.69b | 694.80 ± 17.62ab | 679.28 ± 23.35a |
| procyanidin B2 | 0.12 ± 0.01a | ND | 0.18 ± 0.01b | 0.24 ± 0.01c |
| trans-piceid | ND | 0.22 ± 0.01 | ND | ND |
| Number of polyphenolic compounds | 30 | 25 | 29 | 30 |
| Sum of individual polyphenolic compound content | 905.62 ± 25.85c | 802.24 ± 20.05b | 748.84 ± 18.87a | 739.24 ± 24.18a |
Different letters in same row indicate significant differences (p < 0.05). ND, not detected; DES-UAE, W-UAE, EtOH-UAE, MetOH-UAE: ultrasonic-assisted extraction with deep eutectic solvent (ChCl-EG), ultrapure water, 80% ethanol and 70% methanol, respectively.
Moreover, the highest sum of individual flavonoid content and sum of individual polyphenolic compound content were obtained by DES-UAE, which were 1.16-fold and 1.13-fold higher than those obtained by W-UAE, respectively (p < 0.05). However, the highest sum of individual phenolic acid content was obtained by W-UAE, followed by DES-UAE, MetOH-UAE, and EtOH-UAE in sequence (p < 0.05).
Among phenolic acid compounds, 2-hydroxybenzoic acid, protocatechualdehyde, p-coumaric acid, ferulic acid, caffeic acid and trans-cinnamic acid were extracted by all methods. The first five were the main components of phenolic acids, and the sum of their contents ranged from 94.54% to 96.96% of the sum of individual phenolic acid content. The 2-hydroxybenzoic acid content differed among the extracts obtained by different extraction methods and ranged from 38.88 ± 0.79 to 44.26 ± 1.26 μg/g DW. Compared with DES-UAE, W-UAE showed an insignificant 2-hydroxybenzoic acid content (p > 0.05), but the others showed significantly lower 2-hydroxybenzoic acid contents (p < 0.05). 2-hydroxybenzoic acid accounted for 66.28%–72.19% of the sum of individual phenolic acid content and 4.66%–5.52% of the sum of individual polyphenolic compound content. Bordoloi et al. [8] reported that phenolic acid compounds identified by HPLC-DAD from the ethanolic extract of P. scandens accounted for 100% of the sum of individual polyphenolic compound content, whereas the flavonoid compounds accounted for 0%.
Among flavonoid compounds, hyperoside, rutin, quercitrin, isoquercitrin, guaiaverin, kaempferol-3-O-rutinoside, astragaline, narcissin, apigenin, diosmetin, and cyanidin were extracted by all methods. Rutin, guaiaverin, kaempferol-3-O-rutinoside, narcissin were the main components of flavonoid compounds and the sum of their contents accounted for 88.17%–94.67% of the sum of individual flavonoid content and 79.95%–88.40% of the sum of individual polyphenolic compound content. These results revealed that rutin, guaiaverin, kaempferol-3-O-rutinoside, and narcissin were the main polyphenolic compounds of P. scandens. The highest rutin content was found in the extracts obtained by DES-UAE, followed by W-UAE, EtOH-UAE, and MetOH-UAE (p < 0.05). While the highest guaiaverin content was found in the extracts obtained by DES-UAE, EtOH-UAE, and MetOH-UAE (p > 0.05). There was no significant difference in kaempferol-3-O-rutinoside content among the extracts obtained by all extraction methods (p > 0.05). The highest narcissin content was found in the extracts obtained by DES-UAE and W-UAE (p > 0.05).
