Abstract
Chronic hepatitis B virus (HBV) infection remains difficult to cure due to the persistent, self‐replenishing nature of the viral genome and impaired host immune responses. Current treatment goals for chronic hepatitis B (CHB) are to prevent or significantly delay liver‐related adverse outcomes and death, and two types of treatments are available: nucleos(t)ide analogues (NAs) and interferons (IFNs). NAs effectively suppress HBV replication, and IFNs improve serological response rates, thereby decreasing the risk of adverse outcomes. However, their efficacy in attaining serological responses, especially functional cure (i.e., loss of serum hepatitis B surface antigen), is very limited. Various strategies such as stopping antiviral therapy or combining therapies have been investigated to enhance response, but efficacy is only modestly improved. Importantly, the development of novel direct‐acting antivirals and immunomodulators is underway to improve treatment efficacy and enhance rates of functional cure. The present review provides an overview of the treatment goals and indications, the possibility of expanding indications, and the safety and efficacy of different treatment strategies involving established and/or novel therapies as we continue our search for a cure.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ADV
adefovir dipivoxil
- ALT
alanine aminotransferase
- APASL
Asian Pacific Association for the Study of the Liver
- ASO
antisense oligonucleotide
- cccDNA
covalently closed circular DNA
- CHB
chronic hepatitis B
- CAM
capsid assembly modulator
- EASL
European Association for the Study of the Liver
- ETV
entecavir
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HDV
hepatitis D virus
- NA
nucleos(t)ide analogue
- NAP
nucleic acid polymer
- PD‐1
programmed death 1
- PEG‐IFN‐α
pegylated interferon alpha
- pgRNA
pregenomic RNA
- siRNA
small interfering RNA
- TDF
tenofovir disoproxil fumarate
- TLR
toll‐like receptor
Chronic hepatitis B virus (HBV) infection remains a serious global public health concern affecting an estimated 257 million people worldwide, and together with hepatitis C, contributing to a death toll of 1.3 million.( 1 ) The search for the holy grail of HBV “cure” has been inspired by the success of hepatitis C therapy. A cure for chronic HBV infection poses multiple obstacles: the persistent, self‐replenishing nature of the covalently closed circular (cccDNA), defective innate and adaptive immune responses, and integration of HBV DNA into the host genome. It is most plausible that a cure for chronic HBV infection would require both antiviral agents and immunomodulators.
Current chronic hepatitis B (CHB) treatment aims to prevent or significantly delay liver‐related morbidity and mortality. Because a complete cure with the eradication of cccDNA is not a realistic goal with the current therapeutic options, a functional cure, defined as durable loss of hepatitis B surface antigen (HBsAg) with or without seroconversion, is considered the optimal treatment endpoint, as it is associated with significantly improved patient outcomes.( 2 ) Two types of treatment are currently available for CHB: (pegylated) interferons ([PEG]IFNs) and nucleos(t)ide analogues (NAs). Of the approved NAs, entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide are preferred as first‐line therapy because of their high barrier to HBV resistance. NAs are oral antiviral drugs that are highly effective in suppressing HBV replication; however, HBsAg loss is attained in only a small percentage. (PEG)IFN‐α can induce higher rates of durable serological response from a finite duration of therapy but has numerous side effects. Thus, various treatment strategies such as combining and stopping NA therapies, and novel therapeutic agents targeting different steps in the HBV life cycle, have been developed to improve treatment efficacy and achieve HBsAg loss.
Treatment Indications
Treatment indications are largely based on the following factors: serum HBV‐DNA levels, serum alanine aminotransferase (ALT) levels, and severity of the liver disease (Fig. 1). International clinical practice guidelines unanimously recommend treatment for patients with: ( 1 ) hepatitis B e antigen (HBeAg)–positive or HBeAg‐negative active CHB, defined by HBV DNA ≥ 20,000 IU/mL or ≥2,000 IU/mL, elevated ALT, and/or moderate to severe liver necroinflammation or fibrosis( 2 ); and compensated or decompensated cirrhosis with detectable HBV DNA, regardless of ALT values.( 3 , 4 , 5 )
FIG. 1.
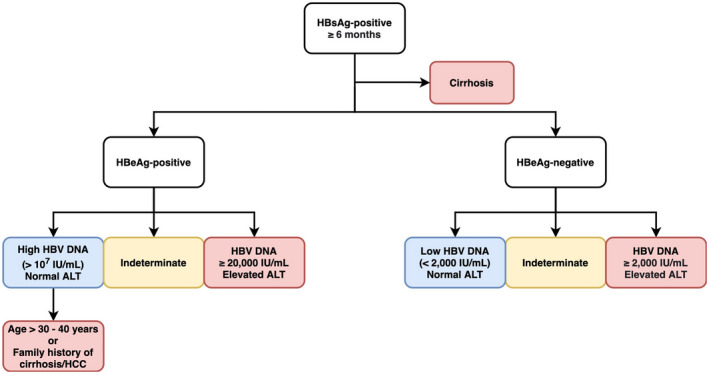
Treatment indications of CHB infection. Color‐coded by recommendations (red: Treat; blue: Do not treat, monitor ALT and HBV‐DNA levels every 3‐6 months, and annual serology tests; yellow: Monitor, exclude other causes of ALT elevation, and assess disease severity, treating if significant hepatic fibrosis or inflammation).
Expanding Treatment Indications
Immune‐Tolerant Patients
Treatment is generally not recommended for patients with HBeAg‐positive chronic HBV infection (i.e., immune‐tolerant), characterized by high HBV‐DNA levels (>7 log10 IU/mL) and normal ALT levels.( 3 , 4 , 5 ) Although monitoring to identify transition to the HBeAg‐positive active CHB is the preferred approach, studies have challenged this approach, primarily due to concerns regarding the association of high levels of HBV DNA and delayed HBeAg seroconversion with an increased risk of disease progression and hepatocellular carcinoma (HCC).( 6 , 7 , 8 ) However, a study from Korea recently reported that the cumulative incidence of HCC at 10 years was 1.9% in immune‐tolerant (HBV DNA > 20,000 IU/mL and normal ALT) patients without significant fibrosis.( 9 ) Of note, this study has been criticized due to its lenient definition of immune tolerance, which led to inclusion of many patients in an intermittently active disease state. Thus, whether HCC risk can be further reduced with antiviral therapy remains unknown.
Treatment studies in immune‐tolerant patients show low rates of serological responses. In a phase 2 study in which patients received TDF with emtricitabine or placebo, although more than half of patients attained virological response, few patients achieved HBeAg seroconversion, and none cleared HBsAg by week 192.( 10 ) Data from the two recent trials from the Hepatitis B Research Network support these findings. Only 3% of children achieved the primary endpoint (HBeAg loss with HBV DNA ≤ 1,000 IU/mL) and cleared HBsAg at 48 weeks after receiving ETV and PEG‐IFN‐α combination therapy.( 11 ) In the parallel trial of adult patients, none met the primary endpoint and all experienced virological relapse after treatment cessation.( 12 )
Several studies have suggested that immune‐tolerant patients could be at risk for significant fibrosis despite having normal ALT levels.( 13 , 14 ) However, it is important to assess whether patients are truly immune‐tolerant throughout the study duration.( 15 ) In a retrospective Korean study, an increased risk of HCC, liver transplantation, or death was seen in untreated immune‐tolerant patients compared with NA‐treated immune‐active patients.( 16 ) However, the median age of the immune‐tolerant group was 38 years, and 26% had HBV DNA < 7 log10 IU/mL, suggesting that a proportion had transitioned out of the immune‐tolerant phase, a conclusion further supported by the finding that almost 70% of the available liver samples from the patients with HCC had stage 3‐4 fibrosis. A prospective study from Hong Kong using paired biopsies demonstrated that those remaining in the immune‐tolerant phase experience minimal fibrosis progression during 5‐year follow‐up.( 17 )
Despite some conflicting results, there is growing consensus that the risk of disease progression and HCC increases with older age in immune‐tolerant patients and the major Western guidelines (American Association for the Study of Liver Diseases [AASLD] and European Association for the Study of the Liver [EASL]) suggest considering treating patients older than 30‐40 years of age. Without long‐term follow‐up studies evaluating clinical outcomes after antiviral therapy, there is insufficient evidence to recommend treatment in all immune‐tolerant patients, as potential harms (e.g., side effects, cost, development of HBV resistance) may outweigh benefits. Given the low rates of HBsAg loss in this group when treated with current therapies, this is an important target group for future antiviral therapies aimed at enhancing HBsAg loss.
