Summary
Human endogenous retrovirus-H long terminal repeat-associating 2 (HHLA2) is a newly emerging immune checkpoint that belongs to B7 family. HHLA2 has a co-stimulatory receptor transmembrane and immunoglobulin domain containing 2 (TMIGD2) and a newly discovered co-inhibitory receptor killer cell Ig-like receptor, three Ig domains, and long cytoplasmic tail (KIR3DL3), which endows it with both immunostimulant and immunosuppression functions in cancer development. In this review, we summarize the HHLA2 expression profile in human cancers, its association with cancer prognosis and clinical features, and its dual roles in regulating cancer immune response through up-to-date literatures. Furthermore, we highlight that precision cancer immunotherapy through manipulating HHLA2-KIR3DL3/TMIGD2 interaction is a promising antitumour strategy.
Keywords: HHLA2, TMIGD2, KIR3DL3, Immune checkpoint, Cancer immunotherapy
Background
Immune checkpoint blockade (ICB) has become a revolutionary approach to antitumour therapy in the past few decades. Via releasing the break signal and potentiating the host's immune system, ICB therapy has been used to treat numerous human cancer types such as melanoma, non-small cell lung cancer, breast cancer, renal cell carcinoma, etc. And it has received prominent responses in a portion of patients.1,2 However, there are still a large amount of patients (80%) with clinically advanced cancers showing primary or secondary resistance in varying degrees to ICB therapy.3 Some patients treated with programmed cell death 1 (PD-1)/programmed death ligand 1 (PD-L1) monoclonal antibodies (mAbs) are reported to acquire hyperprogression.4,5 In addition, although improved quality of life is observed in patients treated with ICB vs. non-ICB regimens, a recent meta-analysis illustrates that drug toxicity and some side effects such as dyspnoea and insomnia from ICB therapy are prevailing and more severe than that caused by conventional oncology agents.6 Therefore, it is urgent to identify alternative effective immune checkpoint pathways for potential antitumour strategies.
Recently, studies on a promising immune checkpoint, Human endogenous retrovirus-H long terminal repeat-associating 2 (HHLA2), are flourishing because its co-inhibitory receptor, killer cell Ig-like receptor, three Ig domains, and long cytoplasmic tail (KIR3DL3), is newly identified.7,8 This makes HHLA2-KIR3DL3 pathway a novel potential target for cancer immunotherapy. HHLA2, also known as B7-H7/B7y, is a type Ι transmembrane protein that belongs to B7 family.9,10 It is initially identified in the process of screening for sequence of human endogenous retroviruses (HERV) long terminal repeat (LTR).11 Since the LTR sequence is integrated in higher primates, HHLA2 has been discovered in primate lineage such as humans, chimpanzees and gorillas, but not in rodents (laboratory mouse and rat), which is unique in B7 family.12 Using sequence analyses, putative HHLA2 orthologs are found to be expressed in a wide range of species, such as Heterocephalus glaber, giant panda, fish, monkey, frog, etc., and may serve evolutionally conserved functions.10
Mainly expressed on the surface of antigen presenting cells (APCs) and tumour cells, HHLA2 plays both positive and negative roles in cancer immune response.10,13 Early studies have illustrated that HHLA2 interacts with transmembrane and immunoglobulin domain containing 2 (TMIGD2, also called CD28H), and serves as an immunostimulatory checkpoint.12 Previously our group, for the first time, have discovered that HHLA2 is a protective factor in pancreatic cancer development using 136 patients’ tissue, and have confirmed that HHLA2-TMIGD2 is a co-stimulatory pathway via in vitro and in vivo experiments.14 However, other in vitro experiments also demonstrate that HHLA2 enhances both CD and CD T cell proliferation and cytokine production, and it is observed in numerous cancer types that high expression of HHLA2 is associated with worse prognoses and pathological conditions.10 It has long been estimated that there is an unknown co-inhibitory HHLA2 receptor balancing HHLA2-TMIGD2 co-stimulatory signal so that HHLA tumours can exhibit distinct immunology features in different cancer types. It is not until recently when Wei et al. and Bhatt et al. independently discovered KIR3DL3 as a co-inhibitory receptor of HHLA2 did scientists eventually complement the research gap of HHLA2-involved immunosuppressive pathway.7,8 Therefore, in this review, we summarize the expression profile and clinical features of HHLA2 based on current literatures, and discuss the dual immunological roles of HHLA2 in human cancer development, highlighting that precise immunotherapeutic targeting of HHLA2 and its receptors may provide promising strategies for malignancy treatment.
Biological structure and expression profile of HHLA2
HHLA2 consists of an N-terminal signal peptide, a transmembrane region, six potential N-linked glycosylation sites, a 49 amino acids in length cytoplasmic tail with no recognizable motif, and three extracellular Ig domains (two IgV domain and one IgC domain), while most other B7 family members contain only one IgC domain and one IgV domain, and B7-H3 has two copies of IgC-IgV domains in succession.9,15 The HHLA2 protein shares significant homology with other human B7 proteins (23–33% amino acid similarity and 10–18% amino acid identity), among which B7x (also called B7-H4/B7S1) and B7-H3 phylogenetically possess the most similarity with HHLA2 and they together form a subgroup (Group III) within the B7 family.10
Using HHLA2 mAbs from mice, it is obvserved that in immune system, HHLA2 is abundantly expressed on CD monocytes, and can be upregulated on B cells by lipopolysaccharide and IFN-γ stimulation. However, for T cells, both CD and CD T cells do not express HHLA2 and are irresponsive after anti-CD3 stimulation.10 Despite the fact that HHLA2 mRNA is widely expressed in human healthy tissues, its protein remains restrictedly expressed, mostly in the epithelium of human kidney, gut, breast and gallbladder, as well as in trophoblastic cells of the placenta.16 On the contrary, HHLA2 protein is broadly observed in human cancers from ovary, breast, thyroid, lung, pancreas, bladder, liver, oesophagus, prostate, kidney, colon and melanoma, with scarce expression on corresponding normal tissues.17
Regulation of HHLA2 expression
Given that HHLA2 is overexpressed on tumour cells of various cancer types with heterogeneous prognostic roles, revealing the molecular mechanisms of HHLA2 expression is critical for inventing new strategies for cancer treatment. Hitherto, regulatory factors of HHLA2 expression can be categorized as gene copy number amplification, epigenetic modification, transcription regulation, and inflammatory stimulation. In breast cancer, HHLA2 gene alterations were observed in 18.8% and 23% of all cases utilizing TCGA, most of which were amplifications or gains of gene copies. Since HHLA2 was upregulated in 56% of a cohort (N = 50) with triple negative breast cancer (TNBC), HHLA2 expression was possibly correlated with gene copy number gain.17 A recent study investigating gene copy number variations of HHLA2 and the kidney renal clear cell carcinoma (KIRC) patients’ prognoses showed that there is no significant association.16 They pointed out that epigenetic modification (such as DNA hypomethylation and post-transcription regulation by microRNAs) may be responsible for the HHLA2 upregulation in KIRC. They also discovered 15 transcription factors involved in the regulation of HHLA2 expression via multiple public databases, and verified that SMAD in monocyte and BATF in B lymphocyte could interact with HHLA2 DNA to potentially regulate HHLA2 expression in KIRC through chip-seq data from Cistrome database.16 HHLA2 gene was differentially methylated in naïve T cells and activated CD T cells, and could be transcriptionally upregulated by CD137 agonist monoclonal antibody through differential DNA methylation.18 In silico analyses also reported that hypomethylation of HHLA2 gene promoter was associated with upregulated HHLA2 expression and favorable prognoses in patients with papillary renal cell carcinoma and KIRC.19, 20, 21 Upon inflammatory stimulation by LPS/IFN-γ, HHLA2 was induced on CDB cells.10 In tumour cells, Wang et al. recently illustrated that HHLA2 could also be upregulated by IFN-γ in hepatocellular carcinoma (HCC).22 Mechanistically, IFN-γ could promote interferon regulatory factor 1 expression, which then transcriptionally activated HHLA2 expression by directly interacting with HHLA2 gene promoter region. By contrast, Bhatt et al. observed that in renal cell carcinoma, HHLA2 expression was not affected by IFN-γ or other cytokines in the tumour microenvironment, suggesting a heterogeneous responses to inflammatory stimulation across different human cancer types.8 Other genes such as LRP1B, CHAC1, and MAGEB5 were reported to be associated with HHLA2 co-expression and co-regulation, yet further experimental confirmations should be done.23, 24, 25 These above studies manifested that HHLA2 expression might be regulated through diverse pathways across various cancer types. Therefore, more in vitro and in vivo experiments should be performed to unveil the pervasive and detailed regulatory mechanisms of HHLA2 expression.
