Abstract
Hepatocellular carcinoma (HCC) disproportionately affects racial, ethnic, and low socioeconomic status (SES) populations. However, the interaction between race, ethnicity, and neighborhood SES in HCC prognosis is not well explored. This study evaluates the interaction between race and ethnicity and neighborhood SES on curative treatment utilization and overall survival among patients with HCC in the United States. We conducted a retrospective cohort study of 13,874 patients aged ≥65 years diagnosed with HCC from 2001 through 2015 using the Surveillance, Epidemiology, and End Results Medicare‐linked database. We performed multivariable logistic regression to examine the association between race, ethnicity, and curative treatment receipt across SES. We also evaluated the association between curative treatment receipt and overall survival using a Cox proportional hazards model. Among 13,874 patients, only 2,617 (18.9%) patients received curative treatment. Overall, Black patients had lower odds of receiving curative treatment than White patients (odds ratio [OR], 0.76; 95% confidence interval [CI], 0.64‐0.91). When stratified by neighborhood SES, Black patients living in high‐poverty neighborhoods had lower odds of curative treatment receipt (OR, 0.64; 95% CI, 0.49‐0.84) and worse survival (hazard ratio, 1.13; 95% CI, 1.02‐1.25). Conversely, Hispanic and Asian patients had similar curative treatment receipt compared to White patients across all socioeconomic levels. Conclusion: Disparities in curative treatment receipt and overall survival are pronounced between Black and White patients. Black–White disparities appear to be moderated by neighborhood SES and are particularly evident among those living in high‐poverty neighborhoods.

Abbreviations
- CI
confidence interval
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- ICD
International Classification of Diseases
- MAFLD
metabolic‐associated fatty liver disease
- NCI
National Cancer Institute
- OR
odds ratio
- SEER
Surveillance, Epidemiology and End Results
- SES
socioeconomic status
Hepatocellular carcinoma (HCC) results in over 700,000 deaths globally every year and is one of the fastest rising causes of cancer‐related mortality in the United States.( 1 ) The 5‐year survival for HCC remains below 20%. Prognosis markedly differs by tumor stage at diagnosis.( 2 ) Patients with early stage HCC are eligible for curative surgical therapy, such as resection or liver transplantation, and can achieve 5‐year survival rates exceeding 60%.( 3 ) Conversely, median survival is typically 1‐2 years for those with a more advanced tumor burden.( 4 )
HCC disproportionately affects racial and ethnic minorities and low socioeconomic status (SES) populations, with significantly higher HCC incidence and mortality rates in Black and Hispanic patients than non‐Hispanic White patients.( 4 , 5 , 6 ) However, fewer studies examine racial and ethnic and socioeconomic disparities in HCC prognosis, including overall survival. A prior systematic review found curative treatment is often underused in clinical practice, with only 22% of all patients with HCC and 59% of patients with early stage HCC undergoing curative treatment.( 7 ) However, only five studies in this systematic review described racial, ethnic, or socioeconomic disparities in treatment receipt.( 7 ) Similarly, a recent systematic review found Black patients with HCC had lower odds of early tumor detection and worse overall survival than non‐Hispanic White patients, although the study did not directly address the interaction between race–ethnicity and SES. Although race, ethnicity, and SES are interrelated, they may impact health outcomes distinctly and have additive contributions to observed health disparities. Studies in other cancer types, including lung, ovarian, breast, prostate, and colorectal cancer, have shown that lower neighborhood SES is independently associated with worse survival.( 8 , 9 , 10 , 11 , 12 ) However, there are few if any data examining the interaction between race, ethnicity, and neighborhood SES in patients with HCC.( 13 )
Therefore, we performed a retrospective cohort study to characterize the interaction of racial, ethnic, and neighborhood socioeconomic disparities in curative treatment use and overall survival in the United States among a large population‐based sample of patients with HCC.
Materials and Methods
Data Sources
We performed a retrospective cohort study using the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) Medicare data between the years 2001 and 2015. SEER is an epidemiological surveillance program that collects data on incident cancer cases from population‐based cancer registries covering 34.6% of the United States.( 14 ) The linked SEER‐Medicare database combines these two population‐based databases providing information on diagnosis, survival, demographics, and health services utilization of patients with cancer from Medicare eligibility until death.( 15 ) This study protocol was reviewed and deemed not human subjects research by the Institutional Review Board at Texas A&M University.
Study Population
We included all Medicare beneficiaries aged 65 years and older who were diagnosed with HCC (International Classification of Diseases [ICD] for Oncology, Third Edition, histology code 8170 and site code C22.0 for liver) between 2001 and 2015.( 16 ) Only patients with diagnostically confirmed HCC (positive histology, cytology, laboratory test, positive radiology tests) were included. We excluded patients who (1) were not continuously enrolled in Medicare Part A and B 1 year before and after HCC diagnosis; (2) were enrolled in health maintenance organizations (HMOs)( 15 , 17 ); (3) had missing characteristics that could not be imputed( 17 ); (4) died within 30 days after HCC diagnosis; or (5) were diagnosed with other cancers 1 year before HCC diagnosis (Supporting Fig. S1).
Study Variables
Outcomes
The primary outcome of interest was the receipt of curative treatment. Curative treatment was defined as liver transplantation, surgical resection, or local ablation and was identified from Medicare data using the ICD, Ninth and Tenth Revision, Clinical Modification (ICD‐9 and ICD‐10‐Procedure Coding System), and Current Procedure Terminology codes within 12 months after HCC diagnosis.( 18 ) Our secondary outcome was overall survival, defined as the time from HCC diagnosis (in months) to the date of death from any cause.
Neighborhood‐Level SES
Census tract poverty level (CPL) was abstracted from the SEER Patient Entitlement and Diagnosis Summary File (PEDSF) and used as a proxy for neighborhood‐level SES, defined as the proportion of the population living in poverty in the patient’s residential census tract at the time of HCC diagnosis. We used 2000 US Census tract data for diagnosis years 2000‐2005 and 2010 US Census tract data for diagnosis years 2006‐2015 and categorized CPL for each patient as follows: high‐poverty neighborhoods (20% to 100% poverty), moderate‐poverty neighborhoods (10% to less than 20% poverty), and low‐poverty neighborhoods (0% to less than 10% poverty), as described in the literature.( 12 , 19 , 20 )
Race, Ethnicity, and Other Sociodemographic Characteristics
SEER PEDSF was used to abstract information on race and ethnicity, age, sex, geographic region (Northeast, West, Midwest, and South), year of diagnosis, and census tract‐level educational attainment. Race and ethnicity variable was categorized as non‐Hispanic White (White), non‐Hispanic Black (Black), Hispanic, Asian/Pacific Islander (Asian), and “other/unknown.” Educational attainment was defined as the proportion of the population 25 years or older with at least 12 years of education.
