Abstract
Objectives: In this retrospective multicenter study, we evaluated the safety of SARS-CoV-2 vaccination in patients harboring autoantibodies targeting neuronal surface and/or synaptic antigens.
Methods: From eight Italian Neurology Units, we included patients with: a) serum and/or CSF positivity for specific neuronal autoantibodies; b) a compatible neurological syndrome; and c) available follow-up ≥6 weeks after vaccination with any of the approved SARS-CoV-2 vaccines. Demographics, clinical data, and information regarding previous SARS-CoV-2 infection and vaccination were collected. Disease relapses were considered “post-infectious” or “post-vaccination” when occurring within 6 weeks from infection/vaccination.
Results: We included 66 patients; 7/66 (11%) had a previous history of SARS-CoV-2 infection and 1/7 (14%) had post-infection relapses. BNT162b2-Pfizer-BioNTec was administered in 55 cases (83.3%) and mRNA-1273-Moderna in 11 (16.7%). The median number of doses administered per patient was 2 (1–3) and >50% of patients did not experience side effects. Five patients (8%) had post-vaccination relapses (seizure 3/5); 4/5 improved after immunotherapy, while one did not receive immunotherapy and worsened. Patients with post-vaccination relapses had higher disability scores at vaccination (p = 0.025), a trend favoring Leucine-rich glioma-inactivated protein 1 LGI1 glutamic acid decarboxylase 65 (GAD65) antibodies (p = 0.054) and shorter time from last relapse (p = 0.057).
Discussion: Our data support the safety of SARS-CoV-2 vaccines in patients with neurological disorders associated with antibodies to neuronal and synaptic antigens.
Keywords: Autoimmune encephalitis, CNS autoantibodies, SARS-CoV-2, Vaccination, Safety
The safety of SARS-CoV-2 vaccines has already been proven in some inflammatory and autoimmune CNS conditions including multiple sclerosis (Di Filippo et al., 2021), aquaporin-4-IgG seropositive neuromyelitis optica, and myelin oligodendrocyte glycoprotein-IgG associated disease (Dinoto et al., 2021).
Recently, single reports described immune-mediated encephalitis as a rare complication of SARS-CoV-2 vaccination (Kaulen et al., 2022; Zuhorn et al., 2021). In agreement, previous studies have shown that other vaccinations, particularly that of Japanese yellow fever, have been associated with antibody-mediated disorders, such as anti-N-Methyl-d-Aspartate receptor (NMDAR) encephalitis (Guedes et al., 2021).
However, no studies have specifically investigated the safety profile of SARS-CoV-2 vaccines in patients with neurological disorders associated with antibodies to neuronal and synaptic antigens.
Methods
We performed a multicenter retrospective study including patients from eight Neurology Units (Supplementary Table 1) with: a) serum and/or cerebrospinal fluid (CSF) positivity for autoantibodies directed against surface/synaptic neuronal antigens; b) a compatible clinical phenotype; and c) ≥6 weeks of follow-up after receiving at least one dose of any approved SARS-CoV-2 vaccines.
Demographic and clinical data were retrospectively collected. Detailed data related to vaccinations were obtained at each center through a review of clinical charts, phone interviews and neurological evaluations and merged in an anonymized shared database. Disease relapses were defined as “post-infection” or “post-vaccination” by the treating physicians as worsening or new-onset of neurological symptoms attributable to the antibody-associated neurological disorder occurring within 6 weeks from SARS-CoV-2 infection/vaccination. Relapse severity was rated by the Clinical Assessment Scale for Autoimmune Encephalitis (CASE), and the modified Rankin Scale (mRS).
Continuous and categorical variables were reported as median (range) and number (%). Comparisons were made with Fisher's exact test, Mann Whitney U, as appropriate. P-values <0.05 were considered statistically significant (IBM SPSS 26).
Results
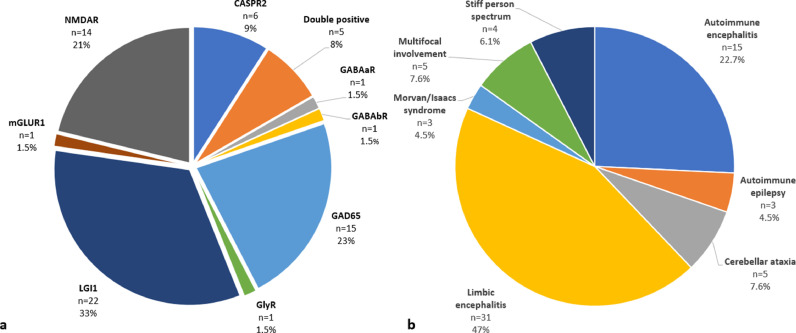
A total of 66 patients were included. Demographic and clinical data are summarized in Fig. 1 and Table 1 .