Typically, the polyphenol profiles depend on extraction methods, including extraction solvents, assisted extraction technology, and extraction parameters used. Traditional and eco-friendly solvents coupled with/without UAE gave significant different polyphenolic compositions of Morinda citrifolia L. leaves [24], [27]. Su et al. [30] found that different extraction solvents and different extraction methods contributed to the variations of free and bound polyphenol profiles of litchi pulp, respectively. The present study found that polyphenol profiles of P. scandens among all extraction methods were greatly different, which was consistent with the previous studies [23], [29], [30]. The difference in polarity of extraction solvents mainly contributed to variations of polyphenol profiles, as well as TFC and antioxidant capacity of the extracts [23], [27], [29], [30]. In the present study, with the same assisted extraction technology UAE and the identical extraction parameters, four extraction solvents (ChCl-EG, ultrapure water, 80% ethanol and 70% methanol) showed different polyphenol profiles of P. scandens, along with varying TFC and antioxidant activities, demonstrating the important effect of extraction solvents on extraction efficiency for polyphenols. Compared to ultrapure water, 80% ethanol and 70% methanol, ChCl-EG had a different polarity, and a strong hydrogen-bond basicity, effectively facilitating intermolecular interactions between DESs and cellulose strands of plants [25], [26], [27]. Among all extraction methods, DES-UAE exhibited the highest sum of individual flavonoid content and sum of individual polyphenolic compound content, as well as highest TFC and FRAP value, indicating that DES-UAE was more efficient than other methods. Moreover, UAE, considered as a green extraction technology, played a pertinent role in improving extraction rates of phytochemicals by disrupting the cell-wall structure of plants using acoustic cavitation [28], [27], [22]. Therefore, the ChCl-EG coupled with UAE was efficient in extracting polyphenols from P. scandens.
4. Conclusions
The present study revealed significant differences in the TFC and antioxidant activities among the 16 DESs coupled with UAE, and wherein choline chloride chloride and ethylene glycol at a 1:2 M ratio showed the highest extractability. DES-UAE optimization using a two-level factorial experiment followed by response surface methodology revealed the optimum parameters (water content in DES of 49.2%, the actual ultrasonic power of 72.4 W, and ultrasonic time of 9.7 min). The experimental TFC and antioxidant capacity closely matched the predicted results. Furthermore, there were significant differences in TFC, antioxidant capacity, and ployphenol profiles among DES-UAE and other extraction methods. 30 individual polyphenolic compounds were found in the extracts obtained by DES-UAE. Additionally, DES-UAE showed the highest sum of individual polyphenolic compound content, as well as the highest TFC and FRAP value. These results revealed the high extractability of DES-UAE.
CRediT authorship contribution statement
Yuxin Liu: Methodology, Resources, Project administration, Writing – original draft. Wang Zhe: Investigation, Project administration. Ruifen Zhang: Writing – review & editing, Funding acquisition. Ziting Peng: Data curation, Software. Yuxi Wang: Software, Supervision. Heqi Gao: Software, Supervision. Zhiqiang Guo: Validation, Writing – review & editing, Formal analysis. Juan Xiao: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The study was supported by the Natural Science Foundation of Hainan province of China (320RC510), the Key Research & Development project of Hainan province of China (ZDYF2021XDNY197), the National Natural Science Foundation of China (31960483), the Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (2021KJ117), the Special Fund for Scientific Innovation Strategy-Construction of High Level Academy of Agriculture Science (R2020PY-JG011) and Open Fund of Key Laboratory of Food Nutrition and Functional Food of Hainan Province (KF202009).
References
- 1.Duarte L., Gasaly N., Poblete-Aro C., Uribe D., Echeverria F., Gotteland M., Garcia-Diaz D.F. Polyphenols and their anti-obesity role mediated by the gut microbiota: a comprehensive review. Rev. Endocr. Metab. Dis. 2021;22(2):367–388. doi: 10.1007/s11154-020-09622-0. [DOI] [PubMed] [Google Scholar]