Clinical Characteristics
Liver disease etiology was identified using Medicare data and was hierarchically categorized as hepatitis C virus (HCV), hepatitis B virus (HBV), alcohol‐related liver disease, other liver diseases (hemochromatosis, disorders of copper metabolism, porphyria), metabolic‐associated fatty liver disease (MAFLD), and no identifiable liver diseases. The severity of liver dysfunction was assessed by the presence of ascites (ICD‐9: 789.51, 789.59 and ICD‐10 code R18.0, R18.8) or hepatic encephalopathy (ICD‐9: 572.2 and ICD‐10 code K72.90, K72.91) at least 12 months before HCC diagnosis by using Medicare claims. We used diagnosis and procedure codes in the year preceding HCC diagnosis to calculate the National Cancer Institute (NCI) comorbidity index as a measure of noncancer comorbidity.( 21 , 22 ) Receipt of abdominal ultrasound within 1 year before HCC diagnosis was captured as a proxy for screening from outpatient and physician/supplier claims data. Patients with early stage HCC were defined as patients with unifocal lesion ≤5 cm with no evidence of vascular invasion or distant metastases. We conducted a sensitivity analysis using SEER stage, classified as localized, regional, or distant.
Statistical Analysis
Chi‐squared tests were used to compare characteristics of the study population by receipt of curative treatment. Multivariable logistic regression with time‐fixed effects was performed to examine the impact of race and ethnicity on receipt of curative treatment across socioeconomic strata. We calculated robust standard errors to account for clustering at the census tract level. Survival time was measured in months from HCC diagnosis to death from any cause. People who were alive on December 31, 2017, were censored on that date. We estimated overall survival by race and ethnicity across the socioeconomic strata using Kaplan‐Meier analysis. Log‐rank tests were used to compare survival distributions by race, ethnicity, and SES. We then performed univariable and multivariable Cox proportional hazards analyses for each SES subgroup to examine the association between race, ethnicity, and survival across socioeconomic strata. We reported the associations from our multivariable models as adjusted odds ratios (ORs) and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs). All P values were two‐sided with a statistical significance P < 0.05. We conducted a subgroup analysis among patients with early stage HCC. All statistical analyses were performed using Stata version 16.1 (StataCorp, College Station, TX).
Results
A total of 46,998 patients were diagnosed with HCC between 2001 and 2015 (Supporting Fig. S1). We excluded 25,084 patients (12.1% Black, 5.8% Hispanic) due to lack of continuous enrollment in Medicare Part A and B or enrollment in HMOs; 4,653 patients with missing sociodemographic information; 2,901 patients who died within 30 days after HCC diagnosis (11.3% Black, 4.6% Hispanic); and 486 patients with other cancers 1 year before HCC diagnosis. There were 13,874 patients with HCC who remained eligible for inclusion in the final sample set (Supporting Fig. S1).
Baseline patient characteristics are detailed in Table 1. The median age was 75 years, and over two thirds (68.0%) of patients were men. The cohort was racially diverse (69.1% Whites, 8.4% Blacks, 12.1% Asians, and 4.1% Hispanics) and had socioeconomic diversity, with 46.8% of patients residing in low‐poverty neighborhoods, 29.9% in moderate‐poverty neighborhoods, and 23.3% in high‐poverty neighborhoods. Most (61.0%) patients did not receive ultrasound‐based screening within 1 year before HCC diagnosis, although screening was higher (52.6%) among those with early stage HCC. Blacks had lower receipt of ultrasound in the year before HCC diagnosis than Whites and Hispanics (33.8% vs. 36.7% and 46.9%, respectively). Although more than half (52.5%) of the patients had localized SEER stage, only one fifth (17.7%) were detected with a unifocal HCC ≤5 cm without vascular invasion or distant metastases.
TABLE 1.
Characteristics of Patients diagnosed With HCC (2001‐2015)
| Overall (n = 13,874) | Early stage HCC* (n = 2,457) | |||
|---|---|---|---|---|
| n (%) | n (%) | |||
| Curative treatment | ||||
| Not received | 11,257 | 81.10% | 1,546 | 62.90% |
| Received | 2,617 | 18.90% | 911 | 37.10% |
| Age at diagnosis | ||||
| 65‐69 years | 3,438 | 24.80% | 757 | 30.80% |
| 70‐74 years | 3,665 | 26.40% | 677 | 27.60% |
| 75‐79 years | 3,244 | 23.40% | 523 | 21.30% |
| 80 years and over | 3,527 | 25.40% | 500 | 20.40% |
