Abstract
Background
People living with HIV (PLWH) are at increased risk of re-activation of latent tuberculosis infection (LTBI). Although UK and international guidelines identify this group as a priority for LTBI screening and treatment, data on attitudes of PLWH to this policy recommendation are lacking.
Methods
A five-point, Likert-style questionnaire was administered to PLWH to assess views and intentions towards accepting LTBI screening and treatment. Subsequent interferon-γ release assay (IGRA) testing was offered, and chemoprophylaxis if required. Influencing demographic and psychological associations with planned, and actual, testing and treatment uptake were assessed using multivariable logistic regression.
Results
444 out of 716 (62%) patients responded. 417 out of 437 (95.4%) expressed intention to accept LTBI testing. The only significant association was the perceived importance of testing to the individual (adjusted odds ratio (aOR) 8.98, 95% CI 2.55–31.67). 390 out of 393 (99.2%) accepted appropriate IGRA screening; 41 out of 390 (10.5%) were positive. 397 out of 431 (92.1%) expressed intention to accept chemoprophylaxis, associated with perceived importance of treatment (aOR 3.52, 95% CI 1.46–8.51), a desire to have treatment for LTBI (aOR 1.77, 95% CI 0.99–3.15) and confidence in taking treatment (aOR 3.77, 95% CI 1.84–7.72). Of those offered chemoprophylaxis, 36 out of 37 (97.3%) accepted and 34 out of 36 (94.4%) completed treatment. There were no correlates with actual screening acceptance.
Conclusions
LTBI is common amongst PLWH, highlighting the importance of robust screening and treatment programmes. This study shows that screening and treatment for LTBI is highly acceptable to PLWH and provides strong, objective evidence for policy-makers developing guidelines in this cohort.
Short abstract
This detailed exploration of the views of people living with HIV in the UK through a prospective questionnaire cohort study is the first of its kind in the published literature, and shows that latent TB screening and treatment is overwhelmingly supported https://bit.ly/3HmhmRy
Introduction
Despite advances in, and the widespread use of, highly active antiretroviral therapy (HAART), people living with HIV infection (PLWH) continue to be at an increased risk of developing active tuberculosis (TB) as a result of re-activation of latent tuberculosis infection (LTBI) [1, 2]. Globally the risk of developing TB is up to 30 times higher for PLWH than for those who are HIV negative [3], and increased mortality is noted even in low TB incidence countries [4]. In order to reduce the risk of active TB in this population, LTBI screening and treatment with chemoprophylaxis [3, 5] are prioritised for PLWH in national and international guidelines [6–9]. However, our previous work has shown that in the UK there remains significant heterogeneity in LTBI screening of PLWH [10].
Despite the consensus on LTBI diagnosis and prevention in this population, there is little data on the factors determining planned and actual behaviour with respect to LTBI testing and treatment uptake in PLWH. Information such as this is highly critical and of direct relevance to policy-makers and clinicians if LTBI screening is to be scaled up and implemented into routine clinical, programmatic practice.
Therefore, we aimed to address this gap in the evidence-base by prospectively evaluating the planned, and actual, behaviours of PLWH, with regard to LTBI testing and treatment.
Methods
We undertook a prospective study at two HIV clinics in the UK (University Hospitals Leicester NHS Trust, Leicester, and Coventry and Warwickshire Partnership NHS Trust, Coventry). Potential participants were given a personalised invitation letter, a study information leaflet, and a booklet that explained TB and LTBI, interferon-γ release assay (IGRA) testing, chemoprophylaxis and potential side-effects. Patients were asked to read these documents and then complete the questionnaire if they wished to participate. All patients were subsequently offered IGRA testing, and LTBI chemoprophylaxis, where clinically appropriate.
Study population, participants and methods of screening
Between April 2014 and September 2017 (inclusive), PLWH who were attending HIV services at one of the two participating centres were eligible to take part. Inclusion criteria were: male and female adult patients (≥16 years); currently receiving care for HIV from one of the two centres; from all ethnic backgrounds; irrespective of antiretroviral treatment or other medical comorbidities. In Leicester, patients with any CD4 count were included. In Coventry, only patients with CD4 counts below 500 were included, as funding for IGRA testing in that centre was dependent upon individuals meeting the National Institute for Health and Care Excellence (NICE) guidance in force at the time, which recommended screening only at CD4 counts below 500 [11]. We excluded: known previous diagnosis of active or latent TB; residence in prison; a lack of capacity to consent to participate; non-English speakers and/or those unable to read English where no translator was available in clinic; or where the treating clinician felt that participation would be detrimental to the wellbeing of the patient (such as acute distress associated with HIV diagnosis).
All sequential, eligible patients were approached as they attended for clinic follow-up, with no randomisation. The participant information leaflet made it apparent that individuals would be offered screening irrespective of whether they wished to participate in the questionnaire study.
