Abstract
Pulmonary artery pseudoaneurysms are an uncommon diagnosis and have been minimally described in the coronavirus (COVID-19) literature. In our case, a 31-year-old man presented with severe acute hypoxic respiratory failure, stress cardiomyopathy, and combined septic and cardiogenic shock secondary to COVID-19 pneumonia and Streptococcus anginosus bacteremia. The patient had perfusing granulomas eroding into the pulmonary vasculature, causing impending hemothorax. Thoracic surgical procedures for infectious pulmonary artery pseudoaneurysms or perfusing granulomas in patients who have had COVID-19 should be performed selectively and with thoughtful perioperative planning to prevent the life-threatening complications of rupture and bleeding.
COVID-19–associated coronavirus is known to cause thrombosis and hemorrhage through pathophysiologic mechanisms of endothelial disruption and dysfunction.1 Streptococcus anginosus (SAG), often detected in the mouth, upper respiratory tract, and gastrointestinal tract, is known to produce pulmonary abscesses.2 Male patients with comorbidities are a common cohort in which respiratory SAG infection occurs.3
A previously healthy 31-year-old man presented with severe acute hypoxic respiratory failure, stress cardiomyopathy, and combined septic and cardiogenic shock secondary to COVID-19 pneumonia and SAG bacteremia.
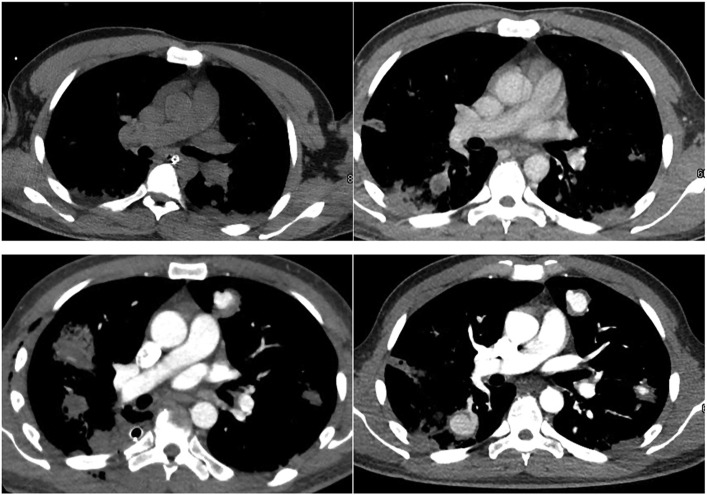
Initial chest computed tomography (CT) scan demonstrated bilateral diffuse nodules, concern for septic emboli with early central cavitation, acute granulomas vs lung abscesses. Subsequent CT angiography showed contrast material filling many of the nodular cavities, concerning for erosion into adjacent pulmonary arteries or pseudoaneurysm formation. The images provided in Figure 1 show the chronologic evolution of the septic emboli over time. These imaging findings are uncommon and have been minimally described in the COVID literature. This case report highlights complex surgical decision-making and brings awareness to the potential for significance of bleeding complications in a critically ill COVID-19 patient with SAG bacteremia and imaging findings of perfusing nodules.
Figure 1.
Chronologic evolution of septic emboli (clockwise from top left).
The patient recovered from stress cardiomyopathy, with normalization of ejection fraction, and hypoxic respiratory failure with minimal oxygen requirements. The persistence and increase in size of the perfusing granulomas brought into question the optimal management of these lesions. The options considered were watchful waiting with serial imaging, prophylactic catheter-directed embolization of expanding and peripheral perfusing nodules, and anatomic parenchymal resection of the lung with the most peripheral and expanding nodules. Watchful waiting was not performed because of concern for free rupture of the peripheral perfused lesions into the pleural cavity. Catheter-directed embolization could not be performed at any level less than a lobar embolization. As a result, we thought that the most prudent course was to undertake an anatomic lung resection of the right lower lobe and the posterior segment of the right upper lobe on the basis of CT angiography.
On hospital day 16, the patient was transported to the operating room and intubated with a left-sided double-lumen endotracheal tube. The patient had a spike in systemic pressures with associated hemoptysis through the endotracheal tube. He became hypoxic with a decrease in oxygen saturation to the upper 80s. Bronchoscopy demonstrated bleeding predominantly in the right upper lobe bronchus. With careful suctioning, cold saline, and dilute epinephrine irrigation, we were able to isolate the bleeding to the posterior segment of the right upper lobe. The bleeding ceased, and we cleared the right tracheobronchial tree of blood. The left tracheobronchial tree remained unaffected by the hemoptysis event. His oxygenation improved, and we monitored him for hemodynamic stability for 30 minutes and then proceeded to position him in the left lateral decubitus position for the right-sided thoracotomy and planned right lower lobectomy with addition of posterior segmentectomy of the right upper lobe.
