Abstract
Objective:
To examine the association between hearing impairment and cognitive function after traumatic brain injury (TBI).
Setting:
A total of 18 Level I trauma centers throughout the United States in the Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) study.
Participants:
From February 2014 to June 2018, 2,697 participants with TBI were enrolled in TRACK-TBI. Key eligibility criteria included external force trauma to the head, presentation to a participating Level I trauma center, and receipt of a clinically indicated head computerized tomography (CT) scan within 24 hours of injury. A total of 1,267 participants were analyzed in the study, with 216 participants with hearing impairment and 1,051 participants without hearing impairment. Those with missing or unknown hearing status or cognitive assessment were excluded from analysis.
Design:
Prospective, observational cohort study.
Main Measures:
Hearing impairment at 2 weeks post-TBI was based on self-report. Participants who indicated worse hearing in one or both ears were defined as having hearing impairment, while those who denied worse hearing in either ear were defined as not having hearing impairment and served as the reference group. Cognitive outcomes at 6 months post-TBI included executive functioning and processing speed, as measured by the Trail Making Test (TMT) B/A and the Wechsler Adult Intelligence Scale, Fourth Edition, Processing Speed Index Subscale (WAIS-IV PSI), respectively.
Results:
TBI-related hearing impairment had a small but significantly greater TMT B/A ratio than without TBI-related hearing impairment (mean difference (B) = 0.25; 95% CI = 0.07 – 0.43; p = 0.005). No significant mean differences on WAIS-IV PSI scores were found between participants with and without TBI-related hearing impairment (B = 0.36; 95% CI = −2.07 – 2.60; p = 0.825).
Conclusion:
We conclude that TBI-related hearing impairment at 6 months post-injury was significantly associated with worse executive functioning but not cognitive processing speed.
Keywords: Traumatic brain injury, hearing, cognition, TRACK-TBI
Introduction
Auditory dysfunction includes tinnitus, dizziness, and hearing impairment, and is a common sequela of traumatic brain injury (TBI).1,2 An estimated 8% to 67% of people with TBI experience hearing loss or impairment, and its prevalence depends on both the severity of TBI in the sample, as well as diagnosis through subjective assessments (i.e., self-report) or objective audiometric testing.2–8 Sensorineural hearing loss is the most pervasive type of hearing loss in individuals with TBI,1 with an estimated greater than 90% of cases attributed to this etiology.8 Other types of TBI-related hearing loss include conductive and mixed hearing loss, which can result from tympanic membrane perforation, hemotympanum, or ossicular discontinuity.9–12 Hearing loss can also be caused by cranial impact injuries without TBI, such as a ruptured eardrum or damage to the middle ear. An important limitation of current research is that much of the evidence on hearing loss following TBI was collected in military veterans and service members, who have a high prevalence of potentially confounding noise-related hearing loss and tinnitus.13
Hearing loss is associated with a small but significant decline in cognition, particularly among older adults with age-related hearing deficits.14–18 Affected cognitive domains include global cognition, attention, processing speed, short- and long-term memory, and executive function.18,19 Additionally, the direct consequences of TBI injury on cognitive function may include alterations in arousal, slowed processing speed, difficulty multitasking, and reduced cognitive stamina.20 TBI also affects memory, executive function, and attention systems, particularly when more severe injuries result in greater frontal and temporal damage.21 Despite evidence from epidemiologic studies reporting an association between age-related hearing loss and cognition, the relationship between TBI-related hearing loss and cognition is not well-understood. Among the few published studies that have examined hearing and cognitive function in TBI,22–24 major limitations exist such as small sample size and lack of longitudinal follow-up.
The objective of the study was to analyze whether hearing impairment shortly following TBI contributes to worse longer-term cognitive outcomes among participants in the longitudinal Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) study. We hypothesized that participants with TBI-related hearing impairment at 2 weeks post-TBI would perform worse on executive function and processing speed at 6 months post-TBI as compared with a reference group of participants without TBI-related hearing impairment at 2 weeks post-TBI.
Methods
Study design and participants
TRACK-TBI is a multi-center, prospective observational cohort study conducted at 18 Level I trauma centers throughout the US.25,26 A total of 2,697 participants with TBI were enrolled from February 26, 2014 to June 15, 2018. Written consent was obtained from all participants or their legal representatives. Inclusion criteria for TRACK-TBI have been published previously,26 and include external force trauma to the head, presentation to a participating Level I trauma center, and receipt of a clinically indicated head computerized tomography (CT) scan within 24 hours of injury, with adequate visual acuity and hearing for testing. Participants were excluded if they were prisoners, pregnant, on a psychiatric hold, participating in an interventional trial, non-English or non-Spanish speaking, or had low likelihood of follow-up, major debilitating mental health disorders, neurologic disease, or significant pre-existing conditions that would interfere with follow-up. Participants under 17 years of age were excluded from this analysis as they received a different outcome battery. Approval for the study was received from the institutional review board at the lead site (University of California, San Francisco) and at each participating site.
Assessment of hearing impairment
Information on pre-TBI hearing impairment, and self- or surrogate-reported post-TBI hearing function was collected by participant or surrogate interviews. At the initial 2-week study visit, participants were asked, “Since your injury, has your hearing been worse in either ear?” Participants responded by indicating, “No,” “Yes, worse in the left ear,” “Yes, worse in the right ear,” or “Yes, worse in both ears.” To evaluate a potential deleterious effect of TBI-related hearing impairment, participants at the 2-week interview who indicated worse hearing in one or both ears were combined into the exposure group representing those who had hearing impairment. Participants at the 2-week interview who denied worse hearing in either ear were classified as the reference group, representing those without hearing impairment after TBI. Participants with missing or unknown hearing status at 2 weeks were excluded from analysis.