Inactive Carriers
HBeAg‐negative chronic HBV infection (i.e., inactive CHB phase) is characterized by the absence of HBeAg, undetectable or low (<2,000 IU/mL) HBV‐DNA levels, and persistently normal or near‐normal ALT. Current guidelines do not recommend treatment of inactive carriers but suggest regular follow‐up every 3‐6 months or 6‐12 months. It is important to distinguish between true inactive carriers and HBeAg‐negative patients with chronic hepatitis with low‐level activity. Quantification of HBsAg <1,000 IU/mL has been shown to aid the identification of true inactive carriers who are at extremely low risk of transitioning to active disease and HBV‐related adverse outcomes.( 18 , 19 , 20 )
Among inactive carriers, disease progression is uncommon in individuals without advanced fibrosis.( 21 , 22 , 23 ) In a study of 4,376 inactive carriers without cirrhosis at baseline, cirrhosis and HCC were documented in 0.7%‐0.9% and 0.1%‐0.4% patients, respectively, during a mean follow‐up of 13 years.( 24 ) The REVEAL study from Taiwan similarly reported low annual incidence rates of HCC (0.06%) and liver‐related death (0.04%) in inactive carriers followed for 13 years.( 25 )
For those inactive carriers with significant fibrosis, there is a risk of HCC development despite low‐level viremia and persistently normal ALT. In a retrospective study of HBeAg‐negative patients with persistently low HBV‐DNA levels (<2,000 IU/mL), 14% with cirrhosis on radiologic examinations, the cumulative incidences of HCC at 7 years were 2.6% and 15.2% among patients without and with cirrhosis, respectively.( 26 ) These data underlie guideline recommendations to generally treat all patients with compensated or decompensated cirrhosis, regardless of the level of HBV‐DNA or ALT level( 3 , 5 ) (conditional for Asian Pacific Association for the Study of the Liver [APASL] if hepatic decompensation is seen( 4 )).
Few studies have investigated the effect of antiviral therapy in inactive carriers. In a prospective cohort study of 144 inactive carriers, a significantly higher proportion of patients achieved HBsAg loss following PEG‐IFN‐α treatment with or without adefovir dipivoxil (ADV) than no treatment.( 27 ) Nonetheless, the study was limited by a lack of randomization, short follow‐up, and per‐protocol analysis assuming full compliance. In contrast, combination therapy with PEG‐IFN‐α plus ADV or TDF was shown to be ineffective in inducing HBsAg loss in another trial of 134 HBeAg‐negative patients with HBV DNA <2,000 IU/mL.( 28 ) A recent study provided evidence that short‐term PEG‐IFN‐α therapy can induce HBsAg loss in most inactive carriers with very low HBsAg levels (<20 IU/mL).( 29 )
PEG‐IFN‐α‐based therapy may theoretically be a desirable therapeutic option to attain HBsAg loss for inactive carriers. However, considering its low response rates, toxicity, and because inactive carriers without significant fibrosis generally have an excellent prognosis( 30 , 31 ) with spontaneous HBsAg loss occurring at an annual rate of 1.0%‐1.2%,( 23 , 32 ) such treatment is not deemed necessary. All guidelines recommend regular monitoring to identify transition to an immune‐active phase and/or significant fibrosis progression, in which treatment should be initiated.
Novel Approaches Using Approved Therapies
Currently approved antivirals effectively induce HBV‐DNA suppression; however, their efficacy in attaining serological responses, especially functional cure, is suboptimal. Therefore, various strategies using approved therapies have been devised to enhance HBeAg and HBsAg loss (Fig. 2).
FIG. 2.
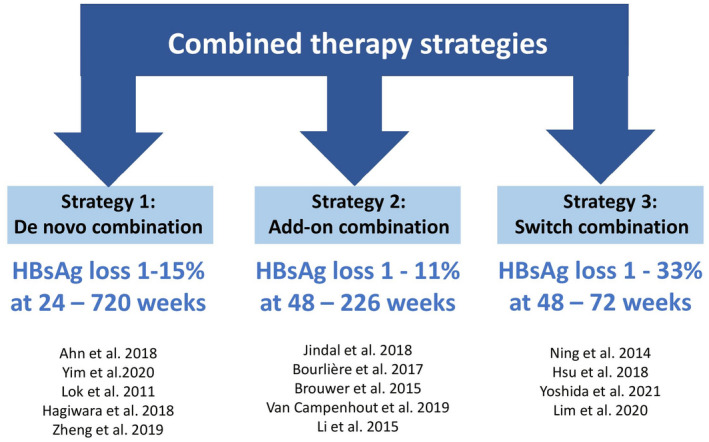
Combination strategies with approved therapies aiming for a functional cure of CHB infection.
Combination Therapy
Combination therapy of two different NAs or NA plus PEG‐IFN‐α has the potential to enhance treatment efficacy with shorter treatment duration.( 33 , 34 ) Broadly, there are three combination approaches with NAs and PEG‐IFN‐α: (1) “de novo,” when both drugs are started at the same time; (2) “add‐on,” when a patient already on treatment with one drug has a second drug added; and (3) “switch,” when one therapy is switched to another, with or without some period of overlap. Available studies are quite heterogeneous in terms of patient populations, specific drug combinations, dose and duration of therapy, and study outcomes examined. A common finding across these studies is that rates of HBeAg and HBsAg loss are modestly enhanced with PEG‐IFN‐α‐based strategies compared with NA monotherapy.
De Novo NA Combination
Few studies have evaluated combinations of NAs. In a study of 379 treatment‐naïve HBeAg‐positive and HBeAg‐negative patients treated with ETV, with or without TDF, there was no significant difference in HBV‐DNA suppression, HBeAg seroconversion, and HBsAg loss between the groups at 96 weeks, although the rate of HBV‐DNA suppression was more rapid with combination therapy in those with high baseline HBV‐DNA levels.( 35 ) In a study by Chan et al., which evaluated TDF plus emtricitabine combination in HBeAg‐positive patients with high levels of HBV DNA but normal ALT, improved rates of viral suppression but not HBeAg seroconversion were seen with the combination versus TDF alone.( 10 ) These results raise the question of whether NA combinations may be more effective in patients with very high viral loads, but ultimately no benefits to achieving HBeAg or HBsAg loss have been shown.
De Novo Combination of PEG‐IFN‐α Plus NA
Results from early pivotal trials suggest that PEG‐IFN‐α plus NA de novo combination therapy leads to more frequent and earlier loss of HBsAg than NA alone but offers little advantage over PEG‐IFN‐α alone. In a study of 814 HBeAg‐positive patients with CHB treated with PEG‐IFN‐α plus lamivudine, or PEG‐IFN‐α or lamivudine alone, PEG‐IFN‐α‐based therapy was superior to lamivudine monotherapy in inducing higher rates of HBeAg and HBsAg seroconversion, HBV‐DNA suppression at 24 weeks following treatment.( 36 ) However, no significant difference was noted between combination therapy and PEG‐IFN‐α monotherapy. Similarly, another trial involving 307 HBeAg‐positive patients showed that PEG‐IFN‐α plus lamivudine combination therapy compared with PEG‐IFN‐α monotherapy initially led to improved rates of HBeAg loss and viral suppression at the end of treatment but comparable rates at 26 weeks after treatment due to relapse.( 37 ) However, a more recent randomized controlled trial of 740 patients receiving TDF plus PEG‐IFN‐α de novo combination therapy (48 weeks) or TDF or PEG‐IFN‐α monotherapy found cumulative rates of HBsAg loss at 72 weeks were 9.1%, 0% and 2.8%, respectively, suggesting that combination therapy may be superior to PEG‐IFN‐α monotherapy in inducing a functional cure.( 38 ) A recent study from the Hepatitis B Research Network of 201 patients with CHB also reported a higher rate of HBsAg loss of 4.4% at 192 weeks among patients treated with TDF in combination with PEG‐IFN‐α (24 weeks) versus 1.0% among those treated with TDF alone, although statistically insignificant (P = 0.20).( 39 ) Cumulatively, these data suggest that PEG‐IFN‐α plus NA de novo combination therapy increases rates of HBsAg loss compared with NA alone and potentially PEG‐IFN‐α monotherapy also, although the enhancement may be more pronounced with newer NAs (e.g., TDF). Therefore, PEG‐IFN‐α remains a therapeutic option, as it is the only well‐characterized, approved immunomodulator capable of improving serological response from a finite duration compared with long‐term NA therapy.
