Abstract
Internuclear distances represent one of the main structural parameters in molecular structure determination using solid-state NMR spectroscopy, complementing chemical shifts and orientational restraints. Although a large number of magic-angle-spinning (MAS) NMR techniques have been available for distance measurements, traditional 13C and 15N NMR experiments are inherently limited to distances of a few angstroms due to the low gyromagnetic ratios of these nuclei. Recent development of fast MAS triple-resonance 19F and 1H NMR probes has stimulated the design of MAS NMR experiments that measure distances in the 1–2 nm range with high sensitivity. This review describes the principles and applications of these multiplexed multidimensional correlation distance NMR experiments, with an emphasis on 19F- and 1H-based distance experiments. Representative applications of these long-distance NMR methods to biological macromolecules as well as small molecules are reviewed.
Graphical Abstract
1. Introduction
Structural studies of biomolecules using nuclear magnetic resonance (NMR) spectroscopy provide chemical shifts, orientation restraints, and inter-atomic distance restraints. Whereas chemical shifts reveal the chemical structure of functional groups and the three-dimensional structure in terms of bond torsion angles, distance restraints define the relative positions of atoms in space. In high-resolution magic-angle-spinning (MAS) solid-state NMR spectroscopy1, distances between two atomic nuclei are measured through nuclear spin dipole-dipole coupling, ω12 ∝ γ1γ2/r3 . This dipolar coupling scales inversely with the cube of the distance r, and is proportional to the nuclear gyromagnetic ratio, γ, which determines the size of the magnetic dipole moment of the nucleus. Distance information is either encoded qualitatively in peak intensities in multidimensional correlation MAS NMR spectra2–4, or measured quantitatively using dipolar recoupling experiments under MAS5. However, in biological molecules, some of the most common spin-1/2 nuclei such as 13C and 15N have relatively small γ, which makes it difficult to measure weak dipolar couplings for the structurally informative, long, distances within the relaxation time of the nuclear spins. The most common distance range that is measurable in 13C and 15N solid-state NMR experiments is less than 7–8 Å (Fig. 1A, B). A recent computational study found that, with distance restraints of < 7 Å, 9–15 distances per amino acid residue are required to obtain an accurate structural model of single-domain proteins6. Thus, for a moderately sized, 100-residue protein, over 1500 short-range distances would be required to determine an accurate structure. For multi-domain proteins or oligomeric proteins, even this large number of short-range distance restraints are insufficient to produce accurate structures, and additional data from other techniques such as cryoEM are required to define the relative positions and orientations of different domains. Therefore, long-range distance restraints are critical for determining the structures of multi-domain proteins and protein-protein complexes. Long-range distances also provide information about protein conformational changes in response to ligand binding, pH changes, and ion concentration changes, which are common in biology.
Figure 1.
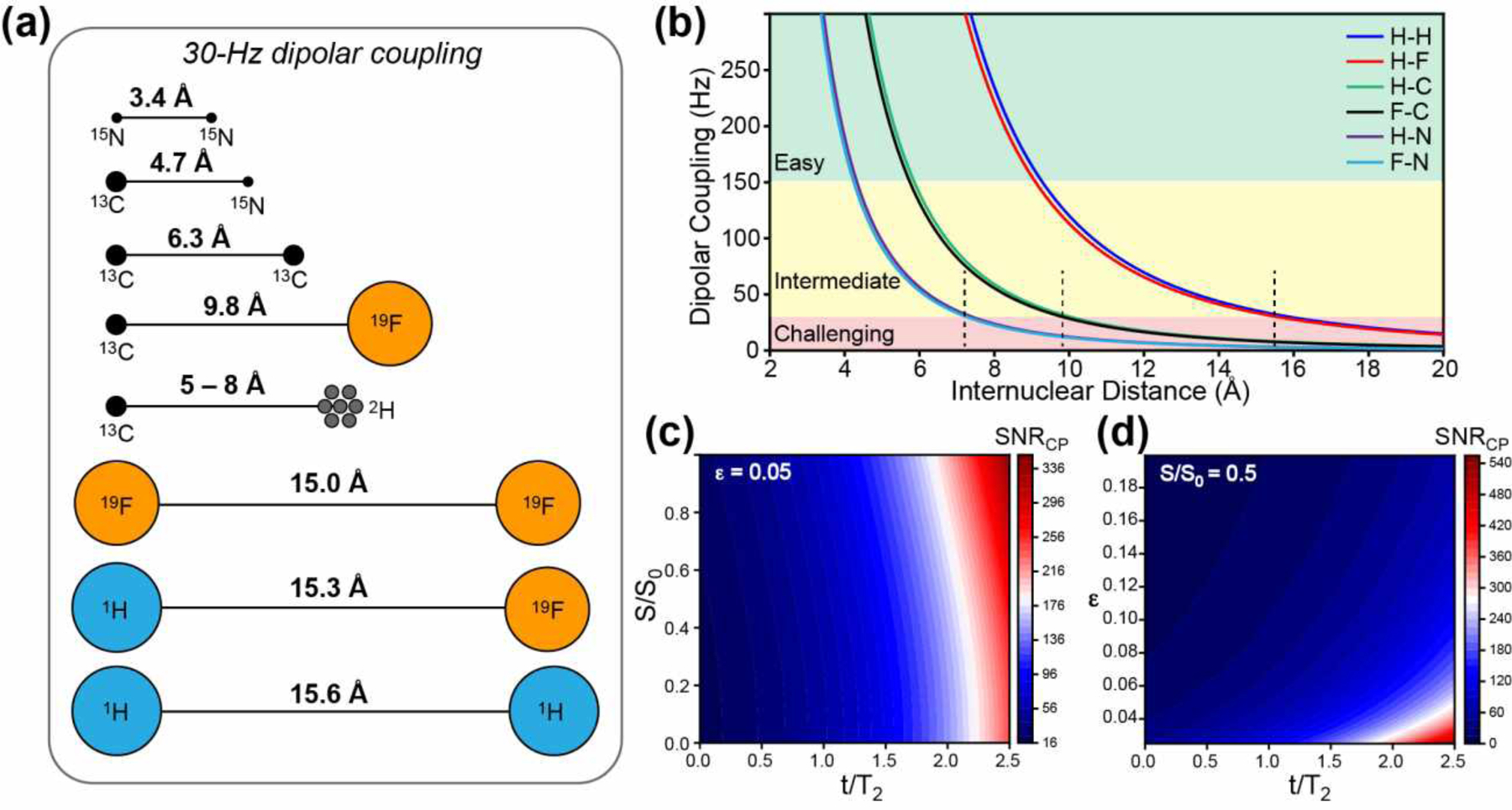
Distance rulers in solid-state NMR spectroscopy. (a) Schematic representation of distances corresponding to a 30-Hz dipolar coupling between NMR-active nuclei that are commonly studied in biological systems. (b) Dipolar coupling strength as a function of internuclear distance for common spin pairs. The longest distances measurable for the intermediate difficulty regime of 30–150 Hz are marked by dashed lines. These distance upper bounds are ~7 Å for 1H-15N and 19F-15N spin pairs, ~10 Å for 1H-13C and 19F-13C spin pairs, and ~15 Å for 1H-1H and 1H-19F spin pairs. (c) Required CP sensitivity as a function of the REDOR dephasing value S/S0 and T2-parameterized REDOR mixing time t/T2, if an uncertainty ε of 0.05 is desired for the measured S/S0 value. (d) Required CP sensitivity as a function of the desired uncertainty ε and t/T2 for an S/S0 value of 0.5.
A number of approaches have been developed in the last decade to extend the distance reach of solid-state NMR spectroscopy. One approach is to measure paramagnetic relaxation enhancements and pseudocontact shifts caused by stable radicals or paramagnetic metal ions. This paramagnetic NMR approach can be exploited to provide information about distances above ~2 nm, where the uncertainty in the position of the flexible paramagnetic tag is small compared to the distance of interest. Paramagnetic NMR has been reviewed recently7–9 and will not be discussed in the current review. Instead, this review focuses on the recent development of 19F and 1H solid-state NMR techniques for measuring long distances. 1H and 19F are the two highest-γ spin-1/2 nuclei among stable isotopes and can be readily exploited in MAS NMR experiments to measure distances to ~2 nm. We review multidimensional 19F NMR techniques and 1H-detected NMR techniques that are tailored to high magnetic fields and fast MAS conditions for distance measurement. We also review 13C and 15N NMR techniques that provide long distances through 1H-mediated polarization transfer. We survey recent applications of these 19F, 1H, 13C and 15N long-distance techniques to membrane proteins, amyloid fibrils, and many biomolecular complexes. For 19F NMR distance measurements, we also review some earlier applications to biomolecular systems to provide a more historical perspective.
2. Heteronuclear 19F-Based Distance Measurements
2.1. Long-Range 19F Heteronuclear Distance NMR Techniques
Rotational-echo double-resonance (REDOR) is one of the most robust heteronuclear dipolar recoupling techniques in MAS solid-state NMR10,11. By applying two 180° pulses per rotation period to the observed and dephasing nuclei, one reintroduces distance-dependent dipolar couplings (Fig. 2A). This dipolar recoupling interferes with the formation of a spin echo, whose T2-normalized intensity can be written as S/S0, where S and S0 denote the intensities of the dephased and control spectra, respectively. Since the invention of REDOR, a large number of heteronuclear and homonuclear dipolar recoupling sequences have been proposed and demonstrated in MAS NMR12–14. However, REDOR remains arguably the most widely used distance-measuring technique due to its simplicity and lack of scaling factors for the recoupled dipolar interaction. As a pulsed technique, REDOR has a relatively large recoupling bandwidth unless quadrupolar nuclei with large spectral widths are involved. Pulse imperfections in long REDOR pulse trains can be compensated for experimentally and taken into account in numerical simulations15–17. The simplicity of the REDOR pulse sequence also allows density operator propagation to be readily calculated for multi-spin systems18,19. These features make REDOR the method of choice for measuring heteronuclear distances.
Figure 2.
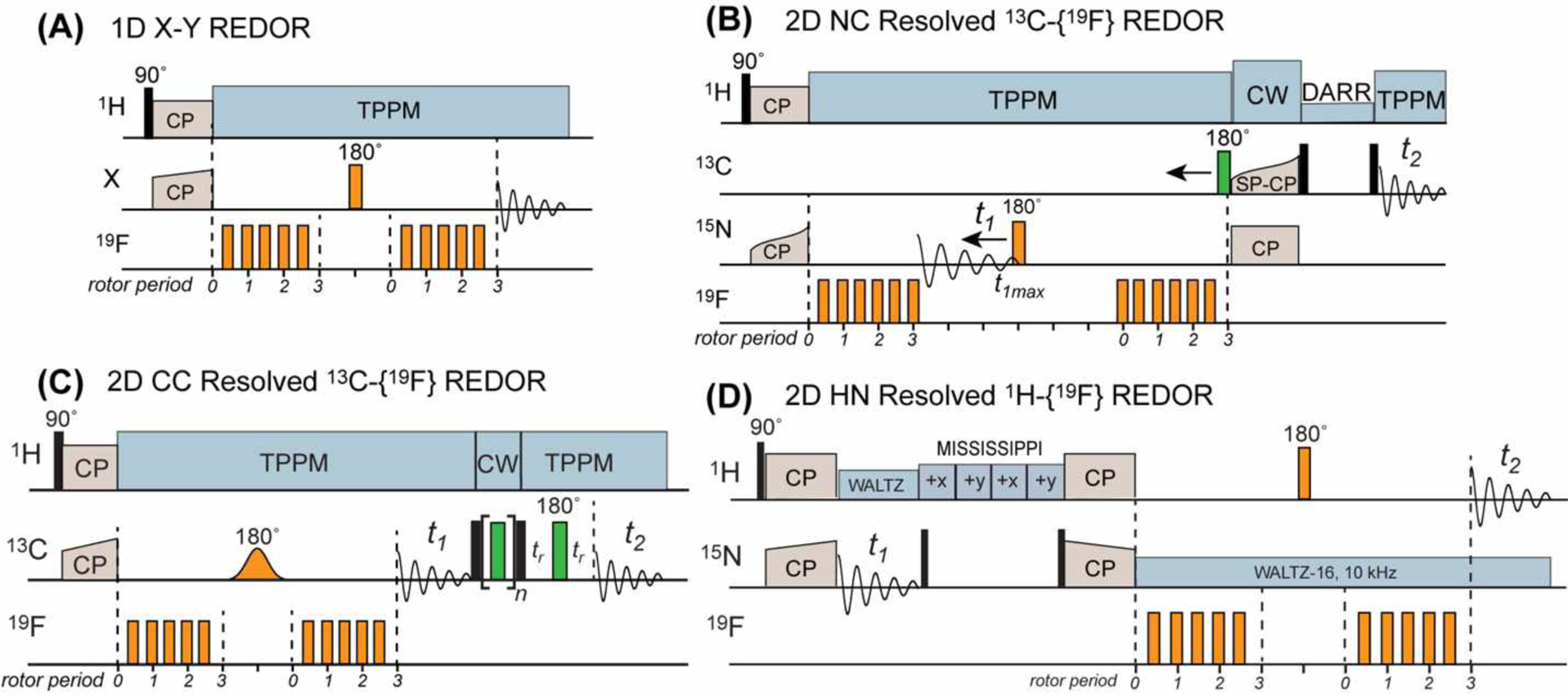
19F-based REDOR pulse sequences for measuring long distances. (A) Standard 1D X-19F REDOR, where the X nucleus can be 13C, 31P, 15N, or other nuclei10. For simplicity, the version of REDOR with most 180° pulses on the 19F channel is shown, but other variations such as alternating 180° pulses on the two channels can also be implemented. (B) 2D 15N-13C resolved 13C-19F REDOR (FRESH) pulse sequence21. (C) 2D 13C-13C resolved 13C-19F REDOR pulse sequence22. The 13C polarization transfer is implemented using RFDR here, but other polarization transfer schemes such as Dipolar-Assisted Rotational Resonance (DARR) can also be used. An optional pre-detection Hahn echo allows the measurement of spectra with a clean baseline. (D) 2D 15N-1H resolved 1H-19F REDOR23.
The feasibility of long-distance measurement by solid-state NMR is determined by two factors: the buildup time for the dipolar coupling of interest and the nuclear spin relaxation time. Fig. 1B shows the dipolar coupling constant as a function of distances for common heteronuclear spin-1/2 pairs in solid-state NMR. Using 30 Hz as the lower bound of readily measurable dipolar couplings, heteronuclear distances involving 15N cannot be measured beyond ~7 Å, whereas distances involving 19F and 1H can be measured to ~15 Å. The signal-to-noise ratio of the cross-polarization (CP) spectra (SNRCP) that is required to measure REDOR distances with sufficient precision depends on the T2 relaxation time of the observed spin and the desired precision of the S/S0 values. Based on the propagated uncertainty ε of the S/S0 value, it can be shown that . Therefore, the higher the desired precision (small ε), the larger the required CP sensitivity. The shorter the T2, the larger the required CP sensitivity (Fig. 1C, D).
To increase the dipolar coupling strength for a given distance, Schaefer and coworkers have long exploited the high-γ 19F spin. An early example was the measurement of 13C-19F distances in the 143 kDa α2β2 tetrameric enzyme, tryptophan synthase. 13C-labeling of Tyr and 4-19F labeling (4F) of Phe20 allowed the authors to monitor four Tyr-Phe pairs in the β subunit that have 13C-19F distances of less than 8 Å. Binding of the ligand, Ser, caused only minor changes to 13C-19F REDOR dephasing, with an estimated distance change of less than 1 Å, indicating that ligand binding did not perturb the conformation of an indole tunnel in the enzyme. This pioneering work was conducted on a 4.7 Tesla NMR spectrometer (with a 19F Larmor frequency of 188 MHz) using a quadruple-resonance (1H, 19F, 13C and 15N tuned) MAS probe, and the sample was spun at 5 kHz. Both 13C-observed 19F-dephased REDOR spectra and the reverse 19F-observed 13C-dephased REDOR spectra were measured.
In the 25 years since this study, increases in magnetic field strengths and MAS probes have led to significant advances in the 19F REDOR NMR methodology. 19F REDOR and Transferred-Echo Double Resonance (TEDOR) experiments have now been demonstrated for MAS frequencies of 20–40 kHz and at a magnetic field of 14.1 Tesla, corresponding to a 19F Larmor frequency of 564 MHz21–24. The 1D REDOR pulse sequence has been extended to two dimensions, in order to extract many dipolar couplings from a single pair of 2D spectra, thus massively speeding up distance measurement and structure determination.
The first 2D-resolved 19F REDOR experiment was reported by Rienstra and coworkers21. Using a 3.2 mm MAS probe simultaneously tuned to 1H, 19F, 13C, and 15N, the authors resolved the 15N and 13C chemical shifts in 2D NcaCX spectra while encoding the 15N-19F dipolar couplings by REDOR (Fig. 2B). In this so-called FRESH experiment, the 15N REDOR 180° pulse is moved to define the t1 period of a constant-time 15N chemical shift period while retaining 15N-19F REDOR dipolar recoupling. The experiment was demonstrated on 13C, 15N-labeled GB1 containing a single fluorine at 5F-W43. Some of the strongest cross-peaks in the REDOR difference (ΔS) spectra correspond to distances of 4.7–7.7 Å, and weaker cross-peaks corresponding to distances of 9–12 Å were also observed. The protein had a relatively low 19F incorporation level of ~40%, which reduced the spectral sensitivity. Nevertheless, the eleven measured distances were found to be in good agreement with the high-resolution structure of this model protein.
Since the FRESH technique requires a quadruple-resonance HFCN probe, which is not widely available, Hong and coworkers introduced a triple-resonance 2D 13C-13C resolved 13C-19F REDOR technique in 201822. The 13C-19F REDOR mixing period is inserted before the 13C t1 evolution time, thus encoding the C-F distances of carbons whose chemical shifts are manifested in the indirect dimension of the 2D spectrum (Fig. 2C). In this work, 13C-13C correlation was implemented using radiofrequency-driven recoupling (RFDR)25, although many other polarization transfer sequences can also be used. Compared to the 15N-19F FRESH experiment, this 2D CC-resolved 13C-19F REDOR method has an intrinsically longer distance reach due to the 2.5-fold larger γ of 13C than 15N. The 13C-19F REDOR period features a selective Gaussian 13C 180° pulse to remove the 13C-13C J-coupling and allow mixing times of ~10 ms to be used to measure long 13C-19F distances. These authors used 5F-W43 labeled GB1 and triply 3F-Tyr labeled GB1 to demonstrate this 2D CC resolved 13C-19F REDOR technique. Compared to the FRESH study, the authors increased the fluorine incorporation level to >95% by increasing the glyphosate concentration and optimizing the timing of the glyphosate addition26. With these improvements, the authors observed significant dipolar dephasing for many residues within 10 ms of REDOR mixing, and extracted 35 13C-19F distances of 5–10 Å from the 2D 13C-19F REDOR spectra. The authors also investigated the effects of the 19F chemical shift anisotropy (CSA) on REDOR dephasing using numerical simulations. At MAS frequencies that are smaller than the 19F CSA, additional oscillations are observed in the REDOR dephasing that are absent in the universal REDOR curve10,11,27 obtained under vanishing CSA. The large 19F CSA also reduces the amplitude of REDOR dephasing to the first minimum. Fast MAS ameliorates these deleterious effects, restoring a nearly universal REDOR curve. Experimental parameters such as the radiofrequency (rf) field strength (ω1) and the MAS rate (ωr) are readily accounted for in the simulations.
An even longer distance ruler than 15N-19F and 13C-19F REDOR is 1H-19F REDOR, which exploits the two highest-γ nuclei among stable isotopes. Hong and coworkers first demonstrated this approach in 200428 by detecting the 1H-19F REDOR dephasing of amide protons through their directly-bonded nitrogens. In this proof-of-concept study, a slow MAS frequency of 3.3 kHz was used, therefore 1H-1H homonuclear decoupling was essential for prolonging the proton T2 relaxation time. The experiment was demonstrated on the model peptide formyl-MLF29,30, which incorporated 4F-Phe and 15N-labeled Leu. The authors measured an HN-F distance of 7.7 Å using REDOR mixing times up to ~6 ms. Because the 1H-19F dipolar couplings are scaled down by the homonuclear decoupling sequence, and the imperfection of homonuclear decoupling shortens 1H T2, the advantage of using the high-γ 1H spin for distance measurement is diminished. Thus, this slow-MAS 1H-19F REDOR approach increased the distance reach by a modest margin compared to 13C-19F REDOR.
The bottleneck of 1H homonuclear decoupling was decisively removed by the recent technological development of fast-MAS probes and the ensuing high-sensitivity 1H-detected MAS NMR without complex 1H homonuclear decoupling sequences. Combining the 2D-resolved REDOR approach with 1H detection, Hong and coworkers introduced a 1H-detected 1H-19F REDOR technique to measure many nanometer-range distances with high sensitivity23. The experiment inserts the 1H-19F REDOR period into a 2D 15N-1H heteronuclear correlation (HETCOR) pulse sequence. Amide protons that are close to the fluorine manifest intensities in the 2D REDOR difference (ΔS=S0-S) spectra (Fig. 2D). By combining the high-γ 1H and 19F spins, this 2D REDOR experiment significantly extends the distance reach compared to a 1H-detected 1H-13C REDOR experiment31. The authors demonstrated this experiment on perdeuterated and 13C,15N (CDN)-labeled GB1 that was back-exchanged with protons. A single fluorine at 5F-W43 served as the dephasing nucleus. The average 1H coherence lifetime was 4.2 ms at 30 kHz MAS and nearly doubled to 7.2 ms at 40 kHz MAS, underscoring the importance of fast MAS for measuring long distances. Amide protons that are within ~0.8 nm of the fluorine showed rapid dipolar dephasing to zero within 8 ms, while amide protons that are further than 1.3 nm from the fluorine still exhibited sizeable REDOR dephasing of S/S0 ~0.8 within 10 ms. 1H detection yielded high spectral sensitivity, with signal-to-noise ratios of 100–200 : 1 in the 2D control (S0) spectra. Therefore, this 1H-detected 2D 1H-19F REDOR technique allows rapid and multiplexed measurement of many nanometer-long distances. The main requirement of this technique is that the sample needs to be structurally sufficiently homogeneous to yield well-resolved 2D 15N-1H correlation spectra. The technique can in principle be extended to three dimensions to further resolve the signals. Such an extension will require quadruple-resonance HFCN probes.
2.2. Applications of 19F Heteronuclear Distance Measurements to Biomolecules
2.2.1. Antimicrobial Peptides
The rapid spread of antibiotic-resistant bacteria has stimulated considerable efforts in understanding the mechanisms of action of antimicrobial compounds. Solid-state NMR spectroscopy is well suited for providing molecular insights into the structures and dynamics of these compounds. Many cationic antimicrobial peptides are produced by the innate immune system of animals32,33 and are thought to operate by disrupting the membranes of microorganisms, but their detailed mechanisms of action were poorly understood. Schaefer and coworkers applied 19F REDOR NMR to investigate an antimicrobial peptide called K3, (KIAGKIA)3 (Fig. 3A)34,35. 13C-19F REDOR data of mixed 13C and 19F labeled peptides showed that the peptide forms parallel α-helical dimers in the membrane. By incorporating acyl-chain fluorinated DPPC and DPPG lipids, the authors measured the distances of the lipid tail from site-specifically labeled peptide carbonyls and from the lipid headgroups using 19F-13C and 19F-31P REDOR, respectively. These data indicate that the α-helical peptide is in close contact with the lipid tail. Moreover, the lipid tail – lipid headgroup 19F-31P distances decreased markedly from 9.9 Å in the peptide-free membrane to 7.6 Å in the peptide-bound membrane, indicating that the peptide increased membrane disorder (Fig. 3B, C). Based on these distance results, the authors proposed a toroidal-pore model for the mechanism of membrane permeabilization by this peptide.
Figure 3.
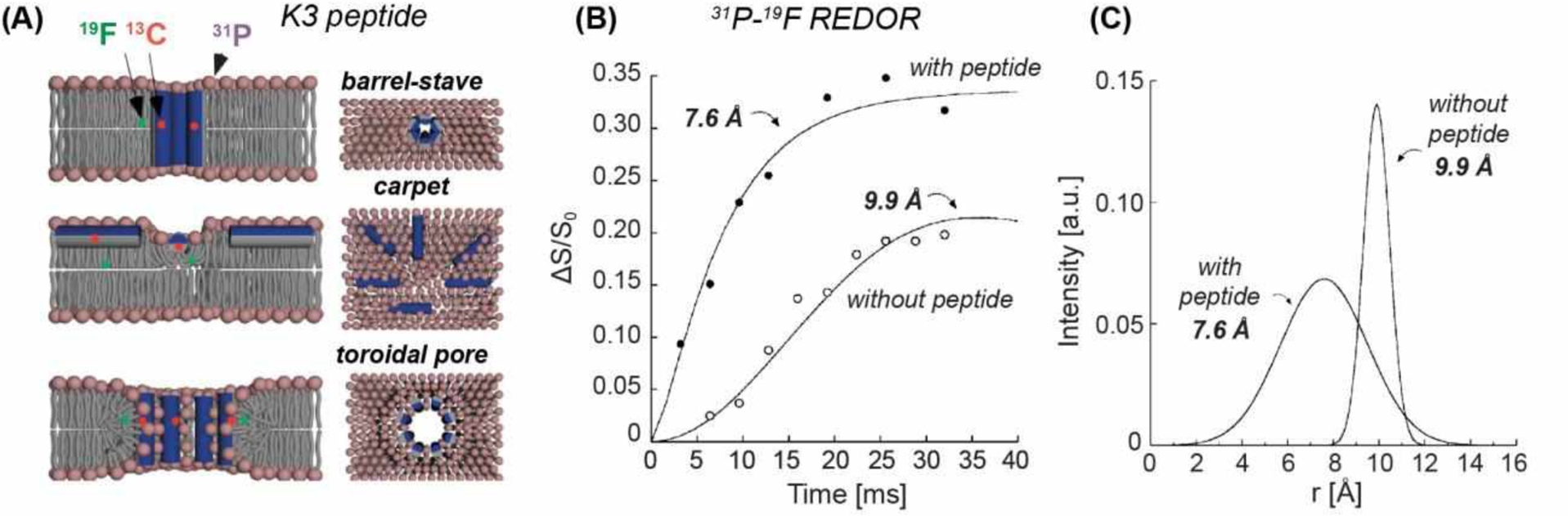
31P-19F REDOR for studying the structure of the antimicrobial peptide K334. (A) Structural and mechanistic models of K3 in lipid membranes. The barrel-stave mode shows minimal membrane disorder and a small pore. The carpet model shows more membrane disorder but no pore. The toroid model shows both membrane disorder and a large pore. (B) 31P-19F REDOR dephasing curves between the lipid headgroup 31P and the fluorinated lipid tail. Peptide binding shortened the P-F distance from 9.9 Å to 7.6 Å. (C) Comparison of the distance distributions between the peptide-free and peptide-bound lipid membranes. K3 binding increased the distance distribution, indicating increased membrane disorder. These data support the toroidal-pore model as the mechanism of membrane disruption by K3.34,35
Hong and coworkers employed 19F REDOR NMR to determine the oligomeric structure of protegrin-1 (PG-1), a β-hairpin antimicrobial peptide36. 19F Centerband-Only Detection of Exchange (CODEX) data showed that PG-1 forms dimers in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) bilayers. To determine whether these dimers are packed in a parallel or antiparallel fashion, and whether the dimer interface consists of the same strand or different strands of the β-hairpin, these authors incorporated 13C and 19F labels at the two ends of the C-terminal β-strand of the hairpin. The measured intermolecular 13C-19F REDOR dephasing indicates a 13C-19F distance of ~10 Å. This supports a parallel packing model in which the C-terminal strands of two β-hairpins face each other, while ruling out antiparallel models. Therefore, 13C-19F distance measurement was instrumental for constraining the oligomeric structure of the PG-1 dimers in lipid membranes.
19F REDOR was also used to elucidate the binding site of a glycopeptide antibiotic in the cell walls of intact whole cells of Staphyloccus aureus37. Fluorinated oritavancin is a vancomycin analog that is effective against vancomysin-resistant enterococci. Schaefer and coworkers used 15N-13C TEDOR to suppress natural abundance 13C signals and selectively detect only the signals of D-Ala 13C that are adjacent to 15N-labeled Gly in the cell wall peptidoglycan. The selected d-Ala 13C signal was then subject to 19F REDOR dephasing to measure the distance from oritavancin. The observed distance of 7.4 Å with a Gaussian distribution of 1.6 Å helped to constrain a molecular model of the peptidoglycan stems in complex with the drug.