Questionnaire design
An elicitation study to determine possible beliefs around LTBI testing and treatment was not performed. However, other studies on LTBI chemoprophylaxis were examined and common themes extracted, forming the basis of our questionnaire (available in the supplementary material), which was designed around the psychological model of behaviour change, the Theory of Planned Behaviour. This model proposes that the intention of an individual to carry out certain behaviours is dependent upon three variables: the individual's overall attitude towards the behaviour; the actual or perceived influence of others (subjective norms); and the perceived control that the individual feels that they have over the behaviour. The theory has been widely utilised to investigate the acceptability of other health-related interventions [12–15].
The questionnaire asked a series of questions pertaining to the planned intentions of accepting screening/treatment, and potential influencing factors which had been identified from the literature. A five-point Likert-scale ranging from 1 (Strongly agree) through 2 (Agree), 3 (Uncertain), 4 (Disagree) and 5 (Strongly disagree) was used. Additional demographic questions and whether individuals had previously been diagnosed with, or treated for, active or latent TB were included. There was a free text box to enable simple thematic analysis of general comments related to LTBI testing and treatment. Readability indices were obtained for written patient materials and all scored between 7.3 and 8.6 on the Flesch–Kincaid Grade.
Data collection
Demographic data were collected from the questionnaire and hospital records. We used a slightly modified version of the World Bank analytical grouping [16] to cohort countries together into specific regions of birth (all patients in the Europe/Central Asia group were actually from Europe). Ethnicity was coded according to the national NHS data dictionary [17].
IGRA testing and clinical management of positive results
IGRA testing varied slightly between the two sites according to which tests were available locally. Coventry used T-SPOT®.TB tests for all patients, whereas Leicester used QuantiFERON-TB® Gold In-Tube Test (QFN-GIT) (and more latterly QuantiFERON-TB® Gold Plus (QFT-Plus)) for those with CD4 counts ≥200, and T-SPOT®.TB tests in conjunction with QuantiFERON-TB® tests for those with CD4 counts <200. All positive or borderline positive tests were classified as being positive, and clinical review took place before LTBI treatment was offered. The choice of treatment regime was determined by the treating clinician and was either 6 months of isoniazid monotherapy (32 out of 36 patients) or 3 months of rifampicin/isoniazid (4 out of 36 patients). Clinicians were blinded to the questionnaire responses.
Statistical analysis
Assuming an estimated total population size of 1000 subjects meeting the inclusion criteria, of whom a hypothesised proportion of 50% indicate that they would accept screening for LTBI (with a margin of error of 5% around this proportion) and a design effect (deff) of 1, a total of 278 subjects would be required to be recruited. Assuming a response rate of 50%, we would inflate the sample size to 556.
Demographic characteristics were summarised using median for age and proportions/percentages for categorical variables; comparisons were made using the non-parametric Mann–Whitney U-test and Pearson's chi-square test (or Fishers exact test if appropriate), respectively.
Responses to individual Likert-scale questions were excluded if none, or more than one, of the response options was ticked. We described the distribution of responses as proportions to gain an overall understanding of the views and beliefs of patients with respect to LTBI screening and treatment. Factors including age, sex, region of birth, known TB contact and responses to the relevant Likert-style questions were tested for their association with the planned intention and actual behaviour to take up LTBI testing and treatment, using separate logistic regression models after amalgamating “strongly agree” and “agree” into one group, and the remainder into a separate group. Univariable and multivariable associations of demographic and influencing factors from the Theory of Planned Behaviour domains associated with testing or treatment for LTBI were reported as crude, and adjusted odds ratios (aOR) and 95% confidence intervals. Multivariable models were adjusted for factors associated with the relevant outcome in univariable logistic regression models (p≤0.05). Statistical analyses were performed using Stata v14.0 (2015; StataCorp, College Station, TX, USA).
Consent and ethics approval
Ethics approval was granted by the National Research Ethics Service (NRES) Committee North West – Greater Manchester South (reference number 13/NW/0895). As per our ethics approval, consent was presumed if the patient completed at least one question on the questionnaire [18] and returned it.
Results
Response rate
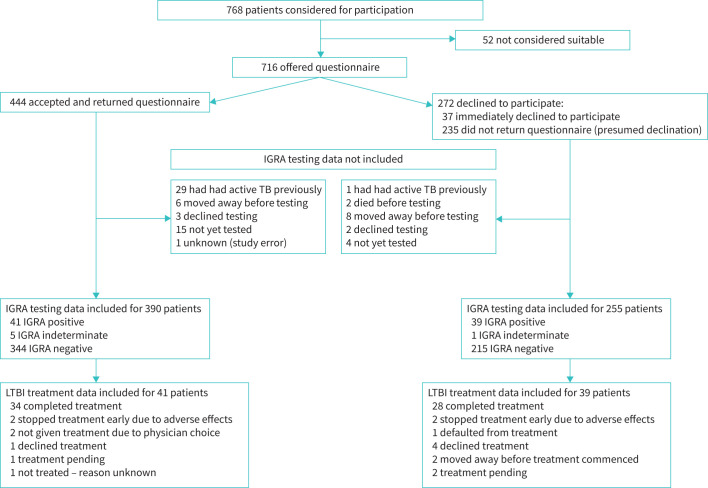
716 individuals were offered the questionnaire. 272 out of 716 (38%) declined immediately or failed to return the questionnaire and were presumed to have declined to participate (figure 1). Overall 444 out of 716 (62%) participated. Responses to the Likert-scale questions are available in the supplementary material.
