Abstract
We have previously shown that gamma interferon (IFN-γ) is a useful adjunct to therapy of experimental systemic cryptococcosis in normal mice. To better emulate AIDS patients, SCID mice were infected intravenously with Cryptococcus neoformans. Mice received no therapy, 3 mg of amphotericin B (AmB) per kg of body weight, or 105 U of IFN-γ alone (prophylactically and therapeutically or only therapeutically) or with AmB. In the first experiment, >75% of the mice survived. Therapy with AmB alone was efficacious compared to no therapy in all organs. Both regimens of IFN-γ alone were efficacious in the brain and lungs, and the combination of AmB and IFN-γ showed significant synergy in the kidneys. AmB alone cured 40% of mice of infection, whereas the combination regimens cured >50% of the mice and 90% of the brain infections. In a second study, IFN-γ again proved efficacious alone, and when given with AmB its efficacy was improved. Therapeutic IFN-γ alone was effective only in the liver compared to no therapy, and the combination regimen, although highly effective, showed no significant synergy. In a third experiment, AmB alone or in combination with IFN-γ prolonged survival compared to no therapy or IFN-γ alone. The combination regimen showed significant synergy over AmB alone in the brain, liver, kidneys, and lungs. AmB alone cured no mice of infections in more than two organs, whereas AmB in combination with IFN-γ cured 55% of infections in three or more organs. These results indicate that IFN-γ has therapeutic efficacy in severely immunodeficient animals, especially in combination with AmB. Significant synergistic activity was noted in all organs except the spleen. Overall, IFN-γ has utility as an adjunctive therapy against systemic cryptococcosis in the severely immunocompromised host.
Cryptococcal meningitis is a fungal disease that requires therapeutic intervention, whether it is manifested in immunocompetent or immunocompromised patients (18). Although currently available antifungal therapies have been shown to be beneficial, relapses while on therapy and mortality are not uncommon (8, 9, 12). Thus, the improvement of therapeutic options is of prime importance in the successful treatment of this disease. One potential therapeutic option involves the use of cytokines as an adjunct to conventional antifungal therapy (18, 23).
Previous studies have demonstrated that adjunctive cytokine therapy with interleukin-12 or gamma interferon (IFN-γ) could improve the outcome of experimental murine cryptococcosis (5, 7, 15, 17, 23). In addition, synergistic efficacy has been demonstrated by the addition of either interleukin-12, an inducer of IFN-γ, or IFN-γ to a regimen of conventional therapy (5, 15, 17). However, these studies were done with nonimmunocompromised animals, whereas the majority of patients with meningeal cryptococcosis are immunocompromised.
In the present study, we have examined the utility of IFN-γ alone or in combination with amphotericin B (AmB) therapy against systemic cryptococcosis established in a severely immunocompromised host, namely, the SCID mouse. This animal has severe combined immunodeficiency with no functional B or T cells and most closely emulates the patient with AIDS (4). Our rationale for choosing this model was to determine whether immunomodulation with IFN-γ alone or as an adjunct to conventional AmB treatment would have therapeutic efficacy in a host incapable of a normal cell-mediated or humoral immune response. Were IFN-γ to prove beneficial in this system, it might provide a clinical option for the treatment of cryptococcal meningitis in immunocompromised patient populations.
(These studies were presented in part to the 4th Congress of the European Confederation of Medical Mycology held in Glasgow, Scotland, in May 1998.)
MATERIALS AND METHODS
Mice.
The animals used in this experiment were 6-week-old male C.B-17 scid/scid (SCID) mice. These mice were purchased from Taconic, Germantown, N.Y. Mice were housed in sterile microisolator cages and were provided sterilized chow and sterilized water ad libitum. Strict protocols were followed in handling these mice to reduce the possibility of animals contracting opportunistic infections. Mice were housed five per cage. All cages were changed at least twice weekly.
Experiment 1.
Six groups of 10 mice each were randomly assigned to therapy groups. The groups were mice receiving no therapy, mice receiving 100,000 U of IFN-γ (recombinant murine IFN-γ supplied by Genentech, Inc., South San Francisco, Calif.) given intravenously (i.v.) in 0.25 ml either therapeutically or prior to infection and then therapeutically, and mice receiving 3.0 mg of AmB (Pharma-Tek, Inc., Huntington, N.Y.) per kg of body weight given intraperitoneally in 0.20 ml alone or in combination with one of the two IFN-γ regimens. IFN-γ was administered i.v. on the basis of prior data in our laboratory, which indicated better efficacy by this route than by a subcutaneous route in normal animals (data not shown).