13C-19F REDOR was used to investigate the self-assembly and sterol interaction of the antifungal compound, amphotericin B (AmB), in lipid bilayers. Murata and coworkers mixed 13C-labeled AmB with fluorinated AmB and conducted 13C-19F REDOR experiments to measure AmB-AmB separations in POPC bilayers38. The data show that the AmB-AmB separations are longer in ergosterol-containing POPC membranes compared to cholesterol-containing membranes. This result suggests that AmB forms a complex with ergosterol in lipid bilayers while self-aggregating and phase-separating from cholesterol in cholesterol-containing membranes. By combining fluorinated AmB with skip 13C-labeled ergosterol and C26, C27-labeled ergosterol39, the authors further measured AmB-ergosterol 13C-19F distances. The resulting distance distributions indicate heterogeneous AmB-ergosterol interactions, with both head-to-head and head-to-tail interactions possible.
2.2.2. Membrane Proteins
13C-19F REDOR has been fruitfully applied to α-helical membrane proteins to elucidate their structure and dynamics in lipid bilayers4,40. The influenza A M2 protein (AM2) is an acid-activated proton channel that is responsible for viral uncoating after endocytosis41,42. Proton selectivity and gating of this protein are accomplished by two residues, His37 and Trp41, respectively. Hong and coworkers measured the sidechain conformations and separations between these two functional residues using 13C-labeled His37 and 5F-Trp41 and 13C-19F REDOR43. The REDOR dephasing is slower at high pH than at low pH, indicating that the imidazole ring and the indole ring approach each other more closely in the acidic open state of the channel compared to the neutral closed state of the channel. These results led to the model that cation-π interactions at low pH between His37 and Trp41 may be partially responsible for the orders of magnitude smaller proton flux (~1000 s−1) of the channel compared to the measured microscopic histidine proton exchange rates of ~105 s−1 44.
Long-range heteronuclear 19F distances have also been used to probe the oligomeric structures of membrane proteins. Intermolecular distances in these systems are often too long to measure using low-γ nuclei such as 13C and 15N. Using 13C-19F REDOR experiments of mixed 13C and 19F labeled proteins, Hong and coworkers measured interhelical contacts that helped to constrain the high-resolution structures of the influenza BM2 proton channel45 and the SARS-CoV-2 envelope (E) cationic channel46. For the tetrameric BM2, they incorporated 4F-Phe at the native Phe5 and Phe20, and mixed it with 13C-labeled protein at a 1:1 ratio. REDOR dephasing of the 13C signals by 19F yielded distances of 4–8 Å. Moreover, the interhelical distances are longer for the low-pH open state of the channel than for the high-pH closed state, indicating that the four-helix bundle is more loosely packed in the open state (Fig. 4A–C). The final high-resolution structures of the closed and open BM2 channel show that the four-helix bundle activates in a scissor-like fashion, with the helices becoming more tilted and more separated from each other in the open state.
Figure 4.
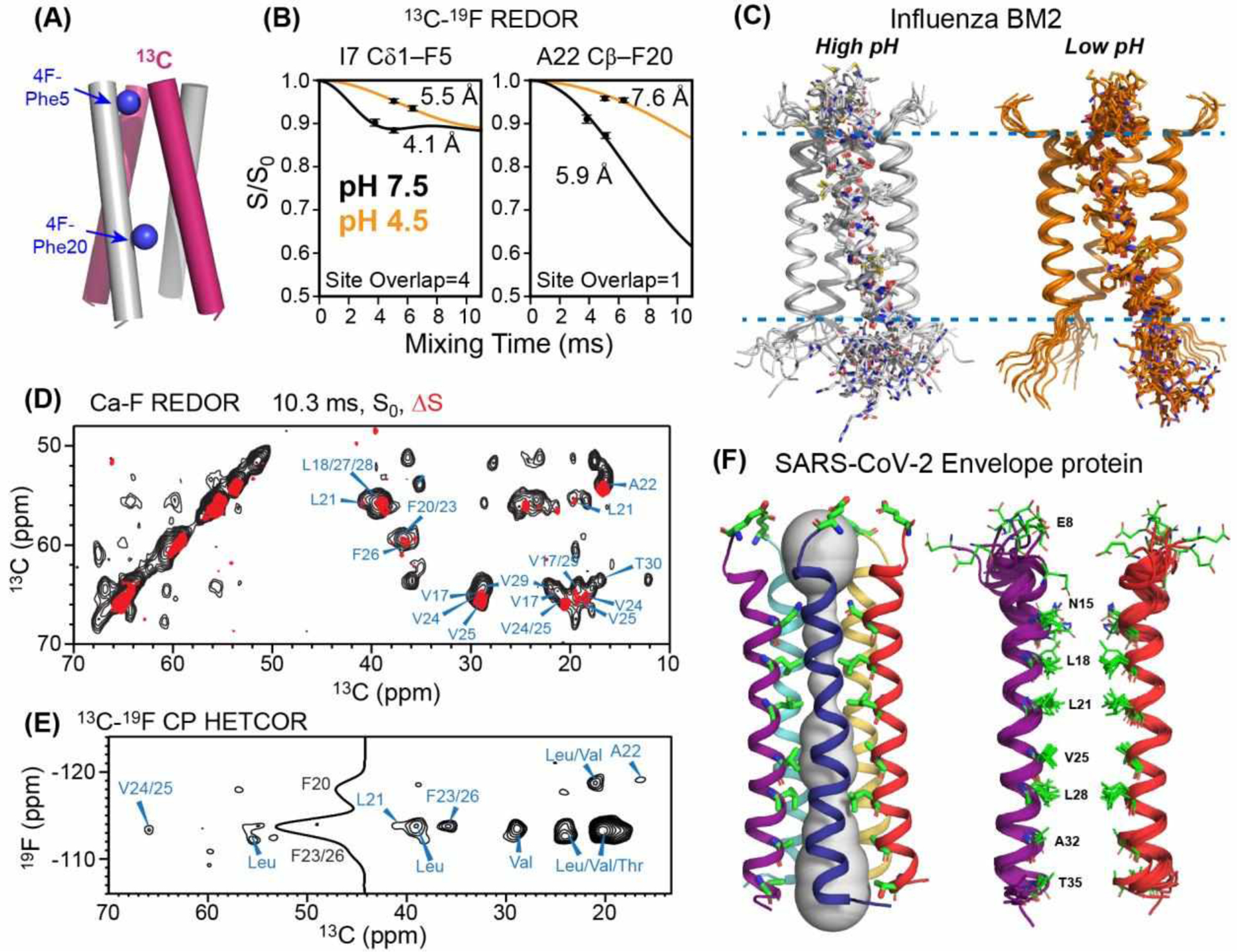
19F-based heteronuclear distance measurements to determine membrane protein structures. (A) Mixed 13C and 19F labeling strategy for measuring interhelical distances in influenza BM2 tetramers45. (B) Representative 13C-19F REDOR data of BM2. The interhelical distances increase from high pH to low pH, indicating that the four-helix bundle is loosened at low pH. (C) BM2 tetramer structures at high and low pH (PDB 6PVR and 6PVT). (D) 2D 13C-13C resolved 13C-19F REDOR spectra of the SARS-CoV-2 envelope protein TMD in lipid bilayers. Control spectrum (S0, black) is overlaid with the difference spectrum (ΔS, red) to show which residues are the closest to the fluorinated Phe residues. (E) 2D 13C-19F HETCOR spectrum to assign the fluorinated Phe residues that are close to each 13C. (F) ETM structure determined in part using these 13C-19F distance restraints. The pentameric helical bundle (left) has a highly hydrophobic pore, as shown for two helices on the right for clarity46 (PDB 7K3G).
The same 13C-19F REDOR strategy was also used to determine the pentameric structure of the SARS-CoV-2 E protein. Solution NMR studies of detergent-bound E since the first SARS epidemic in 2003 yielded inconsistent information about the helical bundle structure, partly due to the scarcity of interhelical distance restraints47,48. Hong and coworkers measured interhelical distances using mixed 13C and 19F labeled proteins and 2D CC-resolved 13C-19F REDOR46. These experiments were carried out on the transmembrane peptide of E (ETM) bound to lipid bilayers that mimic the membrane of the endoplasmic reticulum Golgi intermediate compartment (ERGIC). Because ETM contains three native and regularly spaced Phe residues (F20, F23 and F26), the authors also measured 2D 13C-19F HETCOR spectra to assign the 19F signals. These data yielded 35 interhelical C-F distances, which helped to constrain the high-resolution structure of ETM in lipid bilayers (Fig. 4D–F). The authors also probed the binding site of fluorinated amantadine using 13C-19F REDOR, and found that the drug binds the N-terminus of the channel, in the same location as hexamethylene amiloride. The de novo structure determination of this SARS-CoV-2 membrane protein, carried out during the first six months of the COVID-19 pandemic, demonstrates the power of 19F-based MAS NMR spectroscopy for protein structure determination.
In another study, 13C-19F REDOR was used to investigate the three-dimensional fold and topology of the HIV-1 fusion protein, gp4149. Despite considerable efforts, the structure of the transmembrane domain (TMD) and a membrane-proximal external region (MPER) of gp41 has been controversial in the literature. One discrepancy pertains to whether the MPER is kinked from the TMD or forms a continuous helix. Hong and coworkers incorporated 13C-labeled residues into the TMD and fluorinated Trp678 into the MPER, and measured 13C-19F distances using REDOR. The continuous helix model would predict longer 13C-19F distances (11–13 Å) than the kinked helix model (9–10 Å). The authors found significant dipolar dephasing for I686 and L684 Cα, which correspond to distances of 9–10 Å from the Trp678 sidechain49. Thus, these REDOR data indicate that the MPER-TMD adopts a helix-turn-helix topology in lipid bilayers. Combined with 19F CODEX data (see section 3.4.3 below), these results led to a trimeric umbrella-like fold, which differs from structures found in micelles and bicelles50,51, suggesting the influence of the membrane environment on gp41 assembly.
2.2.3. Nucleic Acids
Nucleic acids are much less studied than proteins by NMR due to challenges associated with their low sequence complexity and the resulting spectral overlap. 31P-19F REDOR52 is an effective probe of nucleic acid structures because of the presence of the high-γ 31P in the nucleic acid backbone and the feasibility of fluorination reactions of aromatic substrates. Drobny and coworkers pioneered the approach of incorporating phosphorothioate (pS) and fluorinated nucleotides such as 2’-deoxy-5-fluorouridine and 2’-deoxy-2’-fluorouridine to measure nanometer 31P-19F distances in DNA and RNAs53. The substitution of a single non-bridging oxygen atom by a sulfur shifts the 31P chemical shift downfield by ~55 ppm, thus giving a well-resolved reporter of the nucleotide without perturbing the backbone conformation54.
Using this 31P-19F REDOR technique, Drobny and coworkers investigated the minor groove width of a DNA oligonucleotide duplex upon binding by a peptide antibiotic, distamycin55. The DNA duplex contains a 2’F-adenine in one chain and a phosphorothioate tag 3.5 residues away from the fluorinated base pair in the other chain. 31P-19F REDOR data showed that the minor groove width is 9.4 Å in the absence of the drug. However, addition of distamycin to a 1 : 1 ratio caused a major distance decrease to 7.0 Å, whereas addition of the drug to a 2:1 ratio caused a major distance increase to 13.6 Å. Thus, these 31P-19F REDOR data revealed a striking structural change of the DNA upon drug binding.
The same approach of phosphorothioate tagging and nucleotide fluorination was also applied to study structural changes of the RNA, HIV TAR, upon binding to the viral regulatory protein tat56. By installing 31P and 19F labels at A27 and U23, respectively, Drobny and coworkers measured 31P-19F distances, and found that tat binding dramatically shortened the distance from 10.3 Å to 6.6 Å (Fig. 5). Thus, 31P-19F REDOR yields insights into the impact of the protein on the conformation of the HIV TAR.
Figure 5.
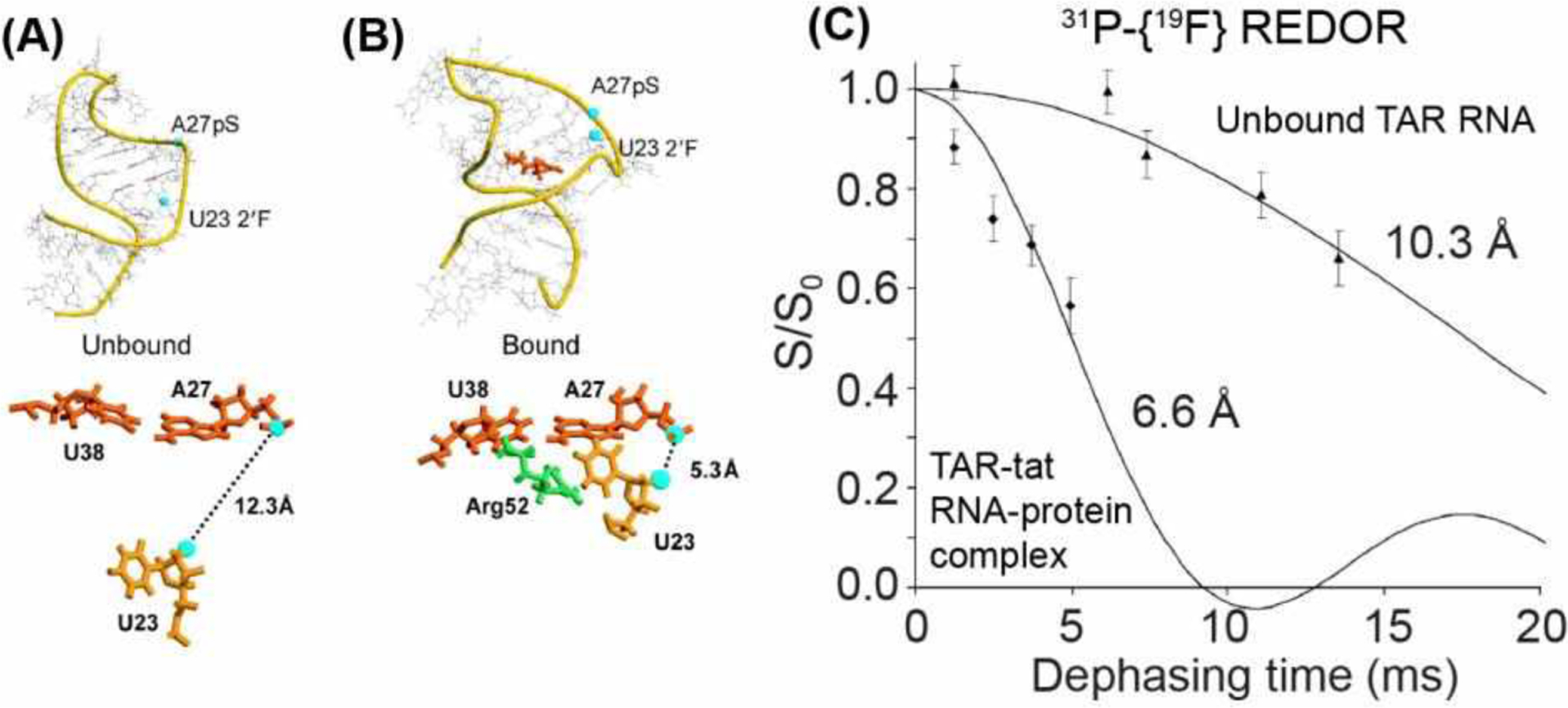
31P-19F REDOR for distance measurements in the tat-TAR complex56. (A) Solution NMR model of TAR RNA (PDB 1ANR), showing locations of the pS-tagged A27 and the fluorodeoxyuridine at U23. (B) Model of tat-bound TAR RNA (PDB 1ANJ), showing a decrease of the 31P-19F distance. The structural model shows that binding of R52 in tat changes the RNA conformation. (C) 31P-19F REDOR dephasing indicates that tat binding shortened the distance between pS-A27 and fluoro-U2356.
Additional studies of protein-nucleic acid complexes explored the flexibility of orthogonal 13C, 15N-labeling of the protein and fluorination of the oligonucleotide. For example, Drobny and coworkers investigated the interactions between the tat protein and TAR RNA using a 13C, 15N-Arg labeled peptide and 5-19F-uracil labeled RNA57. REDOR dephasing of the 13C and 15N signals by 19F yielded distances of 5.6 and 6.6 Å for the Arg Cζ and CO and 15N-19F distances of 4.3 – 6.7 Å. These distances constrained the position of R52 with respect to U23 of the RNA, in good agreement with a solution NMR structure of the tat-TAR complex.
Stivers, Schaefer and coworkers used 31P-19F REDOR to investigate nucleotide binding by a DNA repair enzyme, uracil DNA glycosylase (UDG)58. They introduced a difluorophenyl nucleotide, an analog of uracil, into a DNA duplex, and measured its binding to UDG. These 31P-19F distances can vary from 6 Å to 18 Å, depending on the conformation of uracil. The measured distances indicate a 50 : 50 mixture of B-form DNA and an out-flipped state for the nucleotide. Together with chemical shift and biochemical data, these results led to the conclusion that the difluorophenyl nucleotide forms a metastable intermediate with the enzyme, but the lack of hydrogen bonding precluded the formation of a catalytically productive Michaelis complex.
2.2.4. Biological Assemblies and Protein-Ligand Complexes
19F REDOR NMR has been extensively used to study protein-ligand complexes. An early application of 31P-19F REDOR was to the enzyme 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase bound to two ligands, shikimate 3-phosphate (S3P) and glyphosate (Glp)59. EPSP catalyzes an intermediate step in the biosynthesis of aromatic amino acids via the shikimate pathway. The protein contains only two Trp residues, which were biosynthetically labeled as 6F-Trp, while both ligands contain native 31P atoms, whose chemical shifts are resolved by 12 ppm. Schaefer and coworkers measured 31P-19F distances between each Trp and the ligands. The measured distances of 8.5 Å to 16 Å indicate that the C-shaped apo-enzyme clamps down on the ligands to form a globular ternary complex. This study demonstrated how nanometer distances can give insight into the structure of large protein-ligand complexes.
19F REDOR has proven to be useful for constraining the oligomeric structures of large membrane receptors. The dimeric bacterial chemotaxis receptor forms clusters in lipid membranes whose structures were not known. Thompson and coworkers tested two structural models, a linear array ‘hedgerow’ model and a clustered ‘trimer-of-dimers’ model, using 13C-19F REDOR60. 4F-Phe and 13C-labeled residues were introduced into the protein. The 13C-19F REDOR data yielded inter-dimer distances that are longer than predicted by both models, thus providing constraints to the protein structure.
19F REDOR has been applied to study cholesterol interaction with influenza AM261. Employing isooctyl tail-fluorinated cholesterol and site-specifically 13C-labeled peptides, Hong and coworkers measured 13C-19F REDOR spectra. The data show that cholesterol binds AM2 in the presence of an amphipathic helix (AH), but does not bind the transmembrane (TM) helix in the absence of the AH. In AH-containing peptides, the REDOR S/S0 values decayed to a minimum of 0.5, indicating an unexpected protein : cholesterol binding stoichiometry of 2 : 1. Simulations of the REDOR data yielded distances of 7.0 to 9.3 Å, which provided crucial constraints to the structural model of the AM2-cholesterol complex in the membrane.
Intermolecular 13C-19F REDOR was also applied to understand the interaction between cholesterol and the HIV-1 fusion protein, gp4162. A gp41 peptide, MPER-TMD, was fluorinated at two aromatic residues, 4F-F673 and 5F-W680, and was combined with biosynthetic 13C-labeled cholesterol in a virus-mimetic lipid bilayer. The latter was obtained from a mutant yeast strain that replaced ergosterol synthesis enzymes with cholesterol synthesis enzymes63,64. 13C-19F REDOR data indicate that the cholesterol C9 and C17 atoms approach the F673 sidechain with a distance of 7.0 Å. Moreover, a cholesterol recognition motif (CRAC) is not necessary for this complexation. By comparing protein 13C-detected and cholesterol 19F-detected REDOR data, these authors found that three cholesterol molecules bind each gp41 trimer at the protein/cholesterol molar ratio of 1:7 used in these samples.
When no structure information is available, REDOR based on site-specific isotopic labeling is inefficient for studying protein-ligand binding. Instead, the 2D multiplexed 1H-19F REDOR technique is much more efficient for structure determination. This approach was recently demonstrated by Hong, Henzler-Wildman, and coworkers for structure determination of the bacterial transporter, EmrE65. EmrE confers multidrug resistance to bacteria, but has eluded high-resolution structure determination for decades due to its conformational plasticity. Using 2D 1H-15N resolved 1H-19F REDOR experiments of CDN-labeled protein bound to a fluorinated ligand, tetraphenylphosphonium (TPP+), the authors measured 214 protein-ligand distances from 5.8 Å to 12 Å. These distances constrained a 2.1 Å structure of the dimeric protein in dimyristoyl-phosphatidylcholine (DMPC) bilayers (Fig. 6A–C). Moreover, the 1H-19F distances showed that TPP lies closer to one subunit of the protein than the other, thus explaining the asymmetric proton dissociation constant of the proton-binding residue, Glu14.
Figure 6.
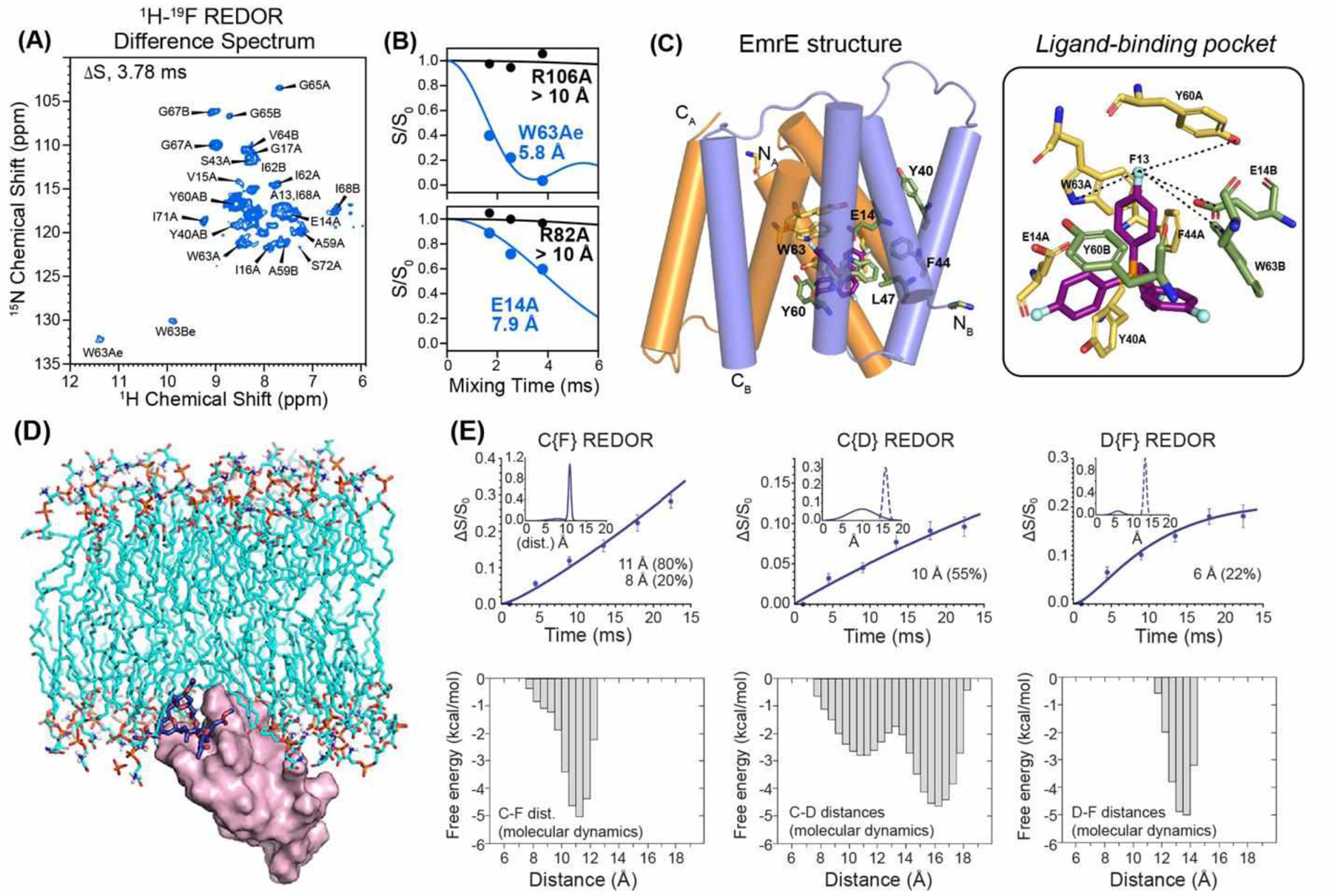
19F NMR for studying ligand binding to proteins. (A) 2D NH-resolved 1H-19F REDOR difference (ΔS) spectrum of EmrE in DMPC bilayers65. The difference peaks indicate residues in close proximity to the fluorinated ligand, F4-TPP+. (B) Representative 1H-19F REDOR dephasing curves and best-fit simulations. (C) Distance-restrained structure of TPP-bound EmrE. Key residues in the binding pocket are shown on the right (PDB 7JK8). (D) Model of Protein Kinase C (magenta) and a Bryostatin analog (dark blue sticks)66. (E) 13C-19F, 13C-2H and 2H-19F REDOR dephasing curves and best-fit simulations of the bryostatin analog. The corresponding distance distributions obtained from molecular dynamics (MD) simulations are shown below each REDOR data panel66.
In some cases, the ligand conformation when bound to its protein target is unknown and can be defined using 19F REDOR NMR. Bryostatin 1 is a protein kinase C (PKC) modulator that has shown promise in oncology, neurology, and infectious diseases. Cegelski, Schaefer and coworkers investigated the structure of a bryostatin analog, bound to the PKC receptor in lipid membranes by measuring 13C-19F, 13C-2H, and 2H-19F REDOR spectra of a triply 13C, 2H, and 19F labeled analog67. The measured distance distributions (Fig. 6D, E)66 show a long-distance component of 10–13.5 Å that coexists with a minor component of a ~6 Å distance. The latter cannot arise from intramolecular dephasing and must be attributed to intermolecular PKC dimers. MD simulations based on these distance restraints led to a model of the membrane-bound PKC-bryolog complex, and suggested that design of future PKC modulators should consider the conformational flexibility of the ligand.
2.3. 19F-Based Heteronuclear Correlation NMR Experiments
While 19F REDOR NMR provides important long-range distance constraints, in systems containing multiple fluorines, 19F resonance assignment becomes necessary. This assignment can be achieved by correlating 19F chemical shifts with 13C or other nuclei in 2D or 3D HETCOR spectra. Polenova and coworkers recently demonstrated a 2D 19F-13C HETCOR experiment using 19F-13C cross polarization (CP) for polarization transfer and dynamic nuclear polarization (DNP) for sensitivity enhancement (Fig. 7A)68. Since no DNP probe that simultaneously tunes 1H, 19F, and X was available at the time of this study, these authors tuned the 1H channel of an HXY probe to 19F. Even in the absence of 1H decoupling, by using a MAS rate of 24 kHz and high-power 19F and 13C pulses, the authors obtained sufficiently resolved spectra. Absolute 19F sensitivity enhancement factors of 12–29 were obtained. The authors demonstrated this DNP 2D 19F-13C HETCOR experiment on 5F-Trp labeled HIV-1 capsid tube assembly. The observed 19F-13C correlations correspond to distances of 8–11 Å, including both intramolecular and intermolecular contacts.
Figure 7.