Cognitive evaluation
The outcome of interest for this analysis, cognitive function, was assessed at 6 months using a battery of neuropsychological tests. Measurements analyzed here included the Trail Making Test (TMT)27,28 and the Wechsler Adult Intelligence Scale, Fourth Edition, Processing Speed Index Subscale (WAIS-IV PSI).29 TMT is a 2-part timed test. Parts A and B both assess visual scanning and motor speed, and Part B further assesses executive functioning. To derive a purer index of executive functioning separate from visual processing and motor speed, we used the ratio of times between Part B and Part A (B/A).30–32 WAIS-IV PSI is a summary measure of non-verbal processing speed consisting of 2 non-verbal tasks (symbol search and coding) that require visual attention and motor speed. The composite score is scalar and ranges from 50 to 150 to correspond to the 0.1 to 99.9 percentile of performance within normative age bands. Neuropsychological tests that involve hearing or listening tasks, such as RAVLT, were excluded from the study because hearing impairment may confound performance on these tests.33
Statistical analysis
Descriptive statistics were presented as mean and standard deviation (SD) for continuous variables, and as counts and percentages for categorical variables. Group differences in demographics and injury characteristics across hearing impairment status were assessed by Pearson’s chi-squared test for categorical variables, and by t-test or Mann-Whitney U test for continuous variables. Demographic and injury characteristics included age, sex, education, race, pre-injury history of subjective hearing impairment, loss of consciousness, post-traumatic amnesia, emergency department admission Glasgow Coma Scale (GCS) score, and presence of intracranial pathology on initial head CT scan. Boxplots were used to show unadjusted means for values of TMT B/A and WAIS-IV PSI at 6 months by TBI-related hearing impairment status at 2 weeks. Multivariable linear regression was performed to examine the association between TBI-related hearing impairment at 2 weeks and cognitive function at 6 months, adjusting for demographic and injury characteristics, such as age, sex, education, race, pre-injury history of subjective hearing impairment, loss of consciousness, post-traumatic amnesia, GCS score, and presence of CT intracranial pathology. The adjusted mean difference (B) and 95% confidence interval (CI) were used to quantify the average difference in outcome measures between TBI-related hearing impairment groups. Participants without TBI-related hearing impairment served as a reference group. A standardized effect size was also estimated using Cohen’s d.34 Sensitivity analyses included analyzing TMT Part A and Part B times individually to assess cognitive processing speed and executive function, respectively, associated with TBI-related hearing impairment. We also conducted multiple imputation by chained equations in order to address missing outcome data in the cognitive tests, as well as missing information on TBI-related hearing function.35
Secondary analyses included the extent of TBI-related hearing impairment as an ordinal variable with 3 categories to evaluate for a potential graded relationship with cognitive function. Participants with hearing impairment in both ears were classified into 1 group, and those with hearing impairment in a single ear only were classified into another group. Participants without hearing impairment at 2 weeks post-TBI served as a reference group. We also stratified associations between TBI-related hearing impairment and each cognitive domain based on severity of TBI injury determined by admission GCS score (<13 classified as moderate-to-severe TBI; ≥13 classified as mild TBI), as well as the presence versus absence of CT intracranial pathology. To account for multiple comparisons due to the 2 outcomes examined, a Bonferroni-adjusted significance level of P <0.025 was used in order to reject the null hypothesis. All analyses were conducted using Stata version 14 (StataCorp, College Station, TX).
Results
Study sample
In total, 2,552 participants at least 17 years of age with TBI were eligible to participate in the study. Figure 1 shows the number of participants who were missing information on self-reported hearing impairment at 2 weeks and cognitive measures at 6 months, leaving 1,267 participants available in the analytic sample. Demographic and injury-related characteristics of the sample showed that 66.5% of participants were male and 76.7% were Caucasian, with the preponderance (97.3%) reporting no history of hearing loss at baseline (Table 1). Average age of participants in the sample was 40.7 years (17 – 90 years). At 2 weeks, 216 participants reported TBI-related hearing impairment (17.0%) compared with 1,051 participants who did not report TBI-related hearing impairment (83.0%). Those with hearing impairment had significantly lower education and were more likely to have intracranial pathology on CT scan.
Figure 1.
