Abstract
Background
In the fibrin‐forming process, thrombin cleaves fibrinogen to fibrin, which form fibrils and then fibers, producing a gel‐like clot. Thrombin also activates coagulation factor XIII (FXIII), which crosslinks fibrin γ‐chains and α‐chains, stabilizing the clot. Many proteins bind to fibrin, including FXIII, an established regulation of clot structure, and platelet glycoprotein VI (GPVI), whose contribution to clot function is largely unknown. FXIII is present in plasma, but the abundant FXIII in platelet cytosol becomes exposed to the surface of strongly activated platelets.
Objectives
We determined if GPVI interacts with FXIII and how this might modulate clot formation.
Methods
We measured interactions between recombinant proteins of the GPVI extracellular domain: GPVI‐dimer (GPVI‐Fc2) or monomer (GPVIex) and FXIII proteins (nonactivated and thrombin‐activated FXIII, FXIII subunits A and B) by ELISA. Binding to fibrin clots and fibrin γ‐chain crosslinking were analyzed by immunoblotting.
Results
GPVI‐dimer, but not GPVI‐monomer, bound to FXIII. GPVI‐dimer selectively bound to the FXIII A‐subunit, but not to the B‐subunit, an interaction that was decreased or abrogated by the GPVI‐dimer–specific antibody mFab‐F. The GPVI‐dimer–FXIII interaction decreased the extent of γ‐chain crosslinking, indicating a role in the regulation of clot formation.
Conclusions
This is the first report of the specific interaction between GPVI‐dimer and the A‐subunit of FXIII, as determined in an in vitro system with defined components. GPVI‐dimer–FXIII binding was inhibitory toward FXIII‐catalyzed crosslinking of fibrin γ‐chains in fibrin clots. This raises the possibility that GPVI‐dimer may negatively modulate fibrin crosslinking induced by FXIII, lessening clot stability.
Keywords: crosslinking, factor XIII, fibrin, fibrin clot, GPVI, GPVI‐dimer, γ‐chain, γ‐dimer
Essentials.
Platelet receptor glycoprotein VI (GPVI)‐dimer binds to collagen and fibrin.
GPVI‐dimers, but not monomers, specifically bind to coagulation factor XIII (FXIII).
GPVI‐dimer binds to FXIII via the A‐subunit and binds noncompetitively with FXIII to fibrin clots.
GPVI‐dimers decrease fibrin γ‐chain crosslinking by FXIII and may modulate clot stability.
1. INTRODUCTION
Platelet collagen‐receptor glycoprotein VI (GPVI) occurs as both monomers and constitutively present homodimers, the functional form. 1 , 2 GPVI‐dimers bind to exposed subendothelial collagen fibers in injured vessels, initiating a signaling cascade leading to platelet activation, aggregate formation, and thrombus formation. GPVI‐dimer level is increased in activated platelets, 2 and GPVI‐dimer clustering in activated platelets brings associated signaling molecules in closer proximity, enhancing signaling. 3 Activated platelets present a membrane surface complex that stimulates the coagulation reaction, increasing active thrombin production. 4 These multiple processes culminate in formation of a platelet thrombus, 5 , 6 which is stabilized by fibrin production due to thrombin generation on the surface of activated platelets.
GPVI has also been suggested to be a fibrin(ogen) receptor, and fibrin fiber formation from fibrinogen enhances collagen‐induced platelet activation. 7 , 8 Whether GPVI can actually bind fibrinogen remains controversial. 9 , 10 We recently demonstrated that GPVI‐dimers, not ‐monomers, bind to fibrin fibers in clots. 9 Both collagen‐initiated platelet activation and coagulation pathways are necessary to form a stable blood clot, comprising many platelets, fibrin fibers, and coagulation factors, ensuring effective hemostasis and wound repair. Clot retraction which contributes to wound closure depends on the integrin αIIbβ3‐fibrin interaction, which allows the platelet cytoskeleton to draw fibrin fibers together. 11 Coagulation factor XIII (FXIII) 12 was also reported to be involved in clot retraction, 13 , 14 although the mechanisms involved are yet not well defined.
Glycoprotein VI also binds to other proteins, including laminin, 15 adiponectin, 16 and extracellular matrix metalloproteinase inducer, 17 so during the course of our studies on the GPVI‐fibrin interaction, we explored whether it interacts with FXIII, part of the plasma milieu in which thrombus formation occurs.