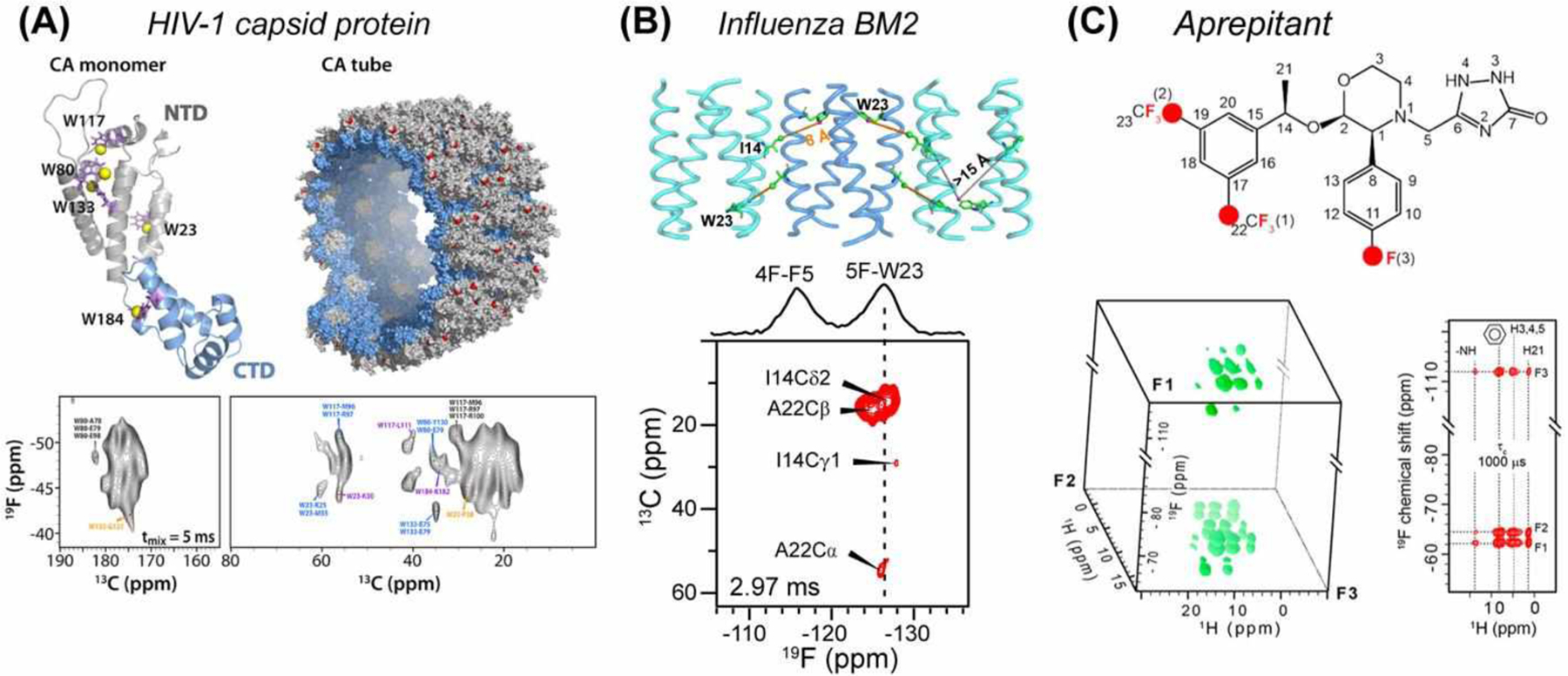
2D and 3D 19F-X HETCOR experiments for structure determination. (A) HIV-1 capsid protein (CA) structure, showing 5F-Trp residues in yellow in the monomer (PDB 4XFX) and red in the capsid tubes68 (PDB 3J4F). DNP sensitivity-enhanced 2D 19F-13C HETCOR spectra were measured using 19F-13C CP. Many 13C-19F correlations for distances > 7 Å were observed. (B) Clustering of the influenza BM2 tetramers in lipid bilayers is revealed by cross peaks between 13C-labeled Ile14 and 5F-Trp23 in 2D 13C-19F HETCOR spectra24. (C) 3D 19F-1H-1H experiments for studying the pharmaceutical drug aprepitant69. The 3D spectrum is shown on the left, while a 2D plane extracted from the F2 chemical shift of 8.1 ppm is shown on the right.
13C-19F HETCOR spectra can also be obtained using REDOR as the polarization transfer building block. Hong and coworkers showed that TEDOR transfer from 13C to 19F for detection has higher efficiency and sensitivity compared to out-and-back sequences and compared to 13C-detected TEDOR24. Applied to the influenza BM2 TM peptide, they observed cross peaks between 13C-labeled Ile14 and 5F-Trp23, which can only arise from antiparallel packed BM2 tetramers (Fig. 7B).
In addition to 13C-19F HETCOR, 1H-19F HETCOR experiments were introduced recently. By combining 1H-19F CP with 1H-1H or 19F-19F RFDR recoupling, Su and coworkers conducted 3D 19F-1H-1H (FHH) and 19F-19F-1H (FFH) correlation experiments under 60 kHz MAS (Fig. 7C)69. Since both 1H and 19F are 100% abundant, these experiments do not require isotopic enrichment and are thus well suited to studies of pharmaceutical compounds. These authors demonstrated these experiments on aprepitant in its crystalline form and as a nanoparticulate formulation, EMEND. They obtained high spectral sensitivity and resolution by 1H detection under 65 kHz MAS. These 19F-1H HETCOR spectra showed that the structure of the active pharmaceutical ingredients (API) is similar between the crystalline form and the drug formulation. Moreover, the 3D FHH spectra show correlations between the aromatic fluorine and one of the CF3 groups, giving information about the conformation of the API.
2.4. 19F Heteronuclear NMR for Pharmaceutical Sciences
Over 30% of pharmaceutical compounds contain fluorines70, making 19F NMR well suited for molecular structural characterization. Considerable efforts in the pharmaceutical industry center on the improvement of drug delivery strategies and product stability, where understanding the physiochemical properties of API’s and excipients is crucial for drug formulation. 19F NMR is a sensitive technique for understanding the interactions of API’s with excipients, and for probing the local structure in amorphous solid drug products. In crystalline pharmaceuticals, characterizing the packing of the drug product is important for maintaining product quality profiles.
Fast MAS 19F-1H HETCOR experiments were recently applied to posaconazole (POSA), the API in an antifungal drug. Su and coworkers detected intermolecular 1H-19F correlation signals under 60 kHz MAS, consistent with the presence of both “head-to-head” and “head-to-tail” packing in the crystalline lattice71. The authors also studied an amorphous form of POSA using 19F-1H HETCOR, 13C-19F CP, and 13C-19F REDOR72. The 13C-19F REDOR data indicate intermolecular distances of 3–5 Å for “head-to-tail” contacts. Interestingly, one 13C-19F distance increased from 3.3 Å to 6.2 Å between the crystalline and amorphous forms, suggesting that the amorphous form of the drug lacks the head-to-head packing. 19F NMR was also used to probe the interactions between POSA and an excipient, hypromellose acetate succinate (HPMCAS), to understand the structural basis of the stability of amorphous solid dispersions (ASD)73. Using a symmetry-based sequence, SR412, for 13C-19F dipolar recoupling, Su and coworkers measured an intermolecular distance of 6.0 Å in the ASD, indicating head-to-tail packing. 13C-19F REDOR data of fluorinated POSA and a 13C-carboxyl group in HPMCAS showed a distance of 4.3 Å, suggesting the possible interactions that stabilize the ASD. These studies demonstrate the power of 19F solid-state NMR for giving molecular insights into the structures of pharmaceutical compounds.
3. Homonuclear 19F-19F Distance Techniques
Complementary to heteronuclear 19F NMR, homonuclear 19F-19F dipolar NMR is an effective approach for measuring nanometer distances when multiple fluorines are present in a system. Due to the high gyromagnetic ratio of 19F, 19F-19F dipolar couplings are 14-fold stronger than 13C-13C dipolar couplings for the same distance. This enables 19F-19F distances up to ~2 nm to be measured, either qualitatively as cross peaks in 2D spectra or more quantitatively as buildup rates or decay rates. Homonuclear 19F-19F distances can be measured using two NMR approaches: isotropic spin exchange and anisotropic spin exchange.
3.1. Principle of Isotropic 19F Spin Exchange
When multiple fluorines are spectrally resolved, 2D 19F-19F correlation experiments allow the extraction of qualitative distance information from cross-peak intensities. Conventional spin diffusion experiments such as proton-driven spin diffusion (PDSD) and combined -driven spin diffusion (CORD)74 have been applied to 19F-19F distance measurements75,76 Under relatively fast MAS rates of 20 kHz or higher, this 19F isotropic spin exchange is more efficient with 1H irradiation (CORD and DARR) than without (PDSD). Because 19F labels are usually sparse in a biomolecular system, these 19F-19F cross peak intensities largely encode direct distances. This differs from 13C-13C spin exchange in uniformly 13C-labeled proteins, where cross peaks reflect relayed polarization transfer and dipolar truncation effects77, thus limiting the accuracy of the distance extraction. If multiple 19F spins happen to be in close proximity, for example for a multi-fluorinated aromatic ring, then care should be taken to account for dipolar truncation effects in interpreting the 19F-19F cross peak intensities75,78.
The first analytical treatment for quantifying 19F-19F distances from isotropic spin exchange was shown by Hong and coworkers75. They calibrated spin exchange rates (ksd) using structurally known model compounds for 19F spectra measured under 25–35 kHz MAS at a moderately high magnetic field of 14.1 T. For spin exchange in the weak-coupling limit, the product of ksd with the square of isotropic shift difference (kSD Δδiso2) depends on the distance as 1/r6:
Here f0 is a phenomenological constant in the overlap integral between the normalized single-quantum lineshapes of the two spins; Δδiso is the isotropic chemical shift difference; and ωd is the dipolar coupling. By measuring cross-peak intensity buildup rates of model compounds with known F-F distances (Fig. 8A), one can obtain the constant c. 2D spectra of model compounds containing both aromatic fluorines and aliphatic CF3 show that CF3-F polarization transfer was more efficient than F-F polarization transfer, reflecting the stronger dipolar coupling of three closely spaced fluorines in the trifluoromethyl group than a single F to a remote fluorine (Fig. 8B).
Figure 8.
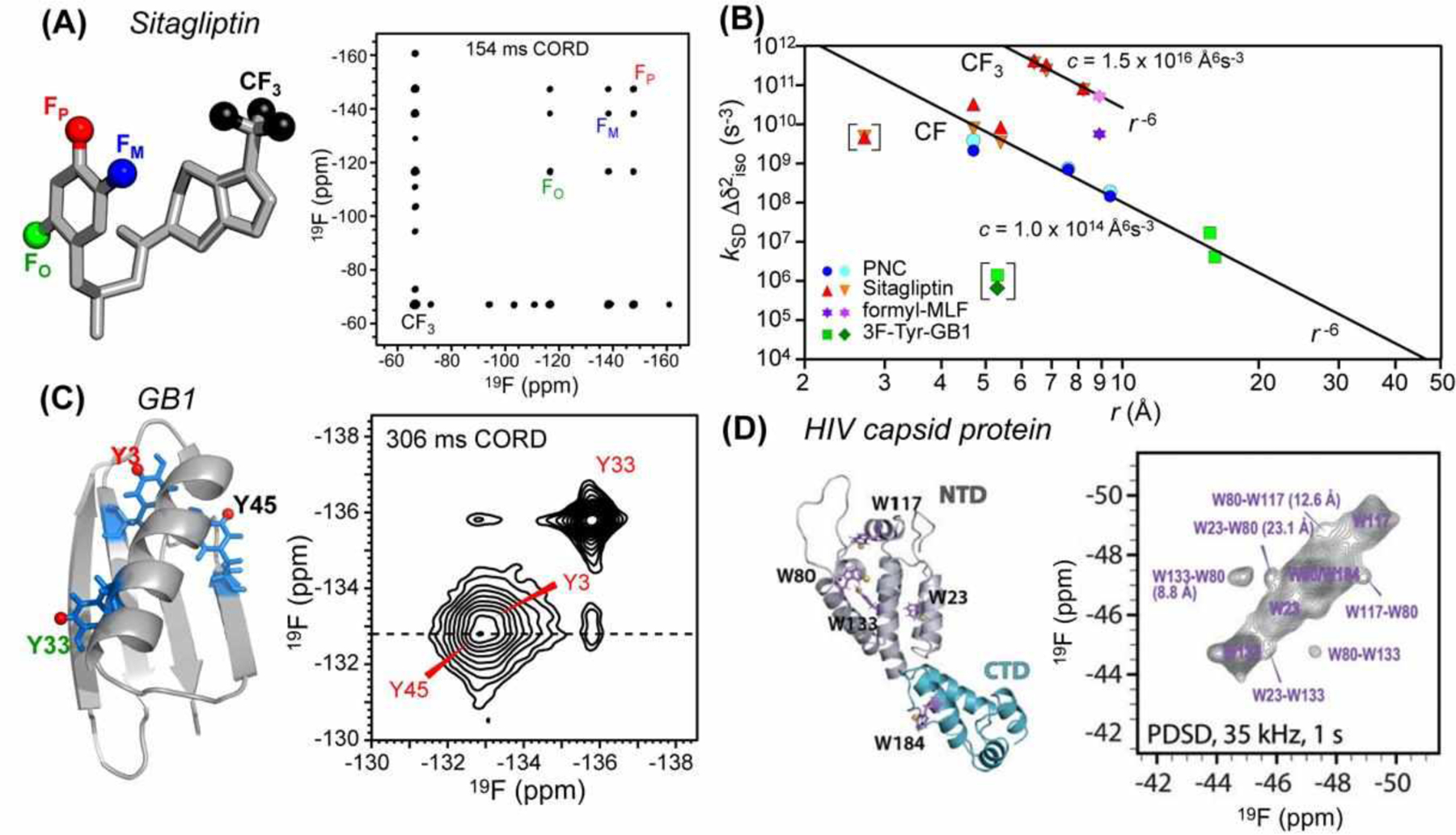
19F-19F distance measurement using isotropic spin exchange. (A) Chemical structure of sitagliptin and its 2D 19F spin diffusion spectrum measured with 154 ms CORD mixing75. (B) Chemical-shift corrected spin-exchange rates as a function of 19F-19F distances75. (C) 3F-Tyr-labeled GB1 (PDB 2JSV) and its 2D 19F correlation spectrum, measured under 25 kHz MAS with 306 ms CORD mixing75. (D) 5F-Trp labeled HIV-1 CA monomer (PDB 4XFX), showing 5-19F-Trp residues as purple sticks. 2D 19F-19F correlation spectrum of the tubular assemblies was measured under 35 kHz MAS using 1 s spin diffusion76.
The effectiveness of this isotropic 19F spin exchange for distance measurement in proteins was demonstrated by Hong and coworkers on the microcrystalline protein GB175 and by Polenova and coworkers on the HIV-1 capsid protein tubular assemblies76. Fast MAS rates of 25–60 kHz and high magnetic fields of 14.1 T and 19.96 T were used for these studies, to obtain high spectral sensitivity and resolution. 3F-Tyr labeled GB1 and 5F-Trp labeled HIV-1 CA proteins were produced by adding glyphosate to the bacterial culture prior to protein expression. For GB1, inter-Tyr cross peaks for distances of 0.5 nm to 1.6 nm were observed using 19F CORD spin diffusion with mixing times up to ~300 ms under 25 kHz MAS (Fig. 8C). The measured cross peak buildup time constants follow the 1/r6 dependence after correcting for chemical shift differences. Thus, 19F spin exchange can be analyzed to give semi-quantitative distances. For the HIV capsid protein, inter-Trp cross peaks for distances up to 2 nm were observed, using PDSD spin diffusion with mixing times up to 1 s under 35 kHz MAS.
3.2. Applications of 19F Isotropic Spin Exchange to Structural Biology
3.2.1. HIV-1 Capsid Protein
Polenova and coworkers applied the 2D 19F correlation NMR approach to measure long distances in the HIV-1 capsid protein tubular assemblies76. The 25.6 kDa CA oligomerizes into hexameric subunits (Fig. 8D) arranged in a hexagonal lattice. 2D 19F-19F correlation spectra of 5F-Trp labeled CA measured at 19.96 T show narrow 19F linewidths of 0.3–1.0 ppm even without 1H decoupling for MAS frequencies of 35 to 60 kHz. With 1 s 19F spin diffusion at 35 kHz MAS, multiple 19F-19F correlations were observed, among which the W23-W80 cross peak corresponds to the longest distance of 23 Å (Fig. 8D). Applying DNP68, these authors showed that the 19F spectral sensitivity can be enhanced up to 100-fold. This was achieved in CA samples containing 22 mM of the biradical AMUPol and spun at 24 kHz MAS. Polarization transfer from the unpaired electrons of the biradical to 19F and the subsequent transfer among 19F spins are efficient, as shown by the similar sensitivity enhancements for 19F and 1H. Because the 19F spin density is low, the 19F DNP buildup times are much longer (9.5–14.4 s) than the 1H buildup times (0.9–4.3 s). The 19F buildup times also show little dependence on the biradical concentration, consistent with a lack of relayed polarization transfer. This DNP condition was also used to obtain high-sensitivity 2D 19F-13C HETCOR spectra in the absence of 1H decoupling.
3.2.2. Protein-Lipid Interactions
Homonuclear 19F NMR, like its heteronuclear analog, is well suited to characterize small-molecule interactions in biological membranes. Hong and coworkers recently applied 2D 19F-19F isotropic spin exchange to investigate cholesterol clustering in lipid membranes79. An isooctyl tail fluorinated cholesterol, F7-cholesterol, contains two CF3 groups and a CF moiety. Interestingly, the 19F spectra resolve two CF3 peaks (−76 ppm and −78 ppm) at high cholesterol concentrations but only one CF3 peak (−76 ppm) at low concentrations. 19F CODEX experiments (see section 3.4.4 below) indicate that the −76 ppm peak results from cholesterol dimers whereas the −78 ppm peak arises from cholesterol tetramers. 2D 19F-19F correlation spectra measured with 40 ms CORD revealed cross peaks between these two CF3 signals, indicating tail-to-tail contacts between cholesterol dimers and tetramers. This study represents the first observation of oligomeric cholesterol assemblies in phospholipid bilayers.
Fluorinated cholesterol also allowed the study of cholesterol binding to the HIV fusion protein, gp41, using 2D 19F-19F correlation NMR62. By combining F7-cholesterol with 4F-Phe and 5F-Trp labeled gp41, cholesterol-gp41 contacts were observed as cross peaks in 2D 19F correlation spectra. These spectra were measured using 500 ms CORD mixing under 10 kHz MAS on a 9.4 T magnet. The MPER-cholesterol cross peaks indicate that the cholesterol tail lies within 2 nm of the surface-bound MPER helix. Together with 13C-19F REDOR data, these led to the proposal that three cholesterol molecules bind each gp41 trimer, and suggest that the MPER-TMD helix-turn-helix structure might sequester cholesterol for function.
3.3. Principle of Anisotropic 19F Spin Exchange
Multiple 19F spins of the same residue in a homo-oligomeric assembly have the same isotropic chemical shift, but usually have different anisotropic chemical shifts due to the different orientations of the 19F chemical shift tensors. Distances between these 19F spins can be measured using the Centerband-Only Detection of Exchange (CODEX) technique, originally developed by Schmidt-Rohr and coworkers for studying slow motion.80,81 The 19F spin diffusion CODEX experiment82,83 encodes anisotropic spin exchange between orientationally inequivalent 19F spins through a stimulated spin echo. Rotor-synchronized 180° pulses are applied to recouple the 19F CSA under MAS (Fig. 9A). Two trains of 180° pulses before and after a mixing period (tm) create a stimulated echo. If spin diffusion between fluorines with different chemical shift tensor orientations occurs during tm, then it changes the 19F CSA, thus preventing the complete refocusing of the stimulated echo. The echo intensity decrease thus provides information about the distance-dependent dipolar coupling. The CODEX experiment is conducted in pairs, with the control spectrum (S0) correcting for T1 relaxation effects for the exchange spectrum (S) through the intensity ratio, S/S0 (Fig. 9B). At sufficiently long mixing times, the magnetization is equally distributed among n orientationally distinct spins, giving an equilibrium intensity of 1/n. Therefore, the equilibrium CODEX intensity reflects the oligomeric number, whereas the rate of CODEX intensity decay encodes the internuclear 19F-19F distance (Fig. 9C).
Figure 9.
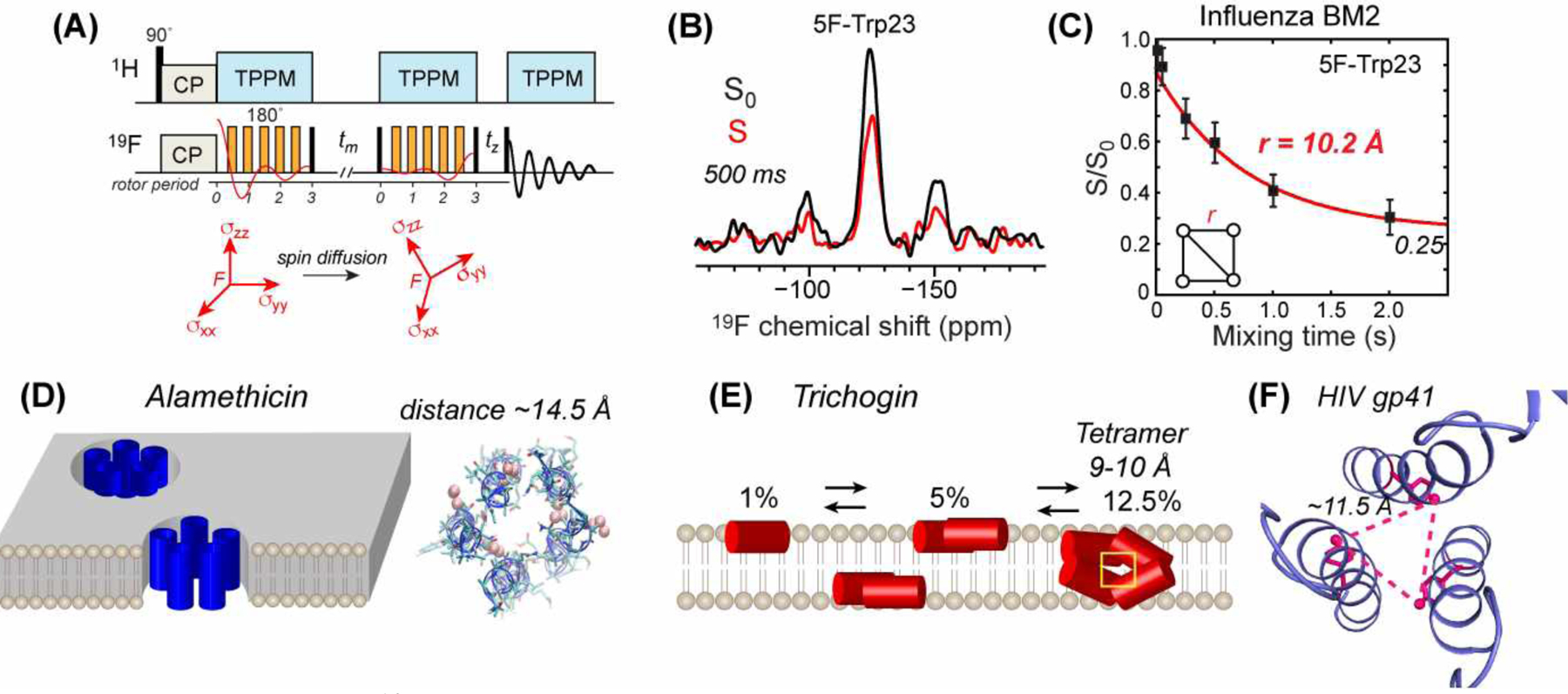
Principles of 19F spin diffusion CODEX and its applications to oligomeric membrane peptides and proteins. (A) 19F CODEX pulse sequence and the principle of anisotropic spin diffusion between nuclei with different chemical shift tensor orientations83. (B) Representative 19F CODEX control (S0) and dephased (S) spectra, shown for 5F-Trp23 labeled influenza BM2 peptide87. (C) 19F CODEX decay curve of 5F-Trp23 in BM2 shows an equilibrium S/S0 value of 0.25, indicating that the peptide assembles into tetramers in lipid bilayers. Best-fit simulation gives a nearest-neighbor 19F-19F distance of 10.2 Å87. (D) Application of 19F CODEX to alamethicin found that the peptide forms pentameric helical bundles at high concentrations88. (E) Application of 19F CODEX to trichogin revealed concentration-dependent oligomeric structures89. (F) Application of 19F CODEX to the HIV-1 fusion protein, gp41, constrained the trimer structure of the MPER-TMD segment49 (PDB 6DLN).
The 19F CODEX decay curves can be quantified using an exchange-matrix formalism to extract distances82. The principle of this formalism is the same as the isotropic spin exchange analysis, but takes into account the multi-spin nature of the problem. As before, the only adjustable parameter in this distance extraction is the overlap integral between the lineshapes of the two spins, which depends on the 19F CSA and MAS frequency. The value of this overlap integral can be calibrated using structurally known model compounds, and was found to vary between 15 and 40 μs for MAS frequencies below 35 kHz and at magnetic field strengths of 9.4 T and 14.1 T75,82. At these MAS rates, anisotropic spin exchange is more efficient in the absence of 1H irradiation (i.e. PDSD) than in the presence (e.g. DARR), in contrast to isotropic spin exchange. This indicates that 1H-19F recoupling broadens the spectral lineshapes, thus reducing the overlap integral. To ensure that this 19F CODEX technique detects spin diffusion instead of millisecond timescale motion, the experiments must be conducted at low temperature where molecular motions are frozen. For membrane proteins, 19F CODEX is typically conducted well below the phase transition temperature of the lipid membrane.
3.4. Application of 19F CODEX to Biomolecules
3.4.1. Influenza M2 Proton Channels
19F spin diffusion CODEX has been extensively applied to the tetrameric M2 channels of influenza A and B viruses41,42. These studies incorporated fluorines into native Phe and Trp residues as 4F-Phe and 5F-Trp. All studies found that the 19F CODEX echo intensities decayed to 0.25, thus directly showing the tetrameric nature of these peptides in lipid bilayers (Fig. 9C).
In addition to confirming the tetrameric nature of the peptides, 19F CODEX has provided mechanistic insights into the conformation of the gating Trp residue in M2 channels. Both influenza AM2 and BM2 contain an HxxxW motif, which is essential for the proton selectivity and channel gating. 5F-Trp incorporated into the M2 peptides was used to probe the structural dynamics of this functional motif. 19F CODEX experiments of 5F-Trp41 in AM284 yielded a nearest-neighbor 19F-19F distance of ~11 Å in DMPC bilayers. This distance constrained the Trp41 sidechain to the t90 rotamer (χ1 ~ 180°, χ2 ~ 90°). Together with a His37 Nδ1–Trp Cγ distance of 3.8 Å85, these F-F distances implied a His37 sidechain conformation of t-160, which points the imidazole ring to the pore. These interhelical Trp41 distances in DMPC bilayers were relatively insensitive to pH. Subsequent experiments on AM2 bound to a virus-mimetic membrane refined these interhelical F-F distances to 11.3 Å and 12.4 Å at high and low pH, respectively43. These values indicate that the Trp41 χ2 angle differs by 15–20° between high pH and low pH. Moreover, C-H dipolar couplings measured at high temperature indicate that the Trp41 sidechain undergoes larger-amplitude torsional fluctuations at low pH than at high pH. This implies periodic close contacts between the cationic His37 imidazolium and the Trp41 indole at low pH. Thus, these data support a dynamic gating model for the HxxxW motif of the M2 channel.
Influenza BM2 is a functional analogue of AM2 but exhibits little sequence homology. Although the amantadine class of antiviral drugs inhibit AM2, they are ineffective against BM2. 19F CODEX of BM2’s TM peptide was measured using 4F-Phe586. The CODEX decay curves were found to be bimodal, centered at 7 Å and 15 Å, suggesting a rectangular geometry for the four-spin cluster. The long-distance component indicates a wide N-terminal pore, which is consistent with the lack of binding of amantadine to the BM2 channel. Further studies of BM2 at different pH found that the gating residue, Trp23, has pH-dependent interhelical distances (Fig. 9C)87. The distances increased from 9.4 Å at pH 7.5 to 10.5 Å at pH 4.5. This channel pore widening was attributed to electrostatic repulsion between the cationic His19 at low pH. Thus, 19F CODEX data revealed subtle structural differences between AM2 and BM2 proton channels.
3.4.2. Antimicrobial Peptides and Cell-Penetrating Peptides
Antimicrobial peptides (AMPs) and cell-penetrating peptides (CPPs) modulate the physical properties of lipid membranes, to cause membrane permeation for host defense and to transport cargo into the cell. Both AMPs and CPPs are cationic and Arg-rich peptides, and share common features in many proposed mechanisms of action32,33. 19F CODEX NMR has been useful for determining the oligomeric structure of these membrane-active peptides.
The first application of 19F CODEX to AMP was to study the β-hairpin peptide PG-183. Hong and coworkers introduced 4F-Phe at a native Phe12 in the C-terminal strand, and measured an equilibrium CODEX intensity (S/S0) of 0.56. This result indicates that PG-1 oligomerizes into at least dimers, with two C-terminal strands in close proximity. Additional 13C-15N, 1H-13C and 13C-19F REDOR experiments revealed that the two peptides are aligned in parallel36. Interestingly, when 4F-phenylglycine (Phg) was introduced at residue 7 in the N-terminal strand, the 19F CODEX data also showed intensity decays, with an equilibrium value of 0.45, indicating that the N-terminal strand is also dimerized90. Thus, the β-hairpins assemble into a larger oligomer like a β-barrel in the membrane. Simulations of the CODEX decays yielded 19F-19F distances of 9.0 Å for Phg7 at the N-N interface and 6.5 Å for Phe12 at the C-C interface. These distances and other biochemical data led to a structural model of a transmembrane β-barrel across the 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) / 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) membrane. Additional 13C-31P REDOR data of Arg-labeled PG-1 indicate that the POPE/POPG membranes exhibit significant defects where some of the lipid headgroups are inserted into the membrane interior to form salt bridges with the guanidinium sidechains of Arg residues91. These results indicate that PG-1 forms a toroidal pore to disrupt the bacterial membrane.