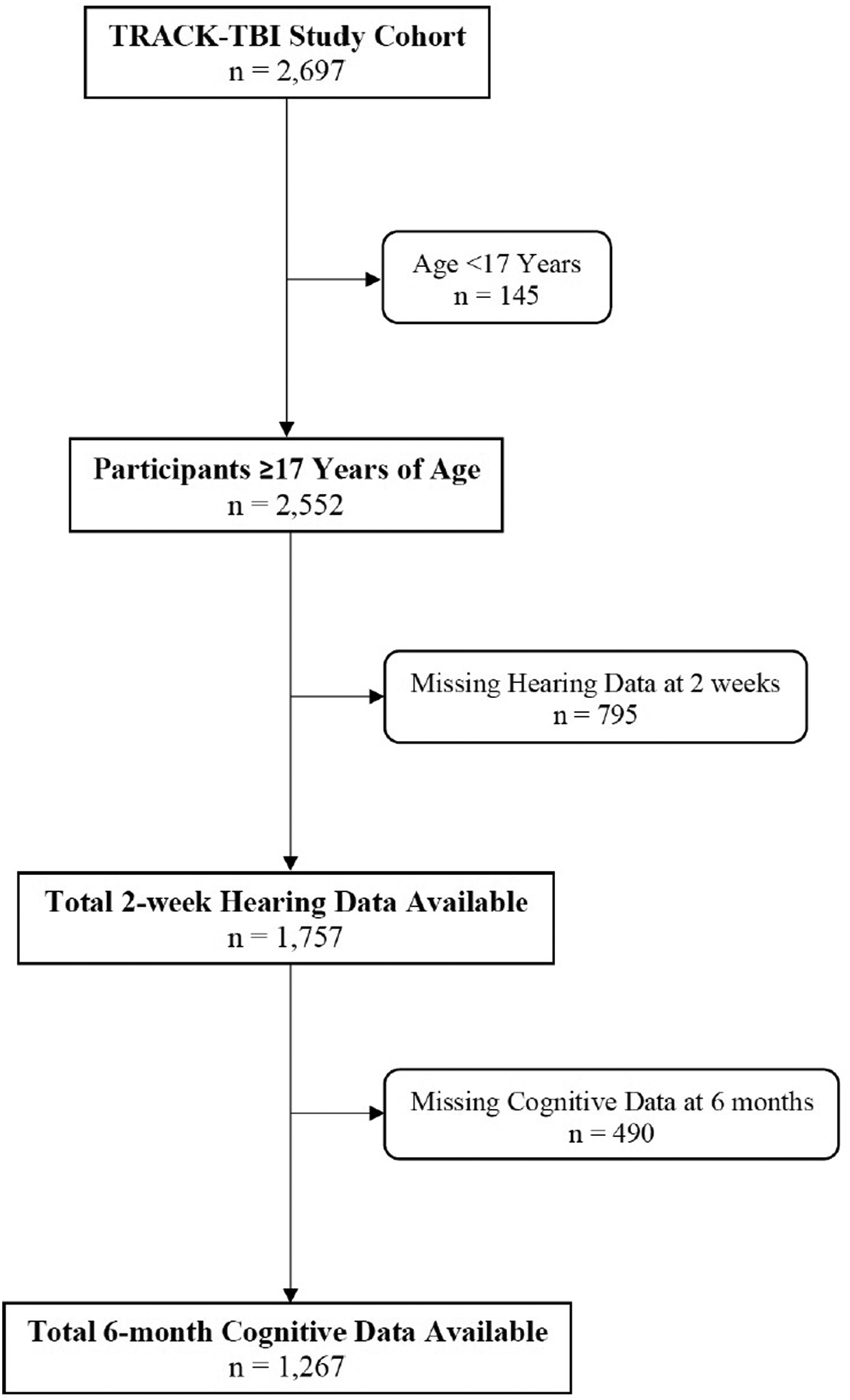
Identification and follow up of participants with TBI from the TRACK-TBI study. Flow chart of inclusion of participants with available information on TBI-related hearing impairment and cognition. TBI, traumatic brain injury: TRACK-TBI, Transforming Research and Clinical Knowledge in TBI
Table 1.
Demographic and clinical characteristics by hearing status at 2-weeks post-TBI
| Variable | Overall (n = 1,267) | No hearing impairment (n = 1,051) | Hearing impairment (n = 216) | P |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (Standard deviation) | 40.7 (17.1) | 40.8 (17.3) | 40.7 (16.2) | 0.97 |
| Sex | ||||
| Male, n (%) | 843 (66.5) | 690 (65.6) | 153 (70.8) | 0.14 |
| Female, n (%) | 424 (33.5) | 361 (34.4) | 63 (29.2) | |
| Education (years) | ||||
| Mean (Standard deviation) | 13.8 (2.8) | 13.9 (2.8) | 13.3 (2.6) | <0.01 |
| Race | ||||
| Caucasian, n (%) | 967 (76.7) | 801 (76.6) | 166 (77.2) | 0.84 |
| Non-Caucasian, n (%) | 294 (23.3) | 245 (23.4) | 49 (22.8) | |
| Pre-injury history of hearing loss | ||||
| Yes, n (%) | 34 (2.7) | 29 (2.8) | 5 (2.3) | 0.71 |
| No, n (%) | 1,232 (97.3) | 1,021 (97.2) | 211 (97.7) | |
| Loss of consciousness | ||||
| Yes, n (%) | 983 (85.8) | 815 (85.7) | 168 (86.6) | 0.74 |
| No, n (%) | 162 (14.2) | 136 (14.3) | 26 (13.4) | |
| Post-traumatic amnesia | ||||
| Yes, n (%) | 885 (77.2) | 733 (76.5) | 152 (80.4) | 0.44 |
| No, n (%) | 227 (19.8) | 196 (20.5) | 31 (16.4) | |
| Suspected, n (%) | 35 (3.0) | 29 (3.0) | 6 (3.2) | |
| ED arrival GCS | ||||
| Mean (Standard deviation) | 14.1 (2.4) | 14.2 (2.3) | 13.8 (2.8) | 0.07 |
| GCS 3–8, n (%) | 65 (5.1) | 50 (4.8) | 15 (6.9) | 0.19 |
| GCS 9–12, n (%) | 32 (2.5) | 24 (2.3) | 8 (3.7) | |
| GCS 13–15, n (%) | 1,170 (92.3) | 977 (93.0) | 193 (89.4) | |
| CT intracranial pathology | ||||
| Yes, n (%) | 475 (38.2) | 352 (34.0) | 123 (59.1) | <0.01 |
| No, n (%) | 768 (61.8) | 683 (66.0) | 85 (40.9) |
Abbreviations: TBI, Traumatic Brain Injury; ED, Emergency Department; GCS, Glasgow Coma Scale; CT, Computerized Tomography