Experiments 2 and 3.
Four groups of 10 mice each were randomly assigned to therapy groups. Mice received either no therapy, 100,000 U of IFN-γ (Genentech) given i.v. in 0.25 ml, or 3.0 mg of AmB (Pharma-Tek, Inc.) per kg given intraperitoneally in 0.20 or 0.25 ml alone or in combination with the IFN-γ regimen.
In all three experiments, IFN-γ was diluted in sterile saline prior to dosing and AmB was diluted in 5% dextrose water. Mice receiving AmB were given six doses on an every-other-day (QOD) schedule beginning on day 1 postinfection. Mice receiving pretreatment with IFN-γ were dosed on a QOD schedule on days 7, 5, 3, and 1 prior to infection. Therapeutic IFN-γ was given on a QOD schedule beginning on day 1 postinfection and continuing through day 27 of infection, for total of 14 doses of IFN-γ.
Infection model.
Cryptococcus neoformans strain 9759 (serotype A) was grown for the preparation of an infecting inoculum as described previously (4, 6, 13, 14). The numbers of yeast cells were estimated by hemacytometer count, and the cells were serially diluted in sterile saline to the number desired for infection. Plating onto Sabouraud's agar plus chloramphenicol was done to determine the number of viable yeast cells in the inoculum. On day 0, all mice were infected i.v. with viable C. neoformans given i.v. in a 0.25-ml volume (4). Mice received 2,000 yeast cells in experiment 1, 3,000 yeast cells in experiment 2, and 5,000 yeast cells in experiment 3, as determined by hemacytometer counts.
One to three days after the cessation of therapy, all surviving mice were euthanatized by CO2 asphyxiation. Various organs were removed aseptically, weighed, and homogenized in 5 ml of saline. Organ homogenates were serially diluted, and samples were plated for the determination of the number of viable C. neoformans cells remaining in the entire organ. The number of CFU in each organ was determined and expressed as the log10 number of CFU per organ. All data are presented as the log10 geometric mean number of CFU from surviving mice. A value of 0 indicates that the number of CFU in the organ was below the detection limit of the assay, which is approximately 5 to 10 CFU per organ. Statistical analyses of comparative burdens of C. neoformans recovered from the organs were done using a Mann-Whitney U test (GB-STAT, version 6.0; Dynamic Microsystems, Inc., Silver Spring, Mad.), with an arbitrary value of log10 7 assigned to data points missing because of the death of an animal due to infection. This ensures that death is considered a worse outcome than survival with any amount of organism burden. Analyses of comparative survival were done by day of death using a Wilcoxon rank sum test.
RESULTS
Experiment 1.
Over the course of the 29 days of experimental infection, 7 of 9 untreated control mice survived. The two deaths occurred on days 21 and 29 postinfection. A single mouse succumbed to infection on day 25 postinfection in the group receiving pretreatment and therapeutic IFN-γ alone. No other mice died of infection with any other regimen. Because of the low mortality in the untreated control group, no survival advantage could be demonstrated for any treatment regimen.
The primary parameter used to evaluate the efficacy of IFN-γ therapy in this study was the comparative burden of C. neoformans recovered from the organs at day 29 postinfection. The log10 geometric mean burdens and the 95% confidence intervals of these burdens are presented in Table 1. In the brain, kidneys, and lungs, the untreated controls had the highest mean burdens of C. neoformans, whereas in the livers and spleens, mice given IFN-γ alone on a pretreatment and therapeutic schedule had higher mean burdens than did the untreated controls. Thus, all treatment regimens showed some efficacy in reducing the mean burden of yeast cells in three or more organs.
TABLE 1.