19F CODEX was used to investigate the concentration-dependent self-assembly of the antibiotic peptide, alamethicin. This 19-residue peptaibol, rich in α-aminoisobutyric acid (Aib) residues, forms α-helical bundles of varying sizes. At high concentrations alamethicin can form ion-conducting TM pores. Bechinger and coworkers incorporated a CF3 group into alamethicin and conducted the 19F CODEX experiment under 15 kHz MAS on a 500 MHz spectrometer. The CODEX equilibrium value of the POPC-bound alamethicin showed a sensitive dependence on the peptide/lipid (P/L) molar ratio. At a P/L of 1:13, an equilibrium value of 0.186 was obtained, while at a lower P/L of 1:30, the equilibrium value increased to ~0.5. Therefore, alamethicin associates into pentamers and dimers at high and low concentrations, respectively88 (Fig. 9D). This study also determined the overlap integral of CF3 groups, yielding a 10-fold higher value of 450 μs compared to the values for CF groups, consistent with the faster isotropic spin exchange observed between CF3 and F groups75.
The same authors applied 19F CODEX to a 10-residue analog of alamethicin, trichogen, to understand its membrane-bound structure and assembly. Trichogin adopts a membrane-surface topology at low concentrations89 but induces ion leakage from liposomes in a concentration-dependent manner. 19F CODEX experiments on POPC-bound trichogin found a tetramer with F-F distances of 9–9.5 Å at a high P/L of 1:8. In comparison, EPR data showed dimer formation at a lower P/L of 1:20 (Fig. 9E). Oriented membrane 15N chemical shifts indicate that most dimers and tetramers are oriented parallel to the membrane surface, but at high concentrations (P/L = 1:8), a small population with non-parallel orientation is also present, indicating that the tetramers are disordered.
Human defensins are cationic disulfide-bonded AMPs that are an essential component of the innate immune system. 19F CODEX experiments were used to investigate the oligomeric structure of the human neutrophil peptide 1 (HNP-1) in DMPC/DMPG bilayers92. A 4F-Phg4 labeled peptide yielded a CODEX equilibrium value of 0.66, indicating that the protein is partly dimerized. Several structural models of this basket-shaped HNP-1 dimer in the membrane are possible, differing in their membrane insertion depths, orientation in the membrane, and the positions of the four Arg sidechains from the membrane surface. Combining 13C-31P REDOR of 13C-labeled Arg and lipid phosphate headgroups and membrane topology information obtained from lipid-protein 1H spin diffusion data, Hong and coworkers found that HNP-1 adopts a dimer pore structure in which the polar top of the peptide faces an aqueous pore whereas the hydrophobic bottom faces the lipids. Among the four Arg residues, R25 forms a hydrogen-bonded guanidinium-phosphate complex to stabilize the structure.
While cationic AMPs disrupt microbial membranes for their action, cationic CPPs preserve the membrane integrity while carrying biological cargos into the cell. Understanding the mechanism of this membrane crossing is of both fundamental interest and practical interest in utilizing these peptides as drug-delivery agents. 19F CODEX data of a CPP, penetratin, in DMPC/DMPG bilayers showed a trimeric assembly of three β-strands93. The measured F-F distance of 6 Å and 10 Å support antiparallel packing of these β-strands.
3.4.3. Viral Fusion Proteins
The TM domain of viral fusion proteins is important for fusing the viral and cell membranes, but its structural details are not well understood because of the hydrophobic nature of these peptides. 19F CODEX of HIV-1 and parainfluenza virus 5 (PIV5) fusion proteins have provided information about the oligomeric structure of these α-helical peptides. Biophysical studies of gp41 in different membrane-mimetic environments had previously led to discrepant structural models in the literature. Using 19F CODEX, Hong and coworkers showed that the gp41 MPER-TMD peptide forms trimeric helical bundles in virus-mimetic lipid bilayers (Fig. 9F)49. The intermolecular F-F distances between 5F-Trp residues in the MPER (W680 and W678) and between 4F-F699 in the TMD range from 11 to 12 Å. These CODEX data, together with membrane insertion data and REDOR distances, constrained a helix-turn-helix structure for each protomer of the trimer. In this assembly, the MPER helix lies on the membrane surface whereas the TMD spans the membrane.
19F CODEX experiments were also applied to the TM peptide of the PIV5 fusion protein F to determine its membrane-bound structure94. By placing 4F-Phe residues at three positions of the helix (L493F, L500F, and L504F) and measuring the CODEX decays, Hong and coworkers found interhelical distances of 8.2 to 10.5 Å. Together with crosslinking data, these distances constrained the interhelical packing of the helical bundle, and suggest that the leucine-rich central segment of this TM peptide is the trimerization core of the protein.
3.4.4. Cholesterol Clusters in Lipid Membranes
19F CODEX experiments are instrumental for proving the existence of small cholesterol clusters in lipid bilayers. Using isooctyl-tail fluorinated cholesterol, F7-cholesterol, and a 2D extension of the 19F CODEX experiment, Hong and coworkers found that cholesterol associates into dimers and tetramers in lipid bilayers79. Due to the presence of two CF3 groups and a CF group with different chemical shifts, 2D CODEX was necessary to distinguish intermolecular anisotropic spin exchange from intramolecular isotropic exchange. The latter is manifested as cross peaks between CF3 and CF peaks, whereas the former is manifested as decay of the diagonal intensities between the 2D CODEX S0 and S spectra. 19F spectra measured on a 17% and 44% cholesterol membrane showed two CF3 chemical shifts. The major component’s diagonal peak exhibited an equilibrium S/S0 value of 0.23 in the 2D CODEX spectra, whereas the minor component, present only at high cholesterol concentration, showed an equilibrium value of 0.12. Since each cholesterol contains two CF3 groups, these values indicate cholesterol dimers and tetramers, respectively. Together with 13C CODEX data and MD simulations, these results led to experimentally constrained structures of small cholesterol clusters, and suggest that dimers are the basic structural unit of cholesterol in lipid bilayers under a wide range of biologically relevant conditions.
3.5. 19F-19F Distance Measurements in Oriented Membranes
While MAS experiments account for the majority of distance measurement techniques in solid-state NMR, static 19F NMR has also been explored for distance measurement. Ulrich and coworkers incorporated two fluorine labels into membrane peptides and used a Carr-Purcell-Meiboom-Gill (CPMG) experiment to measure well resolved 19F-19F dipolar splittings in static 19F spectra of the oriented peptide95. By placing two rigid 4F-Phg labels in the antimicrobial peptide PGLa and aligning it in DMPC bilayers, they obtained distance-dependent 19F −19F dipolar splittings. When the two 4F-Phg labels are separated by four and seven residues, they observed splittings of 363 Hz and 79 Hz, respectively, which correspond to F-F distances of 6.6 and 11.0 Å. Thus, if high membrane orientational order can be achieved, this static 19F NMR is a simple and effective approach for measuring distances.
3.6. 19F-19F Homonuclear Dipolar Recoupling Techniques
At MAS frequencies above ~40 kHz, spin diffusion is no longer effective for polarization transfer, thus calling for homonuclear recoupling approaches for measuring 19F-19F distances. RFDR is one of the simplest homonuclear dipolar recoupling sequences25,96, with only one 180° pulse per rotor period, and has been well explored for 19F NMR. McDermott and coworkers first demonstrated this 19F RFDR approach under 12–13 kHz MAS on a 400 MHz spectrometer (9.4 T)97. Fluorinated small molecules were synthesized with known distances and geometries to study the effects of internuclear distances and CSA tensor orientations on 19F spin exchange. They measured significant 19F spin exchange for distances up to ~12 Å using mixing times up to 35 ms. The CSA tensor orientation had significant effects on the magnetization exchange. But a distance precision of 1 Å was still achievable for shorter distances and 2 Å for longer distances.
Schmidt-Rohr and coworkers employed 2D 19F-19F RFDR experiments under fast MAS to investigate the perfluorinated polymer nafion98. Using 30 kHz MAS at 9.4 T, these authors obtained high-sensitivity 2D 19F-19F RFDR spectra of the polymer, from which they assigned the O, S and C-bonded CF3, CF2, and CF groups. The study was simplified by the lack of hydrogens in the polymer, which allowed the 1H channel to be tuned to fluorine without the need for 1H pulses. Fast MAS is highly beneficial for suppressing the CSA spinning sidebands.
Hong and coworkers investigated the effects of MAS frequencies, rf pulse duty cycle, 19F CSA tensor orientations, and multi-spin effects on 19F RFDR spin exchange99. Under 25 kHz MAS, RFDR polarization transfer rates were found to be two orders of magnitude faster compared to 19F spin diffusion. The RFDR buildup time constants were several milliseconds for distances of 3 – 10 Å, whereas the corresponding spin diffusion buildup time constants are several hundreds of milliseconds. However, relaxation during the RFDR mixing period is also faster compared to T1 relaxation during the spin diffusion mixing period. Therefore the relative rates of cross-peak buildup and relaxation decay should be examined to ascertain which technique has higher overall sensitivity. Under very fast MAS, relaxation during RFDR is sufficiently slowed down that RFDR outperforms spin diffusion for 19F-19F distance measurement. At MAS frequencies of 20–40 kHz, the 19F 180° pulse occupies 15–30% of the rotation period using typical rf field strengths. Under these conditions, cross-peak buildup rates depend on both the 19F CSA tensor orientation and the inter-fluorine distance. Above 40 kHz MAS, numerical simulations showed that the cross-peak buildup rates have a much smaller dependence on the CSA tensor orientation, thus allowing distances to be extracted more accurately. Fast MAS also reduced the 19F relaxation loss in the absence of 1H decoupling. These advantages allow 19F-19F distances up to ~1 nm to be measured with a precision better than 0.5 Å. Numerical simulations indicate that δ-pulse RFDR at MAS frequencies of ~25 kHz encodes both distance and tensor orientation, whereas finite-pulse RFDR (fpRFDR)100 at the MAS frequency of 60 kHz yields primarily distances, thus simplifying the analysis. These conclusions were similarly reached by Polenova and coworkers, who examined the effects of multi-spin 19F-19F dipolar coupling and phase cycling on the performance of 19F RFDR under 60 – 111 kHz MAS101 102. Using fluorinated aromatic compounds with defined distances, these authors found that fpRFDR efficiently transfers polarization without requiring large chemical shift differences. Application of this 19F RFDR experiment to the HIV-1 capsid protein76 at 40 and 60 kHz MAS yielded cross peaks for fluorines separated by 9–23 Å, demonstrating the efficiency of 19F RFDR spin exchange for distance measurement.
4. 1H-1H Distance Measurements Under Fast MAS
As the most abundant as well as the highest-γ stable spin-1/2 nucleus in organic and biological molecules, 1H has long been the most sensitive nuclear spin for NMR. Its high γ also makes 1H-1H dipolar coupling a potential long-distances probe. However, 1H NMR of solids have long been hampered by the severe line broadening caused by the extensive 1H-1H dipolar network. In the last decade, two technological advances have largely overcome this barrier to distance measurement. The first advance is to reduce the proton density of biomolecules by perdeuteration followed by back-exchange in protonated solvents103,104, and the second advance is to spin samples at very fast MAS frequencies of ~100 kHz105–107. These advances have led to the introduction of pulse sequences tailored to 1H-1H distance measurements, which are often implemented with high-sensitivity 1H detection in high-dimensional correlation experiments.
4.1. 1H-1H Distance Measurement Techniques
The simplest approach for measuring qualitative 1H-1H distances in solid-state NMR is Proton Spin Diffusion (PSD), as no rf pulses are applied during the mixing time (Fig. 10A). However, the spin diffusion mechanism is rapidly attenuated with increasing MAS frequencies. Thus, 1H-1H recoupling is necessary under the fast MAS condition where most high-resolution 1H spectra are measured. Recently, several 1H homonuclear recoupling techniques tailored to fast MAS have been examined or introduced for distance measurements108–111. These include fpRFDR, DREAM, SERP, AM-MIRROR, and BASS-SD.
Figure 10.
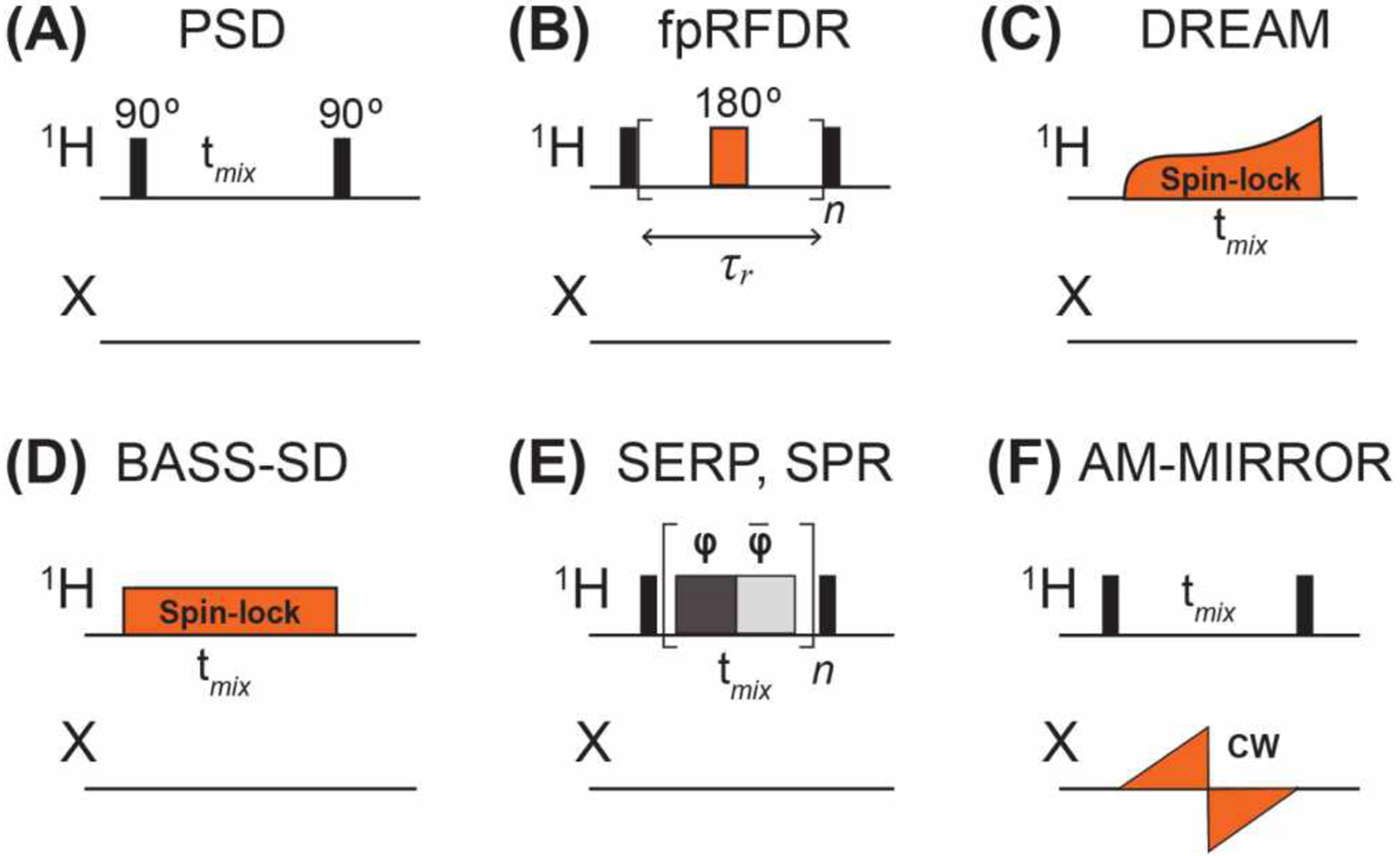
Selected pulse sequence elements for measuring 1H-1H distances under fast MAS. (A) 1H spin diffusion (PSD). (B) The finite-pulse RFDR (fpRFDR) sequence involves a single 180° pulse per rotor period100. (C) The DREAM sequence uses an amplitude-modulated 1H pulse whose field strength is swept across the HORROR condition, ω1=ωr/2116. (D) The BASS-SD sequence involves a 1H spin-lock pulse at a field strength that spans the spectral region of interest to cause band-selective polarization transfer119. (E) The SERP and SPR sequences comprise a series of rotor-synchronized phase-modulated pulses. SERP is based on a symmetry scheme where each pulse series block matches the rotation period120,121. SPR employs a user-defined CNn symmetry scheme where the block length equals n times the rotation period122. (F) Reverse AM-MIRROR123,124 comprises a sawtooth-shaped pulse on the X channel whose amplitude is defined by the chemical shift bandwidth of the X nucleus.
The RFDR experiment25,100 (Fig. 10B), uses rotor-synchronized 180° pulses to induce longitudinal magnetization transfer. With only one pulse per rotor period, this experiment has a relatively modest rf demand, and can be implemented even at fast MAS frequencies of ~100 kHz. Under fast MAS, the finite-pulse RFDR recoupling mechanism dominates100. 1H-1H distance measurements were first demonstrated by Zilm and Rienstra on model proteins ubiquitin104 and GB1112 that were CDN-labelled and back-exchange with H2O to install amide protons. Correlation with 15N and 13C chemical shifts in 2D and 3D experiments resolved the resonances. In the initial demonstrations of this approach, MAS rates of 20 kHz and 39 kHz were used for ubiquitin and GB1, respectively. For GB1, 517 1H-1H distance restraints up to 5.5 Å were measured and allowed de novo structure calculation, giving good agreement with the crystal structure112. Differences mainly lie in the sidechains, for which few constraints were available since only exchangeable HN and HO protons were measured in the perdeuterated protein. Reif, Linser and coworkers proposed 3D and 4D 15N-resolved 1H-1H RFDR experiments and demonstrated them on perdeuterated and 25% back-exchanged α-spectrin SH3113,114. The sparse 1H system allowed the measurement of distances up to 13 Å between amide HN and sidechain methyl protons even at a moderate MAS frequency of 20 and 24 kHz. When the perdeuterated protein was 100% back-exchanged with protons, then spinning to 55 kHz was necessary to give well-resolved 1H spectra. When even faster MAS rates of 100 kHz are used for protonated proteins, RFDR still performs well, giving 1H-1H distance restraints of up to 5.5 Å in GB1 and in a bacteriophage coat protein,115.
Although RFDR is simple to implement, its efficiency decreases with increasing MAS rates. Moreover, at ultrafast MAS, high RF field strengths close to 100 kHz become necessary to fit the 180° pulse into each rotor period. Meier and coworkers employed the DREAM (Dipolar Recoupling Enhanced by Amplitude Modulation) experiment (Fig. 10C)116, originally developed for 13C NMR, to bypass these limitations. DREAM recouples homonuclear dipolar interactions using an adiabatic pulse whose field strength is centered at the Homonuclear Rotary Resonance (HORROR) condition of ω1=ωr/2. Because of its double-quantum nature, the sign of DREAM cross-peaks alternates between positive and negative, depending on whether a 1H-1H correlation results from direct or relayed dipolar transfer. The DREAM recoupling method was incorporated into a 4D 1H-13C resolved HSQC-DREAM-HSQC experiment to measure 1H-1H correlations in methyl labelled ubiquitin. This DREAM experiment was first employed at 55 kHz MAS117 and subsequently at 100 kHz MAS118. In the latter study, distances up to 7 Å were measured using mixing times of 8 ms.
BASS-SD (Band Selective Spectral Spin-Diffusion)119 is a selective zero-quantum recoupling experiment in which a weak spin-lock pulse is applied to achieve selective polarization transfer only within the HN band, the Hα band, or the methyl proton band (Fig. 10D). Using protonated GB1, Agarwal and coworkers showed that BASS-SD selectively transferred magnetization between like protons that are 6 Å apart under 111 kHz MAS. Compared to RFDR, the BASS-SD yields higher signal sensitivity and less rf demand. However, the distance constraints are more qualitative, and the method is limited to short mixing times due to T1ρ relaxation.
Other selective 1H-1H recoupling techniques for fast MAS were recently introduced by Agarwal and coworkers. The SERP (Selective Recoupling of Protons) experiment124 uses phase-modulated pulses to generate double-quantum dipolar Hamiltonians, and is sensitive to 1H-1H distances up to 5 Å in fully protonated molecules (Fig. 10E). Quantitative dipolar oscillations are observed for short distances of ~3 Å120, as shown on thymol at 68 kHz MAS125. SPR (Selective Phase-optimized Recoupling) is similar to SERP and was recently introduced for MAS rates higher than 150 kHz122. 1H-1H correlations for distances over 11 Å were observed in the model compound pioglitazone hydrochloride.
The reverse AM-MIRROR (Amplitude Modulated Mixed Rotational and Rotary Resonance) experiment was introduced by Ernst and coworkers to achieve chemical-shift compensated broadband 1H spin diffusion under fast MAS126. Here the 1H channel has the same simple pair of 90° pulses as in PSD, but additionally a weak adiabatic pulse is applied on the heteronuclear channel (Fig. 10F). Compared to BASS-SD, AM-MIRROR avoids severe signal loss during the spin-lock pulse. The method was demonstrated on ubiquitin, and long distances of up to 10 Å were measured with a mixing time of 20 ms under 100 kHz MAS. Since the AM-MIRROR recoupling field strength is independent of the MAS frequency, the technique does not require high rf amplitudes under fast MAS.
4.2. Applications of Long-Range 1H Distance Measurements to Structural Biology
These 1H-1H distance techniques have been increasingly applied to large, heterogeneous, and insoluble biomolecules under fast MAS frequencies of 60–150 kHz127. These systems include viral proteins, macromolecular assemblies, amyloid fibrils, membrane-bound proteins, and drug formulations128–133. Here, we highlight a few studies where high-sensitivity 1H-detected measurements of 1H-1H distances provided crucial structural restraints for these biomolecules.
4.2.1. Viral Proteins
Pintacuda and co-workers solved the de novo structure of the 14 kDa Acinetobacter phage 205 coat protein (AP205CP) using 1H-detected NMR (Fig. 11A)115. AP205CP is a dimeric protein that constitutes the basic subunit of the icosahedral capsid of the phage. Even with full protonation, sedimented microcrystals of AP205CP yielded narrow 1H linewidths of 0.15–0.20 ppm when spun under 100 kHz on a 1 GHz NMR spectrometer. The presence of sidechain protons in addition to amide HN allowed the measurement of 1H-1H correlations using 3D hNHH and hCHH experiments and RFDR mixing. In total, the authors obtained 410 intramolecular distances and 104 intermolecular distances up to 5.5 Å, which constrained the structure of this dimeric protein. The same approach was also applied to other viral proteins, including the hepatitis B virus nucleocapsid core and envelope proteins134,135, and the hepatitis C virus nonstructural protein 4B bound to lipid bilayers136.
Figure 11.
Long-range 1H-1H distance measurements to determine virus protein structures. (A) Solid-state NMR structures of the viral capsid protein AP205CP (PDB 5JZR)115. (B) Intermolecular contacts of gp17.1 were measured using 1H-1H distance contacts in tailored labelled samples using RFDR mixing138. Reproduced with permission from reference138. Copyright 2018 Wiley.
Using 1H-detected fast MAS NMR, Griffin and coworkers determined the structure of the S31N mutant of the influenza AM218–60 protein in diphytanoylphosphocholine bilayers137. To obtain interhelical distance constraints, the authors conducted 15N-13C TEDOR experiments on mixed 15N and 13C labeled samples as well as 1H-1H RFDR experiments under 60 kHz MAS. The latter involved 1H exchanged, 15N labeled and ILV-methyl 13C-labeled proteins. A 4D HCHHCH experiment with 8 ms fpRFDR and a 3D hNHH experiment with 3.3 ms fpRFDR were conducted to give methyl-methyl and HN-HN distance restraints. The authors obtained nine inter-helical contacts, which constrained the sidechain conformations of the functionally important His37 and Trp41 residues in this proton channel.
Lange and co-workers investigated the oligomeric structure of gp17.1138,139, a protein component of the extensive tail tubes from the SPP1 virus140. To probe the dimerization interface, the authors mixed 1H-exchanged 2H, 15N-gp17.1 with equal amounts of isoleucine methyl-labeled (Ile-1Hδ−13Cδ)-(2H, 12C)-gp17.1. The latter was obtained from α-ketobutyrate-4-13C-3,3-d2 precursors141. 1H-detected 3D HNhH spectra with 10 ms RFDR mixing provided seven unambiguous interchain contacts between methyl and amide HN protons (Fig. 11B). This result was subsequently corroborated by mutagenesis data, where deletion of the identified residues prevented oligomerization. Here the extensive deuteration and site-specific labeling of the protein obviated the need for very fast MAS, and 40 kHz MAS and a 900 MHz magnet sufficed to give well-resolved hNH and hCH spectra.
4.2.2. Membrane Proteins
For large membrane proteins, sparse 1H labeling is an effective approach for distance measurements, by reducing both spectral congestion and 1H linewidths. Weingarth and coworkers introduced amino-acid specific 1H labeling into a perdeuterated protein to measure long-range interactions between non-exchangeable aliphatic protons142. This proton cloud strategy consists of adding 13C, 15N-labeled and protonated amino acids to E. coli minimal media prepared in D2O (Fig. 12A). This strategy was first demonstrated on Val, Leu-labeled ubiquitin under 60 kHz MAS on an 800 MHz spectrometer. Distances up to 6 Å between Val and Leu sidechain protons were observed with 75 ms 1H spin diffusion (Fig. 12A). Since proton clouds do not require re-protonation at exchangeable sites, which can be difficult to achieve for membrane-bound proteins, this approach is particularly useful for studying membrane proteins. This was demonstrated on BamA, a 52 kDa β-barrel assembly (Fig 12B)142.
Figure 12.
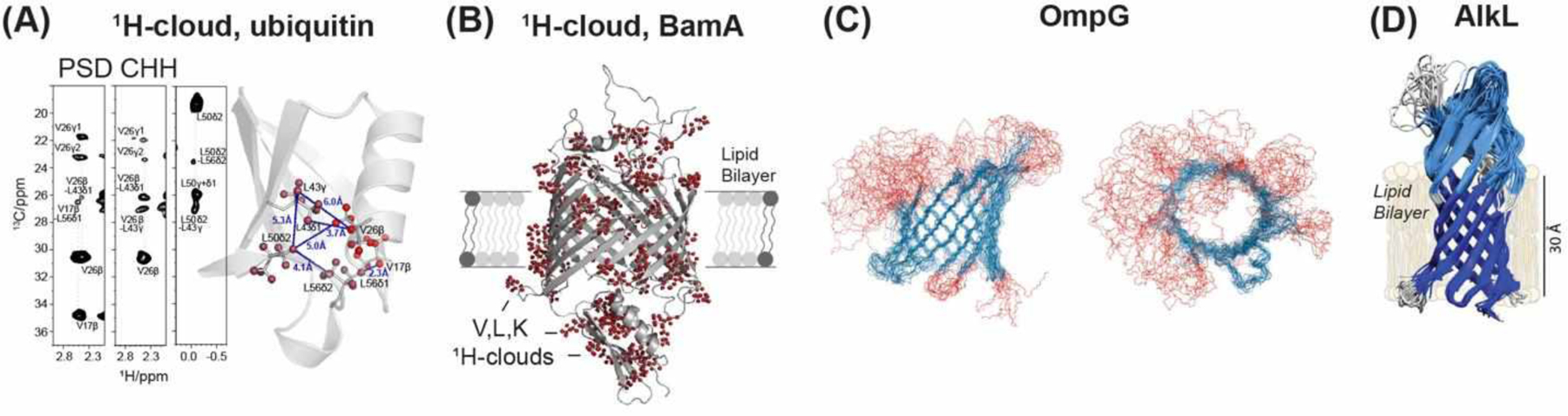
1H-1H distance experiments restrain the structure of membrane proteins. (A) Residue-specific 1H-labeling (proton clouds) greatly reduces spectral congestion, as demonstrated on ubiquitin142. (B) Application of the proton cloud approach to structural studies of BamA142 (PDB 4K3B). (C) Solid-state NMR structure of the β-barrel OmpG (PDB 5MWV) in E. coli lipid extracts143. (D) Solid-state NMR structure of the β-barrel AlkL (PDB 6QWR) in DMPC bilayers, determined using distance restraints obtained from 1H-1H RFDR and BASS-SD144.
Oschkinat and coworkers determined the structure of the 14-stranded 34 kDa β-barrel formed by the outer membrane protein G (OmpG)143. Using 3D hNHH and hNhhNH experiments with 2 ms RFDR mixing under 60 kHz MAS, the authors measured 102 HN- HN distances. The protein was CDN-labeled followed by 1H exchange and was bound to E. coli lipid extracts (Fig. 12C). These HN-HN distances helped to restrain a high-resolution structure of the protein using the ARIA145 and CNS146 software.