Plasma FXIII is an inactive tetramer of two A‐ and two B‐subunits (FXIIIA2B2), until it is cleaved by thrombin in the presence of calcium, which removes the activation peptide from subunit A, freeing it from the complex, and converting it to the active form (FXIIIAa, where "a" designates an active form) that functions as a transglutaminase. 18 Platelet cytosol also contains abundant FXIII as active FXIIIA2, which is further activated by thrombin to FXIIIAa. Platelet FXIII was reported to be involved in clot retraction, platelet spreading and adhesion (as reviewed in Muszbek et al. 18 ); and platelet FXIII was shown to crosslink several proteins to the platelet cytoskeleton, suggesting that it may be involved in platelet morphological change. 19 However, FXIII deficiency does not affect platelet aggregation, indicating that it does not contribute to this process. 14 Although FXIII is not exposed on the surface of resting platelets, it is surface expressed upon strong platelet activation. 12 FXIIIAa crosslinks fibrin γ‐chains, forming γ‐dimers (γ‐γ), and further crosslinks γ‐ and α‐chains, forming higher‐molecular‐weight products. Crosslinked fibrin clots would be more stable and more resistant to fibrinolysis.
Platelets have been shown to participate in numerous coagulation processes, including fibrin formation, and therefore in this study we asked whether fibrin‐binding GPVI‐dimer may interact with FXIII, found in both platelets and plasma. We demonstrate that GPVI‐dimers specifically and directly interact with FXIII, and its inhibitory effect on γ‐γ fibrin crosslinking suggests that this interaction may modulate fibrin clot integrity.
2. MATERIALS AND METHODS
2.1. Materials
Table 1 shows the FXIII proteins (and their abbreviations) used in this study and their affinity constants (KD) for binding to GPVI‐dimer.
TABLE 1.
Human FXIII proteins and their affinities to GPVI‐dimer
| Material (abbreviations) | Details | KD (μM) |
|---|---|---|
|
Plasma FXIIIa (FXIIIA2B2) |
Tetrameric, not activated | 0.216 ± 0.067 |
|
FXIII A‐subunitb (rh‐FXIIIA2) |
Recombinant (rh) Not fully activated form |
0.366 ± 0.047 |
|
FXIII A‐subunit (activated)b (rh‐FXIIIAa) |
Recombinant (rh) Thrombin‐activated form of rh‐FXIIIA2 |
0.140 ± 0.011 |
|
FXIII B‐subunitb (rh‐FXIIIB2) |
Recombinant (rh) | Too low to be determined |
The FXIII proteins were obtained from the following suppliers: aFisher Scientific, Leicestershire, UK; and bZedira, Darmstadt, Germany. Binding affinities (KD) were calculated from the binding curves shown in Figure 1B; data are expressed as values of the mean ± SEM (n = 8). The binding affinity of the fully activated form of the FXIII A‐subunit is comparable to GPVI‐dimer’s affinity to collagen type III (0.183 ± 0.026).
Abbreviations: FVIII, factor VIII; GPVI, glycoprotein VI; SEM, standard error of the mean.
PK1 fibrinogen (FXIII‐, plasminogen‐, fibronectin‐free; Enzyme Research Laboratories, UK); recombinant proteins of human GPVI‐extracellular domain: GPVI‐Fc2 (dimer) and GPVIex (monomer), developed by Moroi and Jung, were previously described. 9 The following antibodies were used: rabbit monoclonal antibody against human FXIIIa (EPR1360) and rabbit polyclonal anti‐fibrinogen γ‐chain antibody (AB96532) (Abcam, Cambridge, England); 1G5 (mouse monoclonal anti‐GPVI; Biocytex, Marseille, France); AlexaFluor‐647–conjugated streptavidin (Jackson Immunoresearch Laboratories, West Grove, PA, USA); and IRDye 800CW anti‐human antibody, IRDye 800CW anti‐rabbit antibody, and IRDye 680RD anti‐rabbit antibody (Li‐Cor, Lincoln, NE, USA).