- 2.Wang R.M., Yao L.L., Lin X., Hu X.P., Wang L. Exploring the potential mechanism of Rhodomyrtus tomentosa (Ait.) Hassk fruit phenolic rich extract on ameliorating nonalcoholic fatty liver disease by integration of transcriptomics and metabolomics profiling. Food Res. Int. 2022;151 doi: 10.1016/j.foodres.2021.110824. [DOI] [PubMed] [Google Scholar]
- 3.Ahmadifar E., Yousefi M., Karimi M., Fadaei Raieni R., Dadar M., Yilmaz S., Dawood M.A.O., Abdel-Latif H.M.R. Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: an overview. Rev. Fish. Sci. Aquac. 2021;29(4):478–511. [Google Scholar]
- 4.Rambaran T.F., Nordstrm A. Medical and pharmacokinetic effects of nanopolyphenols: a systematic review of clinical trials. Food Front. 2021;1:1–13. doi: 10.1002/fft2.72. [DOI] [Google Scholar]
- 5.College J.N.M. Shanghai Scientific and Technological Press; Shanghai: 2006. Directory of Chinese Materia Medica; pp. 1698–1699. [Google Scholar]
- 6.Xiao M., Fu X., Ni Y., Chen J., Jian S., Wang L., Lie L., Du G. Protective effects of Paederia scandens extract on rheumatoid arthritis mouse model by modulating gut microbiota. J. Ethnopharmacol. 2018;226(15):97–104. doi: 10.1016/j.jep.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 7.L. Wang, Y. Jiang, T. Han, C. Zheng, L. Qin, A phytochemical, pharmacological and clinical profile of Paederia foetida and P. scandens, Nat. Prod. Commun. 9 (6) (2014) 879–886, https://doi.org/10.1111/jtxs.12070. [PubMed]
- 8.Bordoloi M., Bordoloi P.K., Dutta P.P., Singh V., Nath S., Narzary B., Bhuyan P.D., Rao P.G., Barua I.C. Studies on some edible herbs: Antioxidant activity, phenolic content, mineral content and antifungal properties. J. Funct. Foods. 2016;23:220–229. doi: 10.1016/j.jff.2016.02.028. [DOI] [Google Scholar]
- 9.Chen Y.F., Huang Y., Tang W.Z., Qin L.P., Zheng H.C. Antinociceptive activity of paederosidic acid methyl ester (pame) from the n-butanol fraction of paederia scandens in mice. Pharmacol. Biochem. Be. 2009;93(2):97–104. doi: 10.1016/j.pbb.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Hou S.X., Zhu W.J., Pang M.Q., Jeffry J., Zhou L.L. Protective effect of iridoid glycosides from paederia scandens (lour.) merrill (rubiaceae) on uric acid nephropathy rats induced by yeast and potassium oxonate. Food Chem. Toxicol. 2014;64:57–64. doi: 10.1016/j.fct.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 11.Zhu W., Pang M., Dong L., Huang X., Wang S., Zhou L. Anti-inflammatory and immunomodulatory effects of iridoid glycosides from paederia scandens (lour.) merrill (rubiaceae) on uric acid nephropathy rats. Life Sci. 2012;91:369–376. doi: 10.1016/j.lfs.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Yang T., Kong B., Gu J.W., Kuang Y.Q., Cheng L., Yang W.T., Cheng J.M., Ma Y., Yang X.K. Anticonvulsant and sedative effects of paederosidic acid isolated from Paederia scandens (Lour.) Merrill. in mice and rats. Pharmacol. Biochem. Be. 2013;111:97–101. doi: 10.1016/j.pbb.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Peng W., Qiu X.Q., Shu Z.H., Liu Q.C., Hu M.B., Han T., Rahman K., Qin L.P., Zheng C.J. Hepatoprotective activity of total iridoid glycosides isolated from Paederia scandens (lour.) Merr. var. tomentosa, J. Ethnopharmacol. 2015;174:317–321. doi: 10.1016/j.jep.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 14.Kumar V., Al-Abbasi F.A., Ahmed D., Verma A., Mujeeb M., Anwar F. Paederia foetida linn. inhibits adjuvant induced arthritis by suppression of pge2 and cox-2 expression via nuclear factor-κb. Food Funct. 2015;6(5):1652–1666. doi: 10.1039/c5fo00178a. [DOI] [PubMed] [Google Scholar]
- 15.Borgohain M.P., Chowdhury L., Ahmed S., Bolshette N., Devasani K., Das T.J., Mohapatra A., Lahkar M. Renoprotective and antioxidative effects of methanolic Paederia foetida leaf extract on experimental diabetic nephropathy in rats. J. Ethnopharmacol. 2017;198:451–459. doi: 10.1016/j.jep.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 16.Zhuang C., Wang X., Miao L., Zhou H., Wu T. Chemical constituents of paederia scandens. Chem. Nat. Compd. 2013;49(2):379–380. doi: 10.1007/s10600-013-0614-0. [DOI] [Google Scholar]
- 17.N. Ishikura Z.-Q. Yang K. Yoshitama K. Kurosawa Flavonol Glycosides from Paederia scandens var. mairei 45 11-12 1990 1081 1084.