Andreas and coworkers determined the structures of another β-barrel membrane protein, AlkL, using fully protonated samples and 1H-detected NMR. The 8-stranded protein is a member of the OmpW family and imports hydrophobic molecules such as alkanes and terpenoids across the outer membrane of Gram-negative bacteria. These authors investigated the AlkL structure in both DMPC bilayers and octyl glucoside micelles using solid-state and solution NMR, respectively144 (Fig. 12D). For the bilayer-bound protein, 1H-1H distance restraints were measured using 4D HNNH experiments with fpRFDR and BASS-SD mixing schemes. These experiments yielded 769 1H-1H restraints, many of which constrain the packing of the β-strands in the membrane. Full protonation of the protein is crucial for obtaining sidechain distances. The bilayer-bound structure shows a narrow TM pore that suggests that substrates are not transported in a straight path. Instead, the presence of several openings near the membrane surface suggests a dynamic lateral release model. This was supported by MD simulations and NMR relaxation data and chemical shift perturbations in the presence of the substrate octane and carvone.
Structural and mechanistic studies of membrane proteins can benefit from NMR spectral editing. Exchanging solvent-exposed NH groups with D2O is a straightforward approach to render these residues invisible while keeping solvent-inaccessible residues in the TM domain visible from 1H-detected experiments. This approach is especially well suited for examining the membrane topology of and water cavities in membrane proteins147,148. Conversely, water-exposed residues can be selectively detected by water magnetization transfer experiments in protonated solvents149. The water magnetization can be selected using a 1H T2 filter, then transferred to the protein104,150. Water contact with proteins has been studied under 55–60 kHz MAS for the anion channel hVDAC151, AlkL144, GlpG protease152, and the potassium channel KcsA153. In the case of KcsA, residue-specific correlations between the protein and ordered water molecules were detected in a 2D HhN experiment with 1.2 ms PSD. Weingarth and coworkers showed that these ordered water molecules play an important role in regulating channel gating154. Well-resolved 1H chemical shifts are also informative for identifying structurally and functionally relevant hydrogen bonds in KcsA154, the influenza M2 proton channel155,156, human carbonic anhydrase II157, nucleic acids158, and amyloid fibrils159.
4.2.3. Amyloid Fibrils
Fast MAS 1H-detected NMR spectroscopy has also been used to investigate amyloid protein structure. Loquet, Habenstein and co-workers identified key molecular properties that modulate the aggregation of functional amyloids160. The authors determined the structure of the fungal amyloid protein, HELLF, based on 1H-1H distance restraints measured under 110 kHz MAS on a 1 GHz NMR spectrometer. The fibrils displayed a single molecular conformation featuring a rigid β-solenoid fold with a 1H linewidth of 150–200 Hz. A total of 143 long-range intramolecular 1H-1H contacts were obtained from 3D hCHH, HhCH, and HhNH experiments with RFDR mixing. Intermolecular 1H contacts were obtained using an HhNH experiment on an equimolar mixture of unlabeled and protonated HELLF mixed with perdeuterated and 1H-exchanged 15N-labeled HELLF (Fig. 13A). Interestingly, although HELLF shares a similar structure to the HET-s protein161 and co-exists in the same fungus, Podospora anserina, these two prions do not cross-aggregate. After generating several HELLF mutants that exhibited the same three-dimensional fold but very different cross-aggregation propensities, the authors concluded that the overall protein fold is insufficient to induce prion strains but that crucial sidechain positions may be important for determining the cross-seeding propensity and prion specificity.
Figure 13.
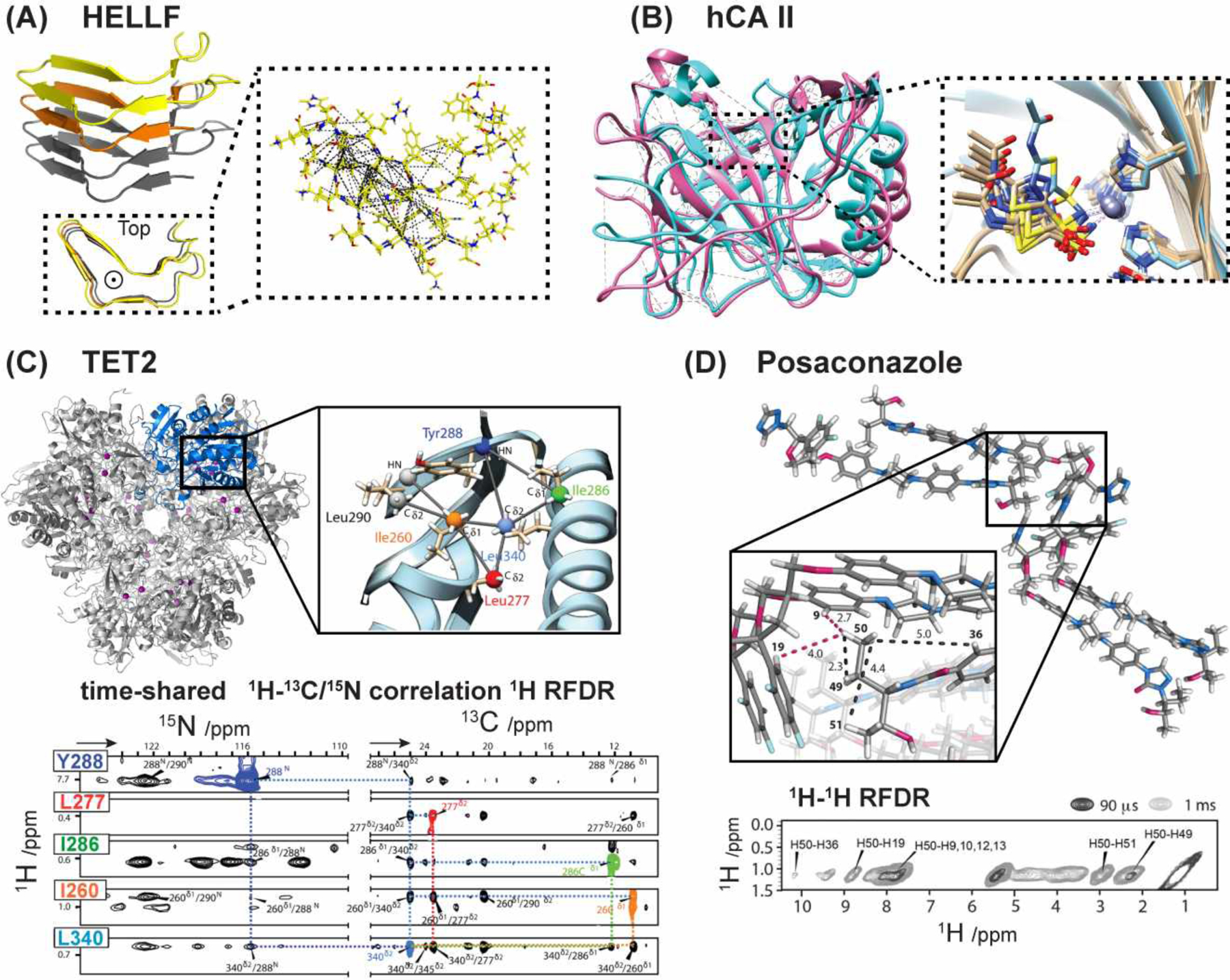
1H-1H distance restraints obtained under fast MAS for determining the supramolecular structures. (A) Structure of the HELLF (209–277) amyloid protein (PDB 6EKA). 1H-1H distance restraints helped to define the rigid core160. (B) Solid-state NMR structure of the hCAII enzyme (magenta, PDB 6QEB) overlaid with the crystal structure (cyan, PDB 3HS4). The inhibitor aceazolamide binds the active site of the enzyme167. (C) Structure of the TET2 enzyme (PDB 6R8N) obtained from integrated analyses of NMR and cryo-EM data174. Representative 3D 1H RFDR spectra and distance restraints are shown. (D) NMR crystallography of the antifungal compound Posaconazole175. Representative 2D 1H-1H RFDR spectra and measured distance restraints are shown.
4.2.4. Protein-Ligand Complexes
The high spectral resolution provided by fast MAS and high magnetic fields brings new opportunities to investigate large enzymatic complexes. Recent studies have investigated the mechanisms162, substrate-binding163–165, and dynamics166 of enzymes such as ATPases, peptidoglycan-crosslinking enzymes, and aminopeptidases. Linser and co-workers studied the structure and function of human carbonic anhydrase II (hCAII), a 29 kDa enzyme responsible for hydrating CO2 to bicarbonate167. Using fully protonated 15N, 13C-labeled hCAII and 111 kHz MAS on an 800 MHz NMR, they conducted time-shared 3D Hh(N/C)H experiments with 1.53 ms 1H RFDR mixing. These experiments yielded important 1H-1H sidechain restraints (Fig. 13B). In particular, the tautomeric structures and protonation states of the zinc-coordinating histidine residues, H94, H96 and H119, were observed in the 1H-detected spectra. 15N spin-lock relaxation dispersion experiments additionally showed that the active site is dynamic and is modulated by a water hydrogen-bonding network157.
Fast MAS 1H-detected NMR experiments have contributed to the studies of lipid-protein interactions. Although 1H spin diffusion slows at fast MAS, NOE-mediated transfer from the lipid acyl chains to the protein remains appreciable104. Therefore, lipid-protein correlations can be measured with 1H detection, as shown in an HhNH experiment for AlkL in phosphocholine membranes at 55 kHz MAS151. This 1H-detected lipid-protein correlation technique was advantageous for investigating the mode of action of membrane-active antibiotics168. Complementarily, chemical shift perturbations in 1H-detected spectra allowed the identification of the binding sites of antibiotics148,169. For dynamic species such as phospholipids and disordered domains in membrane proteins, 1H-detected 2D 13C-1H INEPT correlation spectra obtained at moderate MAS frequencies also provide useful structural information170. Finally, fast MAS allowed the investigations of protein-nucleic acid interactions through chemical shift perturbations in 1H-detected correlation spectra171,172 and through 31P-detected NHHP experiments173.
4.2.5. Integrative Approaches
The complexity of biomolecular machineries compels the integration of solid-state NMR spectroscopy with complementary techniques such as X-ray crystallography and cryo-EM139,164,165,176. Schanda and co-workers demonstrated an integrative NMR and cryo-EM approach for determining the three-dimensional structure of large biomolecular assemblies174. The central idea is to use secondary structure information from NMR and 1H-1H distance restraints from backbone amides and methyl groups to iteratively refine low-resolution cryoEM maps to high resolution. Using this integrative approach, these authors determined the structure and supramolecular assembly of TET2, a 468 kDa homo-dodecametric aminopeptidase, to better than 1 Å precision and accuracy. Intramolecular 1H-1H distance restraints of TET2 subunits were measured using time-shared 3D h(N/C)hh(N/C)H experiments with 5 ms 1H RFDR mixing (Fig. 13C) under 55 kHz MAS. The resulting structure provided information about important loop regions of the protein that was not accessible from X-ray crystallography and that was important for understanding the enzyme activity.
4.2.6. Small Molecules and Material Sciences
Ultrafast MAS has not only been applied to biomolecular studies but has also opened new avenues of research in chemical, materials, and pharmaceutical sciences. Enhanced 1H spectral resolution enables the characterization of pharmaceutical compounds71,177,178 and organic compounds at natural abundance71,130,179. Su and coworkers employed 1H-1H RFDR experiments at 110 kHz MAS to investigate intermolecular interactions between active pharmaceutical ingredients and polymeric excipients180. These studies aid in the formulation of better amorphous solid dispersions to improve therapeutic efficacy. In materials science, fast MAS 1H NMR is a powerful approach for characterizing heterogeneous catalysts and for tracking protons during chemical reactions. Wiegand and coworkers used 110 kHz MAS and 2D 1H spin diffusion experiments to characterize the hydrogenation products of Frustrated Lewis Pairs compounds181. Wimperis and coworkers used 75 kHz MAS to explore the 1H environment in silica and epoxy-silica materials and evaluate the hydration and structural integrity of the in situ immobilized biocatalysts with hCA II182. In biomaterials, Ramamoorthy and co-workers applied 110 kHz MAS to gain insight into bone structure183. These authors powdered bovine cortical bone samples into 0.75 mm rotors to investigate the molecular interactions between collagen and water molecules. Fast MAS rendered collagen backbone HN signals visible and resolved the sidechain 1H signals. 2D 1H fpRFDR experiments were used to show that water interactions with collagen backbone HN differ qualitatively from water interactions with sidechain protons.
NMR crystallography benefitted from fast MAS, as 1H-1H dipolar couplings and 1H chemical shift anisotropy can now be measured in natural abundance organic compounds184,185. Polenova and co-workers characterized the structure of posaconazole, an antifungal agent175. 1H-1H distance contacts were obtained from 3D 1H-1H-1H DQSQ-RFDR experiments with 0.09–1.0 ms mixing. These spectra were measured under 111 kHz MAS on a 700 MHz NMR spectrometer (Fig. 13D). This approach yielded intra- and intermolecular 1H restraints up to 6 Å, which defined the supramolecular packing of the compound.
5. 13C and 15N NMR for Long-Distance Measurements
5.1. 13C-13C Spin Diffusion Experiments
13C spin diffusion NMR has long been the mainstay for measuring long-range distance restraints for structure characterization. These experiments are typically run in a 2D manner beginning with 1H-13C CP, followed by 13C t1 evolution, then storage of the 13C magnetization along the z-axis to allow 13C spin diffusion. This period can last hundreds of milliseconds to detect long-range correlations. Finally, the 13C magnetization is returned to the transverse plane for detection. What distinguishes different spin diffusion techniques is the type of 1H irradiation pulses during the 13C mixing period. In the simplest 13C spin diffusion experiment, PDSD186, no 1H irradiation is applied during the mixing period. This PDSD experiment is easy to set up, requires no optimization, and allows mixing times as long as several seconds to be used due to the lack of rf pulses.
However, even at moderate MAS frequencies of ~20 kHz, 13C spin diffusion by PDSD is no longer efficient because of the diminishing zero-quantum overlap integral between the two carbons187. Therefore, many improved mixing sequences have been developed to increase the efficiency of 13C magnetization transfer. 1H irradiation at an rf field strength (ω1H) that matches the MAS frequency ωr with certain ratios can broaden the overlap integral, thus speeding up spin diffusion. The Dipolar-Assisted Rotary Resonance (DARR) technique uses the matching condition, ω1H = ωr, to give broadband and efficient spin diffusion188,189. The phase-alternated recoupling irradiation scheme (PARIS) alternates the phase of 1H irradiation between +x and −x every half a rotor period or two rotor periods. Unlike DARR, the PARIS 1H rf field strength does not need to fulfill any matching condition190. Therefore, weaker rf fields can be used, which are advantageous for fast MAS conditions where 1H irradiation for hundreds of milliseconds can cause significant sample heating. At MAS frequencies of 30 kHz, PARIS was shown to have higher 13C magnetization transfer efficiency than DARR and PDSD. PARIS is also less sensitive to rf field inhomogeneity, thus a larger proportion of the sample in the rotor experiences optimal 1H-13C recoupling. PARIS allows efficient aliphatic-aliphatic transfer but is not sufficiently broadband to promote spin diffusion from aliphatic carbons to aromatic or carbonyl carbons. PARIS-xy addresses this problem by using an orthogonal rf phase cycle during the recoupling period191. The second-order Hamiltonian among analogous nuclei generated by heteronuclear assistance irradiation (SHANGHAI) is another variant of these experiments. This experiment uses a super-cycled dipolar recoupling scheme to achieve efficient polarization transfer over large isotropic chemical shift differences and at high magnetic fields192.
Combined R2nv driven (CORD) spin diffusion is a broadband 1H-13C recoupling technique that promotes efficient spin diffusion over a range of MAS frequencies74. In the CORD experiment, ω1H is equal to ωr for one third of the mixing time but half the MAS frequency for two thirds of the time. Therefore, this experiment requires less rf power than DARR. Demonstrated on uniformly 13C,15N-labeled amino acids and the dynein light chain protein with mixing times up to 500 ms, the CORD experiment was found to be more broadband than PARIS-xy, while providing greater transfer efficiencies than the SHANGHAI experiment.
5.2. Applications of 13C Spin Diffusion to Proteins
Since 13C spin diffusion is an essential element in almost all biological solid-state NMR experiments, we only highlight a small number of its applications. Among amyloid proteins, the α-synuclein fibril that makes up the Lewy bodies and neurites in Parkinson’s disease has been structurally determined using solid-state NMR193. This structure was challenging to solve because the protein is larger (14.5 kDa) than some of the well-studied amyloid peptides such as Aβ, and the amino acid sequence has low complexity. Using DARR mixing times of 50 ms to 500 ms on samples labeled with either 1,3-13C glycerol or 2-13C glycerol, Rienstra and coworkers measured 180 unambiguous and 80 ambiguous long-range 13C-13C distances. These correlations helped to constrain the novel Greek key motif for the fibril core. Of great utility in assigning these long-range correlations was a 3D 15N-13C-13C experiment with 500 ms DARR mixing. The 15N dimension provided spectral resolution to assign the many long-range contacts. These in vitro α-synuclein fibrils are pathogenic to neuronal cells, suggesting that they are relevant to the in vivo fibrils in Parkinson’s disease.
The amyloid fibril formed by the peptide hormone glucagon presents a second case where 13C spin diffusion was instrumental for structure determination. Glucagon functions in blood glucose homeostasis by raising the blood sugar level and is prescribed for treating severe hypoglycemia. However, at pharmaceutically relevant concentrations, glucagon aggregates rapidly from solution. Thus, it has to be formulated as a lyophilized powder, to be mixed with a diluent solution immediately before administration. Hong and coworkers solved the high-resolution structure of glucagon fibrils (Fig. 14A)194. All spectra show peak doubling in a 1:1 intensity ratio, indicating that the cross-β fibrils contain two coexisting β-strand conformations. 13C CORD spin diffusion experiments with mixing times up to 500 ms gave numerous cross peaks between the N- and C-termini. These cross peaks disappear when the isotopically labeled monomers are diluted with unlabeled monomers. Therefore, these N- to C-contacts are intermolecular, indicating that the β-strands pack in an antiparallel fashion along the fibril axis. Additional 1H-assisted 13C-13C correlation spectra (PULSAR) confirm this result (Fig. 14A).
Figure 14.
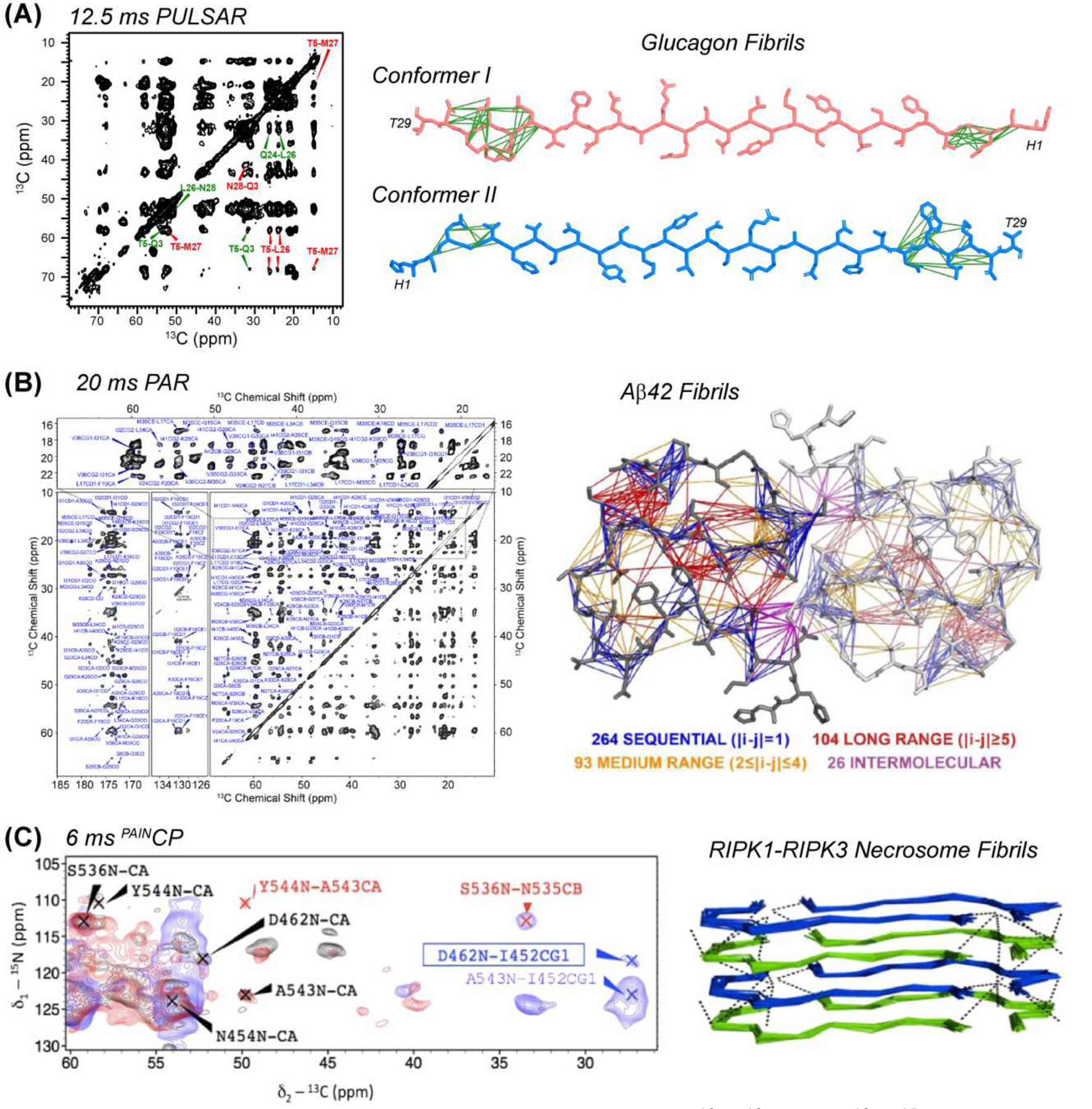
TSAR experiments for measuring long-range 13C-13C and 13C-15N distances to determine amyloid protein structures. (A) 12.5 ms 13C-13C PULSAR spectra of glucagon helped to determine antiparallel packing of this amyloid fibril194,206 (PDB 6NZN). (B) 20 ms 2D 13C PAR spectrum helped to determine the Aβ42 fibril structure211 (PDB 5KK3). (C) 6 ms 2D 15N-13C PAINCP spectrum of RIPK1-RIPK3 necrosome fibrils and the resulting structure217 (PDB 5V7Z).
The HIV-1 capsid that surrounds the viral RNA is made up of ~1,000 copies of the capsid protein. Polenova and coworkers determined a high-resolution structure of the CA in tubular assemblies by combining secondary structure information and distance restraints from solid-state NMR with low-resolution cryoEM maps and molecular dynamics simulations195. Using CORD mixing times of 25, 50, 200, and 500 ms on samples prepared with either 1,6-13C-glucose or 2-13C-glucose, they obtained 414 unambiguous inter-residue contacts. Among these, 166 were medium-range and 219 were long-range in terms of amino acid sequence. These contacts were crucial for determining the high-resolution structure of the CA tubes.
5.3. Third-Spin Assisted Recoupling Experiments
Third-spin-assisted recoupling (TSAR) experiments196,197 were developed by Griffin and coworkers to measure long-range distance restraints between low-γ nuclei. These experiments consist of spin lock of both 1H and the heteronuclei at rf fields that avoid the Hartman-Hahn matching condition and rotary resonance conditions, while fulfilling zero- or double-quantum matching conditions. This class of experiments relies on second-order cross-terms between 1H-A and 1H-B dipolar couplings in the average Hamiltonian to achieve polarization transfer. Therefore, they differ from first-order recoupling experiments such as RFDR96 or TEDOR198,199, which rely on direct 13C-13C and 15N-13C dipolar couplings for polarization transfer. While the first-order average Hamiltonian for TSAR experiments averages to zero, the second-order average Hamiltonian contains trilinear terms of the form HzA±B±, which drive polarization transfer.
The homonuclear TSAR experiment is superior to first-order homonuclear recoupling experiments by avoiding dipolar truncation, a phenomenon that masks the structurally informative long-range weak couplings by short-range strong couplings. Moreover, at high magnetic fields and high MAS frequencies, 13C-13C and 15N-15N spin diffusion in PDSD and DARR experiments becomes increasingly inefficient. In comparison, TSAR experiments continue to perform well under these high-field and fast MAS conditions. The first homonuclear TSAR experiment, called PAR (Proton Assisted Recoupling)197, has been conducted at 20–65 kHz MAS at magnetic fields higher than 20 T200. PAR is also able to provide long-range 15N-15N correlations in proteins201 despite the weakness of 15N-15N dipolar couplings. Sequential 15N-15N cross peaks help resonance assignment, particularly when combined with a 15N-13C SPECIFICCP block to produce a 3D 15N-15N-13C correlation spectrum202. Medium-range 15N-15N correlations obtained using the PAR experiment can help to identify α-helical secondary structure due to the relatively short distances between amide nitrogens in α-helices. Long-range 15N-15N contacts can help to identify the registry of antiparallel β-sheets. The first heteronuclear TSAR experiment is proton-assisted insensitive nuclei cross polarization (PAINCP)196,203, which has also been widely applied for structure determination.
Improved variants of the PAR and PAINCP experiments have also been introduced. One variant combines 13C-13C PAR and 15N-13C PAINCP in one experiment by using simultaneous 1H-13C and 1H-15N CP at the beginning of the experiment204,205. Measuring these two spectra simultaneously gives nearly the same signal-to-noise ratio as measuring them in separate experiments. A careful choice of phase-cycling allows for the heteronuclear 13C-15N cross peaks obtained from PAINCP to be negative, while the homonuclear 13C-13C cross peaks obtained from PAR to be positive. Furthermore, a z-filter during t1 evolution on the 13C channel allows the acquisition of indirect 13C and 15N dimensions with different spectral widths, which is important to fine tune these experiments given the very different chemical shift dispersion between 13C and 15N nuclei.
Recently, Hong and coworkers introduced pulsed variants of the TSAR experiments by replacing the continuous-wave (CW) spin lock on the low-γ channels with pulsed spin-lock. The homonuclear version of these Pulsed TSAR (P-TSAR) experiments is called pulsed proton assisted recoupling (PULSAR)206 while the heteronuclear version is called proton enhanced rotor-echo short pulse irradiation cross-polarization (PERSPIRATIONCP)207. These experiments reduce the rf duty cycle by 80–90% while producing similar polarization transfer as CW-TSAR experiments. When rf heating and probe hardware are the limiting factors in mixing times, these P-TSAR experiments allow for longer mixing times and thus longer-range contacts to be measured. P-TSAR experiments can be described with a simple analytical model206 and are insensitive to rf field strengths. Therefore, they are simpler to optimize than the CW-TSAR experiments208.
5.4. Applications of TSAR Experiments to Structural Biology
5.4.1. Amyloid Proteins
The peptide GNNQQNY is a fragment of the yeast prion protein Sup35p. Solid-state NMR spectra of this amyloid peptide show the presence of three conformers209, whose sidechains are in molecular contact with each other based on PAR and PAINCP spectra of mixed labeled samples measured with 10–14 ms mixing. These intermolecular contacts are attributed to distances of up to 7 Å, and indicate that each conformer makes up a parallel in-register β-sheet, and the different β-sheets associate via sidechain steric zippers. By mapping out which residues interact with each other, these authors identified the smallest building blocks that satisfy all the observed intermolecular correlations.
Tycko and coworkers determined a structure of Aβ40 fibrils seeded from plaques obtained the brains of Alzheimer’s disease patients210. Seeding allows isotopically enriched Aβ monomers to be used to replicate the brain Aβ conformation. Using a 13C PAR experiment with 13 ms mixing, these authors measured many medium- and long-range 13C-13C correlations, which helped to constrain the three-dimensional structure of these AD brain-derived Aβ fibrils.
The 42-residue Aβ42 peptide is more toxic than Aβ40 and is more difficult to study because of its rapid fibrilization kinetics. Griffin, Riek, Meier, Böckmann and coworkers determined the structure of Aβ42 fibrils using 13C-13C PAR and 15N-13C PAINCP experiments in addition to other correlation experiments211,212 (Fig. 14B). A 30 ms 15N-13C PAINCP experiment gave cross peaks with a distance upper limit of ~12 Å, while 13C PAR experiments with 10 and 15 ms mixing gave cross peaks with distance upper limits of 8.5 Å and 10 Å, respectively. The numerous long-range correlations constrained the structure of the fibril, showing a dimer of Aβ42 each adopting a S-shaped fold. Intermolecular contacts between the two molecules were obtained by comparing the 20 ms 13C PAR spectra measured on fully 13C-labeled peptide and 30% 13C-labeled monomers diluted in 70% unlabeled monomers. Intermolecular cross peaks decreased significantly in intensity upon dilution, whereas intramolecular cross peaks retained the same intensities. These distance restraints led to a high-resolution structure with a heavy-atom RMSD of 1.07 Å.
Determining the intermolecular packing and registry of β-strands is essential for amyloid protein structure determination. Mixed 13C and 15N labeled samples can be used to obtain this information by comparing an intermolecular PAINCP experiment with an intramolecular SPECIFICCP-based N-Cα correlation experiment. If the same cross peaks are observed, then this is strong evidence that the fibrils have a parallel in-register packing. This approach was used to show that Aβ40 fibrils bearing the E22Δ Osaka mutation213, Aβ42 fibrils212, and human β-endorphin amyloids214 all have parallel-in-register packing along the fibril axis.