2.2. ELISA to measure GPVI‐Fc2 binding to FXIII proteins
FXIII proteins, collagen (positive control), or bovine serum albumin (BSA; negative control) was incubated with ELISA plate wells (Nunc MaxiSorp, Thermo Fisher Scientific, Waltham, MA, USA) at 10 µg/mL (overnight, 4°C), and blocked with 0.5% BSA. The prepared wells were used for GPVI‐Fc2 (GPVI‐dimer) and GPVIex (GPVI‐monomer) binding assays as previously described. 19 Bound GPVI‐Fc2 and GPVIex were detected by 1G5/IRDye 800CW anti‐mouse antibody and quantified using an Odyssey CLx fluorescence imaging system (Li‐Cor).
2.3. Immunoblotting analysis by clot assay
GPVI‐Fc2 and FXIII binding to fibrin fibers and its effects on fibrin crosslinking were analyzed using a fibrin clot assay. 9 Figure 3A shows a schematic of a typical clot assay experiment. Each reaction mixture containing PK1‐fibrinogen, GPVI‐Fc2, 2 mM Ca2+, and FXIIIA2B2 was clotted by adding thrombin (1 U/mL). Each clot was isolated, washed thoroughly, dissolved in 8 M urea/2% SDS/5 mM 2‐mercaptoethanol and subjected to SDS‐PAGE/western blotting. To detect GPVI‐Fc and FXIII, respectively, the same blot was stained with IRDye 800CW anti‐human antibody and rabbit anti‐FXIII/IRDye 680RD anti‐rabbit antibody; band fluorescence was quantified following fluorescence imaging (Odyssey, Li‐Cor) and normalized to the respective protein band in the original unclotted sample and expressed as a percentage. On a separate blot of the same clot sample, the amounts of fibrin γ‐chain and γ‐dimer (γ‐γ) were detected by rabbit polyclonal anti‐fibrinogen γ‐chain antibody/IRDye 800CW anti‐rabbit antibody and expressed as a percentage of the γ‐chain band in the unclotted sample.
FIGURE 3.

Effects of GPVI‐Fc2 on the binding of FXIII and on γ‐chain crosslinking. Each data value in all the graphs is the mean ± SEM (n = 4). (A) Schematic diagram of the fibrin clot assay, which we previously described in detail. 9 In graphs (B) and (C), clots were formed from FXIII‐free fibrinogen, GPVI‐Fc2 (0, 25, 50, or 100 μg/mL), and FXIIIA2B2 at 0 (●), 1 (■), or 5 (▲) μg/mL and the binding of GPVI‐Fc to fibrin was determined. The amount of GPVI bound to fibrin (expressed as a percent of the control GPVI band (unclotted sample)) was not affected by FXIII. (C). The amount of FXIII bound to fibrin was not affected by GPVI‐Fc2. The fluorescence of the FXIII band was reported as arbitrary units (AU). (D) Amount of fibrin γ‐dimer (γ‐γ, expressed as % of the original γ‐band) in the clots formed in the presence of FXIIIA2B2 (0, 1, or 5 μg/mL) in the absence or presence of 100 μg/mL GPVI‐Fc2. In each two‐bar set, the checkered bars show the effect of adding GPVI‐Fc2 to FXIII at the indicated concentration. The clots formed in the presence of FXIIIA2B2 at 1 μg/mL show a significant decrease in γ‐dimer (P = .0476). (E) Amount of γ‐monomer (expressed as a percentage of the original γ‐band) in the clots formed in the presence of FXIIIA2B2 (0, 1, or 5 μg/mL) in the absence or presence of 100 μg/mL GPVI‐Fc2. In each two‐bar set, the checkered bars show the effect of adding GPVI‐Fc2 to FXIII at the indicated concentration. Adding GPVI‐Fc2 had no significant effect on the amount of γ‐monomer at any FXIIIA2B2 concentration. FVIII, factor VIII; GPVI, glycoprotein VI; SEM, standard error of the mean
To determine if FXIII crosslinks GPVI‐Fc2 to other proteins, GPVI‐Fc2 (50 µg/mL), FXIIIA2B2 (0, 2, or 100 µg/mL), 2 mM Ca2+, and thrombin (1 U/mL), in the presence or absence of PK1‐fibrinogen, were reacted for 1 hour at 37°C. Some samples contained biotinamidopentylamine (1 mM; Sigma‐Aldrich, St. Louis, MO, USA), to incorporate biotin into substrates of FXIII. Immunoblotted membranes were stained by IRDye 800CW anti‐human antibody (Li‐Cor) for GPVI‐Fc and AlexaFluor‐647–conjugated streptavidin for biotin‐incorporated bands and visualized by fluorescence imaging (Odyssey, Li‐Cor).