| Sex | ||||
| Female | 4,442 | 32.00% | 944 | 38.40% |
| Male | 9,432 | 68.00% | 1,513 | 61.60% |
| Race and ethnicity | ||||
| White | 9,594 | 69.20% | 1,593 | 64.80% |
| Black | 1,161 | 8.40% | 189 | 7.70% |
| Asian | 1,675 | 12.10% | 356 | 14.50% |
| Hispanic | 573 | 4.10% | 116 | 4.70% |
| Other/unknown | 871 | 6.30% | 203 | 8.30% |
| Neighborhood‐level SES | ||||
| Low‐poverty neighborhoods | 6,489 | 46.80% | 1,092 | 44.40% |
| Moderate‐poverty neighborhoods | 4,145 | 29.90% | 765 | 31.10% |
| High‐poverty neighborhoods | 3,240 | 23.40% | 600 | 24.40% |
| Census tract education level (mean, SD) | 17.7 | 13.6 | 17.2 | 13.5 |
| Geographic region | ||||
| Northeast | 2,469 | 17.80% | 319 | 13.00% |
| West | 7,377 | 53.20% | 1,497 | 60.90% |
| Midwest | 1,334 | 9.60% | 222 | 9.00% |
| South | 2,694 | 19.40% | 419 | 17.10% |
| Abdominal ultrasound | ||||
| No | 8,463 | 61.00% | 1,165 | 46.40% |
| Yes | 5,411 | 39.00% | 1,292 | 52.60% |
| Unifocal lesion | ||||
| No | 6,603 | 47.60% | ||
| Yes | 2,457 | 17.70% | N/A | N/A |
| Nondeterminable | 4,814 | 34.70% | ||
| SEER stage | ||||
| Localized | 7,290 | 52.50% | ||
| Regional | 3,592 | 25.90% | N/A | N/A |
| Distant | 1,764 | 12.70% | ||
| Unknown | 1,228 | 8.90% | ||
| NCI comorbidity index | ||||
| 0 | 3,186 | 23.00% | 457 | 18.60% |
| 1 | 2,974 | 21.40% | 453 | 18.40% |
| 2 | 2,372 | 17.10% | 681 | 27.70% |
| 3 | 2,312 | 16.70% | 684 | 27.80% |
| 4 | 831 | 6.00% | 210 | 8.50% |
| ≥5 | 2,199 | 15.80% | 576 | 23.40% |
| Liver disease etiology | ||||
| No identifiable liver disease | 2,885 | 20.80% | 281 | 11.40% |
| HCV | 3,589 | 25.90% | 979 | 39.80% |
| HBV | 587 | 4.20% | 176 | 7.20% |
| Alcohol‐related liver disease | 1,379 | 9.90% | 302 | 12.30% |
| Other liver disease † | 244 | 1.80% | 51 | 2.10% |
| MAFLD | 5,190 | 37.40% | 668 | 27.20% |
| Liver dysfunction | ||||
| Presence of hepatic encephalopathy | 815 | 5.90% | 265 | 10.80% |
| Presence of ascites | 1,481 | 10.70% | 457 | 18.60% |
| Year of diagnosis | ||||
| 2001 | 627 | 4.50% | 62 | 2.50% |
| 2002 | 735 | 5.30% | 75 | 3.10% |
| 2003 | 694 | 5.00% | 85 | 3.50% |
| 2004 | 807 | 5.80% | 124 | 5.00% |
| 2005 | 802 | 5.80% | 99 | 4.00% |
| 2006 | 783 | 5.60% | 132 | 5.40% |
| 2007 | 881 | 6.40% | 139 | 5.70% |
| 2008 | 918 | 6.60% | 161 | 6.60% |
| 2009 | 953 | 6.90% | 166 | 6.80% |
| 2010 | 998 | 7.20% | 209 | 8.50% |
| 2011 | 1,068 | 7.70% | 195 | 7.90% |
| 2012 | 1,111 | 8.00% | 224 | 9.10% |
| 2013 | 1,154 | 8.30% | 242 | 9.80% |
| 2014 | 1,146 | 8.30% | 253 | 10.30% |
| 2015 | 1,197 | 8.60% | 291 | 11.80% |
Early stage HCC was defined using the unifocal lesion ≤5 cm without vascular invasion or metastatic spread.
Other liver diseases include hemochromatosis, disorders of copper metabolism, porphyria.
Abbreviation: N/A, not applicable.
Receipt of Curative Treatment
A minority of patients received curative treatment, including 2,617 (18.9%) of the entire cohort of patients. Of the 2,617 who received curative treatment, 68.0% were White, 7.2% were Black, 13.3% were Asian, and 3.3% were Hispanic (Supporting Table S1). Of the 2,457 patients with early stage HCC, 911 (37.1%) received curative treatment; among those who received curative treatment, 62.9% were White, 7.8% were Black, 15.1% were Asian, and 4.2% were Hispanic.
In multivariable analyses (Table 2), men, older patients, and those with higher comorbidity had lower odds of curative treatment receipt. Geographic differences were also observed, with patients living in northeastern and southern regions having higher odds of curative treatment than those in the West. We observed significant racial disparities, with Black patients having lower odds of receiving curative treatment (OR, 0.76; 95% CI, 0.64‐0.91) compared to White patients. Patients in moderate‐poverty neighborhoods also had lower odds of receiving treatment (OR, 0.89; 95% CI, 0.79‐1.00) when compared to patients living in low‐poverty neighborhoods. When stratified by SES, Black patients in high‐poverty neighborhoods continued to have lower odds of curative treatment compared to White patients (OR, 0.64; 95% CI, 0.49‐0.84); however, there were no significant differences in curative treatment receipt between Black and White patients living in low‐poverty (OR, 0.80; 95% CI, 0.54‐1.14) or moderate‐poverty (OR, 0.89; 95% CI, 0.64‐1.23) neighborhoods. No significant disparities in curative treatment receipt were observed for Hispanic and Asian patients in comparison to White patients, irrespective of neighborhood SES.
TABLE 2.