Missingness: Education (1.26% (16/1267) Overall; 1.05% (11/1051) in No hearing impairment; 2.31% (5/216) in Hearing impairment); Race (0.47% (6/1267) Overall; 0.48% (5/1051) in No hearing impairment; 0.46% (1/216) in Hearing impairment); Pre-injury history of hearing loss (0.08% (1/1267) Overall; 0.10% (1/1051) in No hearing impairment; 0% (0/216) in Hearing impairment); Loss of consciousness (9.63% (122/1267) Overall; 9.51% (100/1051) in No hearing impairment; 10.18% (22/216) in Hearing impairment); Post-traumatic amnesia (9.47% (120/1267) Overall; 8.85% (93/1051) in No hearing impairment; 12.50% (27/216) in Hearing impairment); GCS (1.26% (16/1267) Overall; 0.95% (10/1051) in No hearing impairment; 2.78% (6/216) in Hearing impairment); CT pathology (1.89% (24/1267) Overall; 1.52% (16/1051) in No hearing impairment; 3.70% (8/216) in Hearing impairment)
Association between TBI-related hearing impairment and cognition
At 6 months, the mean TMT B/A was 2.69 among those without TBI-related hearing impairment and 2.97 among those with TBI-related hearing impairment, with lower values indicating better performance (Figure 2). The mean WAIS-IV PSI composite score was 101.74 among those without TBI-related hearing impairment and 100.47 among those with TBI-related hearing impairment, with higher scores indicating better performance. After adjusting for demographics and injury characteristics, the association between TBI-related hearing impairment at 2 weeks and TMT B/A at 6 months was significant, but the effect size was small (d = 0.21) (Table 2). The average adjusted TMT B/A was 0.25 greater among those with hearing impairment compared to those without hearing impairment (95% CI = 0.07 – 0.43; p = 0.005). The association between TBI-related hearing impairment at 2 weeks and WAIS-IV PSI at 6 months was not significant. In sensitivity analyses of TMT Parts A and B completion times, we found that TBI-related hearing impairment at 2 weeks was associated with an 8.51-second longer average completion time on TMT Part B compared to no TBI-related hearing impairment (95% CI = 1.30 – 15.73; p = 0.021), whereas the group difference in TMT Part A was not significant (Supplemental Table 1). Results from the imputation-based analysis did not differ appreciably from the primary complete case analysis (Supplemental Table 2).
Figure 2.
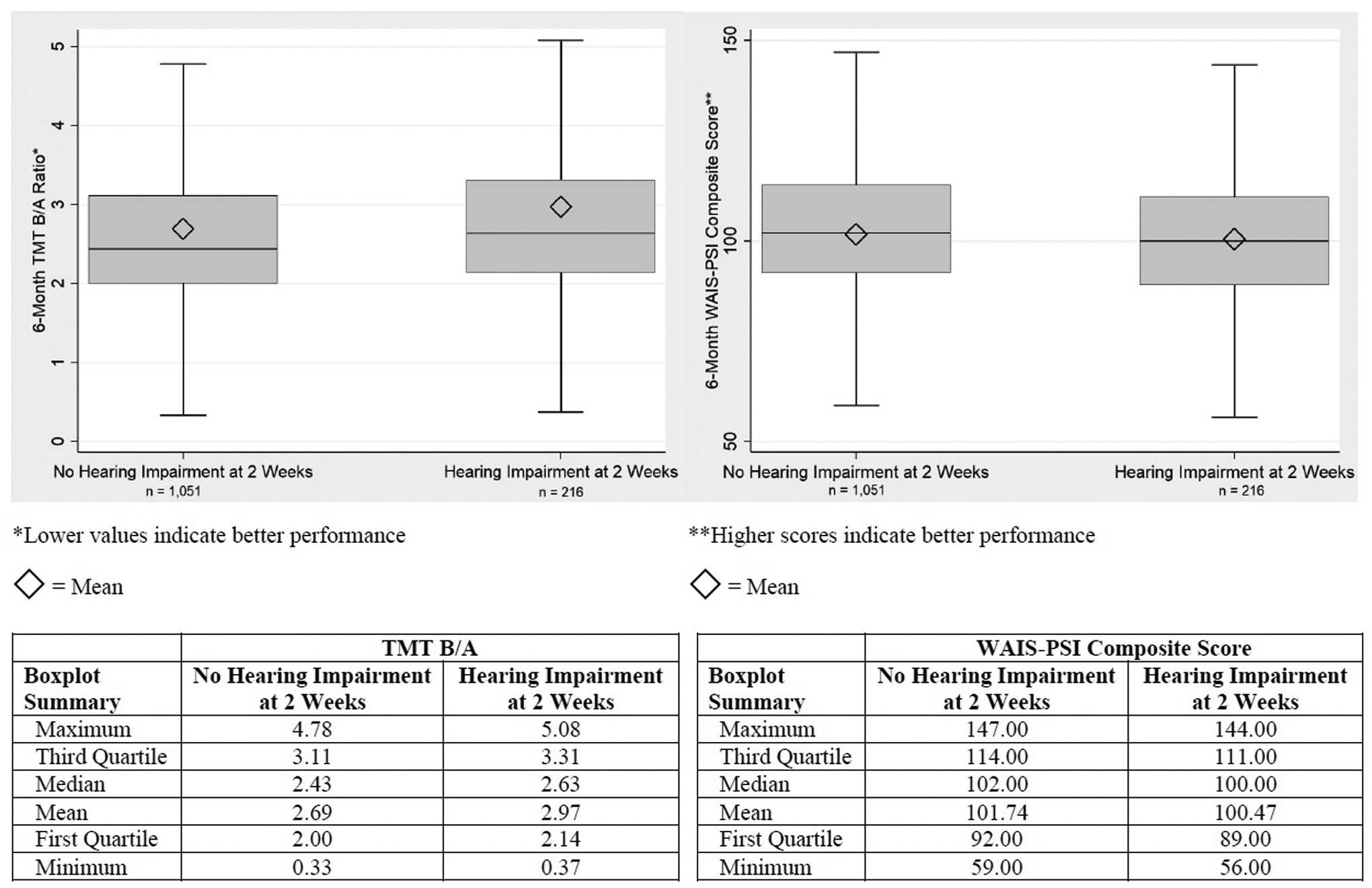
Comparison of 6-month TMT B/A ratio and WAIS-PSI composite score means across hearing status at 2-weeks post-TBI. TMT, Trail Making Test: WAIS-PSI, Wechsler Adult Intelligence Scale, fourth edition, Processing Speed Index; TBI, traumatic brain injury
Table 2.