- 18.Kurihara T., Kikuchi M., Shigenori S. Studies on the constituents of fruits of Paederia chinensis HANCE. Yakugaku. Zasshi. 1975;95:1380–1383. doi: 10.1248/yakushi1947.95.11_1380. [DOI] [PubMed] [Google Scholar]
- 19.Cai M.L., Zhang Q.L., Zheng X.T., Zhai J.J., Peng C.L. Comparison of leaves and stems of paederia scandens (lour.) merr. in tolerance to low temperature. Photosynthetica. 2020;58 doi: 10.32615/ps.2020.034. [DOI] [Google Scholar]
- 20.García A., Rodríguez-Juan E., Rodríguez-Gutierrez G., Rios J.J., Fernandez-Bolanos J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs) Food Chem. 2016;197:554–561. doi: 10.1016/j.foodchem.2015.10.131. [DOI] [PubMed] [Google Scholar]
- 21.Li S.J., Wang R.M., Hu X.P., Li C.F., Wang L. Bio-affinity ultra-filtration combined with HPLC-ESI-qTOF-MS/MS for screening potential α-glucosidase inhibitors from Cerasus humilis (Bge.) Sok. leaf-tea and in silico analysis. Food Chemistry. 2022;373 doi: 10.1016/j.foodchem.2021.131528. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh Y.-H., Li Y., Pan Z., Chen Z., Lu J., Yuan J., Zhu Z., Zhang J. Ultrasonication-assisted synthesis of alcohol-based deep eutectic solvents for extraction of active compounds from ginger. Ultrason. Sonochem. 2019;63 doi: 10.1016/j.ultsonch.2019.104915. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H., Zhang J., Li C., Liu S., Wang L. Morinda citrifolia L. leaves extracts obtained by traditional and eco-friendly extraction solvents: Relation between phenolic compositions and biological properties by multivariate analysis. Ind. Crop. Prod. 2020;153 doi: 10.1016/j.indcrop.2020.112586. [DOI] [Google Scholar]
- 24.Patil S.S., Pathak A., Rathod V.K. Optimization and kinetic study of ultrasound assisted deep eutectic solvent based extraction: A greener route for extraction of curcuminoids from Curcuma longa. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunha S.C., Fernandes J. Extraction techniques with deep eutectic solvents, Trac-Trend. Anal. Chem. 2018;105:225–239. doi: 10.1016/j.trac.2018.05.001. [DOI] [Google Scholar]
- 26.Zhang Q., Karine D., Royer S., Jérme F. Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev. 2012;41(21):7108–7146. doi: 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
- 27.Kumar K., Srivastav S., Sharanagat V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhargava N., Mor R.S., Kumar K., Sharanagat V.S. Advances in application of ultrasound in food processing: a review. Ultrason. Sonochem. 2020;70 doi: 10.1016/j.ultsonch.2020.105293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.L. Wu L. Li S. Chen L.u. Wang X. Lin Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: Optimization, comparison and antioxidant activity Sep. Purif. Technol. 247 2020 10.1016/j.seppur.2020.117014. 2k 117014 117014.