The peptide Ac-IHVHLQI-CONH2 self assembles into amyloid fibrils that coordinate Zn2+ to catalyze ester hydrolysis215. Hong and coworkers determined the structure of these Zn2+-bound amyloid fibrils using solid-state NMR216. A 2D 13C PAR experiment with 15 ms mixing yielded intermolecular contacts between V3 and I7 sidechains and between I1 and L5 sidechains, indicating that the hydrophobic faces of the β-sheets pack in an antiparallel fashion. In the final high-resolution structure, these intermolecular contacts correspond to distances of 8–10 Å.
Receptor-interacting protein kinases 1 (RIPK1) and 3 (RIPK3) are involved in neurodegenerative diseases, cancer, and immune defense. Amyloid fibrils made of RIPK1 and RIPK3 are important for signaling during necroptosis. McDermott and coworkers investigated the hetero-amyloid formed by alternating layers of RIPK1 and RIPK3217. TSAR experiments were used to determine the structure of the monomers as well as the packing of the monomers within the fibrils. 13C PAR experiments with 12 and 15 ms mixing provided intermolecular contacts that established the parallel and alternating stacking of the monomers along the fibril axis. A 6 ms PAINCP experiment helped to define the monomer bend and the registry of inter-residue contacts (Fig. 14C).
5.4.2. Nucleic Acids and Carbohydrates
Unambiguous assignment of Watson-Crick base pairs is a prerequisite for determining the three-dimensional structure of RNAs. Methods that directly probe the NH-N hydrogen bond are ideal for identifying such interactions, but have not been commonly used due to the difficulty of measuring 15N-15N correlations. 15N-15N PAR offers a promising avenue for measuring inter-nucleotide correlations because of the presence of a proton between two 15N nuclei. Wang and coworkers identified G-C Watson-Crick base pairs in the dimerization initiation site of HIV-1 (DIS-HIV-1)158. This experiment begins with 1H-13C CP, followed by 13C-15N SPECIFICCP to 15N with chemical shifts greater than 140 ppm to select for the nitrogens involved in Watson-Crick base pairs. The 15N chemical shift encoding was followed by 7 ms 15N-15N PAR mixing, after which the 15N magnetization was transferred back to 1H for high-sensitivity detection.
The carbohydrate structure and interactions in fungal cell walls are poorly understood. Using solid-state NMR, Wang and coworkers investigated the cell walls of intact A. fumigatus fungi that were grown in 13C and 15N-enriched media218. Using a 15 ms 13C-13C PAR experiment, they identified 23 long-range intermolecular cross peaks, which correspond to distances of 5–10 Å. This experiment helped to identify which carbohydrates are in close proximity to each other. Most of the contacts were seen between chitin and α-1,3-glucans, while some contacts occur between chitin-α-1,3-glucan complexes and β-glucans. Using a DNP sensitivity-enhanced 15N-15N PAR experiment, these authors observed correlations between the three allomorphs of chitin. These results indicate that the three allomorphs coexist within each microfibril rather than being separated into different domains.
5.5. CHHC and NHHC Distance Techniques
Due to the high proton density and strong 1H-1H dipolar couplings in biological molecules, 1H spin diffusion is highly efficient, and is not hampered by high magnetic fields and fast MAS frequencies in the same way that 13C spin diffusion is. Thus, 1H spin diffusion is attractive for distance measurements at moderate MAS frequencies. Baldus and coworkers introduced a CHHC technique for distance measurements using 1H spin diffusion219. The experiment begins with 1H-13C CP and 13C t1 evolution to encode the 13C chemical shift. The 13C polarization is then transferred back to 1H via a short CP step, after which 1H spin diffusion is allowed to occur during a mixing period. Finally, a short CP back to 13C allows detection. This experiment takes advantage of the spectral resolution afforded by 13C while probing proton-proton distances. This experiment can be flexibly adapted to different heteronuclei to yield, for example, the NHHC experiment. When two different low-γ nuclei are used, the detection nucleus is typically the one with the higher γ in order to maximize the experimental sensitivity.
5.6. Application of the XHHY Experiments to Structural Biology
5.6.1. Membrane Proteins and Protein Assemblies
Anabaena sensory rhodopsin (ASR) is a microbial retinal-binding photoreceptor, and forms trimers of heptahelical monomers in lipid bilayers. Using 1,3 13C-labeled protein and a 2D CHHC experiment with 0.25 ms mixing, Ladizhansky and coworkers measured 14 interhelical 1H-1H distances with an upper bound of 5 Å in DMPC/DMPA membrane-bound protein220 (Fig. 15A). Combined with interhelical 13C-13C correlations obtained from PDSD spectra, they determined the three-dimensional structure of this seven-TM helix protein to a backbone RMSD of 0.8 Å.
Figure 15.
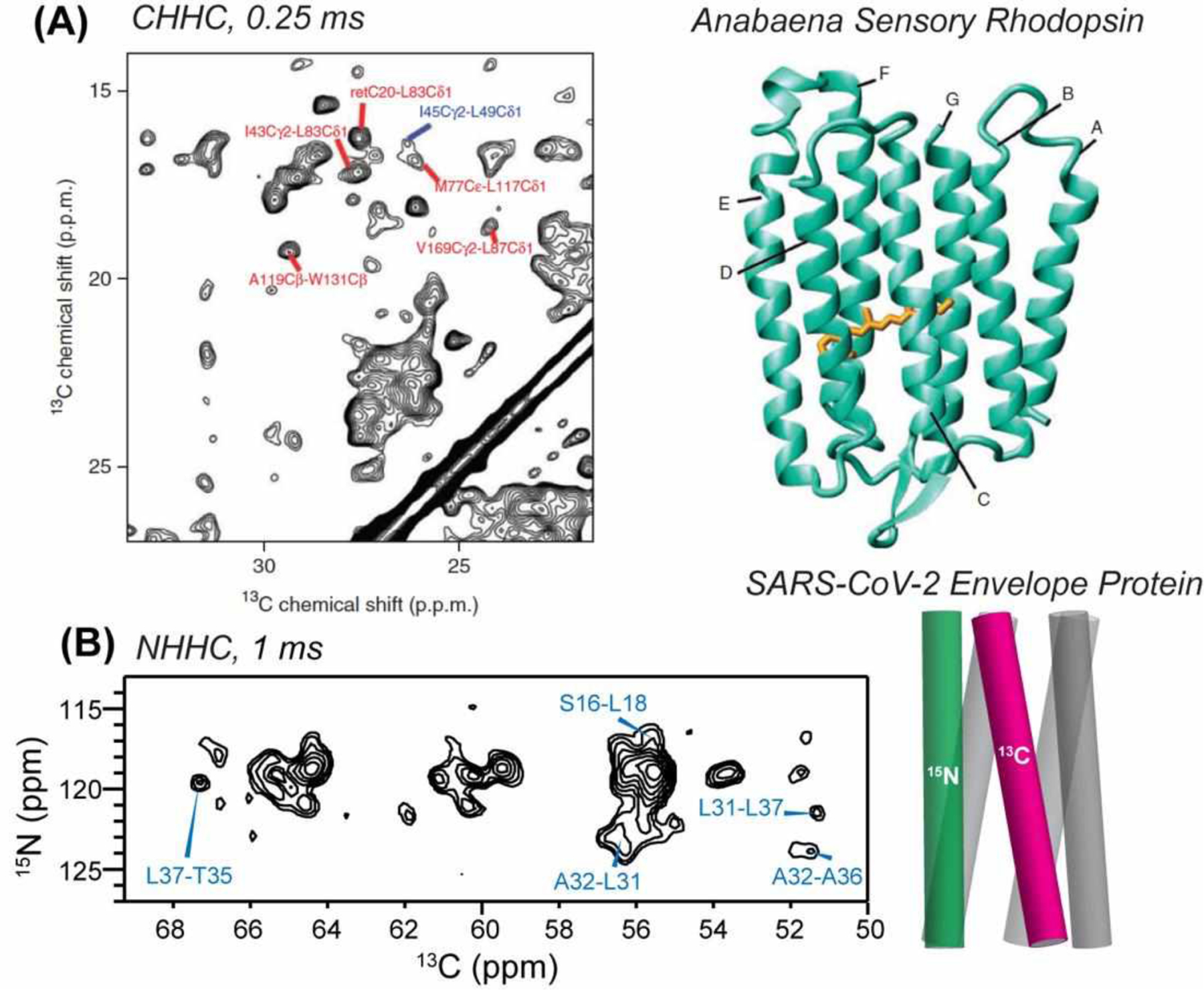
CHHC and NHHC distance experiments for studying membrane protein structure. (A) 0.25 ms 2D CHHC spectrum of Anabaena sensory rhodopsin220 (PDB 2M3G). Unambiguous long-range distance restraints are assigned in red while medium-range distance restraints are assigned in blue. (B) 0.5 ms (in red) and 1.0 ms (in blue) 2D NHHC spectrum of the SARS-CoV-2 envelope protein46. 13C-labeled and 15N-labeled monomers were mixed at a 1:1 ratio to detect interhelical contacts.
NHHC experiments can be readily applied to oligomeric membrane proteins to measure intermolecular distance restraints. Mixing 13C-labeled monomers with 15N-labeled monomers ensures that all 13C-15N cross peaks are intermolecular. This approach was used to determine the structure of the TM domain of the SARS-CoV-2 envelope protein46. The protein forms a cation-selective channel that is important for the virus’s pathogenicity. By mixing 13C-labeled and 15N-labeled monomers at a 1:1 ratio and conducting NHHC experiments with mixing times of 0.5 ms and 1.0 ms, Hong and coworkers obtained 52 inter-helical contacts (Fig. 15B). Using a distance upper bound of 9.0 Å and 11.5 Å for the 0.5 and 1.0 ms mixing times, they calculated the structure of the five-helix bundle. The resulting closed-state structure shows a tight and narrow pore, stabilized by methyl-rich Ile, Val and Leu residues at the helix-helix interface and by three regularly spaced Phe residues.
Molecular assemblies such as molecular motors, bacterial secretion systems, and cytoskeletal filaments can be challenging to study structurally because of their large sizes and their structural disorder. Although cryoEM is ideal for obtaining the global fold of these molecular complexes, it often does not give sufficient resolution. Solid-state NMR is complementary to cryoEM in providing atomic-level structural information. Lange, Sgourakis and coworkers integrated cryoEM with solid-state NMR to obtain a high-resolution structure of the Shigella type-III secretion needle221. Using a 7.7 Å resolution cryoEM structure and 996 solid-state NMR distance restraints obtained from PDSD experiments with 300 ms to 850 ms mixing, they determined the structure of this secretion needle to an RMSD of 0.4 Å. This structure was cross-validated with 96 unambiguous medium- and long-range 1H-1H restraints obtained from 0.25 ms NHHC and CHHC experiments and ambiguous restraints obtained from PDSD experiments. Less than 5% of these validation restraints were violated in the cross-validation step, supporting the accuracy of their high-resolution structure.
5.6.2. Amyloid Proteins
Identifying the mode of intermolecular packing is an important step in determining the high-resolution structure of amyloid fibrils. In an antiparallel β-sheet, Hα atoms on residues that are aligned along the hydrogen-bonded fibril axis are only 2.1 Å from each other. Thus, this intermolecular distance is even shorter than intramolecular Hα-Hα contacts. Using a CHHC experiment with a short 1H-1H mixing period of 0.2 ms, Tycko and coworkers showed that an antiparallel β-sheet can be unambiguously identified by strong 13Cα−13Cα intermolecular cross peaks222. Hong and coworkers successfully applied this approach to show that the peptide hormone glucagon forms an antiparallel hydrogen-bonded amyloid fibril with a well-defined registry194. Antiparallel β-sheets are rare among amyloid proteins, therefore the CHHC approach is complementary to the 15N-13C PAINCP experiment for determining the structure of parallel hydrogen-bonded β-sheets.
The bacterium Caulobacter Crescentus produces bactofilin filaments made of the BacA protein, which are important for the stability and survival of the bacterium. Purified BacA polymerizes and forms single filaments, filament bundles, and two-dimensional semi-crystalline sheets. Lange and coworkers characterized these bactofilin filaments using electron microscopy and solid-state NMR223. They found that the rigid core of the filaments spans a conserved DUF583 domain, while the N- and C-termini are mobile. Chemical shifts indicate at least 10 β-strands in this domain, with no evidence for α-helical conformation. Mass-per-length measurements showed that each monomer spans about 2.7 nm along the fibril axis. This is inconsistent with a cross-β architecture, which would consist of integer multiples of monomers every 0.48 nm. This suggests a β-helical architecture, similar to that of the prion protein HET-s161. Using a homology model based on the crystal structure of the Mycobacterium tuberculosis acetyltransferase G1mU224, Lange and coworkers these authors proposed that BacA adopts a β-helical structure in which the flat faces of the triangular assembly consist of β-strands, as identified from secondary chemical shifts. This model is supported by 8 long-range contacts measured in a 0.2 ms NHHC experiment and 12 long-range contacts measured in a 200 ms 13C-13C PDSD experiment. These long-range contacts provided strong evidence for the β-helical structure of these filaments.
Huntington’s disease is a neurodegenerative disease caused by the formation of an extended polyQ sequence that is prone to aggregation. Van der Wel and coworkers investigated the structure of the amyloid fibrils formed by a huntingtin mutant with a 44-residue polyQ domain225. Chemical shifts and torsion angle data indicate a β-sheet conformation. Interestingly, peak doubling with a 1:1 intensity ratio was observed, indicating that two conformations of Gln coexist in these fibrils. NCACX and NCOCX spectra show that these two forms exist in uniform segments, i.e. individual β-strands are made up of one form or the other. 2D 13C-13C PDSD experiments with 250 ms mixing on isotopically diluted samples show intermolecular cross peaks between the two conformers, suggesting that these fibrils contain a β-hairpin that places the two conformers in an antiparallel orientation along the fibril axis. 2D NHHC experiments with 0.25 ms mixing on mixed labeled proteins in which one quarter of the monomers were 13C-labeled while three quarters were 15N-labeled also showed intermolecular contacts between the two forms. Moreover, cross peaks involving sidechain 15N are more intense than cross peaks involving backbone 15N. This provides evidence for interdigitation of the sidechains to form an intermolecular steric zipper.
5.6.3. Nucleic Acids
RNA, DNA, and ribonucleoprotein proteins (RNPs) are ubiquitous in biology, but their structures are much less studied than proteins. Carlomagno and coworkers applied solid-state NMR to investigate the structure of a 26-residue Box C/D RNA from Pyrococcus Furiosus in complex with the L7Ae protein226. Due to the short 1H-1H distances associated with base pairs, the NHHC experiment is well suited for determining which bases are paired with each other. Using NHHC and CHHC experiments with mixing times of 0.1–0.2 ms, they obtained 27 unambiguous inter-nucleotide contacts with distances up to 5 Å. These proton-based distance restraints, together with 15N-13C distance restraints from TEDOR and 13C-13C restraints from PDSD experiments, helped to determine a three-dimensional structure of the RNA, giving a final structural ensemble with a heavy-atom RMSD of 0.9 Å.
Primases synthesize single-stranded DNA that can be extended by DNA polymerases. Allain and coworkers determined the high-resolution structure of the 115-residue helix bundle domain (HBD) of the pRN1 archaeal primase in complex with DNA 5’-CTGTGCTCA-3’ and ATP, where the GTG nucleic acids make up the binding motif that interacts with the protein227. They found that the binding of two nucleotide triphosphates causes an allosteric change in the protein that makes DNA binding sequence-specific. Solid-state NMR CHHP and NHHP experiments provided intermolecular distance restraints from Lys and Arg residues on the protein to the DNA and ATP. Thus, the XHHY technique can be extended to other nuclei such as 31P to obtain important distance restraints for structure investigation.
6. Concluding Remarks and Outlook
This review shows the broad range of solid-state NMR techniques that are now available to biophysicists, chemists, and materials scientists to measure inter-atomic distances to ~2 nm. 19F MAS NMR plays a prominent role in these long-distance measurements. It should be mentioned that 19F NMR has also been widely used in the solution state to probe structure, dynamics, and ligand binding228–231. These nanometer distances are especially valuable for revealing the molecular structures of partially disordered biomolecular assemblies, including membrane-bound proteins, amyloid proteins, protein-protein complexes, protein-nucleic acid complexes, and carbohydrates in cell walls. To harvest the full power of these distance NMR techniques, one should combine multinuclear detection, fast MAS, high magnetic fields, and multidimensional correlation. The technological barrier for producing ultrahigh magnetic fields has been recently overcome with the commercialization of high-temperature superconducting magnets at field strengths above 28 Tesla. Therefore, we envision that the next step in the widespread application of these distance NMR experiments is the development of more versatile and powerful NMR probes. These probes should be able to simultaneously tunable to four or five nuclear spins, including 1H, 19F, 13C, 15N, and 31P. By illuminating the 1–2 nm distance range, which used to fall in the gap between the detection range of traditional NMR experiments and the detection range of other techniques such as paramagnetic relaxation enhancement NMR, electron paramagnetic resonance and fluorescence spectroscopy, these long-distance solid-state NMR techniques promise to make unique and significant contributions to biophysical chemistry.
Acknowledgements
The 2D 13C-19F and 1H-19F REDOR NMR techniques reviewed here were developed with the support of NIH grants P41 GM132079, GM088204 and GM066976 to M.H. A.A.S is partially supported by an MIT School of Science Sloan Fund. J.M-S gratefully acknowledges a Rubicon Fellowship 452020132 sponsored by the Dutch Research Council (NWO). M.D.G. was supported by an NIH Ruth L. Kirschstein Individual National Research Service Award (1F31AI133989).
Biographies
Alexander A. Shcherbakov is a Ph.D. candidate in the laboratory of Professor Mei Hong at the Massachusetts Institute of Technology (MIT). He received his B.S. degree with honors in Biochemistry and Chemistry and a minor in Mathematics at the University of Washington in Seattle. There he worked in the laboratory of Professor Niels Andersen studying the thermodynamics and kinetics of β-sheet peptide folding using solution NMR and circular dichroism. As a graduate student, Alexander developed fast MAS 19F solid-state NMR techniques for rapid and high-sensitivity measurement of nanometer-length inter-atomic distances. He applied these techniques to the structure determination of several membrane proteins, including influenza B M2, SARS CoV-2 E, and the E. coli. multidrug-resistance protein EmrE.
João Medeiros-Silva studied biochemistry at NOVA University Lisbon and obtained his Ph.D. cum laude in Molecular Life Sciences from Utrecht University. There he investigated the structure and dynamics of potassium channels, antibiotic peptides, and protein-lipid interactions in the laboratory of Professor Markus Weingarth. In 2020 he joined the Hong laboratory at MIT as a Rubicon Postdoctoral Fellow, where he employs advanced solid-state NMR techniques to investigate the structure and biophysics of SARS-CoV-2 membrane proteins and other systems in their native membrane environment.
Nhi Tran received her Ph.D. in Physical Chemistry at the University of Florida in Gainesville under the supervision of Professor Joanna Long. Her research there centered on the application of solid-state NMR and dynamic nuclear polarization to characterize the structure and mechanism of pulmonary surfactant peptides and the investigation of lipid membrane dynamics. As a postdoctoral associate in the group of Professor Mei Hong, Nhi applied 19F solid-state NMR techniques to investigate the structure and oligomeric assembly of membrane proteins involved in virus-cell membrane fusion. Currently, Nhi is a Process Engineer at Intel Corporation.
Martin D. Gelenter received his Ph.D. in Chemistry from MIT under the supervision of Professor Mei Hong. His research focused on studying the structure and aggregation of glucagon fibrils, characterizing the interaction of water with the influenza A M2 proton channel, and developing solid-state NMR techniques for studying the structure and dynamics of biomacromolecules. Currently he is a postdoctoral research fellow in the laboratory of Dr. Ad Bax at the National Institutes of Health. His research at NIH utilizes pressure-jump solution state NMR to study protein folding and misfolding.
Mei Hong is a Professor of Chemistry at the Massachusetts Institute of Technology. She obtained her Ph.D. from the University of California at Berkeley in 1996, where she developed and applied variable-angle spinning solid-state NMR techniques to study the conformation and dynamics of phospholipid membranes in the laboratory of Professor Alex Pines. After a one-year period as an NIH postdoctoral fellow in the laboratory of Bob Griffin at MIT, she went to the University of Massachusetts at Amherst and developed selective and extensive isotopic labeling strategies and multidimensional MAS NMR methods to achieve protein resonance assignment. She established her own laboratory at Iowa State University in 1999, became a full professor in 2005, and held the first John D. Corbett Professorship before returning to MIT in 2014. The Hong group develops and applies solid-state NMR spectroscopy to elucidate the molecular structure, dynamics, and mechanism of action of membrane proteins and amyloid proteins important for human health, as well as the structure and dynamics of plant cell walls.
Footnotes
The authors declare no competing financial interest.