FIGURE 1.
Flowchart of questionnaire participation and outcomes of latent tuberculosis infection (LTBI) screening and treatment. IGRA: interferon-γ release assay; TB: tuberculosis; LTBI: latent tuberculosis infection.
Language translator
Only four respondents indicated that they had needed the assistance of a translator. An English speaker was required by a further three respondents to help them understand and complete the questionnaire.
Demographics
Table 1 details the demographic data of the questionnaire respondents and non-respondents. 52.5% were black African and the cohort was relatively young, with median age 42 years. There was no significant difference between the cohorts in terms of age, sex or ethnicity.
TABLE 1.
Demographics for questionnaire respondents and non-respondents
| Variable | Questionnaire accepted | Questionnaire declined | p-value |
| Subjects n | 444 | 272 | |
| Male sex | 239 (53.8) | 140 (51.5) | 0.539 |
| Median age | 42 | 43 | 0.229 |
| Ethnicity | |||
| White | 150 (33.8) | 77 (28.3) | 0.125 |
| Black | 234 (52.7) | 163 (60) | 0.056 |
| Asian | 39 (8.8) | 28 (10.3) | 0.504 |
| Mixed/other | 21 (4.7) | 4 (1.5) | 0.023 |
| Country of birth # | |||
| Sub-Saharan Africa | 249 (57.11) | - | |
| Europe | 159 (36.47) | - | |
| South Asia | 17 (3.9) | - | |
| Other | 11 (2.52) | - | |
| Reported previous TB ¶ | 29 (6.5) | - | |
| Reported previous LTBI | 0 (0) | - | |
| Acceptance of appropriate IGRA testing | 390/393 (99.2) | 255/257 (99.2) | 0.9483 |
| Acceptance of LTBI treatment if LTBI diagnosed and treatment advised | 36/37 (97.3) | 31/35 (88.6) | 0.15 |
| Completion of treatment if LTBI treatment started | 34/36 (94.4) | 28/31 (90.3) | 0.5 |
Data expressed as n (%) unless otherwise indicated. TB: tuberculosis; LTBI: latent tuberculosis infection; IGRA: interferon-γ release assay. “Other” includes East Asia and Pacific, Latin America and Caribbean, Middle East and North Africa, and North America. #: 436 respondents stated their country of birth. ¶: excluded from IGRA testing.
Previous TB/LTBI
29 out of 444 (6.5%) reported that they had been previously treated for active TB, although this information had not been recorded in their medical notes. These individuals were excluded from IGRA testing. No respondents indicated that they had had prior LTBI diagnosis or treatment.
Views about, and intention to accept, IGRA testing
Intention to accept LTBI screening was tested by the response to the first questionnaire statement, “I plan to accept a blood test for latent TB”. 417 out of 437 (95.4%) strongly agreed or agreed with this, and the result was similar (390 out of 408, 95.6%) after excluding those with previous active TB.
There was agreement that it was important to test and important to know whether one had LTBI by 393 out of 435 (90.3%) and 412 out of 433 (95.2%), respectively, and a desire to know whether they had LTBI was expressed by 402 out of 429 (93.7%). However, only 107 out of 422 (25.4%) agreed with the statement that they were at risk of having been exposed to TB previously.
There was a hierarchy in the proportions of respondents who felt that other individuals would wish them to undergo testing, ranging from 307 out of 430 (71.4%) for their clinic doctor, to 232 out of 427 (54.3%) for other significant individuals in their life, down to 165 out of 428 (38.6%) for others attending the same clinic. High proportions of individuals expressed uncertainty as to whether these individuals would wish them to test. 99 out of 424 (23.3%) felt that they would experience prejudice if they underwent testing.
There was high agreement with the concept of having control over the testing process and being able to decline the test (392 out of 435 (90.1%) and 395 out of 434 (91%), respectively).
Views about, and intention to accept, LTBI chemoprophylaxis
Intention to accept chemoprophylaxis (if advised) was tested by the response to the twelfth questionnaire statement, “I plan to take treatment for latent TB…” and agreed to by 397 out of 431 (92.1%). Similar to the intention to undergo testing, there was high agreement that treatment was important (415 out of 432, 96.1%), but low agreement with the statement that they were at risk of developing active TB (101 out of 407, 24.8%). The behavioural norms trends were also similar to the intention to undergo testing, with the respondents agreeing that their clinic doctor, other significant individuals and other clinic patients would wish them to receive treatment in 388 out of 431 (90%), 318 out of 425 (74.8%) and 233 out of 429 (54.3%) cases, respectively. Concern about prejudice was low at 90 out of 419 (21.5%) and perceived control over treatment declination was high at 401 out of 429 (93.5%).