Associations between TBI-related hearing impairment and 6-month cognitive outcome measures
| Outcome Measure | B (95% Confidence Interval)* | p-value | d |
|---|---|---|---|
| TMT B/A | 0.25 (0.07 – 0.43) | 0.005 | 0.21 |
| WAIS-PSI Composite Score | 0.26 (−2.07 – 2.60) | 0.825 | 0.01 |
Abbreviations: TBI, Traumatic Brain Injury; B, Mean Difference between Hearing Impairment and No Hearing Impairment; d, Cohen’s effect size; TMT, Trail Making Test; WAIS-PSI, Wechsler Adult Intelligence Scale, fourth edition, Processing Speed Index
Adjusted for age (years), sex (male vs. female), race (Caucasian vs. non-Caucasian), education (years), pre-injury history of hearing loss (yes vs. no), loss of consciousness (yes vs. no), post-traumatic amnesia (yes vs. no. vs. suspected), ED arrival GCS (per-unit increase), CT intracranial pathology (yes vs. no)
Extent of TBI-related hearing impairment-dependent associations with cognition
Average TMT B/A was 0.37 greater among participants with impairment in both ears compared to those without TBI-related hearing impairment (95% CI = 0.07 – 0.66; p = 0.014) (Table 3). Impairment in only 1 ear was in the same direction, but not significantly associated with TMT B/A. Similar to the non-significant findings comparing any hearing impairment versus no hearing impairment groups, WAIS-IV PSI was not significantly different between groups with no hearing impairment, impairment in 1 ear, and impairment in 2 ears.
Table 3.
Associations between extent of TBI-related hearing impairment and 6-month cognitive outcome measures
| Hearing Impairment in One Ear Only (n = 149) | |||
|---|---|---|---|
| Outcome Measure | B (95% Confidence Interval)* | p-value | d |
| TMT B/A | 0.20 (−0.01 – 0.40) | 0.062 | 0.17 |
| WAIS-PSI Composite Score | 0.86 (−1.89 – 3.61) | 0.539 | 0.05 |
| Hearing Impairment in Both Ears (n = 67) | |||
| Outcome Measure | B (95% Confidence Interval)* | p-value | d |
| TMT B/A | 0.37 (0.07 – 0.66) | 0.014 | 0.31 |
| WAIS-PSI Composite Score | −1.00 (−4.86 – 2.85) | 0.611 | 0.06 |
Abbreviations: TBI, Traumatic Brain Injury; B, Mean Difference between Hearing Impairment and No Hearing Impairment; d, Cohen’s effect size; TMT, Trail Making Test; WAIS-PSI, Wechsler Adult Intelligence Scale, fourth edition, Processing Speed Index
Adjusted for age (years), sex (male vs. female), race (Caucasian vs. non-Caucasian), education (years), pre-injury history of hearing loss (yes vs. no), loss of consciousness (yes vs. no), post-traumatic amnesia (yes vs. no. vs. suspected), ED arrival GCS (per-unit increase), CT intracranial pathology (yes vs. no)
Differential associations between TBI-related hearing impairment and cognition by TBI severity
Among participants with mild TBI (GCS 13 – 15), TBI-related hearing impairment was associated with a 0.23 greater average adjusted TMT B/A compared to no TBI-related hearing impairment (95% CI = 0.07 – 0.40; p = 0.005) (Supplemental Table 3). TBI-related hearing impairment was not significantly associated with WAIS-IV PSI. Among participants with moderate-to-severe TBI (GCS <13), TBI-related hearing impairment was more strongly associated with TMT B/A and WAIS-IV PSI, though the number of participants in this subgroup was small (n = 97), with wide confidence intervals around estimates and non-significant effects (BTMT B/A = 0.42; 95% CI = −0.65 – 1.49; p = 0.436; BWAIS- IV PSI = 7.93; 95% CI = −0.94 – 16.80; p = 0.079). When stratifying participants by CT pathology across all GCS, TBI-related hearing impairment was significantly associated with TMT B/A (B = 0.38; 95% CI = 0.16 – 0.60; p = 0.001) among those with CT intracranial pathology (Supplemental Table 4). The association between TBI-related hearing impairment and WAIS-IV PSI was nominally significant (p = 0.029). TBI-related hearing impairment was not significantly associated with TMT B/A or WAIS-IV PSI among participants without intracranial pathology on CT.
