Abstract
Ischemic stroke due to internal carotid artery occlusion is a potential devastating condition. More frequently the occlusions are embolic in nature, but sometimes they are caused by arterial dissection and their treatment is a challenge. We describe an illustrative case where a young patient with middle cerebral artery stroke caused by carotid artery dissection was submitted to endovascular treatment of mechanical thrombectomy and stenting, giving an excellent outcome. We believe that tandem approach is a treatment of choice in these cases.
Introduction
Ischemic stroke due to large vessel occlusion (LVO) is a dramatic condition with severe risk of disability and death [1]. Most LVOs show an embolic nature. Only a small proportion of all LVOs show a dissective nature [2]. Internal carotid artery (ICA) dissection is a very uncommon condition, accounting less than 2% of ischemic strokes, but it is the most common cause of strokes in younger patients, under 50 years of age, accounting 10%-25% of stroke in this demographic [3,4]. Although some dissections can be traumatic, in most cases, dissection is considered to be idiopathic [5].
The management of stroke caused by carotid dissection can be challenging. In this report, we present a patient with stroke, due to acute internal carotid artery dissection, that were admitted to our stroke unit and treated with mechanical thrombectomy and extensive stenting with a very good outcome. We believe that mechanical thrombectomy and stenting can be a treatment of choice in these cases.
Case report
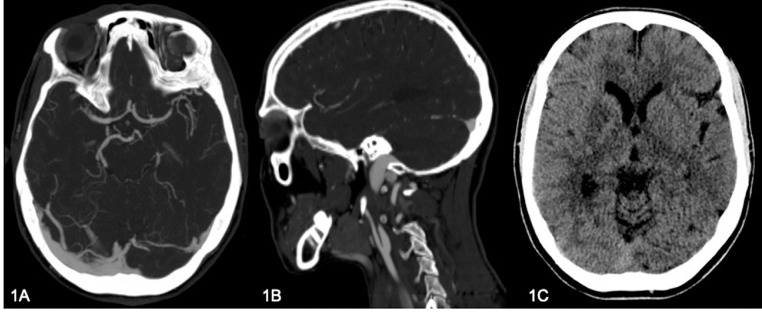
A 42-year-old woman presented to the emergency room with acute onset of aphasia, hemineglect and left hemiparesis. CT and multiphase ACT exams documented the occlusion of right internal carotid artery beyond the bulb and the occlusion of right M1, with intermediate grade leptomeningeal collaterals to the right middle cerebral artery (MCA) territory (Fig. 1A, B).
Fig. 1.
CT angiogram shows on axial plane (A) a middle cerebral arteries asymmetry for occlusion of right M1, while on sagittal plane (B) it shows dissective occlusion of right internal carotid artery (“flame sign”). (C) CT scan documenting a subtle hypodense area in the right lenticular region.
Once arrived to our unit the patient was submitted to CT perfusion examination that revealed the presence of a small ischemic core in the lenticular region, visible as a subtle hypodense area in CT scan (Fig. 1C), within a wider hypoperfused area (penumbra) in the superficial territory of MCA, so she was promptly transported to the angiography room.
Right common carotid artery injection documented a regular dissection of the internal carotid artery beyond the bulb (Fig. 2A, B) with complete vessel occlusion, considered as grade IV of Colorado University classification, that well characterizes severity of the disease, anatomical features and potential complications [13,14].
Fig. 2.
DSA study shows (A) dissective occlusion of right internal carotid artery with (B) stasis in venous phase and (C) ipsilateral middle cerebral artery (tandem) occlusion.
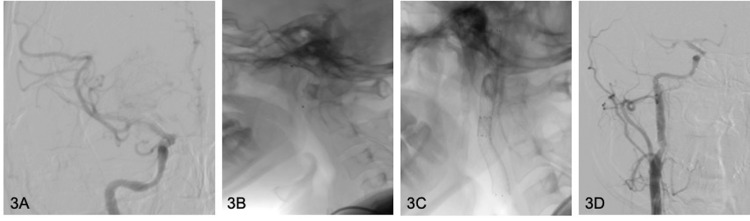
The injection beyond the dissection confirmed ipsilateral M1 occlusion (Fig. 2C). Since the dissected vessel was no easy and safe to navigate, we decided to treat the extra-cranial segment first, so, once systemic heparinization and 500mg iv Flectadol loading were administrated, we proceeded with pta and double overlap stenting (Protegé 7-5 × 40 mm and 8-6 × 40 mm) on Spider FX 6.0 mm monorail system (ev3) (Fig. 3B, C, D). The control angiogram showed antegrade flow and restoration of the normal caliber of the internal carotid artery. Subsequently, right middle cerebral artery was accessed with a 0.060in x 132 mm AXS Catalyst 6 over a 18 Rebar microcatheter and a 3-minutes attempt of mechanical thrombectomy and aspiration was performed, utilizing two 60mL VacLok vacuum pressure syringes through the AXS Catalyst 6 catheter and 8F JR4 Launcher (Medtronic), respectively. The subsequent angiogram documented thrombolysis in cerebral infarction (TICI 3) recanalization (Fig. 3A). Post-procedural DynaCT showed no hemorrhagic complications and the patient regained strength while still on the table.