Factors associated with intention to accept LTBI testing and treatment
Factors significant on univariable analysis were taken forward into multivariable analysis. The only significant association with the intention to accept LTBI testing (table 2) was the importance of testing to the individual (aOR 8.98, 95% CI 2.55–31.67).
TABLE 2.
Univariate and multivariable regression analysis for factors associated with intention to accept latent tuberculosis (TB) infection screening
| Variable | Intent to accept testing | Unadjusted OR (95% CI) (univariate analysis) | p-value | Adjusted OR (95% CI) (multivariable analysis) | p-value |
| Age | 1.03 (0.98–1.08) | 0.21 | |||
|
Sex Male Female |
225/235 (95.74) | 1 | |||
| 192/202 (95.05) | 0.85 (0.35–2.09) | 0.73 | |||
|
World Bank region of birth Europe Sub-Saharan Africa South Asia and Other |
154/158 (97.47) | 1 | |||
| 230/243 (94.65) | 0.46 (0.15–1.44) | 0.18 | |||
| 27/28 (96.43) | 0.70 (0.08–6.52) | 0.76 | |||
|
Known TB contact No Yes |
317/332 (95.48) | 1 (1–1) | |||
| 89/92 (96.74) | 1.40 (0.40–4.96) | 0.60 | |||
| It is important that I have a blood test for latent TB | 21.22 (7.66–58.79) | <0.001 | 8.98 (2.55–31.67) | 0.001 | |
| It is important that I know whether I have latent TB or not | 9.16 (4.30–19.53) | <0.001 | 1.76 (0.53–5.81) | 0.36 | |
| I am at risk of having caught TB in the past | 1.77 (1.12–2.79) | 0.01 | 0.90 (0.43–1.89) | 0.78 | |
| I want to know whether I have latent TB | 7.63 (3.77–15.44) | <0.001 | 2.27 (0.66–7.76) | 0.19 | |
| My clinic doctor would expect me to be tested for latent TB | 3.96 (2.37–6.60) | <0.001 | 1.69 (0.57–5.01) | 0.35 | |
| Other people attending the clinic would expect me to be tested for latent TB | 2.10 (1.35–3.27) | 0.001 | 0.70 (0.24–2.02) | 0.51 | |
| I know people who would be prejudiced against me if I had a test for latent TB | 1.33 (0.87–2.02) | 0.19 | |||
| Other significant people in my life would expect me to be tested for latent TB | 1.99 (1.34–2.95) | 0.001 | 1.40 (0.67–2.94) | 0.37 | |
| I would feel able to tell my doctor if I did not want to have a test for latent TB | 1.63 (1.06–2.50) | 0.027 | 1.00 (0.26–3.88) | 0.10 | |
| It is up to me whether or not to have a test for latent TB | 1.65 (1.14–2.39) | 0.007 | 1.25 (0.41–3.81) | 0.70 |
Data expressed as N/n (%) unless otherwise indicated.
Intention to accept chemoprophylaxis (table 3) was determined by the belief that treatment was important (aOR 3.52, 95% CI 1.46–8.51), a desire to have treatment for LTBI (aOR 1.77, 95% CI 0.99–3.15) and being confident in the ability to take treatment (aOR 3.77, 95% CI 1.84–7.72).
TABLE 3.
Univariate and multivariable regression analysis for intention to accept latent tuberculosis (TB) infection treatment
| Variable | Intent to accept treatment | Unadjusted OR (95% CI) (univariate analysis) | p-value | Adjusted OR (95% CI) (multivariable analysis) | p-value |
| Age | 1.01 (0.97–1.05) | 0.51 | |||
|
Sex Male Female |
221/234 (94.44) | 1 | |||
| 176/197 (89.94) | 0.49 (0.24–1.01) | 0.05 | 0.70 (0.29–1.72) | 0.44 | |
|
World Bank region of birth Europe Sub-Saharan Africa South Asia and Other |
148/157 (94.27) | 1 | |||
| 220/241 (91.29) | 0.64 (0.28–1.43) | 0.27 | |||
| 26/27 (96.30) | 1.58 (0.19–13.00) | 0.67 | |||
|
Known TB contact No Yes |
304/331 (91.84) | 1 | |||
| 82/89 (92.13) | 1.04 (0.44–2.47) | 0.93 | |||
| I am at risk of developing active TB | 2.08 (1.40–3.10) | <0.001 | 1.46 (0.86–2.50) | 0.17 | |
| It is important for me to have treatment if I have latent TB | 5.82 (3.23–10.51) | <0.001 | 3.52 (1.46–8.51) | 0.005 | |
| I want to have treatment for latent TB | 3.31 (2.22–4.95) | <0.001 | 1.77 (0.99–3.15) | 0.05 | |
| My clinic doctor would expect me to take treatment for latent TB if she/he recommended it | 4.09 (2.49–6.71) | <0.001 | 1.66 (0.65–4.28) | 0.29 | |
| Other people attending the clinic would expect me to take treatment for latent TB | 1.75 (1.26–2.43) | 0.001 | 0.60 (0.208–1.31) | 0.20 | |
| Other significant people in my life would expect me to take treatment for latent TB | 2.05 (1.51–2.79) | <0.001 | 0.97 (0.47–1.99) | 0.93 | |
| I know people who would be prejudiced against me if I took treatment for latent TB | 1.14 (0.83–1.57) | 0.41 | |||
| I am confident that I could take the tablets every day for 6 months | 5.54 (3.27–9.39) | <0.001 | 3.77 (1.84–7.72) | <0.001 | |
| Knowing the possible side-effects of the tablets makes it more difficult for me to decide about taking the treatment | 0.82 (0.60–1.13) | 0.23 | |||
| It is up to me to decide whether or not to have this treatment | 0.92 (0.60–1.41) | 0.71 | |||
| I would feel able to tell my doctor if I did not want to have this treatment | 0.92 (0.54–1.545) | 0.75 | |||
| Being pregnant or trying to get pregnant makes it more difficult for me to decide about taking the treatment (leave this question blank if not appropriate) | 0.58 | ||||
| Females only analysed (n=75) | 0.85 (0.47–1.53) |
Data expressed as N/n (%) unless otherwise indicated.