Recovery of C. neoformans from organs of surviving SCID mice treated with IFN-γ or AmB alone or in combination in experiment 1
| Treatment | No. of mice alive/no. cured (no. total) | Log10 geometric mean CFU of C. neoformans per organ (no. of organs free of infection) (95% confidence interval) recovered from:
|
||||
|---|---|---|---|---|---|---|
| Brain | Spleen | Liver | Kidney | Lung | ||
| None | 7/0 (9) | 3.02 (1) (1.5–4.6) | 0.57 (3) (0–1.2) | 4.34 (0) (3.6–5.1) | 2.68 (0) (1.7–3.7) | 2.36 (0) (1.6–3.1) |
| Therapeutic IFN-γ alone | 10/0 (10) | 1.69 (4)a (0.5–2.9) | 0.39 (8) (0–1.0) | 3.99 (0)a (3.6–4.3) | 2.21 (1) (1.4–3.0) | 0.65 (7)a (0–1.5) |
| Prophylactic and therapeutic IFN-γ alone | 10/0 (10) | 0.84 (5)a (0–1.7) | 0.90 (6) (0–2.3) | 4.45 (0) (4–4.9) | 2.19 (1) (1.2–3.2) | 0.96 (3)a (0.1–1.8) |
| AmB alone | 10/4 (10) | 0.73 (8)a (0–2.2) | 0.19 (9)a (0–0.6) | 1.11 (6)a (0–2.2) | 0.99 (5)a (0.02–2.0) | 0.40 (9)a (0–1.3) |
| Therapeutic IFN-γ plus AmB | 10/5 (10) | 0.79 (8)a (0–2.0) | 0.31 (9)a (0–1.0) | 0.97 (6)a (0.03–1.9) | 0 (10)ab | 0 (10)a |
| Prophylactic and therapeutic IFN-γ plus AmB | 9/6 (9) | 0 (9)a | 0.15 (9)a (0–0.5) | 0.49 (7)a (0–1.3) | 0 (9)ab | 0.15 (8)a (0–0.5) |
P < 0.05 to 0.001 versus controls dependent on comparison.
P < 0.05 versus AmB alone.
The significance of the apparent treatment efficacies was determined by comparison of the burdens between treatment groups. Therapy with AmB alone was efficacious compared to no treatment in all five organs in reducing the burden of organisms. Therapy with IFN-γ alone by either dosing schedule also proved effective. However, this activity was significant only in the brain and lungs for both regimens and in the liver for the therapeutic regimen of IFN-γ. The addition of IFN-γ to the AmB regimen further increased the efficacy of the treatment in all organs. However, statistically significant synergistic activity was noted only in the kidneys.
With respect to cure (defined as no detectable infection in the organs assayed), only mice given a regimen which included AmB were free of detectable C. neoformans in all five organs. AmB alone cleared the infection in 40% of the treated animals, whereas AmB plus therapeutic IFN-γ cleared it in 50% of the mice. Administration of IFN-γ as a pretreatment and then therapeutically in combination with AmB cleared 66% of the treated mice of infection. All were free of infection in the brain and kidney.
Experiment 2.
Over the course of the 28 days of experimental infection, 6 of 10 untreated control mice survived. The deaths occurred on days 15, 17, 24, and 26 postinfection. No other mice died of infection with any other regimen. Because of the low mortality in the untreated control group, no survival advantage could be demonstrated for any treatment regimen.
The mean burdens of C. neoformans were highest in the untreated control group and the IFN-γ-treated group (Table 2). In all organs, except the liver, those animals given IFN-γ carried higher, but not significant, mean burdens than did surviving mice that had received no treatment. AmB treatment alone was efficacious in all five organs in reducing the burden of organisms. Therapy with IFN-γ alone proved significantly effective only in the liver. The combination regimen of IFN-γ and AmB was more efficacious than AmB alone in organs other than the brain, but this did not attain statistical significance.
TABLE 2.
Recovery of C. neoformans from organs of surviving SCID mice treated with IFN-γ or AmB alone or in combination in experiment 2
| Treatment | No. of mice alive/no. cured (no. total) | Log10 geometric mean CFU of C. neoformans per organ (no. of organs free of infection) (95% confidence interval) recovered from:
|
||||
|---|---|---|---|---|---|---|
| Brain | Spleen | Liver | Kidney | Lung | ||
| None | 6/0 (10) | 2.23 (3) (0–4.6) | 2.88 (0) (2.5–3.2) | 4.72 (0) (4.6–4.8) | 1.36 (1) (0.5–2.2) | 0.89 (2) (0–2.1) |
| IFN-γ alone | 10/0 (10) | 3.90 (2) (1.7–6.0) | 3.50 (0) (2.8–4.2) | 4.55 (0)a (3.9–5.2) | 1.87 (3) (0.4–3.3) | 1.44 (7) (0.2–2.6) |
| AmB | 10/2 (10) | 0.35 (6)a (0–0.7) | 0.27 (9)a (0–0.9) | 0.22 (8)a (0–0.6) | 0.15 (8)a (0–0.4) | 0.18 (9)a (0–0.6) |
| IFN-γ + AmB | 10/6 (10) | 1.51 (7) (0–3.3) | 0.12 (9)a (0–0.4) | 0.39 (8)a (0–1.1) | 0 (10)a | 0 (10)a |
P < 0.05 to 0.001 versus controls dependent on comparison.