Role of HHLA2 in cancer development
Associations between HHLA2, prognoses, and clinical features
Overexpressed in multiple human cancer types, the prognostic role of HHLA2 remains controversial (Table 1). Several studies indicated that overexpression of HHLA2 in tumour cells was associated with unfavorable clinical outcomes and shorter survival rate in patients with prostate cancer,26 neuroendocrine tumours,27 hepatocellular carcinoma,28, 29, 30 lung adenocarcinoma,31, 32, 33, 34 gastric cancer,35 oral squamous cell carcinoma,36 bladder urothelial carcinoma,37 intrahepatic cholangiocarcinoma,38 colorectal carcinoma,39,40 osteosarcoma,41 and triple negative breast cancer.17 On the contrary, HHLA2 was a protective factor predicting low mortality rate in patients with pancreatic cancer,14,42,43 epithelial ovarian cancer,44 malignant glioma,45 and recurrent or unresectable advanced gastric cancer.46 The discrepancy could plausibly result from the dual role of HHLA2 as both inhibitory and stimulatory immune checkpoint that switch to predominant in different cancer types, further affecting the clinical outcome. The paradoxical clinical outcomes could be observed even in the same type of cancer such as gastric cancer and KIRC. In terms of gastric cancer, Shimonosono et al. only detected HHLA2 mRNA expression levels in patients’ blood samples, while Wei et al. detected both HHLA2 mRNA and protein expression levels in patients’ tumour samples.35,46 This may explain the different outcomes of HHLA2 prognostic predictions. In terms of KIRC, Chen et al. reported that higher HHLA2 level was associated with poorer overall survival (OS) rate, and verified in human KIRC cell lines that the invasion and the migration ability of tumour cells were significantly hindered after HHLA2 knockdown.47 However, Zhen et al. observed that HHLA2 was a positive prognostic factor, which contradicted with Chen et al.48 The different databases and various sizes of cohorts used in these studies might explain the contradictory prognostic roles of HHLA2 in KIRC. Additionally, non-immunological roles of HHLA2 in cancer deveplopment also contributed to the complexity of HHLA2 functions in tumour microenvironment. Tumour cell-expressed HHLA2 might bind to endothelium-expressed TMIGD2 to augment angiogenesis.49 In vitro experiments also reported that HHLA2 silence led to decreased phosphorylation level of EGFR/MAPK/ERK signalling pathway in lung cancer.50 Further verifications in vitro and in vivo are needed to elaborate the immune and non-immune functions of HHLA2, and further investigations focusing on the association between KIRC subtypes and the HHLA2 functions will help understand the heterogeneity of HHLA2 immune function and prognostic value.
Table 1.
Summary of HHLA2 detection as prognostic biomarker in human cancers.
| Tumour type | Year | Research object/Numbers | HHLA2 expression | Conclusions | Refs. |
|---|---|---|---|---|---|
| Prostate cancer | 2021 | Patient tumour samples, N = 239 | Tumour cells | High HHLA2 expression was an independent prognostic predictor for prostate cancer, and was negatively correlated with CD TILs | 26 |
| Neuroendocrine tumours | 2021 | Patient tumour samples, N = 37 | Tumour cells | High HHLA2 expression was correlated with high tumour grade and metastasis | 27 |
| Colorectal cancer | 2021 | Patient tumour samples, N = 214 | Tumour cells | HHLA2 expression was low in colorectral cancer and appeared to have no influence on clinical outcomes. | 40 |
| Hepatocellular carcinoma | 2021 | Patient tumour samples, N = 205 | Peri-tumour region of HCC tissues | HHLA2 expression in the peri‑tumour region was an independent prognostic factor for OS, and was negatively correlated with PD-L1 | 28 |
| Patient tumour samples, N = 55 | Tumour cells | Higher expression of HHLA2 protein was associated with advanced cancer stage, tumour differentiation, and invasion of adjacent structures | 29 | ||
| Patient tumour samples, N = 202 | Tumour cells | HHLA2 level was a independent worse prognostic factor and affected the tumour microenvironment | 30 | ||
| Epithelial ovarian cancer | 2021 | Patient tumour samples, N = 64 | Tumour cells | HHLA2 was correlated with high CD TIL levels and tumour differentiation; and predicted improved survival in ovarian cancer | 44 |
| Lung adenocarcinoma | 2021 | Patient tumour samples, N = 62 | Tumour cells | HHLA2 expression was an prognostic factor for PFS, and was positively correlated with EGFR overexpression | 31 |
| 2020 | Patient tumour samples, N = 167 | Tumour cells | Elevated HHLA2 expression level was associated with short DFS, and was independently correlated with EGFR status | 32 | |
| 2017 | Patient tumour samples, N = 392 (training cohort) & 287 (validation cohort) | Tumour cells | HHLA2 expression was positively associated with EGFR mutation, high TILs, and decreased OS (statistically non-significant) | 33 | |
| Cervical adenocarcinoma | 2021 | Patient tumour samples, N = 76 | Tumour cells | OS and DFS were higher in the HHLA2 high-expression group, but there was no statistically significant difference | 73 |
| Kidney renal clear cell carcinoma | 2020 | Patient tumour samples, N = 206 (training cohort) & 197 (validation cohort) | Tumour cells | HHLA2 expression was significantly associated with microvascular invasion, necrosis, TNM stage, and advanced Fuhrman nuclear, and indicated shorter PFS and OS | 72 |
| Patient tumour samples, N = 250 | Tumour cells | HHLA2 expression predicted a favourable survival outcome | 48 | ||
| 2019 | Patient tumour samples, N = 87 | Tumour cells | Higher expression of HHLA2 was significantly associated with advanced TNM stage and lager tumour size, and prediected better OS | 47 | |
| Patient tumour samples, N = 92 | Tumour cells | High HHLA2 expression was associated with poor OS | 74 | ||
| Gastric cancer | 2020 | Patient tumour samples, N = 124 | Tumour cells | High HHLA2 expression was correlated with deep tumour invasion, advanced clinical stage, metastasis and short OS | 35 |
| 2018 | Patient blood specimens, N = 111 | Peripheral blood mononuclear cell | HHLA2 mRNA in patients’ blood samples expression was significantly related to better OS | 46 | |