Actual LTBI testing, cascade of care and treatment uptake
IGRA testing uptake amongst questionnaire respondents was 390 out of 393 (99.2%) for whom testing was appropriate. Questionnaire respondents and non-respondents did not differ in acceptance of IGRA testing (p=0.95) (table 1).
41 out of 390 (10.5%) IGRA screened respondents had a positive IGRA and 40 out of 41 (97.5%) were diagnosed with LTBI after clinical and radiological assessment. 34 out of 36 (94.4%) of those offered treatment completed chemoprophylaxis as determined by the treating clinician.
Questionnaire respondents and non-respondents did not differ in their acceptance of LTBI treatment (if offered), or completion of treatment (p=0.15 and p=0.5, respectively) (table 1).
Factors associated with actual LTBI testing and treatment uptake
Acceptance of LTBI testing was not associated with any demographic factor or questionnaire response on multivariable regression analysis (table 4). The numbers accepting and completing chemoprophylaxis were too small to enable any meaningful regression analysis to be conducted.
TABLE 4.
Univariate and multivariable regression analysis for factors associated with actual acceptance of latent tuberculosis (TB) infection screening
| Variable | Tested | Unadjusted OR (95% CI) (univariate analysis) | p-value | Adjusted OR (95% CI) (multivariable analysis) | p-value |
| Age | 1.03 (0.98–1.07) | 0.26 | |||
| Sex | |||||
| Male | 215/227 (94.71) | 1 | |||
| Female | 175/188 (93.09) | 0.75 (0.33–1.69) | 0.49 | ||
| World Bank region of birth | |||||
| Europe | 146/156 (93.59) | 1 | |||
| Sub-Saharan Africa | 215/225 (95.56) | 1.47 (0.60–3.63) | 0.40 | ||
| South Asia and Other | 22/26 (84.62) | 0.38 (0.11–1.31) | 0.12 | ||
| Known TB contact | |||||
| No | 299/319 (93.73) | 1 | |||
| Yes | 79/84 (94.05) | 1.06 (0.38–2.90) | 0.92 | ||
| I plan to accept a blood test for latent TB | 1.73 (1.05–2.83) | 0.03 | 0.82 (0.34–2.00) | 0.66 | |
| It is important that I have a blood test for latent TB | 1.72 (1.08–2.75) | 0.02 | 0.80 (0.31–2.12) | 0.66 | |
| It is important that I know whether I have latent TB or not | 2.04 (1.22–3.41) | 0.006 | 1.26 (0.49–3.24) | 0.63 | |
| I am at risk of having caught TB in the past | 1.26 (0.83–1.89) | 0.28 | |||
| I want to know whether I have latent TB | 2.41 (1.51–3.85) | <0.001 | 1.93 (0.92–4.05) | 0.08 | |
| My clinic doctor would expect me to be tested for latent TB | 1.77 (1.18–2.67) | 0.006 | 1.44 (0.85–2.43) | 0.18 | |
| Other people attending the clinic would expect me to be tested for latent TB | 1.31 (0.90–1.93) | 0.16 | |||
| I know people who would be prejudiced against me if I had a test for latent TB | 1.21 (0.83–1.75) | 0.32 | |||
| Other significant people in my life would expect me to be tested for latent TB | 1.36 (0.96–1.92) | 0.08 | |||
| I would feel able to tell my doctor if I did not want to have a test for latent TB | 1.33 (0.87–2.04) | 0.19 | |||
| It is up to me whether or not to have a test for latent TB | 1.55 (1.09–2.21) | 0.02 | 1.36(0.88–2.11) | 0.17 |
Data expressed as N/n (%) unless otherwise indicated.