PEG‐IFN‐α Add‐on to NA
Studies on the addition of PEG‐IFN‐α to NA monotherapy in HBeAg‐positive patients have reported higher rates of HBeAg seroconversion, but this advantage becomes less evident with long‐term follow‐up. In the ARES study, 175 HBeAg‐positive patients with CHB on ETV were randomized to receive a PEG‐IFN‐α add‐on for 24 weeks or continue ETV alone. More patients achieved HBeAg seroconversion in the combination versus ETV continuation arm at week 96 (26% vs. 13%, P = 0.04).( 40 ) However, with long‐term follow‐up to 226 weeks, response rates equilibrated.( 41 ) In the PEGON study, 82 HBeAg‐positive patients treated with ≥1 year of ETV or TDF were randomized to receive PEG‐IFN‐α add‐on for 48 weeks or continue NA monotherapy.( 42 ) HBeAg seroconversion with HBV DNA < 200 IU/mL were seen in 7 of 39 (18%) of the combination group versus 3 of 38 (8%) of the monotherapy group (P = 0.31) at week 96. Rates of HBsAg decline > 0.5 log10 IU/mL in the two groups were equal (both 13%). Li et al. assessed the effect of adding PEG‐IFN‐α for 48 weeks in HBeAg‐positive patients on long‐term ETV therapy without HBeAg seroconversion; at 48 weeks, HBeAg loss was noted in 44% versus 6% among those who had continued ETV therapy (P < 0.01).( 43 )
In the PEGAN study of 185 HBeAg‐negative patients with undetectable HBV DNA on NA therapy for ≥1 year, HBsAg loss occurred in 7 of 90 (7.8%) in the PEG‐IFN‐α add‐on versus 3 of 93 (3.2%) in the NA monotherapy group at 96 weeks (P = 0.15).( 44 ) In the HERMES study, 72 genotype D, HBeAg‐negative patients under NA‐induced viral suppression had PEG‐IFN‐α added for 48 weeks, which resulted in ≥50% HBsAg declines in 67.4% and 50.9% of patients at week 48 and 96, respectively.( 45 ) Despite the seemingly high response rates, only 1 patient lost HBsAg. Although these findings suggest an increased rate of HBsAg loss with PEG‐IFN‐α add‐on therapy, such small increases in response rates are not considered sufficient to advocate for the use of this combination in HBeAg‐negative or HBeAg‐positive patients.
Switch
The last combinatory approach involves switching between NA and PEG‐IFN‐α therapy. In the OSST trial, 192 HBeAg‐positive patients virally suppressed on ETV were assigned to switch to PEG‐IFN‐α or continue ETV for 48 weeks.( 46 ) The switch group had higher rates of HBeAg seroconversion (14.9% vs. 6.1%; P = 0.05) and HBsAg loss (9.5% vs. 0%; P < 0.01) compared with those who continued treatment with ETV. However, the PEG‐IFN‐α switch group had less sustained HBV‐DNA suppression (72.0% vs. 98.7%; P < 0.01) compared with the continuation group, and ALT flares and viral rebounds were seen in the switch group. It is also important to note that the study cohort was a highly selected group of individuals with low HBeAg levels. In another study of 280 HBeAg‐positive treatment‐naïve patients randomized to 4 weeks of placebo, or ETV or ADV alone, followed by a switch to PEG‐IFN‐α monotherapy, no significant differences in HBeAg seroconversion or HBsAg reduction were observed between the placebo and switch groups at 48 weeks.( 47 ) The recent SWAP study compared continued NA therapy to the switch approach to PEG‐IFN‐α versus add‐on in 253 patients with CHB with undetectable HBV DNA on ≥1 year of NA therapy.( 48 ) HBeAg loss and/or >1 log10 IU/mL HBsAg decline at 24 weeks following PEG‐IFN‐α was attained in 3.9%, 33.3% and 26.7%, and HBsAg loss occurred in 0%, 7.8%, and 10.1% of the NA monotherapy control, switch, and add‐on groups, respectively. Both PEG‐IFN‐α‐based strategies (switch and add‐on) resulted in higher response rates versus NA monotherapy (P < 0.01); however, the add‐on strategy was preferred due to improved safety and comparable efficacy. Of note, several factors may be associated with response to therapy, including HBV genotype, baseline HBsAg levels, and duration of NA pretreatment.
Stopping NA Therapy
Withdrawal of NA treatment is considered a strategy to enhance HBsAg loss. In recent years, a significant number of studies have evaluated NA discontinuation as a therapeutic strategy to achieve a functional cure, although most are retrospective studies.
Current treatment guidelines recommend considering NA discontinuation in selected HBeAg‐positive patients after achieving durable HBeAg seroconversion. AASLD and EASL guidance recommend ≥1 year of consolidation therapy after HBeAg seroconversion, whereas the APASL recommends up to 3 years of consolidation therapy (Fig. 3A).( 3 , 4 , 5 ) Longer consolidation therapy has been associated with lower rates of virological and clinical relapse, but the optimal duration has not been empirically evaluated. Moreover, whether NA discontinuation accelerates HBsAg loss compared with continuous long‐term therapy remains unclear. Thus, the primary reason to stop NAs in HBeAg‐positive patients after seroconversion would be to achieve disease remission with finite therapy. For HBeAg‐negative patients, the AASLD guidance recommends against discontinuation before HBsAg loss, whereas the EASL guidance indicates withdrawal can be considered in select patients after ≥3 years of HBV‐DNA suppression and APASL states withdrawal can be undertaken after ≥2 years with undetectable HBV DNA (Fig. 3B).( 3 , 4 , 5 ) Both retrospective and prospective studies have evaluated clinical relapse and HBsAg loss after discontinuation of therapy (Table 1). Comparison between studies is hampered by differing definitions of relapse and retreatment criteria and heterogeneity of study populations.
FIG. 3.
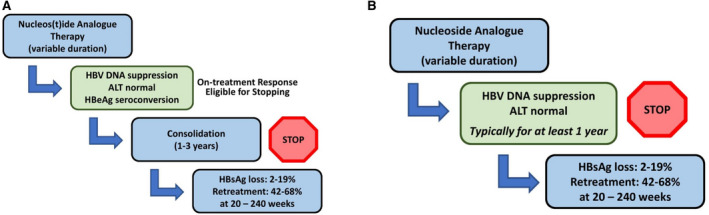
Discontinuation of NA treatment in patients with HBeAg‐positive CHB infection (A) and HBeAg‐negative CHB infection (B).
TABLE 1.
Predictors of HBsAg loss in HBeAg‐negative patients
| Source | Country | N | Age (Mean) | Male (%) | NA Used | Follow‐up (Weeks) | HBsAg Loss (%) | Predictors of HBsAg Loss |
|---|---|---|---|---|---|---|---|---|
| Berg et al.( 49 ) | Germany | 42 | 45 | 33 | TDF | 144 | 10 | N/A |
| Jeng et al.( 52 ) | Taiwan | 691 | 52 | 86 | ETV, TDF, ADV, LMV | 2‐614 | 6.1 | Time to undetectable HBV DNA < 12 weeks |
| HBsAg reduction > 1 log10 IU/mL | ||||||||
| HBsAg level < 100 IU/mL at EOT | ||||||||
| Hung et al.( 59 ) | Taiwan | 73 | 52 | 78 | LMV, ETV, TBV | 48‐288 | 27.4 | HBsAg level at EOT |
| HBsAg decline from baseline to EOT | ||||||||
| Discontinuing vs. continuing NA | ||||||||
| Papatheodoridi et al.( 57 ) | Greece | 57 | 60 | 65 | ETV, TDF | 76 | 21.5 | HBsAg level at EOT |
| ALT level at month 1 | ||||||||
| IP10 level at month 1 | ||||||||
| HBsAg level at month 1 | ||||||||
| Yao et al.( 58 ) | Taiwan | 119 | 52 | 79 | LMV, ETV | 48‐312 | 37 | HBV genotype C vs. B |
| HBsAg level at EOT | ||||||||
| Liem et al.( 51 ) | North America | 45 | 49 | 58 | TDF, ETV | 72 | 2.2 | N/A |
Abbreviations: EOT, end of treatment; IP10, IFN‐γ‐induced protein 10; N/A, not applicable.