Fig. 3.
(A) Final DSA control documents TICI III revascularization of right middle cerebral artery. B shows positioning of the endovascular protection system Spider FX before stent releasing. (C, D) Correct opening and positioning of two overlapped carotid stents with consequent restored caliber of right internal carotid artery.
The day after the procedure no neurological symptoms were detected.
1-month follow-up MRI revealed a small malacic area in right nucleo-capsular region as result of ischemic insult (Fig. 4 and 5). At discharge, the modified Rankin scale (mRS) and NIHSS were both 0.
Fig. 4.
Axial DWI and ADC map images, acquired 1 month after the procedure, document malacic area in right caudate region, as result of ischemic insult.
Fig. 5.
Coronal MIP TOF sequence of same exam as Figure 4 documenting regular signal and caliber of intracranial arterial vessels.
Discussion
ICA dissection occurs when a mural hematoma develops in one or more layers of the vase wall through a tear. Hematoma expansion may occur in the direction of the intima causing luminal narrowing, or along the direction of the adventitia, resulting in pseudoaneurysm formation [6]. Most dissections occur spontaneously. The second most common causative factor is head or neck trauma, especially in car accident victims [5,7]
Spontaneous dissections may be preceded by minor trauma: chiropractic manipulation, yoga, coughing, sneezing and whiplash injuries [8]. There is some evidence that antecedent infection predisposes to dissection [9]. In relation to a genetic multifactorial predisposition, connective tissue disease and arteriopathy (particularly, Ehlers– Danlos syndrome) have been reported [10,11]. Traumatic dissections occur after major penetrating or non-penetrating trauma, such as road traffic accidents [12].
The vast majority of strokes associated with internal carotid artery dissection occur as a result of arterial-to-arterial thromboembolism arising from the dissected region, with most affecting the ipsilateral middle cerebral artery territory. Most of these strokes are cortical in nature, but a small proportion of patients develop lacunar infarcts, suggesting micro embolism as a potential mechanism. In contrast, approximately 5% of ischemic strokes associated with carotid dissection occur at border zones between arterial territories, suggesting a hemodynamic mechanism in some cases [15], [16], [17]. These border-zone infarcts may occur as a result of hypoperfusion, resulting from narrowing or occlusion of the dissected vessel, or occlusion of the ostium of a branch of the dissected vessel by the dissection flap [18].
The importance of making a timely diagnosis is paramount to minimize potential morbidity and mortality of the disease.
The diagnosis of dissection is based on radiologic findings in ultrasound, CT, computed tomography angiography (CTA), magnetic resonance imaging (MRI), and magnetic resonance angiography (MRA); however, digital subtraction arteriography (DSA) is still considered the gold standard [7].
The optimal treatment strategy in internal carotid artery dissection remains a subject of debate. Similar to basilar artery occlusion treatment, no official protocols have yet been established [19].
A randomized feasibility study comparing the efficacy and safety of anticoagulation (unfractionated heparin or low-molecular weight heparin followed by warfarin) and antiplatelet agents (one or a combination of aspirin, dipyridamole or clopidogrel) found no significant difference between the 2 approaches [20].
In large strokes, antiplatelet agents may be favored to avoid the risk of hemorrhagic transformation associated with anticoagulation. Likewise, in cases of intracranial arterial dissection, when pseudoaneurysm formation may be complicated by subarachnoid hemorrhage, antiplatelet agents may be preferred. Conversely, anticoagulation may be preferred if a thrombus is detected in the arterial lumen, or if there are multiple embolic infarcts ipsilateral to the dissected vessel [21].
There is no clear consensus regarding surgical management of dissections.