Odds of Curative Treatment Receipt Among Patients With HCC
| Base Model n = 13,874 OR (95% CI) | Low‐Poverty Neighborhoods n = 6,489 OR (95% CI) | Moderate‐Poverty Neighborhoods n = 4,145 OR (95% CI) | High‐Poverty Neighborhoods n = 3,240 OR (95% CI) | |
|---|---|---|---|---|
| Age at diagnosis | ||||
| 65‐69 years | Ref. | Ref. | Ref. | Ref. |
| 70‐74 years | 0.88 (0.78, 0.99) | 0.82 (0.69, 0.97) | 0.99 (0.78, 1.24) | 0.91 (0.72, 1.17) |
| 75‐79 years | 0.67 (0.59, 0.76) | 0.62 (0.52, 0.74) | 0.75 (0.59, 0.96) | 0.71 (0.54, 0.93) |
| 80 years and over | 0.44 (0.38, 0.51) | 0.40 (0.33, 0.49) | 0.53 (0.41, 0.68) | 0.41 (0.30, 0.56) |
| Male | 0.82 (0.74, 0.91) | 0.82 (0.71, 0.95) | 0.97 (0.81, 1.17) | 0.68 (0.55, 0.84) |
| Race and ethnicity | ||||
| White | Ref. | Ref. | Ref. | Ref. |
| Black | 0.76 (0.64, 0.91) | 0.80 (0.56, 1.14) | 0.89 (0.64, 1.23) | 0.64 (0.49, 0.84) |
| Asian | 1.04 (0.90, 1.21) | 1.01 (0.81, 1.26) | 1.22 (0.92, 1.62) | 0.95 (0.68, 1.31) |
| Hispanic | 0.92 (0.72, 1.17) | 0.73 (0.43, 1.24) | 0.64 (0.39, 1.04) | 1.29 (0.89, 1.87) |
| Other/Unknown | 1.19 (1.00, 1.42) | 1.30 (1.02, 1.64) | 1.23 (0.88, 1.73) | 0.93 (0.59, 1.45) |
| Neighborhood‐level SES | ||||
| Low‐poverty neighborhoods | Ref. | _ | _ | _ |
| Moderate‐poverty neighborhoods | 0.89 (0.79, 1.00) | |||
| High‐poverty neighborhoods | 1.03 (0.89, 1.20) | |||
| Census tract education level | 0.99 (0.98, 0.99) | 0.98 (0.97, 0.99) | 0.99 (0.98, 1.00) | 0.99 (0.98, 1.00) |
| Geographic region | ||||
| West | Ref. | Ref. | Ref. | Ref. |
| Northeast | 1.46 (1.28, 1.66) | 1.34 (1.15, 1.57) | 1.66 (1.25, 2.19) | 1.83 (1.25, 2.67) |
| Midwest | 1.10 (0.93, 1.30) | 1.00 (0.80, 1.26) | 1.23 (0.90, 1.67) | 1.33 (0.93, 1.91) |
| South | 1.21 (1.07, 1.38) | 1.17 (0.95, 1.43) | 1.30 (1.04, 1.61) | 1.27 (0.97, 1.65) |
| Unifocal lesion | ||||
| No | Ref. | Ref. | Ref. | Ref. |
| Yes | 2.64 (2.37, 2.94) | 2.34 (1.99, 2.75) | 2.96 (2.40, 3.65) | 2.94 (2.37, 3.65) |
| Nondeterminable | 0.66 (0.59, 0.73) | 0.65 (0.56, 0.76) | 0.71 (0.57, 0.88) | 0.61 (0.47, 0.78) |
| NCI comorbidity index | ||||
| 0 | Ref. | Ref. | Ref. | Ref. |
| 1 | 0.95 (0.82, 1.11) | 0.87 (0.71, 1.06) | 0.95 (0.73, 1.25) | 1.21 (0.90, 1.63) |
| 2 | 1.02 (0.88, 1.18) | 1.00 (0.82, 1.22) | 1.05 (0.79, 1.38) | 1.04 (0.76, 1.42) |
| 3 | 1.00 (0.86, 1.16) | 0.95 (0.78, 1.17) | 0.96 (0.73, 1.28) | 1.20 (0.87, 1.66) |
| 4 | 0.90 (0.73, 1.11) | 0.85 (0.63, 1.14) | 1.05 (0.72, 1.52) | 0.85 (0.51, 1.41) |
| ≥5 | 0.62 (0.53, 0.73) | 0.63 (0.50, 0.79) | 0.57 (0.41, 0.77) | 0.69 (0.49, 0.95) |
| Liver disease etiology | ||||
| HCV | Ref. | Ref. | Ref. | Ref. |
| HBV | 1.32 (1.07, 1.64) | 1.18 (0.88, 1.59) | 1.52 (1.01, 2.28) | 1.45 (0.90, 2.35) |
| Alcohol‐related liver disease | 0.61 (0.51, 0.72) | 0.69 (0.54, 0.88) | 0.61 (0.44, 0.86) | 0.42 (0.28, 0.63) |
| Other liver disease | 0.98 (0.72, 1.33) | 1.21 (0.81, 1.80) | 0.96 (0.53, 1.75) | 0.41 (0.17, 0.98) |
| MAFLD | 0.75 (0.66, 0.84) | 0.76 (0.64, 0.91) | 0.81 (0.65, 1.01) | 0.66 (0.52, 0.85) |
| No identifiable liver disease | 0.57 (0.49, 0.65) | 0.64 (0.52, 0.79) | 0.48 (0.36, 0.65) | 0.49 (0.36, 0.68) |
| Liver dysfunction | ||||
| Presence of hepatic encephalopathy | 0.87 (0.71, 1.06) | 0.82 (0.62, 1.10) | 0.93 (0.64, 1.35) | 0.94 (0.62, 1.43) |
| Presence of ascites | 1.00 (0.85, 1.17) | 1.04 (0.82, 1.30) | 1.20 (0.89, 1.61) | 0.74 (0.53, 1.03) |
| Year of diagnosis | ||||
| 2001 | Ref. | Ref. | Ref. | Ref. |
| 2002 | 1.19 (0.87, 1.64) | 1.33 (0.87, 2.05) | 1.30 (0.72, 2.36) | 0.78 (0.37, 1.64) |
| 2003 | 1.15 (0.83, 1.60) | 1.13 (0.73, 1.74) | 1.01 (0.53, 1.92) | 1.52 (0.76, 3.04) |
| 2004 | 1.05 (0.78, 1.43) | 0.92 (0.61, 1.40) | 1.26 (0.70, 2.27) | 1.34 (0.67, 2.68) |
| 2005 | 1.14 (0.84, 1.55) | 1.02 (0.66, 1.56) | 1.11 (0.61, 2.02) | 1.60 (0.84, 3.04) |
| 2006 | 0.99 (0.73, 1.35) | 1.10 (0.73, 1.66) | 0.84 (0.45, 1.56) | 0.97 (0.48, 1.97) |
| 2007 | 1.00 (0.74, 1.34) | 1.02 (0.68, 1.54) | 0.93 (0.52, 1.66) | 1.03 (0.54, 1.99) |
| 2008 | 1.02 (0.76, 1.38) | 1.18 (0.79, 1.77) | 1.00 (0.57, 1.76) | 0.62 (0.30, 1.27) |
| 2009 | 0.95 (0.71, 1.28) | 0.96 (0.64, 1.46) | 0.73 (0.41, 1.31) | 1.29 (0.67, 2.46) |
| 2010 | 0.90 (0.67, 1.21) | 0.99 (0.65, 1.50) | 0.77 (0.44, 1.36) | 0.87 (0.46, 1.63) |
| 2011 | 0.96 (0.71, 1.30) | 1.00 (0.66, 1.52) | 0.89 (0.50, 1.58) | 1.00 (0.53, 1.86) |
| 2012 | 0.90 (0.67, 1.20) | 0.95 (0.62, 1.44) | 0.74 (0.42, 1.32) | 1.06 (0.58, 1.93) |
| 2013 | 1.03 (0.77, 1.37) | 1.03 (0.68, 1.56) | 0.90 (0.51, 1.58) | 1.17 (0.63, 2.16) |
| 2014 | 0.86 (0.64, 1.16) | 0.86 (0.57, 1.29) | 0.70 (0.39, 1.26) | 1.08 (0.57, 2.05) |
| 2015 | 1.05 (0.79, 1.41) | 1.00 (0.67, 1.50) | 0.90 (0.51, 1.60) | 1.38 (0.74, 2.55) |
Abbreviation: Ref., reference.