| Pancreatic cancer | 2020 | Patient tumour samples, N = 122 | Tumour cells | High HHLA2 expression was significantly associated with improved post-operative cancer-specific survival and delayed cancer recurrence | 42 |
| 2019 | Patient tumour samples, N = 136 | Tumour cells | Patients with high HHLA2 expression had significantly longer OS than those with low HHLA2 expression | 14 | |
| 2019 | Patient tumour samples, N = 92 | Tumour cells | HHLA2 expression was significantly associated with better survival | 43 | |
| Oral squamous cell carcinoma | 2019 | Patient tumour samples, N = 210 | Tumour cells | High HHLA2 or TMIGD2 expression predicted poor prognosis | 36 |
| Bladder urothelial carcinoma | 2019 | Patient tumour samples, N = 212 | Tumour cells | HHLA2 expression was significantly correlated with tumour grade, tumour stage, tumour size, and lymph node metastasis, and can independently predict unfavourable prognosis | 37 |
| Intrahepatic cholangiocarcinoma | 2019 | Patient tumour samples, N = 153 (training cohort) & 65 (validation cohort) | Tumour cells | HHLA2 was an independent prognostic indicator for shorter OS | 38 |
| Colorectal carcinoma | 2018 | Patient tumour samples, N = 63 | Tumour cells | HHLA2 acted as an independent prognostic factor for shorter OS | 39 |
| Osteosarcoma | 2016 | Patient tumour samples, N = 62 | Tumour cells | HHLA2 expression was associated with poor survival and metastasis | 41 |
| Triple negative breast cancer | 2015 | Patient tumour samples, N = 50 | Tumour cells | HHLA2 expression was associated with lymph node metastasis and advanced stage | 17 |
TILs: tumour-infiltrating lymphocytes; OS: overall survival; PD-L1: programmed cell death-ligand 1; PFS: progression free suvival; EGFR: epidermal growth factor receptor; DFS: disease free suvival.
HHLA2 as a stimulatory immune checkpoint
The immunostimulatory role of HHLA2 was initially revealed when Zhu et al. found its ligand CD28 homolog (CD28H, also known as TMIGD2). It is worth mentioning that Zhu et al. renamed HHLA2 as B7-H5, which is now generally referred to V-domain immunoglobulin suppressor of T cell activation (VISTA).13 Today, HHLA2 are referred to B7-H7 or B7y in most studies in order to distinguish with VISTA, which our group have previously summarized.51 Zhu and his colleges discovered HHLA2 as a ligand to CD28H via high-throughput screening of over 2300 transmembrane proteins, and further verified that CD28H-Ig fusion protein could directly interact with HHLA2 transfectants (vice versa). Via immunizing mice with a HHLA2-Ig fusion protein, they generated HHLA2 mAb, clone 2D3, by which HHLA2 was proved to stimulate allogeneic T-cell proliferation in vitro and in vivo utilizing humanized NSG mice model.13 Meanwhile, another group also independently identified TMIGD2 as a receptor of HHLA2.17 Our group verified HHLA2-TMIGD2 co-stimulatory role in pancreatic cancer and proved that HHLA2 was a protective factor in pancreatic cancer development.14 Since HHLA2 was only expressed in primates and vacant in mice, Janakiram et al. deduced that its ligand should also be exclusively expressed in primates due to co-evolution. Furthermore, they analysed the sequence of TMIGD2 and discovered that it was the same molecule with CD28H and immunoglobulin-containing and proline-rich receptor-1 (IGPR-1), which was known as an adhesion molecule involved in cell migration, tumour chemosensitivity, autophagy, mechanosensing and angiogenesis.52, 53, 54, 55, 56 TMIGD2 (or CD28H, or IGPR-1), containing an extracellular IgV-like domain with two possible glycosylation sites, a transmembrane region, and a cytoplasmic tail with tyrosine residues, was observed on naïve T cells, primary natural killer (NK) cells, innate lymphoid cells, plasmacytoid dendritic cells, and tissue resident T cells, but lost on T regulatory cells, monocytes and B cells.12,57,58 With T cell activation and differentiation, TMIGD2 expression gradually decreased (from 97.5% to 26.4%); Only half of the memory T cells were detected TMIGD2 positive, while only 26.4% terminally differentiated T cells were TMIGD2 positive.13 Similar phenomenon also occurred in NK cells, where TMIGD2 showed diminished expression after NK cell activation.59 TMIGD2 was also expressed on human endothelial and epithelial cells, promoting angiogenesis in human cancers. Whether tumour angiogenesis function of TMIGD2 involves HHLA2 on APCs warrants further investigation.60
HHLA2 functioned as an immunostimulatory checkpoint via binding to TMIGD2 on naïve T cells and NK cells (Figure 1). It was reported that agonistic TMIGD2 mAb strongly augmented T-cell proliferation and cytokine production including IL-17, IL-5, IL-10, IFN-γ, and TNF-α. In the meantime, HHLA2-blocking mAb 2D3 could substantially abrogate T-cell proliferation and cytokine secretion both in vitro and in vivo.13 Similar to B7/CD28 co-stimulation, proliferation and activation of T cells induced by HHLA2/TMIGD2 co-stimulation and TCR crosslinking were via a signalling cascade involving serine‑threonine kinase AKT phosphorylation.13 Apart from binding to TMIGD2 on naïve T cells, HHLA2 enhanced cellular cytotoxicity effect of NK cells by interacting with TMIGD2 on primary NK cells. NK cell activation could be suppressed by interactions of NKG2A on NK cells and nonclassical MHC-I antigen HLA-E.61 It was reported that HHLA HLA- tumour cells were lysed by NK cells with TMIGD2 chimeric antigen receptor (TMIGD2-CAR) since NKG2A/HLA-E inhibition signal was overwhelmed by HHLA2-TMIGD2 signal.59 This shed light on the possibility of targeting HHLA tumours by engineering NK cells with TMIGD2-CAR to potentiate the antitumour efficiency. Interactions of HHLA2 on tumour cells and TMIGD2 on NK cells also triggered selective degranulation of CD NK cells, and degranulation of cytotoxic granules led to cytotoxic effect against tumour cells.59 Taken together, HHLA2 was an activator of both NK cells and T cells through binding with its receptor TMIGD2, and functioned as an immunostimulator in multiple cancer types.