Grade V is considered to be lethal. It is widely accepted that low grade [1] dissections with no bleeding and minor or transient ischemic symptoms can be treated with 3-6 months of antithrombotic therapy with anticoagulant or antiplatelet agents [22]. To our understanding, as the pharmacologic treatment of higher-grade dissections carries a very low success rate, the approach in these cases should be more aggressive that is surgical or endovascular [23], [24]. There are 2 main methods of retrieving a thrombus from an artery: aspiration or using stent retrievers [25], [26].
The RECOST Study [27] analyzed all carotid artery dissection tandem occlusion strokes and isolated anterior circulation occlusions. All patients were selected for endovascular treatment based on clinical radiological mismatch, NIHSS >7 and DWI-ASPECTS >5, within 6 hours of onset. For carotid artery dissection, the revascularization procedure first consisted of distal recanalization by a stent retriever into the intracranial vessel. After evaluation of the circle of Willis, internal carotid artery stent placement was performed only in case of insufficiency. The efficacy, safety and clinical outcome of the carotid artery dissection treatment were compared with the results of the isolated anterior circulation occlusion cohort. Mechanical endovascular treatment of carotid artery dissection and occlusion is safe and effective at occlusion of the isolated anterior circulation.
Conclusion
Mechanical thrombectomy and artery stenting can be beneficial in patients with acute, high grade extracranial carotid artery dissections with artery occlusions and distal embolic complications.
Compliance with Ethical Standards
Guarantor
The scientific guarantor of this publication is A.G.
Statistics and Biometry
One of the authors (F.T.) has significant statistical expertise.
Ethical Approval
Institutional Review Board approval was obtained.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Patient consent: Written informed consent was obtained from all subjects (patient) in this study.
References
- 1.Rennert R.C., Wali A.R., Steinberg J.A., Santiago-Dieppa D.R., Olson S.E., Pannell J.S., et al. Epidemiology, natural history, and clinical presentation of large vessel ischemic stroke. Neurosurgery. 2019;85:S4–S8. doi: 10.1093/neuros/nyz042. Suppl. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandra A., Suliman A., Angle N. Spontaneous dissection of the carotid and vertebral arteries: The 10-year UCSD experience. Ann. Vasc. Surg. 2007;21:178–185. doi: 10.1016/j.avsg.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344:898–906. doi: 10.1056/NEJM200103223441206. [DOI] [PubMed] [Google Scholar]
- 4.Stoker TB, Evans NR, Warburton EA. Internal carotid artery dissection. Br J Hosp Med (Lond) 2016;77(12):708–711. doi: 10.12968/hmed.2016.77.12.708. Dec 2PMID: 27937023. [DOI] [PubMed] [Google Scholar]
- 5.Fusca M.R., Harrigan M.R. Cerebrovascular dissections—A review part I: Spontaneous dissections. Neurosurgery. 2011;68:242–257. doi: 10.1227/NEU.0b013e3182012323. [DOI] [PubMed] [Google Scholar]
- 6.Lee VH, Brown RD, Jr., Mandrekar JN, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67(10):1809–1812. doi: 10.1212/01. wnl.0000244486.30455.71. [DOI] [PubMed] [Google Scholar]
- 7.Mehdi E., Aralasmak A., Toprak H., Yıldız S., Kurtcan S., Kolukisa M., et al. Craniocervical dissections: radiologic findings, pitfalls, mimicking diseases: a pictorial review. Curr. Med. Imaging Rev. 2018;14:207–222. doi: 10.2174/1573405613666170403102235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caso V, Paciaroni M, Bogousslavsky J. Environmental factors and cervical artery dissection. Front Neurol Neurosci. 2005;20:44–53. doi: 10.1159/000088134. [DOI] [PubMed] [Google Scholar]
- 9.Grau AJ, Brandt T, Buggle F, Orberk E, Mytilineos J, Werle E, et al. Association of cervical artery dissection with recent infection. Arch Neurol. 1999;56(7):851–856. doi: 10.1001/archneur.56.7.851. [DOI] [PubMed] [Google Scholar]
- 10.Brandt T, Orberk E, Weber R, Werner I, Busse O, Müller BT, et al. Pathogenesis of cervical artery dissections: association with connective tissue abnormalities. Neurology. 2001;57(1):24–30. doi: 10.1212/wnl.57.1.24. [DOI] [PubMed] [Google Scholar]