As expected, patients with early stage HCC had 2.64 times higher odds (95% CI, 2.37‐2.94) of receiving curative treatment than patients presenting with larger tumor burden. Among patients with early stage HCC, older age, higher comorbidity index, and alcohol‐related liver disease had lower odds of curative treatment receipt (Supporting Table S2). We did not observe significant racial and socioeconomic disparities between Black and White patients irrespective of the SES. However, we observed that Hispanic patients in high‐poverty neighborhoods had higher odds of receiving curative treatment when compared to White patients (OR, 1.92; 95% CI, 1.03‐3.56). In contrast, there were no significant differences in curative treatment receipt between Hispanic and White patients living in low‐poverty (OR, 0.58; 95% CI, 0.22‐1.56) or moderate‐poverty (OR, 0.73; 95% CI, 0.34‐1.55) neighborhoods.
Overall Survival
Median survival for the entire cohort was 11 (interquartile range [IQR], 4‐33) months. Median survival was 10, 9, 17, and 10 months for White, Black, Asian, and Hispanic patients, respectively. Overall unadjusted survival, stratified by race, ethnicity, and SES, for the cohort is illustrated in Figs. 1 and 2A‐D.
FIG. 1.
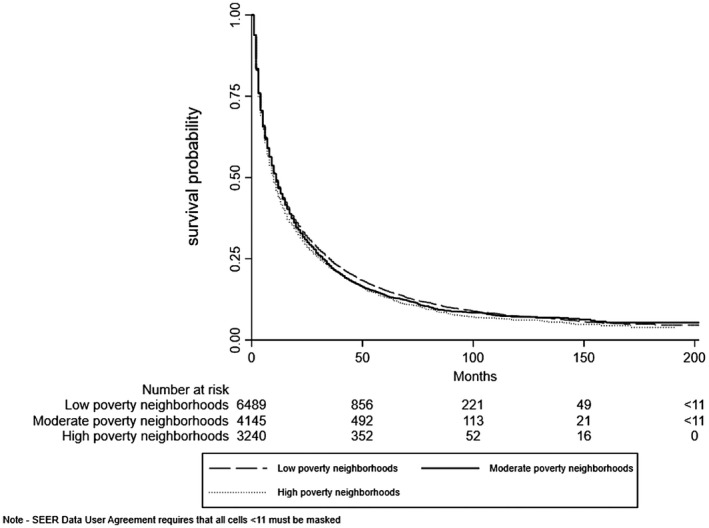
Overall unadjusted survival by SES.
FIG. 2.
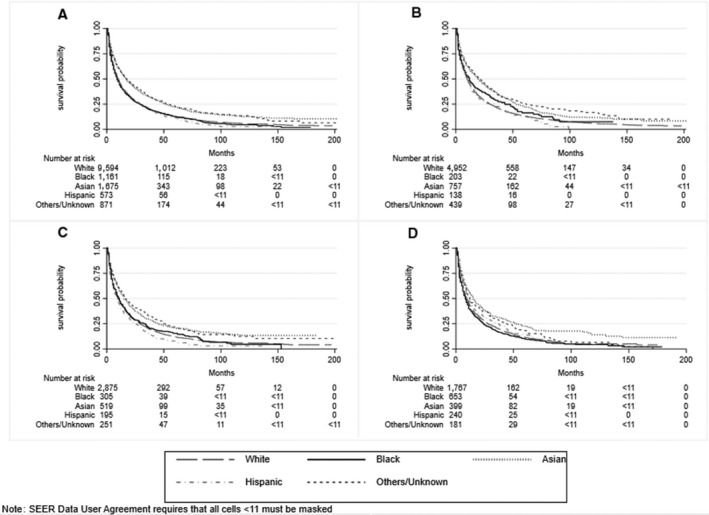
Overall unadjusted survival, stratified by race/ethnicity. (A) Entire cohort, (B) patients living in low‐poverty areas, (C) patients living in moderate‐poverty areas, (D) patients living in high‐poverty areas.
Multivariable Cox proportional hazards model identified several sociodemographic and clinical predictors of overall survival (Table 3). Older patients (age >70 years), those living in the Midwest and South, those with higher comorbidity, and patients with ascites had worse survival than their counterparts. As expected, early stage HCC detection (HR, 0.57; 95% CI, 0.54‐0.60) and curative treatment receipt (HR, 0.42; 95% CI, 0.40‐0.44) were both associated with improved survival.
TABLE 3.