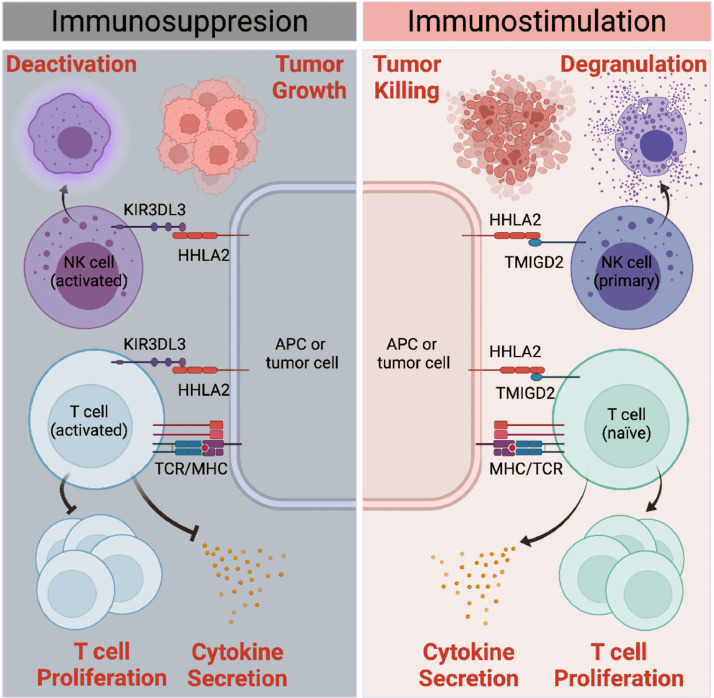
Figure 1.
Dual roles of HHLA2 in regulating immune response in tumour microenvironment. Co-stimulatory receptor (TMIGD2) and co-inhibitory receptor (KIR3DL3) of HHLA2 express on naïve T/NK cells and activated T/NK cells, respectively, and they interact with HHLA2 in a spatial-and-temporal-distinct way. Via binding to TMIGD on naïve T cells and primary NK cells, HHLA2 promotes T cells proliferation and cytokine secretion including IL-17, IL-5, IL-10, IFN-γ, and TNF-α, enhancing both NK cells and T cells lysing function to augment tumour killing. With T/NK cell activation, TMIGD2 expression level gradually decreases while KIR3DL3 expression thrives. When binding to KIR3DL3 on activated T/NK cells, HHLA2 abrogates T cell proliferation and cytokine production including IL-5, IL-10, IL-13, IL-17A, IL-22, TNF-α, and IFN-γ. HHLA2- TMIGD2 interaction also promotes NK cell degranulation, while HHLA2-KIR3DL3 interaction markedly decreases the NK cell degranulation, affecting tumour growth in a NK cell-dependent way.
HHLA2 as an inhibitory immune checkpoint
Despite the fact that TMIGD2 had been the first identified receptor of HHLA2, early studies had discovered a contradictory role of HHLA2 inhibiting T cell proliferation and function. Zhao et al. performed a pioneering work in 2013 illustrating that proliferation of both CD and CD T cells, as well as 7 out of 13 cytokines (IL-5, IL-10, IL-13, IL-17A, IL-22, TNF-α, and IFN-γ) secreted by T cells upon TCR signalling, were inhibited by HHLA2-Ig when incubated with anti-CD3.10 Wang et al. also reported that IL-2 production by T cells was inhibited dose-dependently upon HHLA2-Ig stimulation.62 Additionally, in the co-culture experiment, cytokine production of T lymphocytes from different donors exhibited dramatic variations, suggesting the heterogeneity roles and individualized response of HHLA2.10 They did not refer TMIGD2 as the T cell co-inhibitory ligand of HHLA2 since TMIGD2 was only observed on T cells before repetitive activation and thus was unlikely to participate into tumour immune evasion. Via investigating global transcriptomic changes in T lymphocytes after HHLA2-Ig stimulation, a recent study observed fold changes of key genes expression involved in T cell activation were remarkably lower than those after B7–1 and OKT3 (an anti-CD3 reagent) stimulation, and HHLA2 blockade could augment T cell proliferation and activation.63 They indicated that HHLA2 was insufficient to reach the threshold of T cell activation and served as a break signal in human cancers, yet they were unable to verify whether the coinhibitory function of HHLA2 is TMIGD2-dependent as well. Given that numerous studies separately discovered unfavourable prognosis correlated to HHLA2 expression across multiple human cancers, there was a long-held estimation that an unidentified co-inhibitory receptor of HHLA2 was expressed on activated T cells which HHLA2 on tumour cells or APCs could interact with to protect tumours form immune surveillance.64, 65, 66, 67
The search for the co-inhibitory receptor of HHLA2 had never been stopped. In 2017′s Cold Spring Harbour Asia Conference on Precision Cancer Biology, Zang et al. first brought up that KIR3DL3 was another receptor of HHLA2. Since then, HHLA2-KIR3DL3 pathway was in active investigation. In 2020, two successive but independent high-quality studies portrayed a human immunoglobulin superfamily (IgSF) interactome via high-throughput screening of cell surface protein interactions, indicating that KIR3DL3 and HHLA2 were a pair of receptor-ligand.68,69 Very recently, Wei et al. and Bhatt et al. separately and simultaneously identified that KIR3DL3 was an inhibitory receptor of HHLA2 and KIR3DL3-HHLA2 axis was an immunosuppressive pathway in cancer immunity, making HHLA2-related studies step into a new chapter.7,8
KIR3DL3 was a killer cell immunoglobulin-like receptors (KIR) family protein that contained three extracellular domains (D0, D1, D2), one transmembrane region, and one cytoplasmic tail with an immunoreceptor tyrosine-based inhibitory motif (ITIM).70 Consistent with HHLA2 and TMIGD2, KIR3DL3 was observed only in primates and lost in rodents possibly due to co-evolution. KIR3DL3 predominantly expressed on activated and differentiated T cells and CDCD NK cells while TMIGD2 predominantly expressed on naïve T cells and CDCD NK subset.7 The mutually exclusive expressions of TMIGD2 and KIR3DL3 endowed their ligand HHLA2 with different functions in spatial and temporal distinct circumstances in cancer immunity. In a very recent study, Wei et al. confirmed that the IgC-IgV2 domains of HHLA2 interacted with the D0 domain of KIR3DL3, and co-culturing 3T3 cells-expressed HHLA2 with KIR3DL3-Ig had no effect on TMIGD2 binding to HHLA2 (vice versa), indicating that KIR3DL3 and TMIGD2 were non-competitive and they interacted with distinct regions on HHLA2 simultaneously.7 Utilizing multiple human cancer cell lines and humanized mouse models, they discovered that inhibition of KIR3DL3 augmented NK cell-mediated antitumour efficiency and sensitized HHLA tumour cells to NK cell killing. Further mechanism investigations revealed that ITIM in KIR3DL3 in NK cells could recruit Src homology region 2 domain-containing phosphatase-1/2, and deactivate downstream Vav guanine nucleotide exchange factor 1, extracellular signal-regulated kinase, protein kinase B, and nuclear factor kB pathway.7 They also illustrated that KIR3DL3 could attenuate CD T cells lysis function with or without TCR engagement in vitro.7 Taken together, evidences indicated that HHLA2 acted as an inhibitory immune checkpoint through binding with KIR3DL3 in NK cells and T cells (Figure 1). Blockade of KIR3DL3 in HHLA human tumours was a promising alternative way of enhancing antitumour immunity.