In the FINITE trial, 43 HBeAg‐negative European patients without cirrhosis were randomized to stop or continue TDF after ≥3.5 years of viral suppression for up to 144 weeks.( 49 ) Of those who discontinued TDF, 13 of 21 (62%) remained off therapy to 144 weeks; the rest qualified to restart therapy either due to HBV flares or persistent viremia. At week 144, 4 of 21 (19%) patients had HBsAg loss off treatment versus 0% among those who remained on TDF (P = 0.02). Similarly, in the recent STOP‐NUC trial of 158 HBeAg‐negative European patients, 10.3% of patients in the NA discontinuation arm achieved HBsAg loss versus 0% in the continue‐ arm (P < 0.01), and 67.9% of those who stopped remained without an indication for retreatment at week 96.( 50 ) Although these findings suggest a potential for patients to discontinue NA therapy, other studies have demonstrated less favorable results. In a randomized trial from Canada of 67 patients who had achieved virological suppression on ≥1 year of NA therapy, remission (HBV‐DNA level < 2,000 IU/mL and normal ALT) was seen in 13 of 45 (29%) versus 18 of 22 (82%) in the stop and continue groups, respectively, with no difference in HBsAg loss rates.( 51 ) In Taiwan, lower rates of HBsAg loss and limited HBsAg declines were also found when compared with European studies. Jeng et al. followed 691 Asian patients for a median of 155 weeks after NA discontinuation and found an annual incidence of HBsAg loss of 1.78% versus 0.15% in those continuing NA.( 52 ) Other prospective cohort studies have reported rates of HBsAg loss ranging from 1%‐20% with off‐treatment follow‐up duration ranging from 1 to 5 years.( 53 , 54 ) These studies also highlight that a significant proportion of patients who stop antiviral therapy experience serious flares with clinical relapse. Liver‐related mortality following NA withdrawal has been reported in 0.2%‐1.6% of patients.( 55 ) Both AASLD and EASL guidelines counsel against discontinuation of NAs in patients with cirrhosis—primarily due to concerns for hepatic decompensation associated with flares and clinical relapse.( 3 , 5 )
HBsAg levels at the time of treatment withdrawal have been most consistently associated with HBsAg loss (Table 1), but optimal cutoffs have not been determined.( 52 , 57 , 58 , 59 ) In the RETRACT‐B meta‐analysis using individual patient data, rates of HBsAg loss were significantly higher among Caucasians versus Asians and patients with HBsAg levels < 100 IU/mL at the end of therapy.( 56 ) As the criteria used to define relapse vary by study, retreatment rates may allow for greater insight into the frequency of “clinically relevant” relapse. Retreatment rates range from 22%‐53% with off‐treatment follow‐up periods of ≥1.5 years.( 53 , 54 ) However, it is important to note that retreatment rates could also be affected by other factors such as reimbursement criteria, especially in Asia. The overall modest rate of HBsAg loss under withdrawal of NAs and the lack of validated predictors of harms (severe flares) and benefits (HBsAg loss or inactive CHB) have led to heterogeneity in the guidelines on when to consider stopping treatment in CHB patients. At this time, NA withdrawal should only be considered in those without cirrhosis, under close monitoring.
Emerging HBV Curative Therapies
There are numerous new HBV drugs in the pipeline. There are two main therapeutic strategies in the approach to HBV cure: (1) direct‐acting antivirals targeting various stages of the HBV life cycle and (2) immunomodulators that aim to improve host immune response (Fig. 4). Here, we focus on novel agents in phase 2/3 trials with published data on human subjects, as these agents are further down the pipeline and have the potential to reach clinical care sooner (Table 2).
FIG. 4.
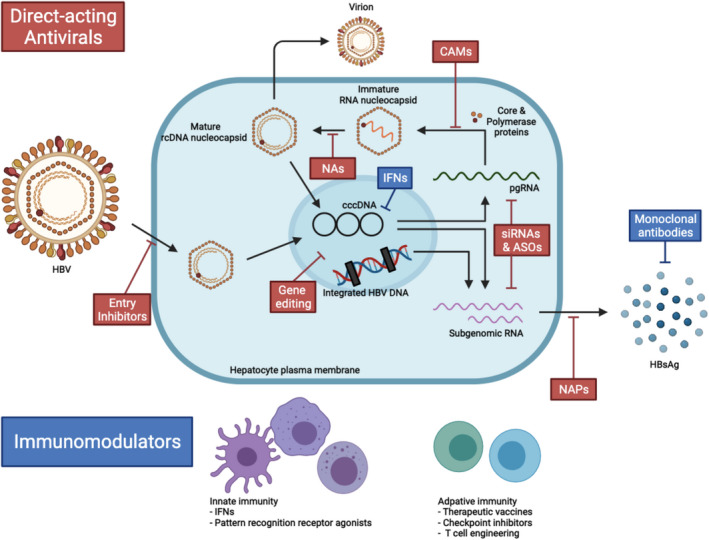
Targets for novel direct‐acting antivirals and immunomodulators in chronic HBV infection. Abbreviation: rcDNA, relaxed circular DNA.
TABLE 2.
Summary of Safety and Efficacy of Novel Therapies for CHB Infection With Phase 2/3 Trial Data
| Drug | Type | Delivery | Efficacy | Safety |
|---|---|---|---|---|
| Bulevirtide (Hepcludex/Myrcludex B)q | HBV entry inhibitor | Subcutaneous injection |
|
|
| VIR‐2218 (ALN HBV02) | siRNA | Subcutaneous injection |
|
|
| RG6346 (DCR‐HBVS) | siRNA | Subcutaneous injection |
|
|
| JNJ‐3989 (ARO‐HBV) | siRNA | Subcutaneous injection |
|
|
| GSK3228836 (IONIS‐HBVRx) | Antisense oligonucleotide | Subcutaneous injection |
|
|
| JNJ‐56136379 | CAM | Oral |
|
|
| ABI‐H0731 | CAM | Oral |
|
|
| GLS4 (Morphothiadin) | CAM | Oral |
|
|
| REP 2139/2165 | NAPs; HBsAg secretion inhibitor | Intravenous infusion |
|
|
| Selgantolimod (GS‐9688) | TLR‐8 agonist | Oral |
|
|
| GS‐4774 | Therapeutic vaccine | Subcutaneous injection |
|
|
| NASVAC | Therapeutic vaccine | Nasal spray |
|
|
Direct‐Acting Antivirals
Inhibiting HBV Entry
Inhibiting HBV and/or hepatitis D virus (HDV) entry via sodium taurocholate co‐transporting polypeptide (NTCP) receptor blocks infection of new hepatocytes, which is hypothesized to subsequently deplete the cccDNA pool. Bulevirtide (also known as Hepcludex or Myrcludex B) is a first‐in‐class entry inhibitor, primarily studied in HDV‐coinfected patients. In phase 1b/2a and 2 studies, both bulevirtide monotherapy and combination therapy with PEG‐IFN‐α resulted in significant decreases in serum HDV‐RNA levels by 24 weeks of treatment.( 60 , 61 , 62 ) In the open‐label phase 2 study in patients with chronic HDV, 27% of patients achieved undetectable levels of HBsAg at 24 weeks after bulevirtide and PEG‐IFN‐α combination therapy, compared with 0% in the monotherapy arms.( 61 ) It should be noted that increased levels of bile salts were seen with bulevirtide, an anticipated consequence of blocking the NTCP receptor. Bulevirtide as suppressive therapy was conditionally approved by the European Medicines Agency for the treatment of chronic HDV infection in July 2020, setting a milestone for its care, as there is no other therapeutic option available for this disease. Its curative efficacy in chronic HBV infection is still under investigation.
Gene Silencing
Small interfering RNAs (siRNAs) and antisense oligonucleotides (ASOs) have been developed to target viral transcripts and induce their degradation, thereby lowering the viral antigen burden. Several agents have reached phase 2 trials, including the siRNAs, VIR‐2218 (also known as ALN HBV02), RG6346 (DCR‐HBVS), and JNJ‐3989 (ARO‐HBV), and the ASO, GSK3228836 (IONIS‐HBVRx).