Predictors of Overall Survival
| Base Model n = 13,874 HR (95% CI) | Low‐Poverty Neighborhoods n = 6,489 HR (95% CI) | Moderate‐Poverty Neighborhoods n = 4,145 HR (95% CI) | High‐Poverty Neighborhoods n = 3,240 HR (95% CI) | |
|---|---|---|---|---|
| Curative treatment | ||||
| Not received | Ref. | Ref. | Ref. | Ref. |
| Received | 0.42 (0.40, 0.44) | 0.43 (0.40, 0.46) | 0.41 (0.37, 0.45) | 0.42 (0.38, 0.46) |
| Age at diagnosis | ||||
| 65‐69 years | Ref. | Ref. | Ref. | Ref. |
| 70‐74 years | 1.12 (1.06, 1.18) | 1.14 (1.05, 1.23) | 1.12 (1.01, 1.23) | 1.10(1.00, 1.22) |
| 75‐79 years | 1.22 (1.15, 1.29) | 1.30 (1.20, 1.41) | 1.15 (1.04, 1.27) | 1.17 (1.04, 1.30) |
| 80 years and over | 1.32 (1.25, 1.39) | 1.44 (1.33, 1.56) | 1.27 (1.16, 1.40) | 1.19 (1.06, 1.33) |
| Male | 1.03 (0.99, 1.07) | 1.04 (0.98, 1.10) | 1.00 (0.93, 1.07) | 1.07 (0.98, 1.16) |
| Race and ethnicity | ||||
| White | Ref. | Ref. | Ref. | Ref. |
| Black | 1.01 (0.94, 1.08) | 0.87 (0.73, 1.04) | 0.95 (0.82, 1.09) | 1.13 (1.02, 1.25) |
| Asian | 0.79 (0.74, 0.84) | 0.76 (0.69, 0.83) | 0.88 (0.78, 0.98) | 0.75 (0.65, 0.86) |
| Hispanic | 0.97 (0.88, 1.06) | 0.97 (0.82, 1.15) | 1.06 (0.92, 1.23) | 0.92 (0.78, 1.07) |
| Other/unknown | 0.83 (0.77, 0.90) | 0.80 (0.71, 0.90) | 0.83 (0.71, 0.97) | 0.91 (0.78, 1.06) |
| Neighborhood‐level SES | ||||
| Low‐poverty neighborhoods | Ref. | _ | _ | _ |
| Moderate‐poverty neighborhoods | 0.97 (0.92, 1.01) | |||
| High‐poverty neighborhoods | 0.95 (0.89, 1.01) | |||
| Census tract education level | 1.00 (1.00, 1.01) | 1.01 (1.00, 1.01) | 1.01 (1.00, 1.01) | 1.00 (1.00, 1.01) |
| Geographic region | ||||
| West | Ref. | Ref. | Ref. | Ref. |
| Northeast | 0.97 (0.92, 1.02) | 0.96 (0.90, 1.03) | 1.00 (0.90, 1.12) | 0.88 (0.76, 1.03) |
| Midwest | 1.12 (1.04, 1.19) | 1.17 (1.06, 1.29) | 1.09 (0.97, 1.22) | 0.97 (0.84, 1.11) |
| South | 1.11 (1.05, 1.17) | 1.10 (1.00, 1.20) | 1.07 (0.98, 1.16) | 1.12 (1.02, 1.23) |
| Unifocal lesion | ||||
| No | Ref. | Ref. | Ref. | Ref. |
| Yes | 0.57 (0.54, 0.60) | 0.55 (0.51, 0.60) | 0.56 (0.51, 0.62) | 0.58 (0.53, 0.64) |
| Nondeterminable | 1.14 (1.10, 1.19) | 1.16 (1.09, 1.23) | 1.14 (1.05, 1.22) | 1.12 (1.03, 1.21) |
| NCI comorbidity index | ||||
| 0 | Ref. | Ref. | Ref. | Ref. |
| 1 | 1.01 (0.96, 1.07) | 1.00 (0.92, 1.09) | 1.01 (0.91, 1.11) | 1.04 (0.92, 1.17) |
| 2 | 0.93 (0.88, 0.99) | 1.00 (0.91, 1.09) | 0.89 (0.79, 1.00) | 0.86 (0.76, 0.98) |
| 3 | 0.94 (0.88, 1.00) | 1.01 (0.91, 1.11) | 0.86 (0.77, 0.97) | 0.91 (0.80, 1.03) |
| 4 | 1.16 (1.07, 1.26) | 1.21 (1.08, 1.37) | 1.30 (1.12, 1.52) | 0.92 (0.78, 1.09) |
| ≥5 | 1.13 (1.07, 1.21) | 1.23 (1.12, 1.36) | 1.15 (1.03, 1.28) | 0.96 (0.85, 1.08) |
| Liver disease etiology | ||||
| HCV | Ref. | Ref. | Ref. | Ref. |
| HBV | 1.25 (1.18, 1.32) | 1.21 (1.11, 1.32) | 1.32 (1.18, 1.46) | 1.27 (1.13, 1.43) |
| Alcohol‐related liver disease | 0.84 (0.75, 0.93) | 0.86 (0.74, 1.01) | 0.78 (0.64, 0.94) | 0.86 (0.68, 1.08) |
| Other liver disease | 1.14 (1.06, 1.22) | 1.11 (1.00, 1.22) | 1.22 (1.07, 1.39) | 1.13 (0.98, 1.32) |
| MAFLD | 0.97 (0.85, 1.11) | 1.00 (0.82, 1.20) | 1.06 (0.83, 1.36) | 0.70 (0.47, 1.02) |
| No identifiable liver disease | 1.22 (1.16, 1.28) | 1.22 (1.13, 1.31) | 1.19 (1.08, 1.30) | 1.28 (1.15, 1.41) |
| Liver dysfunction | ||||
| Presence of hepatic encephalopathy | 0.97 (0.89, 1.07) | 1.04 (0.91, 1.19) | 0.89 (0.77, 1.04) | 0.96 (0.81, 1.14) |
| Presence of ascites | 1.20 (1.12, 1.28) | 1.19 (1.07, 1.33) | 1.22 (1.08, 1.37) | 1.22 (1.07, 1.40) |
Abbreviation: Ref., reference.
We observed racial, ethnic, and socioeconomic disparities in overall survival. Black patients in high‐poverty neighborhoods had worse survival than White patients (HR, 1.13; 95% CI, 1.02‐1.25). In contrast, we found no significant Black–White disparities in survival in moderate‐poverty (HR, 0.95; 95% CI, 0.82‐1.09) or low‐poverty (HR, 0.87; 95% CI, 0.73‐1.04) neighborhoods. Asian patients had lower mortality than White patients irrespective of SES (low‐poverty neighborhoods HR, 0.76; 95% CI, 0.69‐0.83; moderate‐poverty neighborhoods HR, 0.88; 95% CI, 0.78‐0.98; high‐poverty neighborhoods HR, 0.75; 95% CI, 0.65‐0.86). No significant disparities in overall survival were observed between Hispanic and White patients, irrespective of SES. Among those with early stage HCC, Asian–White disparities persisted across SES strata; however, we found no significant disparities between White and Black or Hispanic patients irrespective of SES (Supporting Table S3).