Comments from respondents
69 individuals wrote comments in the free text box, which were grouped into themes (data not shown). 33 comments were supportive of testing and treatment; 12 indicated a desire for more information on some aspect of LTBI diagnosis/management; and 20 were comments of a general nature, mostly describing personal health experiences. Only four comments were not supportive of testing or treatment.
Discussion
We explored, through the first study in this field, the intentions of PLWH towards accepting testing and treatment for LTBI through the provision of patient information, implementing a questionnaire study and offering IGRA testing. We demonstrated that LTBI screening and treatment is highly acceptable to PLWH in the UK, thereby underpinning the expansion of LTBI screening and treatment as an important public health intervention to reduce TB-associated morbidity and mortality in this high-risk population across many different settings. LTBI prevalence in this study was high, at over 10%, highlighting a real need to ensure robust screening pathways are introduced as part of clinical care. We have previously demonstrated that there is currently limited LTBI screening occurring amongst PLWH in the UK [10]. This is the first study to examine these issues in any real detail, although a range of LTBI chemoprophylaxis acceptance rates of between 17 and 85% in PLWH have been reported from elsewhere in the UK and other low TB incidence countries [19–21].
In our cohort, LTBI testing and chemoprophylaxis uptake was very high. Over 99% of questionnaire respondents accepted IGRA testing, and over 94% of those who were consequently diagnosed with LTBI and for whom chemoprophylaxis was recommended successfully completed treatment. There was reassuringly no statistical difference between patients who participated in the study and those who declined to participate, in terms of acceptance of LTBI testing and chemoprophylaxis uptake. This indicates that our study cohort is representative of our wider HIV cohort. We have demonstrated that operationalising this screening is feasible as part of routine care in the UK, and that it is possible to do this without sustaining the losses noted by others during the cascade of care for LTBI screening and treatment [22].
It is likely that there were multifactorial reasons behind these results, including the fact that all patients were attending regular follow-up in our clinics, screening had been integrated into clinical pathways, and many of our HIV clinicians are infectious diseases clinicians experienced in TB and LTBI management. It is important that all HIV clinicians receive training in the diagnosis and treatment of LTBI as part of the expansion of screening.
Interestingly, 6% of individuals reported having had active TB previously, although this information was not documented in their medical notes. Almost all had had treatment for TB overseas, prior to their arrival in the UK. This serves to highlight the importance of ensuring that a complete medical history is taken by clinics at the point of initiating care of PLWH, in order to avoid unnecessary IGRA testing or inappropriate treatment.
We did not find that the intention to accept screening was influenced by subjective norms, including fear of prejudice, although this has been reported previously [23], or by perceived control over the testing process. Modern approaches to care in terms of shared clinician–patient clinical decision-making may influence the latter. A perception that the testing process was inherently important influenced both the intent to test and to accept chemoprophylaxis. Whether this stems from a true belief that TB is an important disease, or simply because study information had been provided, is unknown.
Notably, low numbers of respondents felt that they were at risk of TB exposure, or of developing active TB, despite the well-known risks in HIV infection [1, 2, 4]. Interestingly, despite this view, high numbers did ultimately accept screening. No specific factors influenced IGRA testing acceptance. There may be some other, unexplored influence, such as the personal interaction with a clinician and a further qualitative study to examine whether, and why, attitudes alter after the clinical encounter, would be interesting. Provision of specific educational materials to enhance understanding of TB epidemiology might also be useful adjuncts for screening programmes endeavouring to achieve high LTBI screening/treatment outcomes amongst PLWH.
Self-belief in the ability to take LTBI treatment was one of the factors significantly correlated with the intention to accept chemoprophylaxis. Over 95% of our HIV cohort is virologically suppressed on HAART, and therefore the ability to adhere faithfully to daily tablet treatment is already tried and tested. Although an increasing number of daily medication doses has negatively influenced adherence in other studies [24], we demonstrated that over 94% of those given LTBI chemoprophylaxis completed treatment. The only two individuals who did not developed adverse effects from treatment which necessitated cessation. This suggests that PLWH who are offered LTBI treatment are confident and motivated in their adherence to medication, and is an important point to consider when contemplating systematic screening.
One of the limitations of our study was that it only included individuals from two centres in the UK. Over half were from sub-Saharan Africa, although other respondents were from a diverse range of cultural backgrounds. It is possible that slightly different results may have been obtained if we had extended the questionnaire to other HIV centres, either nationally or internationally. The patient literature and questionnaire were written as simply as possible in English, in keeping with guidance from the NHS [25] and the National Institutes of Health (NIH) [26], but we could potentially have had the documents translated into different languages, given our diverse ethnic cohort. We had four patients who required the assistance of language translators. Additionally, three patients required the assistance of an English speaker in order to help them understand and complete the questionnaire. This highlights the importance of verbal counselling, alongside written information, in order to ensure that patients from a wide variety of socioeconomic and educational backgrounds all have the opportunity to provide informed consent for screening.