Precision cancer immunotherapy via manipulating HHLA2-KIR3DL3/TMIGD2 interaction
Based on the recent discovery of HHLA2-KIR3DL3 immunosuppressive pathway during cancer immune escape, blockade of HHLA2/KIR3DL3 without interrupting HHLA2-TMIDG2 co-immunostimulatory signal may serve as a promising ICB strategy for antitumour therapy. In some cases, such as intrahepatic cholangiocarcinoma, expression of HHLA2 is more frequent than PD-L1.38 Of note, HHLA2 is observed to be widely expressed in tumours with low expression of PD-L1.71 Even within tumours, HHLA2 tends to be expressed in regions that scarcely expressing PD-L1.8 The non-overlapping expressions of HHLA2 and PD-L1 make HHLA2 an alternative immune checkpoint mediating tumour immune escape independent of PD1/PD-L1 axis, and targeting HHLA2 and its receptors exhibits great potential for patients with resistance to PD1/PD-L1 blockade. In limited circumstances such as colorectal cancer and KIRC, however, HHLA2 was observed to be co-expressed with PD-L1 and together they served as unfavourable prognostic biomarkers.40,72 Hitherto, antibodies against HHLA2 are still in early stages, where HHLA2 mAbs are generated through immunizing mouse since rodents do not express HHLA2, and most HHLA2 mAbs block its binding with both KIR3DL3 and TMIGD2. Therefore, specific inhibition of HHLA2-KIR3DL3 interaction while leaving HHLA2-TMIGD2 pathway unaffected might be a wise and promising strategy against cancer. Current HHLA2 antibodies that exclusively inhibit HHLA2-KIR3DL3 pathway and spare the HHLA2-TMIGD2 costimulatory signal were rare and non-commercialized (e.g., 2C4 and 6D10 from Bhatt et al.). Another option was to inhibit KIR3DL3. Despite its polymorphism, the D0 domain of KIR3DL3 was comparatively conserved and was the binding site of HHLA2. Similar with HHLA2 mAbs, KIR3DL3 mAbs have currently been in its initial phase where only Wei et al. generated anti-KIR3DL3 clone 26E10 without hindering HHLA2 from binding to TMIGD2.7 This calls for the development of humanized and commercialized mAbs that exclusively target HHLA2-KIR3DL3 pathway. Future investigations concerning KIR3DL3 polymorphism effects on its binding affinity to HHLA2 and subsequent clinical outcomes in human cancers should also be implemented.
Numerous studies have been performed using ICB alone or together with small-molecule inhibitors, demonstrating their mechanisms and therapeutic potentials in various mouse models. However, only a few of them prove to be just as effective in human clinical trials. The genetic differences between primates and rodents are apparently an influence factor to blame. Distinct from other B7 family members and their receptors, HHLA2-KIR3DL3/TMIGD2 interaction is only observed in primates and lost in rodents. Though in vivo experiments are confronted with more complex technical requirements, it is a noteworthy opportunity to invent novel ICB therapy targeting HHLA2 and its receptors because HHLA2-involved immune escape may possibly be the critical immune response not needed in rodent but vital in human beings. Via multiple humanized mouse models, Wei et al. has done an elaborate and excellent work demonstrating that inhibition of HHLA2-KIR3DL3 interaction effectively promotes NK cell lysing function. The four mouse models utilized were: (1) NSG mice were first subcutaneously injected with HHLA or HHLA Raji cells, then dealt with KIR3DL NK92 cells, followed by mIgG1 or 26E10; (2) NSG mice were first subcutaneously injected with HCC827 cell line, then intratumourally dealt with KIR3DL primary NK cell line, followed by mIgG1 or 26E10; (3) NSG mice were first intraperitoneally injected with luciferase-transduced HCC827 cells, then intraperitoneally dealt with KIR3DL primary NK cell line, followed by mIgG1 or 26E10; (4) NSG mice were first intravenously inoculated with luciferase-transduced HCC827 cells, then dealt with KIR3DL primary NK cell line, followed by mIgG1 or 26E10.7 This suggests that, cell-line-derived xenograft (CDX) model and patient-derived xenograft (PDX) model, which are commonly used in cancer immunology research, cannot fully mimic all immune responses in human cancers because HHLA2-KIR3DL3/TMIGD2 pathway plays a vital role in HHLA tumours but neither HHLA2 nor its receptors have murine orthologs. Therefore, further studies using humanized mouse models or even primate models to abrogate HHLA2-KIR3DL3 signal or to augment HHLA2-TMIGD2 signal are welcome. Combination therapies involving manipulation of HHLA2-KIR3DL3/TMIGD2 interaction are also intriguing.
To the best of our knowledge, hitherto there is no completed or in progress clinical trial directly targeting HHLA2 or its receptors. Nevertheless, HHLA2 was used as an immune checkpoint marker together with PD-L1, B7x, and B7-H3 in clinical trial NCT04514484 to help measure the therapeutic efficacy of cabozantinib and nivolumab in treating patients with advanced cancer and human immunodeficiency virus, while its receptor KIR3DL3 served as one of the KIR types in clinical trial NCT04882605 to predict KIR-based NK alloreactivity to donor/recipient couples. Phase I clinical trial directly targeting HHLA2-KIR3DL3 co-inhibitory pathway is urgently expected.8 More prospective clinical trials investigating the preliminary efficacy and safety of HHLA2-KIR3DL3 inhibition alone or in combination with other ICB therapies are in desperate demand to promote bench-to-bedside transformation.
Conclusions
As a novel immune checkpoint predominantly expressed in tumour cells and APCs in primates, HHLA2 functions as both an immunosuppressive and an immunostimulatory checkpoint in human cancer development (Figure 1), and its expression in tumours correlates with clinical outcomes varying from different cancer types. Most importantly, newly-discovered HHLA2-KIR3DL3 immunosuppressive pathway has filled the research gap of HHAL2 and provided promising immunotherapeutic targets for treating cancer. In this review, we summarize the HHLA2 expression profile and its association with patients’ prognoses and clinical features through up-to-date literatures. We further reveal the molecular mechanisms of HHLA2 expression as well as stimulatory and inhibitory immune checkpoint roles in human cancers, highlighting that HHLA2-KIR3DL3/TMIGD2 pathway can be manipulated to potentiate host's immune response against cancer. Though targeting HHLA2/KIR3DL3 is a prospective approach for cancer immunotherapy, more studies using humanized mouse models or non-rodent models, as well as clinical trials containing novel anti-HHLA2/KIR3DL3 therapeutic strategies alone or in combination with other oncology agents should be further explored.