VIR‐2218 is designed to silence all HBV transcripts from both cccDNA and integrated DNA. In an ongoing phase 2 trial, two monthly doses of the drug immediately led to significant dose‐dependent reductions in serum HBsAg levels (mean −1.4 log10 IU/mL with the highest dose) at week 24.( 63 ) JNJ‐3989 also has been shown to induce ≥1‐log reduction of HBsAg at the nadir in 85% of patients on NA therapy; however, due to gradual rebounds, only 34% of patients showed a sustained effect at 48 weeks after the last dose.( 64 ) In a phase 1b/2a study, RG6346, added to NA, induced HBsAg reductions ≥1.5 log10 IU/mL in 75% of patients.( 65 ) At 1 month following treatment, mean reductions ranged between 1.4 and 1.8 log10 IU/mL in the three dose groups studied. The effects were largely maintained for up to day 488 in patients who entered conditional follow‐up by achieving ≥1‐log reduction. No clinically significant ALT elevations were observed with these siRNAs, especially among those receiving NAs.
GSK3228836 is an ASO that targets all HBV RNAs for degradation and requires more frequent dosing than siRNAs. In a phase 2a study involving patients with CHB on stable NA regimen and NA‐naïve patients with CHB, GSK3228836 resulted in robust declines in serum HBsAg compared with placebo.( 66 ) In the NA‐naïve group, 3 of 12 patients had HBsAg reductions >3 log10 IU/mL (2 achieved HBsAg < 0.05 IU/mL). In the NA‐treated group, all 3 patients who remained on the study drug had HBsAg reductions > 3 log10 IU/mL (2 achieved HBsAg < 0.05 IU/mL). Asymptomatic and self‐resolving ALT flares associated with HBsAg reductions were observed in both NA‐naïve and experienced patients after therapy.( 66 )
The off‐treatment sustainability of the HBsAg responses with siRNAs and ASOs remains to be investigated. In the absence of an effective immune response, HBsAg declines may be considered artificial and thus prone to relapse without proper immune control. These compounds are given subcutaneously, and apart from injection site reaction, side effects are limited thus far.
Capsid Assembly Modulation
The HBV core protein forms viral capsids that contain pregenomic RNA (pgRNA), which is reverse‐transcribed to viral DNA. Capsid assembly modulators (CAMs) are inhibitors that target the core protein to induce the formation of aberrant nucleocapsids (class I) or empty nucleocapsids (class II). CAMs thus interfere with the encapsidation of pgRNA, production of virions and extracellular RNA, and HBV‐DNA replication, and inhibition of de novo cccDNA formation.( 67 , 68 )
JNJ‐56136379, ABI‐H0731, and GLS4 (Morphothiadin) are currently the furthest along in development. Interim results from the phase 2 trial of JNJ‐6379 given in combination with an NA led to more profound HBV DNA and/or HBV‐RNA reductions over NA monotherapy in both previously untreated and virally suppressed patients at week 24.( 69 ) ABI‐H0731 plus NA given for 24 weeks was also shown to induce more rapid and profound declines in HBV‐RNA and HBV‐DNA levels by the end of treatment compared with NA alone.( 70 ) Prolonged treatment demonstrated continued declines in HBV DNA and RNA but minimal effect on HBsAg levels.( 71 ) Similarly, GLS4 given together with ritonavir and ETV versus ETV monotherapy led to significantly greater reductions in HBV DNA among previously untreated patients at week 12.( 72 ) HBV‐RNA declines were more profound in all patients (whether untreated or NA‐suppressed) treated with GLS4/ritonavir and ETV versus ETV alone. CAMs are given orally and are generally well‐tolerated. Nevertheless, overall, CAMs have a minimal effect on HBsAg levels, do not give a sustained off‐treatment response, and are prone to resistance. For such reasons, CAMs will have to be given in combination with other compounds to aim for a functional cure.
Inhibiting HBsAg Secretion
REP 2139 and REP 2165 are nucleic acid polymers (NAPs) that are considered to block the release of HBsAg from infected hepatocytes. In patients coinfected with HBV/HDV, REP 2139 plus PEG‐IFN‐α therapy resulted in significant HBsAg declines accompanied by HDV‐RNA clearance.( 73 ) A phase 2 trial of REP 2139 and REP 2165 demonstrated that the addition of either one to a backbone therapy of TDF and PEG‐IFN‐α was effective in attaining HBsAg levels < 1 IU/mL in 14 of 20 and seroconversion in 11 of 20 patients with CHB in the first 24 weeks.( 74 ) HBsAg declines occurred immediately and rapidly after the introduction of NAPs. Eventually, all patients in the control arm crossed over to triple combination therapy. By the end of treatment, 24 of 40 subjects achieved HBsAg < 0.05 IU/mL, which persisted throughout 48 weeks of off‐treatment follow‐up in 14 subjects. One patient experienced viral rebound accompanied by hepatic decompensation at off‐treatment week 12. NAPs were frequently associated with ALT flares, which were reported to be asymptomatic and related to profound HBsAg declines. Additional studies are needed to investigate the mechanism of action, the safety with regard to flares, the sustainability of response, and the potential intracellular accumulation of HBsAg in humans.
Immunomodulators
A hallmark of chronic HBV infection is a weak immune response, and more specifically, T‐cell exhaustion, characterized by impaired proliferative capacity and effector functions and increased expression of inhibitory molecules such as programmed death 1 (PD‐1). The need for a robust immune response for sustained viral control has driven the development of immunomodulators that aim to improve immune response and subsequent treatment response rates without significant side effects. Although most of the immune‐modulatory approaches are not very successful in inducing functional control on their own, they may be much more effective in the setting of combination with direct‐acting antivirals, which strongly inhibit viral replication and viral protein production.
Pattern Recognition Receptors
Toll‐like receptors (TLRs) are the most studied pattern recognition receptors in HBV infection that play a key role in sensing the initial invasion and activating innate immune responses. Selgantolimod (GS‐9688) is an oral TLR‐8 agonist that has been shown in preclinical studies to induce the production of antiviral cytokines,( 75 ) but in a phase 1b study did not lead to significant HBsAg declines in neither virally suppressed patients with CHB nor patients with viremic CHB.( 76 ) Similarly, phase 2 interim results showed that selgantolimod induced only modest HBsAg declines in virally suppressed patients at week 48.( 77 ). Although this TLR agonist can be given orally and has limited side effects, the therapeutic window of such compounds may be limited, hampering the balance between efficacy and potential side effects, such as flares.
Therapeutic Vaccines
The idea behind therapeutic vaccination is to reinvigorate immune responses by introducing modified HBV antigens. GS‐4774, a yeast‐based T‐cell vaccine, was able to induce a profound immune reaction as indicated by increased cytokine production, but this was not associated with any significant changes in HBsAg levels in virally suppressed patients.( 78 ) In another phase 2 trial involving previously untreated patients, GS‐4774 was safe and well‐tolerated, with rare and self‐limiting ALT flares.( 79 ) However, GS‐4774 plus TDF similarly did not induce significant HBsAg declines by 48 weeks. GS‐4774 had potent stimulatory effects on CD8+ T cells, but the overall HBV‐specific T‐cell response failed to induce substantial antiviral effects. NASVAC is another therapeutic vaccine that is nasally administered. Modest HBsAg reductions (median 0.16‐0.30 log10 IU/mL) were seen at 18 months following treatment; 2 (9%) and 4 (12%) cases of HBsAg loss were observed among NA‐suppressed and treatment‐naïve patients remaining in the study, respectively.( 80 ) However, this open‐label trial included no controls. To date, therapeutic vaccines have been shown to have only minimal effects on viral markers.
Checkpoint Inhibition
In patients with chronic HBV infection, T cells overexpress inhibitory receptors, namely, PD‐1. PD‐1 blockade has been associated with increased T‐cell response in vitro.( 81 ) In a pilot study of nivolumab, a PD‐1 inhibitor, a mean HBsAg decline of −0.30 log10 IU/mL was seen at week 12 in the higher dose group, and 1 patient achieved HBsAg loss.( 82 ) The study also included a nivolumab plus GS‐4774 combination arm; the addition of GS‐4774 had negligible effects on HBsAg levels (mean −0.16 log10 IU/mL). A note of caution is warranted, as immune‐related adverse events, including autoimmune‐like hepatitis, have been associated with checkpoint inhibitors.( 83 )
Conclusion
Current therapies for chronic HBV infection are safe and well‐tolerated and effectively induce viral suppression. However, the challenges in reaching a functional cure remain difficult to overcome. Combination strategies using NAs and PEG‐IFN‐α achieve only modest enhancement in rates of HBsAg loss. Stopping antiviral therapy in patients who have been under long‐term viral suppression requires further studies to identify the patients best served by this approach to maximize benefit over risk. As chronic HBV infection poses multiple obstacles to achieving a cure, an HBV cure likely requires durable, concerted action from different classes of agents with various virological and immunological targets, and such trials with new HBV drugs are underway. With collaborative efforts, we can anticipate moving forward on the road to a cure.