Fig. 1.
(a) antibody positivity and (b) clinical phenotype of included patients. Double positive patients harbored the following antibodies: CASPR2+LGI1 n = 2; GABAbR+GAD65 n = 1; GAD65+AChR n = 1; IgLON5+GAD65 n = 1. Those with multifocal involvement had: autoimmune encephalitis and myasthenia gravis n = 1; stiff person syndrome and cerebellar ataxia n = 1; overlapping features between IgLON5 and GAD65 (double positive patient) n = 1; limbic encephalitis and chorea n = 1; cerebellar ataxia and progressive encephalomyelitis with rigidity and myoclonus n = 1. NMDAR: N-Methyl-d-Aspartate receptor, CASPR2: contactin-associated protein-like 2, GABAaR: gamma-aminobutyric acid A receptor, GABAbR: gamma-aminobutyric acid B receptor, GAD65: glutamic acid decarboxylase 65, GlyR: glycine receptor, LGI1: leucine-rich glioma-inactivated protein 1, mGLUR: metabotropic glutamate receptor 1, AChR: acetylcholine receptor, IgLON5: immunoglobulin-like cell adhesion molecule 5.
Table 1.
Demographic, clinical, SARS-CoV-2 infection and vaccination data of included patients (n=66).
| Age at vaccination (years) | 62 (17-85) |
| Sex | Male 30 (45.5%) Female 36 (55.5%) |
| Clinical features | Cognitive disturbances 41 (62.1%) Altered consciousness 23 (34.8%) Psychiatric disturbances 43 (65.2%) Focal CNS symptoms 6 (9.1%) PNS involvement 13 (19.7%) Movement disorders 19 (28.8%) Dysautonomia 22 (33.3%) Seizures 47 (71.2%) |
| Disease course | Monophasic 36 (54.5%) Relapsing 18 (27.3%) Progressive 12 (18.2%) |
| Paraneoplastic disease | 9 (13.6%) |
| Underlying malignancy | Ovarian teratoma 7 (77.8%) Thymoma 2 (22.2%) |
| Other immunological triggers | Post-vaccination 0 Post-infectious 3 (4.5%) |
| Number of flares | 1 (1-10) |
| Disease duration at first vaccine dose (months) | 63.3 (2-298) |
| Time from last relapse at first vaccine dose (months) | 38.5 (0-298) |
| Ongoing immunotherapy at vaccination | None 34 (51.5%) Oral steroids 12 (17.9%) Intravenous immunoglobulins 3 (4.5%) Azathioprine 6 (9.1%) Mycophenolate Mofetil 3 (4.5%) Rituximab 5 (7.6%) Tocilizumab 1 (1.5%) Rituximab+oral steroids 1 (1.5%) Azathioprine+oral steroids 1 (1.5%) |
| CASE at vaccination | 2 (0-10) |
| mRS at vaccination | 1 (0-4) |
| Previous history of SARS-CoV-2 infection | 7 (10.6%) |
| SARS-CoV-2 infection severity | Asymptomatic 3 (42.9%) Mild symptoms without hospital admission 4 (57.1%) |
| Flares after SARS-CoV-2 infection | 1/7 (14.3%) |
| Clinical features of post-infectious flares | Worsening of psychiatric disturbances, tremor, and seizure (GAD65) |
| Time from SARS-CoV-2 infection to flares, days | 31 |
| Outcome of SARS-CoV-2-related flares | No improvement 1 (100%) |
| SARS-CoV-2 vaccine | BNT162b2-Pfizer-BioNTech 55 (83.3%) mRNA-1273-Moderna 11 (16.7%) |
| Number of doses | 2 (1-3)* |
| Side effects at first dose | No side effects 35 (53%) Pain at injection site 16 (24.2%) Fatigue 3 (4.5%) Fever 1 (1.5%) Flu-like symptoms 4 (6.1%) Headache 1 (1.5%) Herpes reactivation 1 (1.5%) More than one side effects 2 (3%) Relapse 3 (4.5%) |
| Side effects at second dose | No side effects 37 (63.8%) Pain at injection site 9 (15.5%) Fatigue 1 (1.7%) Fever 3 (5.2%) Flu-like symptoms 4 (6.9%) More than one side effects 1 (1.5%) Relapse 2 (3.4%) |
| Relapses after SARS-CoV-2 vaccination | 5 (7.6%) |
| Clinical features of post-vaccination relapses | Worsening of ataxia: 1 (mGLUR1) Seizures: 2 (both LGI1) Seizure + altered mental status: 1 (GAD65) Abnormal behavior + movement disorders: 1 (GAD65) |
| Time from vaccination to relapse, days | 7 (2-45) |
| Outcome of vaccination-related relapses | Worsening 1 (20%) Improved 3 (60%) Complete recovery 1 (20%) |
| Follow-up duration (from last vaccine dose), months | 7 (1.5-11) |
Data are expressed as median (range) and number (percentage), as appropriate.