Outstanding questions
With the inhibitory receptor of HHLA2 newly discovered in 2021 filling the research gap of HHLA2-involved immunosuppressive pathway, HHLA2 has become an attractive immune checkpoint with both positive and negative effect on tumour immune response. Targeting HHLA2-involved immune regulatory pathways has shown huge potential in broadening cancer immunotherapy mainly because: 1. HHLA2 and PD-L1 expression are non-overlapping in most cancer types, making HHLA2 an alternative immune checkpoint that exhibits great potential for patients with resistance to PD1/PD-L1 blockade. 2. HHLA2-KIR3DL3/TMIGD2 interaction is only observed in primates and lost in rodents. HHLA2-involved immune escape may possibly be the critical immune response not needed in rodent but vital in human beings. To develop more effective cancer immunotherapies, first, humanized mouse models or even primate models to abrogate HHLA2-KIR3DL3 signal or to augment HHLA2-TMIGD2 signal are welcome. Second, development of humanized and commercialized mAbs that exclusively target HHLA2-KIR3DL3/TMIGD2 pathway must be exhaustively investigated. Lastly, combination therapies involving manipulation of HHLA2-KIR3DL3/TMIGD2 interaction are also intriguing.
Search strategy and selection criteria
Data for this Review were identified by searches of MEDLINE, PubMed and Web of Science using the search terms “HHLA2”, “B7-H7”, “B7y”, “B7-H5”, “TMIGD2”, and “KIR3DL3”. Abstracts and reports from meetings were included only when they related directly to previously published work. Only articles published in English between 1998 and 2021 were included.
Contributors
Xueli Bai and Tingbo Liang provided direction and guidance throughout the preparation of this manuscript, and shared senior authorship; Honggang Ying and Jian Xu contributed equally by writing the manuscript and preparing the figure and the table; Honggang Ying and Xiaozhen Zhang discussed and revised the manuscript. All authors discussed and approved the final manuscript.
Declaration of interests
All authors report grants from National Natural Science Foundation of China, grants from Fundamental Research Funds for the Zhejiang Provincial Universities, grants from Key Research and Development Program of Zhejiang Province, grants from Key Program of Medical Scientific Research Foundation of Zhejiang Province, during the conduct of the study.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (81871925 and 82071867 to X.B., 81830089 and U20A20378 to T.L.), the Fundamental Research Funds for the Zhejiang Provincial Universities (2021XZZX031 to X.B.), Key Research and Development Program of Zhejiang Province (2020C03117 to X.B.), and Key Program of Medical Scientific Research Foundation of Zhejiang Province (2019C03019 to T.L.). None of the above funders were involved in paper design, data collection, data analysis, interpretation, or writing of the paper. We confirm that this article is not paid to write by a pharmaceutical company or other agency. Authors were not precluded from accessing data in the study, and they accept responsibility to submit for publication.
Contributor Information
Tingbo Liang, Email: liangtingbo@zju.edu.cn.
Xueli Bai, Email: shirleybai@zju.edu.cn.
References
- 1.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian S.L., Weiner G.J., Pardoll D.M. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29(36):4828–4836. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews L.P., Marciscano A.E., Drake C.G., Vignali D.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276(1):80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champiat S., Dercle L., Ammari S., et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 5.de Miguel M., Calvo E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell. 2020;38(3):326–333. doi: 10.1016/j.ccell.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez B.D., Eisel S.L., Bowles K.E., et al. Meta-analysis of quality of life in cancer patients treated with immune checkpoint inhibitors. J Natl Cancer Inst. 2021;113(9):1113–1265. doi: 10.1093/jnci/djab171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei Y., Ren X., Galbo P.M., et al. KIR3DL3-HHLA2 is a human immunosuppressive pathway and a therapeutic target. Sci. Immunol. 2021;6(61):eabf9792. doi: 10.1126/sciimmunol.abf9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt R.S., Berjis A., Konge J.C., et al. KIR3DL3 is an inhibitory receptor for HHLA2 that mediates an alternative immunoinhibitory pathway to PD1. Cancer Immunol Res. 2021;9(2):156–169. doi: 10.1158/2326-6066.CIR-20-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flajnik M.F., Tlapakova T., Criscitiello M.F., Krylov V., Ohta Y. Evolution of the B7 family: co-evolution of B7H6 and NKp30, identification of a new B7 family member, B7H7, and of B7′s historical relationship with the MHC. Immunogenetics. 2012;64(8):571–590. doi: 10.1007/s00251-012-0616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao R., Chinai J.M., Buhl S., et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci USA. 2013;110(24):9879–9884. doi: 10.1073/pnas.1303524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mager D.L., Hunter D.G., Schertzer M., Freeman J.D. Endogenous retroviruses provide the primary polyadenylation signal for two new human genes (HHLA2 and HHLA3) Genomics. 1998;59:255–263. doi: 10.1006/geno.1999.5877. [DOI] [PubMed] [Google Scholar]
- 12.Janakiram M., Shah U.A., Liu W., Zhao A., Schoenberg M.P., Zang X. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol Rev. 2017;276(1):26–39. doi: 10.1111/imr.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Y., Yao S., Iliopoulou B.P., et al. B7-H5 costimulates human T cells via CD28H. Nat Commun. 2013;4:2043. doi: 10.1038/ncomms3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Q., Wang J., Chen W., et al. B7-H5/CD28H is a co-stimulatory pathway and correlates with improved prognosis in pancreatic ductal adenocarcinoma. Cancer Sci. 2019;110(2):530–539. doi: 10.1111/cas.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung K., Choi I. Emerging Co-signaling networks in T cell immune regulation. Immune Netw. 2013;13(5):184–193. doi: 10.4110/in.2013.13.5.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B., Ran Z., Liu M., Ou Y. Prognostic significance of potential immune checkpoint member HHLA2 in human tumors: a comprehensive analysis. Front Immunol. 2019;10:1573. doi: 10.3389/fimmu.2019.01573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janakiram M., Chinai J.M., Fineberg S., et al. Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res. 2015;21(10):2359–2366. doi: 10.1158/1078-0432.CCR-14-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aznar M.A., Labiano S., Diaz-Lagares A., et al. CD137 (4-1BB) costimulation modifies DNA methylation in CD8(+) T cell-relevant genes. Cancer Immunol Res. 2018;6(1):69–78. doi: 10.1158/2326-6066.CIR-17-0159. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z., Wan Y., Yang M., et al. Identification of methylation-driven genes related to the prognosis of papillary renal cell carcinoma: a study based on the cancer genome atlas. Cancer Cell Int. 2020;20:235. doi: 10.1186/s12935-020-01331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh N.P., Vinod P.K. Integrative analysis of DNA methylation and gene expression in papillary renal cell carcinoma. Mol Genet Genom. 2020;295(3):807–824. doi: 10.1007/s00438-020-01664-y. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D., Wang Y., Hu X. Identification and comprehensive validation of a DNA methylation-driven gene-based prognostic model for clear cell renal cell carcinoma. DNA Cell Biol. 2020;39(10):1799–1812. doi: 10.1089/dna.2020.5601. [DOI] [PubMed] [Google Scholar]