Discussion
In this analysis of the SEER‐Medicare database, we found that less than one fifth of patients with HCC received curative treatment, including less than one third of those with early stage HCC, leading to a poor median overall survival of only 11 months. Further, we observed statistically significant racial, ethnic, and neighborhood socioeconomic disparities in receipt of curative treatment for HCC. Black patients were significantly less likely to undergo curative treatment and have worse overall survival than White patients, whereas we did not observe Hispanic–White disparities in curative treatment receipt or overall survival. Notably, disparities in curative treatment receipt were less marked among those with early stage HCC than all patients, suggesting observed disparities were in part driven by differences in tumor burden at diagnosis.
The striking Black–White disparities in HCC prognosis identified in our study are consistent with published studies and parallel the conclusions from a recent systematic review.( 7 ) Our study extends the published literature by examining the intersection of race, ethnicity, and SES in HCC prognosis in a large population‐based patient sample. Notably, despite the study cohort representing an insured population of Medicare enrollees, we found Black–White disparities in treatment and survival appear to be moderated by SES as we observed these disparities only in high‐poverty neighborhoods and not in moderate‐poverty or low‐poverty neighborhoods. These data provide further context in our understanding of the interplay between racial, ethnic, and neighborhood socioeconomic disparities in HCC prognosis; this is critical as we move from a model of simply describing health disparities to understanding why disparities exist and developing interventions to promote health equity.
The root causes of HCC curative treatment disparities are complex and likely related to a combination of factors at the individual (e.g., misconceptions about cancer treatment, mistrust, transportation barriers, caregiver burden), provider (e.g., implicit and/or explicit biases), and system (e.g., hospital volume and facilities) levels.( 23 ) Furthermore, all these factors may be intertwined with and exacerbated by individual and neighborhood‐level poverty and inextricably linked to health care access. Our study also highlights that simply having health insurance does not remove all barriers as disparities in guideline‐concordant HCC care exist even among those with equal health coverage (in this case Medicare enrollees).( 24 , 25 , 26 ) Further, insured patients with limited financial means may still have difficulty affording out‐of‐pocket costs for medications and clinic visits. Patients living in high‐poverty neighborhoods may also have other noninsurance‐related barriers that can result in missed visits and postponed care or shortages of physicians and subspecialists in medically underserved areas.( 27 , 28 , 29 , 30 ) In particular, the availability of liver transplantation and hepatic resection may be limited in these areas.( 31 )
Differential access to health care may not wholly explain racial and ethnic disparities in prognosis and subsequent receipt of curative treatment. For instance, there is increasing evidence highlighting the role of epigenetic effects and chronic stress from racism and poverty, leading to immunologic changes that may impact cancer biology and prognosis.( 32 , 33 ) Several studies have suggested lower HCC surveillance receipt in racial–ethnic minorities and more advanced tumor burden at diagnosis.( 13 , 24 , 28 , 30 ) Although recent data suggest variation in tumor growth patterns, there are no ethnic disparities in the frequency of common somatic mutations associated with HCC (e.g., catenin beta 1 [CTNNB1]) and no convincing data demonstrating racial and ethnic disparities in tumor biology and growth patterns.( 32 , 33 ) Compared to other racial–ethnic groups, Asians are more likely to have underlying HBV infection, which can cause HCC in the absence of cirrhosis and may facilitate curative surgical resection. Recent data suggest Black patients may develop HCC at earlier stages of fibrosis, outside of traditional surveillance criteria, which may be one of the reasons they present at more advanced HCC stages.( 34 ) Although our study highlights the complexity of racial and ethnic disparities, particularly the intersection with race–ethnicity and SES, further studies are needed to evaluate these sociodemographic disparities mediating pathways.
Strengths of our study include a large population‐based patient sample and novel analysis characterizing the interaction between race, ethnicity, and neighborhood SES and its impact on curative treatment use and survival. Further, linkage to the Medicare database provided us with some clinical information not included in SEER (e.g., liver disease etiology, ascites/encephalopathy), more detailed treatment data, and an improvement over using one or the other data alone. We acknowledge that our study also has limitations. Our analysis included older patients, limiting generalizability to younger patients who may be more likely to undergo curative therapies.( 35 ) Although SEER is extensive population‐based data, it does not include all geographic regions in the United States, limiting generalizability given the geographic variation in HCC treatment receipt and prognosis. While we had information on the presence of ascites and/or hepatic encephalopathy indicating the presence of underlying liver dysfunction, SEER‐Medicare does not contain laboratory data to allow for more precise quantification of liver dysfunction (e.g., to allow for calculation of Model for End‐Stage Liver Disease score and/or Child‐Pugh score), data on performance status, or sufficient tumor characteristics to determine Milan criteria. These are all factors that influence the likelihood of curative treatment and risk of mortality in patients with HCC. We characterized disparities in curative treatment receipt but did not examine receipt of palliative locoregional or systemic therapies, which can prolong survival, albeit to a smaller degree than curative options. We also acknowledge that our results should be interpreted cautiously due to heterogeneity within a race and ethnic group. For example, Asians and Pacific Islanders include ethnicities with stark differences and should not be mistaken for a monolith.
In conclusion, our study highlights that Black–White disparities persist in curative treatment use and overall survival among patients with HCC. This disparity appears to be moderated by neighborhood‐level SES, with the most significant differences noted among persons from high‐poverty areas. Future studies are needed to identify intervention targets to reduce disparities in HCC prognosis.
Supporting information
Fig S1
Table S1‐3
Supported by the Population Informatics Lab, the Texas Virtual Data Library at Texas A&M University, and the National Institutes of Health (R01 MD012565 to A.S.).
The data underlying this article cannot be shared publicly because the National Cancer Institute does not permit others to use the data except for collaborators at our institution involved with this research as described in our research proposal. However, the data can be obtained through https://healthcaredelivery.cancer.gov/seermedicare/obtain/ by paying the cost mentioned.