3. RESULTS AND DISCUSSION
3.1. GPVI‐Fc2 specifically binds to FXIII
GPVI‐Fc2 binds with higher affinity to crosslinked fibrin than to noncrosslinked fibrin, 9 suggesting that FXIII may affect its binding or crosslinked fibrin would show a higher affinity to GPVI‐Fc2. An ELISA assay was used to determine whether GPVI‐Fc2 and FXIII proteins are capable of direct interaction, as shown in Figure 1A (relative levels of binding at fixed GPVI‐Fc2 concentration) and Figure 1B (concentration‐dependency curves for calculation of KD (dissociation constant) values summarized in Table 1). rh‐FXIIIAa (0.140 ± 0.011 µM) had the highest affinity, which was similar to the KD for collagen type‐III (0.183 ± 0.026 µM). Although subunit B (rh‐FXIIIB2) shows some binding to GPVI‐Fc2 at 50 μg/mL (Figure 1A, chartreuse bar), its concentration‐dependency curve (Figure 1B, chartreuse squares) indicates that it scarcely binds to GPVI‐Fc2, having a nearly linear binding curve, reminiscent of a nonspecific interaction, precluding calculation of KD. These data indicate that the activated form of subunit A binds most strongly to GPVI‐Fc2. In contrast, GPVI‐monomer (GPVIex) does not bind to any form of FXIII (Figure 1C) and binds only weakly to collagen‐III, indicating that GPVI must be in its dimeric form to bind to FXIII.
FIGURE 1.

Analysis of GPVI binding to FXIII by ELISA. GPVI‐Fc2 binding to factor XIII proteins FXIIIA2B2, rh‐FXIIIAa, rh‐FXIIIA2, rh‐FXIIIB2 and the positive control collagen type‐III were determined by ELISA. The binding of GPVI‐Fc2 to FXIIIs were detected by anti‐GPVI antibody 1G5/ IR Dye 800CW anti‐mouse antibody. (A) Relative binding of GPVI‐Fc2 (50 µg/mL) to FXIII proteins coated at 10 µg/mL. The binding is expressed as fluorescence strength (arbitrary units, AU). (B) Concentration‐dependence of GPVI‐Fc2 binding. FXIIIA2B2 (orange ●), rh‐FXIIIA2 (pink ▲), rh‐FXIIIAa (purple ♦); rh‐FXIIIB2 (chartreuse □); collagen‐III (blue o); BSA (navy ■). The obtained KDs are described in Table 1. (C) GPVI‐Fc2 (GPVI‐dimer) and GPVIex (GPVI‐monomer) binding to FXIII proteins, with collagen‐III as a positive control. GPVI‐Fc2 or GPVIex was 50 µg/mL and the wells were coated with a FXIII protein or collagen type III (Col‐III) at 10 μg/mL. The P value (n = 3) comparing GPVI‐Fc2 binding to GPVIex binding for each FXIII protein is shown above each set of bars. GPVI‐Fc2 bound to col‐III and all FXIII proteins except for FXIII B‐subunit. GPVIex did not bind to any of the FXIII proteins and only weakly bound to col‐III. (D) Effects of GPVI‐dimer−specific antibody (mFab‐F) on GPVI‐Fc2 binding to FXIIIA2B2, rh‐FXIIIA2, rh‐FXIIIAa (thrombin activated rh‐FXIIIA2), rh‐FXIIIB2, and col‐III. The P value (n = 4) comparing no mFab‐F addition and addition of mFab‐F (+fab) of each FXIII protein is shown above each set of bars. The addition of mFab‐F (200 µg/mL) dramatically decreased or abrogated the binding of GPVI‐Fc2 to all the FXIIIs. FVIII, factor VIII; GPVI, glycoprotein VI
3.2. FXIII binding to GPVI‐Fc2 abrogated by inhibitory GPVI‐dimer–specific antibody
Figure 1D shows that although the binding of GPVI‐Fc2 to collagen is moderately inhibited by high concentration of the GPVI‐dimer–specific antibody mFab‐F (200 µg/mL), this concentration severely decreases or abrogates GPVI‐Fc2 binding to all the FXIII‐related proteins. This suggests that FXIII may bind near the collagen‐binding site of GPVI‐Fc2 since GPVI‐Fc2, not GPVIex, binds specifically to collagen fibers 2 and its binding to FXIII is also dimer‐ specific (Figure 1C). Addition of ZED A108, FXIII‐transglutaminase inhibitor (Zedira, Darmstadt, Germany), had no effect on GPVI‐dimer binding to FXIII (data not shown), showing that the transglutaminase activity of FXIII is not involved in the binding interaction, suggesting that GPVI‐dimer–bound FXIII may retain enzymatic activity for a small substrate like biotinamidopentylamine.