- 22.Wang R., Guo H., Tang X., et al. Interferon gamma-induced interferon regulatory factor 1 activates transcription of HHLA2 and induces immune escape of hepatocellular carcinoma cells. Inflammation. 2021;45(1):308–330. doi: 10.1007/s10753-021-01547-3. [DOI] [PubMed] [Google Scholar]
- 23.Li D., Liu S., Xu J., et al. Ferroptosis-related gene CHAC1 is a valid indicator for the poor prognosis of kidney renal clear cell carcinoma. J Cell Mol Med. 2021;25(7):3610–3621. doi: 10.1111/jcmm.16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F., Hou W., Liang J., Zhu L., Luo C. LRP1B mutation: a novel independent prognostic factor and a predictive tumor mutation burden in hepatocellular carcinoma. J Cancer. 2021;12(13):4039–4048. doi: 10.7150/jca.53124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Achinko D.A., Dormer A., Narayanan M., Norman E.F. Targeted immune epitope prediction to HHLA2 and MAGEB5 protein variants as therapeutic approach to related viral diseases. BMC Immunol. 2021;22(1):49. doi: 10.1186/s12865-021-00440-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Q., Li K., Lai Y., et al. B7 score and T cell infiltration stratify immune status in prostate cancer. J Immunother Cancer. 2021;9(8):e002455. doi: 10.1136/jitc-2021-002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan Z., Gardiner J.C., Maggi E.C., et al. B7 immune-checkpoints as targets for the treatment of neuroendocrine tumors. Endocr Relat Cancer. 2021;28(2):135–149. doi: 10.1530/ERC-20-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y., Huang Z., Yu X., Li Z., Zheng L., Xu J. HHLA2 expression is associated with poor survival in patients with hepatocellular carcinoma. Biologics. 2021;15:329–341. doi: 10.2147/BTT.S325019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo M., Xiong Y., Lin Y., Liang R., Li Y., Ge L. H long terminal repeat-associating 2 (HHLA2) is a biomarker of advanced stage hepatocellular carcinoma and promotes tumor cell development in vitro. Med Sci Monit. 2021;27 doi: 10.12659/MSM.930215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo M., Lin Y., Liang R., Li Y., Ge L. Clinical significance of the HHLA2 protein in hepatocellular carcinoma and the tumor microenvironment. J Inflamm Res. 2021;14:4217–4228. doi: 10.2147/JIR.S324336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrag M.S., Ibrahim E.M., El-Hadidy T.A., Akl M.F., Elsergany A.R., Abdelwahab H.W. Human endogenous retrovirus-h long terminal repeat- associating protein 2 (HHLA2) is a novel immune checkpoint protein in lung cancer which predicts survival. Asian Pac J Cancer Prev. 2021;22(6):1883–1889. doi: 10.31557/APJCP.2021.22.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y., Hu R., Li X., et al. B7-H4 and HHLA2, members of B7 family, are aberrantly expressed in EGFR mutated lung adenocarcinoma. Pathol Res Pract. 2020;216(10) doi: 10.1016/j.prp.2020.153134. [DOI] [PubMed] [Google Scholar]
- 33.Cheng H., Janakiram M., Borczuk A., et al. HHLA2, a new immune checkpoint member of the B7 family, is widely expressed in human lung cancer and associated with EGFR mutational status. Clin Cancer Res. 2017;23(3):825–832. doi: 10.1158/1078-0432.CCR-15-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L., Luo X., Cheng C., Amos C.I., Cai G., Xiao F. A gene expression-based immune signature for lung adenocarcinoma prognosis. Cancer Immunol Immunother. 2020;69(9):1881–1890. doi: 10.1007/s00262-020-02595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei L., Tang L., Chang H., Huo S., Li Y. HHLA2 overexpression is a novel biomarker of malignant status and poor prognosis in gastric cancer. Hum Cell. 2020;33(1):116–122. doi: 10.1007/s13577-019-00280-2. [DOI] [PubMed] [Google Scholar]
- 36.Xiao Y., Li H., Yang L.L., et al. The expression patterns and associated clinical parameters of human endogenous retrovirus-H long terminal repeat-associating protein 2 and transmembrane and immunoglobulin domain containing 2 in oral squamous cell carcinoma. Dis Markers. 2019;2019 doi: 10.1155/2019/5421985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin G., Ye H., Wang J., Chen S., Chen X., Zhang C. Immune checkpoint human endogenous retrovirus-h long terminal repeat-associating protein 2 is upregulated and independently predicts unfavorable prognosis in bladder urothelial carcinoma. Nephron. 2019;141(4):256–264. doi: 10.1159/000495887. [DOI] [PubMed] [Google Scholar]
- 38.Jing C.Y., Fu Y.P., Yi Y., et al. HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. J Immunother Cancer. 2019;7(1):77. doi: 10.1186/s40425-019-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Z., Dong W. Overexpression of HHLA2, a member of the B7 family, is associated with worse survival in human colorectal carcinoma. Onco Targets Ther. 2018;11:1563–1570. doi: 10.2147/OTT.S160493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W., Acuna-Villaorduna A., Kuan K., et al. B7-H3 and PD-L1 expression are prognostic biomarkers in a multi-racial cohort of patients with colorectal cancer. Clin Colorectal Cancer. 2021;20(2):161–169. doi: 10.1016/j.clcc.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Koirala P., Roth M.E., Gill J., et al. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci Rep. 2016;6:31154. doi: 10.1038/srep31154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boor P.P.C., Sideras K., Biermann K., et al. HHLA2 is expressed in pancreatic and ampullary cancers and increased expression is associated with better post-surgical prognosis. Br J Cancer. 2020;122(8):1211–1218. doi: 10.1038/s41416-020-0755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan H., Qiu W., Koehne de Gonzalez A.K., et al. HHLA2 is a novel immune checkpoint protein in pancreatic ductal adenocarcinoma and predicts post-surgical survival. Cancer Lett. 2019;442:333–340. doi: 10.1016/j.canlet.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu G., Shi Y., Ling X., et al. HHLA2 predicts better survival and exhibits inhibited proliferation in epithelial ovarian cancer. Cancer Cell Int. 2021;21(1):252. doi: 10.1186/s12935-021-01930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi Y., Deng G., Xu P., et al. HHLA2 is a novel prognostic predictor and potential therapeutic target in malignant glioma. Oncol Rep. 2019;42(6):2309–2322. doi: 10.3892/or.2019.