Discussion
In this large cohort of Level I trauma center patients with TBI, we found a small but significant association between TBI-related hearing impairment and executive dysfunction. These data reflect a possible decrease in higher-level cognitive skills at 6 months post-TBI among those with, versus without, TBI-related hearing impairment at 2 weeks post-TBI.
The existing research on auditory complications following TBI and associations with cognition is limited. In a study of 22 patients with a closed head injury, 80% who had impairment in central auditory function performed worse than expected on 1 or more cognitive tests that examined working memory capacity, verbal information processing speed, phonological processing, and verbal inference-making ability, compared with 44% who did not have central auditory processing disorders.22 Another study with 9 participants with TBI and 6 control participants without TBI found a significant correlation between speech processing in the presence of 2 simultaneous talkers and standardized measures of processing speed, as well as a correlation between processing speed and subjective assessment of listening effort while performing various speech-in-noise tasks.23 A study using a matched-groups design with 33 participants, 13 of whom had mild TBI and 20 of whom had no history of TBI or other neurological disorder, found differential associations between performance on monaural and binaural speech-in-speech and speech-in-noise tasks and cognitive ability, measured by tests of attention, processing speed, and working memory.24 Finally, a case study of an adult patient with TBI and mild neurosensory hearing loss reported that auditory training was not associated with improvement in long-term cognitive function.36 Our study extends these findings by demonstrating, in a well-characterized and large sample of participants with TBI, hearing impairment was significantly associated with worse executive functioning compared with no hearing impairment. Interestingly, when stratifying participants based on hearing impairment in 1 ear only or both ears, the association between TBI-related hearing impairment and executive functioning was significant only among participants who reported bilateral hearing impairment. In the subgroup with moderate-to-severe TBI, associations between hearing impairment and executive functioning and processing speed were medium-to-large in magnitude, but were not significant, perhaps as an artifact of the small sample size of this subgroup. Moreover, only participants with CT intracranial pathology showed a significant association between TBI-related hearing impairment and executive functioning. This may hint at a graded relationship between TBI severity and hearing-related cognitive impairment, although a larger sample of moderate-to-severe TBI patients with an early assessment of hearing loss would be needed to further investigate this possibility.
Although several theories have been proposed, mainly in the context of age-related hearing loss, the underlying mechanism of the hearing-cognition relationship is not well-understood. Some proposed mechanisms that may apply to individuals with TBI-related hearing impairment include sensory degradation/deprivation, cognitive resource allocation/depletion, and social isolation/depression.37 The sensory degradation or deprivation hypothesis postulates that hearing impairment results in reduced input to the brain, leading to neural degradation and impaired function.38,39 The cognitive resource allocation or depletion hypothesis centers on the detrimental effect that degraded sensory signals may have on higher-order thinking and memory due to rearrangement of cognitive resources necessary for signal processing.40,41 Finally, hearing impairment may lead to social isolation and depression which, in turn, increases risk of cognitive dysfunction.42,43 It is possible, too, that the mechanism is a combination of any or all of these factors in the context of impairment due to trauma-related structural injury.44 In addition to conductive and sensorineural hearing loss, hearing impairment resulting from TBI can produce central auditory processing deficits. The shearing or stretching forces from trauma can disrupt the parallel organization of the brainstem and reduce the ability to localize sound or to make small frequency and temporal distinctions in the presence of competing stimuli.45 This reduction in binaural processing can result in a decreased ability of the auditory system to distinguish between multiple sound sources or to maintain speech recognition ability in the presence of background noise. Concurrent damage to the auditory pathway and central auditory system from TBI may, in part, be responsible for the greater impact on hearing loss among individuals with TBI compared to those without TBI. Most of the evidence regarding the effects of TBI, particularly related to blast exposure, on the central auditory system come from animal studies; however, these findings suggest that the effects of sensory degradation and cognitive load both impact the hearing-cognition relationship following TBI exposure.
This study has a number of strengths, including the prospective longitudinal evaluation, large sample size, and standardization of cognitive outcome assessment. However, important limitations exist, including possible misclassification of hearing impairment status due to use of self-reported hearing impairment. The use of self-report hearing loss has been validated against audiometry and shown to have moderate-to-good accuracy,46–48 but does not reflect performance in patients with TBI. Studies examining the concordance between subjective hearing loss versus objective hearing loss following TBI are limited and further research is needed. There may also be discrepancies in self-reported versus surrogate-reported hearing problems, with greater variability depending on severity.49 Additionally, although we used TMT B/A to derive a purer score of executive functioning, some studies conclude that in individuals with TBI, the B/A ratio score does not appear to enhance the clinical utility of the TMT.50 This concern does not appear to apply to our study, given that we found similar findings for TMT Part B completion time as for TMT B/A. Models were adjusted for key potential confounders of the association between hearing impairment and cognition, but residual or unmeasured confounding may still exist, and therefore results should be interpreted as associations rather than causal relationships. We lacked sample size among those with moderate-to-severe TBI to adequately power studies of the association between TBI-related hearing impairment and cognition in this subgroup. Finally, our results may not be generalizable to the broader TBI population since the TRACK-TBI study enrolled only participants seen at academic Level I trauma centers across the United States who received head CT scans and those who could provide self-reported hearing impairment at 2 weeks post-TBI, which means more severely injured individuals were not included in the study.