Our data indicate that the active form of GPVI, GPVI‐dimer, exclusively binds to FXIII A‐subunit and complex FXIIIA2B2, while showing little interaction with subunit B. Notably, FXIII subunit B, not subunit A, was reported to bind to fibrinogen and stimulate activation of FXIII on fibrin. 21 This means that platelet FXIIIA2 could bind to GPVI‐dimers since it is exposed on the cell surface of strongly activated platelets. 22 , 23 Mitchell et al 23 showed that platelet FXIII‐induced fibrin crosslinking occurs when no plasma FXIII is present, suggesting that active FXIII is exposed on the platelet surface, performing its function to crosslink fibrin chains. FXIII of activated platelets localizes in sphingomyelin‐rich rafts, 13 and GPVI was also present in rafts on the platelet membrane. 24 This raises the tantalizing possibility that GPVI‐dimer on the platelet membrane may serve as a receptor for platelet FXIII. Since the A‐subunit is an active subunit, the interaction with GPVI might also influence the transglutaminase activity of FXIII from plasma or platelets, modulating the crosslinking of fibrin clots.
3.3. GPVI is not a principal substrate of FXIII
It can be hypothesized that FXIII might crosslink GPVI to fibrin since GPVI binds more to crosslinked fibrin than to noncrosslinked fibrin. 9 We tested this using reaction mixtures comprising GPVI‐Fc2, FXIIIA2B2, thrombin (1 U/mL), 2 mM Ca2+, and biotinamidopentylamine in the presence or absence of FXIII‐free fibrinogen and determined if the transglutaminase activity of FXIII can transfer the biotinyl moiety from biotinamidopentylamine to GPVI‐dimer, which would indicate that GPVI‐dimer is a substrate of FXIII. Figure 2A shows a western blot simultaneously stained for GPVI‐Fc (green) and proteins with incorporated biotin (red). In the sample with a high FXIII concentration (100 µg/mL; lane 4, Figure 2A), an obvious biotinylated protein band is seen at ≈80 kDa, and there is only a very faint band on the position of GPVI‐Fc that can be seen in Figure 2B (lane 4), which only shows the biotin staining. Since the molecular weights of FXIII A‐ and B‐subunit are 83 and 80 kD, respectively, the strong red band at around 80 kD suggests the incorporation of biotin to FXIII. Crosslinking of the FXIII A‐subunit to a fibrin clot was previously reported. 25 The weak biotinylation of a protein that migrates at the GPVI position (lane 4, panel B) suggests some GPVI‐crosslinking may occur at high FXIII concentrations. In the fibrin clot, biotin was incorporated to fibrin α‐chain, γ‐γ dimer, and higher‐molecular‐weight bands, but little GPVI‐Fc (green) was associated with bands with molecular weight higher than GPVI‐Fc, suggesting that GPVI‐Fc would not be crosslinked to fibrin under our experimental conditions where FXIII is used at concentrations of 1–5 μg/mL. This figure indicates that crosslinking occurred predominantly in the fibrin γ‐ and α‐chains.
FIGURE 2.