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimonosono M., Arigami T., Yanagita S., et al. The association of human endogenous retrovirus-H long terminal repeat-associating protein 2 (HHLA2) expression with gastric cancer prognosis. Oncotarget. 2018;9(31):22069–22078. doi: 10.18632/oncotarget.25179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L., Zhu D., Feng J., et al. Overexpression of HHLA2 in human clear cell renal cell carcinoma is significantly associated with poor survival of the patients. Cancer Cell Int. 2019;19:101. doi: 10.1186/s12935-019-0813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z., Liu J., Zhang C., et al. Over-expression and prognostic significance of HHLA2, a new immune checkpoint molecule, in human clear cell renal cell carcinoma. Front Cell Dev Biol. 2020;8:280. doi: 10.3389/fcell.2020.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janakiram M., Chinai J.M., Zhao A., Sparano J.A., Zang X. HHLA2 and TMIGD2: new immunotherapeutic targets of the B7 and CD28 families. Oncoimmunology. 2015;4(8) doi: 10.1080/2162402X.2015.1026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun W., Li S., Tang G., et al. HHLA2 deficiency inhibits non-small cell lung cancer progression and THP-1 macrophage M2 polarization. Cancer Med. 2021;10(15):5256–5269. doi: 10.1002/cam4.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang X., Zhang X., Li E., et al. VISTA: an immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J Hematol Oncol. 2020;13(1) doi: 10.1186/s13045-020-00917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahimi N., Rezazadeh K., Mahoney J.E., Hartsough E., Meyer R.D. Identification of IGPR-1 as a novel adhesion molecule involved in angiogenesis. Mol Biol Cell. 2012;23(9):1646–1656. doi: 10.1091/mbc.E11-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho R.X., Tahboub R., Amraei R., et al. The cell adhesion molecule IGPR-1 is activated by and regulates responses of endothelial cells to shear stress. J Biol Chem. 2019;294(37):13671–13680. doi: 10.1074/jbc.RA119.008548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amraei R., Alwani T., Ho R.X., Aryan Z., Wang S., Rahimi N. Cell adhesion molecule IGPR-1 activates AMPK connecting cell adhesion to autophagy. J Biol Chem. 2020;295(49):16691–16699. doi: 10.1074/jbc.RA120.014790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y.H.W., Meyer R.D., Bondzie P.A., et al. IGPR-1 is required for endothelial cell-cell adhesion and barrier function. J Mol Biol. 2016;428(24 Pt B):5019–5033. doi: 10.1016/j.jmb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woolf N., Pearson B.E., Bondzie P.A., et al. Targeting tumor multicellular aggregation through IGPR-1 inhibits colon cancer growth and improves chemotherapy. Oncogenesis. 2017;6(9):e378. doi: 10.1038/oncsis.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crespo J., Vatan L., Maj T., Liu R., Kryczek I., Zou W. Phenotype and tissue distribution of CD28H(+) immune cell subsets. Oncoimmunology. 2017;6(12) doi: 10.1080/2162402X.2017.1362529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tian Y., Sun Y., Gao F., et al. CD28H expression identifies resident memory CD8 + T cells with less cytotoxicity in human peripheral tissues and cancers. Oncoimmunology. 2019;8(2) doi: 10.1080/2162402X.2018.1538440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhuang X., Long E.O. CD28 homolog is a strong activator of natural killer cells for lysis of B7H7(+) tumor cells. Cancer Immunol Res. 2019;7(6):939–951. doi: 10.1158/2326-6066.CIR-18-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun L., Amraei R., Rahimi N. NEDD4 regulates ubiquitination and stability of the cell adhesion molecule IGPR-1 via lysosomal pathway. J Biomed Sci. 2021;28(1):35. doi: 10.1186/s12929-021-00731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Long E.O., Kim H.S., Liu D., Peterson M.E., Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J., Manick B., Wu G., Hao R. Biofunctions of three new B7 family members (IRM7P.486) J Immunol. 2014;192(1):126.11. [Google Scholar]
- 63.Rieder S.A., Wang J., White N., et al. B7-H7 (HHLA2) inhibits T-cell activation and proliferation in the presence of TCR and CD28 signaling. Cell Mol Immunol. 2021;18(6):1503–1511. doi: 10.1038/s41423-020-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao Y., Freeman G.J. A New B7: CD28 family checkpoint target for cancer immunotherapy: HHLA2. Clin Cancer Res. 2015;21(10):2201–2203. doi: 10.1158/1078-0432.CCR-14-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ni L., Dong C. New B7 family checkpoints in human cancers. Mol Cancer Ther. 2017;16(7):1203–1211. doi: 10.1158/1535-7163.MCT-16-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sankin A., Narasimhulu D., John P., Gartrell B., Schoenberg M., Zang X. The expanding repertoire of targets for immune checkpoint inhibition in bladder cancer: what lies beneath the tip of the iceberg, PD-L1. Urol Oncol. 2018;36(10):459–468. doi: 10.1016/j.urolonc.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang X., Liu G., Li Y., Pan Y. Immune checkpoint: the novel target for antitumor therapy. Genes Dis. 2021;8(1):25–37. doi: 10.1016/j.gendis.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verschueren E., Husain B., Yuen K., et al. The immunoglobulin superfamily receptome defines cancer-relevant networks associated with clinical outcome. Cell. 2020;182(2):329–344. doi: 10.1016/j.cell.2020.06.007. e19. [DOI] [PubMed] [Google Scholar]
- 69.Wojtowicz W.M., Vielmetter J., Fernandes R.A., et al. A human IgSF cell-surface interactome reveals a complex network of protein-protein interactions. Cell. 2020;182(4):1027–1043. doi: 10.1016/j.cell.2020.07.025. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leaton L.A., Shortt J., Kichula K.M., et al. Conservation, extensive heterozygosity, and convergence of signaling potential all indicate a critical role for KIR3DL3 in higher primates. Front Immunol. 2019;10:24. doi: 10.3389/fimmu.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng H., Borczuk A., Janakiram M., et al. Wide expression and significance of alternative immune checkpoint molecules, B7x and HHLA2, in PD-L1-negative human lung cancers. Clin Cancer Res. 2018;24(8):1954–1964. doi: 10.1158/1078-0432.CCR-17-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Q.H., Li K.W., Chen X., et al. HHLA2 and PD-L1 co-expression predicts poor prognosis in patients with clear cell renal cell carcinoma. J Immunother Cancer. 2020;8(1):e000157. doi: 10.1136/jitc-2019-000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Byun J.M., Cho H.J., Park H.Y., et al. The clinical significance of HERV-H LTR -associating 2 expression in cervical adenocarcinoma. Medicine. 2021;100(1):e23691. doi: 10.1097/MD.0000000000023691. (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen D., Chen W., Xu Y., et al. Upregulated immune checkpoint HHLA2 in clear cell renal cell carcinoma: a novel prognostic biomarker and potential therapeutic target. J Med Genet. 2019;56(1):43–49. doi: 10.1136/jmedgenet-2018-105454. [DOI] [PubMed] [Google Scholar]