References
- (1).Reif B; Ashbrook SE; Emsley L; Hong M, Solid-State NMR Spectroscopy. Nat. Rev. Methods Primers 2021, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).McDermott AE, Structure and Dynamics of Membrane Proteins by Magic Angle Spinning Solid-State NMR. Annu. Rev. Biophys 2009, 38, 385–403. [DOI] [PubMed] [Google Scholar]
- (3).Comellas G; Rienstra CM, Protein Structure Determination by Magic-Angle Spinning Solid-State NMR, and Insights into the Formation, Structure, and Stability of Amyloid Fibrils. Annu. Rev. Biophys 2013, 42, 515–536. [DOI] [PubMed] [Google Scholar]
- (4).Mandala VS; Williams JK; Hong M, Structure and Dynamics of Membrane Proteins from Solid-State NMR. Ann. Rev. Biophys 2018, 47, 201–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Griffin R, Dipolar Recoupling in MAS Spectra of Biological Solids. Nature Struct. Biol 1998, 5, 508–512. [DOI] [PubMed] [Google Scholar]
- (6).Russell RW; Fritz MP; Kraus J; Quinn CM; Polenova T; Gronenborn AM, Accuracy and Precision of Protein Structures Determined by Magic Angle Spinning NMR Spectroscopy: For Some ‘with a Little Help from a Friend’. J. Biomol. NMR 2019, 73, 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Clore MG; Iwahara J, Theory, Practice, and Applications of Paramagnetic Relaxation Enhancement for the Characterization of Transient Low-Population States of Biological Macromolecules and Their Complexes. Chem. Rev 2009, 109, 4108–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Sengupta I; Nadaud PS; Jaroniec CP, Protein Structure Determination with Paramagnetic Solid-State NMR Spectroscopy. Acc. Chem. Res 2013, 46, 2117–2126. [DOI] [PubMed] [Google Scholar]
- (9).Clore MG, Practical Aspects of Paramagnetic Relaxation Enhancement in Biological Macromolecules; Qin PZ;Warncke K, Eds.; Academic Press, 2015; Vol. 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Gullion T; Schaefer J, Rotational Echo Double Resonance NMR. J. Magn. Reson 1989, 81, 196–200. [DOI] [PubMed] [Google Scholar]
- (11).Pan Y; Gullion T; Schaefer J, Determination of C-N Internuclear Distances by Rotational-Echo Double-Resonance NMR of Solids. J. Magn. Reson 1990, 90, 330. [Google Scholar]
- (12).Levitt MH, Symmetry in the Design of NMR Multiple-Pulse Sequences. J. Chem. Phys 2008, 128, 052205. [DOI] [PubMed] [Google Scholar]
- (13).De Paëpe G, Dipolar Recoupling in Magic Angle Spinning Solid-State Nuclear Magnetic Resonance. Annu. Rev. Phys. Chem 2012, 63, 661–684. [DOI] [PubMed] [Google Scholar]
- (14).Brinkmann A; Kentgens AP, Proton-Selective 17o-1H Distance Measurements in Fast Magic-Angle-Spinning Solid-State NMR Spectroscopy for the Determination of Hydrogen Bond Lengths. J. Am. Chem. Soc 2006, 128, 14758–14759. [DOI] [PubMed] [Google Scholar]
- (15).Sinha N; Schmidt-Rohr K; Hong M, Compensation for Pulse Imperfections in Rotational-Echo Double-Resonance NMR by Composite Pulses and Exorcycle. J. Magn. Reson 2004, 168, 358–365. [DOI] [PubMed] [Google Scholar]
- (16).Gullion T; Schaefer J, Elimination of Resonance Offset Effects in Rotational-Echo Double Resonance NMR. J. Magn. Reson 1991, 92, 439–442. [Google Scholar]
- (17).Weldeghiorghis TK; Schaefer J, Compensating for Pulse Imperfections in REDOR. J. Magn. Reson 2003, 165, 230–236. [DOI] [PubMed] [Google Scholar]
- (18).Bak M; Rasmussen T; Nielsen NC, Simpson: A General Simulation Program for Solid-State NMR Spectroscopy. J. Magn. Reson 2000, 147, 296–330. [DOI] [PubMed] [Google Scholar]
- (19).Veshtort M; Griffin RG, Spinevolution: A Powerful Tool for the Simulation of Solid and Liquid State NMR Experiments. J. Magn. Reson 2006, 178, 248–282. [DOI] [PubMed] [Google Scholar]
- (20).McDowell LM; Lee M; McKay RA; Anderson KS; Schaefer J, Intersubunit Communication in Tryptophan Synthase by Carbon-13 and Fluorine-19 REDOR NMR. Biochemistry 1996, 35, 3328–3334. [DOI] [PubMed] [Google Scholar]
- (21).Graesser DT; Wylie BJ; Nieuwkoop AJ; Franks WT; Rienstra CM, Long-Range 19F-15N Distance Measurements in Highly-13C, 15N-Enriched Solid Proteins with 19F-Dephased REDOR Shift (Fresh) Spectroscopy. Magn. Reson. Chem 2007, 45, S129–S134. [DOI] [PubMed] [Google Scholar]
- (22).Shcherbakov AA; Hong M, Rapid Measurement of Long-Range Distances in Proteins by Multidimensional 13C–19F REDOR NMR under Fast Magic-Angle Spinning. J. Biomol. NMR 2018, 71, 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Shcherbakov AA; Mandala VS; Hong M, High-Sensitivity Detection of Nanometer 1H–19F Distances for Protein Structure Determination by 1H-Detected Fast MAS NMR. J. Phys. Chem. B 2019, 123, 4387–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Shcherbakov AA; Roos M; Kwon B; Hong M, Two-Dimensional (19)F-(13)C Correlation NMR for (19)F Resonance Assignment of Fluorinated Proteins. J. Biomol. NMR 2020, 74, 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Bennett AE; Griffin RG; Ok JH; Vega S, Chemical Shift Correlation Spectroscopy in Rotating Solids: Radio Frequency‐Driven Dipolar Recoupling and Longitudinal Exchange. J. Chem. Phys 1992, 96, 8624–8627. [Google Scholar]
- (26).Kim HW; Perez JA; Ferguson SJ; Campbell ID, The Specific Incorporation of Labelled Aromatic Amino Acids into Proteins through Growth of Bacteria in the Presence of Glyphosate. Application to Fluorotryptophan Labelling to the H(+)-Atpase of Escherichia Coli and NMR Studies. FEBS Lett. 1990, 272, 34–36. [DOI] [PubMed] [Google Scholar]
- (27).Gullion T, Measuring 13C-2D Dipolar Couplings with a Universal REDOR Dephasing Curve. J. Magn. Reson 2000, 146, 220–222. [DOI] [PubMed] [Google Scholar]
- (28).Wi S; Sinha N; Hong M, Long-Range 1H-19F Distance Measurement in Peptides by Solid-State NMR. J. Am. Chem. Soc 2004, 126, 12754–12755. [DOI] [PubMed] [Google Scholar]
- (29).Hong M; Griffin RG, Resonance Assignment for Solid Peptides by Dipolar-Mediated 13C/15N Correlation Solid-State NMR. J. Am. Chem. Soc 1998, 120, 7113–7114. [Google Scholar]
- (30).Rienstra CM; Tucker-Kellogg L; Jaroniec CP; Hohwy M; Reif B; McMahon MT; Tidor B; Lozano-Perez T; Griffin RG, De Novo Determination of Peptide Structure with Solid-State Magic-Angle Spinning NMR Spectroscopy. Proc. Natl. Acad. Sci. USA 2002, 99, 10260–10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Ghosh M; Rienstra CM, 1H-Detected REDOR with Fast Magic-Angle Spinning of a Deuterated Protein. J. Phys. Chem. B 2017, 121, 8503–8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Su Y; Li S; Hong M, Cationic Membrane Peptides: Atomic-Level Insight of Structure-Activity Relationships from Solid-State NMR. Amino Acids 2013, 44, 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Hong M; Su Y, Structure and Dynamics of Cationic Membrane Peptides and Proteins: Insights from Solid-State NMR. Protein Sci. 2011, 20, 641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Toke O; Lee Maloy W; Kim SJ; Blazyk J; Schaefer J, Secondary Structure and Lipid Contact of a Peptide Antibiotic in Phospholipid Bilayers by REDOR. Biophys. J 2004, 87, 662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Toke O; O’Connor RD; Weldeghiorghis TK; Maloy WL; Glaser RW; Ulrich AS; Schaefer J, Structure of (Kiagkia)3 Aggregates in Phospholipid Bilayers by Solid-State NMR. Biophys. J 2004, 87, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Mani R; Tang M; Wu X; Buffy JJ; Waring AJ; Sherman MA; Hong M, Membrane-Bound Dimer Structure of a B-Hairpin Antimicrobial Peptide from Rotational-Echo Double-Resonance Solid-State NMR. Biochemistry 2006, 45, 8341–8349. [DOI] [PubMed] [Google Scholar]
- (37).Cegelski L; Steuber D; Mehta AK; Kulp DW; Axelsen PH; Schaefer J, Conformational and Quantitative Characterization of Oritavancin–Peptidoglycan Complexes in Whole Cells of Staphylococcus Aureus by in Vivo 13C and 15N Labeling. J. Mol. Biol 2006, 357, 1253–1262. [DOI] [PubMed] [Google Scholar]
- (38).Umegawa Y; Matsumori N; Oishi T; Murata M, Ergosterol Increases the Intermolecular Distance of Amphotericin B in the Membrane-Bound Assembly as Evidenced by Solid-State NMR. Biochemistry 2008, 47, 13463–13469. [DOI] [PubMed] [Google Scholar]
- (39).Umegawa Y; Adachi T; Matsumori N; Murata M, Possible Conformation of Amphotericin B Dimer in Membrane-Bound Assembly as Deduced from Solid-State NMR. Bioorg. Med. Chem 2012, 20, 5699–5704. [DOI] [PubMed] [Google Scholar]
- (40).Hong M, Oligomeric Structure, Dynamics, and Orientation of Membrane Proteins from Solid-State NMR. Structure 2006, 14, 1731–1740. [DOI] [PubMed] [Google Scholar]
- (41).Hong M; DeGrado WF, Structural Basis for Proton Conduction and Inhibition by the Influenza M2 Protein. Protein Sci. 2012, 21, 1620–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Pinto LH; Lamb RA, The M2 Proton Channels of Influenza A and B Viruses. J. Biol. Chem 2006, 281, 8997–9000. [DOI] [PubMed] [Google Scholar]
- (43).Williams, Jonathan K; Zhang Y; Schmidt-Rohr K; Hong M, Ph-Dependent Conformation, Dynamics, and Aromatic Interaction of the Gating Tryptophan Residue of the Influenza M2 Proton Channel from Solid-State NMR. Biophys. J 2013, 104, 1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Hu F; Luo W; Hong M, Mechanisms of Proton Conduction and Gating by Influenza M2 Proton Channels from Solid-State NMR. Science 2010, 330, 505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Mandala VS; Loftis AR; Shcherbakov AA; Pentelute BL; Hong M, Atomic Structures of Closed and Open Influenza B M2 Proton Channel Reveal the Conduction Mechanism. Nat. Struct. Mol. Biol 2020, 27, 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Mandala VS; McKay MJ; Shcherbakov AA; Dregni AJ; Kolocouris A; Hong M, Structure and Drug Binding of the Sars-Cov-2 Envelope Protein Transmembrane Domain in Lipid Bilayers. Nat. Struct. Mol. Biol 2020, 27, 1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Surya W; Li Y; Torres J, Structural Model of the Sars Coronavirus E Channel in Lmpg Micelles. Biochim. Biophys. Acta Biomembr 2018, 1860, 1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Pervushin K; Tan E; Parthasarathy K; Lin X; Jiang FL; Yu D; Vararattanavech A; Soong TW; Liu DX; Torres J, Structure and Inhibition of the Sars Coronavirus Envelope Protein Ion Channel. PLoS Pathog. 2009, 5, e1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Kwon B; Lee M; Waring AJ; Hong M, Oligomeric Structure and Three-Dimensional Fold of the Hiv Gp41 Membrane-Proximal External Region and Transmembrane Domain in Phospholipid Bilayers. J. Am. Chem. Soc 2018, 140, 8246–8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Chiliveri SC; Louis JM; Ghirlando R; Baber JL; Bax A, Tilted, Uninterrupted, Monomeric Hiv-1 Gp41 Transmembrane Helix from Residual Dipolar Couplings. J. Am. Chem. Soc 2018, 140, 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Dev J; Park D; Fu Q; Chen J; Ha HJ; Ghantous F; Herrmann T; Chang W; Liu Z; Frey G et al. , Structural Basis for Membrane Anchoring of Hiv-1 Envelope Spike. Science 2016, 353, 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Pan Y, 31P-19F Rotational-Echo, Double-Resonance Nuclear Magnetic Resonance Experiment on Fluoridated Hydroxyapatite. Solid State Nucl. Magn. Reson 1995, 5, 263–268. [DOI] [PubMed] [Google Scholar]
- (53).Merritt ME; Sigurdsson ST; Drobny GP, Long-Range Distance Measurements to the Phosphodiester Backbone of Solid Nucleic Acids Using 31P−19F REDOR NMR. J. Am. Chem. Soc 1999, 121, 6070–6071. [Google Scholar]
- (54).Cruse WBT; Salisbury SA; Brown T; Cosstick R; Eckstein F; Kennard O, Chiral Phosphorothioate Analogues of B-DNA: The Crystal Structure of Rp-D¦Gp(S)Cpgp(S)Cpgp(S)C¦. J. Mol. Biol 1986, 192, 891–905. [DOI] [PubMed] [Google Scholar]
- (55).Olsen GL; Louie EA; Drobny GP; Sigurdsson ST, Determination of DNA Minor Groove Width in Distamycin‐DNA Complexes by Solid‐State NMR. Nucleic Acids Res. 2003, 31, 5084–5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Olsen GL; Edwards TE; Deka P; Varani G; Sigurdsson ST; Drobny GP, Monitoring Tat Peptide Binding to Tar Rna by Solid-State 31P–19F REDOR NMR. Nucleic Acids Res. 2005, 33, 3447–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Huang W; Varani G; Drobny GP, 13C/15N−19F Intermolecular REDOR NMR Study of the Interaction of Tar Rna with Tat Peptides. J. Am. Chem. Soc 2010, 132, 17643–17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Jiang YL; McDowell LM; Poliks B; Studelska DR; Cao C; Potter GS; Schaefer J; Song F; Stivers JT, Recognition of an Unnatural Difluorophenyl Nucleotide by Uracil DNA Glycosylase. Biochemistry 2004, 43, 15429–15438. [DOI] [PubMed] [Google Scholar]
- (59).Studelska DR; Klug CA; Beusen DD; McDowell LM; Schaefer J, Long-Range Distance Measurements of Protein Binding Sites by Rotational-Echo Double-Resonance NMR. J. Am. Chem. Soc 1996, 118, 5476–5477. [Google Scholar]
- (60).Fowler DJ; Weis RM; Thompson LK, Kinase-Active Signaling Complexes of Bacterial Chemoreceptors Do Not Contain Proposed Receptor−Receptor Contacts Observed in Crystal Structures. Biochemistry 2010, 49, 1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Elkins MR; Williams JK; Gelenter MD; Dai P; Kwon B; Sergeyev IV; Pentelute BL; Hong M, Cholesterol-Binding Site of the Influenza M2 Protein in Lipid Bilayers from Solid-State NMR. Proc. Natl. Acad. Sci. U. S. A 2017, 114, 12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Kwon B; Mandal T; Elkins MR; Oh Y; Cui Q; Hong M, Cholesterol Interaction with the Trimeric Hiv Fusion Protein Gp41 in Lipid Bilayers Investigated by Solid-State NMR Spectroscopy and Molecular Dynamics Simulations. J. Mol. Biol 2020, 432, 4705–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Elkins MR; Sergeyev IV; Hong M, Determining Cholesterol Binding to Membrane Proteins by Cholesterol 13C Labeling in Yeast and Dynamic Nuclear Polarization NMR. J. Am. Chem. Soc 2018, 140, 15437–15449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Souza CM; Schwabe TM; Pichler H; Ploier B; Leitner E; Guan XL; Wenk MR; Riezman I; Riezman H, A Stable Yeast Strain Efficiently Producing Cholesterol Instead of Ergosterol Is Functional for Tryptophan Uptake, but Not Weak Organic Acid Resistance. Metab. Eng 2011, 13, 555–569. [DOI] [PubMed] [Google Scholar]
- (65).Shcherbakov AA; Hisao G; Mandala VS; Thomas NE; Soltani M; Salter EA; Davis JH; Henzler-Wildman KA; Hong M, Structure and Dynamics of the Drug-Bound Bacterial Transporter Emre in Lipid Bilayers. Nat. Commun 2021, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Yang H; Staveness D; Ryckbosch SM; Axtman AD; Loy BA; Barnes AB; Pande VS; Schaefer J; Wender PA; Cegelski L, REDOR NMR Reveals Multiple Conformers for a Protein Kinase C Ligand in a Membrane Environment. ACS Cent. Sci 2018, 4, 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Loy BA; Lesser AB; Staveness D; Billingsley KL; Cegelski L; Wender PA, Toward a Biorelevant Structure of Protein Kinase C Bound Modulators: Design, Synthesis, and Evaluation of Labeled Bryostatin Analogues for Analysis with Rotational Echo Double Resonance NMR Spectroscopy. J. Am. Chem. Soc 2015, 137, 3678–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Lu M; Wang M; Sergeyev IV; Quinn CM; Struppe J; Rosay M; Maas W; Gronenborn AM; Polenova T, 19F Dynamic Nuclear Polarization at Fast Magic Angle Spinning for NMR of Hiv-1 Capsid Protein Assemblies. J. Am. Chem. Soc 2019, 141, 5681–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Lu X; Skomski D; Thompson KC; McNevin MJ; Xu W; Su Y, Three-Dimensional NMR Spectroscopy of Fluorinated Pharmaceutical Solids under Ultrafast Magic Angle Spinning. Anal. Chem 2019, 91, 6217–6224. [DOI] [PubMed] [Google Scholar]
- (70).Inoue M; Sumii Y; Shibata N, Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Lu X; Lu X; Tsutsumi Y; Huang C; Xu W; Byrn SR; Templeton AC; Buevich AV; Amoureux JP; Amoureux JP et al. , Molecular Packing of Pharmaceuticals Analyzed with Paramagnetic Relaxation Enhancement and Ultrafast Magic Angle Pinning NMR. Phys. Chem. Chem. Phys 2020, 22, 13160–13170. [DOI] [PubMed] [Google Scholar]
- (72).Lu X; Huang C; Li M; Skomski D; Xu W; Yu L; Byrn SR; Templeton AC; Su Y, Molecular Mechanism of Crystalline-to-Amorphous Conversion of Pharmaceutical Solids from (19)F Magic Angle Spinning NMR. J. Phys. Chem. B 2020, 124, 5271–5283. [DOI] [PubMed] [Google Scholar]
- (73).Lu X; Li M; Huang C; Lowinger MB; Xu W; Yu L; Byrn SR; Templeton AC; Su Y, Atomic-Level Drug Substance and Polymer Interaction in Posaconazole Amorphous Solid Dispersion from Solid-State NMR. Mol. Pharm 2020, 17, 2585–2598. [DOI] [PubMed] [Google Scholar]
- (74).Hou G; Yan S; Trébosc J; Amoureux JP; Polenova T, Broadband Homonuclear Correlation Spectroscopy Driven by Combined R2(N)(V) Sequences under Fast Magic Angle Spinning for NMR Structural Analysis of Organic and Biological Solids. J. Magn. Reson 2013, 232, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Roos M; Wang T; Shcherbakov AA; Hong M, Fast Magic-Angle-Spinning 19F Spin Exchange NMR for Determining Nanometer 19F–19F Distances in Proteins and Pharmaceutical Compounds. J. Phys. Chem. B 2018, 122, 2900–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Wang M; Lu M; Fritz MP; Quinn CM; Byeon IL; Byeon CH; Struppe J; Maas W; Gronenborn AM; Polenova T, Fast Magic-Angle Spinning 19F NMR Spectroscopy of Hiv-1 Capsid Protein Assemblies. Angew. Chem. Int. Ed. Engl 2018, 57, 16375–16379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Bayro MJ; Huber M; Ramachandran R; Davenport TC; Meier BH; Ernst M; Griffin RG, Dipolar Truncation in Magic-Angle Spinning NMR Recoupling Experiments. J. Chem. Phys 2009, 130, 114506–114506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Roos M; Mandala VS; Hong M, Determination of Long-Range Distances by Fast Magic-Angle-Spinning Radiofrequency-Driven 19F–19F Dipolar Recoupling NMR. J. Phys. Chem. B 2018, 122, 9302–9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Elkins MR; Bandara A; Pantelopulos GA; Straub JE; Hong M; Gerling UIM; Salwiczek M; Cadicamo CD; Erdbrink H; Czekelius C et al. , Direct Observation of Cholesterol Dimers and Tetramers in Lipid Bilayers. J. Phys. Chem. B 2021, 5, 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).deAzevedo ER; Bonagamba TJ; Hu W; Schmidt-Rohr K, Centerband-Only Detection of Exchange: Efficient Analysis of Dynamics in Solids by NMR. J. Am. Chem. Soc 1999, 121, 8411–8412. [Google Scholar]
- (81).deAzevedo ER; Hu WG; Bonagamba TJ; Schmidt-Rohr K, Principles of Centerband-Only Detection of Exchange in Solid-State Nuclear Magnetic Resonance, and Extension to Four-Time Centerband-Only Detection of Exchange. J. Chem. Phys 2000, 112, 8988–9001. [Google Scholar]
- (82).Luo W; Hong M, Determination of the Oligomeric Number and Intermolecular Distances of Membrane Protein Assemblies by Anisotropic 1H-Driven Spin Diffusion NMR Spectroscopy. J. Am. Chem. Soc 2006, 128, 7242–7251. [DOI] [PubMed] [Google Scholar]
- (83).Buffy JJ; Waring AJ; Hong M, Determination of Peptide Oligomerization in Lipid Bilayers Using 19F Spin Diffusion NMR. J. Am. Chem. Soc 2005, 127, 4477–4483. [DOI] [PubMed] [Google Scholar]
- (84).Luo W; Mani R; Hong M, Side-Chain Conformation of the M2 Transmembrane Peptide Proton Channel of Influenza A Virus from 19F Solid-State NMR. J. Phys. Chem. B 2007, 111, 10825–10832. [DOI] [PubMed] [Google Scholar]
- (85).Nishimura K; Kim S; Zhang L; Cross TA, The Closed State of a H+ Channel Helical Bundle Combining Precise Orientational and Distance Restraints from Solid State NMR. Biochemistry 2002, 41, 13170–13177. [DOI] [PubMed] [Google Scholar]
- (86).Williams JK; Shcherbakov AA; Wang J; Hong M, Protonation Equilibria and Pore-Opening Structure of the Dual-Histidine Influenza B Virus M2 Transmembrane Proton Channel from Solid-State NMR. J. Biol. Chem 2017, 292, 17876–17884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Kwon B; Roos M; Mandala VS; Shcherbakov AA; Hong M; Lane Gilchrist M; Monde K; Tomita Y; Iwashita T; Nakanishi K et al. , Elucidating Relayed Proton Transfer through a His–Trp–His Triad of a Transmembrane Proton Channel by Solid-State NMR. J. Mol. Biol 2019, 431, 2554–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Salnikov ES; Raya J; De Zotti M; Zaitseva E; Peggion C; Ballano G; Toniolo C; Raap J; Bechinger B, Alamethicin Supramolecular Organization in Lipid Membranes from 19F Solid-State NMR. Biophys. J 2016, 111, 2450–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Salnikov ES; De Zotti M; Bobone S; Mazzuca C; Raya J; Siano AS; Peggion C; Toniolo C; Stella L; Bechinger B, Trichogin Ga Iv Alignment and Oligomerization in Phospholipid Bilayers. Chembiochem 2019, 20, 2141–2150. [DOI] [PubMed] [Google Scholar]
- (90).Mani R; Cady SD; Tang M; Waring AJ; Lehrer RI; Hong M, Membrane-Dependent Oligomeric Structure and Pore Formation of a Beta-Hairpin Antimicrobial Peptide in Lipid Bilayers from Solid-State NMR. Proc. Natl. Acad. Sci. U. S. A 2006, 103, 16242–16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Tang M; Waring AJ; Hong M, Intermolecular Packing and Alignment in an Ordered B-Hairpin Antimicrobial Peptide Aggregate from 2D Solid-State NMR. J. Am. Chem. Soc 2005, 127, 13919–13927. [DOI] [PubMed] [Google Scholar]
- (92).Zhang Y; Lu W; Hong M, The Membrane-Bound Structure and Topology of a Human A-Defensin Indicate a Dimer Pore Mechanism for Membrane Disruption. Biochemistry 2010, 49, 9770–9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Su Y; Doherty T; Waring AJ; Ruchala P; Hong M, Roles of Arginine and Lysine Residues in the Translocation of a Cell-Penetrating Peptide from (13)C, (31)P, and (19)F Solid-State NMR. Biochemistry 2009, 48, 4587–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Lee M; Yao H; Kwon B; Waring AJ; Ruchala P; Singh C; Hong M, Conformation and Trimer Association of the Transmembrane Domain of the Parainfluenza Virus Fusion Protein in Lipid Bilayers from Solid-State NMR: Insights into the Sequence Determinants of Trimer Structure and Fusion Activity J. Mol. Biol 2018, 430, 695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Grage SL; Xu X; Schmitt M; Wadhwani P; Ulrich AS, (19)F-Labeling of Peptides Revealing Long-Range NMR Distances in Fluid Membranes. J. Phys. Chem. Lett 2014, 5, 4256–4259. [DOI] [PubMed] [Google Scholar]
- (96).Bennett AE; Rienstra CM; Griffiths JM; Zhen W; Lansbury PT Jr.; Griffin RG, Homonuclear Radio Frequency-Driven Recoupling in Rotating Solids. J. Chem. Phys 1998, 108, 9463–9479. [Google Scholar]
- (97).Lane Gilchrist M; Monde K; Tomita Y; Iwashita T; Nakanishi K; McDermott AE, Measurement of Interfluorine Distances in Solids. J. Magn. Reson 2001, 152, 1–6. [DOI] [PubMed] [Google Scholar]
- (98).Chen Q; Schmidt-Rohr K, 19F and 13C NMR Signal Assignment and Analysis in a Perfluorinated Ionomer (Nafion) by Two-Dimensional Solid-State NMR. Macromolecules 2004, 37, 5995–6003. [Google Scholar]
- (99).Roos M; Mandala VS; Hong M, Determination of Long-Range Distances by Fast Magic-Angle-Spinning Radiofrequency-Driven (19)F-(19)F Dipolar Recoupling NMR. J. Phys. Chem. B 2018, 122, 9302–9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Ishii Y, 13C-13C Dipolar Recoupling under Very Fast Magic Angle Spinning in Solid-State Nuclear Magnetic Resonance: Applications to Distance Measurements, Spectral Assignments, and High-Throughput Secondary-Structure Determination. J. Chem. Phys 2001, 114, 8473–8483. [Google Scholar]
- (101).Quinn CM; Zadorozhnyi R; Struppe J; Sergeyev IV; Gronenborn AM; Polenova T, Fast 19F Magic-Angle Spinning Nuclear Magnetic Resonance for the Structural Characterization of Active Pharmaceutical Ingredients in Blockbuster Drugs. Anal. Chem 2021, 93, 13029–13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Fritz M; Kraus J; Quinn CM; Yap GPA; Struppe J; Sergeyev IV; Gronenborn AM; Polenova T, Measurement of Accurate Interfluorine Distances in Crystalline Organic Solids: A High-Frequency Magic Angle Spinning NMR Approach. J. Phys. Chem. B 2019, 123, 10680–10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Reif B, Ultra-High Resolution in MAS Solid-State NMR of Perdeuterated Proteins: Implications for Structure and Dynamics. J. Magn. Reson 2012, 216, 1–12. [DOI] [PubMed] [Google Scholar]
- (104).Paulson EK; Morcombe CR; Gaponenko V; Dancheck B; Byrd RA; Zilm KW, High-Sensitivity Observation of Dipolar Exchange and Noes between Exchangeable Protons in Proteins by 3D Solid-State NMR Spectroscopy. J. Am. Chem. Soc 2003, 125, 14222–14223. [DOI] [PubMed] [Google Scholar]
- (105).Barbet-Massin E; Pell AJ; Retel JS; Andreas LB; Jaudzems K; Franks WT; Nieuwkoop AJ; Hiller M; Higman V; Guerry P et al. , Rapid Proton-Detected NMR Assignment for Proteins with Fast Magic Angle Spinning. J. Am. Chem. Soc 2014, 136, 12489–12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Penzel S; Smith AA; Agarwal V; Hunkeler A; Org ML; Samoson A; Böckmann A; Ernst M; Meier BH, Protein Resonance Assignment at MAS Frequencies Approaching 100 kHz: A Quantitative Comparison of J-Coupling and Dipolar-Coupling-Based Transfer Methods. J. Biomol. NMR 2015, 63, 165–186. [DOI] [PubMed] [Google Scholar]
- (107).Andreas LB; Le Marchand T; Jaudzems K; Pintacuda G, High-Resolution Proton-Detected NMR of Proteins at Very Fast MAS. J. Magn. Reson 2015, 253, 36–49. [DOI] [PubMed] [Google Scholar]
- (108).Ji Y; Liang L; Bao X; Hou G, Recent Progress in Dipolar Recoupling Techniques under Fast MAS in Solid-State NMR Spectroscopy; Elsevier B.V, 2021; Vol. 112. [DOI] [PubMed] [Google Scholar]
- (109).Vasa SK; Rovó P; Linser R, Protons as Versatile Reporters in Solid-State NMR Spectroscopy. Acc. Chem. Res 2018, 51, 1386–1395. [DOI] [PubMed] [Google Scholar]
- (110).Zhang R; Mroue KH; Ramamoorthy A, Proton-Based Ultrafast Magic Angle Spinning Solid-State NMR Spectroscopy. Acc. Chem. Res 2017, 50, 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Duong NT; Raran-Kurussi S; Nishiyama Y; Agarwal V, Can Proton-Proton Recoupling in Fully Protonated Solids Provide Quantitative, Selective and Efficient Polarization Transfer? J. Magn. Reson 2020, 317, 106777–106777. [DOI] [PubMed] [Google Scholar]
- (112).Zhou DH; Shea JJ; Nieuwkoop AJ; Franks WT; Wylie BJ; Mullen C; Sandoz D; Rienstra CM, Solid-State Protein-Structure Determination with Proton-Detected Triple-Resonance 3D Magic-Angle-Spinning NMR Spectroscopy. Angew. Chem. Int. Ed. Engl 2007, 46, 8380–8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Grohe K; Nimerovsky E; Singh H; Vasa SK; Söldner B; Vögeli B; Rienstra CM; Linser R, Exact Distance Measurements for Structure and Dynamics in Solid Proteins by Fast-Magic-Angle-Spinning NMR. Chem. Commun 2019, 55, 7899–7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Linser R; Bardiaux B; Higman V; Fink U; Reif B, Structure Calculation from Unambiguous Long-Range Amide and Methyl 1H-1H Distance Restraints for a Microcrystalline Protein with MAS Solid-State NMR Spectroscopy. J. Am. Chem. Soc 2011, 133, 5905–5912. [DOI] [PubMed] [Google Scholar]
- (115).Andreas LB; Jaudzems K; Stanek J; Lalli D; Bertarelloa A; Marchand TL; De Paepe DC; Kotelovica S; Akopjana I; Knott B et al. , Structure of Fully Protonated Proteins by Proton-Detected Magic- Angle Spinning NMR. Proc. Natl. Acad. Sci. U. S. A 2016, 113, 9187–9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Verel R; Ernst M; Meier BH, Adiabatic Dipolar Recoupling in Solid-State NMR: The Dream Scheme. J. Magn. Reson 2001, 150, 81–99. [DOI] [PubMed] [Google Scholar]
- (117).Huber M; Hiller S; Schanda P; Ernst M; Böckmann A; Verel R; Meier BH, A Proton-Detected 4D Solid-State NMR Experiment for Protein Structure Determination. Chemphyschem 2011, 12, 915–918. [DOI] [PubMed] [Google Scholar]