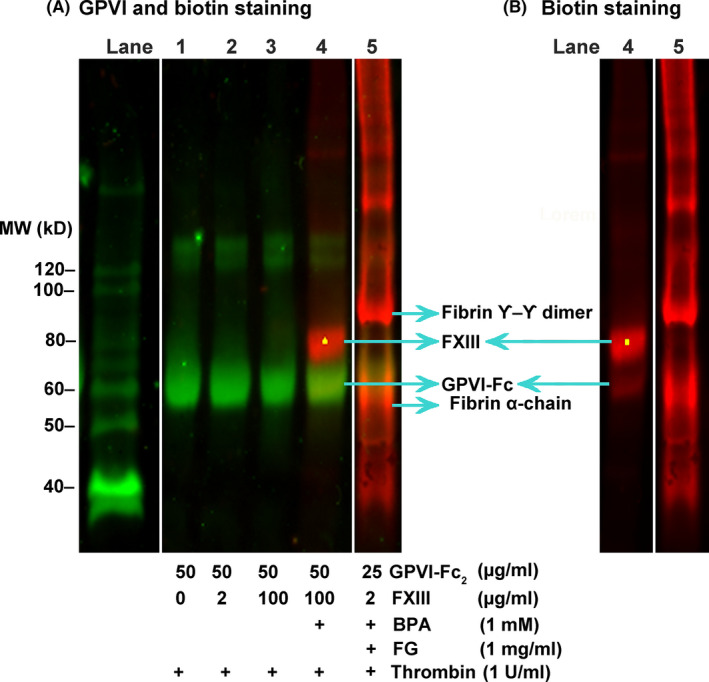
Analysis of factor XIII enzymatic activity. (A) In lanes 1‐4 (specific components in the reaction are given in the table below the blot), thrombin (1 U/mL) was added to a mixture of GPVI‐Fc2 (50 µg/mL) and FXIIIA2B2 and then allowed to react for 1 hour at 37°C. The samples were then analyzed by immunoblotting. In lane 5, GPVI‐Fc2 (25 µg/mL) and FXIIIA2B2 (2 µg/mL) is reacted in the presence of FXIII‐free fibrinogen (1 mg/mL) and the formed clot is isolated and similarly analyzed by immunoblotting. In addition, the reactions in lanes 4 and 5 are reacted in the presence of biotinamidopentylamine (1 mM) and incorporated biotin is detected by AlexaFluor 647–conjugated streptavidin (red). In this blot, GPVI‐Fc is detected by IRDye800CW anti‐human antibody (green). (B) Lanes 4 and 5 of the same western blot as panel A, showing only the biotin incorporation (AlexaFluor 647–conjugated streptavidin staining); the other lanes do not show any red staining. FVIII, factor VIII; GPVI, glycoprotein VI
3.4. GPVI‐dimer–FXIII interaction in fibrin clots
We determined if FXIII might affect GPVI‐dimer binding to fibrin fibers and vice versa using our clot binding assay, which determines fibrin‐bound proteins by western blotting (Figure 3A: assay schematic and detailed in ref. 9). FXIII‐free fibrinogen is converted to fibrin by thrombin under different concentrations of FXIIIA2B2 (0, 1, 5 µg/mL) and GPVI‐Fc2 (0, 25, 50, 100 µg/mL). FXIII and GPVI‐Fc2 bound to fibrin were detected by staining the western blots with anti‐Fc and anti‐FXIIIA antibodies, respectively. Figure 3B (fibrin‐bound GPVI‐Fc vs GPVI‐Fc2) shows that the dose‐dependent increase in amount of GPVI‐Fc bound to fibrin is not affected by the amount of FXIII added to the clotting mixture, and the amount of FXIII bound to fibrin does not change with the concentrations of GPVI‐Fc2 in the clots (Figure 3C, fibrin‐bound FXIII vs GPVI‐Fc2). In this experiment, fibrinogen is converted to fibrin and FXIIIA2B2 is also activated to FXIIIAa by thrombin. The anti‐FXIIIAa antibody employed is specific for FXIIIA2, so this result demonstrates the fibrin‐specific binding of FXIIIA2. Bymes et al 21 proposed a model where the FXIII B‐subunit binds to fibrinogen, which stimulates FXIIIA2 binding to fibrin D‐domain after fibrin formation and FXIII activation. GPVI‐dimer also binds to fibrin(ogen) D‐domain. 20 This suggests that both proteins bind to the fibrin D‐domain but as our results show, GPVI and FXIII independently interact with fibrin. However, since GPVI‐Fc2 and FXIII can bind to each other, they could be in close proximity on the fibrin fiber.