Potential conflict of interest: Dr. Terrault consults and received grants from Gilead, Roche‐Genentech, GlaxoSmithKline, and consulting from EXIGO, ENYO, and Moderna. Dr. Janssen consults and received grants from Gilead, GlaxoSmithKline, Janssen, Roche, and Vir. He consults for Aligos, Antios, Arbutus, Eiger, VBI Vaccines, and Viroclinics. He received grants from AbbVie.
References
Author names in bold designate shared co‐first authorship.
- 1. World Health Organization . Global Hepatitis Report. 2017. https://www.who.int/publications/i/item/global‐hepatitis‐report‐2017. Accessed June 30, 2021. [Google Scholar]
- 2. Anderson RT, Choi HSJ, Lenz O, Peters MG, Janssen HLA, Mishra P, et al. Association between seroclearance of hepatitis B surface antigen and long‐term clinical outcomes of patients with chronic hepatitis B virus infection: systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2021;19:463‐472. [DOI] [PubMed] [Google Scholar]
- 3. Terrault NA, Lok ASF, McMahon BJ, Chang K‐M, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HLY, Chen CJ, et al. Asian‐Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lampertico P, Agarwal K, Berg T, Buti M, Janssen HLA, Papatheodoridis G, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370‐398. [DOI] [PubMed] [Google Scholar]
- 6. Chen C, Lee W, Yang H, Chang H, Jen C, Iloeje UH, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology 2011;141:1240‐1248. [DOI] [PubMed] [Google Scholar]
- 7. Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med 2004;116:829‐834. [DOI] [PubMed] [Google Scholar]
- 8. Chen YG, Chu CM, Liaw YF. Age‐specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology 2010;51:435‐444. [DOI] [PubMed] [Google Scholar]
- 9. Lee HA, Lee HW, Kim IH, Park SY, Sinn DH, Yu JH, et al. Extremely low risk of hepatocellular carcinoma development in patients with chronic hepatitis B in immune‐tolerant phase. Aliment Pharmacol Ther 2020;52:196‐204. [DOI] [PubMed] [Google Scholar]
- 10. Chan HLY, Chan CK, Hui AJ, Chan S, Poordad F, Chang T‐T, et al. Effects of tenofovir disoproxil fumarate in hepatitis B e antigen‐positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA. Gastroenterology 2014;146:1240‐1248. [DOI] [PubMed] [Google Scholar]
- 11. Rosenthal P, Ling SC, Belle SH, Murray KF, Rodriguez‐Baez N, Schwarzenberg SJ, et al. Combination of entecavir/peginterferon alfa‐2a in children with hepatitis B e antigen‐positive immune tolerant chronic hepatitis B virus infection. Hepatology 2019;69:2326‐2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feld JJ, Terrault NA, Lin HHS, Belle SH, Chung RT, Tsai N, et al. Entecavir and peginterferon Alfa‐2a in adults with hepatitis B e antigen‐positive immune‐tolerant chronic hepatitis B virus infection. Hepatology 2019;69:2338‐2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, et al. Virologic and histologic features of chronic hepatitis B virus‐infected asymptomatic patients with persistently normal ALT. Gastroenterology 2008;134:1376‐1384. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen MH, Garcia RT, Trinh HN, Lam KD, Weiss G, Nguyen HA, et al. Histological disease in Asian‐americans with chronic hepatitis B, high hepatitis B virus DNA, and normal alanine aminotransferase levels. Am J Gastroenterol 2009;104:2206‐2213. [DOI] [PubMed] [Google Scholar]
- 15. Zhou K, Terrault N. Immune tolerant HBV and HCC: time to revise our tolerance levels for therapy? AME Med J 2018;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim G‐A, Lim Y‐S, Han S, Choi J, Shim JH, Kim KM, et al. High risk of hepatocellular carcinoma and death in patients with immune‐tolerant‐phase chronic hepatitis B. Gut 2018;67:945‐952. [DOI] [PubMed] [Google Scholar]
- 17. Hui C‐K, Leung N, Yuen S‐T, Zhang H‐Y, Leung K‐W, Lu L, et al. Natural history and disease progression in Chinese chronic hepatitis B patients in immune‐tolerant phase. Hepatology 2007;46:395‐401. [DOI] [PubMed] [Google Scholar]
- 18. Liu J, Yang H‐I, Lee M‐H, Jen C‐L, Batrla‐Utermann R, Lu S‐N, et al. Serum levels of hepatitis B surface antigen and DNA can predict inactive carriers with low risk of disease progression. Hepatology 2016;64:381‐389. [DOI] [PubMed] [Google Scholar]
- 19. Tseng T‐C, Liu C‐J, Yang H‐C, Su T‐H, Wang C‐C, Chen C‐L, et al. Serum hepatitis B surface antigen levels help predict disease progression in patients with low hepatitis B virus loads. Hepatology 2013;57:441‐450. [DOI] [PubMed] [Google Scholar]
- 20. Brunetto MR, Oliveri F, Colombatto P, Moriconi F, Ciccorossi P, Coco B, et al. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology 2010;139:483‐490. [DOI] [PubMed] [Google Scholar]
- 21. Wong GLH, Chan HLY, Yu Z, Chan HY, Tse CH, Wong VWS. Liver fibrosis progression is uncommon in patients with inactive chronic hepatitis B: a prospective cohort study with paired transient elastography examination. J Gastroenterol Hepatol 2013;28:1842‐1848. [DOI] [PubMed] [Google Scholar]
- 22. Villeneuve J‐P, Desrochers M, Infante‐Rivard C, Willems B, Raymond G, Bourcier M, et al. A long‐term follow‐up study of asymptomatic hepatitis B surface antigen‐positive carriers in Montreal. Gastroenterology 1994;106:1000‐1005. [DOI] [PubMed] [Google Scholar]
- 23. Manno M, Cammà C, Schepis F, Bassi F, Gelmini R, Giannini F, et al. Natural history of chronic HBV carriers in Northern Italy: morbidity and mortality after 30 years. Gastroenterology 2004;127:756‐763. [DOI] [PubMed] [Google Scholar]
- 24. Tai DI, Lin SM, Sheen IS, Chu CM, Lin DY, Liaw YF. Long‐term outcome of hepatitis B e antigen‐negative hepatitis B surface antigen carriers in relation to changes of alanine aminotransferase levels over time. Hepatology 2009;49:1859‐1867. [DOI] [PubMed] [Google Scholar]
- 25. Chen J, Yang H, Iloeje UH, You S, Lu S, Wang Li–Yu, et al. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver‐related death. Gastroenterology 2010;138:1747‐1754. [DOI] [PubMed] [Google Scholar]
- 26. Paik N, Sinn DH, Lee JH, Oh IS, Kim JH, Kang W, et al. Non‐invasive tests for liver disease severity and the hepatocellular carcinoma risk in chronic hepatitis B patients with low‐level viremia. Liver Int 2018;38:68‐75. [DOI] [PubMed] [Google Scholar]
- 27. Cao Z, Liu Y, Ma L, Lu J, Jin YI, Ren S, et al. A potent hepatitis B surface antigen response in subjects with inactive hepatitis B surface antigen carrier treated with pegylated‐interferon alpha. Hepatology 2017;66:1058‐1066. [DOI] [PubMed] [Google Scholar]
- 28. de Niet A, Jansen L, Stelma F, Willemse SB, Kuiken SD, Weijer S, et al. Peg‐interferon plus nucleotide analogue treatment versus no treatment in patients with chronic hepatitis B with a low viral load: a randomised controlled, open‐label trial. Lancet Gastroenterol Hepatol 2017;2:576‐584. [DOI] [PubMed] [Google Scholar]
- 29. Zeng Q‐L, Yu Z‐J, Shang J, Xu G‐H, Sun C‐Y, Liu NA, et al. Short‐term peginterferon‐induced high functional cure rate in inactive chronic hepatitis B virus carriers with low surface antigen levels. Open Forum Infect Dis 2020;7:ofaa208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fattovich G, Olivari N, Pasino M, D’Onofrio M, Martone E, Donato F. Long‐term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut 2008;57:84‐90. [DOI] [PubMed] [Google Scholar]