- (118).Agarwal V; Penzel S; Szekely K; Cadalbert R; Testori E; Oss A; Past J; Samoson A; Ernst M; Böckmann A et al. , De Novo 3D Structure Determination from Sub-Milligram Protein Samples by Solid-State 100 kHz MAS NMR Spectroscopy. Angew. Chem. Int. Ed. Engl 2014, 53, 12253–12256. [DOI] [PubMed] [Google Scholar]
- (119).Jain MG; Lalli D; Stanek J; Gowda C; Prakash S; Schwarzer TS; Schubeis T; Castiglione K; Andreas LB; Madhu PK et al. , Selective 1H-1H Distance Restraints in Fully Protonated Proteins by Very Fast Magic-Angle Spinning Solid-State NMR. J. Phys. Chem. Lett 2017, 8, 2399–2405. [DOI] [PubMed] [Google Scholar]
- (120).Jain MG; Mote KR; Madhu PK, NMR Crystallography at Fast Magic-Angle Spinning Frequencies: Application of Novel Recoupling Methods. Crystals 2019, 9, 231. [Google Scholar]
- (121).Potnuru LR; Duong NT; Sasank B; Raran-Kurussi S; Nishiyama Y; Agarwal V, Selective (1)H-(1)H Recoupling Via Symmetry Sequences in Fully Protonated Samples at Fast Magic Angle Spinning. J. Magn. Reson 2021, 328, 107004. [DOI] [PubMed] [Google Scholar]
- (122).Zhang Z; Oss A; Org ML; Samoson A; Li M; Tan H; Su Y; Yang J, Selectively Enhanced 1H-1H Correlations in Proton-Detected Solid-State NMR under Ultrafast MAS Conditions. J. Phys. Chem. Lett 2020, 11, 8077–8083. [DOI] [PubMed] [Google Scholar]
- (123).Wittmann JJ; Hendriks L; Meier BH; Ernst M, Controlling Spin Diffusion by Tailored Rf-Irradiation Schemes. Chem. Phys. Lett 2014, 608, 60–67. [Google Scholar]
- (124).Duong NT; Raran-Kurussi S; Nishiyama Y; Agarwal V, Quantitative 1H-1H Distances in Protonated Solids by Frequency-Selective Recoupling at Fast Magic Angle Spinning NMR. J. Phys. Chem. Lett 2018, 9, 5948–5954. [DOI] [PubMed] [Google Scholar]
- (125).Potnuru LR; Duong NT; Ahlawat S; Raran-Kurussi S; Ernst M; Nishiyama Y; Agarwal V, Accuracy of 1H-1H Distances Measured Using Frequency Selective Recoupling and Fast Magic-Angle Spinning. J. Chem. Phys 2020, 153. [DOI] [PubMed] [Google Scholar]
- (126).Wittmann JJ; Agarwal V; Hellwagner J; Lends A; Cadalbert R; Meier BH; Ernst M, Accelerating Proton Spin Diffusion in Perdeuterated Proteins at 100 khz MAS. J. Biomol. NMR 2016, 66, 233–242. [DOI] [PubMed] [Google Scholar]
- (127).Schledorn M; Malär AA; Torosyan A; Penzel S; Klose D; Oss A; Org ML; Wang S; Lecoq L; Cadalbert R et al. , Protein NMR Spectroscopy at 150 kHz Magic-Angle Spinning Continues to Improve Resolution and Mass Sensitivity. Chembiochem 2020, 21, 2540–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Kotschy J; Linser R, Proton-Detected Solid-State NMR and Its Applications to Membrane Proteins; IOP Publishing, 2020. [Google Scholar]
- (129).Li M; Xu W; Su Y, Solid-State NMR Spectroscopy in Pharmaceutical Sciences. Trends in Analytical Chemistry 2021, 135, 116152. [Google Scholar]
- (130).Kobayashi T; Mao K; Paluch P; Nowak-Krõl A; Sniechowska J; Nishiyama Y; Gryko DT; Potrzebowski MJ; Pruski M, Study of Intermolecular Interactions in the Corrole Matrix by Solid-State NMR under 100 kHz MAS and Theoretical Calculations. Angew. Chem. Int. Ed. Engl 2013, 52, 14108–14111. [DOI] [PubMed] [Google Scholar]
- (131).Schütz AK, Solid-State NMR Approaches to Investigate Large Enzymes in Complex with Substrates and Inhibitors. Biochem. Soc. Trans 2021, 49, 131–144. [DOI] [PubMed] [Google Scholar]
- (132).Schubeis T; Le Marchand T; Andreas LB; Pintacuda G, 1H Magic-Angle Spinning NMR Evolves as a Powerful New Tool for Membrane Proteins. J. Magn. Reson 2018, 287, 140–152. [DOI] [PubMed] [Google Scholar]
- (133).Lecoq L; Fogeron ML; Meier BH; Nassal M; Böckmann A, Solid-State NMR for Studying the Structure and Dynamics of Viral Assemblies. Viruses 2020, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).David G; Fogeron ML; Schledorn M; Montserret R; Haselmann U; Penzel S; Badillo A; Lecoq L; André P; Nassal M et al. , Structural Studies of Self-Assembled Subviral Particles: Combining Cell-Free Expression with 110 kHz MAS NMR Spectroscopy. Angew. Chem. Int. Ed. Engl 2018, 57, 4787–4791. [DOI] [PubMed] [Google Scholar]
- (135).Lecoq L; Schledorn M; Wang S; Smith-Penzel S; Malär AA; Callon M; Nassal M; Meier BH; Böckmann A, 100 kHz MAS Proton-Detected NMR Spectroscopy of Hepatitis B Virus Capsids. Front. Mol. Biosci 2019, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Jirasko V; Lakomek NA; Penzel S; Fogeron ML; Bartenschlager R; Meier BH; Böckmann A, Proton-Detected Solid-State NMR of the Cell-Free Synthesized A-Helical Transmembrane Protein Ns4b from Hepatitis C Virus. Chembiochem 2020, 21, 1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Andreas LB; Reese M; Eddy MT; Gelev V; Ni QZ; Miller EA; Emsley L; Pintacuda G; Chou JJ; Griffin RG, Structure and Mechanism of the Influenza A M218–60 Dimer of Dimers. J. Am. Chem. Soc 2015, 137, 14877–14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Zinke M; Fricke P; Lange S; Zinn-Justin S; Lange A, Protein−Protein Interfaces Probed by Methyl Labeling and Proton-Detected Solid-State NMR Spectroscopy. Chemphyschem 2018, 19, 2457–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Zinke M; Sachowsky KAA; Öster C; Zinn-Justin S; Ravelli R; Schröder GF; Habeck M; Lange A, Architecture of the Flexible Tail Tube of Bacteriophage Spp1. Nat. Commun 2020, 11, 5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Zinke M; Fricke P; Samson C; Hwang S; Wall JS; Lange S; Zinn-Justin S; Lange A, Bacteriophage Tail-Tube Assembly Studied by Proton-Detected 4D Solid-State NMR. Angew. Chem. Int. Ed. Engl 2017, 56, 9497–9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Gardner KH; Kay LE, Production and Incorporation of 15N, 13C, 2H (1H-Δ1 Methyl) Isoleucine into Proteins for Multidimensional NMR Studies. J. Am. Chem. Soc 1997, 119, 7599–7600. [Google Scholar]
- (142).Sinnige T; Daniëls M; Baldus M; Weingarth M, Proton Clouds to Measure Long-Range Contacts between Nonexchangeable Side Chain Protons in Solid-State NMR. J. Am. Chem. Soc 2014, 136, 4452–4455. [DOI] [PubMed] [Google Scholar]
- (143).Retel JS; Nieuwkoop AJ; Hiller M; Higman VA; Barbet-Massin E; Stanek J; Andreas LB; Franks WT; Van Rossum BJ; Vinothkumar KR et al. , Structure of Outer Membrane Protein G in Lipid Bilayers. Nat. Commun 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Schubeis T; Le Marchand T; Daday C; Kopec W; Movellan KT; Stanek J; Schwarzer TS; Castiglione K; de Groot BL; Pintacuda G et al. , A B-Barrel for Oil Transport through Lipid Membranes: Dynamic NMR Structures of Alkl. Proc. Natl. Acad. Sci. U. S. A 2020, 117, 21014–21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Bardiaux B; Malliavin T; Nilges M, Aria for Solution and Solid-State NMR. Methods Mol. Biol 2012, 831, 453–483. [DOI] [PubMed] [Google Scholar]
- (146).Brünger AT; Adams PD; Clore GM; Delano WL; Gros P; Grossekunstleve RW; Jiang JS; Kuszewski J; Nilges M; Pannu NS et al. , Crystallography & NMR System: A New Software Suite for Macromolecular Structure Determination. Acta Crystallogr. D 1998, 54, 905–921. [DOI] [PubMed] [Google Scholar]
- (147).Medeiros-Silva J; Mance D; Daniëls M; Jekhmane S; Houben K; Baldus M; Weingarth M, 1H-Detected Solid-State NMR Studies of Water-Inaccessible Proteins in Vitro and in Situ. Angew. Chem. Int. Ed. Engl 2016, 55, 13606–13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Medeiros-Silva J; Jekhmane S; Paioni AL; Gawarecka K; Baldus M; Swiezewska E; Breukink E; Weingarth M, High-Resolution NMR Studies of Antibiotics in Cellular Membranes. Nat. Commun 2018, 9, 3963–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Huster D; Yao XL; Hong M, Membrane Protein Topology Probed by 1H Spin Diffusion from Lipids Using Solid-State NMR Spectroscopy. J. Am. Chem. Soc 2002, 124, 874–883. [DOI] [PubMed] [Google Scholar]
- (150).Lesage A; Gardiennet C; Loquet A; Verel R; Pintacuda G; Emsley L; Meier BH; Böckmann A, Polarization Transfer over the Water-Protein Interface in Solids. Angew. Chem. Int. Ed. Engl 2008, 47, 5851–5854. [DOI] [PubMed] [Google Scholar]
- (151).Najbauer EE; Movellan KT; Schubeis T; Schwarzer T; Castiglione K; Giller K; Pintacuda G; Becker S; Andreas LB, Probing Membrane Protein Insertion into Lipid Bilayers by Solid-State NMR. Chemphyschem 2019, 20, 302–310. [DOI] [PubMed] [Google Scholar]
- (152).Shi C; Öster C; Bohg C; Li L; Lange S; Chevelkov V; Lange A, Structure and Dynamics of the Rhomboid Protease Glpg in Liposomes Studied by Solid-State NMR. J. Am. Chem. Soc 2019, 141, 17314–17321. [DOI] [PubMed] [Google Scholar]
- (153).Weingarth M; Van Der Cruijsen EAW; Ostmeyer J; Lievestro S; Roux B; Baldus M, Quantitative Analysis of the Water Occupancy around the Selectivity Filter of a K+ Channel in Different Gating Modes. J. Am. Chem. Soc 2014, 136, 2000–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Jekhmane S; Medeiros-Silva J; Li J; Kümmerer F; Müller-Hermes C; Baldus M; Roux B; Weingarth M, Shifts in the Selectivity Filter Dynamics Cause Modal Gating in K + Channels. Nat. Commun 2019, 10, 123–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Hong M; Fritzsching KJ; Williams JK, Hydrogen-Bonding Partner of the Proton-Conducting Histidine in the Influenza M2 Proton Channel Revealed from 1H Chemical Shifts. J. Am. Chem. Soc 2012, 134, 14753–14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Movellan KT; Wegstroth M; Overkamp K; Leonov A; Becker S; Andreas LB, Imidazole-Imidazole Hydrogen Bonding in the Ph-Sensing Histidine Side Chains of Influenza A M2. J. Am. Chem. Soc 2020, 142, 2704–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Singh H; Vasa SK; Jangra H; Rovó P; Päslack C; Das CK; Zipse H; Schäfer LV; Linser R, Fast Microsecond Dynamics of the Protein-Water Network in the Active Site of Human Carbonic Anhydrase Ii Studied by Solid-State NMR Spectroscopy. J. Am. Chem. Soc 2019, 141, 19276–19288. [DOI] [PubMed] [Google Scholar]
- (158).Yang Y; Xiang S; Liu X; Pei X; Wu P; Gong Q; Li N; Baldus M; Wang S, Proton-Detected Solid-State NMR Detects the Inter-Nucleotide Correlations and Architecture of Dimeric Rna in Microcrystals. Chem. Commun 2017, 53, 12886–12889. [DOI] [PubMed] [Google Scholar]
- (159).Wiegand T; Malär AA; Cadalbert R; Ernst M; Böckmann A; Meier BH, Asparagine and Glutamine Side-Chains and Ladders in Het-S(218–289) Amyloid Fibrils Studied by Fast Magic-Angle Spinning NMR. Front. Mol. Biosci 2020, 7, 261–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Daskalov A; Martinez D; Coustou V; Mammeri NE; Mélanie B; Andreas LB; Bardiaux B; Stanek J; Noubhani A; Kauffmann B et al. , Structural and Molecular Basis of Cross-Seeding Barriers in Amyloids. Proc. Natl. Acad. Sci. U. S. A 2020, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Wasmer C; Lange A; Van Melckebeke H; Siemer AB; Riek R; Meier BH, Amyloid Fibrils of the Het-S(218–289) Prion Form a Beta Solenoid with a Triangular Hydrophobic Core. Science 2008, 319, 1523–1526. [DOI] [PubMed] [Google Scholar]
- (162).Rydzek S; Shein M; Bielytskyi P; Schütz AK, Observation of a Transient Reaction Intermediate Illuminates the Mechanochemical Cycle of the Aaa-Atpase P97. J. Am. Chem. Soc 2020, 142, 14472–14480. [DOI] [PubMed] [Google Scholar]
- (163).Schanda P; Triboulet S; Laguri C; Bougault CM; Ayala I; Callon M; Arthur M; Simorre JP, Atomic Model of a Cell-Wall Cross-Linking Enzyme in Complex with an Intact Bacterial Peptidoglycan. J. Am. Chem. Soc 2014, 136, 17852–17860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Kosol S; Gallo A; Griffiths D; Valentic TR; Masschelein J; Jenner M; de los Santos ELC; Manzi L; Sydor PK; Rea D et al. , Structural Basis for Chain Release from the Enacyloxin Polyketide Synthase. Nat. Chem 2019, 11, 913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Felix J; Weinhäupl K; Chipot C; Dehez F; Hessel A; Gauto DF; Morlot C; Abian O; Gutsche I; Velazquez-Campoy A et al. , Mechanism of the Allosteric Activation of the Clpp Protease Machinery by Substrates and Active-Site Inhibitors. Sci. Adv 2019, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Gauto DF; Macek P; Barducci A; Fraga H; Hessel A; Terauchi T; Gajan D; Miyanoiri Y; Boisbouvier J; Lichtenecker R et al. , Aromatic Ring Dynamics, Thermal Activation, and Transient Conformations of a 468 Kda Enzyme by Specific 1H-13C Labeling and Fast Magic-Angle Spinning NMR. J. Am. Chem. Soc 2019, 141, 11183–11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Vasa SK; Singh H; Grohe K; Linser R, Assessment of a Large Enzyme–Drug Complex by Proton-Detected Solid-State NMR Spectroscopy without Deuteration. Angew. Chem. Int. Ed. Engl 2019, 58, 5758–5762. [DOI] [PubMed] [Google Scholar]
- (168).Shukla R; Medeiros-Silva J; Parmar A; Vermeulen BJA; Das S; Paioni AL; Jekhmane S; Lorent J; Bonvin AMJJ; Baldus M et al. , Mode of Action of Teixobactins in Cellular Membranes. Nat. Commun 2020, 11, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Öster C; Walkowiak GP; Hughes DE; Spoering AL; Peoples AJ; Catherwood AC; Tod JA; Lloyd AJ; Herrmann T; Lewis K et al. , Structural Studies Suggest Aggregation as One of the Modes of Action for Teixobactin. Chem. 2018, 9, 8850–8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Ward ME; Ritz E; Ahmed MAM; Bamm VV; Harauz G; Brown LS; Ladizhansky V, Proton Detection for Signal Enhancement in Solid-State NMR Experiments on Mobile Species in Membrane Proteins. J. Biomol. NMR 2015, 63, 375–388. [DOI] [PubMed] [Google Scholar]
- (171).Xiang SQ; le Paige UB; Horn V; Houben K; Baldus M; van Ingen H, Site-Specific Studies of Nucleosome Interactions by Solid-State NMR Spectroscopy. Angew. Chem. Int. Ed. Engl 2018, 57, 4571–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Lacabanne D; Boudet J; Malär AA; Wu P; Cadalbert R; Salmon L; Allain FHT; Meier BH; Wiegand T, Protein Side-Chain-DNA Contacts Probed by Fast Magic-Angle Spinning NMR. J. Phys. Chem. B 2020, 124, 11089–11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Wiegand T; Schledorn M; Malär AA; Cadalbert R; Däpp A; Terradot L; Meier BH; Böckmann A, Nucleotide Binding Modes in a Motor Protein Revealed by 31P- and 1H-Detected MAS Solid-State NMR Spectroscopy. Chembiochem 2020, 21, 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Gauto DF; Estrozi LF; Schwieters CD; Effantin G; Macek P; Sounier R; Sivertsen AC; Schmidt E; Kerfah R; Mas G et al. , Integrated NMR and Cryo-Em Atomic-Resolution Structure Determination of a Half-Megadalton Enzyme Complex. Nat. Commun 2019, 10, 2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Struppe J; Quinn CM; Sarkar S; Gronenborn AM; Polenova T, Ultrafast 1H MAS NMR Crystallography for Natural Abundance Pharmaceutical Compounds. Mol. Pharm 2020, 17, 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Stöppler D; Macpherson A; Smith-Penzel S; Basse N; Lecomte F; Deboves H; Taylor RD; Norman T; Porter J; Waters LC et al. , Insight into Small Molecule Binding to the Neonatal Fc Receptor by X-Ray Crystallography and 100 kHz Magic-Angle-Spinning NMR. PLoS Biol. 2018, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Li M; Lu X; Xu W; Troup GM; McNevin MJ; Nie H; Su Y, Quantifying Pharmaceutical Formulations from Proton Detected Solid-State NMR under Ultrafast Magic Angle Spinning. J. Pharm. Sci 2020, 109, 3045–3053. [DOI] [PubMed] [Google Scholar]
- (178).Hirsh DA; Wijesekara AV; Carnahan SL; Hung I; Lubach JW; Nagapudi K; Rossini AJ, Rapid Characterization of Formulated Pharmaceuticals Using Fast MAS 1H Solid-State NMR Spectroscopy. Mol. Pharm 2019, 16, 3121–3132. [DOI] [PubMed] [Google Scholar]
- (179).Nishiyama Y, Fast Magic-Angle Sample Spinning Solid-State NMR at 60–100khz for Natural Abundance Samples. Solid State Nucl. Magn. Reson 2016, 78, 24–36. [DOI] [PubMed] [Google Scholar]
- (180).Li M; Meng F; Tsutsumi Y; Amoureux J-P; Xu W; Lu X; Zhang F; Su Y, Understanding Molecular Interactions in Rafoxanide–Povidone Amorphous Solid Dispersions from Ultrafast Magic Angle Spinning NMR. Mol. Pharm 2020, 17, 2196–2207. [DOI] [PubMed] [Google Scholar]
- (181).Malär AA; Dong S; Kehr G; Erker G; Meier BH; Wiegand T, Characterization of H2-Splitting Products of Frustrated Lewis Pairs: Benefit of Fast Magic-Angle Spinning. Chemphyschem 2019, 20, 672–679. [DOI] [PubMed] [Google Scholar]
- (182).Varghese S; Halling PJ; Häussinger D; Wimperis S, Two-Dimensional 1H and 1H-Detected NMR Study of a Heterogeneous Biocatalyst Using Fast MAS at High Magnetic Fields. Solid State Nucl. Magn. Reson 2018, 92, 7–11. [DOI] [PubMed] [Google Scholar]
- (183).Mroue KH; Nishiyama Y; Kumar Pandey M; Gong B; McNerny E; Kohn DH; Morris MD; Ramamoorthy A, Proton-Detected Solid-State NMR Spectroscopy of Bone with Ultrafast Magic Angle Spinning. Sci. Rep 2015, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Damron JT; Kersten KM; Pandey MK; Mroue KH; Yarava JR; Nishiyama Y; Matzger AJ; Ramamoorthy A, Electrostatic Constraints Assessed by 1H MAS NMR Illuminate Differences in Crystalline Polymorphs. J. Phys. Chem. Lett 2017, 8, 4253–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Paluch P; Trébosc J; Amoureux JP; Potrzebowski MJ, 1H-31P Cpvc NMR Method under Very Fast Magic Angle Spinning for Analysis of Dipolar Interactions and Dynamics Processes in the Crystalline Phosphonium Tetrafluoroborate Salts. Solid State Nucl. Magn. Reson 2017, 87, 96–103. [DOI] [PubMed] [Google Scholar]
- (186).Szeverenyi NM; Sullivan MJ; Maciel GE, Observation of Spin Exchange by Two-Dimensional Fourier Transform 13C Cross Polarization-Magic-Angle Spinning. J. Magn. Reson 1982, 47, 462–475. [Google Scholar]
- (187).Meier BH, Polarization Transfer and Spin Diffusion in Solid-State NMR. Adv. Magn. Opt. Reson 1994, 18, 1–115. [Google Scholar]
- (188).Takegoshi K; Nakamura S; Terao T, 13C–1H Dipolar-Assisted Rotational Resonance in Magic-Angle Spinning NMR. Chem. Phys. Lett 2001, 344, 631–637. [Google Scholar]
- (189).Takegoshi K; Nakamura S; Terao T, 13C–1H Dipolar-Driven 13C–13C Recoupling without 13C RF Irradiation in Nuclear Magnetic Resonance of Rotating Solids. J. Chem. Phys 2003, 118, 2325–2341. [Google Scholar]
- (190).Weingarth M; Demco DE; Bodenhausen G; Tekely P, Improved Magnetization Transfer in Solid-State NMR with Fast Magic Angle Spinning. Chem. Phys. Lett 2009, 469, 342–348. [Google Scholar]
- (191).Weingarth M; Bodenhausen G; Tekely P, Broadband Magnetization Transfer Using Moderate Radio-Frequency Fields for NMR with Very High Static Fields and Spinning Speeds. Chem. Phys. Lett 2010, 488, 10–16. [Google Scholar]
- (192).Hu B; Lafon O; Trébosc J; Chen Q; Amoureux JP, Broad-Band Homo-Nuclear Correlations Assisted by 1H Irradiation for Bio-Molecules in Very High Magnetic Field at Fast and Ultra-Fast MAS Frequencies. J. Magn. Reson 2011, 212, 320–329. [DOI] [PubMed] [Google Scholar]
- (193).Tuttle MD; Comellas G; Nieuwkoop AJ; Covell DJ; Berthold DA; Kloepper KD; Courtney JM; Kim JK; Barclay AM; Kendall A et al. , Solid-State NMR Structure of a Pathogenic Fibril of Full-Length Human A-Synuclein. Nat. Struct. Mol. Biol 2016, 23, 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Gelenter MD; Smith KJ; Liao SY; Mandala VS; Dregni AJ; Lamm MS; Tian Y; Xu W; Pochan DJ; Tucker TJ et al. , The Peptide Hormone Glucagon Forms Amyloid Fibrils with Two Coexisting B-Strand Conformations. Nat. Struct. Mol. Biol 2019, 26, 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Lu M; Russell RW; Bryer AJ; Quinn CM; Hou G; Zhang H; Schwieters CD; Perilla JR; Gronenborn AM; Polenova T, Atomic-Resolution Structure of Hiv-1 Capsid Tubes by Magic-Angle Spinning NMR. Nat. Struct. Mol. Biol 2020, 27, 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Lewandowski JR; De Paëpe G; Griffin RG, Proton Assisted Insensitive Nuclei Cross Polarization. J. Am. Chem. Soc 2007, 129, 728–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).De Paëpe G; Lewandowski JR; Loquet A; Böckmann A; Griffin RG, Proton Assisted Recoupling and Protein Structure Determination. J. Chem. Phys 2008, 129, 245101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Hing AW; Vega S; Schaefer J, Transferred-Echo Double-Resonance NMR. J. Magn. Reson 1992, 96, 205–209. [Google Scholar]
- (199).Jaroniec CP; Filip C; Griffin RG, 3D Tedor NMR Experiments for the Simultaneous Measurement of Multiple Carbon-Nitrogen Distances in Uniformly (13)C,(15)N-Labeled Solids. J. Am. Chem. Soc 2002, 124, 10728–10742. [DOI] [PubMed] [Google Scholar]
- (200).Lewandowski JR; De Paëpe G; Eddy MT; Struppe J; Maas W; Griffin RG, Proton Assisted Recoupling at High Spinning Frequencies. J. Phys. Chem. B 2009, 113, 9062–9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).Lewandowski JR; De Paëpe G; Eddy MT; Griffin RG, (15)N-(15)N Proton Assisted Recoupling in Magic Angle Spinning NMR. J. Am. Chem. Soc 2009, 131, 5769–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Donovan KJ; Silvers R; Linse S; Griffin RG, 3D MAS NMR Experiment Utilizing through-Space (15)N-(15)N Correlations. J. Am. Chem. Soc 2017, 139, 6518–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (203).De Paëpe G; Lewandowski JR; Loquet A; Eddy M; Megy S; Böckmann A; Griffin RG, Heteronuclear Proton Assisted Recoupling. J. Chem. Phys 2011, 134, 095101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (204).Nielsen AB; Székely K; Gath J; Ernst M; Nielsen NC; Meier BH, Simultaneous Acquisition of Par and Pain Spectra. J. Biomol. NMR 2012, 52, 283–288. [DOI] [PubMed] [Google Scholar]
- (205).Lamley JM; Lewandowski JR, Simultaneous Acquisition of Homonuclear and Heteronuclear Long-Distance Contacts with Time-Shared Third Spin Assisted Recoupling. J. Magn. Reson 2012, 218, 30–34. [DOI] [PubMed] [Google Scholar]
- (206).Gelenter MD; Dregni AJ; Hong M, Pulsed Third-Spin-Assisted Recoupling NMR for Obtaining Long-Range (13)C-(13)C and (15)N-(13)C Distance Restraints. J. Phys. Chem. B 2020, 124, 7138–7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Gelenter MD; Hong M, Efficient (15)N-(13)C Polarization Transfer by Third-Spin-Assisted Pulsed Cross-Polarization Magic-Angle-Spinning NMR for Protein Structure Determination. J. Phys. Chem. B 2018, 122, 8367–8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Donovan KJ; Jain SK; Silvers R; Linse S; Griffin RG, Proton-Assisted Recoupling (Par) in Peptides and Proteins. J. Phys. Chem. B 2017, 121, 10804–10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (209).Lewandowski JR; van der Wel PC; Rigney M; Grigorieff N; Griffin RG, Structural Complexity of a Composite Amyloid Fibril. J. Am. Chem. Soc 2011, 133, 14686–14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (210).Lu JX; Qiang W; Yau WM; Schwieters CD; Meredith SC; Tycko R, Molecular Structure of B-Amyloid Fibrils in Alzheimer’s Disease Brain Tissue. Cell 2013, 154, 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (211).Colvin MT; Silvers R; Ni QZ; Can TV; Sergeyev I; Rosay M; Donovan KJ; Michael B; Wall J; Linse S et al. , Atomic Resolution Structure of Monomorphic Aβ42 Amyloid Fibrils. J. Am. Chem. Soc 2016, 138, 9663–9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (212).Wälti MA; Ravotti F; Arai H; Glabe CG; Wall JS; Böckmann A; Güntert P; Meier BH; Riek R, Atomic-Resolution Structure of a Disease-Relevant Aβ(1–42) Amyloid Fibril. Proc. Natl. Acad. Sci. U. S. A 2016, 113, E4976–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (213).Schütz AK; Vagt T; Huber M; Ovchinnikova OY; Cadalbert R; Wall J; Güntert P; Böckmann A; Glockshuber R; Meier BH, Atomic-Resolution Three-Dimensional Structure of Amyloid B Fibrils Bearing the Osaka Mutation. Angew. Chem. Int. Ed. Engl 2015, 54, 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (214).Seuring C; Verasdonck J; Gath J; Ghosh D; Nespovitaya N; Wälti MA; Maji SK; Cadalbert R; Güntert P; Meier BH et al. , The Three-Dimensional Structure of Human B-Endorphin Amyloid Fibrils. Nat. Struct. Mol. Biol 2020, 27, 1178–1184. [DOI] [PubMed] [Google Scholar]
- (215).Rufo CM; Moroz YS; Moroz OV; Stohr J; Smith TA; Hu XZ; DeGrado WF; Korendovych IV, Short Peptides Self-Assemble to Produce Catalytic Amyloids. Nat. Chem 2014, 6, 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (216).Lee M; Wang T; Makhlynets OV; Wu Y; Polizzi NF; Wu H; Gosavi PM; Stöhr J; Korendovych IV; DeGrado WF et al. , Zinc-Binding Structure of a Catalytic Amyloid from Solid-State NMR. Proc. Natl. Acad. Sci. U. S. A 2017, 114, 6191–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (217).Mompeán M; Li W; Li J; Laage S; Siemer AB; Bozkurt G; Wu H; McDermott AE, The Structure of the Necrosome Ripk1-Ripk3 Core, a Human Hetero-Amyloid Signaling Complex. Cell 2018, 173, 1244–1253.e1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (218).Kang X; Kirui A; Muszyński A; Widanage MCD; Chen A; Azadi P; Wang P; Mentink-Vigier F; Wang T, Molecular Architecture of Fungal Cell Walls Revealed by Solid-State NMR. Nat. Commun 2018, 9, 2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).Lange A; Luca S; Baldus M, Structural Constraints from Proton-Mediated Rare-Spin Correlation Spectroscopy in Rotating Solids. J. Am. Chem. Soc 2002, 124, 9704–9705. [DOI] [PubMed] [Google Scholar]
- (220).Wang S; Munro RA; Shi L; Kawamura I; Okitsu T; Wada A; Kim SY; Jung KH; Brown LS; Ladizhansky V, Solid-State NMR Spectroscopy Structure Determination of a Lipid-Embedded Heptahelical Membrane Protein. Nat. Methods 2013, 10, 1007–1012. [DOI] [PubMed] [Google Scholar]
- (221).Demers JP; Habenstein B; Loquet A; Kumar Vasa S; Giller K; Becker S; Baker D; Lange A; Sgourakis NG, High-Resolution Structure of the Shigella Type-Iii Secretion Needle by Solid-State NMR and Cryo-Electron Microscopy. Nat. Commun 2014, 5, 4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Tycko R; Ishii Y, Constraints on Supramolecular Structure in Amyloid Fibrils from Two-Dimensional Solid-State NMR Spectroscopy with Uniform Isotopic Labeling. J. Am. Chem. Soc 2003, 125, 6606–6607. [DOI] [PubMed] [Google Scholar]
- (223).Vasa S; Lin L; Shi C; Habenstein B; Riedel D; Kühn J; Thanbichler M; Lange A, B-Helical Architecture of Cytoskeletal Bactofilin Filaments Revealed by Solid-State NMR. Proc. Natl. Acad. Sci. U. S. A 2015, 112, E127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Zhang Z; Bulloch EM; Bunker RD; Baker EN; Squire CJ, Structure and Function of Glmu from Mycobacterium Tuberculosis. Acta Crystallogr. D 2009, 65, 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (225).Hoop CL; Lin HK; Kar K; Magyarfalvi G; Lamley JM; Boatz JC; Mandal A; Lewandowski JR; Wetzel R; van der Wel PC, Huntingtin Exon 1 Fibrils Feature an Interdigitated B-Hairpin-Based Polyglutamine Core. Proc. Natl. Acad. Sci. U. S. A 2016, 113, 1546–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (226).Marchanka A; Simon B; Althoff-Ospelt G; Carlomagno T, Rna Structure Determination by Solid-State NMR Spectroscopy. Nat. Commun 2015, 6, 7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (227).Boudet J; Devillier JC; Wiegand T; Salmon L; Meier BH; Lipps G; Allain FH, A Small Helical Bundle Prepares Primer Synthesis by Binding Two Nucleotides That Enhance Sequence-Specific Recognition of the DNA Template. Cell 2019, 176, 154–166.e113. [DOI] [PubMed] [Google Scholar]
- (228).Gakh YG; Gakh AA; Gronenborn AM, Fluorine as an NMR Probe for Structural Studies of Chemical and Biological Systems. Magn. Reson. Chem 2000, 38, 551–558. [Google Scholar]
- (229).Kitevski-LeBlanc JL; Prosser RS, Current Applications of 19F NMR to Studies of Protein Structure and Dynamics. Prog. Nucl. Magn. Reson. Spectrosc 2012, 62, 1–33. [DOI] [PubMed] [Google Scholar]
- (230).Sharaf NG; Gronenborn AM, 19F-Modified Proteins and 19F-Containing Ligands as Tools in Solution NMR Studies of Protein Interactions; Kelman Z, Ed.; Academic Press, 2015; Vol. 565. [DOI] [PubMed] [Google Scholar]
- (231).Arntson KE; Pomerantz WCK, Protein-Observed Fluorine NMR: A Bioorthogonal Approach for Small Molecule Discovery. J. Med. Chem 2016, 59, 5158–5171. [DOI] [PubMed] [Google Scholar]