Similar to the results of the first experiment, AmB alone cleared infection in 20% of the treated animals, whereas AmB plus IFN-γ cleared the infection in 60% of the mice. However, by Fisher's exact test this comparative rate of cure was not significantly different (P = 0.085).
Experiment 3.
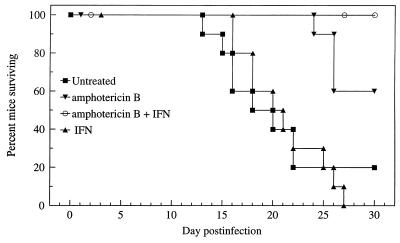
Over the course of the 30-day experimental infection, 2 of 10 untreated control mice survived. In comparison, 6 of 10 mice given AmB alone, 0 of 10 mice given IFN-γ alone, and 9 of 9 mice given the combination regimen of AmB and IFN-γ survived (1 mouse died of an injection-related trauma and was not included in any analysis) (Fig. 1). Statistical comparison showed that both AmB and the combination regimen significantly prolonged survival in comparison with no treatment or IFN-γ alone (P < 0.01 to 0.001). IFN-γ alone did not significantly prolong survival, nor was synergy demonstrated with the combination regimen, i.e., IFN-γ plus AmB was equivalent to AmB alone, and IFN-γ alone was equivalent to no treatment (P < 0.05).
FIG. 1.
Cumulative mortality of mice infected with C. neoformans and treated with AmB or IFN-γ alone or in combination in experiment 3.
The comparative burdens of C. neoformans recovered from the organs at day 30 postinfection are presented in Table 3. AmB treatment alone was efficacious in only the spleen and liver in reducing the burden of organisms. The addition of IFN-γ to the AmB regimen increased the efficacy of the treatment in all organs except the spleen (Table 3). Statistically significant synergistic activity was noted in the brain, liver, kidneys, and lungs with the AmB and IFN-γ regimen.
TABLE 3.
Recovery of C. neoformans from organs of surviving SCID mice treated with IFN-γ or AmB alone or in combination in experiment 3
| Treatment | No. of mice alive/no. cured (no. alive) | Log10 geometric mean CFU of C. neoformans per organ (no. of organs free of infection) (95% confidence interval) recovered from:
|
||||
|---|---|---|---|---|---|---|
| Brain | Spleen | Liver | Kidney | Lung | ||
| None | 2/0 (10) | 0 (2) | 3.08 (0) (2.5–3.2) | 4.13 (0) (0–11) | 1.32 (0) (0–4.8) | 0.60 (0) (0–8.2) |
| IFN-γ alone | 0/0 (10) | |||||
| AmB | 6/0 (10) | 5.79 (0) (3.7–7.9) | 1.46 (1)a (0.5–2.4) | 2.04 (1)a (0.9–3.2) | 1.18 (2) (0.2–2.2) | 1.10 (2) (0–2.4) |
| IFN-γ plus AmB | 9/1 (9) | 3.82 (3)ab (1.6–6.1) | 1.55 (2)a (0.6–2.4) | 0.74 (6)ab (0–1.6) | 0.28 (7)ab (0–0.7) | 0.37 (5)ab (0.02–0.7) |
P < 0.05 to 0.001 versus controls dependent on comparison.
P < 0.05 to 0.01 versus AmB alone dependent on comparison.
AmB alone cleared none of the treated animals in more than two organs, whereas AmB plus IFN-γ cleared 55% of the mice of detectable infection in three or more organs; one mouse was free of infection in all five organs.
DISCUSSION
There exists a substantial body of literature dealing with the involvement of IFN-γ in host resistance to C. neoformans (1–3, 5, 7, 10, 11, 15, 16, 19–22, 24). Both in vitro and in vivo studies have shown IFN-γ to play a role in host resistance to this organism. In our previous studies, we have shown that in immunocompetent mice the administration of IFN-γ in combination with AmB, but not fluconazole, significantly improves the host's capacity to restrict the proliferation of the organism, especially in the brain, in a synergistic manner (17).