- 30.Su D., Zhang R., Hou F., Zhang M., Guo J., Huang F., Deng Y., Wei Z. Comparison of the free and bound phenolic profiles and cellular antioxidant activities of litchi pulp extracts from different solvents. BMC Complem. Altern. Med. 2014;14:9. doi: 10.1186/1472-6882-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guimarães J.T., Almeida P.P., Brito M.L., Cruz B.O., Costa S., Almeida N., Ito R.V., Mota J.C., Bertolo M., Morais S.T.B., Neto R.P.C., Inês B., Tavares M., Souto F., Junior S.B., Cimentel P.T., Stockler-Pinto M.B., Freitas M.Q., Cruz A.G. In vivo functional and health benefits of a prebiotic soursop whey beverage processed by high-intensity ultrasound: study with healthy Wistar rats. Food Chem. 2022 doi: 10.1016/j.foodchem.2022.132193. [DOI] [PubMed] [Google Scholar]
- 32.Gil-Martín E., Forbes-Hernández T., Alejandro R., Cianciosi D., Giampieri F., Battino M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022;378:31918. doi: 10.1016/j.foodchem.2021.131918. [DOI] [PubMed] [Google Scholar]
- 33.Martínez-Patiño J.C., Gullón B., Romero I., Ruiz E., Brnčić M., Žlabur J.Š., Castro E. Optimization of ultrasound-assisted extraction of biomass from olive trees using response surface methodology. Ultrason. Sonochem. 2019;51:487–495. doi: 10.1016/j.ultsonch.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 34.Chel-Guerrero L.D., Oney-Montalvo J.E., Rodríguez-Buenfil I.M. Phytochemical characterization of by-products of habanero pepper grown in two different types of soils from Yucatán. Mexico. Plants (Basel) 2021;10:779–786. doi: 10.3390/plants10040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H.F., Shao S.J., Xin X.R., Wang M., Wei J.L. Research on extraction and antibacterial activity of flavonoids in potato peel. J. North. Univ. China. 2017;38:6660–6665. doi: 10.3969/j.issn.1673-3193.2017.06.018. [DOI] [Google Scholar]
- 36.Xiao J., Zhang R., Wu Y., Wu C., Jia X., Dong L., Liu L., Chen Y., Bai Y., Zhang M. Rice bran phenolic extract protects against alcoholic liver injury in mice by alleviating intestinal microbiota dysbiosis, barrier dysfunction, and liver inflammation mediated by the endotoxin-TLR4-NF-κB pathway. J. Agric. Food Chem. 2020;68(5):1237–1247. doi: 10.1021/acs.jafc.9b04961. [DOI] [PubMed] [Google Scholar]
- 37.Lambert M., Meudec E., Verbaere A., Mazerolles G., Wirth J., Masson G. A high-throughput UHPLC-QqQ-MS method for polyphenol profiling in rose wines. Molecules. 2015;20:7890–7914. doi: 10.3390/molecules20057890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arivalagan M., Roy T.K., Yasmeen A.M., Pavithra K.C., Jwala P.N., Shivasankara K.S., Manikantan M.R., Hebbar K.B., Kanade S.R. Extraction of phenolic compounds with antioxidant potential from coconut (cocos nucifera l.) testa and identification of phenolic acids and flavonoids using uplc coupled with tqd-ms/ms, LWT–Food. Sci. Technol. 2018;92:116–126. [Google Scholar]
- 39.Luo D., Mu T., Sun H. Profiling of phenolic acids and flavonoids in sweet potato (Ipomoea batatas L.) leaves and evaluation of their anti-oxidant and hypoglycemic activities. Food Bioscience. 2021;39:100801. [Google Scholar]
- 40.Flamini R., Vedova A.D., Cancian D., Panighel A., Rosso M.D. GC/MS-positive ion chemical ionization and MS/MS study of volatile benzene compounds in five different woods used in barrel making. J. Mass Spectrom. 2010;42(5):641–646. doi: 10.1002/jms.1193. [DOI] [PubMed] [Google Scholar]
- 41.Mattonai M., Parri E., Querci D., Degano I., Ribechini E. Development and validation of an HPLC-DAD and HPLC/ESI-MS2 method for the determination of polyphenols in monofloral honeys from tuscany (italy) Microchem. J. 2015;126:220–229. doi: 10.1016/j.microc.2015.12.013. [DOI] [Google Scholar]
- 42.Ivanescu B., Vlase L., Corciova A., Lazar M.I. HPLC-DAD-MS study of polyphenols from artemisia absinthium, a. Annua, and a. Vulgaris. Chem. Nat. Compd. 2010;46:468–470. doi: 10.1007/s10600-010-9648-8. [DOI] [Google Scholar]