- 11.Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009;40(6):e459–e466. doi: 10.1161/strokeaha.108.534669. [DOI] [PubMed] [Google Scholar]
- 12.Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009;8(7):668–678. doi: 10.1016/s1474-4422(09)70084-5. [DOI] [PubMed] [Google Scholar]
- 13.Biffl W.L., Moore E.E., Offner P.J., Brega K.E., Franciose R.J., Burch J.M. Blunt carotid arterial injuries: implications of a new grading scale. J. Trauma Inj. Infect. Crit. Care. 1999;47:845–853. doi: 10.1097/00005373-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Biffl W.L., Moore E.E., Offner P.J., Burch J.M. Blunt carotid and vertebral arterial injuries. World J. Surg. 2001;25:1036–1043. doi: 10.1007/s00268-001-0056-x. [DOI] [PubMed] [Google Scholar]
- 15.Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29(12):2646–2648. doi: 10.1161/01.str.29.12.2646. [DOI] [PubMed] [Google Scholar]
- 16.Molina CA, Alvarez-Sabin J, Schonewille W, Montaner J, Rovira A, Abilleira S, et al. Cerebral microembolism in acute spontaneous internal carotid artery dissection. Neurology. 2000;55(11):1738–1740. doi: 10.1212/wnl.55.11.1738. [DOI] [PubMed] [Google Scholar]
- 17.Benninger DH, Georgiadis D, Kremer C, Studer A, Nedeltchev K, Baumgartner RW. Mechanism of ischemic infarct in spontaneous carotid dissection. Stroke. 2004;35(2):482–485. doi: 10.1161/01.str.0000109766.27393.52. [DOI] [PubMed] [Google Scholar]
- 18.Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci. 2015;2(4) doi: 10.5812/archneurosci.26670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tortora M, Tortora F, Guida A, Buono G, Marseglia M, Tarantino M, et al. Basilar Artery Occlusion (BAO) revascularization after more than 12 hours from the onset of symptoms with excellent outcome: Report of a case. Radiol Case Rep. 2022;17(4):1300–1304. doi: 10.1016/j.radcr.2022.01.064. Feb 17PMID: 35242256; PMCID: PMC8857565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machet A, Fonseca AC, Oppenheim C, Touzé E, Meder JF, Mas JL, et al. Does anticoagulation promote mural hematoma growth or delayed occlusion in spontaneous cervical artery dissections? Cerebrovasc Dis. 2013;35(2):175–181. doi: 10.1159/000346592. [DOI] [PubMed] [Google Scholar]
- 21.Engelter ST, Brandt T, Debette S, Caso V, Lichy C, Pezzini A, et al. Antiplatelets vs anticoagulation in cervical artery dissection. Stroke. 2007;38(9):2605–2611. doi: 10.1161/strokeaha.107.489666. [DOI] [PubMed] [Google Scholar]
- 22.ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Stroke. 2011;42:e420–e463. doi: 10.1161/STR.0b013e3182112d08. [DOI] [PubMed] [Google Scholar]
- 23.Lyrer P., Engelter S.T. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst. Rev. 2010;10 doi: 10.1002/14651858.CD000255.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavallée P.C., Mazighi M., Saint-Maurice J.-P., Meseguer E., Abboud H., Klein I.F., et al. Stent-assisted endovascular thrombolysis vs intravenous thrombolysis in internal carotid artery dissection with tandem internal carotid and middle cerebral artery occlusion. Stroke. 2007;38:2270–2274. doi: 10.1161/STROKEAHA.106.481093. [DOI] [PubMed] [Google Scholar]
- 25.Mourand I., Brunel H., Vendrell J.-F., Thouvenot E., Bonafé A. Endovascular stent-assisted thrombolysis in acute occlusive carotid artery dissection. Neuroradiology. 2010;52:135–140. doi: 10.1007/s00234-009-0597-5. [DOI] [PubMed] [Google Scholar]
- 26.Procházka V., Jonszta T., Czerny D., Krajca J., Roubec M., Hurtikova E., et al. Comparison of mechanical thrombectomy with contact aspiration, stent retriever, and combined procedures in patients with large-vessel occlusion in acute ischemic stroke. Med. Sci. Monit. 2018;24:9342–9353. doi: 10.12659/MSM.913458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costalat V, Lobotesis K, Machi P, Mourand I, Maldonado I, Heroum C, et al. Prognostic factors related to clinical outcome following thrombectomy in ischemic stroke (RECOST study). 50 patients prospective study. Eur J Radiol. 2012;81(12):4075–4082. doi: 10.1016/j.ejrad.2012.07.012. Decdoi: 10.1016/j.ejrad.2012.07.012. Epub 2012 Aug 30. PMID: 22940230. [DOI] [PubMed] [Google Scholar]