- 31. Cho J‐Y, Paik Y‐H, Sohn W, Cho HC, Gwak G‐Y, Choi MS, et al. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut 2014;63:1943‐1950. [DOI] [PubMed] [Google Scholar]
- 32. Chu CM, Liaw YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long‐term follow‐up. Hepatology 2007;45:1187‐1192. [DOI] [PubMed] [Google Scholar]
- 33. Micco L, Peppa D, Loggi E, Schurich A, Jefferson L, Cursaro C, et al. Differential boosting of innate and adaptive antiviral responses during pegylated‐interferon‐alpha therapy of chronic hepatitis B. J Hepatol 2013;58:225‐233. [DOI] [PubMed] [Google Scholar]
- 34. Boni C, Laccabue D, Lampertico P, Giuberti T, Viganò M, Schivazappa S, et al. Restored function of HBV‐specific T cells after long‐term effective therapy with nucleos(t)ide analogues. Gastroenterology 2012;143:963‐973. [DOI] [PubMed] [Google Scholar]
- 35. Lok AS, Trinh H, Carosi G, Akarca US, Gadano A, Habersetzer F, et al. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide‐nave patients with chronic hepatitis B. Gastroenterology 2012;143:619‐628. [DOI] [PubMed] [Google Scholar]
- 36. Lau GKK, Piratvisuth T, Kang XL, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon alfa‐2a, lamivudine, and the combination for HBeAg‐positive chronic hepatitis B. N Engl J Med 2005;30:2682‐2695. [DOI] [PubMed] [Google Scholar]
- 37. Janssen HLA, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, et al. Pegylated interferon alfa‐2b alone or in combination with lamivudine for HBeAg‐positive chronic hepatitis B: a randomised trial. Lancet 2005;365:123‐129. [DOI] [PubMed] [Google Scholar]
- 38. Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, et al. Combination of tenofovir disoproxil fumarate and peginterferon α‐2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology 2016;150:134‐144. [DOI] [PubMed] [Google Scholar]
- 39. Terrault N, Lok AS, Wahed A, Wong DKH, Khalili M, Fried MW. Randomized trial of 192 weeks of tenofovir with or without peginterferon alfa for the first 24 weeks followed by protocolized withdrawal in adults with chronic hepatitis B. Hepatology 2020;72:15A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brouwer WP, Xie Q, Sonneveld MJ, Zhang N, Zhang Q, Tabak F, et al. Adding pegylated interferon to entecavir for hepatitis B e antigen‐positive chronic hepatitis B: a multicenter randomized trial (ARES study). Hepatology 2015;61:1512‐1522. [DOI] [PubMed] [Google Scholar]
- 41. van Campenhout MJH, Brouwer WP, Xie Q, Guo S, Chi H, Qi X, et al. Long‐term follow‐up of patients treated with entecavir and peginterferon add‐on therapy for HBeAg‐positive chronic hepatitis B infection: ARES long‐term follow‐up. J Viral Hepat 2019;26:109‐117. [DOI] [PubMed] [Google Scholar]
- 42. Chi H, Hansen BE, Guo S, Zhang NP, Qi X, Chen L, et al. Pegylated interferon alfa‐2b add‐on treatment in hepatitis b virus envelope antigen‐positive chronic hepatitis B patients treated with nucleos(t)ide analogue: a randomized, controlled trial (PEGON). J Infect Dis 2017;215:1085‐1093. [DOI] [PubMed] [Google Scholar]
- 43. Li GJ, Yu YQ, Chen SL, Fan P, Shao LY, Chen JZ, et al. Sequential combination therapy with pegylated interferon leads to loss of hepatitis B surface antigen and hepatitis B e Antigen (HBeAg) seroconversion in HBeAg‐positive chronic hepatitis B patients receiving long‐term entecavir treatment. Antimicrob Agents Chemother 2015;59:4121‐4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bourlière M, Rabiega P, Ganne‐Carrie N, Serfaty L, Marcellin P, Barthe Y, et al. Effect on HBs antigen clearance of addition of pegylated interferon alfa‐2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen‐negative chronic hepatitis B and sustained undetectable plasma hepatitis. Lancet Gastroenterol Hepatol 2017;2:177‐188. [DOI] [PubMed] [Google Scholar]
- 45. Lampertico P, Brunetto MR, Craxì A, Gaeta GB, Rizzetto M, Rozzi A, et al. Add‐on peginterferon alfa‐2a to nucleos(t)ide analogue therapy for Caucasian patients with hepatitis B ‘e’ antigen‐negative chronic hepatitis B genotype D. J Viral Hepat 2019;26:118‐125. [DOI] [PubMed] [Google Scholar]
- 46. Ning Q, Han M, Sun Y, Jiang J, Tan D, Hou J, et al. Switching from entecavir to PegIFN alfa‐2a in patients with HBeAg‐positive chronic hepatitis B: a randomized open‐label trial (OSST trial). J Hepatol 2014;61:777‐784. [DOI] [PubMed] [Google Scholar]
- 47. Hsu CW, Su WW, Lee CM, Peng CY, Chuang WL, Kao JH, et al. Phase IV randomized clinical study: peginterferon alfa‐2a with adefovir or entecavir pre‐therapy for HBeAg‐positive chronic hepatitis B. J Formos Med Assoc 2018;117:588‐597. [DOI] [PubMed] [Google Scholar]
- 48. Lim SG, Yang WL, Ngu JH, Chang J, Tan J, Ahmed T, et al. Switching to or add‐on peginterferon in patients on nucleos(t)ide analogues for chronic hepatitis B: the SWAP RCT. Clin Gastroenterol Hepatol 2021. 10.1016/j.cgh.2021.04.031. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49. Berg T, Simon K‐G, Mauss S, Schott E, Heyne R, Klass DM, et al. Long‐term response after stopping tenofovir disoproxil fumarate in non‐cirrhotic HBeAg‐negative patients—FINITE study. J Hepatol 2017;67:918‐924. [DOI] [PubMed] [Google Scholar]
- 50. van Bömmel F, Stein K, Heyne R, Möller H, Petersen J, Buggisch P, et al. Response to discontinuation of long‐term nucleos(t)ide analogue treatment in HBeAg negative patients: results of the Stop‐NUC trial. J Hepatol 2020;73:S118. [Google Scholar]
- 51. Liem KS, Fung S, Wong DK, Yim C, Noureldin S, Chen J, et al. Limited sustained response after stopping nucleos(t)ide analogues in patients with chronic hepatitis B: results from a randomised controlled trial (Toronto STOP study). Gut 2019;68:2206‐2213. [DOI] [PubMed] [Google Scholar]
- 52. Jeng W‐J, Chen Y‐C, Chien R‐N, Sheen I‐S, Liaw Y‐F. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen‐negative chronic hepatitis B. Hepatology 2018;68:425‐434. [DOI] [PubMed] [Google Scholar]
- 53. Tout I, Lampertico P, Berg T, Asselah T. Perspectives on stopping nucleos(t)ide analogues therapy in patients with chronic hepatitis B. Antiviral Res 2021;185:104992. [DOI] [PubMed] [Google Scholar]
- 54. van Bömmel F, Berg T. Stopping long‐term treatment with nucleos(t)ide analogues is a favourable option for selected patients with HBeAg‐negative chronic hepatitis B. Liver Int 2018;38:90‐96. [DOI] [PubMed] [Google Scholar]
- 55. Liem KS, Gehring AJ, Feld JJ, Janssen HLA. Challenges with stopping long‐term nucleos(t)ide analogue therapy in patients with chronic hepatitis B. Gastroenterology 2020;158:1185‐1190. [DOI] [PubMed] [Google Scholar]
- 56. Hirode G, Choi HSJ, Su T‐H, Wong GL‐H, Seto W‐K, Van Hees S. HBsAg loss is higher among Caucasians compared to Asians after stopping nucleos(t)ide analogue therapy: results from a large, global, multi‐ethnic cohort of patients with chronic hepatitis B (RETRACT‐B STUDY). Hepatology 2020;72:19A.31654573 [Google Scholar]
- 57. Papatheodoridi M, Hadziyannis E, Berby F, Zachou K, Testoni B, Rigopoulou E, et al. Predictors of hepatitis B surface antigen loss, relapse and retreatment after discontinuation of effective oral antiviral therapy in noncirrhotic HBeAg‐negative chronic hepatitis B. J Viral Hepat 2020;27:118‐126. [DOI] [PubMed] [Google Scholar]