The question of whether IFN-γ would have utility in the treatment of systemic cryptococcosis in a severely immunocompromised host has been addressed in the present studies. We have demonstrated that IFN-γ indeed shows therapeutic efficacy in severely immunodeficient animals. Although the results from the three experiments are not in exact accord with one another, all are indicative of IFN-γ having therapeutic efficacy against systemic cryptococcosis, particularly against meningeal infection. In each experiment, mice given the combination regimen of IFN-γ and AmB carried lower mean burdens of yeast cells in the brain, which is the main target organ in this model, than did untreated controls. Thus, the efficacy of IFN-γ was demonstrated when given alone as well as in combination with conventional AmB therapy. In some instances, the efficacy of sole IFN-γ treatment was not different from that of conventional AmB treatment.
It is important to note that the combination regimens effected complete cures in the greatest number of animals (experiments 1 and 2) and in the greatest number of organs (experiments 1 to 3). However, it should be noted that the cure rates observed in these studies were very likely influenced by the severity of the infections. In the two studies in which few mice succumbed to infection, both AmB and IFN-γ alone or in combination showed efficacy. However, in the rapidly fatal disease established in the third experiment, fewer cures of mice or individual organs occurred. Thus, the question of efficacy in the setting of severe immunodeficiency with meningeal disease was best answered by the results from the third experiment, in which significant synergistic efficacy in prolongation of survival as well as clearing of brain infection was observed. This increased efficacy was not limited to the brain but was found in all other organs except the spleen. The greater severity of infection in the third experiment also reduced the apparent efficacy of sole AmB or sole IFN-γ therapy and allowed for a clearer demonstration of the increased activity of the combination regimen.
The results of this study showing efficacy of treatment by IFN-γ alone are in contrast to previous data for normal mice which indicated that IFN-γ given alone was unable to cause a significant reduction in organism burden in any organ (15, 17; K. V. Clemons and D. A. Stevens, XIII Int. Soc. Human Animal Mycoses, abstr. S89, p. 63, 1997). One possible explanation for this difference likely is related to the use in the present study of immunodeficient SCID mice, which are known to have lower naturally occurring levels of IFN-γ due to T-cell defects and thus probably respond to a larger degree to the exogenous IFN-γ.
Overall, these studies are suggestive of the potential use of immunomodulation using IFN-γ as an adjunctive therapy against cryptococcosis. These results provide a rationale for IFN-γ therapy in the immunocompromised patient.
ACKNOWLEDGMENT
These studies were funded in part by a grant from Genentech, Inc.
REFERENCES
- 1.Aguirre K, Havell E A, Gibson G W, Johnson L L. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformans in the central nervous system of mice. Infect Immun. 1995;63:1725–1731. doi: 10.1128/iai.63.5.1725-1731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brummer E, Nassar F, Stevens D A. Effect of macrophage colony-stimulating factor on anticryptococcal activity of bronchoalveolar macrophages: synergy with fluconazole for killing. Antimicrob Agents Chemother. 1994;38:2158–2161. doi: 10.1128/aac.38.9.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brummer E, Stevens D A. Macrophage colony-stimulating factor induction of enhanced macrophage anticryptococcal activity: synergy with fluconazole for killing. J Infect Dis. 1994;170:173–179. doi: 10.1093/infdis/170.1.173. [DOI] [PubMed] [Google Scholar]
- 4.Clemons K V, Azzi R, Stevens D A. Experimental systemic cryptococcosis in SCID mice. J Med Vet Mycol. 1996;34:331–335. doi: 10.1080/02681219680000561. [DOI] [PubMed] [Google Scholar]