We performed a posterolateral thoracotomy through the fifth intercostal space with the harvest of an intercostal muscle flap. The right lower lobe reverberated with pulsatility and a thrill. There were many visible nodules on the surface. With minimal lung manipulation, to avoid rupture of perfusing nodules before vascular isolation of the lobe, the fissure over the lower lobe pulmonary artery was opened. The fissural dissection was challenging because of inflammation. The basilar and superior segmental branches to the lower lobe were circumferentially isolated and divided using an Endo GIA vascular stapler (Medtronic). The fissure was then completed posteriorly, and the posterior recurrent pulmonary artery to the upper lobe was identified and ligated. The superior segmental bronchus and the basilar bronchus were divided separately because of their position in relation to the middle lobe. The inferior pulmonary vein was then divided, and an intercostal muscle flap was used to cover the bronchial stump to support healing. A single 28F chest tube was placed, and the thoracotomy was closed in the standard fashion.
The patient was kept intubated overnight and extubated without event the next morning. Postoperatively, the patient remained hemodynamically stable without further hemoptysis. The chest tube was removed by the third postoperative day. Final permanent pathologic examination revealed the vascular lesions to be “perfusing granulomas.” On hospital day 28, the patient was discharged home in good condition and prescribed long-term antibiotics.
Comment
In this case report, we describe a patient with perfusing granulomas eroding into the pulmonary vasculature, causing hemoptysis in the operating room and impending hemothorax. This has been described only once and not in the COVID literature to date.4 , 5 This report highlights complex decision-making and brings awareness to the potentially fatal vascular complications that can occur in the pulmonary system in a critically ill COVID-19 patient with associated bacterial infection with SAG.
The existing clinical correlate to the CT findings in the report is pulmonary artery pseudoaneurysms. Given the paucity of data regarding perfusing granulomas, it would seem prudent to manage them clinically like pulmonary artery pseudoaneurysms. The management of each patient should be individualized. Medical management of COVID-19 with treatment of associated bacterial and fungal causes should be undertaken. Risk stratification of the patient with regard to cardiopulmonary disease severity and baseline comorbidities should be made to establish probability of morbidity and death in conjunction with the risk of operative management of perfusing granulomas. Presentation with hemoptysis or pleural rupture warrants immediate treatment and is likely to have a high mortality.
Serial imaging with contrast-enhanced CT angiography is useful in determining size, location, and progression of the lesions. The frequency of scans is unclear and should vary according to the characteristics of the lesions. Consideration should be given to prompt surgical management of lesions close to the pleural surface or rapidly expanding ones, given risk of life-threatening hemorrhage.
Consultation with the interventional radiology service is important to determine whether embolization could be effective as a less invasive but definitive therapy or as a temporizing treatment in the setting of massive hemorrhage and shock. Surgical resection offers definitive therapy, ranging from segmental resection to lobectomy or pneumonectomy, depending on the distribution of concern for perfused granulomas. Anesthetic and airway management considerations center on feasibility of single-lung ventilation if pulmonary artery pressures rise with lung isolation. Controlling systemic and pulmonary artery pressures at normal or slightly less than normal is important to avoid bleeding complications before vascular control of the affected lung. To that point, minimal lung manipulation is critical until the arterial supply has been controlled.
Lung resection for infectious perfusing granulomas in patients who have had COVID-19 should be performed selectively and with thoughtful perioperative planning to prevent the life-threatening complications of rupture and bleeding.
Acknowledgments
Funding sources
The authors have no funding sources to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Khurram R., Karia P., Naidu V., Quddus A., Woo W.L., Davies N. Pulmonary artery pseudoaneurysm secondary to COVID-19 treated with endovascular embolisation. Eur J Radiol Open. 2021;8:100346. doi: 10.1016/j.ejro.2021.100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang S., Li M., Fu T., Shan F., Jiang L., Shao Z. Clinical characteristics of infections caused by Streptococcus anginosus group. Sci Rep. 2020;10:9032. doi: 10.1038/s41598-020-65977-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noguchi S., Yatera K., Kawanami T., et al. The clinical features of respiratory infections caused by the Streptococcus anginosus group. BMC Pulm Med. 2015;15:133. doi: 10.1186/s12890-015-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeza I., Romera I., Fortuño J.R. Massive hemoptysis due to Aspergillus-related pulmonary artery pseudoaneurysm in a patient with COVID-19 pneumonia. Med Intensiva (Engl Ed) 2023;47:184–185. doi: 10.1016/j.medin.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desnos C., Boussouar S., Hekimian G., Redheuil A., Combes A. Spontaneous hemothorax in 4 COVID-19 ARDS patients on VV-ECMO revealing pulmonary artery aneurysms. Crit Care. 2020;24:638. doi: 10.1186/s13054-020-03359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]