- 58. Yao CC, Hung CH, Hu TH, Lu SN, Wang JH, Lee CM, et al. Incidence and predictors of HBV relapse after cessation of nucleoside analogues in HBeAg‐negative patients with HBsAg ≤ 200 ≥IU/mL. Sci Rep 2017;7:1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hung CH, Wang JH, Lu SN, Hu TH, Lee CM, Chen CH. Hepatitis B surface antigen loss and clinical outcomes between HBeAg‐negative cirrhosis patients who discontinued or continued nucleoside analogue therapy. J Viral Hepat 2017;24:599‐607. [DOI] [PubMed] [Google Scholar]
- 60. Bogomolov P, Alexandrov A, Voronkova N, Macievich M, Kokina K, Petrachenkova M, et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: first results of a phase Ib/IIa study. J Hepatol 2016;65:490‐498. [DOI] [PubMed] [Google Scholar]
- 61. Wedemeyer H, Schoneweis K, Bogomolov P, Voronkova N, Chulanov V, Stepanova T. Final results of a multicenter, open‐label phase 2 clinical trial (MYR203) to assess safety and efficacy of myrcludex B in cwith PEG‐interferon Alpha 2a in patients with chronic HBV/HDV coinfection. J Hepatol 2019;70:e81. [Google Scholar]
- 62. Wedemeyer H, Schöneweis K, Bogomolov PO, Chulanov V, Stepanova T, Viacheslav M, et al. 48 weeks of high dose (10 mg) bulevirtide as monotherapy or with peginterferon alfa‐2a in patients with chronic HBV/HDV coinfection. J Hepatol 2020;73:S52. [Google Scholar]
- 63. Gane E, Lim Y‐S, Tangkijvanich P, O’Beirne J, Lim TH, Bakardjiev A, et al. Preliminary safety and antiviral activity of VIR‐2218, an X‐targeting HBV RNAi therapeutic, in chronic hepatitis B patients. J Hepatol 2020;73:S50. [Google Scholar]
- 64. Gane E, Locarnini S, Lim TH, Strasser S, Sievert W, Cheng W, et al. Short‐term treatment with RNA interference therapy, JNJ‐3989, results in sustained hepatitis B surface antigen suppression in patients with chronic hepatitis B receiving nucleos(t)ide analogue treatment. J Hepatol 2020;73:S20. [Google Scholar]
- 65. Yuen M‐F, Lim TH, Kim W, Tangkijvanich P, Yoon J‐H, Sievert W. HBV RNAi inhibitor RG6346 in phase 1b–2a trial was safe, well‐tolerated, and resulted in substantial and durable reductions in serum HBsAg levels. Hepatology 2020;72:LO9. [Google Scholar]
- 66. Yuen M‐F, Heo J, Jang JW, Yoon J‐H, Kweon YO, Park S‐J, et al. Hepatitis B virus (HBV) surface antigen (HBsAg) inhibition with ISIS 505358 in chronic hepatitis B (CHB) patients on stable nucleos (t)ide analogue (NA) regimen and in NA‐naive CHB patients: phase 2a, randomized, double‐blind, placebo‐controlled study. J Hepatol 2020;73:S19‐S57. [Google Scholar]
- 67. Lam AM, Ren S, Espiritu C, Kelly M, Lau V, Zheng L, et al. Hepatitis B virus capsid assembly modulators, but not nucleoside analogs, inhibit the production of extracellular pregenomic RNA and spliced RNA variants. Antimicrob Agents Chemother 2017;61:e680‐e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Berke JM, Dehertogh P, Vergauwen K, Mostmans W, Vandyck K, Raboisson P, et al. Antiviral properties and mechanism of action studies of the hepatitis B virus capsid assembly modulator JNJ‐56136379. Antimicrob Agents Chemother 2020;64:e02439‐e2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Janssen H, Hou J, Asselah T, Chan H, Zoulim F, Tanaka Y, et al. Efficacy and safety results of the phase 2 JNJ‐56136379 JADE study in patients with chronic hepatitis B: interim week 24 data. J Hepatol 2020;73:S129. [Google Scholar]
- 70. Fung S, Sulkowski M, Lalezari J, Schiff ER, Dieterich D, Hassanein T, et al. Antiviral activity and safety of the hepatitis B core inhibitor ABI H0731 administered with a nucleos(t)ide reverse transcriptase inhibitor in patients with HBeAg‐negative chronic hepatitis B infection. J Hepatol 2020;73:S51. [Google Scholar]
- 71. Yuen M‐F, Agarwal K, Ma X, Nguyen T, Schiff ER, Hann H‐W, et al. Antiviral activity and safety of the hepatitis B core inhibitor ABIH0731 administered with a nucleos(t)ide reverse transcriptase inhibitor in patients with HBeAg‐positive chronic hepatitis B infection in a long‐term extension study. J Hepatol 2020;73:S140. [Google Scholar]
- 72. Zhang M, Zhang J, Tan Y, Xin Y, Gao H, Zheng S, et al. Efficacy and safety of GLS4/ritonavir combined with entecavir in HBeAg‐positive patients with chronic hepatitis B: interim results from phase 2b, multi‐center study. J Hepatol 2020;73:S878. [Google Scholar]
- 73. Bazinet M, Pântea V, Cebotarescu V, Cojuhari L, Jimbei P, Albrecht J, et al. Safety and efficacy of REP 2139 and pegylated interferon alfa‐2a for treatment‐naive patients with chronic hepatitis B virus and hepatitis D virus co‐infection (REP 301 and REP 301‐LTF): a non‐randomised, open‐label, phase 2 trial. Lancet Gastroenterol Hepatol 2017;2:877‐889. [DOI] [PubMed] [Google Scholar]
- 74. Bazinet M, Pântea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, et al. Safety and efficacy of 48 weeks REP 2139 or REP 2165, tenofovir disoproxil, and pegylated interferon alfa‐2a in patients with chronic HBV infection naïve to nucleos(t)ide therapy. Gastroenterology 2020;158:2180‐2194. [DOI] [PubMed] [Google Scholar]
- 75. Amin OE, Colbeck EJ, Daffis S, Khan S, Ramakrishnan D, Pattabiraman D, et al. Therapeutic potential of TLR8 agonist GS‐9688 (selgantolimod) in chronic hepatitis B: re‐modelling of antiviral and regulatory mediators. Hepatology 2021;74:55‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gane EJ, Kim HJ, Visvanathan K, Kim YJ, Nguyen A‐H, Wallin JJ, et al. Safety, pharmacokinetics, and pharmacodynamics of the oral TLR8 agonist selgantolimod in chronic hepatitis B [in press]. Hepatology 2021;74:1737‐1749. [DOI] [PubMed] [Google Scholar]
- 77. Gane E, Dunbar PR, Brooks A, Zhao Y, Tan S, Lau A, et al. Efficacy and safety of 24 weeks treatment with oral TLR8 agonist, selgantolimod, in virally‐suppressed adult patients with chronic hepatitis B: a phase 2 study. J Hepatol 2020;73:S52. [Google Scholar]
- 78. Lok AS, Pan CQ, Han S‐H, Trinh HN, Fessel WJ, Rodell T, et al. Randomized phase II study of GS‐4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J Hepatol 2016;65:509‐516. [DOI] [PubMed] [Google Scholar]
- 79. Boni C, Janssen HLA, Rossi M, Yoon SK, Vecchi A, Barili V, et al. Combined GS‐4774 and tenofovir therapy can improve HBV‐specific T‐cell responses in patients with chronic hepatitis. Gastroenterology 2019;157:227‐241. [DOI] [PubMed] [Google Scholar]
- 80. Yoshida O, Imai Y, Shiraishi K, Tokumoto Y, Sanada T. HBsAg reduction by nasal administration of a therapeutic vaccine containing HBsAg and HBcAg (NASVAC) in patients with chronic HBV infection: the results of 18 months follow up. Hepatology 2020;72:60A. [Google Scholar]
- 81. Bengsch B, Martin B, Thimme R. Restoration of HBV‐specific CD8+ T cell function by PD‐1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol 2014;61:1212‐1219. [DOI] [PubMed] [Google Scholar]
- 82. Gane E, Verdon DJ, Brooks AE, Gaggar A, Nguyen AH, Subramanian GM, et al. Anti‐PD‐1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: a pilot study. J Hepatol 2019;71:900‐907. [DOI] [PubMed] [Google Scholar]
- 83. Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug‐induced liver injury. Mod Pathol 2018;31:965‐973. [DOI] [PubMed] [Google Scholar]