- 5.Clemons K V, Brummer E, Stevens D A. Cytokine treatment of central nervous system infection: efficacy of interleukin-12 alone and synergy with conventional antifungal therapy in experimental cryptococcosis. Antimicrob Agents Chemother. 1994;38:460–464. doi: 10.1128/aac.38.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemons K V, Stevens D A. Comparison of Fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosis. Antimicrob Agents Chemother. 1998;42:899–902. doi: 10.1128/aac.42.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decken K, Köhler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately M K, Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denning D W, Tucker R M, Hanson L H, Hamilton J R, Stevens D A. Itraconazole therapy for cryptococcal meningitis and cryptococcosis. Arch Intern Med. 1989;149:2301–2308. [PubMed] [Google Scholar]
- 9.Denning D W, Tucker R M, Hanson L H, Stevens D A. Itraconazole in opportunistic mycoses: cryptococcosis and aspergillosis. J Am Acad Dermatol. 1990;23:602–607. doi: 10.1016/0190-9622(90)70262-g. [DOI] [PubMed] [Google Scholar]
- 10.Harrison T S, Levitz S M. Role of IL-12 in peripheral blood mononuclear cell responses to fungi in persons with and without HIV infection. J Immunol. 1996;156:4492–4497. [PubMed] [Google Scholar]
- 11.Hoag K A, Lipscomb M F, Izzo A A, Street N E. IL-12 and IFN-γ-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 12.Hostetler J, Denning D W, Stevens D A. US experience with itraconazole in Aspergillus, Cryptococcus and Histoplasma infections in the immunocompromised host. Chemotherapy. 1992;38(Suppl. 1):12–22. doi: 10.1159/000239048. [DOI] [PubMed] [Google Scholar]
- 13.Hostetler J S, Clemons K V, Hanson L H, Stevens D A. Efficacy and safety of amphotericin B colloidal dispersion compared with those of amphotericin B deoxycholate suspension for treatment of disseminated murine cryptococcosis. Antimicrob Agents Chemother. 1992;36:2656–2660. doi: 10.1128/aac.36.12.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hostetler J S, Hanson L H, Stevens D A. Effect of hydroxypropyl-β-cyclodextrin on efficacy of oral itraconazole in disseminated murine cryptococcosis. J Antimicrob Chemother. 1993;32:459–463. doi: 10.1093/jac/32.3.459. [DOI] [PubMed] [Google Scholar]
- 15.Joly V, Saint-Julien L, Carbon C, Yeni P. In vivo activity of interferon-gamma in combination with amphotericin B in the treatment of experimental cryptococcosis. J Infect Dis. 1994;170:1331–1334. doi: 10.1093/infdis/170.5.1331. [DOI] [PubMed] [Google Scholar]
- 16.Lipovsky M M, Juliana A E, Gekker G, Hu S, Hoepelman A I M, Peterson P K. Effect of cytokines on anticryptococcal activity of human microglial cells. Clin Diagn Lab Immunol. 1998;5:410–411. doi: 10.1128/cdli.5.3.410-411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz J E, Clemons K V, Stevens D A. Enhancement of antifungal chemotherapy by interferon-gamma in experimental systemic cryptococcosis. J Antimicrob Chemother. 2000;46:437–442. doi: 10.1093/jac/46.3.437. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mody C H, Tyler C L, Sitrin R G, Jackson C, Toews G B. Interferon-γactivates rat alveolar macrophages for anticryptococcal activity. Am J Respir Cell Mol Biol. 1991;5:19–26. doi: 10.1165/ajrcmb/5.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Nassar F, Brummer E, Stevens D A. Effect of in vivo macrophage colony-stimulating factor on fungistasis of bronchoalveolar and peritoneal macrophages against Cryptococcus neoformans. Antimicrob Agents Chemother. 1994;38:2162–2164. doi: 10.1128/aac.38.9.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nassar F, Brummer E, Stevens D A. Macrophage colony-stimulating factor (M-CSF) induction of enhanced anticryptococcal activity in human monocyte-derived macrophages: synergy with fluconazole for killing. Cell Immunol. 1995;164:113–118. doi: 10.1006/cimm.1995.1149. [DOI] [PubMed] [Google Scholar]
- 22.Salkowski C A, Balish E. A monoclonal antibody to gamma interferon blocks augmentation of natural killer cell activity induced during systemic cryptococcosis. Infect Immun. 1991;59:486–493. doi: 10.1128/iai.59.2.486-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens D A, Domer J E, Ashman R B, Blackstock R, Brummer E. Immunomodulation in mycoses. J Med Vet Mycol. 1994;32(Suppl. 1):253–265. doi: 10.1080/02681219480000881. [DOI] [PubMed] [Google Scholar]
- 24.Zhang T, Kawakami K, Qureshi M H, Okamura H, Kurimoto M, Saito A. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–3599. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]