The effect of GPVI‐Fc2 on FXIII‐catalyzed crosslinking was determined by the fibrin clot assay and quantitating bands on the western blot stained by γ‐chain–specific antibody. On the blots, γ‐and γ‐γ were identified by their molecular weight and the amounts calculated as percent of the amount of γ‐chain in the original (nonclotted) sample. As shown in Figure 3D, adding FXIII at 1 μg/mL increases γ‐dimer, and addition of GPVI‐Fc2 decreases it (middle set of bars), but this effect was not evident at higher FXIII concentration (rightmost set of bars). Since the enzymatic activity of FXIII is not involved in the binding to GPVI‐Fc2, the inhibitory activity of GPVI‐Fc2 would be due to steric hinderance. In contrast, adding GPVI‐Fc2 had no significant effect on the amount of γ‐monomer (Figure 3E).
4. CONCLUSION
Using an in vitro system of defined components, we demonstrated for the first time that GPVI‐dimer specifically interacts with the FXIIIAa (activated A‐subunit of FXIII), and FXIII and GPVI‐dimer independently bind to fibrin at proximal sites in the fibrin D‐domain. This suggests that the binding site for FXIII on GPVI‐dimer is different from its fibrin‐binding site and the binding site for GPVI‐dimer on FXIIIA2 is different from its fibrin‐binding site. We can hypothesize that the specific binding of GPVI‐dimer to FXIIIAa demonstrated in our study may negatively modulate the crosslinking activity of FXIIIAa and thus affect clot stability. The occurrence of this interaction between GPVI‐dimer and FXIII in the physiological context, in plasma and/or FXIII expressed on the activated platelet, and its function must be assessed in future work.
RELATIONSHIP DISCLOSURE
None of the authors have any conflicts of interests to report.
AUTHOR CONTRIBUTIONS
SMJ and MM designed and performed experiments, provided reagents, analyzed data, wrote the manuscript, and made the figures. II designed and performed experiments and read and made suggestions about the manuscript. RWF critically read the manuscript and made suggestions.
ACKNOWLEDGMENTS
This study was supported by a Project Grant from the British Heart Foundation (SP/13/7/30575 to SMJ and RWF). II is funded by a NIHR Academic Clinical Lectureship (RG85316), was supported by a British Heart Foundation Cambridge Centre of Research Excellence fellowship (RE/13/6/30180) and an Addenbrookes Charitable Trust grant.
Moroi M, Induruwa I, Farndale RW, Jung SM. Factor XIII is a newly identified binding partner for platelet collagen receptor GPVI‐dimer—An interaction that may modulate fibrin crosslinking. Res Pract Thromb Haemost. 2022;6:e12697. doi: 10.1002/rth2.12697
Handling Editor: Prof. Yotis Senis
Contributor Information
Isuru Induruwa, Email: ii231@cam.ac.uk, @iamthatiz.
Richard W. Farndale, Email: rwf10@cam.ac.uk.
Stephanie M. Jung, Email: ladysci1024@circus.ocn.ne.jp.
REFERENCES
- 1. Jung SM, Tsuji K, Moroi M. Glycoprotein (GP) VI dimer as a major collagen‐binding site of native platelets: direct evidence obtained with dimeric GPVI‐specific Fabs. J Thromb Haemost. 2009;7:1347‐1355. [DOI] [PubMed] [Google Scholar]
- 2. Jung SM, Moroi M, Soejima K, et al. Constitutive dimerization of Glycoprotein VI (GPVI) in resting platelets is essential for binding to collagen and activation in flowing blood. J Biol Chem. 2012;287:30000‐30013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poulter NS, Pollitt AY, Owen DM, et al. Clustering of glycoprotein VI(GPVI) dimers upon adhesion to collagen as a mechanism to regulate GPVI signaling in platelets. J Thromb Haemost. 2017;15:549‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watson SP, Herbert JMJ, Pollitt AY. GPVI and CLEC‐2 in hemostasis and vascular integrity. J Thromb Haemost. 2010;8:1457‐1467. [DOI] [PubMed] [Google Scholar]
- 5. Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest. 2005;115:3355‐3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farndale RW, Slatter DA, Siljander PRM, Jarvis GE. Platelet receptor recognition and cross‐talk in collagen‐induced activation of platelets. J Thromb Haemost. 2007;5:220‐229. [DOI] [PubMed] [Google Scholar]