- 43.Ertas A., Yener I. A comprehensive study on chemical and biological profiles of three herbal teas in Anatolia; rosmarinic and chlorogenic acids. S. Afr. J. Bot. 2020;130:274–281. doi: 10.1016/j.sajb.2020.01.008. [DOI] [Google Scholar]
- 44.Akhtar N., Thadhani V.M., Ul Haq F., Khan M.N., Ali S., Musharraf S.G. Rapid identification and quantification of bioactive metabolites in processed camellia sinensis samples by uhplc-esi-ms/ms and evaluation of their antioxidant activity. J. Ind. Eng. Chem. 2020;90:419–426. [Google Scholar]
- 45.Cai Y., Wu L., Lin X., Hu X., Wang L. Phenolic profiles and screening of potential α-glucosidase inhibitors from Polygonum aviculare L. leaves using ultra-filtration combined with HPLC-ESIqTOF-MS/MS and molecular docking analysis. Ind. Crop. Prod. 2020;154 doi: 10.1016/j.indcrop.2020.112673. [DOI] [Google Scholar]
- 46.Olate-Gallegos C., Barriga A., Vergara C., Fredes C., García P., Giménez B. Identifification of polyphenols from Chilean brown seaweeds extracts by LC-DAD-ESI-MS/MS. J. Aqua. Food Prod. T. 2019;28:375–391. doi: 10.1080/10498850.2019.1594483. [DOI] [Google Scholar]
- 47.A. Plazonić F. Bucar Ž. Maleš A. Mornar B. Nigović N. Kujundžić Identification and Quantification of Flavonoids and Phenolic Acids in Burr Parsley (Caucalis platycarpos L.), Using High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ionization Mass Spectrometry Molecules 14 7 2466 2490. [DOI] [PMC free article] [PubMed]
- 48.Pereira C.A.M., Yariwake J.H., Mccullagh M. Distinction of the glycosylflavone isomer pairs orientin/isoorientin and vitexin/isovitexin using HPLC-MS exact mass measurement and in-source CID. Phytochem. Analysis. 2005;16(5):295–301. doi: 10.1002/pca.820. [DOI] [PubMed] [Google Scholar]
- 49.Kim S.-B., Lee T., Lee H.S., Song C.K., Cho H.-J., Kim D.-D., Maeng H.-J., Yoon I.-S. Development and validation of a highly sensitive lc–ms/ms method for the determination of acacetin in human plasma and its application to a protein binding study. Arch. Pharm. Res. 2016;39(2):213–220. doi: 10.1007/s12272-015-0697-1. [DOI] [PubMed] [Google Scholar]
- 50.Chen X., Xu L., Guo S., Wang Z., Jiang L., Wang F., Zhang J., Liu B. Profiling and comparison of the metabolites of diosmetin and diosmin in rat urine, plasma and feces using UHPLC-LTQ-Orbitrap MSn. J. Chromatogr. B. 2019;1124:58–71. doi: 10.1016/j.jchromb.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 51.P.X. Chen, Y. Tang, M.F. Marcone, P.K. Pauls, B. Zhang, R. Liu, R. Tsao, Characterization of free, conjugated and bound phenolics and lipophilic antioxidants in regular- and non-darkening cranberry beans (Phaseolus vulgaris L.), Food Chem. 185 (2015) 298–308, http://dx.doi.org/10.1016/j.foodchem. 2015.03.100. [DOI] [PubMed]
- 52.Zainal-Abidin M.H., Hayyan M., Hayyan A., N.S.J., kumar, New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 979. 2017:1–23. doi: 10.1016/j.aca.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Khezeli T., Daneshfar A., Sahraei R. A green ultrasonic-assisted liquid-liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of ferulic, caffeic and cinnamic acid from olive, almond, sesame and cinnamon oil. Talanta. 2016;150:577–585. doi: 10.1016/j.talanta.2015.12.077. [DOI] [PubMed] [Google Scholar]
- 54.Cui Q., Peng X., Yao X.-H., Wei Z.-F., Luo M., Wang W., Zhao C.-J., Fu Y.-J., Zu Y.-G. Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep. Purif. Technol. 2015;150:63–72. doi: 10.1016/j.seppur.2015.06.026. [DOI] [Google Scholar]
- 55.Sang Q., Jia Q., Zhang H., Lin C., Zhao X., Zhang M., Wang Y., Hu P. Chemical profiling and quality evaluation of Zhishi-Xiebai-Guizhi Decoction by UPLC-Q-TOF-MS and UPLC fingerprint. J. Pharmaceut. Biomed. 2021;194 doi: 10.1016/j.jpba.2020.113771. [DOI] [PubMed] [Google Scholar]
- 56.Berger A., Preinfalk A., Robien W., Brecker L., Valant-Vetschera K., Schinnerl J. New reports on flavonoids, benzoic- and chlorogenic acids as rare features in the Psychotria alliance (Rubiaceae) Biochem. Systemat. Ecol. 2016;66:145–153. doi: 10.1016/j.bse.2016.02.027. [DOI] [Google Scholar]