CASE: Clinical Assessment Scale for Autoimmune Encephalitis, mRS: modified Rankin Scale, mGLUR1: metabotropic glutamate receptor 1, GAD65: glutamic acid decarboxylase 65, LGI1: leucine-rich glioma-inactivated protein 1.
12 patients received a booster dose of vaccination (data not shown).
Seven (10.6%) patients had a previous history of SARS-CoV-2 infection, and 4 of them (57.1%) experienced mild flu-like symptoms while the remaining 3 were asymptomatic. One patient with glutamic acid decarboxylase 65 (GAD65) antibodies worsened (with psychiatric disturbances, tremor and seizures) 31 days after the infection without improvement after treatment with benzodiazepines and levetiracetam.
Regarding vaccination, the median number of doses administered was 2 (1–3); 55 (83.3%) patients received BNT162b2-Pfizer-BioNTech, whilst 11 (16.7%) mRNA-1273-Moderna. Around half of the patients did not experience side effects (53% and 63.8% after the first and second dose, respectively). Among the side effects, pain at injection site was the most frequently reported (24.2% and 15.5% after the first and second dose, respectively).
Five patients (5/66, 7.6%) experienced post-vaccination relapses a median of 7 days after vaccination (range, 2–45), and 2/5 were receiving chronic immunotherapy at the time of vaccination (all oral steroids). Clinical features of relapses included seizures (n = 2), worsening of ataxia (n = 1), seizure + altered mental status (n = 1), and abnormal behavior + movement disorders (n = 1). All patients who experienced seizures (3/3) were already affected by epilepsy as part of their autoimmune disorder. At last follow-up, outcome was as follow: complete recovery (n = 1), partial improvement (n = 3), worsening (n = 1). Four patients received intravenous steroids while one subject was not treated and worsened. The GAD65 positive patient who had a flare after SARS-CoV-2 infection also experienced a relapse after vaccination. Individual data of relapsing patients are reported in Supplementary Table 2.
When comparing patients with and without post-vaccination relapses, relapsing cases had higher CASE scores at vaccination (p = 0.021) and a trend favoring Leucine-rich glioma-inactivated protein 1 LGI1 GAD65 antibodies positivity (p = 0. 054) and a shorter time from last relapse (p = 0.057), Supplementary Table 3.
Discussion
Our multicenter retrospective study shows that SARS-CoV-2 vaccination is safe in patients with neurological disorders associated with antibodies to neuronal and synaptic antigens since 1. vaccine-related side effects occur in about half of patients but are typically mild and showed a rate comparable with other autoimmune neurological conditions (Dinoto et al., 2021; I Lotan et al., 2021); 2. post-vaccination relapses rarely occur in these conditions (7.6%); 3. post-vaccination relapses generally show a favorable outcome to immunotherapy.