A further limitation of this study was that we had an overall response rate of only 62% from patients approached to participate. Previous work indicates that response rates may be increased through the use of digital questionnaires, small monetary incentives, provision of a pre-notification letter about the forthcoming questionnaire and a reminder [27–29]. Nevertheless, there were no significant differences in terms of acceptance of screening and treatment between the cohorts of questionnaire respondents and non-respondents, thus suggesting that our study cohort is representative of PLWH who attend for care at our centres.
In our study, meticulous planning and constant oversight of the IGRA testing process ensured that as many individuals as possible were given the opportunity to undergo LTBI screening as part of clinical care. Without this logistical control, we feel that far fewer patients would have undergone LTBI screening, and it is a potential barrier to the same level of screening being replicated elsewhere.
In summary, we have demonstrated that LTBI screening and treatment is viewed positively by PLWH in the UK and that this positive intention translates to high levels of screening and chemoprophylaxis completion rates. This has important consequences for public health and clinical teams implementing more widespread LTBI screening programmes in low TB burden countries. The results of this study provide strong, objective evidence for policy-makers developing guidelines on latent TB prevention in this high-risk cohort.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00442-2021.supplement (170.3KB, pdf)
Acknowledgements
Particular thanks go to the HIV clinical staff at the Leicester Royal Infirmary and the Integrated Sexual Health Service, Coventry, who were supportive of the research and helped with the distribution of the questionnaire, screening and treatment, with special mention of Satyajit Das (Coventry), Jyoti Dhar, Caroline Williams and Adrian Palfreeman (Leicester). Additional thanks go to the laboratory staff of the microbiology and immunology departments of both hospitals for processing the interferon-γ release assay samples.
Provenance: Submitted article, peer reviewed.
This article has supplementary material available from openres.ersjournals.com
Data availability: Anonymised data from this trial is available by reasonable request.
Conflict of interest: H.A. White has nothing to disclose.
Conflict of interest: H. Okhai has nothing to disclose.
Conflict of interest: A. Sahota has nothing to disclose.
Conflict of interest: J. Maltby has nothing to disclose.
Conflict of interest: I. Stephenson has nothing to disclose.
Conflict of interest: H. Patel has nothing to disclose.
Conflict of interest: P.M. Hefford has nothing to disclose.
Conflict of interest: M.J. Wiselka has nothing to disclose.
Conflict of interest: M. Pareek has nothing to disclose.
Support statement: H.A. White received a registrar research award that was coawarded by Gilead/British HIV Association (awarded in 2013). Charitable funds from the University Hospitals of Leicester were received towards the cost of printing the patient questionnaire materials. The cost of interferon-γ release assay testing and latent tuberculosis infection treatment was borne by the HIV departments of both hospitals as part of clinical care. M. Pareek is supported by the National Institute for Health Research (NIHR Post-Doctoral Fellowship PDF-2015-08-102). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Grant AD, Bansi L, Ainsworth J, et al. Tuberculosis among people with HIV infection in the United Kingdom: opportunities for prevention? AIDS 2009; 23: 2507–2515. doi: 10.1097/QAD.0b013e3283320dfd [DOI] [PubMed] [Google Scholar]
- 2.Gupta RK, Rice B, Brown AE, et al. Does antiretroviral therapy reduce HIV-associated tuberculosis incidence to background rates? A national observational cohort study from England, Wales, and Northern Ireland. Lancet HIV 2015; 2: 243–E251. doi: 10.1016/S2352-3018(15)00063-6 [DOI] [PubMed] [Google Scholar]
- 3.Winter JR, Adamu AL, Gupta RK, et al. Tuberculosis infection and disease in people living with HIV in countries with low tuberculosis incidence. Int J Tuberc Lung Dis 2018; 22: 713–722. doi: 10.5588/ijtld.17.0672 [DOI] [PubMed] [Google Scholar]
- 4.Zenner D, Abubakar I, Conti S, et al. Impact of TB on the survival of people living with HIV infection in England, Wales and Northern Ireland. Thorax 2015; 70: 566–573. doi: 10.1136/thoraxjnl-2014-206452 [DOI] [PubMed] [Google Scholar]
- 5.Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2010; 2010: CD000171. doi: 10.1002/14651858.CD000171.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organisation. Towards tuberculosis elimination; an action framework for low incidence countries. 2014. www.who.int/publications/i/item/9789241507707.
- 7.Rosales-Klintz S, Bruchfeld J, Haas W, et al. Guidance for programmatic management of latent tuberculosis infection in the European Union/European Economic Area. Eur Respir J 2019; 53: 1802077. doi: 10.1183/13993003.02077-2018 [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence. Tuberculosis. Prevention, diagnosis, management and service organisation. NG33. 2016. www.nice.org.uk/guidance/ng33 Date last updated: 12 September 2019.
- 9.British HIV Association. British HIV Association guidelines for the management of tuberculosis in adults living with HIV 2018. 2021. www.bhiva.org/TB-guidelines.