Conclusion
This prospective observational study of participants with TBI indicated that hearing impairment at 2 weeks post-TBI was significantly associated with worse executive functioning at 6 months, particularly for individuals with acute intracranial findings on clinical head CT scans and those with hearing impairment in both ears. These findings, along with the strongest associations observed among TBI patients with hearing impairment in both ears, suggest that degradation of incoming auditory signals may be, in part, responsible for impaired cognition. Future studies are needed to investigate: (1) the nature of the subjective hearing loss reported at 2 weeks; (2) whether objective measures can differentiate the underlying cause(s) at that point in time; (3) how well subjectively reported hearing loss following TBI correlates with more objective audiological assessment of hearing; and (4) whether the self-reported hearing loss persists beyond 2 weeks post-TBI exposure.
Supplementary Material
Acknowledgements:
We thank Jason Barber, MS, University of Washington, for his assistance with obtaining the data files and Kimberly Boase, University of Washington, for her advice on use of neuropsychological tests in TRACK-TBI. We also acknowledge other TRACK-TBI Investigators: Neeraj Badjatia, MD, University of Maryland; Ramon Diaz-Arrastia, MD PhD, University of Pennsylvania; Ann-Christine Duhaime, MD, MassGeneral Hospital for Children; V Ramana Feeser, MD, Virginia Commonwealth University; Etienne Gaudette, PhD, University of Southern California; Shankar Gopinath, MD, Baylor College of Medicine; C. Dirk Keene, MD PhD, University of Washington; Christopher Madden, MD, UT Southwestern; Randall Merchant, PhD, Virginia Commonwealth University; Pratik Mukherjee, MD PhD, University of California, San Francisco; Laura B. Ngwenya, MD, PhD, University of Cincinnati; David Okonkwo, MD PhD, University of Pittsburgh; Claudia Robertson, MD, Baylor College of Medicine; David Schnyer, PhD, UT Austin; Sabrina Taylor, PhD, University of California, San Francisco; Mary Vassar, RN MS, University of California, San Francisco; John K. Yue, MD, University of California, San Francisco; Ross Zafonte, Harvard Medical School.
Conflicts of Interest and Source of Funding:
The TRACK-TBI study was supported by the National Institute of Neurological Disorders and Stroke (NINDS) grant number U01 NS06090 and US Department of Defense grant number W81XWH-14-2-0176. One Mind provided funding for TRACK-TBI patient stipends and support to clinical sites. Dr. Nelson’s effort was also funded by NINDS grant number R01 NS110856. For the remaining authors none were declared.
References
- 1.Lew HL, Jerger JF, Guillory SB, Henry JA. Auditory dysfunction in traumatic brain injury. J Rehabil Res Dev 2007;44:921–928. [DOI] [PubMed] [Google Scholar]
- 2.Fausti SA, Wilmington DJ, Gallun FJ, Myers PJ, Henry JA. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. J Rehab Res Dev 2009;46:797–810. [DOI] [PubMed] [Google Scholar]
- 3.Lew HL, Garvert DW, Pogoda TK, et al. Auditory and visual impairments in patients with blast-related traumatic brain injury: effect of dual sensory impairment on Functional Independence Measure. J Rehabil Res Dev 2009;46:819–826. [DOI] [PubMed] [Google Scholar]
- 4.Lew HL, Pogoda TK, Baker E, et al. Prevalence of dual sensory impairment and its association with traumatic brain injury and blast exposure in OEF/OIF veterans. J Head Trauma Rehabil 2011;26:489–496. [DOI] [PubMed] [Google Scholar]
- 5.Penn C, Watermeyer J, Schie K. Auditory disorders in a South African paediatric TBI population: some preliminary data. Int J Audiol 2009;48:135–143. [DOI] [PubMed] [Google Scholar]
- 6.Vadala R, Giugni E, Pezzella FR, Sabatini U, Bastianello S. Progressive sensorineural hearing loss, ataxia and anosmia as manifestation of superficial siderosis in post traumatic brain injury. Neurol Sci 2013;34:1259–1262. [DOI] [PubMed] [Google Scholar]
- 7.Swan AA, Nelson JT, Swiger B, et al. Prevalence of hearing loss and tinnitus in Iraq and Afghanistan Veterans: A Chronic Effects of Neurotrauma Consortium study. Hear Res 2017;349:4–12. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths MV. The incidence of auditory and vestibular concussion following minor head injury. J Laryngol Otol 1979;93:253–265. [DOI] [PubMed] [Google Scholar]
- 9.Carroll LJ, Cassidy JD, Holm L, Kraus J, Coronado VG; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004:113–125. [DOI] [PubMed] [Google Scholar]
- 10.Schell A, Kitsko D. Audiometric outcomes in pediatric temporal bone trauma. Otolaryngol Head Neck Surg 2016;154:175–180. [DOI] [PubMed] [Google Scholar]
- 11.Burchhardt DM, David J, Eckert R, Robinette NL, Carron MA, Zuliani GF. Trauma patterns, symptoms, and complications associated with external auditory canal fractures. Laryngoscope 2015:125:1579–1582. [DOI] [PubMed] [Google Scholar]
- 12.Kanavati O, Salamat AA, Tan TY, Hellier W. Bilateral temporal bone fractures associated with bilateral profound sensorineural hearing loss. Postgrad Med J 2016;92:302–303. [DOI] [PubMed] [Google Scholar]
- 13.Humes LE, Joellenbeck LM, Durch JS. Noise and military service: implications for hearing loss and tinnitus. The National Academies Press; 2006. [Google Scholar]