Potential conflict of Interest: Dr. Singal consults for Genentech, AstraZeneca, Bayer, Eisai, Exelixis, Bristol‐Myers Squibb, Roche, Exact Sciences, Wako, Glycotest, and GRAIL. Dr. Rich consults for AstraZeneca. The other authors have nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Kulik L, El‐Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 2019;156:477‐491.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol 2020;18:2650‐2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore) 2017;96:e5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014;109:542‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ha J, Yan M, Aguilar M, Bhuket T, Tana MM, Liu B, et al. Race/ethnicity‐specific disparities in cancer incidence, burden of disease, and overall survival among patients with hepatocellular carcinoma in the United States. Cancer 2016;122:2512‐2523. [DOI] [PubMed] [Google Scholar]
- 6. Shebl FM, Capo‐Ramos DE, Graubard BI, McGlynn KA, Altekruse SF. Socioeconomic status and hepatocellular carcinoma in the United States. Cancer Epidemiol Biomarkers Prev 2012;21:1330‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta‐analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther 2013;38:703‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peterson CE, Rauscher GH, Johnson TP, Kirschner CV, Freels S, Barrett RE, et al. The effect of neighborhood disadvantage on the racial disparity in ovarian cancer‐specific survival in a large hospital‐based study in Cook County, Illinois. Front. Public Health 2015;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Freeman VL, Ricardo AC, Campbell RT, Barrett RE, Warnecke RB. Association of census tract‐level socioeconomic status with disparities in prostate cancer‐specific survival. Cancer Epidemiol Biomarkers Prev 2011;20:2150‐2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area‐socioeconomic and race‐ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987‐2005). Cancer Epidemiol Biomarkers Prev 2009;18:121‐131. [DOI] [PubMed] [Google Scholar]
- 11. Johnson AM, Hines RB, Johnson JA, Bayakly AR. Treatment and survival disparities in lung cancer: the effect of social environment and place of residence. Lung Cancer 2014;83:401‐407. [DOI] [PubMed] [Google Scholar]
- 12. Pruitt SL, Davidson NO, Gupta S, Yan Y, Schootman M. Missed opportunities: racial and neighborhood socioeconomic disparities in emergency colorectal cancer diagnosis and surgery. BMC Cancer 2014;14:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2019;17:551‐559.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Institutes of Health, National Cancer Institute . Overview of the SEER program. https://seer.cancer.gov/about/overview.html. Published 2020. Accessed December 2020.
- 15. National Institutes of Health, National Cancer Institute . SEER‐Medicare: brief description of the SEER‐Medicare database. https://healthcaredelivery.cancer.gov/seermedicare/overview/. Published May 2019. Accessed March 2020.
- 16. National Institutes of Health, National Cancer Institute . SEER‐Medicare: number of cancer cases for selected cancers appearing in the data. https://healthcaredelivery.cancer.gov/seermedicare/aboutdata/cases.html. Published February 2021. Accessed May 2021.
- 17. Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER‐Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40(Suppl.):IV3‐IV18. [DOI] [PubMed] [Google Scholar]
- 18. Davila JA, Kramer JR, Duan Z, Richardson PA, Tyson GL, Sada YH, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology 2013;57:1858‐1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ojinnaka CO, Luo W, Ory MG, McMaughan D, Bolin JN. Disparities in surgical treatment of early‐stage breast cancer among female residents of Texas: the role of racial residential segregation. Clin Breast Cancer 2017;17:e43‐e52. [DOI] [PubMed] [Google Scholar]
- 20. Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area‐based socioeconomic measures—the public health disparities geocoding project. Am J Public Health 2003;93:1655–1671. 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Institutes of Health, National Cancer Institute . NCI comorbidity index overview. https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity.html. Updated September 2021. Accessed March 2021.
- 22. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258‐1267. [DOI] [PubMed] [Google Scholar]
- 23. Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, et al. Approaching health disparities from a population perspective: the National Institutes Of Health Centers for Population Health and Health Disparities. Am J Public Health 2008;98:1608‐1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi DT, Kum H‐C, Park S, Ohsfeldt RL, Shen YU, Parikh ND, et al. Hepatocellular carcinoma screening is associated with increased survival of patients with cirrhosis. Clin Gastroenterol Hepatol 2019;17:976‐987.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mokdad AA, Murphy CC, Pruitt SL, Mansour JC, Marrero JA, Singal AG, et al. Effect of hospital safety net designation on treatment use and survival in hepatocellular carcinoma. Cancer 2018;124:743‐751. [DOI] [PubMed] [Google Scholar]
- 26. Rich NE, Carr C, Yopp AC, Marrero JA, Singal AG. Racial and ethnic disparities in survival among patients with hepatocellular carcinoma in the United States: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2020. 10.1016/j.cgh.2020.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li X, Sundquist J, Zöller B, Sundquist K. Neighborhood deprivation and lung cancer incidence and mortality: a multilevel analysis from Sweden. J Thorac Oncol 2015;10:256‐263. [DOI] [PubMed] [Google Scholar]
- 28. Singal AG, Li X, Tiro J, Kandunoori P, Adams‐Huet B, Nehra MS, et al. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am J Med 2015;128:e1‐e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singal AG, Chan V, Getachew Y, Guerrero R, Reisch JS, Cuthbert JA. Predictors of liver transplant eligibility for patients with hepatocellular carcinoma in a safety net hospital. Dig Dis Sci 2012;57:580‐586. [DOI] [PubMed] [Google Scholar]
- 30. Rao A, Rich NE, Marrero JA, Yopp AC, Singal AG. Diagnostic and therapeutic delays in patients with hepatocellular carcinoma. J Natl Compr Canc Netw 2021;28:1‐14. [DOI] [PubMed] [Google Scholar]
- 31. Zhou K, Pickering TA, Gainey CS, Cockburn M, Stern MC, Liu L, et al. Presentation, management, and outcomes across the rural‐urban continuum for hepatocellular carcinoma. JNCI Cancer Spectr 2021;5. 10.1093/jncics/pkaa100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nathani P, Gopal P, Rich N, Yopp A, Yokoo T, John B, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta‐analysis. Gut 2021;70:401‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rich NE, John BV, Parikh ND, Rowe I, Mehta N, Khatri G, et al. Hepatocellular carcinoma demonstrates heterogeneous growth patterns in a multicenter cohort of patients with cirrhosis. Hepatology 2020;72:1654‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shaltiel T, Zheng S, Siderides C, Gleeson EM, Carr J, Pletcher ER, et al. Hepatitis C‐positive Black patients develop hepatocellular carcinoma at earlier stages of liver disease and present with a more aggressive phenotype. Cancer 2021;127:1395‐1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yan M, Ha J, Aguilar M, Liu B, Frenette CT, Bhuket T, et al. Older patients with hepatocellular carcinoma have more advanced disease, lower rates of treatment, and lower survival. J Clin Gastroenterol 2017;51:378‐383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐3