- 10.White HA, Miller RF, Pozniak AL, et al. Latent tuberculosis infection screening and treatment in HIV: insights from evaluation of UK practice. Thorax 2017; 72: 180–182. doi: 10.1136/thoraxjnl-2016-209063 [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Clinical Excellence. Tuberculosis – Clinical diagnosis and management of tuberculosis, and measures for its prevention and control. Clinical Guideline 117. 2011. www.nice.org.uk/guidance/cg117.
- 12.Andrew BJ, Mullan BA, de Wit JB, et al. Does the theory of planned behaviour explain condom use behaviour among men who have sex with men? A meta-analytic review of the literature. AIDS Behav 2016; 20: 2834–2844. doi: 10.1007/s10461-016-1314-0 [DOI] [PubMed] [Google Scholar]
- 13.Juraskova I, O'Brien M, Mullan B, et al. HPV vaccination and the effect of information framing on intentions and behaviour: an application of the theory of planned behaviour and moral norm. Int J Behav Med 2012; 19: 518–525. doi: 10.1007/s12529-011-9182-5 [DOI] [PubMed] [Google Scholar]
- 14.Mtenga SM, Exavery A, Kakoko D, et al. Social cognitive determinants of HIV voluntary counselling and testing uptake among married individuals in Dar es Salaam Tanzania: theory of Planned Behaviour (TPB). BMC Public Health 2015; 15: 213. doi: 10.1186/s12889-015-1545-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Zhu Y, Zhang L, et al. What factors influence exclusive breastfeeding based on the theory of planned behaviour. Midwifery 2018; 62: 177–182. doi: 10.1016/j.midw.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 16.The World Bank. How does the World Bank classify countries? datahelpdesk.worldbank.org/knowledgebase/articles/378834-how-does-the-world-bank-classify-countries Date last accessed: 25 June 2018.
- 17.NHS Data Model and Dictionary Service. NHS Data Model and Dictionary Services. Ethnic category code. 2019. www.datadictionary.nhs.uk/data_elements/ethnic_category.html
- 18.National Patient Safety Agency, NHS Health Research Authority. National Patient Safety Agency, NHS Research Ethics Service. Information sheets and consent forms – Guidance for researchers and reviewers. 2011. www.lancashirecare.nhs.uk/media/Publications/R_and_D/Guidance/Template%20Consent%20Forms%20and%20Information%20Sheets.pdf Date last accessed: 25 June 2018.
- 19.Sultan B, Benn P, Mahungu T, et al. Comparison of two interferon-gamma release assays (QuantiFERON-TB Gold In-Tube and T-SPOT.TB) in testing for latent tuberculosis infection among HIV-infected adults. Int J STD AIDS 2013; 24: 775–779. doi: 10.1177/0956462413486459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kall MM, Coyne KM, Garrett NJ, et al. Latent and subclinical tuberculosis in HIV infected patients: a cross-sectional study. BMC Infect Dis 2012; 12: 107. doi: 10.1186/1471-2334-12-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gow N, Briggs S, Nisbet M. Screening for latent tuberculous infection in people living with HIV infection in Auckland, New Zealand. Int J Tuberc Lung Dis 2017; 21: 1008–1012. doi: 10.5588/jtld.17.0103 [DOI] [PubMed] [Google Scholar]
- 22.Alsdurf H, Hill PC, Matteelli A, et al. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16: 1269–1278. doi: 10.1016/S1473-3099(16)30216-X [DOI] [PubMed] [Google Scholar]
- 23.Munseri PJ, Talbot EA, Mtei L, et al. Completion of isoniazid preventive therapy among HIV-infected patients in Tanzania. Int J Tuberc Lung Dis 2008; 12: 1037–1041. [PubMed] [Google Scholar]
- 24.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001; 23: 1296–1310. doi: 10.1016/S0149-2918(01)80109-0 [DOI] [PubMed] [Google Scholar]
- 25.Department of Health. NHS Toolkit for producing patient information. 2003; Version 2.0. https://wessexahsn.org.uk/img/projects/NHS%20Toolkit%20for%20producing%20patient%20information%20v2%20(2003)-1489154340.pdf.
- 26.National Institutes of Health. How to write easy-to-read health materials. 2017. https://www.scribd.com/document/261199628/How-to-Write-Easy-To-Read-Health-Materials-MedlinePlus Date last accessed: 10 November 2016.
- 27.Ebert JF, Huibers L, Christensen B, et al. Paper- or web-based questionnaire invitations as a method for data collection: cross-sectional comparative study of differences in response rate, completeness of data, and financial cost. J Med Internet Res 2018; 20: e24. doi: 10.2196/jmir.8353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koitsalu M, Eklund M, Adolfsson J, et al. Effects of pre-notification, invitation length, questionnaire length and reminder on participation rate: a quasi-randomised controlled trial. BMC Med Res Methodol 2018; 18: 3. doi: 10.1186/s12874-017-0467-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robb KA, Gatting L, Wardle J. What impact do questionnaire length and monetary incentives have on mailed health psychology survey response? Br J Health Psychol 2017; 22: 671–685. doi: 10.1111/bjhp.12239 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00442-2021.supplement (170.3KB, pdf)