- 7. Mammadova‐Bach E, Ollivier V, Loyau S, et al. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood. 2015;126:683‐691. [DOI] [PubMed] [Google Scholar]
- 8. Alshehri OM, Hughes CE, Montague S, et al. Fibrin activates GPVI in human and mouse platelets. Blood. 2015;126:1601‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moroi M, Induruwa I, Farndale RW, Jung SM. Dimers of the platelet collagen receptor glycoprotein VI bind specifically to fibrin fibers during clot formation, but not to intact fibrinogen. J Thromb Haemost. 2021;19:2056‐2067. [DOI] [PubMed] [Google Scholar]
- 10. Mangin PH, Onselaer M‐B, Receveur N, et al. Immobilized fibrinogen activates human platelets through glycoprotein VI. Haematologica. 2018;103:898‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haling JR, Monkley SJ, Critchley DR, Petrich BG. Talin‐dependent integrin activation is required for fibrin clot retraction by platelets. Blood. 2011;117:1719‐1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitchell JL, Lionikiene AS, Fraser SR, Whyte CS, Booth NA, Mutch NJ. Functional factor XIII‐A is exposed on the stimulated platelet surface. Blood. 2014;124:3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kasahara K, Kaneda M, Miki T, et al. Clot retraction is mediated by factor XIII‐dependent fibrin‐αIIbβ3‐myosin axis in platelet sphingomyelin‐rich membrane rafts. Blood. 2013;122:3340‐3348. [DOI] [PubMed] [Google Scholar]
- 14. Kasahara K, Souri M, Kaneda M, Mild T, Yamamoto N, Ichinose A. Impaired clot retraction in factor XIII A subunit‐deficient mice. Blood. 2010;115:1277‐1279. [DOI] [PubMed] [Google Scholar]
- 15. Inoue O, Suzuki‐Inoue K, McCarty OJT, et al. Laminin stimulates spreading of platelets through integrin α6β1‐dependent activation of GPVI. Blood. 2006;107:1405‐1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riba R, Hughes CE, Graham A, Watson SP, Naseem M. Globular adiponectin induces platelet activation through the collagen receptor GPVI‐Fc receptor g chain complex. J Thromb Haemost. 2008;6:1012‐1020. [DOI] [PubMed] [Google Scholar]
- 17. Seizer P, Borst O, Langer HF, et al. EMMPRIN (CD147) is a novel receptor for platelet GPVI and mediates platelet rolling via GPVI‐EMMPRIN interaction. Thromb Haemost. 2009;101:682‐686. [DOI] [PubMed] [Google Scholar]
- 18. Muszbek L, Bereczky Z, Bagoly Z, Komaromi I, Katona E. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev. 2011;91:931‐972. [DOI] [PubMed] [Google Scholar]
- 19. Serrano K, Devine DV. Intracellular factor XIII crosslinks platelet cytoskeletal elements upon platelet activation. Thromb Haemost. 2002;88:315‐320. [PubMed] [Google Scholar]
- 20. Induruwa I, Moroi M, Bonna A, et al. Platelet collagen receptor glycoprotein VI‐dimer recognizes fibrinogen and fibrin through their D‐domains, contributing to platelet adhesion and activation during thrombus formation. J Thromb Haemost. 2018;16:389‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bymes JR, Wilsonm C, Boutelle AM, et al. The interaction between fibrinogen and zymogen FXIII‐A2B2 is mediated by fibrinogen residues gamma 390–396 and the FXIII‐B subunits. Blood. 2016;128:1969‐1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mitchell JL, Mutch NJ. Novel aspects of platelet factor XIII function. Thromb Res. 2016;14152:S17‐S21. [DOI] [PubMed] [Google Scholar]
- 23. Mitchell JL, Mutch NJ. Let’s cross‐link: diverse functions of the promiscuous cellular transglutaminase factor XIII‐A. J Thromb Haemost. 2019;17:19‐30. [DOI] [PubMed] [Google Scholar]
- 24. Ezumi Y, Kodama K, Uchiyama T, Takayama H. Constitutive and functional association of the platelet collagen receptor glycoprotein VI‐Fc receptor γ‐chain complex with membrane rafts. Blood. 2002;99:3250‐3255. [DOI] [PubMed] [Google Scholar]
- 25. Nikolajsen CL, Dyrlund TF, Poulsen ET, Enghild JJ, Scavenius C. Coagulation factor XIIIa substrates in human plasma. Identification and incorporation into the clot. J Biol Chem. 2014;289:6526‐6534. [DOI] [PMC free article] [PubMed] [Google Scholar]