- 14.Deal JA, Betz J, Yaffe K, et al. ; the Health ABC Study Group. Hearing impairment and incident dementia and cognitive decline in older adults: the Health ABC Study. J Gerontol A Biol Sci Med Sci 2017;72:703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman B, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology 2011;25:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci 2011;66A:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognition decline among older adults. JAMA Intern Med 2013;173:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2018;144:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yumba WK. Cognitive processing speed, working memory, and the intelligibility of hearing aid-processed speech in persons with hearing impairment. Front Psychol 2017;8:1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lux WE. A neuropsychiatric perspective on traumatic brain injury. J Rehabil Res Dev 2007;44:951–962. [DOI] [PubMed] [Google Scholar]
- 21.Myers PJ, Wilmington DJ, Gallun FJ, et al. Hearing impairment and traumatic brain injury among soldiers: special considerations for the audiologist. Semin Hear 2009;30:5–27. [Google Scholar]
- 22.Bergemalm PO, Lyxell B. Appearances are deceptive? Long-term cognitive and central auditory sequalae from closed head injury. Int J Audiol 2005;44:39–49. [DOI] [PubMed] [Google Scholar]
- 23.Krause MO, Kennedy MRT, Nelson PB. Masking release, processing speed and listening effort in adults with traumatic brain injury. Brain Inj 2014;28:1473–1484. [DOI] [PubMed] [Google Scholar]
- 24.Hoover EC, Souza PE, Gallun FJ. Auditory and cognitive factors associated with speech-in-noise complaints following mild traumatic brain injury. J Am Acad Audiol 2017;28:325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manley GT, MacDonald CL, Markowitz A, et al. The Traumatic Brain Injury Endpoints Development (TED) Initiative: progress on a public-private regulatory collaboration to accelerate diagnosis and treatment of traumatic brain injury. J Neurotrauma 2017;34:2721–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yue JK, Vassar MJ, Lingsma HF, et al. Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma 2013;30:1831–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Army Individual Test Battery. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Office; 1994. [Google Scholar]
- 28.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Therapy and clinical interpretation. Tucson, AZ: Neuropsychological Press; 1985. [Google Scholar]
- 29.Wechsler D Wechsler Adult Intelligence Scale, fourth edition (WAIS-IV). San Antonio, TX: The Psychological Corporation; 2008. [Google Scholar]
- 30.Salthouse TA. What cognitive abilities are involved in trail-making performance? Intelligence 2011;39:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switch paradigm. J Clin Exp Neuropsychol 2000;22:518–528. [DOI] [PubMed] [Google Scholar]
- 32.Christidi F, Kararizou E, Triantafyllou N, Anagnostouli M, Zalonis I. Derived Trail Making Test indices: demographics and cognitive background variables across the adult life span. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2015;22:667–678. [DOI] [PubMed] [Google Scholar]
- 33.Dupuis K, Pichora-Fuller MK, Chasteen AL, Marchuk V, Singh G, Smith SL. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2015;22:413–437. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York, NY: Academic Press; 1988. [Google Scholar]
- 35.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 36.Murphy CFB, Fillippini R, Palma D, Zalcman TE, Lima JP, Schochat E. Auditory training and cognitive functioning in adult with traumatic brain injury. Clinics (Sao Paulo) 2011;66:713–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fulton SE, Lister JJ, Bush ALH, Edwards JD, Andel R. Mechanisms of the hearing-cognition relationship. Semin Hear 2015;36:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. J Acoust Soc Am 1995;97:593–608. [DOI] [PubMed] [Google Scholar]
- 39.Schwaber MK, Garraghty PE, Kaas JH. Neuroplasticity of the adult primate auditory cortex following cochlear hearing loss. Am J Otol 1993;14:252–258. [PubMed] [Google Scholar]
- 40.Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging 2009;24:761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci 2011;31:12638–12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gopinath B, Hickson L, Schneider J, et al. Hearing-impaired adults are at increased risk of experiencing emotional distress and social engagement restrictions five years later. Age Ageing 2012;41:618–623. [DOI] [PubMed] [Google Scholar]
- 43.Wilson RS, Barnes LL, Aggarwal NT, et al. Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology 2010;75:990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li KZ, Lindenberger U. Relations between aging sensory/sensorimotor and cognitive functions. Neurosci Biobehav Res 2002;26:777–783. [DOI] [PubMed] [Google Scholar]
- 45.Gallun FJ, Lewis MS, Folmer RL, et al. Implications of blast exposure for central auditory function: a review. J Rehab Res Dev 2012;49:1059–1074. [DOI] [PubMed] [Google Scholar]
- 46.Nondahl DM, Cruickshanks KJ, Wiley TL, Tweed TS, Klein R, Klein BE. Accuracy of self-reported hearing loss. Audiology 1998;37:295–301. [DOI] [PubMed] [Google Scholar]
- 47.Vermiglio AJ, Soli SD, Fang X. An argument for self-report as a reference standard in audiology. J Am Acad Audiol 2018;29:206–222. [DOI] [PubMed] [Google Scholar]
- 48.Sindhusake D, Mitchell P, Smith W, et al. Validation of self-reported hearing loss: the Blue Mountains Hearing Study. Int J Epidemiol 2001;30:1371–1378. [DOI] [PubMed] [Google Scholar]
- 49.Hart T, Whyte J, Polansky M, et al. Concordance of patient and family report of neurobehavioral symptoms at 1 year after traumatic brain injury. Arch Phys Med Rehabil 2003;84:204–213. [DOI] [PubMed] [Google Scholar]
- 50.Martin TA, Hoffman NM, Donders J. Clinical utility of the trail making test ratio score. Appl Neuropsychol 2003;10:163–169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