Of note, additional factors have to be considered as possible contributors to the few relapses herein observed after vaccination. In this context, seizures were a common manifestation in post-vaccination relapses and always occurred in patients with a known history of epilepsy. Since an increase in seizure frequency after vaccination has also been reported in a minority of patients with non-autoimmune epilepsy, the possibility that systemic/structural rather than autoimmune factors might be the leading cause of this post-vaccine symptom should be considered (Steriade et al., 2020; Lu et al., 2022). The association between pre-vaccination disease severity, which represents a risk factor for remote symptomatic seizures, and the occurrence of post-vaccination worsening further reinforces this hypothesis. Similarly, the worsening of ataxia and movement disorder associated with psychiatric disturbances after vaccination may be related to the progressive course of the underlying condition (in one patient with ataxia with metabotropic glutamate receptor 1 (mGLUR1) antibodies, and in one patient with ataxia and progressive encephalomyelitis with rigidity and myoclonus with GAD65 antibodies, respectively).
In accordance, the incidence of relapses did not increase after vaccination in other neurological autoimmune disorders, even though transient worsening of neurological symptoms may occur without evidence of disease activity (I Lotan et al., 2021; I Lotan et al., 2021).
Although most of our patients showed a favorable response after treatment with immunotherapy, which might suggest an autoimmune nature of the event, the lack of further confirmatory investigations (such as brain magnetic resonance imaging scans or CSF analysis) prevent a definitive discrimination between the occurrence of a transient worsening and a definite immune-mediated relapse.
Our study is limited by the small sample size, the retrospective design, the predominant administration of mRNA-based vaccination, and the lack of evaluation of CNS antibody titers before and after vaccination.
Despite these limits, our data supports the safety of SARS-CoV-2 vaccination in patients with antibody-mediated disorders and may help clinicians to properly inform patients with these rare conditions about the overwhelming benefits of vaccination.
Author contributors
clinical data collection (AD, MG, RI, SoM, VD, AF, MZ, ES, FP, GTM, RB, LZ, SF, SaM), data generation and interpretation (AD, SaM), drafting the manuscript (AD, SaM, SF), results interpretation (AD, SaM, SF), revising the manuscript for intellectual content (AD, MG, RI, SoM, VD, AF, MZ, ES, FP, GTM, RB, LZ, SF, SaM).
Funding source
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
Data are available from the corresponding author on reasonable request.
Ethics approval
Approval was obtained from the ethics committee of University Verona (56COVIDCESC).
Consent to participate
Informed consent was acquired.
Declaration of Competing Interest
The authors report no competing interests in relation to this study.
Acknowledgements
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2022.103827.
Appendix. Supplementary materials
References
- Di Filippo M., Cordioli C., Malucchi S., et al. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2021 doi: 10.1136/jnnp-2021-327200. jnnp-2021-327200. [DOI] [PubMed] [Google Scholar]
- Dinoto A., Sechi E., Ferrari S., et al. Risk of disease relapse following COVID-19 vaccination in patients with AQP4-IgG-positive NMOSD and MOGAD. Mult. Scler. Relat. Disord. 2021 doi: 10.1016/j.msard.2021.103424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes B.F., Ribeiro A.F., Pinto L.F., et al. Potential autoimmune encephalitis following yellow fever vaccination: a report of three cases. J. Neuroimmunol. 2021;355 doi: 10.1016/j.jneuroim.2021.577548. [DOI] [PubMed] [Google Scholar]
- Kaulen L.D., Doubrovinskaia S., Mooshage C., et al. Neurological autoimmune diseases following vaccinations against SARS-CoV-2: a case series. Eur. J. Neurol. 2022;29:555–563. doi: 10.1111/ene.15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan I., Romanow G., Levy M. Patient-reported safety and tolerability of the COVID-19 vaccines in persons with rare neuroimmunological diseases. Mult. Scler. Relat. Disord. 2021;55 doi: 10.1016/j.msard.2021.103189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan I., Wilf-Yarkoni A., Friedman Y., et al. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): early experience from a tertiary MS center in Israel. Eur. J. Neurol. 2021;28:3742–3748. doi: 10.1111/ene.15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Zhang Q., Xiao J., et al. COVID-19 vaccine take-up rate and safety in adults with epilepsy: data from a multicenter study in China. Epilepsia. 2022;63:244–251. doi: 10.1111/epi.171388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade C., Britton J., Dale R.C., et al. Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated epilepsy: conceptual definitions. Epilepsia. 2020;61:1341–1351. doi: 10.1111/epi.16571. [DOI] [PubMed] [Google Scholar]
- Zuhorn F., Graf T., Klingebiel R., et al. Postvaccinal encephalitis after ChAdOx1 nCov-19. Ann. Neurol. 2021;90:506–511. doi: 10.1002/ana.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author on reasonable request.